1. Introduction
Thyroid cancer is rare in childhood, accounting for
1.5-3.0% of carcinomas in children and adolescents. Nevertheless,
it is the most common malignant neoplasms of the endocrine system
in this age group (1). Globally,
the annual incidence of the disease in children varies between 0.5
and 10.0 cases per 100,000 (2,3).
Notably, data from the National Cancer Institute Surveillance,
Epidemiology, and End Results (SEER) and a North American
population-based study show increases in the incidence in patients
<20 years of age, at a 2.0% ratio per year (4,5).
The two most common histological types of thyroid
cancer are papillary thyroid carcinoma (PTC) and follicular thyroid
carcinoma (FTC), comprising differentiated thyroid cancers (DTCs).
In children and adolescents, DTC is responsible for >95% of
thyroid cancers, with PTC accounting for ~90% of cases (6,7).
The contribution of other histological types, such as medullary,
poorly differentiated or anaplastic thyroid cancer, is minor given
their rarity in the paediatric population (8). Medullary carcinoma is most commonly
diagnosed in the setting of prophylactic thyroidectomies for
carriers of RET mutations in multiple endocrine neoplasia syndromes
(9).
DTC is an indolent neoplasm with low morbidity and
mortality rates. The prognosis in children and adolescents is
excellent, even in cases of advanced disease (6,10,11). A North American population study
conducted between 1992 and 2014 found 20-year survival rates of
99.7 and 96.3% for PTC and FTC, respectively, regardless of the
disease stage (12).
Notwithstanding, a subgroup of patients presents an aggressive
clinical course, with increased morbidity and mortality (13-17). Currently, a significant challenge
in the management of DTC is identifying patients at high risk of
unfavourable outcomes. In this context, the identification of
prognostic factors to improve risk assessment is essential for
proper management.
The present article aims to critically review and
update the current concepts of DTC management in children and
adolescents, with an emphasis on clinical presentation, treatment,
risk assessment, follow-up and future perspectives.
2. Clinical presentation and treatment
The most common clinical presentation of DTC is a
palpable nodule of the thyroid gland (13). It may also be diagnosed due to
cervical adenopathy with or without a detectable thyroid nodule or
as an incidental finding on non-thyroid imaging exams (18). Occasionally, however, the
diagnosis follows the detection of distant metastases, most
frequently in the lungs (19).
PTC usually presents with bilateral (30%) and
multicentric (65%) tumours and cervical lymph node metastases
(20-23). Haematogenous dissemination may
occur in up to 25% of cases but is usually associated with
significant metastases to the cervical region (18,20,24). The most common PTC variants are
classical, solid, follicular and diffuse sclerosing (25). Conversely, FTC usually presents as
a unique tumour and shows increased potential for
haematogenous-related metastases to the lungs and bones in the
initial presentation (21). In
contrast to PTC, cervical lymph node metastases are rare in FTC
(13).
DTC in children is a distinct disease from that
observed in adults, with particularities in the pathophysiology,
clinical presentation and long-term outcomes (10,13). Recently, the American Thyroid
Association (ATA), accounting for these differences, published
specific guidelines for thyroid nodule and DTC for children
(13). DTC clinical presentation
in children is usually more extensive than in adults (10,18). Tumour sizes tend to be larger,
with earlier involvement of the thyroid capsule and adjacent
tissues (26,27). Lymph node involvement is present
in 40-90% of children, compared with 20-50% of adults, and the
prevalence of distant metastases is 20-30% in children, compared
with 2-5% in the adult population (18,28). The most prevalent sites of distant
metastases in children are the lungs, bone and central nervous
system (21). Notably, the same
histological variants, such as the diffuse sclerosing and
follicular variants of PTC, are more common in younger children
(<10 years old) (29).
Children are considerably less likely to die from DTC (≤2%
long-term cause-specific mortality) than adults, which is partially
explained by the differences in the molecular pathology of the
tumour (18,20,21).
The ATA recommendation for initial treatment in
paediatric DTC consists of total thyroidectomy, followed by
radioactive iodine (RAI) and suppressive therapy with levothyroxine
(13). The recommendation of
total thyroidectomy is based on the high incidence of multifocal
and bilateral disease, as well as an increased risk of recurrence
in paediatric patients who undergo subtotal thyroidectomy or
lobectomy (18,23). It should be emphasized that the
two primary contemporary objectives of DTC management in the
paediatric population are to maintain the low specific mortality of
the disease and reduce the potential complications of treatment. A
critical point in this process is an improved understanding of the
clinical characteristics that predict response to these therapies
and identify those who will benefit from more aggressive
treatment.
Paediatric patients with advanced DTC disease also
seem to present a better response to therapy. A systematic review
that evaluated 112 paediatric patients with pulmonary metastasis
observed a complete or partial response to RAI treatment in 47.3
and 38.3%, respectively (30),
contrasting with 44% stable disease in the adult population
(31).
The reported rate of disease-free survival at 10
years of follow-up varies in paediatric advanced DTC from 67-70%
(21,32). Similar results were found in
adults, as shown by two cohorts with 768 and 357 patients with DTC
that demonstrated disease-free survival rates of 67.4 and 71.7%,
respectively (33,34). However, paediatric patients with
persistent disease usually present a more stable course, resulting
in a more favourable progression-free survival (21,30).
3. Risk stratification
Due to the low mortality rates, one of the most
critical steps in the evaluation of children and adolescents with
DTC is risk stratification for persistent/recurrent disease
(35). Several prognostic
factors, such as age extremes, larger tumours, multicentricity,
extrathyroidal extension, lymph node metastasis, vascular invasion
and postoperative thyroglobulin (POTg) levels, are well established
in the adult population (36).
These factors are also used in young patients with DTC. Overall,
these characteristics are split into patient (age and sex) and
tumour-related factors (histological type, size, multifocality,
disease extension, staging, lymph node and distant metastasis and
completeness of initial surgery).
Role of individual prognostic
factors
Several studies have evaluated the association of
patient factors, such as sex and age, and disease outcomes with
conflicting results (23,24,32,37-40). Certain studies found an
association between younger age and the risk of persistent disease
(23,38,40), whereas others have failed to find
such an association (24,32,37,39). Males are more likely to have a
poorer prognosis based on certain studies (24,39,40); however, other studies did not
confirm these findings (23,32,37,38). The majority of studies showed no
association between tumour size, histological type or
extrathyroidal invasion, with a risk of persistent disease
(23,24,37-40), but conflicting results have been
reported on multifocality and tumour staging (24,32,37-40). Nevertheless, the majority of
studies have shown an association between lymph node and distant
metastasis with persistent disease (24,32,37,39). Of note, a study that evaluated
prognostic factors in a population of 65 patients with DTC under
the age of 20 years showed that lymph node and distant metastases
were the only predictors for persistent disease (37). However, Mihailovic et al
(38) observed different results
in a population of 51 patients with DTC of the same age group. They
found that diagnosis at a younger age, less radical primary surgery
and tumour multifocality were also strong predictors for disease
recurrence.
Different risk stratification systems combining
several risk factors have been proposed to predict the outcome of
patients with DTC. In general, these systems aim to estimate
recurrence risk and mortality, guide follow-up and treatment, and
ensure effective communication with patients and diverse
professionals while permitting benchmarking (36,41). However, the current systems have
limitations, particularly for paediatric patients. Factors
affecting disease recurrence/persistence and survival prediction
are distinct. Additionally, these tools have poor performance in
predicting outcomes for patients in the early stages of disease,
considered low risk (primarily stages I and II), which comprise the
majority of patients with DTC (26,42). Moreover, they employ only
information regarding disease presentation, but do not incorporate
response to treatment and have not been validated for several
populations, including paediatric patients (41). As illustrated in Table I and discussed below, there is
conflicting evidence on the performance of these prognostic factors
in children and adolescents (23,24,32,37-40).
 | Table IEvaluation of the association between
prognostic factors and persistent disease in children and
adolescents with differentiated thyroid carcinoma. |
Table I
Evaluation of the association between
prognostic factors and persistent disease in children and
adolescents with differentiated thyroid carcinoma.
| Jarzab et
al, | Wada et
al, | Vaisman et
al, | Mihailovic et
al, | Verburg et
al, | Pires et
al, | Zanella et
al, |
|---|
| Factor | 2000 | 2009 | 2011 | 2014 | 2015 | 2016 | 2018 |
| Patient
Factors | | | | | | | |
| Age | Y | N | N | Y | Y | N | N |
| Sex | N | Y | N | N | Y | Y | N |
| Tumor Factors | | | | | | | |
| Size | NE | N | N | N | NE | NE | Y |
| Multifocality | NE | Y | N | Y | NE | N | N |
| Histological
type | N | N | NE | N | N | N | N |
| Extrathyroidal
invasion | NE | N | NE | NE | NE | N | NE |
| Tumor staging | NE | Y | NE | N | N | NE | Y |
| Lymph node
metastases | N | Y | Y | N | N | Y | Y |
| Distant
metastases | NE | NE | Y | N | Y | Y | Y |
| Treatment
factors | | | | | | | |
| Initial
surgery | Y | NE | NE | Y | NE | NE | NE |
| Post-operative
factors | | | | | | | |
| sPOTg | NE | NE | NE | NE | NE | NE | Y |
| ATA Risk | NE | NE | NE | NE | NE | NE | Y |
| DRS | NE | NE | NE | NE | NE | NE | Y |
TNM/American Joint committee on cancer
(TNM/AJCC)
The TNM/AJCC staging system is the most commonly
used staging system, and is recommended by the ATA DTC paediatric
guidelines (13,36). This system is focused on
predicting mortality. It includes as variables the age of the
patient at diagnosis (stratified around 55 years), the size of the
tumour, and the presence of lymph node and distant metastases.
Adult patients are classified into four stages, with a progressive
decline in survival for stages I, II, III and IV. Due to the age at
which patients are stratified, children and adolescents are
classified only in stages I and II (with or without distant
metastases, respectively), limiting the discriminatory factor in
determining the prognosis for this population. Patients classified
as TNM/AJCC I have a survival rate close to 100% (43).
The primary criticisms of this system are the lack
of inclusion of variables known to influence the evolution and
prognosis of patients such as, histological type/subtype and
treatment-related data, and its inability to predict outcomes other
than mortality (such as recurrences and persistent disease). The
TNM/AJCC is updated periodically, and the 8th edition is the most
recent version (Table II)
(44).
 | Table IIa. TNM staging of DTC. |
Table II
a. TNM staging of DTC.
| Tx | Primary tumor
cannot be assessed |
|---|
| T0 | No evidence of
primary tumor |
| T1a | Tumor ≤1 cm in
greatest dimension limited to thyroid |
| T1b | Tumor >1 cm but
≤2 cm in greatest dimension, limited to thyroid |
| T2 | Tumor >2 cm but
≤4 cm in greatest dimension, limited to thyroid |
| T3a | Tumor >4 cm
limited to thyroid |
| T3b | Gross
extrathyroidal extension invading only strap muscles (sternohyoid,
sternothyroid, thyrohyoid, or omohyoid muscles) from a tumor of any
size |
| T4a | Gross
extrathyroidal extension invading subcutaneous soft tissues,
larynx, trachea, esophagus, or recurrent laryngeal nerve from a
tumor of any size |
| T4b | Gross
extrathyroidal extension invading prevertebral fascia or encasing
carotid artery or mediastinal vessels from a tumor of any size |
| Nx | Regional lymph
nodes cannot be assessed |
| N0a | One or more
cytological or histologically confirmed benign lymph node |
| N0b | No radiologic or
clinical evidence of locoregional lymph node metastasis |
| N1a | Metastasis to level
VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian or
upper mediastinal) lymph nodes |
| N1b | Metastasis to
unilateral, bilateral, or contralateral lateral neck lymph nodes
(levels I, II, III, IV, or V) or retropharyngeal lymph nodes |
| M0 | No distant
metastasis |
| M1 | Distant
metastasis |
| Stage | Age <55
years |
| I | Any T, Any N,
M0 |
| II | Any T, Any N,
M1 |
ATA risk stratification in children and
adolescents with DTC
Since DTC mortality rates in children and
adolescents are very low, systems that capture the likelihood of
relapse or persistent disease in the long-term follow-up are
essential for defining the therapeutic strategies in this
population. The ATA risk stratification incorporates a system that
addresses the risk of persistent cervical disease and identifies
which patients should undergo imaging to assess the presence of
distant metastases (13). In this
system, the patient is categorized into three levels of risk: Low,
intermediate or high (Table
III). However, its value is limited since it only considers
histopathological data and does not consider the response to
therapy.
 | Table IIIa. ATA risk classification in children
and adolescents with DTC. |
Table III
a. ATA risk classification in children
and adolescents with DTC.
| Risk | Definition |
|---|
| Low | Disease grossly
confined to the thyroid with N0/Nx disease or patients with
incidental N1a disease (microscopic metastasis to a small number of
central neck lymph nodes) |
| Intermediate | Extensive N1a or
minimal N1b disease |
| High | Regionally
extensive disease (extensive N1b) or locally invasive disease (T4
tumors), with or without distant metastasis |
Dynamic risk stratification (DRS),
including the response to therapy in predicting disease
outcome
The classification systems based on
clinicopathological features use information from the patient's
initial assessment for categorization of risk, without changes in
this classification over time (13,44). The use of response to initial
treatment has been advocated to estimate the risk of recurrence and
death (45-50). This new modality risk
stratification was termed DRS, based on the observation that a
patient's risk may change over time, according to new data gathered
during follow-ups (45). In this
system, patients are classified into four categories: Excellent,
biochemical incomplete, structural incomplete and indeterminate
response (Table IV) (46,47).
 | Table IVa. Dynamic risk stratification. |
Table IV
a. Dynamic risk stratification.
| Response | Definition |
|---|
| Excellent | Nonstimulated Tg
level <0.2 ng/ml or stimulated Tg level <1 ng/ml and
undetectable TgAc and negative imaging |
| Biochemical
incomplete | Nonstimulated Tg
level >1 ng/ml or stimulated Tg level >10 ng/ml or increasing
TgAc levels and negative imaging |
| Structural
incomplete | Structural or
functional evidence of disease regardless of Tg or TgAc |
| Indeterminate | Nonspecific
findings on imaging studies or faint uptake in thyroid bed on RAI
scanning or nonstimulated Tg level 0.2-1 ng/ml or stimulated Tg
level 1-10 ng/ml or TgAc levels stable or declining in the absence
of structural or functional disease |
The utility of DRS has been shown in several DTC
cohorts (46-48). A study by Vaisman et al
(48) showed that patients with
an excellent response after the initial therapy had a risk of only
1.4% for persistent/recurrent disease (48). Conversely, amongst patients with
persistent structural disease, only 9% were classified as excellent
response, even after several additional therapies.
However, whilst DRS has been validated in the adult
DTC population, the assessment of its role in children and
adolescent management is still limited (49,50). Indeed, the current ATA guidelines
for children with DTC do not suggest the use of DRS for children
(13). Lazar et al
(49) evaluated DRS in a cohort
of 54 patients with a median age at diagnosis of 13.9 years and a
median follow-up of 8.8 years. They found that patients classified
as having an excellent response after the initial treatment
presented a favourable prognosis: 82.9% of them remained classified
as excellent at follow-up. Conversely, all patients with an
incomplete response after the initial therapy remained with
persistent disease. Sung et al (50) recruited a cohort of 77 paediatric
patients with DTC and demonstrated that DRS was useful in
predicting disease outcome at follow-up. When compared to the group
with an excellent response, the risk of persistent/recurrent
disease was significantly higher in patients with an indeterminate
or incomplete structural response. Recently, our group conducted a
multicentre study involving four institutions to evaluate DRS in
children and adolescents (32). A
total of 66 patients with a diagnosis of DTC before 18 years of age
were included. In this study, a multivariate analysis including
tumour size, lymph node and distant metastasis, ATA paediatric risk
stratification and DRS was performed. The results showed that DRS
was the only predictor of persistent/recurrent disease, with odds
ratios (confidence intervals) of 35.2 (3.7-762.5), 54.9
(2.5-3,933.1) and 13.9 (1.1-313.7) for indeterminate, biochemically
persistent and structurally persistent disease, respectively.
Postoperative staging
For the majority of patients, the initial
postoperative evaluation is performed ~3 months after surgery
(13). This assessment aims to
evaluate persistent locoregional disease and identify patients who
may benefit from RAI dosing, such as those with known or suspected
distant metastases (13).
Low-risk patients should undergo thyroglobulin (Tg) measurement
using levothyroxine (Tg-T4) and cervical ultrasound. In turn, in
patients at intermediate and high risk, the addition of stimulated
Tg (sTg) for improved risk stratification and determination of the
need for RAI treatment is useful. Thus, a more individualized and
conservative approach to treatment and postoperative staging can
reduce unnecessary exposure to RAI in children with no evidence of
disease, in whom the risks of routine therapy with RAI probably
outweigh the benefits. Additionally, certain patients will require
additional imaging techniques, such as neck and chest computed
tomography (CT), especially those with detectable Tg-T4. The value
of PET/CT has been poorly studied in this population and is not
routinely recommended for children (13).
Role of stimulated (s)POTg
Serum Tg levels serve as a marker of recurrent
disease, and ultrasensitive serum Tg assays are considered the most
sensitive method for the detection of residual thyroid cancer
(13,51). Measurement of serum Tg levels is
critical for the management of paediatric patients with DTC, both
at the initial postoperative staging and during long-term follow-up
(13). Therefore, monitoring Tg
under levothyroxine therapy (Tg-T4) is the ideal approach to
evaluate disease recurrence or progression (13). Interestingly, the Tg levels may be
higher in children than in adults with a similar extent of disease
(52).
The role of sPOTg as a prognostic factor for DTC in
the paediatric population has been recently addressed by several
studies. The first study included 32 children and adolescents
diagnosed with DTC <18 years old and found that the ideal
cut-off value for the prediction of excellent response was 31.5
ng/ml, with a sensitivity and specificity of 100% (53). Similar results were observed in a
larger sample of 66 young patients: A cut-off of 37.8 ng/ml showed
81% sensitivity and 100% specificity (32). More recently, a Chinese study with
118 paediatric patients (<20 years old) evaluated the prognostic
factor of pre-ablation sPOTg and found that the ideal cut-off to
predict disease-free status was 17.8 ng/ml, with a negative
predictive value of 96.8% (54).
Anti-thyroglobulin antibodies (TgAc)
TgAc are present in ~25% of patients with DTC, and
their positivity may determine laboratory interference with Tg
measurement (55). As the
concentrations of TgAc respond to changes in circulating Tg antigen
levels and thus indirectly represent changes in thyroid tissue
mass, TgAc levels may serve as a surrogate tumour marker for DTC
(13,55). As a result, it is recommended to
evaluate TgAc levels in all patients with DTC during their
follow-up (56).
Most studies in adult populations have reported that
recurrence, persistence, or a rising trend in postoperative TgAc
concentrations are significant risk factors for persistent or
recurrent disease (57). However,
it is not known whether a positive TgAc value correlates with
disease extension/invasiveness or prognosis (58). A decline in TgAc levels suggests a
decreasing disease burden, considering an average of 3 years to
eliminate TgAc after cure of DTC (59). A significant increase in TgAc may
indicate disease progression, and this should be assessed in more
detail. Similar to Tg measurements, the trend in TgAc
concentrations is more relevant for disease detection than a single
determination (58).
4. Perspectives: Precision medicine
Several genetic markers have been proposed as
prognostic factors for children and adolescents with PTC (60,61). These advances in the molecular
profile of paediatric DTC may help with individualized management,
potentially improving diagnosis and treatment (62,63).
Genetic alterations in effectors of the
mitogen-activated protein kinase signalling pathway (MAPK) are the
most well associated with the development and aggressiveness of DTC
(63). The intracellular MAPK
signalling pathway serves a central role in cell growth, division,
proliferation, differentiation and apoptosis. Data from The Cancer
Genome Atlas Research showed that the most frequent genes involved
in DTC pathogenesis were, in descending order, BRAF, RAS, RET/PTC
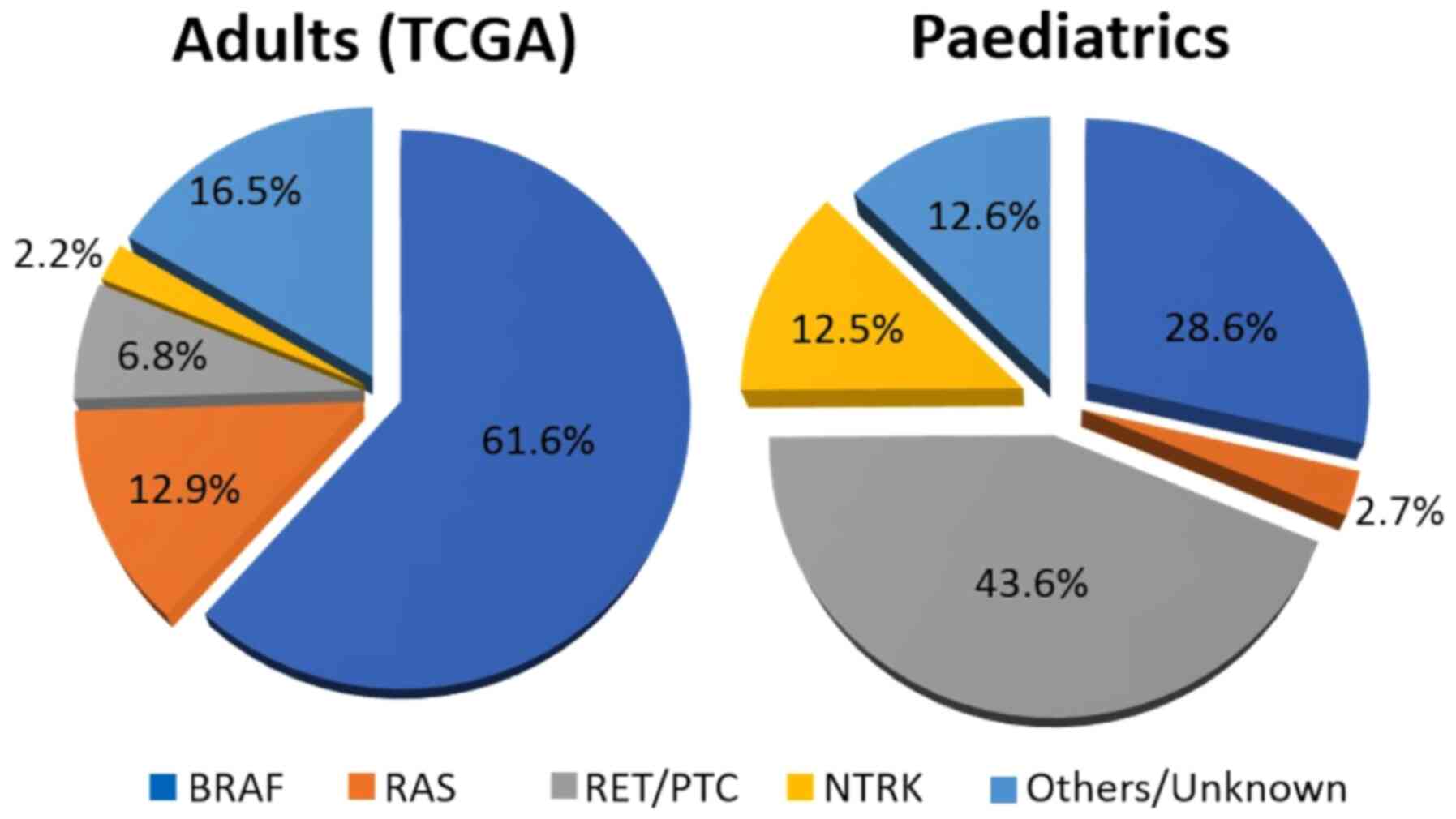
and neurotrophic tyrosine kinase type (NTRK) (Fig. 1) (64). Nevertheless, it should be noted
that the study included nearly 500 patients with DTC, but only nine
were under the age of 20 at diagnosis. The results from small
cohorts of children and adolescents show that the prevalence of
mutations in this population differs from that observed in adults
(Fig. 1) (65-88). Differences in the molecular tumour
profile may be one of the reasons for an improved response to RAI
in children with PTC. This may also partially explain their low
mortality rates and rare progression to undifferentiated tumours.
However, studies on this matter have shown conflicting results
regarding the prevalence of genetic mutations, and their role as
prognostic factors for paediatric DTC remains uncertain (Table V) (65-88).
 | Table VStudies evaluating DTC pediatric
mutations, prevalence and outcomes. |
Table V
Studies evaluating DTC pediatric
mutations, prevalence and outcomes.
| Author, year | Country | N | Mutation, %
| Outcome |
|---|
| RAS | RET/PTC | BRAF | NTRK |
|---|
| Nikiforov et
al, 1997 | USA | 38a/23 | - | 77a/65 | - | - | NE |
| Fenton et
al, 1999 | USA | 31 | 6.5 | - | - | - | NA |
| Fenton et
al, 2000 | USA | 33 | - | 45 | - | - | NA |
| Kumagai et
al, 2004 | Japan | 15a/31 | 0a/0 | 15a/31 | 0a/3.2 | - | NA |
| Penko et al,
2005 | USA | 14 | 0 | 58 | 0 | - | NE |
| Nikiforova et
al, 2005 | Ukraine | 34 | - | 71a | 0a | - | NE |
| Rosenbaum et
al, 2005 | USA | 20 | - | - | 20 | - | NA |
| Sassolas et
al, 2012 | France | 27 | 3.7 | 29.6 | 7.4 | - | NA |
| Ricarte-Filho et
al, 2013 | Sweden | 26a/27 | 0a/7.4 | 57.6a/25.9 | 0a/25.9 | 11.5a/7,4 | NE |
| Henke et al,
2014 | USA | 27 | - | - | 63 | - | NA |
| Givens et
al, 2014 | USA | 19 | - | - | 36.8 | - | NA |
| Prasad et
al, 2016 | USA | 27 | 0 | 22 | 48 | 26 | NTRK-disease
extension; aggressive histology |
| Alzahrani et
al, 2016 | Saudi Arabia | 53 | - | - | 22.6 | - |
Persistent/recurrent disease |
| Nikita et
al, 2016 | USA | 28 | 3.6 | 21.4 | 32.1 | - | BRAF-young
patients |
| Onder et al,
2016 | Turkey | 50 | - | - | 30 | - | Local
Recurrence/DFS |
| Gertz et al,
2016 | USA | 13 | 0 | 15 | 31 | - | NA |
| Ballester et
al, 2016 | USA | 25 | 0 | 24 | 40 | 3.7 | NE |
| Picarsic et
al, 2016 | USA | 18 | 16.5 | 16.6 | 16.6 | 22.6 | NTRK-aggressive
histology |
| Cordioli et
al, 2017 | Brazil | 35 | 0 | 37 | 9 | 9 | RET-size;
multifocality
BRAF-size |
| Geng et al,
2017 | China | 48 | - | - | 35.4 | - | Age <10 years;
multifocality; disease extension |
| Poyrazoglu et
al, 2017 | Turkey | 56 | - | - | 25 | - | NA |
| Hardee et
al, 2017 | USA | 50 | - | - | 48 | - | NA |
| Alzahrani et
al, 2017 | Saudi Arabia | 79 | 2.5 | - | 26.4 | - | NA |
| Wasserman et
al, 2018 | Canada | 30 | - | 23 | 16 | - | NE |
RET PTC
The proto-oncogene RET, located on chromosome
10q11.2, encodes a tyrosine kinase receptor (89,90). At least 12 types of RET/PTC
rearrangements have been described, with types 1 and 3 being the
most common (89,90). In the paediatric population,
RET/PTC mutations are the most common type of mutations, ranging
from 15-77% based on different studies (65, 67-70,72,73,76,78,80-83,88).
Several studies have examined the role of RET/PTC as
a prognostic factor in paediatric DTC patients. Whilst the majority
of studies have failed to demonstrate an association (67,68,72,76,78,80,82), a recent Brazilian study reported
an association between RET/PTC3, larger tumour size and
multifocality (83).
BRAF
BRAF kinase, whose encoding gene is located on
chromosome 7, is the most potent activator of the MAPK pathway
(89,90). Over 40 mutations of the BRAF gene
have been identified, with the T1799A mutation being the most
common (89,90). This missense mutation, due to a
somatic transversion of thymine to adenine at position 1,799 in
exon 15, results in the substitution of a valine amino acid for
glutamic acid at position 600 (BRAFV600E). In children and
adolescents, this is the second most prevalent mutation, found in
~28% of cases, with prevalence ranging from 0-68% (68-88).
The association of the BRAFV600E mutation with
disease outcome in paediatric patients is still controversial.
Alzahrani et al (77)
evaluated 55 children and adolescents with DTC and found that
persistent/recurrent thyroid cancer was more prevalent in patients
with the BRAFV600E mutation (66.7 vs. 34.1%) and more pronounced in
patients with classic PTC (77.8% vs. 33.3%). Onder et al
(79) observed that the classic
architecture with multicentricity and local recurrence was
correlated with BRAFV600E mutation (79). In contrast, several studies found
no association between BRAFV600E mutation and disease prognosis
(68,71,72,74-76,80,82,85-87).
NTRK
The NTRK1 receptor gene, located on chromosome 1,
encodes the high-affinity nerve growth factor receptor and is
activated via the MAPK pathway (73). ETV6-NTRK3 is the result of an
interchromosomal translocation (12; 15) (p13; q25) that juxtaposes
exons 1-4 of ETV6 to exons 12-18 of NTRK3 (73). This gene has recently been studied
and is gaining importance due to its high prevalence (~12%, ranging
from 7-26%) being the third most common in the paediatric
population. Moreover, studies have shown an association between
NTRK fusions and worse clinical outcomes (73,76,81-83). Compared with BRAF mutations, the
presence of fusion genes has been associated with larger tumours
(2.2 vs. 1.5 cm), aggressive histology (84% vs. 0%), and lymph
vascular invasion (92.3% vs. 46.1%) (76).
RAS
RAS genes encode highly related G proteins, which
serve a central role in intracellular signal transduction by
activating the MAPK and other signalling pathways, such as PI3K/AKT
(89,90). Amino acid modifying mutations of
RAS generally occur at codons 12, 13 or 61 of H-RAS, K-RAS or N-RAS
proteins (89,90).
Mutations in the RAS gene were the first studied in
the DTC paediatric population. RAS mutations are much less
prevalent in paediatric patients than in the adult population, with
an estimated rate of 2.7% (prevalence range, 0-16%) (66,68,69,72,73,76,78,80-83,87). No associations have been reported
between RAS mutations and disease presentation in paediatric DTC
(66,72,78,82,87).
Targeted therapy
Despite the excellent prognosis of DTC in paediatric
patients, a small subset of this population may show progressive
and RAI refractory disease (13-17). In such a case, systemic therapy
should be considered (13).
Identifying tumour molecular profiles may be critical in selecting
the most appropriate therapy (Fig.
2) (91). Of note, the
majority of the current knowledge of targeted kinase inhibitors in
this population is based on case reports and anecdotal clinical
experience (8,13). In addition, long-term side effects
in the paediatric age group are unknown (91). Two drugs have been approved by the
Food and Drug Administration (FDA) for DCT refractory disease,
namely, sorafenib and levantinib, although several other drugs are
in clinical trials (8,92,93). Anti-neoplastic therapy in children
should be performed in centres experienced with the use of these
therapeutic agents in paediatric patients (13).
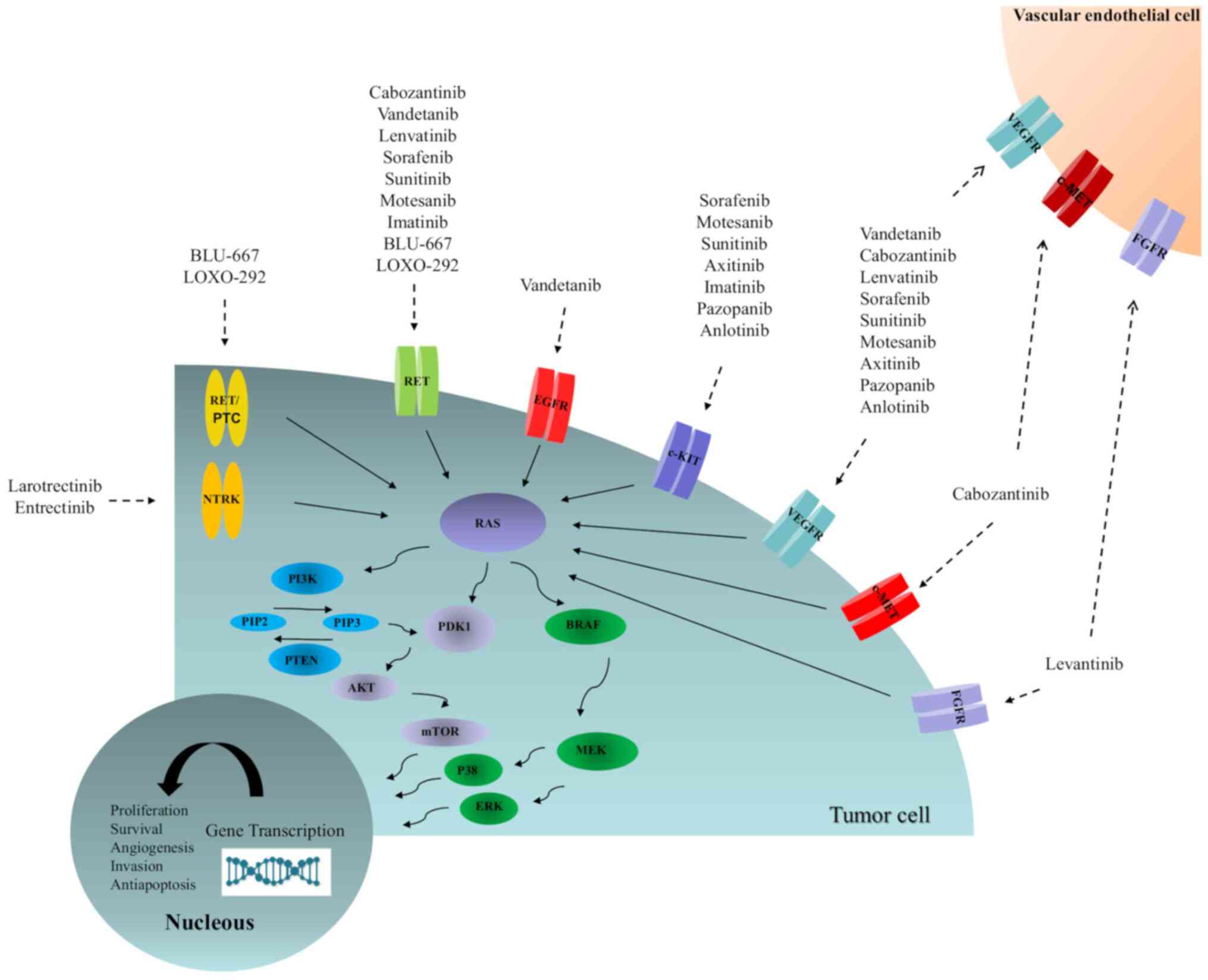 | Figure 2Schematic of the activated pathways
responsible for the proliferation and progression of thyroid
cancer, as well as molecular targeted-related compounds. AKT, v-akt
murine thymoma viral oncogene homologue; BRAF,
serine/threonine-protein kinase B-Raf; c-KIT, tyrosine-protein
kinase Kit; c-MET, hepatocyte growth factor; EGFR, epidermal growth
factor receptor; ERK, extracellular signal-regulated kinase; FGFR,
fibroblast growth factor receptor; MEK, mitogen-activated protein
kinase kinase; mTOR, mammalian target of rapamycin; p38,
mitogen-activated protein kinase; PDK1, pyruvate dehydrogenase
kinase isozyme 1; PI3K, phosphatidylinositol-3 kinase; PIP2,
phosphatidylinositol (4,5) biphosphate; PIP3,
phosphatidylinositol 3,4,5-triphosphate; PTEN, phosphatase and
tensin homologue; NTRK, neurotrophic tyrosine kinase; RAS, rat
sarcoma viral oncogene homologue; RET, rearranged during
transfection; RET/PTC, rearranged during transfection/papillary
thyroid cancer; VEGFR, vascular endothelial growth factor
receptor. |
Sorafenib therapy has been reported in three
patients with lung metastatic and progressive disease (14-16). The first case was a 14-year-old
girl who experienced a significant reduction in lung metastasis
after 2 months of sorafenib therapy, although with side effects,
such as cutaneous toxicity and neutropenia (14). The second patient was an
8-year-old boy with hypoxaemia and a need for mechanical
ventilation (15). The patient
was weaned off mechanical ventilation, and CT showed regression of
the pulmonary metastasis after 2 months of therapy. The third case
was an 11-year-old boy who showed stable disease after 24 months of
sorafenib use (16).
Treatment with levantinib has been used in a small
series of paediatric patients with extensive bilateral metastatic
pulmonary disease, including one patient who previously used
sorafenib (17). All three
patients had respiratory distress requiring oxygen therapy. After a
few weeks of treatment with levantinib, all patients were
successfully weaned off oxygen. The drug was well tolerated, and
proteinuria was the only major adverse effect. Two patients had
stable disease at 11 and 23 months after the initiation of
levantinib. The third patient switched treatment to a
tumour-specific target.
More recently, larotrectinib, a highly selective
inhibitor of tropomyosin receptor kinase, was approved by the FDA
for patients with solid tumours harbouring NTRK fusions in adult
and paediatric populations (94).
The drug was tested in a phase I/II clinical trial, which included
24 children with solid tumours and two with PTC. These two patients
showed stable disease for >7 months of follow-up (94,95). In another phase II study, 55
adolescent and adult patients were included, with five diagnosed
with thyroid cancer. Of these, four patients presented with a
partial response, and one showed complete response (94).
5. Conclusions
In conclusion, the incidence of DTC has been
increasing in recent years. The disease has an excellent prognosis
in the paediatric population, despite a more aggressive clinical
presentation than in adults. Nevertheless, a few patients will
present progressive disease and require closer attention and
additional therapy. Early identification of patients at high risk
is a fundamental step in the therapeutic strategy. The risk
stratification systems TNM, ATA and DRS are particularly useful in
this regard. The advent of molecular markers may offer additional
help in individualizing management. Preliminary reports of targeted
therapy in paediatric patients with DTC with progressive disease
have shown encouraging results, but appropriate clinical trials are
still necessary.
Availability of data and materials
Not applicable.
Author's contributions
ABZ, RSS, LW, JMD, and ALM contributed to the
conception of the subject of the review and writing the manuscript.
ABZ was responsible for the literature review. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competition interests
The authors declare that they have no competing
interests.
Acknowledgments
We would like to thank Dr Alceu Migliavacca, Dr
José Ricardo Guimarães and Dr Diego Mossmann surgeons at the
Hospital de Clínicas de Porto Alegre, for the surgical management
of the patients.
Funding
This work was supported by funding from Conselho Nacional de
Desenvolvimento Científico e Tecnológico (grant no.
CNPq-150270/2019-4), Fundo de Incentivo a Pesquisa (FIPE-2020-0108)
and Programa de Apoio a Núcleos de Excelência (PRONEX)/Fundação de
Amparo à Pesquisa do Estado do Rio Grande do Sul
(FAPERGS-16/2551-0000486-2).
References
|
1
|
Enemoto Y, Enomoto K, Uchino S, Shibuya H,
Watanabe S and Noguchi S: Clinical features, treatment and
long-term outcome of papillary thyroid cancer in children and
adolescents without radiation exposure. World J Surg. 36:1241–1246.
2012. View Article : Google Scholar
|
|
2
|
Bleyer A, Leary O, Barr M and Ries LAG:
Cancer epidemiology in older adolescents and young adults 15 to 29
years of age, including SEER incidence and survival 1975-2000.
National Cancer Institute; Bethesda, MD: (NIH Pub No. 06-5767).
2006
|
|
3
|
Ministry of Health, National Cancer
Institute, Brazilian Society of Pediatric Oncology: Childhood and
adolescents cancer in Brazil: Data from mortality and
population-based registries. https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//childhood-adolescent-cancer-2009.pdf.
|
|
4
|
Holmes L, Hossain J and Opara F: Pediatric
thyroid carcinoma incidence and temporal trends in the USA
(1973-2007): Race or shifting diagnostic paradigm? ISRN Oncol.
2012:9061972012.PubMed/NCBI
|
|
5
|
Golpanian S, Perez EA, Tashiro J, Lew JI,
Sola JE and Hogan AR: Pediatric papillary thyroid carcinoma:
Outcomes and survival predictors in 2504 patients. Pedriatr Surg
Int. 32:201–208. 2016. View Article : Google Scholar
|
|
6
|
Vaisman F, Corbo R and Vaisman M: Thyroid
carcinoma in children and adolescents-systematic review of the
literature. J Thyroid Res. 845362:2011.
|
|
7
|
Park S, Jeong JS, Ryu HR, Lee CR, Park JH,
Kang SW, Jeong JJ, Nam KH, Chung KY and Park CS: Differentiated
thyroid carcinoma of children and adolescents: 27-year experience
in the Yonsei University Health System. J Korean Med Sci.
28:693–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paulson VA, Rudzinski ER and Hawkins DS:
Thyroid cancer in the pediatric population. Genes (Basel).
10:7232019. View Article : Google Scholar
|
|
9
|
Ceolin L, Duval MADS, Benini AF, Ferreira
CV and Maia AL: Medullary thyroid carcinoma beyond surgery:
Advances, challenges, and perspectives. Endocr Relat Cancer.
26:R499–R518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Markovina S, Grigsby PW, Schwarz JK,
DeWees T, Moley JF, Siegel BA and Perkins SM: Treatment approach,
surveillance, and outcome of well-differentiated thyroid cancer in
childhood and adolescence. Thyroid. 24:1121–1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tuttle RM, Vaisman F and Tronko MD:
Clinical presentation and clinical outcomes in Chernobyl-related
pediatric thyroid cancers: What do we know now? What can we expect
in the future? Clin Oncol (R Coll Radiol). 23:268–275. 2011.
View Article : Google Scholar
|
|
12
|
Massimino M, Evens DB, Podda M, Spinelli
C, Collini P, Pizzi N and Bleyer A: Thyroid cancer in adolescents
and young adults. Pediatr Blood Cancer. 65:e270252018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Francis GL, Waguespack SG, Bauer AJ,
Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID,
Luster M, et al: Management guidelines for children with thyroid
nodules and differentiated thyroid cancer. Thyroid. 25:716–759.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waguespack SG, Sherman SI, Williams MD,
Clayman GL and Herzog CE: The successful use of Sorafenib to treat
pediatric papillary thyroid carcinoma. Thyroid. 19:407–412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iyer P, Mayer JLR and Ewig JM: Response to
Sorafenib in a pediatric patient with papillary thyroid carcinoma
with diffuse nodular pulmonary disease requiring mechanical
ventilation. Thyroid. 24:169–174. 2014. View Article : Google Scholar
|
|
16
|
Higuchi Y, Motoky T, Ishida H, Kanamitsu
K, Washio K, Oyama T, Noda T, Tsurumaru Y, Okada A, Tsukahara H and
Shimada A: Sorafenib treatment for papillary thyroid carcinoma with
diffuse lung metastases in a child with autism spectrum disorder: A
Case Report. BMC Cancer. 17:7752017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahajan P, Dawrant J, Kheradpour A,
Quintanilla NM, Lopez ME, Orth RC, Athanassaki I and Venkatramani
R: Response to Lenvatinib in children with papillary thyroid
carcinoma. Thyroid. 28:1450–1454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Welch Dinauer CA, Tuttle RM, Robie DK,
McClellan DR, Svec RL, Adair C and Francis GL: Clinical features
associated with metastasis and recurrence of differentiated thyroid
cancer in children, adolescents and young adults. Clin Endocrinol
(Oxf). 49:619–628. 1998. View Article : Google Scholar
|
|
19
|
Vassilopoulou-Sellin R, Klein MJ, Smith
TH, Samaan NA, Frankenthaler RA, Goepfert H, Cangir A and Haynie
TP: Pulmonary metastases in children and young adults with
differentiated thyroid cancer. Cancer. 71:1348–1352. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demidchik YE, Demidchik EP, Reiners C,
Biko J, Mine M, Saenko VA and Yamashita S: Comprehensive clinical
assessment of 740 cases of surgically treated thyroid cancer in
children of Belarus. Ann Surg. 243:525–532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newman KD, Black T, Heller G, Azizkhan RG,
Holcomb GW III, Sklar C, Vlamis V, Haase GM and La Quaglia MP:
Differentiated thyroid cancer: Determinants of disease progression
in patients <21 years of age at diagnosis: A report from the
surgical discipline committee of the Children's cancer group. Ann
Surg. 227:533–541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wada N, Sugino K, Mimura T, Nagahama M,
Kitagawa W, Shibuya H, Ohkuwa K, Nakayama H, Hirakawa S, Rino Y, et
al: Pediatric differentiated thyroid carcinoma in stage I: Risk
factor analysis for disease free survival. BMC Cancer. 9:3062009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jarzab B, Handkiewicz Junak D, Wloch J,
Kalemba B, Roskosz J, Kukulska A and Puch Z: Multivariate analysis
of prognostic factors for differentiated thyroid carcinoma in
children. Eur J Nucl Med. 27:833–841. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wada N, Sugino K, Mimura T, Nagahama M,
Kitagawa W, Shibuya H, Ohkuwa K, Nakayama H, Hirakawa S, Yukawa N,
et al: Treatment strategy of papillary thyroid carcinoma in
children and adolescents: Clinical significance of the initial
nodal manifestation. Ann Surg Oncol. 16:3442–3449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koo JS, Hong S and Park CS: Diffuse
sclerosing variant is a major subtype of papillary thyroid
carcinoma in the young. Thyroid. 19:1225–1231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugg SL, Ezzat S, Rosen IB, Freeman JL and
Asa SL: Distinct multiple RET/PTC gene rearrangements in multifocal
papillary thyroid neoplasia. J Clin Endocrinol Metab. 83:4116–4122.
1998.PubMed/NCBI
|
|
28
|
Zaydfudim V, Feurer ID, Griffin MR and
Phay JE: The impact of lymph node involvement on survival in
patients with papillary and follicular thyroid carcinoma. Surgery.
144:1070–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leboulleux S, Baudin E, Hartl DW, Travagli
JP and Schlumberger M: Follicular cell-derived thyroid cancer in
children. Horm Res. 63:145–151. 2005.PubMed/NCBI
|
|
30
|
Pawelczak M, David R, Franklin B, Kessler
M, Lam L and Shah B: Outcomes of children and adolescents with
well-differentiated thyroid carcinoma and pulmonary metastases
following 131I Treatment: A systematic review. Thyroid.
20:1095–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabra MM, Grewal RK, Tala H, Larson SM and
Tuttle RM: Clinical outcomes following empiric radioiodine therapy
in patients with structurally identifiable metastatic follicular
cell-derived thyroid carcinoma with negative diagnostic but
positive post-therapy 131I whole-body scans. Thyroid. 22:877–883.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zanella AB, Scheffel RS, Nava CF, Golbert
L, Meyer ELS, Punales M, Gonçalves I, Dora JM and Maia AL: Dynamic
risk stratification in the follow-up of children and adolescents
with differentiated thyroid cancer. Thyroid. 28:1285–1292. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scheffel RS, Zanella AB, Antunes D, Dora
JM and Maia AL: Low recurrence rates in a cohort of differentiated
thyroid carcinoma patients: A referral center experience. Thyroid.
25:883–889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park S, Kim WG, Song E, Oh HS, Kim M, Kwon
H, Jeon MJ, Kim TY, Shong YK and Kim WB: Dynamic risk
stratification for predicting recurrence in patients with
differentiated thyroid cancer treated without radioactive iodine
remnant ablation therapy. Thyroid. 27:524–530. 2017. View Article : Google Scholar
|
|
35
|
Krajewska J, Jarzab M, Czarniecka A,
Roskosz J, Kukulska A, Handkiewicz-Junak D, Puch Z, Wygoda Z,
Paliczka-Cieślik E, Kropińska A, et al: Ongoing risk stratification
for differentiated thyroid cancer (DTC)-stimulated serum
thyroglobulin (Tg) before radioiodine (RAI) ablation, the most
potent risk factor of cancer recurrence in M0 patients. Endokrynol
Pol. 67:2–11. 2016. View Article : Google Scholar
|
|
36
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association Management
Guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroi. Thyroid.
26:1–133. 2016. View Article : Google Scholar :
|
|
37
|
Vaisman F, Bulzico D, Pessoa CHCN,
Bordallo MA, Mendonça UB, Dias FL, Coeli CM, Corbo R and Vaisman M:
Prognostic factors of a good response to initial therapy in
children and adolescents with differentiated thyroid cancer.
Clinics (Sao Paulo). 66:281–286. 2011. View Article : Google Scholar
|
|
38
|
Mihailovic J, Nikoletic K and Srbovan D:
Recurrent disease in juvenile differentiated thyroid carcinoma:
Prognostic factors, treatments, and outcomes. J Nucl Med.
55:710–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pires BP, Alves PA, Bordallo MA, Bulzico
DA, Lopes FPPL, Farias T, Dias F, Lima RA, Gisler ICS, Coeli CM, et
al: Prognostic factors for early and long-term remission in
pediatric differentiated thyroid cancer: The role of sex, age,
clinical presentation and the newly proposed American Thyroid
Association risk stratification system. Thyroid. 26:1480–1487.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Verburg FA, Mäder U, Luster M, Hänscheid H
and Reiners C: Determinants of successful ablation and complete
remission after total thyroidectomy and I131 therapy of pediatric
differentiated thyroid cancer. Eur J Nucl Med Mol Imaging.
42:1390–1398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rosário PW, Ward LS, Carvalho GA, Graf H,
Maciel RM, Maciel LM, Maia AL and Vaisman M; Sociedade Brasileira
de Endocrinologia e Metabologia: Thyroid nodules and differentiated
thyroid cancer: Update on the Brazilian consensus. Arq Bras
Endocrinol Metabol. 57:240–264. 2013.In En, Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hannequin P, Liehn JC and Delisle MJ:
Multifactorial analysis of survival in thyroid cancer. Pitfalls of
applying the results of published studies to another population.
Cancer. 58:1749–175. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
DeGroot LJ, Kaplan EL, Straus FH and
Shukla MS: Does the method of management of papillary thyroid
carcinoma make a difference in outcome? World J Surg. 18:123–130.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tuttle RM, Morris LF, Haugen B, et al:
Thyroid-Differentiated and Anaplastic Carcinoma (Chapter 73). AJCC
Cancer Staging Manual. Amin MB, Edge S, Greene F, et al: 8th
edition. Springer International Publishing; New York, NY: 2017,
View Article : Google Scholar
|
|
45
|
Tala H and Tuttle RM: Contemporary
post-surgical management of differentiated thyroid carcinoma. Clin
Oncol (R Coll Radiol). 22:419–429. 2010. View Article : Google Scholar
|
|
46
|
Tuttle RM, Tala H, Shah J, Leboeuf R,
Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA and Shaha A:
Estimating risk of recurrence in differentiated thyroid cancer
after total thyroidectomy and radioactive iodine remnant ablation:
Using response to therapy variables to modify the initial estimates
predicted by the new American Thyroid Association Staging System.
Thyroid. 20:1341–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Momesso DP and Tuttle RM: Update on
differentiated thyroid cancer staging. Endocrinol Metab Clin North
Am. 43:401–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vaisman F, Momesso D, Bulzico DA, Pessoa
CH, Dias F, Corbo R, Vaisman M and Tuttle RM: Spontaneous remission
in thyroid cancer patients after biochemical incomplete response to
initial therapy. Clin Endocrinol (Oxf). 77:132–138. 2012.
View Article : Google Scholar
|
|
49
|
Lazar L, Lebhental Y, Segal K, Steinmetz
A, Strenov Y, Cohen M, Yaniv I, Yackobovitch-Gavan M and Phillip M:
Pediatric thyroid cancer: Post-operative classifications and
response-to-initial-therapy as prognostic factors. J Clin
Endocrinol Metab. 101:1970–1979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sung TY, Jeon MJ, Lee YH, Lee YM, Kwon H,
Yoon JH, Chung KW, Kim WG, Song DE and Hong SJ: Initial and dynamic
risk stratification of pediatric patients with differentiated
thyroid cancer. J Clin Endocrinol Metab. 102:793–800. 2017.
|
|
51
|
Mazzaferri EL, Robbins RJ, Spencer CA,
Braverman LE, Pacini F, Wartofsky L, Haugen BR, Sherman SI, Cooper
DS, Braunstein GD, et al: A consensus report of the role of serum
thyroglobulin as a monitoring method for low-risk patients with
papillary thyroid carcinoma. J Clin Endocrinol Metab. 88:1433–1441.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hanscheid H, Verburg FA, Biko J, Diessl S,
Demidchik YE, Drozd V and Reiners C: Success of the post-operative
131I therapy in young Belarusian patients with differentiated
thyroid cancer after Chernobyl depends on the radiation absorbed
dose to the blood and the thyroglobulin level. Eur J Nucl Med Mol
Imaging. 38:1296–1302. 2011. View Article : Google Scholar
|
|
53
|
Zanella A, Scheffel RS, Pasa MW, Dora JM
and Maia AL: Role of post-operative stimulated thyroglobulin as
prognostic factor for differentiated thyroid cancer in children and
adolescents. Thyroid. 27:787–792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu L, Zhang X, Tian T, Huang R and Liu B:
Prognostic value of pre-ablation stimulated thyroglobulin in
children and adolescents with differentiated thyroid cancer.
Thyroid. 30:1017–1024. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Verburg FA, Luster M, Cupini C, Chiovato
L, Duntas L, Elisei R, Feldt-Rasmussen U, Rimmele H, Seregni E,
Smit JW, et al: Implications of thyroglobulin antibody positivity
in patients with differentiated thyroid cancer: A clinical position
statement. Thyroid. 23:1211–1225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Spencer CA: Clinical review: Clinical
utility of thyroglobulin antibody (TgAb) measurements for patients
with differentiated thyroid cancers (DTC)). J Clin Endocrinol
Metab. 96:3615–3627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Spencer CA, Takeuchi M, Kazarosyan M, Wang
CC, Guttler RB, Singer PA, Fatemi S, LoPresti JD and Nicoloff JT:
Serum thyroglobulin autoantibodies: Prevalence, influence on serum
thyroglobulin measurement, and prognostic significance in patients
with differentiated thyroid carcinoma. J Clin Endocrinol Metab.
83:1121–1127. 1998.PubMed/NCBI
|
|
58
|
Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY,
Kim JM, Ryu JS, Gong G, Hong SJ and Shong YK: Change of serum
antithyroglobulin antibody levels is useful for prediction of
clinical recurrence in thyroglobulin-negative patients with
differentiated thyroid carcinoma. J Clin Endocrinol Metab.
93:4683–4689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chiovato L, Latrofa F, Braverman LE,
Pacini F, Capezzone M, Masserini L, Grasso L and Pinchera A:
Disappearance of humoral thyroid autoimmunity after complete
removal of thyroid antigens. Ann Intern Med. 139:346–351. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tufano RP, Teixeira GV, Bishop J, Carson
KA and Xing M: BRAF mutation in papillary thyroid cancer and its
value in tailoring initial treatment: A systematic review and
meta-analysis. Medicine (Baltimore). 91:274–286. 2012. View Article : Google Scholar
|
|
62
|
Bauer AJ: Molecular genetics of thyroid
cancer in children and adolescents. Endocrinol Metab Clin North Am.
46:389–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hsiao SJ and Nikiforov YE: Molecular
approach to thyroid cancer diagnosis. Endocr Relat Cancer.
21:T301–T313. 2014.PubMed/NCBI
|
|
64
|
Cancer Genome Atlas Research Network:
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nikiforov YE, Rowland JM, Bove KE,
Monforte-Munoz H and Fagin JA: Distinct pattern of RET oncogene
rearrangements in morphological variants of radiation-induced and
sporadic thyroid papillary carcinomas in children. Cancer Res.
57:1690–1694. 1997.PubMed/NCBI
|
|
66
|
Fenton C, Anderson J, Lukes Y, Dinauer CA,
Tuttle RM and Francis GL: Ras mutations are uncommon in sporadic
thyroid cancer in children and Young adults. J Endocrinol Invest.
22:781–789. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fenton C, Lukes Y, Nicholson D, Dinauer
CA, Francis GL and Tuttle RM: The RET/PTC mutations are common in
sporadic papillary thyroid carcinoma of Children and young adults.
J Clin Endocrinol Metab. 85:1170–1175. 2000.PubMed/NCBI
|
|
68
|
Kumagai A, Namba H, Saenko VA, Ashizawa K,
Ohtsuru A, Ito M, Ishikawa N, Sugino K, Ito K, Jeremiah S, et al:
Low frequency of BRAF T1796A mutation in childhood thyroid
carcinomas. J Clin Endocrinol Metab. 89:4280–4284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Penko K, Livezey J, Fenton C, Patel A,
Nicholson D, Flora M, Oakley K, Tuttle RM and Francis G: BRAF
mutations are uncommon in papillary thyroid cancer of Young
patients. Thyroid. 15:320–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nikiforova MN, Ciampi R, Salvatore G,
Santoro M, Gandhi M, Knauf JA, Thomas GA, Jeremiah S, Bogdanova TI,
Tronko MD, et al: Low prevalence of BRAF mutations in
radiation-induced thyroid tumors in contrast to sporadic papillary
carcinomas. Cancer Lett. 209:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rosenbaum E, Hosler G, Zahurak M, Cohen Y,
Sidransky D and Westra WH: Mutational activation of BRAF is not a
major event in sporadic childhood papillary thyroid carcinoma. Mod
Pathol. 18:898–902. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sassolas G, Hafdi-Nejjari Z, Ferraro A,
Decaussin-Petrucci M, Rousset B, Borson-Chazot F, Borbone E, Berger
N and Fusco A: Oncogenic alterations in papillary thyroid cancers
of young patients. Thyroid. 22:17–26. 2012. View Article : Google Scholar
|
|
73
|
Ricarte-Filho JC, Li S, Garcia-Rendueles
ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova
T, Thomas GA, et al: Identification of kinase fusion oncogenes in
post-Chernobyl radiation-induced thyroid cancers. J Clin Invest.
123:4935–4944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Henke LE, Perkins SM, Pfeifer JD, Ma C,
Chen Y, DeWees T and Grigsby PW: BRAF V600E mutational status in
pediatric thyroid cancer. Pediatr Blood Cancer. 61:1168–1172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Givens DJ, Buchmann LO, Agarwal AM,
Grimmer JF and Hunt JP: BRAF V600E does not predict aggressive
features of pediatric papillary thyroid carcinoma. Laryngoscope.
24:E389–E393. 2014. View Article : Google Scholar
|
|
76
|
Prasad ML, Vyas M, Horne MJ, Virk RK,
Morotti R, Liu Z, Tallini G, Nikiforova MN, Christison-Lagay ER,
Udelsman R, et al: NTRK fusion oncogenes in pediatric papillary
thyroid carcinoma in northeast United Sates. Cancer. 122:1097–1107.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alzahrani AS, Qasem E, Murugan AK,
Al-Hindi HN, AlKhafaji D, Almohanna M, Xing M, Alhomaidah D and
AlSwailem M: Uncommon TERT promoter mutations in pediatric thyroid
cancer. Thyroid. 26:235–241. 2016. View Article : Google Scholar
|
|
78
|
Nikita ME, Jiang W, Cheng SM, Hantash FM,
McPhaul MJ, Newbury RO, Phillips SA, Reitz RE, Waldman FM and
Newfield RS: Mutational analysis in pediatric thyroid cancer and
correlations with age, ethnicity and clinical presentation.
Thyroid. 6:227–234. 2016. View Article : Google Scholar
|
|
79
|
Onder S, Ozturk Sari S, Yegen G, Sormaz
IC, Yilmaz I, Poyrazoglu S, Sanlı Y, Giles Senyurek Y, Kapran Y and
Mete O: Classic architecture with multicentricity and local
recurrence, and absence of TERT promoter mutations are correlates
of BRAF (V600E) harboring pediatric papillary thyroid carcinomas.
Endocr Pathol. 27:153–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gertz RJ, Nikiforov Y, Rehrauer W,
McDaniel L and Lloyd RV: Mutation in BRAF and other members of the
MAPK pathway in papillary thyroid carcinoma in the pediatric
population. Arch Pathol Lab Med. 140:134–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ballester LY, Sarabia SF, Sayeed H, Patel
N, Baalwa J, Athanassaki I, Hernandez JA, Fang E, Quintanilla NM,
Roy A and López-Terrada DH: Integrating molecular testing in the
diagnosis and management of children with thyroid lesions. Pediatr
Dev Pathol. 19:94–100. 2016. View Article : Google Scholar
|
|
82
|
Picarsic JL, Buryk MA, Ozolek J,
Ranganathan S, Monaco SE, Simons JP, Witchel SF, Gurtunca N, Joyce
J, Zhong S, et al: Molecular characterization of sporadic pediatric
thyroid carcinoma with the DNA/RNA Thyro/Seq v2 next-generation
sequencing assay. Pediatr Dev Pathol. 19:115–122. 2016. View Article : Google Scholar
|
|
83
|
Cordioli MI, Moraes L, Bastos AU, Besson
P, Alves MTS, Delcelo R, Monte O, Longui C, Cury AN and Cerutti JM:
Fusion oncogenes are the main genetic events found in the sporadic
papillary thyroid carcinomas from children. Thyroid. 27:182–188.
2017. View Article : Google Scholar
|
|
84
|
Geng J, Wang H, Liu Y, Tai J, Jin Y, Zhang
J, He L, Fu L, Qin H, Song Y, et al: Correlation between BRAF V600E
mutation and clinicopathological features in pediatric papillary
thyroid carcinoma. Sci China Life Sci. 60:729–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Poyrazoglu S, Bundak R, Bas F, Yeğen G,
Şanlı Y and Darendeliler F: Clinicopathological characteristics of
papillary thyroid cancer in children with emphasis on pubertal
status and association with BRAFV600E mutation. J Clin
Res Pedriatr Endocrinolol. 9:185–193. 2017. View Article : Google Scholar
|
|
86
|
Hardee S, Prasad ML, Hui P, Dinauer CA and
Morotti RA: Pathologic characteristics, natural history, and
prognostic implications of BRAF V600E mutation in pediatric thyroid
carcinoma. Pediatr Dev Pathol. 20:206–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Alzahrani AS, Murugan AK, Qasem E,
Alswailem M, Al-Hindi H and Shi Y: Single point mutations in
pediatric differentiated thyroid cancer. Thyroid. 27:189–196. 2017.
View Article : Google Scholar
|
|
88
|
Wasserman JD, Sabbaghian N, Fahiminiya S,
Chami R, Mete O, Acker M, Wu MK, Shlien A, de Kock L and Foulkes
WD: DICER1 mutations are frequent in adolescent-onset papillary
thyroid carcinoma. J Clin Endocrinol Metab. 103:2009–2015. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Romitti M, Ceolin L, Siqueira DR, Ferreira
CV, Wajner SM and Maia AL: Signaling pathways in follicular
cell-derived thyroid carcinomas (review). Int J Oncol. 42:19–28.
2013. View Article : Google Scholar
|
|
90
|
Rangel-Pozzo A, Sisdelli S, Cordiolo MIV,
Vaisman F, Caria P, Mai S and Cerutti JM: Genetic landscape of
papillary thyroid carcinoma and nuclear architecture: An overview
comparing pediatric and adult populations. Cancers (Basel).
12:E31462020. View Article : Google Scholar
|
|
91
|
Prasad PK, Mahajan P, Hawkins DS,
Mostoufi-Moab S and Venkatramani R: Management of pediatric
differentiated thyroid cancer: An overview for the pediatric
oncologist. Pediatr Blood Cancer. 67:e281412020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in locally advanced or metastatic,
radioactive iodine-refractory, differentiated thyroid cancer: A
randomized, double-blind, Phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Eng J Med. 372:621–630. 2015. View Article : Google Scholar
|
|
94
|
Drilon A, Laetsch TW, Kummar S, DuBois SG,
Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo
AS, et al: Eficacy of Larotrectinib in TRK fusion-positive cancers
in adults and children. N Engl J Med. 378:731–739. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Laetsch TW, DuBois SG, Mascarenhas L,
Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL,
Rudzinski E, Feraco AM, et al: Larotrectinib for pediatric solid
tumours harbouring NTRK gene fusions: Phase 1 results from a
multicentre, open-label, phase 1/2 study. Lancet Oncol. 19:705–714.
2018. View Article : Google Scholar : PubMed/NCBI
|