Globally CRC is one of most common malignancies,
ranking as the third most frequently diagnosed type of cancer and
the second cause of cancer-related mortality worldwide (1). CRC stems from acquiring genetic and
epigenetic alterations over the course of several years referred to
as the adenoma-carcinoma sequence (2). CRC is a multifaceted and
heterogenous disease and its etiology emerges from an interaction
between the host and the environment. The role of microbes among
environmental factors in carcinogenesis has been recognized.
Infectious agents have been estimated to cause >15% of all
cancers; for example, Helicobacter pylori causes gastric
cancer, hepatitis B and C viruses cause hepatocellular cancer and
papilloma virus causes cervical cancer. The scientific community
has begun to study the role of the host-microbe interaction in
cancer progression. Microbiota refers to the diverse community of
microorganisms present in a specific environment. This has emerged
as an important environmental factor for gastrointestinal cancer
and CRC. The gut microbiome consists of a large population of
bacteria (>100 billion) interacting with intestinal cells of the
host, affecting immunity and the metabolome (3). Microbial composition varies along
the gastrointestinal tract; 70% of microbes are located in the
colorectum where interaction and crosstalk take place (4). The large intestine, particularly the
colon, is more prone to developing cancer as compared to the small
intestine, due to the heavy colonization of the microorganism.
Cancer incidence is 12-fold more in the colon as compared to the
remainder of the gastrointestinal tract (5).
The microbiota is important for normal physiological
functions, such as energy harvest (6) and the immune maturity of the gut
(7). Alterations in relative
abundance can modulate the balance, leading to pathological
conditions, such as obesity, metabolic disorders and autoimmune
disease (8-10). Certain microbes and their
metabolites may create a microenvironment that is more favorable to
cancer growth (11). Emerging
evidence suggests that the gut microbiota plays an important role
in the initiation and progression of CRC (12). Previous research with germ-free
animals have demonstrated a role for the microbiota in a number of
models of carcinogenesis (13).
The association between the microbiota, inflammation and CRC is
well understood; patients with inflammatory bowel disease (IBD) are
known to be more susceptible to CRC progression. The composition
and diversity of microorganisms varies among different individuals,
depending on diet, antibiotic/medicine consumption and chemical
exposure (14). Dietary habits
and lifestyle are well-established risk factors known to alter the
gut microbiota. Alterations in diet have been found to affect the
microbiota (15). The gut
microbiota releases various metabolites that may have beneficial or
damaging effects on the host. The production of metabolites, such
as short-chain fatty acids (SCFAs), polyphenols, vitamins and
polyamines lead to the pathogenesis of human disease. Recent
findings have reported that SCFAs, specifically butyrate, play a
critical role in immunomodulatory functions. Alterations in SCFA
levels and other amino acid metabolites have been known to play an
important role in cancer progression and metastasis (16). The biosynthesis of chemical
carcinogens by microorganisms, such as N-nitroso compounds and
acetaldehyde are among the potential mechanisms through which the
microbiota may play a role in cancer progression. Resarch on the
gut microbiota has provided a new direction and hope for the early
detection of CRC, specifically during the early stages, increasing
the 5-year survival rate, as compared to the late stages (17). The detection of alterations in
certain microorganisms has provided a promising strategy for the
early diagnosis of CRC (17).
Some microbes have been shown to exert a protective effect against
CRC by metabolite production, immune tolerance and outcompete with
detrimental microbes (18). The
better understanding of the microbiota and host interaction would
provide novel opportunities for the early detection of CRC and
therapeutics targeting the microbiota. The present review focuses
on the role of the microbiota associated with CRC development.
A pyrosequencing-based strategy adopted to study
microbial dysbiosis in patients with CRC explained that the
elevation in the population of Bacteroides fragilis (B.
fragilis) can be linked to the development of cancer in the
colon (25). B. fragilis
is known to be an enterotoxigenic strain producing a bacterial
toxin (metalloprotease) known as the B. fragilis toxin (BFT)
responsible for the virulent properties of the strain (26). In the study by Ahn et al
the taxonomic analyses of the gut microbiota was carried out by the
amplification of 16srRNA sequences obtained from fecal bacterial
DNA; it was observed that the increased abundance of the anaerobic
Gram-negative genera, Fusobacterium and
Porphyromonas, led to an increased risk of CRC development
in the individuals (27).
Fusobacterium nucleatum (F. nucleatum) was found in
abundance in the fecal samples of patients with CRC and various
clinical and metagenomic studies have highlighted a significant
role of F. nucleatum in the inflammatory response of IBD and
CRC (27,28). Along the similar lines of research
i.e., based on the sequencing of 16srRNA, the clinical study
performed by Wu et al revealed that Campylobacter and
Fusobacterium species were relatively more abundant in CRC
samples along with several other families, such as Enterococcaceae,
Staphylococcaceae and Eubacteriaceae, exerting pathogenic effects
in the colon (28). Another
anaerobic commensal bacterium predominant in the gut microbiota is
Escherichia coli (E. coli) and a previous study on
mice inoculated with colon cancer associated E. coli strains
reported an increase in the colonization of tumor-associated and
mucosa-associated E. coli strains in patients with CRC
(29). E. coli strains
obtained from CRC samples were found to express certain
toxin-producing genes, such as colibactin, which confer bacterial
cells with properties to induce DNA damage and genomic instability,
subsequently causing colorectal carcinogenesis (30). The statistical study by Zhang
et al also revealed an elevation in the numbers of
Devosia in the gut microbiota of patients with CRC (31).
Apart from elevated levels of several pathogenic
microbes in the mucosal and fecal samples of patients with CRC, Ahn
et al in their study, also highlighted a decrease in the
population of Gram-positive strains of Clostridia,
specifically Coprococcus, increasing the risk of
inflammation in the colon followed by tumorigenesis (27). Some butyrate-producing genera,
such as Roseburia, Faecalibacterium and Eubacterium
were significantly reduced in the microbiota obtained from CRC
samples, suggesting that dysbiosis in the fecal microbiota can
serve as a marker for CRC detection (28,31). Another notable observation for the
dysbiosis of the colon microbiota was made by Marchesi et al
where upon investigating the tumor tissues of patients with CRC, a
significant reduction in the population of
Enterobacteriaceae was identified, namely in species such as
Citrobacter, Shigella, Serratia, Salmonella and
Kulyvera spp. (32).
Lactic acid bacteria (LAB), such as Bifidobacterium and
Lactobacilli have also been found to play a negative role in
CRC (33). An imbalance in the
microbial population can alter the micro-environment of the
intestine, which cause an imbalance in crucial intrinsic and
extrinsic factors, resulting in the initiation of cancerous growth.
A summary of the bacteria playing a role in CRC development is
provided in Table I.
To date, various metagenomic studies performed on
clinical samples obtained from individuals suffering from CRC have
pointed towards the abundance of pathogenic bacterial strains of
F. nucleatum, Bacteroides fragilis and E.
coli. On the contrary, a significant reduction in the
population of butyrate-producing strains belonging to the class
Clostridia, are also considered to be a contributing factor
to CRC. Thus, the understanding of the metabolites produced by
these species and their mechanisms of action is critical in order
to reach to better diagnostic and therapeutic conclusions and to
assist in devising novel treatment strategies.
This genotoxic compound generates double-strand
breaks in DNA causing damage to several portions of DNA, rendering
it unstable and this instability of the genome paves the way for
the upregulation of expression of oncogenes, such as c-Myc, which
results in the formation of adenomas and the development of CRC
(56,67).
Apart from these bacterial species mentioned above,
there are several other microbes predominantly found in the fecal
and mucosal samples of patients with CRC, and play a key role in
pathogenesis and carcinogenesis. Microbiota analysis of healthy
individuals indicated that several species of LAB namely,
Lactobacillus acidophilus, L. casei, L. rhamnosus
exert anti-inflammatory effects in maintaining gut homeostasis
(75,76). L. rahmnosus was found to
reduce the levels of β-catenin and NF-κB p65 proteins, and to
induce the expression of tumor suppressor gene p53 and
anti-apoptotic factor BAX in colon epithelial cell, thus preventing
CRC (76).
Bifidobacterium, another LAB, exerts a negative effect on
CRC proliferation due to its property to reduce β-glucuronidase
activity in the gut which enhances the chemotherapeutic efficacy of
CPT-11, hence providing a beneficiary role in the treatment of CRC
(77). The detailed role of these
commensal LABs in the prevention of CRC has yet to be identified
and can be explored in the near future in order to gain better
insight into potential treatment strategies for CRC. The abundance
of Parvimonas micra has been found in the stool of patients
with CRC. P. micra was found to inhibit the NOD2 signaling,
giving rise to an inflammatory and pro-tumorigenic microenvironment
(78). Xu et al found that
P. micra abundance was elevated in patients with CRC and was
low in healthy individuals and patients with colorectal adenoma
(79). The overabundance of
Porphyromonas gingivalis has been found in patients with CRC
(80). Yang et al reported
an association between Prevotella intermedia with a higher
risk of CRC development (81).
Another study identified P. intermedia in a multinational
cohort of fecal samples of CRC (82). Gemella morbillorum has been
found to regulate IL-12 production and thereby, immunoregulation in
CRC. Other species of Gemella regulate the protective
function of the adaptive immune response at the mucosal surface by
cleaving IgA1 (83,84). Still, however, no direct role of
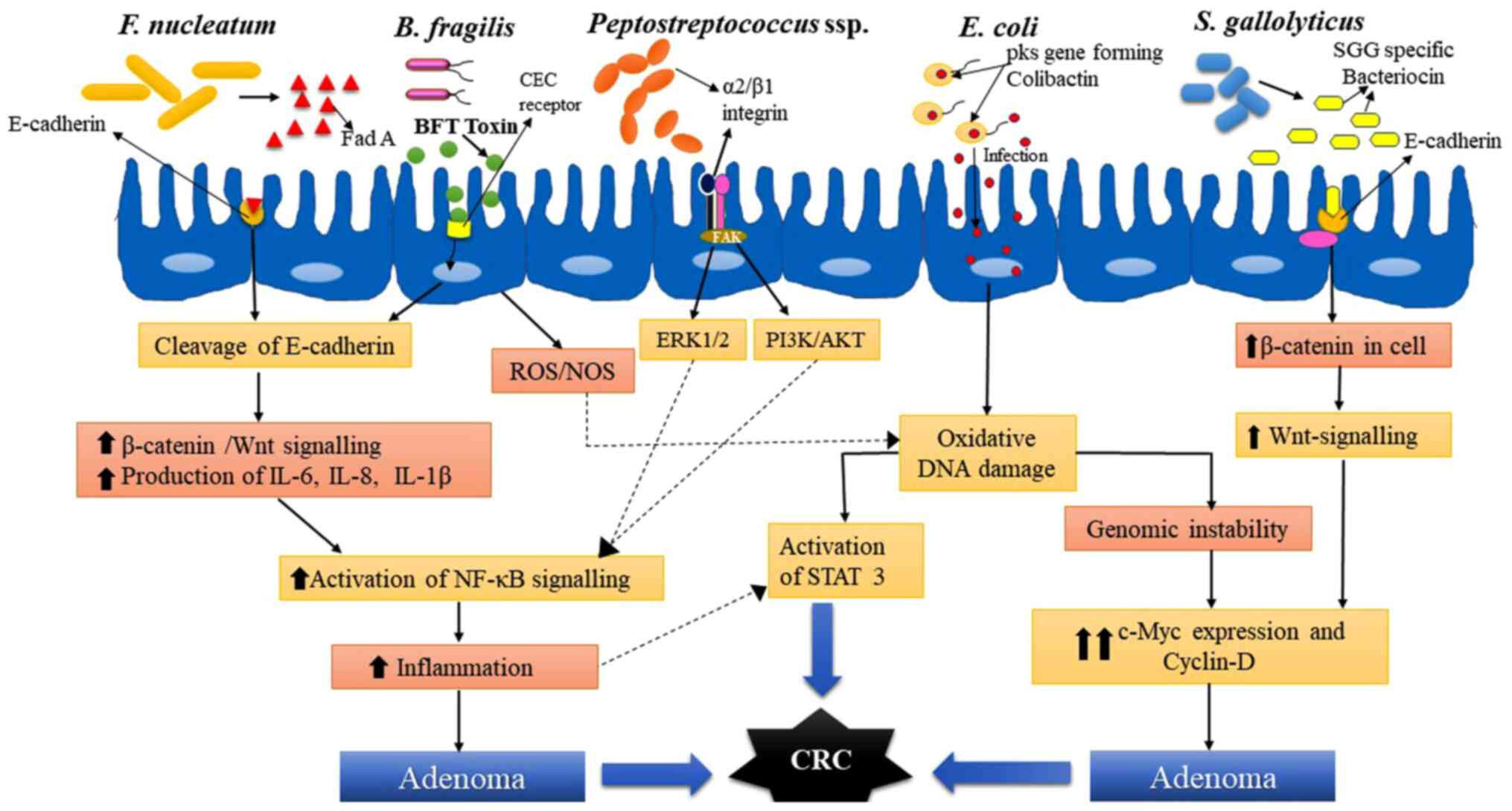
this bacteria has been reported in CRC development. A schematic
diagram of the mechanisms of action of major species of bacteria
involved in the development of CRC in humans is presented in
Fig. 1.
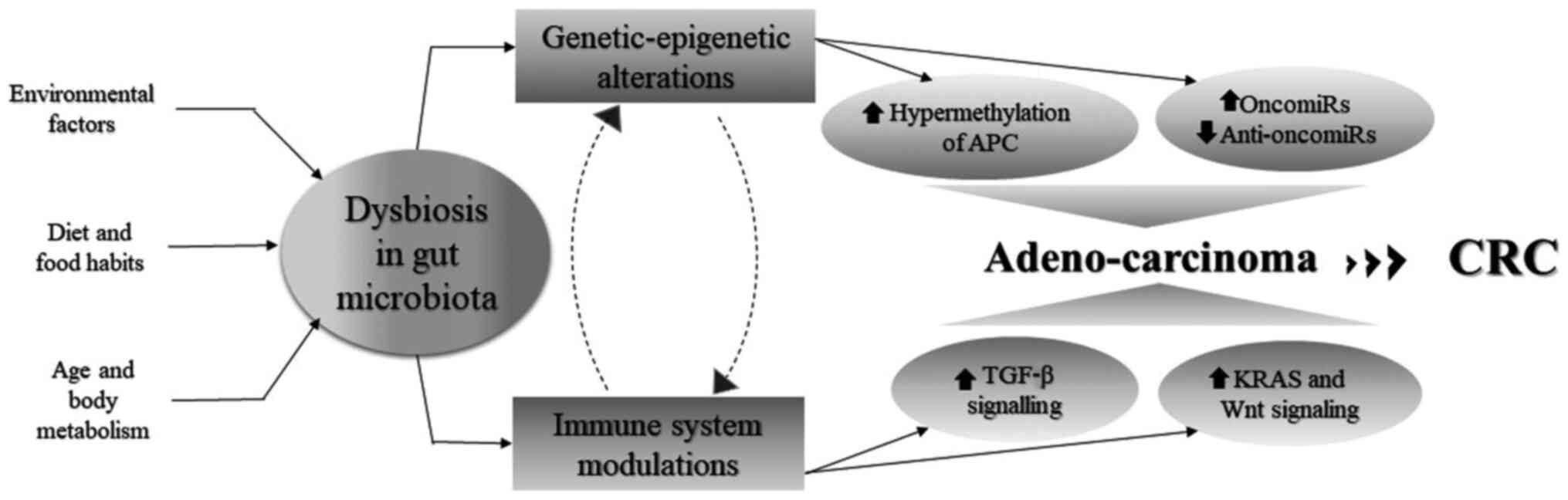
CRC can be caused due to the cumulative effects of
several genetic, epigenetic and environmental factors, which
modulate microbial composition in the gut, altering the metabolite
profile and the immune response of the body accordingly. CRC is not
a single-step process, but rather a culmination of several steps
which involve a number of changes in the genetic and epigenetic
machinery of the host. The development of CRC begins with the
transition of a normal epithelium into the hyperproliferative
epithelium, which eventually leads to the loss of its structure and
function, leading to a condition known as hyperplasia followed by
dysplasia, leading to the formation of adenomas. These adenomas are
non-malignant in nature and are known as polyps (85). In this section, the host-microbe
interaction in the context of epigenomic modifications and immune
system alterations is discussed.
Allen and Sears highlighted the impact of the
dysbiosis of the gut microbiome on genome and epigenomics of the
colon epithelial cells which increased cell proliferation and tumor
formation, growth and metastasis (86). DNA methylation patterns and
histone marks on several promoters and enhancers were found to be
dysregulated, leading to the downregulation of tumor suppressor
genes (TSGs) and the upregulation of oncogenes. Several of the
miRNAs (oncomiRs and anti-oncomiRs) and long non-coding RNAs have
also been found to be dysregulated and associated with CRC
(86). B. fragilis has
been found to induce the hypermethylation of several TSGs, such as
Hoxa5, Polg, Runx1, Runx3, CD37, Stx11, Tceb2, Lgr6, Cdx1 and Fut4,
causing carcinoma development in the colon and rectum (86). Xia et al identified F.
nucleatum and Hungatella hathewayi to be highly
associated with the upregulation of DNA methyltransferases which
cause the hypermethylation of promoter of TSG CDX2 and MLH1
(87). In a previous study, the
APC gene and DNA mismatch repair (MMR) system were the prime
genetic factors identified, which if altered or silenced, led to
the development of CRC (88). In
an extensive clinical analysis performed by Gagnière et al
the MMR pathway was shown to be most affected by entero-pathogenic
E. coli. They reported that pks+ E.
coli strains downregulated the expression of MLH1 genes and
inhibited the formation of MLH1 MMR protein in T-84 cells, which
led to MSI and tumorigenesis (89). In addition, methylation in APC and
INK4a TSG also promoted tumor development in the case of
colitis-associated CRC (90-92). Streptococcus species have
also been identified to be associated with APC gene
hypermethylation (87).
Apart from methylation, dysregulation in the
expression of miRNAs serves as a major epigenetic marker
responsible for CRC. F. nucleatum is often found to increase
RASA1 expression by inhibiting miR-21 expression, thus leading to
chronic inflammation in the intestine, initiating carcinogenesis
(93). F. nucleatum has
also been found to downregulate the expression of miR-4802 and
miR-18a, which lead to resistance against chemotherapeutic drugs
administered to patients suffering from CRC (94). Several other oncomiRs and
anti-oncomiRs were analyzed from fecal samples and gut mucosa,
which revealed differential miRNA profiles in the presence of
different gut microbiome, and this variation in miRNA profiles can
also serve as a fingerprint for the detection and diagnosis of CRC
(95,96).
The gut microbiota is known to contribute immensely
towards the maintenance of the immune system. Fluctuations in the
dynamic equilibrium of this microbiota composition leads to defects
in the immune system, resulting in inflammation and in tumor
initiation. Genotoxins from B. fragilis and
pks+ E. coli are known to induce
inflammation in colon epithelial cells and this inflammatory
response by the host induces genetic and epigenetic alterations,
which contribute to CRC development (97). Mutations in the tumor suppressor
p53 gene are commonly associated with cancer initiation, as well as
progression, and they have been found to prolong the effects of
NF-κB signaling, which generates the inflammatory response in cells
(98). Inflammation triggers
oxidative stress, increasing DNA damage and causing mutations in
genes, such as APC, directing the Wnt signaling pathway and KRAS,
which initiates adenoma formation and is followed by the loss of
chromosome 18q and mutations in TP53, resulting in CSI and in the
formation of carcinomas (99,100). Inflammation in cells often
regulates the production of chemokines and cytokine-driven
signaling pathways, such as the NF-κB, PI3K, Akt and ERK pathways.
These signaling pathways are responsible for the initiation of
tumorigenesis by either upregulating Wnt signaling, which promotes
cell proliferation or by inhibiting apoptosis (101). F. nucleatum has also been
found to enhance pro-inflammatory markers and the infiltration of
CD11b+ myeloid immune cells and few macrophages,
activating Th17 cells and TGFβ signaling, which promotes tumor
initiation and angiogenesis (102).
The composition of microbes in the host are a
continuous influence on the environment and in order to protect
themselves, microbes belonging to specific species tend to form
mucosal biofilms. These microbial biofilms exert a protective
function for the microbiota from immune factors present in the host
(104). The study by Dejea et
al demonstrated that biofilms were characteristically found in
almost all the patients with right-sided CRC (105). The microbial composition of
these biofilms was then further studied and it was predominantly
found that microbes belonged to Bacteroides, Fusobacteria,
Clostridia, Bifidobacterium and E. coli (104,105). These biofilms in the colon have
been found to exert pro-carcinogenic effects by disrupting
E-cadherin, and enhancing IL-6, Ki63 and pSTAT3 expression in the
colon epithelium (105), more
specifically on the right side. The presence of these biofilms
often results in a poor prognosis (100). Thus, it can be conveniently
concluded that biofilms can serve as a signature biomarker for
CRC.
The present review thus far discussed several
microbial species which act as a driving force behind the
occurrence of CRC. This dysbiosis in the microbiota is frequently
studied by obtaining fecal samples. The metabolomic study of fecal
dysbiosis has pointed towards the utility of microbial dysbiosis as
a signature biomarker for the early prognosis and diagnosis of CRC
(106). The most pathogenic
bacterial strains identified as driving microbes for CRC, F.
nucleatum and B. fragilis, can serve as fecal biomarkers
for the early diagnosis of CRC (107,108), since elevated levels of these
bacterial species have been found to be associated with an
elevation in the levels of major inflammatory mediators (109). F. nucleatum has been
found to increase the levels of β-catenin and TGF-β, whereas B.
fragilis upregulates the expression of NF-κB, COX-2 and MMP-9,
which can be indicative of early signs of CRC. Faecalibacterium
prausnitzii (F. prausnitzii) was found in low levels in
the fecal microbiota of patients with CRC and was responsible for
low levels of β-catenin. Hence, the detection of F. nucleatum,
B. fragilis and F. prausnitzii in fecal samples of
individuals can help detect signs of CRC at an early stage. In
addition, the analysis of these inflammatory mediators along with
immunohistochemical markers, such as enhanced KRAS expression and
decreased MLH1 expression can serve as effective diagnostic markers
for early prognosis of CRC (109). Wu et al carried out a
16srRNA based meta-analysis on fecal biomarkers for CRC and adenoma
and identified 24 biomarkers sorted into three clusters, out of
which first and third cluster were found to have heterogenous
population of bacteria and second cluster had relatively homogenous
population comprising mainly of members of Clostridiales
order (110). These clusters
were claimed to be distinguishing biomarkers between adenoma and
cancer in the colon and rectum (110). Microbes belonging to genera
Porphyromonas, Parvimonas, Hungatella and Bacteroides
were CRC-associated biomarkers, whereas Streptococcus
thermophilus TH1435, Roseburia intestinalis, Blautia
faecis and Eubacterium ruminantium were found to be
adenoma-associated biomarkers (110). miRNAs are often found to mediate
the crosstalk between microbes and the immune system. Hence,
detecting fecal miRNAs and their analysis can prove to be
beneficial in the prognosis of CRC and may also provide insight
into the early diagnosis, as these fecal miRNAs play a significant
role in the fecal dysbiosis of the microbial population (111-113). These fecal miRNAs interact with
the microbiota and have been found to help F. nucleatum and
E. coli invade host intestinal cells, disrupting the
intestinal homeostasis (114). A
preliminary investigation in this context was performed by Li et
al to find a conclusive association between the differential
expression of several oncomiRs and anti-oncomiRs, and distinctive
microbiome profiles in CRC specimens (115). Fecal miRNAs can be upregulated,
as well as downregulated under the influence of bacterial
metabolites and virulence factors, exerting a carcinogenic effect
(116). Among several
dysregulated miRNAs, the ones which are upregulated were miR-17-92
cluster, miR-20a, miR-135, miR-144, miR-221 and miR-92a, and those
found to be downregulated were miR-29a, miR-224, miR-143, miR-145
and miR-4478, significantly contributing to CRC and serving as
non-invasive fecal biomarkers for the early diagnostics and
therapeutic implication in CRC.
CRC is not a single-step process occurring due to
one particular pathway or one individual bacterial strain, but
rather an amalgamation of several epigenetic and immunomodulated
cascades, which are initiated due to an increased abundance and
synergistic effect of characteristic 'Driver and Passenger'
bacteria i.e., F. nucleatum, B. fragilis, E. coli, E.
faecalis and Streptococcus spp. Therefore, there are
several treatment strategies devised and several of these are on
their way from 'bench to bedside' in order to combat CRC. These
therapeutic strategies can be broadly classified into chemotherapy
and immunotherapy, following two different approaches but reaching
to a similar conclusion i.e., a cure for CRC. Chemotherapy involves
the administration of potent anticancer drugs (5-fluorouracil,
oxaliplatin, irinotecan, etc.) in combination, to eliminate or
inhibit tumor cells. Targeted therapeutics have been approved by
the FDA for the treatment of CRC, such as cetuximab, a monoclonal
antibody against EGFR, bevacizumab, inhibitor of angiogenesis and
capecitabine, inhibitor of DNA synthesis (94). The efficacy of these
chemotherapies are often found to be affected by gut
microbiota-induced toxicity and chemoresistance (117-119); for instance, F. nucleatum
renders CRC resistant to the chemotherapeutic drugs, oxaliplatin
and 5-FU. This chemoresistance by F. nucleatum was observed
on colorectal cell lines in vivo and the induction of
autophagy by F. nucleatum has been found to be the prime
reason behind it (119). A
previous study found that F. nucleatum was also involved in
the risk of recurrence following neoadjuvant chemoradiotherapy in
locally advanced rectal cancer (120). Within the tumor
microenvironment, the intestinal microbiota was found to regulate
the functions of myeloid derived cells, thereby affecting the
response to chemotherapy against cancer (85). Since the gut microbiota was often
observed to interfere and affect the efficacy of anticancer drugs
through its interaction with immune cells (121-123), there is an urgent need to
identify alternatives to chemotherapy.
Immunotherapy is a biological method of motivating
the immune system to fight by stimulating or suppressing body's own
immune cells as per requirement in order to elicit an immune
response. Now that microbes have been found to play a key role in
development, as well as in the diagnosis of CRC, it was
hypothesized by several groups that protective bacterial species if
restored/maintained in the intestine, can serve as bio-therapeutic
in triggering the host immune response against virulence factors
generated by drivers of CRC. Several clinical trials have
highlighted the antitumor potential of L. acidophilus and
Bifidobacterium, and suggested their utility as a probiotic
in the treatment of CRC, where it can be administered orally to
patients. This will help restore the balance of commensal microbial
genera in the gut and preventing intestinal toxicity (79). The study by Sivan et al
detailed the potency of Bifidobacterium as a probiotic and
its efficacy as a biotherapeutic agent (121). Bifidobacterium in the
gut, on its oral administration to patients with CRC, reduced tumor
growth to an extent similar to that obtained by treatment with
anti-PD-L1 therapy, and when the probiotic in combination with
therapy was used for treatment, Bifidobacterium enhanced the
response of anti-PD-L1 antibody therapy and abolished tumor growth
(121). Another immune
checkpoint therapy involves targeting anti-CTLA4 in the intestine
to induce the maturation and activation of dendritic cells and
exert antitumor effects; several gut microbiota species are known
to have a positive impact on this immunotherapy (122). A positive impact of the gut
microbiota in several other immune therapies have been deduced
which target induction of CD8+ T-lymphocytes,
macrophages and the activation of dendritic cell, and major
histocompatibility complex (MHC) driven pathways in order to exert
antitumor effects (123). The
restoration of the commensal gut microbiota population beneficial
for intestinal epithelia, can trigger several immunoregulatory
pathways and minimize adverse effects of immunotherapy. Thus, it
may prove to be crucial in treatment of CRC and can pave the way
for the development of novel therapeutic approaches (22). Another therapeutic approach
involves the bioengineering of bacteria to trigger the immune
response in the host. Cancer-invading bacterial cells were
engineered, such that they produce a short hairpin segment of RNA
which can effectively interact with β-catenin to suppress Wnt
signaling and inhibit tumor formation in colorectal tissue
(124). Thus, the human gut
microbiota plays a crucial role in determining the efficacy and
toxicity potential of a treatment, and also provides a great future
prospect for developing novel biotherapeutics using gut microbiota
and its metabolites for the treatment of CRC. A summary of novel
therapeutic strategies for the treatment of CRC and their possible
mechanisms of action is presented in Table II.
Several studies have revealed that the microbiota
composition has been altered in benign lesions and in malignant
tumors of the colon and rectum. Moreover, in patients with CRC, a
dysbiosis in the gut microbiota has been found as compared to
healthy controls. An enrichment in pro-inflammatory microbiota and
the depletion in butyrate-producing bacteria has been noted. The
dysbiosis of the gut microbiota in CRC results in the impairment of
the intestinal epithelial barrier function, the activation of
pro-inflammatory responses, genotoxin synthesis and toxic
metabolite generation. The gut microbiota should be considered as a
prime factor that can contribute to both CRC initiation and
development. Dysbiosis can be avoided by the intake of dietary
components that can alter the cancer-associated microbiome and
suppress intestinal inflammation. This strategy can improve the
cancer therapeutic response and prevent the progression of CRC.
Furthermore, once the microbiome composition of a given patient is
characterized, using a personalized medical approach, a desired
bacterial equilibrium could be restored using pre and probiotics
and a tailored phage therapeutics. Identifying the mechanistic
pathways through which the gut microbiota influences CRC would help
in devising more effective strategies for the treatment of CRC.
Therefore, targeting metabolome by drugs or diet modulation and
developing immunogenic peptides against cell surface proteins would
improve the therapeutic efficacy for CRC and overall survival.
Not applicable.
DA, MA, RA and SKS conceived the study, and drafted
and wrote the manuscript. MKP, JKS and TBT were involved in the
critical review of the manuscript. SKS, RA and MKP revised and
edited the manuscript. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors state that they have no competing
interests.
The present study was supported by the Deanship of
Scientific Research, King Saud University, for funding through the
Vice Deanship of Scientific Research Chairs.
No funding was received.
|
1
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keum N and Giovannucci E: Global burden of
colorectal cancer: Emerging trends, risk factors and prevention
strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helmink BA, Khan MAW, Hermann A,
Gopalakrishnan V and Wargo JA: The microbiome, cancer, and cancer
therapy. Nat Med. 25:377–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dahmus JD, Kotler DL, Kastenberg DM and
Kistler CA: The gut microbiome and colorectal cancer: A review of
bacterial pathogenesis. J Gastrointest Oncol. 9:769–777. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gagnière J, Raisch J, Veziant J, Barnich
N, Bonnet R, Buc E, Bringer MA, Pezet D and Bonnet M: Gut
microbiota imbalance and colorectal cancer. World J Gastroenterol.
22:501–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini
V, Mardis ER and Gordon JI: An obesity-associated gut microbiome
with increased capacity for energy harvest. Nature. 444:1027–1031.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung H, Pamp SJ, Hill JA, Surana NK,
Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR,
et al: Gut immune maturation depends on colonization with a
host-specific microbiota. Cell. 149:1578–1593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
John GK and Mullin GE: The gut microbiome
and obesity. Corr Oncol Rep. 18:452016. View Article : Google Scholar
|
|
9
|
Dabke K, Hendrick G and Devkota S: The gut
microbiome and metabolic syndrome. J Clin Invest. 129:4050–4057.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H, Liu M, Cao J, Li X, Fan D, Xia Y, Lu
X, Li J, Ju D and Zhao H: The dynamic interplay between the gut
microbiota and autoimmune diseases. J Immunol Res.
2019:75460472019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kostic AD, Chun E, Robertson L, Glickman
JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold
GL, et al: Fusobacterium nucleatum potentiates intestinal
tumorigenesis and modulates the tumor-immune microenvironment. Cell
Host Microbe. 14:207–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ternes D, Karta J, Tsenkova M, Wilmes P,
Haan S and Letellier E: Microbiome in colorectal cancer: How to get
from Meta-omics to Mechanism. Trends Microbiol. 28:401–423. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwabe RF and Jobin C: The microbiome and
cancer. Nat Rev Cancer. 13:800–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Costello EK, Lauber CL, Hamady M, Fierer
N, Gordon JI and Knight R: Bacterial community variation in human
body habitats across space and time. Science. 326:1694–1697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu GD, Chen J, Hoffmann C, Bittinger K,
Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R,
et al: Linking long-term dietary patterns with gut microbial
enterotypes. Science. 334:105–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhat MI and Kapila R: Dietary metabolites
derived from gut microbiota: Critical modulators of epigenetic
changes in mammals. Nutr Rev. 75:374–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong SH, Kwong TNY, Chow TC, Luk AKC, Dai
RZW, Nakatsu G, Lam TYT, Zhang L, Wu JCY, Chan FKL, et al:
Quantitation of faecal Fusobacterium improves faecal immunochemical
test in detecting advanced colorectal neoplasia. Gut. 66:1441–1448.
2017. View Article : Google Scholar :
|
|
18
|
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong
SH, Ng SC, Chan FKL, Sung JJY and Yu J: Enteric fungal microbiota
dysbiosis and ecological alterations in colorectal cancer. Gut.
68:654–662. 2019. View Article : Google Scholar :
|
|
19
|
Ley RE, Peterson DA and Gordon JI:
Ecological and evolutionary forces shaping microbial diversity in
the human intestine. Cell. 124:837–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tlaskalová-Hogenová H, Stepánková R,
Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková
H, Rossmann P, Bártová J, Sokol D, et al: Commensal bacteria
(normal microflora), mucosal immunity and chronic inflammatory and
autoimmune diseases. Immunol Lett. 93:97–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang
X, Jia W, Cai S and Zhao L: Structural segregation of gut
microbiota between colorectal cancer patients and healthy
volunteers. ISME J. 6:320–329. 2012. View Article : Google Scholar :
|
|
22
|
Zackular JP, Rogers MA, Ruffin MT IV and
Schloss PD: The human gut microbiome as a screening tool for
colorectal cancer. Cancer Prev Res (Phila). 7:1112–1121. 2014.
View Article : Google Scholar
|
|
23
|
Yu YN and Fang JY: Gut microbiota and
colorectal cancer. Gastrointest Tumors. 2:26–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong SH and Yu J: Gut microbiota in
colorectal cancer: Mechanisms of action and clinical applications.
Nat Rev Gastroenterol Hepatol. 16:690–704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobhani I, Tap J, Roudot-Thoraval F,
Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J and
Furet JP: Microbial dysbiosis in colorectal cancer (CRC) patients.
PLoS One. 6:e163932011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sears CL: Enterotoxigenic bacteroides
fragilis: A rogue among symbiotes. Clin Micobiol Rev. 22:349–369.
2009. View Article : Google Scholar
|
|
27
|
Ahn J, Sinha R, Pei Z, Dominianni C, Wu J,
Shi J, Goedert JJ, Hayes RB and Yang L: Human gut microbiome and
risk for colorectal cancer. J Natl Cancer Inst. 105:1907–1911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu N, Yang X, Zhang R, Li J, Xiao X, Hu Y,
Chen Y, Yang F, Lu N, Wang Z, et al: Dysbiosis signature of fecal
microbiota in colorectal cancer patients. Microb Ecol. 66:462–470.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonnet M, Buc E, Sauvanet P, Darcha C,
Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D and
Darfeuille-Michaud A: Colonization of the human gut by E coli and
colorectal cancer risk. Clin Cancer Res. 20:859–867. 2014.
View Article : Google Scholar
|
|
30
|
Buc E, Dubois D, Sauvanet P, Raisch J,
Delmas J, Darfeuille-Michaud A, Pezet D and Bonnet R: High
prevalence of mucosa-associated E coli producing cyclomudulin and
genotoxin in colon cancer. PLoS One. 8:e569642013. View Article : Google Scholar
|
|
31
|
Zhang H, Chang Y, Zheng Q, Zhang R, Hu C
and Jia W: Altered intestinal microbiota associated with colorectal
cancer. Front Med. 13:461–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marchesi JR, Dutilh BE, Hall N, Peters
WHM, Roelofs R, Boleji A and Tjalsma H: Towards the human
colorectal cancer microbiome. PLoS One. 6:e204472011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zou S, Fang L and Lee MH: Dysbiosis of Gut
microbiota in promoting the development of colorectal cancer.
Gastroenterol Rep (Oxf). 6:1–12. 2018. View Article : Google Scholar
|
|
34
|
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G
and Han YW: Fusobacterium nucleatum promotes colorectal
carcinogenesis by modulating E-cadherin/β-catenin signaling via its
FadA adhesin. Cell Host Microbe. 14:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gur C, Ibrahim Y, Isaacson B, Yamin R,
Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N,
Coppenhagen-Glazer S, et al: Binding of the Fap2 protein of
Fusobacterium nucleatum to human inhibitory receptor TIGIT protects
tumors from immune cell attack. Immunity. 42:344–355. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abed J, Emgard JE, Zamir G, Faroja M,
Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, et al:
Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma
enrichment by binding to tumor-expressed gal-GalNAc. Cell Host
Microbe. 20:215–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma CT, Luo HS, Gao F, Tang QC and Chen W:
Fusobacterium nucleatum promotes the progression of colorectal
cancer by interacting with E-cadherin. Oncol Lett. 16:2606–2612.
2018.PubMed/NCBI
|
|
38
|
Kourtidis A, Lu R, Pence LJ and
Anastasiadis PZ: A central role for cadherin signaling in cancer.
Exp Cell Res. 358:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim WK, Kwon Y, Jang M, Park M, Kim J, Cho
S, Jang DG, Lee WB, Jung SH, Choi HJ, et al: β-catenin activation
down-regulates cell-cell junction-related genes and induces
epithelial-to-mesenchymal transition in colorectal cancer. Sci Re.
9:184402019.
|
|
40
|
Yu MR, Kim HJ and Park HRF: Fusobacterium
nucleatum accelerates the progression of colitis-associated
colorectal cancer by promoting EMT. Cancers (Basel). 12:27282020.
View Article : Google Scholar
|
|
41
|
Guo P, Tian Z, Kong X, Yang L, Shan X,
Dong B, Ding X, Jing X, Jiang C, Jiang N and Yu Y: FadA promotes
DNA damage and progression of Fusobacterium nucleatum-induced
colorectal cancer through up-regulation of chk2. J Exp Clin Cancer
Res. 39:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Okita Y, Koi M, Takeda K, Ross R,
Mukherjee B, Koeppe E, Stoffel EM, Galanko JA, McCoy AN, Keku TO,
et al: Fusobacterium nucleatum infection correlates with two types
of microsatellite alterations in colorectal cancer and triggers DNA
damage. Gut Pathol. 12:462020. View Article : Google Scholar
|
|
43
|
Sayed IM, Chakraborty A, Abd El-Hafeez AA,
Sharma A, Sahan AZ, Huang WJM, Sahoo D, Ghosh P, Hazra TK and Das
S: The DNA Glycosylase NEIL2 suppresses
Fusobacterium-infection-induced inflammation and DNA damage in
colonic epithelial cells. Cells. 9:19802020. View Article : Google Scholar :
|
|
44
|
Guo S, Chen J, Chen F, Zeng Q, Liu WL and
Zhang G: Exosomes derived from Fusobacterium nucleatum-infected
colorectal cancer cells facilitate tumour metastasis by selectively
carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut. Nov 10–2020.Epub
ahead of print. View Article : Google Scholar
|
|
45
|
Lin R, Han C, Ding Z, Shi H, He R, Liu J,
Qian W, Zhang Q, Fu X, Deng X, et al: Knock down of BMSC-derived
Wnt3a or its antagonist analogs attenuate colorectal carcinogenesis
induced by chronic Fusobacterium nucleatum infection. Cancer Lett.
495:165–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Q, Yu C, Yue C and Liu X and Liu X:
Fusobacterium nucleatum produces cancer stem cell characteristics
via EMT-resembling variations. Int J Clin Exp Pathol. 13:1819–1828.
2020.PubMed/NCBI
|
|
47
|
Wu S, Rhee KJ, Zhang M, Franco A and Sears
CL: Bacteroides fragilis toxin stimulates intestinal epithelial
cell shedding and gamma-secretase dependent E-cadherin cleavage. J
Cell Sci. 120:1944–1952. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tian X, Liu Z, Niu B, Zhang J, Lee SR,
Zhao Y, Harris DC and Zheng G: E-cadherin/beta-catenin complex and
the epithelial barrier. J Biomed Biotechnol. 2011:5673052011.
View Article : Google Scholar
|
|
49
|
Sears CL, Geis AL and Housseau F:
Bacteroides fragilis subverts mucosal biology: From symbiont to
colon carcinogenesis. J Clin Invest. 124:4166–4172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nistal E, Fernández-Fernández N, Vivas S
and Olcoz JL: Factors determining colorectal cancer: The role of
the intestinal microbiota. Front Oncol. 5:2202015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zamani S, Taslimi R, Sarabi A, Jasemi S,
Sechi LA and Feizabadi MM: Enterotoxigenic Bacteroides fragilis: A
possible etiological candidate for bacterially-induced colorectal
precancerous and cancerous lesions. Frontier Cell Infect Microbiol.
9:4492020. View Article : Google Scholar
|
|
52
|
Liu QQ, Li CM, Fu LN, Wang HL, Tan J, Wang
YQ, Sun DF, Gao QY, Chen YX and Fang JY: Enterotoxigenic
Bacteroides fragilis induces the stemness in colorectal cancer via
upregulating histone demethylase JMJD2B. Gut Microbes.
12:17889002020. View Article : Google Scholar :
|
|
53
|
Hwang S, Lee CG, Jo M, Park CO, Gwon SY,
Hwang S, Yi HC, Lee SY, Eom YB, Karim B and Rhee KJ:
Enterotoxigenic Bacteroides fragilis infection exacerbates
tumorigenesis in AOM/DSS mouse model. Int J Med Sci. 17:145–152.
2020. View Article : Google Scholar :
|
|
54
|
Roberti MP, Yonekura S, Duong CPM, Picard
M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann CHK, Amon
L, et al: Chemotherapy-induced ileal crypt apoptosis and the ileal
microbiome shape immunosurveillance and prognosis of proximal colon
cancer. Nat Med. 26:919–931. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bao Y, Tang J, Qian Y, Sun T, Chen H, Chen
Z, Sun D, Zhong M, Chen H, Hong J, et al: Long noncoding RNA BFAL1
mediates enterotoxigenic Bacteroides fragilis-related
carcinogenesis in colorectal cancer via the RHEB/mTOR pathway. Cell
Death Dis. 10:6752019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arthur JC, Perez-Chanona E, Muhlbauer M,
Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B,
Rogers AB, et al: Intestinal inflammation targets cancer-inducing
activity of the microbiota. Science. 338:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tjalsma H, Boleij A, Marchesi JR and
Dutilh BE: A bacterial driver-passenger model for colorectal
cancer: Beyond the usual suspects. Nat Rev Microbiol. 10:575–582.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Swidsinski A, Khilkin M, Kerjaschki D,
Schreiber S, Ortner M, Weber J and Lochs H: Association between
intraepithelial Escherichia coli and colorectal cancer.
Gastroenterology. 115:281–286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Martin HM, Campbell BJ, Hart CA, Mpofu C,
Nayar M, Singh R, Englyst H, Williams HF and Rhodes JM: Enhanced
Escherichia coli adherence and invasion in Crohn's disease and
colon cancer. Gastroenterology. 127:80–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Darfeuille-Michaud A, Neut C, Barnich N,
Lederman E and Di Martino P: Presence of adherent Escherichia coli
strains in ileal mucosa of patients with Crohn's disease.
Gastroenterology. 115:1405–1413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Darfeuille-Michaud A, Boudeau J, Bulois P,
Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie
L and Colombel JF: High prevalence of adherent-invasive Escherichia
coli associated with ileal mucosa in Crohn's disease.
Gastroenterology. 127:412–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lax AJ: Opinion: Bacterial toxins and
cancer-a case to answer? Nat Rev Microbiol. 3:343–349. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Thelestam M and Frisan T: Cytolethal
distending toxins. Rev Physiol Biochem Pharmacol. 152:111–133.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Falzano L, Filippini P, Travaglione S,
Miraglia AG, Fabbri A and Fiorentini C: Escherichia coli cytotoxic
necrotizing factor 1 blocks cell cycle G2/M transition in
uroepithelial cells. Infect Immun. 74:3765–3772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Malorni W and Fiorentini C: Is the Rac
GTPase-activating toxin CNF1 a smart hijacker of host cell fate?
FASEB J. 20:606–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jubelin G, Chavez CV, Taieb F, Banfield
MJ, Samba-Louaka A, Nobe R, Nougayrède JP, Zumbihl R, Givaudan A,
Escoubas JM and Oswald E: Cycle inhibiting factors (CIFs) are a
growing family of functional cyclomodulins present in invertebrate
and mammal bacterial pathogens. PLoS One. 4:e48552009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nougayrede JP, Homburg S, Taieb F, Boury
M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt
U and Oswald E: Escherichia coli induces DNA double-strand breaks
in eukaryotic cells. Science. 313:848–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cuevas-Ramosa G, Petita CR, Marcqa I,
Bourya M, Oswalda E and Nougayrède JP: Escherichia coli induces DNA
damage in vivo and triggers genomic instability in mammalian cells.
Proc Natl Acad Sci USA. 107:11357–11542. 2010.
|
|
69
|
Kwong TNY, Wang X, Nakatsu G, Chow TC,
Tipoe T, Dai RZW, Tsoi KKK, Wong MCS, Tse G, Chan MTV, et al:
Association between bactereia from specific microbes and subsequent
diagnosis of colorectal cancer. Gastroenterology. 155:383–390.e8.
2018. View Article : Google Scholar
|
|
70
|
Tsoi H, Chu ESH, Zhang X, Sheng J, Nakatsu
G, Ng SC, Chan AWH, Chan FKL, Sung JJY and Yu J: Peptostreptococcus
anaerobius induces intracellular cholesterol biosynthesis in colon
cells to induce proliferation and causes dysplasia in mice.
Gastroenterology. 152:1419–1433.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Purcell RV, Visnovska M, Biggs PJ,
Schmeier S and Frizelle FA: Distinct gut microbiome patterns
associate with consensus molecular subtypes of colorectal cancer.
Sci Rep. 7:115902017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Long X, Wong CC, Tong L, Chu ESH, Ho Szeto
C, Go MYY, Coker OO, Chan AWH, Chan FKL, Sung JJY and Yu J:
Peptostreptococcus anaerobius promotes colorectal carcinogenesis
and modulates tumour immunity. Nat Microbiol. 4:2319–2330. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kumar R, Herold JL, Schady D, Davis J,
Kopetz S, Martinez-Moczygemba M, Murray BE, Han F, Li Y, Callaway
E, et al: Streptococcus gallolyticus Subsp gallolyticus promotes
colorectal tumor development. PLoS Pathog. 13:e10064402017.
View Article : Google Scholar
|
|
74
|
Aymeric L, Donnadieu F, Mulet C, du Merle
L, Nigro G, Saffarian A, Bérard M, Poyart C, Robine S, Regnault B,
et al: Colorect a l ca ncer sp eci f ic cond itions promote
Streptococcus gallolyticus gut colonization. Proc Natl Acad Sci
USA. 115:E283–E291. 2018. View Article : Google Scholar
|
|
75
|
Konstantinov SR, Kuipers EJ and
Peppelenbosch MP: Functional genomic analysis of Gut microbiota for
CRC screening. Nat Rev Gastroenterol Hepatol. 10:741–745. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gamallat Y, Meyiah A, Kuugbee ED, Hago AM,
Chiwala G, Awadasseid A, Bamba D, Zhang X, Shang X, Luo F and Xin
Y: Lactobacillus rhamnosus induced epithelial cell apoptosis,
ameliorates inflammation and prevents colon cancer development in
an animal model. Biomed Pharmacother. 83:536–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wallace BD, Wang H, Lane KT, Scott JE,
Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S and Redinbo
MR: Alleviating cancer drug toxicity by inhibiting a bacterial
enzyme. Science. 330:831–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Marchesan J, Jiao YZ, Schaff RA, Hao J,
Morelli T, Kinney JS, Gerow E, Sheridan R, Rodrigues V, Paster BJ,
et al: TLR4, NOD1 and NOD2 mediate immune recognition of putative
newly identified periodontal pathogens. Mol Oral Microbiol.
31:243–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xu J, Yang M, Wang D, Zhang S, Yan S, Zhu
Y and Chen W: Alteration of the abundance of Parvimonas micra in
the gut along the adenoma-carcinoma sequence. Oncol Lett.
20:1062020. View Article : Google Scholar :
|
|
80
|
Allali I, Boukhatem N, Bouguenouch L,
Hardi H, Boudouaya HA, Cadenas MB, Ouldim K, Amzazi S,
Azcarate-Peril MA and Ghazal H: Gut microbiome of Moroccan
colorectal cancer patients. Med Microbiol Immunol (Berl).
207:211–225. 2018. View Article : Google Scholar
|
|
81
|
Yang Y, Cai Q, Shu XO, Steinwandel MD,
Blot WJ, Zheng W and Long J: Prospective study of oral microbiome
and colorectal cancer risk in low-income and African American
populations. Int J Cancer. 144:2381–2389. 2019. View Article : Google Scholar :
|
|
82
|
Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao
L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH and Yu J:
Multi-cohort analysis of colorectal cancer metagenome identified
altered bacteria across populations and universal bacterial
markers. Microbiome. 6:702018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sobrinho AR: Cytokine production in
response to endodontic infection in germ-free mice. Oral Microbiol
Immunol. 17:344–353. 2002. View Article : Google Scholar
|
|
84
|
Lomholt JA and Kilian M: Immunoglobulin A1
protease activity in Gemella haemolysans. J Clin Microbiol.
38:2760–2762. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Montalban-Arques A and Scharl M:
Intestinal microbiota and colorectal carcinoma: Implications for
pathogenesis, diagnosis, and therapy. EBioMedicine. 48:648–655.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Allen J and Sears CL: Impact of the gut
microbiome on the genome and epigenome of colon epithelial cells:
Contributions to colorectal cancer development. Genome Med.
11:112019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xia X, Wu WKK, Wong SH, Liu D, Kwong TNY,
Nakatsu G, Yan PS, Chuang YM, Chan MW, Coker OO, et al: Bacteria
pathogens drive host colonic epithelial cell promoter
hypermethylation of tumor suppressor genes in colorectal cancer.
Microbiome. 8:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sobhani I, Rotkopf H and Khazaie K:
Bacteria-related changes in host DNA methylation and risk for CRC.
Gut Microbes. 12:e18008982020. View Article : Google Scholar
|
|
89
|
Gagnière J, Bonnin V, Jarrousse AS,
Cardamone E, Agus A, Uhrhammer N, Sauvanet P, Déchelotte P, Barnich
N, Bonnet R, et al: Interactions between microsatellite instability
and human gut colonization by Escherichia coli in colorectal
cancer. Clin Sci (Lond). 131:471–485. 2017. View Article : Google Scholar
|
|
90
|
Foran E, Garrity-Park MM, Mureau C, Newell
J, Smyrk TC, Limburg PJ and Egan LJ: Upregulation of DNA
methyltransferase-mediated gene silencing, anchorage-independent
growth, and migration of colon cancer cells by interleukin-6. Mol
Cancer Res. 8:471–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li Y, Deuring J, Peppelenbosch MP, Kuipers
EJ, de Haar C and van der Woude CJ: IL-6-induced DNMT1 activity
mediates SOCS3 promoter hypermethylation in ulcerative
colitis-related colorectal cancer. Carcinogenesis. 3:1889–1896.
2012. View Article : Google Scholar
|
|
92
|
Hartnett L and Egan LJ: Inflammation, DNA
methylation and colitis-associated cancer. Carcinogenesis.
33:723–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hashemi Goradel N, Heidarzadeh S,
Jahangiri S, Farhood B, Mortezaee K, Khanlarkhani N and Neghadari
B: Fusobacterium nucleatum and colorectal cancer: A mechanistic
overview. J Cell Physiol. 234:2337–2344. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yu T, Guo F, Yu Y, Sun T, Ma D, Han J,
Qian Y, Kryczek I, Sun D, Nagarsheth N, et al: Fusobacterium
nucleatum promotes chemoresistance to colorectal cancer by
modulating autophagy. Cell. 170:548–563.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tarallo S, Ferrero G, Gallo G, Francavilla
A, Clerico G, Realis Luc A, Manghi P, Thomas AM, Vineis P, Segata
N, et al: Altered fecal small RNA profiles in colorectal cancer
reflected gut microbiome composition in stool samples. mSystems.
4:200289–19. 2019. View Article : Google Scholar
|
|
96
|
Yuan C, Steer CJ and Subramanian S:
Host-microRNA-Microbiota interaction in colorectal cancer. Genes
(Basel). 10:2702019. View Article : Google Scholar
|
|
97
|
Kang M and Martin A: Microbiome and
colorectal cancer: Unraveling host-microbiota interactions in
colitis-associated colorectal cancer development. Semin Immunol.
13:3–13. 2017. View Article : Google Scholar
|
|
98
|
Cooks T, Pateras IS, Tarcic O, Solomon H,
Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld
N, et al: Mutant p53 prolongs NF-κB activation and promotes chronic
inflammation and inflammation-associated colorectal cancer. Cancer
Cell. 23:634–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lasry A, Zinger A and Ben-Neriah Y:
Inflammatory networks underlying colorectal cancer. Nat Immunol.
17:230–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ruskov H, Burcharth J and Pommergaard HC:
Linking gut microbiota to colorectal cancer. J Cancer. 8:3378–3395.
2017. View Article : Google Scholar
|
|
101
|
Grivennikov SI: Inflammation and
colorectal cancer: Colitisassociated neoplasia. Semin Immunopathol.
35:229–244. 2013. View Article : Google Scholar
|
|
102
|
Coussens LM and Pollard JW: Leukocytes in
mammary development and cancer. Cold Spring Harb Perspect Biol.
3:a0032852011. View Article : Google Scholar
|
|
103
|
Chen T, Li Q, Wu J, Wu Y, Peng W, Li H,
Wang J, Tang X, Peng Y and Fu X: Fusobacterium nucleatum promotes
M2 polarization of macrophages in the microenvironment of
colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol
Immunother. 67:1635–1646. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Drewes JL, Housseau F and Sears CL:
Sporadic colorectal cancer: Microbial contributors to disease
prevention, development and therapy. Br J Cancer. 115:273–280.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dejea CM, Wick EC, Hechenbleikner EM,
White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC,
Borisy GG, Lazarev M, et al: Microbiota organization is a distinct
feature of proximal colorectal cancers. Proc Natl Acad Sci USA.
111:18321–18326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Villéger R, Lopès A, Veziant J, Gagnière
J, Barnich N, Billard E, Boucher D and Bonnet M: Microbial markers
in colorectal cancer detection and/or prognosis. World J
Gastroenterol. 24:2327–2347. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kostic AD, Gevers D, Pedamallu CS, Michaud
M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et
al: Genomic analysis identifies association of Fusobacterium with
colorectal carcinoma. Genome Res. 22:292–298. 2012. View Article : Google Scholar :
|
|
108
|
Yu J, Feng Q, Wong SH, Zhang D, Liang QY,
Qin Y, Tang L, Zhao H, Stenvang J, Li Y, et al: Metagenomic
analysis of faecal microbiome as a tool towards targeted
non-invasive biomarkers for colorectal cancer. Gut. 66:70–78. 2017.
View Article : Google Scholar
|
|
109
|
Wei Z, Cao S, Liu S, Yao Z, Sun T, Li Y,
Li J, Zhang D and Zhou Y: Could gut microbiota serve as prognostic
biomarker associated with colorectal cancer patients' survival? A
pilot study on relevant mechanism. Oncotarget. 7:46158–46170. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wu Y, Ziao N, Zhu R, Zhang Y, Wu D, Wang
AJ, Fang S, Tao L, Li Y, Cheng S, et al: Identification of
microbial markers across populations in early detection of
colorectal cancer. bioRixv. https://doi.org/10.1101/2020.08.16.253344.
|
|
111
|
Liu S, da Cunha AP, Rezende RM, Cialic R,
Wei Z, Bry L, Comstock LE, Gandhi R and Weiner HL: The host shapes
the gut Microbiota via fecal MicroRNA. Cell Host Microbe. 19:32–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yuan C, Burns MB, Subramanian S and
Blekhman R: Interaction between Host MicroRNAs and the Gut
Microbiota in Colorectal Cancer. mSystems. 3:e00205–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Sarshar M, Scribano D, Ambrosi C, Palamara
AT and Masotti A: Fecal microRNAs as innovative biomarkers of
intestinal diseases and effective players in Host-Microbiome
interactions. Cancers (Basel). 12:21742020. View Article : Google Scholar
|
|
114
|
Yang T, Owen JL, Lightfoot YL, Kladde MP
and Mohamadzadeh M: Microbiota impact on the epigenetic regulation
of colorectal cancer. Trends Mol Med. 19:714–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li M, Chen WD and Wang YD: The roles of
the gut microbiotamiRNA interaction in the host pathophysiology.
Mol Med. 26:1012020. View Article : Google Scholar
|
|
116
|
Geller LT, Barzily-Rokni M, Danino T,
Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee
K, et al: Potential role of intratumor bacteria in mediating tumor
resistance to the chemotherapeutic drug gemcitabine. Science.
357:1156–1160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Serna G, Ruiz-Pace F, Hernando J, Alonso
L, Fasani R, Landolfi S, Comas R, Jimenez J, Elez E, Bullman S, et
al: Fusobacterium nucleatum persistance and risk of recurrence
after preoperative treatment in locally advanced rectal cancer. Ann
Oncol. 31:1366–1375. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Iida N, Dzutsev A, Stewart CA, Smith L,
Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S,
et al: Commensal bacteria control cancer response to therapy by
modulating the tumor microenvironment. Science. 342:967–970. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Viaud S, Saccheri F, Mignot G, Yamazaki T,
Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ,
et al: The intestinal microbiota modulates the anticancer immune
effects of cyclophosphamide. Science. 342:971–976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Xu X and Zhang X: Effects of
cyclophosphamide on immune system and gut microbiota in mice.
Microbiol Res. 171:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley JM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
activity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Vétizou M, Pitt JM, Daillère R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Uribe-Herranz M, Bittinger K, Rafail S,
Guedan S, Pierini S, Tanes C, Ganetsky A, Morgan MA, Gill S, Tanyi
JL, et al: Gut microbiota modulates adoptive cell therapy via CD8α
dendritic cells and IL-12. JCI Insight. 3:e949522018. View Article : Google Scholar
|
|
124
|
Hold GL: Gastrointestinal microbiota and
colon cancer. Dig Dis. 34:244–250. 2016. View Article : Google Scholar : PubMed/NCBI
|