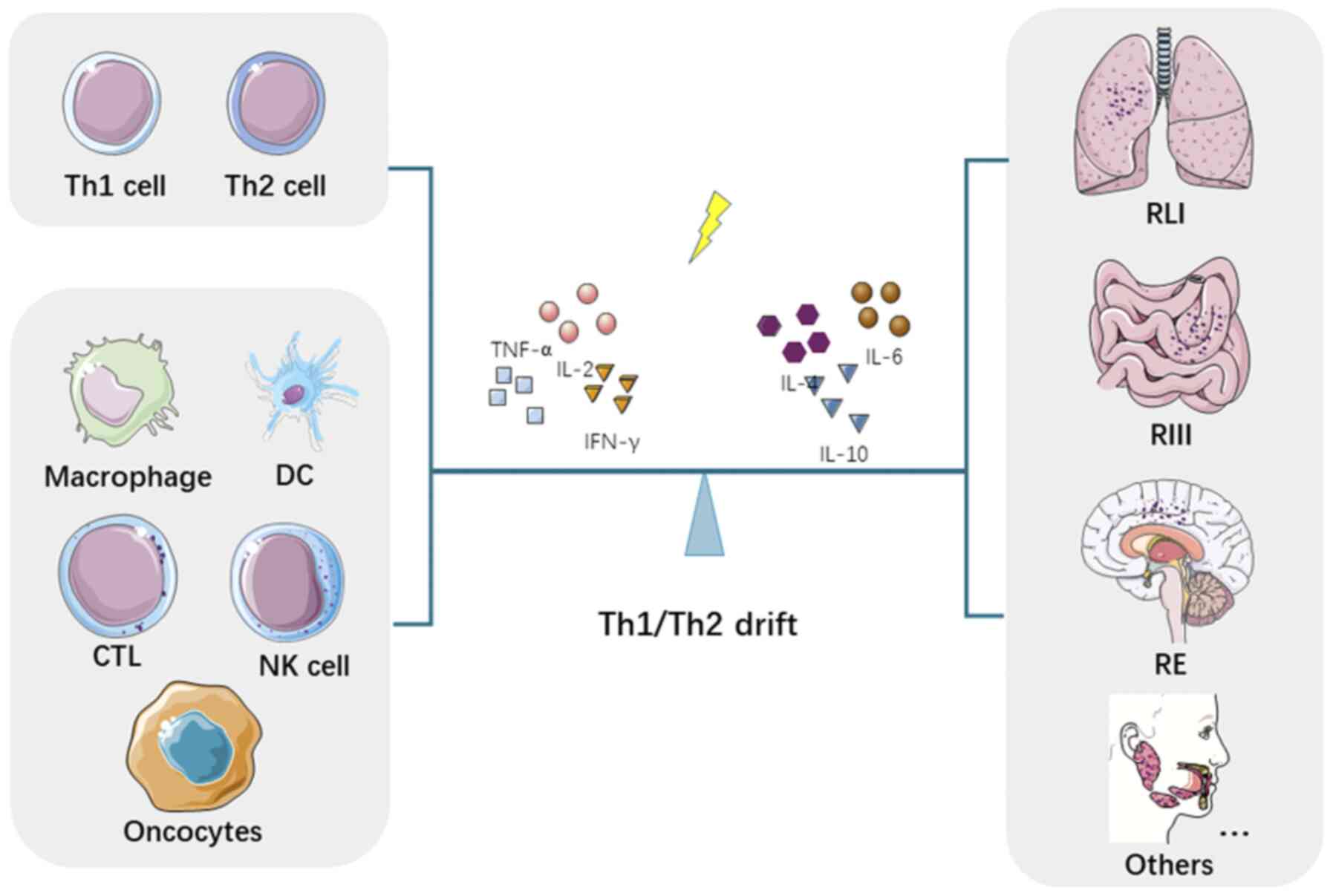
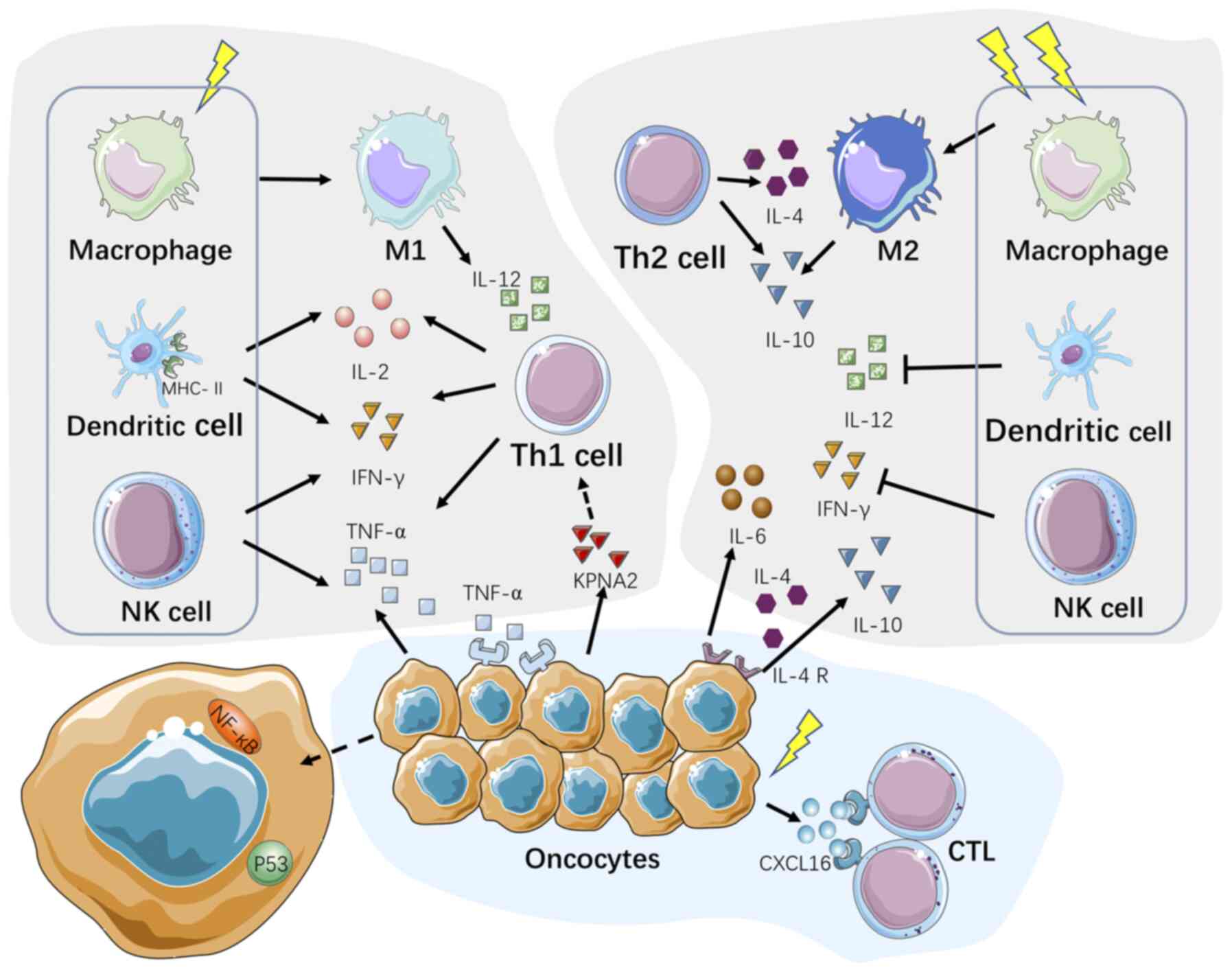
|
1
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4+ T cell help in cancer
immunology and immunotherapy. Nat Rev Immunol. 18:635–647. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Linehan WM and Ricketts CJ: The cancer
genome atlas of renal cell carcinoma: Findings and clinical
implications. Nat Rev Urol. 16:539–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skinnider BF and Mak TW: The role of
cytokines in classical Hodgkin lymphoma. Blood. 99:4283–4297. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Fan H and Jiang S: CD4(+) T-cell
subsets in transplantation. Immunol Rev. 252:183–191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masjedi A, Hashemi V, Hojjat-Farsangi M,
Ghalamfarsa G, Azizi G, Yousefi M and Jadidi-Niaragh F: The
significant role of interleukin-6 and its signaling pathway in the
immunopathogenesis and treatment of breast cancer. Biomed
Pharmacother. 108:1415–1424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu J and Paul WE: CD4 T cells: Fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan YY: GATA3: A master of many trades in
immune regulation. Trends Immunol. 35:233–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maazi H and Akbari O: Type two innate
lymphoid cells: The Janus cells in health and disease. Immunol Rev.
278:192–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STAT1-induced
regulator of IL-12R expression in naïve CD4+ T cells.
Nat Immunol. 3:549–557. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian L, Goldstein A, Wang H, Ching Lo H,
Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, et
al: Mutual regulation of tumour vessel normalization and
immunostimulatory reprogramming. Nature. 544:250–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Darawish Y, Li W, Yamanishi K, Pencheva
M, Oka N, Yamanishi H, Matsuyama T, Tanaka Y, Minato N and Okamura
H: Frontline Science: IL-18 primes murine NK cells for
proliferation by promoting protein synthesis, survival, and
autophagy. J Leukoc Biol. 104:253–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta S and Gollapudi S: Molecular
mechanisms of TNF-alpha-induced apoptosis in naïve and memory T
cell subsets. Autoimmun Rev. 5:264–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vadevoo SMP, Kim JE, Gunassekaran GR, Jung
HK, Chi L, Kim DE, Lee SH, Im SH and Lee B: IL4 receptor-targeted
proapoptotic peptide blocks tumor growth and metastasis by
enhancing antitumor immunity. Mol Cancer Ther. 16:2803–2816. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oft M: Immune regulation and cytotoxic T
cell activation of IL-10 agonists-preclinical and clinical
experience. Semin Immunol. 44:1013252019. View Article : Google Scholar
|
|
18
|
Urosevic M and Dummer R: HLA-G and IL-10
expression in human cancer-different stories with the same message.
Semin Cancer Biol. 13:337–342. 2003. View Article : Google Scholar
|
|
19
|
Sallusto F, Lenig D, Mackay CR and
Lanzavecchia A: Flexible programs of chemokine receptor expression
on human polarized T helper 1 and 2 lymphocytes. J Exp Med.
187:875–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annunziato F, Galli G, Cosmi L, Romagnani
P, Manetti R, Maggi E and Romagnani S: Molecules associated with
human Th1 or Th2 cells. Eur Cytokine Netw. 9(3 Suppl): S12–S16.
1998.
|
|
21
|
Annunziato F, Manetti R, Tomasévic I,
Guidizi MG, Biagiotti R, Giannò V, Germano P, Mavilia C, Maggi E
and Romagnani S: Expression and release of LAG-3-encoded protein by
human CD4+ T cells are associated with IFN-gamma production. FASEB
J. 10:769–776. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szabo SJ, Dighe AS, Gubler U and Murphy
KM: Regulation of the interleukin (IL)-12R beta 2 subunit
expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med.
185:817–824. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loetscher P, Uguccioni M, Bordoli L,
Baggiolini M, Moser B, Chizzolini C and Dayer JM: CCR5 is
characteristic of Th1 lymphocytes. Nature. 391:344–345. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin S, Rottman JB, Myers P, Kassam N,
Weinblatt M, Loetscher M, Koch AE, Moser B and Mackay CR: The
chemokine receptors CXCR3 and CCR5 mark subsets of T cells
associated with certain inflammatory reactions. J Clin Invest.
101:746–754. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu D, Chan WL, Leung BP, Hunter D, Schulz
K, Carter RW, McInnes IB, Robinson JH and Liew FY: Selective
expression and functions of interleukin 18 receptor on T helper
(Th) type 1 but not Th2 cells. J Exp Med. 188:1485–1492. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo
D, Sozzani S, Mantovani A and Sinigaglia F: Selective up-regulation
of chemokine receptors CCR4 and CCR8 upon activation of polarized
human type 2 Th cells. J Immunol. 161:5111–5115. 1998.PubMed/NCBI
|
|
28
|
Cosmi L, Annunziato F, Galli MIG, Maggi
RME, Nagata K and Romagnani S: CRTH2 is the most reliable marker
for the detection of circulating human type 2 Th and type 2 T
cytotoxic cells in health and disease. Eur J Immunol. 30:2972–2979.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Groux H, Sornasse T, Cottrez F, de Vries
JE, Coffman RL, Roncarolo MG and Yssel H: Induction of human T
helper cell type 1 differentiation results in loss of IFN-gamma
receptor beta-chain expression. J Immunol. 158:5627–5631.
1997.PubMed/NCBI
|
|
30
|
Jourdan P, Abbal C, Noraz N, Hori T,
Uchiyama T, Vendrell JP, Bousquet J, Taylor N, Pène J and Yssel H:
IL-4 induces functional cell-surface expression of CXCR4 on human T
cells. J Immunol. 160:4153–4157. 1998.PubMed/NCBI
|
|
31
|
Xu D, Chan WL, Leung BP, Huang Fp, Wheeler
R, Piedrafita D, Robinson JH and Liew FY: Selective expression of a
stable cell surface molecule on type 2 but not type 1 helper T
cells. J Exp Med. 187:787–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Del Prete G, De Carli M, D'Elios MM,
Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E and
Romagnani S: CD30-mediated signaling promotes the development of
human T helper type 2-like T cells. J Exp Med. 182:1655–1661. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sallusto F, Mackay CR and Lanzavecchia A:
Selective expression of the eotaxin receptor CCR3 by human T helper
2 cells. Science. 277:2005–2007. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weinstein JS, Laidlaw BJ, Lu Y, Wang JK,
Schulz VP, Li N, Herman EI, Kaech SM, Gallagher PG and Craft J:
STAT4 and T-bet control follicular helper T cell development in
viral infections. J Exp Med. 215:337–355. 2018. View Article : Google Scholar :
|
|
35
|
Christodoulopoulos P, Cameron L, Nakamura
Y, Lemière C, Muro S, Dugas M, Boulet LP, Laviolette M, Olivenstein
R and Hamid Q: TH2 cytokine-associated transcription factors in
atopic and nonatopic asthma: Evidence for differential signal
transducer and activator of transcription 6 expression. J Allergy
Clin Immunol. 107:586–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng W and Flavell RA: The transcription
factor GATA-3 is necessary and sufficient for Th2 cytokine gene
expression in CD4 T cells. Cell. 89:587–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaplan MH, Schindler U, Smiley ST and
Grusby MJ: Stat6 is required for mediating responses to IL-4 and
for development of Th2 cells. Immunity. 4:313–319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ho IC, Hodge MR, Rooney JW and Glimcher
LH: The proto-oncogene c-maf is responsible for tissue-specific
expression of interleukin-4. Cell. 85:973–983. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han SK, Song JY, Yun YS and Yi SY: Effect
of gamma radiation on cytokine expression and cytokine-receptor
mediated STAT activation. Int J Radiat Biol. 82:686–697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ridnour LA, Cheng RY, Weiss JM, Kaur S,
Soto-Pantoja DR, Basudhar D, Heinecke JL, Stewart CA, DeGraff W,
Sowers AL, et al: NOS inhibition modulates immune polarization and
improves radiation-induced tumor growth delay. Cancer Res.
75:2788–2799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Attar M, Molaie Kondolousy Y and Khansari
N: Effect of high dose natural ionizing radiation on the immune
system of the exposed residents of Ramsar Town, Iran. Iran J
Allergy Asthma Immunol. 6:73–78. 2007.PubMed/NCBI
|
|
42
|
Karkanitsa L, Mitskevitch P, Uss A,
Ostapenko V and Dainiak N: Elevated levels of cytokine gene
expression in leukemic hemopoietic cells of belorussians exposed to
ionizing radiation (IR) following the chernobyl catastrophe. Blood.
96:295A2000.
|
|
43
|
Han SK, Song JY, Yun YS and Yi SY: Ginsan
improved Th1 immune response inhibited by gamma radiation. Arch
Pharm Res. 28:343–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kunwar A, Bag PP, Chattopadhyay S, Jain VK
and Priyadarsini KI: Anti-apoptotic, anti-inflammatory, and
immunomodulatory activities of 3,3′-diselenodipropionic acid in
mice exposed to whole body γ-radiation. Arch Toxicol. 85:1395–1405.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Li B, Jia X, Ma Y, Gu Y, Zhang P,
Wei Q, Cai J, Cui J, Gao F and Yang Y: Radiation-induced decrease
of CD8+ dendritic cells contributes to Th1/Th2 shift. Int
Immunopharmacol. 46:178–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mishra S, Patel DD, Bansal DD and Kumar R:
Semiquinone glucoside derivative provides protection against
γ-radiation by modulation of immune response in murine model.
Environ Toxicol. 31:478–488. 2016. View Article : Google Scholar
|
|
47
|
Malhotra P, Adhikari M, Mishra S, Singh S,
Kumar P, Singh SK and Kumar R: N-acetyl tryptophan glucopyranoside
(NATG) as a countermeasure against gamma radiation-induced
immunosuppression in murine macrophage J774A.1 cells. Free Radic
Res. 50:1265–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nadella V, Ranjan R, Senthilkumaran B,
Qadri SSYH, Pothani S, Singh AK, Gupta ML and Prakash H:
Podophyllotoxin and rutin modulate M1 (iNOS+) macrophages and
mitigate lethal radiation (LR) induced inflammatory responses in
mice. Front Immunol. 10:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu XD, Ma SM and Liu SZ: Effects of 0.075
Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice.
Phys Med Biol. 48:2041–2049. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao H, Dong Z, Gong X, Dong J, Zhang Y,
Wei W, Wang R and Jin S: Effects of various radiation doses on
induced T-helper cell differentiation and related cytokine
secretion. J Radiat Res. 59:395–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karimi G, Balali-Mood M, Alamdaran SA,
Badie-Bostan H, Mohammadi E, Ghorani-Azam A, Sadeghi M and
Riahi-Zanjani B: Increase in the Th1-cell-based immune response in
healthy workers exposed to low-dose radiation-immune system status
of radiology staff. J Pharmacopuncture. 20:107–111. 2017.PubMed/NCBI
|
|
52
|
Cho SJ, Kang H, Hong EH, Kim JY and Nam
SY: Transcriptome analysis of low-dose ionizing radiation-impacted
genes in CD4+ T-cells undergoing activation and
regulation of their expression of select cytokines. J
Immunotoxicol. 15:137–146. 2018. View Article : Google Scholar
|
|
53
|
Bogdándi EN, Balogh A, Felgyinszki N,
Szatmári T, Persa E, Hildebrandt G, Sáfrány G and Lumniczky K:
Effects of low-dose radiation on the immune system of mice after
total-body irradiation. Radiat Res. 174:480–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Elhadary AA, Marzook EA and Abdelmonem HA:
Evaluation of the level of gamma radiation dose on some immune
system parameters against cancer. Biosci J. 35:307–316. 2019.
View Article : Google Scholar
|
|
55
|
Ghazy AA, Abu El-Nazar SY, Ghoneim HE,
Taha AR and Abouelella AM: Effect of murine exposure to gamma rays
on the interplay between Th1 and Th2 lymphocytes. Front Pharmacol.
6:742015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu X, Liu Z, Wang D, Han Y, Hu S, Xie Y,
Liu Y, Zhu M, Guan H, Gu Y and Zhou PK: Effects of low dose
radiation on immune cells subsets and cytokines in mice. Toxicol
Res (Camb). 9:249–262. 2020. View Article : Google Scholar
|
|
57
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar
|
|
58
|
Arpinati M, Green CL, Heimfeld S, Heuser
JE and Anasetti C: Granulocyte-colony stimulating factor mobilizes
T helper 2-inducing dendritic cells. Blood. 95:2484–2490. 2000.
View Article : Google Scholar
|
|
59
|
Jutel M and Akdis CA: T-cell subset
regulation in atopy. Curr Allergy Asthma Rep. 11:139–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Merrick A, Errington F, Milward K,
O'Donnell D, Harrington K, Bateman A, Pandha H, Vile R, Morrison E,
Selby P and Melcher A: Immunosuppressive effects of radiation on
human dendritic cells: Reduced IL-12 production on activation and
impairment of naive T-cell priming. Br J Cancer. 92:1450–1458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Clerici M, Shearer GM and Clerici E:
Cytokine dysregulation in invasive cervical carcinoma and other
human neoplasias: Time to consider the TH1/TH2 paradigm. J Natl
Cancer Inst. 90:261–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lappin MB and Campbell JD: The Th1-Th2
classification of cellular immune responses: Concepts, current
thinking and applications in haematological malignancy. Blood Rev.
14:228–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Backer RA, Diener N and Clausen BE:
Langerin+CD8+ dendritic cells in the splenic
marginal zone: Not so marginal after all. Front Immunol.
10:7412019. View Article : Google Scholar
|
|
64
|
Prendergast KA, Daniels NJ, Petersen TR,
Hermans IF and Kirman JR: Langerin+ CD8α+
dendritic cells drive early CD8+ T cell activation and
IL-12 production during systemic bacterial infection. Front
Immunol. 9:9532018. View Article : Google Scholar
|
|
65
|
Yu N, Wang S, Song X, Gao L, Li W, Yu H,
Zhou C, Wang Z, Li F and Jiang Q: Low-dose radiation promotes
dendritic cell migration and IL-12 production via the ATM/NF-kappaB
pathway. Radiat Res. 189:409–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shigematsu A, Adachi Y, Koike-Kiriyama N,
Suzuki Y, Iwasaki M, Koike Y, Nakano K, Mukaide H, Imamura M and
Ikehara S: Effects of low-dose irradiation on enhancement of
immunity by dendritic cells. J Radiat Res. 48:51–55. 2007.
View Article : Google Scholar
|
|
67
|
Murray PJ: Macrophage polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar
|
|
68
|
Orecchioni M, Ghosheh Y, Pramod AB and Ley
K: Macrophage polarization: Different gene signatures in M1(LPS+)
vs. classically and M2(LPS-) vs. alternatively activated
macrophages. Front Immunol. 10:10842019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiratori H, Feinweber C, Luckhardt S,
Wallner N, Geisslinger G, Weigert A and Parnham MJ: An in vitro
test system for compounds that modulate human inflammatory
macrophage polarization. Eur J Pharmacol. 833:328–338. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nadella V, Singh S, Jain A, Jain M,
Vasquez KM, Sharma A, Tanwar P, Rath GK and Prakash H: Low dose
radiation primed iNOS + M1macrophages modulate angiogenic
programming of tumor derived endothelium. Mol Carcinog.
57:1664–1671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Knoops L, Haas R, de Kemp S, Majoor D,
Broeks A, Eldering E, de Boer JP, Verheij M, van Ostrom C, de Vries
A, et al: In vivo p53 response and immune reaction underlie highly
effective low-dose radiotherapy in follicular lymphoma. Blood.
110:1116–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Nguy S, Alothman S, Alqunaibit D, Avanzi A, Daley D, Barilla R, et
al: Radiation therapy induces macrophages to suppress T-cell
responses against pancreatic tumors in mice. Gastroenterology.
150:1659–1672.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Okubo M, Kioi M, Nakashima H, Sugiura K,
Mitsudo K, Aoki I, Taniguchi H and Tohnai I: M2-polarized
macrophages contribute to neovasculogenesis, leading to relapse of
oral cancer following radiation. Sci Rep. 6:275482016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fu E, Liu T, Yu S, Chen X, Song L, Lou H,
Ma F, Zhang S, Hussain S, Guo J, et al: M2 macrophages reduce the
radiosensitivity of head and neck cancer by releasing HB-EGF. Oncol
Rep. 44:698–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Reading JL, Gálvez-Cancino F, Swanton C,
Lladser A, Peggs KS and Quezada SA: The function and dysfunction of
memory CD8+ T cells in tumor immunity. Immunol Rev.
283:194–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Crespo J, Sun H, Welling TH, Tian Z and
Zou W: T cell anergy, exhaustion, senescence, and stemness in the
tumor microenvironment. Curr Opin Immunol. 25:214–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y,
Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al: Therapeutic
effects of ablative radiation on local tumor require
CD8+ T cells: Changing strategies for cancer treatment.
Blood. 114:589–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lugade AA, Moran JP, Gerber SA, Rose RC,
Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma
tumors increases the generation of tumor antigen-specific effector
cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Takeshima T, Chamoto K, Wakita D, Ohkuri
T, Togashi Y, Shirato H, Kitamura H and Nishimura T: Local
radiation therapy inhibits tumor growth through the generation of
tumor-specific CTL: Its potentiation by combination with Th1 cell
therapy. Cancer Res. 70:2697–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chattopadhyay S and Chakraborty NG:
Continuous presence of Th1 conditions is necessary for longer
lasting tumor-specific CTL activity in stimulation cultures with
PBL. Hum Immunol. 66:884–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harada M, Matsueda S, Yao A, Noguchi M and
Itoh K: Vaccination of cytotoxic T lymphocyte-directed peptides
elicited and spread humoral and Th1-type immune responses to
prostate-specific antigen protein in a prostate cancer patient. J
Immunother. 28:368–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yokouchi H, Chamoto K, Wakita D, Yamazaki
K, Shirato H, Takeshima T, Dosaka-Akita H, Nishimura M, Yue Z,
Kitamura H and Nishimura T: Combination tumor immunotherapy with
radiotherapy and Th1 cell therapy against murine lung carcinoma.
Clin Exp Metastasis. 24:533–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Terrén I, Orrantia A, Vitallé J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hodgins JJ, Khan ST, Park MM, Auer RC and
Ardolino M: Killers 2.0: NK cell therapies at the forefront of
cancer control. J Clin Invest. 129:3499–3510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wei H, Zheng X, Lou D, Zhang L, Zhang R,
Sun R and Tian Z: Tumor-induced suppression of interferon-gamma
production and enhancement of interleukin-10 production by natural
killer (NK) cells: Paralleled to CD4+ T cells. Mol
Immunol. 42:1023–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang G, Kong Q, Wang G, Jin H, Zhou L, Yu
D, Niu C, Han W, Li W and Cui J: Low-dose ionizing radiation
induces direct activation of natural killer cells and provides a
novel approach for adoptive cellular immunotherapy. Cancer Biother
Radiopharm. 29:428–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cheda A, Wrembel-Wargocka J, Lisiak E,
Nowosielska EM, Marciniak M and Janiak MK: Single low doses of X
rays inhibit the development of experimental tumor metastases and
trigger the activities of NK cells in mice. Radiat Res.
161:335–340. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Miller GM, Andres ML and Gridley DS: NK
cell depletion results in accelerated tumor growth and attenuates
the antitumor effect of total body irradiation. Int J Oncol.
23:1585–1592. 2003.PubMed/NCBI
|
|
90
|
Park HR, Jung U and Jo SK: Impairment of
natural killer (NK) cells is an important factor in a weak Th1-like
response in irradiated mice. Radiat Res. 168:446–452. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zarcone D, Tilden AB, Lane VG and Grossi
CE: Radiation sensitivity of resting and activated nonspecific
cytotoxic cells of T lineage and NK lineage. Blood. 73:1615–1621.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou L, Zhang X, Li H, Niu C, Yu D, Yang
G, Liang X, Wen X, Li M and Cui J: Validating the pivotal role of
the immune system in low-dose radiation-induced tumor inhibition in
Lewis lung cancer-bearing mice. Cancer Med. 7:1338–1348. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhou J, Zhang J, Lichtenheld MG and
Meadows GG: A role for NF-kappa B activation in perforin expression
of NK cells upon IL-2 receptor signaling. J Immunol. 169:1319–1325.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar
|
|
95
|
Demaria S, Golden EB and Formenti SC: Role
of local radiation therapy in cancer immunotherapy. JAMA Oncol.
1:1325–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Simon PS, Bardhan K, Chen MR, Paschall AV,
Lu C, Bollag RJ, Kong FC, Jin J, Kong FM, Waller JL, et al: NF-κB
functions as a molecular link between tumor cells and Th1/Tc1 T
cells in the tumor microenvironment to exert radiation-mediated
tumor suppression. Oncotarget. 7:23395–23415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Matsumura S, Wang B, Kawashima N,
Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti
SC, Dustin ML and Demaria S: Radiation-induced CXCL16 release by
breast cancer cells attracts effector T cells. J Immunol.
181:3099–3107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Song KH, Jung SY, Kang SM, Kim MH, Ahn J,
Hwang SG, Lee JH, Lim DS, Nam SY and Song JY: Induction of
immunogenic cell death by radiation-upregulated karyopherin alpha 2
in vitro. Eur J Cell Biol. 95:219–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Song KH, Jung SY, Park JI, Ahn J, Park JK,
Um HD, Park IC, Hwang SG, Ha H and Song JY: Inhibition of
karyopherin-α2 augments radiation-induced cell death by perturbing
BRCA1-mediated DNA repair. Int J Mol Sci. 20:28432019. View Article : Google Scholar
|
|
100
|
Huettner C, Paulus W and Roggendorf W:
Messenger RNA expression of the immunosuppressive cytokine IL-10 in
human gliomas. Am J Pathol. 146:317–322. 1995.PubMed/NCBI
|
|
101
|
Hao C, Parney IF, Roa WH, Turner J, Petruk
KC and Ramsay DA: Cytokine and cytokine receptor mRNA expression in
human glioblastomas: Evidence of Th1, Th2 and Th3 cytokine
dysregulation. Acta Neuropathol. 103:171–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen B, Alvarado DM, Iticovici M, Kau NS,
Park H, Parikh PJ, Thotala D and Ciorba MA: Interferon-induced IDO1
mediates radiation resistance and is a therapeutic target in
colorectal cancer. Cancer Immunol Res. 8:451–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hanania AN, Mainwaring W, Ghebre YT,
Hanania NA and Ludwig M: Radiation-induced lung injury: Assessment
and management. Chest. 156:150–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Giuranno L, Ient J, De Ruysscher D and
Vooijs MA: Radiation-induced lung injury (RILI). Front Oncol.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Stenmark MH, Cai XW, Shedden K, Hayman JA,
Yuan S, Ritter T, Ten Haken RK, Lawrence TS and Kong FM: Combining
physical and biologic parameters to predict radiation-induced lung
toxicity in patients with non-small-cell lung cancer treated with
definitive radiation therapy. Int J Radiat Oncol Biol Phys.
84:e217–e222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rübe CE, Rodemann HP and Rübe C: The
relevance of cytokines in the radiation-induced lung reaction.
Experimental basis and clinical significance. Strahlenther Onkol.
180:541–549. 2004.In German. View Article : Google Scholar
|
|
107
|
Arpin D, Perol D, Blay JY, Falchero L,
Claude L, Vuillermoz-Blas S, Martel-Lafay I, Ginestet C, Alberti L,
Nosov D, et al: Early variations of circulating interleukin-6 and
interleukin-10 levels during thoracic radiotherapy are predictive
for radiation pneumonitis. J Clin Oncol. 23:8748–8756. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Büttner C, Skupin A, Reimann T, Rieber EP,
Unteregger G, Geyer P and Frank KH: Local production of
interleukin-4 during radiation-induced pneumonitis and pulmonary
fibrosis in rats: Macrophages as a prominent source of
interleukin-4. Am J Respir Cell Mol Biol. 17:315–325. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen Y, Rubin P, Williams J, Hernady E,
Smudzin T and Okunieff P: Circulating IL-6 as a predictor of
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 49:641–648.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tang Y, Yang L, Qin W, Yi M, Liu B and
Yuan X: Validation study of the association between genetic variant
of IL4 and severe radiation pneumonitis in lung cancer patients
treated with radiation therapy. Radiother Oncol. 141:86–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Y, Guan X, Liu W, Chen HL, Truscott J,
Beyatli S, Metwali A, Weiner GJ, Zavazava N, Blumberg RS, et al:
Helminth-induced production of TGF-β and suppression of
graft-versus-host disease is dependent on IL-4 production by host
cells. J Immunol. 201:2910–2922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Groves AM, Johnston CJ, Misra RS, Williams
JP and Finkelstein JN: Effects of IL-4 on pulmonary fibrosis and
the accumulation and phenotype of macrophage subpopulations
following thoracic irradiation. Int J Radiat Biol. 92:754–765.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Han G, Zhang H, Xie CH and Zhou YF:
Th2-like immune response in radiation-induced lung fibrosis. Oncol
Rep. 26:383–388. 2011.PubMed/NCBI
|
|
114
|
Paun A, Bergeron ME and Haston CK: The
Th1/Th17 balance dictates the fibrosis response in murine
radiation-induced lung disease. Sci Rep. 7:115862017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xu L, Xiong S, Guo R, Yang Z, Wang Q, Xiao
F, Wang H, Pan X and Zhu M: Transforming growth factor β3
attenuates the development of radiation-induced pulmonary fibrosis
in mice by decreasing fibrocyte recruitment and regulating
IFN-gamma/IL-4 balance. Immunol Lett. 162:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chiang CS, Liu WC, Jung SM, Chen FH, Wu
CR, McBride WH, Lee CC and Hong JH: Compartmental responses after
thoracic irradiation of mice: Strain differences. Int J Radiat
Oncol Biol Phys. 62:862–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang C, Zhao H, Li BL, Fu-Gao, Liu H, Cai
JM and Zheng M: CpG-oligodeoxynucleotides may be effective for
preventing ionizing radiation induced pulmonary fibrosis. Toxicol
Lett. 292:181–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang Y, Liu W, Liu H, Yang Y, Cui J,
Zhang P, Zhao H, He F, Cheng Y, Ni J, et al: Grape seed
pro-anthocyanidins ameliorates radiation-induced lung injury. J
Cell Mol Med. 18:1267–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen J, Wang Y, Mei Z, Zhang S, Yang J, Li
X, Yao Y and Xie C: Radiation-induced lung fibrosis in a
tumor-bearing mouse model is associated with enhanced Type-2
immunity. J Radiat Res. 57:133–141. 2016. View Article : Google Scholar :
|
|
120
|
Oh K, Seo MW, Kim YW and Lee DS:
Osteopontin potentiates pulmonary inflammation and fibrosis by
modulating IL-17/IFN-γ-secreting T-cell ratios in bleomycin-treated
mice. Immune Netw. 15:142–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lei L, Zhao C, Qin F, He ZY, Wang X and
Zhong XN: Th17 cells and IL-17 promote the skin and lung
inflammation and fibrosis process in a bleomycin-induced murine
model of systemic sclerosis. Clin Exp Rheumatol. 34(Suppl 100):
S14–S22. 2016.
|
|
122
|
Li Y, Zou L, Yang X, Chu L, Ni J, Chu X,
Guo T and Zhu Z: Identification of lncRNA, MicroRNA, and
mRNA-associated CeRNA network of radiation-induced lung injury in a
mice model. Dose Response. 17:15593258198910122019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hauer-Jensen M, Denham JW and Andreyev HJ:
Radiation enteropathy-pathogenesis, treatment and prevention. Nat
Rev Gastroenterol Hepatol. 11:470–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zheng J, Wang J, Pouliot M, Authier S,
Zhou D, Loose DS and Hauer-Jensen M: Gene expression profiling in
non-human primate jejunum, ileum and colon after total-body
irradiation: A comparative study of segment-specific molecular and
cellular responses. BMC Genomics. 16:9842015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Huang Z, Epperly M, Watkins SC,
Greenberger JS, Kagan VE and Bayır H: Necrostatin-1 rescues mice
from lethal irradiation. Biochim Biophys Acta. 1862:850–856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kim JS, Ryoo SB, Heo K, Kim JG, Son TG,
Moon C and Yang K: Attenuating effects of granulocyte-colony
stimulating factor (G-CSF) in radiation induced intestinal injury
in mice. Food Chem Toxicol. 50:3174–3180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kim JS, Yang M, Lee CG, Kim SD, Kim JK and
Yang K: In vitro and in vivo protective effects of granulocyte
colony-stimulating factor against radiation-induced intestinal
injury. Arch Pharm Res. 36:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Symon Z, Goldshmidt Y, Picard O, Yavzori
M, Ben-Horin S, Alezra D, Barshack I and Chowers Y: A murine model
for the study of molecular pathogenesis of radiation proctitis. Int
J Radiat Oncol Biol Phys. 76:242–250. 2010. View Article : Google Scholar
|
|
129
|
Sha H, Gu Y, Shen W, Zhang L, Qian F, Zhao
Y, Li H, Zhang T and Lu W: Rheinic acid ameliorates
radiation-induced acute enteritis in rats through PPAR-γ/NF-κB.
Genes Genomics. 41:909–917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lu L, Li W, Sun C, Kang S, Li J, Luo X, Su
Q, Liu B and Qin S: Phycocyanin ameliorates radiation-induced acute
intestinal toxicity by regulating the effect of the gut microbiota
on the TLR4/Myd88/NF-κB pathway. JPEN J Parenter Enteral Nutr.
44:1308–1317. 2020. View Article : Google Scholar
|
|
131
|
Wei YL, Xu JY, Zhang R, Zhang Z, Zhao L
and Qin LQ: Effects of lactoferrin on X-ray-induced intestinal
injury in Balb/C mice. Appl Radiat Isot. 146:72–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Radwan RR and Karam HM: Resveratrol
attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR
signaling pathway. Environ Toxicol. 35:223–230. 2020. View Article : Google Scholar
|
|
133
|
Wang H, Sun RT, Li Y, Yang YF, Xiao FJ,
Zhang YK, Wang SX, Sun HY, Zhang QW, Wu CT and Wang LS: HGF gene
modification in mesenchymal stem cells reduces radiation-induced
intestinal injury by modulating immunity. PLoS One.
10:e01244202015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H
and Jin X: Multi-therapeutic effects of human adipose-derived
mesenchymal stem cells on radiation-induced intestinal injury. Cell
Death Dis. 4:e6852013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Linard C, Strup-Perrot C, Lacave-Lapalun
JV and Benderitter M: Flagellin preconditioning enhances the
efficacy of mesenchymal stem cells in an irradiation-induced
proctitis model. J Leukoc Biol. 100:569–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Akpolat M, Gulle K, Topcu-Tarladacalisir
Y, Safi Oz Z, Bakkal BH, Arasli M and Ozel Turkcu U: Protection by
L-carnitine against radiation-induced ileal mucosal injury in the
rat: Pattern of oxidative stress, apoptosis and cytokines. Int J
Radiat Biol. 89:732–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bessout R, Demarquay C, Moussa L, René A,
Doix B, Benderitter M, Sémont A and Mathieu N: TH17 predominant
T-cell responses in radiation-induced bowel disease are modulated
by treatment with adipose-derived mesenchymal stromal cells. J
Pathol. 237:435–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Balentova S and Adamkov M: Molecular,
cellular and functional effects of radiation-induced brain injury:
A review. Int J Mol Sci. 16:27796–27815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Deng Z, Sui G, Rosa PM and Zhao W:
Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling
pathway in microglial cells. PLoS One. 7:e367392012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xue J, Dong JH, Huang GD, Qu XF, Wu G and
Dong XR: NF-κB signaling modulates radiation-induced microglial
activation. Oncol Rep. 31:2555–2560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Dong X, Luo M, Huang G, Zhang J, Tong F,
Cheng Y, Cai Q, Dong J, Wu G and Cheng J: Relationship between
irradiation-induced neuro-inflammatory environments and impaired
cognitive function in the developing brain of mice. Int J Radiat
Biol. 91:224–239. 2015. View Article : Google Scholar
|
|
142
|
Chen LJ, Zhang RG, Yu DD, Wu G and Dong
XR: Shenqi fuzheng injection ameliorates radiation-induced brain
injury. Curr Med Sci. 39:965–971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xin N, Li YJ, Li X, Wang X, Li Y, Zhang X,
Dai RJ, Meng WW, Wang HL, Ma H, et al: Dragon's blood may have
radioprotective effects in radiation-induced rat brain injury.
Radiat Res. 178:75–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chiang CS, Hong JH, Stalder A, Sun JR,
Withers HR and McBride WH: Delayed molecular responses to brain
irradiation. Int J Radiat Biol. 72:45–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Vozenin-Brotons MC, Gault N, Sivan V,
Tricaud Y, Dubray B, Clough K, Cosset JM, Lefaix JL and Martin M:
Histopathological and cellular studies of a case of cutaneous
radiation syndrome after accidental chronic exposure to a cesium
source. Radiat Res. 152:332–337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lee JW, Zoumalan RA, Valenzuela CD, Nguyen
PD, Tutela JP, Roman BR, Warren SM and Saadeh PB: Regulators and
mediators of radiation-induced fibrosis: Gene expression profiles
and a rationale for Smad3 inhibition. Otolaryngol Head Neck Surg.
143:525–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Blétry O and Somogyi A: Do the interferons
have an antifibrotic action? The internist's point of view. Rev Med
Interne. 23(Suppl 4): 511s–515s. 2002.In French. View Article : Google Scholar
|
|
148
|
Peter RU, Gottlöber P, Nadeshina N, Krähn
G, Braun-Falco O and Plewig G: Interferon gamma in survivors of the
Chernobyl power plant accident: New therapeutic option for
radiation-induced fibrosis. Int J Radiat Oncol Biol Phys.
45:147–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Oliva D, Nilsson M, Strandéus M, Andersson
BÅ, Sharp L, Laytragoon-Lewin N and Lewin F: Individual genetic
variation might predict acute skin reactions in women undergoing
adjuvant breast cancer radiotherapy. Anticancer Res. 38:6763–6770.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Takeda I, Kizu Y, Yoshitaka O, Saito I and
Yamane GY: Possible role of nitric oxide in radiation-induced
salivary gland dysfunction. Radiat Res. 159:465–470. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Moura JF, Mota JM, Leite CA, Wong DV,
Bezerra NP, Brito GA, Lima V, Cunha FQ and Ribeiro RA: A novel
model of megavoltage radiation-induced oral mucositis in hamsters:
Role of inflammatory cytokines and nitric oxide. Int J Radiat Biol.
91:500–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chung YL, Lee MY and Pui NN: Epigenetic
therapy using the histone deacetylase inhibitor for increasing
therapeutic gain in oral cancer: Prevention of radiation-induced
oral mucositis and inhibition of chemical-induced oral
carcinogenesis. Carcinogenesis. 30:1387–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Epperly MW, Gretton JA, DeFilippi SJ,
Greenberger JS, Sikora CA, Liggitt D and Koe G: Modulation of
radiation-induced cytokine elevation associated with esophagitis
and esophageal stricture by manganese superoxide
dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res.
155:2–14. 2001. View Article : Google Scholar
|
|
154
|
Moreb J and Zucali JR: The therapeutic
potential of interleukin-1 and tumor necrosis factor on
hematopoietic stem cells. Leuk Lymphoma. 8:267–275. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Boniver J, Humblet C, Rongy AM, Delvenne
C, Delvenne P, Greimers R, Thiry A, Courtoy R and Defresne MP:
Cellular aspects of the pathogenesis of radiation-induced thymic
lymphomas in C57 BL mice (review). In Vivo. 4:41–43.
1990.PubMed/NCBI
|