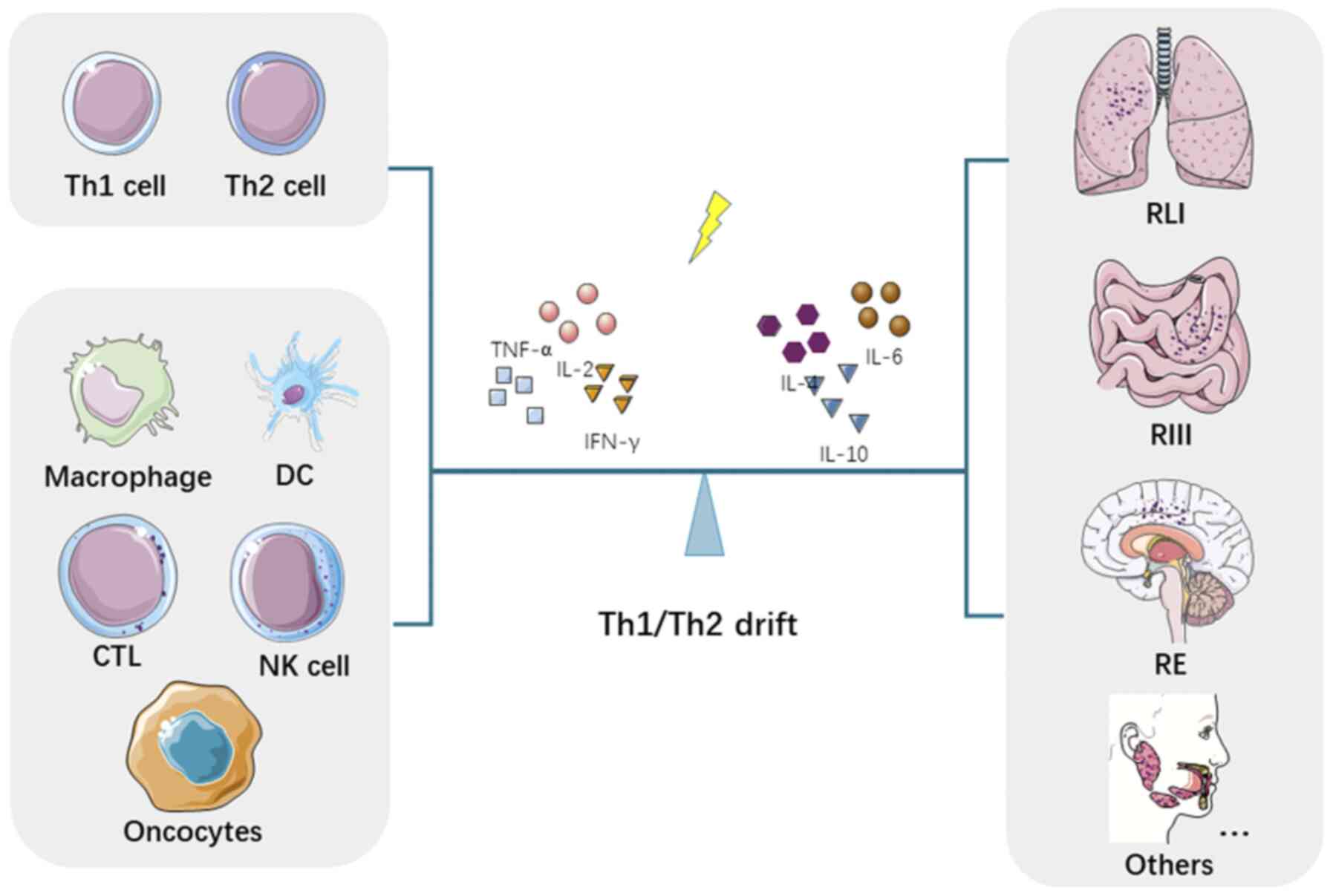
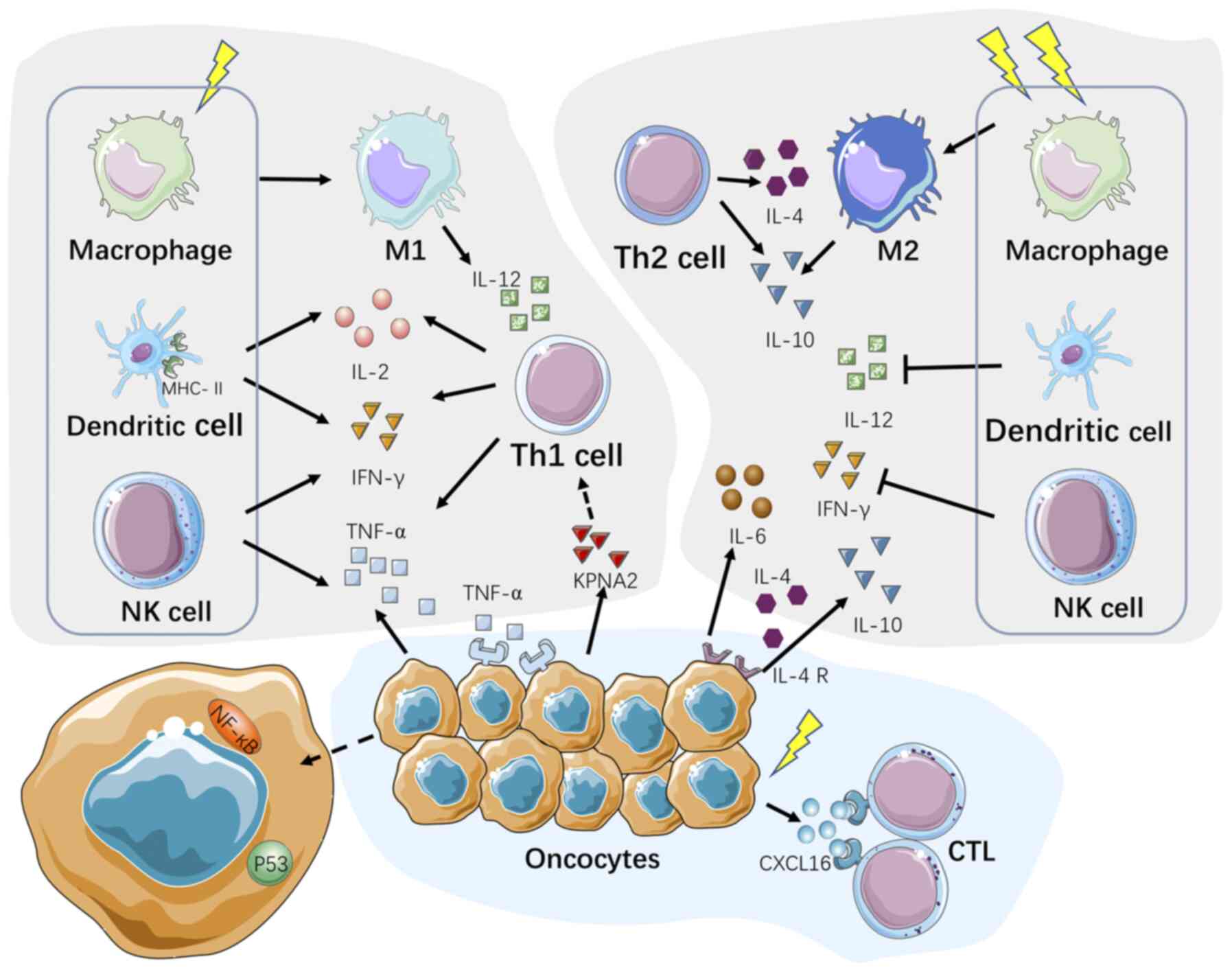
Over the past few decades, there have been
significant advances made in immunotherapy for malignant tumors,
from adaptive immunocyte modification to novel immune target
discovery (1). T helper (Th)
cells have been the subject of intensive research on the tumor
immune microenvironment (TIME), as they are involved in cellular
immunity together with cytotoxic T lymphocytes (CTLs) (2,3). T
helper type 1 (Th1) and type 2 (Th2) cells have been found to
sustain a functional balance in the normal immune system, while the
alterations in cell polarization and cytokine imbalance, referred
to as the Th1/Th2 shift, have been associated with numerous
immunity-related diseases, as well as malignant tumors (4,5).
Radiotherapy is one of the cornerstones of
therapeutic strategies for various tumors. Radiation destroys the
double DNA strands of susceptible tumor cells during meiosis,
without affecting surrounding tissues to the same extent. It has
also been reported that radiation may have a distinct impact on the
TIME during the course of prolonged clinical observation (6). Local irradiation markedly alters the
immunogenic status of the tumor cells and their ability to elicit
an immune response, enhances the initiation of CD8+ T
cells and notably augments the secretion of antitumor cytokines
(7).
Previous studies (discussed below) have shed light
on the impact of the Th1/Th2 shift in the presence of ionizing
radiation (IR). Furthermore, the potential role of the Th1/Th2
shift in tumorigenesis and tumor progression has been attracting
the attention of researchers. Therefore, the aim of the present
review was to summarize the specific implications and effects of
radiation on the Th1/Th2 shift in tumor tissues, from molecular
mechanisms to clinical impact. The causative association between
radiotherapy and immune response was particularly emphasized and
highlighted. The outline of this review is presented in Fig. 1.
In general, cytokines produced by Th1 cells serve as
suppressors against a tumor-promoting microenvironment. Th1-derived
IFN-γ induced by IL-12 from antigen-presenting cells has been
reported to stimulate the transcription of T-bet in Th1 cells,
upregulating IL-12Rβ signals through the JAK/STAT1 pathway, as a
positive feedback loop of the IFN-γ cascade (11). IFN-γ has an anti-angiogenic
function in the tumor environment, preventing tumor cells from
further infiltration and metastasis (12). Low-dose IL-2 binds to the IL-2
immunoreceptor β on the surface of natural killer (NK) cells,
thereby enhancing the phosphorylation of STAT3 and STAT5, followed
by the overexpression of cyclin B1, leading to selective NK cell
proliferation (13). TNF-α, as a
multifunctional cytokine, plays crucial roles in inflammation,
apoptosis and cell survival. The binding of TNF-α to its receptors
triggers cell apoptosis through the caspase cascade, NF-κB
activation and receptor-interacting protein recruitment (14). In addition, TNF-α targets the
tumor vasculature by destroying the vascular lining and causing
hyperpermeability (15). On the
other hand, cytokines secreted by Th2 cells are immunosuppressive
and promote tumor immune evasion in the TIME. For example, IL-4
combines with IL-4R to form an IL-4/IL-4Rα1 complex, and
phosphorylates STAT6, thereby increasing apoptotic resistance and
colonization of tumor cells (16). In addition to promoting
inflammation, IL-10 has been reported to suppress the expression of
major histocompatibility complex (MHC) I and the proliferation of
CD8+ T cells, markedly decreasing the cytotoxic effects
(17). Notably, IL-10 from tumor
cells was observed to abrogate the oncolytic activity of CTLs via
activating human leukocyte antigen-G (18).
In addition to differentially secreted humoral
factors derived from Th1/Th2 cell populations, both subtypes have
been found to be characterized by specific surface protein markers
throughout molecular experiments (19,20). IL-18R, IL-12Rβ2, C-C motif
chemokine receptor (CCR)5 and C-X-C motif chemokine receptor
(CXCR)3, along with lymphocyte activation gene-3, T-cell
immunoglobulin and mucin-domain containing-3, have been documented
to be highly expressed on Th1 cells (20-26). The Th2 cell population has
specific identifiers, such as CD30, CCR3, CCR4, CXCR4,
prostaglandin D2 receptor 2, IFN-γRβ and IL-1 receptor-like 1
(19,27-33). Moreover, a Th1/Th2 immune shift
occurs accordingly under the influence of different transcriptional
factors. T-box transcription factor 21 and STAT4 induce a type 1
shift, while c-Maf and GATA binding protein-3 (GATA-3) induce a
type 2 functional cascade (34-38). Due to the lack of affirmatory
surface identifiers, Th1/Th2 groups are still defined predominantly
based on the representative cytokines they produce.
Depending on the inhibitory roles of the various
cytokines, the Th2 shift in the TIME favors a tumor-supporting
environment, resulting in tumor immunological resistance.
In addition to the direct damage of DNA double
strands and the induction of reactive oxygen species in tumor
cells, IR also modulates the molecular balance from immunocytes in
the TIME, rendering tumor cells more susceptible or tolerant to IR.
Accumulating evidence has uncovered the role of the Th1/Th2 shift
induced by IR in the TIME, which consists of tumor cells and
immunocytes, including NK cells, macrophages, CTLs and dendritic
cells (DCs). The impact of IR on the Th1/Th2 imbalance and its
ability to interact with tumor-associated immunocytes, achieving an
improved antitumoral immune response to radiotherapy, are reviewed
below.
Various doses of IR mediate a distinct Th1/Th2
cytokine imbalance. High-dose IR (HDIR, ≥2 Gy) induces a Th2 shift
(Table II) (39-56). Irradiation at 5 Gy notably
promotes the secretion of Th2 cytokines, including IL-4, IL-5 and
IL-10, most likely through the upregulation of the transcription
factor, GATA-3 and c-Maf. The mRNA and protein levels of
Th1-secreted molecules, such as IFN-γ and IL-12, are inhibited by
the suppression of the STAT signaling pathway in murine splenocytes
(39). Similar effects of the Th2
shift were previously observed in tumor-bearing mice with HDIR at
10 Gy. Tumor growth delay was significantly extended after IL-10
suppression in a manner similar to the function of nitric oxide
synthase (NOS) inhibitors, leading to immune-enhanced Th1
polarization (40). Furthermore,
the exposure of the human immune system to natural HDIR favors a
shift to a type 2 response (41,42), with an evidently higher Th2
cytokine production and lower serum antioxidant levels, confirming
the IR-induced Th2 shift. On the other hand, potent radioprotectors
have been found to reverse the Th2 cytokine shift by IR.
Specifically, a combination comprising 3,3′-diselenodipropionic
acid, semiquinone glucoside derivative, G-003M, Ginsan
polysaccharide, N-acetyl tryptophan glucoside and Fms-like tyrosine
kinase 3 ligand, was confirmed to prevent Th1/Th2 imbalance in the
TIME, mainly through oxidative stress alleviation and reduction of
inflammatory cell infiltration (43-48). Previous results indicated a shift
towards Th2 in the TIME mediated by HDIR (39-47,50); however, molecular experiments are
required to elucidate the underlying mechanisms.
Taken together, these findings indicate that HDIR
leads to an immunosuppressive Th2 shift response, while LDIR
affects the Th1/Th2 balance with no certain defined effect in a
dose- and time-dependent manner. Optimizing the dose and duration
of radiotherapy may inhibit immunosuppressive Th2 response and
promote a Th1 shift. Identification of potential translational
radioprotectors may effectively reverse the Th2 shift of HDIR in
the clinical setting. Overcoming these obstacles will help to
overcome the limitations of radiotherapy.
In the presence of IR, tumor cells as well as
multiple immunocytes, including DCs, macrophages, CTLs and NK
cells, were reported to partially contribute to the modification of
Th1/Th2 shift (Fig. 2).
Macrophage plasticity has been attracting increasing
attention due to the polarized activation and differentiation in
divergent environments (67).
Classically activated M1-like macrophages (IL-12high,
IL-10low) primed by Th1-secreted factors (IFN-γ,
granulocyte/macrophage-colony-stimulating factor) exert antitumor
effects and suppress tumor progression (68). On the other hand, tumor-associated
macrophages (TAMs) are characterized by an M2 phenotype
(IL-10high, IL-12low) promoted by
Th2-secreted cytokines (IL-4 and IL-13) and have been found to be
associated with a poor prognosis in cancer (69,70). LDIR facilitates the polarization
of TAMs towards the M1 phenotype, potentially suppressing
angiogenic responses in endothelial NOS-positive endothelial cells,
due to the presence of Th1 cytokines and downregulation of
hypoxia-inducible factor-1 (71).
Furthermore, very low-dose IR has been shown to upregulate the
expression of a whole set of biological functional genes associated
with macrophage activation and Th1 immunity in patients with
follicular lymphoma (72).
However, HDIR has been reported to deteriorate avascular hypoxia,
substantially favoring polarization towards the M2 phenotype
(73,74), reducing radiosensitivity through
heparin-binding epidermal growth factor and accelerated
neovasculogenesis, thereby leading to tumor relapse (75). Notably, HDIR has been shown to
induce IL-10 oversecretion by M2 macrophages, which is reversed
into Th1 immune polarization by NOS inhibitor administration,
indicating the participation of M2 macrophages in the Th2 shift
(40). A similar activated
Th1-type cytokine shift has been observed following irradiation
with 20 Gy via inhibition of the NK-1 receptors expressed on the
surface of macrophages (47).
Hence, promoting IR-induced M1 polarization likely improves the
efficacy of radiotherapy and restores the Th1/Th2 balance.
CTLs eliminate tumor cells through both secretory
(perforin, lymphotoxin, granzyme and TNF-related protein) and
non-secretory (Fas ligand and tumor necrosis factor-related
apoptosis-inducing ligand) mechanisms (76,77). In a previous study, the curative
effects of IR on tumor-bearing mice were eliminated by anti-CD8
monoclonal antibody treatment, confirming the dominant antitumor
role of CTLs (78). In another
study, in a B16-F0 tumor model, IR (15 or 5×3 Gy) was reported to
boost the numbers of tumor-specific CTLs that secrete IFN-γ at the
tumor site (79). Of note, the
combination of local irradiation and Th1 cell therapy (CpG or
recombinant IL-12 or anti-IL-4 antibody), which promote the
Th1-type microenvironment, induced the proliferation of
tumor-specific CTLs and tumor regression (80-83). Therefore, further investigation of
the molecular interactions between CTLs and Th1 cells during
radiotherapy will expand the current knowledge on antitumor
cellular immunity and promote the application of this combination
therapy in the clinical setting.
NK cells recognize and kill tumor cells through the
activating and inhibitory receptors on their surface (84,85). It was previously reported that the
numbers of DX5+IFN-γ+ NK cells significantly
decreased, while the numbers of DX5+IL-10+
and DX5+IL-4+ NK cells markedly increased
during tumor progression, partly confirming the Th2 shift in the
TIME (86). NK cells respond with
various functional alterations after being exposed to IR at various
doses. LDIR (75-150 mGy) has been shown to increase the
proliferation and the levels of cytotoxic effectors of NK cells,
including IFN-γ and TNF-α, possibly through the p38/MAPK signaling
pathway (87). LDIR stimulates
the cytolytic function of NK cells in vivo, leading to the
suppression of tumor metastases in animal models (53,88). In a similar manner, the
LDIR-induced activation of NK cells has been found to be involved
in the antitumor effect of total body irradiation (TBI) (89). On the other hand, the depletion of
NK cells following HDIR (5 Gy) with TBI has been shown to lead to a
decrease in the levels of Th1-type cytokines in mice, while the
injection of NK cells in TBI mice was shown to normalize the IFN-γ
levels (90), indicating the
contribution of NK cells to the Th1 shift. In addition, NK cells
display morphological changes and functional impairment following
HDIR (30 Gy), although they retained their ability to bind to
targets on tumor cells. However, IL-2 pre-treatment has been shown
to maintain the cytotoxic function of NK cells (53,91,92), which is likely associated with
NF-κB activation triggered by IL-2/IL-2 receptor binding (93). Collectively, the impact of IR on
NK cells varies widely according to the radiation dose, promoting
cytolytic function at low doses and abating IFN-γ secretion at high
doses. Therefore, the combination of optimal clinical irradiation
dose together with IL-2, which preserves NK cell activity, may
promote Th1 immunity and maintain the antitumor function of NK
cells.
IR destroys tumor cells via both directly breaking
DNA strands and activating tumor-suppressor genes, as well as
programming the TIME (94,95).
It has been reported that tumor-derived TNF-α in the presence of IR
induces the restoration of p53 targets and a rapid re-activation of
p65/p50 NF-κB complexes in an autocrine manner (72,96), thus triggering tumor cell death.
Furthermore, human breast cancer cells exposed to IR have been
shown to produce CXC ligand 16 to recruit
CD8+CXCR6+ T cells to the tumor site
(97). Similarly, IR-enhanced
karyopherin α2 release by colorectal cancer cells increased the
expression of TNF-α and IL-12 in DCs, promoting Th1/Th17
differentiation (98,99). On the other hand, tumor cells
modify the TIME to create favorable, tumor-promoting conditions.
For example, glioma cells secrete Th2 cytokines, including IL-6 and
IL-10, to abrogate cytotoxic antitumor immune responses (100). Similarly, IL-4 receptor
expression has been shown to increase to accommodate enhanced IL-4
in the TIME of glioblastomas (101). In addition, IR has been shown to
upregulate indoleamine 2,3 dioxygenase 1 in colorectal cancer,
which blocks the Th1 shift in the TIME and leads to radioresistance
(102). Combined with TIME
modification agents, IR enables the optimization of immune-mediated
tumor destruction and minimizes radiotolerance through promoting a
Th1 shift.
A considerable number of studies have revealed that
the Th1/Th2 shift is involved in the clinical and biological damage
of different organs and tissues in patients following radiotherapy,
including radiation-induced lung injury (RLI), radiation-induced
intestinal injury (RIII), radiation encephalopathy (RE), as well as
other severe complications.
Numerous studies have highlighted the differential
roles of Th1- and Th2-type cytokines in RLI, which include
radiation pneumonitis (RP) and radiation fibrosis (RF), occurring
within and beyond 3 months following radiotherapy, respectively
(103,104). The balance of Th1/Th2 is
confirmed to determine the direction and outcome of lung
inflammation following lung irradiation (105-116). RP is closely associated with Th1
shift, while RF is more likely associated with Th2 shift.
A number of pro-inflammatory Th2 cytokines,
including IL-4, IL-6 and TGF-β, have been reported to be positively
associated with RP (105-107).
For example, TGF-β, a known key factor involved in inflammation and
fibrosis, was found to be markedly upregulated in mice with RP (15
Gy, single dose) via the TGF-β-Smad2/3 pathway (106). In addition, IL-4 was
substantially increased in the lungs of irradiated rats within 3
weeks following the administration of a single dose of 20 Gy, at
both the transcriptional and translational levels (108). Furthermore, Th2 cytokines,
including IL-4, IL-6 and IL-10, have been found to be independent
predictive factors for the incidence of RP (all P<0.05) by
prospective clinical studies in patients with lung cancer (107,109,110). As regards RF, which is a
long-term radiation-induced complication, IL-4 has been reported to
play a key role through enhancing collagen synthesis by fibroblasts
and inducing the production of TGF-β, leading to irreversible lung
injury (111). Furthermore, IL-4
enhances and maintains macrophage activation to promote RF
(112). In a similar manner,
thoracic HDIR at 12 Gy has been shown to promote the secretion of
IL-13 and Arginase-1 through GATA-3 upregulation in vivo,
supporting the causative role of Th2 cytokines in pulmonary
fibrosis (113). On the other
hand, Th1 factors exert a protective function against RF. For
example, obvious RF has been observed in IFN-γ-/- mice
following whole-thorax irradiation with 18 Gy compared with
C57BL/6J (IFN-γ+/+) mice (114). Additionally, the upregulated
IFN-γ and downregulated IL-4 levels have been shown to contribute
to a deceleration of the fibrotic process when the Th2 shift was
partially reversed by TGF-β3 in RF (115). As regards RP, increased IFN-γ
levels at 2-3 months following thoracic irradiation have been
observed in RP rats of different strains, indicating the role of
Th1 cytokines (116). Further
investigations of the TGF/Smad pathway identified preclinical RLI
protectors, such as CpG-oligodeoxynucleotides and grape seed
pro-anthocyanidins (117-119),
successfully modifying the Th1-dominant microenvironment to
alleviate RLI. In addition, the Th17 cell subpopulation was found
to accelerate post-irradiation inflammation and fibrosis in the
lung (120,121). Both RF and overt neutrophil
infiltration have been shown to be averted following the
downregulation of the IL6/TGF-β/IL-17 pathway in irradiated
IL17-/- mice (114,122).
Thus, IFN-γ has been confirmed to suppress
radiation-induced fibrosis while enhancing the inflammatory
response, and Th2 cytokines act as both pro-inflammatory and
pro-fibrosis factors during irradiation. Further studies are
required to elucidate the interaction between the novel Th17
subpopulation and the Th1/Th2 shift in RLI. A promising preventive
strategy for RLI may be reversing the Th2 shift with potential
transformable radiation protectors.
RIII often arises as a complication of radiotherapy
in patients with pelvic, abdominal, or retroperitoneal tumors and
is attributed to the injury of radiation-sensitive stem cells in
the intestinal epithelium (123). It is currently considered that
each individual cytokine, rather than a class of cytokines, plays a
specific role in RIII. Th1/Th2 factors may be basically divided
into two categories, namely the pro-RIII type cytokines, including
TNF-α, IFN-γ, IL-1β and IL-6, and the anti-RIII cytokine, IL-10.
For example, a TBI trial performed on rhesus macaque monkeys
demonstrated that the TNF-α cascade and the upregulation of
matrix-dissociated genes were associated with severe intestinal
inflammation and mucosal barrier disruption (124,125). These pathological changes may be
normalized by granulocyte colony-stimulating factor (126,127). Furthermore, the findings from a
novel brachytherapy mouse model revealed a marked increase in IL-1β
and IL-6 levels, as high as 100- to 300-fold, following irradiation
with 5.5-8 Gy (128), and both
cytokines were of notable predictive value for radiation-induced
proctitis based on receiver operating characteristic curve analysis
(128). The suppression of NF-κB
with specific radioprotectors, targeting either the peroxisome
proliferator activated receptor-γ/NF-κB or the Toll-like receptor
4/MYD88 innate immune signal transduction adaptor/NF-κB axes, has
been shown to contribute to a decrease in the levels of the
pro-inflammatory cytokines, IL-6 and TNF-α, in RIII (129-131), which has also been shown to be
attenuated through the PI3K/AKT/mTOR pathway (132). In the clinical setting,
mesenchymal stem cell (MSC) transplantation has been reported to
alleviate RIII by increasing IL-10 and reducing TNF-α and IFN-γ
levels in serum (133-136). However, another study stated
that the predominant Th17 rather than the Th1/Th2 population was
inhibited by adipose-derived MSCs in RIII (137). Therefore, Th1 (TNF-α and IFN-γ)
and Th2 (IL-1β, IL-6, IL-10) cytokines play key roles in RIII and
may serve as reliable RIII predictors. Further research on Th17
cells may shed more light on the mechanism underlying the
development of RIII.
RE is a complication of radiotherapy for
craniofacial tumors, and often presents as a series of pathological
and morphological alterations of brain structure. Microglial
activation has been considered as a potential contributor to
inflammatory responses in RE (138). Previous studies have revealed
that the induction of the NF-κB and MEK/ERK1/2 signaling pathways
may trigger microglial activation after cranial radiation therapy,
leading to an increase in the levels of inflammatory factors, such
as IL-1β, TNF-α and IL-6 in microglia (139-141). In addition, the abnormal
elevation of TNF-α has been found to coincide with the occurrence
of neurological abnormalities at 2-3 and 6 months following
irradiation in vivo (142). On the contrary, the inhibition
of TNF-α and IFN-γ has been shown to prevent severe neurological
damage in rats by suppressing hippocampal neuronal apoptosis
(143). The observation that
patients suffering from less prominent cognitive function
impairment after cranial radiotherapy exhibit higher levels of
anti-inflammatory IL-10 in serum (144) has suggested the potential use of
cytokines against RE. Thus, further preclinical studies are
required to investigate the alleviation of microglial activity as
well as the promotion of Th2 polarization in vivo.
Cutaneous radiation syndrome (CRS), which is
characterized by extensive inflammatory response, fibrosis or,
ultimately, necrosis of the skin, mostly occurs as a consequence of
HDIR. The TGF-β/Smad3 pathway mediates inflammation in CRS
(145,146). IFN-γ therapy has been observed
to ameliorate cutaneous fibrosis, most likely through TGF-β
inhibition (147). A clinical
randomized trial confirmed that low-dose IFN-γ administration
induced a significant reduction in fibrosis in patients with IR
overexposure (148).
Furthermore, blood-based single-nucleotide polymorphism (SNP)
analysis revealed a possible association between SNPs in the IFN-γ
gene (rs2069705) and acute radiation-induced skin reactions in
patients with breast cancer undergoing adjuvant radiotherapy
(149).
TNF-α has been found to be implicated as a potential
contributor and underlying target in radiation-induced salivary
dysfunction and oral mucositis, as it increased nitric oxide levels
in salivary gland epithelial cells and disrupted salivary gland
function (150-152). In addition, in radiation-induced
esophagitis, manganese superoxide dismutase (SOD2)-plasmid/liposome
treatment 24 h prior to irradiation markedly decreased the mRNA
levels of cytokines (IL-1, TNF-α and IFN-γ) in C3H/HeNsd mice and
inhibited apoptosis and micro-ulceration (153). Of note, IL-1 and TNF-α
pre-treatment protected hematopoietic cells against lethal
cytotoxicity from HDIR, mostly through the production of a specific
antioxidant enzyme, SOD2 (154).
Exogenous IFN-γ and TNF-α were reported to mimic the effects of
bone marrow transplantation on the suppression of radiation
lymphedema (155). Taken
together, the aforementioned findings indicate that Th1 cytokines,
such as IFN-γ and TNF-α, markedly promote radiation-related
inflammation, but reduce fibrosis, myelosuppression and radiation
lymphedema.
The modulation of the Th1/Th2 balance in the tumor
micro- environment has prominent immunoregulatory properties and
interferes with tumor progression. An increasing number of
molecular-centric studies indicate that IR may modify the Th1/Th2
shift based on different irradiation doses. The combination of
clinically transformable Th1/Th2 modulators and IR at the proper
dose and fraction may help design practical and effective antitumor
therapies. Hopefully, such treatment will benefit patients with
unsatisfactory prognosis and radiation-induced complications via
modulating the cross- talk of immunocytes and Th1/Th2 cytokines in
the presence of irradiation. Further investigations on the
regulatory roles of Th cells in the TIME will improve the
comprehensive understanding of the possible applicability of
immunoradiotherapy in the treatment of malignant tumors.
Not applicable.
JL, ZZ, YG and CX retrieved and summarized the
relevant literature and drafted the manuscript. QW, JC, XL and JiZ
revised the draft of the manuscript. YL, WS, ZH and JuZ created the
figures and tables. YG and CX confirmed the authenticity of all the
raw data. All the authors (JL, ZZ, QW, JC, XL, JiZ, YL, WS, ZH,
JuZ, YG and CX) contributed to manuscript revision, and have read
and approved the final version.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Dr Yingming Sun
(Sanming First Hospital) for providing guidance and assistance with
the writing of the manuscript.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81773236, 81800429 and 81972852),
the Key Research and Development Project of Hubei Province (grant
no. 2020BCA069), the Natural Science Foundation of Hubei Province
(grant no. 2020CFB612), Health Commission of Hubei Province Medical
Leading Talent Project, Health Commission of Hubei Province
Scientific Research Project (grant nos. WJ2019H002 and WJ2019Q047),
Young and Middle-Aged Medical Backbone Talents of Wuhan (grant no.
WHQG201902), Application Foundation Frontier Project of Wuhan
(grant no. 2020020601012221), and Zhongnan Hospital of Wuhan
University Science, Technology and Innovation Seed Fund (grant nos.
znpy2019001, znpy2019048 and ZNJC201922), and the Chinese Society
of Clinical Oncology Top Alliance Tumor Immune Research Fund (no.
Y-JS2019-036).
|
1
|
Binnewies M, Roberts EW, Kersten K, Chan
V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI,
Ostrand-Rosenberg S, Hedrick CC, et al: Understanding the tumor
immune microenvironment (TIME) for effective therapy. Nat Med.
24:541–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4+ T cell help in cancer
immunology and immunotherapy. Nat Rev Immunol. 18:635–647. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Linehan WM and Ricketts CJ: The cancer
genome atlas of renal cell carcinoma: Findings and clinical
implications. Nat Rev Urol. 16:539–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skinnider BF and Mak TW: The role of
cytokines in classical Hodgkin lymphoma. Blood. 99:4283–4297. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Fan H and Jiang S: CD4(+) T-cell
subsets in transplantation. Immunol Rev. 252:183–191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masjedi A, Hashemi V, Hojjat-Farsangi M,
Ghalamfarsa G, Azizi G, Yousefi M and Jadidi-Niaragh F: The
significant role of interleukin-6 and its signaling pathway in the
immunopathogenesis and treatment of breast cancer. Biomed
Pharmacother. 108:1415–1424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu J and Paul WE: CD4 T cells: Fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan YY: GATA3: A master of many trades in
immune regulation. Trends Immunol. 35:233–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maazi H and Akbari O: Type two innate
lymphoid cells: The Janus cells in health and disease. Immunol Rev.
278:192–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Afkarian M, Sedy JR, Yang J, Jacobson NG,
Cereb N, Yang SY, Murphy TL and Murphy KM: T-bet is a STAT1-induced
regulator of IL-12R expression in naïve CD4+ T cells.
Nat Immunol. 3:549–557. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian L, Goldstein A, Wang H, Ching Lo H,
Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N, et
al: Mutual regulation of tumour vessel normalization and
immunostimulatory reprogramming. Nature. 544:250–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Darawish Y, Li W, Yamanishi K, Pencheva
M, Oka N, Yamanishi H, Matsuyama T, Tanaka Y, Minato N and Okamura
H: Frontline Science: IL-18 primes murine NK cells for
proliferation by promoting protein synthesis, survival, and
autophagy. J Leukoc Biol. 104:253–264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta S and Gollapudi S: Molecular
mechanisms of TNF-alpha-induced apoptosis in naïve and memory T
cell subsets. Autoimmun Rev. 5:264–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vadevoo SMP, Kim JE, Gunassekaran GR, Jung
HK, Chi L, Kim DE, Lee SH, Im SH and Lee B: IL4 receptor-targeted
proapoptotic peptide blocks tumor growth and metastasis by
enhancing antitumor immunity. Mol Cancer Ther. 16:2803–2816. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oft M: Immune regulation and cytotoxic T
cell activation of IL-10 agonists-preclinical and clinical
experience. Semin Immunol. 44:1013252019. View Article : Google Scholar
|
|
18
|
Urosevic M and Dummer R: HLA-G and IL-10
expression in human cancer-different stories with the same message.
Semin Cancer Biol. 13:337–342. 2003. View Article : Google Scholar
|
|
19
|
Sallusto F, Lenig D, Mackay CR and
Lanzavecchia A: Flexible programs of chemokine receptor expression
on human polarized T helper 1 and 2 lymphocytes. J Exp Med.
187:875–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Annunziato F, Galli G, Cosmi L, Romagnani
P, Manetti R, Maggi E and Romagnani S: Molecules associated with
human Th1 or Th2 cells. Eur Cytokine Netw. 9(3 Suppl): S12–S16.
1998.
|
|
21
|
Annunziato F, Manetti R, Tomasévic I,
Guidizi MG, Biagiotti R, Giannò V, Germano P, Mavilia C, Maggi E
and Romagnani S: Expression and release of LAG-3-encoded protein by
human CD4+ T cells are associated with IFN-gamma production. FASEB
J. 10:769–776. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szabo SJ, Dighe AS, Gubler U and Murphy
KM: Regulation of the interleukin (IL)-12R beta 2 subunit
expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med.
185:817–824. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loetscher P, Uguccioni M, Bordoli L,
Baggiolini M, Moser B, Chizzolini C and Dayer JM: CCR5 is
characteristic of Th1 lymphocytes. Nature. 391:344–345. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin S, Rottman JB, Myers P, Kassam N,
Weinblatt M, Loetscher M, Koch AE, Moser B and Mackay CR: The
chemokine receptors CXCR3 and CCR5 mark subsets of T cells
associated with certain inflammatory reactions. J Clin Invest.
101:746–754. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu D, Chan WL, Leung BP, Hunter D, Schulz
K, Carter RW, McInnes IB, Robinson JH and Liew FY: Selective
expression and functions of interleukin 18 receptor on T helper
(Th) type 1 but not Th2 cells. J Exp Med. 188:1485–1492. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo
D, Sozzani S, Mantovani A and Sinigaglia F: Selective up-regulation
of chemokine receptors CCR4 and CCR8 upon activation of polarized
human type 2 Th cells. J Immunol. 161:5111–5115. 1998.PubMed/NCBI
|
|
28
|
Cosmi L, Annunziato F, Galli MIG, Maggi
RME, Nagata K and Romagnani S: CRTH2 is the most reliable marker
for the detection of circulating human type 2 Th and type 2 T
cytotoxic cells in health and disease. Eur J Immunol. 30:2972–2979.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Groux H, Sornasse T, Cottrez F, de Vries
JE, Coffman RL, Roncarolo MG and Yssel H: Induction of human T
helper cell type 1 differentiation results in loss of IFN-gamma
receptor beta-chain expression. J Immunol. 158:5627–5631.
1997.PubMed/NCBI
|
|
30
|
Jourdan P, Abbal C, Noraz N, Hori T,
Uchiyama T, Vendrell JP, Bousquet J, Taylor N, Pène J and Yssel H:
IL-4 induces functional cell-surface expression of CXCR4 on human T
cells. J Immunol. 160:4153–4157. 1998.PubMed/NCBI
|
|
31
|
Xu D, Chan WL, Leung BP, Huang Fp, Wheeler
R, Piedrafita D, Robinson JH and Liew FY: Selective expression of a
stable cell surface molecule on type 2 but not type 1 helper T
cells. J Exp Med. 187:787–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Del Prete G, De Carli M, D'Elios MM,
Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E and
Romagnani S: CD30-mediated signaling promotes the development of
human T helper type 2-like T cells. J Exp Med. 182:1655–1661. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sallusto F, Mackay CR and Lanzavecchia A:
Selective expression of the eotaxin receptor CCR3 by human T helper
2 cells. Science. 277:2005–2007. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weinstein JS, Laidlaw BJ, Lu Y, Wang JK,
Schulz VP, Li N, Herman EI, Kaech SM, Gallagher PG and Craft J:
STAT4 and T-bet control follicular helper T cell development in
viral infections. J Exp Med. 215:337–355. 2018. View Article : Google Scholar :
|
|
35
|
Christodoulopoulos P, Cameron L, Nakamura
Y, Lemière C, Muro S, Dugas M, Boulet LP, Laviolette M, Olivenstein
R and Hamid Q: TH2 cytokine-associated transcription factors in
atopic and nonatopic asthma: Evidence for differential signal
transducer and activator of transcription 6 expression. J Allergy
Clin Immunol. 107:586–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng W and Flavell RA: The transcription
factor GATA-3 is necessary and sufficient for Th2 cytokine gene
expression in CD4 T cells. Cell. 89:587–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaplan MH, Schindler U, Smiley ST and
Grusby MJ: Stat6 is required for mediating responses to IL-4 and
for development of Th2 cells. Immunity. 4:313–319. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ho IC, Hodge MR, Rooney JW and Glimcher
LH: The proto-oncogene c-maf is responsible for tissue-specific
expression of interleukin-4. Cell. 85:973–983. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han SK, Song JY, Yun YS and Yi SY: Effect
of gamma radiation on cytokine expression and cytokine-receptor
mediated STAT activation. Int J Radiat Biol. 82:686–697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ridnour LA, Cheng RY, Weiss JM, Kaur S,
Soto-Pantoja DR, Basudhar D, Heinecke JL, Stewart CA, DeGraff W,
Sowers AL, et al: NOS inhibition modulates immune polarization and
improves radiation-induced tumor growth delay. Cancer Res.
75:2788–2799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Attar M, Molaie Kondolousy Y and Khansari
N: Effect of high dose natural ionizing radiation on the immune
system of the exposed residents of Ramsar Town, Iran. Iran J
Allergy Asthma Immunol. 6:73–78. 2007.PubMed/NCBI
|
|
42
|
Karkanitsa L, Mitskevitch P, Uss A,
Ostapenko V and Dainiak N: Elevated levels of cytokine gene
expression in leukemic hemopoietic cells of belorussians exposed to
ionizing radiation (IR) following the chernobyl catastrophe. Blood.
96:295A2000.
|
|
43
|
Han SK, Song JY, Yun YS and Yi SY: Ginsan
improved Th1 immune response inhibited by gamma radiation. Arch
Pharm Res. 28:343–350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kunwar A, Bag PP, Chattopadhyay S, Jain VK
and Priyadarsini KI: Anti-apoptotic, anti-inflammatory, and
immunomodulatory activities of 3,3′-diselenodipropionic acid in
mice exposed to whole body γ-radiation. Arch Toxicol. 85:1395–1405.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Li B, Jia X, Ma Y, Gu Y, Zhang P,
Wei Q, Cai J, Cui J, Gao F and Yang Y: Radiation-induced decrease
of CD8+ dendritic cells contributes to Th1/Th2 shift. Int
Immunopharmacol. 46:178–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mishra S, Patel DD, Bansal DD and Kumar R:
Semiquinone glucoside derivative provides protection against
γ-radiation by modulation of immune response in murine model.
Environ Toxicol. 31:478–488. 2016. View Article : Google Scholar
|
|
47
|
Malhotra P, Adhikari M, Mishra S, Singh S,
Kumar P, Singh SK and Kumar R: N-acetyl tryptophan glucopyranoside
(NATG) as a countermeasure against gamma radiation-induced
immunosuppression in murine macrophage J774A.1 cells. Free Radic
Res. 50:1265–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nadella V, Ranjan R, Senthilkumaran B,
Qadri SSYH, Pothani S, Singh AK, Gupta ML and Prakash H:
Podophyllotoxin and rutin modulate M1 (iNOS+) macrophages and
mitigate lethal radiation (LR) induced inflammatory responses in
mice. Front Immunol. 10:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu XD, Ma SM and Liu SZ: Effects of 0.075
Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice.
Phys Med Biol. 48:2041–2049. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao H, Dong Z, Gong X, Dong J, Zhang Y,
Wei W, Wang R and Jin S: Effects of various radiation doses on
induced T-helper cell differentiation and related cytokine
secretion. J Radiat Res. 59:395–403. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karimi G, Balali-Mood M, Alamdaran SA,
Badie-Bostan H, Mohammadi E, Ghorani-Azam A, Sadeghi M and
Riahi-Zanjani B: Increase in the Th1-cell-based immune response in
healthy workers exposed to low-dose radiation-immune system status
of radiology staff. J Pharmacopuncture. 20:107–111. 2017.PubMed/NCBI
|
|
52
|
Cho SJ, Kang H, Hong EH, Kim JY and Nam
SY: Transcriptome analysis of low-dose ionizing radiation-impacted
genes in CD4+ T-cells undergoing activation and
regulation of their expression of select cytokines. J
Immunotoxicol. 15:137–146. 2018. View Article : Google Scholar
|
|
53
|
Bogdándi EN, Balogh A, Felgyinszki N,
Szatmári T, Persa E, Hildebrandt G, Sáfrány G and Lumniczky K:
Effects of low-dose radiation on the immune system of mice after
total-body irradiation. Radiat Res. 174:480–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Elhadary AA, Marzook EA and Abdelmonem HA:
Evaluation of the level of gamma radiation dose on some immune
system parameters against cancer. Biosci J. 35:307–316. 2019.
View Article : Google Scholar
|
|
55
|
Ghazy AA, Abu El-Nazar SY, Ghoneim HE,
Taha AR and Abouelella AM: Effect of murine exposure to gamma rays
on the interplay between Th1 and Th2 lymphocytes. Front Pharmacol.
6:742015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu X, Liu Z, Wang D, Han Y, Hu S, Xie Y,
Liu Y, Zhu M, Guan H, Gu Y and Zhou PK: Effects of low dose
radiation on immune cells subsets and cytokines in mice. Toxicol
Res (Camb). 9:249–262. 2020. View Article : Google Scholar
|
|
57
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar
|
|
58
|
Arpinati M, Green CL, Heimfeld S, Heuser
JE and Anasetti C: Granulocyte-colony stimulating factor mobilizes
T helper 2-inducing dendritic cells. Blood. 95:2484–2490. 2000.
View Article : Google Scholar
|
|
59
|
Jutel M and Akdis CA: T-cell subset
regulation in atopy. Curr Allergy Asthma Rep. 11:139–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Merrick A, Errington F, Milward K,
O'Donnell D, Harrington K, Bateman A, Pandha H, Vile R, Morrison E,
Selby P and Melcher A: Immunosuppressive effects of radiation on
human dendritic cells: Reduced IL-12 production on activation and
impairment of naive T-cell priming. Br J Cancer. 92:1450–1458.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Clerici M, Shearer GM and Clerici E:
Cytokine dysregulation in invasive cervical carcinoma and other
human neoplasias: Time to consider the TH1/TH2 paradigm. J Natl
Cancer Inst. 90:261–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lappin MB and Campbell JD: The Th1-Th2
classification of cellular immune responses: Concepts, current
thinking and applications in haematological malignancy. Blood Rev.
14:228–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Backer RA, Diener N and Clausen BE:
Langerin+CD8+ dendritic cells in the splenic
marginal zone: Not so marginal after all. Front Immunol.
10:7412019. View Article : Google Scholar
|
|
64
|
Prendergast KA, Daniels NJ, Petersen TR,
Hermans IF and Kirman JR: Langerin+ CD8α+
dendritic cells drive early CD8+ T cell activation and
IL-12 production during systemic bacterial infection. Front
Immunol. 9:9532018. View Article : Google Scholar
|
|
65
|
Yu N, Wang S, Song X, Gao L, Li W, Yu H,
Zhou C, Wang Z, Li F and Jiang Q: Low-dose radiation promotes
dendritic cell migration and IL-12 production via the ATM/NF-kappaB
pathway. Radiat Res. 189:409–417. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shigematsu A, Adachi Y, Koike-Kiriyama N,
Suzuki Y, Iwasaki M, Koike Y, Nakano K, Mukaide H, Imamura M and
Ikehara S: Effects of low-dose irradiation on enhancement of
immunity by dendritic cells. J Radiat Res. 48:51–55. 2007.
View Article : Google Scholar
|
|
67
|
Murray PJ: Macrophage polarization. Annu
Rev Physiol. 79:541–566. 2017. View Article : Google Scholar
|
|
68
|
Orecchioni M, Ghosheh Y, Pramod AB and Ley
K: Macrophage polarization: Different gene signatures in M1(LPS+)
vs. classically and M2(LPS-) vs. alternatively activated
macrophages. Front Immunol. 10:10842019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiratori H, Feinweber C, Luckhardt S,
Wallner N, Geisslinger G, Weigert A and Parnham MJ: An in vitro
test system for compounds that modulate human inflammatory
macrophage polarization. Eur J Pharmacol. 833:328–338. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nadella V, Singh S, Jain A, Jain M,
Vasquez KM, Sharma A, Tanwar P, Rath GK and Prakash H: Low dose
radiation primed iNOS + M1macrophages modulate angiogenic
programming of tumor derived endothelium. Mol Carcinog.
57:1664–1671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Knoops L, Haas R, de Kemp S, Majoor D,
Broeks A, Eldering E, de Boer JP, Verheij M, van Ostrom C, de Vries
A, et al: In vivo p53 response and immune reaction underlie highly
effective low-dose radiotherapy in follicular lymphoma. Blood.
110:1116–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Seifert L, Werba G, Tiwari S, Giao Ly NN,
Nguy S, Alothman S, Alqunaibit D, Avanzi A, Daley D, Barilla R, et
al: Radiation therapy induces macrophages to suppress T-cell
responses against pancreatic tumors in mice. Gastroenterology.
150:1659–1672.e5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Okubo M, Kioi M, Nakashima H, Sugiura K,
Mitsudo K, Aoki I, Taniguchi H and Tohnai I: M2-polarized
macrophages contribute to neovasculogenesis, leading to relapse of
oral cancer following radiation. Sci Rep. 6:275482016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fu E, Liu T, Yu S, Chen X, Song L, Lou H,
Ma F, Zhang S, Hussain S, Guo J, et al: M2 macrophages reduce the
radiosensitivity of head and neck cancer by releasing HB-EGF. Oncol
Rep. 44:698–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Reading JL, Gálvez-Cancino F, Swanton C,
Lladser A, Peggs KS and Quezada SA: The function and dysfunction of
memory CD8+ T cells in tumor immunity. Immunol Rev.
283:194–212. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Crespo J, Sun H, Welling TH, Tian Z and
Zou W: T cell anergy, exhaustion, senescence, and stemness in the
tumor microenvironment. Curr Opin Immunol. 25:214–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y,
Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al: Therapeutic
effects of ablative radiation on local tumor require
CD8+ T cells: Changing strategies for cancer treatment.
Blood. 114:589–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lugade AA, Moran JP, Gerber SA, Rose RC,
Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma
tumors increases the generation of tumor antigen-specific effector
cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Takeshima T, Chamoto K, Wakita D, Ohkuri
T, Togashi Y, Shirato H, Kitamura H and Nishimura T: Local
radiation therapy inhibits tumor growth through the generation of
tumor-specific CTL: Its potentiation by combination with Th1 cell
therapy. Cancer Res. 70:2697–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Chattopadhyay S and Chakraborty NG:
Continuous presence of Th1 conditions is necessary for longer
lasting tumor-specific CTL activity in stimulation cultures with
PBL. Hum Immunol. 66:884–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Harada M, Matsueda S, Yao A, Noguchi M and
Itoh K: Vaccination of cytotoxic T lymphocyte-directed peptides
elicited and spread humoral and Th1-type immune responses to
prostate-specific antigen protein in a prostate cancer patient. J
Immunother. 28:368–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yokouchi H, Chamoto K, Wakita D, Yamazaki
K, Shirato H, Takeshima T, Dosaka-Akita H, Nishimura M, Yue Z,
Kitamura H and Nishimura T: Combination tumor immunotherapy with
radiotherapy and Th1 cell therapy against murine lung carcinoma.
Clin Exp Metastasis. 24:533–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Terrén I, Orrantia A, Vitallé J,
Zenarruzabeitia O and Borrego F: NK cell metabolism and tumor
microenvironment. Front Immunol. 10:22782019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hodgins JJ, Khan ST, Park MM, Auer RC and
Ardolino M: Killers 2.0: NK cell therapies at the forefront of
cancer control. J Clin Invest. 129:3499–3510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wei H, Zheng X, Lou D, Zhang L, Zhang R,
Sun R and Tian Z: Tumor-induced suppression of interferon-gamma
production and enhancement of interleukin-10 production by natural
killer (NK) cells: Paralleled to CD4+ T cells. Mol
Immunol. 42:1023–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang G, Kong Q, Wang G, Jin H, Zhou L, Yu
D, Niu C, Han W, Li W and Cui J: Low-dose ionizing radiation
induces direct activation of natural killer cells and provides a
novel approach for adoptive cellular immunotherapy. Cancer Biother
Radiopharm. 29:428–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cheda A, Wrembel-Wargocka J, Lisiak E,
Nowosielska EM, Marciniak M and Janiak MK: Single low doses of X
rays inhibit the development of experimental tumor metastases and
trigger the activities of NK cells in mice. Radiat Res.
161:335–340. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Miller GM, Andres ML and Gridley DS: NK
cell depletion results in accelerated tumor growth and attenuates
the antitumor effect of total body irradiation. Int J Oncol.
23:1585–1592. 2003.PubMed/NCBI
|
|
90
|
Park HR, Jung U and Jo SK: Impairment of
natural killer (NK) cells is an important factor in a weak Th1-like
response in irradiated mice. Radiat Res. 168:446–452. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zarcone D, Tilden AB, Lane VG and Grossi
CE: Radiation sensitivity of resting and activated nonspecific
cytotoxic cells of T lineage and NK lineage. Blood. 73:1615–1621.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou L, Zhang X, Li H, Niu C, Yu D, Yang
G, Liang X, Wen X, Li M and Cui J: Validating the pivotal role of
the immune system in low-dose radiation-induced tumor inhibition in
Lewis lung cancer-bearing mice. Cancer Med. 7:1338–1348. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhou J, Zhang J, Lichtenheld MG and
Meadows GG: A role for NF-kappa B activation in perforin expression
of NK cells upon IL-2 receptor signaling. J Immunol. 169:1319–1325.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Herrera FG, Bourhis J and Coukos G:
Radiotherapy combination opportunities leveraging immunity for the
next oncology practice. CA Cancer J Clin. 67:65–85. 2017.
View Article : Google Scholar
|
|
95
|
Demaria S, Golden EB and Formenti SC: Role
of local radiation therapy in cancer immunotherapy. JAMA Oncol.
1:1325–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Simon PS, Bardhan K, Chen MR, Paschall AV,
Lu C, Bollag RJ, Kong FC, Jin J, Kong FM, Waller JL, et al: NF-κB
functions as a molecular link between tumor cells and Th1/Tc1 T
cells in the tumor microenvironment to exert radiation-mediated
tumor suppression. Oncotarget. 7:23395–23415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Matsumura S, Wang B, Kawashima N,
Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti
SC, Dustin ML and Demaria S: Radiation-induced CXCL16 release by
breast cancer cells attracts effector T cells. J Immunol.
181:3099–3107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Song KH, Jung SY, Kang SM, Kim MH, Ahn J,
Hwang SG, Lee JH, Lim DS, Nam SY and Song JY: Induction of
immunogenic cell death by radiation-upregulated karyopherin alpha 2
in vitro. Eur J Cell Biol. 95:219–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Song KH, Jung SY, Park JI, Ahn J, Park JK,
Um HD, Park IC, Hwang SG, Ha H and Song JY: Inhibition of
karyopherin-α2 augments radiation-induced cell death by perturbing
BRCA1-mediated DNA repair. Int J Mol Sci. 20:28432019. View Article : Google Scholar
|
|
100
|
Huettner C, Paulus W and Roggendorf W:
Messenger RNA expression of the immunosuppressive cytokine IL-10 in
human gliomas. Am J Pathol. 146:317–322. 1995.PubMed/NCBI
|
|
101
|
Hao C, Parney IF, Roa WH, Turner J, Petruk
KC and Ramsay DA: Cytokine and cytokine receptor mRNA expression in
human glioblastomas: Evidence of Th1, Th2 and Th3 cytokine
dysregulation. Acta Neuropathol. 103:171–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen B, Alvarado DM, Iticovici M, Kau NS,
Park H, Parikh PJ, Thotala D and Ciorba MA: Interferon-induced IDO1
mediates radiation resistance and is a therapeutic target in
colorectal cancer. Cancer Immunol Res. 8:451–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hanania AN, Mainwaring W, Ghebre YT,
Hanania NA and Ludwig M: Radiation-induced lung injury: Assessment
and management. Chest. 156:150–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Giuranno L, Ient J, De Ruysscher D and
Vooijs MA: Radiation-induced lung injury (RILI). Front Oncol.
9:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Stenmark MH, Cai XW, Shedden K, Hayman JA,
Yuan S, Ritter T, Ten Haken RK, Lawrence TS and Kong FM: Combining
physical and biologic parameters to predict radiation-induced lung
toxicity in patients with non-small-cell lung cancer treated with
definitive radiation therapy. Int J Radiat Oncol Biol Phys.
84:e217–e222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rübe CE, Rodemann HP and Rübe C: The
relevance of cytokines in the radiation-induced lung reaction.
Experimental basis and clinical significance. Strahlenther Onkol.
180:541–549. 2004.In German. View Article : Google Scholar
|
|
107
|
Arpin D, Perol D, Blay JY, Falchero L,
Claude L, Vuillermoz-Blas S, Martel-Lafay I, Ginestet C, Alberti L,
Nosov D, et al: Early variations of circulating interleukin-6 and
interleukin-10 levels during thoracic radiotherapy are predictive
for radiation pneumonitis. J Clin Oncol. 23:8748–8756. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Büttner C, Skupin A, Reimann T, Rieber EP,
Unteregger G, Geyer P and Frank KH: Local production of
interleukin-4 during radiation-induced pneumonitis and pulmonary
fibrosis in rats: Macrophages as a prominent source of
interleukin-4. Am J Respir Cell Mol Biol. 17:315–325. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen Y, Rubin P, Williams J, Hernady E,
Smudzin T and Okunieff P: Circulating IL-6 as a predictor of
radiation pneumonitis. Int J Radiat Oncol Biol Phys. 49:641–648.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Tang Y, Yang L, Qin W, Yi M, Liu B and
Yuan X: Validation study of the association between genetic variant
of IL4 and severe radiation pneumonitis in lung cancer patients
treated with radiation therapy. Radiother Oncol. 141:86–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Y, Guan X, Liu W, Chen HL, Truscott J,
Beyatli S, Metwali A, Weiner GJ, Zavazava N, Blumberg RS, et al:
Helminth-induced production of TGF-β and suppression of
graft-versus-host disease is dependent on IL-4 production by host
cells. J Immunol. 201:2910–2922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Groves AM, Johnston CJ, Misra RS, Williams
JP and Finkelstein JN: Effects of IL-4 on pulmonary fibrosis and
the accumulation and phenotype of macrophage subpopulations
following thoracic irradiation. Int J Radiat Biol. 92:754–765.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Han G, Zhang H, Xie CH and Zhou YF:
Th2-like immune response in radiation-induced lung fibrosis. Oncol
Rep. 26:383–388. 2011.PubMed/NCBI
|
|
114
|
Paun A, Bergeron ME and Haston CK: The
Th1/Th17 balance dictates the fibrosis response in murine
radiation-induced lung disease. Sci Rep. 7:115862017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xu L, Xiong S, Guo R, Yang Z, Wang Q, Xiao
F, Wang H, Pan X and Zhu M: Transforming growth factor β3
attenuates the development of radiation-induced pulmonary fibrosis
in mice by decreasing fibrocyte recruitment and regulating
IFN-gamma/IL-4 balance. Immunol Lett. 162:27–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chiang CS, Liu WC, Jung SM, Chen FH, Wu
CR, McBride WH, Lee CC and Hong JH: Compartmental responses after
thoracic irradiation of mice: Strain differences. Int J Radiat
Oncol Biol Phys. 62:862–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang C, Zhao H, Li BL, Fu-Gao, Liu H, Cai
JM and Zheng M: CpG-oligodeoxynucleotides may be effective for
preventing ionizing radiation induced pulmonary fibrosis. Toxicol
Lett. 292:181–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Huang Y, Liu W, Liu H, Yang Y, Cui J,
Zhang P, Zhao H, He F, Cheng Y, Ni J, et al: Grape seed
pro-anthocyanidins ameliorates radiation-induced lung injury. J
Cell Mol Med. 18:1267–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen J, Wang Y, Mei Z, Zhang S, Yang J, Li
X, Yao Y and Xie C: Radiation-induced lung fibrosis in a
tumor-bearing mouse model is associated with enhanced Type-2
immunity. J Radiat Res. 57:133–141. 2016. View Article : Google Scholar :
|
|
120
|
Oh K, Seo MW, Kim YW and Lee DS:
Osteopontin potentiates pulmonary inflammation and fibrosis by
modulating IL-17/IFN-γ-secreting T-cell ratios in bleomycin-treated
mice. Immune Netw. 15:142–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lei L, Zhao C, Qin F, He ZY, Wang X and
Zhong XN: Th17 cells and IL-17 promote the skin and lung
inflammation and fibrosis process in a bleomycin-induced murine
model of systemic sclerosis. Clin Exp Rheumatol. 34(Suppl 100):
S14–S22. 2016.
|
|
122
|
Li Y, Zou L, Yang X, Chu L, Ni J, Chu X,
Guo T and Zhu Z: Identification of lncRNA, MicroRNA, and
mRNA-associated CeRNA network of radiation-induced lung injury in a
mice model. Dose Response. 17:15593258198910122019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hauer-Jensen M, Denham JW and Andreyev HJ:
Radiation enteropathy-pathogenesis, treatment and prevention. Nat
Rev Gastroenterol Hepatol. 11:470–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zheng J, Wang J, Pouliot M, Authier S,
Zhou D, Loose DS and Hauer-Jensen M: Gene expression profiling in
non-human primate jejunum, ileum and colon after total-body
irradiation: A comparative study of segment-specific molecular and
cellular responses. BMC Genomics. 16:9842015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Huang Z, Epperly M, Watkins SC,
Greenberger JS, Kagan VE and Bayır H: Necrostatin-1 rescues mice
from lethal irradiation. Biochim Biophys Acta. 1862:850–856. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kim JS, Ryoo SB, Heo K, Kim JG, Son TG,
Moon C and Yang K: Attenuating effects of granulocyte-colony
stimulating factor (G-CSF) in radiation induced intestinal injury
in mice. Food Chem Toxicol. 50:3174–3180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kim JS, Yang M, Lee CG, Kim SD, Kim JK and
Yang K: In vitro and in vivo protective effects of granulocyte
colony-stimulating factor against radiation-induced intestinal
injury. Arch Pharm Res. 36:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Symon Z, Goldshmidt Y, Picard O, Yavzori
M, Ben-Horin S, Alezra D, Barshack I and Chowers Y: A murine model
for the study of molecular pathogenesis of radiation proctitis. Int
J Radiat Oncol Biol Phys. 76:242–250. 2010. View Article : Google Scholar
|
|
129
|
Sha H, Gu Y, Shen W, Zhang L, Qian F, Zhao
Y, Li H, Zhang T and Lu W: Rheinic acid ameliorates
radiation-induced acute enteritis in rats through PPAR-γ/NF-κB.
Genes Genomics. 41:909–917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lu L, Li W, Sun C, Kang S, Li J, Luo X, Su
Q, Liu B and Qin S: Phycocyanin ameliorates radiation-induced acute
intestinal toxicity by regulating the effect of the gut microbiota
on the TLR4/Myd88/NF-κB pathway. JPEN J Parenter Enteral Nutr.
44:1308–1317. 2020. View Article : Google Scholar
|
|
131
|
Wei YL, Xu JY, Zhang R, Zhang Z, Zhao L
and Qin LQ: Effects of lactoferrin on X-ray-induced intestinal
injury in Balb/C mice. Appl Radiat Isot. 146:72–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Radwan RR and Karam HM: Resveratrol
attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR
signaling pathway. Environ Toxicol. 35:223–230. 2020. View Article : Google Scholar
|
|
133
|
Wang H, Sun RT, Li Y, Yang YF, Xiao FJ,
Zhang YK, Wang SX, Sun HY, Zhang QW, Wu CT and Wang LS: HGF gene
modification in mesenchymal stem cells reduces radiation-induced
intestinal injury by modulating immunity. PLoS One.
10:e01244202015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H
and Jin X: Multi-therapeutic effects of human adipose-derived
mesenchymal stem cells on radiation-induced intestinal injury. Cell
Death Dis. 4:e6852013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Linard C, Strup-Perrot C, Lacave-Lapalun
JV and Benderitter M: Flagellin preconditioning enhances the
efficacy of mesenchymal stem cells in an irradiation-induced
proctitis model. J Leukoc Biol. 100:569–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Akpolat M, Gulle K, Topcu-Tarladacalisir
Y, Safi Oz Z, Bakkal BH, Arasli M and Ozel Turkcu U: Protection by
L-carnitine against radiation-induced ileal mucosal injury in the
rat: Pattern of oxidative stress, apoptosis and cytokines. Int J
Radiat Biol. 89:732–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Bessout R, Demarquay C, Moussa L, René A,
Doix B, Benderitter M, Sémont A and Mathieu N: TH17 predominant
T-cell responses in radiation-induced bowel disease are modulated
by treatment with adipose-derived mesenchymal stromal cells. J
Pathol. 237:435–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Balentova S and Adamkov M: Molecular,
cellular and functional effects of radiation-induced brain injury:
A review. Int J Mol Sci. 16:27796–27815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Deng Z, Sui G, Rosa PM and Zhao W:
Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling
pathway in microglial cells. PLoS One. 7:e367392012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xue J, Dong JH, Huang GD, Qu XF, Wu G and
Dong XR: NF-κB signaling modulates radiation-induced microglial
activation. Oncol Rep. 31:2555–2560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Dong X, Luo M, Huang G, Zhang J, Tong F,
Cheng Y, Cai Q, Dong J, Wu G and Cheng J: Relationship between
irradiation-induced neuro-inflammatory environments and impaired
cognitive function in the developing brain of mice. Int J Radiat
Biol. 91:224–239. 2015. View Article : Google Scholar
|
|
142
|
Chen LJ, Zhang RG, Yu DD, Wu G and Dong
XR: Shenqi fuzheng injection ameliorates radiation-induced brain
injury. Curr Med Sci. 39:965–971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xin N, Li YJ, Li X, Wang X, Li Y, Zhang X,
Dai RJ, Meng WW, Wang HL, Ma H, et al: Dragon's blood may have
radioprotective effects in radiation-induced rat brain injury.
Radiat Res. 178:75–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chiang CS, Hong JH, Stalder A, Sun JR,
Withers HR and McBride WH: Delayed molecular responses to brain
irradiation. Int J Radiat Biol. 72:45–53. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Vozenin-Brotons MC, Gault N, Sivan V,
Tricaud Y, Dubray B, Clough K, Cosset JM, Lefaix JL and Martin M:
Histopathological and cellular studies of a case of cutaneous
radiation syndrome after accidental chronic exposure to a cesium
source. Radiat Res. 152:332–337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Lee JW, Zoumalan RA, Valenzuela CD, Nguyen
PD, Tutela JP, Roman BR, Warren SM and Saadeh PB: Regulators and
mediators of radiation-induced fibrosis: Gene expression profiles
and a rationale for Smad3 inhibition. Otolaryngol Head Neck Surg.
143:525–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Blétry O and Somogyi A: Do the interferons
have an antifibrotic action? The internist's point of view. Rev Med
Interne. 23(Suppl 4): 511s–515s. 2002.In French. View Article : Google Scholar
|
|
148
|
Peter RU, Gottlöber P, Nadeshina N, Krähn
G, Braun-Falco O and Plewig G: Interferon gamma in survivors of the
Chernobyl power plant accident: New therapeutic option for
radiation-induced fibrosis. Int J Radiat Oncol Biol Phys.
45:147–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Oliva D, Nilsson M, Strandéus M, Andersson
BÅ, Sharp L, Laytragoon-Lewin N and Lewin F: Individual genetic
variation might predict acute skin reactions in women undergoing
adjuvant breast cancer radiotherapy. Anticancer Res. 38:6763–6770.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Takeda I, Kizu Y, Yoshitaka O, Saito I and
Yamane GY: Possible role of nitric oxide in radiation-induced
salivary gland dysfunction. Radiat Res. 159:465–470. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Moura JF, Mota JM, Leite CA, Wong DV,
Bezerra NP, Brito GA, Lima V, Cunha FQ and Ribeiro RA: A novel
model of megavoltage radiation-induced oral mucositis in hamsters:
Role of inflammatory cytokines and nitric oxide. Int J Radiat Biol.
91:500–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chung YL, Lee MY and Pui NN: Epigenetic
therapy using the histone deacetylase inhibitor for increasing
therapeutic gain in oral cancer: Prevention of radiation-induced
oral mucositis and inhibition of chemical-induced oral
carcinogenesis. Carcinogenesis. 30:1387–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Epperly MW, Gretton JA, DeFilippi SJ,
Greenberger JS, Sikora CA, Liggitt D and Koe G: Modulation of
radiation-induced cytokine elevation associated with esophagitis
and esophageal stricture by manganese superoxide
dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res.
155:2–14. 2001. View Article : Google Scholar
|
|
154
|
Moreb J and Zucali JR: The therapeutic
potential of interleukin-1 and tumor necrosis factor on
hematopoietic stem cells. Leuk Lymphoma. 8:267–275. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Boniver J, Humblet C, Rongy AM, Delvenne
C, Delvenne P, Greimers R, Thiry A, Courtoy R and Defresne MP:
Cellular aspects of the pathogenesis of radiation-induced thymic
lymphomas in C57 BL mice (review). In Vivo. 4:41–43.
1990.PubMed/NCBI
|