Anaplastic large cell lymphoma (ALCL) is a highly
aggressive type of T cell lymphoma (TCL) that is characterized by
CD30 expression and accounts for ~2% of adult non-Hodgkin's
lymphoma (NHL) (1) and 15% of
pediatric NHL cases (2).
According to the 2017 World Health Organization classification
(3), ALCL is divided into
anaplastic lymphoma kinase-positive (ALK+) ALCL,
ALK-negative (ALK-) ALCL, primary cutaneous ALCL and
breast implant-associated ALCL; the first two types are
collectively referred to as systemic ALCL (sALCL). ALK+
ALCL accounts for 60-85% of all sALCL cases, and all express ALK
fusion proteins, which are transcribed and translated by ALK fusion
genes. The most common fusion gene is nucleophosmin (NPM)-ALK,
which is formed by translocation of the ALK gene on chromosome 2p23
and the NPM gene on chromosome 5q35 (4). Another 10-20% of ALK+
ALCL cases contain variant ALK fusions, such as
5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP
cyclohydrolase-ALK (5), TRK-fused
gene-ALK (6), tropomyosin 3-ALK
(7) and moesin-ALK (8). The majority of patients with ALCL
are males presenting with B symptoms and advanced-stage disease.
Patients with ALK+ ALCL are usually aged <60 years
and are younger than patients with ALK- ALCL (9). Due to the rarity of the disease,
most of the evidence describing the outcomes and various treatment
options for patients with sALCL comes from retrospective studies of
invasive lymphoma or TCL. The most effective therapeutic option for
sALCL remains under investigation. Although the outcome of ALCL is
more favorable than that of peripheral T-cell lymphoma (PTCL)-not
otherwise specified (9), the
overall survival (OS) rate and progression-free survival (PFS) rate
have barely improved in the past 30 years (10), and the outcomes of relapsed or
refractory (R/R) patients are not optimistic. With the deepening of
our understanding of ALCL biology, an increasing number of
potential therapeutic targets and immune checkpoints are being
discovered and tested to improve the prognosis. The traditional
therapeutic strategies and emerging immunotherapies and targeted
therapies of sALCL are presented in this review.
At present, sALCL is mostly treated with the
anthracycline-based cyclophosphamide, vincristine, doxorubicin and
prednisone (CHOP) regimen, but there is no consensus on the
standard regimen for sALCL. With this treatment strategy, the
prognosis of patients with ALK+ ALCL is significantly
superior to that of patients with ALK- ALCL. The German
High-Grade Non-Hodgkin Lymphoma Study Group evaluated the outcomes
of 343 patients with TCL, including 191 patients with ALCL (113
ALK- and 78 ALK+). Patients were separated
into CHOP and etoposide plus CHOP (CHOEP) regimen groups. For
patients aged ≤60 and those with normal lactate dehydrogenase
levels, the 3-year event-free survival (EFS) rate of the CHOEP
regimen was significantly superior to that of the CHOP regimen, but
OS rate was not improved. In elderly patients, the addition of
etoposide had no obvious effect on prognosis (11). Another pooled analysis of 263
patients with ALK+ ALCL found that CHOEP improved both
PFS and OS rates. Furthermore, the 5-year cumulative incidence of
relapse (IR)/progression was clearly decreased following
etoposide-based induction (37 vs. 17%) (12). This study only included patients
with ALK+ ALCL and had a longer follow-up period, which
may be the reason for the benefits observed in not only PFS rate,
but also OS rate.
In addition to CHOP or CHOP-like regimens, a number
of studies have been conducted on alternative therapies, such as
hyperfractionated cyclophosphamide, vincristine, Adriamycin,
dexamethasone/methotrexate and cytarabine (hyper CVAD/MA) (13,14), hyper-CHOP (14) and etoposide, ifosfamide and
cisplatin-reinforced doxorubicin, bleomycin, vinblastine and
dacarbazine (15). Except for
hyper CVAD/MA, which is controversial with regards to improving PFS
rate (13,14), none of the other treatments
exhibited better outcomes. Although intensive regimens may have a
higher response rate, they also lead to a higher toxicity compared
with traditional chemotherapy. In addition, due to the rarity of
the disease, none of these studies analyzed the individual survival
outcomes of ALCL, which needs to be verified by more rigorous
randomized controlled trials.
To date, CHOP remains the recommended first-line
treatment option for patients with sALCL. Young patients (≤60 years
old) have a more favorable EFS following the CHOEP regimen, while
dose-intensive CHOP or alternative treatments have not been
reported to have superior outcomes (Table I). Although patients with ALCL
have a more favorable OS and EFS than other patients with PTCL, the
effect of traditional chemotherapy has seen little improvement over
the past 30 years.
Certain retrospective and prospective studies have
shown that consolidative ASCT improves the outcomes of patients
with PTCL, including those with ALCL (Table II) (18-25). In addition, based on the results
of recent studies, the National Comprehensive Cancer Network (NCCN)
guidelines (26) and the clinical
practice recommendation of the American Society for Blood and
Marrow Transplantation (27)
recommend ASCT for most PTCL subtypes that reach the first CR
(CR1), including ALK- ALCL, but not ALK+
ALCL. However, these studies have certain limitations, as all three
prospective studies were single-arm phase II studies and did not
use a contemporaneous control group. In addition, patients
undergoing ASCT tend to be young and in a generally good condition,
but with a more aggressive disease state. In addition, these
studies had inconsistent responses to first-line chemotherapy [100%
CR or partial response (PR)/CR] and different histological types
(including or not including ALK+ patients). Furthermore,
several recent studies, including a retrospective study (28) and prospective cohort study
(29), have shown that ASCT could
not improve OS rate in patients with ALK- ALCL,
highlighting the need for randomized controlled studies to
determine the role of ASCT as first-line consolidation therapy.
The role of ASCT and allogeneic HSCT (allo-HSCT) in
patients with R/R ALCL remains unclear. Only a few studies have
analyzed the impact of HSCT on the survival of patients with R/R
ALCL (Table III). The
effectiveness of HSCT as consolidation therapy in R/R ALCL was
studied in two retrospective analyses conducted by the European
Society for Blood and Marrow Transplantation (30,31). The 3-year cumulative IR, PFS and
OS rates were 34, 64 and 73% for patients who had undergone ASCT
(30), and 40, 53 and 74% for
patients receiving allo-HSCT, respectively (31). The 5-year treatment-related
mortality (TRM), IR, OS and EFS rates were 25, 28, 54 and 50%,
respectively, in a retrospective study that enrolled 24 patients
with R/R ALCL who had undergone allo-HSCT (32). In addition, the 5-year OS rate of
patients receiving reduced-intensity condition (RIC) regimens (n=8)
was reported to be superior to that of patients receiving
myeloablative conditioning regimens (n=30) (100 vs. 49%; P=0.018),
which may be associated with the lower TRM of the RIC regimen
(33). In addition, the
retrospective nature of the study and the small number of patients
may have had an impact on the results.
One large multicenter retrospective study from the
International Blood and Bone Marrow Transplantation Research Center
compared the effect of ASCT and allo-HSCT in 112 patients with ALCL
(61 ASCT and 51 allo-HSCT) (34).
The results revealed that ASCT had a higher 3-year OS rate (62 vs.
33%; P=0.0088) and a lower TRM rate (5 vs. 32%; P<0.001)
compared with allo-HSCT in patients beyond CR1, but PFS rate and
recurrence/progression were not significantly different between the
two groups. However, an important limitation of that study was that
ALK status was indeterminate.
According to the NCCN guidelines, allo-HSCT or
HDC/ASCT can be used in patients with R/R ALCL who achieve PR/CR
following second-line treatment. However, further in-depth studies
comparing allo-HSCT and HDC/ASCT specifically in ALCL are necessary
to prove their efficiency. In addition, decreasing the mortality
rate and the incidence of side effects of HSCT is also a challenge
that needs to be overcome.
With the development of biomedicine and the
reinvention of tumor therapy, targeted therapy and immunotherapy
have gradually become the focus of tumor therapy research and are
now playing an increasingly important role in the treatment of
tumors, including ALCL. The role of drugs such as brentuximab
vedotin (BV), histone deacetylase (HDAC) inhibitors, ALK inhibitors
and programmed cell death protein 1 (PD-1)/programmed death-ligand
1 (PD-L1) inhibitors in ALCL have already been widely studied
(Table IV), and the ongoing
trials are summarized in Table
V.
BV (SGN-35) is an antibody-drug conjugate (ADC)
against CD30 antigen, expressed in HL and ALCL. BV consists of
three parts: An anti-CD30 monoclonal antibody cAC10, antimitotic
drug microtubule polymerization monomethyl auristatin E (MMAE) and
valine-citrulline dipeptide as a linker (35). CD30 is a member of the tumor
necrosis factor receptor superfamily (36); its expression is limited in normal
tissues but high in malignant tumors, such as ALCL and HL (37). Due to the targeting effect of
antibodies, BV specifically binds to CD30 on the surface of
malignant cells and induces cytotoxicity through MMAE-induced cell
cycle arrest (38). This ADC
combines the targeting selectivity of monoclonal antibodies with
the high cytotoxicity of MMAE, achieving high antitumor activity
and low toxicity and side effects compared with traditional
chemotherapy (39).
BV was approved by the Food and Drug Administration
(FDA) in August 2011 for the treatment of patients with sALCL who
have experienced failure of first-line multiagent chemotherapy at
least once (40). The NCCN
guidelines also recommend BV as the preferred option as second-line
treatment for R/R patients, regardless of whether a transplant is
intended (26). A multicenter
study conducted in Italy evaluated the effectiveness of BV in 40
patients with R/R sALCL (18 ALK+ and 22
ALK-). Of those, 31 (77.5%) achieved a favorable
response after a median of four cycles of single-agent BV, with an
overall response rate (ORR) of 62.5% (45% CR) (41). In addition to retrospective
studies, prospective trials have also confirmed the efficacy of BV
in ALCL. A phase 2 study (NCT00866047) showed a CR rate of 66% with
an estimated 5-year OS and PFS rate of 60 and 39%, respectively
(42). According to the present
analysis, BV has good developmental and application prospects, with
great potential to be used in the treatment of R/R ALCL.
Although patients with R/R ALCL have a high response
rate to BV treatment and a good prognosis, disease progression
still occurs in some patients following BV monotherapy-induced
remission. The effect of BV retreatment has been explored in
previous studies. A prospective study (NCT00947856) involving 8
patients with sALCL reported a ORR of 88% (63% CR) to BV
retreatment and controllable safety (43). A retrospective multicenter study
reported similar results (ORR, 70%; CR, 60%) (44). These two studies suggested that BV
might be a promising option for the retreatment of patients with
R/R ALCL.
Given the side effects and unimproved efficacy of
traditional chemotherapy, as well as the excellent results of BV in
patients with R/R ALCL, Fanale et al (45) evaluated the efficacy of front-line
BV in combination with cyclophosphamide, doxorubicin and prednisone
(BV + CHP) in untreated patients with primary tumors. The study
showed an estimated 5-year PFS and OS rate of 47 and 79%,
respectively, and continued CR was maintained in 56% (9/16) of
patients with ALCL until the end of the study, which indicated that
frontline BV + CHP could yield durable benefits and might be a
curative treatment option for some untreated patients with primary
tumors. Given these promising results, ECHELON-2(NCT01777152), a
large multicenter phase 3 double-blind randomized trial, was
launched to compare the efficacy and safety of BV + CHP and CHOP in
452 untreated patients with CD30+ PTCL (70% sALCL)
(46). After 6 or 8 cycles of
treatment, the CR rate and ORR of the BV + CHP group were superior
to those of the CHOP group. In addition, BV + CHP also showed a
higher 3-year PFS rate (57.1 vs. 44.4%) and a lower risk of death
(hazard ratio, 0.66; P=0.0244). This favorable result prompted the
FDA to approve BV for patients with previously untreated sALCL in
combination with chemotherapy (47).
The most important adverse event (AE) of BV is
peripheral neuropathy (PN), which has an incidence rate of >50%;
however, in previous studies, a significant portion of patients
showed regression or improvement, and there were no fatal AEs
associated with BV during the treatment (42,45,46). Furthermore, ECHELON-2 showed that
the incidence and severity of PN were similar between the BV + CHP
and CHOP groups (46), which
meant that BV may not increase the incidence of PN while still
improving the curative effect.
Overall, the efficacy of BV in patients with R/R
ALCL is considerable, and its retreatment effect on these patients
is also worthy of further study. Compared with traditional
chemotherapy, BV + CHP can achieve superior therapeutic outcomes
without significantly increasing toxicity and it is emerging as a
new treatment option for patients with newly diagnosed ALCL. There
are several ongoing trials on BV in ALCL (NCT01909934, NCT03766516
and NCT03947255) (Table V).
In addition to BV, other US FDA-approved drugs for
patients with R/R PTCL include pralatrexate, romidepsin and
belinostat (48). Pralatrexate is
a novel targeted folic acid agent that can competitively inhibit
dihydrofolate reductase, ultimately leading to DNA replication
error and tumor cell apoptosis (49).
In a large phase 2 study, PROPEL, patients with R/R
PTCL who received a median of three previous systemic treatments
achieved an ORR of 29% [11% CR/unconfirmed CR (CRu), 18% PR] and a
median duration of response (DOR) of 10.1 months (50). Based on the relatively good and
consistent reactivity, in 2009, pralatrexate became the first drug
to be approved by the FDA for patients with R/R PTCL. In an
international case-matched control analysis of the PROPEL study,
the median OS time of the PROPEL group was superior to that of the
control group (15.24 vs. 4.07 months). In patients with ALCL, the
OS curves of the two groups coincided, suggesting that pralatrexate
might have a similar efficacy to that of treatment previously used
in ALCL (51). The addition of
new drugs to conventional cytotoxic drugs has the potential to
yield unexpected clinical benefits for patients, such as the
aforementioned successful discovery of CHOEP and BV + CHP; for that
reason, some clinical centers are experimenting with different drug
combinations. Advani et al (52) studied the effects of
cyclophosphamide, etoposide, vincristine and prednisone alternating
with pralatrexate as first-line treatment for patients with PTCL,
but the results did not show a higher response rate or more
favorable survival compared with the traditional CHOP regimen
(NCT01336933).
Romidepsin, belinostat and chidamide are HDAC
inhibitors that act at the epigenetic level to induce tumor cell
cycle arrest and apoptosis. Results from three phase II clinical
studies showed that among patients with R/R PTCL who had previously
received a median of two or three systemic therapies, the ORR was
25% (15% CR), 25.8% (10.8% CR) and 28% (14% CR), and the median DOR
was 17, 13.6 and 9.9 months, following treatment with romidepsin
(53), belinostat (54) and chidamide (55), respectively. The most common AEs
of grade 3 or higher were hematological toxicities (e.g.,
thrombocytopenia and neutropenia), with the lowest incidence
observed following treatment with belinostat. Due to the poor
survival of patients with R/R PTCL and the relatively effective and
persistent reactivity of romidepsin and belinostat shown in those
studies, these drugs were approved by the US FDA. Chidamide, a
novel benzoamide-like HDAC inhibitor, was approved by the Chinese
FDA in December 2014 for R/R PTCL and is currently only available
in China. In a multicenter real-world study, the ORR of chidamide
monotherapy was 39% and reached 51% when combined with chemotherapy
(56). In addition, a long-term
follow-up study of the romidepsin trial showed a median DOR of 28
months for patients who responded to treatment, and a median PFS
time of 29 months for those who achieved CR/CRu, and the continuous
application of romidepsin did not increase its toxicity (57). Therefore, romidepsin can enable
patients to obtain an effective, long-lasting and relatively safe
response, laying an important foundation for its further study in
patients with PTCL.
The efficacy of these three drugs in combination
with conventional chemotherapy in first-line treatment was also
studied separately. In the phase 2 trial of romidepsin in
combination with CHOP, the ORR was 68% (51% CR), with a 30-month
PFS and OS rate of 41.0 and 70.7%, respectively (NCT01280526)
(58), indicating that the
combination appears to be a feasible option. However, the higher
toxicity of the romidepsin group, with grade 3-4 neutropenia in 89%
of patients, and at least one serious AE in 68% of patients, should
not be ignored. In addition, the existence of cardiotoxicity needs
to be determined. An ongoing multicenter phase 3 randomized
controlled study (NCT01796002) is currently comparing the efficacy
of CHOP with that of romidepsin plus CHOP. In the belinostat
combined with CHOP study, the ORR was 86% (71% CR) at the maximum
tolerated dose of belinostat, and the incidence of AEs was similar
to that following treatment with belinostat alone, suggesting that
the combination would not cause additional toxicity (59). Chidamide, when combined with the
CHOEP regimen, had an ORR of 60.2% (40.7% CR) and a 3-year PFS rate
of 32.8% in 113 newly diagnosed PTCL patients who received 20 mg
chidamide twice a week. The AEs were also at a controllable level
(60).
However, all of these studies were non-randomized,
single-arm trials, with a small patient population and without a
separate analysis of the basic characteristics and survival
outcomes of the patients with ALCL. Therefore, the efficacy of
these drugs in ALCL needs to be further confirmed. At present, the
drugs can be considered for patients who are R/R and have no
response to treatment with BV; however, their role in combination
with chemotherapy in first-line treatment needs to be further
studied.
ALK is a tyrosine kinase receptor that belongs to
the insulin receptor superfamily. Under normal circumstances, the
expression of ALK is limited to neurogenic cells in humans, while
the translocation of the ALK chromosome leads to its expression and
continuous activation. The expression of ALK can be found in
ALK+ ALCL, inflammatory myofibroblastoma and non-small
cell lung cancer (NSCLC). Fusions of ALK have a clear carcinogenic
potential, since their abnormal tyrosine kinase activity can
promote cell proliferation and survival (61-63). The carcinogenic effect of ALK is
mediated by triggering a large number of downstream molecules of
the intracellular signaling cascade. Therefore, ALK can be used as
an ideal therapeutic target for ALK+ disease. At
present, there are three generations of ALK inhibitors; the first
generation is crizotinib, the second generation contains seritinib,
alitinib and brigatinib, and the third generation is lorlatinib
(64). In 2011, crizotinib was
approved by the FDA for the treatment of patients with
ALK+ NSCLC (40); it
has also been tried out in patients with ALCL and ALK+
mutations in recent years and a series of clinical trials are
underway.
The efficacy and safety of crizotinib in patients
with advanced or R/R ALCL have also been demonstrated in two
clinical trials (66,67). One enrolled 26 patients with
ALK+ ALCL, aged 1-21 years, who received 280 or 165
mg/m2 crizotinib twice daily and had an ORR of 88% (CR
81%) (66). The positive results
led to the FDA approval of crizotinib for use in children aged ≥1
years old or young adults with R/R ALK+ ALCL in 2021
(68). Another study showed that
in 18 patients with R/R ALCL, ORR and CR were 52.9 and 47.1%,
respectively, with a median DOR of 2.6 years (67). Although 88.6% of the patients
experienced AEs, most were grade 1 and there were no fatal AEs.
Several case reports also reported that crizotinib
alone or combined with HSCT had a good therapeutic effect in R/R
ALCL (69-74). Alitinib, a second-generation ALK
inhibitor, has also been reported to induce CR in patients with
ALK+ ALCL (75). All
these studies suggested that ALK inhibitors could improve the
prognosis of patients with ALK+ ALCL, but there is also
a report of rapid recurrence following the withdrawal of crizotinib
(76); therefore, long-term
follow-ups are still required to evaluate the efficacy and safety
of these drugs.
Patients with R/R ALCL usually have a poor
prognosis, and there is no consensus on the treatment that should
be administered to these patients. Although research and clinical
practice have confirmed that BV has a favorable effect on patients
with R/R ALCL, its long-term AEs, such as PN, cannot be ignored. In
recent studies, crizotinib has been shown to have significant and
durable antitumor effects and a good tolerance in patients with R/R
ALCL, which suggests its potential as a treatment option. However,
it can only be used in ALK+ patients and is currently
only tentatively used in R/R patients. The matter of recurrence
following drug withdrawal, and whether crizotinib can be used as
first-line treatment, require further study.
Even following BV treatment or HSCT, the prognosis
of patients with R/R ALCL has been reported to remain poor
(77); for that reason, novel
drugs have gradually started to be used for the treatment of these
patients. In addition to the BV, pralatrexate, HDAC inhibitors and
ALK inhibitors aforementioned, other emerging therapies, such as
PD-1/PD-L1 inhibitors, PDGFRB inhibitors and other NPM-ALK fusion
protein inhibitors, have also been widely studied and found to have
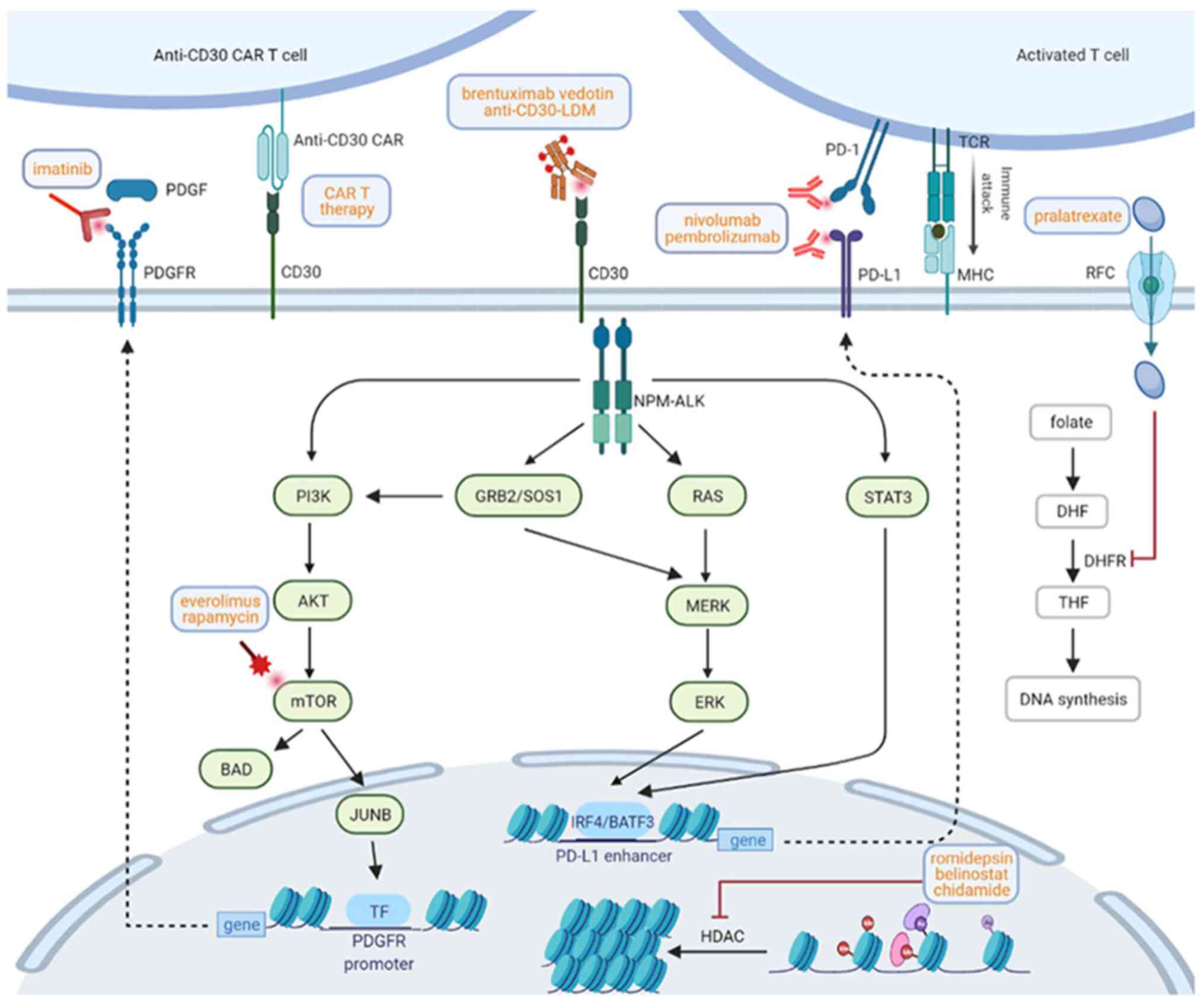
potential benefits. The signal transduction pathways,
immunotherapies and targeted therapies in ALCL are illustrated in
Fig. 1.
PDGF is a peptide regulatory factor that stimulates
tissue cell growth and plays a biological role by phosphorylating
and activating PDGFR and initiating the PDGF/PDGFR signaling
pathway. ALK can induce the expression of JunB and JUN through the
PI3K/Akt/mTOR signaling pathways (78,79), and then bind to and activate the
PDGFRB promoter. The expression and activation of PDGFR is a key
event in lymphoma cell survival and proliferation. A previous study
(80) showed that the therapeutic
inhibition of PDGFR significantly prolonged the survival time of
NPM-ALK transgenic mice. The study also reported the case of a
27-year-old patient with stage III ALK+ ALCL, who was
unresponsive to conventional chemotherapy and relapsed following
ASCT (80). After 10 days of
treatment with 400 mg/day imatinib, a kind of PDGFR inhibitor, the
tumor marker levels decreased and the patient rapidly achieved CR,
which was maintained for 22 months. A follow-up study showed that
there was no recurrence 1 year after the withdrawal of imatinib
(81). These results suggested
that PDGFR inhibitors may be an effective treatment for
NPM-ALK+ ALCL. In addition, PDGFRs exists not only in
NPM-ALK+ TCL but also in ALK- ALCL.
Therefore, the application of PDGFR inhibitors is worthy of further
study not only in patients with ALK+ ALCL but also in
those with ALK- ALCL.
In addition, studies have shown that the combination
of alectinib and everolimus (97)
or the combination of crizotinib and everolimus (98) can have a synergistic effect on the
growth of ALK+ ALCL cells, indicating that the
combination of ALK inhibitors and mTOR inhibitors is worthy of
further clinical investigation.
PD-1 is a receptor protein on the surface of
activated T cells and a member of the immunoglobulin superfamily.
PD-L1 protein is the ligand of PD-1 and is expressed in a variety
of tumor cells. The combination of the two proteins transmits a
negative regulatory signal to T cells, inhibits T-cell activation,
proliferation and cytokine production, induces T-cell apoptosis and
enables the immune escape of tumor cells (99-101). The FDA has approved PD-1/PD-L1
inhibitors for the treatment of lung cancer, Hodgkin's lymphoma and
other tumors (102-104).
These studies provide a theoretical basis for the
application of PD-1/PD-L1 inhibitors in R/R ALCL. In two previous
case reports, 2 patients with ALK+ ALCL who relapsed
after chemotherapy and ALK inhibitor treatment (one of whom also
underwent allo-HSCT) obtained a sustained CR following treatment
with nivolumab (3 mg/kg/2 weeks) (110,111). Another patient (112) with stage IV ALK- ALCL
who experienced relapse following chemotherapy, ASCT, allo-HSCT and
BV treatment, was administered pembrolizumab (2 mg/kg; 1 dose of
each at weeks 1 and 6, then once every 3 weeks) and obtained a CR
after the third dose. These reports provide a clinical basis for
the application of anti-PD1/PD-L1 therapy in R/R ALCL. A clinical
trial (NCT03703050) evaluating the response of patients with
progressive R/R ALK+ ALCL to nivolumab or its use as
consolidative therapy in patients with a CR after relapse is
currently recruiting.
As a major breakthrough in immunology, the emergence
of CAR-T therapy has significant implications for the treatment of
R/R B-cell NHL (113,114). However, its development in
T-cell malignancies is fraught with difficulties and challenges,
due to targetable antigens, cell fratricide and other issues
(115). The presence of CAR-T
cells that could recognize CD30 offers hope for patients with ALCL.
As previously described, CD30 is widely expressed in ALCL cells.
Several clinical trials on the efficacy, safety or optimum dose of
CD30 CAR-T therapy in patients with R/R CD30+ lymphocyte
malignancies (including ALCL) are ongoing (NCT04526834,
NCT04008394, NCT03049449, NCT04083495 and NCT03383965) (Table V).
The MYC proto-oncogene, basic helix-loop-helix
transcription factor gene family and its products can promote cell
proliferation, differentiation and transformation, and play an
important role in a variety of tumors, while ALCL can induce the
expression of MYC protein in several ways (116,117). In addition, MYC can regulate
antitumor immunity by controlling the expression of PD-L1 in ALCL
(118,119). The inhibition of MYC had a toxic
effect on the DEL ALK+ ALCL cell line, suggesting that
use of the MYC inhibitor may be an effective breakthrough in the
treatment of ALCL in the future (117).
Lidamycin (LDM) is a highly effective enadiyne
antitumor antibiotic that kills tumor cells by directly acting on
DNA chains to cause DNA damage (120). The LDM molecule is composed of
an apoprotein and highly active enadiyne chromophore (121). Anti-CD30-LDM, obtained by
coupling LDP with CD30 by genetic engineering, is a new type of ADC
that has exhibited strong tumor-targeting and antitumor effects in
CD30+ ALCL cells and a Karpas299 mouse model, with no
obvious toxicity to normal tissue (122). Further experiments showed that
the combination of crizotinib and anti-CD30-LDM had a significant
synergistic inhibitory effect on ALK+ ALCL cells in
vitro and in vivo (123).
Other agents, such as KRCA-0008 (a co-inhibitor of
wild-type ALK and crizotinib-resistant ALK mutant) (124), ZYY (a novel ALK inhibitor)
(125) and tyrosine kinase 2 (a
member of the JAK family of tyrosine kinases) (126) inhibitors, have been studied in
ALCL and achieved good responses in a series of in vivo and
in vitro trials, providing new options for the treatment of
ALCL and the potential to improve patient prognosis.
ALCL is a type of lymphoma with a low incidence,
which makes carrying out clinical research challenging. Traditional
chemotherapy, such as the CHOP regimen, is still recommended for
the first-line treatment of ALCL due to its acceptable AEs and
relatively satisfactory treatment effects and survival rate
compared with intensive chemotherapy. HSCT can be administered to
certain patients and improve their survival, but the increased AEs
and TRM cannot be ignored, particularly with regard to allo-HSCT.
Targeted therapy and immunotherapy, such as ALK inhibitors, HDAC
inhibitors, and BV, have exhibited high response rates and
favorable survival rates for patients with ALK+ or
CD30+ R/R ALCL, but their application as first-line
therapy requires further exploration. In addition, other novel
drugs, such as mTOR inhibitors, PDGFR inhibitors and PD-1/PD-L1
inhibitors, have been proven to be effective in certain case
reports, offering options for R/R patients. However, the relatively
high price is an important reason to limit the widespread
application of immunotherapy. In addition, how to evaluate and
determine which immunotherapy regimen to use and how to predict its
effectiveness, as well as the AEs and drug resistance of
immunotherapy, all need further research.
Not applicable.
XS wrote the manuscript, XF provided the main
outline and direction of the manuscript, and helped to revise the
content of the manuscript. DL and YL summarized the data and
tables, and helped to revise the manuscript. XW provided the latest
ideas and polished the manuscript. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was funded by the National Natural Science Foundation
(grant nos. 82070203, 81770210, 81473486 and 81270598), the Key
Research and Development Program of Shandong Province (grant no.
2018CXGC1213), the Technology Development Projects of Shandong
Province (grant no. 2017GSF18189), the Translational Research Grant
of NCRCH (grant nos. 2021WWB02 and 2020ZKMB01), the Technology
Projects of Jinan (grant nos. 201704092 and 202019044), the Taishan
Scholars Program of Shandong Province, Shandong Provincial
Engineering Research Center of Lymphoma, and Academic Promotion
Programme of Shandong First Medical University (grant no.
2019QL018).
|
1
|
Al-Hamadani M, Habermann TM, Cerhan JR,
Macon WR, Maurer MJ and Go RS: Non-Hodgkin lymphoma subtype
distribution, geodemographic patterns, and survival in the US: A
longitudinal analysis of the National cancer data base from 1998 to
2011. Am J Hematol. 90:790–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alessandri AJ, Pritchard SL, Schultz KR
and Massing BG: A population-based study of pediatric anaplastic
large cell lymphoma. Cancer. 94:1830–1835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swerdlow SH, Harris NL, Jaffe ES, Pileri
SA, Stein H and Thiele J: WHO classification of tumours of
hematopoietic and lymphoid tissues. 2. 4th edition. International
Agency for Research on Cancer (IARC); Lyon: 2017
|
|
4
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer KG, Shapiro DN, Saltman DL and Look AT: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's
lymphoma. Science. 263:1281–1284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Z, Cools J, Marynen P, Cui X, Siebert
R, Gesk S, Schlegelberger B, Peeters B, De Wolf-Peeters C,
Wlodarska I and Morris SW: Inv(2)(p23q35) in anaplastic large-cell
lymphoma induces constitutive anaplastic lymphoma kinase (ALK)
tyrosine kinase activation by fusion to ATIC, an enzyme involved in
purine nucleotide biosynthesis. Blood. 95:2144–2149. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández L, Pinyol M, Hernández S, Beà S,
Pulford K, Rosenwald A, Lamant L, Falini B, Ott G, Mason DY, et al:
TRK-fused gene (TFG) is a new partner of ALK in anaplastic large
cell lymphoma producing two structurally different TFG-ALK
translocations. Blood. 94:3265–3268. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamant L, Dastugue N, Pulford K, Delsol G
and Mariamé B: A new fusion gene TPM3-ALK in anaplastic large cell
lymphoma created by a (1;2)(q25;p23) translocation. Blood.
93:3088–3095. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tort F, Pinyol M, Pulford K, Roncador G,
Hernandez L, Nayach I, Kluin-Nelemans HC, Kluin P, Touriol C,
Delsol G, et al: Molecular characterization of a new ALK
translocation involving moesin (MSN-ALK) in anaplastic large cell
lymphoma. Lab Invest. 81:419–426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savage KJ, Harris NL, Vose JM, Ullrich F,
Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne
RD, et al: ALK-anaplastic large-cell lymphoma is clinically and
immunophenotypically different from both ALK+ ALCL and peripheral
T-cell lymphoma, not otherwise specified: Report from the
International peripheral t-cell lymphoma project. Blood.
111:5496–5504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larose H, Burke GAA, Lowe EJ and Turner
SD: From bench to bedside: The past, present and future of therapy
for systemic paediatric ALCL, ALK. Br J Haematol. 185:1043–1054.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmitz N, Trumper L, Ziepert M, Nickelsen
M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A and
Pfreundschuh M: Treatment and prognosis of mature T-cell and
NK-cell lymphoma: An analysis of patients with T-cell lymphoma
treated in studies of the German high-grade non-hodgkin lymphoma
study group. Blood. 116:3418–3425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sibon D, Nguyen DP, Schmitz N, Suzuki R,
Feldman AL, Gressin R, Lamant L, Weisenburger DD, Rosenwald A,
Nakamura S, et al: ALK-positive anaplastic large-cell lymphoma in
adults: An individual patient data pooled analysis of 263 patients.
Haematologica. 104:e562–e565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abramson JS, Feldman T, Kroll-Desrosiers
AR, Muffly LS, Winer E, Flowers CR, Lansigan F, Nabhan C, Nastoupil
LJ, Nath R, et al: Peripheral T-cell lymphomas in a large US
multicenter cohort: Prognostication in the modern era including
impact of frontline therapy. Ann Oncol. 25:2211–2217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Escalon MP, Liu NS, Yang Y, Hess M, Walker
PL, Smith TL and Dang NH: Prognostic factors and treatment of
patients with T-cell non-Hodgkin lymphoma: The M D Anderson cancer
center experience. Cancer. 103:2091–2098. 2005. View Article : Google Scholar
|
|
15
|
Simon A, Peoch M, Casassus P, Deconinck E,
Colombat P, Desablens B, Tournilhac O, Eghbali H, Foussard C,
Jaubert J, et al: Upfront VIP-reinforced-ABVD (VIP-rABVD) is not
superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma.
Results of the randomized phase III trial GOELAMS-LTP95. Br J
Haematol. 151:159–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sibon D, Fournier M, Brière J, Lamant L,
Haioun C, Coiffier B, Bologna S, Morel P, Gabarre J, Hermine O, et
al: Long-term outcome of adults with systemic anaplastic large-cell
lymphoma treated within the groupe d'etude des lymphomes de
l'adulte trials. J Clin Oncol. 30:3939–3946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brugières L, Quartier P, Le Deley MC,
Pacquement H, Perel Y, Bergeron C, Schmitt C, Landmann J, Patte C,
Terrier-Lacombe MJ, et al: Relapses of childhood anaplastic
large-cell lymphoma: Treatment results in a series of 41 children-a
report from the French society of pediatric oncology. Ann Oncol.
11:53–58. 2000. View Article : Google Scholar
|
|
18
|
Corradini P, Tarella C, Zallio F, Dodero
A, Zanni M, Valagussa P, Gianni AM, Rambaldi A, Barbui T and
Cortelazzo S: Long-term follow-up of patients with peripheral
T-cell lymphomas treated up-front with high-dose chemotherapy
followed by autologous stem cell transplantation. Leukemia.
20:1533–1538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
d'Amore F, Relander T, Lauritzsen GF,
Jantunen E, Hagberg H, Anderson H, Holte H, Österborg A, Merup M,
Brown P, et al: Up-front autologous stem-cell transplantation in
peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 30:3093–3099.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reimer P, Rudiger T, Geissinger E,
Weissinger F, Nerl C, Schmitz N, Engert A, Einsele H,
Müller-Hermelink HK and Wilhelm M: Autologous stem-cell
transplantation as first-line therapy in peripheral T-cell
lymphomas: Results of a prospective multicenter study. J Clin
Oncol. 27:106–113. 2009. View Article : Google Scholar
|
|
21
|
Wilhelm M, Smetak M, Reimer P, Geissinger
E, Ruediger T, Metzner B, Schmitz N, Engert A, Schaefer-Eckart K
and Birkmann J: First-line therapy of peripheral T-cell lymphoma:
Extension and long-term follow-up of a study investigating the role
of autologous stem cell transplantation. Blood Cancer J.
6:e4522016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez J, Conde E, Gutierrez A, Arranz
R, León A, Marín J, Bendandi M, Albo C and Caballero MD: The
results of consolidation with autologous stem-cell transplantation
in patients with peripheral T-cell lymphoma (PTCL) in first
complete remission: The Spanish lymphoma and autologous
transplantation group experience. Ann Oncol. 18:652–657. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He XH, Li B, Zou SM, Dong M, Zhou SY, Yang
JL, Xue LY, Yang S, Liu P, Qin Y, et al: Efficacy of peripheral
blood stem cell transplantation versus conventional chemotherapy on
anaplastic large-cell lymphoma: A retrospective study of 64
patients from a single center. Chin J Cancer. 31:532–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehta N, Maragulia JC, Moskowitz A, Hamlin
PA, Lunning MA, Moskowitz CH, Zelenetz A, Matasar MJ, Sauter C,
Goldberg J and Horwitz SM: A retrospective analysis of peripheral
T-cell lymphoma treated with the intention to transplant in the
first remission. Clin Lymphoma Myeloma Leuk. 13:664–670. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ellin F, Landstrom J, Jerkeman M and
Relander T: Real-world data on prognostic factors and treatment in
peripheral T-cell lymphomas: A study from the Swedish lymphoma
registry. Blood. 124:1570–1577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horwitz SM, Ansell S, Ai WZ, Barnes J,
Barta SK, Clemens MW, Dogan A, Goodman AM, Goyal G, Guitart J, et
al: NCCN Guidelines Insights: T-Cell Lymphomas, Version 1.2021. J
Natl Compr Canc Netw. 18:1460–1467. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kharfan-Dabaja MA, Kumar A, Ayala E,
Hamadani M, Reimer P, Gisselbrecht C, d'Amore F, Jantunen E, Ishida
T, Bazarbachi A, et al: Clinical practice recommendations on
indication and timing of hematopoietic cell transplantation in
Mature T cell and NK/T cell lymphomas: An International
collaborative effort on behalf of the guidelines committee of the
American society for blood and marrow transplantation. Biol Blood
Marrow Transplant. 23:1826–1838. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SI, Horwitz SM, Foss FM, Pinter-Brown
LC, Carson KR, Rosen ST, Pro B, His ED, Federico M, Gisselbrecht C,
et al: The role of autologous stem cell transplantation in patients
with nodal peripheral T-cell lymphomas in first complete remission:
Report from COMPLETE, a prospective, multicenter cohort study.
Cancer. 125:1507–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fossard G, Broussais F, Coelho I, Bailly
S, Nicolas-Virelizier E, Toussaint E, Lancesseur C, Le Bras F,
Willems E, Tchernonog E, et al: Role of up-front autologous
stem-cell transplantation in peripheral T-cell lymphoma for
patients in response after induction: An analysis of patients from
LYSA centers. Ann Oncol. 29:715–723. 2018. View Article : Google Scholar
|
|
30
|
Domingo-Domenech E, Boumendil A, Climent
F, Sengeloev H, Wahlin B, Wattad W, Arat M, Finel H, Schapp N,
Ganser A, et al: Autologous hematopoietic stem cell transplantation
for relapsed/refractory systemic anaplastic large cell lymphoma. A
retrospective analysis of the lymphoma working party (LWP) of the
EBMT. Bone Marrow Transplant. 55:796–803. 2020. View Article : Google Scholar
|
|
31
|
Domingo-Domenech E, Boumendil A, Climent
F, Socié G, Kroschinsky F, Finel H, Vandenbergue E, Nemet D,
Stelljes M, Bittenbring JT, et al: Allogeneic hematopoietic stem
cell transplantation for patients with relapsed/refractory systemic
anaplastic large cell lymphoma. A retrospective analysis of the
lymphoma working party of the European society for blood and marrow
transplantation. Bone Marrow Transplant. 55:633–640. 2020.
View Article : Google Scholar
|
|
32
|
Fukano R, Mori T, Kobayashi R, Mitsui T,
Fujita N, Iwasaki F, Suzumiya J, Chin M, Goto H, Takahashi Y, et
al: Haematopoietic stem cell transplantation for relapsed or
refractory anaplastic large cell lymphoma: A study of children and
adolescents in Japan. Br J Haematol. 168:557–563. 2015. View Article : Google Scholar
|
|
33
|
Fukano R, Mori T, Fujita N, Kobayashi R,
Mitsui T, Kato K, Suzuki R, Suzumiya J, Fukuda T, Shindo M, et al:
Successful outcome with reduced-intensity condition regimen
followed by allogeneic hematopoietic stem cell transplantation for
relapsed or refractory anaplastic large-cell lymphoma. Int J
Hematol. 110:723–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith SM, Burns LJ, van Besien K,
Lerademacher J, He W, Fenske TS, Suzuki R, Hsu JW, Schouten HC,
Hale GA, et al: Hematopoietic cell transplantation for systemic
mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 31:3100–3109.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katz J, Janik JE and Younes A: Brentuximab
Vedotin (SGN-35). Clin Cancer Res. 17:6428–6436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith CA, Farrah T and Goodwin RG: The TNF
receptor superfamily of cellular and viral proteins: Activation,
costimulation, and death. Cell. 76:959–962. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Younes A and Kadin ME: Emerging
applications of the tumor necrosis factor family of ligands and
receptors in cancer therapy. J Clin Oncol. 21:3526–3534. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ansell SM: Brentuximab vedotin: Delivering
an antimitotic drug to activated lymphoma cells. Expert Opin
Investig Drugs. 20:99–105. 2011. View Article : Google Scholar
|
|
39
|
Shustov A and Soma L: Anaplastic large
cell lymphoma: Contemporary concepts and optimal management. Cancer
Treat Res. 176:127–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
2011 Notifications. https://www.fda.gov/drugs/resources-information-approved-drugs/2011-notifications.
Journal 2021. U.S Food and Drug Adminstration; 2018
|
|
41
|
Broccoli A, Pellegrini C, Di Rocco A,
Puccini B, Patti C, Gini G, Mannina D, Tani M, Rusconi C, Romano A,
et al: Italian real-life experience with brentuximab vedotin:
Results of a large observational study of 40 cases of
relapsed/refractory systemic anaplastic large cell lymphoma.
Haematologica. 102:1931–1935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pro B, Advani R, Brice P, Bartlett NL,
Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M,
Connors JM, et al: Five-year results of brentuximab vedotin in
patients with relapsed or refractory systemic anaplastic large cell
lymphoma. Blood. 130:2709–2717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bartlett NL, Chen R, Fanale MA, Brice P,
Gopal A, Smith SE, Advani R, Matous JV, Ramchandren R, Rosenblatt
JD, et al: Retreatment with brentuximab vedotin in patients with
CD30-positive hematologic malignancies. J Hematol Oncol. 7:242014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fukuhara N, Yamamoto G, Tsujimura H, Chou
T, Shibayama H, Yanai T, Shibuya K and Izutsu K: Retreatment with
brentuximab vedotin in patients with relapsed/refractory classical
Hodgkin lymphoma or systemic anaplastic large-cell lymphoma: A
multicenter retrospective study. Leuk Lymphoma. 61:176–180. 2020.
View Article : Google Scholar
|
|
45
|
Fanale MA, Horwitz SM, Forero-Torres A,
Bartlett NL, Advani RH, Pro B, Chen RW, Davies A, Illidge T,
Uttarwar M, et al: Five-year outcomes for frontline brentuximab
vedotin with CHP for CD30-expressing peripheral T-cell lymphomas.
Blood. 131:2120–2124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Horwitz S, O'Connor OA, Pro B, Illidge T,
Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F,
Domingo-Domenech E, et al: Brentuximab vedotin with chemotherapy
for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global,
double-blind, randomised, phase 3 trial. Lancet. 393:229–240. 2019.
View Article : Google Scholar :
|
|
47
|
U.S. Food and Drug (FDA): FDA approves
first-line treatment for peripheral T-cell lymphoma under new
review pilot. FDA; Silver Spring, MD: 2018, https://www.fda.gov/news-events/press-announcements/fda-approves-first-line-treatment-peripheralt-cell-lymphoma-under-new-review-pilot.
Accessed November 16, 2018.
|
|
48
|
Vu K and Ai W: Update on the treatment of
anaplastic large cell lymphoma. Curr Hematol Malig Rep. 13:135–141.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Malik SM, Liu K, Qiang X, Sridhara R, Tang
S, McGuinn WD Jr, Verbois SL, Marathe A, Williams GM, Bullock J, et
al: Folotyn (pralatrexate injection) for the treatment of patients
with relapsed or refractory peripheral T-cell lymphoma: U.S. food
and drug administration drug approval summary. Clin Cancer Res.
16:4921–4927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
O'Connor OA, Pro B, Pinter-Brown L,
Bartlett N, Popplewell L, Coiffier B, Lechowicz MJ, Savage KJ,
Shustov AR, Gisselbrecht C, et al: Pralatrexate in patients with
relapsed or refractory peripheral T-cell lymphoma: Results from the
pivotal PROPEL study. J Clin Oncol. 29:1182–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
O'Connor OA, Marchi E, Volinn W, Shi J,
Mehrling T and Kim WS: Strategy for assessing new drug value in
orphan diseases: An international case match control analysis of
the PROPEL study. JNCI Cancer Spectr. 2:pky0382018. View Article : Google Scholar
|
|
52
|
Advani RH, Ansell SM, Lechowicz MJ, Beaven
AW, Loberiza F, Carson KR, Evens AM, Foss F, Horwitz S, Pro B, et
al: A phase II study of cyclophosphamide, etoposide, vincristine
and prednisone (CEOP) Alternating with Pralatrexate (P) as front
line therapy for patients with peripheral T-cell lymphoma (PTCL):
Final results from the T-cell consortium trial. Br J Haematol.
172:535–544. 2016. View Article : Google Scholar
|
|
53
|
Coiffier B, Pro B, Prince HM, Foss F,
Sokol L, Greenwood M, Caballero D, Borchmann P, Morschhauser F,
Wilhelm M, et al: Results from a pivotal, open-label, phase II
study of romidepsin in relapsed or refractory peripheral T-cell
lymphoma after prior systemic therapy. J Clin Oncol. 30:631–636.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
O'Connor OA, Horwitz S, Masszi T, Van Hoof
A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S,
et al: Belinostat in patients with relapsed or refractory
peripheral T-Cell Lymphoma: Results of the pivotal phase II BELIEF
(CLN-19) study. J Clin Oncol. 33:2492–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi Y, Dong M, Hong X, Zhang W, Feng J,
Zhu J, Yu L, Ke X, Huang H, Shen Z, et al: Results from a
multicenter, open-label, pivotal phase II study of chidamide in
relapsed or refractory peripheral T-cell lymphoma. Ann Oncol.
26:1766–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi Y, Jia B, Xu W, Li W, Liu T, Liu P,
Zhao W, Zhang H, Sun X, Yang H, et al: Chidamide in relapsed or
refractory peripheral T cell lymphoma: A multicenter real-world
study in China. J Hematol Oncol. 10:692017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Coiffier B, Pro B, Prince HM, Foss F,
Sokol L, Greenwood M, Caballero D, Morschhauser F, Wilhelm M,
Pinter-Brown L, et al: Romidepsin for the treatment of
relapsed/refractory peripheral T-cell lymphoma: Pivotal study
update demonstrates durable responses. J Hematol Oncol. 7:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dupuis J, Morschhauser F, Ghesquières H,
Tilly H, Casasnovas O, Thieblemont C, Ribrag V, Bossard C, Le Bras
F, Bachy E, et al: Combination of romidepsin with cyclophosphamide,
doxorubicin, vincristine, and prednisone in previously untreated
patients with peripheral T-cell lymphoma: A non-randomised, phase
1b/2 study. Lancet Haematol. 2:e160–e165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Johnston PB, Cashen AF, Nikolinakos PG,
Beaven AW, Barta SK, Bhat G, Hasal SJ, De Vos S, Oki Y, Deng C and
Foss FM: Belinostat in combination with standard cyclophosphamide,
doxorubicin, vincristine and prednisone as first-line treatment for
patients with newly diagnosed peripheral T-cell lymphoma. Exp
Hematol Oncol. 10:152021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang W, Su L, Liu L, Gao Y, Wang Q, Su H,
Song Y, Zhang H, Shen J, Jing H, et al: The combination of
chidamide with the CHOEP regimen in previously untreated patients
with peripheral T-cell lymphoma: A prospective, multicenter, single
arm, phase 1b/2 study. Cancer Biol Med. Mar 23–2021.Epub ahead of
print. View Article : Google Scholar
|
|
61
|
Jäger R, Hahne J, Jacob A, Egert A,
Schenkel J, Wernert N, Schorle H and Wellmann A: Mice transgenic
for NPM-ALK develop non-Hodgkin lymphomas. Anticancer Res.
25:3191–3196. 2005.PubMed/NCBI
|
|
62
|
Chiarle R, Gong JZ, Guasparri I, Pesci A,
Cai J, Liu J, Simmons WJ, Dhall G, Howes J, Piva R and Inghirami G:
NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and
plasma cell tumors. Blood. 101:1919–1927. 2003. View Article : Google Scholar
|
|
63
|
Kuefer MU, Look AT, Pulford K, Behm FG,
Pattengale PK, Mason DY and Morris SW: Retrovirus-mediated gene
transfer of NPM-ALK causes lymphoid malignancy in mice. Blood.
90:2901–2910. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Waqar SN and Morgensztern D: Lorlatinib: A
new-generation drug for ALK-positive NSCLC. Lancet Oncol.
19:1555–1557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gambacorti Passerini C, Farina F, Stasia
A, Redaelli S, Ceccon M, Mologni L, Messa C, Guerra L, Giudici G,
Sala E, et al: Crizotinib in advanced, chemoresistant anaplastic
lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst.
106:djt3782014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mossé YP, Voss SD, Lim MS, Rolland D,
Minard CG, Fox E, Adamson P, Wilner K, Blaney SM and Weigel BJ:
Targeting ALK with crizotinib in pediatric anaplastic large cell
lymphoma and inflammatory myofibroblastic tumor: A children's
oncology group study. J Clin Oncol. 35:3215–3221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gambacorti-Passerini C, Orlov S, Zhang L,
Braiteh F, Huang H, Esaki T, Horibe K, Ahn JS, Beck JT, Edenfield
WJ, et al: Long-term effects of crizotinib in ALK-positive tumors
(excluding NSCLC): A phase 1b open-label study. Am J Hematol.
93:607–614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
U.S. Food and Drug (FDA): FDA approves
crizotinib for children and young adults with relapsed or
refractory, systemic anaplastic large cell lymphoma. FDA; Silver
Spring, MD: 2021, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-crizotinib-children-and-young-adults-relapsed-orrefractory-systemic-anaplastic-large.
Accessed January 15, 2021.
|
|
69
|
Mahuad CV, Repáraz Mde L, Zerga ME,
Aizpurua MF, Casali C and Garate G: Three years sustained complete
remission achieved in a primary refractory ALK-positive anaplastic
T large cell lymphoma treated with crizotinib. Rare Tumors.
8:62662016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
John TD, Naik S, Leung K, Sasa G, Martinez
C and Krance RA: Allogeneic hematopoietic cell transplant following
crizotinib monotherapy for relapsed/refractory anaplastic large
cell lymphoma. Pediatr Transplant. 22:e132102018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ordemann R, Stöhlmacher J,
Beuthien-Baumann B, Platzek I, van den Hoff J, Kroschinsky F,
Middeke JM, Platzbecker U, Zietz C, Bornhäuser M and Ehninger G:
Use of targeted therapy for refractory ALK-positive anaplastic
large cell lymphoma as a bridging strategy prior to allogeneic
transplantation. Ann Hematol. 92:125–127. 2013. View Article : Google Scholar
|
|
72
|
Cleary JM, Rodig S, Barr PM, Shinagare AB,
Clark JW, Shapiro GI and Armand P: Crizotinib as salvage and
maintenance with allogeneic stem cell transplantation for
refractory anaplastic large cell lymphoma. J Natl Compr Canc Netw.
12:323–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shelikhova LN, Fominykh VV, Abramov DS,
Myakova NV, Maschan MA and Maschan AA: Use of crizotinib for
refractory ALK-positive lymphomas. Ter Arkh. 89:51–56. 2017.
|
|
74
|
Sun X, Fang X and Jiang Y: Successful
combination of crizotinib and hematopoietic stem cell
transplantation in relapsed ALK-positive ALCL. Indian J Cancer.
58:108–111. 2021.PubMed/NCBI
|
|
75
|
Reed DR, Hall RD, Gentzler RD, Volodin L,
Douvas MG and Portell CA: Treatment of refractory ALK rearranged
anaplastic large cell lymphoma with alectinib. Clin Lymphoma
Myeloma Leuk. 19:e247–e250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gambacorti-Passerini C, Mussolin L and
Brugieres L: Abrupt relapse of ALK-Positive lymphoma after
discontinuation of crizotinib. N Engl J Med. 374:95–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chihara D, Wong S, Feldman T, Fanale MA,
Sanchez L, Connors JM, Savage KJ and Oki Y: Outcome of patients
with relapsed or refractory anaplastic large cell lymphoma who have
failed brentuximab vedotin. Hematol Oncol. 37:35–38. 2019.
View Article : Google Scholar
|
|
78
|
Mathas S, Hinz M, Anagnostopoulos I,
Krappmann D, Lietz A, Jundt F, Bommert K, Mechta-Grigoriou F, Stein
H, Dörken B and Scheidereit C: Aberrantly expressed c-Jun and JunB
are a hallmark of Hodgkin lymphoma cells, stimulate proliferation
and synergize with NF-kappa B. EMBO J. 21:4104–4113. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Staber PB, Vesely P, Haq N, Ott RG, Funato
K, Bambach I, Fuchs C, Schauer S, Linkesch W, Hrzenjak A, et al:
The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces
JUNB transcription via ERK1/2 and JunB translation via mTOR
signaling. Blood. 110:3374–3383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Laimer D, Dolznig H, Kollmann K, Vesely
PW, Schlederer M, Merkel O, Schiefer AI, Hassler MR, Heider S,
Amenitsch L, et al: PDGFR blockade is a rational and effective
therapy for NPM-ALK-driven lymphomas. Nat Med. 18:1699–1704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Laimer-Gruber D: Blockade of the PDGF
receptor: A new and effective therapy option for NPM-ALK-dependent
lymphoma. Pathologe. 35(Suppl 2): S185–S186. 2014. View Article : Google Scholar
|
|
82
|
Slupianek A, Nieborowska-Skorska M, Hoser
G, Morrione A, Majewski M, Xue L, Morris SW, Wasik MA and Skorski
T: Role of phosphatidylinositol 3-kinase-Akt pathway in
nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis.
Cancer Res. 61:2194–2199. 2001.PubMed/NCBI
|
|
83
|
Bai RY, Ouyang T, Miething C, Morris SW,
Peschel C and Duyster J: Nucleophosmin-anaplastic lymphoma kinase
associated with anaplastic large-cell lymphoma activates the
phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway.
Blood. 96:4319–4327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Slupianek A and Skorski T: NPM/ALK
downregulates p27Kip1 in a PI-3K-dependent manner. Exp Hematol.
32:1265–1271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rassidakis GZ, Feretzaki M, Atwell C,
Grammatikakis I, Lin Q, Lai R, Claret FX, Medeiros LJ and Amin HM:
Inhibition of Akt increases p27Kip1 levels and induces cell cycle
arrest in anaplastic large cell lymphoma. Blood. 105:827–829. 2005.
View Article : Google Scholar
|
|
86
|
Chiarle R, Simmons WJ, Cai H, Dhall G,
Zamo A, Raz R, Karras JG, Levy DE and Inghirami G: Stat3 is
required for ALK-mediated lymphomagenesis and provides a possible
therapeutic target. Nat Med. 11:623–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Q, Raghunath PN, Xue L, Majewski M,
Carpentieri DF, Odum N, Morris S, Skorski T and Wasik MA:
Multilevel dysregulation of STAT3 activation in anaplastic lymphoma
kinase-positive T/null-cell lymphoma. J Immunol. 168:466–474. 2002.
View Article : Google Scholar
|
|
88
|
Marzec M, Kasprzycka M, Liu X, Raghunath
PN, Wlodarski P and Wasik MA: Oncogenic tyrosine kinase NPM/ALK
induces activation of the MEK/ERK signaling pathway independently
of c-Raf. Oncogene. 26:813–821. 2007. View Article : Google Scholar
|
|
89
|
Marzec M, Kasprzycka M, Liu X, El-Salem M,
Halasa K, Raghunath PN, Bucki R, Wlodarski P and Wasik MA:
Oncogenic tyrosine kinase NPM/ALK induces activation of the
rapamycin-sensitive mTOR signaling pathway. Oncogene. 26:5606–5614.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Vega F, Medeiros LJ, Leventaki V, Atwell
C, Cho-Vega JH, Tian L, Claret FX and Rassidakis GZ: Activation of
mammalian target of rapamycin signaling pathway contributes to
tumor cell survival in anaplastic lymphoma kinase-positive
anaplastic large cell lymphoma. Cancer Res. 66:6589–6597. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gao J, Yin M, Zhu Y, Gu L, Zhang Y, Li Q,
Jia C and Ma Z: Prognostic significance and therapeutic potential
of the activation of anaplastic lymphoma kinase/protein kinase
B/mammalian target of rapamycin signaling pathway in anaplastic
large cell lymphoma. BMC cancer. 13:4712013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li JF, Li GD, Gu L, Liu WP, Li FY, Liao DY
and Ma ZG: Study on activation of AKT/mTOR pathway in anaplastic
large cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. 29:649–653.
2008.In Chinese.
|
|
93
|
Jundt F, Raetzel N, Müller C, Calkhoven
CF, Kley K, Mathas S, Lietz A, Leutz A and Dörken B: A rapamycin
derivative (everolimus) controls proliferation through
down-regulation of truncated CCAAT enhancer binding protein {beta}
and NF-{kappa}B activity in Hodgkin and anaplastic large cell
lymphomas. Blood. 106:1801–1807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Witzig TE, Reeder C, Han JJ, LaPlant B,
Stenson M, Tun HW, Macon W, Ansell SM, Habermann TM, Inwards DJ, et
al: The mTORC1 inhibitor everolimus has antitumor activity in vitro
and produces tumor responses in patients with relapsed T-cell
lymphoma. Blood. 126:328–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kim SJ, Shin DY, Kim JS, Yoon DH, Lee WS,
Lee H, Do YR, Kang HJ, Eom HS, Ko YH, et al: A phase II study of
everolimus (RAD001), an mTOR inhibitor plus CHOP for newly
diagnosed peripheral T-cell lymphomas. Ann Oncol. 27:712–718. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chumsri S, Zhao M, Garofalo M, Burger A,
Hamburger A, Zhao F and Rapoport A: Inhibition of the mammalian
target of rapamycin (mTOR) in a case of refractory primary
cutaneous anaplastic large cell lymphoma. Leuk Lymphoma.
49:359–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kim D, Koh Y and Yoon SS: Synergistic
effect of alectinib and everolimus on ALK-positive anaplastic large
cell lymphoma growth inhibition. Anticancer Res. 40:1395–1403.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xu W, Kim JW, Jung WJ, Koh Y and Yoon SS:
Crizotinib in combination with everolimus synergistically inhibits
proliferation of anaplastic lymphoma kinase-positive anaplastic
large cell lymphoma. Cancer Res Treat. 50:599–613. 2018. View Article : Google Scholar
|
|
99
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
U.S. Food and Drug (FDA): FDA expands
pembrolizumab indication for first-line treatment of NSCLC (TPS
≥1%). FDA; Silver Spring, MD: 2019, https://www.fda.gov/drugs/fda-expands-pembrolizumabindication-first-line-treatment-nsclc-tps-1.
Accessed April 11, 2019.
|
|
103
|
U.S. Food and Drug (FDA): FDA extends
approval of pembrolizumab for classical Hodgkin lymphoma. FDA;
Silver Spring, MD: 2020, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-extends-approval-pembrolizumab-classical-hodgkin-lymphoma.
Accessed November 10, 2020.
|
|
104
|
Durvalumab (Imfinzi). https://www.fda.gov/drugs/resourcesinformation-approved-drugs/durvalumab-imfinzi.
Journal. 2017
|
|
105
|
Yamamoto R, Nishikori M, Tashima M, Sakai
T, Ichinohe T, Takaori-Kondo A, Ohmori K and Uchiyama T: B7-H1
expression is regulated by MEK/ERK signaling pathway in anaplastic
large cell lymphoma and Hodgkin lymphoma. Cancer Sci.
100:2093–2100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang JP, Song Z, Wang HB, Lang L, Yang
YZ, Xiao W, Webster DE, Wei W, Barta SK, Kadin ME, et al: A novel
model of controlling PD-L1 expression in ALK+ anaplastic
large cell lymphoma revealed by CRISPR screening. Blood.
134:171–185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:20852–20857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shen J, Li S, Medeiros LJ, Lin P, Wang SA,
Tang G, Yin CC, You MJ, Khoury JD, Iyer SP, et al: PD-L1 expression
is associated with ALK positivity and STAT3 activation, but not
outcome in patients with systemic anaplastic large cell lymphoma.
Mod Pathol. 33:324–333. 2020. View Article : Google Scholar
|
|
109
|
Kong J, Dasari S and Feldman AL: PD-L1
expression in anaplastic large cell lymphoma. Mod Pathol.
33:1232–1233. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rigaud C, Abbou S, Minard-Colin V,
Geoerger B, Scoazec JY, Vassal G, Jaff N and Heuberger L:
Valteau-Couanet D and Brugieres L: Efficacy of nivolumab in a
patient with systemic refractory ALK+ anaplastic large cell
lymphoma. Pediatr Blood Cancer. 65:e269022018. View Article : Google Scholar
|
|
111
|
Hebart H, Lang P and Woessmann W:
Nivolumab for refractory anaplastic large cell lymphoma: A case
report. Ann Intern Med. 165:607–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chan TS, Khong PL and Kwong YL:
Pembrolizumab for relapsed anaplastic large cell lymphoma after
allogeneic haematopoietic stem cell transplantation: Efficacy and
safety. Ann Hematol. 95:1913–1915. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Schuster SJ, Bishop MR, Tam CS, Waller EK,
Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin
JR, et al: Tisagenlecleucel in adult relapsed or refractory diffuse
large B-cell lymphoma. N Engl J Med. 380:45–56. 2019. View Article : Google Scholar
|
|
115
|
Rogers AM and Brammer JE: Hematopoietic
cell transplantation and adoptive cell therapy in peripheral T cell
lymphoma. Curr Hematol Malig Rep. 15:316–332. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lollies A, Hartmann S, Schneider M, Bracht
T, Weiß AL, Arnolds J, Klein-Hitpass L, Sitek B, Hansmann ML,
Küppers R and Weniger MA: An oncogenic axis of STAT-mediated BATF3
upregulation causing MYC activity in classical Hodgkin lymphoma and
anaplastic large cell lymphoma. Leukemia. 32:92–101. 2018.
View Article : Google Scholar
|
|
117
|
Weilemann A, Grau M, Erdmann T, Merkel O,
Sobhiafshar U, Anagnostopoulos I, Hummel M, Siegert A, Hayford C,
Madle H, et al: Essential role of IRF4 and MYC signaling for
survival of anaplastic large cell lymphoma. Blood. 125:124–132.
2015. View Article : Google Scholar
|
|
118
|
Casey SC, Tong L, Li Y, Do R, Walz S,
Fitzgerald KN, Gouw AM, Baylot V, Gütgemann I, Eilers M and Felsher
DW: MYC regulates the antitumor immune response through CD47 and
PD-L1. Science. 352:227–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Atsaves V, Tsesmetzis N, Chioureas D, Kis
L, Leventaki V, Drakos E, Panaretakis T, Grander D, Medeiros LJ,
Young KH and Rassidakis GZ: PD-L1 is commonly expressed and
transcriptionally regulated by STAT3 and MYC in ALK-negative
anaplastic large-cell lymphoma. Leukemia. 31:1633–1637. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shao RG and Zhen YS: Enediyne anticancer
antibiotic lidamycin: Chemistry, biology and pharmacology.
Anticancer Agents Med Chem. 8:123–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Shao RG and Zhen YS: Relationship between
the molecular composition of C1027, a new macromolecular antibiotic
with enediyne chromophore, and its antitumor activity. Yao Xue Xue
Bao. 30:336–342. 1995.In Chinese.
|
|
122
|
Wang R, Li L, Zhang S, Li Y, Wang X, Miao
Q and Zhen Y: A novel enediyne-integrated antibody-drug conjugate
shows promising antitumor efficacy against CD30+
lymphomas. Mol Oncol. 12:339–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang R, Li L, Duan A, Li Y, Liu X, Miao Q,
Gong J and Zhen Y: Crizotinib enhances anti-CD30-LDM induced
antitumor efficacy in NPM-ALK positive anaplastic large cell
lymphoma. Cancer Lett. 448:84–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Hwang J, Song I, Lee K, Kim HR, Hong EH,
Hwang JS, Ahn SH and Lee J: KRCA-0008 suppresses ALK-positive
anaplastic large-cell lymphoma growth. Invest New Drugs.
38:1282–1291. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Shen J, Wang J, Du J, Wang L, Zhou X,
Chang X, Li Z, Zhai X, Zuo D and Wu Y: A novel ALK inhibitor ZYY
inhibits Karpas299 cell growth in vitro and in a mouse xenograft
model and induces protective autophagy. Toxicol Appl Pharmacol.
383:1147812019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Prutsch N, Gurnhofer E, Suske T, Liang HC,
Schlederer M, Roos S, Wu LC, Simonitsch-Klupp I, Alvarez-Hernandez
A, Kornauth C, et al: Dependency on the TYK2/STAT1/MCL1 axis in
anaplastic large cell lymphoma. Leukemia. 33:696–709. 2019.
View Article : Google Scholar
|