Ovarian cancer represents the eighth most frequently
diagnosed tumor and the seventh most lethal cancer in women leading
to almost 185,000 deaths annually worldwide (1). Despite the improvement of screening
strategies and the advancement of anticancer surgical and
pharmacological treatments, ovarian cancer is still considered one
of the most commonly diagnosed and aggressive urogenital female
tumors, with a 5-year relative survival rate of 93% and 5-year
cause-specific survival rates of 82, 71, 66 and 43% for
endometrioid, mucinous, clear cell carcinoma and serous ovary
carcinoma, respectively (2,3).
The majority of ovarian cancer cases are epithelial, which accounts
for 85-90% of all diagnosed ovarian tumors. This type of tumor
usually affects women aged between 55 and 65 years old (4); contrariwise, germ cell ovarian
cancer accounts for ~5% of all diagnosed tumors with an average age
of onset of 20 years old (4).
Several risk factors have been recognized for
ovarian cancer. It was demonstrated that the risk of developing
ovarian cancer increases significantly with age and in particular
after menopause, probably due to hormonal imbalance (5). In this regard, it was observed that
post-menopause hormone therapies, based on the administration of
estrogens alone or in combination with progesterone, significantly
increased the risk of developing ovarian cancer (relative risk,
1.53; confidence interval, 1.40-1.66) (6). Strictly associated with menopause
and hormone imbalance risk factors, weight gain and obesity have
also been associated with an increased risk of ovarian cancer
(7). Of note, obesity represents
one of the main important modifiable risk factors for different
tumors. In patients suffering from ovarian cancer, it was also
demonstrated that obesity negatively affects the prognosis of
patients leading to therapeutic failure and worse overall survival
time (7). As widely described for
other tumors such as breast and prostate cancer (8,9),
besides these physiological variations of hormone levels,
occupational and environmental risk factors as well as endocrine
disruptors and other chemical substances have been associated with
the development of ovarian cancer (10-13).
Other well-recognized risk factors are gene
mutations and hereditary syndromes that represent the most notable
predisposing causes for the development of ovarian cancer (14). A growing body of literature has
demonstrated that individuals harboring germline mutations
affecting BRCA1 and BRCA2 genes have an increased
risk of breast and ovarian cancer (15-18). Overall, ~25% of ovarian cancer
tumors are positive for BRCA1 or BRCA2 mutations
(19). As explained in the
following sections, the evaluation of such mutations is important
for the choice of anticancer pharmacological treatments (20,21). Other hereditary syndromes related
to ovarian cancer include hereditary non-polyposis colon cancer
syndrome, Peutz-Jeghers syndrome and adenine DNA glycosylase
(MUTYH)-associated polyposis syndrome affecting several mismatch
repair genes (including MSH2, MSH6 and MLH1),
STK11 and MUTYH (22-24), respectively.
Other controversial and not yet ascertained risk
factors are represented by tobacco smoking, androgens, diet and
talc powder. For all these risk factors, observational,
case-control, retro- and prospective studies have generated
conflicting results thus limiting the awareness about the causative
effects of these factors (25).
It was demonstrated that tobacco smoking is associated with the
development of mucinous ovarian cancer, but it does not increase
susceptibility to other types of ovarian tumors (26,27). Unconvincing data have been
obtained for the association between powder use in the genital area
and the risk of ovarian cancer. In this context, some studies
highlighted a slightly increased risk of ovarian cancer in women
using talc powder in the genital area (28-30). However, a recent observational
study on 250,000 women observed for 11 years has demonstrated that
the use of powder does not significantly increase the incidence of
ovarian cancer (28-30).
Besides these risk factors, numerous studies have
also identified some protective factors able to reduce the
incidence of ovarian cancer. Among these factors, pregnancy and
breastfeeding are both associated with a reduced risk of developing
this tumor. In particular, a significantly reduced risk for ovarian
cancer has been observed in women carrying full-term pregnancies
before 26 years old (31). In
addition, the increased number of full-term pregnancies, together
with the time of breastfeeding, is associated with a lower risk of
ovarian cancer (31). Finally,
the use of oral contraceptives for birth control seems to play an
important protective role against ovarian cancer with higher
protective effects the longer the treatments are administered
(32). In this context, other
birth control strategies, including intrauterine devices and tubal
ligation, have also been associated with a reduced risk of ovarian
cancer (33).
During the early stages, ovarian cancer is not
associated with clinical symptoms, therefore the diagnosis of this
tumor is often delayed. Mild ovarian cancer symptoms may be often
confused with other benign pathologies, including gastrointestinal
disorders, urogenital infections and benign ovarian lesions
(including ovarian cysts, teratomas and fibromas) (34). However, unlike benign diseases,
ovarian cancer symptoms are persistent and worsen over time
(34). Generally, moderate or
severe symptoms are associated with the spread of the disease in
adjacent anatomical regions. Among these symptoms, the most
frequently observed are pelvic distension, abdominal and pelvic
pain and urgent or frequent urination (34). Other symptoms may include pain
during sex, back pain, constipation, altered menstruation, fatigue
and weight loss (35). The
correct self-assessment of these symptoms by the patients may
improve the timing of diagnosis allowing the gynecological surgeon
and oncologist to intervene promptly by increasing the patient
response to treatments (36).
Regarding ovarian cancer staging, two main staging
systems are used worldwide for ovarian cancer, which are the
International Federation of Gynecology and Obstetrics (FIGO 2018)
system and the American Joint Committee on Cancer (AJCC 8th
edition) system both based on the Tumor-Node-Metastasis (TNM)
parameters (37,38). Table
I shows both FIGO and AJCC staging in terms of the pathological
characteristics of tumors: Tumor dimension (T), lymph node
involvement (N) and presence of distant metastasis (M) (Table I).
At present, several diagnostic strategies are
available to make a correct and timely diagnosis of ovarian cancer
when recurrent symptoms are observed. The first step for a correct
diagnosis of ovarian cancer is based on the collection of patient's
medical history and on a correct physical exam performed by a
gynecologist with expertise in gynecological oncology (39,40). The aim of these procedures is the
collection of all relevant data about the presence of pre-existing
conditions or risk factors that could increase the risk of
developing ovarian cancer. In particular, as previously mentioned,
the presence of a family member with ovarian cancer or the presence
of hereditary syndromes and genetic mutations may lead the
clinician to make a diagnosis of suspected ovarian cancer in the
presence of specific abdominal symptoms. In the same manner, the
physical examination of the abdomen and pelvis is of fundamental
importance to observe pelvic mass, ascites or abdominal distension
suggestive of ovarian cancer (41). The physical examination could
include a rectovaginal exam performed with empty bladder to
evaluate the presence of abdominal or pelvic masses. However,
although important and easy to perform, physical investigations
have a low sensitivity and a low specificity, especially in
overweight patients or in presence of small tumors, as abdominal or
pelvic distention may be caused by other benign pathologies
(42,43).
After the physical examination, patients with
suspected ovarian cancer are subjected to various laboratory and
imaging tests useful to detect the presence of the tumor, its
severity and extent (41). Among
the most used laboratory tests both for preventive and diagnostic
purposes is the evaluation of blood tumor markers, namely cancer
antigen (CA) 125 and human epididymis protein 4 (HE4), alongside
the normal hematochemical parameters (red and white blood cells
count, platelets and hemoglobin). In particular, CA 125 is
considered the main predictive serum biomarker for ovarian cancer
as it is elevated in 50% of patients with early-stage ovarian
cancer and in over 80% of all patients with this tumor (44).
Regarding HE4, this marker is evaluated together
with CA 125 as it appears to be elevated in a significant fraction
of patients with ovarian cancer negative for CA 125 (45,46). Therefore, the use of HE4 is of
fundamental importance in screening strategies to intercept all
those ovarian carcinomas negative for other tumor biomarkers. The
evaluation of these two markers, together with the evaluation of
six symptoms predicting the presence of ovarian cancer (pelvic
pain, abdominal pain, urinary urgency/frequency, increased
abdominal size, bloating and difficulty eating/feeling full) showed
a significant improvement in diagnostic accuracy from 83.8 to 98.5%
(46). Other tumor biomarkers,
such as serum α-fetoprotein and quantitative β-human chorionic
gonadotropin (β-hCG), are less used and are used for the diagnosis
of germ cell ovarian cancer (47).
After these preliminary assessments, women who
present symptoms and biomarkers predictive for ovarian cancer
undergo imaging tests, including ultrasound, computed tomography
(CT) scan, magnetic resonance imaging (MRI) scan and positron
emission tomography (PET) scan (48).
Generally, the first imaging test to be performed is
a transvaginal ultrasonography. Several studies have shown how
transvaginal ultrasonography is able to distinguish benign lesions
from tumors with an excellent rate of accuracy (pooled sensitivity
and specificity of 92 and 88%, respectively) thus allowing the
clinician to evaluate the structure and vascularization of the
ovarian parenchyma, the presence of cysts or masses and any ascitic
effusions (49-52).
Both CT, MRI and PET are not widely used for the
diagnosis of ovarian cancer but for the evaluation of the extent of
the tumor and the possible presence of distant metastases.
Specifically, CT scan can be used to perform biopsies of suspected
metastases in a procedure called CT-guided needle biopsy (53,54). Meanwhile, PET and MRI are mostly
used to evaluate the spread of diseases in neighboring lymph node
stations and in distant organs, such as the medulla and brain,
through the use of radiotracers or contrast agents (for example
gadolinium) (55).
Finally, after tumor diagnosis, it is essential to
perform molecular tests and genetic counseling to determine the
presence of relevant mutations in tumor specimens useful for
prognostic and therapeutic purposes (56-60). As aforementioned, the most
frequent mutations observed in ovarian cancer are those affecting
the BRCA1 and BRCA2 genes as well as other mutations
within STK11, MSH2, MSH6, MLH1, PMS6
and MUTYH (56,57). Besides molecular evaluations,
previous studies have demonstrated that immunohistochemical
investigations are fundamental both for diagnostic and prognostic
purposes for different abdominal tumors, including that of ovaries
(58-60).
Over the decades, the therapeutic options for the
treatment of advanced ovarian cancer have been improved
significantly through the development of more precise and less
invasive surgical techniques as well as the availability of novel
effective drugs able to extend the life expectancy of patients,
especially for metastatic ovarian cancer (61,62). Different studies have demonstrated
that in the last 20-35 years there was a significant improvement in
the survival rates of patients with ovarian cancer; however, some
reports have shown that the advancements of the anticancer
treatment have not ameliorated the long-term survival and the cure
rate of ovarian cancer (63-65). In particular, a recent study
showed that both incidence and 5-year survival rates have improved
in the last 30 years. Indeed, the 5-year survival rate increased
from 39.3% in the 80s to 45.4% observed in 2012; similarly, the
survival time was also improved passing from 34 months observed in
1983 to 52 months observed in 2012, highlighting how the latest
treatments have improved the survival time of patients with ovarian
cancer (62).
At present, surgery represents the gold standard for
the treatment of ovarian cancer. Ovariectomy and adnexectomy are
used for both staging, debulking and treatment of early ovarian
cancer, thus being curative in such tumors limited to the ovaries
(66,67). Ovarian cancer surgery can be
performed by open surgery with midline incision or by minimally
invasive surgery (MIS). MIS, performed by laparoscopic surgery, is
generally performed for newly diagnosed tumors limited to one or
both ovaries and to the pelvic cavity without metastatic
dissemination (68). However, MIS
is generally used only in structured centers equipped with
experienced gynecological surgeons (68). In the case of advanced tumors
(stages II, III and IV), open surgery is always used in order to
perform extended cytoreduction (debulking) aimed at eliminating all
cancer lesions with a thickness of >1 cm (69,70). Briefly, in both MIS and open
surgery, the first steps consist in the collection of ascitic fluid
and in the execution of peritoneal lavage used for
immunocytochemistry evaluations useful to establish the presence of
tumor cells in the peritoneal cavity (71). Subsequently, surgeons check the
entire peritoneal cavity to assess the absence of any suspicious
extra ovary lesions. In the case of no suspicious masses, biopsies
from different parts of the peritoneal cavity (paracolic gutters,
pelvis and diaphragm) should be obtained to exclude cancer
dissemination (71). After these
preliminary steps, surgeons can remove the primary tumor through
bilateral salpingo-oophorectomy, hysterectomy, omentectomy and
lymph node dissection (both pelvic and paraaortic nodes). To avoid
post-surgery cancer dissemination, the tumor has to be removed
encapsulated. In the case of young patients (20-45 years old) with
monolateral stage IA and IC ovarian cancer that would maintain
fertility, the surgeon could opt for unilateral ovariectomy and
adnexectomy, thus preserving the contralateral ovary and uterus
(71).
Overall, the main objectives of ovarian cancer
surgery are the removal of the primary tumor and the maximal
debulking of pelvic and peritoneal masses. In presence of advanced
tumors, the clinicians can opt for neoadjuvant chemotherapy (NAC)
followed by debulking surgery. If the NAC plus surgery approach is
chosen, tumor biopsies are collected before chemotherapy to assess
the molecular features of the tumors (through immunohistochemistry
or molecular tests) thus allowing administration of appropriate
anticancer drugs (72). In those
patients undergoing NAC and debulking surgery, a whole-abdominal
radiation treatment should be applied if residual disease is still
observed after a second-look laparotomy; however, this approach
needs to be carefully evaluated to avoid bowel toxicity (73,74).
Besides surgery, anticancer pharmacological
treatments are the best therapeutic option for the management of
ovarian cancer. Over the years, several chemotherapeutic agents
have been used for the treatment of ovarian cancer. Thanks to the
evolution of anticancer pharmacological treatments, it is now
possible to effectively treat the different histological and
molecular subtypes of ovarian cancer, contributing to the
improvement of the quality of life and life expectancy of these
patients (2,75).
After surgery, chemotherapy can be optionally
administered in patients with low-grade tumors (stage IA or IB),
while the first-line treatment for ovarian cancer with more
advanced stages is based on the administration of platinum-based
chemotherapy. Indeed, the first-line regimen consists of the
administration of intravenous platinum/taxane every three weeks for
six cycles (76). The same
compounds are usually administered also in patients with stage
III/IV ovarian cancer undergoing NAC protocols for three cycles
followed by debulking surgery plus six additional cycles of
platinum/taxane (76,77).
Thus, for >20 years, the first-line treatment for
ovarian cancer has been based on the administration of carboplatin
(used instead of cisplatin because it is less toxic and equally
effective) and paclitaxel administered every three weeks in a
six-cycle schedule. The preferred route of administration is the
intravenous systemic one, although several studies have also
proposed intraperitoneal administration, which has not given
improved results in terms of improvement of progression-free
survival (PFS) (78,79). Similarly, several trials have
investigated the beneficial effects of paclitaxel weekly
administration compared with the conventional 3-week schedule;
however, this therapeutic option is not widely used as conflicting
data have been generated in three different clinical studies (JGOG
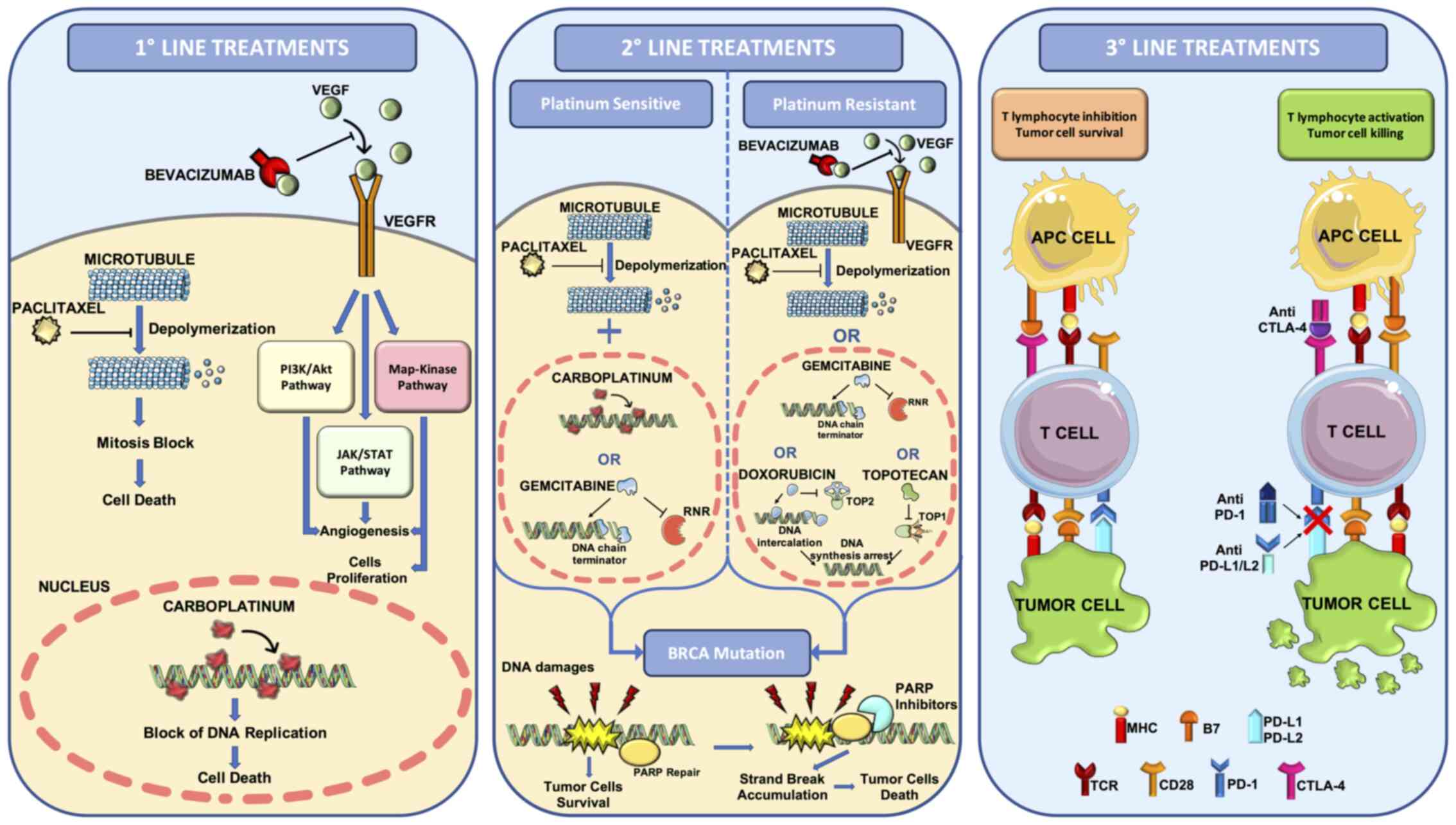
3016, GOG 262 and MITO 7) (80-82) (Fig.
1).
More recently, the introduction of anticancer
targeted therapies has improved the efficacy of first-line
treatments for patients with ovarian cancer who can benefit from
treatments based on the administration of carboplatin, paclitaxel
and bevacizumab (83,84). Bevacizumab is a monoclonal
antibody against the pro-angiogenetic factor VEGF-A that has
prolonged the PFS and OS time of patients, especially of those
patients with advanced tumors (83). In particular, it was demonstrated
that the prolonged administration of 15 mg/kg 3-weekly of
bevacizumab up to 15 months together with standard
carboplatin/paclitaxel chemotherapy is associated with a prolonged
PFS time; however, due to the expensive cost of treatments and the
related gastrointestinal and vascular toxicities, novel protocols
based on a low dose of bevacizumab for 30 months is still under
evaluation and it is awaiting approval as a therapeutic standard
for this tumor (76,85,86) (Fig.
1).
After the first-line chemo- and targeted therapy,
the patients can completely respond to treatments or develop a
relapse. In the case of a partial or complete response, patients
can undergo maintenance chemotherapy with the same drugs used in
the first-line treatment to improve PFS (87,88).
In the case of tumor recurrence, patients are
treated with a second-line treatment that is different depending on
whether the tumor is resistant or sensitive to platinum compounds
(89,90). Tumor recurrence can be observed
through biochemical (increased expression of CA125 and other
biomarkers) or clinical (imaging techniques) examinations (91) after which patients are assigned to
standard treatment for recurrent disease or to experimental
clinical trials using novel drugs or different drug combinations
(92,93).
For patients with ovarian cancer developing a
platinum-resistant disease, the second-line treatments consist of
single non-platinum-based therapies using different agents,
including docetaxel, paclitaxel, topotecan and gemcitabine, with a
therapeutic efficacy ranging from 19 to 27% of the treated patients
(71). Similar percentages of
response have been obtained treating ovarian cancer relapse with
bevacizumab (therapeutic response observed in ~20% of patients)
(94). More recently, in absence
of severe adverse events, combined therapies with bevacizumab plus
one agent among doxorubicin, topotecan and paclitaxel have shown a
significant improvement of OS in patients with platinum-resistant
recurrent disease (95) (Fig. 1).
In the case of platinum-sensitive recurrence, there
are different therapeutic options based on the administration of
several drug combinations including carboplatin plus paclitaxel (or
docetaxel with weakly or 3-weeks administration), carboplatin plus
gemcitabine and bevacizumab, carboplatin plus liposomal doxorubicin
and cisplatin plus gemcitabine (96). In addition, patients with ovarian
cancer who are platinum-sensitive are often eligible for novel
clinical trials assessing the efficacy of novel agents or combined
therapies. Among these trials, recent evidence has demonstrated the
therapeutic efficacy of poly (ADP-ribose) polymerase (PARP)
inhibitors in both platinum-sensitive and -resistant ovarian cancer
harboring BRCA1 or BRCA2 mutations. In particular, patients with
platinum-sensitivity with complete or partial response to at least
two lines of treatments can benefit from olaparib single-agent
maintenance therapy improving their PFS from 5.5 to 19.1 months
(97). Similarly, olaparib
single-therapy can be used also in patients with platinum-resistant
BRCA mutated ovarian cancer who failed three or more lines
of chemotherapy (98) (Fig. 1).
As aforementioned, some patients with ovarian cancer
can benefit from novel first-line and second-line treatments based
on the administration of selective inhibitors of PARP. PARP
proteins are a family of 17 enzymes involved in numerous cellular
processes, and in particular PARP-1 and PARP-2 play a crucial role
in DNA damage repair (99,100).
The development of PARP inhibitors has represented
the turning point in the treatment of ovarian cancer, both in the
first-line and in case of tumor recurrence, high-lighting the
importance of studying the molecular profile of tumors to improve
the selection of patients eligible for these innovative treatments.
Indeed, these drugs, including olaparib, rucaparib, niraparib and
talazoparib, find application in tumors with germline mutations
affecting BRCA1 and BRCA2 or in advanced ovarian
cancer refractory to three or more lines of treatment (101).
The therapeutic efficacy of PARP inhibitors has been
demonstrated also in patients without BRCA mutations. The
NOVA trial based on the administration of niraparib in patients
with ovarian cancer demonstrated that all patients can benefit from
this treatment, although improved results were obtained for
patients with homologous recombination deficiency (HRD) compared
with patients without mutations affecting the HR system (105).
In December 2016, the Food and Drug Administration
launched an accelerated approval process for the use of rucaparib
as a single agent for the treatment of patients with ovarian cancer
at an advanced stage and with a BRCA mutation (germline or
somatic) who had been previously treated with two or more lines of
chemotherapy (109). In
addition, rucaparib is also used as second-line maintenance therapy
in patients with platinum-sensitivity with or without
BRCA1/2 mutations as reported in the Ariel 3 Trial (100).
Besides these conventional therapies, novel
approaches for the treatment of advanced or metastatic ovarian
cancer are being developed and studied. Modest results have been
obtained in several clinical trials assessing the efficacy of
immune checkpoint inhibitors (ICIs) already used for the treatment
of several advanced and metastatic tumors (110-112). In particular, the administration
of anti-PD-1 (nivolumab or pembrolizumab) or anti-PD-L1
(atezolizumab) in advanced ovarian cancer has a good response only
in 10-15% of patients (113-115). Similar results have been
obtained with the single administration of the anti-CTLA-4 ICI
ipilimumab, which is effective only in a small fraction of patients
who has previously received an anticancer therapeutic vaccine
(116). Overall, single-agent
ICI administration shows limited efficacy in advanced ovarian
cancer, therefore novel protocols assessing the concomitant
administration of ipilimumab and nivolumab have been proposed
(117). Such studies have
demonstrated an improved and longer response rate in patients
treated with two ICIs compared with patients treated with nivolumab
alone, thus replacing the single-agent ICI regimens (117). A recent review of the literature
collected all the completed and ongoing clinical trials using
different combinations of ICIs, selective inhibitors or
chemotherapeutic agents showing encouraging and conflicting results
based on the clinical and molecular features of the patients
enrolled (118) (Fig. 1).
Other investigated therapeutic options for advanced
ovarian cancers are represented by therapeutic vaccines, adoptive
cellular therapy, T cell transfer and chimeric antigen receptor
T-cell therapy; however, further clinical studies are needed to
assess the efficacy and safety of these further treatments
(119).
Finally, several treatments are available as
maintenance or palliative therapy for disseminated and metastatic
ovarian cancer. Similarly, VEGFR inhibitors, including pazopanib,
nintedanib and cediranib, are often used for the treatment of
recurrent platinum-resistant ovarian cancer (100). In line with these treatments,
VEGF inhibitors such as aflibercept are used in case of malignant
ascites showing an improvement of time to next paracentesis but not
of OS (120).
Despite the availability of all these surgical and
pharmacological treatments, the prognosis of patients with ovarian
cancer is often poor. To improve the quality of life and life
expectancy of these patients, it is necessary to opt for
therapeutic choices that take into account the patient's
comorbidities, the adverse effects of therapies and the patient's
age. Therefore, the management of the patient with ovarian cancer
is extremely complex and requires the convergence of different
professional skills to ensure high standards of care.
The main decision criterion for second-line
treatments is the definition of platinum-sensitivity or resistance.
Sensitivity to platinum-based treatments must be assessed after a
period of at least 6 months; however, there is a linear
relationship between PFI and platinum sensitivity, therefore the
evaluation of PFI is of primary importance in future therapeutic
choices and must be considered as a continuous variable in the
decision-making process leading to the new therapy. Furthermore,
PFI will be used as a parameter for the eligibility of patients in
novel clinical trials, therefore the evaluation of PFI cannot be
limited to a fixed 6-month window but should be evaluated
periodically.
Overall, platinum-based therapy remains the most
effective therapy in the management of epithelial ovarian cancer,
and primary PFI provides relevant prognostic and predictive
information. A significant fraction of patients receives different
lines of platinum-based therapy, thus evaluating the interval of
time after the most recent line can provide prognostic information
about acquired resistance and clonal evolution of the tumor due to
intervening non-platinum treatments. Over the years different
non-platinum agents have been integrated into conventional therapy;
this led to the need of new prognostic and predictive markers to
make the best treatment decisions for the management of recurrence
(121).
As aforementioned, the symptomatology, diagnosis,
staging and treatments of ovarian cancer are extremely complex and
require the convergence of various specialists able to provide the
gynecological oncologist with a clinical picture as detailed as
possible, useful for designing the appropriate therapeutic protocol
for each patient. Therefore, at present, the approach to the
patient with advanced ovarian cancer should be multidisciplinary.
This includes a team of experts who follow the patient step by step
during the diagnosis, surgical therapy, pharmacological therapy,
rehabilitation and follow-up, creating a collaborative network
where the patient is at the center and can benefit of high
standards of care in the perspective of personalized medicine and
patient-centered care (122).
In this context, over the decades, great
advancements in the management of patients with ovarian cancer have
occurred, passing from a linear approach to care, where the patient
is treated by individual specialists without communication between
them, to a multidisciplinary and integrated approach where
different specialists share clinical information and chose the best
therapeutic options together (123,124).
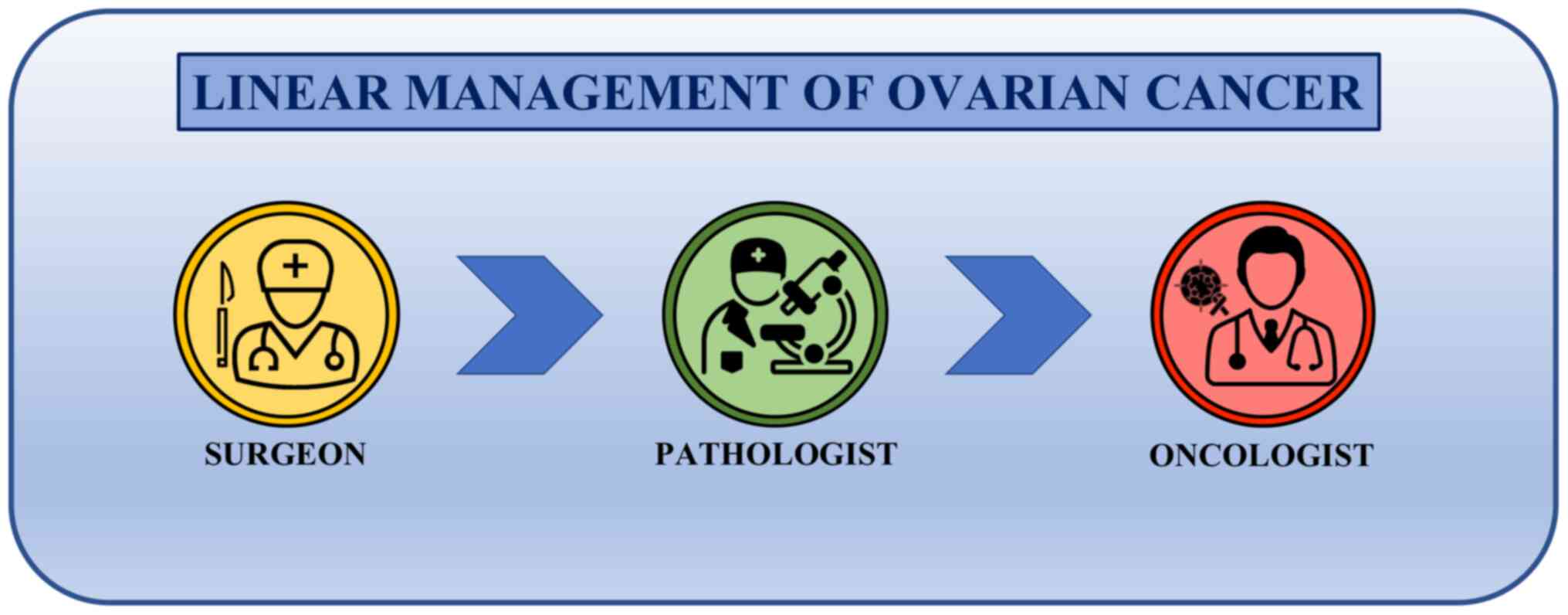
Until 30 years ago, the therapeutic approach
followed a linear trend where the main stakeholders of cancer
management were the surgeon, who operated the surgical resection of
the tumor, the pathologist, who made the histological diagnosis,
and the medical oncologist, who dealt with the therapeutic schedule
to be administered (Fig. 2).
Although other professionals participated in the clinical
management of ovarian cancer (including gynecologists, radiologists
and laboratory technicians), they did not actively take part in the
clinical-therapeutic decisions. In addition, the interactions
between the patient, the surgeon, the pathologist and the
oncologist rarely occurred and each of these three professional
figures made therapeutic choices without first discussing with
colleagues (125,126).
Since the late 80s, some studies have highlighted
the benefits of the multidisciplinary management of patients with
cancer in terms of diagnosis, therapeutic response, survival and
quality of life, suggesting that an integrated approach to cancer
could lead to improved outcomes for patients (127-129). With regards ovarian cancer, it
was demonstrated that a collegial discussion can lead all the
specialists to evaluate the diagnostic-therapeutic areas beyond
those of their own competence, leading to an increase of awareness
in the number of potential treatments available and expected
pitfalls thus improving the effectiveness of treatments (130,131).
The development of multidisciplinary teams has
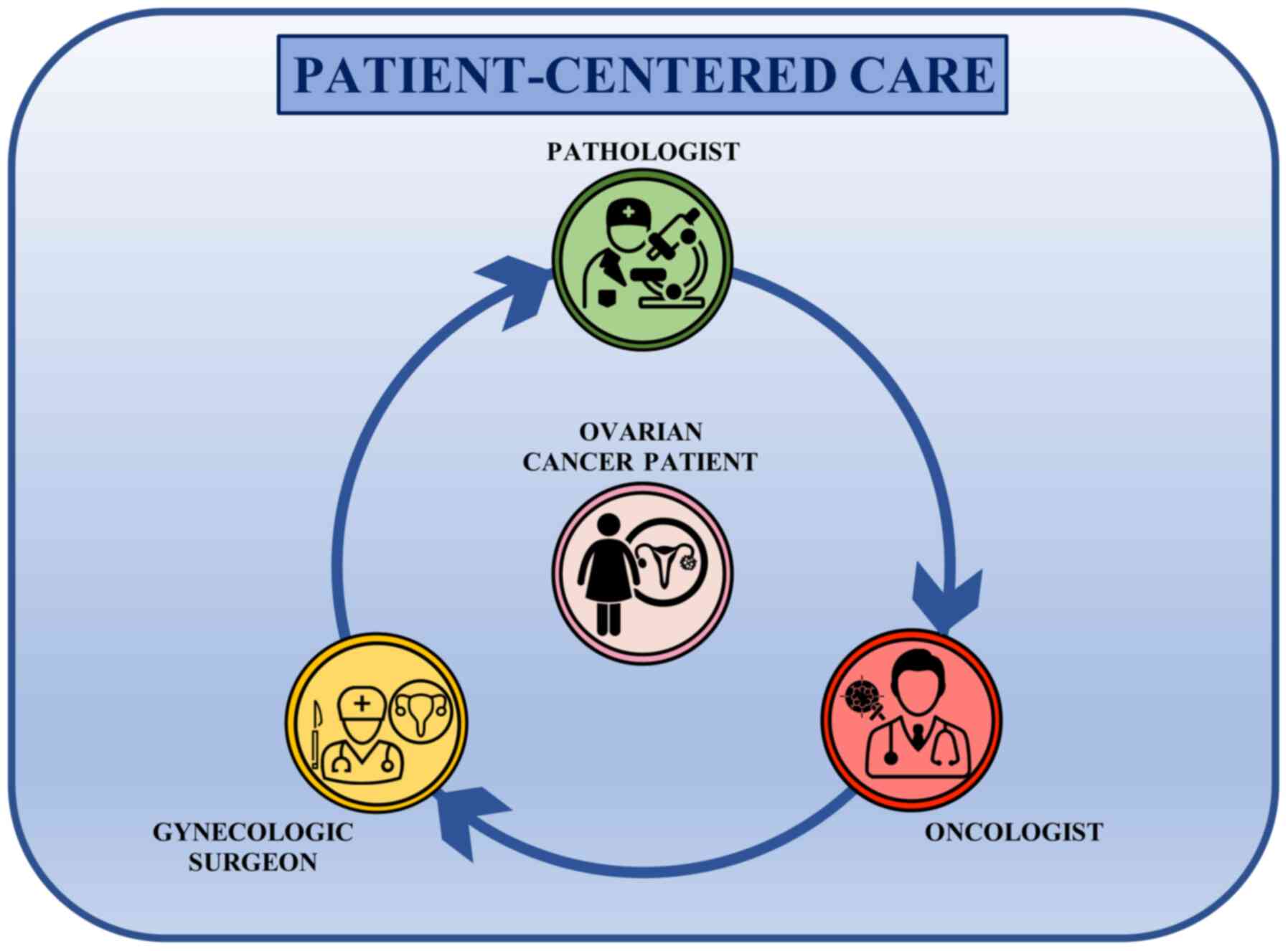
changed the previous linear approach to patients into a circular
one. Indeed, the main players of ovarian cancer management are now
working together, comparing their clinical findings each other in a
patient-centered care approach where the patients are in the middle
of a circular decision-making pathway receiving information and
therapeutic options shared between the gynecological surgeon, the
pathologist and the medical oncologist (Fig. 3). This circular approach to
ovarian cancer treatment has introduced different therapeutic
opportunities that are continuously evaluated and re-elaborated
according to the clinical information received from the different
specialists involved in the collaborative care network. Therefore,
this approach results in a improved management of ovarian cancer
and patient awareness about the status of the disease, as well as
greater confidence in the therapeutic options that they will
undergo (132).
Although patient-centered care has significantly
improved the standard of ovarian cancer care, several studies have
demonstrated that patients treated in specialized structures where
multidisciplinary teams operate have an improved prognosis compared
with patients treated in non-specialized centers (133-135). A possible explanation of this
trend could be related to the well-organized approach to treatment
in specialized hospitals with a high volume of patients with
ovarian cancer per year where the components of the
multi-disciplinary team meet together weekly to discuss the
periodic clinical, laboratory and instrumental findings useful to
take appropriated and shared clinical decision. Of note, despite
the undoubted advantages of a multidisciplinary team in terms of
the quality of the assistance provided, this type of
interdisciplinary display requires appropriate organization, time
for periodic meetings, willingness to collaborate and adequate IT
support; it is useful to share medical records and clinical data,
favoring a continuous constructive debate for the better management
of each patient (136).
Only a structured center can offer well-structured
multidisciplinary teams able to address all of the patient needs.
In this context, the Mercado et al (137) study shows that patients treated
in referral centers and treated by expert physicians have a 40%
higher survival compared with patients treated in a peripheral
center.
As surgery represents the gold standard for the
treatment of ovarian cancer, it is well established that patients
treated in experienced centers benefit from maximum cytoreductive
surgical resection which positively correlates with the overall
survival of patients (135,138). Besides the importance of
surgery, the discussion of clinical cases in the multidisciplinary
team does not end with the diagnosis and surgical resection of
tumor masses, but it takes place at every decision-making point,
especially in case of recurrent diseases (139). In these cases, the interaction
of the various specialists can lead to the design of novel and
effective therapeutic strategies tailored to each patient (139). Thus, such strategies may involve
new surgical interventions in the peritoneal cavity or other body
districts, which requires the expertise of different types of
surgeons, including urologists, vascular surgeons and general
surgeons, or could lead to novel anticancer treatments using both
chemo- and radiation therapies when distant metastases are observed
(140,141).
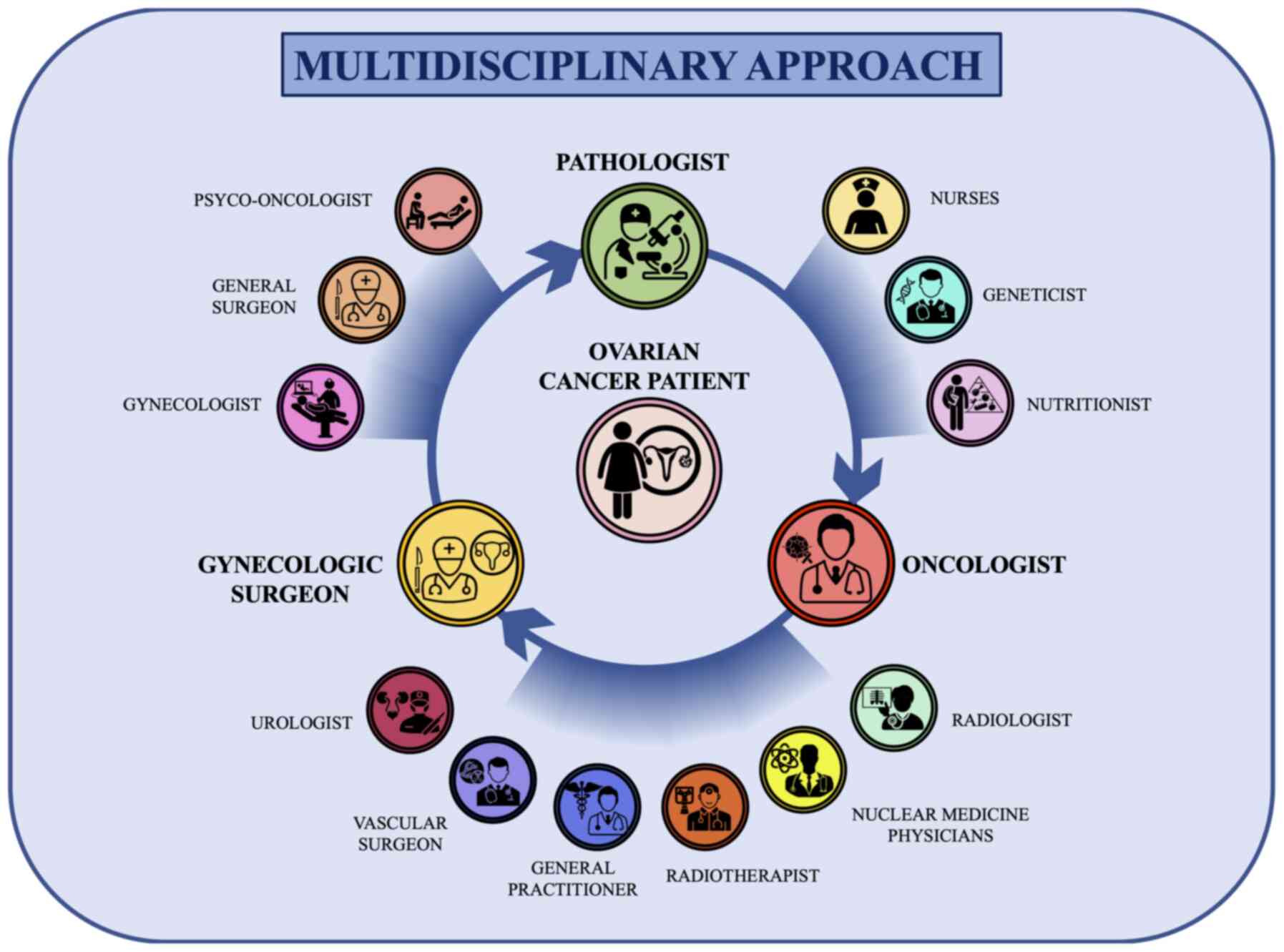
It is important to note that each specialist within
the multidisciplinary team has a fundamental role in the diagnostic
or therapeutic process. Indeed, the use of imaging techniques
performed by the radiologist is fundamental to formulate a
diagnosis of suspected ovarian cancer and to establish the
localization of lesions (142,143). In the same manner, the precise
histological and biomolecular evaluation of ovarian cancer is now
essential for modern cancer treatments (144,145). In this context, the pathologist,
geneticist and molecular biologist are fundamental for the
assessment of grading, histotyping and molecular typing of ovarian
cancer (146,147). In addition, the
multidisciplinary network of specialists is further enriched by the
inclusion of breast specialists and nutritionists. In particular,
breast specialists intervene in the case of BRCA1- and
BRCA2-positive ovarian cancer who could develop a secondary
neoplasm affecting the breast (148,149); while nutritionists are now a key
professional figure in medical oncology departments. In fact,
several studies have demonstrated that nutrition represents an
important protective factor against the development of tumors
(150) but also represents an
effective therapeutic intervention for patients with cancer
(151,152). In this context, several studies
have demonstrated that dietary and lifestyle interventions during
cancer treatments can ameliorate the adherence to treatment as well
as patient quality of life and prognosis [hazard ratio (HR) for
physical activity, 0.60; 95% CI, 0.39-0.92; P=0.02; HR for highest
vs. lowest tertile of quality diet, 0.73; 95% CI, 0.55 to 0.97;
P=0.03] (153-156). These data, together with the
clinical features of patients allow clinicians to determine the
best therapeutic approach as well as to predict the prognosis and
outcomes of patients (157).
The importance of a multidisciplinary team for the
management of ovarian cancer has emerged during the COVID-19
pandemic where patients with ovarian cancer have experienced
difficulties in accessing medical treatment (158). Indeed, due to the spread of
infection, patients with cancer have experienced delays in
treatment or missed some therapies with a negative impact on the
treatment response (159). In
addition, patients with cancer are considered vulnerable
individuals with an increased risk of COVID-19 infection and severe
symptomatology (160). In this
context, the multidisciplinary team involved in the management of
ovarian cancer has improved novel telemedicine strategies useful to
monitor patients with ovarian cancer at a distance, thus following
the progression of the disease and patient health status during the
treatment. In addition, thanks to the patient-centered circular
multidisciplinary network the information is easily transferred
among specialists, thus increasing the speed of the implementation
of therapeutic strategies and follow-up visits (161-163).
In recent years, the management of ovarian cancer
has been significantly improved through the introduction of novel
pharmacological treatments and mini-invasive surgical techniques.
Besides these advancements, a multidisciplinary approach for the
treatment of ovarian cancer has significantly improved the quality
of life and prognosis of patients. Overall, a multidisciplinary
team is able to face clinical, molecular, pathological and
psychological issues of patients with ovarian cancer, ensuring a
high standard of care supporting the process of personalized
medicine. The importance of a multidisciplinary team and periodic
meetings lays also on the constant improvement of molecular,
biological and therapeutic knowledge in the field of ovarian cancer
care. Indeed, the active discussion performed within a
multidisciplinary team improves the adoption of the best therapies
for patients as well as the efficacy of treatments.
Not applicable.
LF, GSca and PS conceived the manuscript, performed
bibliographic research and wrote the article. VL, GG and AL
performed the bibliographic research and prepared the table and
figures. GSci and ABD provided critical revisions. All authors
provided critical revisions and read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shafrir AL, Rice MS, Gupta M, Terry KL,
Rosner BA, Tamimi RM, Hecht JL and Tworoger SS: The association
between reproductive and hormonal factors and ovarian cancer by
estrogen-α and progesterone receptor status. Gynecol Oncol.
143:628–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Alonzo M, Bounous VE, Villa M and Biglia
N: Current evidence of the oncological benefit-risk profile of
hormone replacement therapy. Medicina (Kaunas). 55:5732019.
View Article : Google Scholar
|
|
7
|
Craig ER, Londoño AI, Norian LA and Arend
RC: Metabolic risk factors and mechanisms of disease in epithelial
ovarian cancer: A review. Gynecol Oncol. 143:674–683. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fenga C, Gangemi S, Di Salvatore V,
Falzone L and Libra M: Immunological effects of occupational
exposure to lead (Review). Mol Med Rep. 15:3355–3360. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falzone L, Marconi A, Loreto C, Franco S,
Spandidos DA and Libra M: Occupational exposure to carcinogens:
Benzene, pesticides and fibers (Review). Mol Med Rep. 14:4467–4474.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amir S, Shah STA, Mamoulakis C, Docea AO,
Kalantzi OI, Zachariou A, Calina D, Carvalho F, Sofikitis N,
Makrigiannakis A and Tsatsakis A: Endocrine disruptors acting on
estrogen and androgen pathways cause reproductive disorders through
multiple mechanisms: A review. Int J Environ Res Public Health.
18:14642021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ianoşi S, Ianoşi G, Neagoe D, Ionescu O,
Zlatian O, Docea AO, Badiu C, Sifaki M, Tsoukalas D, Tsatsakis AM,
et al: Age-dependent endocrine disorders involved in the
pathogenesis of refractory acne in women. Mol Med Rep.
14:5501–5506. 2016. View Article : Google Scholar
|
|
12
|
Del Pup L, Mantovani A, Cavaliere C,
Facchini G, Luce A, Sperlongano P, Caraglia M and Berretta M:
Carcinogenetic mechanisms of endocrine disruptors in female cancers
(Review). Oncol Rep. 36:603–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rachoń D: Endocrine disrupting chemicals
(EDCs) and female cancer: Informing the patients. Rev Endocr Metab
Disord. 16:359–364. 2015. View Article : Google Scholar
|
|
14
|
Shulman LP and Dungan JS: Cancer genetics:
Risks and mechanisms of cancer in women with inherited
susceptibility to epithelial ovarian cancer. Cancer Treat Res.
156:69–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomes R, Spinola PD, Brant AC, Matta BP,
Nascimento CM, de Aquino Paes SM, Bonvicino CR, Dos Santos AC and
Moreira MA: Prevalence of germline variants in consensus
moderate-to-high-risk predisposition genes to hereditary breast and
ovarian cancer in BRCA1/2-negative Brazilian patients. Breast
Cancer Res Treat. 185:851–861. 2021. View Article : Google Scholar
|
|
16
|
Hodgson A and Turashvili G: Pathology of
hereditary breast and ovarian cancer. Front Oncol. 10:5317902020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jara L, Morales S, de Mayo T,
Gonzalez-Hormazabal P, Carrasco V and Godoy R: Mutations in BRCA1,
BRCA2 and other breast and ovarian cancer susceptibility genes in
Central and South American populations. Biol Res. 50:352017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tedaldi G, Tebaldi M, Zampiga V, Danesi R,
Arcangeli V, Ravegnani M, Cangini I, Pirini F, Petracci E, Rocca A,
et al: Multiple-gene panel analysis in a case series of 255 women
with hereditary breast and ovarian cancer. Oncotarget.
8:47064–47075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanley GE, McAlpine JN, Miller D, Huntsman
D, Schrader KA, Blake Gilks C and Mitchell G: A population-based
analysis of germline BRCA1 and BRCA2 testing among ovarian cancer
patients in an era of histotype-specific approaches to ovarian
cancer prevention. BMC Cancer. 18:2542018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madariaga A, Lheureux S and Oza AM:
Tailoring ovarian cancer treatment: Implications of BRCA1/2
mutations. Cancers (Basel). 11:4162019. View Article : Google Scholar
|
|
21
|
Ashour M and Ezzat Shafik H: Frequency of
germline mutations in BRCA1 and BRCA2 in ovarian cancer patients
and their effect on treatment outcome. Cancer Manag Res.
11:6275–6284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsaousis GN, Papadopoulou E, Apessos A,
Agiannitopoulos K, Pepe G, Kampouri S, Diamantopoulos N, Floros T,
Iosifidou R, Katopodi O, et al: Analysis of hereditary cancer
syndromes by using a panel of genes: Novel and multiple pathogenic
mutations. BMC Cancer. 19:5352019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakonechny QB and Gilks CB: Ovarian cancer
in hereditary cancer susceptibility syndromes. Surg Pathol Clin.
9:189–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishioka Y, Kobayashi K, Sagae S, Sugimura
M, Ishioka S, Nagata M, Terasawa K, Tokino T and Kudo R: Mutational
analysis of STK11 gene in ovarian carcinomas. Jpn J Cancer Res.
90:629–632. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLemore MR, Miaskowski C, Aouizerat BE,
Chen LM and Dodd MJ: Epidemiological and genetic factors associated
with ovarian cancer. Cancer Nurs. 32:281–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Faber MT, Kjær SK, Dehlendorff C,
Chang-Claude J, Andersen KK, Høgdall E, Webb PM and Jordan SJ;
Australian Cancer Study (Ovarian Cancer); Australian Ovarian Cancer
Study Group; et al Cigarette smoking and risk of ovarian cancer: A
pooled analysis of 21 case-control studies. Cancer Causes Control.
24:989–1004. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Modugno F, Ness RB and Cottreau CM:
Cigarette smoking and the risk of mucinous and nonmucinous
epithelial ovarian cancer. Epidemiology. 13:467–471. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Brien KM, Tworoger SS, Harris HR,
Anderson GL, Weinberg CR, Trabert B, Kaunitz AM, D'Aloisio AA,
Sandler DP and Wentzensen N: Association of powder use in the
genital area with risk of ovarian cancer. JAMA. 323:49–59. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fletcher NM, Harper AK, Memaj I, Fan R,
Morris RT and Saed GM: Molecular basis supporting the association
of talcum powder use with increased risk of ovarian cancer. Reprod
Sci. 26:1603–1612. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berge W, Mundt K, Luu H and Boffetta P:
Genital use of talc and risk of ovarian cancer: A meta-analysis.
Eur J Cancer Prev. 27:248–257. 2018. View Article : Google Scholar
|
|
31
|
Troisi R, Bjørge T, Gissler M, Grotmol T,
Kitahara CM, Myrtveit Saether SM, Ording AG, Sköld C, Sørensen HT,
Trabert B and Glimelius I: The role of pregnancy, perinatal factors
and hormones in maternal cancer risk: A review of the evidence. J
Intern Med. 283:430–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Havrilesky LJ, Moorman PG, Lowery WJ,
Gierisch JM, Coeytaux RR, Urrutia RP, Dinan M, McBroom AJ,
Hasselblad V, Sanders GD and Myers ER: Oral contraceptive pills as
primary prevention for ovarian cancer: A systematic review and
meta-analysis. Obstet Gynecol. 122:139–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Z, Gao Y, Wen W, Li H, Zheng W, Shu
XO and Beeghly-Fadiel A: Contraceptive methods and ovarian cancer
risk among Chinese women: A report from the shanghai women's health
study. Int J Cancer. 137:607–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goff B: Symptoms associated with ovarian
cancer. Clin Obstet Gynecol. 55:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bankhead CR, Kehoe ST and Austoker J:
Symptoms associated with diagnosis of ovarian cancer: A systematic
review. BJOG. 112:857–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rossing MA, Wicklund KG, Cushing-Haugen KL
and Weiss NS: Predictive value of symptoms for early detection of
ovarian cancer. J Natl Cancer Inst. 102:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kehoe S: FIGO staging in ovarian carcinoma
and histological subtypes. J Gynecol Oncol. 31:e702020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tokunaga H, Shimada M, Ishikawa M and
Yaegashi N: TNM classification of gynaecological malignant tumours,
eighth edition: Changes between the seventh and eighth editions.
Jpn J Clin Oncol. 49:311–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chua KJC, Patel RD, Trivedi R, Greenberg
P, Beiter K, Magliaro T, Patel U and Varughese J: Accuracy in
referrals to gynecologic oncologists based on clinical presentation
for ovarian mass. Diagnostics (Basel). 10:1062020. View Article : Google Scholar
|
|
40
|
Barnes D, Rivera R, Gibson S, Craig C,
Cragun J, Monk B and Chase D: The utility of patient reported data
in a gynecologic oncology clinic. Gynecol Oncol Res Pract. 5:42018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Funston G, Van Melle M, Baun ML, Jensen H,
Helsper C, Emery J, Crosbie EJ, Thompson M, Hamilton W and Walter
FM: Variation in the initial assessment and investigation for
ovarian cancer in symptomatic women: A systematic review of
international guidelines. BMC Cancer. 19:10282019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu JH and Zanotti KM: Management of the
adnexal mass. Obstet Gynecol. 117:1413–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chan KK, Tam KF, Tse KY and Ngan HY: The
role of regular physical examination in the detection of ovarian
cancer recurrence. Gynecol Oncol. 110:158–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scholler N and Urban N: CA125 in ovarian
cancer. Biomark Med. 1:513–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dochez V, Caillon H, Vaucel E, Dimet J,
Winer N and Ducarme G: Biomarkers and algorithms for diagnosis of
ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res.
12:282019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Andersen MR, Goff BA, Lowe KA, Scholler N,
Bergan L, Drescher CW, Paley P and Urban N: Use of a symptom index,
CA125, and HE4 to predict ovarian cancer. Gynecol Oncol.
116:378–383. 2010. View Article : Google Scholar
|
|
47
|
Bastani A, Asghary A, Heidari MH and
Karimi-Busheri F: Evaluation of the sensitivity and specificity of
serum level of prostasin, CA125, LDH, AFP, and hCG+β in epithelial
ovarian cancer patients. Eur J Gynaecol Oncol. 38:418–424.
2017.
|
|
48
|
Fischerova D and Burgetova A: Imaging
techniques for the evaluation of ovarian cancer. Best Pract Res
Clin Obstet Gynaecol. 28:697–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
van Nagell JR Jr and Hoff JT: Transvaginal
ultrasonography in ovarian cancer screening: Current perspectives.
Int J Womens Health. 6:25–33. 2013. View Article : Google Scholar :
|
|
50
|
Zhang X, Meng X, Dou T and Sun H:
Diagnostic accuracy of transvaginal ultrasound examination for
assigning a specific diagnosis to adnexal masses: A meta-analysis.
Exp Ther Med. 20:2652020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Meng XF, Zhu SC, Sun SJ, Guo JC and Wang
X: Diffusion weighted imaging for the differential diagnosis of
benign vs. malignant ovarian neoplasms. Oncol Lett. 11:3795–3802.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiao JJ, Yu J, Yu Z, Li N, Song C and Li
M: Contrast-enhanced ultrasonography in differential diagnosis of
benign and malignant ovarian tumors. PLoS One. 10:e01188722015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sahdev A: CT in ovarian cancer staging:
How to review and report with emphasis on abdominal and pelvic
disease for surgical planning. Cancer Imaging. 16:192016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thabet A, Somarouthu B, Oliva E, Gervais
DA, Hahn PF and Lee SI: Image-guided ovarian mass biopsy: Efficacy
and safety. J Vasc Interv Radiol. 25:1922–1927.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Khiewvan B, Torigian DA, Emamzadehfard S,
Paydary K, Salavati A, Houshmand S, Werner TJ and Alavi A: An
update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J
Nucl Med Mol Imaging. 44:1079–1091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao C, Li S, Zhao M, Zhu H and Zhu X:
Prognostic values of DNA mismatch repair genes in ovarian cancer
patients treated with platinum-based chemotherapy. Arch Gynecol
Obstet. 297:153–159. 2018. View Article : Google Scholar :
|
|
57
|
Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya
K, Oyama T, Mochizuki H and Omata M: Multigene panel analysis
identified germline mutations of DNA repair genes in breast and
ovarian cancer. Mol Genet Genomic Med. 3:459–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Missaoui N, Salhi S, Bdioui A, Mestiri S,
Abdessayed N, Mokni M and Yacoubi MT: Immunohistochemical
characterization improves the reproducibility of the histological
diagnosis of ovarian carcinoma. Asian Pac J Cancer Prev.
19:2545–2551. 2018.PubMed/NCBI
|
|
59
|
Zlatian OM, Comănescu MV, Roşu AF, Roşu L,
Cruce M, Găman AE, Călina CD and Sfredel V: Histochemical and
immunohistochemical evidence of tumor heterogeneity in colorectal
cancer. Rom J Morphol Embryol. 56:175–181. 2015.PubMed/NCBI
|
|
60
|
Docea AO, Mitruţ P, Grigore D, Pirici D,
Călina DC and Gofiţă E: Immunohistochemical expression of TGF beta
(TGF-β), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal
variant of gastric adenocarcinomas. Rom J Morphol Embryol. 53(Suppl
3): S683–S692. 2012.
|
|
61
|
Lee JY, Kim S, Kim YT, Lim MC, Lee B, Jung
KW, Kim JW, Park SY and Won YJ: Changes in ovarian cancer survival
during the 20 years before the era of targeted therapy. BMC Cancer.
18:6012018. View Article : Google Scholar
|
|
62
|
Wu J, Sun H, Yang L, Deng Y, Yan Y, Wang
S, Yang G and Ma H: Improved survival in ovarian cancer, with
widening survival gaps of races and socioeconomic status: A period
analysis, 1983-2012. J Cancer. 9:3548–3556. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Timmermans M, Sonke GS, Van de Vijver KK,
van der Aa MA and Kruitwagen RF: No improvement in long-term
survival for epithelial ovarian cancer patients: A population-based
study between 1989 and 2014 in the Netherlands. Eur J Cancer.
88:31–37. 2018. View Article : Google Scholar
|
|
64
|
Nagle CM, Francis JE, Nelson AE, Zorbas H,
Luxford K, de Fazio A, Fereday S, Bowtell DD, Green AC and Webb PM:
Reducing time to diagnosis does not improve outcomes for women with
symptomatic ovarian cancer: A report from the Australian ovarian
cancer study group. J Clin Oncol. 29:2253–2258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Engel J, Eckel R, Schubert-Fritschle G,
Kerr J, Kuhn W, Diebold J, Kimmig R, Rehbock J and Hölzel D:
Moderate progress for ovarian cancer in the last 20 years:
Prolongation of survival, but no improvement in the cure rate. Eur
J Cancer. 38:2435–2445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Martín-Cameán M, Delgado-Sánchez E, Piñera
A, Diestro MD, De Santiago J and Zapardiel I: The role of surgery
in advanced epithelial ovarian cancer. Ecancermedicalscience.
10:6662016.PubMed/NCBI
|
|
67
|
Vinotha T, Anitha T, Ajit S, Rachel C and
Abraham P: The role of completion surgery in ovarian cancer. J
Obstet Gynaecol India. 66(Suppl 1): S435–S440. 2016. View Article : Google Scholar
|
|
68
|
Fagotti A, Perelli F, Pedone L and Scambia
G: Current recommendations for minimally invasive surgical staging
in ovarian cancer. Curr Treat Options Oncol. 17:32016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hall M, Savvatis K, Nixon K, Kyrgiou M,
Hariharan K, Padwick M, Owens O, Cunnea P, Campbell J, Farthing A,
et al: Maximal-effort cytoreductive surgery for ovarian cancer
patients with a high tumor burden: Variations in practice and
impact on outcome. Ann Surg Oncol. 26:2943–2951. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shih KK and Chi DS: Maximal cytoreductive
effort in epithelial ovarian cancer surgery. J Gynecol Oncol.
21:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Suh DH, Chang SJ, Song T, Lee S, Kang WD,
Lee SJ, Roh JW, Joo WD, Yoon JH, Jeong DH, et al: Practice
guidelines for management of ovarian cancer in Korea: A Korean
society of gynecologic oncology consensus statement. J Gynecol
Oncol. 29:e562018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim SR, Kotsopoulos J, Sun P, Bernardini
M, Laframboise S, Ferguson S, Rosen B, Narod SA and May T: The
impacts of neoadjuvant chemotherapy and of cytoreductive surgery on
ten-year survival from advanced ovarian cancer. Int J Gynaecol
Obstet. 2017.Epub ahead of print.
|
|
73
|
Choi N, Chang JH, Kim S and Kim HJ:
Radiation for persistent or recurrent epithelial ovarian cancer: A
need for reassessment. Radiat Oncol J. 35:144–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Dowdy SC, Metzinger DS, Gebhart JB,
Srivatsa P, Haddock MG, Suman VJ and Podratz KC: Salvage
whole-abdominal radiation therapy after second-look laparotomy or
secondary debulking surgery in patients with ovarian cancer.
Gynecol Oncol. 96:389–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shimada T, Saito T, Shimokawa M, Shimamoto
K, Matsushita S, Yamaguchi S, Ariyoshi K and Okadome M: Improvement
in the prognosis of ovarian cancer in the era before addition of
molecular targeting therapy. Jpn J Clin Oncol. 47:494–498. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ledermann JA: First-line treatment of
ovarian cancer: Questions and controversies to address. Ther Adv
Med Oncol. 10:17588359187682322018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sato S and Itamochi H: Neoadjuvant
chemotherapy in advanced ovarian cancer: Latest results and place
in therapy. Ther Adv Med Oncol. 6:293–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chambers LM, Son J, Radeva M and
DeBernardo R: Evaluation of non-completion of intraperitoneal
chemotherapy in patients with advanced epithelial ovarian cancer. J
Gynecol Oncol. 30:e932019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yoon JY, Koo YJ, Kim MJ, Kim TJ, Lim KT
and Lee KH: Survival outcomes and toxicity of intraoperative
intraperitoneal chemotherapy in advanced epithelial ovarian cancer.
Obstet Gynecol Sci. 57:484–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chan JK, Brady MF, Penson RT, Huang H,
Birrer MJ, Walker JL, DiSilvestro PA, Rubin SC, Martin LP, Davidson
SA, et al: Weekly vs. Every-3-week paclitaxel and carboplatin for
ovarian cancer. N Engl J Med. 374:738–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pignata S, Scambia G, Katsaros D, Gallo C,
Pujade-Lauraine E, De Placido S, Bologna A, Weber B, Raspagliesi F,
Panici PB, et al: Carboplatin plus paclitaxel once a week versus
every 3 weeks in patients with advanced ovarian cancer (MITO-7): A
randomised, multicentre, open-label, phase 3 trial. Lancet Oncol.
15:396–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Katsumata N, Yasuda M, Isonishi S,
Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S,
Terauchi F, et al: Long-term results of dose-dense paclitaxel and
carboplatin versus conventional paclitaxel and carboplatin for
treatment of advanced epithelial ovarian, fallopian tube, or
primary peritoneal cancer (JGOG 3016): A randomised, controlled,
open-label trial. Lancet Oncol. 14:1020–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Marchetti C, Muzii L, Romito A and
Benedetti Panici P: First-line treatment of women with advanced
ovarian cancer: Focus on bevacizumab. Onco Targets Ther.
12:1095–1103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Komiyama S, Kato K, Inokuchi Y, Takano H,
Matsumoto T, Hongo A, Asai-Sato M, Arakawa A, Kamiura S, Tabata T,
et al: Bevacizumab combined with platinum-taxane chemotherapy as
first-line treatment for advanced ovarian cancer: A prospective
observational study of safety and efficacy in Japanese patients
(JGOG3022 trial). Int J Clin Oncol. 24:103–114. 2019. View Article : Google Scholar
|
|
85
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al: A phase 3 trial of bevacizumab in
ovarian cancer. N Engl J Med. 365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qian X, Qin J, Pan S, Li X, Pan Y and Ma
S: Maintenance therapy in ovarian cancer with targeted agents
improves PFS and OS: A systematic review and meta-analysis. PLoS
One. 10:e01390262015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Hess LM, Rong N, Monahan PO, Gupta P,
Thomaskutty C and Matei D: Continued chemotherapy after complete
response to primary therapy among women with advanced ovarian
cancer: A meta-analysis. Cancer. 116:5251–5260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Baert T, Ferrero A, Sehouli J, O'Donnell
DM, González-Martín A, Joly F, van der Velden J, Blecharz P, Tan
DSP, Querleu D, et al: The systemic treatment of recurrent ovarian
cancer revisited. Ann Oncol. Mar 3–2021.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lindemann K, Gao B, Mapagu C, Fereday S,
Emmanuel C, Alsop K, Traficante N; Australian Ovarian Cancer Study
Group; Harnett PR, Bowtell DDL and DeFazio A: Response rates to
second-line platinum-based therapy in ovarian cancer patients
challenge the clinical definition of platinum resistance. Gynecol
Oncol. 150:239–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Guo B, Lian W, Liu S, Cao Y and Liu J:
Comparison of diagnostic values between CA125 combined with CA199
and ultrasound combined with CT in ovarian cancer. Oncol Lett.
17:5523–5528. 2019.PubMed/NCBI
|
|
92
|
Mancari R, Cutillo G, Bruno V, Vincenzoni
C, Mancini E, Baiocco E, Bruni S, Vocaturo G, Chiofalo B and Vizza
E: Development of new medical treatment for epithelial ovarian
cancer recurrence. Gland Surg. 9:1149–1163. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kozłowska E, Vallius T, Hynninen J,
Hietanen S, Färkkilä A and Hautaniemi S: Virtual clinical trials
identify effective combination therapies in ovarian cancer. Sci
Rep. 9:186782019. View Article : Google Scholar
|
|
94
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: A
gynecologic oncology group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bamias A, Gibbs E, Khoon Lee C, Davies L,
Dimopoulos M, Zagouri F, Veillard AS, Kosse J, Santaballa A, Mirza
MR, et al: Bevacizumab with or after chemotherapy for
platinum-resistant recurrent ovarian cancer: Exploratory analyses
of the AURELIA trial. Ann Oncol. 28:1842–1848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Luvero D, Milani A and Ledermann JA:
Treatment options in recurrent ovarian cancer: Latest evidence and
clinical potential. Ther Adv Med Oncol. 6:229–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dockery LE, Tew WP, Ding K and Moore KN:
Tolerance and toxicity of the PARP inhibitor olaparib in older
women with epithelial ovarian cancer. Gynecol Oncol. 147:509–513.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kaufman B, Shapira-Frommer R, Schmutzler
RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G,
Stemmer SM, Hubert A, et al: Olaparib monotherapy in patients with
advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol.
33:244–250. 2015. View Article : Google Scholar
|
|
99
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI
|
|
100
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018. View Article : Google Scholar :
|
|
101
|
Jiang X, Li W, Li X, Bai H and Zhang Z:
Current status and future prospects of PARP inhibitor clinical
trials in ovarian cancer. Cancer Manag Res. 11:4371–4390. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Banerjee S, Gonzalez-Martin A, Harter P,
Lorusso D, Moore KN, Oaknin A and Ray-Coquard I: First-line PARP
inhibitors in ovarian cancer: Summary of an ESMO Open-Cancer
Horizons round-table discussion. ESMO Open. 5:e0011102020.
View Article : Google Scholar
|
|
104
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pujade-Lauraine E, Ledermann JA, Selle F,
Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A,
Pignata S, et al: Olaparib tablets as maintenance therapy in
patients with platinum-sensitive, relapsed ovarian cancer and a
BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet Oncol. 18:1274–1284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomised phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Balasubramaniam S, Beaver JA, Horton S,
Fernandes LL, Tang S, Horne HN, Liu J, Liu C, Schrieber SJ, Yu J,
et al: FDA approval summary: Rucaparib for the treatment of
patients with deleterious BRCA mutation-associated advanced ovarian
cancer. Clin Cancer Res. 23:7165–7170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Leonardi GC, Candido S, Falzone L,
Spandidos DA and Libra M: Cutaneous melanoma and the immunotherapy
revolution (Review). Int J Oncol. 57:609–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Vivarelli S, Falzone L, Grillo CM,
Scandurra G, Torino F and Libra M: Cancer management during
COVID-19 pandemic: Is immune checkpoint inhibitors-based
immunotherapy harmful or beneficial? Cancers (Basel). 12:22372020.
View Article : Google Scholar
|
|
112
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:14722019. View Article : Google Scholar
|
|
113
|
Liu JF, Gordon M, Veneris J, Braiteh F,
Balmanoukian A, Eder JP, Oaknin A, Hamilton E, Wang Y, Sarkar I, et
al: Safety, clinical activity and biomarker assessments of
atezolizumab from a Phase I study in advanced/recurrent ovarian and
uterine cancers. Gynecol Oncol. 154:314–322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Varga A, Piha-Paul S, Ott PA, Mehnert JM,
Berton-Rigaud D, Morosky A, Yang P, Ruman J and Matei D:
Pembrolizumab in patients with programmed death ligand 1-positive
advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol Oncol.
152:243–250. 2019. View Article : Google Scholar
|
|
115
|
Hamanishi J, Mandai M, Ikeda T, Minami M,
Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S,
et al: Safety and antitumor activity of anti-PD-1 antibody,
nivolumab, in patients with platinum-resistant ovarian cancer. J
Clin Oncol. 33:4015–4022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hodi FS, Butler M, Oble DA, Seiden MV,
Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, et al:
Immunologic and clinical effects of antibody blockade of cytotoxic
T lymphocyte-associated antigen 4 in previously vaccinated cancer
patients. Proc Natl Acad Sci USA. 105:3005–3010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zamarin D, Burger RA, Sill MW, Powell DJ
Jr, Lankes HA, Feldman MD, Zivanovic O, Gunderson C, Ko E, Mathews
C, et al: Randomized phase II trial of nivolumab versus nivolumab
and ipilimumab for recurrent or persistent ovarian cancer: An NRG
oncology study. J Clin Oncol. 38:1814–1823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Palaia I, Tomao F, Sassu CM, Musacchio L
and Benedetti Panici P: Immunotherapy for ovarian cancer: Recent
advances and combination therapeutic approaches. Onco Targets Ther.
13:6109–6129. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Gotlieb WH, Amant F, Advani S, Goswami C,
Hirte H, Provencher D, Somani N, Yamada SD, Tamby JF and Vergote I:
Intravenous aflibercept for treatment of recurrent symptomatic
malignant ascites in patients with advanced ovarian cancer: A phase
2, randomised, double-blind, placebo-controlled study. Lancet
Oncol. 13:154–162. 2012. View Article : Google Scholar
|
|
121
|
McGee J, Bookman M, Harter P, Marth C,
McNeish I, Moore KN, Poveda A, Hilpert F, Hasegawa K, Bacon M, et
al: Fifth ovarian cancer consensus conference: Individualized
therapy and patient factors. Ann Oncol. 28:702–710. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Aletti GD, Garbi A, Messori P, Achilarre
MT, Zanagnolo V, Rizzo S, Alessi S, Bocciolone L, Landoni F, Biffi
R, et al: Multidisciplinary approach in the management of advanced
ovarian cancer patients: A personalized approach. Results from a
specialized ovarian cancer unit. Gynecol Oncol. 144:468–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pillay B, Wootten AC, Crowe H, Corcoran N,
Tran B, Bowden P, Crowe J and Costello AJ: The impact of
multidisciplinary team meetings on patient assessment, management
and outcomes in oncology settings: A systematic review of the
literature. Cancer Treat Rev. 42:56–72. 2016. View Article : Google Scholar
|
|
124
|
Junor EJ, Hole DJ and Gillis CR:
Management of ovarian cancer: Referral to a multidisciplinary team
matters. Br J Cancer. 70:363–370. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gleeson M, Meiser B, Barlow-Stewart K,
Trainer AH, Tucker K, Watts KJ, Friedlander M and Kasparian N:
Communication and information needs of women diagnosed with ovarian
cancer regarding treatment-focused genetic testing. Oncol Nurs
Forum. 40:275–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Stead ML, Brown JM, Fallowfield L and
Selby P: Lack of communication between healthcare professionals and
women with ovarian cancer about sexual issues. Br J Cancer.
88:666–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Selby P, Gillis C and Haward R: Benefits
from specialised cancer care. Lancet. 348:313–318. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
August DA, Carpenter LC, Harness JK,
Delosh T, Cody RL, Adler DD, Oberman H, Wilkins E, Schottenfeld D
and McNeely SG: Benefits of a multidisciplinary approach to breast
care. J Surg Oncol. 53:161–167. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Vinciguerra V, Degnan TJ, Sciortino A,
O'Connell M, Moore T, Brody R, Budman D, Eng M and Carlton D: A
comparative assessment of home versus hospital comprehensive
treatment for advanced cancer patients. J Clin Oncol. 4:1521–1528.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Heudel PE, Devouassoux-Shisheboran M,
Taieb S, Genestie C, Selle F, Morice P, Rouzier R and Ray-Coquard
I: Multidisciplinary management of advanced ovarian cancer for an
optimal therapeutic strategy. Eur J Gynaecol Oncol. 38:175–180.
2017.PubMed/NCBI
|
|
131
|
Bjørn SF, Schnack TH, Lajer H, Christensen
IJ, Lundvall L, Thomsen LN and Høgdall C: Classification of ovarian
cancer surgery facilitates treatment decisions in a gynecological
multi-disciplinary team. Int J Gynecol Cancer. 27:382–389. 2017.
View Article : Google Scholar
|
|
132
|
Pozzar RA and Berry DL: Patient-centered
research priorities in ovarian cancer: A systematic review of
potential determinants of guideline care. Gynecol Oncol.
147:714–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Moterani VC, Tiezzi DG, de Andrade JM and
Candido Dos Reis FJ: Analysis of the relationship between hospital
characteristics and survival in ovarian cancer: A historical
cohort. J Surg Oncol. 122:1802–1807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wright JD, Chen L, Hou JY, Burke WM,
Tergas AI, Ananth CV, Neugut AI and Hershman DL: Association of
hospital volume and quality of care with survival for ovarian
cancer. Obstet Gynecol. 130:545–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Vernooij F, Heintz AP, Coebergh JW,
Massuger LF, Witteveen PO and van der Graaf Y: Specialized and
high-volume care leads to better outcomes of ovarian cancer
treatment in the Netherlands. Gynecol Oncol. 112:455–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ke KM, Blazeby JM, Strong S, Carroll FE,
Ness AR and Hollingworth W: Are multidisciplinary teams in
secondary care cost-effective? A systematic review of the
literature. Cost Eff Resour Alloc. 11:72013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mercado C, Zingmond D, Karlan BY, Sekaris
E, Gross J, Maggard-Gibbons M, Tomlinson JS and Ko CY: Quality of
care in advanced ovarian cancer: The importance of provider
specialty. Gynecol Oncol. 117:18–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Long B, Chang J, Ziogas A, Tewari KS,
Anton-Culver H and Bristow RE: Impact of race, socioeconomic
status, and the health care system on the treatment of
advanced-stage ovarian cancer in California. Am J Obstet Gynecol.
212:468.e1–9. 2015. View Article : Google Scholar
|
|
139
|
Scott R, Hawarden A, Russell B and
Edmondson RJ: Decision-making in gynaecological oncology
multidisciplinary team meetings: A cross-sectional, observational
study of ovarian cancer cases. Oncol Res Treat. 43:70–77. 2020.
View Article : Google Scholar
|
|
140
|
Rausei S, Uccella S, D'Alessandro V,
Gisone B, Frattini F, Lianos G, Rovera F, Boni L, Dionigi G and
Ghezzi F: Aggressive surgery for advanced ovarian cancer performed
by a multidisciplinary team: A retrospective analysis on a large
series of patients. Surg Open Sci. 1:43–47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Burton E, Chase D, Yamamoto M, de Guzman
J, Imagawa D and Berman ML: Surgical management of recurrent
ovarian cancer: The advantage of collaborative surgical management
and a multidisciplinary approach. Gynecol Oncol. 120:29–32. 2011.
View Article : Google Scholar
|
|
142
|
Forstner R, Meissnitzer M and Cunha TM:
Update on imaging of ovarian cancer. Curr Radiol Rep. 4:312016.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Guerriero S, Saba L, Alcazar JL, Pascual
MA, Ajossa S, Perniciano M, Piras A, Sedda F, Peddes C, Fabbri P,
et al: Past, present and future ultrasonographic techniques for
analyzing ovarian masses. Womens Health (Lond). 11:369–383. 2015.
View Article : Google Scholar
|
|
144
|
Avril S: Histopathological markers of
treatment response and recurrence risk in ovarian cancers and
borderline tumors. Pathologe. 38(Suppl 3): S180–S191. 2017.
View Article : Google Scholar
|
|
145
|
Rojas V, Hirshfield KM, Ganesan S and
Rodriguez-Rodriguez L: Molecular characterization of epithelial
ovarian cancer: Implications for diagnosis and treatment. Int J Mol
Sci. 17:21132016. View Article : Google Scholar
|
|
146
|
Ramalingam P: Morphologic,
immunophenotypic, and molecular features of epithelial ovarian
cancer. Oncology (Williston Park). 30:166–176. 2016.
|
|
147
|
Lalwani N, Prasad SR, Vikram R, Shanbhogue
AK, Huettner PC and Fasih N: Histologic, molecular, and cytogenetic
features of ovarian cancers: Implications for diagnosis and
treatment. Radiographics. 31:625–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kim H, Choi DH, Park W, Im YH, Ahn JS,
Park YH, Nam SJ, Kim SW, Lee JE, Yu JH, et al: The association
between non-breast and ovary cancers and BRCA mutation in first-
and second-degree relatives of high-risk breast cancer patients: A
large-scale study of Koreans. Hered Cancer Clin Pract. 17:12019.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Pruthi S, Gostout BS and Lindor NM:
Identification and management of women with BRCA mutations or
hereditary predisposition for breast and ovarian cancer. Mayo Clin
Proc. 85:1111–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Di Furia L, Rusciano MR, Nardini L, Rossi
P, Giammarchi C, Vittori E, Tilocca S, Russo FL, Montuori P,
Triassi M, et al: A nutritional approach to the prevention of
cancer: From assessment to personalized intervention. Transl Med
UniSa. 13:33–41. 2016.PubMed/NCBI
|
|
151
|
Zheng B, Shen H, Han H, Han T and Qin Y:
Dietary fiber intake and reduced risk of ovarian cancer: A
meta-analysis. Nutr J. 17:992018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Crane TE, Khulpateea BR, Alberts DS,
Basen-Engquist K and Thomson CA: Dietary intake and ovarian cancer
risk: A systematic review. Cancer Epidemiol Biomarkers Prev.
23:255–273. 2014. View Article : Google Scholar :
|
|
153
|
Montagnese C, Porciello G, Vitale S,
Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M,
Falzone L, et al: Quality of life in women diagnosed with breast
cancer after a 12-month treatment of lifestyle modifications.
Nutrients. 13:1362020. View Article : Google Scholar
|
|
154
|
Porciello G, Montagnese C, Crispo A,
Grimaldi M, Libra M, Vitale S, Palumbo E, Pica R, Calabrese I,
Cubisino S, et al: Mediterranean diet and quality of life in women
treated for breast cancer: A baseline analysis of DEDiCa
multicentre trial. PLoS One. 15:e02398032020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hansen JM, Nagle CM, Ibiebele TI, Grant
PT, Obermair A, Friedlander ML, DeFazio A and Webb PM; Ovarian
Cancer Prognosis Lifestyle Study Group: A healthy lifestyle and
survival among women with ovarian cancer. Int J Cancer.
147:3361–3369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Thomson CA, E Crane T, Wertheim BC,
Neuhouser ML, Li W, Snetselaar LG, Basen-Engquist KM, Zhou Y and
Irwin ML: Diet quality and survival after ovarian cancer: Results
from the women's health initiative. J Natl Cancer Inst. 106. pp.
dju3142014, View Article : Google Scholar
|
|
157
|
Peres LC, Cushing-Haugen KL, Anglesio M,
Wicklund K, Bentley R, Berchuck A, Kelemen LE, Nazeran TM, Gilks
CB, Harris HR, et al: Histotype classification of ovarian
carcinoma: A comparison of approaches. Gynecol Oncol. 151:53–60.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Jacome LS, Deshmukh SK, Thulasiraman P,
Holliday NP and Singh S: Impact of COVID-19 pandemic on ovarian
cancer management: Adjusting to the new normal. Cancer anag Res.
13:359–366. 2021.
|
|
159
|
Vivarelli S, Falzone L, Torino F,
Scandurra G, Russo G, Bordonaro R, Pappalardo F, Spandidos DA,
Raciti G and Libra M: Immune-checkpoint inhibitors from cancer to
COVID-19: A promising avenue for the treatment of patients with
COVID-19 (Review). Int J Oncol. 58:145–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tsatsakis A, Calina D, Falzone L, Petrakis
D, Mitrut R, Siokas V, Pennisi M, Lanza G, Libra M, Doukas SG, et
al: SARS-CoV-2 pathophysiology and its clinical implications: An
integrative overview of the pharmacotherapeutic management of
COVID-19. Food Chem Toxicol. 146:1117692020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zimmerman BS, Seidman D, Berger N,
Cascetta KP, Nezolosky M, Trlica K, Ryncarz A, Keeton C, Moshier E
and Tiersten A: Patient perception of telehealth services for
breast and gynecologic oncology care during the COVID-19 pandemic:
A single center survey-based study. J Breast Cancer. 23:542–552.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Mancebo G, Solé-Sedeño JM, Membrive I,
Taus A, Castells M, Serrano L, Carreras R and Miralpeix E:
Gynecologic cancer surveillance in the era of SARS-CoV-2
(COVID-19). Int J Gynecol Cancer. 5:ijgc-2020–001942. 2020.
|
|
163
|
Frey MK, Ellis AE, Zeligs K, Chapman-Davis
E, Thomas C, Christos PJ, Kolev V, Prasad-Hayes M, Cohen S, Holcomb
K and Blank SV: Impact of the coronavirus disease 2019 pandemic on
the quality of life for women with ovarian cancer. Am J Obstet
Gynecol. 223:725.e1–725.e9. 2020. View Article : Google Scholar
|