Introduction
Head and neck squamous cell carcinomas (HNSCC)
develop from the mucosal linings of the upper aerodigestive tract,
including the nasal cavity and paranasal sinuses, nasopharynx,
hypopharynx, larynx, trachea, oral cavity and oropharynx. Notably,
squamous cell carcinoma (SCC) is the most frequent malignant tumor
of the head and neck region (1).
The incidence rate of HNSCC is higher among males and individuals
aged >50 years (2), and is often
associated with a number of environmental and lifestyle risk
factors, such as alcohol consumption, UV light exposure, tobacco
smoking and human papillomavirus infection (3). Moreover, betel chewing is also
associated with the development of oral cancer in individuals in
Southeast Asia (4). The treatment
of SCC includes surgery, radiation, chemotherapy, immunotherapy and
gene therapy (5); however, the
incidence rates continue to increase (6).
Arsenic is a natural element found on the Earth's
crust that exhibits both metallic and non-metallic properties
(7), which is further classified
based on its valence state. Notably, inorganic arsenic is
considered more toxic than organic arsenic (8), and for numerous years, it has been
used as a pesticide due to its high toxicity. Moreover, arsenic
trioxide (ATO), originally used as an ingredient in Traditional
Chinese Medicine, was shown to exert antitumor effects in patients
with acute promyelocytic leukemia (APL) in 1997 (9). Further studies have demonstrated that
ATO induces malignant cell apoptosis in numerous types of cancer,
including APL (10), multiple
myeloma (11) and lung cancer
(12). The inorganic arsenic
compound, arsenic hexoxide, has also demonstrated anticancer
properties in MCF-7 breast cancer cells (13). In addition, this organic arsenic
derivative has been shown to be safe and effective in the treatment
of hematologic and solid tumors in preclinical models (14). Thus, both inorganic and organic
arsenic compounds have exhibited potential in the treatment of
tumor progression both in vitro and in vivo.
There are a number of mechanisms underlying cell
death, including autophagy, apoptosis and necrosis, which occur as
cells sense environmental stresses or intracellular signals
(15–17). Both autophagy and apoptosis are
characterized as programmed cell death (18), and defective apoptosis is considered
a major causative factor in the progression of cancer (19). The features of apoptosis are
predominantly morphological, such as cell shrinkage, plasma
membrane blebbing, DNA fragmentation and chromatin condensation
(20,21). At the molecular level, extrinsic and
intrinsic apoptotic pathways are activated in response to original
stimuli, such as granyzme and perforin, and the regulatory
aspartate-specific cysteine protease (caspase) cascade (22).
The extrinsic apoptotic pathway is also referred to
as the death receptor pathway, which is induced by death receptor
(DR)3, DR4, DR5, tumor necrosis factor (TNF) receptor 1 and
Fas/CD95 when bound to a specific ligand, such as TNF (23). After binding, the trimerized
receptor recruits associated signaling molecules by interacting
with the death domain to induce the cleavage of procaspase-8,
initiating the protease cascade to cleave targets inside cells,
causing apoptotic cell death (22,24).
On the other hand, the intrinsic pathway is dependent on the
decreasing mitochondrial membrane potential (25). Under conditions of stress, such as
DNA damage, UV exposure or hypoxia, cytochrome c is released from
the mitochondrial intermembrane space to the cytosol. In turn,
cytochrome c binds to apoptotic protease activating factor 1 to
form a complex referred to as the apoptosome, which recruits
procaspase-9 (26). Active
caspase-9 subsequently cleaves procaspase-3, which is released to
the cytosol, affecting proteolytic degradation upon target
substrates (26). Both apoptotic
pathways stimulate effector caspases, which initiate poly
ADP-ribose polymerase (PARP) cleavage and delay cellular DNA repair
function. Moreover, numerous studies have demonstrated that ATO
stimulates tumor cell apoptosis by downregulating Bcl-2 expression
and activating the caspase cascade (22,27,28).
It has previously been established that MAPKs play
crucial roles in regulating cell death associated with apoptosis
(29). MAPKs contain three family
members: ERK1 and 2, p38 MAPKs and c-Jun NH2-terminal kinase (JNK1,
2 and 3) (29). Moreover, MAPKs
promote either cell survival or death, depending on the cell type
and stimulus (29,30). It has previously been reported that
the activation of JNK and ERK enhances ovarian carcinoma cell
apoptosis with cisplatin (30).
Furthermore, the results of a previous study demonstrated that ATO
activated JNK and p38 to induce human cervical cancer cell death
through the mitochondrial apoptotic cascade (31).
Of note, the authors have previously published a
study on the anticancer effects of arsenic compounds on OEC-M1
gingival epidermal carcinoma cells (32). It was found that the arsenic
compounds induced the apoptosis of OEC-M1 cells via the MAPK and
caspase pathways (32). The present
study aimed to examine the potential anticancer properties of the
arsenic compounds in a different type of oral cancer,
hypopharyngeal SCC, using FaDu cells (33). FaDu cells were treated with both
sodium arsenite (NaAsO2) and dimethyl arsenic acid
(DMA), and cell viability, cell cycle progression, signaling
pathways and apoptosis were investigated. The findings of the
present study may provide a novel theoretical basis for the
treatment of oral cancers.
Materials and methods
Chemicals, reagents and
antibodies
NaAsO2, DMA, PI, high-glucose DMEM,
staurosporine, penicillin-streptomycin, MTT and RNase A were
purchased from Sigma-Aldrich; Merck KGaA. Trypsin-EDTA and FBS and
were purchased from AG Scientific, Inc. Tris base, potassium
chloride, HEPES and sodium chloride were obtained from J.T. Baker.
Potassium dihydrogen phosphate (KH2PO4),
sodium bicarbonate (NaHCO3) and disodium hydrogen
phosphate (Na2HPO4) were purchased from
Honeywell Riedel-de Haen. An Annexin V-FITC apoptosis detection kit
was purchased from Strong Biotech Corporation. Tween-20, sodium
hydroxide, DMSO, hydrochloric acid and SDS were purchased from
Sigma-Aldrich; Merck KGaA. Donkey anti-rabbit IgG (cat. no.
NEF81200-1EA) conjugated to HRP was purchased from PerkinElmer,
Inc. An ECL detection kit was purchased from MilliporeSigma. A
Micro BCA protein assay kit was purchased from Thermo Fisher
Scientific, Inc. Antibodies against phosphorylated (p)-p38 (cat.
no. 9215), p38 (cat. no. 9212), p-ERK1/2 (cat. no. 9101), ERK1/2
(cat. no. 9102), p-JNK (cat. no. 9251), JNK (cat. no. 9252),
cleaved PARP (cat. no. 9542), cleaved caspase-8 (cat. no. 9429),
cleaved caspase-3 (cat. no. 9661), cleaved caspase-9 (cat. no.
9509) PARP and β-actin (cat. no. 58169; 1:5,000) were obtained from
Cell Signaling Technology, Inc.
Cells and cell culture
FaDu human oral cancer cells (hypopharyngeal SCC;
HTB-43) purchased from ATCC (33)
were used in the present study. FaDu cells were maintained in
high-glucose DMEM supplemented with NaHCO3 (24 mM),
HEPES (25 mM), 10% heat-inactivated FBS and 100 U/ml penicillin
plus 100 µg/ml streptomycin (pH 7.4) in a humidified atmosphere at
37°C containing 95% air with 5% CO2 (34).
Morphological analysis
A total of 4.5×105 FaDu cells were plated
in a 6-cm Petri dish in 2 ml culture medium. At ~70% confluency,
the cells were treated with NaAsO2 (0.1, 1, 10, 25, 50
and 100 µM) or DMA (0.1, 1, 2, 5, 10, 25, 50 and 100 mM) for 24 h.
All the aforementioned concentrations have been used in previous
studies to exert apoptotic effects on testicular and oral cancer
cells (32,35). Changes in cell morphology were
examined using an Olympus CK40 light microscope, and recorded using
an Olympus DP20 digital camera (Olympus Corporation).
MTT assay
A total of 8×103 FaDu cells were plated
in 96-well plates with 100 µl culture medium per well. At ~80%
confluence, cells were treated with NaAsO2 (0.1, 1, 10,
25, 50 and 100 µM) or DMA (0.1, 1, 2, 5, 10, 25, 50 and 100 mM) for
24 h. MTT was added at a final concentration of 0.5 mg/ml and
incubated at 37°C for 4 h. The medium was subsequently discarded
and 50 µl DMSO were added to each well to dissolve the crystals for
20 min in the dark by shaking the plate (36–38).
The absorbance values were confirmed at λ=570 nm using the VersaMax
ELISA reader (Molecular Devices, LLC).
Cell cycle progression analysis
To determine the effects of NaAsO2 and
DMA on FaDu cell apoptosis, cell cycle progression was determined
using flow cytometry with PI staining. A total of
4.5×105 FaDu cells were plated in a 6-cm Petri dish in 2
ml culture medium. At ~70% confluency, the cells were treated with
NaAsO2 (0.1, 1, 10, 25, 50 and 100 µM) or DMA (0.1, 1,
2, 5, 10, 25, 50 and 100 mM) for 24 h. The cells were subsequently
collected using trypsin and centrifuged at 400 × g and 4°C for 12
min. Following centrifugation, the cells were washed with isoton II
and fixed with 70% ethanol at −20°C for ~2 h. The cells were then
washed with isoton II again and subsequently harvested by
centrifugation at 400 × g for 12 min at 4°C. Isoton II mixed with
100 µg/ml RNase and 40 µg/ml PI were used to resuspend the cell
pellets for 30 min at 25°C. A flow cytometer (FACScan; Becton,
Dickinson and Company) was used to analyze the stained cells with
excitation set at λ=488 nm, which would highlight the G1 phase DNA
content in normal cells that are diploid, as DNA synthesis
increases in the G2/M phase. However, sub-G1 phase cells exhibit a
reduced DNA content and are hypodiploid, which indicates cell
apoptosis (39–41). The percentages of cells in the
sub-G1, S and G2/M phase were further analyzed using FACStation
v6.1× and Modfit LT v3.3 software (BD Biosciences).
Annexin V/PI double staining
assay
Following treatment with NaAsO2 or DMA as
aforementioned, the FaDu cells were collected using trypsin and
subsequently washed with 2 ml medium. Following centrifugation at
160 × g for 10 min at 4°C, cold isoton II was used to resuspend
pellets prior to centrifugation again at 400 × g for 12 min at 4°C.
The pellets were subsequently mixed for 15 min with 100 µl staining
solution (Annexin V-FITC apoptosis detection kit; Strong Biotech).
A FACScan flow cytometer (Becton, Dickinson and Company) was used
to analyze the stained cells at >600-nm band pass filter for PI
detection, and λ=488 nm excitation using 515-nm band pass filter
for FITC detection. The plots comprise four quadrants, which
include negative cells, PI-positive cells (necrosis), Annexin
V-positive cells (early apoptosis) and Annexin V/PI double-positive
cells (late apoptosis) (42,43).
The percentage of cells in the four quadrants were analyzed using
FACStation v6.1× software. In addition, cells were also treated
with staurosporine (Sigma-Aldrich; Merck KGaA) and these were
considered as a positive control.
Western blot analysis
A total of 6×105 FaDu cells were plated
in a 60-mm dish. At ~70% confluency, the cells were treated with 10
and 25 µM NaAsO2, or 10 and 25 mM DMA for 3, 6, 12 and
24 h. The cell medium was transferred to a 15-ml tube and
centrifuged at 1,500 × g for 10 min at 4°C. The attached FaDu cells
were lysed with 100 µl lysis buffer containing proteinase inhibitor
(cat. no. P8340; Sigma-Aldrich; Merck KGaA). The pellets were
subsequently resuspended with 10 µl lysis buffer, blended into cell
lysates and centrifuged again at 12,000 × g for 12 min at 4°C. The
supernatants were then harvested and stored at −80°C until further
use. The protein concentration of the cell lysates was determined
using a Micro BCA assay (44,45).
For western blot analysis, ~30 µg lysates per lane were resolved on
a 12% SDS-PAGE gel with standard running buffer (24 mM Tris/HCl,
0.19 M glycine, 0.5% SDS, pH 8.3) at 25°C, and were subsequently
transferred to PVDF membranes at 4°C. The membranes were then
blocked with 4% milk at room temperature for 60 min, and incubated
with the following primary antibodies against p-p38 (1:1,000), p38
(1:4,000), p-ERK1/2 (1:4,000), ERK1/2 (1:4,000), p-JNK (1:4,000),
JNK (1:1,000), cleaved PARP (1:1,000), cleaved caspase-8 (1:1,000),
cleaved caspase-3 (1:1,000), cleaved caspase-9 (1:1,000) and
β-actin (1:5,000) (all antibody details are as aforementioned)
overnight at 4°C. The membranes were then washed with 0.1% TBS
Tween-20 and incubated with HRP-conjugated secondary antibodies
(donkey anti-rabbit IgG; 1:2,000) for 1 h at room temperature. The
membranes were visualized using an ECL detection kit and UVP EC3
BioImaging Systems (Analytik Jena AG) (33,34).
Band semi-quantification was performed using ImageJ software
version 1.50 (National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± SEM of three
independent experiments. Significantly statistical differences
between control and treatment groups were examined using one-way
ANOVA followed by Tukey's post hoc test, using GraphPad Prism 6
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Arsenic compounds induce morphological
changes in FaDu cells
The FaDu cells were plated in a 6-cm Petri dish with
either 0, 0.1, 1, 10, 25, 50 and 100 µM NaAsO2 or 0,
0.1, 1, 2, 5, 10, 25, 50 and 100 mM DMA for 24 h. The morphological
differences were subsequently examined under a light microscope. In
the control group, the FaDu cells were firmly attached to the Petri
dish and formed healthy polygonal shapes (Fig. 1A). However, following treatment with
NaAsO2, cells floated in the medium and acquired a more
rounded shape in a concentration-dependent manner. Notably, the
shapes of the attached cells were irregular, indicating cell death
(Fig. 1B-E).
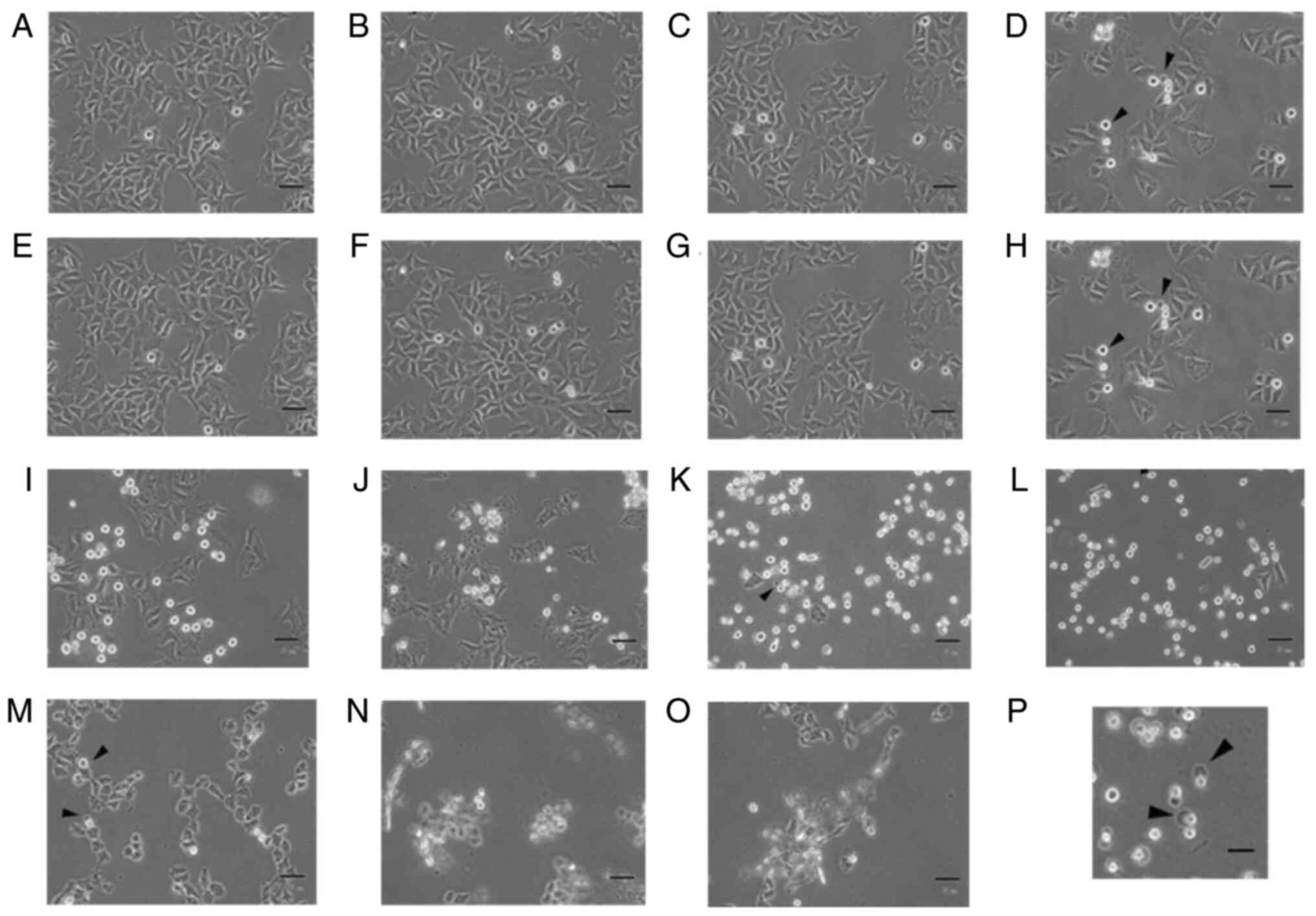 | Figure 1.Arsenic compounds induce
morphological changes in FaDu cells. FaDu cells were treated with
(A) plain medium, (B) 0.1, (C) 1, (D) 10, (E) 25, (F) 50 and (G)
100 µM sodium arsenite or (H) 0.1, (I) 1, (J) 2, (K) 5, (L) 10, (M)
25, (N) 50 and (O) 100 mM dimethyl arsenic acid for 24 h,
respectively. Morphological differences were examined by light
microscopy (scale bar, 50 µm). Arrowheads indicate cells with
membrane blebbing. (P) Enlarged image (4-fold) from (G) of cells
with membrane blebbing (scale bar, 200 µm). |
Moreover, cells that were treated with ≤50 and 100
µM NaAsO2 exhibited notable blebbing in the plasma
membrane, which is a characteristic of cell apoptosis (Fig. 1F and G). Treatment with 0.1 to 2 mM
DMA also caused the morphological rounding of the FaDu cells
(Fig. 1H-J), and increasing
concentrations of DMA at 5 and 10 mM induced the rounding of the
majority of cells (Fig. 1K and L).
Furthermore, following treatment with 50 and 100 mM DMA, cells
floated in the cell medium (Fig. 1N and
O). Notably, following treatment with 25 mM DMA, the morphology
of the FaDu cells was reversed, and the cells exhibited a shriveled
membrane (Fig. 1M). The results of
the present study demonstrated that treatment with both
NaAsO2 and DMA induced abnormal morphological changes of
the FaDu cells in a concentration-dependent manner.
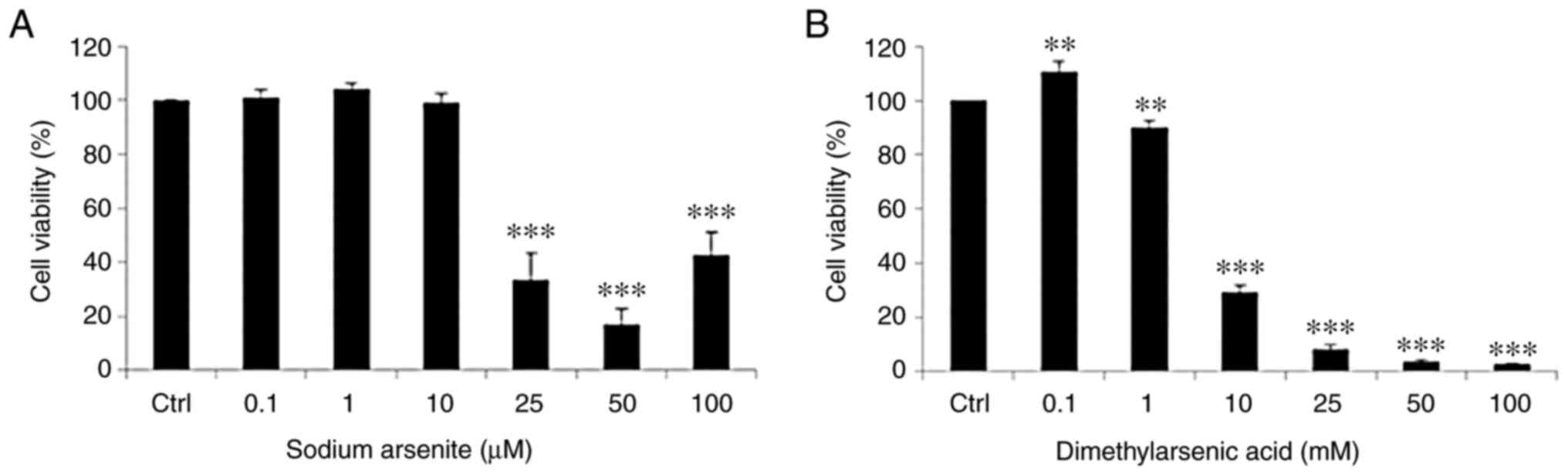
Arsenic compounds suppress FaDu cell
viability
Following treatment with the arsenic compounds, the
levels of FaDu cell viability were determined using MTT assays.
FaDu cells were treated with 0, 0.1, 1, 10, 25, 50 and 100 µM
NaAsO2 or 0.1, 1, 2, 5, 10, 25, 50 and 100 mM DMA for 24
h. The results of the present study demonstrated that treatment
with 25, 50 and 100 µM NaAsO2 markedly suppressed FaDu
cell viability, and treatment with DMA significantly decreased FaDu
cell viability in a concentration-dependent manner. The survival
rate of the FaDu cells was significantly decreased following
treatment with NaAsO2 from 25–100 µM, and following
treatment with DMA from 1-100 mM (Fig.
2A and B).
The concentration of DMA that was required to reduce
the level of FaDu cell viability to 50% was ~1,000-fold higher than
the concentration of NaAsO2. Therefore,
NaAsO2 exerted an increased level of cytotoxicity in
FaDu cells than DMA.
Arsenic compounds modulate FaDu cell
cycle progression
To investigate the effects of NaAsO2 and
DMA on cell apoptosis, FaDu cells were treated with these arsenic
compounds and subsequently examined using flow cytometric analysis.
Briefly, the FaDu cells were treated with NaAsO2 (0,
0.1, 1, 10, 25, 50 and 100 µM) or DMA (0, 0.1, 1, 10, 25, 50 and
100 mM) for 24 h, and the effects of the compounds on cell cycle
regulation were determined (Figs. 3
and 4). The results of previous
studies have revealed that DNA fragmentation in the sub-G1 phase
cells is recognized as cell apoptosis (39,46).
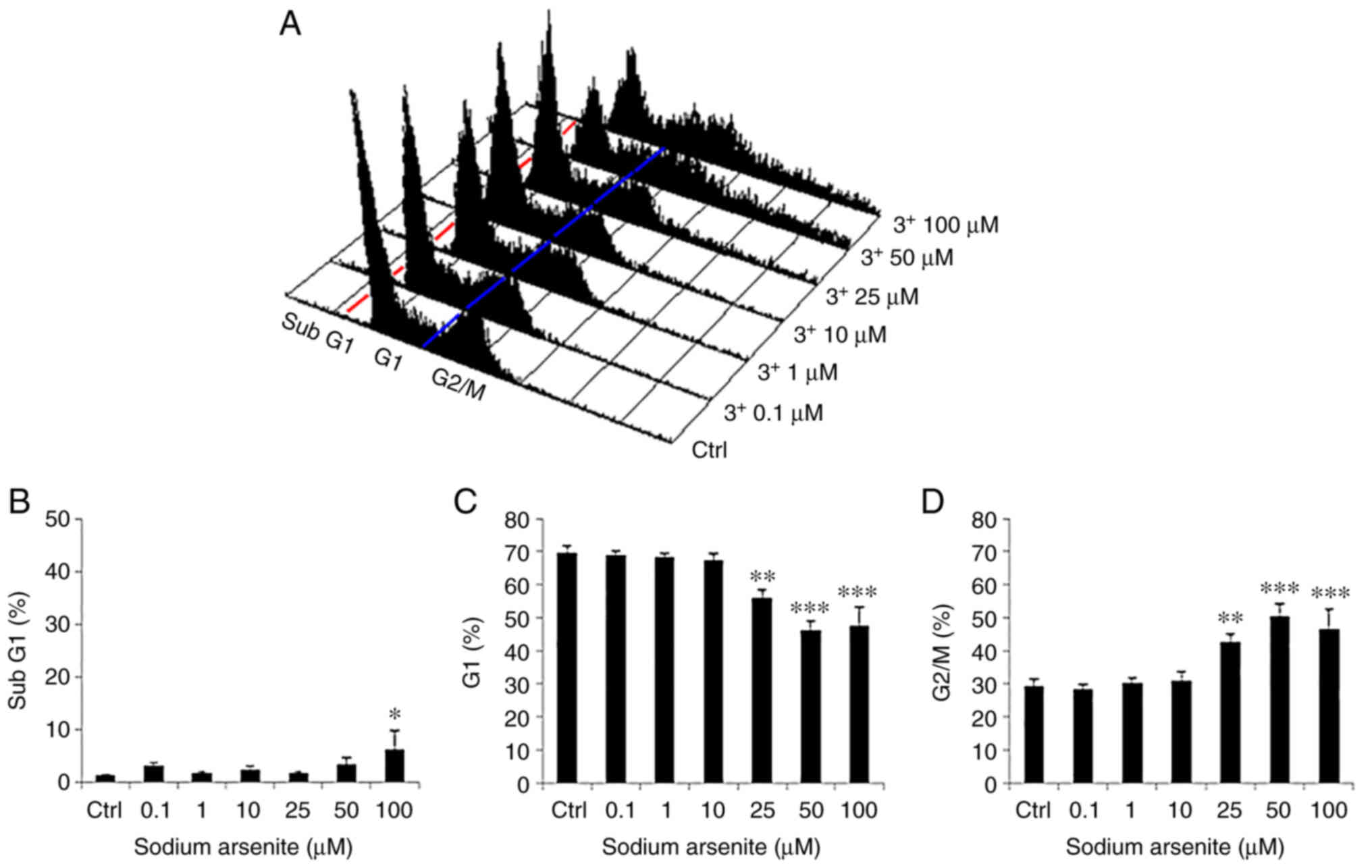 | Figure 3.Sodium arsenite modulates the cell
cycle progression of FaDu cells. (A) FaDu cells were treated with
various concentrations of sodium arsenite (0–100 µM) for 24 h, and
then fixed/stained with PI and evaluated using flow cytometry.
Cells in the sub-G1 phase with less DNA content, compared to normal
cells, indicate apoptosis. Red and blue lines are plotted to
illustrate the changes of sub-G1 (left to red line), G0/G1 (between
red and blue lines) and G2/M phases (right to blue line) in the
different treatment groups. Percentages of (B) sub-G1, (C) G1, and
(D) G2/M phase cells are illustrated, respectively. Results are
presented as the mean ± SEM of three separate experiments
(*P<0.05, **P<0.01 and ***P<0.001, significant differences
compared to the control group). Ctrl, control. |
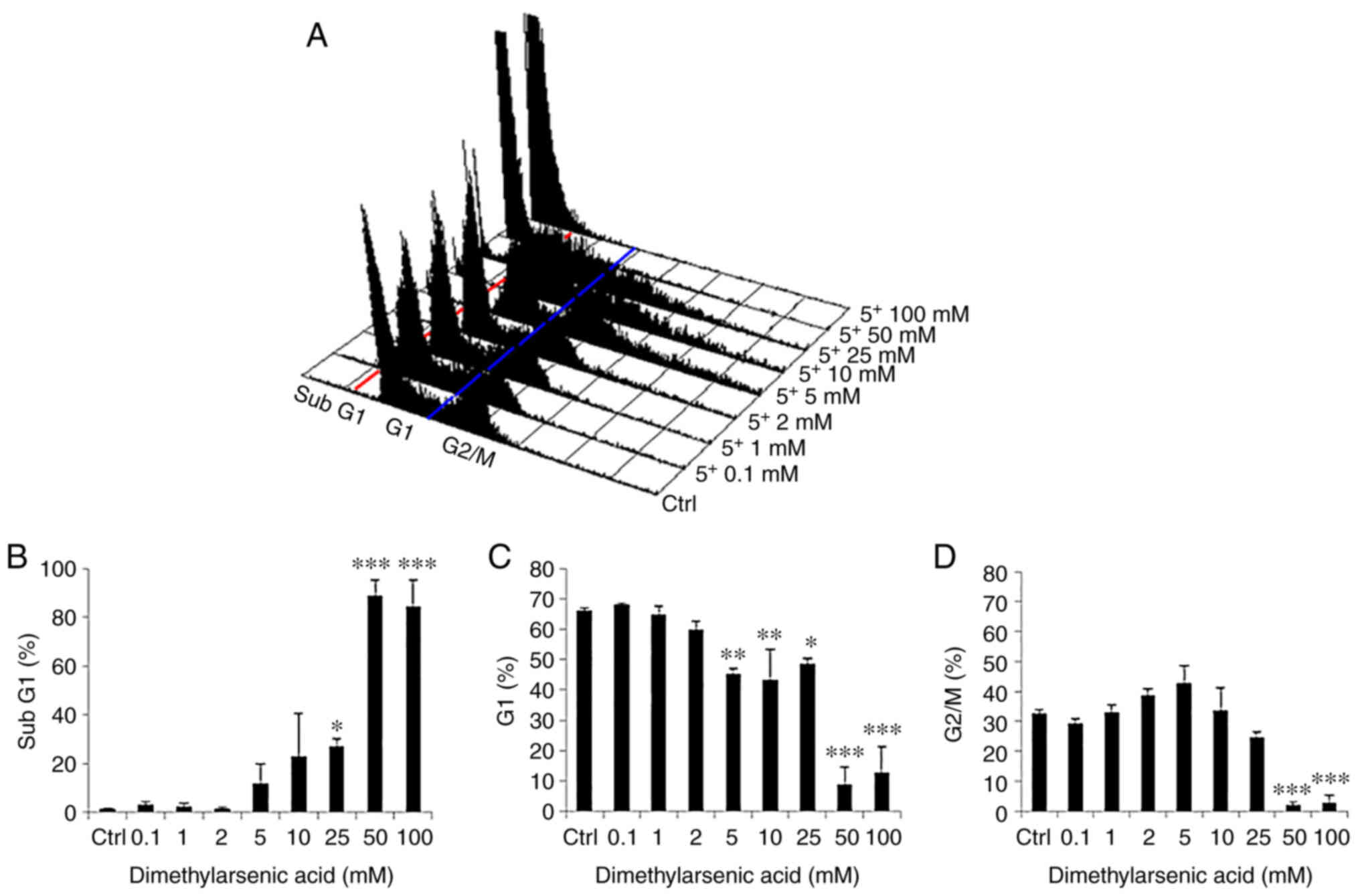 | Figure 4.Dimethyl arsenic acid modulates the
cell cycle progression of FaDu cells. (A) FaDu cells were treated
with various concentrations of DMA (0–100 mM) for 24 h, and then
fixed/stained with PI and evaluated using flow cytometry. Cells in
the sub-G1 phase with less DNA content, compared to normal cells,
indicate apoptosis. Red and blue lines were plotted to illustrate
the changes of subG1 (left to red line), G0/G1 (between red and
blue lines) and G2/M phases (right to blue line) in the different
treatment groups. Percentages of (B) sub-G1, (C) G1, and (D) G2/M
phase cells are illustrated, respectively. Results are presented as
the mean ± SEM of three separate experiments (*P<0.05,
**P<0.01 and ***P<0.001, significant differences compared to
the control group). Ctrl, control. |
The results of the present study demonstrated a
significant increase in the percentage of cells in the sub-G1 phase
following treatment with NaAsO2 at 100 µM for 24 h
(Fig. 3A and B). Moreover, the
percentage of cells in the G1 phase decreased with the increasing
concentration of NaAsO2 from 25–100 µM for 24 h
(Fig. 3A and C). In addition, the
percentage of FaDu cells undergoing G2/M phase arrest was markedly
increased with the increasing concentration of NaAsO2
from 25-100 µM for 24 h (Fig. 3A and
D). The results of a previous study demonstrated that G2/M
phase arrest led to cell apoptosis (47).
Moreover, treatment with increasing concentrations
of DMA from 25–100 mM led to a notable increase in the percentage
of cells in the sub-G1 phase (Fig. 4A
and B). In addition, the increasing concentration of DMA from
5-100 mM significantly decreased the percentage of cells in the G1
phase (Fig. 4A and C). Furthermore,
DMA at 50 and 100 mM significantly decreased the percentage of
cells in the G2/M phase (Fig. 4A and
D).
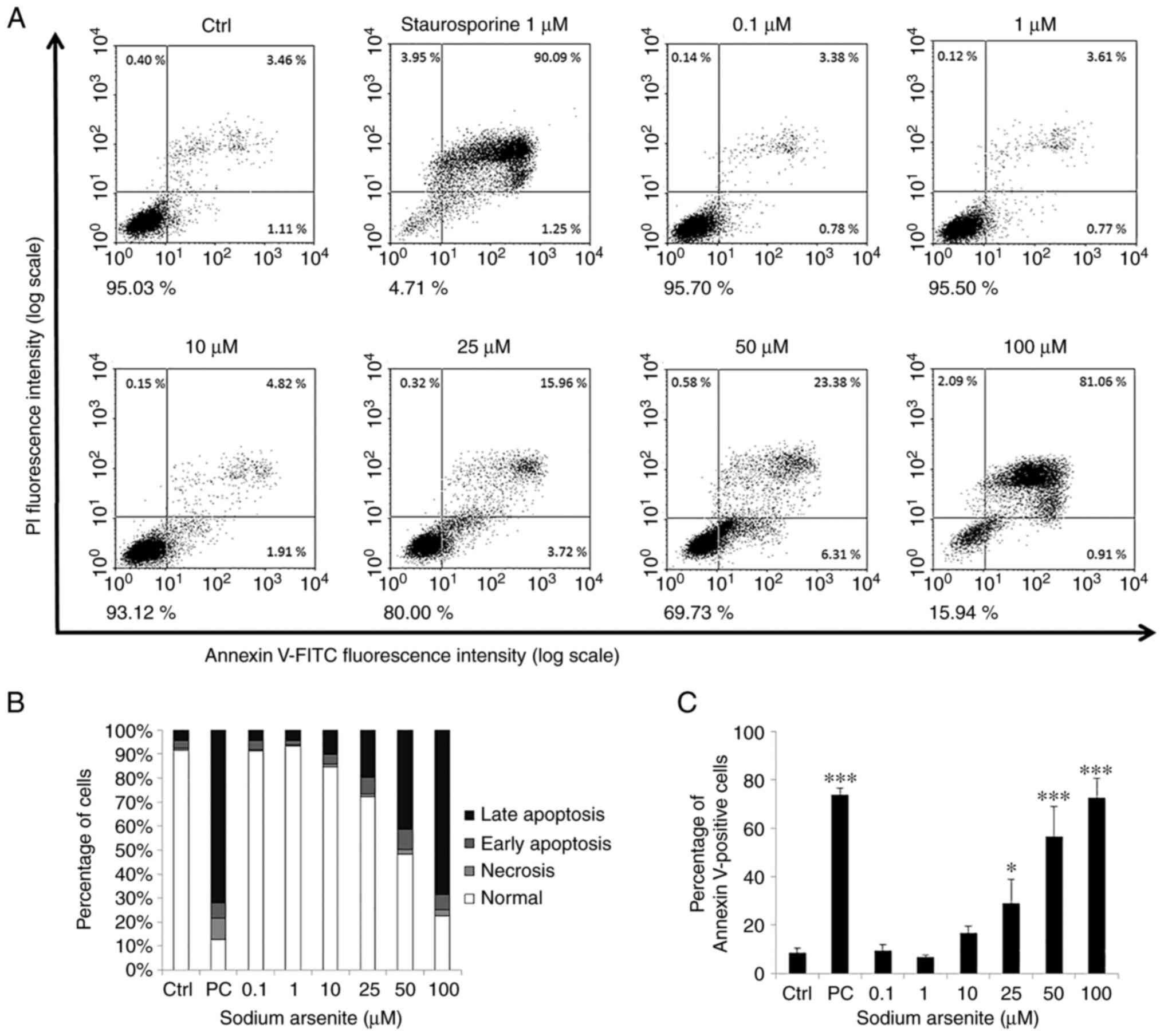
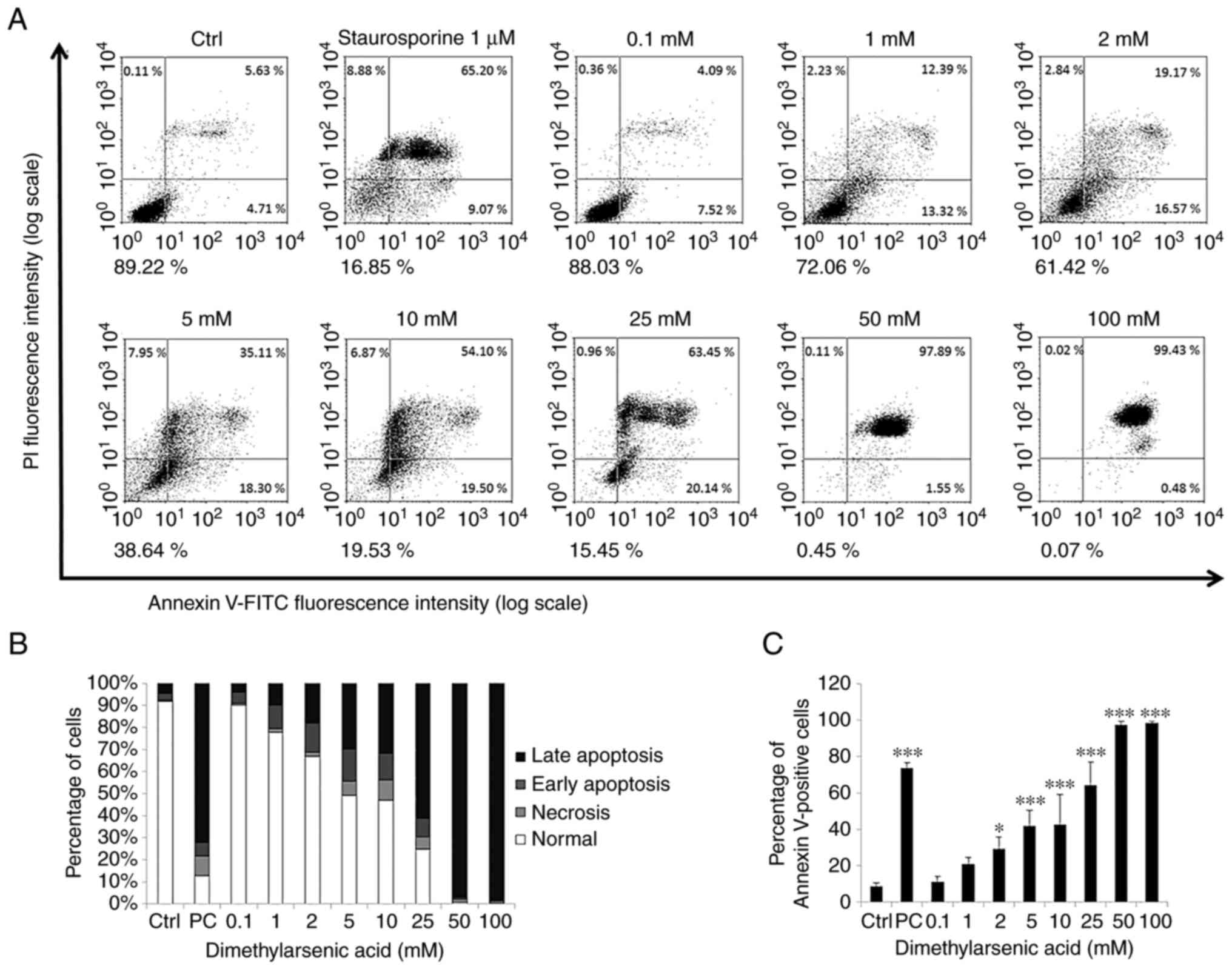
Arsenic compounds induce FaDu cell
apoptosis
To investigate the effects of the arsenic compounds
on FaDu cell apoptosis, an Annexin V and PI double staining assay
was carried out in the present study. It has previously been
established that the percentage of negative (viable), PI-positive
(necrosis), Annexin V-positive (early apoptosis) and
double-positive (late apoptosis) cells are shown in four quadrants
to determine cell apoptotic phenomena (42).
The results of the present study demonstrated that
treatment with NaAsO2 (25–100 µM) and DMA (2–100 mM) for
24 h significantly promoted the apoptosis of the FaDu cells (early
plus late apoptosis). Moreover, the number of Annexin V-positive
cells increased following treatment with the arsenic compounds in a
concentration-dependent manner (Figs.
5 and 6). Collectively, these
results demonstrated that both NaAsO2 and DMA promoted
FaDu cell apoptosis.
Arsenic compounds activate extrinsic
and intrinsic caspase pathways to induce FaDu cell apoptosis
To investigate whether arsenic compound-induced cell
deaths are involved in extrinsic (death receptor) or intrinsic
(mitochondrial) apoptotic pathways, western blot analysis was
performed to determine the expression levels of cleaved caspase-9,
caspase-8, caspase-3 and cleaved PARP. The results of the present
study demonstrated that treatment with 10 and 25 µM
NaAsO2 for 24 h induced the expression of cleaved
caspase-8, −9 and −3, as well as the substrate of activated
caspase, PARP, in the FaDu cells (Fig.
7). In addition, following treatment with 2 mM DMA for 12 h,
the expression levels of cleaved caspase-3 and cleaved PARP were
significantly increased, and treatment with 1 and 2 mM DMA for 24 h
significantly increased the expression levels of cleaved caspase-9,
−8, −3 and PARP, compared with the control group in the FaDu cells
(Fig. 8). These data suggested that
long-term treatment with NaAsO2 and DMA may stimulate
caspase-8, −9, −3 and PARP expression to activate both death
receptor and mitochondrial apoptotic pathways in FaDu cells.
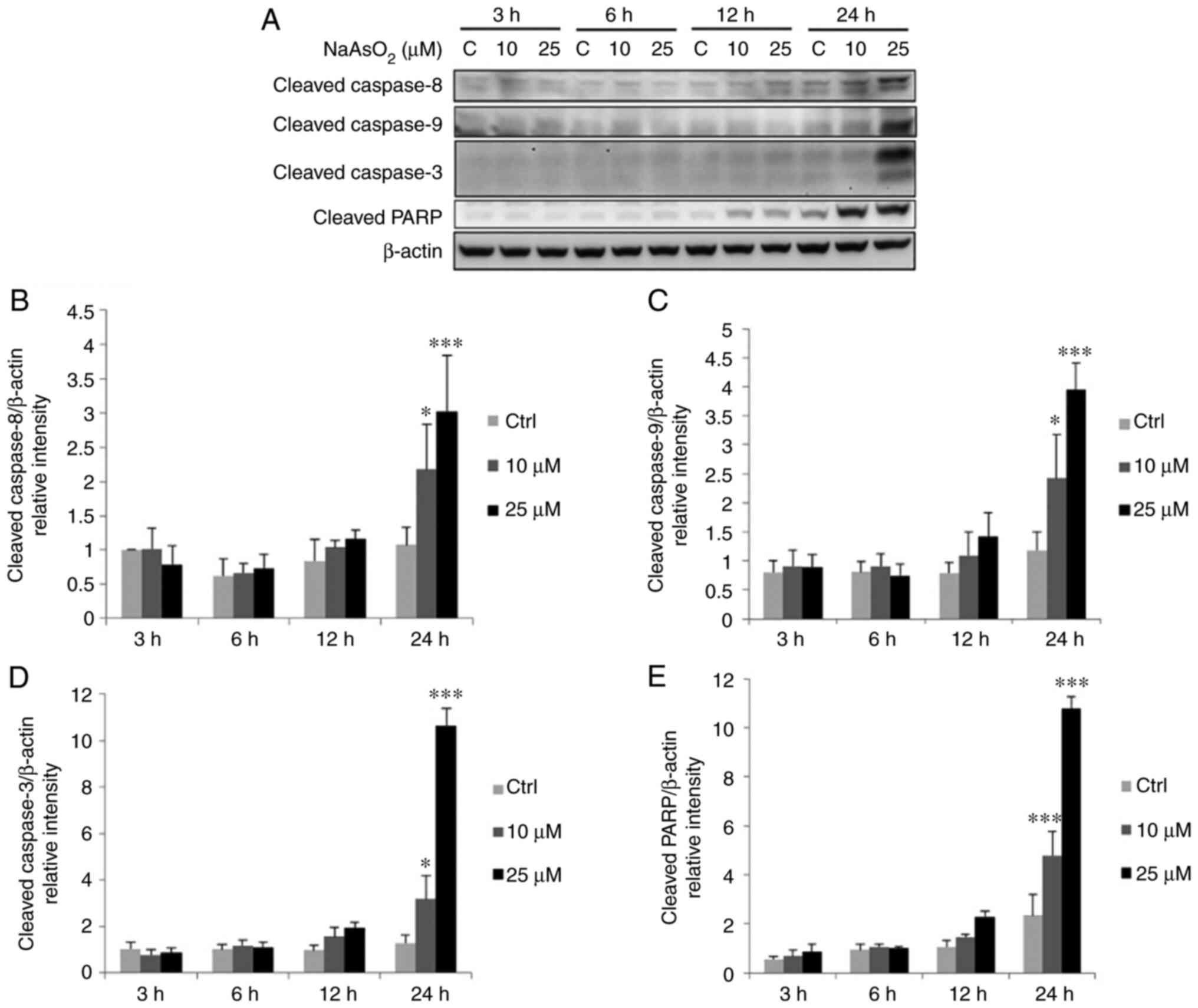 | Figure 7.NaAsO2 promotes the
cleavage of caspase-8, −9, −3 plus PARP in FaDu cells. FaDu cells
were treated with NaAsO2 (0, 10 and 25 µM for 3, 6, 12
and 24 h, respectively). (A) The cleavage of caspase-8 (43 kDa), −9
(35/37 kDa), −3 (17/19 kDa) plus PARP (85–90 kDa) was examined
using western blot analysis. Integrated optical intensities of
cleaved (B) caspase-8, (C) caspase-9, (D) caspase-3, and (E) PARP
were standardized by β-actin (43 kDa) among all lanes. Results are
presented as the mean ± SEM of three separate experiments
(*P<0.05 and ***P<0.001, significant differences compared to
the control group). Ctrl, control. NaAsO2, sodium
arsenite. |
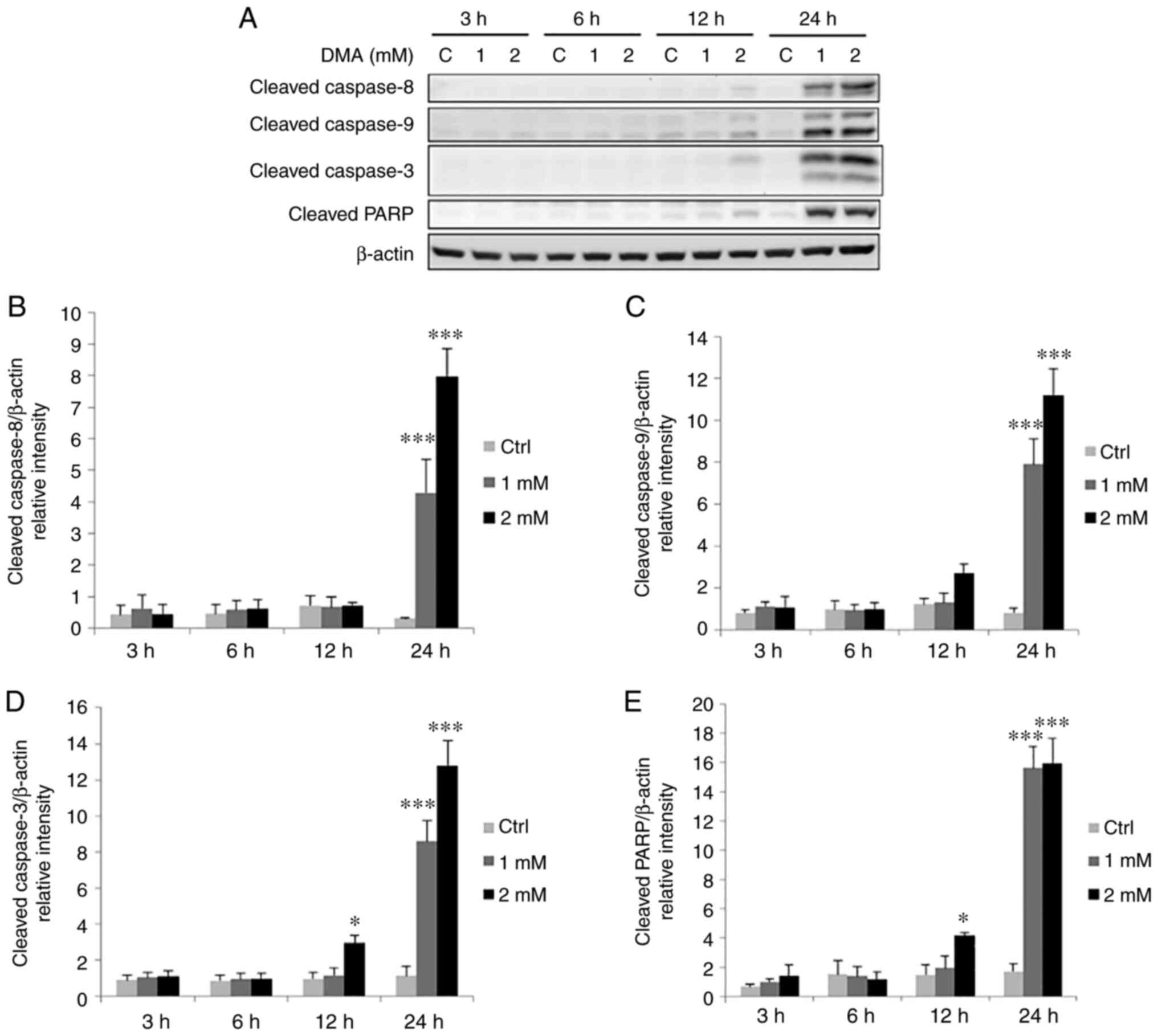 | Figure 8.DMA promotes the cleavage of
caspase-8, −9, −3 plus PARP in FaDu cells. FaDu cells were treated
with DMA (0, 1 and 2 mM for 3, 6, 12 and 24 h, respectively). (A)
The cleavage of caspase-8 (43 kDa), −9 (35/37 kDa), −3 (17/19 kDa)
plus PARP (85~90 kDa) was examined using western blot analysis.
Integrated optical intensities of cleaved (B) caspase-8, (C)
caspase-9, (D) caspase-3, and (E) PARP were standardized by β-actin
(43 kDa) among all lanes. Results are presented as the mean ± SEM
of three separate experiments (*P<0.05 and ***P<0.001,
significant differences compared to the control group). Ctrl,
control; DMA, dimethyl arsenic acid. |
Arsenic compounds activate MAPK
pathways to induce FaDu cell apoptosis
Numerous studies have demonstrated that MAPK
pathways regulate cell mitosis, proliferation, survival, apoptosis,
differentiation and gene expression (29,31,37).
In the present study, to investigate the potential role of MAPK
pathways in the induction of apoptosis following treatment with
arsenic compounds, western blot analysis was performed to analyze
the phosphorylation levels of JNK, ERK1/2 and p38.
The results revealed that treatment with 25 µM
NaAsO2 for 3 h significantly increased the
phosphorylation levels of JNK, and treatment with 25 µM
NaAsO2 for 3, 12 and 24 h significantly increased the
phosphorylation levels of ERK1/2 in FaDu cells (Fig. 9). Moreover, treatment with 1 and 2
mM DMA for 24 h significantly increased the phosphorylation levels
of JNK, and treatment with 1 and 2 mM DMA for 12 and 24 h
significantly increased the phosphorylation levels of ERK1/2
(Fig. 10). The phosphorylation
status of p38 were not altered following treatment with both
arsenic compounds in the FaDu cells.
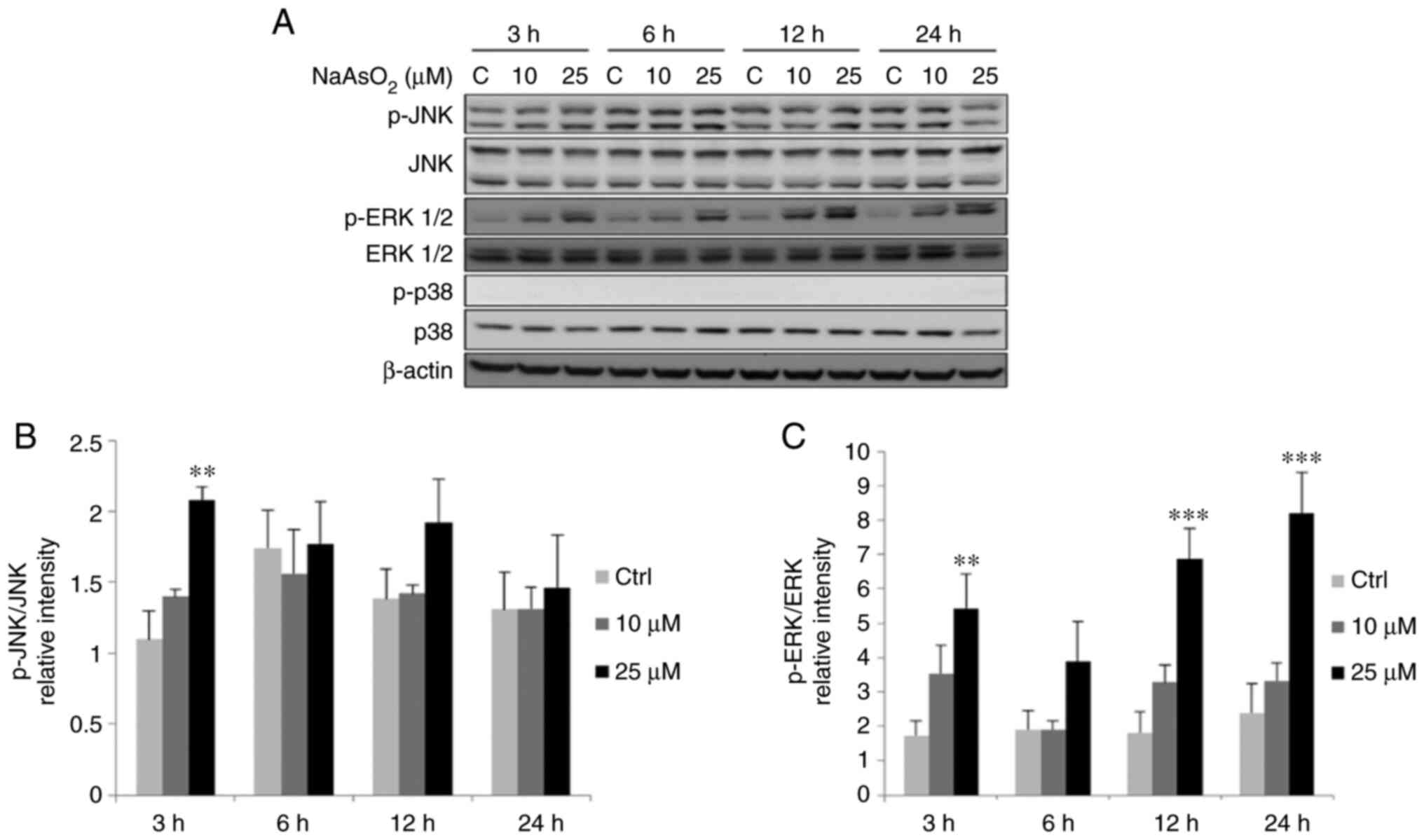 | Figure 9.NaAsO2 induces the
phophorylation of MAPK pathways in FaDu cells. FaDu cells were
treated with NaAsO2 (0, 10 and 25 µM for 3, 6, 12 and 24
h, respectively). (A) Phosphorylated-JNK (46/54 kDa), total JNK
(46/54 kDa), phosphorylated-ERK1/2 (42/44 kDa), total ERK1/2 (42/44
kDa), phosphorylated-p38 (43 kDa) and total p38 (43 kDa) were
examined using western blot analysis. Integrated optical
intensities of (B) p-JNK and (C) p-ERK proteins were standardized
with total forms of JNK and ERK, respectively. Results are
presented as the mean ± SEM of three separate experiments
(**P<0.01 and ***P<0.001, significant differences compared to
the control group). Ctrl, control; NaAsO2, sodium
arsenite. |
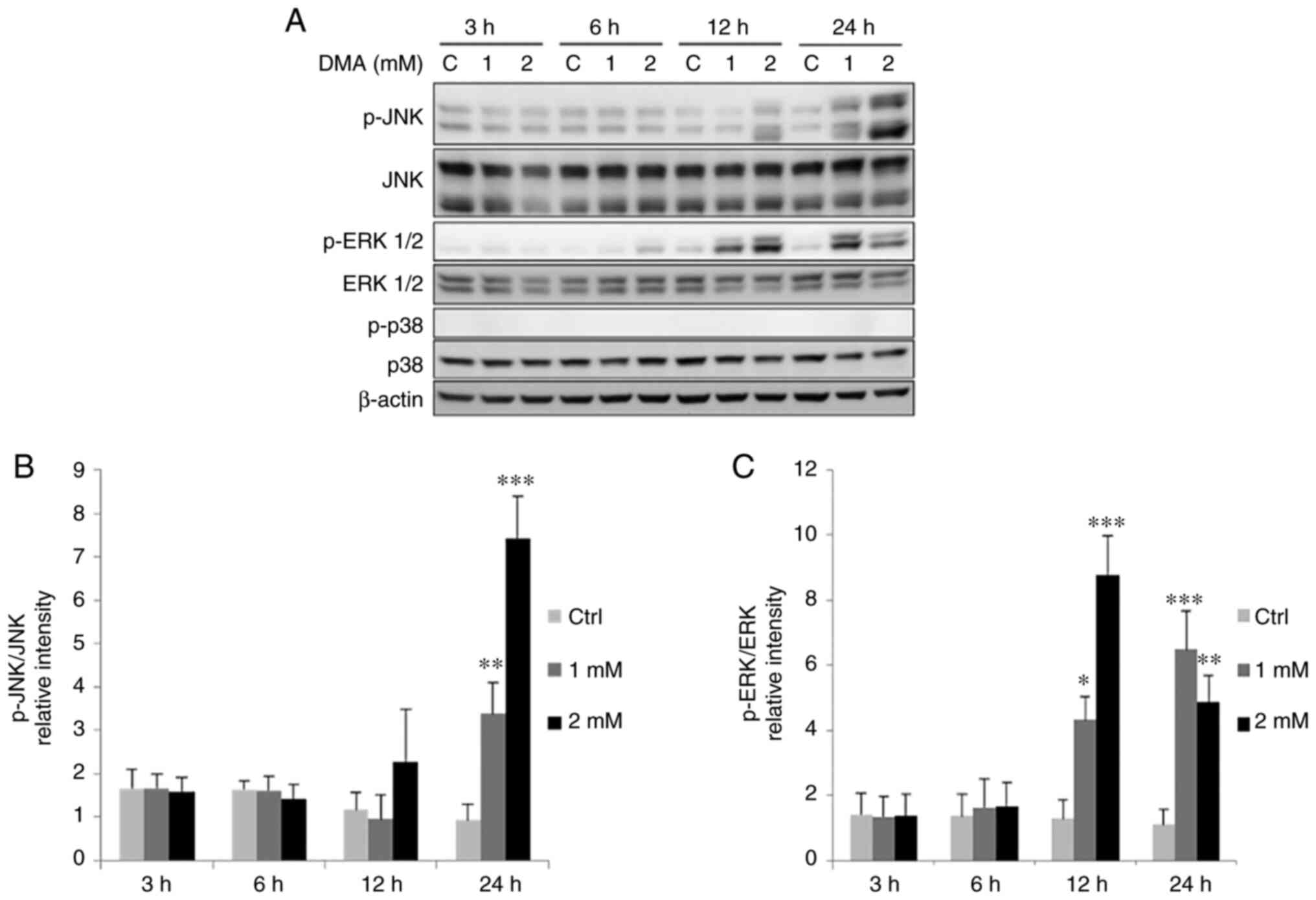 | Figure 10.DMA induces the phosphorylation of
MAPK pathways in FaDu cells. FaDu cells were treated with DMA (0, 1
and 2 mM for 3, 6, 12 and 24 h, respectively). (A)
Phosphorylated-JNK (46/54 kDa), total JNK, phosphorylated-ERK1/2
(42/44 kDa), total ERK1/2 (42/44 kDa), phosphorylated-p38 (43 kDa)
and total p38 were examined using western blot analysis. Integrated
optical intensities of (B) p-JNK and (C) p-ERK proteins were
standardized with total forms of JNK and ERK, respectively. Results
are presented as the mean ± SEM of three separate experiments
(*P<0.05, **P<0.01 and ***P<0.001, significant differences
compared to the control group). Ctrl, control; DMA, dimethyl
arsenic acid. |
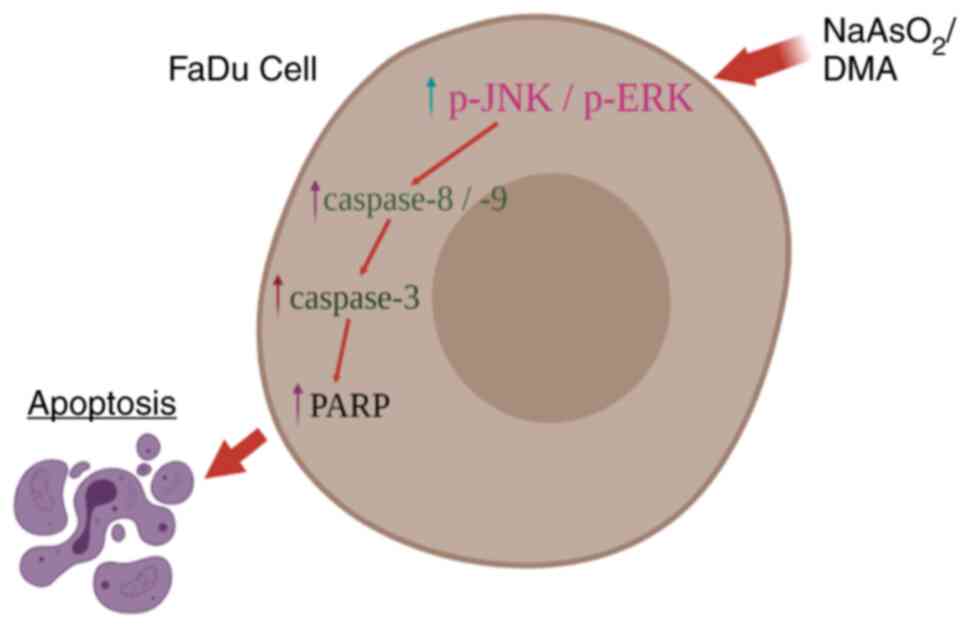
In summary, the aforementioned results illustrate
that NaAsO2 and DMA activate JNK and ERK
phosphorylation, but not p38, to induce caspase cascade and to thus
stimulate FaDu cancer cell apoptosis (Fig. 11).
Discussion
ATO initially demonstrated anticancer properties in
patients with APL (48).
Additionally, arsenic compounds have demonstrated a level of
efficiency in treating numerous types of cancers, such as
pancreatic, colon and breast cancers (13,49,50) by
inducing cell apoptosis. However, the effects of arsenic compounds
in the treatment of oral cavity cancers, and the underlying
regulating mechanisms remain to be fully elucidated. Oral cancers
are often refractory solid tumors, and the majority of oral cancers
are classified as SCC. In Taiwan, the incidence and mortality rates
increase each year (6). Treatment
options remain limited; thus, the development of novel therapeutic
strategies is required. In the present study, NaAsO2 and
DMA both demonstrated a capability to promote the apoptosis of FaDu
oral cancer cells.
Numerous previous studies have demonstrated that the
cytoskeleton is targeted by NaAsO2 and DMA (51,52),
and the degree of morphological alterations modulating the cell
skeleton is associated with drug dosages and treatment time
(53). The results of the present
study revealed that treatment with NaAsO2 and DMA
induced a number of morphological changes in the FaDu cells.
Following treatment with 25 and 50 µM NaAsO2, and 1 and
2 mM DMA, the attached cells stretched along the border, forming
large exposed cell surfaces. Compared with cells with abalone-like
shapes following treatment with 25 and 50 µM NaAsO2, the
DMA-treated cells appeared to be similar to the control group,
although they exhibited rough edges. Following treatment with 5 and
10 mM DMA, the cells were attached to the Petri dish, and the
majority of cells appeared to be rounded in shape. Moreover,
following treatment with 50 and 100 mM DMA, cells floated in
clusters. However, the mechanisms underlying arsenic the regulatory
effects of the compounds on cell morphology remain to be fully
elucidated. Of note, these results indicate that changes associated
with the cytoskeleton are dependent on both drug type and
concentration.
When specific checkpoint requirements are met by
upstream events, the cell cycle continues to the next phase. Under
conditions of cell damage or stress, cells can arrest in a specific
phase and undergo programmed cell death (54). The results of a previous study
demonstrated that arsenite induced microtubule network disruption,
causing abnormalities in spindles to induce mitotic cell apoptosis
(55). Moreover, arsenic-induced
cellular mitotic arrest may be an essential step in the activation
of apoptotic pathways among different human tumor cells (56). Another study demonstrated a clear
association between G2/M arrest and the apoptosis of ovarian
carcinoma cells in response to DNA damage (57). In the present study,
NaAsO2 and DMA notably led to G2/M phase arrest, and
increased the percentage of cells in the sub-G1 phase, indicating
that both arsenic compounds promoted FaDu cell apoptosis. Moreover,
the results of the present study demonstrated that arsenic-induced
apoptosis was associated with aberrant cell cycle redistribution.
The Annexin V/PI double staining assay further verified that
apoptosis was induced by NaAsO2 and DMA in a
concentration-dependent manner.
Apoptosis is a crucial process for cell homeostasis,
and resisting apoptosis leads to cancer development (58). Previous studies have demonstrated
that apoptosis is initiated by an extrinsic or intrinsic death
signal to activate caspase cascades (59,60).
The results of a previous study revealed that ATO stimulated
laryngeal cancer cell apoptosis by decreasing the mRNA expression
of survivin and inhibiting caspase activation (61). Previous studies have also
demonstrated that ATO induces apoptosis in myeloma and gastric
cancers by stimulating caspase-9, −8 and −3 (11,62).
The results of the present study demonstrated that
NaAsO2 and DMA significantly induced the expression of
cleaved caspase-8, −9, 3 and PARP in FaDu cells. Consistent with
the results of previous studies (11,32,35),
these results indicate that arsenic compounds activate extrinsic
and intrinsic apoptotic pathways in oral cavity cancer cells. The
results of previous studies have also demonstrated that
arginine-glycine-aspartate peptides, granzyme B and endoplasmic
reticulum stress are responsible for direct caspase-3 activation,
and the induction of apoptosis (63–65).
Moreover, the results of the present study revealed that following
treatment with 2 mM DMA for 12 h, caspase-3 activation occurred
before the activation of both caspase-8 and −9, indicating that
alternative signaling pathways require further investigation.
It has previously been established that apoptotic
pathways are closely associated with numerous cellular mechanisms,
and MAPK signaling pathways respond to different cellular stimuli
to induce cell apoptosis (29,30).
MAPK signaling pathways stimulate survival or induce apoptosis
depending on the stimuli type, cell types and the latency of MAPK
activation (29,30). JNKs (also known as stress-activated
protein kinases) are ubiquitously expressed in response to growth
factors or numerous types of stress (66,67).
The results of a previous study demonstrated that JNK activation
mediated ATO-induced APL cell apoptosis (68). The ERK pro-apoptotic function is
also stimulated by various antitumor compounds (69,70).
In addition, another study revealed that the activation of JNK1/2
and ERK was associated with the ATO-induced apoptosis of human
mesothelioma cells (71). However,
the involvement of p38 in cell apoptosis remains unclear, as it can
reduce the expression levels of caspase-8 and −3 in human
neutrophils (72). Furthermore, p38
activation mediates the apoptosis of endothelial cells by
activating caspase-3 and suppressing Bcl-x(L) (73). The results of the present study
demonstrated that the phosphorylation levels of JNK and ERK1/2 were
increased following treatment with NaAsO2 and DMA in
FaDu cells. However, the levels of p-p38 were not detected in the
FaDu cells following treatment with any arsenic compound. Notably,
the results of the present study demonstrated that treatment with
DMA activated caspases and PARP at 12 and 24 h, and stimulated MAPK
at 3 h, highlighting that that MAPK activation was initially
activated by DMA to induce the apoptosis of FaDu oral cancer
cells.
The results of previous studies have revealed the
anticancer effects of arsenic compounds through apoptotic cascades
among different cell types (32,35,49,50,74).
Previous studies have also demonstrated that arsenic compounds
induce tumor cell deaths through cell cycle arrest, the production
of reactive oxygen species, DNA methylation and the reduction of
stem cell markers (75,76). Thus, further investigations into the
anticancer mechanisms underlying arsenic compounds in FaDu cells
are required. Moreover, the use of a control cell line for
comparison with FaDu cells, in vivo experiments using an
animal model and clinical trials are all required to increase the
reliability of the results. Although further investigations are
required, the present study provides a novel theoretical basis for
the use of arsenic compounds in the clinical treatment on oral
cancers.
Collectively, these results suggested that both
NaAsO2 and DMA stimulated FaDu cell apoptosis by
activating apoptotic pathways, highlighting their antitumor effects
in FaDu cells. Moreover, these arsenic compounds activated JNK and
ERK phosphorylation, but not p38, modulating the induction of the
caspase cascade to stimulate the apoptosis in FaDu cancer cells
(Fig. 11). Thus, both arsenic
compounds possess the potential for antitumor therapy in oral
cancers and their efficiency is demonstrated in FaDu oral cancer
cells.
In a previous study, the authors treated OEC-M1
cells with the arsenic compounds (32). Both NaAsO2 and DMA
induced cell apoptosis through extrinsic and intrinsic apoptotic
pathways, exhibiting potential antitumor effects in the OEC-M1 oral
cancer cells; similar effects were observed in the present study
with the FaDu cells. In addition, the levels of phosphorylated JNK
and ERK1/2 were elevated by NaAsO2 and DMA in both the
OEC-M1 (35) and FaDu cells. Of
note, the levels of phosphorylated p38 were hardly detectable in
the FaDu cells with either of the arsenic compounds; however, the
expression of p-p38 did increase in the OEC-M1 cells following
treatment with NaAsO2 and DMA (32). These data illustrate that arsenic
compounds activate different MAPK pathways to induce apoptosis
between FaDu and OEC-M1 oral cavity cells. These differences
activating MAPK pathways between FaDu and OEC-M1 are worthy of
further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of Science and
Technology (MOST) of Taiwan, R.O.C. (grant nos.
MOST-107-2320-B-471-001, MOST-110-2314-B-218-001, MOST
106-2320-B-006-MY3 and MOST 110-2320-6-B-025-MY3), the Shu-Zen
Junior College of Medicine and Management (grant nos. SZPT10800008,
SZPT10902009 and SZPT11002012), and Chi-Mei Medical Center,
Liouying (grant no. CLFHR11025, Taiwan, R.O.C.).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request, which adheres to the FAIR principles (https://www.go-fair.org/fair-principles/), including
the fundamental principles of Findability, Accessibility,
Interoperability, and Reusability.
Authors' contributions
SZW, YYL, CYC, YKW and YPL established and conducted
the experiments, analyzed the results and wrote the manuscript. BMH
and HYC participated in the study design and were also involved in
the statistical analysis of the results, and revised the
manuscript. SZW, HYC and BMH confirm the authenticity of all the
raw data. All authors have read and approved the final version of
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
David MC, Randal SW and Stephen YL: Head
and neck cancer. Cancer. 113 (Suppl 7):S1911–S1932. 2008.
View Article : Google Scholar
|
|
2
|
Laraway DC, Lakshmiah R, Lowe D, Roe B and
Rogers SN: Quality of life in older people with oral cancer. Br J
Oral Maxillofac Surg. 50:715–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabio JM, Pasquau J and Jiménez-Alonso J:
Human papillomavirus infection as a risk factor for squamous-cell
carcinoma of the head and neck. N Engl J Med. 345:376–377. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM
and Tsai CC: Betel quid chewing, cigarette smoking and alcohol
consumption related to oral cancer in Taiwan. J Oral Pathol Med.
24:450–453. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernier J and Cooper JS: Chemoradiation
after surgery for high-risk head and neck cancer patients: How
strong is the evidence? Oncologist. 10:215–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CJ, You SL, Lin LH, Hsu WL and Yang
YW: Cancer epidemiology and control in Taiwan: A brief review. Jpn
J Clin Oncol. 32 (Suppl):S66–S81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandal BK and Suzuki KT: Arsenic round the
world: A review. Talanta. 58:201–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calatayud M, Devesa V and Vélez D:
Differential toxicity and gene expression in Caco-2 cells exposed
to arsenic species. Toxicol Lett. 218:70–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yedjou C, Tchounwou P, Jenkins J and
McMurray R: Basic mechanisms of arsenic trioxide (ATO)-induced
apoptosis in human leukemia (HL-60) cells. J Hematol Oncol.
3:282010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Q, Hilsenbeck S and Gazitt Y: Arsenic
trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or
G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and
synergy with APO2/TRAIL. Blood. 101:4078–4087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandegary A, Torshabi M, Seyedabadi M,
Amirheidari B, Sharif E and Ghahremani MH: Indomethacin-enhanced
anticancer effect of arsenic trioxide in A549 cell line:
Involvement of apoptosis and phospho-ERK and p38 MAPK pathways.
Biomed Res Int. 2013:2375432013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim MJ, Jung JH, Lee WS, Yun JW, Lu JN, Yi
SM, Kim HJ, Chang SH, Kim GS, Hong SC and Ha WS: Arsenic hexoxide
enhances TNF-α-induced anticancer effects by inhibiting NF-κB
activity at a safe dose in MCF-7 human breast cancer cells. Oncol
Rep. 31:2305–2311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mann KK, Wallner B, Lossos IS and Miller
WH Jr: Darinaparsin: A novel organic arsenical with promising
anticancer activity. Expert Opin Investig Drugs. 18:1727–1734.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaeschke H and Bajt ML: Intracellular
signaling mechanisms of acetaminophen-induced liver cell death.
Toxicol Sci. 89:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuwabara M, Asanuma T, Niwa K and Inanami
O: Regulation of cell survival and death signals induced by
oxidative stress. J Clin Biochem Nutr. 43:51–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson TR, Johnston PG and Longley DB:
Anti-apoptotic mechanisms of drug resistance in cancer. Curr Cancer
Drug Targets. 9:307–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasibhatla S and Tseng B: Why target
apoptosis in cancer treatment? Mol Cancer Ther. 2:573–580.
2003.PubMed/NCBI
|
|
21
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O' Reilly E, Tirincsi A, Logue SE and
Szegezdi E: The Janus face of death receptor signaling during tumor
immunoediting. Front Immunol. 7:4462016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu H, Li J, Yuan W, Hao S, Wang M, Wang F
and Xuan H: Bioactive components and mechanisms of poplar propolis
in inhibiting proliferation of human hepatocellular carcinoma HepG2
cells. Biomed Pharmacother. 144:1123642021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patwardhan GA, Beverly LJ and Siskind LJ:
Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr.
48:153–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akao Y, Nakagawa Y and Akiyama K: Arsenic
trioxide induces apoptosis in neuroblastoma cell lines through the
activation of caspase 3 in vitro. FEBS Lett. 455:59–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen GQ, Zhu J, Shi XG, Ni JN, Zhong HJ,
Si GY, Jin XL, Tang W, Li XS, Xong SM, et al: In vitro studies on
cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia: As2O3 induces NB4
cell apoptosis with downregulation of Bcl-2 expression and
modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayakawa J, Ohmichi M, Kurachi H, Ikegami
H, Kimura A, Matsuoka T, Jikihara H, Mercola D and Murata Y:
Inhibition of extracellular signal-regulated protein kinase or
c-Jun N-terminal protein kinase cascade, differentially activated
by cisplatin, sensitizes human ovarian cancer cell line. J Biol
Chem. 274:31648–31654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang YH and Lee SJ: The role of p38 MAPK
and JNK in arsenic trioxide-induced mitochondrial cell death in
human cervical cancer cells. J Cell Physiol. 217:23–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Foo NP, Ko CL, Chu CY, Wang CY, So EC and
Huang BM: Arsenic compounds activate the MAPK and caspase pathways
to induce apoptosis in OEC-M1 gingival epidermal carcinoma. Oncol
Rep. 44:2701–2714. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu WC, Hsiao JR, Lian YY, Lin CY and Huang
BM: The apoptotic effect of cordycepin on human OEC-M1 oral cancer
cell line. Cancer Chemother Pharmacol. 60:103–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mu YF, Chen YH, Chang MM, Chen YC and
Huang BM: Arsenic compounds induce apoptosis through caspase
pathway activation in MA-10 Leydig tumor cells. Oncol Lett.
18:944–954. 2019.PubMed/NCBI
|
|
36
|
Berridge MV and Tan AS: Characterization
of the cellular reduction of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT):
Subcellular localization, substrate dependence, and involvement of
mitochondrial electron transport in MTT reduction. Arch Biochem
Biophys. 303:474–482. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang FC, Wang SC, So EC, Chang MM, Wong
KL, Cheng KS, Chen YC and Huang BM: Propofol may increase caspase
and MAPK pathways, and suppress the Akt pathway to induce apoptosis
in MA-10 mouse Leydig tumor cells. Oncol Rep. 41:3565–3574.
2019.PubMed/NCBI
|
|
38
|
Kang FC, Chen YC, Wang SC, So EC and Huang
BM: Propofol induces apoptosis by activating caspases and the MAPK
pathways, and inhibiting the Akt pathway in TM3 mouse Leydig
stem/progenitor cells. Int J Mol Med. 46:439–448. 2020.PubMed/NCBI
|
|
39
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang MM, Lai MS, Hong SY, Pan BS, Huang
H, Yang SH, Wu CC, Sun HS, Chuang JI, Wang CY and Huang BM:
FGF9/FGFR2 increase cell proliferation by activating ERK1/2,
Rb/E2F1, and cell cycle pathways in mouse Leydig tumor cells.
Cancer Sci. 109:3503–3518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang MM, Pan BS, Wang CY and Huang BM:
Cordycepin-induced unfolded protein response-dependent cell death,
and AKT/MAPK-mediated drug resistance in mouse testicular tumor
cells. Cancer Med. 8:3949–3964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Engeland M, Ramaekers FC, Schutte B
and Reutelingsperger CP: A novel assay to measure loss of plasma
membrane asymmetry during apoptosis of adherent cells in culture.
Cytometry. 24:131–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lan YY, Chen YH, Liu C, Tung KL, Wu YT,
Lin SC, Wu CH, Chang HY, Chen YC and Huang BM: Role of JNK
activation in paclitaxel-induced apoptosis in human head and neck
squamous cell carcinoma. Oncol Lett. 22:7052021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang MM, Hong SY, Yang SH, Wu CC, Wang CY
and Huang BM: Anti-cancer effect of cordycepin on FGF9-induced
testicular tumorigenesis. Int J Mol Sci. 21:83362020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang MM, Wu SZ, Yang SH, Wu CC, Wang CY
and Huang BM: FGF9/FGFR1 promotes cell proliferation,
epithelial-mesenchymal transition, M2 macrophage infiltration and
liver metastasis of lung cancer. Transl Oncol. 14:1012082021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shu CH, Yang WK, Shih YL, Kuo ML and Huang
TS: Cell cycle G2/M arrest and activation of cyclin-dependent
kinases associated with low-dose paclitaxel-induced sub-G1
apoptosis. Apoptosis. 2:463–470. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tyagi AK, Singh RP, Agarwal C, Chan DC and
Agarwal R: Silibinin strongly synergizes human prostate carcinoma
DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest,
and apoptosis. Clin Cancer Res. 8:3512–3519. 2002.PubMed/NCBI
|
|
48
|
Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen
SJ and Chen Z: Arsenic trioxide, a therapeutic agent for APL.
Oncogene. 20:7146–7153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakagawa Y, Akao Y, Morikawa H, Hirata I,
Katsu K, Naoe T, Ohishi N and Yagi K: Arsenic trioxide-induced
apoptosis through oxidative stress in cells of colon cancer cell
lines. Life Sci. 70:2253–2269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Ding X and Adrian TE: Arsenic
trioxide induces apoptosis in pancreatic cancer cells via changes
in cell cycle, caspase activation, and GADD expression. Pancreas.
27:174–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li W and Chou IN: Effects of sodium
arsenite on the cytoskeleton and cellular glutathione levels in
cultured cells. Toxicol Appl Pharmacol. 114:132–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ochi T, Nakajima F and Fukumori N:
Different effects of inorganic and dimethylated arsenic compounds
on cell morphology, cytoskeletal organization, and DNA synthesis in
cultured Chinese hamster V79 cells. Arch Toxicol. 72:566–573. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zuk A, Targosz-Korecka M and Szymonski M:
Effect of selected drugs used in asthma treatment on morphology and
elastic properties of red blood cells. Int J Nanomedicin.
6:249–257. 2011. View Article : Google Scholar
|
|
54
|
Hartwell LH and Weinert TA: Checkpoints:
Controls that ensure the order of cell cycle events. Science.
246:629–634. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yih LH, Wu YC, Hsu NC and Kuo HH: Arsenic
trioxide induces abnormal mitotic spindles through a PIP4KIIγ/Rho
pathway. Toxicol Sci. 128:115–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ling YH, Jiang JD, Holland JF and
Perez-Soler R: Arsenic trioxide produces polymerization of
microtubules and mitotic arrest before apoptosis in human tumor
cell lines. Mol Pharmacol. 62:529–538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Concin N, Stimpfl M, Zeillinger C, Wolff
U, Hefler L, Sedlak J, Leodolter S and Zeillinger R: Role of p53 in
G2/M cell cycle arrest and apoptosis in response to
gamma-irradiation in ovarian carcinoma cell lines. Int J Oncol.
22:51–57. 2003.PubMed/NCBI
|
|
58
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nguyen TTM, Gillet G and Popgeorgiev N:
Caspases in the developing central nervous system: Apoptosis and
beyond. Front Cell Dev Biol. 9:7024042021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cheng B, Yang X, Han Z, An L and Liu S:
Arsenic trioxide induced the apoptosis of laryngeal cancer via
down-regulation of survivin mRNA. Auris Nasus Larynx. 35:95–101.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho
CH, Lai KC, Lin MC, Kung HF and Lam SK: Arsenic trioxide induces
apoptosis in human gastric cancer cells through up-regulation of
p53 and activation of caspase-3. Int J Cancer. 91:173–179. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Buckley CD, Pilling D, Henriquez NV,
Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN,
Lord JM and Salmon M: RGD peptides induce apoptosis by direct
caspase-3 activation. Nature. 397:534–539. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Goping IS, Barry M, Liston P, Sawchuk T,
Constantinescu G, Michalak KM, Shostak I, Roberts DL, Hunter AM,
Korneluk R and Bleackley RC: Granzyme B-induced apoptosis requires
both direct caspase activation and relief of caspase inhibition.
Immunity. 18:355–365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hitomi J, Katayama T, Taniguchi M, Honda
A, Imaizumi K and Tohyama M: Apoptosis induced by endoplasmic
reticulum stress depends on activation of caspase-3 via caspase-12.
Neurosci Lett. 357:127–130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Minden A, Lin A, McMahon M, Lange-Carter
C, Dérijard B, Davis RJ, Johnson GL and Karin M: Differential
activation of ERK and JNK mitogen-activated protein kinases by
Raf-1 and MEKK. Science. 266:1719–1723. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Westwick JK, Bielawska AE, Dbaibo G,
Hannun YA and Brenner DA: Ceramide activates the stress-activated
protein kinases. J Biol Chem. 270:22689–22692. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Davison K, Mann KK, Waxman S and Miller WH
Jr: JNK activation is a mediator of arsenic trioxide-induced
apoptosis in acute promyelocytic leukemia cells. Blood.
103:3496–3502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim YH, Lee DH, Jeong JH, Guo ZS and Lee
YJ: Quercetin augments TRAIL-induced apoptotic death: Involvement
of the ERK signal transduction pathway. Biochem Pharmacol.
75:1946–1958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tewari R, Sharma V, Koul N and Sen E:
Involvement of miltefosine-mediated ERK activation in glioma cell
apoptosis through Fas regulation. J Neurochem. 107:616–627. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Eguchi R, Fujimori Y, Takeda H, Tabata C,
Ohta T, Kuribayashi K, Fukuoka K and Nakano T: Arsenic trioxide
induces apoptosis through JNK and ERK in human mesothelioma cells.
J Cell Physiol. 226:762–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Alvarado-Kristensson M, Melander F,
Leandersson K, Rönnstrand L, Wernstedt C and Andersson T: p38-MAPK
signals survival by phosphorylation of caspase-8 and caspase-3 in
human neutrophils. J Exp Med. 199:449–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Grethe S, Ares MP, Andersson T and
Pörn-Ares MI: p38 MAPK mediates TNF-induced apoptosis in
endothelial cells via phosphorylation and downregulation of
Bcl-x(L). Exp Cell Res. 298:632–642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim D, Park NY, Kang K, Calderwood SK, Cho
DH, Bae IJ and Bunch H: Arsenic hexoxide has differential effects
on cell proliferation and genome-wide gene expression in human
primary mammary epithelial and MCF7 cells. Sci Rep. 11:37612021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sönksen M, Kerl K and Bunzen H: Current
status and future prospects of nanomedicine for arsenic trioxide
delivery to solid tumors. Med Res Rev. 42:374–398. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Eyvani H, Moghaddaskho F, Kabuli M, Zekri
A, Momeny M, Tavakkoly-Bazzaz J, Alimoghaddam K, Ghavamzadeh A and
Ghaffari SH: Arsenic trioxide induces cell cycle arrest and alters
DNA methylation patterns of cell cycle regulatory genes in
colorectal cancer cells. Life Sci. 167:67–77. 2016. View Article : Google Scholar : PubMed/NCBI
|