Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a deadly
disease with a poor prognosis and the fourth most common cause of
cancer-associated death in developed countries (1-3).
Despite the indisputable progress in elucidating its pathology and
mechanisms, the acquired knowledge has always been minimally
translated into successful cancer treatments.
Given the importance of the interaction between
cancer cells and the surrounding environment, PDAC progression may
be sketched out as a combination of a series of phenomena ranging
from tumor stemness, epithelial-mesenchymal transition (EMT),
secretion of autocrine and paracrine factors and finally to the
modification of the metabolic machinery. These aspects are tightly
intertwined. On one hand, EMT is necessary for tumor spreading,
acquisition of chemoresistance and mitochondrial dysfunction
(4); on the other hand, the
presence of cancer stem cells is associated with tumor spreading,
relapse and EMT, suggesting that both EMT and stemness are strictly
related events in tumor progression.
The orphan nuclear receptor transcription factor
chicken ovalbumin upstream promoter transcription factor II
(COUP-TFII), also known as nuclear receptor 2 family 2 (NR2F2),
belongs to the orphan nuclear receptor family and has an important
role in cell fate determination, neuronal and vascular
organogenesis and stemness (5-11).
Alterations of COUP-TFII expression and transcription activity are
associated with epithelial cancer progression (12-18); furthermore, its contribution to
the development of PDAC was recently highlighted (18). The role of COUP-TFII in cancer is
complex: It acts both as an oncogene and as a tumor suppressor by
controlling different pathways, such as angiogenesis, TGFβ
signaling, telomere lengthening and cancer metabolism. The evidence
that COUP-TFII deletion has little impact on adult physiological
functions indicates that this receptor may be a potential
therapeutic target in oncology (19).
COUP-TFII is prototyped by the steroid hormone
receptor; however, it has been recently demonstrated that different
COUP-TFII variants are expressed in epithelial cells and one of
these variants, COUP-TFII_V2, lacks the DNA-binding domain (DBD)
(10,20). Although the absence of DBD
suggests a lack of transcription activity, the role of this
receptor has remained elusive.
To the best of our knowledge, the involvement of
COUP-TFII_V2 in cancer biology has not been previously reported;
therefore, the present study was performed to elucidate the role of
this recently discovered variant in the complex scenario of PDAC
progression, indicating its crucial implication in cancer
invasiveness and chemoresistance.
Materials and methods
Cell culture, transfection and
commercially available plasmids
Pancreatic cell lines (PANC-1, BxPC3, CAPAN-2,
MiaPaca2, hTERT-HPNE, PL-45 and Su.86.86) were obtained from the
American Tissue Type Collection and cultured as previously
described (18). Specifically,
PANC-1, MiaPaca2,CAPAN-2 and PL-45 were cultured in DMEM high
glucose (MilliporeSigma) with 10% Fetal Bovine Serum (FBS;
Euroclone); Su.86.86 and BxPC3 cells were cultured in RPMI-1640
(MilliporeSigma)/10% FBS; hTERT-HPNE were cultured in 75% DMEM
(MilliporeSigma) (with 2 mM L-glu and 1.5 g/l sodium bicarbonate,
both from MilloporeSigma)/25% Medium M3 Base (Incell Corp.) with 5%
FBS, 10 ng/ml human recombinant EGF (Thermo Fisher Scientific,
Inc.), 5.5 mM D-glucose (1 g/l; MilliporeSigma) and 750 ng/ml
puromycin (Thermo Fisher Scientific, Inc.). The transfections of
plasmids were performed with FuGENE HD (Promega Corporation)
according to the manufacturer's protocol. Short hairpin (sh)RNA for
COUP-TFII (shNR2F2) and negative control shRNA (shNEG) are
described in (18); shNR2F2
covers the same target sequence of a small interfering (si)RNA for
COUP-TFII (Hs_NR2F2_6, cat. no. s103649065; Qiagen GmbH).
COUP-TFII_V2-GFP (cat. no. RG226609) and COUP-TFII_V2 (cat. no.
4453629) plasmids were from OriGene Technologies, Inc.
EGFP-COUP-TFII_V1 plasmid
To produce the N-terminal enhanced green
fluorescence protein (EGFP)-tagged COUP-TFII_V1 a 1.5 Kb
EcoRI/XhoI fragment of the COUP-TFII_V1 cDNA was
amplified from the plasmid pCR3.1-COUP-TFII by PCR with PFU Ultra
II (Agilent Technologies) using the following primers: Forward,
5′-C AT GAA TTC GGC AAT GGT AG-3′ and reverse, 5′-TAG AAG GCA CAG
TCG AGG-3′. Thermocycling conditions were as per the manufacturer's
instructions with annealing at 54°C for 30 sec and extension at
72°C for 30 sec (×35 cycles); reaction mixtures were prepared as
per the manufacturer's protocol. After digestion with EcoRI
and XhoI enzymes (New England Biolabs, Inc.) and gel
purification of the PCR product, the COUP-TFII cDNA was ligated in
the EcoRI/SalI sites of pEGFP-C1 (GenBank accession
no. U55763; cat. no. 6084-1; Clontech). The absence of errors due
to PCR amplification was confirmed by standard Sanger DNA
sequencing. The pCR3.1-COUP-TFII plasmid was a kind gift of
Professor M. Vasseur-Cognet [INSERM, U1016; Department of
Endocrinology, Metabolism and Cancer, Cochin Institute, CNRS (UMR
8104), Paris, France].
COUP-TFII_V2-NLS plasmid
COUP-TFII_V2 was fused at the C-terminal end with a
nuclear localization signal derived from SV40 virus (-PKKKRKV-) to
force nuclear localization. In brief, an EcoRI/MluI
COUP-TFII fragment, obtained by double enzymatic digestion with
EcoRI and MluI (New England Biolabs, Inc) from
plasmid RG226609 (OriGene Technologies, Inc.), was cloned between
the EcoRI and XhoI sites of the pcDNA3.1 plasmid
(cat. no. V79020; Thermo Fisher Scientific, Inc.) and the nuclear
localization signal (NLS) was inserted in frame as a double-strand
(ds) DNA in the MluI/XhoI restriction sites. Tag
presence was detected by restriction digestion with the newly
inserted SpeI site and confirmed by standard Sanger DNA
sequencing. For COUP-TFII_V2NLS-EGFP, the ds oligo corresponding to
the NLS was directly cloned in the MluI/XhoI
restriction sites of the plasmid RG226609. The NLS was obtained by
annealing the oligos NLS-[forward (for)/reverse (rev)] for V2-NLS
and NLS-GFP (for/rev) for V2-NLS-GFP (Table SI).
Time-lapse experiment
Time-lapse experiments were performed on a Leica
AM600 inverted microscope equipped with a microscope miniculture
incubator (CTI-control 3400 digital) and Temp control (37-2
digital), both from Leica Microsystems GmbH. Transfected cells were
plated on 35-mm µ-Dishes (Ibidi GmbH) for observation;
usually, cells were followed for 16 h and images were acquired
every 5 min. The average speed and linearity were measured with Icy
v.2.0.3 (icy.bioimageanalysis.org).
Cell viability, apoptosis, mitochondrial
membrane potential and cell senescence
Cell viability, apoptosis and mitochondrial
potential were evaluated with the 'Cell Count and Viability', with
the 'Annexin V & Cell Death kit' and with the 'MitoPotential
kit', respectively, on a Guava Muse cell Analyzer (Luminex
Corporation) following the manufacturer's protocols. Cellular
senescence was histochemical determined with the Senescence
detection kit (BioVision) as percentage of senescence-associated
ß-gal expressing cells.
Wound healing and invasiveness
A wound-healing assay was performed by plating
PANC-1 clones in the chambers of µ-Dish culture inserts
(Ibidi GmbH) for live cell analysis. After reaching confluence, the
inserts were removed and images of the same areas were acquired
immediately (time 0) and then after 4, 24 and 48 h. Captured images
were then analyzed with ImageJ 1.52r inside Icy 2.0.3. To measure
the wound width, its margins were determined by thresholding the
images and then a straight vertical line was drawn for each margin;
the wound gap was then measured as the distance between these two
lines. Chemo-invasiveness was performed as described previously
(18). In brief, cells suspended
in serum-free medium were loaded in the top chambers of 12
multiwell Boyden chambers (Neuro Probe Inc.) at 104
cells/filter and complete medium was added to the lower chamber as
a chemo-attractant. Upper and lower chambers were separated by an 8
µm pore polycarbonate membrane, coated with
Matrigel® (Biomap snc). After 6 h of incubation at 37°C
with 5% CO2, the Boyden chambers were disassembled, the
cells were removed from the upper side of the membranes with a
cotton bud, the filters stained with Diff-Quick stain (Biomap snc)
according to manufacturer's protocol and the cells that had
transgressed to the lower surface were counted with a Leica DM4000B
Microscope (Leica Microsystems GmbH).
3D growth and clonogenic assay
A clonogenic assay was performed as described in
(18). In brief, 104
cells were mixed with DMEM/10% FBS containing 0.3% agarose and were
layered over a solid base of 0.5% agarose in the same medium. After
15 days of incubation at 37°C, colonies were stained and
counted.
Spheroid growth was achieved by seeding 2,500 cells,
resuspended in 100 µl complete cell culture medium
supplemented with 0.24% MethoCell (Merck KGaA) in each well of a
96-well round-bottom low-adhesion cell culture plate (Greiner
Bio-One GmbH). Seeded cells were grown under standard temperature
and CO2 conditions until the formation of spheroids
(usually 5 days).
Isolation of cell clones
COUP-TFII_V2 and COUP-TFII_V1 PANC-1 cells were
obtained by serial dilution of PANC-1 cells transiently transfected
with COUP-TFII_V2 or COUP-TFII_V1 plasmids. Transfections were
performed with FuGENE HD transfection reagent (Promega Corporation)
according to manufacturer's protocol. Serial dilutions were
performed 48 h after transfection; selection of transfected cells
was carried out exposing the transfected cells to 3.2 mg/ml G418
(Invitrogen; Thermo Fisher Scientific, Inc.). Similarly, MOCK
PANC-1 cells were obtained after transfection with pcDNA3.1(+)
plasmid and PANC-V2NLS were obtained transfecting the PANC-1 cells
with the COUP-TFII_V2NLS plasmid. The expression of the COUP-TFIIs
was verified by western blot analysis.
Patients
A total of 43 PDAC tissues were obtained after
written informed consent from patients that underwent surgical
resection at the Surgery Unit of Careggi University Hospital
(Firenze, Italy) between February 2013 and December 2018. The study
was approved by Careggi University Hospital Ethical Committee
(Firenze, Italy; no. 0028114). Inclusion criteria were admission to
surgery for PDAC; exclusion criteria were the absence of consent
and the presence of comorbidities that may influence survival. The
diagnosis was performed after surgery. Of the 43 PDAC samples
collected, three were discarded due to the absence of amplification
of the housekeeping genes and two had no detectable COUP-TFII_V2
expression (Table I). The median
follow-up of the study population was 412.5 days and the median
survival time was 644 days; 23 subjects were female and 17 male,
with a median age of 70 years. All patients received gemcitabine as
adjuvant therapy and none received any neo-adjuvant treatments.
 | Table ICOUP-TFII-V2 expression in primary
samples. |
Table I
COUP-TFII-V2 expression in primary
samples.
| Item | n | COUP-TFII_V2
relative quantities, mean (min; max); median | P-value |
|---|
| Sex | | | 0.988 |
| Male | 16 | 1.02 (0.001; 11.2);
0.048 | |
| Female | 22 | 14.5 (0.002; 313);
0.039 | |
| Age, years | | | 0.895 |
| <70 | 18 | 17.7 (0.001; 313);
0.03 | |
| ≥70 | 20 | 0.792 (0.002;
11.2); 0.055 | |
| T-stage | | | 0.247 |
| 1 | 1 | 0.002 | |
| 2 | 12 | 27.1 (0.002; 313);
0.059; | |
| 3 | 24 | 0.415 (0.001;
4.26); 0.028 | |
| Nodal status | | | 0.028 |
| N0 | 8 | 0.0238 (0.002;
0.099); 0.011 | |
| N+ | 30 | 11.2 (0.001; 313);
0.06 | |
|
Differentiation | | | 0.008 |
| Low | 6 | 0.008 (0.001;
0.014); 0.008 | 0.014 vs.
medium |
| Moderate | 29 | 11.6 (0.002; 313);
0.06 | |
| High | 3 | 0.016 (0.002;
0.039); 0.006 | |
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted with TRI-Reagent
(MilliporeSigma) or with RNeasy mini kit (Qiagen). Subsequently,
100 ng to 1 µg RNA was reverse transcribed with the
High-capacity RNA-to-cDNA master mix (Thermo Fisher Scientific,
Inc.), whereas qPCR was performed with GoTaq qPCR Master Mix
(Promega Corporation) on an AbiPrism 7000 (Thermo Fisher
Scientific, Inc.). Quantification of relative expression was
performed with the 2−∆∆Cq method with DataAssist
software 3.01 (RRID:SCR_014969; Thermo Fisher Scientific, Inc.) or
with LinRegPCR 2020.0 (https://www.medischebiologie.nl/files/). GAPDH and
RPL13A were used as internal controls and their resulting geometric
mean was used for normalization. The primers are listed in Table SII.
Western blot analysis,
COUP-TFII_V2-specific antibody and proteomics
SDS-Page western blot was performed as previously
described (18,21). Relative quantification of western
blot data was performed with the Gel analysis function of ImageJ
1.52r inside Icy 2.0.3. Immunoprecipitation (IP) was performed with
'Protein A/G PLUS-Agarose Immunoprecipitation reagent' (cat. no.
sc-2003; Santa Cruz Biotechnology, Inc.), according to the
manufacturer's instruction. Nuclear extraction was performed with
the 'Nuclear extraction kit' (cat. no. 2900; Chemicon
International) according to the manufacturer's instructions. A
rabbit polyclonal COUP-TFII_V2 antibody was generated by Genecust
Europe using the N-terminal region of V2 as the antigen; its
reactivity and specificity were tested by western blot,
immunohistochemistry (IHC) and immunofluorescence (IF); the
COUP-TFII_V2 antibody was used at the following dilutions: 1:50
(WB), 1:25 (IHC) and 1:100 (IF). Antibodies used for WB were as
follows: COUP-TFII_V1 (cat. no. ab41859; 1:500 dilution; Abcam),
COUP-TFII (cat. no ab50487; 1:1,000 Abcam), β-catenin (cat. no.
sc-7963; 1:1,000; Santa Cruz Biotechnology, Inc.); ERK (cat. no.
sc-94; 1:100; Santa Cruz Biotechnology, Inc.); phosphorylated
(P)-ERK (cat. no. sc-7383; 1:200; Santa Cruz Biotechnology, Inc.);
AKT (cat. no. 9272; 1:1,000; Cell Signaling Technology, Inc.);
P-AKT (cat. no. 9271; 1:1,000; Cell Signaling Technology, Inc.);
AMPK (cat. no. 2603; 1:1,000; Cell Signaling Technology, Inc.);
P-AMPK (cat. no. 4188; 1:1,000; Cell Signaling Technology, Inc.);
forkhead box (FOX)O3a (cat. no. ab47409; 1:1,000; Abcam); P-FOXO3a
(cat. no. ab47285; 1:1,000; Abcam); P-GSK3 (cat. no. 8566; 1:1,000;
Cell Signaling Technology, Inc.); GSK3 (cat. no. 5676; 1:1,000;
Cell Signaling Technology, Inc.); vimentin (cat. no. M0725;
1:1,000; DAKO); GAPDH (cat. no. G8759; 1:10,000; MilliporeSigma);
BRG1 (cat. no. ab70558; 1:1,000; Abcam); Histone H3 (cat. no.
Ab10799, 1:1,000, Abcam); HSP70 (cat. no. sc-24; 1:1,000; Santa
Cruz Biotechnology, Inc.); FAK (cat. no. sc-558; 1:200; Santa Cruz
Biotechnology, Inc.); Vinculin (cat. no. V9131; 1:1,000;
MilliporeSigma); P21 (cat. no. ab7960; 1:200; Abcam); pP21 (cat.
no. ab47300; 1:500; Abcam); RhoA (cat. no. sc-418; 1:100; Santa
Cruz Biotechnology, Inc.); Ubiquitin (cat. no. SPA-203; 1:1,000;
Stressgene Corp.); β-tubulin (cat. no. T5201, 1:500, Sigma).
Proteomics experiments and Differential in Gel
Expression (DIGE) were performed as previously described (22). Specifically, total protein was
extracted from subconfluent PANC-1 clones (MOCK, COUP-TFII_V1 and
COUP-TFII_ V2). From each clone, three protein extracts were
combined. Fifty micrograms of cyanine 3 (Cy3)- or Cy5-labeled
proteins were electrofocused on Dry Strip gel pH 3-10 nl immobiline
strips (Cytiva) together with 50 µg of a Cy2-labeled pool of
proteins coming from the three clones. Differentially expressed
proteins were then identified by mass spectrometry (22). Gene ontology (GO) and Reactome
pathway analysis were performed with the Cytoscape
(RRID:SCR_003032) ClueGO plugin (RRID:SCR_005748) (23). The ontology database was updated
to the version published in August 2019.
IHC, IF and collagen staining
IHC and IF with antibodies to COUP-TFII_V1 (cat. no.
AB41859; RRID:AB_742211) and cytokeratin (CK)19 (cat. no. AB15463;
RRID:AB_2281021; both from Abcam) were performed as previously
described (18,24). The custom-made primary antibody
for COUP-TFII_V2 was used at 1:25 dilution in PBS containing 2% BSA
(MilliporeSigma) and 0.01% Triton X-100 (Sigma). IHC for α-smooth
muscle actin was performed with the monoclonal clone A4 (DAKO;
Agilent Technologies, Inc.) diluted 1:100 in PBS with 2% BSA and
0.01% Triton X-100 following the previously described protocol; the
sections were incubated with an M.O.M. kit (Vector Laboratories,
Inc.) prior to incubation with the primary antibody. For β-catenin
IF the same antibody used in western blot was used diluted 1:50;
SMAD2/3P IF was performed with a 1:100 dilution of a rabbit
polyclonal antibody (cat. no. ab272332; Abcam); actin filaments
were decorated with Alexa Fluor 633 phalloidin (Molecular Probes
cat. no. A22284); cell nuclei were stained with DAPI (cat. no.
10236276001; Roche). Collagen was stained with standard Sirius Red
staining. Sirius Red was quantified with ImageJ 1.52r under Icy
2.0.3 on images with RGB color settings; the selection of stained
areas was achieved with the function 'Color threshold'.
Expression in primary tissues
COUP-TFII_V2 expression in primary PDAC tissues was
determined by RT-qPCR of RNA extracted from 4-5 30 µm-tick
formalin-fixed and sucrose-protected tumor cryo-sections; the
expression of COUP-TFII_V2 was confirmed by IHC on selected
samples. For IHC, the custom anti-COUP-TFII_V2 rabbit polyclonal
antibody was used diluted 1:25 in PBS with 2% BSA and 0.01% Triton
X-100; incubation with the antibody was performed overnight (O/N)
at 4°C in a humified chamber. Prior to primary antibody incubation,
sections were preblocked with 2.5% NGS for 1 h at room temperature
(ImmPRESS® HRP Horse Anti-Rabbit IgG Polymer Detection
Kit, Peroxidase, RRID:AB_2336529; Vectorlabs). Citrate buffer (pH
6.0) was used for antigen retrieval (100°C for 10 min) before
primary antibody incubation and pre-blocking of tissue sections; an
anti-rabbit HRP polymer contained in the above-mentioned kit was
used as the secondary antibody; sections were incubated with the
polymer for 30 min at room temperature in a humified chamber.
Semiquantitative scoring of PDAC was performed on 22 samples by two
expert pathologists. Points were given according to the intensity
of the staining (no staining, 0 points; low, 1 point; moderate, 2
points; and strong, 3 points); and the percentage of cells
expressing the nuclear receptor (<10%, 1 point; ≥10% and
<50%, 2 points; ≥50 and <80%, 3 points; ≥80%, 4 points). The
final score was multiplicative and PADC expression was considered
low when the final score was <6. Cohen's kappa coefficient of
concordance (25) between IHC
semi-quantitative analysis and qPCR was calculated in R with the
library 'vcd'. Results of the concordance analysis between the qPCR
score and IHC score are provided in Fig. S1.
In vivo experiment
Athymic nude male mice (Fo×1nu/nu; age, 6
weeks; body weight, 22 g) were purchased from Harlan and kept under
sterile conditions under a 12-h light/dark cycle with chow and
water provided ad libitum. PANC-1 clones (MOCK, PANC-V1 and
PANC-V2) were harvested, suspended at a concentration of
106 cells/50 µl PBS and injected into the tail of
the pancreas; during the surgical procedure, the mice were
anesthetized with an intraperitoneal injection of ketamine/xylazine
(100 and 10 mg/kg, respectively). A total of 15 animals were used,
5 in each experimental group. After surgery, the animals were
checked daily. At two weeks after the surgery, the mice were
euthanized by cervical dislocation; death was verified by the
absence of heartbeat and respiration. Thereafter, the pancreas and
adjacent organs were collected for histology evaluation. The tumor
score was based on a variation of the score reported in (26); final score was additive; the score
point allocation system is provided in Table SIII. All experiments were
performed following the guidance for the use of laboratory animals
and were approved by the appointed authority under Italian law
(Ministry of Health; Rome, Italy; no. 853/2015-PR) (27). None of the mice was found dead and
no animal reached the humane endpoints of the experiment, which
were weight loss >20%, no movement for >24 h, ulceration at
the site of the surgical procedure with organ exposure and
breathing difficulties (e.g. apnea).
Oligo annealing
Labeled nucleotide corresponding to the COUP-TFII
binding site in the sodium-hydrogen exchanger (NHE) pump promoter
were synthesized by MilliporeSigma and purified by HPLC (oligo
sequences are provided in Table
SIV). Oligos were resuspended at 1 mg/ml in molecular
biology-grade water. For the annealing, 30 ng of each set of oligos
per gel shift reaction were diluted in annealing buffer (100 mM
Tris HCl pH 8.0, 100 mM EDTA pH 8.0, 20 mM NaCl, 5 mM
MgCl2) and denatured at 95°C for 3 min in a thermomixer
(Eppendorf), then allowed to slowly cool to RT. Annealed oligos
were stored at −20°C.
Gel shift
Gel shift experiments were performed according to
the protocol described in (22)
with a Cy3-labeled oligonucleotide corresponding to the COUP-TFII
binding site in the NHE promoter (28). Total proteins were extracted from
PANC-1 cells 48 h after transfection with a control plasmid,
COUP-TFII_V1 or COUP-TFII_V2. For the binding reaction, 50
µg of proteins were incubated for 1 h at 4°C in a binding
reaction with Cy3-labeled oligos (Table SIV) (no differences in binding to
labeled oligos were detected among incubation times ranging from 1
h to O/N, data not shown). For gel shift, total protein extracts
were pre-incubated O/N at 4°C with 0.1 mg/ml of the same
COUP-TFII_V1 mouse monoclonal antibody used for IF, western blot
and IHC; the following day, the labeled oligonucleotide was added
to the reaction mix for 1 h at 4°C. The product mixtures were then
run on 5% non-denaturing Tris-borate EDTA (TBE, 45 mM Tris-borate
buffer with 1 mM EDTA)-polyacrylamide gel and run with TBE buffer
for 10 h at 4°C.
NHE reporter and Gli transcription factor
activity assay
NHE reporter and Gli activity were evaluated with
the Dual Luciferase Assay system (Promega Corporation). Briefly,
cells were transfected with NHE reporter plasmid or with Gli
reporter, together with a minimal promoter Renilla reporter
plasmid (Promega Corporation) (at a 1:100 ratio with respect to the
target plasmids). The transfections were carried out in suspension
with FuGENE HD (Promega Corporation) and 10,000 cells/well were
plated in 96 wells plates. Readout was performed with a Glomax 96
microplate luminometer Dual injector system (Promega Corporation)
48 h after transfection.
Statistical analysis
Each experiment was performed in triplicates unless
otherwise stated. Statistical analysis was performed with R 4.1
(www.r-project.org; RRID:SCR_001905) and Jamovi
2.0 (www.jamovi.org; RRID:SCR_016142).
Differences in experimental results were evaluated by Student's
t-test or the Mann-Whitney U test (Wilcoxon Rank-sum test),
abbreviated as 'Wilcoxon' when reported in the figures, for
parametric and non-parametric data, respectively. When comparing
multiple groups, ANOVA followed by Tukey's highly-significant
differences post-hoc test was used for parametric data, while the
Kruskal-Wallis test followed by the Conover-Iman test was used for
pairwise comparisons of non-parametric data (29). Normality of data distribution was
assessed with the Shapiro-Wilk normality test. Hierarchical
clustering was performed using R with the 'pheatmap' library.
Survival and Cox analyses for this study cohort were performed with
the 'survival', 'survminer' and 'ggforest' libraries. Cohen's
concordance was calculated with the 'vcd' library in R. Values are
expressed as the mean ± standard deviation unless otherwise stated.
P<0.05 was considered to indicate statistical significance.
Principal component analysis (PCA) was performed with 'prcomp'
under R 4.1. The graphical representations of the experimental
results were generated with the 'ggpubr' and 'ggplot2' R libraries
and final images were mounted with Scribus 1.5.4 (https://www.scribus.net) or Inkscape 1.0 (https://www.inkscape.org).
Results
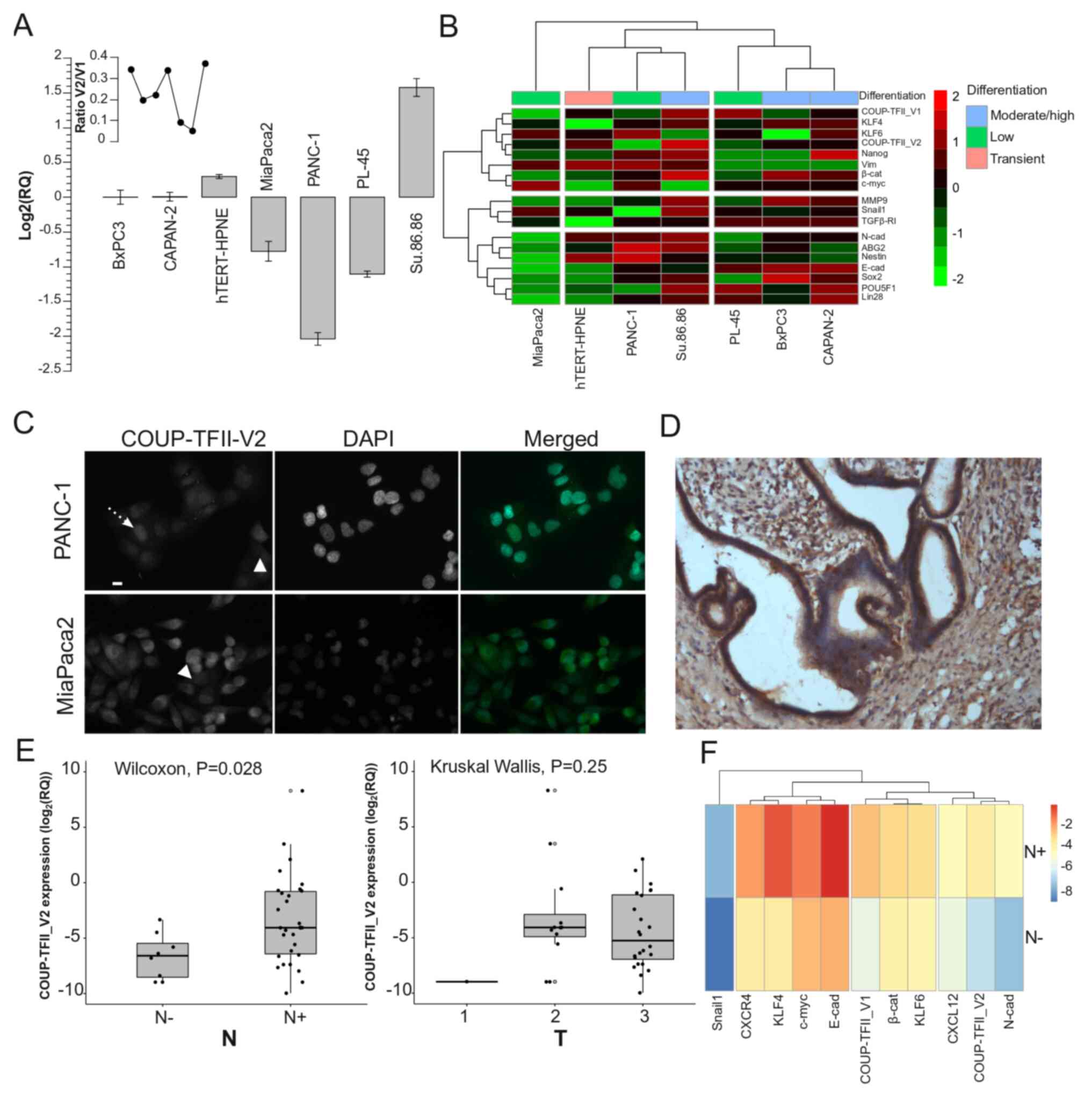
COUP-TFII_V2 is highly expressed in PDAC
and affects patient survival
As a previously published study by our group
indicated that human PDAC cells express COUP-TFII_V1 (18), the present study sought to
demonstrate the expression of COUP-TFII_V2 in human PDAC cells. As
presented in Fig. 1A,
COUP-TFII_V2 mRNA was detectable in all tested PDAC cell lines
(from the well-differentiated and K-RAS wild-type BxPC3 to the
poorly differentiated PANC-1). The expression was lower in PANC-1
and higher in Su.86.86 cells. Gene clustering suggested that
COUP-TFII_V1 and COUP-TFII_V2 share expression patterns similar to
Kruppel-like factor (KLF)4, KLF6 and NANOG (Fig. 1B), while the PCA-biplot indicated
a correlation of COUP-TFII_V1 and V2 with SNAIL1; furthermore, PCA
suggested that the receptors are a characterizing factor of the
metastatic cell line Su.86.86 (Fig.
S2A). These data suggest that both isoforms are associated with
stemness and metastatic potential. Of note, COUP-TFII_V2 expression
was not limited to the nucleus but spanned to the cytosol (Figs. 1C and S2B-D), whereas V1 was strictly
nuclear-specific (Fig. S2E)
(5,18). Furthermore, COUP-TFII_V2
localization exhibited cell-to-cell variation, having a stronger
cytoplasmic expression in certain cells (Figs. 1C and S2B). In primary tumors, COUP-TFII_V2
was expressed in the cancer cells (Figs. 1D and S3A-H) and its mRNA expression was
predictive of advanced disease (Table
I). Specifically, COUP-TFII_V2 expression was significantly
higher in patients with lymph node metastasis (Figs. 1E and S3I), suggesting that this nuclear
receptor may be associated with tumor spreading. No specific
associations with age, sex or other clinicopathological
characteristics were observed (Table
I). When confronting the average gene expression between N0 and
N+ patients, the hierarchical clustering indicated a close
association of COUP-TFII_V2 with N-cadherin and C-X-C motif
chemokine ligand (CXCL)12, while COUP-TFII_V1 was linked to
β-catenin and KLF4 (Fig. 1F).
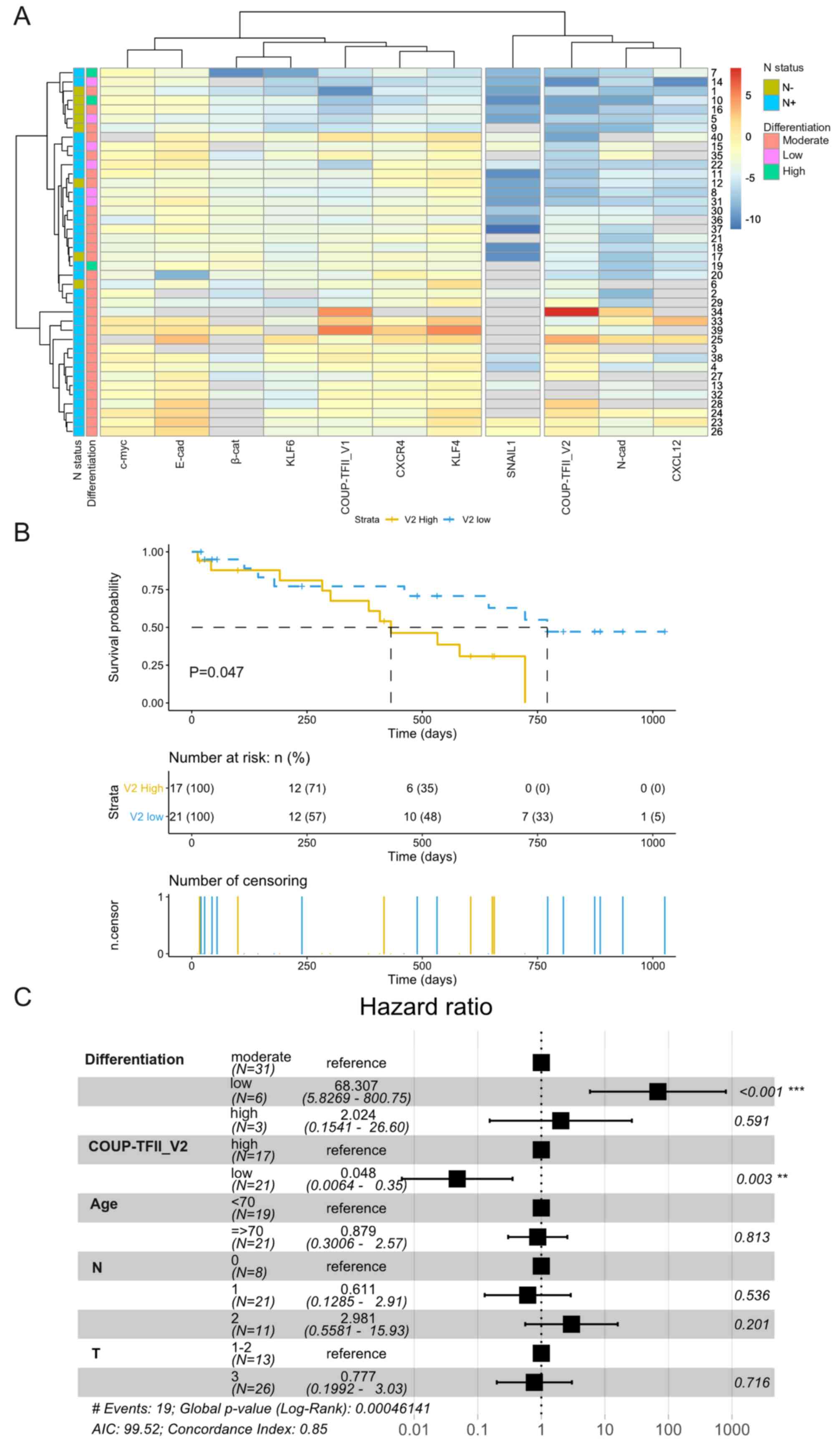
Considering each PDAC patient separately, V1 was associated with
the expression of CXCR4 and KLF4, whereas V2 was associated with
CXCL12 and N-cadherin (Fig. 2A);
similar results were obtained with a correlation analysis of gene
expression (Fig. S4).
Kaplan-Meier survival analysis indicated that patients with high
expression of COUP-TFII_V2 had a significantly shorter overall
survival (432 vs. 771 days, P=0.047) (Fig. 2B) and Cox multivariate analysis
demonstrated that patients with low expression of COUP-TFII_V2 or a
higher degree of differentiation had a lower hazard ratio (Fig. 2C), confirming the results of the
Cox univariate analysis (Table
SV). The influence of clinical parameters on the survival in
the study population is provided in Fig. S5.
COUP-TFII isoforms reciprocally regulate
their expression and cell localization
To determine the role of COUP-TFII_V2, PDAC cell
lines were transiently transfected with this receptor isoform. In
MiaPaca2 and PANC-1, overexpression of COUP-TFII_V2 reduced the
mRNA and protein levels of COUP-TFII_V1, mimicking the effects of
siRNA silencing (Figs. 3A and B
and S6A-E). Of note,
overexpression of COUP-TFII_V1 consistently decreased V2 expression
(Fig. 3B), suggesting a negative
feedback loop between the isoforms. In addition,
Co-immunoprecipitation demonstrated that the variants are
associated (Fig. S6F). Indeed,
COUP-TFII_V2 overexpression delocalized COUP-TFII_V1 in the cytosol
as demonstrated by IF in hTERT-HPNE (Fig. 3C). This result was confirmed by
co-transfection experiments in PANC-1 cells with the
EGFP-COUP-TFII_V1 and V2 isoforms (Fig. S6G). Furthermore, the interaction
of COUP-TFII_V2 with COUP-TFII_V1 reduced the ability of the latter
to bind the DNA, as demonstrated by Gel shift and NHE reporter
activity (Fig. S7).
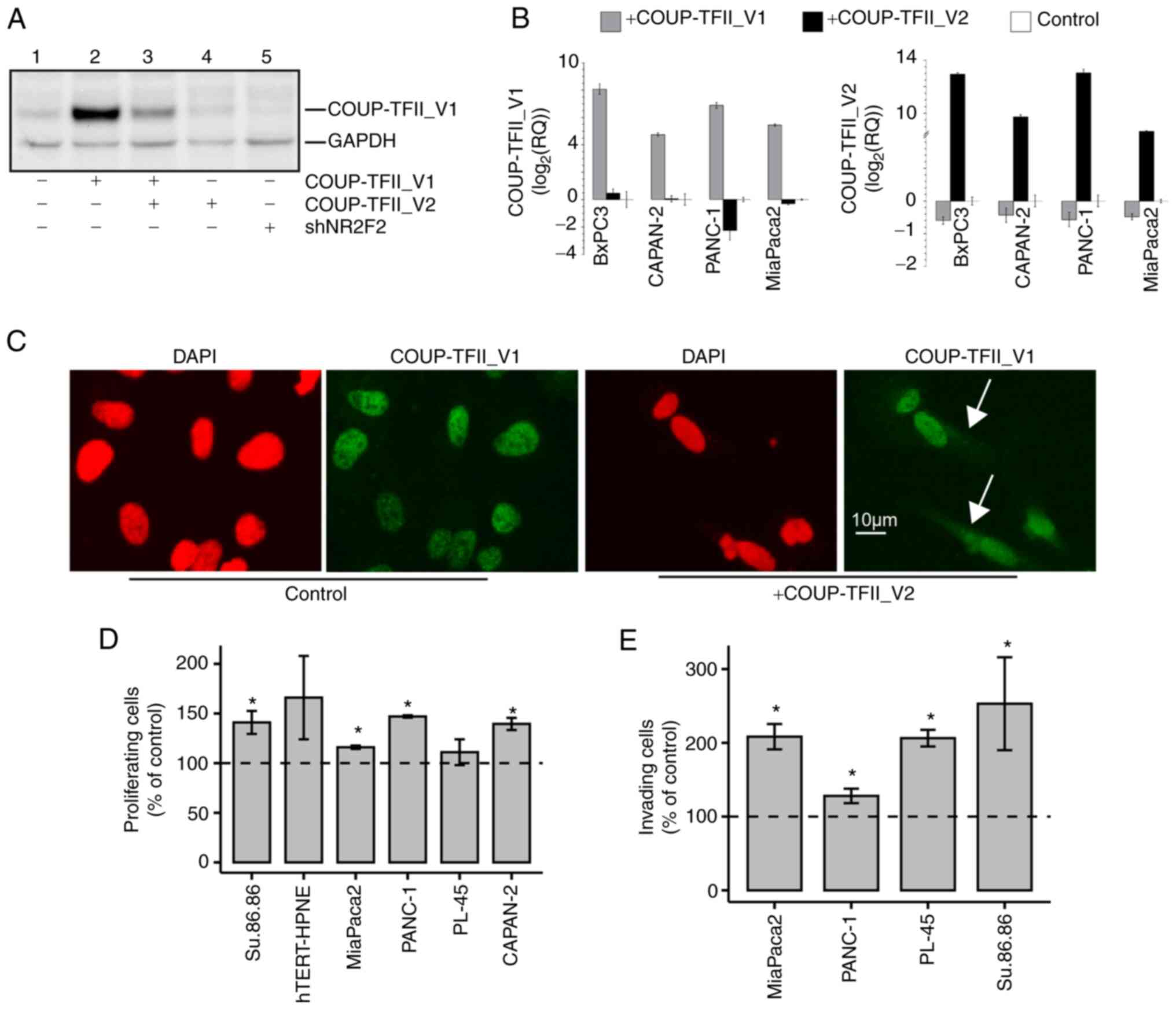 | Figure 3(A) Western blot analysis of PANC-1
cells transiently transfected with a fixed amount of COUP-TFII_V1
plasmid with and without COUP-TFII_V2 co-transfection (lanes 2-3)
or COUP-TFII_V2 alone (lane 4) or a plasmid for COUP-TFII shRNA
(shNR2F2, lane 5). Co-transfection of COUP-TFII_V2 reduced the
expression of COUP-TFII_V1, with an effect similar to that obtained
with the shRNA. Cells in lanes 1-4 were co-transfected with shNEG.
(B) COUP-TFII_V1 (left panel) and COUP-TFII_V2 (right panel)
expression detected by reverse transcription-quantitative PCR in
pancreatic ductal adenocarcinoma cells. Cells were transfected with
COUP-TFII_V1- or COUP-TFII_V2-expressing plasmids for 72 h prior to
RNA extraction. (C) COUP-TFII_V1 immunofluorescence in
COUP-TFII_V2-overexpressing cells. In hTERT-HPNE cells,
COUP-TFII_V2 expression modified the cellular morphology and
COUP-TFII_V1 compartmentalization, causing its cytosolic
localization (scale bar, 10 µm). (D) Cell viability tests
were performed at 48 h (hTERT-HPNE, PANC-1, MiaPaca2, Su.86.86) or
72 h (PL-45 and CAPAN-2) after COUP-TFII_V2 transfection. Data are
normalized to viable cells in pcDNA3.1-transfected cells. (E) The
invasiveness of MiaPaca2, PL-45 and PANC-1 cells was increased
after COUP-TFII_V2 overexpression. Data are normalized to invading
cells in controls. Values are expressed as the mean ± standard
deviation. *P<0.05 vs. control. shRNA, short hairpin
RNA; shNR2F2, shRNA targeting NR2F2 (COUP-TFII); shNEG, negative
control shRNA; NR2F2, nuclear receptor 2 family 2; TF,
transcription factor. |
COUP-TFII-V2 confers drug resistance and
increases anchorage-independent growth and cell motility
COUP-TFII_V2 overexpression had a modest effect on
proliferation in PANC-1, MiaPaca2, CAPAN-2 and Su.86.86 cells;
however, transient COUP-TFII_V2 overexpression increased cell
invasion in all tested lines (Fig. 3D
and E). Consequently, the present study further focused on
PANC-1 cells that express less of the COUP-TFII_V2 isoform and
PANC-1 clones overexpressing COUP-TFII_V1 (PANC-V1) or COUP-TFII_V2
(PANC-V1) isoforms or simply resistant to G418 (MOCK) were
generated. In these models, COUP-TFII-V2 overexpression
significantly limited the effect of chemotherapy (Fig. 4A and B). The IC50 of
gemcitabine for PANC-V2 was higher than that for the control and
V1-expressing cells (IC50: 1, 0.130 and 0.036 µM
for PANC-V2, MOCK and PANC-V1, respectively). The higher
sensitivity of V1-expressing cells to gemcitabine was indirectly
confirmed by western blot analysis, as a greater reduction of
COUP-TFII_V1 compared to COUP-TFII_V2 protein levels was observed
after gemcitabine treatment in cells overexpressing COUP-TFII_V1 or
V2 isoforms, respectively (Fig.
S8A). In addition, PANC-V2 exhibited higher proliferation than
MOCK or PANC-V1 cells and they formed more colonies in soft agar,
suggesting increased anchorage-independent growth and
tumorigenicity (Fig. 4C and D).
The increased chemoresistance and proliferation of V2-expressing
cells was maintained during the 3D growth (Figs. 4E and S8B and C). Time-lapse experiments with
PANC-1 transfected with EGFP-COUP-TFII proteins indicated that only
COUP-TFII_V2 significantly increased cellular motility (0.37±0.02
vs. 0.26±0.017 µm/sec, COUP-TFII_V2 vs. control, P<0.01)
(Fig. 4F). Similar results were
obtained when tracking GFP-positive cells (1/10 of total
transfection) in co-transfection experiments with untagged
COUP-TFII_V1, COUP-TFII_V2 or empty plasmid (data not shown). These
results were further confirmed by the wound-healing assay (Figs. 4G and S8D).
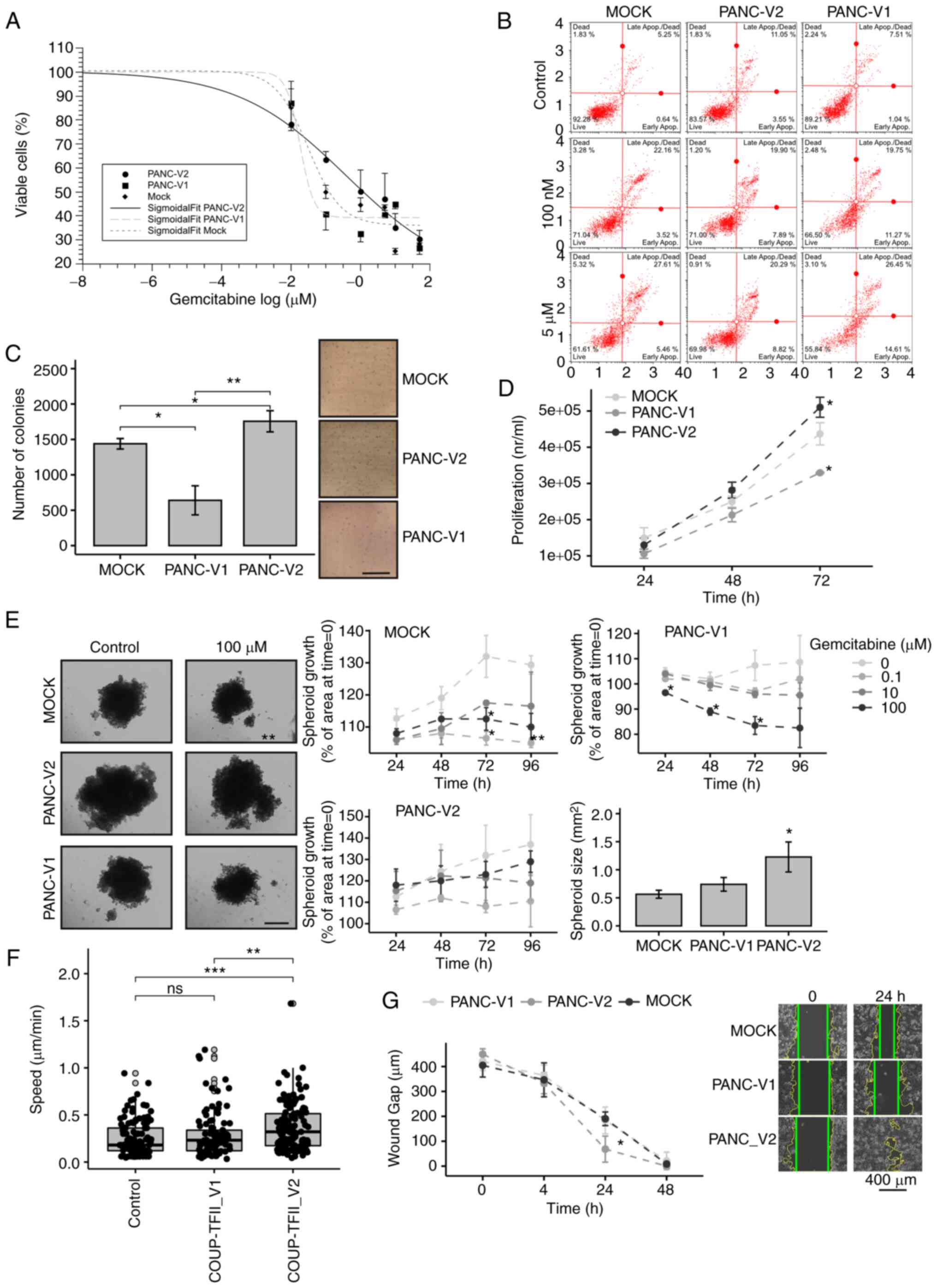 | Figure 4Biological effects of COUP-TFII_V2
expression. (A) MOCK, PANC-V1 and PANC-V2 cells were treated for 48
h with increasing concentrations of gemcitabine. Data are reported
on a log scale and the dose-response curves were fitted with a
Sigmoidal Boltzmann equation. (B) Annexin staining performed on
PANC-1 clones treated for 24 h with gemcitabine indicated a
significant increase in apoptosis of PANC-V1 and MOCK cells. (C)
Colony-formation assay of PANC-1 clones (scale bar, 5 mm). (D)
Conversely to PANC-V1, PANC-V2 cells had a slightly increased
proliferation that became significant at 72 h after plating. (E)
PANC-V2 spheroids were bigger and more gemcitabine-resistant than
their MOCK counterparts (scale bar, 500 mm). (F) Cell motility
(cell velocity, expressed as µm/sec) of cells transfected
with enhanced green fluorescence protein-COUP-TFII_V1 or
-COUP-TFII_V2 plasmids; increased motility of COUP-TFII_V2 cells
compared to GFP (control) and COUP-TFII_V1 transfected cells was
apparent. Gray-filled circles mark the position of outliers. (G)
Wound-healing assay of PANC-1 clones. Photomicrographs are
representative images at 0 and 24 h. Intermediate images are
provided in Fig. S8. Wound
margins were outlined in yellow, whereas the straight green lines
mark the wound edges used for the distance measurement (scale bar,
400 mm). Values are expressed as the mean ± standard deviation.
*P<0.05; **P<0.01;
***P<0.001. ns, no significance; PANC-V1, PANC-1 cell
line overexpressing COUP-TFII_V1; PANC-V2, PANC-1 cell line
overexpressing COUP-TFII_V2; MOCK, PANC-1 cell line resistant to
G418; TF, transcription factor. |
Increased COUP-TFII_V2 expression
facilitates tumor growth and spreading in vivo
To evaluate the different contributions of the two
COUP-TFII isoforms in tumor progression, MOCK, PANC-V1 and PANC-V2
cells were implanted in the pancreas of nude mice (Fig. 5A-D). After 2 weeks, the tumor
engraftment ratio was 4/5, 3/5 and 5/5 for MOCK, PANC-V1 and
PANC-V2 mice, respectively. Of note, 4 out of 5 PANC-V2 xenografts
exhibited liver metastasis compared to just 1 in the PANC-MOCK and
none in the PANC-V1 group; local diffusion to the adjacent tissues,
such as the spleen, was instead present in almost all engrafted
animals and the maximum tumor size observed was 18 mm (data not
shown). Accordingly, the tumor score and tumor weight were
significantly higher for PANC-V2 tumors (P<0.05 vs. PANC-V1 and
MOCK tumor-bearing mice) (Fig.
5B). Immunostaining for PCNA and activated Caspase 3/7 of the
tumors indicated that proliferation and apoptosis were
significantly altered in PANC-V2-bearing mice (Fig. 5C and D). No clear differences in
stromal reaction (as demonstrated by Sirius Red Collagen staining
and quantification) and stromal infiltration were detected
(Fig. S9).
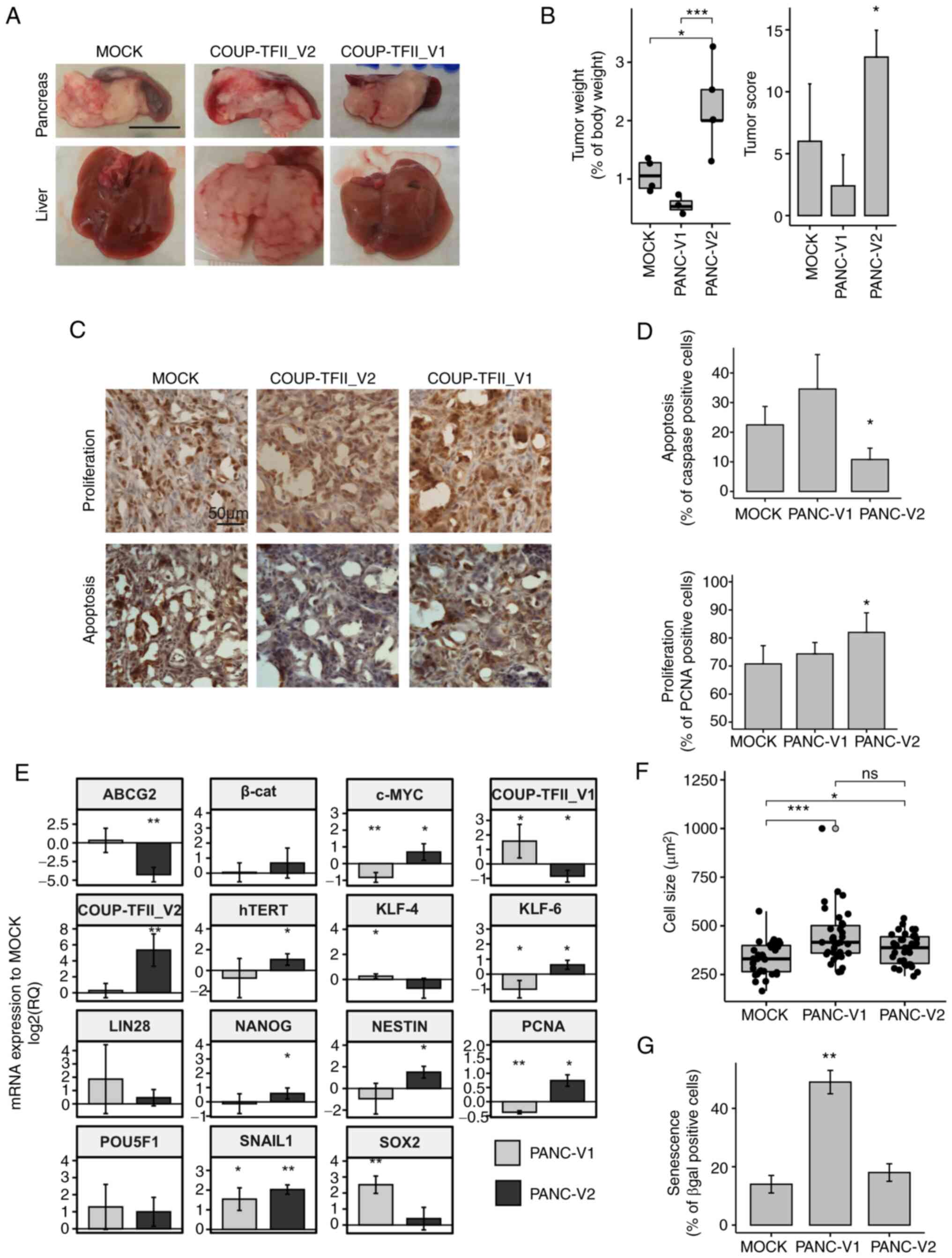 | Figure 5Regulation of gene expression and
in vivo experiments. (A) Representative photomicrographs of
livers and xenograft tumors demonstrating the metastasizing
potential of PANC-V2 cells in the liver (scale bar, 1 cm). (B)
Tumor weight and tumor score. (C) Representative
immunohistochemistry images for PCNA and activated caspase 3/7
(scale bar, 50 µm). (D) Quantitative analysis of
proliferation and apoptosis. (E) Reverse transcription-quantitative
PCR analysis of genes involved in stemness. Values are expressed as
the mean ± standard deviation of at least 3 biological replicates.
(F) Cell size of PANC-V1, PANC-V2 and MOCK cells. Outliers are
indicated by light gray dots. (G) Cellular senescence determined as
the percentage of β-gal positive cells. Values are expressed as
mean ± standard deviation. ns, no significance;
*P<0.05; **P<0.01;
***P<0.001 (vs. MOCK when not indicated). PANC-V1,
PANC-1 cell line overexpressing COUP-TFII_V1; PANC-V2, PANC-1 cell
line overexpressing COUP-TFII_V2; MOCK, PANC-1 cell line resistant
to G418; TF, transcription factor; β-cat, β-catenin; PCNA,
proliferating cell nuclear antigen; KLF4, Kruppel-like factor 4;
hTERT, human telomerase reverse transcriptase; ABCG2, ATP binding
cassette Subfamily G member 2. |
COUP-TFII_V2 influences stemness and
senescence
In consideration of the role of COUP-TFII in the
stemness and cell fate determination (29), it was assessed whether
COUP-TFII_V2 affects cell stemness. COUP-TFII_V2 specifically
increased the expression of NANOG, Nestin, c-Myc and human
telomerase reverse transcriptase, genes that are downregulated or
not altered in V1 (Fig. 5E). On
the other hand, COUP-TFII_V1 is associated with increased SOX2 and
KLF4 and decreased expression of KLF6, which are associated with a
higher tumor potential in PDAC (30-33). Similar results were obtained in
transiently transfected BxPC3, MiaPaca2, CAPAN-2 and PANC-1 cells
and in cells growing in 3D (Fig.
S10A and B). These differences were associated with
significantly increased senescence and cell size of
COUP-TFII_V1-expressing cells (Figs.
5F and G and S10C).
Proteome of COUP-TFII-expressing
cells
To better characterize the function of COUP-TFII
isoforms, an exploratory proteomic analysis was performed on
PANC-V2 and PANC-V1 against the MOCK background. According to DIGE
proteomics, 175 protein spots were altered in PANC-V1 and 147 in
PANC-V2, compared to the MOCK cells. GO analysis (Figs. 6A and S11, Tables SVI and SVII) suggested that for
COUP-TFII_V2, the GO terms are linked to regulation of cell
metabolism, cytoskeleton and cell cycle/apoptosis. On the contrary,
COUP-TFII_V1-specific GO terms point to small GTPase, response to
stress and stimuli [e.g., TNF, interleukins (ILs), Hedgehog (HH)
and Heatshock protein (HSP)], secretion of angiogenic factors (such
as VEGF), cell-to-cell interactions and IL signaling (Tables SVIII and SIX). Reactome analysis
confirmed the GO ontologies suggesting that COUP-TFII_V2 is
associated with glycolysis, apoptotic cleavage of cellular proteins
and cytoskeleton, whereas COUP-TFII_V1 is associated with response
to stress, formation of cell-to-cell junction, Rho activity and HH
signaling (Tables SVII and
SIX).
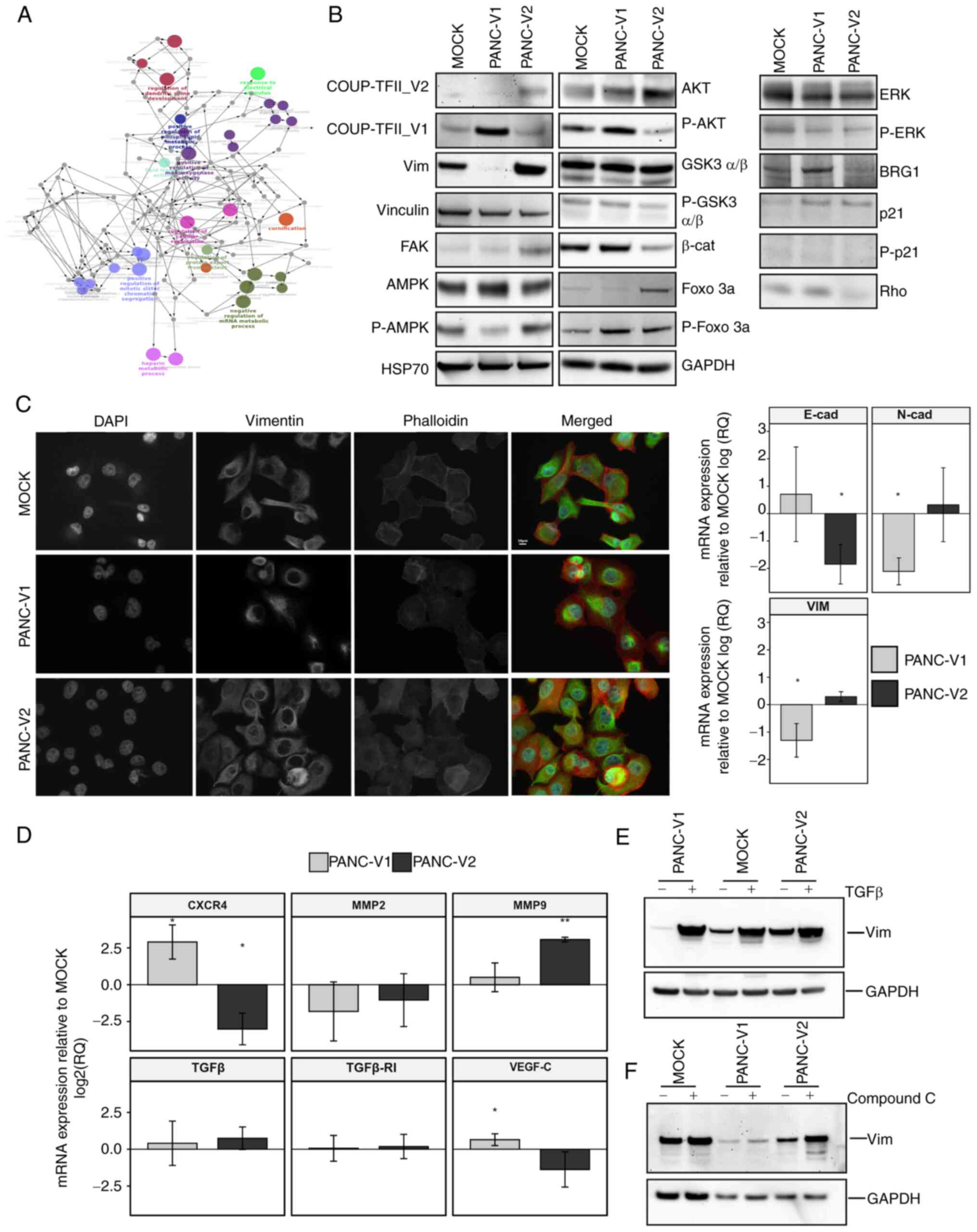 | Figure 6Signaling, gene expression and
response to stimuli. (A) Network of Gene Ontology terms from ClueGO
analysis of PANC-V2 cells compared to MOCK. A magnified version of
this panel is reported as Fig.
S15. (B) Representative western blot of PANC-V1, PANC-V2 and
MOCK confluent cells. (C) Immunofluorescence analysis of vimentin
and actin intermediate filaments and RT-qPCR for EMT markers
N-cadherin, E-cadherin and vimentin. RT-qPCR data were normalized
to the MOCK group. Scale bar, 10 µm. (D) RT-qPCR analysis of
genes linked to cell homing and modification of the
microenvironment. (E) Induction of EMT mediated by TGFβ in PANC-V1,
PANC-V2 and MOCK groups. (F) Representative western blot of
vimentin regulation after treatment with the AMPK inhibitor
Compound C. Values are expressed as the mean ± standard deviation.
*P<0.05; **P<0.01 (vs. MOCK). PANC-V1,
PANC-1 cell line overexpressing COUP-TFII_V1; PANC-V2, PANC-1 cell
line overexpressing COUP-TFII_V2; MOCK, PANC-1 cell line resistant
to G418; TF, transcription factor; RT-qPCR, reverse
transcription-quantitative PCR; EMT, epithelial to mesenchymal
transition; E-cad, E-cadherin; vim, vimentin; β-cat, β-catenin;
FOXO 3a forkhead box O3a; GSK3, glycogen synthase kinase 3; FAK,
focal adhesion kinase. |
COUP-TFII_V2 regulates EMT
Proteomic data, alongside the present in
vitro results, suggested that COUP-TFII_V2 may be implicated in
the regulation of EMT. In agreement with this, western blot
analysis indicated increased vimentin expression in PANC-V2 cells
(Fig. 6B). Furthermore, IF
suggested that COUP-TFII_V2 expression, compared to COUP-TFII_V1,
resulted in well-organized actin intermediate filaments and
decreased expression of the epithelial marker E-cadherin.
Conversely, the mesenchymal markers N-cadherin and vimentin were
decreased in PANC-V1 (Fig. 6C).
However, TGFβ and TGFβ-receptor I, considered to be associated with
EMT, were not altered in cells overexpressing either of the two
isoforms (Fig. 6D). Unexpectedly,
PANC-V1 cells strongly responded to TGFβ and had higher levels of
SMAD2/3P, as suggested by IF (Figs.
6E and S12A and B).
Conversely, MOCK and PANC-V2 exhibited only a modest increase in
vimentin and a slight alteration of cell shape after TGFβ treatment
(Figs. 6E and S12A). Of note, VEGF-C, MMP9 and the
cell-homing receptor CXCR4 were differentially expressed between
PANC-V1 and PANC-V2 (Fig. 6D). In
accordance with the alteration of the cytoskeleton and EMT, a
reduction of β-catenin in PANC-V2 was observed (Fig. 6B); furthermore, the IF experiments
pointed to a higher nuclear localization of β-catenin in PANC-V1
cells (Fig. S12C). The reduced
protein levels of β-catenin in PANC-V2 may be explained by the
decreased phosphorylation of AKT and the ensuing reduction of the
inhibitory phosphorylation of its downstream target glycogen
synthase kinase (GSK)3β; In addition, FOXO3a phosphorylation was
also increased in PANC-V1, confirming the higher AKT activity in
these cells (Figs. 6B and
S13A). Similar results were
obtained following transient transfection of the MiaPaca2, PL-45
and CAPAN-2 cell lines (Fig.
S13B).
COUP-TFII isoforms differentially
influence cell metabolism via AMPK pathway
In accordance with the isoform-dependent effect on
cell metabolism emerged from the proteomic analysis, a reduction of
AMPK phosphorylation was observed in COUP-TFII_V1-expressing cells.
Using the mitochondrial potential as a surrogate of the cells'
energetic condition, as reported in Fig. S13C, it was observed that PANC-V2
mitochondria were more depolarized compared to PANC-V1, a result
that agrees with AMPK expression. Different PANC-1 clones treated
with the AMPK inhibitor compound C exhibited an isoform-dependent
increase of vimentin expression and induction of a mesenchymal-like
phenotype (Figs. 6F and S12A), suggesting that AMPK-mediated EMT
depends on the expression of COUP-TFII isoforms. In agreement with
AMPK expression, Rho A, which is negatively regulated by AMPK, is
decreased by COUP-TFII_V2 and increased by COUP-TFII_V1 (Fig. 6B). Of note, expression of RhoA is
associated with cytoskeleton remodeling and inhibition of AMPK
(34,35). Furthermore, proteomic data point
to a modification of HH signaling after the expression of COUP-TFII
receptors. It was previously indicated that HH may stimulate Rho A
GTPase and Gli transcription factor activity is downregulated by
AMPK (36,37). Confirming these data, Gli activity
was increased in PANC-V1 cells (Fig.
S13D); in addition, LiCl (an inhibitor of GSK3β and HH)
increased apoptosis in PANC-V1 but not PANC-V2 cells, suggesting
that V2-expressing cells do not depend on HH for survival (Fig. S13E).
The function of V2 is linked to its
cellular localization
COUP-TFII_V2 localization was observed to be
associated with a modification of cell shape and acquisition of EMT
characteristics (Fig. 7). After
COUP-TFII_V2-EGFP transfection, MiaPaca2 and PANC-1 EGFP-positive
cells exhibited differences in cell shape linked to the level of
nuclear/cytoplasmic expression of COUP-TFII_V2 (Figs. 7A and B and S14). Accordingly, cells with mostly
cytosolic COUP-TFII_V2 exhibited a pronounced mesenchymal-like
phenotype (e.g., well-organized cytoskeleton or cell protrusion),
while this was reduced in cells with nuclear COUP-TFII_V2,
suggesting that its nuclear exclusion may potentiate EMT. To
validate the hypothesis that nuclear COUP-TFII_V2 limits EMT,
COUP-TFII_V2 was modified by adding an NLS to obtain the
COUP-TFII_V2NLS. As indicated in Figs. 7C-F and S14, COUP-TFII_V2NLS reduced cell
proliferation, cell invasion, motility and drug resistance,
compared to wild-type COUP-TFII_V2-transfected cells. Of note,
unlike COUP-TFII_V2, COUP-TFII_V2NLS failed to reduce the
expression of COUP-TFII_V1.
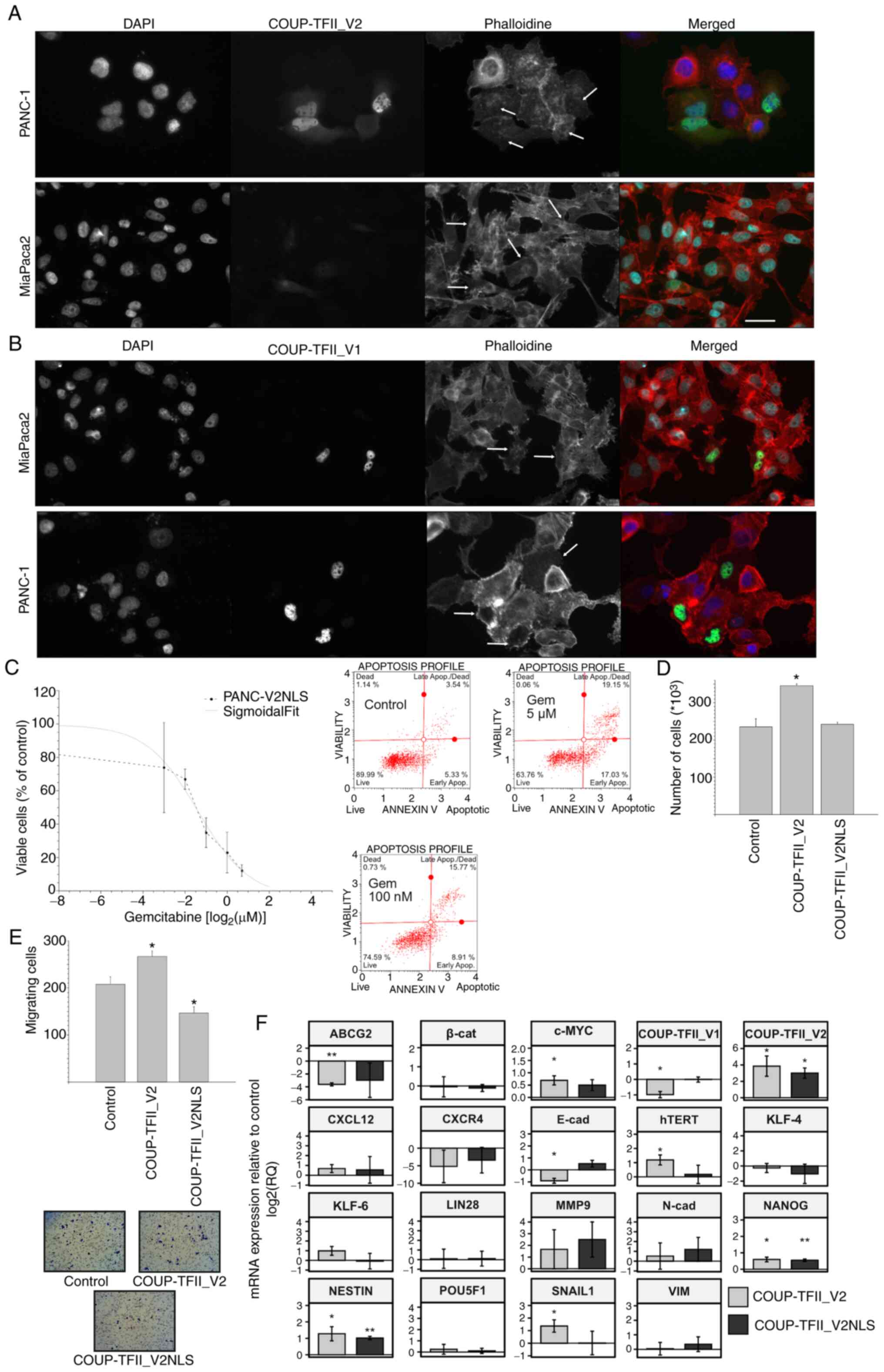 | Figure 7Nuclear localization of COUP-TFII_V2.
(A and B) Immunofluorescence images for β-actin in PANC-1 and
MiaPaca2 cells transfected with (A) COUP-TFII_V2-EGFP or (B)
COUP-TFII_V1-EGFP. Arrows point to cells with either more nuclear
or more cytoplasmic COUP-TII_V2 and of different phenotypes, more
visible in MiaPaca2 cells. Cells expressing COUP-TFII_V1-EGFP are
usually flat (scale bar, 10 µm). (C) Response of PANC-V2NLS
to gemcitabine indicates an IC50 similar to that of MOCK cells
(upper panel). The lower panel is a representative annexin plot of
PANC-V2NLS cells treated with gemcitabine as presented in Fig. 4B. (D) COUP-TFII_V2NLS had no
significant effect on cell proliferation. (E) Chemo-invasion of
PANC-1 cells transfected with COUP-TFII_V2 or COUP-TFII_V2NLS,
indicating that nuclear V2 reduces the chemoinvasiveness of PDAC
cells (original magnification, ×100). (F) Reverse
transcription-quantitative PCR was used to assess gene expression
in PANC-1 transfected with COUP-TFII_V2 or COUP-TFII_V2NLS for 48
h. Values are expressed as mean ± standard deviation.
*P<0.05; **P<0.01 (vs. Control). MOCK,
PANC-1 cell line resistant to G418; TF, transcription factor;
PANC-V2NLS, PANC-1 cell line overexpressing a COUP-TFII_V2 with an
exogenous nuclear localization signal; EGFP, enhanced green
fluorescence protein. VIM, vimentin; N-cad, N-cadherin; β-cat,
β-catenin; hTERT, human telomerase reverse transcriptase; ABCG2,
ATP binding cassette subfamily G member 2; COUP-TFII_V2NLS,
COUP-TFII_V2 with added nuclear localization sequence. |
Discussion
PDAC is associated with the accumulation of
mutations, epigenetic modifications and alterations of cellular
pathways. Although K-RAS is the most frequently altered gene,
several studies have indicated that nuclear receptors are widely
implicated in cancer development. Nuclear receptors may regulate
processes ranging from metastatization to the modulation of the
tumor microenvironment (38) with
significant effects on tumor progression. COUP-TFII is a nuclear
receptor that may act as tumor suppressor or oncogene, depending on
the cellular context (15,16).
A previous study by our group demonstrated that COUP-TFII, in the
context of PDAC, acts as an oncogene (18). In the last decade, a DBD-lacking
isoform of COUP-TFII was described, but its mechanisms of action
have remained elusive and were not previously evaluated in cancer
(10,20). The present study indicated that
both COUP-TFII isoforms are predictive of PDAC development but the
results point to different actions of the receptors, suggesting a
complex regulation. The present results indicated that COUP-TFII_V2
was expressed in primary pancreatic cancers and in cell lines and
comparatively high expression was associated with an increased risk
of death and development of metastasis. In addition, COUP-TFII_V2
expression was associated with increased tumor growth and spreading
in nude mice. According to the association between COUP-TFII_V2 and
advanced tumor stages, the hierarchical clustering in cell lines
and patients links the receptor to EMT. Acquisition of a
mesenchymal-like phenotype has a central role in the dissemination
process of cancer cells and EMT-regulated mechanisms function as
prognostic predictors in patients with PDAC (39). COUP-TFII_V2-induced EMT is
probably mediated by the AKT and GSK3 axis, which may be
consequential to PPARγ transcriptional control (40), and it is associated with
alterations of β-catenin, E-cadherin-to-N-cadherin switch and with
the modulation of HH pathways. Of note, it appears that, to exert
its effect on cadherin expression, V2 requires the presence of
mutated K-RAS, as suggested by the opposite regulation of cadherins
in K-RAS wild-type BxPC3 cells compared to CAPAN-2 cells, that are
K-RAS mutated. Phosphorylation by AKT has inhibitory effects on
FOXO3a and GSK3β (41,42), and accordingly, the reduced
phosphorylation of FOXO3a in V2 cells may explain the increased
proliferation and the increase in the mRNA of the FOXO3a-downstream
target c-myc. GSK3 belongs to the APC complex that degrades
β-catenin (43) and when GSK3β is
phosphorylated, the proteasome degradation of β-catenin is reduced
(44). Accordingly, β-catenin is
decreased in V2-expressing cells, whereas in PANC-V1 cells that
exhibit higher phosphorylation of AKT, β-catenin expression is
increased. AMPK is a major sensor of the cellular metabolic
condition and it may reverse or induce EMT (45). In PANC-V1 cells that exhibit signs
of decreased EMT compared to MOCK and PANC-V2 cells, P-AMPKTyr172
was decreased; however, inhibition of AMPK induced an increase in
vimentin and it changed the cells' morphology. These results
suggest a putative inhibitory role of AMPK on EMT, a hypothesis
that is also supported by the higher expression of Rho A in PANC-V1
cells. Increased expression of Rho A is associated with relaxation
of the contractile fibers and with the switch of cell motility from
a mesenchymal to an ameboid-like movement (34,35). In this context, it may be
speculated that cancer cells may switch the movement behavior quite
rapidly in response to extracellular stimuli regulating the
COUP-TFII_V2 or V1 expression or their cellular location.
Furthermore, the reduced phosphorylation of AMPK, coupled with Rho
A expression and higher mitochondrial polarization, suggest that
V1-expressing cells may produce ATP more efficiently than V2. Of
note, the higher V2 mitochondrial stress may lead to a higher
apoptosis rate but it may also be associated with the production of
reactive oxygen species that favors the K-RAS-mediated
tumorigenesis (46). Taken
together, mitochondrial polarization, AMPK and HH activation, which
is linked to metabolic rewiring (47), may suggest that the nuclear
receptor may be linked with the Warburg effect, but further
experiments will be required to confirm this hypothesis. Given that
PANC-V1 cells proliferate at a slower pace but do not exhibit any
reduction of tumor growth in vivo, it may be assumed that
COUP-TFII_V1 is responsible for local growth and regulates the
tumor micro-environment, whereas COUP-TFII_V2 facilitates tumor
spreading. Indeed, expression of COUP-TFII_V1 is associated with
increased senescence paralleled by increased secretion of VEGF-C
and alteration of IL and chemokine expressions, in line with a
secretive-senescence phenotype that facilitates local tumor
progression (48). The idea of
different roles of the isoforms is strengthened by the existence of
a regulatory feedback expression and by the COUP-TFII_V2-mediated
regulation of the COUP-TFII_V1 transcriptional activity. Of note,
COUP-TFII_V2 also influences COUP-TFII_V1 nuclear-specific
localization. Of note, COUP-TFII_V2, which is clearly nuclear and
cytosolic, exhibits a distinctive pattern of expression not
previously described for any NR2F. How COUP-TFII_V2 shuttles
between the two cell compartments is currently elusive, but its
localization has clear effects on the cell phenotype. Confining the
receptor to the nucleus reduces its ability to induce EMT and
influences its activity on cell signaling. Indeed, the exclusive
nuclear localization of COUP-TFII_V2 alters both COUP-TFII_V1
transcription and the consequent expression of stemness genes. It
is conceivable that pancreatic tumor cells may respond to different
microenvironmental conditions regulating COUP-TFII isoform
expression in order to facilitate tumor progression. In this
context, it may be speculated that the cells during the initial
cancer growth may primarily express COUP-TFII_V1 to create a
permissive microenvironment promoting cell-cell interaction,
neo-angiogenesis (5) and
regulation of immune response, then switching to a higher
expression of COUP-TFII_V2, essentially for EMT and tumor
spreading. Accordingly, the increase of MMP-9 mRNA after
COUP-TFII_V2 overexpression may explain the higher invasiveness of
tumor cells. Indeed, this gelatinase, alongside MMP-2, is a major
factor in extracellular matrix degradation and it has been linked
to the progression of PDAC and several other tumor types. In PDAC,
MMP-9 is abnormally overexpressed but its association with survival
is limited. Furthermore, in experimental models, promising results
have been obtained with MMP-2 and MMP-9 inhibitors (49,50), indicating that these gelatinases
are involved in PDAC progression. However, in the model of the
present study, no alteration of collagen deposition was observed,
as may have been expected from an increase in MMP-9; this
discrepancy indicates that MMP-9 alone is not sufficient to explain
the increased invasiveness of COUP-TFII_V2-overexpressing cells,
given that systemic downregulation of MMP-9 may trigger metastasis
in PDAC (51). Of note, nuclear
receptor activities are not always mutually exclusive, and a
certain degree of overlapped functions is demonstrated by similar
effects on specific genes. Although a direct binding of
COUP-TFII_V2 to DNA may be clearly excluded, a plausible
explanation of similar effects is that COUP-TFII_V2 may act as bait
for repressors in the context of specific genes, hence increasing
the activity of COUP-TFII_V1 or facilitate the action of completely
different transcription factors that remain unidentified (17,29). Of note, the first exon of
COUP-TFII_V2 is located upstream of the known COUP-TFII_V1 promoter
(9) and another putative
functional promoter exists upstream of the first exon of
COUP-TFII_V2. This gene organization suggests that COUP-TFII_V2 may
not be an isoform of COUP-TFII but a completely new gene.
The present study has certain limitations. First of
all, the patients were recruited only in one centre, which limited
the population size, and they all underwent adjuvant therapy with
gemcitabine; consequently, no information was provided on the
effect of COUP-TFII_V2 on survival in the presence of new
chemotherapeutic regimens, such as Folfirinox. Furthermore, the
in vitro results were obtained almost exclusively under
overexpression conditions, whereas modulation by drugs, currently
unavailable, may have been more similar to the physiological
conditions; besides, it may have provided more useful information
for future clinical applications. Furthermore, although various
results of the present study were validated in numerous cell lines,
AsPC1, which is one of the most aggressive pancreatic cancer cell
lines, was not used; in the future, it may be worthwhile to use
this cell line both in vitro and in vivo as a PDAC
cancer model to enhance the knowledge on the function of
COUP-TFII_V2. Finally, the regulatory factors causing the
expression of either COUP-TFII_V1 or COUP-TFII_V2 were not
identified, which may be pursued in the future.
In conclusion, the present study was the first to
describe the effect of a novel DBD-lacking variant of COUP-TFII
that was able to directly (by mediating the transcriptional
activity) or indirectly (independently from transcriptional
activity) act on pancreatic cancer cells' plasticity. The present
results point to an efficient interaction between the COUP-TFII
isoforms that confers an advantage in terms of PDAC progression and
dissemination. The recent demonstration that COUP-TFII is modulated
by small molecules (52) suggests
that this nuclear receptor system is druggable and potentially a
new therapeutic target for PDAC.
Supplementary Data
Availability of data and materials
Proteomic data are deposited in PRIDE (https://www.ebi.ac.uk/pride/; no. PXD 030264). Other
data are available from the corresponding author upon reasonable
request.
Authors' contributions
SiP created the cell models, performed most of the
in vitro experiments and WB and wrote the manuscript. SaP
created the V2NLS construct, supported in experiments with V2NLS
and performed the in vivo experiments together with MT. ST
contributed by performing part of the main and preliminary
experiments. EC and GM performed the proteomics analysis. IS
performed part of the in vitro experiments and reviewed the
manuscript. EG and LA provided the statistical analysis for primary
samples. LB enrolled the patients and collected the primary
samples. TM processed the primary samples, performed the RT-qPCR
analysis and reviewed the manuscript. GD and CG processed the
primary samples and performed the RT-qPCR analysis. LP performed
part of the RT-qPCR analysis of in vitro experiments. SM
contributed to analyzing the data, critically reviewed the results
and corrected the main manuscript. AG defined the general goal of
the research, supervised the project, enrolled the patients,
reviewed the results and wrote the manuscript. All authors read and
approved the final manuscript. SM and AG checked and approved the
authenticity of the raw data.
Ethics approval and consent to
participate
Tissue and data collections and analysis were
approved by, performed following and in agreement with the criteria
of the Ethical Committee of the Azienda Ospedaliero-Universitaria
di Careggi (Florence, Italy; no. 0028114). All experiments on
animal models were performed following the guidance of the use of
laboratory animals and approved by the appointed authority under
Italian law (Ministry of Health, Rome, Italy; no. 853/2015-PR).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank Professor M. Vasseur-Cognet
[INSERM, U1016; Department of Endocrinology, Metabolism and Cancer,
Cochin Institute, CNRS (UMR 8104), Paris, France] for the kind gift
of the COUP-TFII_V1 plasmid and Dr H. Sasaki (Laboratory of
Embryonic Induction, RIKEN Center for Development Biology, Kobe,
Japan) for the Gli reporter plasmid.
Funding
This work was supported by Fondo per gli Investimenti della
Ricerca di Base (grant no. RBAP 10MY35_002), by Fondazione CR di
Firenze (grant no. 2013.0673) and by FiorGen ONLUS to AG. AG is an
Investigator Grant recipient of the 'AIRC Foundation for Cancer
Research' (grant no. IG 2017-20590).
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polvani S, Tarocchi M, Tempesti S, Bencini
L and Galli A: Peroxisome proliferator activated receptors at the
crossroad of obesity, diabetes, and pancreatic cancer. World J
Gastroenterol. 22:2441–2459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guerra F, Guaragnella N, Arbini AA, Bucci
C, Giannattasio S and Moro L: Mitochondrial dysfunction: A novel
potential driver of epithelial-to-mesenchymal transition in cancer.
Front Oncol. 7:2952017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ceni E, Mello T, Polvani S, Vasseur-Cognet
M, Tarocchi M, Tempesti S, Cavalieri D, Beltrame L, Marroncini G,
Pinzani M, et al: The orphan nuclear receptor COUP-TFII coordinates
hypoxia-independent proangiogenic responses in hepatic stellate
cells. J Hepatol. 66:754–764. 2017. View Article : Google Scholar
|
|
6
|
Chen X, Qin J, Cheng CM, Tsai MJ and Tsai
SY: COUP-TFII is a major regulator of cell cycle and notch
signaling pathways. Mol Endocrinol. 26:1268–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis RB, Curtis CD and Griffin CT: BRG1
promotes COUP-TFII expression and venous specification during
embryonic vascular. Development. 140:1272–1281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naka H, Nakamura S, Shimazaki T and Okano
H: Requirement for COUP-TFI and II in the temporal specification of
neural stem cells in CNS development. Nat Neurosci. 11:1014–1023.
2008. View Article : Google Scholar
|
|
9
|
Okamura M, Kudo H, Wakabayashi K, Tanaka
T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne
TF, et al: COUP-TFII acts downstream of Wnt/beta-catenin signal to
silence PPARgamma gene expression and repress adipogenesis. Proc
Natl Acad Sci USA. 106:5819–5824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosa A and Brivanlou AH: A regulatory
circuitry comprised of miR-302 and the transcription factors OCT4
and NR2F2 regulates human embryonic stem cell differentiation. EMBO
J. 30:237–248. 2011. View Article : Google Scholar :
|
|
11
|
Scroyen I, Bauters D, Vranckx C and Lijnen
HR: The anti-adipogenic potential of COUP-TFII is mediated by
downregulation of the notch target gene hey1. PLoS One.
10:e01456082015. View Article : Google Scholar
|
|
12
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C and Deng A: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boudot A, Kerdivel G, Lecomte S, Flouriot
G, Desille M, Godey F, Leveque J, Tas P, Le Dréan Y and Pakdel F:
COUP-TFI modifies CXCL12 and CXCR4 expression by activating EGF
signaling and stimulates breast cancer cell migration. BMC Cancer.
14:4072014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin J, Wu SP, Creighton CJ, Dai F, Xie X,
Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, et al: COUP-TFII
inhibits TGF-β-induced growth barrier to promote prostate
tumorigenesis. Nature. 493:236–240. 2013. View Article : Google Scholar
|
|
15
|
Qin J, Tsai SY and Tsai MJ: The critical
roles of COUP-TFII in tumor progression and metastasis. Cell
Biosci. 4:582014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu M, Qin J, Tsai SY and Tsai MJ: The role
of the orphan nuclear receptor COUP-TFII in tumorigenesis. Acta
Pharmacol Sin. 36:32–36. 2015. View Article : Google Scholar :
|
|
17
|
Polvani S, Pepe S, Milani S and Galli A:
COUP-TFII in health and disease. Cells. 9:1012019. View Article : Google Scholar
|
|
18
|
Polvani S, Tarocchi M, Tempesti S, Mello
T, Ceni E, Buccoliero F, D'Amico M, Boddi V, Farsi M, Nesi S, et
al: COUP-TFII in pancreatic adenocarcinoma: Clinical implication
for patient survival and tumor progression. Int J Cancer.
134:1648–1658. 2014. View Article : Google Scholar
|
|
19
|
Lin FJ, Qin J, Tang K, Tsai SY and Tsai
MJ: Coup d'Etat: An orphan takes control. Endocr Rev. 32:404–421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamazaki T, Suehiro JI, Miyazaki H, Minami
T, Kodama T, Miyazono K and Watabe T: The COUP-TFII variant lacking
a DNA-binding domain inhibits the activation of the Cyp7a1 promoter
through physical interaction with COUP-TFII. Biochem J.
452:345–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guasti L, Crociani O, Redaelli E, Pillozzi
S, Polvani S, Masselli V, Mello T, Galli A, Amedei A, Wymore RS, et
al: Identification of a posttranslational mechanism for the
regulation of hERG1 K+ channel expression and hERG1
current density in tumor cells. Mol Cell Biol. 28:5043–5060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galli A, Ceni E, Mello T, Polvani S,
Tarocchi M, Buccoliero F, Lisi F, Cioni L, Ottanelli V, Foresta V,
et al: Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis
B virus-transgenic mice by peroxisome proliferator-activated
receptor gamma-independent regulation of nucleophosmin. Hepatology.
52:493–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polvani S, Calamante M, Foresta V, Ceni E,
Mordini A, Quattrone A, D'Amico M, Luchinat C, Bertini I and Galli
A: Acycloguanosyl 5′-thymidyltriphosphate, a thymidine analogue
prodrug activated by telomerase, reduces pancreatic tumor growth in
mice. Gastroenterology. 140:709–720.e9. 2011. View Article : Google Scholar
|
|
25
|
Lassman AB, Roberts-Rapp L, Sokolova I,
Song M, Pestova E, Kular R, Mullen C, Zha Z, Lu X, Gomez E, et al:
Comparison of biomarker assays for EGFR: Implications for precision
medicine in patients with glioblastoma. Clin Cancer Res.
25:3259–3265. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hennig R, Ventura J, Segersvard R, Ward E,
Ding XZ, Rao SM, Jovanovic BD, Iwamura T, Talamonti MS, Bell RH Jr
and Adrian TE: LY293111 improves efficacy of gemcitabine therapy on
pancreatic cancer in a fluorescent orthotopic model in athymic
mice. Neoplasia. 7:417–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Research Council (US) Committee
for the Update of the Guide for the Care and use of Laboratory
Animals Guide for the care and use of laboratory animals. 8th
edition. National Academies Press (US); Washington, DC: 2011,
https://www.ncbi.nlm.nih.gov/books/NBK54050/.
View Article : Google Scholar
|
|
28
|
Fernandez-Rachubinski F and Fliegel L:
COUP-TFI and COUP-TFII regulate expression of the NHE through a
nuclear hormone responsive element with enhancer activity. Eur J
Biochem. 268:620–634. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu SP, Yu CT, Tsai SY and Tsai MJ: Choose
your destiny: Make a cell fate decision with COUP-TFII. J Steroid
Biochem Mol Biol. 157:7–12. 2016. View Article : Google Scholar :
|
|
30
|
Herreros-Villanueva M, Zhang JS, Koenig A,
Abel EV, Smyrk TC, Bamlet WR, de Narvajas AAM, Gomez TS, Simeone
DM, Bujanda L and Billadeau DD: SOX2 promotes dedifferentiation and
imparts stem cell-like features to pancreatic cancer cells.
Oncogenesis. 2:e612013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hartel M, Narla G, Wente MN, Giese NA,
Martignoni ME, Martignetti JA, Friess H and Friedman SL: Increased
alternative splicing of the KLF6 tumour suppressor gene correlates
with prognosis and tumour grade in patients with pancreatic cancer.
Eur J Cancer. 44:1895–1903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ganguly K, Krishn SR, Rachagani S, Jahan
R, Shah A, Nallasamy P, Rauth S, Atri P, Cox JL, Pothuraju R, et
al: Secretory Mucin 5AC promotes neoplastic progression by
augmenting KLF4-mediated pancreatic cancer cell stemness. Cancer
Res. 81:91–102. 2021.
|
|
33
|
Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J,
Zhu Z, Gao Y and Xie K: A novel KLF4/LDHA signaling pathway
regulates aerobic glycolysis in and progression of pancreatic
cancer. Clin Cancer Res. 20:4370–4380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sedgwick AE, Clancy JW, Olivia Balmert M
and D'Souza-Schorey C: Extracellular microvesicles and invadopodia
mediate non-overlapping modes of tumor cell invasion. Sci Rep.
5:147482015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Čermák V, Gandalovičová A, Merta L, Harant
K, Rösel D and Brábek J: High-throughput transcriptomic and
proteomic profiling of mesenchymal-amoeboid transition in 3D
collagen. Sci Data. 7:1602020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polizio AH, Chinchilla P, Chen X, Kim S,
Manning DR and Riobo NA: Heterotrimeric Gi proteins link Hedgehog
signaling to activation of Rho small GTPases to promote fibroblast
migration. J Biol Chem. 286:19589–19596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Magno L, Basile A, Coni S, Manni S,
Sdruscia G, D'Amico D, Antonucci L, Infante P, De Smaele E, Cucchi
D, et al: The energy sensor AMPK regulates hedgehog signaling in
human cells through a unique Gli1 metabolic checkpoint. Oncotarget.
7:9538–9549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Polvani S, Tarocchi M, Tempesti S and
Galli A: Nuclear receptors and pathogenesis of pancreatic cancer.
World J Gastroenterol. 20:12062–12081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sato M, Matsumoto M, Saiki Y, Alam M,
Nishizawa H, Rokugo M, Brydun A, Yamada S, Kaneko MK, Funayama R,
et al: BACH1 promotes pancreatic cancer metastasis by repressing
epithelial genes and enhancing epithelial-mesenchymal transition.
Cancer Res. 80:1279–1292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Polvani S, Tarocchi M and Galli A: PPARγ
and oxidative stress: Con(β) catenating NRF2 and FOXO. PPAR Res.
2012:6410872012. View Article : Google Scholar
|
|
41
|
Lizcano JM and Alessi DR: The insulin
signalling pathway. Curr Biol. 12:R236–R238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Santo EE, Stroeken P, Sluis PV, Koster J,
Versteeg R and Westerhout EM: FOXO3a is a major target of
inactivation by PI3K/AKT signaling in aggressive neuroblastoma.
Cancer Res. 73:2189–2198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F,
Maestro R, et al: Multifaceted roles of GSK-3 and Wnt/β-catenin in
hematopoiesis and leukemogenesis: Opportunities for therapeutic
intervention. Leukemia. 28:15–33. 2014. View Article : Google Scholar
|
|
44
|
Kim JG, Kim MJ, Choi WJ, Moon MY, Kim HJ,
Lee JY, Kim J, Kim SC, Kang SG, Seo GY, et al: Wnt3A induces GSK-3β
Phosphorylation and β-catenin accumulation through RhoA/ROCK. J
Cellu Physiol. 232:1104–1113. 2017. View Article : Google Scholar
|
|
45
|
Chou CC, Lee KH, Lai IL, Wang D, Mo X,
Kulp SK, Shapiro CL and Chen CS: AMPK reverses the mesenchymal
phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a
signaling axis. Cancer Res. 74:4783–4795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lim JK and Leprivier G: The impact of
oncogenic RAS on redox balance and implications for cancer
development. Cell Deat Dis. 10:9552019. View Article : Google Scholar
|
|
47
|
Teperino R, Amann S, Bayer M, McGee SL,
Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G,
et al: Hedgehog partial agonism drives warburg-like metabolism in
muscle and brown fat. Cell. 151:414–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Faget DV, Ren Q and Stewart SA: Unmasking
senescence: Context-dependent effects of SASP in cancer. Nature Rev
Cancer. 19:439–453. 2019. View Article : Google Scholar
|
|
49
|
Awasthi N, Mikels-Vigdal AJ, Stefanutti E,
Schwarz MA, Monahan S, Smith V and Schwarz RE: Therapeutic efficacy
of anti-MMP9 antibody in combination with nab-paclitaxel-based
chemotherapy in pre-clinical models of pancreatic cancer. J Cell
Mol Med. 23:3878–3887. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie J, Zhou X, Wang R, Zhao J, Tang J,
Zhang Q, Du Y and Pang Y: Identification of potential diagnostic
biomarkers in MMPs for pancreatic carcinoma. Medicine (Baltimore).
100:e261352021. View Article : Google Scholar
|
|
51
|
Grünwald B, Vandooren J, Gerg M, Ahomaa K,
Hunger A, Berchtold S, Akbareian S, Schaten S, Knolle P, Edwards
DR, et al: Systemic ablation of MMP-9 triggers invasive growth and
metastasis of pancreatic cancer via deregulation of IL6 expression
in the bone marrow. Mol Cancer Res. 14:1147–1158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Cheng CM, Qin J, Xu M, Kao CY, Shi
J, You E, Gong W, Rosa LP, Chase P, et al: Small-molecule inhibitor
targeting orphan nuclear receptor COUP-TFII for prostate cancer
treatment. Sci Adv. 6:eaaz80312020. View Article : Google Scholar : PubMed/NCBI
|