Introduction
Glioblastoma (GB) is the most common and fatal brain
tumor, as it involves a grave prognosis (1). According to the World Health
Organization (WHO) classification, GB is designated as grade IV
astrocytoma either generated anew (primary GB) or recrudesced from
a residual low-grade tumor (secondary GB) (2-4). GB
is characterized by rapid cell proliferation, infiltrative
migration, and aggressive invasion into adjacent brain parenchyma.
Although existing research has developed progressive surgical
resection accompanied by numerous tryout therapies, the mean
survival time of patients with GB is still <1 year from
diagnosis (5-7).
Neuropilin-1 (NRP-1) is a cell surface co-receptor
of vascular endothelial growth factor (VEGF)-A165 with
fetal liver kinase 1 (Flk-1) in endothelial cells that is mainly
recognized for its function in the development of blood vessels
(8,9). Recent studies have revealed that
NRP-1 is highly expressed in various types of cancers, including
GB, and stimulates malignancy (10-13).
NRP-1 plays a critical role in tumor development by activating
tumor angiogenesis (14,15). It has also been suggested that
NRP-1 can be modified by adding glycosaminoglycan (GAG) chains in
endothelial cells and various cancer cells (13,16).
GAGs are fundamental components of proteoglycans and the
extracellular matrix (ECM) that contribute to the regulation of
cell proliferation, differentiation, and paracrine cell-matrix
interaction, and its chondroitin-sulfated modification or
derestriction is correlated with clinical outcomes in various
malignant tumors (17,18). Although sporadic studies, as
aforementioned, have presented the expression patterns of NRP-1,
the biological significance of NRP-1 expression pattern in
malignant GB remains ambiguous.
In a previous study by the authors it was reported
that various malignant tumors including GB have differing
sensitivities to VEGF-A signaling (19). The aim of the present study was to
further investigate the mechanism responsible for the differential
dependency of GB to VEGF-A signaling and create an effective
strategy for the blockade of GB via silencing of VEGF-A
signaling.
Materials and methods
Overview
To investigate the association between differential
dependency of VEGF-A signaling and NRP-1 expression type in GB
cells, experimental verifications such as in vitro, in
vivo, and patient sample analyses were performed. For the
experimental preparation, a number of GB cell lines and a human
primary astrocyte cell line were used. To elucidate the expression
pattern of NRP-1 and its association with sensitivity to VEGF-A
signaling in GB cells, in vitro and in vivo
experiments were performed such as proliferation assays, si-RNA
transfection against Flt-1, Flk-1, NRP-1 and VEGF-A, sandwich
enzyme-linked immunosorbent assay (ELISA), western blot analysis,
immunoprecipitation, immunohistochemistry, and a xenograft model.
To verify the association between chondroitin-sulfated NRP-1
expression and insensitivity to VEGF-A signaling in GB cells,
chondroitinase ABC (Ch-ABC) was used in vitro and in
vivo. To confirm clinical significance, immunohistochemical
analysis using a tissue microarray study of patients with GB was
designed and the association between chondroitin sulfate expression
and prognosis of patients with GB was verified by a pathologist
blinded to the sample identities.
Gene expression analysis
The microarray expression profiles were obtained
from the public microarray database Gene Expression Omnibus (GEO)
at NCBI (https://www.ncbi.nlm.nih.gov/geo/) in the previously
described manner (20). A total of
four datasets were used including GSE16011 (21), GSE4290 (22), GSE4271 (23), and GSE2727. All data were
normalized by Robust Multichip Average (RMA) method (21). BrainArray probe annotation was
used, and the values of several probes for one gene were averaged
into a value for the gene as previously described (22). The statistical significance of the
differences in each expression was assessed using the Student's
t-test, where unequal variance was assumed if the P-value of
one-way ANOVA was <0.05, and where equal variance was assumed
otherwise.
Cell culture and reagents
U251-MG, U373-MG, CRT-MG, and LN215-MG cells were
kindly provided by Professor Etty N. Benvenite (University of
Alabama, Birmingham, USA) and the human primary astrocytes were
kindly provided by Professor In-Hong Choi (Yonsei University,
Seoul, Korea). The human primary astrocytes were prepared according
to a previously described procedure (23). The human umbilical vein endothelial
cells (HUVECs; CRL-1730) were purchased from ATCC. A172 (KCLB no.
21620), SNU-466 (KCLB no. 00466), SNU-489 (KCLB no. 00489), SNU-626
(KCLB no. 00626), and SNU-1105 (KCLB no. 01105) were obtained from
the Korean Cell Line Bank (KCLB). LN215-MG and U251-MG cells were
grown in DMEM (Gibco-BRL; Thermo Fisher Scientific, Inc.) and A172,
CRT-MG, SNU-466, SNU-489, SNU-626, SNU-1105, and U373-MG cells were
grown in RPMI-1640 medium (Gibco-BRL; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco-BRL; Thermo
Fisher Scientific, Inc.) and penicillin-streptomycin 100 mg/l
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere containing 5 % CO2. HUVECs were
grown in EGM-2 Bulletkit medium (Lonza Group, Ltd.) at 37°C in a
humidified atmosphere containing 5% CO2. All experiments
were performed using HUVECs within 3-7 passages. U373-MG cells were
authenticated using short tandem repeat (STR) analysis by Macrogen,
Inc. Antibodies of Flk-1 (cat. no. sc-6251) and Flt-1 (cat. no.
sc-271789) were obtained from Santa Cruz Biotechnology, Inc.
Phosphorylated (p)-FAK (Y397; cat. no. 8556), FAK (cat. no. 71433),
p-AKT (Ser473; cat. no. 4060), AKT (cat. no. 9272), NRP-1 (cat. no.
3725), caspase-3 (cat. no. 9662), cleaved caspase-3 (cat. no.
9664), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat.
no. 5174) were purchased from Cell Signaling Technology Inc. 4G10
(cat. no. 05-321), anti-phosphotyrosine antibody, and p-Flt-1
(Y1213; #07-758) were obtained from MilliporeSigma. SU1498 (cat.
no. SML1193), a VEGFR inhibitor, and Ch-ABC (cat. no. SAE0150) were
purchased from Sigma-Aldrich; Merck KGaA. Recombinant VEGF-A (cat.
no. 293-VE) was obtained from R&D Systems Inc.
Small interfering RNA (siRNA)
transfection
Transfection of siRNAs was performed using
Effectene® transfection reagent (Qiagen GmbH), in
U251-MG, U373-MG, LN215-MG, and SNU-466 cells as previously
described (24,25). The siRNA oligonucleotides (100
pmole/µl) that encode VEGF-A, Flt-1, Flk-1, NRP-1, and
scrambled control were obtained from Bioneer Corporation (Daejeon,
Korea). The sequences of the siRNAs were as follows: VEGF-A, 5′-AAA
UGU GAA UGC AGA CCA A-3′-dTdT; FLT-1; 5′-GAC UCU CUU CUG GCU CCU
A-3′-dTdT; FLK-1, 5′-CUC CUA AUG AGA GUU CCU U-3′-dTdT; NRP-1,
5′-GUC CGA AUC AAG CCU GCA A-3′-dTdT; and scrambled control, 5′-CCU
ACG CCA AUU UCG U-3′-dTdT. U251-MG, U373-MG, LN215-MG, and SNU-466
cells (1×105 cells/well) were seeded in six-well plates
(Nunc S/A; Nalge Nunc International), and after 18 h, transfection
was performed with 2 µl si-RNAs or scrambled si-RNA and 208
µl Effectene reagent (200 µl of buffer; 3 µl
of enhancer; 5 µl of Effectene) in a final volume of 2,500
µl RPMI-1640 medium containing 10% serum without
antibiotics. The cells were then maintained an additional 72 h at
37°C in a humidified incubator containing 5% CO2. The
efficacy of siRNA transfection was confirmed by western blot
analysis of the corresponding proteins.
Assessment of cell death
To evaluate cell viability for the treatment of
exogenous VEGF-A and SU1498, WST-1 reagent (cat. no. 89-024-504;
Nalgene; Thermo Fisher Scientific, Inc.) was used as previously
described (25). After 30 min of
incubation at room temperature, the absorbance was measured at 450
nm using a microplate reader (Bio-Rad Laboratories, Inc.). To
assess cell death for the combination treatment of SU1498 and
Ch-ABC, lactate dehydrogenase (LDH) assay (cat. no. J2380; Promega
Corporation) was conducted according to the manufacturer's
protocol.
Western blot analysis
To evaluate Flt-1, Flk-1, and NRP-1 expression in
nine GB cell lines and human primary astrocytes, western blotting
was performed as previously described (25). For whole cell lysates, cells were
lysed in M-PER lysis buffer (cat. no. 78501; Thermo Fisher
Scientific, Inc.) containing protease and phosphatase inhibitors.
The total quantity of protein was determined with BCA Protein Assay
kit (cat. no. 23227; Thermo Fisher Scientific, Inc.). Cell lysates
(20 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
nitrocellulose membrane (cat. no. 10600003; Amersham Biosciences;
Cytiva). The membrane was blocked using 5% (w/v) bovine serum
albumin and 0.2% (v/v) Tween-20 in Tris-buffered saline (T-TBS) for
2 h at room temperature and then incubated with diluted antibodies
(1:2,000 specific for NRP-1 or 1:1,000 for Flt-1 and Flk-1),
overnight at 4°C. After each incubation with primary antibodies,
the membranes were washed three times with T-TBS buffer for 10 min
each prior to incubation with 1:5,000 secondary antibody (goat-anti
rabbit IgG-HRP, cat. no. sc-2004 for NRP-1; goat-anti mouse
IgG-HRP, cat. no. sc-2005 for Flt-1 and Flk-1; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. To visualize
bands on an X-ray film, SuperSignal West Pico PLUS Chemiluminescent
Substrate (cat. no. 34580; Thermo Fisher Scientific, Inc.) was
used. Bands were assessed by densitometric analysis using ImageJ
software (ver. 1.46r; National Institutes of Health). To ensure
equal protein loading, GAPDH was used as the reference.
ELISA
The Human VEGF Duoset® ELISA Development
System (cat. no. DY293B; R&D Systems, Inc.) was used with
cultured supernatants collected from approximately 70-80% confluent
GB cells or HUVECs according to the manufacturer's
instructions.
Immunoprecipitation
SNU-466, LN215-MG, U251-MG, and U373-MG cells were
seeded in a 60-mm dish. After 2 days, the cells were lysed in lysis
buffer (1% NP-40, 20 mM Tris-Cl, 137 mM NaCl, 2 mM EDTA, mixtures
of proteinase inhibitor, and phosphatase inhibitor). Next, the
lysate aliquots (1 mg/1 ml lysis buffer) were incubated with
anti-NRP-1 (1:200), anti-Flt-1 (1:200), or normal IgG (1:200)
antibodies, overnight at 4°C, followed by the addition of 20
µl of Pierce Protein A Agarose (cat. no. 20333; Thermo
Scientific, Inc.) for 6 h at 4°C. Bead-bound protein was washed
five times with T-TBS (2 min at 4°C; 2,000 × g). Bound proteins
were boiled with 2X Laemmli sample buffer (cat. no. 1610737;
Bio-Rad Laboratories, Inc.) for 10 min and then subjected to
SDS-PAGE, and assessed using western blotting.
Migration assay
Migration assays were performed using 24-Transwell
plates (Corning Costar, Inc.) according to a previously described
method (26). Briefly, SNU-466 and
LN215-MG cells (5×105 cells/well) were seeded in
six-well plates (Nunc A/S; Nalge Nunc International). After 24 h,
the cells were starved under 0.2% serum containing RPMI-1640 medium
for 12 h. Detached SNU-466 and LN215-MG cells (1×105
cells/1 ml 0.2% serum containing RPMI-1640 medium) were exposed
with VEGF-A or Ch-ABC for 4 h. Polycarbonate filters were
pre-coated with 10 mg/l fibronectin (cat. no. F0895; Sigma-Aldrich;
Merck KGaA) in phosphate-buffered saline (PBS) for 1 h at room
temperature. The lower chamber was filled with 500 µl of 10%
fetal bovine serum containing RPMI-1640 medium. VEGF-A- or
Ch-ABC-treated cells (2×104 cells/200 µl) were
applied to the upper chamber for 6 h at 37°C, and then the cells on
the upper surface of the filter were removed with a cotton swab.
The filters were fixed with 4% paraformaldehyde (cat. no.
sc-281692; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature and stained with 1% crystal violet solution (cat. no.
V5265; Sigma-Aldrich; Merck KGaA) for 6 h at room temperature. The
absorbance of the eluted dye using 10% (v/v) acetic acid (cat. no.
135-16; Thermo Fisher Scientific, Inc.) was measured at 560 nm
using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad
Laboratories, Inc.).
Preparation and interpretation of tissue
microarray (TMA) of patients with GB
Tissue samples of patients were preserved in
paraffin blocks after surgery. Pathologic diagnosis was performed
with samples from 19 patients (12 males and 7 females) who had been
diagnosed with GB. The samples were acquired from August 1st 2017
to July 31st 2021 at Korea University Guro Hospital (Seoul,
Republic of Korea). The inclusion criteria was a sample of patients
who underwent surgery, and samples of children under 18 years of
age or elderly patients over 75 years of age were excluded. The
mean age of the patient group was 61.4±12.3 years, and the median
age was 64 years. TMA slides consisting of 57 total tissue samples
from 2 to 3 different tumor sites per patient were prepared as
previously described (27). After
immunostaining, a pathologist provided readings on the level and
presence of VEGF-A and NRP-1 through a blind review. The staining
intensity was scored from none or '1+' (very weak positive) to '4+'
(very strong positive). This study received ethical approval (IRB
no. 2017GR330) from the Institutional Review Board of Korea
University Guro Hospital (Seoul, Korea), including molecular
characterization and related prognosis analysis, and written
consent was obtained from patients prior to sample collection.
Xenograft tumor model
Animal care and handling procedures were performed
in accordance with the Animal Research Committee Guidelines of
KAIST and Korea University College of Medicine. All animal
experiments were approved by the Institutional Animal Care and Use
Committee of the KAIST (IACUC No. KA2013-13) and the Korea
University College of Medicine (IACUC No. KOREA-2019-0123). A total
of 36 female BALB/c nude mice (aged 4-5 weeks; weighing 20-25 g)
were obtained from Orient Bio, Inc. All mice were raised in an
animal room in a laboratory animal resource center throughout the
experiments under the following conditions: Controlled humidity,
50-60%; temperature, 25°C; 12-h light/dark cycle and were provided
with free access to food and water. The mice in all groups were
intraperitoneally injected with 1×107 LN215-MG cells or
3×107 SNU-466 cells. The condition and behavior of the
nude mice was monitored every 2 days. The mice would be humanely
euthanized if they experienced unrelieved pain or distress, based
on the euthanasia criteria. The groups were as follows: Group 1,
PBS-treated mice (Mock, n=4); group 2, mice treated with Ch-ABC
alone (n=4); group 3, mice treated with SU1498 alone (SU1498, n=4);
and group 4, mice treated Ch-ABC with SU1498 (Ch-ABC/SU1498, n=6).
The tumor volume and body weight were monitored throughout the
study period. In all experiments, tumor dimensions were measured
using calipers, and the tumor volume was calculated using the
following formula: V=0.523 LW2 (L=length and W=width).
When the tumors reached an average size of approximately 200
mm3 in LN215-MG xenograft models or of approximately 100
mm3 in SNU-466 xenograft models, the mice were
intraperitoneally injected with Ch-ABC (10 mU/ml), SU1498 (10
mg/kg), or combined Ch-ABC (10 mU/ml)/SU1498 (10 mg/kg) every 3
days for 2 weeks (from day post injection, DPI 30 to DPI 42) in
established mice care conditions. If the tumor volume reached 400
mm3, sacrifice of the mice was planned, however none of
the tumors of the mice in the present experiment reached that size
during the experiment. The method of euthanasia used for the mice
was CO2 asphyxiation followed by cervical dislocation
(CO2 was introduced into the chamber at a rate of 30-70%
of the chamber volume per min to minimize distress). After
euthanizing the mice, all tumors were harvested for western blot
analysis.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). A two-tailed unpaired Student's t-test and Mann-Whitney test
were used to determine the levels of significance for comparisons
between two independent samples. Multiple group comparisons were
performed using one-way analysis of variance (ANOVA) with Tukey's
post hoc test, and patient survival and hazard ratio were analyzed
with the log-rank (Mantel-Cox) test and the Mantel-Haenszel method.
All data were analyzed using SPSS 12.0K for Windows (SPSS, Inc.)
and GraphPad Prism 7 software (GraphPad Software, Inc.). P-values
<0.05 were considered to indicate statistically significant
differences.
Results
Expression of VEGF-A and its receptors in
GB cells
In previous studies by the authors it was reported
that GB cells have different sensitivities to VEGF-A signaling and
that silencing VEGF-A signaling can specifically induce a
significant anticancer effect in VEGF-A signaling-sensitive cells
through inhibition of the autocrine signaling pathway (19,26).
To further evaluate the underlying molecular mechanisms of these
notable features, a sufficient number of GB cells were used.
Analyzing the microarray data from the public database GEO yielded
that the mRNA levels of VEGF-A, Flk-1, and
NRP-1, but not of Flt-1, were significantly higher in
GB tissues than in normal brain (Fig.
1A). In particular, the mRNA levels of NRP-1, VEGF-A, and Flk-1
were significantly associated with tumor grade, by contrast, the
mRNA level of Flt-1 was not associated with tumor grade (Figs. 1B and S1). To verify the clinical significance
between VEGF-A and NRP-1 expression, a TMA analysis of patients
with GB was performed (Fig. 1C).
The expression level of VEGF-A in samples from patients with GB was
found to be positively associated with NRP-1 expression status
(Fig. 1D, left; NRP-1 low,
0.75±0.75; NRP-1 high, 1.75±1.04; P<0.04) and vice versa
(Fig. 1D, right; VEGF-A low,
2.00±0.71; VEGF-A high, 3.14±0.90; P<0.02). However, the in
vitro expression patterns of Flt-1 and Flk-1 were variable and
commonly demonstrated high expression in all nine GB cells compared
to human primary astrocytes. NRP-1, which is approximately 120 kDa
in molecular size, was highly expressed in SNU-489, SNU-1105,
U251-MG, and U373-MG, while its expression was negative or low in
A172 and SNU-626. It should be noted that in the immunoblot
analysis, the NRP-1 band was uniquely shifted to a higher molecular
size in SNU-466, CRT-MG, and LN215-MG cells (Fig. 1E). Significantly higher expression
levels of secreted VEGF-A were also observed in the nine GB cells
compared with HUVECs (Fig.
1F).
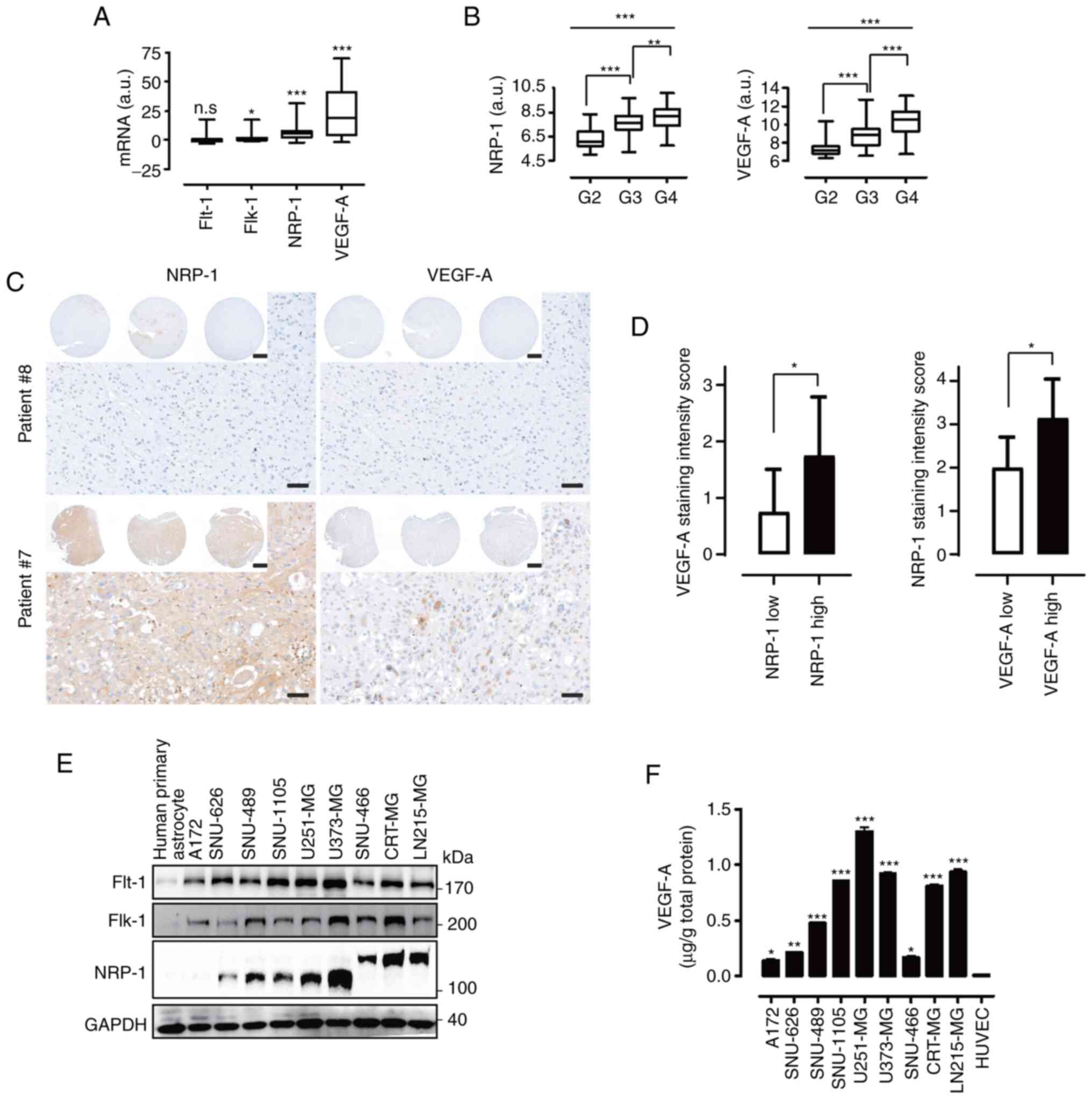 | Figure 1Expression levels of VEGF-A and its
receptors in GB cells. (A) mRNA expression levels of Flt-1,
Flk-1, NRP-1, and VEGF-A in brain tumors
(n=144) compared with normal brain samples (n=330) analyzed using
the GEO database (P-value evaluated with Student's t-test according
to brain tumors vs. normal brain samples; *P<0.05 and
***P<0.001). (B) Transcriptional levels of
NRP-1 and VEGF-A in various grades of brain tumors
analyzed using the GEO database (G indicates the tumor grade, n=3;
Tukey's post hoc test was applied to significant group effects in
ANOVA, P<0.0001; P-value evaluated with Student's t-test
according to G2 vs. G3 and G3 vs. G4; **P<0.01 and
***P<0.001, G2; n=24, G3; n=85, G4; n=159). (C)
Representative tissue microarray analysis images of patients with
GB for NRP-1 and VEGF-A (scale bar, 50 µm; inlet image scale
bar, 500 µm). (D) Left, comparison of VEGF-A staining
intensity scores between the NRP-1 low-expression group (n=12) and
high-expression group (n=8). Right, comparison of NRP-1 staining
intensity scores between the VEGF-A low-expression group (n=13) and
high-expression group (n=7). (E) Flt-1, Flk-1, and NRP-1 expression
levels in nine GB cells and human primary astrocytes evaluated by
western blot analysis. GAPDH was measured as a control. (F) ELISA
was performed to quantify the expression levels of VEGF-A using the
cultured supernatants from nine GB cells and HUVECs (P-value by
two-tail t-tests; *P<0.05, **P<0.01 and
***P<0.001). VEGF-A, vascular endothelial growth
factor-A; GB, glioblastoma; Flt-1, FMS related receptor tyrosine
kinase 1; Flk-1, fetal liver kinase 1; NRP-1, neuropilin-1; GEO,
Gene Expression Omnibus; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; HUVECs, human umbilical vein endothelial cells; n.s,
not significant. |
Association between sensitivity of VEGF-A
signaling and the expression pattern of NRP-1 in GB cells
To validate the association between the sensitivity
to autocrine VEGF-A signaling and the expression pattern of VEGF-A
involving molecules, nine GB cells were incubated in the absence or
presence of recombinant VEGF-A or SU1498, which is a
pharmacological inhibitor against VEGF-A signaling. The viability
was found to be increased by exogenous VEGF-A treatment in SNU-489,
SNU-1105, U251-MG, and U373-MG cells, while the viability was not
affected in A172, SNU-626, SNU-466, CRT-MG, and LN215-MG cells
(Fig. 2A). A similar result was
obtained using exogenous SU1498 treatment in GB cells (Fig. 2B). To achieve a more detailed
investigation, two VEGF-A signaling-sensitive GB cell lines
(U251-MG and U373-MG) and two VEGF-A signaling-resistant GB cell
lines (SNU-466 and LN215-MG) were selected, and then the effect of
transfection with si-VEGF-A or knockdown of VEGFRs on cell death
was determined to elucidate the biological function of VEGF-A and
its receptors in GB cells. The efficacy of knockdown of VEGF-A and
VEGFRs was demonstrated by western blot analysis (Fig. 2C). The reduction in VEGF-A
expression by si-VEGF-A transfection induced significant cell death
in U251-MG and U373-MG cells, while the same treatment had a weak
or negative cell death effect on SNU-466 and LN215-MG cells.
Silencing the expression of VEGFRs by siRNA transfection also
significantly induced cell death in U251-MG and U373-MG cells, but
it had little effect in SNU-466 and LN215-MG cells (Fig. 2D). To further evaluate the effects
of VEGF-A signaling in a different type of GB cells in vivo,
VEGF-A signaling-sensitive U251-MG and VEGF-A signaling-resistant
LN215-MG xenograft models were developed. To this end, tumor-laden
mice were subcutaneously injected with control buffer or 10 mg/kg
SU1498 when the tumors reached an average size of approximately 150
mm3. U251-MG xenograft tumors treated with control
buffer were found to grow to an average size of 379.19±93.36
mm3 in 42 days after transplantation, while those
treated with 10 mg/kg SU1498 grew to an average size of
200.43±46.72 mm3 in 42 days post-transplantation
(Fig. 2E). There was no
significant difference in weight loss between the two groups (data
not shown). By contrast, treatment with control buffer or 10 mg/kg
SU1498 had no effect in LN215-MG xenograft models (Fig. 2F). No weight loss was detected in
the LN215-MG xenograft models either (data not shown).
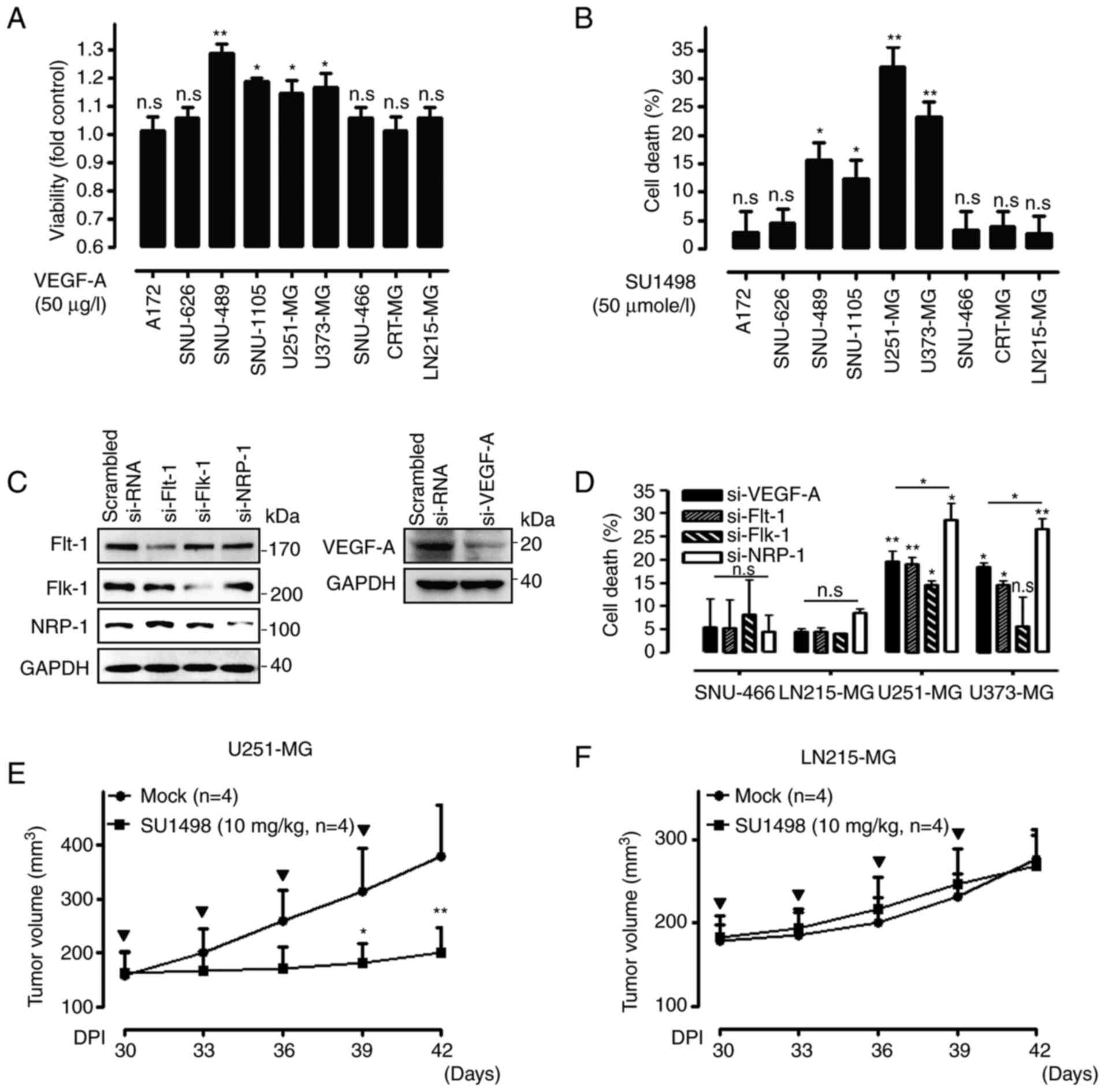 | Figure 2Effect of VEGF-A signaling in
association with the expression patterns of NRP-1, on GB cells. (A
and B) Nine GB cells were incubated in the absence or presence of
exogenous VEGF-A (50 µg/l, left) or SU1498 (50
µmole/l, right), a VEGF receptor inhibitor, for 72 h, after
which they were examined for cell viability using WST-1 assay and
cell death using LDH assay (n=3; Tukey's post hoc test was applied
to significant group effects in ANOVA, P<0.0001; asterisks
indicate significant differences compared with the control group,
*P<0.05 and **P<0.01). (C) Efficacy of
Flt-1, Flk-1, NRP-1, and VEGF-A siRNA transfections assessed by
western blot analysis. GAPDH was used as a control. (D) Cell death
was assessed 72 h after transient transfection with siRNAs against
VEGF-A, Flt-1, Flk-1, and NRP-1 using an LDH assay (n=3; Tukey's
post hoc test was applied to significant group effects in ANOVA,
P<0.0001; asterisks indicate significant differences compared to
0% inhibition; *P<0.05 and **P<0.01).
(E) Effect of SU1498 (10 mg/kg) on the tumor volumes of U251-MG
xenograft models (control group, n=4; SU1498 10 mg/kg, n=4)
measured for 42 days using the following formula: V=0.523
LW2 (L=length and W=width). Bold arrows indicate the
time-points of SU1498 injection (Tukey's post-hoc test was used to
determine significant group effects in ANOVA, P<0.0001;
asterisks indicate significant differences between the control
group and the SU1498-injected group; *P<0.05 and
**P<0.01). (F) Effect of SU1498 (10 mg/kg) on the
tumor volumes of LN215-MG xenograft models (control group, n=4;
SU1498 10 mg/kg, n=4) measured for 42 days using the formula
aforementioned, V=0.523 LW2. Bold arrows indicate the time-points
of SU1498 injection. VEGF-A, vascular endothelial growth factor-A;
GB, glioblastoma; NRP-1, neuropilin-1; LDH, lactate dehydrogenase;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Flt-1, FMS related
receptor tyrosine kinase 1; Flk-1, fetal liver kinase 1; n.s.,
non-significant; si-RNA or si-, small interfering RNA. |
Differential intracellular signaling in
VEGF-A-sensitive or VEGF-A-resistant GB cells
To elucidate the molecular mechanisms responsible
for the differing sensitivity to VEGF-A signaling in GB cells,
signal transduction pathways were examined following treatment with
exogenous VEGF-A or SU1498. Incubation with exogenous VEGF-A was
demonstrated to increase phosphorylation of various tyrosine
residues in a time-dependent manner; in addition, treatment with
SU1498 inhibited phosphorylation of tyrosine residues of various
proteins compared to their basal levels in U251-MG cells (Fig. 3A). By contrast, treatment with
SU1498 was not associated with any alteration of phosphotyrosine
levels in LN215-MG cells (Figs. 3B
and S2A). To analyze the
mechanism of VEGF-A signaling in further detail, the
phosphorylation levels of Flt-1, Flk-1, FAK, and AKT were examined
following treatment with exogenous VEGF-A or SU1498. Treatment with
VEGF-A induced the phosphorylation of Flt-1, FAK, and AKT in
U251-MG cells, but not Flk-1, while SU1498 treatment produced the
exact opposite effect in a time-dependent manner (Fig. 3C). By contrast, incubation with
VEGF-A or SU1498 did not affect intracellular signaling in LN215-MG
cells (Figs. 3D and S2B).
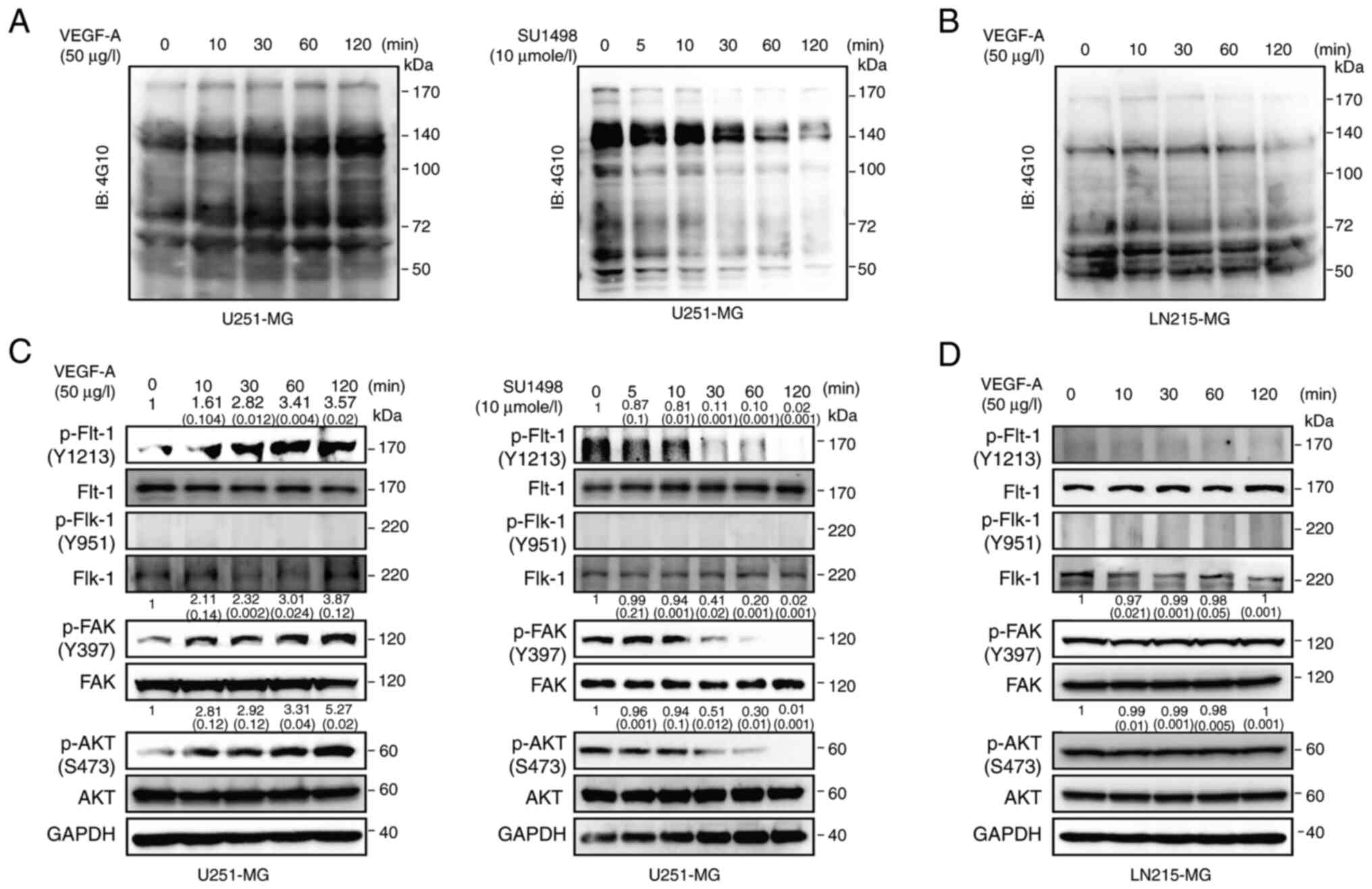 | Figure 3Intracellular signaling of
VEGF-A-sensitive or VEGF-A-resistant GB cells. (A) U251-MG cells
incubated with VEGF-A (50 µg/l) or SU1498 (10
µmole/l) for varying time-points, after which the cell
lysates were subjected to western blot analysis using antibodies
specific for total phosphotyrosine kinase, 4G10. (B) LN215-MG cells
incubated with VEGF-A (50 µg/l) for varying time-points,
after which the cell lysates were subjected to western blot
analysis using antibodies specific for total phosphotyrosine
kinase, 4G10. (C) U251-MG cells incubated with VEGF-A (50
µg/l) or SU1498 (10 µmole/l) for varying time-points,
after which the cell lysates were subjected to western blot
analysis using antibodies specific for p-Flt-1 (Y1213), total
Flt-1, p-Flk-1 (Y951), total Flk-1, p-FAK (Y397), total FAK, p-AKT
(S473), total AKT, and GAPDH. The relative pixel intensities of the
target molecules were assessed by densitometric analysis using
ImageJ analysis software. Data are representative of three
individual experiments. (D) LN215-MG cells incubated with VEGF-A
(50 µg/l) for varying time-points, after which the cell
lysates were subjected to western blot analysis using antibodies
specific for p-Flt-1 (Y1213), total Flt-1, p-Flk-1 (Y951), total
Flk-1, p-FAK (Y397), total FAK, p-AKT (S473), total AKT, and GAPDH.
The relative pixel intensities of the target molecules were
assessed by densitometric analysis using ImageJ analysis software.
Data are representative of three individual experiments. VEGF-A,
vascular endothelial growth factor-A; GB, glioblastoma; p-,
phosphorylated; Flt-1, FMS related receptor tyrosine kinase 1;
Flk-1, fetal liver kinase 1; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
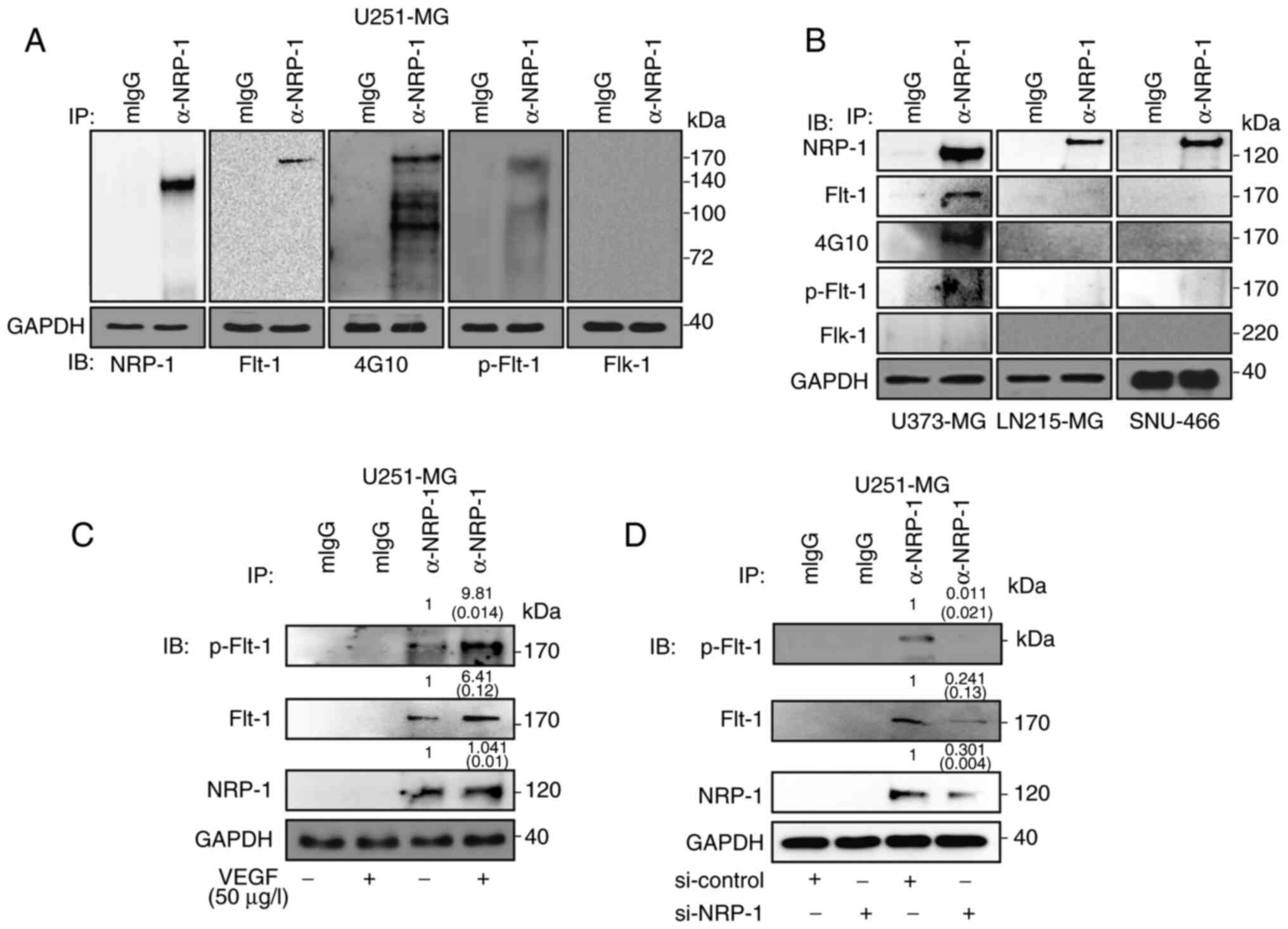
Physical interaction between wild-type
NRP-1 and Flt-1 in VEGF-A signaling-sensitive GB cells
Since research has reported that the function of
NRP-1 is associated with Flt-1 and Flk-1 (8,9), the
molecular interaction between VEGFRs and NRP-1 was next examined
using immunoprecipitation. In VEGF-A signaling-sensitive U251-MG
and U373-MG cells, NRP-1 exclusively interacted with functional
Flt-1, whereas no molecular interaction was observed in VEGF-A
signaling-resistant LN215-MG and SNU-466 cells (Fig. 4A and B). The interaction between
NRP-1 and Flt-1 was intensified by exogenous VEGF-A treatment
(Fig. 4C), whereas the reduction
in NRP-1 expression by si-NRP-1 transfection weakened its
interaction with Flt-1 (Fig.
4D).
Restoring VEGF-A signaling after
eliminating chondroitin sulfate modification of NRP-1 in VEGF-A
signaling-resistant GB cells
It was next demonstrated whether NRP-1 could be
modified by chondroitin sulfate using Ch-ABC, which is an enzyme
that cleaves GAG from membrane surface proteoglycans in LN215-MG
cells. Treatment with Ch-ABC effectively removed the higher
molecular weight band with a concomitant increase of 120 kDa NRP-1.
The phosphorylation levels of FAK and AKT were also increased by
Ch-ABC treatment in LN215-MG cells (Fig. 5A). Furthermore, an interaction
between NRP-1 and Flt-1 was observed after eliminating chondroitin
sulfate modification of NRP-1 in LN215-MG cells, and Flt-1 which
interacted with NRP-1 was found to be phosphorylated (Fig. 5B). To investigate the molecular
function of chondroitin sulfate modification of NRP-1 in VEGF-A
signaling-resistant GB cells, SNU-466 and LN215-MG cells were
incubated in the absence or presence of Ch-ABC, and then malignant
features were assessed. Proliferation and migration stimulated by
VEGF-A signaling were recovered by combined treatment with Ch-ABC
in SNU-466 and LN215-MG cells (Fig.
5C). The significance of combination treatment with VEGF-A and
Ch-ABC was validated by immunoblot analysis. Combined treatment
with VEGF-A and Ch-ABC increased the phosphorylation of FAK and AKT
relative to that of VEGF-A treatment in LN215-MG cells (Fig. 5D). In adition, eliminating the
chondroitin sulfate modification with Ch-ABC significantly
increased the cytotoxicity of SU1498 in SNU-466 and LN215-MG cells
in a dose-dependent manner (Fig.
5E). The significant effect of combination treatment with
SU1498 and Ch-ABC was also confirmed by immunoblot analysis
(Fig. 5F).
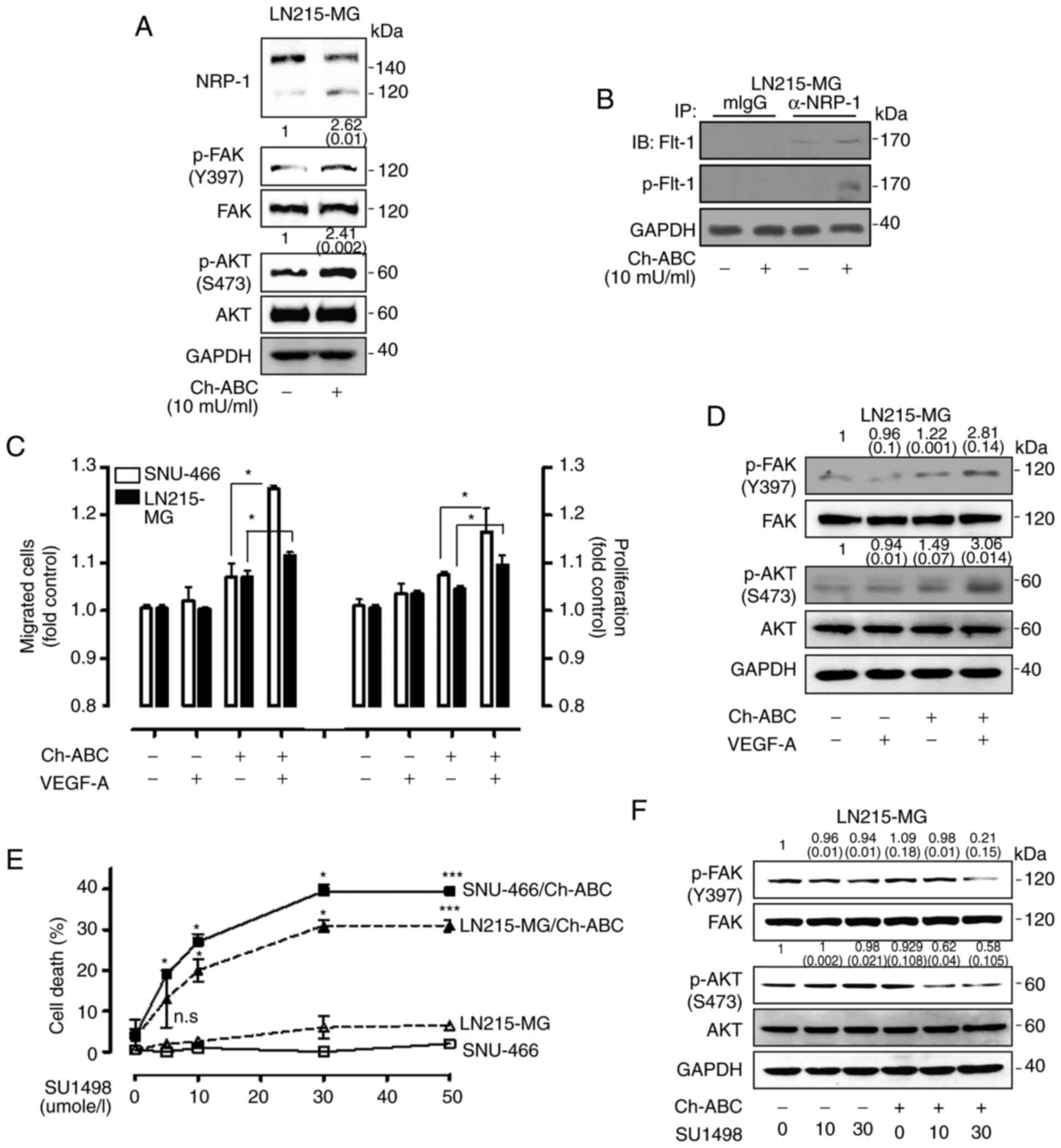 | Figure 5Effect of eliminating chondroitin
sulfate modification on NRP-1 in GB cells. (A) Elimination of
chondroitin sulfate modification on NRP-1 using Ch-ABC (10 mU/ml)
treatment in LN215-MG cells, after which the cell lysates were
subjected to western blot analysis using antibodies specific for
NRP-1, p-FAK (Y397), total FAK, p-AKT (S473), and total AKT. GAPDH
was used as a control. The relative pixel intensities of the target
molecules were assessed by densitometric analysis using ImageJ
analysis software. Data are representative of three individual
experiments. (B) An increase in interaction between NRP-1 and Flt-1
was determined by immunoprecipitation using 1% NP-40 lysis buffer
in LN215-MG cells following Ch-ABC treatment. The relative pixel
intensities of the target molecules were assessed by densitometric
analysis using ImageJ analysis software. GAPDH was used as a
control. Data are representative of three individual experiments.
(C) SNU-466 and LN215-MG cells incubated with or without Ch-ABC in
the absence or presence of VEGF-A (50 µg/l) for 4 h
(migration) or 72 h (proliferation). VEGF-mediated cell migration
was assessed using Transwell migration assay (left) and
proliferation was evaluated by WST-1 assay (right) (P-values were
evaluated with Student's t-tests). (D) LN215-MG pretreated with or
without Ch-ABC for 24 h in the absence or presence of VEGF-A (50
µg/l) for 120 min, after which the LN215-MG cell lysates
were subjected to western blot analysis using antibodies specific
for p-FAK (Y397), total FAK, p-AKT (S473), and total AKT. GAPDH was
used as a control. The relative pixel intensities of the target
molecules were assessed by densitometric analysis using ImageJ
analysis software. Data are representative of three individual
experiments. (E) SNU-466 and LN215-MG cells were incubated with
SU1498 in a dose-dependent manner in the absence or presence of
Ch-ABC for 24 h. Cell death was assessed using an LDH assay (n=3;
Tukey's post hoc test was applied to significant group effects in
ANOVA; *P<0.05 and ***P<0.005). (F)
LN215-MG cells were incubated with SU1498 in a dose-dependent
manner in the absence or presence of Ch-ABC for 24 h, after which
the cell lysates were subjected to western blot analysis using
antibodies specific for p-FAK (Y397), total FAK, p-AKT (S473), and
total AKT. GAPDH was used as a control. The relative pixel
intensities of the target molecules were assessed by densitometric
analysis using ImageJ analysis software. Data are representative of
three individual experiments. NRP-1, neuropilin-1; GB,
glioblastoma; Ch-ABC, chondrotinase ABC; p-, phosphorylated; Flt-1,
FMS related receptor tyrosine kinase 1; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; VEGF-A, vascular endothelial growth
factor-A. |
In vivo effect of eliminating chondroitin
sulfate modification in VEGF-A signaling-resistant GB cells
To further validate the restoration of VEGF-A
signaling by eliminating chondroitin sulfate modification in
VEGF-A-resistant GB cells in vivo, LN215-MG and SNU-466
xenograft models were developed. When their tumors reached an
average size of approximately 200 mm3, tumor-laden mice
were subcutaneously injected with either control buffer, 10 mU/ml
Ch-ABC, 10 mg/kg SU1498, or a combination of SU1498 and Ch-ABC. At
42 days after transplantation, LN215-MG xenograft tumors treated
with control buffer had grown to an average size of 290.74±12.53
mm3 whereas those treated with both 10 mU/ml Ch-ABC and
10 mg/kg SU1498 had grown to an average size of 227.43±16.65
mm3. Administration of 10 mU/ml Ch-ABC or 10 mg/kg
SU1498 had no effect in LN215-MG xenograft models (Fig. 6A). In addition, there were no
differences in weight loss between groups (data not shown). SNU-466
xenograft tumors treated with control buffer grew to an average
size of 234.33±13.77 mm3 in 42 days after
transplantation while those treated with a combination of 10 mU/ml
Ch-ABC and 10 mg/kg SU1498 grew to an average size of 193.67±14.67
mm3 in the same time-frame. Treatment with 10 mU/ml
Ch-ABC or 10 mg/kg SU1498 had no effect in SNU-466 xenograft models
(Fig. 6B). There was no
significant difference in weight loss either (data not shown). To
ascertain the in vivo anti-growth effect, caspase-3-mediated
apoptosis in the LN215-MG xenograft model was investigated.
Concurrent treatment with 10 mU/ml Ch-ABC and 10 mg/kg SU1498 was
shown to induce cleavage of caspase-3 in VEGF-A-resistant the
LN215-MG xenograft model (Fig.
6C). Based on the in vivo findings indicating that
chondroitin sulfate modifications may affect GB cell malignancies,
a TMA analysis of patients with GB was conducted to determine
whether the level of chondroitin sulfate expression could affect
the prognosis of patients GB (Fig.
6D). As revealed in Fig. 6E,
the group with high expression of chondroitin sulfate exhibited
poor progression-free survival compared with the group with low
expression (P<0.02; hazard ratio, 4.938; 95% confidence
interval, 1.34-18.16), although there was no statistical difference
in overall survival (Fig.
S3).
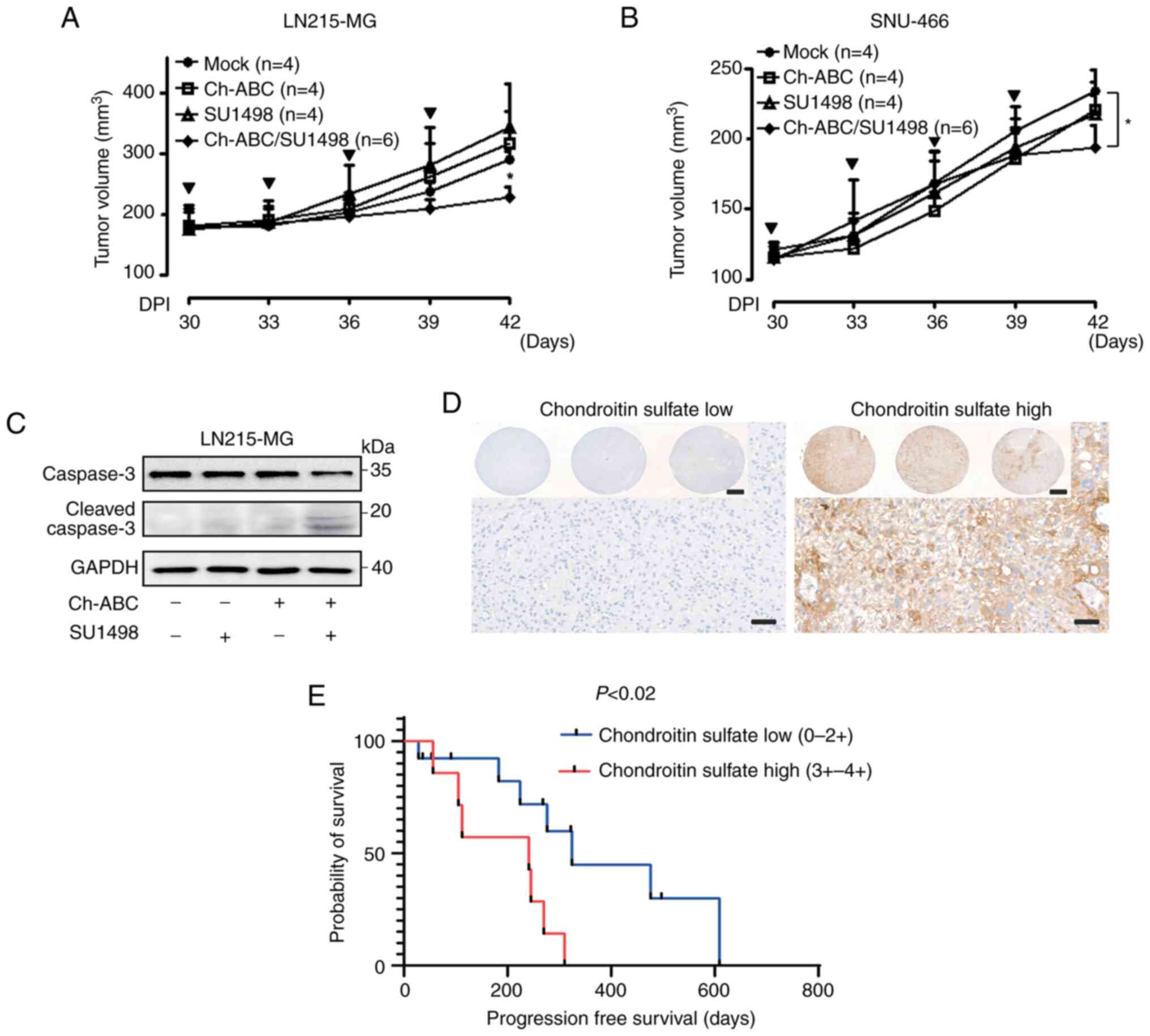 | Figure 6Resensitization of VEGF-A signaling
through the elimination of chondroitin sulfate modification in GB
cells. (A) Effect of Ch-ABC (10 mU/ml), SU1498 (10 mg/kg), or
Ch-ABC/SU1498 (10 mU/ml/10 mg/kg) on the tumor volumes of LN215-MG
xenograft models (control group, n=4; Ch-ABC, n=4; SU1498, n=4; and
Ch-ABC/SU1498, n=6) measured for 42 days using the following
formula: V=0.523 LW2 (L=length and W=width). Bold arrows indicate
the time-points of Ch-ABC, SU1498, or Ch-ABC/SU1498 injection
(Tukey's post-hoc test was used to determine significant group
effects in ANOVA, P<0.0001; asterisks indicate significant
differences between the control group and the Ch-ABC, SU1498, or
Ch-ABC/SU1498 group; *P<0.05). (B) Effect of Ch-ABC
(10 mU/ml), SU1498 (10 mg/kg), or Ch-ABC/SU1498 (10 mU/ml/10 mg/kg)
on the tumor volumes of SNU-466 xenograft models (control group,
n=4; Ch-ABC, n=4; SU1498, n=4; and Ch-ABC/SU1498, n=6) measured for
42 days using the aforementioned formula: V= 0.523 LW2.
Bold arrows indicate the time-points of Ch-ABC, SU1498, or
Ch-ABC/SU1498 injection (Tukey's post-hoc test was used to
determine significant group effects in ANOVA, P<0.0001;
asterisks indicate significant differences between the control
group and the Ch-ABC, SU1498, or Ch-ABC/SU1498 group;
*P<0.05). (C) Lysates derived from LN215-MG xenograft
tumor samples were assessed for caspase-3, cleaved caspase-3, and
GAPDH using western blot analysis. Data are representative of three
individual experiments. (D) Representative tissue microarray
analysis images of chondroitin sulfate expression of patients with
GB (scale bar, 50 µm; inlet image scale bar, 500 µm).
(E) Association between chondroitin sulfate expression and
progression-free survival of GB patients. The survival analysis of
19 patients with GB was performed by integrating the clinical data
of patients with GB with their chondroitin sulfate expression
levels. VEGF-A, vascular endothelial growth factor-A; GB,
glioblastoma; Ch-ABC, chondrotinase ABC. |
Discussion
In the present study, the associations between the
expression types of NRP-1 and the susceptibility of GB cells to
VEGF-A signaling were revealed both in vitro and in
vivo. Differential dependency to VEGF-A signaling was
classified according to the interaction between NRP-1 and Flt-1. It
was further confirmed that re-sensitization of VEGF-A signaling
could be achieved by eliminating the chondroitin sulfate
modification of NRP-1 in VEGF-A signaling-resistant GB cells both
in vitro and in vivo. Chondroitin sulfate
modification was also found to be associated with an adverse
prognosis in patients with GB. To the best of our knowledge, this
is the first study to report that anti-VEGF-A therapies that
involve the elimination of chondroitin sulfate modification of
NRP-1, may represent innovative therapeutic approaches for patients
with GB who exhibit no response to treatments targeting the VEGF-A
signaling pathway.
Due to the increasing number of failed clinical
trials attempting to use angiogenesis inhibitors for cancer
therapies, there has been growing interest in the mechanisms
underlying autocrine VEGF-A signaling (28). Research has shown that some
patients with GB temporarily benefit from a single VEGF-A targeting
therapy or combination therapies with antitumor drugs (2). However, the majority of patients with
GB are entirely refractory to anti-VEGF-A therapies. The cause of
this may be attributable to the acquisition of resistance to
anti-VEGF-A therapies due to alternative signaling pathways
(29-32). In the present study, potential
VEGF-A therapies exploiting the autocrine VEGF-A signaling involved
in NRP-1 expression types were revealed. It was demonstrated that
silencing of VEGF-A signaling by siRNA transfection or
pharmacological inhibitor treatment resulted in two types of
outcomes in vitro and in vivo based on the expression
pattern of NRP-1. Furthermore, the differential sensitivity was
accompanied by a convincing intracellular signaling pathway
difference, indicating that prudent grouping of patients with GB to
target VEGF-A signaling should be performed based on the expression
types of NRP-1.
VEGF-A and its receptors were originally
investigated in endothelial cells in angiogenesis (33). Flk-1 (also known as VEGF-R2), has
been defined as a critical signaling receptor for VEGF-A-mediated
mitogenesis, vascular permeability, and angiogenesis due to its
strong tyrosine kinase activity. By comparison, Flt-1 (also known
as VEGF-R1), has weak tyrosine kinase activity, which leads Flt-1
to be a supplemented receptor for Flk-1 in angiogenesis (34). However, several research groups
have suggested that the mediation of Flt-1 by VEGF-A signaling
affects survival in lymphoma, leukemia, prostate cancer, colon
cancer, and neuroblastoma (35-39).
VEGF-A signaling has also been reported to involve a combined
function of Flt-1 and Flk-1 on survival in primary glioblastoma
cells (40). At present, Flt-1 is
the specific receptor associated with autocrine VEGF-A signaling
based on its capability to interact with wild-type NRP-1 in GB
cells.
NRP-1 has been reported to have a functional role in
tumorigenesis and prognosis in various malignant tumors (38,41-44).
Furthermore, research has shown that enhanced NRP-1 expression
promotes glioma progression in vivo through a HGF/SF and
HGFR signaling-independent VEGF-A signaling pathway (10). In addition, NRP-1 has also been
suggested to have a tumorigenesis-suppressive function in
pancreatic cells (43) and was
demonstrated to have improved prognosis in colon cancers (45). The results of the present study
reveal that chondroitin sulfate-modified NRP-1 could numb the
sensitivity to VEGF-A signaling, while the removal of this
chondroitin sulfate modification on NRP-1 led to re-sensitization
to VEGF-A signaling by restoring the interaction with Flt-1, thus
indicating that the existence or non-existence of
chondroitin-sulfated modification on NRP-1 may be a critical factor
for determining the effectiveness of therapies targeting VEGF-A
signaling. To determine the association between the expression
pattern of NRP-1 and sensitivity to VEGF-A signaling more clearly,
it should be considered whether the smeary minor bands of the
western blotting results are a natural property of GB cells or a
minor experimental discrepancy. It has recently been reported that
targeting endogenous VEGF-A or NRP-1 does not directly decrease
tumor cell growth or invasion, and that the anticancer effect is
instead due to an anti-angiogenic effect in endothelial cells
(44,46,47).
However, no studies have examined the role of NRP-1 depending on
the status of its expression pattern. It was also observed that the
co-administration of Ch-ABC and SU1498 had a relatively moderate
in vivo anticancer effect, contrary to the in vitro
results. The limited effect of targeting VEGF-A signaling with the
elimination of chondroitin sulfate modification of NRP-1 may be
attributable to the non-specific elimination by Ch-ABC in LN215-MG
and SNU-466 xenograft models. Thus, to improve this innovative
therapy, a direct reagent or technique for eliminating chondroitin
sulfate modification on NRP-1 should be developed, i.e., 'the
pincer attack'. In addition, derestricted GAGs have been reported
to be correlated with clinical outcomes in various malignant tumors
(18). Consistent with the results
of this previous study, it was revealed that chondroitin sulfate
modification was associated with adverse prognosis in patients with
GB. To further elucidate the association between chondroitin
sulfate modification on NRP-1 and clinical outcomes in patients
with GB, future studies should be performed using a large number of
tissue samples from patients with GB. Based on our preliminary
results associated with detecting the expression type of NRP-1 in
whole blood samples of patients with GB, application of the
technique to determine the association between chondroitin-sulfated
NRP-1 and wild-type NRP-1 expression in whole blood samples from
patients with GB could not be ignored.
Recent studies have suggested that soluble NRP-1 can
be detected both in vitro and in vivo through the
functional role of a metalloprotease such as ADAM10 (48-50).
In agreement with these previous studies, preliminary results
indicating that chondroitin sulfate-modified soluble NRP-1 can be
detected in GB cells were obtained using accessible techniques such
as ELISA and immunoprecipitation (Fig. S4). These encouraging results
suggest that the detection of wild-type NRP-1 and modified NRP-1
could potentially be achieved using human materials such as whole
blood, saliva, and urine, which would lead to widespread practical
applications. The use of this classifying technique of NRP-1
expression pattern from human materials may be an innovative
therapeutic approach achieved using various inhibitors against
VEGF-A signaling for patients with GB.
In summary, the results of the present study provide
a basis that may be of aid in variable or disappointing outcomes
among patients with GB to therapies targeting VEGF-A signaling in
clinical trials. The present study also offers a rationale for
further research to develop innovative strategies targeting VEGF-A
signaling by eliminating the modification from NRP-1.
Supplementary Data
Availability of data and materials
The data generated in the present study are included
in the figures and supplementary figures of this article.
Authors' contributions
JWL and CC conceived and designed the study. JWL,
KChong, and JSL developed the methodology. JWL, KChong, JSL, and CK
contributed to the acquisition of the data, animals, and facilities
as well as the management of the data of patients. JWL, KChong,
JSL, JHK, CK, KChoi, and CC analyzed and interpreted the data
(e.g., statistical analysis, biostatistics, computational
analysis). JWLee, KChong, and CC wrote, reviewed, and/or revised
the manuscript. JWL and CC supervised the study. JWL and KChong
confirm the authenticity of all the raw data. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The patient sample study complied with the
guidelines and protocols approved by the Institutional Review Board
(IRB no. 2017GR330) of Korea University Guro Hospital (Seoul,
Korea). Written informed consent was obtained prior to sample
collection from all the participants who agreed to the use of their
samples in scientific research, and procedures were conducted
according to the Declaration of Helsinki. Animal care and handling
procedures were performed in accordance with Animal Research
Committee's Guidelines of KAIST and Korea University College of
Medicine. All animal experiments were approved by the Institutional
Animal Care and Use Committee of the KAIST (IACUC No. KA2013-13)
and the Korea University College of Medicine (IACUC No.
KOREA-2019-0123).
Patient consent for publication
Not applicable.
Competing interests
CC is the founder and shareholder, and KChoi is a
minor shareholder of ILIAS Biologics, Inc. The other authors
declare that they have no competing interests.
Acknowledgments
We would like to thank Professor Etty N. Benveniste
(University of Alabama, Birmingham, USA) for providing the U251-MG,
U373-MG, CRT-MG, and LN215-MG cells and Professor In-Hong Choi
(Yonsei University, Seoul, Korea) for providing the human normal
astrocytes.
Funding
The present research was supported by the Bio & Medical
Technology Development Program of the National Research Foundation
(NRF) of Korea funded by the Ministry of Science and ICT (grant no.
2016M3A9B6945831) and (grant no. 2017M3A9G8084516). The research
was also supported by the NRF of the Republic of Korea (grant no.
2016R1A6A1A03012862) and (grant no. NRF-2019M3A9E2061791).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westphal M and Lamszus K: The neurobiology
of gliomas: From cell biology to the development of therapeutic
approaches. Nat Rev Neurosci. 12:495–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller CR and Perry A: Glioblastoma. Arch
Pathol Lab Med. 131:397–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newton HB: Primary brain tumors: Review of
etiology, diagnosis and treatment. Am Fam Physician. 49:787–797.
1994.PubMed/NCBI
|
|
8
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu C, Rodriguez ER, Reimert DV, Shu T,
Fritzsch B, Richards LJ, Kolodkin AL and Ginty DD: Neuropilin-1
conveys semaphorin and VEGF signaling during neural and
cardiovascular development. Dev Cell. 5:45–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu B, Guo P, Bar-Joseph I, Imanishi Y,
Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R and Cheng
SY: Neuropilin-1 promotes human glioma progression through
potentiating the activity of the HGF/SF autocrine pathway.
Oncogene. 26:5577–5586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellis LM: The role of neuropilins in
cancer. Mol Cancer Ther. 5:1099–1107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans IM, Yamaji M, Britton G, Pellet-Many
C, Lockie C, Zachary IC and Frankel P: Neuropilin-1 signaling
through p130Cas tyrosine phosphorylation is essential for growth
factor-dependent migration of glioma and endothelial cells. Mol
Cell Biol. 31:1174–1185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frankel P, Pellet-Many C, Lehtolainen P,
D'Abaco GM, Tickner ML, Cheng L and Zachary IC: Chondroitin
sulphate-modified neuropilin 1 is expressed in human tumour cells
and modulates 3D invasion in the U87MG human glioblastoma cell line
through a p130Cas-mediated pathway. EMBO Rep. 9:983–989. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miao HQ, Lee P, Lin H, Soker S and
Klagsbrun M: Neuropilin-1 expression by tumor cells promotes tumor
angiogenesis and progression. FASEB J. 14:2532–2539. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parikh AA, Fan F, Liu WB, Ahmad SA,
Stoeltzing O, Reinmuth N, Bielenberg D, Bucana CD, Klagsbrun M and
Ellis LM: Neuropilin-1 in human colon cancer: Expression,
regulation, and role in induction of angiogenesis. Am J Pathol.
164:2139–2151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shintani Y, Takashima S, Asano Y, Kato H,
Liao Y, Yamazaki S, Tsukamoto O, Seguchi O, Yamamoto H, Fukushima
T, et al: Glycosaminoglycan modification of neuropilin-1 modulates
VEGFR2 signaling. EMBO J. 25:3045–3055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kjellen L and Lindahl U: Proteoglycans:
Structures and interactions. Annu Rev Biochem. 60:443–475. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Theocharis AD, Tsolakis I, Tzanakakis GN
and Karamanos NK: Chondroitin sulfate as a key molecule in the
development of atherosclerosis and cancer progression. Adv
Pharmacol. 53:281–295. 2006. View Article : Google Scholar
|
|
19
|
Lee J, Ku T, Yu H, Chong K, Ryu SW, Choi K
and Choi C: Blockade of VEGF-A suppresses tumor growth via
inhibition of autocrine signaling through FAK and AKT. Cancer Lett.
318:221–225. 2012. View Article : Google Scholar
|
|
20
|
Piccolo SR, Withers MR, Francis OE, Bild
AH and Johnson WE: Multiplatform single-sample estimates of
transcriptional activation. Proc Natl Acad Sci USA.
110:17778–17783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai M, Wang P, Boyd AD, Kostov G, Athey B,
Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, et al: Evolving
gene/transcript definitions significantly alter the interpretation
of GeneChip data. Nucleic Acids Res. 33:e1752005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee J, Lee J, Yun JH, Choi C, Cho S, Kim
SJ and Kim JH: Autocrine DUSP28 signaling mediates pancreatic
cancer malignancy via regulation of PDGF-A. Scientific reports.
7:127602017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J, Lee J, Sim W and Kim JH: Soluble
TGFBI aggravates the malignancy of cholangiocarcinoma through
activation of the ITGB1 dependent PPARγ signalling pathway. Cell
Oncol (Dordr). 45:275–291. 2022. View Article : Google Scholar
|
|
25
|
Lee J, Kim DH and Kim JH: Combined
administration of naringenin and hesperetin with optimal ratio
maximizes the anti-cancer effect in human pancreatic cancer via
down regulation of FAK and p38 signaling pathway. Phytomedicine.
58:1527622019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee J, Lee J, Yun JH, Choi C, Cho S, Kim
SJ and Kim JH: Autocrine DUSP28 signaling mediates pancreatic
cancer malignancy via regulation of PDGF-A. Sci Rep. 7:127602017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reddy SP, Britto R, Vinnakota K, Aparna H,
Sreepathi HK, Thota B, Kumari A, Shilpa BM, Vrinda M, Umesh S, et
al: Novel glioblastoma markers with diagnostic and prognostic value
identified through transcriptome analysis. Clin Cancer Res.
14:2978–2987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: Current progress unresolved
questions and future directions. Angiogenesis. 17:471–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sabir A, Schor-Bardach R, Wilcox CJ,
Rahmanuddin S, Atkins MB, Kruskal JB, Signoretti S, Raptopoulos VD
and Goldberg SN: Perfusion MDCT enables early detection of
therapeutic response to antiangiogenic therapy. AJR Am J
Roentgenol. 191:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chung AS, Lee J and Ferrara N: Targeting
the tumour vasculature: Insights from physiological angiogenesis.
Nat Rev Cancer. 10:505–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29(6 Suppl 16): S10–S14.
2002. View Article : Google Scholar
|
|
35
|
Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z,
Hicklin DJ and Moore MA: Targeting autocrine and paracrine VEGF
receptor pathways inhibits human lymphoma xenografts in vivo.
Blood. 104:2893–2902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karp JE, Gojo I, Pili R, Gocke CD, Greer
J, Guo C, Qian D, Morris L, Tidwell M, Chen H and Zwiebel J:
Targeting vascular endothelial growth factor for relapsed and
refractory adult acute myelogenous leukemias: Therapy with
sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and
bevacizumab. Clin Cancer Res. 10:3577–3585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi L, Robinson WA, Brady BM and Glode LM:
Migration and invasion of human prostate cancer cells is related to
expression of VEGF and its receptors. Anticancer Res. 23:3917–3922.
2003.PubMed/NCBI
|
|
38
|
Bates RC, Goldsmith JD, Bachelder RE,
Brown C, Shibuya M, Oettgen P and Mercurio AM: Flt-1-dependent
survival characterizes the epithelial-mesenchymal transition of
colonic organoids. Curr Biol. 13:1721–1727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Das B, Yeger H, Tsuchida R, Torkin R, Gee
MF, Thorner PS, Shibuya M, Malkin D and Baruchel S: A
hypoxia-driven vascular endothelial growth factor/Flt1 autocrine
loop interacts with hypoxia-inducible factor-1alpha through
mitogen-activated protein kinase/extracellular signal-regulated
kinase 1/2 pathway in neuroblastoma. Cancer Res. 65:7267–7275.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Steiner HH, Karcher S, Mueller MM,
Nalbantis E, Kunze S and Herold-Mende C: Autocrine pathways of the
vascular endothelial growth factor (VEGF) in glioblastoma
multiforme: Clinical relevance of radiation-induced increase of
VEGF levels. J Neurooncol. 66:129–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bachelder RE, Crago A, Chung J, Wendt MA,
Shaw LM, Robinson G and Mercurio AM: Vascular endothelial growth
factor is an autocrine survival factor for neuropilin-expressing
breast carcinoma cells. Cancer Res. 61:5736–5740. 2001.PubMed/NCBI
|
|
42
|
Broholm H and Laursen H: Vascular
endothelial growth factor (VEGF) receptor neuropilin-1's
distribution in astrocytic tumors. APMIS. 112:257–263. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gray MJ, Wey JS, Belcheva A, McCarty MF,
Trevino JG, Evans DB, Ellis LM and Gallick GE: Neuropilin-1
suppresses tumorigenic properties in a human pancreatic
adenocarcinoma cell line lacking neuropilin-1 coreceptors. Cancer
Res. 65:3664–3670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan Q, Chanthery Y, Liang WC, Stawicki S,
Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, et al:
Blocking neuropilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kamiya T, Kawakami T, Abe Y, Nishi M,
Onoda N, Miyazaki N, Oida Y, Yamazaki H, Ueyama Y and Nakamura M:
The preserved expression of neuropilin (NRP) 1 contributes to a
better prognosis in colon cancer. Oncol Rep. 15:369–373.
2006.PubMed/NCBI
|
|
46
|
Hong X, Jiang F, Kalkanis SN, Zhang ZG,
Zhang X, Zheng X, Mikkelsen T, Jiang H and Chopp M: Decrease of
endogenous vascular endothelial growth factor may not affect glioma
cell proliferation and invasion. J Exp Ther Oncol. 6:219–229.
2007.PubMed/NCBI
|
|
47
|
Jia H, Bagherzadeh A, Hartzoulakis B,
Jarvis A, Löhr M, Shaikh S, Aqil R, Cheng L, Tickner M, Esposito D,
et al: Characterization of a bicyclic peptide neuropilin-1 (NP-1)
antagonist (EG3287) reveals importance of vascular endothelial
growth factor exon 8 for NP-1 binding and role of NP-1 in KDR
signaling. J Biol Chem. 281:13493–13502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Swendeman S, Mendelson K, Weskamp G,
Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S and Blobel CP:
VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk
between VEGFR2 and ERK signaling. Circ Res. 103:916–918. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu Y, Xiang H, Liu P, Tong RR, Watts RJ,
Koch AW, Sandoval WN, Damico LA, Wong WL and Meng YG:
Identification of circulating neuropilin-1 and dose-dependent
elevation following anti-neuropilin-1 antibody administration.
MAbs. 1:364–369. 2009. View Article : Google Scholar :
|
|
50
|
Gagnon ML, Bielenberg DR, Gechtman Z, Miao
HQ, Takashima S, Soker S and Klagsbrun M: Identification of a
natural soluble neuropilin-1 that binds vascular endothelial growth
factor: In vivo expression and antitumor activity. Proc Natl Acad
Sci USA. 97:2573–2578. 2000. View Article : Google Scholar : PubMed/NCBI
|