In 2008, the Houchen group proposed that
serine-threonine kinase, doublecortin-like kinase 1 (DCLK1, then
known as DCAMKL-1), was a specific marker protein for intestinal
adenoma stem cells (1), which was
the first of a series of research reports providing evidence that
it may be an effective target for oncology drug development. To
date, DCLK1 has been reported to be a selective marker of several
types of cancer stem cells (CSCs), including those in colon,
breast, pancreas, kidney and esophageal cancers (2,3).
The first high-level evidence for the CSC marker status of DCLK1
came in 2012, when Nakanishi et al (4) reported that DCLK1 does not mark
normal stem cells, but specifically marks CSCs in the adenomatous
polyposis coli (APC) loss-driven APCMin/+ model of
intestinal tumorigenesis. Furthermore, in normal gastrointestinal
epithelia, DCLK1 has been indicated to mark fully differentiated
epithelial tuft cells among several other cell types in the gastric
antrum, bile duct and pancreas (5,6).
Epithelial tuft cells are characterized by microtubule bundles
located at the cell apex and express DCLK1 and acetylated α-tubulin
to take part in regulating the microenvironment (7). DCLK1+ tuft cells have a key role in
tumorigenesis by regulating inflammation of the microenvironment
via expression of proteins such as Cox1, Cox2 and hematopoietic
prostaglandin-D synthase in intestinal cancer (8).
After 20 years of research, DCLK1 is accepted as a
specific marker of tuft cells and several types of CSCs and
evidence of its ability to regulate tumor growth and metastasis has
been provided (9). DCLK1 is
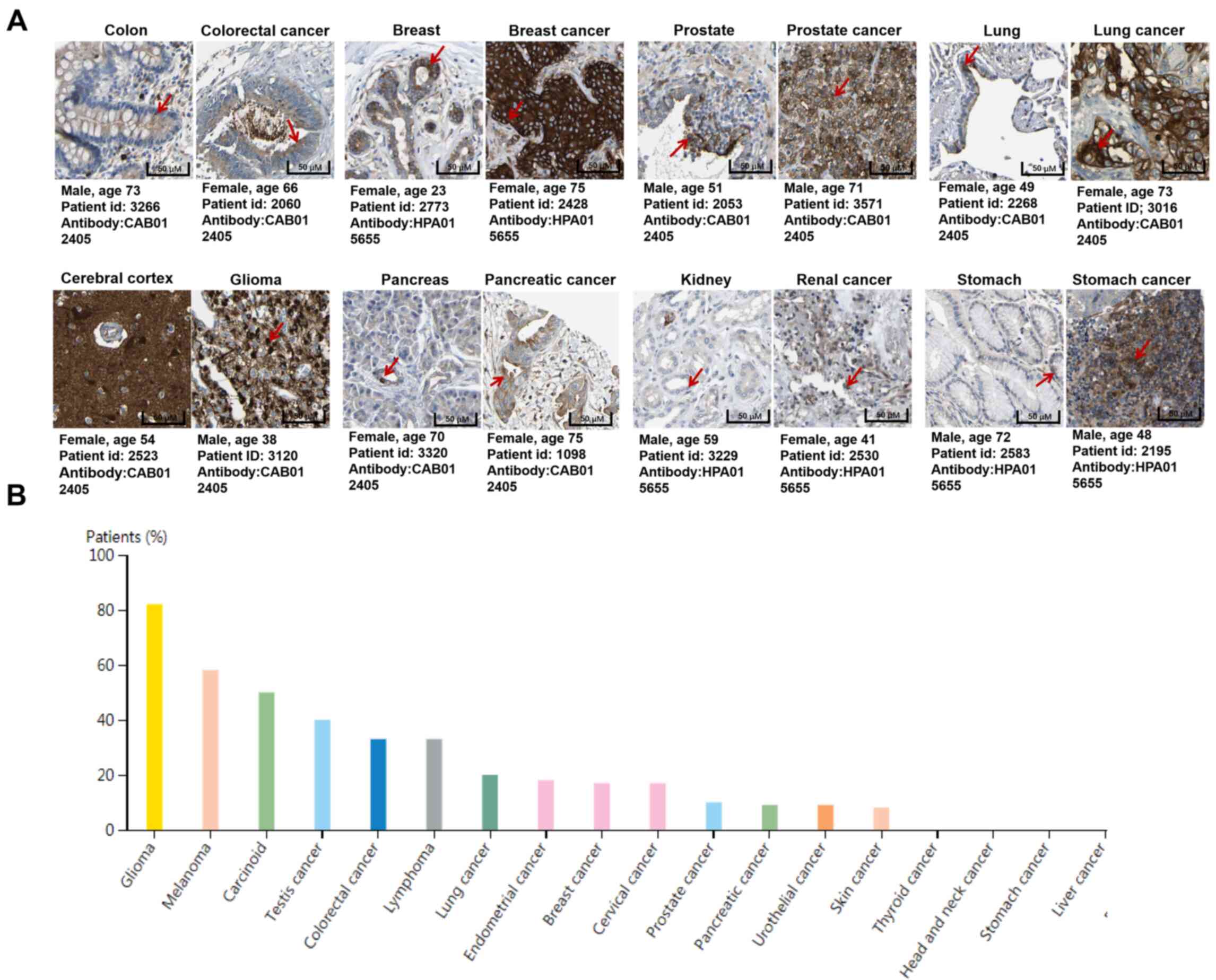
expressed in lung, liver, heart, spleen, thymus, prostate and
intestine and strongly marks specific cell types (Fig. 1) (10–12). In the present review, the
molecular structure and biological mechanisms of DCLK1 in
tumorigenesis and metastasis were summarized and its role in
metabolism was highlighted as a potentially novel area for further
exploration.
The human DCLK1 gene is located on the long arm
13q12.3-q13 of the 13th chromosome, which contains two different
promoter sequences to form splicing variants with different protein
functional domains (13).
Structural domains include two N-terminal doublecortin (DCX)
domains and one C-terminal serine/threonine protein kinase domain,
homologous to the protein kinase superfamily and DCX (14,15). The structural characteristics of
DCX1 are similar to those of DCX, which is able to specifically
bind to microtubules, and DCX2 mainly interacts with microtubules
and their dimers. These two DCX domains allow DCLK1 to bind
microtubules and regulate microtubule aggregation to affect
neuronal migration. The C-terminal domain is similar to calmodulin
dependent kinase II, but lacks a typical calmodulin binding site
(16,17). At present, there are several
splicing variants in the DCLK1 gene that have been identified,
including a full-length type with all domains and a poly-arginine
region, a DCX-like type containing only the microtubule-binding
domains, and a smaller molecular-weight type containing a
phosphoserine-rich region and kinase domain. These variations in
protein domains resulting from alternative splicing and multiple
transcriptional promoter regions are hypothesized to result in
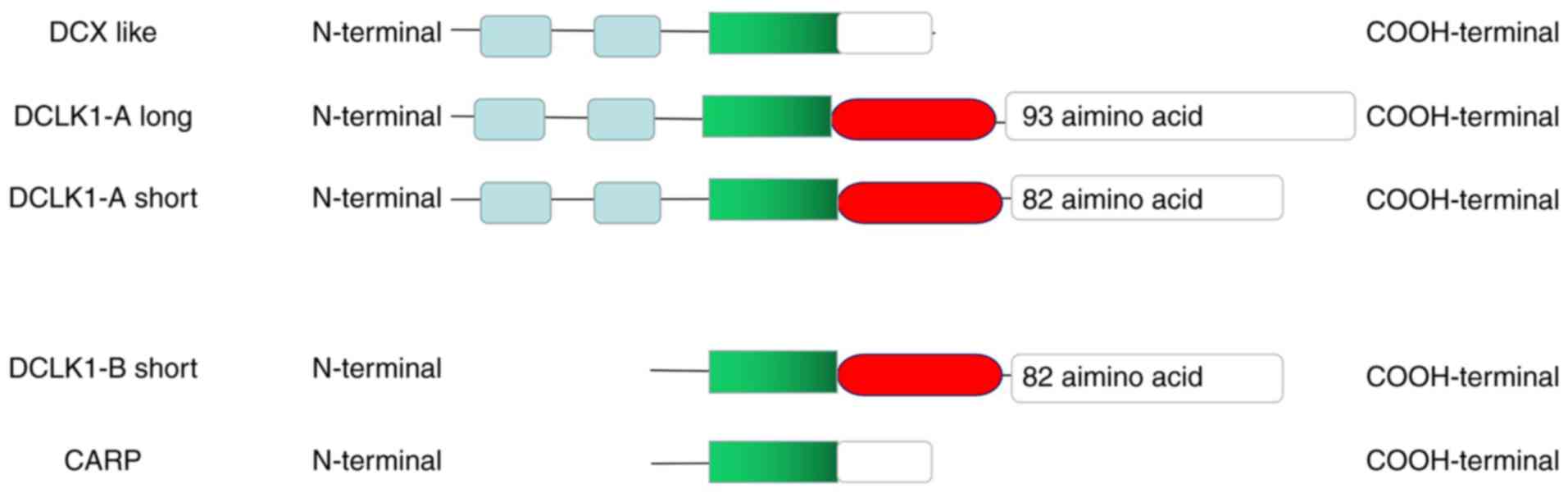
completely different molecular functions (18). Human DCLK1 includes 82- and 52-kDa
isoforms, which are transcribed from an upstream (A, CpG-regulated)
or downstream promoter (B, TATA-box) with differing C-terminal
domains (Fig. 2). The A isoforms
contain N-terminal doublecortin domains to bind to microtubules and
a protein kinase domain, while the B isoforms lack N-terminal
doublecortin domains. Later, DCX-like was identified and only
includes N-terminal DCX domains produced from the A promoter and
Camk-related peptide, a 56 amino acid B-promoter-derived peptide
with unknown function, was also identified (19,20).
The biological activity of DCLK1 in cancer is
different between β-promoter (alternatively termed as DCLK1-S or
DCLK3/4, 45–52 kDa, isoform 3/4) and α-promoter (termed as DCLK1-L
or DCLK1/2, ~82 kDa, isoform 1/2) isoforms. One study determined
that hypermethylation of the α-promoter directly led to the absence
of expression of DCLK1-L in 15 human colon cancer cell lines, and
that the α-promoter was activated by β-catenin and T-cell
factor-4/lymphoid enhancer factor (LEF), while the β-promoter was
activated by NF-κB p65 in cancer cells. In this study, the majority
of human CRCs were reported to express DCLK1-S, which developed an
invasive phenotype and this was associated with unfavorable overall
survival (19). Park et al
(21) identified DCLK1-B
transcription as directly activated by Wnt/β-catenin signaling and
that LEF1 mediates Wnt-induced CSC properties. Sarkar et al
(22) reported that DCLK1-L and
DCLK1-S are in nuclear and mitochondrial fractions, as well as
plasma membrane and cytosolic fractions, but DCLK1-S is in the
nuclei and mitochondria in colon cancer. DCLK1 α-promoter
demonstrated hypermethylation in cholangiocarcinoma, but
hypomethylation in α- and β-promoter regions in renal cell
carcinoma (RCC) (23). Of note,
two mouse models using DCLK1 A-promoter isoforms to drive
Cre-recombinase (DCLK1-CreERT and DCLK1-CreERT2) demonstrated
lineage tracing of CRC tumors in the presence of APC mutation
(24,25). Interestingly, Ge et al
(26) reported that both DCLK1-AS
(isoform 1, 82 kD) and DCLK1-BL (isoform 4) isoforms are able to
efficiently activate epithelial-to-mesenchymal transition (EMT) in
pancreatic ductal adenocarcinoma (PDAC) cell lines. Overall, there
is a significant shortcoming in the literature regarding the
function of DCLK1 isoforms in cancer. The combined evidence
suggests that all major DCLK1 isoforms are oncogenic, but there may
be a variation among different tumor types or even among tumor
subtypes.
The biological function of DCLK1 in tumorigenesis
and metastasis as a marker of tuft cells and CSCs is most
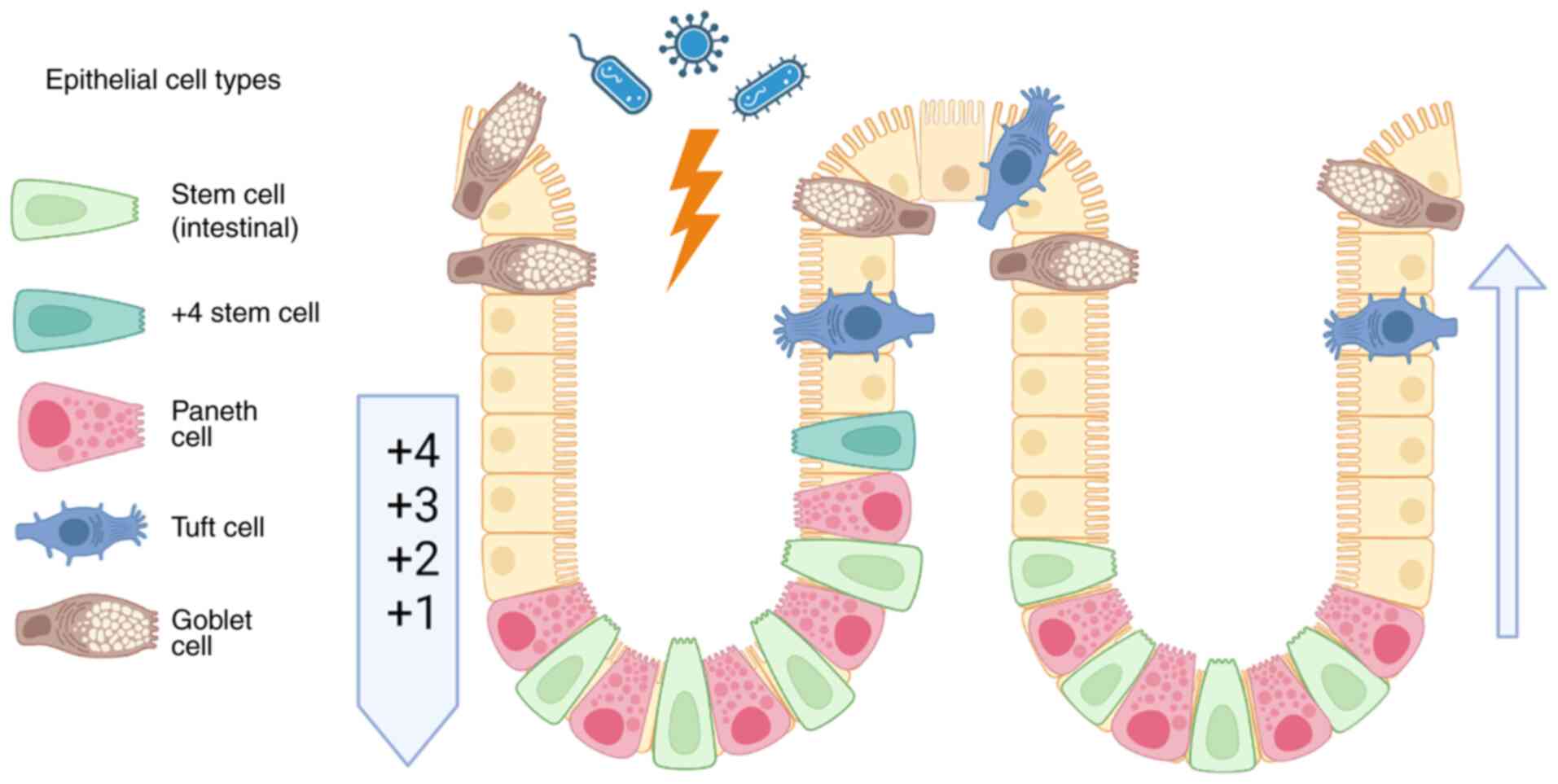
thoroughly studied in CRC. In normal human intestinal tissue, stem
cells are located at the base of the intestinal crypt epithelium,
where they are marked by leucine-rich repeat-containing G
protein-coupled receptor 5 (LGR5) without co-expressing gut
endocrine markers chromogranin A and somatostatin (Fig. 3) (27). DCLK1 marks fully differentiated
intestinal tuft cells located in the crypt and villus (28). Long-lived DCLK1+ tuft cells with
characteristic microvilli feature self-renewal ability and
potential quiescent stem-like functionality (29). Knockdown of the Wnt regulator APC
does not alter this quiescence, but subsequent activation through
inflammation induced by dextran sodium sulfate is sufficient to
initiate colon cancer in Dclk1-Cre/Apcflox/flox
(Dclk1-Cre/Apcflox/flox transgenic mice featuring
knocking out APC gene in DCLK1+ cells) transgenic mice
(30). These findings are
supported in DCLK1-knockout mice where deficiency results in
increased epithelial barrier permeability, higher levels of
pro-inflammatory cytokines and chemokines, decreased levels of LGR5
and dysregulated Wnt/β-Catenin pathway genes in
Villin-Cre/Dclk1flox/flox
(Villin-Cre/Dclk1flox/flox mice featuring deletion of
DCLK1 expression in villin-positive cells) mice (31). In 2009, Gerbe et al
(32) provided conclusive
evidence that DCLK1 was in fact a tuft cell rather than stem cell
marker, as indicated by the position and marker co-expression
(cyclooxygenase enzymes 1/2, advillin and tubulin) of
DCLK1-expressing cells. Lineage tracing studies demonstrated that
DCLK1-positive cells also express colorectal CSC markers, such as
CD133 and CD44 (3,4,33).
Of note, DCLK1-positive normal intestinal epithelial cells isolated
by fluorescence-assisted cell sorting form spheroids that may
assemble into glandular epithelial structures and express multiple
markers of gut epithelial lineages when implanted subcutaneously in
athymic nude mice (27).
Self-renewal and differentiation characteristics of DCLK1-positive
cells and low expression in normal tissue both led researchers to
speculate that they may mark a type of stem cell (34,35), but these findings are no longer
supported in the literature and previous results are likely
artifacts resulting from the existence of rare DCLK1/LGR5
double-positive cells.
Certain studies reported that DCLK1 expression is
significantly higher in CRC tissue and adenomas compared to normal
tissue. In addition, increased levels are seen in distant
metastases and it is closely associated with CRC recurrence
(36,37). Key evidence demonstrated that
DCLK1 specifically marked CSCs in the intestine, which continuously
produce tumor progeny to prompt polyp growth, but there is no
apparent effect on normal tissue after deletion of these cells
(38). Furthermore,
overexpression of DCLK1 was observed to enhance the percentage of
stem-like human CRC cells in vitro (39,40). It has become clear that DCLK1,
while not a bona fide normal stem cell marker, is instead a
key marker of differentiated intestinal epithelial tuft cells
(6,32), which, in the context of mutation
and tumorigenesis, may identify specific CRC stem cells.
A series of reports evidence that DCLK1 regulates
EMT, proliferation and CSC maintenance through the Notch, Ras and
Wnt pathways via interaction with different microRNAs
(miRNAs/miRs). MiR-1291 was observed to directly bind to the
3′-untranslated region sequence of DCLK1 and then inhibited the
stemness and cell cycle through the cyclin-dependent kinase
inhibitors p21WAF1/CIP1 and p27KIP1 (41). The levels of miR-137 and miR-15a
were inversely correlated with high levels of DCLK1 detected in CRC
with larger tumor size, poor differentiation and lymph node
involvement (42). Knockdown of
DCLK1 expression led to downregulation of miR-200a, miR-144 and
miR-let7a along with downregulation of EMT-associated transcription
factors [zinc finger E-box binding homeobox 1 (ZEB1), ZEB2, Snail,
Slug and Twist], c-Myc, KRAS and Notch-1 in human colon cancer
cells (43). In a non-tumorigenic
context, a recent study indicated that miR-195 is able to directly
interact with DCLK1 mRNA, resulting in suppressed function for tuft
and paneth cells in the small intestinal epithelium by inhibiting
DCLK1 translation (44).
In CRC cell lines, Notch pathway-regulated markers
of CRC CSCs [DCLK1, LGR5, aldehyde dehydrogenase 1 family member A1
(ALDH1) and CD44] by JAK2, STAT3 and ERK1/2 phosphorylation and
increased expression of Jagged 1 to promote stemness (45). On the basis of the above findings,
researchers have proposed potential therapeutics to suppress
proliferation, colony formation and reduce the number of DCLK1+
cells via the Notch pathway (46,47). The Wnt pathway promotes elongator
acetyltransferase complex subunit 3 expression and SOX9
translation, which in turn support LGR5(+)/DCLK1(+) intestinal
cancer stem cells in response to intestinal regeneration after
radiation-induced injury (28).
Furthermore, β-catenin nuclear translocation is increased by
overexpression of RNA binding motif protein 3, which induces
stemness in DCLK1(+)/LGR5(+)/CD44(+) CRC cells (48). Wnt/β-catenin pathway and
pluripotency transcription factors c-Myc, KLF transcription factor
4, OCT4 and SOX2 are activated by commensal-polarized macrophages
through a microbiome-induced bystander effect in E.
faecalis-colonized IL10 knockout mice, leading to increases in
the number of DCLK1(+)/CD44(+) cells through gene mutation,
chromosomal instability and endogenous transformation to promote
tumorigenicity (35). Basic
research indicated that compounds, such as γ-mangostin present in
the mangosteen (Garcinia mangostana) fruit, WNT5A agonist
FOXY5 and niclosamide, are able to regulate chemotherapy resistance
and cancer stemness by decreasing the number of DCLK1-positive
cells (21,49,50). KRAS mutation was observed to
upregulate DCLK1 protein levels, which was reversed by inhibiting
KRAS expression (51).
DCLK1 marks a small subpopulation of morphologically
and functionally distinct pancreatic cancer cells, which promote
tumorigenesis in multiple mouse models (52,53). In normal adult pancreas, DCLK1 is
expressed in ductal epithelial cells and islet cells (54) and it is upregulated in murine and
human pancreatic intraepithelial neoplasia (55). It is co-expressed with
neurogenin-3 and somatostatin, and pancreatic stem cell markers,
but not with insulin and glucagon, which mark pancreatic α cells
(24). DCLK1-positive cells
isolated by flow cytometry injected into nude mice give rise to
nodules with a hyperplastic appearance (56). Acetylated tubulin (AcTub), a
marker of differentiation of specific pancreatic intraepithelial
neoplasia, is frequently co-expressed with DCLK1 and regulates
epithelial-mesenchymal transformation of pancreatic cancer cells.
AcTub and DCLK1-marked cells demonstrate a typical tuft cell
morphology with prominent microvilli at the apical surface of the
cell and lead to increased size and number of spheroids in cancer
self-renewal assays (53,57). Furthermore, these cells express
high levels of ABL proto-oncogene 1, non-receptor tyrosine kinase
and insulin-like growth factor 1 receptor, which are drug targets
in clinical cancer therapy (58,59). DCLK1 was also reported to be a
marker of a population of pancreatic cancer-initiating cells with
morphological and molecular features of gastrointestinal tuft cells
(53), which drive pancreatic
tumor growth by immune cell-derived IL17, which in turn regulates
POU class 2 homeobox 3, ALDH1A1 and IL17 receptor C (60). In the pancreas, DCLK1 marks
pancreatic tuft and acinar, but rarely islet cells. DCLK1+ tuft
cells expand in response to chronic injury or chronic inflammation,
and DCLK1+ epithelial cells are a source of acinar-ductal
metaplasia after Kras-G12D mutation. These findings indicated that
DCLK1+ pancreatic cells may act as pancreatic intraepithelial
neoplasia stem cells, but whether or not these arise from
pancreatic DCLK1+ tuft cells or DCLK1+ acinar cells is a matter of
debate (5,61).
In human normal stomach tissue, stem cells are
located in the isthmus of gastric glands and DCLK1-positive cells
were originally located in the gastric stem cell zone. These
DCLK1-positive cells were not able to be labeled by
bromodeoxyuridine, which was consistent with static stem cells
lacking typical cell proliferation ability, suggesting that DCLK1
may be a marker of quiescent stem cells (71). The expression of DCLK1 in gastric
cancer tissues was significantly higher than that in adjacent
normal tissue and significantly correlated with lymph node
metastasis and prognosis (72–75). A recent study suggested that long
non-coding RNA small nucleolar RNA host gene 1 promoted the effects
of DCLK1/Notch1 on the EMT process through regulating miR-15b
expression (76). Small
extracellular vesicle (exosome) isolated from a
DCLK1-overexpressing human gastric cancer cell line promoted the
migration of non-transfected gastric cancer cells in a
kinase-dependent manner (77).
DCLK1 is also a potential biomarker to predict the survival of
patients with gastric cancer (78).
DCLK1 expression progressively increases from
Barrett's esophagus to dysplasia and then to esophageal
adenocarcinoma (2,79). In human esophageal squamous cell
carcinoma (ESCC) cells, DCLK1-S induced MMP2 expression via
MAPK/ERK signaling to activate the EMT (80). Knockdown of DCLK1 inhibited the
progression of ESCC by regulating proliferation, migration and
invasion by suppressing the β-catenin/c-Myc pathway (81). These results indicated that DCLK1
levels are associated with the occurrence and development of
esophageal cancer. In Barrett's esophageal adenocarcinoma, the
expression of DCLK1 and LGR5 are significantly increased in
squamous epithelial cells located at the gastric spout, which
indicates that Barrett's esophageal adenocarcinoma probably comes
from gastric cancer (82,83).
Serum estradiol levels are an important factor of
increased risk of postmenopausal breast cancer. Haakensen et
al (84) detected
differentially expressed genes by analysis of gene microarrays and
indicated that DCLK1 was one of six influenced by serum estradiol.
DCLK1 gene expression was downregulated in breast carcinoma samples
compared with normal tissue samples but did not exhibit any
significantly differential expression between invasive breast
cancer and ductal carcinoma in situ. DCLK1 was not
significant as an independent factor associated with serum
estradiol in a linear regression model. A series of subsequent
studies on DCLK1 expression in breast carcinomas were developed and
the clinical results indicated DCLK1 was associated with
clinicopathological features, estrogen receptor status and
neuroendocrine markers (85). A
cohort study including 1,132 cases reported that DCLK1 levels
varied in several molecular subtypes. Luminal cancers had higher
DCLK1 expression than HER2-overexpression and triple negative
breast cancers (TNBCs). Elevated DCLK1 was associated with a lower
histologic grade, absence of lymphovascular invasion, fibrotic
foci, necrosis and lower pN stage. DCLK1 did not correlate with
other breast CSC markers and stem cell features, but significant
correlations were found with estrogen receptor and neuroendocrine
markers. Zhao et al (86)
used DCLK1 to devise a clinically practical method based on
immunohistochemistry for the molecular subtyping of the mesenchymal
subtype TNBC. Specifically, DCLK1 marked a mesenchymal subtype
enriched in stem cell-related gene signatures and activated
JAK/STAT3 pathway, which is highly correlated with CSC-like breast
cancer cells (86). In support of
these findings, Ramamoorthy et al (87) reported that DCLK1 is downregulated
with ALDH and CD133 downstream of the Notch signaling pathway,
which results in inhibition of TNBC stemness. In breast cancer cell
lines, silencing of DCLK1 decreased the levels of Wnt/β-catenin
pathway proteins such as β-catenin, c-Myc and cyclin D1 to decrease
cell migration and invasion (88). Further basic studies indicated
that DCLK1 is a molecular regulator of breast cancer proliferation,
migration, invasion and a degradome-related metastatic stem-like
profile (88–90). Furthermore, miR-424-5p was
indicated to act as a tumor suppressive miRNA regulating breast
cancer cell proliferation, migration and invasion via binding DCLK1
in vitro (90). In
combination, these findings suggest that DCLK1 is a potential
therapeutic target in breast cancer, but further mechanistic
studies are required.
Only a small number of known markers of CSCs in
kidney cancers is available. Among these are the commonly reported
broad CSC markers ALDH, CD44 and CD133. Ge et al (91) reported that DCLK1 stimulated
essential molecular and functional characteristics of renal CSCs,
including expression of ALDH, self-renewal and resistance to
approved tyrosine kinase inhibitors sunitinib, sorafenib,
everolimus and temsirolimus, suggesting that DCLK1 is a potential
renal CSC marker. Furthermore, they indicated that overexpression
of DCLK1 was a direct regulatory factor in renal clear carcinoma
progression, supporting the notion that DCLK1 is a potential CSC
target to inhibit RCC metastasis in early stages (3). Of note, treatment with a
DCLK1-targeted monoclonal antibody was able to inhibit
tumorigenesis in ACHN renal cancer xenografts, suggesting a
potential therapeutic strategy for this highly chemoresistant
cancer (91). In addition, a
small-molecule kinase inhibitor of DCLK1, DCLK1-IN-1, demonstrated
obvious inhibition of immune checkpoint ligand programmed death
ligand 1 and an apparent increase in immune-mediated cytotoxicity
alone or in combination with anti-programmed death 1 therapy by
suppressing DCLK1 phosphorylation and downregulating pluripotency
factors and CSC- or EMT-associated markers, including c-MET, c-MYC
and N-cadherin in RCC cell lines. These experimental results were
consistent with the analysis of clinical populations in which DCLK1
predicted RCC survival. In addition, its expression was correlated
with reduced CD8+ cytotoxic T-cell infiltration and increased in M2
immunosuppressive macrophage populations (92).
To date, DCLK1 has not been identified to be a
hepatocellular CSC marker. However, the expression of DCLK1 in
chronic hepatitis, cirrhosis and hepatocellular carcinoma was
significantly increased (93).
DCLK1 is mainly expressed in epithelial and stromal cells,
lymphocytes and bile duct cells of liver tissue of patients with
chronic hepatitis C virus infection. Furthermore, the level of
DCLK1 is related to the expression of S100A9 protein (94). S100A9 is a key protein of
pro-inflammatory signaling by binding to advanced glycation end
product receptor and toll-like receptor 4 to activate the NFκB
pathway (95). Upregulation of
DCLK1 may promote the expression of S100A9 protein, while
downregulation of DCLK1 directly reduces the expression of S100A9
protein and reduces signal cell infiltration of inflammatory cells
(96). Ali et al (94) reported that DCLK1 was
overexpressed in liver cells infected with hepatitis C virus and
further results indicated that DCLK1 was involved in the
replication of hepatitis C virus. According to recent findings,
tuft cells express CD300lf (a murine norovirus receptor) and are
virally induced to proliferate through this receptor to improve
murine norovirus infection. Although research in this area is
limited, it is worth considering if tuft cells in the intestine may
similarly take part in the replication of hepatitis C and other
viruses (97). Liver tissues from
patients with cirrhosis and HCC exhibited overexpression of DCLK1,
β-catenin and cleaved E-cadherin. DCLK1-overexpressing hepatoma
cells induced high levels of β-catenin, α-fetoprotein and SOX9,
which led to clonogenicity and dedifferentiated phenotypes
(98). In HCC tumors,
DCLK1-positive cells have characteristics of CSCs and co-express
marker proteins CD133, LGR5, Lin28, AFP and c-Myc (99,100). DCLK1 may be a new target for the
treatment of hepatitis C virus-induced tumorigenesis. However, the
stem cell characteristics of DCLK1 in hepatocellular carcinoma
require confirmation by further research.
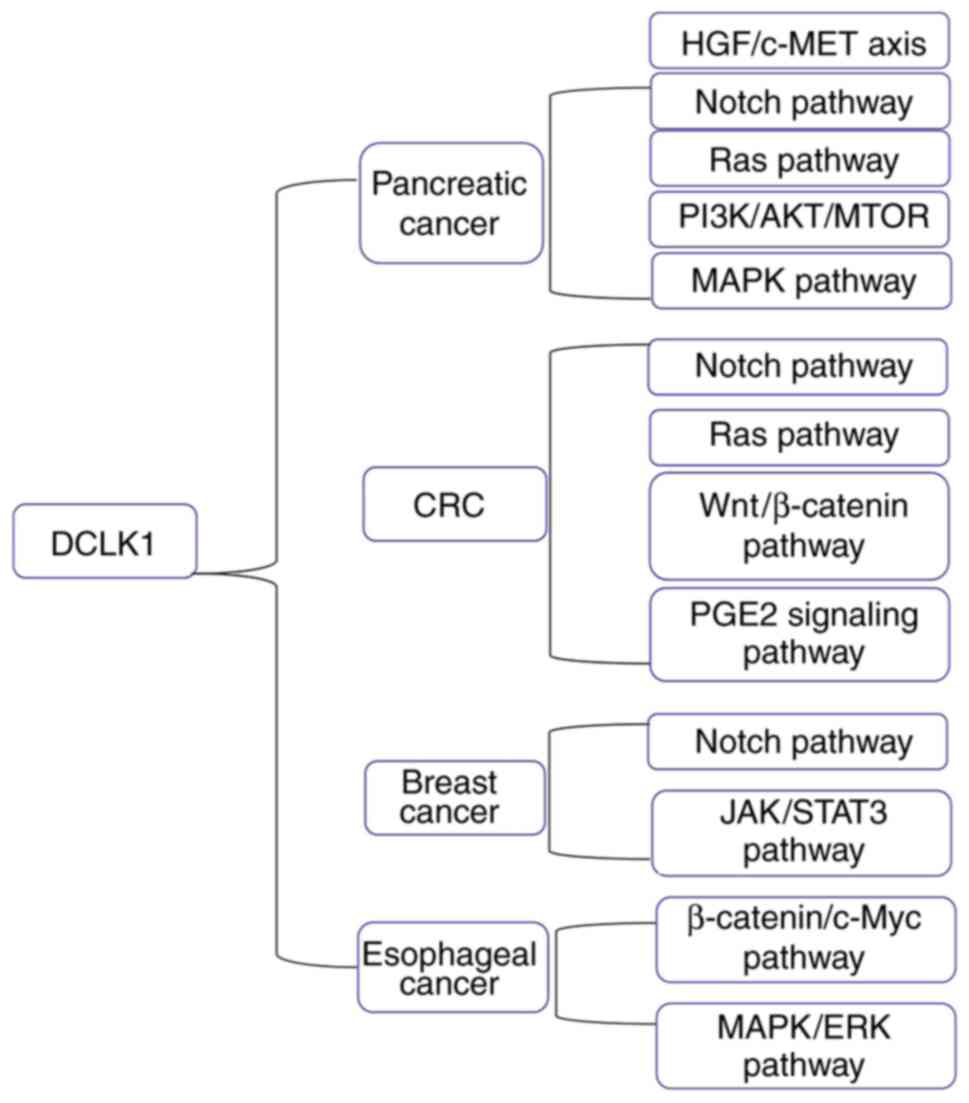
All related signaling pathways of DCLK1 in different
types of cancer are illustrated in Fig. 4.
DCLK1 is one of the most important CSC markers due
to its role in promoting tumorigenesis, metastasis, invasion and
drug resistance by supporting self-renewal, stemness properties and
quiescence, with activating signaling pathways including Wnt, Ras
and Notch (101,102). DCLK1 represents a more specific
CSC marker, compared with previously studied markers for
colorectal, pancreatic and possibly other cancer types, such as
gastric cancer, esophageal cancer, breast cancer and renal
carcinoma. Development of drugs targeting DCLK1 has been reported,
including kinase inhibitors LRRK2-IN-1, XMD8-92 and DCLK1-IN-1;
monoclonal antibody CBT-15 (targeting DCLK1′s extracellular
C-terminus); and chimeric antigen receptor T-cell therapy (CAR-T_
CBT-511) (91,103–105). These drugs exhibited anti-tumor
effects via regulating EMT, angiogenesis, proliferation, migration,
invasion, apoptosis, cell cycle, DNA damage and stemness in several
different cancer types (106)
(Table I).
Notch, Wnt/β-catenin and RAS pathways are closely
related to DCLK1 in regulating stemness, tumorigenesis, metastasis
and drug resistance of several different cancer types. At present,
increasing attention is paid to the energy metabolism of CSCs, as
the common antitumor treatments aiming to decrease tumor size or
reduce proliferating tumor cells may fail to target CSCs, which
accounts for this therapeutic treatment resistance (107). Furthermore, the metabolic type
for CSCs is primarily dominated by oxidative phosphorylation but
not glycolysis, as CSCs consume more oxygen, produce higher levels
of ATP and increase mitochondrial mass and membrane potential
compared with the bulk of differentiated cancer cells, which rely
on glycolysis. The limited but emerging data in this field suggest
the importance of further investigation of the relationship between
DCLK1 and DCLK1+ CSCs and metabolism (108).
Despite promising findings regarding DCLK1-targeted
agents, successfully targeting DCLK1 and avoiding toxicity and
other concerns will require a thorough exploration of the roles of
DCLK1 in other biological aspects. In 2013, Verissimo et al
(109) first reported that
knockdown of DCL, a splice variant of DCLK1, is related to reduced
mitochondrial activity, which significantly decreased tumor growth
in neuroblastoma xenografts. In this study, DCL affected oxidative
phosphorylation by interacting with the mitochondrial outer
membrane protein outer membrane protein 25/synaptojanin 2 binding
protein. However, DCL lacks the kinase domain and kinase catalytic
and autoinhibitory activity present in other prominent DCLK1
isoforms (110). However, new
evidence suggests that DCLK1 may also be important in conditions of
altered metabolism. First, MCF-7 breast cancer cells deregulated
the metabolism by triggering transcriptomic reprogramming closely
related to DCLK1 levels (111).
These findings suggest accelerated dedifferentiation towards a more
stem-like state and that DCLK1 may be a key part of this process.
Coincidentally, in a non-cancer context, an isoform of DCLK1,
candidate plasticity gene 16 (CPG16; also known as DCLK1-BL or
DCLK1-Short), was identified as a negative regulator of insulin
gene expression, which was increased by long-term exposure of
pancreatic β-cells to a high-glucose medium (112). In addition, CPG16 suppressed the
jun dimerization protein 2-mediated upregulation of insulin
promoter activity in a kinase activity-dependent manner under
glucotoxic conditions (113). Of
note, Zhao et al (114)
reported that glycolysis promotes the expression of DCLK1 and
maintains the CSC and EMT phenotypes via maintenance of low
reactive oxygen species levels in gemcitabine-resistant Patu8988
pancreatic cancer cells. Together, these findings suggest that
DCLK1 may be a key target of glucose metabolism inhibiting drugs
such as metformin, which may be helpful in decreasing the incidence
of cancer. The limited but emerging data in this field suggest the
importance of further investigation of the relationship between
DCLK1 function and metabolism.
DCLK1 as a marker of tuft cells and CSCs is closely
related to tumorigenesis and metastasis in various cancer types,
including gastrointestinal, breast, renal and other cancers. The
DCLK1 isoforms have different functions in the development and
progression of the above cancers. Furthermore, the evidence for the
emergence of tumors related to various signaling pathways has been
linked to DCLK1 in the literature (e.g. Notch, WNT and RAS
signaling pathways). Several drugs have been developed by targeting
the genetic or kinase activity of DCLK1, and in the future,
metabolic regulation via glycolysis and regulation of insulin
expression by targeting DCLK1 is worthy of further study.
It is well known that DCLK1 expression is obviously
significant in melanoma, testicular cancer, lymphoma and
endometrial cancer (9), besides
the above ones, but only a small number of studies have been
performed on them until now. Thus, by including these data, it is
esteemed that other groups in these specific subfields of oncology
may become aware of and consider researching DCLK1 in their
respective projects.
Not applicable.
This research was funded by the CHEN Ke-ji Integrative Medicine
Development Fund (grant nos. CKJ 2021011 and CKJ 2021010), National
Natural Science Foundation of China (grant no. 81873166).
Data sharing is not applicable.
Study conception and design: ZC, ZY and QL;
Searching and selection the literature: QL, HF, HC and JD; writing
the original manuscript: QL, HF, HC; revision of the manuscript:
ZC, ZY and NW. All authors read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
May R, Riehl TE, Hunt C, Sureban SM, Anant
S and Houchen CW: Identification of a novel putative
gastrointestinal stem cell and adenoma stem cell marker,
doublecortin and CaM kinase-like-1, following radiation injury and
in adenomatous polyposis coli/multiple intestinal neoplasia mice.
Stem Cells. 26:630–637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vega KJ, May R, Sureban SM, Lightfoot SA,
Qu D, Reed A, Weygant N, Ramanujam R, Souza R, Madhoun M, et al:
Identification of the putative intestinal stem cell marker
doublecortin and CaM kinase-like-1 in Barrett's esophagus and
esophageal adenocarcinoma. J Gastroenterol Hepatol. 27:773–780.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weygant N, Qu D, May R, Tierney RM, Berry
WL, Zhao L, Agarwal S, Chandrakesan P, Chinthalapally HR, Murphy
NT, et al: DCLK1 is a broadly dysregulated target against
epithelial-mesenchymal transition, focal adhesion, and stemness in
clear cell renal carcinoma. Oncotarget. 6:2193–2205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delgiorno KE, Hall JC, Takeuchi KK, Pan
FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B,
Wright CV, et al: Identification and manipulation of biliary
metaplasia in pancreatic tumors. Gastroenterology. 146:233–244.e5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saqui-Salces M, Keeley TM, Grosse AS, Qiao
XT, El-Zaatari M, Gumucio DL, Samuelson LC and Merchant JL: Gastric
tuft cells express DCLK1 and are expanded in hyperplasia. Histochem
Cell Biol. 136:191–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerbe F, van Es JH, Makrini L, Brulin B,
Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF,
Pignodel C, et al: Distinct ATOH1 and Neurog3 requirements define
tuft cells as a new secretory cell type in the intestinal
epithelium. J Cell Biol. 192:767–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howitt MR, Lavoie S, Michaud M, Blum AM,
Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF,
Osborne LC, et al: Tuft cells, taste-chemosensory cells,
orchestrate parasite type 2 immunity in the gut. Science.
351:1329–1333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Westphalen CB, Quante M and Wang TC:
Functional implication of Dclk1 and Dclk1-expressing cells in
cancer. Small GTPases. 8:164–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi J, Bergstrom K, Fu J, Shan X, McDaniel
JM, McGee S, Qu D, Houchen CW, Liu X and Xia L: Dclk1 in tuft cells
promotes inflammation-driven epithelial restitution and mitigates
chronic colitis. Cell Death Differ. 26:1656–1669. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patel O, Dai W, Mentzel M, Griffin MD,
Serindoux J, Gay Y, Fischer S, Sterle S, Kropp A, Burns CJ, et al:
Biochemical and structural insights into doublecortin-like kinase
domain 1. Structure. 24:1550–1561. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung AS, de Rooy C, Levinger I, Rana K,
Clarke MV, How JM, Garnham A, McLean C, Zajac JD, Davey RA and
Grossmann M: Actin alpha cardiac muscle 1 gene expression is
upregulated in the skeletal muscle of men undergoing androgen
deprivation therapy for prostate cancer. J Steroid Biochem Mol
Biol. 174:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto N, Pilz DT and Ledbetter DH:
Genomic structure, chromosomal mapping, and expression pattern of
human DCAMKL1 (KIAA0369), a homologue of DCX (XLIS). Genomics.
56:179–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burgess HA and Reiner O: Cleavage of
doublecortin-like kinase by calpain releases an active kinase
fragment from a microtubule anchorage domain. J Biol Chem.
276:36397–36403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim MH, Cierpicki T, Derewenda U,
Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J,
Bushweller JH and Derewenda ZS: The DCX-domain tandems of
doublecortin and doublecortin-like kinase. Nat Struct Biol.
10:324–333. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin PT, Gleeson JG, Corbo JC, Flanagan L
and Walsh CA: DCAMKL1 encodes a protein kinase with homology to
doublecortin that regulates microtubule polymerization. J Neurosci.
20:9152–9161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engels BM, Schouten TG, van Dullemen J,
Gosens I and Vreugdenhil E: Functional differences between two DCLK
splice variants. Brain Res Mol Brain Res. 120:103–114. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burgess HA and Reiner O: Alternative
splice variants of doublecortin-like kinase are differentially
expressed and have different kinase activities. J Biol Chem.
277:17696–17705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Connell MR, Sarkar S, Luthra GK, Okugawa
Y, Toiyama Y, Gajjar AH, Qiu S, Goel A and Singh P: Epigenetic
changes and alternate promoter usage by human colon cancers for
expressing DCLK1-isoforms: Clinical Implications. Sci Rep.
5:149832015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walker TL, Yasuda T, Adams DJ and Bartlett
PF: The doublecortin-expressing population in the developing and
adult brain contains multipotential precursors in addition to
neuronal-lineage cells. J Neurosci. 27:3734–3742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SY, Kim JY, Choi JH, Kim JH, Lee CJ,
Singh P, Sarkar S, Baek JH and Nam JS: Inhibition of LEF1-mediated
DCLK1 by niclosamide attenuates colorectal cancer stemness. Clin
Cancer Res. 25:1415–1429. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarkar S, Popov VL, O'Connell MR,
Stevenson HL, Lee BS, Obeid RA, Luthra GK and Singh P: A novel
antibody against cancer stem cell biomarker, DCLK1-S, is
potentially useful for assessing colon cancer risk after screening
colonoscopy. Lab Invest. 97:1245–1261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andresen K, Boberg KM, Vedeld HM, Honne H,
Hektoen M, Wadsworth CA, Clausen OP, Karlsen TH, Foss A, Mathisen
O, et al: Novel target genes and a valid biomarker panel identified
for cholangiocarcinoma. Epigenetics. 7:1249–1257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westphalen CB, Takemoto Y, Tanaka T,
Macchini M, Jiang Z, Renz BW, Chen X, Ormanns S, Nagar K, Tailor Y,
et al: Dclk1 defines quiescent pancreatic progenitors that promote
injury-induced regeneration and tumorigenesis. Cell Stem Cell.
18:441–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaga Y, Fukuda A, Nakanishi Y, Goto N,
Matsumoto Y, Yoshioka T, Maruno T, Chiba T and Seno H: Gene
expression profile of Dclk1+ cells in intestinal tumors.
Dig Liver Dis. 50:1353–1361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ge Y, Liu H, Zhang Y, Liu J, Yan R, Xiao
Z, Fan X, Huang X and An G: Inhibition of DCLK1 kinase reverses
epithelial-mesenchymal transition and restores T-cell activity in
pancreatic ductal adenocarcinoma. Transl Oncol. 17:1013172022.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
May R, Sureban SM, Hoang N, Riehl TE,
Lightfoot SA, Ramanujam R, Wyche JH, Anant S and Houchen CW:
Doublecortin and CaM kinase-like-1 and
leucine-rich-repeat-containing G-protein-coupled receptor mark
quiescent and cycling intestinal stem cells, respectively. Stem
Cells. 27:2571–2579. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ladang A, Rapino F, Heukamp LC, Tharun L,
Shostak K, Hermand D, Delaunay S, Klevernic I, Jiang Z, Jacques N,
et al: Elp3 drives Wnt-dependent tumor initiation and regeneration
in the intestine. J Exp Med. 212:2057–2075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leppänen J, Helminen O, Huhta H, Kauppila
JH, Miinalainen I, Ronkainen VP, Saarnio J, Lehenkari PP and
Karttunen TJ: Doublecortin-like kinase 1-positive enterocyte-a new
cell type in human intestine. APMIS. 124:958–965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Westphalen CB, Asfaha S, Hayakawa Y,
Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H,
Muley A, et al: Long-lived intestinal tuft cells serve as colon
cancer-initiating cells. J Clin Invest. 124:1283–1295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu D, Weygant N, May R, Chandrakesan P,
Madhoun M, Ali N, Sureban SM, An G, Schlosser MJ and Houchen CW:
Ablation of doublecortin-like kinase 1 in the colonic epithelium
exacerbates dextran sulfate sodium-induced colitis. PLoS One.
10:e01342122015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerbe F, Brulin B, Makrini L, Legraverend
C and Jay P: DCAMKL-1 expression identifies Tuft cells rather than
stem cells in the adult mouse intestinal epithelium.
Gastroenterology. 137:2179–2181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eini L, Naseri M, Karimi-Busheri F,
Bozorgmehr M, Ghods R and Madjd Z: Primary colonospheres maintain
stem cell-like key features after cryopreservation. J Cell Physiol.
235:2452–2463. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chandrakesan P, Yao J, Qu D, May R,
Weygant N, Ge Y, Ali N, Sureban SM, Gude M, Vega K, et al: Dclk1, a
tumor stem cell marker, regulates pro-survival signaling and
self-renewal of intestinal tumor cells. Mol Cancer. 16:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Yang Y and Huycke MM:
Commensal-infected macrophages induce dedifferentiation and
reprogramming of epithelial cells during colorectal carcinogenesis.
Oncotarget. 8:102176–102190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gagliardi G, Goswami M, Passera R and
Bellows CF: DCLK1 immunoreactivity in colorectal neoplasia. Clin
Exp Gastroenterol. 5:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vedeld HM, Skotheim RI, Lothe RA and Lind
GE: The recently suggested intestinal cancer stem cell marker DCLK1
is an epigenetic biomarker for colorectal cancer. Epigenetics.
9:346–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takiyama A, Tanaka T, Kazama S, Nagata H,
Kawai K, Hata K, Otani K, Nishikawa T, Sasaki K, Kaneko M, et al:
DCLK1 expression in colorectal polyps increases with the severity
of dysplasia. In Vivo. 32:365–371. 2018.PubMed/NCBI
|
|
39
|
Ahmed I, Roy BC, Raach RT, Owens SM, Xia
L, Anant S, Sampath V and Umar S: Enteric infection coupled with
chronic Notch pathway inhibition alters colonic mucus composition
leading to dysbiosis, barrier disruption and colitis. PLoS One.
13:e02067012018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mirzaei A, Tavoosidana G, Modarressi MH,
Rad AA, Fazeli MS, Shirkoohi R, Tavakoli-Yaraki M and Madjd Z:
Upregulation of circulating cancer stem cell marker, DCLK1 but not
Lgr5, in chemoradiotherapy-treated colorectal cancer patients.
Tumour Biol. 36:4801–4810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Yokoyama Y, Hirose H, Shimomura Y,
Bonkobara S, Itakura H, Kouda S, Morimoto Y, Minami K, Takahashi H,
et al: Functional assessment of miR-1291 in colon cancer cells. Int
J Oncol. 60:132022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Razi S, Sadeghi A, Asadi-Lari Z, Tam KJ,
Kalantari E and Madjd Z: DCLK1, a promising colorectal cancer stem
cell marker, regulates tumor progression and invasion through
miR-137 and miR-15a dependent manner. Clin Exp Med. 21:139–147.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sureban SM, May R, Mondalek FG, Qu D,
Ponnurangam S, Pantazis P, Anant S, Ramanujam RP and Houchen CW:
Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144
and inhibits colorectal cancer tumor growth via a Notch-1 dependent
mechanism. J Nanobiotechnology. 9:402011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwon MS, Chung HK, Xiao L, Yu TX, Wang SR,
Piao JJ, Gorospe M and Wang JY: MicroRNA-195 regulates Tuft cell
function in the intestinal epithelium by altering translation of
DCLK1. Am J Physiol Cell Physiol. 320:C1042–C1054. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Neradugomma NK, Subramaniam D, Tawfik OW,
Goffin V, Kumar TR, Jensen RA and Anant S: Prolactin signaling
enhances colon cancer stemness by modulating Notch signaling in a
Jak2-STAT3/ERK manner. Carcinogenesis. 35:795–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ahmed I, Roy BC, Subramaniam D, Ganie SA,
Kwatra D, Dixon D, Anant S, Zargar MA and Umar S: An ornamental
plant targets epigenetic signaling to block cancer stem cell-driven
colon carcinogenesis. Carcinogenesis. 37:385–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ponnurangam S, Dandawate PR, Dhar A,
Tawfik OW, Parab RR, Mishra PD, Ranadive P, Sharma R, Mahajan G,
Umar S, et al: Quinomycin A targets Notch signaling pathway in
pancreatic cancer stem cells. Oncotarget. 7:3217–3232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Venugopal A, Subramaniam D, Balmaceda J,
Roy B, Dixon DA, Umar S, Weir SJ and Anant S: RNA binding protein
RBM3 increases β-catenin signaling to increase stem cell
characteristics in colorectal cancer cells. Mol Carcinog.
55:1503–1516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Krishnamachary B, Subramaniam D, Dandawate
P, Ponnurangam S, Srinivasan P, Ramamoorthy P, Umar S, Thomas SM,
Dhar A, Septer S, et al: Targeting transcription factor TCF4 by
γ-mangostin, a natural xanthone. Oncotarget. 10:5576–5591. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Osman J, Bellamkonda K, Liu Q, Andersson T
and Sjölander A: The WNT5A agonist Foxy5 reduces the number of
colonic cancer stem cells in a xenograft mouse model of human
colonic cancer. Anticancer Res. 39:1719–1728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hammond DE, Mageean CJ, Rusilowicz EV,
Wickenden JA, Clague MJ and Prior IA: Differential reprogramming of
isogenic colorectal cancer cells by distinct activating KRAS
mutations. J Proteome Res. 14:1535–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qiu W, Remotti HE, Tang SM, Wang E,
Dobberteen L, Lee Youssof A, Lee JH, Cheung EC and Su GH:
Pancreatic DCLK1+ cells originate distinctly from
PDX1+ progenitors and contribute to the initiation of
intraductal papillary mucinous neoplasm in mice. Cancer Lett.
423:71–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bailey JM, Alsina J, Rasheed ZA,
McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N,
Matsui W, et al: DCLK1 marks a morphologically distinct
subpopulation of cells with stem cell properties in preinvasive
pancreatic cancer. Gastroenterology. 146:245–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
May R, Sureban SM, Lightfoot SA, Hoskins
AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S
and Houchen CW: Identification of a novel putative pancreatic
stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J
Physiol Gastrointest Liver Physiol. 299:G303–G310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG
and Houchen CW: DCLK1 regulates pluripotency and angiogenic factors
via microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yao ZX, Qin ML, Liu JJ, Chen XS and Zhou
DS: In vitro cultivation of human fetal pancreatic ductal stem
cells and their differentiation into insulin-producing cells. World
J Gastroenterol. 10:1452–1456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Seeley ES, Carrière C, Goetze T,
Longnecker DS and Korc M: Pancreatic cancer and precursor
pancreatic intraepithelial neoplasia lesions are devoid of primary
cilia. Cancer Res. 69:422–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lee H, Basso IN and Kim DDH: Target
spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic
myeloid leukemia. Int J Hematol. 113:632–641. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang Y, Gao C, Cao F, Wu Y, Chen S, Han
X, Mo J, Qiu Z, Fan W, Zhou P and Shen L: Pan-cancer analysis of
IGF-1 and IGF-1R as potential prognostic biomarkers and
immunotherapy targets. Front Oncol. 11:7553412021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Y, Zoltan M, Riquelme E, Xu H, Sahin
I, Castro-Pando S, Montiel MF, Chang K, Jiang Z, Ling J, et al:
Immune cell production of interleukin 17 induces stem cell features
of pancreatic intraepithelial neoplasia cells. Gastroenterology.
155:210–223.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
DelGiorno KE, Naeem RF, Fang L, Chung CY,
Ramos C, Luhtala N, O'Connor C, Hunter T, Manor U and Wahl GM: Tuft
cell formation reflects epithelial plasticity in pancreatic injury:
Implications for modeling human pancreatitis. Front Physiol.
11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park JT and Leach SD: Zebrafish model of
KRAS-initiated pancreatic cancer. Anim Cells Syst (Seoul).
22:353–359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou B, Irwanto A, Guo YM, Bei JX, Wu Q,
Chen G, Zhang TP, Lei JJ, Feng QS, Chen LZ, et al: Exome sequencing
and digital PCR analyses reveal novel mutated genes related to the
metastasis of pancreatic ductal adenocarcinoma. Cancer Biol Ther.
13:871–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qu D, Weygant N, Yao J, Chandrakesan P,
Berry WL, May R, Pitts K, Husain S, Lightfoot S, Li M, et al:
Overexpression of DCLK1-AL increases tumor cell invasion, drug
resistance, and KRAS activation and can be targeted to inhibit
tumorigenesis in pancreatic cancer. J Oncol. 2019:64029252019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chandrakesan P, Panneerselvam J, May R,
Weygant N, Qu D, Berry WR, Pitts K, Stanger BZ, Rao CV, Bronze MS
and Houchen CW: DCLK1-isoform2 alternative splice variant promotes
pancreatic tumor immunosuppressive M2-macrophage polarization. Mol
Cancer Ther. 19:1539–1549. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu Z, Pang TCY, Liu AC, Pothula SP,
Mekapogu AR, Perera CJ, Murakami T, Goldstein D, Pirola RC, Wilson
JS and Apte MV: Targeting the HGF/c-MET pathway in advanced
pancreatic cancer: A key element of treatment that limits primary
tumour growth and eliminates metastasis. Br J Cancer.
122:1486–1495. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rieder S, Michalski CW, Friess H and
Kleeff J: Insulin-like growth factor signaling as a therapeutic
target in pancreatic cancer. Anticancer Agents Med Chem.
11:427–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sureban SM, May R, Lightfoot SA, Hoskins
AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao
CV, et al: DCAMKL-1 regulates epithelial-mesenchymal transition in
human pancreatic cells through a miR-200a-dependent mechanism.
Cancer Res. 71:2328–2338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bjerknes M, Khandanpour C, Moroy T,
Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martín MG
and Cheng H: Origin of the brush cell lineage in the mouse
intestinal epithelium. Dev Biol. 362:194–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ali Y, Lin Y, Gharibo MM, Gounder MK,
Stein MN, Lagattuta TF, Egorin MJ, Rubin EH and Poplin EA: Phase I
and pharmacokinetic study of imatinib mesylate (Gleevec) and
gemcitabine in patients with refractory solid tumors. Clin Cancer
Res. 13:5876–5882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Giannakis M, Stappenbeck TS, Mills JC,
Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam
M, Brent MR and Gordon JI: Molecular properties of adult mouse
gastric and intestinal epithelial progenitors in their niches. J
Biol Chem. 281:11292–11300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Weygant N, Ge Y, Qu D, Kaddis JS, Berry
WL, May R, Chandrakesan P, Bannerman-Menson E, Vega KJ, Tomasek JJ,
et al: Survival of patients with gastrointestinal cancers can be
predicted by a surrogate microRNA signature for cancer stem-like
cells marked by DCLK1 kinase. Cancer Res. 76:4090–4099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang Y and Huang X: Investigation of
doublecortin and calcium/calmodulin-dependent protein
kinase-like-1-expressing cells in the mouse stomach. J
Gastroenterol Hepatol. 25:576–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Meng QB, Yu JC, Kang WM, Ma ZQ, Zhou WX,
Li J, Zhou L, Cao ZJ and Tian SB: Expression of doublecortin-like
kinase 1 in human gastric cancer and its correlation with
prognosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35:639–644.
2013.(In Chinese). PubMed/NCBI
|
|
75
|
Sureban SM, Qu D and Houchen CW:
Regulation of miRNAs by agents targeting the tumor stem cell
markers DCLK1, MSI1, LGR5, and BMI1. Curr Pharmacol Rep. 1:217–222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu ZQ, He WF, Wu YJ, Zhao SL, Wang L,
Ouyang YY and Tang SY: LncRNA SNHG1 promotes EMT process in gastric
cancer cells through regulation of the miR-15b/DCLK1/Notch1 axis.
BMC Gastroenterol. 20:1562020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Carli ALE, Afshar-Sterle S, Rai A, Fang H,
O'Keefe R, Tse J, Ferguson FM, Gray NS, Ernst M, Greening DW and
Buchert M: Cancer stem cell marker DCLK1 reprograms small
extracellular vesicles toward migratory phenotype in gastric cancer
cells. Proteomics. 21:e20000982021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dai J, Li ZX, Zhang Y, Ma JL, Zhou T, You
WC, Li WQ and Pan KF: Whole genome messenger RNA profiling
identifies a novel signature to predict gastric cancer survival.
Clin Transl Gastroenterol. 10:e000042019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schellnegger R, Quante A, Rospleszcz S,
Schernhammer M, Höhl B, Tobiasch M, Pastula A, Brandtner A, Abrams
JA, Strauch K, et al: Goblet cell ratio in combination with
differentiation and stem cell markers in barrett esophagus allow
distinction of patients with and without esophageal adenocarcinoma.
Cancer Prev Res (Phila). 10:55–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ge Y, Fan X, Huang X, Weygant N, Xiao Z,
Yan R, Liu H, Liu J, An G and Yao J: DCLK1-short splice variant
promotes esophageal squamous cell carcinoma progression via the
MAPK/ERK/MMP2 pathway. Mol Cancer Res. 19:1980–1991. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang L, Zhou S, Guo E, Chen X, Yang J and
Li X: DCLK1 inhibition attenuates tumorigenesis and improves
chemosensitivity in esophageal squamous cell carcinoma by
inhibiting β-catenin/c-Myc signaling. Pflugers Arch. 472:1041–1049.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Whorton J, Sureban SM, May R, Qu D,
Lightfoot SA, Madhoun M, Johnson M, Tierney WM, Maple JT, Vega KJ
and Houchen CW: DCLK1 is detectable in plasma of patients with
Barrett's esophagus and esophageal adenocarcinoma. Dig Dis Sci.
60:509–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Quante M, Bhagat G, Abrams JA, Marache F,
Good P, Lee MD, Lee Y, Friedman R, Asfaha S, Dubeykovskaya Z, et
al: Bile acid and inflammation activate gastric cardia stem cells
in a mouse model of Barrett-like metaplasia. Cancer Cell. 21:36–51.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Haakensen VD, Bjøro T, Lüders T, Riis M,
Bukholm IK, Kristensen VN, Troester MA, Homen MM, Ursin G,
Børresen-Dale AL and Helland Å: Serum estradiol levels associated
with specific gene expression patterns in normal breast tissue and
in breast carcinomas. BMC Cancer. 11:3322011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu YH, Tsang JY, Ni YB, Hlaing T, Chan
SK, Chan KF, Ko CW, Mujtaba SS and Tse GM: Doublecortin-like kinase
1 expression associates with breast cancer with neuroendocrine
differentiation. Oncotarget. 7:1464–1476. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhao S, Ma D, Xiao Y, Li XM, Ma JL, Zhang
H, Xu XL, Lv H, Jiang WH, Yang WT, et al: Molecular subtyping of
triple-negative breast cancers by immunohistochemistry: Molecular
Basis and clinical relevance. Oncologist. 25:e1481–e1491. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ramamoorthy P, Dandawate P, Jensen RA and
Anant S: Celastrol and triptolide suppress stemness in triple
negative breast cancer: Notch as a therapeutic target for stem
cells. Biomedicines. 9:4822021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang YL, Li Y, Ma YG and Wu WY: DCLK1
promotes malignant progression of breast cancer by regulating
Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci.
23:9489–9498. 2019.PubMed/NCBI
|
|
89
|
Liu H, Wen T, Zhou Y, Fan X, Du T, Gao T,
Li L, Liu J, Yang L, Yao J, et al: DCLK1 plays a
metastatic-promoting role in human breast cancer cells. Biomed Res
Int. 2019:10619792019.PubMed/NCBI
|
|
90
|
Wang J, Wang S, Zhou J and Qian Q:
miR-424-5p regulates cell proliferation, migration and invasion by
targeting doublecortin-like kinase 1 in basal-like breast cancer.
Biomed Pharmacother. 102:147–152. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ge Y, Weygant N, Qu D, May R, Berry WL,
Yao J, Chandrakesan P, Zheng W, Zhao L, Zhao KL, et al: Alternative
splice variants of DCLK1 mark cancer stem cells, promote
self-renewal and drug-resistance, and can be targeted to inhibit
tumorigenesis in kidney cancer. Int J Cancer. 143:1162–1175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ding L, Yang Y, Ge Y, Lu Q, Yan Z, Chen X,
Du J, Hafizi S, Xu X, Yao J, et al: Inhibition of DCLK1 with
DCLK1-IN-1 suppresses renal cell carcinoma invasion and stemness
and promotes cytotoxic T-cell-mediated anti-tumor immunity. Cancers
(Basel). 13:57292021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sureban SM, Madhoun MF, May R, Qu D, Ali
N, Fazili J, Weygant N, Chandrakesan P, Ding K, Lightfoot SA and
Houchen CW: Plasma DCLK1 is a marker of hepatocellular carcinoma
(HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a
microRNA-dependent mechanism. Oncotarget. 6:37200–37215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ali N, Chandrakesan P, Nguyen CB, Husain
S, Gillaspy AF, Huycke M, Berry WL, May R, Qu D, Weygant N, et al:
Inflammatory and oncogenic roles of a tumor stem cell marker
doublecortin-like kinase (DCLK1) in virus-induced chronic liver
diseases. Oncotarget. 6:20327–20344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Girotto G, Vuckovic D, Buniello A,
Lorente-Cánovas B, Lewis M, Gasparini P and Steel KP: Expression
and replication studies to identify new candidate genes involved in
normal hearing function. PLoS One. 9:e853522014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Srikrishna G: S100A8 and S100A9: New
insights into their roles in malignancy. J Innate Immun. 4:31–40.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wilen CB, Lee S, Hsieh LL, Orchard RC,
Desai C, Hykes BL Jr, McAllaster MR, Balce DR, Feehley T, Brestoff
JR, et al: Tropism for tuft cells determines immune promotion of
norovirus pathogenesis. Science. 360:204–208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ali N, Nguyen CB, Chandrakesan P, Wolf RF,
Qu D, May R, Goretsky T, Fazili J, Barrett TA, Li M, et al:
Doublecortin-like kinase 1 promotes hepatocyte clonogenicity and
oncogenic programming via non-canonical β-catenin-dependent
mechanism. Sci Rep. 10:105782020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ali N, Allam H, May R, Sureban SM, Bronze
MS, Bader T, Umar S, Anant S and Houchen CW: Hepatitis C
virus-induced cancer stem cell-like signatures in cell culture and
murine tumor xenografts. J Virol. 85:12292–12303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ali N, Allam H, Bader T, May R,
Basalingappa KM, Berry WL, Chandrakesan P, Qu D, Weygant N, Bronze
MS, et al: Fluvastatin interferes with hepatitis C virus
replication via microtubule bundling and a doublecortin-like
kinase-mediated mechanism. PLoS One. 8:e803042013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pattabiraman DR and Weinberg RA: Tackling
the cancer stem cells-what challenges do they pose? Nat Rev Drug
Discov. 13:497–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Brooks MD, Burness ML and Wicha MS:
Therapeutic implications of cellular heterogeneity and plasticity
in breast cancer. Cell Stem Cell. 17:260–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Weygant N, Qu D, Berry WL, May R,
Chandrakesan P, Owen DB, Sureban SM, Ali N, Janknecht R and Houchen
CW: Small molecule kinase inhibitor LRRK2-IN-1 demonstrates potent
activity against colorectal and pancreatic cancer through
inhibition of doublecortin-like kinase 1. Mol Cancer. 13:1032014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ferguson FM, Nabet B, Raghavan S, Liu Y,
Leggett AL, Kuljanin M, Kalekar RL, Yang A, He S, Wang J, et al:
Discovery of a selective inhibitor of doublecortin like kinase 1.
Nat Chem Biol. 16:635–643. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sureban SM, Berahovich R, Zhou H, Xu S, Wu
L, Ding K, May R, Qu D, Bannerman-Menson E, Golubovskaya V and
Houchen CW: DCLK1 monoclonal antibody-based CAR-T cells as a novel
treatment strategy against human colorectal cancers. Cancers
(Basel). 12:542019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Cao Z, Weygant N, Chandrakesan P, Houchen
CW, Peng J and Qu D: Tuft and cancer stem cell marker DCLK1: A new
target to enhance anti-tumor immunity in the tumor
microenvironment. Cancers (Basel). 12:38012020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chae YC and Kim JH: Cancer stem cell
metabolism: Target for cancer therapy. BMB Rep. 51:319–326. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Sancho P, Barneda D and Heeschen C:
Hallmarks of cancer stem cell metabolism. Br J Cancer.
114:1305–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Verissimo CS, Elands R, Cheng S, Saaltink
DJ, ter Horst JP, Alme MN, Pont C, van de Water B, Håvik B,
Fitzsimons CP and Vreugdenhil E: Silencing of doublecortin-like
(DCL) results in decreased mitochondrial activity and delayed
neuroblastoma tumor growth. PLoS One. 8:e757522013. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Patel O, Roy MJ, Kropp A, Hardy JM, Dai W
and Lucet IS: Structural basis for small molecule targeting of
doublecortin like kinase 1 with DCLK1-IN-1. Commun Biol.
4:11052021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Oliveras-Ferraros C, Vazquez-Martin A,
Cuyàs E, Corominas-Faja B, Rodríguez-Gallego E, Fernández-Arroyo S,
Martin-Castillo B, Joven J and Menendez JA: Acquired resistance to
metformin in breast cancer cells triggers transcriptome
reprogramming toward a degradome-related metastatic stem-like
profile. Cell Cycle. 13:1132–1144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nakane T, Ido A, Higuchi T, Todaka H,
Morisawa K, Nagamine T, Fukunaga K, Sakamoto S, Murao K and
Sugiyama Y: Candidate plasticity gene 16 mediates suppression of
insulin gene expression in rat insulinoma INS-1 cells under
glucotoxic conditions. Biochem Biophys Res Commun. 512:189–195.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nakane T, Matsumoto S, Iida S, Ido A,
Fukunaga K, Murao K and Sugiyama Y: Candidate plasticity gene 16
and jun dimerization protein 2 are involved in the suppression of
insulin gene expression in rat pancreatic INS-1 β-cells. Mol Cell
Endocrinol. 527:1112402021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen
Q, Wang C and Yin T: Up-regulation of glycolysis promotes the
stemness and EMT phenotypes in gemcitabine-resistant pancreatic
cancer cells. J Cell Mol Med. 21:2055–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ponnurangam S, Mammen JM, Ramalingam S, He
Z, Zhang Y, Umar S, Subramaniam D and Anant S: Honokiol in
combination with radiation targets notch signaling to inhibit colon
cancer stem cells. Mol Cancer Ther. 11:963–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ahmed I, Roy BC, Rao Jakkula LUM,
Subramaniam D, Dandawate P, Anant S, Sampath V and Umar S:
Infection-induced signals generated at the plasma membrane
epigenetically regulate Wnt signaling in vitro and in vivo. J Biol
Chem. 295:1021–1035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Dandawate P, Subramaniam D, Panovich P,
Standing D, Krishnamachary B, Kaushik G, Thomas SM, Dhar A, Weir
SJ, Jensen RA and Anant S: Cucurbitacin B and I inhibits colon
cancer growth by targeting the Notch signaling pathway. Sci Rep.
10:12902020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Sameri S, Saidijam M, Bahreini F and
Najafi R: Cancer chemopreventive activities of silibinin on
colorectal cancer through regulation of E-cadherin/β-catenin
pathway. Nutr Cancer. 73:1389–1399. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sureban SM, May R, Weygant N, Qu D,
Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen
CB, Wang TC and Houchen CW: XMD8-92 inhibits pancreatic tumor
xenograft growth via a DCLK1-dependent mechanism. Cancer Lett.
351:151–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kato H, Tateishi K, Fujiwara H, Ijichi H,
Yamamoto K, Nakatsuka T, Kakiuchi M, Sano M, Kudo Y, Hayakawa Y, et
al: Deletion of histone methyltransferase G9a suppresses mutant
kras-driven pancreatic carcinogenesis. Cancer Genomics Proteomics.
17:695–705. 2020. View Article : Google Scholar : PubMed/NCBI
|