1. Introduction
Adenoid cystic carcinoma (ACC) is an aggressive
malignancy that usually arises in the salivary glands; it
represents 10% of salivary gland tumors and 1% of head and neck
cancer cases. According to estimates, there are 3-4.5 cases of ACC
for every one million individuals. Although ACC affects individuals
of all ages, its peak occurrence is between the ages of 40 and 60
years, and there is a slight female preponderance (60% female and
40% male) (1). ACC is the most
common malignant tumor of the minor salivary glands, and is also
not uncommon in the sublingual, submandibular and parotid salivary
glands. The lungs, cervix and skin, as well as the glandular tissue
of the breast, lacrimal glands, paranasal sinuses and nasopharynx,
are examples of uncommon locations of this type of tumor (1). A number of factors are considered to
be potential causes of ACC, particularly exposure to ionizing
radiation (2). Notably, an
increased incidence of subsequent salivary gland cancer has been
observed in women diagnosed with breast cancer. However, the
associations between smoking, alcohol consumption and ACC remain
unclear (2).
2. Clinical features
Pain, facial nerve dysfunction and nerve ending
invasion are features found in the majority of patients with ACC
(3). In a previous study, 30% of
patients with ACC were shown to have ulcerations, 48% exhibited
pain and 98% reported a mass; the duration of symptoms ranged from
1 month to 4 years (4). The
symptoms of ACC may differ, depending on the location of the tumor.
The tumor represents a mass in the major salivary glands, and
facial nerve palsy may occur when the tumor is located in the
parotid gland. Tumors are usually common in the palate, and thus
ulcerations and fistula can also be observed. When the tumor occurs
in the larynx, the first presenting symptom may be dyspnea;
however, in tumors occurring in the nose and paranasal sinuses,
epistaxis and eye symptoms, as well as deep facial pain and nasal
obstruction, may be the forefront symptoms (4). With an occurrence rate of 16.1-72.7%,
the common malignant feature of ACC is distant metastasis (DM). The
lungs are the most common metastatic site, accounting for
74.5-94.4% of cases of metastasis (5).
3. Histopathology and diagnostic
imaging
With regard to ACC, according to the histological
appearance, 'cylindromas' are the initial histopathological term. A
minimal cytoplasm and angulated hyperchromatic nuclei can be noted
in ACC cells, and the affected tissue is usually eosinophilic or
clear. Although myoepithelial differentiation predominates, ACC
exhibits biphasic differentiation with both myoepithelial and
secretory glandular elements (6).
ACC exhibits different ratios of the three distinct growth patterns
known as solid, tubular and cribriform. Of these, cribriform is the
most frequent subtype (7).
The preferred test for identifying bone invasion
patterns in ACC is computed tomography. Heterogeneous bone
remodeling and enhancement are often observed in low-grade ACC,
while in high-grade ACC, 'worm'-like and osteolytic lesions,
adjacent bone compression resorption-like changes and bone
destruction are more commonly observed (8).
Magnetic resonance imaging is the primary method for
demonstrating the involvement of the skull base. A 'dural tail
sign' is displayed, while tumor cells spread along nerves to the
anterior cranial fossa, and then consequently affect the dura
mater. Soft meningeal enhancement, nodular enhancement or dural
thickening >5 mm is usually suggestive of dural infiltration
(5,8,9).
4. Treatment
The therapeutic strategy for ACC depends on the
tumor stage and grade. Regardless of the primary tumor site,
surgery is the standard of care for non-metastatic ACC. The primary
goal is a complete surgical excision (10). Irrespective of prior treatment, in
the setting of distant metastatic disease, and resectable,
recurrent locoregional disease, the appropriate treatment is
specified by the American Society of Clinical Oncology guidelines
(5). Under these circumstances, if
the metastatic disease does not exhibit rapid progression or is
considered imminently lethal, treatment should include appropriate
surgical reconstruction and rehabilitation, and palliative care. In
the case that complete surgical resection is feasible and if
following primary tumor treatment, the time to pulmonary relapse is
>36 months, the surgical treatment of oligometastatic disease
should also be considered. According to the literature, patients
treated with post-operative radiotherapy exhibit better local
control (9,11).
Currently, microRNAs (miRNAs/miRs) are attractive
molecular biomarker candidates, as they can be repeatedly extracted
from a variety of biological samples, and are often stable and
tolerant in a wide range of storage conditions. In addition, miRNAs
can be easily detected and accurately quantified by a variety of
widely used standard techniques, such as small RNA sequencing,
microarrays and reverse transcription-quantitative PCR (12). Furthermore, miRNAs could be used as
alternative therapeutic targets. The combination of miRNA
therapeutics and chemotherapy, radiotherapy and immunotherapy has
exhibited promising results (13).
Thus, the development of new forms of miRNAs may provide
significant clinical benefits for cancer patients; however, further
research is needed in this field (13).
5. Biogenesis of miRNAs
As vital post-transcriptional regulators of gene
expression, miRNAs are part of a large family of RNAs, 21 nt in
length, and have significantly improved our understanding of the
post-transcriptional regulation of gene expression. miRNAs control
the activity of 50% of protein-coding genes in mammals, according
to research (14).
The biogenesis and maturation of miRNAs first occurs
in the nucleus, and subsequently, with the aid of proteins and
enzymes, biogenesis and maturation occur in the cytoplasm (15). Briefly, as long primary transcripts
(pri-miRNAs), miRNAs are initially produced by RNA polymerase II in
the nucleus. Drosha and Dicer, two enzymes from the RNase III
family, are bound by pri-miRNAs, which fold into hairpin
structures. In the nucleus, the microprocessor complex is formed by
Drosha with DGCR8 microprocessor complex subunit, and the 70 nt
precursor miRNA (pre-miRNA) hairpin is then liberated by the
primary transcript. The mature miRNA/miRNA duplex is produced by
Dicer when exportin-5 exports pre-miRNA to the cytoplasm (Fig. 1) (16,17).
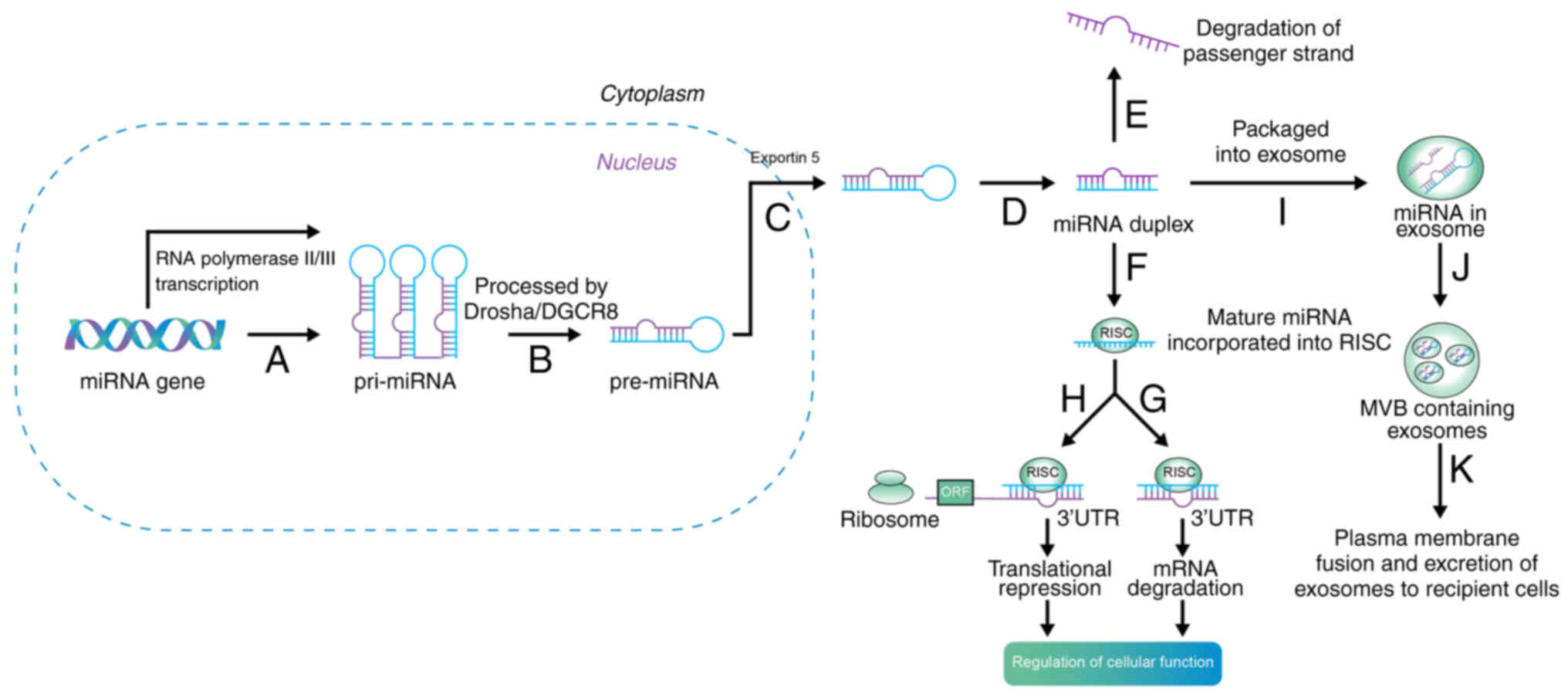 | Figure 1Biogenesis of miRNA. Step A: miRNAs
are initially produced by RNA polymerase II or III in the nucleus,
and transcribed into pri-miRNA. Step B: Drosha and Dicer are bound
by the pri-miRNAs; this microprocessor complex is formed by drosha
with DGCR8, and then pre-miRNA is liberated. Step C: Exportin-5
exports pre-miRNA to the cytoplasm. Step D: The mature miRNA/miRNA
duplex are produced by dicer. Step E: Inactive strands are
degraded. Step F: The incorporation of the mature strand into RISC.
The gene expression is suppressed by RISC with Step G: mRNA
degradation or Step H: translational repression, then regulates
cellular function. Step I: In addition, exosomes can package
miRNAs. Step J: Exosomes are then compartmentalized into an MVB.
Step K: The plasma membrane is fused by the MVB, and then
miRNA-containing exosomes are transferred to recipient cells and
influence gene regulation. miRNA, microRNA; pri-, primary; pre-,
precursor; RISC, RNA-induced silencing complex; mRNA, messenger
RNA; MVB, multivesicular body; 3′UTR, 3′-untranslated region; ORF,
open reading frame; DGCR8, DGCR8 microprocessor complex
subunit. |
To construct the RNA-induced silencing complex
(RISC), transactivation-responsive RNA-binding protein and
Argonaute 2 are bound by mature miRNAs. Although related to RISC,
inactive strands are degraded, and other active strands remain
within the RISC (18). Through
partial complementary sequences, miRNAs can recognize target mRNAs.
The miRNA/mRNA complexes cannot complete protein synthesis when
miRNAs undergo partial base pairing with target mRNAs (19). miRNAs can not only promote
translational repression, by deadenylating the target mRNA poly-A
tail, but can also promote target mRNA degradation. miRNA-based
translational repression is often overwhelmed by the process of
mRNA destabilization in a rapid manner. Dysregulated miRNAs can
affect various intracellular signaling pathways via these two
mechanisms of miRNAs, and thus influence the development of
diseases, including cancer (20).
Since mature miRNAs regulate the expression of
multiple target genes, the dysregulation of miRNAs may cause
abnormal gene expression profiles in cells, which may then lead to
organ injury or even to cancer (20). Furthermore, miRNAs can be well
preserved in a variety of specimens, such as formalin-fixed tissue
blocks, urine and blood plasma or serum. Compared with proteins,
miRNAs are more measurable due to their increased sensitivity
(12). There is currently
increasing interest in creating miRNAs as biomarkers for various
molecular diagnostic applications, such as autoimmune diseases,
cardiovascular diseases and cancer. miRNA profiling has therefore
gained interest from researchers in a variety of biological and
medical research fields (21,22).
Increasingly, studies have indicated that miRNAs and
the biogenesis machinery have a critical influence on the
development of cancer (23,24).
For instance, the dysregulation of miRNA biogenesis enzymes,
tumor-suppressor miRNAs and miRNAs with oncogenic functions are
related to the development of cancer (25). Therefore, miRNAs are crucial for
cancer research. As aforementioned, miRNAs are becoming reliable
biomarkers for disease detection due to their varied
characteristics (they can be repeatedly extracted from a variety of
biological samples, and are often stable and tolerant in a wide
range of storage conditions), and miRNA target-based therapies are
increasingly being used in clinical practice; however, further
research is warranted in this area.
In the present review, through a preliminary
literature search, a large amount of literature reporting on
miRNAs, as well as ACC, was identified; however, no studies were
identified that summarize the mechanisms of miRNAs in ACC. The
present review thus aimed to summarize the miRNAs that have been
experimentally validated and have pathogenic mechanisms in ACC. It
is hoped that the present review may provide a basis for future
research on miRNAs in ACC.
6. miRNAs in ACC
In ACC, the molecular mechanisms underlying the
molecular alterations responsible for oncogenic activity remain
elusive. There are a number of studies reporting the association
between miRNAs and ACC. For example, Kiss et al (26) found that miRNA profiles in breast
and salivary ACC (SACC) differed from those in corresponding normal
tissues. miR-9-5p is considered to be a potential biomarker and
therapeutic target (27).
miR-6835-3p, miR-4676, miR-1180 and certain other miRNAs are
related to the overall survival and recurrence-free survival of
patients with ACC (28). miR-20a
and miR-17 have been shown to be associated with the poor outcomes
of patients (29). miR-375,
miR-150 and miR-455-3p have been identified as aberrantly expressed
miRNAs in SACC (30). Zhao et
al (31) suggested that
miR-29a-3p may bind with AKT serine/threonine kinase 2, and has an
influence on lacrimal gland ACC (LACC). The present review
discusses the miRNAs that have been validated by in vitro or
in vivo studies, and describes their role in ACC (Table I).
 | Table ImiRNAs in ACC. |
Table I
miRNAs in ACC.
| miRNA | Upstream | Downstream | Expression | Function | Prognosis | (Refs.) |
|---|
| miR-130a | MYB | NDRG2 | + | MYB activates the
expression of miR-130a, and then leads to NDRG2 downregulation | Poor | (32) |
| miR-21 | - | PDCD4 Bcl-2 | + | Through modulation
of PDCD4 and Bcl-2 expression, miR-21 can suppress cell apoptosis
and then increase cell proliferation and metastasis | Poor | (39) |
| miR-93-5p | - | BRMS1L | + | May promote EMT,
and by targeting BRMS1L, regulate Wnt signaling | Poor | (48) |
| miR-103-3p | - | TPD52 | + | Metastatic
properties of SACC are maintained by the feedback regulation
between TPD52 and miR-103a-3p | Poor | (50) |
| miR-222 | - | PUMA | + | Promotes the
ability of proliferation and migration of ACC cells | Poor | (53) |
| miR-155 | - | EGFR/NF-κB | + | Facilitates cell
cycle progression and promotes invasion in ACC and that the
EGFR/NF-κB pathway might participate in mediating the effects of
miRNA155 | Poor | (62) |
| miR-320a | - | ITGB3 | − | Inhibits SACC
metastasis by silencing ITGB3 | Favorable | (73) |
| miR-140-5P | - | Survivin | − | Suppresses SACC
cell proliferation and invasion, and induces cell apoptosis by
regulating survivin expression | Favorable | (81) |
| miR-187 | CXCR5 | - | − | CXCR5 facilitates
PNI through downregulating miR-187 | Favorable | (88) |
| miR-101-3p | - | Pim-1 | − | Suppresses cell
proliferation, invasion and enhances chemotherapeutic sensitivity
in SACC by targeting Pim-1 | Favorable | (97) |
| miR-98 | - | N-RAS | − | Possibly acts as a
tumor suppressor in SACC by negatively regulating the oncogene
N-RAS | Favorable | (100) |
| miR-125a-5p | - | P38/MAPK | − | Downregulation of
miR-125a-5p promotes SACC progression through p38 signal
pathway | Favorable | (109) |
| miR-582-5p | - | FOXC1 | − | miR-582-5p can
inhibit invasion and migration in SACC cell lines | Favorable | (113) |
miR-130a
miR-130a is located on chromosome 11 (32). As previously demonstrated, miR-130a
is aberrantly expressed in a number of types of cancer; for
instance, it is overexpressed in esophageal cancer tissue (33), osteosarcoma (34), non-small cell lung cancer (35), basal cell carcinoma (36), adult T-cell leukemia (37) and gastric cancer (38), although it is underexpressed in
chronic lymphocytic leukemia (39), prostate carcinoma (40), glioblastoma (41), hepatocellular carcinoma cells
(42), ovarian cancer (43), breast cancer (44) and cervical cancer (45).
As a member of a transcription factor family, MYB
proto-oncogene, transcription factor (MYB) is associated with human
malignancies, such as melanoma, pancreatic and esophageal cancer
(46). N-myc downstream-regulated
gene 2 (NDRG2), a tumor suppressor gene, can inhibit metastasis,
attenuate tumor progression and increase tumor sensitivity to
anticancer drugs. In various aggressive tumors, NDRG2 is suppressed
and its expression is related to patient prognosis (47). It has been found that NDRG2
expression is downregulated in SACC tissue samples, and in mouse
models, it may promote the metastasis and growth of xenograft
tumors. According to the literature research, by targeting NDRG2,
miR-130a can suppress NDRG2 expression (48). In SACC samples, miR-130a expression
has been inversely linked to NDRG2 in vitro or in
vivo; the overexpression of miR-130a can increase SACC
proliferation and invasion. In addition, miR-130a-modulated cell
invasion, colony formation and proliferation are reversed by the
restoration of NDRG2 expression. Furthermore, through binding to
the miR-130a promoter, MYB activates the expression of miR-130a,
and then leads to NDRG2 downregulation (48). On the whole, the MYB/miR-130a/NDRG2
axis may present an effective strategy for the treatment of
SACC.
miR-21
miR-21 is located on chromosome 17q23.2 (49). miR-21 can affect cell proliferation
through a variety of targets, including phosphatase and tensin
homolog (PTEN), sprouty RTK signaling antagonist 2 and programmed
cell death protein 4 (PDCD4) (50). miR-21 is overexpressed in various
human tumors, such as oral, prostate, breast and colorectal cancer
(51-54). These findings indicate that miR-21
may play a vital role in tumorigenesis.
The role of miR-21 in ACC has also been
investigated. In 2015, Jiang et al (55) found that through the
miR-21/PDCD4/STAT3 pathway, miR-21 may regulate SACC progression.
In SACC cell lines and tissue samples, miR-21 was shown to be
overexpressed and to enhance the cellular capacity for migration
and invasion. As a tumor suppressor gene, PDCD4 is the direct
target of miR-21, and miR-21 can suppress PDCD4. It was also found
that, in SACC samples, the low expression of PDCD4 and the high
expression of phosphorylated (p-)STAT3 were linked to the high
expression of miR-21. In a study by Wang et al (56), miR-21 inhibitor reduced lung
metastatic SACC cell resistance to simvastatin, which was found be
effective against the growth of various types of cancer, including
breast (57), anaplastic thyroid
(58), and lung (59) cancer. Yan et al (60) also indicated that PDCD4, PTEN and
B-cell lymphoma-2 (Bcl-2) may be the potential targets of miR-21,
and through modulation of PDCD4 and Bcl-2 expression, miR-21 can
suppress cell apoptosis, and increase cell proliferation and
metastasis. miR-21 thus has potential for use as a therapeutic
target in SACC.
miR-93-5p
miR-93 is derived from the paralogue of the
miR-17-92 cluster. SMAD family member 7, vascular endothelial
growth factor A, STAT3, SRY-box transcription factor 4, AKT
serine/threonine kinase 3, erb-b2 receptor tyrosine kinase 2,
cyclin B1 and p21 are identified targets of miR-93, suggesting that
through diverse mechanisms, miR-93 may function as a tumor
suppressor (61). miR-93-5p is
associated with a variety of cancer types, including epithelial
ovarian carcinoma (61),
colorectal cancer (62), gastric
cancer (63) and hepatocellular
carcinoma (64).
As part of the Sin3A-histone deacetylase
co-repressor complex, breast cancer metastasis suppressor 1 like
(BRMS1L) may suppress target gene transcription (65). As a mediator downstream of the p53
pathway, BRMS1L can also inhibit the invasion and migration of
cancer cells, which are critical processes in cancer metastasis
(66). In LACC, miR-93-5p enhances
cell tumorigenesis by targeting BRMS1L (67). In LACC tissues, it has been found
that miR-93-5p expression is increased, and miR-93-5p can prevent
the apoptosis of LACC cells; with the overexpression of miR-93-5p,
an evident enhancement of the invasion and migration of LACC cells
has been observed (67). miR-93-5p
targets BRMS1L, and miR-93-5p can then inhibit the protein
expression of BRMS1L. When BRMS1L expression is increased, the
invasive and migratory potential of LACC cells is significantly
inhibited (67). Mutated Wnt
pathway components can also affect cancer and multiple
growth-related pathologies. It has also been indicated that through
BRMS1L, miR-93-5p can regulate Wnt signaling; the elucidation of
the exact mechanisms involved may result in the development of
effective treatment strategies that may markedly decrease the
morbidity and mortality of patients with LACC (67).
miR-103-3p
According to the literature, miR-103a-3p functions
as an oncogene; in gastric cancer, endometrial carcinoma and
hepatocellular carcinoma, miR-103a-3p expression is upregulated
(68). In SACC, by targeting tumor
protein D52 (TPD52), miR-103a-3p promotes metastasis (69). Unlike in healthy tissues,
miR-103a-3p expression is high in SACC tissues. By assessing the
clinicopathological features of 52 patients with SACC, the high
expression of miR-103a-3p was found to be associated with lung
metastasis and local regional recurrence (69). When miR-103a-3p expression was
knocked down, cell migration was suppressed, and cell functions may
be affected via the epithelial-mesenchymal transition process.
TPD52, a member of the TPD52-like protein family, is mapped to
chromosome 8q21. TPD52 exerts differential effects on various tumor
types. In breast and prostate cancer, the expression of TPD52 is
high (70,71), while in lung cancer and
liposarcoma, low expression is observed (72,73).
TPD52 may promote cell invasion, migration, proliferation and
survival; however, research also suggests that TPD52 may act as a
suppressor in the progression of tumors. Compared with that in SACC
tissues, TPD52 expression is markedly higher in healthy tissues,
and in SACC, TPD52 may be the direct target of miR-103a-3p. As
previously demonstrated, when TPD52 is overexpressed, the migration
of SACC-LM (highly metastatic) cells is significantly suppressed,
suggesting that the migration of SACC cells is inhibited by TPD52;
miR-103a-3p overexpression decreases TPD52 expression in SACC cells
when the cellular expression of TPD52 is excessive (69).
These findings indicate that the metastatic
properties of SACC are maintained by the feedback regulation
between TPD52 and miR-103a-3p. In summary, the miR-103a-3p/TPD52
axis may be critical in SACC pathogenesis, and may provide further
insight into potential therapeutic targets or novel biomarkers.
miR-222
Encoding on chromosome X (Xp11.3), miR-221 and
miR-222 are highly homologous, functioning as a cluster
(miR-221/222). Acting as an oncogene, this cluster may overcome the
status of cell quiescence and may promote cell proliferation,
survival and metastasis (74).
miR-222 is involved in colorectal, gastric, prostate, pancreatic,
liver and breast cancer, and thus has potential for use as a
diagnostic and prognostic biomarker (75).
In oral squamous cell carcinoma, miR-222 expression
is positive (76). As previously
demonstrated, in ACC, when miR-222 was knocked down, the
proliferation and migration of ACC cells was inhibited, and
apoptosis was significantly induced (77). Moreover, the expression of p53
upregulated modulator of apoptosis (PUMA) was increased (77). Belonging to the Bcl-2 protein
family, PUMA is a BH3-only protein; PUMA gene mutation or absence
may result in reduced rates of apoptosis, suggesting that PUMA
plays a crucial role in the process of apoptosis (78). miR-222 and PUMA expression are
negatively regulated; thus, the elaboration of their association is
essential for the study of ACC (77).
miR-155
As one of the most multifunctional miRNAs, miR-155
is related to inflammation, immunological modulation and tumor
development (79). miR-155 is
involved in several types of cancer, including gastric (80), lung (81), kidney (82) and breast (83) cancer, and head and neck squamous
cell carcinoma (84). The
expression of miR-155 in malignant pathologies suggests its
possible use as a diagnostic biomarker (85).
As demonstrated in the study by Liu et al
(86), miR-155 plays a critical
role in the invasion and growth of SACC. Compared with that in
normal tissues, miR-155 expression in ACC is markedly increased. By
knocking down miR-155, cell proliferation was inhibited, indicating
that, in SACC, miR-155 may promote cell proliferation and
facilitate cell cycle progression, which suggests the promoting
effects of miR-155 on SACC carcinogenesis. Indeed, the knockdown of
miR-155 markedly inhibited the invasive ability of SACC cells, and
in nude mice, the silencing of miR-155 inhibited the pulmonary
metastasis of SACC cells. In addition, there was a correlation
between miR-155 and the EGFR⁄NF-κB pathway. The EGFR⁄NF-κB pathway
plays a role in the growth and metastasis of several malignant
tumors. EGFR overexpression can induce metastasis, invasion,
angiogenesis and tumorigenesis (87-89).
The EGFR⁄NF-κB pathway may be related to the effects of miR-155 on
ACC carcinogenesis. The mechanisms underlying the interactions
between EGFR⁄NF-κB and miR-155 warrant further investigations. In
addition, through the ubiquitin-like modifier activating enzyme 2
pathway, miR-155 affects SACC metastasis (90).
miR-320a
As a member of the miR-320 family, miR-320a is
located on chromosome 8p21.3 (91,92).
It has been found that miR-320a expression is decreased in various
tumors, and by downregulating target gene expression, miR-320a may
function as a tumor suppressor (93). For instance, in hepatocellular
carcinoma, by targeting high mobility group box 1, miR-320a could
function as a suppressor of tumor and play a critical role in the
invasion-metastasis cascade (94).
In breast cancer, by modulating the expression of Rab protein
Rab11a, miR-320a could also function as a tumor suppressor and
biomarker (95). By targeting ras
association domain family 8, miR-320a enhanced the proliferation
and invasion of epithelial ovarian cancer cells (96). In summary, miR-320a plays a
critical role in cancer.
In the study by Sun et al (97), it was found that in highly
metastatic ACC cells in the lungs, miR-320a was the most markedly
downregulated miRNA. The cells were then transfected with miRNA
mimics to increase miR-320a expression, and this markedly inhibited
the adhesion, invasion and migration of SACC cells. This indicated
that, in SACC cells, reduced miR-320a expression can result in
enhanced invasiveness. Based on a series of measurements, integrin
β3 (ITGB3) was considered to be the target of miR-320a. ITGB3 has
been found in variety of cancer types, such as breast cancer
(98), nasopharyngeal carcinoma
(99) and human non-small cell
lung cancer (100). In SACC
cells, by targeting ITGB3, miR-320a could regulate cell
invasiveness (97). In addition,
through in vivo experiments, the overexpression of miR-320a
in ACC cells was found to suppress metastasis to the liver or lungs
of tumor-bearing mice, which suggested that the metastasis of ACC
xenografts was suppressed by miR-320a overexpression (97). Furthermore, miR-320a overexpression
reduced IGTB3 expression, and by silencing ITGB3, miR-320a
inhibited SACC metastasis (97).
In summary, in metastatic SACC cells, miR-320a is downregulated,
which leads to the overexpression of ITGB3, and the upregulation of
ITGB3 enhances cell invasion and metastasis. miR-320a may thus be a
promising therapeutic target and prognostic biomarker for SACC.
miR-140-5p
miR-140-5p has been reported to function as a tumor
suppressor in hypopharyngeal, biliary tract and colorectal cancer
(101). Rothman et al
(102) demonstrated that the
downregulation of miR-140-5p regulated cellular proliferation and
migration in hepatocellular carcinoma, and lung and breast cancer.
These findings indicate that miR-140-5p plays a vital role in
cancer.
Survivin (BIRC5), a 142-amino acid, 16.5-kDa
protein, is the smallest member of the family of inhibitors of
apoptosis proteins (103).
Compared with the levels in adult differentiated tissues,
overexpression is noted in tumors; in addition, in several human
neoplasms, survivin is related to a poor prognosis. This apoptotic
inhibitor has a notable influence on both the inhibition of cell
death and the promotion of cancer cell survival (104). In SACC, miR-140-5p inhibits
metastasis and progression by targeting survivin (105). miRNA array screening identified
that miR-140-5p expression was decreased in SACC, while the
overexpression of miR-140-5p suppressed cell proliferation and
invasion, and induced apoptosis, inhibiting tumor growth (105). In SACC tissues, the expression of
survivin was found to be high and the overexpression of survivin
was related to a poor prognosis of patients with SACC (105). miR-140-5p could target the
3′-untranslated region of survivin directly, and inversely regulate
survivin. SACC cell proliferation and invasion were suppressed by
the inhibition of survivin, and the inhibition of survivin could
also induce cell apoptosis (105). By contrast, the enforced
expression of survivin could counteract the tumor suppressive
effects of miR-140-5p. It was also illustrated in SACC that
miR-140-5p functions as a tumor suppressor. By regulating survivin
expression, miR-140-5p has the potential to suppress the
proliferation and invasion of SACC cells, inducing cell apoptosis
(105). On the whole, miR-140-5p
has the potential to be a promising target for the treatment of
SACC.
miR-187
The human miR-187 gene is located at 18q12.2. Among
the cancer-related miRNAs, the study of miR-187 has attracted
increasing attention in recent years. It has been found that the
expression of miR-187 varies markedly in various tumor types
(106). For instance, in breast
cancer, as an independent prognostic factor, miR-187 may confer an
increased invasive potential in vitro (107). By targeting disabled homolog-2,
miR-187 can regulate ovarian cancer progression (108), and by targeting FGF9, miR-187 can
suppress lung cancer cell proliferation (109). In summary, miR-187 plays a vital
role in cancer.
As a member of the G-protein coupled receptor
superfamily, C-X-C chemokine receptor type 5 (CXCR5) can evoke
inflammatory responses and promote lymphocyte migration (110). It has been suggested that in a
number of human cancer types, CXCR5 is highly expressed and may be
associated with tumor occurrence, invasion and metastasis (111). In SACC, CXCR5 induces perineural
invasion (PNI) by inhibiting miR-187 (112). In SACC samples, CXCR5 expression
is increased, which may result in SACC-LM cell PNI, invasion and
migration, while the silencing of CXCR5 attenuates migration,
invasion and PNI (112). As the
main cells of peripheral nerves, Schwann cells may be beneficial
for the maintenance of axons and may be fundamental to the
development and survival of nerves. The inhibition of the
expression of CXCR5 leads to a downregulation of Schwann cell
hallmarks (112). As previously
demonstrated, miR-187 is the downstream miRNA of CXCR5. At the
nerve invasion frontier, miR-187 expression exhibits a downward
tendency, and by inhibiting miR-187, CXCR5 can promote Schwann cell
marker expression. By suppressing miR-187, CXCR5 induces the
differentiation of tumor cells into Schwann-like cells,
facilitating the PNI of SACC (112). Thus, miR-187 is essential for the
study of SACC.
miR-101-3p
In various malignancies, miR-101 is one of the
downregulated miRNAs, and the genomic loss of miRNA-101 may confer
a proliferative advantage on cancer (113). miR-101-3p expression has been
found in various types of cancer, including ovarian cancer
(114), cholangiocarcinoma
(115), and gastric (116), breast (117) and bladder cancer (118).
PIM kinase is a part of the family of
serine/threonine kinases (119).
Pim-1 is a proto-oncogene, and the dysregulation of Pim-1 may
result in tumorigenesis and in malignant progression (120). In SACC, miR-101-3p can enhance
chemotherapeutic sensitivity, and can suppress the proliferation
and invasion of cells by targeting Pim-1 (121). Compared with that in normal
parotid glands, miR-101-3p expression in ACC tissues is markedly
reduced. The overexpression of miR-101-3p can inhibit ACC cell
proliferation and invasion, while the silencing of miR-101-3p
reverses the phenomenon, indicating that miR-101-3p may play a
crucial role in the ACC inhibition of progression (121). Moreover, Pim-1 expression is
inversely associated with the expression of miR-101-3p; by directly
downregulating Pim-1, miR-101-3p can inhibit ACC cell invasion and
proliferation (121). In
addition, in cells treated with cisplatin, miR-101-3p expression
was markedly downregulated, and the expression of Pim-1 was notably
increased, indicating that in SACC cells, miR-101-3p may enhance
sensitivity to cisplatin (121).
In summary, miR-101-3p may prove to be a promising therapeutic
target for patients with ACC.
miR-98
As part of the mature let-7 family, miR-98 was
initially found to be downregulated in leukemia cell lines
(122). In nasopharyngeal
carcinoma, it was also found that miR-98 expression was markedly
decreased. In addition, miR-98 was abnormally expressed in various
types of cancer, including lung, breast, colorectal cancer and
glioma. Consequently, miR-98 is widely considered as a tumor
suppressor gene (122,123).
As demonstrated in the study by Liu et al
(124), miR-98 expression was
downregulated in SACC tissues compared with that in adjacent normal
tissues; in highly metastatic ACC-M cell lines, miR-98 expression
was found to be lower, whereas N-Ras expression was higher. As part
of a family of oncoproteins, N-Ras is commonly mutated in cancer,
and mutations in N-Ras can lead to the activation of downstream
serine/threonine kinases, which may enhance cell survival and
cellular transformation, and promote cell cycle progression
(125,126). In SACC, miR-98 is related to
N-Ras, and miR-98 may negatively regulate N-Ras expression,
suggesting that miR-98 may target N-Ras directly (124). miR-98 overexpression can decrease
cell clonogenicity and viability, while N-Ras is evidently related
to tumor size and clinical phase, suggesting that by targeting
N-RAS translation, miR-98 can function as a tumor suppressor
(124). In addition, the
expression levels of p-AKT and p-ERK are decreased by the
overexpression of miR-98, indicating the inactivation of the
RAS/MAPK/ERK and PI3K/AKT pathways (124). Thus, the role of miR-98 in SACC
may be crucial.
miR-125a-5p
miR-125a-5p is part of an evolutionarily conserved
cluster of three miRNA genes within 727 bp of one another on
chr19q13.41 (127). In several
human cancer types, including medulloblastoma, and lung, ovarian
and breast cancer, the expression of miR125a-5p is downregulated
(128). In oral squamous cell
carcinoma, miR-125a-5p expression is also markedly downregulated
(129). These findings indicate
that miR-125a-5p may function as a tumor suppressor.
The p38/MAPK signaling pathway is considered to
regulate various physiological processes, including cell
proliferation, differentiation and apoptosis (130). Recently, increasing evidence has
suggested that p38 signaling is activated during the tumorigenesis
of several human malignancies (131,132). In SACC, by targeting the
p38/JNK/ERK signaling pathway, miR-125a-5p is associated with SACC
progression (133). Firstly, it
was found that miR-125a-5p was downregulated in primary SACC
tissues, and lower miR-125a-5p expression levels were positively
linked to a metastatic phenotype (133). In SACC cells, miR-125a-5p
downregulation markedly promoted migration and invasion, while
miR-125a-5p overexpression markedly inhibited migration and
invasion. It was also shown that the inhibition of miR-125a-5p
upregulated the expression of p-p38/JNK/ERK, while the
overexpression of miR-125a-5p may decrease the activation of p-p38,
JNK and ERK; based on this inference, miR-125a-5p regulates SACC
progression through the p38/JNK/ERK signaling pathway (133). This suggests that miR-125a-5p has
potential for use as a prognostic biomarker and therapeutic target
for SACC.
miR-582-5p
In prostate cancer, gastric cancer, colorectal
carcinoma, hepatocellular carcinoma and bladder cancer, miR-582-5p
functions as a tumor suppressor. However, miR-582-5p does not only
always act as a tumor inhibitor. miR-582-5p can enhance the
survival of glioblastoma stem cells, and the overexpression of
miR-582-5p can promote the growth of prostate cancer (134).
Forkhead box C1 (FOXC1) plays a critical role in the
development of normal embryonic tissues and can regulate the
development of several organs (135). FOXC1 also exerts a notable effect
on tumor development and metastasis (136). In SACC, by targeting FOXC1,
miR-582-5p can inhibit invasion and migration (137). In SACC cell lines and tissues,
miR-582-5p has been found to be significantly downregulated.
Following the overexpression of miR-582-5p by transfection, SACC
cell invasion and metastasis were inhibited, and proliferation was
promoted in vitro (137).
miR-582-5p can target FOXC1, and the expression of FOXC1 is
inversely related to the expression of miR-582-5p. FOXC1 expression
is reduced when miR-582-5p expression is increased, and SACC cell
invasion and migration are markedly inhibited (137). In addition, in a xenograft tumor
model, tumorigenesis and lung metastasis were inhibited by enhanced
miR-582-5p expression (137).
These findings suggest the potential of miR-582-5p as a prognostic
biomarker and therapeutic target for patients with SACC.
In addition to the aforementioned miRNAs, there are
also some experimentally validated miRNAs. For instance, by the
regulation of the miR-146b-5p/ATP-citrate lyase axis, cancer
susceptibility candidate 9 can facilitate the malignant phenotypes
of SACC cells (138). By
targeting mTOR, miR-144-3p inhibits the proliferation and induces
the apoptosis of SACC cells (139). By targeting PTEN, miR-23b-3p may
exert a critical effect by enhancing angiogenesis and local
vascular microleakage (140). In
SACC, miR-5191 expression has been found to be downregulated; an
increase in the expression of miR-5191 led to the inhibition of
tumorigenesis and pulmonary metastasis, indicating that miR-5191
was associated with an improved prognosis (141). The expression of miR-338-5p/3p
was also lower in SACC cell lines, and may impair motility and
invasion by targeting the γ2 chain gene (142). Through sponging with miR-143-3p,
long non-coding RNA ADAMTS9 antisense RNA 2 can promote SACC cell
migration and invasion (143).
From the aforementioned summarized findings, it can be seen that
miRNAs have a critical influence on the development of ACC, and can
be used as biomarkers and therapeutic targets; some miRNAs are
associated with a good prognosis, while others have opposite
functions. Thus, further research on miRNAs in ACC is necessary.
Among the miRNAs aforementioned, miR-6835-3p has hardly been
reported in other cancer types, and perhaps it may be a unique
biomarker, as well as a therapeutic target for ACC; however,
further research into this miRNA is required in order to fully
understand its functions. In addition, miR-5191 has rarely been
reported in other cancer types, and thus the study of this miRNA in
ACC may also prove useful.
7. Conclusions and future perspectives
There is strong evidence in support of the role of
miRNAs in a number of cancer types, including ACC. Along with the
alterations of miRNA biogenesis mechanisms, alterations in the
levels of miRNA several upstream targets, including mutated protein
controls, transcription factors and epigenetic controls, can also
lead to alterations in miRNA levels. Thus, the dysregulation of
miRNAs may lead to oncogenic or tumor-suppressive effects,
consequently influencing the onset, progression and diffusion of
ACC (144).
The incidence of PNI in ACC is ~43.2%, and PNI may
be an independent factor associated with a poor prognosis. DM is
common, with an incidence of 40-50%. The natural history of ACC is
defined by a disease-free window followed by treatment failure,
locoregional recurrence and distant metastasis, with a guarded
prognosis and propensity for indolent progression. Thus, the early
diagnosis of ACC is essential (5).
As there are still some limitations to the currently
available diagnostic methods, and a better prognosis is often
achieved from an early and accurate diagnosis, new diagnostic and
prognostic biomarkers are being developed using miRNAs for patients
with ACC (145). As also
aforementioned in the summary of relevant miRNAs, each miRNA
exhibits alterations in expression, and the majority of these
affect tumor cell invasion, metastasis and proliferation; some can
also influence tumor development by affecting their targets, and
some can affect the therapeutic effects of certain drugs. Thus,
miRNAs can be used as biomarkers for the diagnosis of ACC and to
predict the prognosis of patients with ACC.
In addition, several studies have reported that
other miRNAs also play a role in the diagnosis, progression and
prognosis of ACC (146-148). In bodily fluids, these miRNAs are
circulating and their stability can be easily maintained. Thus,
miRNAs can be non-invasive, promising, affordable, easily
accessible and novel testing tools for patients with ACC with a
personalized management plan. In addition, as miRNAs control
multiple target genes, the combination of several miRNAs may
enhance sensitivity; compared with individual miRNA assays,
circulating and multiple miRNA-based profiles may present a
considerable and effective diagnostic and prognostic tool. This may
reveal how each miRNA affects tumor development and elucidate the
biological effects of miRNA regulation on the multistep process
leading to ACC (144,145,149).
Under conditions of DM or symptomatic locoregional
recurrence, surgery and radiotherapy are not available treatment
options for patients with ACC, but chemotherapy is then suitable
for the disease; thus, future studies are required to focus on the
development and delivery of miRNA-based drugs (150). Further attention needs to be paid
to the control of the off-target effects of miRNA therapeutics,
improvements in miRNA delivery and the optimization of miRNA-based
drug stability. Moreover, in order to improve non-miRNA treatment,
more efforts need be focused on the use of such non-miRNA
treatments (radiotherapy and chemotherapy) linked to miRNAs, which
play a critical role in the regulation of the treatment response
(151).
In conclusion, the present review provides a brief
introduction into ACC and the biogenesis of miRNAs. miRNAs that
have been validated by in vitro or in vivo studies
are presented, and their role in ACC is described. Various miRNAs
exhibit differential expression and regulation in ACC, and can
function as either tumor suppressor genes or oncogenes. The present
review may provide the basis for future research, which is required
to examine the role of miRNAs in ACC.
Availability of data and materials
Not applicable.
Authors' contributions
YL and FG collected the related literature and
drafted the manuscript. YH, JX and XH revised and edited the
manuscript. YW and RC performed revision of the manuscript, and
conducted project administration and funding acquisition. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by Anhui Medical University School of
Stomatology Discipline Construction Follow-up Project (grant no.
2020kqsy02).
References
|
1
|
Nightingale J, Lum B, Ladwa R, Simpson F
and Panizza B: Adenoid cystic carcinoma: A review of clinical
features, treatment targets and advances in improving the immune
response to monoclonal antibody therapy. Biochim Biophys Acta Rev
Cancer. 1875:1885232021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cantù G: Adenoid cystic carcinoma. An
indolent but aggressive tumour. Part A: From aetiopathogenesis to
diagnosis. Acta Otorhinolaryngol Ital. 41:206–214. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang Z, Pan J, Chen J, Wu S, Wu T, Ye H,
Zhang H, Nie X and Huang C: Multicentre clinicopathological study
of adenoid cystic carcinoma: A report of 296 cases. Cancer Med.
10:1120–1127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck-an update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y, Peng Z, Wang Y, Gao K, Liu Y, Fan
R, Zhang H, Xie Z and Jiang W: Current opinions on diagnosis and
treatment of adenoid cystic carcinoma. Oral Oncol. 130:1059452022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jaso J and Malhotra R: Adenoid cystic
carcinoma. Arch Pathol Lab Med. 135:511–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dillon PM, Chakraborty S, Moskaluk CA,
Joshi PJ and Thomas CY: Adenoid cystic carcinoma: A review of
recent advances, molecular targets, and clinical trials. Head Neck.
38:620–627. 2016. View Article : Google Scholar
|
|
8
|
Seshadri M and Rich LJ: Ultrasound guided
generation of PDOX models of adenoid cystic carcinoma.
EBioMedicine. 42:382019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ha H, Keam B, Ock CY, Kim TM, Kim JH,
Chung EJ, Kwon SK, Ahn SH, Wu HG, Sung MW and Heo DS: Role of
concurrent chemoradiation on locally advanced unresectable adenoid
cystic carcinoma. Korean J Intern Med. 36:175–181. 2021. View Article : Google Scholar :
|
|
10
|
Guazzo E, Bowman J, Porceddu S, Webb L and
Panizza B: Advanced adenoid cystic carcinoma of the skull base-the
role of surgery. Oral Oncol. 99:1044662019. View Article : Google Scholar
|
|
11
|
Atallah S, Marc M, Schernberg A, Huguet F,
Wagner I, Mäkitie A and Baujat B: Beyond surgical treatment in
adenoid cystic carcinoma of the head and neck: A literature review.
Cancer Manag Res. 14:1879–1890. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fabris L, Ceder Y, Chinnaiyan AM, Jenster
GW, Sorensen KD, Tomlins S, Visakorpi T and Calin GA: The potential
of MicroRNAs as prostate cancer biomarkers. Eur Urol. 70:312–322.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi
S, Xie H, Peng X, Yin W, Tao Y and Wang X: miRNA-based biomarkers,
therapies, and resistance in cancer. Int J Biol Sci. 16:2628–2647.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JQ, Papp G, Szodoray P and Zeher M:
The role of microRNAs in the pathogenesis of autoimmune diseases.
Autoimmun Rev. 15:1171–1180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar :
|
|
17
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Achkar NP, Cambiagno DA and Manavella PA:
miRNA biogenesis: A dynamic pathway. Trends Plant Sci.
21:1034–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH,
Kaur B and Eltzschig HK: Strategies to modulate MicroRNA functions
for the treatment of cancer or organ injury. Pharmacol Rev.
72:639–667. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pritchard CC, Cheng HH and Tewari M:
MicroRNA profiling: Approaches and considerations. Nat Rev Genet.
13:358–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar :
|
|
23
|
Dragomir MP, Knutsen E and Calin GA:
Classical and noncanonical functions of miRNAs in cancers. Trends
Genet. 38:379–394. 2022. View Article : Google Scholar
|
|
24
|
Hussen BM, Hidayat HJ, Salihi A, Sabir DK,
Taheri M and Ghafouri-Fard S: MicroRNA: A signature for cancer
progression. Biomed Pharmacother. 138:1115282021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kiss O, Tőkés AM, Vranic S, Gatalica Z,
Vass L, Udvarhelyi N, Szász AM and Kulka J: Expression of miRNAs in
adenoid cystic carcinomas of the breast and salivary glands.
Virchows Arch. 467:551–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Auxzilia Preethi K, Chandralekha
Selvakumar S and Sekar D: MicroRNAs and it's targets in the
treatment of salivary adenoid cystic carcinoma. Oral Oncol.
133:1060532022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andreasen S, Tan Q, Agander TK, Hansen
TVO, Steiner P, Bjørndal K, Høgdall E, Larsen SR, Erentaite D,
Olsen CH, et al: MicroRNA dysregulation in adenoid cystic carcinoma
of the salivary gland in relation to prognosis and gene fusion
status: A cohort study. Virchows Arch. 473:329–340. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitani Y, Roberts DB, Fatani H, Weber RS,
Kies MS, Lippman SM and El-Naggar AK: MicroRNA profiling of
salivary adenoid cystic carcinoma: Association of miR-17-92
upregulation with poor outcome. PLoS One. 8:e667782013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown AL, Al-Samadi A, Sperandio M, Soares
AB, Teixeira LN, Martinez EF, Demasi APD, Araújo VC, Leivo I, Salo
T and Passador-Santos F: MiR-455-3p miR-150 and miR-375 are
aberrantly expressed in salivary gland adenoid cystic carcinoma and
polymorphous adenocarcinoma. J Oral Pathol Med. 48:840–845. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao J, Liu X, Lin J, Jiang M, Xu F, Zhang
C, Tang Q, Zhu L, Dong L and Lin T: AKT2 identified as a potential
target of mir-29a-3p via microRNA profiling of patients with high
proliferation lacrimal gland adenoid cystic carcinoma. Exp Eye Res.
219:1090672022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HD, Jiang LH, Sun DW, Li J and Ji
ZL: The role of miR-130a in cancer. Breast Cancer. 24:521–527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ,
Wang TY, Li HC and Wu XN: Differential expression of miRNAs in
esophageal cancer tissue. Oncol Lett. 5:1639–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Yan D, Wu W, Zhu J, Ye W and Shu
Q: MicroRNA-130a promotes the metastasis and epithelial-mesenchymal
transition of osteosarcoma by targeting PTEN. Oncol Rep.
35:3285–3292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XC, Tian LL, Wu HL, Jiang XY, Du LQ,
Zhang H, Wang YY, Wu HY, Li DG, She Y, et al: Expression of
miRNA-130a in nonsmall cell lung cancer. Am J Med Sci. 340:385–388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishihara K, Sasaki D, Tsuruda K, Inokuchi
N, Nagai K, Hasegawa H, Yanagihara K and Kamihira S: Impact of
miR-155 and miR-126 as novel biomarkers on the assessment of
disease progression and prognosis in adult T-cell leukemia. Cancer
Epidemiol. 36:560–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer
A, Lichter P and Seiffert M: miRNA-130a targets ATG2B and DICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boll K, Reiche K, Kasack K, Mörbt N,
Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn
F and Hackermüller J: MiR-130a, miR-203 and miR-205 jointly repress
key oncogenic pathways and are downregulated in prostate carcinoma.
Oncogene. 32:277–285. 2013. View Article : Google Scholar
|
|
41
|
Qiu S, Lin S, Hu D, Feng Y, Tan Y and Peng
Y: Interactions of miR-323/miR-326/miR-329 and
miR-130a/miR-155/miR-210 as prognostic indicators for clinical
outcome of glioblastoma patients. J Transl Med. 11:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Huang L, Zhao Y and Tan W:
Downregulation of miR-130a contributes to cisplatin resistance in
ovarian cancer cells by targeting X-linked inhibitor of apoptosis
(XIAP) directly. Acta Biochim Biophys Sin (Shanghai). 45:995–1001.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long
G and Yang K: MicroRNA-130a inhibits cell proliferation, invasion
and migration in human breast cancer by targeting the RAB5A. Int J
Clin Exp Pathol. 8:384–393. 2015.PubMed/NCBI
|
|
45
|
He L, Wang HY, Zhang L, Huang L, Li JD,
Xiong Y, Zhang MY, Jia WH, Yun JP, Luo RZ and Zheng M: Prognostic
significance of low DICER expression regulated by miR-130a in
cervical cancer. Cell Death Dis. 5:e12052014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ramsay RG and Gonda TJ: MYB function in
normal and cancer cells. Nat Rev Cancer. 8:523–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim G, Lim S and Kim KD: N-myc
downstream-regulated gene 2 (NDRG2) function as a positive
regulator of apoptosis: A new insight into NDRG2 as a tumor
suppressor. Cells. 10:26492021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Zhang CY, Xia RH, Han J, Sun B,
Sun SY and Li J: The MYB/miR-130a/NDRG2 axis modulates tumor
proliferation and metastatic potential in salivary adenoid cystic
carcinoma. Cell Death Dis. 9:9172018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li YF, Jing Y, Hao J, Frankfort NC, Zhou
X, Shen B, Liu X, Wang L and Li R: MicroRNA-21 in the pathogenesis
of acute kidney injury. Protein Cell. 4:813–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bautista-Sánchez D, Arriaga-Canon C,
Pedroza-Torres A, De La Rosa-Velázquez IA, González-Barrios R,
Contreras-Espinosa L, Montiel-Manríquez R, Castro-Hernández C,
Fragoso-Ontiveros V, Álvarez-Gómez RM and Herrera LA: The promising
role of miR-21 as a cancer biomarker and its importance in
RNA-based therapeutics. Mol Ther Nucleic Acids. 20:409–420. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Das PK, Islam F and Lam AK: The roles of
cancer stem cells and therapy resistance in colorectal carcinoma.
Cells. 9:13922020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dioguardi M, Caloro GA, Laino L, Alovisi
M, Sovereto D, Crincoli V, Aiuto R, Coccia E, Troiano G and Lo
Muzio L: Circulating miR-21 as a potential biomarker for the
diagnosis of oral cancer: A systematic review with meta-analysis.
Cancers (Basel). 12:9362020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ribas J and Lupold SE: The transcriptional
regulation of miR-21, its multiple transcripts, and their
implication in prostate cancer. Cell Cycle. 9:923–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li S, Yang X, Yang J, Zhen J and Zhang D:
Serum microRNA-21 as a potential diagnostic biomarker for breast
cancer: A systematic review and meta-analysis. Clin Exp Med.
16:29–35. 2016. View Article : Google Scholar
|
|
55
|
Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu
XX, Han J, Wu YC, Liu X, Zhu X, et al: miR-21 regulates tumor
progression through the miR-21-PDCD4-Stat3 pathway in human
salivary adenoid cystic carcinoma. Lab Invest. 95:1398–1408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang C, Li T, Yan F, Cai W, Zheng J, Jiang
X and Sun J: Effect of simvastatin and microRNA-21 inhibitor on
metastasis and progression of human salivary adenoid cystic
carcinoma. Biomed Pharmacother. 105:1054–1061. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H
and Yang W: Simvastatin induced ferroptosis for triple-negative
breast cancer therapy. J Nanobiotechnology. 19:3112021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen MC, Tsai YC, Tseng JH, Liou JJ, Horng
S, Wen HC, Fan YC, Zhong WB and Hsu SP: Simvastatin inhibits cell
proliferation and migration in human anaplastic thyroid cancer. Int
J Mol Sci. 18:26902017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu X, Pan Y, Ma H and Li W: Simvastatin
inhibits proliferation and induces apoptosis in human lung cancer
cells. Oncol Res. 20:351–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yan F, Wang C, Li T, Cai W and Sun J: Role
of miR-21 in the growth and metastasis of human salivary adenoid
cystic carcinoma. Mol Med Rep. 17:4237–4244. 2018.PubMed/NCBI
|
|
61
|
Chen X, Chen S, Xiu YL, Sun KX, Zong ZH
and Zhao Y: RhoC is a major target of microRNA-93-5P in epithelial
ovarian carcinoma tumorigenesis and progression. Mol Cancer.
14:312015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen X, Liu J, Zhang Q, Liu B, Cheng Y,
Zhang Y, Sun Y, Ge H and Liu Y: Exosome-mediated transfer of
miR-93-5p from cancer-associated fibroblasts confer radioresistance
in colorectal cancer cells by downregulating FOXA1 and upregulating
TGFB3. J Exp Clin Cancer Res. 39:652020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X,
Wang SM, Wu YY, Zhu HB, Wang DX and Yang SM: miR-93-5p/IFNAR1 axis
promotes gastric cancer metastasis through activating the STAT3
signaling pathway. Cancer Lett. 408:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shi X, Liu TT, Yu XN, Balakrishnan A, Zhu
HR, Guo HY, Zhang GC, Bilegsaikhan E, Sun JL, Song GQ, et al:
microRNA-93-5p promotes hepatocellular carcinoma progression via a
microRNA-93-5p/MAP3K2/c-Jun positive feedback circuit. Oncogene.
39:5768–5781. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y,
Su F, Liu Q, Yao H and Song E: BRMS1L suppresses breast cancer
metastasis by inducing epigenetic silence of FZD10. Nat Commun.
5:54062014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koyama R, Tamura M, Nakagaki T, Ohashi T,
Idogawa M, Suzuki H, Tokino T and Sasaki Y: Identification and
characterization of a metastatic suppressor BRMS1L as a target gene
of p53. Cancer Sci. 108:2413–2421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hao J, Jin X, Shi Y and Zhang H: miR-93-5p
enhance lacrimal gland adenoid cystic carcinoma cell tumorigenesis
by targeting BRMS1L. Cancer Cell Int. 18:722018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun Z, Zhang Q, Yuan W, Li X, Chen C, Guo
Y, Shao B, Dang Q, Zhou Q, Wang Q, et al: MiR-103a-3p promotes
tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J
Exp Clin Cancer Res. 39:2502020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fu M, Chen CW, Yang LQ, Yang WW, Du ZH, Li
YR, Li SL and Ge XY: MicroRNA-103a-3p promotes metastasis by
targeting TPD52 in salivary adenoid cystic carcinoma. Int J Oncol.
57:574–586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shehata M, Bièche I, Boutros R,
Weidenhofer J, Fanayan S, Spalding L, Zeps N, Byth K, Bright RK,
Lidereau R and Byrne JA: Nonredundant functions for tumor protein
D52-like proteins support specific targeting of TPD52. Clin Cancer
Res. 14:5050–5060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rubin MA, Varambally S, Beroukhim R,
Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M,
Kuefer R, Fletcher JA, et al: Overexpression, amplification, and
androgen regulation of TPD52 in prostate cancer. Cancer Res.
64:3814–3822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang SJ, Li YJ, Gao B, Li XL, Li YT and He
HY: Long non-coding RNA 00152 slicing represses the growth and
aggressiveness of hemangioma cell by modulating miR-139-5p. Biomed
Pharmacother. 120:1093852019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tennstedt P, Bölch C, Strobel G, Minner S,
Burkhardt L, Grob T, Masser S, Sauter G, Schlomm T and Simon R:
Patterns of TPD52 overexpression in multiple human solid tumor
types analyzed by quantitative PCR. Int J Oncol. 44:609–615. 2014.
View Article : Google Scholar
|
|
74
|
Ravegnini G, Cargnin S, Sammarini G,
Zanotti F, Bermejo JL, Hrelia P, Terrazzino S and Angelini S:
Prognostic role of miR-221 and miR-222 expression in cancer
patients: A systematic review and meta-analysis. Cancers (Basel).
11:9702019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Song J, Ouyang Y, Che J, Li X, Zhao Y,
Yang K, Zhao X, Chen Y, Fan C and Yuan W: Potential value of
miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers
for diseases. Front Immunol. 8:562017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang F, Zhao W, Zhou L, Zhang L, Liu Z
and Yu D: miR-222 regulates the cell biological behavior of oral
squamous cell carcinoma by targeting PUMA. Oncol Rep. 31:1255–1262.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou Z, Zhou L, Jiang F, Zeng B, Wei C,
Zhao W and Yu D: Downregulation of miR-222 induces apoptosis and
cellular migration in adenoid cystic carcinoma cells. Oncol Res.
25:207–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li Y, He Y, Xiang J, Feng L, Wang Y and
Chen R: The functional mechanism of MicroRNA in oral lichen planus.
J Inflamm Res. 15:4261–4274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li Y, Tian Z, Tan Y, Lian G, Chen S, Chen
S, Li J, Li X, Huang K and Chen Y: Bmi-1-induced miR-27a and
miR-155 promote tumor metastasis and chemoresistance by targeting
RKIP in gastric cancer. Mol Cancer. 19:1092020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Van Roosbroeck K, Fanini F, Setoyama T,
Ivan C, Rodriguez-Aguayo C, Fuentes-Mattei E, Xiao L, Vannini I,
Redis RS, D'Abundo L, et al: Combining anti-Mir-155 with
chemotherapy for the treatment of lung cancers. Clin Cancer Res.
23:2891–2904. 2017. View Article : Google Scholar
|
|
82
|
Kulkarni P, Dasgupta P, Hashimoto Y,
Shiina M, Shahryari V, Tabatabai ZL, Yamamura S, Tanaka Y, Saini S,
Dahiya R and Majid S: A lncRNA TCL6-miR-155 interaction regulates
the Src-Akt-EMT network to mediate kidney cancer progression and
metastasis. Cancer Res. 81:1500–1512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bacci M, Giannoni E, Fearns A, Ribas R,
Gao Q, Taddei ML, Pintus G, Dowsett M, Isacke CM, Martin LA, et al:
miR-155 drives metabolic reprogramming of ER+ breast cancer cells
following long-term estrogen deprivation and predicts clinical
response to aromatase inhibitors. Cancer Res. 76:1615–1626. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hess AK, Müer A, Mairinger FD, Weichert W,
Stenzinger A, Hummel M, Budach V and Tinhofer I: MiR-200b and
miR-155 as predictive biomarkers for the efficacy of chemoradiation
in locally advanced head and neck squamous cell carcinoma. Eur J
Cancer. 77:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gulei D, Raduly L, Broseghini E, Ferracin
M and Berindan-Neagoe I: The extensive role of miR-155 in malignant
and non-malignant diseases. Mol Aspects Med. 70:33–56. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liu L, Hu Y, Fu J, Yang X and Zhang Z:
MicroRNA155 in the growth and invasion of salivary adenoid cystic
carcinoma. J Oral Pathol Med. 42:140–147. 2013. View Article : Google Scholar
|
|
87
|
Biswas DK and Iglehart JD: Linkage between
EGFR family receptors and nuclear factor kappaB (NF-kappaB)
signaling in breast cancer. J Cell Physiol. 209:645–652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cascinu S, Verdecchia L, Valeri N, Berardi
R and Scartozzi M: New target therapies in advanced pancreatic
cancer. Ann Oncol. 17(Suppl 5): v148–v152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bianco R, Gelardi T, Damiano V, Ciardiello
F and Tortora G: Rational bases for the development of EGFR
inhibitors for cancer treatment. Int J Biochem Cell Biol.
39:1416–1431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Feng X, Matsuo K, Zhang T, Hu Y, Mays AC,
Browne JD, Zhou X and Sullivan CA: MicroRNA profiling and target
genes related to metastasis of salivary adenoid cystic carcinoma.
Anticancer Res. 37:3473–3481. 2017.PubMed/NCBI
|
|
91
|
Jin Y, Chen X, Gao ZY, Liu K, Hou Y and
Zheng J: The role of miR-320a and IL-1β in human chondrocyte
degradation. Bone Joint Res. 6:196–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shang C, Zhang H, Guo Y, Hong Y, Liu Y and
Xue Y: MiR-320a down-regulation mediates bladder carcinoma invasion
by targeting ITGB3. Mol Biol Rep. 41:2521–2527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xie N, Jia Z and Li L: miR-320a
upregulation contributes to the development of preeclampsia by
inhibiting the growth and invasion of trophoblast cells by
targeting interleukin 4. Mol Med Rep. 20:3256–3264. 2019.PubMed/NCBI
|
|
94
|
Lv G, Wu M, Wang M, Jiang X, Du J, Zhang
K, Li D, Ma N, Peng Y, Wang L, et al: miR-320a regulates high
mobility group box 1 expression and inhibits invasion and
metastasis in hepatocellular carcinoma. Liver Int. 37:1354–1364.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang B, Yang Z, Wang H, Cao Z, Zhao Y,
Gong C, Ma L, Wang X, Hu X and Chen S: MicroRNA-320a inhibits
proliferation and invasion of breast cancer cells by targeting
RAB11A. Am J Cancer Res. 5:2719–2729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang L, Chen H, He F, Zhang S, Li A and
Zhang A and Zhang A: MicroRNA-320a promotes epithelial ovarian
cancer cell proliferation and invasion by targeting RASSF8. Front
Oncol. 11:5819322021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sun L, Liu B, Lin Z, Yao Y, Chen Y, Li Y,
Chen J, Yu D, Tang Z, Wang B, et al: MiR-320a acts as a prognostic
factor and inhibits metastasis of salivary adenoid cystic carcinoma
by targeting ITGB3. Mol Cancer. 14:962015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fuentes P, Sesé M, Guijarro PJ, Emperador
M, Sánchez-Redondo S, Peinado H, Hümmer S, Ramón Y and Cajal S:
ITGB3-mediated uptake of small extracellular vesicles facilitates
intercellular communication in breast cancer cells. Nat Commun.
11:42612020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL,
Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al: Long noncoding RNA
FAM225A promotes nasopharyngeal carcinoma tumorigenesis and
metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and
upregulate ITGB3. Cancer Res. 79:4612–4626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao B, Han H, Chen J, Zhang Z, Li S, Fang
F, Zheng Q, Ma Y, Zhang J, Wu N and Yang Y: MicroRNA let-7c
inhibits migration and invasion of human non-small cell lung cancer
by targeting ITGB3 and MAP4K3. Cancer Lett. 342:43–51. 2014.
View Article : Google Scholar
|
|
101
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rothman AM, Arnold ND, Pickworth JA,
Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M,
Morrell NW, Thomas M, et al: MicroRNA-140-5p and SMURF1 regulate
pulmonary arterial hypertension. J Clin Invest. 126:2495–2508.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kelly RJ, Lopez-Chavez A, Citrin D, Janik
JE and Morris JC: Impacting tumor cell-fate by targeting the
inhibitor of apoptosis protein survivin. Mol Cancer. 10:352011.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Martínez-García D, Manero-Rupérez N,
Quesada R, Korrodi-Gregório L and Soto-Cerrato V: Therapeutic
strategies involving survivin inhibition in cancer. Med Res Rev.
39:887–909. 2019. View Article : Google Scholar
|
|
105
|
Qiao Z, Zou Y and Zhao H: MicroRNA-140-5p
inhibits salivary adenoid cystic carcinoma progression and
metastasis via targeting survivin. Cancer Cell Int. 19:3012019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Peng W, Sha H, Sun X, Zou R, Zhu Y, Zhou G
and Feng J: Role and mechanism of miR-187 in human cancer. Am J
Transl Res. 12:4873–4884. 2020.PubMed/NCBI
|
|
107
|
Mulrane L, Madden SF, Brennan DJ, Gremel
G, McGee SF, McNally S, Martin F, Crown JP, Jirström K, Higgins DG,
et al: miR-187 is an independent prognostic factor in breast cancer
and confers increased invasive potential in vitro. Clin Cancer Res.
18:6702–6713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chao A, Lin CY, Lee YS, Tsai CL, Wei PC,
Hsueh S, Wu TI, Tsai CN, Wang CJ, Chao AS, et al: Regulation of
ovarian cancer progression by microRNA-187 through targeting
disabled homolog-2. Oncogene. 31:764–775. 2012. View Article : Google Scholar
|
|
109
|
Liang Z, Xu J, Ma Z, Li G and Zhu W:
MiR-187 suppresses non-small-cell lung cancer cell proliferation by
targeting FGF9. Bioengineered. 11:70–80. 2020. View Article : Google Scholar :
|
|
110
|
Bagaeva LV, Rao P, Powers JM and Segal BM:
CXC chemokine ligand 13 plays a role in experimental autoimmune
encephalomyelitis. J Immunol. 176:7676–7685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hussain M, Adah D, Tariq M, Lu Y, Zhang J
and Liu J: CXCL13/CXCR5 signaling axis in cancer. Life Sci.
227:175–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang M, Wu JS, Xian HC, Chen BJ, Wang HF,
Yu XH, Pang X, Dai L, Jiang J, Liang XH and Tang YL: CXCR5 induces
perineural invasion of salivary adenoid cystic carcinoma by
inhibiting microRNA-187. Aging (Albany NY). 13:15384–15399. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gui T and Shen K: miRNA-101: A potential
target for tumor therapy. Cancer Epidemiol. 36:537–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasion-metastasis in serous ovarian cancer by competitively
binding miR-101-3p to regulate ZEB1 expression. Mol Cancer.
17:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Xu Y, Yao Y, Jiang X, Zhong X, Wang Z, Li
C, Kang P, Leng K, Ji D, Li Z, et al: SP1-induced upregulation of
lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding
EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangiocarcinoma. J
Exp Clin Cancer Res. 37:812018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cao S, Lin L, Xia X and Wu H: lncRNA
SPRY4-IT1 regulates cell proliferation and migration by sponging
miR-101-3p and regulating AMPK expression in gastric cancer. Mol
Ther Nucleic Acids. 17:455–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang X, Gao D, Fang K, Guo Z and Li L:
Med19 is targeted by miR-101-3p/miR-422a and promotes breast cancer
progression by regulating the EGFR/MEK/ERK signaling pathway.
Cancer Lett. 444:105–115. 2019. View Article : Google Scholar
|
|
118
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar
|
|
119
|
Xu J, Xiong G, Cao Z, Huang H, Wang T, You
L, Zhou L, Zheng L, Hu Y, Zhang T and Zhao Y: PIM-1 contributes to
the malignancy of pancreatic cancer and displays diagnostic and
prognostic value. J Exp Clin Cancer Res. 35:1332016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pang W, Tian X, Bai F, Han R, Wang J, Shen
H, Zhang X, Liu Y, Yan X, Jiang F and Xing L: Pim-1 kinase is a
target of miR-486-5p and eukaryotic translation initiation factor
4E, and plays a critical role in lung cancer. Mol Cancer.
13:2402014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liu XY, Liu ZJ, He H, Zhang C and Wang YL:
MicroRNA-101-3p suppresses cell proliferation, invasion and
enhances chemotherapeutic sensitivity in salivary gland adenoid
cystic carcinoma by targeting Pim-1. Am J Cancer Res. 5:3015–3029.
2015.PubMed/NCBI
|
|
122
|
Zhou H, Huang Z, Chen X and Chen S: miR-98
inhibits expression of TWIST to prevent progression of non-small
cell lung cancers. Biomed Pharmacother. 89:1453–1461. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Huang SD, Yuan Y, Zhuang CW, Li BL, Gong
DJ, Wang SG, Zeng ZY and Cheng HZ: MicroRNA-98 and microRNA-214
post-transcriptionally regulate enhancer of zeste homolog 2 and
inhibit migration and invasion in human esophageal squamous cell
carcinoma. Mol Cancer. 11:512012. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Liu X, Zhang W, Guo H, Yue J and Zhuo S:
miR-98 functions as a tumor suppressor in salivary adenoid cystic
carcinomas. Onco Targets Ther. 9:1777–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Thumar J, Shahbazian D, Aziz SA, Jilaveanu
LB and Kluger HM: MEK targeting in N-RAS mutated metastatic
melanoma. Mol Cancer. 13:452014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang Y, Velho S, Vakiani E, Peng S, Bass
AJ, Chu GC, Gierut J, Bugni JM, Der CJ, Philips M, et al: Mutant
N-RAS protects colorectal cancer cells from stress-induced
apoptosis and contributes to cancer development and progression.
Cancer Discov. 3:294–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bhatlekar S, Manne BK, Basak I, Edelstein
LC, Tugolukova E, Stoller ML, Cody MJ, Morley SC, Nagalla S,
Weyrich AS, et al: miR-125a-5p regulates megakaryocyte proplatelet
formation via the actin-bundling protein L-plastin. Blood.
136:1760–1772. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Nishida N, Mimori K, Fabbri M, Yokobori T,
Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y and Mori M:
MicroRNA-125a-5p is an independent prognostic factor in gastric
cancer and inhibits the proliferation of human gastric cancer cells
in combination with trastuzumab. Clin Cancer Res. 17:2725–2733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu X, Zhao T, Yuan Z and Ge S: MIR600HG
sponges miR-125a-5p to regulate glycometabolism and cisplatin
resistance of oral squamous cell carcinoma cells via mediating
RNF44. Cell Death Discov. 8:2162022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Falcicchia C, Tozzi F, Arancio O,
Watterson DM and Origlia N: Involvement of p38 MAPK in synaptic
function and dysfunction. Int J Mol Sci. 21:56242020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Han J and Sun P: The pathways to tumor
suppression via route p38. Trends Biochem Sci. 32:364–371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liang Y, Ye J, Jiao J, Zhang J, Lu Y,
Zhang L, Wan D, Duan L, Wu Y and Zhang B: Down-regulation of
miR-125a-5p is associated with salivary adenoid cystic carcinoma
progression via targeting p38/JNK/ERK signal pathway. Am J Transl
Res. 9:1101–1113. 2017.PubMed/NCBI
|
|
134
|
Zeng X, Ma X, Guo H, Wei L, Zhang Y, Sun
C, Han N, Sun S and Zhang N: MicroRNA-582-5p promotes
triple-negative breast cancer invasion and metastasis by
antagonizing CMTM8. Bioengineered. 12:10126–10135. 2021. View Article : Google Scholar
|
|
135
|
Han B, Bhowmick N, Qu Y, Chung S, Giuliano
AE and Cui X: FOXC1: An emerging marker and therapeutic target for
cancer. Oncogene. 36:3957–3963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Gilding LN and Somervaille TCP: The
diverse consequences of FOXC1 deregulation in cancer. Cancers
(Basel). 11:1842019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Wang WW, Chen B, Lei CB, Liu GX, Wang YG,
Yi C, Wang YY and Zhang SY: miR-582-5p inhibits invasion and
migration of salivary adenoid cystic carcinoma cells by targeting
FOXC1. Jpn J Clin Oncol. 47:690–698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Xie H, Tang J, Lu L, Li B and Wang M:
CASC9 plays a role in salivary adenoid cystic carcinoma in vitro by
upregulation of ACLY. Oral Dis. 28:352–363. 2022. View Article : Google Scholar
|
|
139
|
Huo F, Zhang C, He H and Wang Y:
MicroRNA-144-3p inhibits proliferation and induces apoptosis of
human salivary adenoid carcinoma cells via targeting of mTOR.
Biotechnol Lett. 38:409–416. 2016. View Article : Google Scholar
|
|
140
|
Hou CX, Sun NN, Han W, Meng Y, Wang CX,
Zhu QH, Tang YT and Ye JH: Exosomal microRNA-23b-3p promotes tumor
angiogenesis and metastasis by targeting PTEN in salivary adenoid
cystic carcinoma. Carcinogenesis. 43:682–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Li Z, Zhang Q, Su H, Li HY, Cao G, Xu JK,
Wang JL, Niu CZ, Zhang F, Yang J and Chen W: miR-5191 acts as a
tumor suppressor in salivary adenoid cystic carcinoma by targeting
Notch-2. Oral Dis. 28:1871–1881. 2022. View Article : Google Scholar
|
|
142
|
Wang S, Zhang L, Shi P, Zhang Y, Zhou H
and Cao X: Genome-wide profiles of metastasis-associated mRNAs and
microRNAs in salivary adenoid cystic carcinoma. Biochem Biophys Res
Commun. 500:632–638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xie S, Yu X, Li Y, Ma H, Fan S, Chen W,
Pan G, Wang W, Zhang H, Li J and Lin Z: Upregulation of lncRNA
ADAMTS9-AS2 promotes salivary adenoid cystic carcinoma metastasis
via PI3K/Akt and MEK/Erk signaling. Mol Ther. 26:2766–2778. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ju R, Huang Y, Guo Z, Han L, Ji S, Zhao L
and Long J: The circular RNAs differential expression profiles in
the metastasis of salivary adenoid cystic carcinoma cells. Mol Cell
Biochem. 476:1269–1282. 2021. View Article : Google Scholar
|
|
147
|
Xu Q, Liu X, Chen W and Zhang Z:
Inhibiting adenoid cystic carcinoma cells growth and metastasis by
blocking the expression of ADAM 10 using RNA interference. J Transl
Med. 8:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Andreasen S: Molecular features of adenoid
cystic carcinoma with an emphasis on microRNA expression. Apmis.
126(Suppl 140): S7–S57. 2018. View Article : Google Scholar
|
|
149
|
Liang H, Gong F, Zhang S, Zhang CY, Zen K
and Chen X: The origin, function, and diagnostic potential of
extracellular microRNAs in human body fluids. Wiley Interdiscip Rev
RNA. 5:285–300. 2014. View Article : Google Scholar
|
|
150
|
Papaspyrou G, Hoch S, Rinaldo A, Rodrigo
JP, Takes RP, van Herpen C, Werner JA and Ferlito A: Chemotherapy
and targeted therapy in adenoid cystic carcinoma of the head and
neck: A review. Head Neck. 33:905–911. 2011. View Article : Google Scholar
|
|
151
|
Le Tourneau C, Razak AR, Levy C, Calugaru
V, Galatoire O, Dendale R, Desjardins L and Gan HK: Role of
chemotherapy and molecularly targeted agents in the treatment of
adenoid cystic carcinoma of the lacrimal gland. Br J Ophthalmol.
95:1483–1489. 2011. View Article : Google Scholar
|















