Introduction
Pancreatic cancer, particularly pancreatic head
cancer, seriously threatens human health, with high malignancy and
poor prognosis (1). At present,
the treatment of pancreatic cancer is still mainly based on
surgical tumor resection. However, the operation is associated with
large trauma and numerous complications, and the postoperative
tumor recurrence rate is high. Furthermore, at the time of
diagnosis, numerous patients have already missed the opportunity of
surgical tumor resection. Thus, the mortality rate is high and the
prognosis is poor for patients with pancreatic cancer (1,2).
Therefore, it is essential to find new treatment options.
Targeted therapy has been used in the clinical
treatment of certain tumors and has achieved a good therapeutic
effect (3-5). However, for numerous tumor types,
particularly pancreatic cancer, effective targeted therapies are
limited. Therefore, further research on pancreatic cancer targeted
therapy is required.
The key to effective targeted therapy lies in the
selection of target molecules. Therefore, to establish effective
targeted therapy for pancreatic cancer, it is first required to
find potential effective target molecules.
Keratin 19 (K19), with a molecular weight of ~40
kDa, is a type I cytokeratin but lacks the common tail domain of
cytokeratin (6). It has been
suggested that K19 is related to the degree of malignancy,
invasiveness and poor prognosis of tumors (7). Indeed, tumors expressing K19 have
stronger invasiveness and worse prognosis (8). Therefore, it was assumed that high
malignancy, strong invasion and metastasis, and poor prognosis of
pancreatic cancer are also related to the expression of K19, and
that K19 may be a promoter and potential effective therapeutic
target for pancreatic cancer. However, research on K19 and
pancreatic cancer is scarce, and the role and mechanism of K19 in
pancreatic cancer remain largely elusive.
In the present study, it was reported that K19 was
significantly overexpressed in pancreatic cancer; it was indicated
to promote pancreatic cancer cell proliferation, tumorigenesis and
metastasis, inhibit tumor cell apoptosis and to be associated with
poor prognosis. K19 was determined to activate the Hedgehog pathway
to promote pancreatic cancer proliferation and metastasis. In other
words, K19 promoted pancreatic cancer progression and poor
prognosis. Mechanistically, these effects were mediated through the
activation of the Hedgehog pathway. K19 may be a novel target
molecule for pancreatic cancer treatment.
Materials and methods
Specimens and clinicopathological data of
patients with pancreatic cancer
Specimens with complete clinicopathological data
were obtained from patients with pancreatic cancer treated by
surgery at Guizhou Provincial People's Hospital (Guiyang, China)
between December 2018 and September 2020. All patients provided
informed consent. The study received the approval from the Ethics
Committee of Guizhou Provincial People's Hospital [Guiyang, China;
no. (2021)253] and was conducted in line with the principles of the
Helsinki declaration. The clinical diagnosis of pancreatic cancer
was based on conventional clinical and histological criteria. The
operation principle for all of the patients was tumor resection. In
addition, pancreatic cancer tissue microarrays (HPanA060CS04,
HPanA120Su02) purchased from Shanghai Outdo Biotech Co., Ltd. were
used. Finally, the tissues of 117 patients with pancreatic cancer,
including 67 males and 50 females with an average age of
63.81±10.14 years, and the follow-up data of 66 patients, including
37 males and 29 females, with a follow-up time of 1-65 months and
an average age of 65.33±9.87 years, were used. The demographic data
and clinicopathological characteristics of these patients are
provided in Table SI.
Cell lines
The commercialized pancreatic cancer cell lines
PANC-1 and MIA-PaCa-2 purchased from Beijing SyngenTech Co., Ltd.
and Wuhan AtaGenix Co., Ltd., respectively, were cultivated in DMEM
(Hyclone; Cytiva) and RPMI1640 (Hyclone; Cytiva) culture medium,
respectively, in a 5% CO2 incubator at 37°C and
saturated humidity of 95%. The culture medium was supplemented with
10% fetal bovine serum (Hyclone; Cytiva), 100 U/ml penicillin and
100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.). The
mycoplasma culture test was confirmed to be negative every
month.
Mice
Male BALB/c nude mice (nu/nu; age, four weeks; body
weight, 15-20 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. The mice were maintained under specific
pathogen-free conditions with a 12-h light/dark cycle, temperature
of 22±2°C and humidity of 40-60%, and had ad libitum access
to a regular chow diet and water.
Cell proliferation assay
A cell suspension (5,000 cells/well) was added into
a 96-well plate and cultured at 37°C and 5% CO2 for 24,
48, 72 and 96 h. Next, 10 µl of Cell Counting Kit-8 (CCK8)
solution (Dojindo Laboratories, Inc.) was added in line with the
kit instructions and incubated at 37°C and 5% CO2 for 4
h. The optical density at 450 nm was detected by a microplate
reader.
Cell transfection
The cells in the exponential growth phase were
inoculated into a 6-well plate (50,000 cells/well) and cultured at
37°C with 5% CO2. Transfection of a lentivirus with an
overexpression or knockdown vector for K19 was performed by
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions the next day and the medium was
replaced with fresh medium 8 h after lentivirus transfection. At 72
h after lentivirus transfection, images of the cells were captured
under the fluorescence microscope. The cells were collected and the
overexpression and knockdown efficiencies were detected by reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis
(Fig. S1).
The short hairpin (sh)RNA sequences (Beijing
SyngenTech Co., Ltd.) used to knock down K19 expression were as
follows: shK19 (knockdown of K19 gene) sense, 5′-GCC GGA CTG AAG
AAT TGA ACC-3′ and antisense, 5′-CGA AGG TTC AAT TCT TCA GTC CGG
C-3′; shCtrl (knockdown control) sense, 5′-AAA CGT GAC ACG TTC GGA
GAA-3′ and antisense, 5′-CGA ATT CTC CGA ACG TGT CAC GTT T-3′.
K19-RNA sequences (Beijing SyngenTech Co., Ltd.) used to
overexpress K19 expression were as follows: K19 (overexpression of
K19 gene, human, NM-002276) included 10,724 DNA bases; Ctrl
(overexpression control, mNeongreen) included 9,581 DNA bases.
RT-qPCR
The transfected cells were collected, RNA was
extracted, the absorbance at 260 nm (A260)/A280 value was measured
and the RNA concentration was estimated. A small amount of RNA was
taken out to determine the RNA quality by agarose gel
electrophoresis. RNAs that met the requirements were used and
denatured at 65°C for 5 min. After the residual genomic DNA of the
product was removed by DNase reaction, RT reagent
[Hifair® II 1st Strand cDNA Synthesis SuperMix for qPCR
(gDNA digester plus); Yeasen Biotechnology (Shanghai) Co., Ltd.]
was added to reverse-transcribe the mRNA and synthesize cDNA
according to the manufacturer's instructions. The primers and the
cDNA products synthesized by RT were used for real-time qPCR
(thermocycling conditions: 95°C for 15 sec, 60°C for 1 min, 95°C
for 15 sec) in an ABI Prism 7300 PCR instrument (Applied
Biosystems, Inc.) and Hieff® qPCR SYBR Green Master Mix
[Low Rox; Yeasen Biotechnology (Shanghai) Co., Ltd.] was used
according to the manufacturer's instructions. The expression level
of GAPDH was calculated according to the 2−ΔΔCq method
(9) and used as a standardization
control. The PCR primers were as follows: K19 sense, 5′-TAG AGG TGA
AGA TCC GCG AC-3′ and anti-sense, 5′-CCG TCT CAA ACT TGG TTC GG-3′;
GAPDH sense, 5′-TCA AGA AGG TGG TGA AGC AGG-3′ and antisense,
5′-TCA AAG GTG GAG GAG TGG GT-3′.
Immunohistochemistry (IHC)
The tissues were routinely dehydrated, embedded in
paraffin and sliced. After baking slices at 60°C overnight, the
tissue sections were successively dewaxed, hydrated, permeated,
sealed, antigen-repaired, incubated with primary antibody at 4°C
overnight and secondary antibody at room temperature for 30 min,
color-rendered, counterstained with hematoxylin and sealed
according to standard protocols. Information about the antibodies
is provided in Table SII.
The proportion of cells with positive staining was
determined as follows: From each slice, 10 high-power vision fields
were randomly selected, the number of positive cells among 100
cells in each high-power vision field was determined, and the
average value of the total number of these 10 high-power vision
fields was then calculated. From the percentage of positive cells,
the positive index was scored as follows: <25%, score 0; 25-50%,
score 1; 50-75%, score 2; >75%, score 3.
The staining intensity was rated score 0 if there
was no color rendering and three levels from light color to deep
color corresponded to scores 1, 2 and 3.
The scores of the positive cell proportion and
staining intensity were multiplied to obtain the following
categories: Scores 0-2, (−); scores 3-4, (+); scores 5-6, (++);
scores 7-9, (+++).
The cell density (positive cell
number/mm2) was determined as follows: From each slice,
10 high-power vision fields (magnification, ×400; area, 0.0788
mm2) were randomly selected, the number of positive
cells in each high-power field was counted and the average value of
the total number of these 10 high-power fields was then taken.
Western blot analysis
Cell lysis and collection of protein were performed
using conventional procedures and detection of total protein
content using a BCA protein concentration detection kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions. The total protein was then subjected to SDS-PAGE (10%
gel), membrane transfer to an immobilion® PVDF membrane,
(Merck KGaA), primary antibody and secondary antibody incubation at
4°C overnight and room temperature for 1 h, respectively, and
imaging with Luminol reagent (Santa Cruz Biotechnology, Inc.).
GAPDH protein was used as a standardization control. Antibody
information is provided in Table
SII.
Colony-formation assay
The cells of each experimental group (200
cells/well) were added into a 6-well plate and three duplicate
wells were set in each group. The cells were cultured at 37°C with
5% CO2. The culture medium was changed every 3 days and
the cells were observed. After 14 days of culture, 4%
paraformaldehyde was added for fixation at room temperature for 10
min. Next, the colonies were stained with crystal violet aqueous
solution (Beyotime Institute of Biotechnology) at room temperature
for 10 min, images were acquired under a microscope and the number
of colonies (clusters of >50 cells) was counted.
Apoptosis assay
Cells of each experimental group (100,000
cells/well) were added into a 24-well plate and three duplicated
wells were set in each group. They were incubated at 37°C with 5%
CO2 for 48 h. The cells were collected, Annexin V and
propidium iodide [Yeasen Biotechnology (Shanghai) Co., Ltd.] were
added and cells were incubated at room temperature in the dark for
15 min and suspended in 1X Binding Buffer [Yeasen Biotechnology
(Shanghai) Co., Ltd.] according to the manufacturer's instructions.
Apoptosis was then detected by flow cytometry.
Cell cycle analysis
The cells of each experimental group (100,000
cells/well) were added into a 24-well plate and each group was set
with three duplicated wells, which were cultured at 37°C with 5%
CO2 for 48 h. The cells were collected and added into
ethanol precooled at -20°C and fixed at 4°C overnight. Next, the
ethanol was removed, 300 µl propidium iodide solution
(Biolegend, Inc.) was added and the cells were incubated away from
light at room temperature for 15 min. The cell cycle was then
detected by flow cytometry.
Transwell assay
Transwell chambers (Corning, Inc.; pore size, 8
µm) were inserted into a 24-well plate, cells of each
experimental group (20,000 cells/200 µl/chamber) were added
to the chamber, and three duplicate wells were set for each group.
Next, 600 µl medium was added into the lower chamber and
plates were cultured at 37°C and 5% CO2 for 24 h. The
cell culture medium and non-migrating cells in the upper chamber
were removed, 4% paraformaldehyde was added for fixation at room
temperature for 10 min and crystal violet aqueous solution
(Beyotime Institute of Biotechnology) was added to stain the cells
that had migrated at room temperature for 10 min. Images of the
cells that had migrated were acquired under a microscope and these
cells were counted by ImageJ software version Fiji (National
Institutes of Health).
The Transwell invasion assay was performed in a
similar manner, with the variation that the filter membranes of the
Transwell chambers were precoated with Matrigel® (BD
Biocoat, Inc.).
Construction of pancreatic cancer cells
with stable overexpression of K19
PANC-1 pancreatic cancer cells in the exponential
growth phase were inoculated into a 6-well plate (50,000
cells/well) and cultured at 37°C with 5% CO2. The next
day, lentivirus overexpressing K19 and their control (Beijing
SyngenTech Co., Ltd.; details provided above) were stably
transfected according to the manufacturer's instructions. At 8 h
after transfection, the medium was renewed with fresh culture
medium. At 72 h after transfection, the cells were screened using 2
µg/ml puromycin (Thermo Fisher Scientific, Inc.) and images
were acquired under a fluorescence microscope. Next, the cells were
cultured to establish pancreatic cancer cells stably overexpressing
K19, and their overexpression efficiency was detected by RT-qPCR
and western blot analysis (10)
(Fig. S2).
Construction of a subcutaneously
transplanted tumor model and an abdominal metastasis tumor model of
pancreatic cancer in nude mice
A subcutaneously transplanted tumor model and an
abdominal metastasis tumor model of pancreatic cancer in nude mice
were constructed via subcutaneously and intraperitoneally injecting
PANC-1 cells stably overexpressing K19 (n=6 mice) and control cells
(n=6 mice) (2×107/ml cells ×0.15 ml/mice). Subsequently,
the condition of the nude mice was observed and their body weight
and tumor size were measured once every 2-3 days. The tumor size
was calculated as follows: Tumor volume (mm3)=1/2 ×
(tumor long diameter x tumor short diameter2). After
reaching the endpoint of the experiment, the mice were euthanized
by cervical dislocation. The endpoints of the experiment included
that animals were dying and unable to move; did not respond to
gentle stimuli; had difficulty breathing; had diarrhoea and gatism;
lost their ability to eat or drink; exhibited obvious anxiety and
restlessness; their body weight was decreased by >20% of that
prior to the start of the experiment; the tumor weight exceeded 10%
of the animal's own body weight; the maximum diameter of the
subcutaneous tumor in mice was close to 20 mm (not >20 mm); the
tumor was ulcerating; the damage area of animal skin was >30% of
the whole body surface; and the skin of the animals was infected
and purulent. Of note, the experiment with the mice with abdominal
metastasis tumors was terminated two weeks after that with the mice
with the subcutaneous transplantation tumor. The tumor was
dissected and tumor size and tumor weight were measured.
Furthermore, the mesentery, liver, lung and tumor tissues of the
mice were dissected for subsequent IHC staining and
hematoxylin-eosin (H&E) staining according to standard
procedures.
TUNEL apoptosis assay
Tumor tissues from subcutaneous transplanted tumors
in mice were routinely dewaxed to water in line with the above IHC
method. The instructions of the TUNEL kit (cat. no. G1501; Wuhan
Servicebio Technology Co., Ltd.) were then followed. The results
were expressed as the number of positive cells.
Remedial experiment
When the cells were cultured and in the exponential
growth phase, 5 µM of glioma-associated oncoprotein 1 (Gli1)
inhibitor (GANT 58; cat. no. HY-13282; MedChemExpress) was added.
Subsequently, the expression of Gli1 and K19 proteins, as well as
the proliferation and invasion of pancreatic cancer cells, were
detected according to the previously mentioned western blot, CCK8
and Transwell invasion assays.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean for three or more independent experiments, and were
analyzed by SPSS version 23.0 (SPSS, Inc.,) and GraphPad Prism
software version 8.0 (GraphPad Software, Inc.). Student's t-test,
the Mann-Whitney U-test, Fisher's exact test, chi-square test and
analysis of variance (ANOVA) were used for assessment of
statistical significance. Student's t-test, a parametric test, was
used for mean comparison between two groups (n<30 and normal
distribution). The Mann-Whitney U-test, a non-parametric test, was
used for mean comparison between two groups, which were not
suitable for Student's t-test. ANOVA, also a parametric test, was
used for mean comparison between two or more groups. For comparison
of multiple groups, one-way ANOVA was used along with post-hoc
multiple-comparisons tests, including least-significant
differences, Student-Newman-Keuls and Bonferroni. The chi-squared
test, a nonparametric test, was used for rate comparison between
two or more groups (n≥40 and theoretical frequency T ≥1). Fisher's
exact test, also a nonparametric test, was used for rate comparison
between two or more groups (n <40 or theoretical frequency T
<1). Survival was calculated using the Kaplan-Meier method and
analyzed by the log-rank test. Significant variables from the
univariate analysis were entered into a multivariate analysis using
a Cox regression model with forward stepwise selection. Statistical
significance was considered if P<0.05.
Results
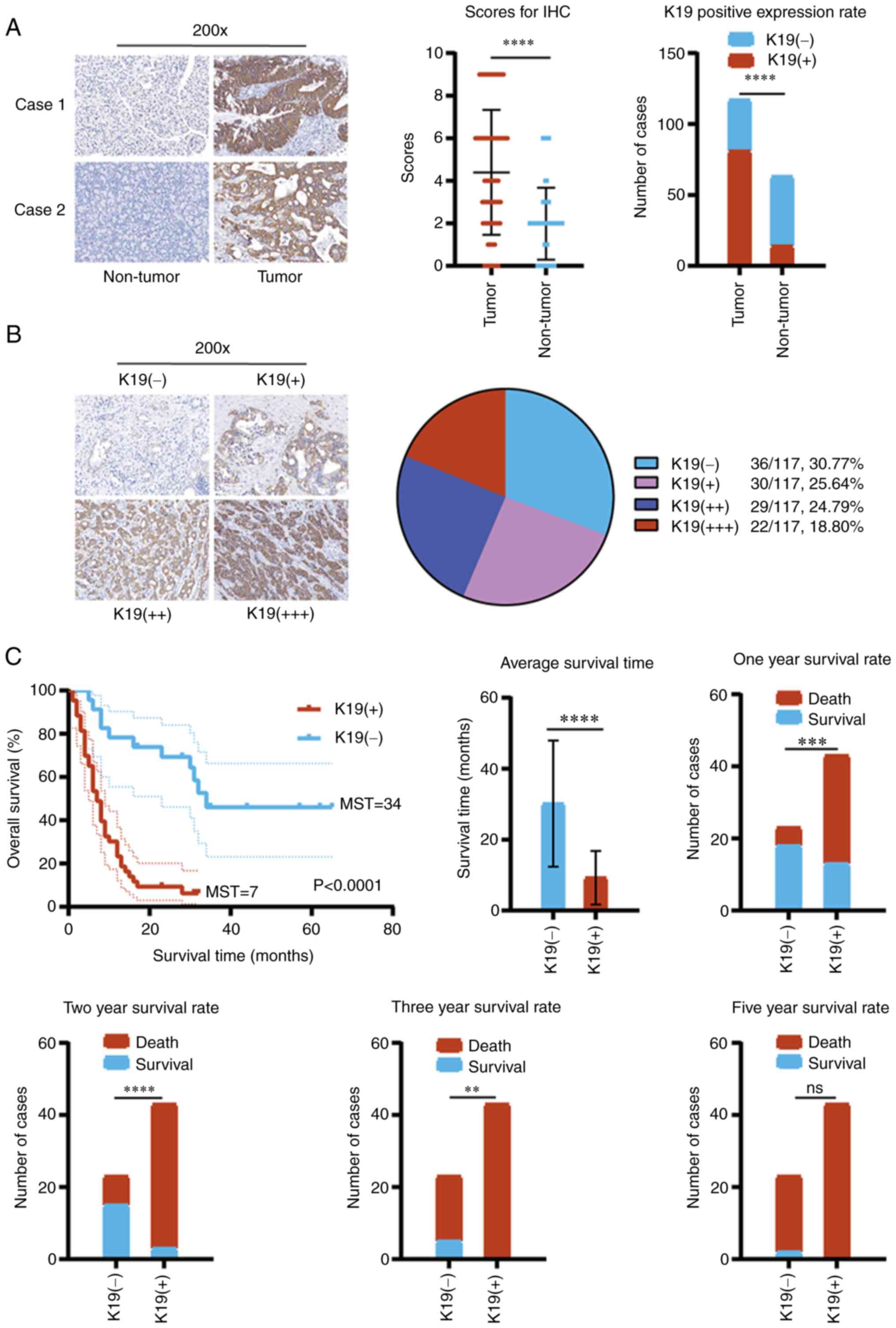
K19 is significantly overexpressed in
pancreatic cancer and is associated with poor prognosis
To observe the effect of K19 on pancreatic cancer,
the expression of K19 in pancreatic cancer and corresponding
paracancerous tissues was first detected by IHC. Compared with
paracancerous tissues, K19 was significantly overexpressed in
pancreatic cancer tissues (Fig.
1A). Of 117 pancreatic cancer samples, 81 (69.23%) were
positive [30 (25.64%) were weakly positive (+), 29 (24.79%) were
moderately positive (++) and 22 (18.80%) were strongly positive
(+++)] and 36 (30.77%) were negative for K19 expression (Fig. 1B). However, there was no
association between K19 expression and the demographic and
clinicopathological data of the patients with pancreatic cancer,
including age, gender, tumor distribution, tumor size, vascular
invasion, tumor metastasis, pathological grading and clinical stage
(Table SIII). However, the
incidence of tumors with a diameter ≤2 cm in the K19-positive group
was significantly lower than that in the K19-negative group
(Table SIII).
Survival analysis indicated that the overall
survival, median survival time and average survival time of
patients with pancreatic cancer expressing K19 were significantly
reduced, compared with those in patients without K19 expression
(Fig. 1C). Furthermore, the
one-year survival rate, two-year survival rate and three-year
survival rate of patients with pancreatic cancer expressing K19
were also significantly reduced, compared with those in patients
without K19 expression (Fig. 1C).
The five-year survival rate of patients with pancreatic cancer
expressing K19 was 0% (Fig. 1C).
Compared with those without K19 expression, no patients with
pancreatic cancer expressing K19 survived for >3 years (Fig. 1C). These data suggested that K19
was associated with poor prognosis of patients with pancreatic
cancer.
In addition, univariate analysis suggested that K19
expression and the pathological grade were unfavorable prognostic
factors for pancreatic cancer (Table
I). Multivariate analysis (Cox regression) indicated that K19
expression was an independent risk factor for poor prognosis of
pancreatic cancer (Table II).
 | Table IUnivariate analysis of survival in
patients with pancreatic cancer. |
Table I
Univariate analysis of survival in
patients with pancreatic cancer.
| Variable | Number of
patients | One-year survival,
% | Two-year survival,
% | Three-year
survival, % | Five-year survival,
% | P-value |
|---|
| Age, years | | | | | | 0.1824 |
| ≤60 | 24 | 63 | 33 | 8 | 4 | |
| >60 | 42 | 38 | 24 | 7 | 2 | |
| Gender | | | | | | 0.4493 |
| Male | 37 | 43 | 22 | 8 | 3 | |
| Female | 29 | 52 | 34 | 7 | 3 | |
| K19 expression | | | | | | 0.0001 |
| Negative | 23 | 78 | 65 | 22 | 9 | |
| Positive | 43 | 30 | 7 | 0 | 0 | |
| Tumor location | | | | | | 0.0822 |
| Pancreatic
head | 36 | 42 | 19 | 0 | 0 | |
| Pancreatic body
and tail | 17 | 59 | 35 | 24 | 12 | |
| Pancreas | 13 | 46 | 38 | 8 | 0 | |
| Tumor size, cm | | | | | | 0.5106 |
| ≥4 | 46 | 50 | 33 | 7 | 4 | |
| >2, <4 | 17 | 35 | 12 | 6 | 0 | |
| ≤2 | 3 | 67 | 33 | 0 | 0 | |
| Vascular
invasion | | | | | | 0.3462 |
| Absent | 50 | 50 | 28 | 8 | 4 | |
| Present | 16 | 38 | 25 | 6 | 0 | |
| Lymph node
metastasis | | | | | | 0.0906 |
| Absent | 43 | 51 | 33 | 12 | 5 | |
| Present | 23 | 39 | 17 | 0 | 0 | |
| Clinical stage | | | | | | 0.1465 |
| I-II | 62 | 50 | 29 | 8 | 3 | |
| III-IV | 4 | 0 | 0 | 0 | 0 | |
| Pathologic
grade | | | | | | 0.0277 |
| I-II | 38 | 61 | 39 | 8 | 3 | |
| III-IV | 28 | 29 | 11 | 7 | 4 | |
 | Table IIIndependent prognostic factors for
survival by multivariate analysis. |
Table II
Independent prognostic factors for
survival by multivariate analysis.
| Variable | Exp(B) (HR) | SE of Exp(B)
(HR) | P-value | 95% CI for Exp(B)
(HR) |
|---|
| K19 expression | 0.200 | 0.381 | <0.001 | 0.200
(0.095-0.422) |
| Pathologic
grade | 1.761 | 0.292 | 0.052 | 1.761
(0.995-3.119) |
| Tumor location | - | - | 0.583 | - |
| Lymph node
metastasis | - | - | 0.316 | - |
| Clinical stage | - | - | 0.291 | - |
| Tumor size | - | - | 0.848 | - |
| Vascular
invasion | - | - | 0.397 | - |
K19 promotes pancreatic cancer
proliferation
To observe the effect of K19 on pancreatic cancer
cell proliferation, K19-shRNA (knockdown of K19) and K19-RNA vector
(overexpression of K19) were first used to knockdown and
overexpress the K19 gene in pancreatic cancer cells, respectively.
Next, CCK8, colony-formation and flow cytometric assays were
performed to examine the proliferation, colony formation and cell
cycle progression of pancreatic cancer cells with knockdown and
overexpression of K19, respectively. Knockdown of K19 inhibited
pancreatic cancer cell proliferation (Fig. 2A), colony formation (Fig. 2B) and cell cycle progression
(Fig. 2C), while overexpression of
K19 promoted tumor cell proliferation (Fig. 2D), colony formation (Fig. 2E) and cell cycle progression
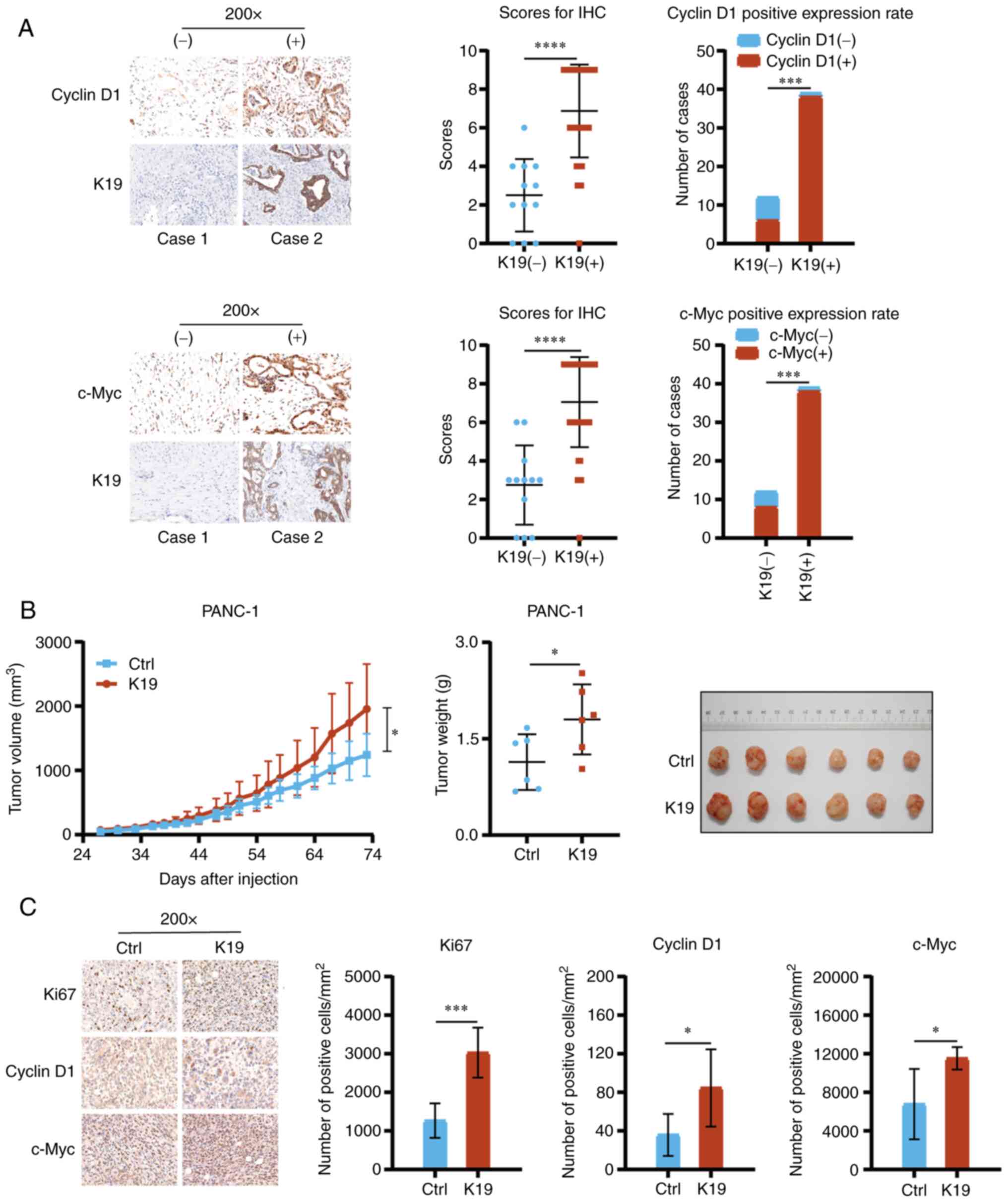
(Fig. 2F). CyclinD1 and c-Myc have
important roles in cancer proliferation (11,12).
Of note, compared with that in pancreatic cancer with negative K19
expression, the expression of CyclinD1 and c-Myc in pancreatic
cancer with positive K19 expression was upregulated (Fig. 3A).
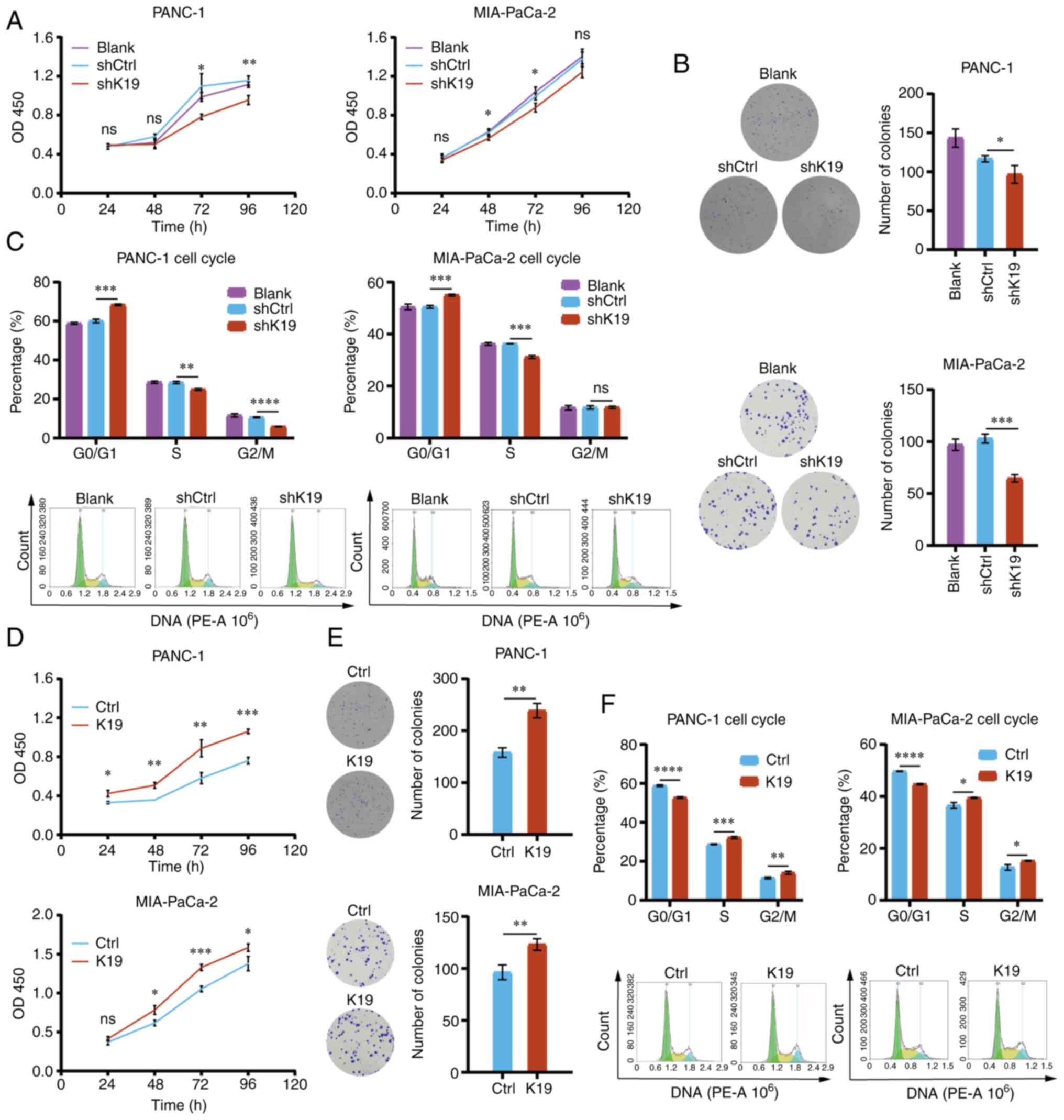 | Figure 2K19 promotes pancreatic cancer cell
proliferation in vitro. (A) Knockdown of K19 inhibited the
proliferation of PANC-1 and MIA-PaCa-2 cells. (B) Knockdown of K19
inhibited the colony formation of PANC-1 and MIA-PaCa-2 cells. (C)
Knockdown of K19 inhibited the cell cycle progression of PANC-1 and
MIA-PaCa-2 cells. (D) Overexpression of K19 promoted the
proliferation of PANC-1 and MIA-PaCa-2 cells. (E) Overexpression of
K19 promoted the colony formation of PANC-1 and MIA-PaCa-2 cells.
(F) Overexpression of K19 promoted the cell cycle progression of
PANC-1 and MIA-PaCa-2 cells. The proliferation, colony formation
and cell cycle progression of pancreatic cancer cells with
knockdown and overexpression of K19 were detected by Cell Counting
Kit-8, clone formation assay and flow cytometry, respectively.
These experiments were repeated three times. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001; ns, no significance; K19, keratin 19;
sh, short hairpin RNA; Ctrl, control; OD, optical density. |
In addition, as presented in Fig. 3B, the tumors in the mice
subcutaneously injected with K19-overexpressing PANC-1 cells grew
more quickly, were larger and weighed more than those in the
control group. Furthermore, the levels of Ki67, CyclinD1 and c-Myc
proteins in the tumors in the mice subcutaneously injected with
K19-overexpressing PANC-1 cells were increased (Fig. 3C). Collectively, the above data
suggested that K19 promoted pancreatic cancer proliferation, at
least in part via enhancing the expression of Ki67, CyclinD1 and
c-Myc.
K19 inhibits pancreatic cancer cell
apoptosis
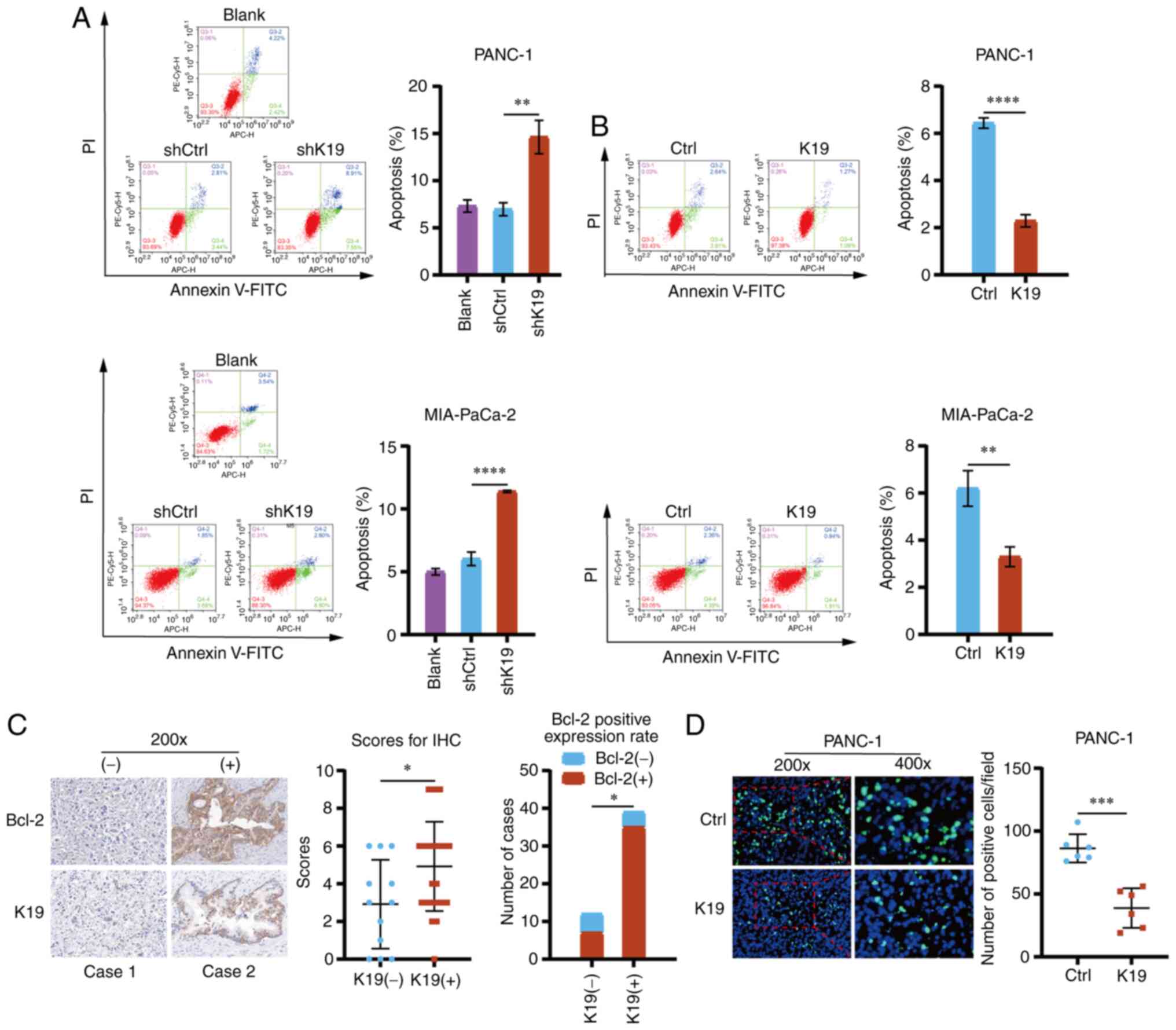
To observe the effect of K19 on pancreatic cancer
cell apoptosis, flow cytometry was performed. The apoptosis of
pancreatic cancer cells with K19 knockdown and K19 overexpression
was detected. Knockdown of K19 promoted pancreatic cancer cell
apoptosis (Fig. 4A), whereas
overexpression of K19 inhibited their apoptosis (Fig. 4B). Bcl-2 has a key role in
regulating apoptosis (13,14); i.e., upregulation of Bcl-2 is able
to inhibit apoptosis, while downregulation of Bcl-2 may induce
apoptosis (15). Therefore, to
observe the effect of K19 on the expression of Bcl-2 in pancreatic
cancer, IHC was used to detect the expression of Bcl-2 in
pancreatic cancer tissues. As indicated in Fig. 4C, Bcl-2 expression in pancreatic
cancer tissues expressing K19 was upregulated as compared with that
in pancreatic cancer tissues without K19 expression.
In addition, the results of the TUNEL apoptosis
assay suggested that overexpression of K19 inhibited pancreatic
cancer apoptosis in the subcutaneous tumors in the mice injected
with PANC-1 cells that overexpressed K19 (Fig. 4D). Collectively, these data
suggested that K19 inhibited pancreatic cancer cell apoptosis.
K19 promotes pancreatic cancer
metastasis
To observe the effect of K19 on pancreatic cancer
metastasis, Transwell migration and invasion assays were performed
to examine the migration and invasion of pancreatic cancer cells
with K19 knockdown and K19 overexpression. As presented in Fig. 5A and B, knockdown of K19 inhibited
the migration and invasion of pancreatic cancer cells, while
overexpression of K19 promoted their migration and invasion
(Fig. 5C and D). MMP2 and MMP9
have important roles in cancer metastasis (16,17).
Of note, compared with pancreatic cancer tissues with negative K19
expression, the expression of MMP2 and MMP9 were upregulated in
pancreatic cancer tissues with positive K19 expression (Fig. 5E).
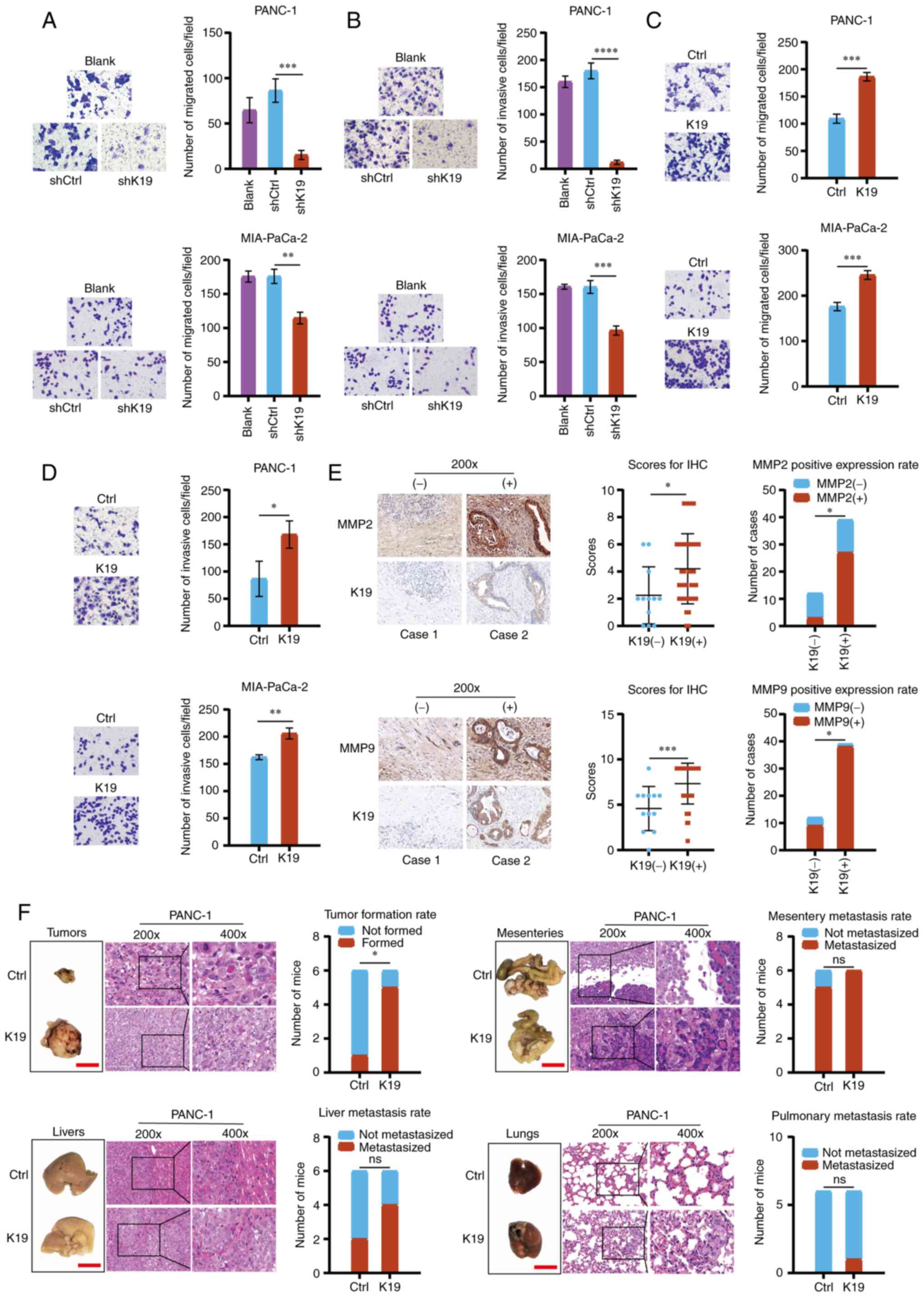 | Figure 5K19 promotes pancreatic cancer
metastasis in vitro and in vivo. (A) Knockdown of K19
inhibited the migration of PANC-1 and MIA-PaCa-2 cells
(magnification, ×200). (B) Knockdown of K19 inhibited the invasion
of PANC-1 and MIA-PaCa-2 cells (magnification, ×200). (C)
Overexpression of K19 promoted the migration of PANC-1 and
MIA-PaCa-2 cells (magnification, ×200). (D) Overexpression of K19
promoted the invasion of PANC-1 and MIA-PaCa-2 cells
(magnification, ×200). The migration and invasion of pancreatic
cancer cells with knockdown of K19 and overexpression of K19 was
detected by Transwell migration and invasion assays. These
experiments were repeated three times. (E) K19 upregulated the
expression of MMP2 and MMP9 in pancreatic cancer tissues
(magnification, ×200). The expression of MMP2 and MMP9 in
pancreatic cancer tissues with K19 positive and K19 negative was
detected by immunohistochemistry. (F) Overexpression of K19
promoted the tumorigenesis and metastasis of peritoneal metastasis
tumors in the mice injected with PANC-1 cells that overexpress K19
(magnification, ×200 in left panels and ×400 in magnified windows
to the right; scale bar in tumor/organ images, 1 cm).
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001; ns, no
significance; K19, keratin 19; Ctrl, control; sh, short hairpin
RNA. |
In addition, overexpression of K19 promoted tumor
formation, mesenteric metastasis, liver metastasis and lung
metastasis in the mice intraperitoneally injected with
K19-overexpressing PANC-1 cells, as compared with the controls
(Fig. 5F). Furthermore, the cell
density, invasion and infiltration ability of tumor cells were
increased in the K19-overexpressing group (H&E; Fig. 5F). Metastatic K19-overexpressing
cells were observed in the mesentery, liver and lung tissues on
H&E staining (Fig. 5F). In
conclusion, the above data indicated that K19 promoted pancreatic
cancer metastasis.
K19 activates the Hedgehog pathway and
promotes the proliferation and metastasis of pancreatic cancer
The Hedgehog pathway has a key role in pancreatic
cancer tumorigenesis and progression (14,18,19).
The activation of the Hedgehog pathway affects the survival,
proliferation, apoptosis, migration and invasion of tumors
(20-24). Therefore, to explore the mechanism
by which K19 affects the progression and prognosis of pancreatic
cancer, the effect of K19 on the Hedgehog pathway in pancreatic
cancer was analyzed. As presented in Figs. 6A and S3A, knockdown of K19 downregulated the
expression of Indian hedgehog (Ihh), transmembrane receptor patched
(PTCH), G protein-coupled-like receptor smoothened (SMO) and
Gli1-the key proteins of the Hedgehog pathway; by contrast,
overexpression of K19 upregulated their expression in pancreatic
cancer cells (Figs. 6B and
S3B). Of note, the expression of
Ihh, PTCH, SMO and Gli1 in pancreatic cancer tissues with positive
K19 expression was also upregulated as compared with that in
pancreatic cancer tissues with negative K19 expression (Fig. 6C). These data indicated that K19
activated the Hedgehog pathway in pancreatic cancer.
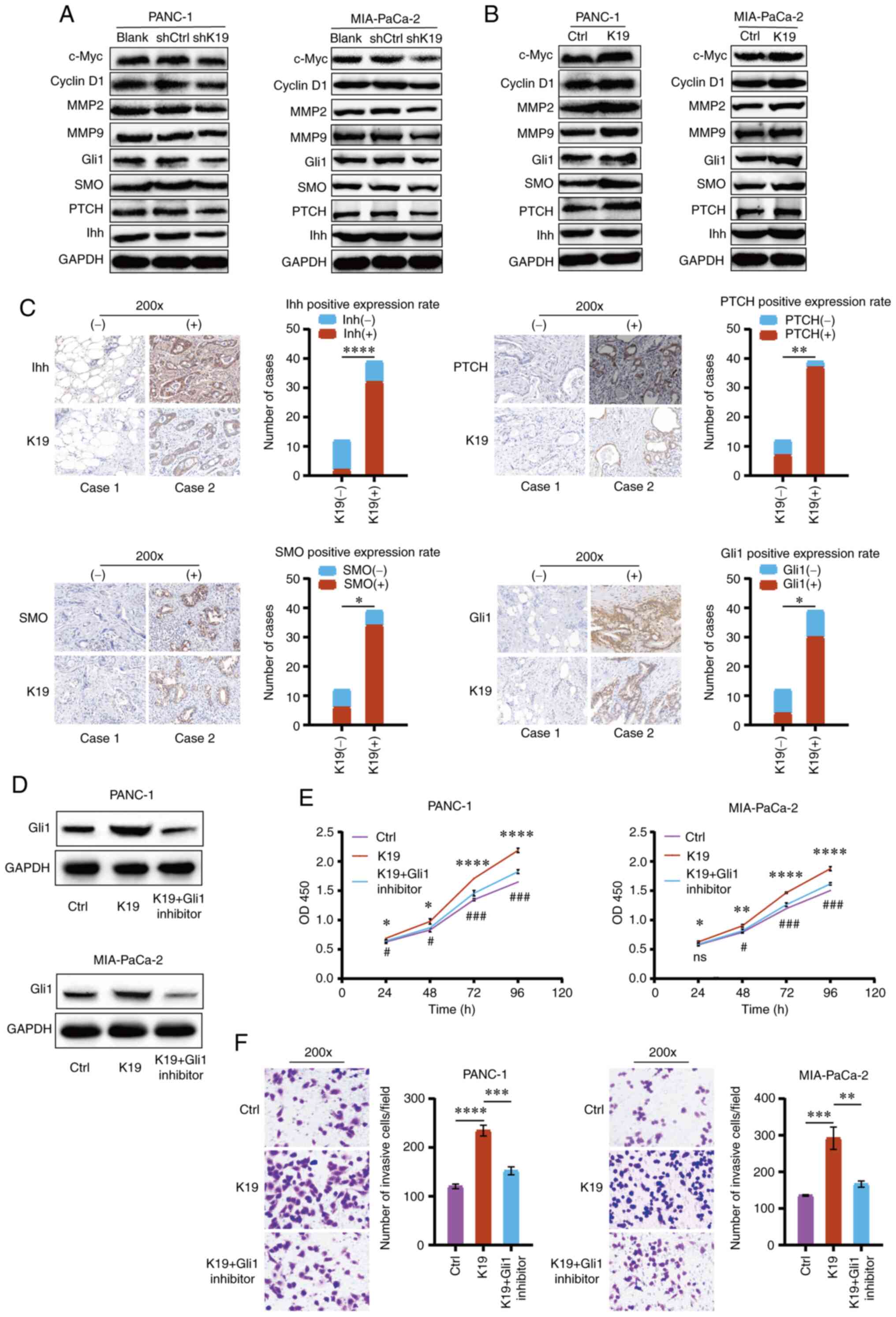 | Figure 6K19 activates the Hedgehog pathway
and promotes the proliferation and metastasis of pancreatic cancer.
(A) Knockdown of K19 downregulated the expression of Ihh, PTCH,
SMO, Gli1, c-Myc, CyclinD1, MMP2 and MMP9 in PANC-1 and MIA-PaCa-2
cells. (B) Overexpression of K19 upregulated the expression of Ihh,
PTCH, SMO, Gli1, c-Myc, CyclinD1, MMP2 and MMP9 in PANC-1 and
MIA-PaCa-2 cells. The expression of Ihh, PTCH, SMO, Gli1, c-Myc,
CyclinD1, MMP2 and MMP9 in pancreatic cancer cells with knockdown
of K19 and overexpression of K19 was detected by western blot. (C)
K19 upregulated the expression of Ihh, PTCH, SMO and Gli1 in
pancreatic cancer tissues (magnification, ×200). The expression of
Ihh, PTCH, SMO and Gli1 in pancreatic cancer tissues with K19
positive and K19 negative was detected by immunohistochemistry. (D)
Overexpression of K19 upregulated the expression of Gli1 in PANC-1
and MIA-PaCa-2 cells, while Gli1 inhibitor reversed it. The
expression of Gli1 in pancreatic cancer cells in K19 overexpression
group, K19 overexpression + Gli1 inhibitor group and control group
was detected by western blot analysis. (E) Overexpression of K19
promoted the proliferation of PANC-1 and MIA-PaCa-2 cells, while
Gli1 inhibitor reversed it. The proliferation of pancreatic cancer
cells in K19 overexpression group, K19 overexpression + Gli1
inhibitor group and control group was detected by a Cell Counting
Kit-8 assay. These experiments were repeated three times. (F)
Overexpression of K19 promoted the invasion of PANC-1 and
MIA-PaCa-2 cells, while Gli1 inhibitor reversed it (magnification,
×200). Invasion of pancreatic cancer cells in K19 overexpression
group, K19 overexpression + Gli1 inhibitor group and control group
was detected by a Transwell invasion assay. These experiments were
repeated three times. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001, K19 overexpression vs. control group or
as indicated; #P<0.05, ###P<0.001, K19
overexpression + Gli1 inhibitor vs. K19 overexpression group. ns,
no significance. K19, keratin 19; Ctrl, control; sh, short hairpin
RNA; OD450, optical density at 450 nm; PTCH, transmembrane receptor
patched; SMO, G protein-coupled-like receptor smoothened; Ihh,
Indian hedgehog; Gli1, glioma-associated oncoprotein 1. |
It is generally thought that Gli1 is a reliable
marker for the activation of the Hedgehog pathway (25). Therefore, to observe whether the
mechanism by which K19 promotes pancreatic cancer progression is
through activating the Hedgehog pathway, a Gli1 inhibitor was used
to block the Hedgehog pathway. As demonstrated in Fig. 6D, overexpression of K19 upregulated
the expression of Gli1, while Gli1 inhibitor reversed this effect.
It was then observed that overexpression of K19 promoted the
proliferation and invasion of pancreatic cancer, while Gli1
inhibitor reversed these effects (Fig.
6E and F). Of note, knockdown of K19 downregulated the
expression of Ihh, PTCH, SMO and Gli1, and also downregulated the
expression of c-Myc, CyclinD1, MMP2 and MMP9 in pancreatic cancer
cells (Figs. 6A and S3A). Overexpression of K19 upregulated
the expression of Ihh, PTCH, SMO and Gli1, and also upregulated the
expression of c-Myc, CyclinD1, MMP2 and MMP9 in pancreatic cancer
cells (Figs. 6B and S3B). Collectively, these data indicated
that K19 activated the Hedgehog pathway to promote pancreatic
cancer progression. In other words, the mechanism by which K19
promoted pancreatic cancer progression was through the activation
of the Hedgehog pathway.
Discussion
Although previous studies have indicated that tumors
expressing K19 have stronger invasiveness and worse prognosis
(7,8), the function and mechanism of K19 in
pancreatic cancer progression and prognosis remain largely elusive.
In the present study, it was indicated that K19 has a critical role
in promoting pancreatic cancer progression and to be associated
with poor prognosis. Specifically, K19 was significantly
overexpressed in pancreatic cancer and an indicator of poor
prognosis. Furthermore, K19 promoted pancreatic cancer
proliferation, tumorigenesis and metastasis, and inhibited its
apoptosis. Mechanistically, these effects were mediated through
activating the Hedgehog pathway.
CyclinD1 and c-Myc inhibit tumor cell apoptosis,
promote their proliferation and cell cycle progression, and have a
key role in tumorigenesis (11,12,26-28).
Of note, in the present study, it was indicated that K19
upregulated the expression of CyclinD1 and c-Myc in pancreatic
cancer, promoted pancreatic cancer proliferation, cell cycle
progression, tumorigenesis and development, and inhibited its
apoptosis. Reducing extracellular matrix and basement membrane is
the key mechanism of tumor invasion and metastasis. MMP2 and MMP9
may reduce extracellular matrix and basement membrane and have a
key role in tumor invasion and metastasis (29,30).
Downregulating or inhibiting MMP2 and MMP9 may inhibit tumor
migration and invasion (31,32).
Of note, in the present study, it was indicated that K19
upregulated MMP2 and MMP9 in pancreatic cancer and promoted
pancreatic cancer cell migration, invasion and metastasis. These
data indicated that K19 may promote pancreatic cancer proliferation
and metastasis to promote pancreatic cancer progression, at least
in part via enhancing the expression of CyclinD1, c-Myc, MMP2 and
MMP9.
The Hedgehog pathway has a crucial role in
pancreatic cancer tumorigenesis and development (18,33).
The activation of the Hedgehog pathway affects the survival,
proliferation, apoptosis, migration and invasion of tumors
(20-24). Ihh, PTCH, SMO and Gli1 are the key
proteins of the Hedgehog pathway (19,34).
The Hedgehog pathway is regulated by the Hedgehog pathway ligands
PTCH and SMO. When the ligands [Sonic hedgehog (Shh), Ihh and
desert hedgehog] are combined with PTCH (PTCH1 and PTCH2), SMO
increases and releases Gli1, Gli2 and Gli3 transcription factors,
which promote transcription of Gli1, Gli2 and Gli3. As a result,
the Hedgehog pathway is activated (15,22,35).
Therefore, to investigate the mechanism by which K19 affects the
progression and prognosis of pancreatic cancer, the expression of
Ihh, PTCH, SMO and Gli1 in pancreatic cancer was observed. Of note,
it was indicated that K19 upregulated the expression of Ihh, PTCH,
SMO and Gli1 in pancreatic cancer tissues and cells. This finding
indicated that K19 activated the Hedgehog pathway. Furthermore, it
was found that the changes of CyclinD1, c-Myc, MMP2 and MMP9 were
consistent with those of Ihh, PTCH, SMO and Gli1. K19 upregulated
the expression of Ihh, PTCH, SMO and Gli1 in pancreatic cancer
tissues and cells, and also upregulated the expression of CyclinD1,
c-Myc, MMP2 and MMP9. These findings suggest that K19 promotes
pancreatic cancer proliferation and metastasis through the
activation of the Hedgehog pathway. Furthermore, it is generally
thought that Gli1 is a reliable marker for the activation of the
Hedgehog pathway (25). Therefore,
to examine whether K19 promotes pancreatic cancer progression
through activating the Hedgehog pathway, a Gli1 inhibitor was used
to block the Hedgehog pathway. It was indicated that Gli1 inhibitor
reversed pancreatic cancer proliferation and invasion induced by
overexpression of K19. Collectively, these data suggest that the
mechanism by which K19 promotes the progression and poor prognosis
of pancreatic cancer is through the activation of the Hedgehog
pathway.
Bcl-2 expression is related to the activation of the
Hedgehog pathway in pancreatic cancer (15). The Hedgehog pathway key protein Shh
inhibits apoptosis through upregulating Bcl-2 expression (36). Upregulation of Gli1 may upregulate
Bcl-2 (37). The Hedgehog pathway
regulates the survival of tumor cells by regulating Bcl-2
expression (38). Of note, in the
present study, it was indicated that K19 upregulated the expression
of Bcl-2 in pancreatic cancer tissues and cells, inhibited their
apoptosis and activated the Hedgehog pathway. These data suggest
that K19 activates the Hedgehog pathway to upregulate Bcl-2
expression and inhibit apoptosis in pancreatic cancer. This is
consistent with previous studies (15,36).
In addition, in the present study, intraperitoneal
injection instead of tail vein injection was chosen when
constructing a metastatic tumor model, mainly because the
intraperitoneal metastatic tumor model is more similar to the
clinical characteristics of pancreatic cancer (39,40).
In the subcutaneously transplanted tumor model, tumor growth was
observed for a long time, mainly because the number of cells
injected was low, so that the tumor grew slowly.
Of note, the present study also had certain
limitations. First, it remains insufficiently clear how K19
activates the Hedgehog pathway to promote progression and poor
prognosis of pancreatic cancer. Furthermore, it remains to be
investigated whether patients with high K19 expression may benefit
from chemotherapy and whether any drug may downregulate and inhibit
K19 in pancreatic cancer to improve progression and prognosis of
pancreatic cancer. These topics are the areas that are currently
being investigated or will be investigated in the future.
In conclusion, K19 promotes pancreatic cancer
progression and poor prognosis. The mechanism involves the
activation of the Hedgehog pathway. K19 may be a novel molecular
target for pancreatic cancer treatment.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
CZ contributed to the design, implementation and
execution of the experiments; acquisition, analysis and
interpretation of data; and writing the manuscript. YX, YR and ML
contributed to the completion of the experiments and acquisition of
data. XG and WL contributed to the design of the experiments, the
interpretation of data and the revision of the manuscript
critically for important intellectual content. CZ and WL checked
and approved the authenticity of the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided informed consent for the
surgical procedure and the obtainment/use of their sample and
clinical data for research. The study received approval from the
Ethics Committee of Guizhou Provincial People's Hospital [Guiyang,
China; no. (2021)253] and was conducted in line with the principles
of the Helsinki declaration. All experimental procedures involving
animals were conducted in accordance with animal protocols approved
by the Laboratory Animal Center of Xiamen University (Xiamen,
China), which was in line with the principles of the ARRIVE
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to Professor Ting Wu from
the School of Medicine, Xiamen University (Xiamen, China) for her
guidance and help with this research work.
Funding
This work was supported by grants from the Natural Science
Foundation of Guizhou Province [grant no. Qian ke he cheng guo
(2019) 4444 to CZ], the Beijing Medical and Health Public Welfare
Foundation (grant no. YWJKJJHKYJJ-B184054 to CZ), the Key Project
of the Science and Technology Ministry of China (grant no.
2017ZXl0203206-005-002 to WL) and the Young and Middle-Aged
Backbone Talent Training Program of Fujian Health Commission (grant
no. 2021GGB036 to ML).
Abbreviations:
|
K19
|
keratin 19
|
|
PTCH
|
transmembrane receptor patched
|
|
SMO
|
G protein-coupled-like receptor
smoothened
|
|
Shh
|
Sonic hedgehog
|
|
Ihh
|
Indian hedgehog
|
|
Gli
|
glioma-associated oncoproteins
|
|
Ctrl
|
control
|
|
sh
|
short hairpin
|
References
|
1
|
Mizrahi JD, Surana R, Valle JW and Shroff
RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain T and Dudeja V: The war against
pancreatic cancer in 2020-advances on all fronts. Nat Rev
Gastroenterol Hepatol. 18:99–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhave P, Pallan L, Long GV, Menzies AM,
Atkinson V, Cohen JV, Sullivan RJ, Chiarion-Sileni V, Nyakas M,
Kahler K, et al: Melanoma recurrence patterns and management after
adjuvant targeted therapy: A multicentre analysis. Br J Cancer.
124:574–580. 2021. View Article : Google Scholar :
|
|
4
|
Chan A, Moy B, Mansi J, Ejlertsen B,
Holmes FA, Chia S, Iwata H, Gnant M, Loibl S, Barrios CH, et al:
Final efficacy results of neratinib in HER2-positive hormone
receptor-positive early-stage breast cancer from the phase III
ExteNET trial. Clin Breast Cancer. 21:80–91.e7. 2021. View Article : Google Scholar
|
|
5
|
Garcia Campelo MR, Lin HM, Zhu Y, Pérol M,
Jahanzeb M, Popat S, Zhang P and Camidge DR: Health-related quality
of life in the randomized phase III trial of brigatinib vs
crizotinib in advanced ALK inhibitor-naive ALK + non-small cell
lung cancer (ALTA-1L). Lung Cancer. 155:68–77. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhee H, Kim HY, Choi JH, Woo HG, Yoo JE,
Nahm JH, Choi JS and Park YN: Keratin 19 expression in
hepatocellular carcinoma is regulated by fibroblast-derived HGF via
a MET-ERK1/2AP1 and SP1 axis. Cancer Res. 78:1619–1631. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee
JE, Cho JY, Yoo JE, Choi JS and Park YN: Human hepatocellular
carcinomas with 'Stemness'-related marker expression: Keratin 19
expression and a poor prognosis. Hepatology. 54:1707–1717. 2011.
View Article : Google Scholar
|
|
8
|
Kawai T, Yasuchika K, Ishii T, Katayama H,
Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Mizumoto M,
et al: Keratin 19, a cancer stem cell marker in human
hepatocellular carcinoma. Clin Cancer Res. 21:3081–3091. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
10
|
Hu S, Chen Z, Gu J, Tan L, Zhang M and Lin
W: TLE2 is associated with favorable prognosis and regulates cell
growth and gemcitabine sensitivity in pancreatic cancer. Ann Transl
Med. 8:10172020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang XK, Zhang YW, Wang CM, Li B, Zhang
TZ, Zhou WJ, Cheng LJ, Huo MY, Zhang CH and He YL: METTL16 promotes
cell proliferation by up-regulating cyclin D1 expression in gastric
cancer. J Cell Mol Med. 25:6602–6617. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao R, Liu Y, Wu C, Li M, Wei Y, Niu W,
Yang J, Fan S, Xie Y, Li H, et al: BRD7 promotes cell proliferation
and tumor growth through stabilization of c-Myc in colorectal
cancer. Front Cell Dev Biol. 9:6593922021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar
|
|
15
|
Chen XL, Cheng QY, She MR, Wang Q, Huang
XH, Cao LQ, Fu XH and Chen JS: Expression of sonic hedgehog
signaling components in hepatocellular carcinoma and
cyclopamine-induced apoptosis through Bcl-2 downregulation in
vitro. Arch Med Res. 41:315–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta GP, Nguyen DX, Chiang AC, Bos PD,
Kim JY, Nadal C, Gomis RR, Manova-Todorova K and Massagué J:
Mediators of vascular remodelling co-opted for sequential steps in
lung metastasis. Nature. 446:765–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiratsuka S, Nakamura K, Iwai S, Murakami
M, Itoh T, Kijima H, Shipley JM, Senior RM and Shibuya M: MMP9
induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bausch D, Fritz S, Bolm L, Wellner UF,
Fernandez-Del-Castillo C, Warshaw AL, Thayer SP and Liss AS:
Hedgehog signaling promotes angiogenesis directly and indirectly in
pancreatic cancer. Angiogenesis. 23:479–492. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richtig G, Aigelsreiter AM, Asslaber M,
Weiland T, Pichler M, Eberhard K, Sygulla S, Schauer S, Hoefler G
and Aigelsreiter A: Hedgehog pathway proteins SMO and GLI
expression as prognostic markers in head and neck squamous cell
carcinoma. Histopathology. 75:118–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng J, Rao M, Wang M, Liang L, Chen Z,
Pang X, Lu W and Sun Z: Triptolide suppresses pancreatic cancer
cell proliferation by inhibiting hedgehog signaling pathway
activity. Sci China Life Sci. 62:1409–1412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lospinoso Severini L, Quaglio D, Basili I,
Ghirga F, Bufalieri F, Caimano M, Balducci S, Moretti M, Romeo I,
Loricchio E, et al: A Smo/Gli multitarget hedgehog pathway
inhibitor impairs tumor growth. Cancers (Basel). 11:15182019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Xiao S, Zhang M, Yang L, Zhong J, Li
B, Li F, Xia X, Li X, Zhou H, et al: A novel protein encoded by
circular SMO RNA is essential for Hedgehog signaling activation and
glioblastoma tumorigenicity. Genome Biol. 22:332021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang C, Zheng X, Ye K, Sun Y, Lu Y, Fan Q
and Ge H: miR-135a inhibits the invasion and migration of
esophageal cancer stem cells through the Hedgehog signaling pathway
by targeting Smo. Mol Ther Nucleic Acids. 19:841–852. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guimaraes VSN, Vidal MTA, de Faro Valverde
L, de Oliveira MG, de Oliveira Siquara, da Rocha L, Coelho PLC,
Soares FA, de Freitas Souza BS, Bezerra DP, Coletta RD, et al:
Hedgehog pathway activation in oral squamous cell carcinoma:
Cancer-associated fibroblasts exhibit nuclear GLI-1 localization. J
Mol Histol. 51:675–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doheny D, Manore SG, Wong GL and Lo HW:
Hedgehog signaling and truncated GLI1 in cancer. Cells. 9:21142020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montalto FI and De Amicis F: Cyclin D1 in
cancer: A molecular connection for cell cycle control, adhesion and
invasion in tumor and stroma. Cells. 9:26482020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yenmiş G, Beşli N, Yaprak Saraç E,
Hocaoğlu Emre FS, Şenol K and Kanıgür G: Metformin promotes
apoptosis in primary breast cancer cells by downregulation of
cyclin D1 and upregulation of P53 through an AMPK-alpha independent
mechanism. Turk J Med Sci. 51:826–834. 2021. View Article : Google Scholar
|
|
28
|
Zhong Y, Yang L, Xiong F, He Y, Tang Y,
Shi L, Fan S, Li Z, Zhang S, Gong Z, et al: Long non-coding RNA
AFAP1-AS1 accelerates lung cancer cells migration and invasion by
interacting with SNIP1 to upregulate c-Myc. Signal Transduct Target
Ther. 6:2402021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mondal S, Adhikari N, Banerjee S, Amin SA
and Jha T: Matrix metalloproteinase-9 (MMP-9) and its inhibitors in
cancer: A minireview. Eur J Med Chem. 194:1122602020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren F, Tang R, Zhang X, Madushi WM, Luo D,
Dang Y, Li Z, Wei K and Chen G: Overexpression of MMP family
members functions as prognostic biomarker for breast cancer
patients: A systematic review and meta-analysis. PLoS One.
10:e01355442015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Wang R, Cheng L and Xu H:
Celastrol inhibit the proliferation, invasion and migration of
human cervical HeLa cancer cells through down-regulation of MMP-2
and MMP-9. J Cell Mol Med. 25:5335–5338. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chuang YC, Hsieh MC, Lin CC, Lo YS, Ho HY,
Hsieh MJ and Lin JT: Pinosylvin inhibits migration and invasion of
nasopharyngeal carcinoma cancer cells via regulation of
epithelial-mesenchymal transition and inhibition of MMP-2. Oncol
Rep. 46:1432021. View Article : Google Scholar :
|
|
33
|
Niyaz M, Khan MS, Wani RA, Shah OJ and
Mudassar S: Sonic Hedgehog protein is frequently up-regulated in
pancreatic cancer compared to colorectal cancer. Pathol Oncol Res.
26:551–557. 2020. View Article : Google Scholar
|
|
34
|
Mohd Ariffin K, Abd Ghani F, Hussin H, Md
Said S, Yunus R, Veerakumarasivam A and Abdullah MA: Hedgehog
signalling molecule, SMO is a poor prognostic marker in bladder
cancer. Malays J Pathol. 43:49–54. 2021.PubMed/NCBI
|
|
35
|
Kasiri S, Chen B, Wilson AN, Reczek A,
Mazambani S, Gadhvi J, Noel E, Marriam U, Mino B, Lu W, et al:
Stromal Hedgehog pathway activation by IHH suppresses lung
adenocarcinoma growth and metastasis by limiting reactive oxygen
species. Oncogene. 39:3258–3275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang H, Yu H, Lin L, Chen J and Zhu P:
Protective effect of sonic hedgehog against oxidized low-density
lipoprotein-induced endothelial apoptosis: Involvement of NF-κB and
Bcl-2 signaling. Int J Mol Med. 45:1864–1874. 2020.PubMed/NCBI
|
|
37
|
Bigelow RL, Chari NS, Unden AB, Spurgers
KB, Lee S, Roop DR, Toftgard R and McDonnell TJ: Transcriptional
regulation of bcl-2 mediated by the sonic hedgehog signaling
pathway through gli-1. J Biol Chem. 279:1197–1205. 2004. View Article : Google Scholar
|
|
38
|
Han ME, Lee YS, Baek SY, Kim BS, Kim JB
and Oh SO: Hedgehog signaling regulates the survival of gastric
cancer cells by regulating the expression of Bcl-2. Int J Mol Sci.
10:3033–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang YJ, Lee CL, Wang Q, Zhou ZW, Yang F,
Jin C and Fu DL: Establishment of an orthotopic pancreatic cancer
mouse model: Cells suspended and injected in Matrigel. World J
Gastroenterol. 20:9476–9485. 2014. View Article : Google Scholar :
|
|
40
|
Tang S, Hang Y, Ding L, Tang W, Yu A,
Zhang C, Sil D, Xie Y and Oupický D: Intraperitoneal siRNA
nanoparticles for augmentation of gemcitabine efficacy in the
treatment of pancreatic cancer. Mol Pharm. 18:4448–4458. 2021.
View Article : Google Scholar : PubMed/NCBI
|