1. Introduction
Although breakthroughs have been made in
pharmaceutical research and development, cancer is still a global
problem and a leading cause of death at present (1,2). The
emergence of immunotherapy, particularly anti-programmed cell death
protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) therapy, which
acts by blocking the immune escape mechanism of cancer cells, has
improved the survival of patients with advanced cancer, especially
when specific molecular targets are lacking (3). For example, the OAK study (4) demonstrated that the median overall
survival (OS) increased from 9.6 in patients receiving chemotherapy
to 13.8 months in patients with advanced non-small cell lung cancer
(NSCLC) receiving immunotherapy. Furthermore, if those patients had
been treated previously, the median duration of response was
notably longer in the atezolizumab group than in the docetaxel
group (16.3 and 6.2 months, respectively). In breast cancer,
KEYNOTE-355 (5) revealed a median
progression-free survival (PFS) of 7.5 months when pembrolizumab
plus chemotherapy was applied compared with 5.6 months when the
placebo plus chemotherapy was used.
Neoadjuvant therapy (NAT) involves the
administration of therapeutic agents prior to surgery. Neoadjuvant
chemotherapy (NACT) is typically applied as a preoperative
treatment for solid tumors, such as head and neck, lung, and breast
cancer (6,7). Evidence has demonstrated that NACT
could improve the prognosis of patients with locally advanced and
borderline resectable solid cancer by reducing tumor burden,
improving the tumor resection rate and controlling micrometastases
(7-9). However, most patients may not
markedly benefit from NACT and may, on the other hand, suffer
strong side effects (10). Given
that immunotherapy is a successful treatment for advanced cancer
(3), a number of studies (11-13)
have explored the feasibility and efficacy of immunotherapy for
perioperative applications in early-stage cancer. In addition to
the advantages of traditional NACT, immunotherapy uniquely
activates tumor-specific T cell function and prolongs postoperative
antitumor immunity, which may be associated with improved survival
in a neoadjuvant setting (14). In
a preclinical study, Liu et al (15) demonstrated that neoadjuvant
immunotherapy was superior to adjuvant immunotherapy in terms of
therapeutic power. Furthermore, immunotherapy is considered an
effective approach to re-activate the function of exhausted
CD8+ T cells and control cancer progression (14). In addition, >100 clinical trials
were searched for in PubMed, Cochrane and Embase (Fig. 1). The present review summarizes the
clinical trial data of neoadjuvant immunotherapy for 11 solid tumor
types [head and neck cancer, breast cancer, lung cancer, esophageal
cancer, gastroesophageal junction and gastric cancer,
hepatocellular carcinoma, renal cancer, colorectal cancer (CC),
bladder cancer, melanoma and Merkel cell carcinoma]. The selection
process is shown in Fig. 1.
2. Neoadjuvant anti-PD-1/PD-L1 therapy
according to cancer types
Overview
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane
(https://www.cochrane.org/) and Embase
(https://www.embase.com/) databases were searched
to obtain comprehensive and reliable literature. Furthermore, the
abstracts of various conferences were screened using Embase. Two
reviewers selected studies for inclusion and extracted data, and
any disagreement was resolved by a third reviewer. The last search
date was March 2022. The reviewers searched for the following
combinations of key words: 'Neoadjuvant therapy'; 'Immunotherapy';
'PD-1/PD-L1'; 'Immune checkpoint inhibitors'; 'pathologic complete
response'; 'pCR'; 'objective response rate'; 'ORR'; 'major
pathologic response'; 'MPR'. In order to reduce the heterogeneity
of different studies, the intention-to-treat (ITT) population of
individual studies was considered as the overall size of the study.
The objective response rate (ORR) in tumor volume was determined
according to Response Evaluation Criteria in Solid Tumors (RECIST)
version 1.1 (16). The major
pathologic response (MPR) was defined as the presence of 10% or
fewer viable tumor cells in the primary tumor, including pathologic
complete response (pCR), which was defined as tumors without any
viable tumor cells in the resected cancer specimen and all sampled
regional lymph nodes (17-19). The extracted metrics included: ORR,
MPR, pCR, treatment-related adverse events (TRAEs), immune-related
adverse events (irAEs), treatment-related surgical delay,
postoperative complications, not radical resection (the condition
in which surgery is completed but the tumor is not completely
removed) and no surgery (the number of individuals who had not
completed the operation). All data were taken from the text of each
reference, and the data were aggregated and displayed in box plots
based on different cancer types and different treatment modalities,
and effects were compared using the midline. All graphics were
generated using GraphPad Prism 8 (GraphPad Software; Dotmatics) and
Adobe Illustrator 2021 (Adobe Systems, Inc.).
Head and neck cancer
NCT03342911 trial
The phase 2, single-arm study NCT03342911 assessed
the ability of nivolumab in combination with chemotherapy in
patients with locally advanced head and neck squamous cell
carcinoma (HNSCC) that was suitable for surgical removal. A total
of 32 patients had treatment on the study regimen followed by
surgery, and 26 patients were either human papillomavirus
(HPV)-negative (24 patients) or HPV unknown but had oral cancer (2
patients). Of these 26 patients, MPR was observed in 17 out of 26
patients (65%) and 35% of patients had a pCR. All had negative
margins at the surgery (11).
NCT02919683 trial
The phase 2, randomized study NCT02919683
investigated the effect of nivolumab combined with ipilimumab as a
neoadjuvant treatment for oral cavity squamous cell carcinoma
(OCSCC). A total of 29 patients were enrolled, of which 14 were
randomly assigned to the nivolumab group (group 1) and 15 to the
dual drugs group (group 2). The ORR was 13% (nivolumab) and 38%
(nivolumab combined with ipilimumab). A total of 4 patients had
exhibited MPR (nivolumab, n=1; nivolumab combined with ipilimumab,
n=3), including 1 patient with a pCR in cohort nivolumab combined
with ipilimumab. No surgical delays occurred. irAEs occurred in 21
(72%) patients, including grade ≥3 events in 2 (nivolumab) and 5
(nivolumab combined with ipilimumab) patients. The median follow-up
time was 14.2 months, and the 1-year PFS rate was 85% (20).
CIAO (NCT03144778) trial
The phase 1, randomized study CIAO
(NCT03144778) indicated the security and effectivity of
durvalumab with or without tremelimumab in patients with stage
II-IVA oropharyngeal squamous cell cancer. Of the 29 patients
enrolled, one patient allocated to durvalumab was found to be
ineligible, and 28 patients were randomly assigned to the
durvalumab (group 1) or durvalumab plus tremelimumab (group 2) for
two cycles prior to surgery groups. A total of 2 (7%) of 28
patients achieved an overall MPR, including 1 patient with a pCR
(7%) in group 1 and 1 patient with a MPR (7%) in group 2. A total
of 12 patients (43%) had an ORR, including 6 patients (40%) in
group 1 and 6 patients (43%) in group 2. A total of 26 patients
(90%) experienced a TRAE, including 4 patients (14%) who
experienced grade 3 TRAEs. The incidence and severity of TRAEs were
similar in the two treatment cohorts (21).
NCT02296684 trial
The phase 2, non-randomized two-group study
NCT02296684 examined the feasibility of pembrolizumab in
HPV-unrelated head and neck cancer that was resectable. In group 1,
36 patients received one dose of neoadjuvant pembrolizumab before
surgery. Grade ≥3 irAEs did not occur in the neoadjuvant stage. The
MPR rate was 6%. In group 2, 29 patients enrolled and 25 patients
received two doses of neoadjuvant pembrolizumab before surgery. MPR
was achieved in 4 patients (14%), including 1 case with a pCR (3%)
(22,23).
NIRT-HNC (NCT03247714) trial
The phase 1b, single-arm study NIRT-HNC
(NCT03247714) investigated the effectiveness of neoadjuvant
nivolumab plus stereotactic body radiation therapy (SBRT) in
locally advanced HPV-positive and HPV-negative HNSCC. To the best
of our knowledge, this was the first report of neoadjuvant
immune-radiotherapy in patients with HNSCC. Patients with resected
HNSCC received nivolumab and SBRT before surgery, followed by
adjuvant nivolumab. A total of 21 of the 24 enrolled patients
completed neoadjuvant treatment. There were no therapy-related
surgical delays and TRAEs of any grade occurred in all patients.
Among the 10 HPV-positive patients treated with nivolumab and SBRT,
the ORR was 50%, the MPR rate was 100% and the pCR rate was 90%.
Among the 5 HPV-negative patients, the ORR was 60%, the MPR rate
was 60% and the pCR rate was 20% (24).
IMCISION (NCT03003637) trial
The phase 1b/2a, non-randomized study IMCISION
(NCT03003637) demonstrated that nivolumab with or without
ipilimumab followed by surgery was an effective and feasible option
for patients with resectable HNSCC. A total of 32 patients with
HNSCC were treated with nivolumab (MONO; n=6) or nivolumab plus
ipilimumab (COMBO; n=26) before surgery. Grade ≥3 irAEs were
observed in 33% of MONO and 38% of COMBO patients. The MPR rate was
31% for patients in the COMBO group and the pCR rate of these
patients was 4%. The MPR rate of patients in the MONO group was
17%. The patients who achieved MPR did not develop recurrent HSNCC
within 24.0 months (25).
NCT03021993 trial
The phase 2, single-arm study NCT03021993 assessed
the effectiveness of nivolumab in patients with OCSCC who were
about to undergo surgery. A total of 12 patients with stage II-IVA
OCSCC were treated with nivolumab followed by definitive surgical
resection. Of the patients eligible for efficacy analysis, 4 had
>30% response, 4 had stable disease and 4 exhibited progression
of disease, resulting in an ORR of 33%. There were no pCR.
Any-grade irAEs were observed in 67% of patients, and no grade ≥3
irAEs were observed. There were no delays in surgery. All patients
obtained negative margins. This work suggested the feasibility and
efficacy of the incorporation of nivolumab for OCSCC in the
neoadjuvant stage (26).
NCT02641093 trial
The phase 2, single-arm study NCT02641093
investigated the ability of neoadjuvant pembrolizumab with adjuvant
radiotherapy with or without cisplatin in patients with HNSCC. Of
the 80 evaluable patients, 6 (8%) had an MPR (27).
CheckMate358 (NCT02488759) trial
The phase 1/2, randomized study CheckMate358
(NCT02488759) investigated the effect of nivolumab or nivolumab
combination therapy in patients who had virus-associated tumors. A
total of 52 patients with stage III-IV resectable HNSCC underwent
neoadjuvant nivolumab treatment (26 HPV-positive; 26 HPV-negative).
In group 1, the ORR and MPR rates were 12 and 6%, respectively. For
group 2, the ORR was 8%, and there was no pCR. Any-grade TRAEs
occurred in 73 and 54% of the HPV-positive and HPV-negative
cohorts, respectively. A total of 5 (19%) and 3 patients (12%)
developed grade ≥3 TRAEs, respectively. The patients had no
treatment-related surgical delays (28).
Breast cancer
NCT02685059 (GeparNuevo) trial
The phase 2, randomized, double-blind,
placebo-controlled study NCT02685059 (GeparNuevo)
investigated the effect of NACT with durvalumab compared with
placebo in patients with non-metastatic triple-negative breast
cancer (TNBC). A total of 88 patients were randomly assigned to the
durvalumab group and 86 patients were assigned to the placebo
group. In the durvalumab arm, 64% of patients completed all
therapies, and durvalumab was discontinued in 23%. A total of 47
out of the 88 patients (53%) treated with durvalumab achieved a
pCR. Overall, 30 patients in the durvalumab arm and 29 patients in
the placebo arm experienced at least one serious irAEs (12).
NCT02622074 (KEYNOTE-173) trial
The phase 1b, randomized study NCT02622074
(KEYNOTE-173) evaluated the efficacy and feasibility of
pembrolizumab in combination with six therapeutic schedules as
preoperative treatment for patients with TNBC. A total of 60
patients were enrolled and dose-limiting toxicities (DLTs) occurred
in 22 patients. The pCR rate across all cohorts was 57% and the ORR
was 88%. irAEs were observed in 18 patients (30%), including grade
3 irAEs in 6 patients (10%) (29).
NCT02489448 trial
The phase 2, single-arm study NCT02489448 assessed
the safety and feasibility of using durvalumab concurrently with
weekly nab-paclitaxel and dose-dense doxorubicin/cyclophosphamide
as neoadjuvant treatment for stage I-III TNBC. A total of 59
patients were enrolled. The ITT population was 55 patients. The pCR
rate was 44% in 55 patients treated with 10 mg/kg durvalumab
recommended in Phase II. The 17 patients who underwent all
therapies had a pCR. In cases that exhibited pCR, there has been no
recurrence over a 20-month median follow-up. Grade ≥3 TRAEs were
observed in 18 patients (31%). There were no perioperative
complications (30).
IMpassion031 (NCT03197935) trial
The phase 3, randomized, double-blind,
placebo-controlled study IMpassion031 (NCT03197935)
evaluated the safety and effect of neoadjuvant treatment with
atezolizumab and nab-paclitaxel followed by doxorubicin and
cyclophosphamide (nab-pac-AC), or placebo with nab-pac-AC in
patients with TNBC who were eligible for surgery. Atezolizumab plus
chemotherapy was randomly assigned to 165 patients, while placebo
plus chemotherapy was administered to 168 patients. The pCR rate
was 58% in the atezolizumab group. TRAEs of all grades occurred in
162 patients (98%) in the atezolizumab group, including grade ≥3
TRAEs in 57%. The number of patients who discontinued atezolizumab
or placebo due to TRAEs was 21 (13%). Atezolizumab plus
chemotherapy was associated with pCR in 53 (69%) of 77 patients
with PD-L1-positive status compared with 37 (49%) of 75 patients in
the placebo plus chemotherapy group (31).
KEYNOTE-522 (NCT03036488) trial
The phase 3, randomized, double-blind,
placebo-controlled study KEYNOTE-522 (NCT03036488)
investigated the efficacy and feasibility of pembrolizumab plus
chemotherapy vs. placebo plus chemotherapy as NAT in patients with
TNBC. Patients were randomly assigned to receive either
pembrolizumab or a placebo, with a 2:1 ratio. A total of 240 of 401
patients (60%) in the pembrolizumab plus chemotherapy group had a
pCR. irAEs occurred in 39% of patients, including grade 3 irAEs in
13% (32).
I-SPY-2 (NCT01042379) trial
The phase 2, multi-arm study I-SPY-2
(NCT01042379) is an adaptively randomized trial. For various
breast cancer biomarker subsets, it aimed to identify novel drug
combinations with higher pCR rates than standard chemotherapy.
There were two results. In early-stage breast cancer, one treatment
option was pembrolizumab plus NACT. A total of 69 women were
randomly selected to receive four cycles of pembrolizumab in
conjunction with weekly paclitaxel, followed by AC. A total of 49
of the women had hormone receptor (HR)-positive tumors, while 29
had triple-negative tumors. The final estimated pCR rates were 44,
30 and 60% for pembrolizumab in the HER2-negative,
HR-positive/HER2-negative and triple-negative cohorts,
respectively. Patients who achieved pCR had a higher event-free
survival rate (33). Another
cohort received durvalumab plus the poly (ADP-ribose) polymerase
inhibitor Olaparib, combined with the standard chemotherapy
[durvalumab/Olaparib/paclitaxel (DOP)] as NAT, which was evaluated
in the phase II I-SPY2 trial. A total of 73 patients were randomly
selected to receive DOP, including 21 patients with TNBC and 52
patients with HR-positive/HER2-negative breast cancer. On average,
durvalumab plus olaparib increased the estimated pCR rates from 27
to 47% in TNBC, from 20 to 37% in HER2-negative cancer, and from 14
to 28% in HR-positive/HER2-negative cancer. In total, 20 patients
(27%) in the experimental group experienced irAEs, including 9
patients (12%) in the DOP arm who had grade ≥3 irAEs (34).
NCT02530489 trial
The phase 2, single-arm study NCT02530489 assessed
the feasibility and efficiency of atezolizumab plus nab-paclitaxel
for the treatment of patients with TNBC. Patients with stage I-III
TNBC showing suboptimal response to 4 cycles of doxorubicin and
cyclophosphamide (AC) received atezolizumab plus nab-paclitaxel as
the second phase of NAT before undergoing surgery followed by
adjuvant atezolizumab. A total of 34 patients were enrolled and 33
patients completed NAT plus atezolizumab plus nab-paclitaxel. The
pCR rate was 30%. Discontinuation of atezolizumab due to irAEs
occurred in 4 patients (12%; nephritis, n=2; adrenal insufficiency,
n=1; hepatitis, n=1) (35).
NCT03366844 trial
The phase 2, single-arm study NCT03366844 assessed
the safety and feasibility of using pembrolizumab with standard
radiation treatment prior to standard treatment for patients with
breast cancer. In 20 patients, preoperative pembrolizumab was
followed by radiotherapy (24 Gy/3 fractions) followed by
standard-of-care treatment (SOC) 3-5 weeks later. The pCR rate was
60%. During the SOC treatment, none of the patients experienced a
significant delay. During pembrolizumab with or without
radiotherapy treatment, there were no toxicities of grade ≥3. Grade
4 colitis that was attributed to pembrolizumab was reported
(36).
NeoTRIPaPDL1 (NCT02620280) trial
The phase 3, randomized study NeoTRIPaPDL1
(NCT02620280) tested the safety and effect of the addition of
atezolizumab to carboplatin and nab-paclitaxel in patients with
TNBC. The study included 280 patients who were randomly divided
into carboplatin and nab-paclitaxel with (n=138) or without
atezolizumab (n=142) groups. The pCR and ORR rates after treatment
with atezolizumab were 49 and 80%, respectively. Notably, 23
patients did not complete the planned therapy. Disease progression
while on treatment was documented in 8 patients (6%) treated with
the atezolizumab regimen. Overall, during treatment, 98% of
patients treated with atezolizumab had at least one TRAE, including
grade ≥3 TRAEs in 78% (37).
GIADA (NCT04659551) trial
The phase 2, single-arm study GIADA
(NCT04659551) evaluated the feasibility and efficacy of
nivolumab combined with epirubicin/cyclophosphamide for luminal
B-like breast cancer treatment in the neoadjuvant setting. pCR was
achieved in 7 out of 43 patients (16%). The ORR was 71%. A total of
9 patients permanently discontinued nivolumab for safety reasons
and 3 patients discontinued nivolumab for other reasons (38).
Lung cancer
checkmate-159 (NCT02259621) trial
The phase 2, single-arm study checkmate-159
(NCT02259621) examined the safety and efficacy of neoadjuvant
nivolumab therapy in patients with high-risk resectable NSCLC. Of
22 patients enrolled, 20 were completely resected.
Treatment-related surgical delays did not occur. The ORR, MPR rate
and pCR rate were 10, 43 and 10%, respectively. A total of 5
patients (23%) experienced TRAEs, and only 1 patient (5%) had a
grade ≥3 TRAE. Postoperative complications occurred in 10 out of 20
patients (50%) and the most common was atrial arrhythmia (6/20;
30%) (39,40). Another article reported the results
of nivolumab + ipilimumab for patients with NSCLC. For the 9
patients enrolled, the ORR was 11%. TRAEs were identified in 6
patients (67%), with 3 patients (33%) experiencing grade ≥3 TRAEs.
A total of 2 patients had a pCR. The study was terminated early due
to toxic side effects (41).
MK3475-223 (NCT02938624) trial
The phase 1, non-randomized study MK3475-223
(NCT02938624) investigated the effectiveness and safety of
neoadjuvant pembrolizumab treatment for early-stage resectable
NSCLC. Of 10 patients who received 2 cycles (21 days apart) of
neoadjuvant pembrolizumab, 4 patients achieved an MPR. DLTs did not
occur in the dose-schedule escalation cohorts (42).
NCT02716038 trial
The phase 2, single-arm study NCT02716038 tested the
effectiveness of nab-paclitaxel + carboplatin + atezolizumab for
the treatment of NSCLC. Of 30 patients enrolled, 29 patients were
taken into the operating theatre, and 26 underwent successful R0
resection. The ORR was 63%. A total of 17 (57%) out of 30 patients
had an MPR, including 33% of patients who had a pCR. To the best of
our knowledge, this was the first published trial of chemotherapy
combined with an anti-PD-L1 antibody in patients with resectable
NSCLC (43).
NADIM (NCT03081689) trial
The phase 2, single-arm study NADIM
(NCT03081689) assessed the feasibility, safety and efficacy of
combined nivolumab with paclitaxel + carboplatin as NAT in
resectable stage IIIA N2-NSCLC. In the postoperative phase, the
patients were treated with nivolumab for adjuvant therapy for 1
year. Of the 46 patients enrolled, 41 patients had surgery. Of the
41 patients who underwent surgery, 12 (29%) exhibited postoperative
complications. The ORR was 76%. TRAEs occurred during the period of
NAT in 43 patients (93%), of which 14 (30%) had grade ≥3 TRAEs. For
the ITT population, 34 (74%) patients achieved an MPR, of whom 26
(57%) had a pCR. The study had a PFS rate of 77% and an OS rate of
90% when follow-up reached 24 months (44).
ChiCTR-OIC-17013726 trial
The phase 1b, single-arm study ChiCTR-OIC-17013726
evaluated the safety and outcome of sintilimab for resectable
NSCLC. Among the 40 patients enrolled, radical resection was
completed in 37 patients. Neoadjuvant TRAEs occurred in 21 patients
(53%), of which 4 patients (10.0%) experienced grade ≥3 neoadjuvant
TRAEs. The ORR was 20%. Among the 40 patients, 15 (38%) achieved
MPR, including 3 patients (8%) with pCR. A total of 2 patients had
treatment-related surgical delays, and 1 patient underwent R2
resection (45).
PRINCEPS (NCT02994576) trial
The phase 2, single-arm study PRINCEPS
(NCT02994576) assessed the feasibility and efficacy of
atezolizumab as NAT in patients with resectable NSCLC. A total of
30 patients with clinical stage IA-IIIA non-N2 NSCLC received one
injection of atezolizumab followed by surgery. The ORR was 7%, and
an MPR was observed in 4 patients (13%). No pCR was observed. A
total of 29 patients underwent R0 resection, and 1 underwent R1
resection (46).
LCMC3 (NCT02927301) trial
The phase 2, single-arm study LCMC3
(NCT02927301) evaluated the efficacy and toxicity of
neoadjuvant and adjuvant atezolizumab in patients with NSCLC that
was suitable for surgical resection. Of 181 patients enrolled, 30
patients (17%) achieved an MPR, including 10 patients with pCR. A
total of 145 out of 159 patients (91%) had complete (R0) resection.
A total of 44 patients (24%) experienced irAEs during neoadjuvant
treatment, including 4 patients (2%) who had irAEs of grade ≥3
(47).
SAKK 16/14 (NCT02572843) trial
The phase 2, single-arm study SAKK 16/14
(NCT02572843) evaluated the efficacy and feasibility of the
addition of durvalumab to standard NACT (with cisplatin/docetaxel)
in primary resectable stage IIIA(N2) NSCLC. In 55 patients, the
surgical resection rate of R0 was 93%, R1 resection was achieved in
3 patients (5%), and R2 resection was achieved in 1 patient (2%).
After neoadjuvant durvalumab, the ORR was 58%. A total of 34
patients (55%) achieved an MPR, including 10 patients (16%) who
achieved pCR. A total of 50 patients (81%) had TRAEs during the
neoadjuvant phase and 8 patients (13%) experienced TRAEs of grade
≥3 (13).
NeoTAP01 (NCT04304248) trial
The phase 2, single-arm study NeoTAP01
(NCT04304248) assessed the safety and efficacy of toripalimab
plus standard chemotherapy (carboplatin +
pemetrexed/nab-paclitaxel) for stage IIIA or T3-4N2 IIIB NSCLC
deemed surgically resectable in the neoadjuvant setting. A total of
33 patients were enrolled, and R0 was achieved in 29 patients who
underwent resection. The ORR was 88%. A total of 20 patients (61%)
of the population intended for treatment achieved MPR, of which 15
patients (46%) achieved pCR. Furthermore, 18% of patients developed
grade ≥3 TRAEs (48).
NEOMUN (NCT03197467) trial
The phase 2, single-arm study NEOMUN
(NCT03197467) investigated the feasibility and safety of
neoadjuvant pembrolizumab therapy for resectable NSCLC. Among the
15 patients enrolled, the ORR was 27%. A total of 4 patients (27%)
achieved an MPR, including 2 patients (13%) with pCR. A total of 5
patients (33%) had TRAEs during neoadjuvant treatment and 3 (20%)
patients had TRAEs of grade 3. The surgery was lobectomy for all
patients and complete tumor resection (R0) was achieved in all 15
patients. The incidence of postoperative complications was 7% (1
patient) due to postoperative common pneumonia (non-treatment
related) (49).
NEOSTAR (NCT03158129) trial
The phase 2, randomized study NEOSTAR
(NCT03158129) investigated the safety and feasibility of
nivolumab or nivolumab plus ipilimumab as preoperative therapy for
NSCLC. Of the 44 patients enrolled, 23 were randomly assigned to
nivolumab monotherapy and 21 to nivolumab + ipilimumab. The ORR in
the ITT population was 22% after nivolumab monotherapy, and 19%
after nivolumab + ipilimumab. For the monotherapy group, the MPR
was 22%, including 2 patients (9%) who achieved pCR. For the
dual-drug group, the MPR and pCR rates were 38 and 29%,
respectively. The complete (R0) resection rate was 100%. Grade ≥3
TRAEs were observed in 13% of patients treated with monotherapy and
10% of patients treated with combined therapy (50).
NCT02904954 trial
The phase 2, randomized study NCT02904954 evaluated
the feasibility and efficacy of SBRT combined with neoadjuvant
durvalumab in patients with early-stage NSCLC. The 60 patients
enrolled were randomly divided into a monotherapy group (n=30) and
a combined group (n=30). It was observed that 1 patient (3%) in the
monotherapy group and 14 patients (47%) in the combined group
achieved ORR. No patient had a radiographic complete response. A
total of 26 patients in each group completed the surgery and the R0
resection rate was 77% (23 of 30) in the monotherapy group and 83%
(25 of 30) in the combined group. MPR was observed in 2 patients
(7%) in the monotherapy group, and no pCR was observed. In the
combined group, 16 patients (53%) had an MPR, including 8 patients
(27%) who had a pCR. Overall, 5 patients (17%) in the monotherapy
group and 6 patients (20%) in the combined group were observed to
have grade 3-4 TRAEs (51).
TOP 1501 (NCT02818920) trial
The phase 2, single-arm study TOP 1501
(NCT02818920) evaluated the safety and efficacy of neoadjuvant
pembrolizumab for resectable NSCLC. Of 35 patients enrolled,
neoadjuvant pembrolizumab was used in 30 patients, and 25 underwent
lung radical resection. A total of 22 patients achieved R0
resection, and MPR was observed in 7 of 30 patients (23%),
including 3 patients (10%) with pCR. Only 1 patient had a delay
attributed to treatment, and 1 patient had a grade of 3 or more
irAE. The most common complication was atrial fibrillation, which
affected 6 patients (24%) (52).
CheckMate 816 (NCT02998528) trial
The phase 3, randomized study CheckMate 816
(NCT02998528) investigated the safety and effectiveness of
nivolumab plus chemotherapy vs. chemotherapy alone and described
nivolumab plus ipilimumab's safety and effectiveness in treating
resectable NSCLC. Of the 179 patients enrolled in the combined
group, 176 patients received treatment. The radiological ORR was
55%. A total of 66 (38%) of the 176 patients achieved an MPR,
including 24% of patients with a pCR. Any-grade TRAEs were observed
in 145 patients (82%). Grade ≥3 TRAEs were observed in 34% of
patients (53).
Esophageal cancer
KEEP-G 03 (NCT03946969) trial
The phase 2, single-arm study KEEP-G 03
(NCT03946969) investigated the safety and feasibility of
sintilimab plus chemotherapy as a neoadjuvant treatment in
esophageal cancer. In the 15 patients enrolled, sintilimab combined
with lipo-paclitaxel, cisplatin and S-1 was injected for two cycles
every 21 days, followed by esophagectomy. All patients completed
neoadjuvant treatment as planned and achieved 100% R0 resection. A
total of 8 patients (53%) achieved an MPR, including 4 patients
(27%) with pCR. Grade ≥3 TRAEs occurred in 35% of patients
(54).
NCT03604991 trial
The phase 2, single-arm study NCT03604991 assessed
the feasibility and efficacy of neoadjuvant pembrolizumab plus
chemoradiotherapy in resectable esophageal squamous cell carcinoma
(ESCC). Of the 20 patients enrolled, 3 patients were still waiting
for surgery, 15 patients underwent esophagectomy, and 9 patients
(53%) achieved pCR (55).
PALACE-1 (NCT03792347) trial
The phase 1, single-arm study PALACE-1
(NCT03792347) investigated the efficacy and safety of
neoadjuvant immunotherapy combined with chemoradiotherapy in
patients with resectable ESCC. Of the 20 patients enrolled, 18
underwent surgery and no treatment-related surgical delay was
observed. The ORR was 90%. A total of 16 patients (80%) achieved an
MPR, including 10 patients (50%) with pCR. All patients had TRAEs
of any grade, including 13 patients (65%) who had grade ≥3 TRAEs.
To the best of our knowledge, this was the first article on the
preoperative administration of two doses of pembrolizumab in
combination with chemoradiotherapy in patients with resectable ESCC
(56).
PERFECT (NCT03087864) trial
The phase 2, single-arm study PERFECT
(NCT03087864) assessed the safety and efficacy of preoperative
therapy with atezolizumab plus chemoradiation (carboplatin,
paclitaxel and radiation) in resectable esophageal adenocarcinoma
(EAC). Of the 40 patients enrolled, 10 patients (25%) achieved pCR.
irAEs were observed in 6 patients (15%), including 2 patients (5%)
with grade ≥3 irAEs. A total of 33 patients proceeded to surgery
and all patients completed R0 resection (57).
ESONICT-1 (ChiCTR2100045659)
trial
The phase 2, single-arm study ESONICT-1
(ChiCTR2100045659) investigated the safety and feasibility of
sintilimab with cisplatin plus albumin-bound paclitaxel for locally
advanced ESCC in a neoadjuvant setting. Among the 30 patients
enrolled, the ORR was 67% and the MPR rate of the primary tumor was
40%, with 4 patients (13%) achieving pCR. A total of 28 patients
(93%) developed TRAEs of any grade, including 1 patient (3%) who
suffered a grade 3 TRAE. For 23 patients who underwent surgery, no
surgery was delayed and R0 resection was achieved in all (58).
NCT03985670 trial
The phase 2, randomized study NCT03985670
investigated the efficacy and safety of neoadjuvant toripalimab in
combination with chemotherapy in treating locally advanced ESCC. A
total of 30 patients were randomized into two groups at a 1:1
ratio, and received chemotherapy plus sequence toripalimab (group
1) or chemotherapy in combination with toripalimab (group 2) at the
same time. In the 30 patients, the ORR was 60%. The ORR of group 1
was 53% (8 of 15), and that of group 2 was 67% (10 of 15). A total
of 11 patients in group 1 and 13 patients in group 2 underwent
radical resection. R0 resection was observed in 24 patients. The
pCR rate was 27 and 7% for groups 1 and 2, respectively. All
patients had TRAEs (59).
ChiCTR1900023880 trial
The phase 2, single-arm study ChiCTR1900023880
assessed the feasibility and efficacy of camrelizumab in
combination with chemotherapy and apatinib as preoperative therapy
for ESCC. Of the 30 patients enrolled, 15 patients (50%) attained
MPR, including 7 patients with pCR (23%). Grade 3 TRAEs were
observed in 11 patients. No grade 4 and grade 5 TRAEs were
mentioned. The most frequent grade 3 TRAE was neutropenia (7/30;
23.3%). No surgery-related mortality was observed (60).
NCT04506138 trial
The phase 2, single-arm study NCT04506138 evaluated
the safety and efficacy of camrelizumab with chemotherapy as
neoadjuvant treatment for resectable ESCC. Of the 46 patients
enrolled, 18 (39%) achieved MPR, including 8 patients (17%) with
pCR. All 46 patients presented with TRAEs of any grade, including 7
patients (7/46; 15%) who experienced grade ≥3 TRAEs. A total of 9
patients were deemed unsuitable for surgery. All patients who
received surgery achieved R0 resection (61).
NIC-ESCC2019 trial
The phase 2, single-arm study NCT04225364 evaluated
the efficacy of camrelizumab plus concurrent chemotherapy as a
neoadjuvant approach for patients with operable ESCC. Of the 56
patients enrolled, 51 patients received surgery and all achieved R0
resection. A total of 34 patients (61%) achieved an ORR. A total of
30 patients (54%) had an MPR, including 16 (29%) with pCR. irAEs of
any grade occurred in 23 patients (41%), including 2 patients (4%)
with grade ≥3 irAEs. Postoperative complications were observed in
14 patients (62).
NCT04177797 trial
The phase 2, single-arm study NCT04177797 assessed
the feasibility and efficacy of toripalimab with carboplatin and
paclitaxel as a preoperative regiment for ESCC. A total of 20
patients were enrolled and underwent toripalimab plus paclitaxel
and carboplatin treatment for 2 cycles (21 days apart), followed by
planned esophagectomy. A total of 16 patients did not experience
treatment-related surgical delay, and among these, 2 patients did
not receive radical resection. In the 20 patients, the ORR was 55%.
The MPR rate was 35%, including 3 patients (15%) with pCR. TRAEs
were observed in all patients (100%), including 4 patients (20%)
with grade ≥3 TRAEs (63).
ChiCTR1900026240 trial
The phase 2, single-arm study ChiCTR1900026240
assessed the safety and efficacy of camrelizumab plus NACT in
patients with resectable ESCC. A total of 60 patients received
intravenous camrelizumab plus nab-paclitaxel and carboplatin. Of
the 60 patients enrolled, 51 patients completed surgery and R0
resection was reached in 50 patients. A total of 24 patients (40%)
achieved ORR. A total of 35 patients (58%) had an MPR, including 20
patients with pCR. irAEs occurred in 27 patients (45%), including 2
patients who had grade 3 irAEs. Overall, postoperative
complications occurred in 24 of 51 (47%) patients (64).
Gastroesophageal junction (GEJ) and
gastric cancer
NCT03044613 trial
The phase 1, single-arm study NCT03044613 assessed
the safety and feasibility of neoadjuvant nivolumab combined with
chemoradiation in patients with local advanced esophageal/GEJ
cancer. Of the 16 patients enrolled, 10 patients underwent surgery
at the cut-off time. pCR was achieved in 4 patients (25%). This
regimen had an acceptable toxicity profile and did not cause delays
in surgery (65).
NCT03939962 trial
The phase 2, single-arm study NCT03939962 evaluated
the feasibility and efficacy of camrelizumab plus 5-fluorouracil,
leucovorin and oxaliplatin (FOLFOX) as preoperative therapy for
patients with gastric and GEJ cancer (GC/GEJC). Of the 16 patients
enrolled, 15 patients underwent an operation and 13 patients had R0
resection. A total of 3 patients (19%) achieved an MPR, including 1
patient (6%) with pCR. The most common grade ≥3 TRAE was
neutropenia (n=3; 19%). No serious TRAEs were observed (66).
NCT02998268 trial
The phase 2, single-arm study NCT02998268
investigated the safety and efficacy of neoadjuvant pembrolizumab
in combination with chemoradiotherapy in locally advanced EAC or
GEJ adenocarcinoma. Of the 31 patients enrolled, an MPR was
achieved in 50% of patients. irAEs of grade ≥3 was not observed.
The 1-year disease-free survival (DFS) rate was 100% for patients
who achieved MPR (67).
NCT03488667 trial
The phase 2, single-arm study NCT03488667 evaluated
the efficacy and tolerability of oxaliplatin + leucovorin + 5-FU
(mFOLFOX6) plus pembrolizumab in patients with resectable
adenocarcinoma of the GEJ and stomach. Of the 35 patients enrolled,
only 32 patients were evaluated for surgery, and 26 had curative
intended operations with R0 resection for all. pCR was observed in
5 patients (16%). Grade ≥3 TRAEs were observed in 19 of all 35
patients (54%) and there were no unexpected toxicities (68).
NCT04890392 trial
The phase 2, single-arm study NCT04890392
investigated the safety and efficacy of neoadjuvant tislelizumab
combined with s-1 plus oxaliplatin in patients with local advanced
GC/GEJC. Of 21 patients enrolled, 13 (62%) achieved an MPR. A total
of 5 patients had a complete tumor response (24%) and 2 patients
were assessed as disease progression (10%). TRAEs occurred in 11
out of 21 patients (52%). Grade ≥3 TRAEs occurred in only 1 of 21
patients (5%) (69).
NCT04065282 trial
The phase 2, single-arm study NCT04065282 evaluated
sintilimab plus CapeOx (oxaliplatin and capecitabine) as
preoperative therapy in patients with resectable G/GEJC. For the 36
patients enrolled, the ORR was 11%. RECIST V.1.1 was not considered
the best measure of efficacy to assess NAT for GC (70). A total of 17 patients (47%)
achieved an MPR, including 7 patients with pCR. All patients
completed planned surgery and 1 patient had R1 resection. A total
of 33 (92%) and 1 (3%) patient experienced TRAEs and irAEs,
respectively. A total of 10 patients (28%) experienced grade 3
TRAEs. The 1-year DFS and OS rates were 90 and 94%, respectively
(70).
Hepatocellular carcinoma (HCC)
NCT03299946 trial
The phase 1b, single-arm study NCT03299946 evaluated
the safety and efficacy of cabozantinib combined with nivolumab in
patients with local advanced HCC. Of 15 patients enrolled, 12
achieved R0 resection, and 5 patients (33%) had an MPR, including 1
patient (7%) with a pCR. The ORR was 7%. TRAEs of any grade were
observed in 14 patients (93%), including 2 patients (13%) with
grade ≥3 TRAEs (71).
NCT03510871 trial
The phase 2, single-arm study NCT03510871
investigated the tolerability and efficacy of preoperative
nivolumab + ipilimumab in patients with resectable HCC. Of 29
patients enrolled, 5 patients (17%) achieved an MPR. The ORR was
24%. The most common all-grade TRAE was hepatitis (48%). Grade ≥3
TRAEs occurred in 12 patients (41%), including 7 patients (24%)
with irAEs (72).
NCT03222076 trial
The phase 2, randomized study NCT03222076 assessed
the safety and feasibility of neoadjuvant nivolumab monotherapy or
nivolumab plus ipilimumab in patients with resectable HCC. The 27
patients enrolled were randomly divided into monotherapy (13
patients) and combination therapy (14 patients) groups. The ORR was
23% for nivolumab monotherapy and 0% for combination therapy. A
total of 3 patients (23%) with monotherapy and 3 patients (21%)
with nivolumab plus ipilimumab therapy had an MPR, including 2
patients (15%) who achieved a pCR with nivolumab treatment, as did
3 patients (21%) in the combined group. No patients experienced
surgery delay. To the best of our knowledge, this was the first
study to investigate nivolumab plus ipilimumab as a neoadjuvant
treatment in patients with local advanced HCC (73).
NCT03916627 trial
The phase 2, single-arm study NCT03916627 evaluated
the clinical activity of neoadjuvant cemiplimab in patients with
resectable HCC. A total of 21 patients were injected two cycles of
cemiplimab every 21 days followed by radical removal. All patients
received planned treatment and 20 patients completed surgery. Of 21
patients enrolled, 3 (14%) had an overall response. A total of 4
patients had marked tumor necrosis, including 3 (14%) who had a
pCR. TRAEs of any grade occurred in 6 patients (29%), including 2
patients (10%) with grade 3 TRAEs. No grade 4 or 5 TRAEs were
reported (74).
Renal cancer
NCT02210117 trial
The phase 1, randomized study NCT02210117 assessed
the safety and clinical activity of nivolumab alone, nivolumab with
bevacizumab or nivolumab combined with ipilimumab in patients with
metastatic renal cell carcinoma (RCC). A total of 105 patients were
randomly assigned (at a ratio of 2:3:2) to receive nivolumab alone,
nivolumab in combination with bevacizumab or nivolumab combined
with ipilimumab, followed by cytoreductive surgery. The ORR was 55,
44 and 43%, respectively. Grade ≥3 TRAEs were observed in 38% of
patients in the nivolumab group, 42% of patients in the nivolumab +
bevacizumab group and 47% of patients in the nivolumab + ipilimumab
group (75).
NCT03680521 trial
The phase 2, single-arm study NCT03680521 evaluated
the safety and efficacy of sitravatinib combined with nivolumab in
patients with resectable clear cell RCC. DLTs led to a dose
de-escalation, resulting in 7 patients treated at 120 mg 1 day
apart (QD) sitravatinib and 13 treated at 80 mg QD. Of 20 patients
enrolled, 2 patients (10%) achieved a partial response. There was 1
delayed surgery due to immune-related thyroiditis. TRAEs of any
grade occurred in 100% of patients, including grade 3 TRAEs in 45%
of patients. No grade 4/5 TRAEs were observed (76).
NCT02575222 trial
The phase 1, single-arm study NCT02575222 evaluated
the feasibility and safety of neoadjuvant nivolumab in patients
with nonmetastatic RCC. Of 17 patients enrolled, all patients
completed three doses of neoadjuvant nivolumab and underwent
surgery without delay. Although none met the radiographic criteria
for an object response, 1 patient was found to have a pathological
partial response. Any-grade irAEs occurred in 10 patients (59%). No
grade ≥3 irAEs occurred. No patient experienced a Clavien grade
≥III postoperative complication (77).
NeoAvAx(NCT03341845) trial
The phase 2, single-arm study
NeoAvAx(NCT03341845) investigated the safety and efficacy of
neoadjuvant avelumab plus axitinib in patients with localized RCC.
Of 40 patients enrolled, 12 patients (30%) achieved a partial
response. There were no treatment-related surgery delays and no
progression. At a median follow-up of 23.5 months, recurrence
occurred in 13 patients (78).
NCT02595918 trial
The phase 1, single-arm study NCT02595918 evaluated
the feasibility and safety of preoperative nivolumab in patients
with localized RCC. All 18 patients completed surgery without
delay, and 17 received at least three nivolumab doses, resulting in
a feasibility rate of 94%. All patients did not develop progressive
disease during the nivolumab administration period. No MPR was
observed. irAEs of any grade were observed in 3 patients (17%),
including 2 patients (11%) with grade 3 irAEs. No grade 4/5 irAEs
were observed (79).
Colorectal cancer (CC)
NICHE (NCT03026140) trial
The phase 2, randomized study NICHE
(NCT03026140) investigated the efficacy, feasibility and
antitumor activity of ipilimumab plus nivolumab with or without
celecoxib in patients with mismatch repair (MMR)-proficient (pMMR)
and MMR-deficient (dMMR) early-stage colon cancer. Of 40 patients
enrolled, 35 patients underwent radical resections within the
predefined 6 weeks and 100% of the resections were radical. A total
of 20 patients (dMMR) and 8 patients (pMMR) received ipilimumab
plus nivolumab, and 7 patients (pMMR) received ipilimumab plus
nivolumab with celecoxib. A total of 19 patients (95%) with dMMR
tumors had an MPR, including 12 (60%) with pCR. A total of 3
patients with pMMR tumors that received ipilimumab plus nivolumab
had an MPR, including 2 patients with pCR. Of the 7 patients with a
pMMR tumor who received celecoxib in addition to ipilimumab +
nivolumab, only 1 patient (14%) achieved an MPR. TRAEs of any grade
occurred in 70% of patients (28 of 40), including 5 patients (13%)
who had grade ≥3 TRAEs. Postoperative complications were observed
in 9 patients (80).
EPOC1504 (NCT02948348) trial
The phase 1b/2, single-arm study EPOC1504
(NCT02948348) assessed the feasibility and efficacy of
preoperative nivolumab plus chemoradiotherapy in patients with
microsatellite stability (MSS) and microsatellite instability-high
(MSI-H) locally advanced rectal cancer (RC). Sequential use of
nivolumab combined with chemoradiotherapy and radical surgery was
well tolerated. In MSS RC, an MPR was observed in 14 patients
(38%), including 11 patients (30%) with a pCR. Among the 5 patients
with MSI-H RC enrolled, pCR was observed in 3 patients (60%). irAEs
of any grade were observed in 3 patients (7%), including 2 patients
(5%) with grade ≥3 irAEs. No treatment-related deaths were observed
(81).
NICOLE (NCT04123925) trial
The phase 2, single-arm study NICOLE
(NCT04123925) evaluated the feasibility and safety of
neoadjuvant nivolumab in patients with unselected MMR early-stage
CC. Of 22 patients enrolled, including 19 pMMR and 3 dMMR cases,
all patients received radical resection without delays. An MPR was
observed in 3 patients (14%) with pMMR, including 1 patient (5%)
with a pCR. No MPR was observed in patients with dMMR. To the best
of our knowledge, this was the first study to assess nivolumab as
preoperative treatment in dMMR and pMMR resectable CC (82).
NRG-GI002 (NCT02921256) trial
The phase 2, randomized study NRG-GI002
(NCT02921256) assessed the feasibility, safety and efficacy of
the addition of pembrolizumab after neoadjuvant chemoradiotherapy
combined with FOLFOX in patients with locally advanced RC. A total
of 185 patients were randomly assigned to the control arm (n=95) or
the pembrolizumab arm (PA; n=90). In the PA group, 69 patients
received radical surgery and the R0 resection was observed in 94%
of patients. A pCR was observed in 22 patients (24%). irAEs were
observed in 35 patients (39%), including 3 patients (3%) with grade
3 irAEs (83).
AVANA (NCT03854799). The phase 2, single-arm
study AVANA (NCT03854799) investigated the role of avelumab
in combination with neoadjuvant chemoradiotherapy in patients with
locally advanced RC. Of 101 patients enrolled, 59 patients (58%)
achieved an MPR, including 22 (22%) with a pCR. The rate of grade
≥3 irAEs was 4% (84).
PANDORA (NCT04083365) trial
The phase 2, single-arm study PANDORA
(NCT04083365) investigated the feasibility and safety of
durvalumab in combination with neoadjuvant chemoradiotherapy in
patients with locally advanced RC. Of 19 patients enrolled, 18
patients underwent surgery. A pCR was observed in 5 patients (26%).
A total of 8 patients (42%) had any-grade irAEs related to
durvalumab, and no grade ≥3 irAEs related to durvalumab treatment
were observed. The study demonstrated that radiotherapy plus
capecitabine followed by durvalumab as a preoperative option had an
acceptable toxicity profile and promising antitumor activity
(85).
Averectal (NCT03503630) trial
The phase 2, single-arm study Averectal
(NCT03503630) evaluated the safety and efficacy of short-course
radiation followed by mFOLFOX6 with avelumab in patients with
locally advanced RC. Of 44 patients enrolled, 40 patients completed
at least 1 treatment cycle and total mesorectal excision. An MPR
was observed in 27 patients (61%), and 15 patients (34%) achieved a
pCR. No grade ≥3 irAEs were reported (86).
R-IMMUNE (NCT03127007) trial
The phase 1b/2, single-arm study R-IMMUNE
(NCT03127007) evaluated the feasibility and safety of
neoadjuvant atezolizumab combined with radio-chemotherapy in
patients with locally advanced RC. Of 26 patients enrolled, six
patients (23%) achieved a pCR. Overall, 151 TRAEs were reported and
20 (13%) were grade ≥3 in 9 patients (35%), including 2 (10%)
anastomotic leakage/infections, 4 (20%) urinary infections, 1 (5%)
renal function impairment and 1 (5%) immune thrombocytopenia
(87).
PICC (NCT03926338) trial
The phase 2, randomized study PICC
(NCT03926338) evaluated the feasibility and efficacy of
neoadjuvant toripalimab with or without celecoxib in patients with
dMMR or MSI-H CC. A total of 34 patients were randomly divided into
either the combination group (n=17) or the monotherapy group (n=17)
at a 1:1 ratio. All patients in both groups received radical
resection within the normal period and 34 (100%) were R0
resections. A total of 16 patients (94%) in the dual drug group and
17 (100%) in the monotherapy group had an MPR, and pCR occurred in
15 patients (88%) in the dual group and 11 patients (65%) in the
monotherapy group. TRAEs of any grade were observed in 11 patients
(65%) in the combination group and 10 patients (59%) in the
monotherapy group, including 1 patient (6%) with grade ≥3 TRAEs in
the combination group (88).
Bladder cancer
PURE-01 (NCT02736266) trial
The phase 2, single-arm study PURE-01
(NCT02736266) investigated the safety and activity of
preoperative pembrolizumab in patients with muscle-invasive bladder
carcinoma (MIBC). The study allowed the presence of predominant
variant histology (VH), defined as involving >50% of the tumor
specimens. Of 114 patients enrolled, VHs were found in 34 patients,
with predominant VHs in 19 patients. In total, 42 patients (37%)
achieved a pCR, including 3 patients (16%) with predominant VH and
39 patients (41%) with other histological categories. A total of 63
patients (55%) achieved tumor downstaging to pT≤1 (89).
ABACUS (NCT02662309) trial
The phase 2, single-arm study ABACUS
(NCT02662309) assessed the feasibility and efficacy of
preoperative atezolizumab in patients with MIBC. Of 95 patients
enrolled, 8 patients did not undergo cystectomy. A total of 58
patients underwent sequential imaging at the preoperative stage.
Radiological progression occurred in 16% of patients, and the ORR
was 22%. An MPR was observed in 34 patients (36%), including 27
patients (28%) with pCR. Among all patients, 42 patients (44%) had
irAEs of any grade, and grade ≥3 irAEs occurred in 10 patients
(11%). No surgery was delayed due to treatment-related toxicities.
Postoperative complications occurred in 53 patients (90,91).
NCT02812420 trial
The phase 1, single-arm study NCT02812420
investigated the safety and efficacy of neoadjuvant durvalumab plus
tremelimumab in cisplatin-ineligible patients with muscle-invasive
bladder cancer (MIBC) identified as having high-risk features. Of
28 patients enrolled, 24 patients completed cystectomy and 2
patients had delays related to toxicities. A pCR (pT0N0) was
observed in 8 patients (29%). irAEs of any grade occurred in 26
patients (93%), including grade ≥3 irAEs in 21% of patients. No
deaths related to therapy occurred (92).
BLASST-2 (NCT03773666) trial
The phase 1, single-arm study BLASST-2
(NCT03773666) evaluated the feasibility and safety of
neoadjuvant durvalumab in patients with MIBC. Of 10 patients
enrolled, 8 patients underwent radical cystectomy. A pCR was
observed in 1 patient (10%). A grade 3 TRAE was reported in 1
patient (10%), with no grade ≥4 TRAE. No DLT was observed (93).
DUTRENEO (NCT03472274) trial
The phase 2, randomized study DUTRENEO
(NCT03472274) prospectively explored the feasibility of
preoperative anti-PD-L1 + anti-cytotoxic T-lymphocyte associated
protein 4 (CTLA4) vs. chemotherapy in patients with MIBC selected
according to a tumor pro-inflammatory IFN-γ signature [tumor immune
score (TIS)]. Patients with MIBC who receive cisplatin are
classified as 'hot' or 'cold' according to the tumor TIS. A total
of 23 patients randomly assigned to the 'hot' arms received
durvalumab plus tremelimumab. A pCR was achieved in 8 patients
(35%). Grade ≥3 irAEs were reported in 5 patients (22%) (94).
NEODURVARIB (NCT03534492) trial
The phase 2, single-arm study NEODURVARIB
(NCT03534492) investigated the feasibility and safety of
neoadjuvant durvalumab plus olaparib in patients with MIBC. Of 29
patients enrolled, 26 underwent radical cystectomy. Partial
response of radiology was observed in 24% of patients. A pCR was
observed in 13 patients (45%). No dose reductions were indicated
(95).
GU14-188 (NCT02365766) trial
The phase 1b/2, non-randomized study GU14-188
(NCT02365766) investigated the safety and efficacy of
neoadjuvant pembrolizumab combined with gemcitabine with or without
cisplatin in patients with cisplatin-eligible/ineligible
muscle-invasive bladder cancer (MIBC). In the cisplatin-eligible
group, 43 patients were enrolled. The pCR rate was 44%. A total of
4 patients did not have radical cystectomy (96). In the cisplatin-ineligible group,
37 patients were enrolled. The pCR rate was 45%. There were 4
patients (11%) with grade 3 irAEs. A total of 3 patients did not
undergo radical cystectomy (97).
NABUCCO (NCT03387761) trial
The phase 1, randomized study NABUCCO
(NCT03387761) evaluated the feasibility and safety of
neoadjuvant nivolumab plus ipilimumab in patients with
locoregionally advanced urothelial cancer (UC). In cohort 1 of the
study, 24 patients were injected two doses of ipilimumab and two
doses of nivolumab, followed by radical surgery. An MPR was
attained in 20 patients (83%), including 11 (46%) with pCR. irAEs
of any grade were reported in all patients (100%), and grade ≥ 3
irAEs occurred in 55% of patients (98). In cohort 2, 30 patients were
randomly divided into arms A and B, 26 underwent radical surgery,
and 24 patients received surgery within 12 weeks. In arm A, 6
patients (40%) had a pCR. In arm B, 1 patient (7%) had a pCR. This
trial suggests that a higher dose of ipilimumab is a more
efficacious neoadjuvant treatment than a low dose of ipilimumab in
UC (99).
NCT02451423 trial
The phase 2, non-randomized study NCT02451423
assessed the best dose of neoadjuvant atezolizumab in treating
patients with bladder cancer that has not spread to other places in
the body. A total of 20 patients were enrolled and sequentially
treated with one (n=6), two (n=5) and three (n=9) cycles of
atezolizumab prior to radical cystectomy. pCR was observed in 1
patient (17%) in the one-dose group and 1 patient (20%) in the
two-dose group. No pCR was observed in the three-dose group. TRAEs
of any grade were observed in 75% of patients, including 10% who
had grade 3 TRAEs. There were no grade 4/5 events (100).
PrE0807 (NCT03532451) trial
The phase 1, non-randomized study PrE0807
(NCT03532451) evaluated the feasibility and safety of nivolumab
with or without lirilumab as a preoperative regimen in patients
with resectable MIBC. Among 43 patients enrolled, 13 patients were
in the group of nivolumab alone (cohort 1) and 30 were in the group
of nivolumab plus lirilumab (cohort 2). The pCR rates for cohorts 1
and 2 were 8 and 18%, respectively. Grade 3 TRAEs occurred in 0% of
patients in cohort 1 and 7% of patients in cohort 2. No grade 4/5
traces occurred (101).
SAKK 06/17 (NCT03406650) trial
The phase 2, single-arm study SAKK 06/17
(NCT03406650) investigated the safety and efficacy of
neoadjuvant durvalumab combined with standard NACT (with
cisplatin/gemcitabine) in patients with MIBC. Of 58 patients
enrolled, 53 underwent resection and R0 resection was completed in
52 patients. A total of 18 patients (31%) achieved a pCR. TRAEs of
grade ≥3 were observed in 75% of patients, and irAEs of grade ≥3
were observed in 8 patients (14%) (102).
LCCC 1520 (NCT02690558) trial
The phase 2, single-arm study LCCC 1520
(NCT02690558) evaluated the safety and efficacy of gemcitabine
and cisplatin in combination with pembrolizumab as a NAT before
radical cystectomy in MIBC. Of 39 patients enrolled, 38 patients
underwent radical cystectomy, and 4 patients did not have R0
resection. No patients had clinical or radiographic progression
before radical cystectomy. pCR was achieved in 14 patients (36%).
All patients experienced at least one TRAE of any grade, and 74% of
patients experienced grade ≥3 TRAEs. Only 1 patient (3%) had a
grade 3 irAE (103).
NCT02989584 trial
The phase 2, single-arm study NCT02989584
investigated the feasibility and safety of atezolizumab followed by
four cycles of gemcitabine and cisplatin (GC) in patients before
radical cystectomy and pelvic lymph node dissection (RC-PLND). Of
44 patients enrolled, 36 patients underwent RC-PLND and no patient
had TRAE-related surgical delays. A total of 27 patients (61%)
achieved non-muscle-invasive downstaging to less than pT2N0,
including 16 patients (36%) with a pCR. Among the 44 patients, 98%
experienced TRAEs, including 26 patients (59%) with a grade ≥3
TRAE. The most common grade ≥3 TRAE was neutropenia (36%). irAEs of
grade 3 occurred in 5 patients (11%). No grade 4 irAEs were
observed (104).
Melanoma
OpACIN (NCT02437279) trial
The phase 1b, randomized study OpACIN
(NCT02437279) examined the safety and efficacy of neoadjuvant
vs. adjuvant nivolumab plus ipilimumab in patients with operable
stage III melanoma. In the neoadjuvant group, 10 patients were
enrolled. All patients in the neoadjuvant treatment arm achieved
planned surgery. The ORR was 40%. An MPR was achieved in 6 patients
(60%), including 3 patients (30%) with a pCR. TRAEs of any grade
were observed in all patients and grade ≥3 TRAEs were observed in 9
patients (90%) in the neoadjuvant arm (105).
NCT02519322 trial
The phase 2, randomized study NCT02519322 assessed
the feasibility and efficacy of preoperative nivolumab with or
without ipilimumab in patients with high-risk resectable melanoma
and evaluated the feasibility of nivolumab in combination with
relatlimab. For the nivolumab vs. combined ipilimumab with
nivolumab group, 23 patients were randomly assigned to monotherapy
(n=12) and combined therapy (n=11). The ORR was 25% with nivolumab
monotherapy and 73% with combined ipilimumab and nivolumab therapy.
A pCR was observed in 3 patients (25%) and 5 patients (45%),
respectively. TRAEs of any grade occurred in 11 patients (92%) with
nivolumab monotherapy and 10 (91%) with combined ipilimumab and
nivolumab therapy, including grade-3 TRAEs occurring in 1 patient
(8%) and 8 patients (73%), respectively. No grade 4 or 5 TRAEs were
observed (106). For the
nivolumab combined with relatlimab group, 30 patients were enrolled
and 29 underwent surgery. The ORR was 57% and the MPR rate was 66%,
including a pCR rate of 59%. The 1-year recurrence-free survival
(RFS) for patients with an MPR was 100% compared with 80% for
patients without an MPR. There were no grade ≥3 TRAEs (107).
OpACIN-neo (NCT02977052) trial
The phase 2, randomized controlled study
OpACIN-neo (NCT02977052) identified a promising dosing
schedule of ipilimumab plus nivolumab. ORR was achieved in 45
patients (52%) overall: 19 patients (63%) in group A, 17 (57%) in
group B and 9 patients (35%) in group C. An MPR was observed in 21
patients (70%) in group A [14 (47%) with a pCR], 19 patients (64%)
in group B [17 (57%) with a pCR] and 12 patients (46%) in group C
[6 (23%) with a pCR]. irAEs of any grade occurred in 29 patients
(97%) in group A, 29 patients (97%) in group B and 26 patients
(100%) in group C. Grade ≥3 irAEs were reported in 12 patients
(40%) in group A, 6 patients (20%) in group B and 13 patients (50%)
in group C. None of the 64 patients who achieved a pathological
response had relapsed after a median follow-up of 32 months,
whereas 9 patients (43%) without pathological response had relapsed
or progressed before surgery (108).
NCT02434354 trial
The phase 1, single-arm study NCT02434354 evaluated
the safety, tolerability and irAE profile of pembrolizumab in
patients with high-risk melanoma, and tumor tissues were collected
from patients before and after receipt of pembrolizumab to examine
how the experimental drug interacted with tumor tissue. Of 27
patients evaluable at the time of cut-off, 8 patients (30%)
achieved an MPR, including 5 patients (19%) with a pCR. All
patients who achieved a complete or MPR did not relapse. Grade 3
irAEs were observed in 6 patients (22%), with no grade 4 events and
no delays in surgical management (109,110).
OrienX010-II-12 (NCT04197882)
trial
The phase 1b, single-arm study OrienX010-II-12
(NCT04197882) evaluated the efficacy and tolerability of
recombinant human granulocyte-macrophage colony-stimulating factor
herpes simplex virus intratumoral injection (OrienX010) combined
with toripalimab as a preoperative regimen in patients with local
advanced melanoma. In the 30 patients enrolled, the ORR was 33% (10
patients). A total of 21 patients exhibited pathologic responses,
including 3 (10%) showing a pCR. All patients had TRAEs and 3
patients (10%) had grade ≥3 TRAEs (111).
HCI102346(NCT03259425) trial
The phase 2, single-arm study
HCI102346(NCT03259425) evaluated the safety and efficacy of
neoadjuvant nivolumab and canerpaturev in resectable stage IIIB,
IIIC and IVM1a melanoma. Of 7 patients enrolled, 6 underwent
surgery after nivolumab and canerpaturev and were evaluable. A pCR
was observed in 5 patients (83%), and R0 resection was achieved in
5 patients. Radiographic complete or partial responses were
observed in 4 patients (67%). At a median follow-up of 27.3 months,
RFS was achieved in 50% of patients (112).
EudraCT 2018-002172-40 trial
The phase 2, single-arm study EudraCT
2018-002172-40 assessed the safety and efficacy of the
ipilimumab/nivolumab combination as primary treatment for patients
with locally advanced or oligometastatic melanoma. Of 35 patients
enrolled, 3 patients are still in NAT and 3 patients were
discontinued before surgery. A total of 6 patients (17%) developed
treatment-related grade ≥3 TRAEs. An MPR was achieved in 18
patients (51%), including 16 (46%) with a pCR. Furthermore, 1
patient died 5 months after the end of therapy due to an ischemic
stroke (113).
NCT02339324 trial
The pilot phase 1b/2, single-arm study NCT02339324
evaluated the safety and efficacy of neoadjuvant pembrolizumab
combined with high-dose IFNa-2b in patients with high-risk
surgically resectable stage III or IV melanoma. Of 30 patients
enrolled, all patients were evaluated for efficacy and underwent
surgery as planned. The ORR was 73% (22 patients). An MPR was
achieved in 17 patients (57%), including 13 (43%) with a pCR. All
patients experienced at least one TRAE of any grade. The most
common grade 3 TRAE was hypophosphatemia, which was observed in 13
patients (43%). At the time of cut-off, the patients with a pCR had
not experienced recurrence (114).
Merkel cell carcinoma (MCC)
CheckMate358 (NCT02488759) trial
The phase 1/2, randomized study CheckMate358
(NCT02488759) investigated the safety and efficacy of nivolumab
monotherapy or nivolumab combination therapy in patients with
virus-associated tumors. In the neoadjuvant MCC cohort, 39 patients
were enrolled and 36 of these patients received the planned doses
of nivolumab. The ORR was 46%. An MPR was achieved in 21 patients
(54%), including 17 patients (44%) with a pCR. Any-grade TRAEs were
reported in 18 patients (46%), including 3 patients (7%) with grade
≥3 TRAEs (115).
3. Benefits and efficacy of neoadjuvant
immunotherapy
A number of studies (7-9) have
demonstrated that NACT is an attractive strategy to improve
postoperative outcomes. Its primary clinical benefits stem from its
ability to reduce the volume of the primary tumor, control
micrometastases and decrease growth factor release caused by
surgery or subsequent wound healing (8,9,116,117). With immunotherapy being hailed as
a potentially practice-changing therapy for advanced cancer,
ongoing studies (20,29,48)
seek to determine the efficacy of neoadjuvant immunotherapy in the
early stages of cancer. The present review demonstrated that
immunotherapy is promising in the neoadjuvant setting. In addition
to the advantages offered by NACT, a unique feature is that it
stimulates tumor-specific T cells against all tumor neoantigens and
attacks the minimally residual tumor cells throughout the body
(14). Therefore, neoadjuvant
immunotherapy may decrease the risk of disease recurrence and
benefit the OS of patients with cancer (15).
Details of the clinical trials investigated in this
review are listed in Table I. A
total of 11 cancer types were included in the analysis, and more
than half of the studies were phase 1/2 single-arm trials.
Therefore, the sample size of most trials was small. The present
review describes seven distinct forms of NAT, including immune
checkpoint inhibitor monotherapy (IO), IO in combination with
another IO, IO in combination with chemotherapy, IO plus
radiotherapy, IO plus chemoradiotherapy, IO in combination with
anti-VEGFR drugs and IO plus other regiments (PARP, COX, Virus and
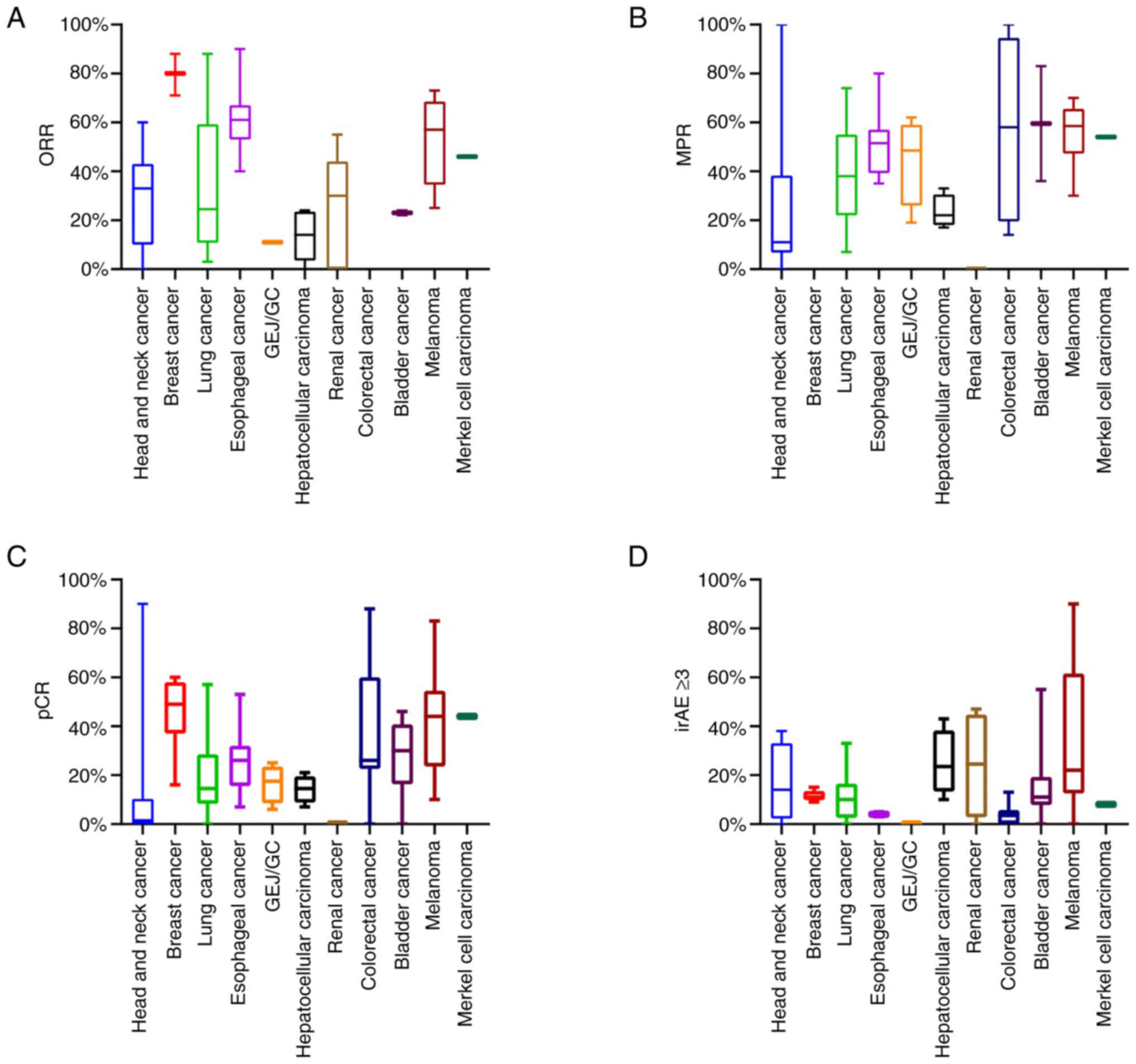
IFN). As shown in Fig. 2A-C,
patients with breast cancer, esophageal cancer and melanoma had a
higher ORR and pCR rate after the neoadjuvant immunotherapy than
patients with head and neck cancer, HCC and lung cancer, which may
be due to the treatment strategy. Regarding the 11 cancer types,
when patients with cancer received immunotherapy combination
therapy, they achieved a higher ORR, MPR and pCR compared with
those with IO (Fig. 3A-C).
Previous studies have reported that chemotherapy enhanced the
efficacy of immunotherapy by directly killing tumor cells or
activating the immune function of the body (118,119). Among all treatment regimens
studied here, regardless of whether radiotherapy was applied, IO
plus chemotherapy achieved a higher ORR, MPR and pCR than IO or IO
+ IO.
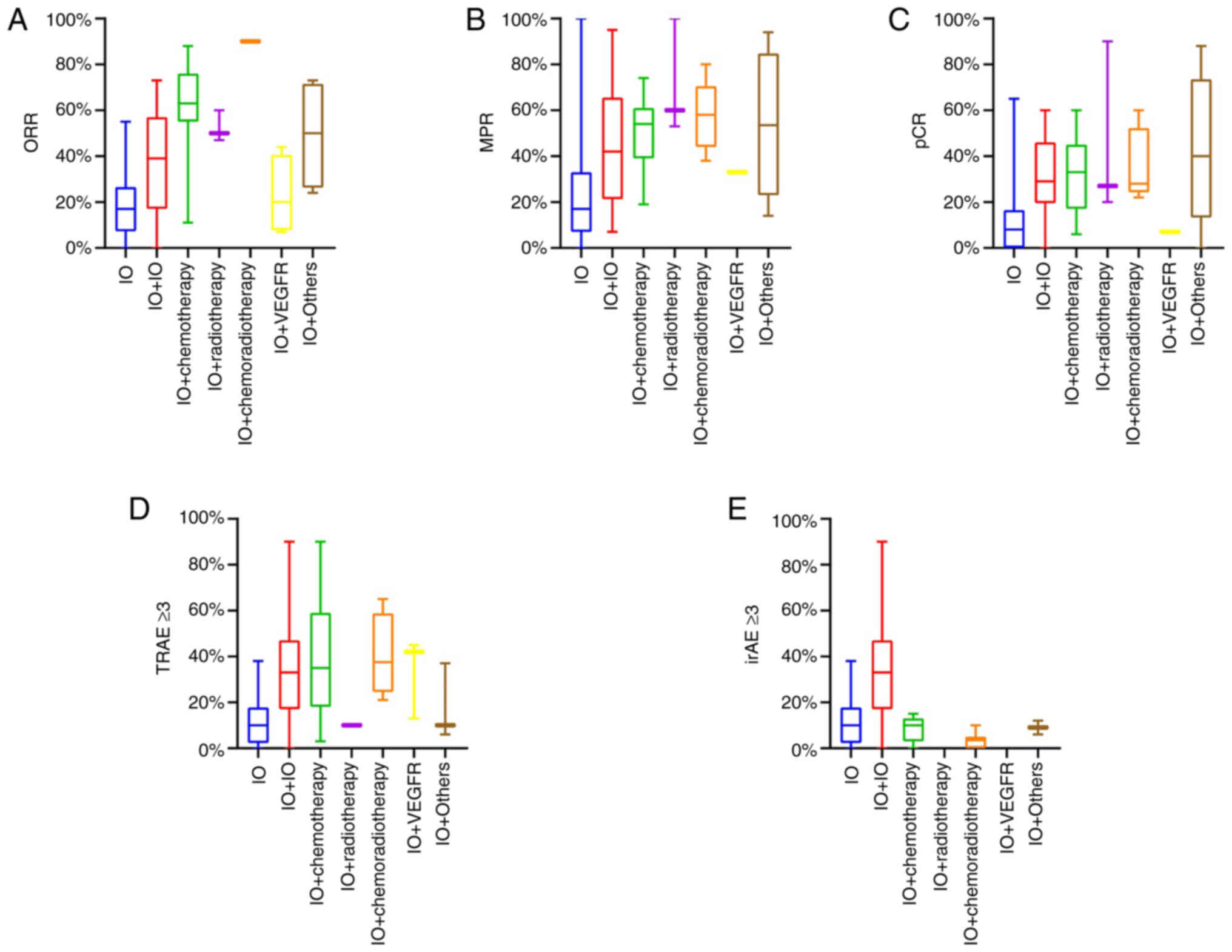 | Figure 3Efficacy and safety (including TRAEs
and irAEs) of different neoadjuvant therapy regimens. (A) ORR. (B)
MPR. (C) pCR. (D) Grade ≥3 TRAEs. (E) Grade ≥3 irAEs. IO, immune
checkpoint inhibitor alone (PD-1 inhibitor or PD-L1 inhibitor
alone); IO+IO, combination of PD-1/PD-L1 inhibitor and cytotoxic
T-lymphocyte associated protein 4 inhibitor or lymphocyte
activation gene-3 inhibitor; IO + chemotherapy, combination of
PD-1/PD-L1 inhibitor and chemotherapy; IO + radiotherapy,
combination of PD-1/PD-L1 inhibitor and radiotherapy; IO +
chemoradiotherapy, combination of PD-1/PD-L1 inhibitor and
chemotherapy plus radiotherapy; IO+VEGFR, combination of PD-1/PD-L1
inhibitor and angiogenesis inhibitor; IO + Others, combination of
PD-1/PD-L1 inhibitor and special drugs; irAE, immune-related
adverse event; MPR, major pathologic response; ORR, objective
response rate; pCR, pathologic complete response; PD-1, programmed
cell death protein 1; PD-L1, programmed death-ligand 1; TRAE,
treatment-related adverse event. |
 | Table ISummary of the studies included in
the present review. |
Table I
Summary of the studies included in
the present review.
| First author/s,
year | Study name | Tumor | Type | ORR, % | MPR, % | pCR, % | TRAE, % | TRAE ≥3, % | irAE, % | irAEs ≥3, % | (Refs.) |
|---|
| Uppaluri et
al, 2020 | NCT02296684-1 | HNSCC | IO | NA | 6 | 0 | NA | 0 | NA | 0 | (22) |
| Uppaluri et
al, 2021 | NCT02296684-2 | HNSCC | IO | NA | 14 | 3 | NA | 3 | NA | 3 | (23) |
| Knochelmann et
al, 2021 | NCT03021993 | OCSCC | IO | 33 | NA | 0 | 67 | 0 | 67 | 0 | (26) |
| Wise-Draper et
al, 2021 | NCT02641093 | HNSCC | IO | NA | 8 | NA | NA | NA | NA | NA | (27) |
| Ferris et
al, 2021 | CheckMate358-1 | HNSCC (HPV+) | IO | 12 | 6 | 0 | 73 | 19 | 73 | 19 | (28) |
| Ferris et
al, 2021 | CheckMate358-2 | HNSCC (HPV-) | IO | 8 | 0 | 0 | 54 | 12 | 54 | 12 | |
| Vos, 2021 | IMCISION-1 | HNSCC | IO | 0 | 17 | 0 | 67 | 33 | 67 | 33 | (25) |
| Ferrarotto et
al, 2020 | CIAO-1 | OPSCC | IO | 40 | 7 | 7 | 93 | 20 | 93 | 20 | (21) |
| Bar et al,
2019 | MK3475-223 | NSCLC | IO | NA | 40 | NA | NA | NA | NA | NA | (42) |
| Gao et al,
2020 |
ChiCTR-OIC-17013726 | NSCLC | IO | 20 | 38 | 8 | 53 | 10 | 53 | 10 | (45) |
| Besse et al,
2020 | PRINCEPS | NSCLC | IO | 7 | 13 | 0 | 3 | 0 | 3 | 0 | (46) |
| Lee et al,
2021 | LCMC3 | NSCLC | IO | NA | 17 | 6 | 24 | 2 | 24 | 2 | (47) |
| Eichhorn et
al, 2021 | NEOMUN | NSCLC | IO | 27 | 27 | 13 | 33 | 20 | 33 | 20 | (49) |
| Tong et al,
2022 | TOP 1501 | NSCLC | IO | NA | 23 | 10 | NA | 3 | NA | 3 | (52) |
| Cascone et
al, 2021 | NEOSTAR-1 | NSCLC | IO | 22 | 22 | 9 | NA | 13 | NA | 13 | (50) |
| Altorki et
al, 2021 | NCT02904954-1 | NSCLC | IO | 3 | 7 | 0 | NA | NA | NA | NA | (51) |
| Forde et al,
2018 and Bott et al, 2019 |
checkmate-159-1 | NSCLC | IO | 10 | 43 | 10 | 23 | 5 | 23 | 5 | (39,40) |
| Kaseb et al,
2022 | NCT03222076-1 | HCC | IO | 23 | 23 | 15 | 77 | 23 | 77 | 23 | (73) |
| Marron et
al, 2022 | NCT03916627 | HCC | IO | 14 | NA | 14 | 29 | 10 | 29 | 10 | (74) |
| Gao et al,
2019 | NCT02210117-1 | RCC | IO | 55 | NA | NA | NA | 38 | NA | 38 | (75) |
| Gorin et al,
2022 | NCT02575222 | RCC | IO | 0 | 0 | 0 | 59 | 0 | 59 | 0 | (77) |
| Carlo et al,
2022 | NCT02595918 | RCC | IO | 0 | 0 | 0 | 17 | 11 | 17 | 11 | (79) |
| Avallone et
al, 2020 | NICOLE | CC | IO | NA | 14 | 5 | NA | NA | NA | NA | (82) |
| Hu et al,
2022 | PICC-1 | CC
(dMMR/MSI-H) | IO | NA | 100 | 65 | 59 | 0 | 59 | 0 | (88) |
| Necchi et
al, 2020 | PURE-01-1 | MIBC (VH) | IO | NA | NA | 16 | NA | NA | NA | NA | (89) |
| Necchi et
al, 2020 | PURE-01-2 | MIBC | IO | NA | NA | 41 | NA | NA | NA | NA | |
| Powles et
al, 2019 and Szabados et al, 2021 | ABACUS | MIBC | IO | 22 | 36 | 28 | 44 | 11 | 44 | 11 | (90,91) |
| Wei et al,
2020 | BLASST-2 | MIBC | IO | NA | NA | 10 | NA | 10 | NA | 10 | (93) |
| Grivas et
al, 2021 | PrE0807-1 | MIBC (Cis-) | IO | NA | NA | 8 | NA | 0 | NA | 0 | (101) |
| Natesan et
al, 2021 | NCT02451423-1 | MIBC (Cis-) | IO | NA | NA | 17 | 75 | 10 | 75 | 10 | (100) |
| Natesan et
al, 2021 | NCT02451423-2 | MIBC (Cis-) | IO | | | 20 | | | | | |
| Natesan et
al, 2021 | NCT02451423-3 | MIBC (Cis-) | IO | | | 0 | | | | | |
| Huang et al,
2018 and Huang et al, 2019 | NCT02434354 | Melanoma | IO | NA | 30 | 19 | NA | 22 | NA | 22 | (109,110) |
| Amaria et
al, 2018 | NCT02519322-1 | Melanoma | IO | 25 | NA | 25 | 92 | 8 | 92 | 8 | (106) |
| Topalian et
al, 2020 | CheckMate358 | MCC | IO | 46 | 54 | 44 | 46 | 8 | 46 | 8 | (115) |
| Schoenfeld et
al, 2020 | NCT02919683-1 | OCSCC | IO | 13 | 7 | 0 | NA | 14 | NA | 14 | (20) |
| Ferrarotto et
al, 2020 | CIAO-2 | OPSCC | IO+IO | 43 | 7 | 0 | 86 | 7 | 86 | 7 | (21) |
| Vos et al,
2021 | IMCISION-2 | HNSCC | IO+IO | 10 | 31 | 4 | 69 | 38 | 69 | 38 | (25) |
| Reuss et al,
2020 |
checkmate-159-2 | NSCLC | IO+IO | 11 | 22 | 22 | 67 | 33 | 67 | 33 | (41) |
| Cascone et
al, 2021 | NEOSTAR-2 | NSCLC | IO+IO | 19 | 38 | 29 | NA | 10 | NA | 10 | (50) |
| Kaseb et al,
2022 | NCT03222076-2 | HCC | IO+IO | 0 | 21 | 21 | 86 | 43 | 86 | 43 | (73) |
| Su et al,
2021 | NCT03510871 | HCC | IO+IO | 24 | 17 | NA | NA | 41 | NA | 24 | (72) |
| Gao et al,
2019 | NCT02210117-3 | RCC | IO+IO | 43 | NA | NA | NA | 47 | NA | 47 | (75) |
| Grivas et
al, 2021 | PrE0807-2 | MIBC (Cis-) | IO+IO | NA | NA | 18 | NA | 7 | NA | 7 | (101) |
| Gao et al,
2020 | NCT02812420 | MIBC (Cis-) | IO+IO | NA | NA | 29 | 93 | 21 | 93 | 21 | (92) |
| Grande et
al, 2020 | DUTRENEO | MIBC (Cis+) | IO+IO | NA | NA | 35 | NA | 22 | NA | 22 | (94) |
| Van Dorp et
al, 2021 | NABUCCO-1 | MIBC | IO+IO | NA | 83 | 46 | 100 | 55 | 100 | 55 | (99) |
| Van Dorp et
al, 2021 | NABUCCO-2 | MIBC | IO+IO | NA | NA | 40 | NA | NA | NA | NA | |
| Van Dorp et
al, 2021 | NABUCCO-3 | MIBC | IO+IO | NA | NA | 7 | NA | NA | NA | NA | |
| Amaria et
al, 2018 | NCT02519322-2 | Melanoma | IO+IO | 73 | NA | 45 | 91 | 73 | 91 | 73 | (106) |
| Amaria et
al, 2021 | NCT02519322-3 | Melanoma | IO+IO | 57 | 66 | 59 | NA | 0 | NA | 0 | (107) |
| Blank et al,
2018 | OpACIN | Melanoma | IO+IO | 40 | 60 | 30 | 100 | 90 | 100 | 90 | (105) |
| Rozeman et
al, 2019 | OpACIN-neo-1 | Melanoma | IO+IO | 63 | 70 | 47 | 97 | 40 | 97 | 40 | (108) |
| Rozeman et
al, 2019 | OpACIN-neo-2 | Melanoma | IO+IO | 57 | 64 | 57 | 97 | 20 | 97 | 20 | |
| Rozeman et
al, 2019 | OpACIN-neo-3 | Melanoma | IO+IO | 35 | 46 | 23 | 100 | 50 | 100 | 50 | |
| Cocorocchio et
al, 2021 | EudraCT
2018-002172-40 | Melanoma | IO+IO | NA | 51 | 46 | NA | 17 | NA | 17 | (113) |
| Schoenfeld et
al, 2020 | NCT02919683-2 | OCSCC | IO+IO | 38 | 20 | 7 | NA | 33 | NA | 33 | (20) |
| Chalabi et
al, 2020 | NICHE-1 | CC (dMMR) | IO+IO | NA | 95 | 60 | NA | NA | NA | NA | (80) |
| Chalabi et
al, 2020 | NICHE-2 | CC (pMMR) | IO+IO | NA | 25 | 25 | NA | NA | NA | NA | |
| Zinner et
al, 2020 | NCT03342911 | HNSCC |
IO+Chemotherapy | NA | 65 | 35 | NA | 35 | NA | NA | (11) |
| Loibl et al,
2019 | GeparNuevo | TNBC |
IO+Chemotherapy | NA | NA | 53 | NA | NA | NA | NA | (12) |
| Schmid et
al, 2020 | KEYNOTE-173 | TNBC |
IO+Chemotherapy | 88 | NA | 57 | 100 | 90 | 30 | 10 | (29) |
| Foldi et al,
2021 | NCT02489448 | TNBC |
IO+Chemotherapy | NA | NA | 44 | NA | 31 | NA | 9 | (30) |
| Mittendorf et
al, 2020 | IMpassion031 | TNBC |
IO+Chemotherapy | NA | NA | 58 | 98 | 57 | 70 | 15 | (31) |
| Schmid et
al, 2020 | KEYNOTE-522 | TNBC |
IO+Chemotherapy | NA | NA | 60 | 99 | 77 | 39 | 13 | (32) |
| Yam et al,
2021 | NCT02530489 | TNBC |
IO+Chemotherapy | NA | NA | 30 | NA | NA | NA | NA | (35) |
| Gianni et
al, 2022 | NeoTRIPaPDL1 | TNBC |
IO+Chemotherapy | 80 | NA | 49 | 98 | 78 | NA | NA | (37) |
| Dieci et al,
2022 | GIADA | BC |
IO+Chemotherapy | 71 | NA | 16 | NA | NA | NA | NA | (38) |
| Nanda et al,
2020 | I-SPY-2-1 | BC (HER2-) |
IO+Chemotherapy | NA | NA | 44 | NA | NA | NA | NA | (33) |
| Rothschild et
al, 2021 | SAKK 16/14 | NSCLC |
IO+Chemotherapy | 58 | 55 | 16 | 81 | 13 | NA | NA | (13) |
| Zhao et al,
2021 | NeoTAP01 | NSCLC |
IO+Chemotherapy | 88 | 61 | 46 | NA | 18 | NA | NA | (48) |
| Shu et al,
2020 | NCT02716038 | NSCLC |
IO+Chemotherapy | 63 | 57 | 33 | NA | NA | NA | NA | (43) |
| Provencio et
al, 2020 | NADIM | NSCLC |
IO+Chemotherapy | 76 | 74 | 57 | 93 | 30 | NA | NA | (44) |
| Forde et al,
2022 | CheckMate 816 | NSCLC |
IO+Chemotherapy | 55 | 38 | 24 | 82 | 34 | NA | NA | (53) |
| Gu et al,
2020 | KEEP-G 03 | ESCC |
IO+Chemotherapy | NA | 53 | 27 | NA | 35 | NA | NA | (54) |
| Zhang et al,
2021 | ESONICT-1 | ESCC |
IO+Chemotherapy | 67 | 40 | 13 | 93 | 3 | NA | NA | (58) |
| Xing et al,
2021 | NCT03985670-1 | ESCC |
IO+Chemotherapy | 53 | NA | 27 | 100 | NA | NA | NA | (59) |
| Xing et al,
2021 | NCT03985670-2 | ESCC |
IO+Chemotherapy | 67 | NA | 7 | 100 | NA | NA | NA | |
| Xu et al,
2022 | NCT04506138 | ESCC |
IO+Chemotherapy | NA | 39 | 17 | 100 | 15 | NA | NA | (61) |
| Liu et al,
2022 | NIC-ESCC2019 | ESCC |
IO+Chemotherapy | 61 | 54 | 29 | 86 | 11 | 41 | 4 | (62) |
| He et al,
2022 | NCT04177797 | ESCC |
IO+Chemotherapy | 55 | 35 | 15 | 100 | 20 | NA | NA | (63) |
| Liu et al,
2022 |
ChiCTR1900026240 | ESCC |
IO+Chemotherapy | 40 | 58 | 33 | 97 | 57 | 45 | 3 | (64) |
| Liu et al,
2020 | NCT03939962 | G/GEJC |
IO+Chemotherapy | NA | 19 | 6 | NA | NA | NA | NA | (66) |
| Sun et al,
2022 | NCT03488667 | G/GEJC |
IO+Chemotherapy | NA | NA | 16 | NA | 54 | NA | NA | (68) |
| Tao et al,
2022 | NCT04890392 | G/GEJC |
IO+Chemotherapy | NA | 62 | NA | 52 | 5 | NA | NA | (69) |
| Jiang et al,
2022 | NCT04065282 | G/GEJC |
IO+Chemotherapy | 11 | 47 | 19 | 92 | 28 | 3 | 0 | (70) |
| Hoimes et
al, 2020 | GU14-188-1 | MIBC (Cis+) |
IO+Chemotherapy | NA | NA | 44 | NA | NA | NA | NA | (96) |
| Kaimakliotis et
al, 2020 | GU14-188-2 | MIBC (Cis-) |
IO+Chemotherapy | NA | NA | 45 | NA | 36 | NA | 11 | (97) |
| Cathomas et
al, 2021 | SAKK 06/17 | MIBC |
IO+Chemotherapy | NA | NA | 31 | NA | 75 | NA | 14 | (102) |
| Rose et al,
2021 | LCCC 1520 | MIBC |
IO+Chemotherapy | NA | NA | 36 | 100 | 74 | NA | 3 | (103) |
| Funt et al,
2022 | NCT02989584 | MIBC |
IO+Chemotherapy | NA | NA | 36 | 98 | 59 | NA | 11 | (104) |
| Altorki et
al, 2021 | NCT02904954-2 | NSCLC | IO+Radio | 47 | 53 | 27 | NA | NA | NA | NA | (51) |
| Leidner et
al, 2021 | NIRT-HNC-1 | HNSCC (HPV+) | IO+Radio | 50 | 100 | 90 | 100 | 10 | NA | NA | (24) |
| Leidner et
al, 2021 | NIRT-HNC-2 | HNSCC (HPV-) | IO+Radio | 60 | 60 | 20 | 100 | NA | NA | NA | |
| McArthur et
al, 2021 | NCT03366844 | TNBC | IO+CRT | NA | NA | 60 | NA | NA | NA | 10 | (36) |
| Kelly et al,
2019 | NCT03044613 | E/GEJC | IO+CRT | NA | NA | 25 | NA | NA | NA | NA | (65) |
| Shah et al,
2021 | NCT02998268 | E/GEJC | IO+CRT | NA | 50 | NA | NA | NA | NA | 0 | (67) |
| Li et al,
2020 | NCT03604991 | ESCC | IO+CRT | NA | NA | 53 | NA | NA | NA | NA | (55) |
| Li et al,
2021 | PALACE-1 | ESCC | IO+CRT | 90 | 80 | 50 | 100 | 65 | NA | NA | (56) |
| van den Ende et
al, 2021 | PERFECT | EA | IO+CRT | NA | NA | 25 | NA | 40 | 15 | 5 | (57) |
| Rahma et al,
2021 | NRG-GI002 | RC | IO+ CRT | NA | NA | 24 | NA | NA | 39 | 3 | (83) |
| Salvatore et
al, 2021 | AVANA | RC | IO+CRT | NA | 58 | 22 | NA | NA | NA | 4 | (84) |
| Tamberi et
al, 2021 | PANDORA | RC | IO+CRT | NA | NA | 26 | NA | 21 | 42 | 0 | (85) |
| Shamseddine et
al, 2021 | Averectal | RC | IO+CRT | NA | 61 | 34 | NA | NA | NA | 0 | (86) |
| Carrasco et
al, 2021 | R-IMMUNE | RC | IO+CRT | NA | NA | 23 | NA | 35 | NA | NA | (87) |
| Yuki et al,
2020 | EPOC1504-1 | RC (MSS) | IO+CRT | NA | 38 | 30 | NA | NA | 7 | 5 | (81) |
| Yuki et al,
2020 | EPOC1504-2 | RC (MSI-H) | IO+CRT | NA | NA | 60 | | | | | |
| Ho et al,
2021 | NCT03299946 | HCC | IO+VEGFR | 7 | 33 | 7 | 93 | 13 | NA | NA | (71) |
| Gao et al,
2019 | NCT02210117-2 | RCC | IO+VEGFR | 44 | NA | NA | NA | 42 | NA | NA | (75) |
| Karam et al,
2021 | NCT03680521 | RCC | IO+VEGFR | 10 | NA | NA | 100 | 45 | NA | NA | (76) |
| Bex et al,
2022 | NeoAvAx | RCC | IO+VEGFR | 30 | NA | NA | NA | NA | NA | NA | (78) |
| Wang et al,
2021 |
ChiCTR1900023880 | ESCC | IO+Chemotherapy+
VEGFR | NA | 50 | 23 | NA | 37 | NA | NA | (60) |
| Pusztai et
al, 2021 | I-SPY-2-2 | BC (HER2-) | IO+Chemotherapy+
PARP | NA | NA | 37 | NA | NA | 27 | 12 | (34) |
| Hu et al,
2022 | PICC-2 | CC
(dMMR/MSI-H) | IO+COX | NA | 94 | 88 | 65 | 6 | 65 | 6 | (88) |
| Rodriguez-Moreno
et al, 2020 | NEODURVARIB | MIBC | IO+PARP | 24 | NA | 45 | NA | NA | NA | NA | (95) |
| Wang et al,
2021 |
OrienX010-II-12 | Melanoma | IO+Virus | 33 | NA | 10 | 100 | 10 | NA | NA | (111) |
| Hyngstrom et
al, 2022 | HCI102346 | Melanoma | IO+Virus | 67 | NA | 83 | NA | NA | NA | NA | (112) |
| Najjar et
al, 2021 | NCT02339324 | Melanoma | IO+IFN | 73 | 57 | 43 | 100 | NA | NA | NA | (114) |
| Chalabi et
al, 2020 | NICHE-3 | CC(pMMR) | IO+IO+COX | NA | 14 | 0 | NA | NA | NA | NA | (80) |
The association between radiological and
pathological responses is shown in Fig. 4A and B. Patients with a higher ORR
were more likely to achieve both a higher MPR and pCR after
surgery. This link was more pronounced when the therapies were
combined, most notably when IO was combined with chemotherapy. MPR
and pCR are partially associated with long-term survival in various
cancer types (NSCLC, breast cancer and melanoma) (120-122). This aforementioned finding
indicates that patients with a higher ORR after neoadjuvant
immunotherapy may also have improved survival. Three clinical
trials [NCT02641093 (27),
NCT03081689 (44) and NCT02437279
(105)], demonstrated that
patients with a higher MPR after surgery had an improved DFS time
compared with those who had no MPR, implying that MPR (or pCR) may
replace DFS as a primary study objective in the future. However, a
caveat of these studies (27,44,105) was its small sample size.
Therefore, further research is required, and the association
between pathological response and survival benefits requires
further evaluation in ongoing clinical trials.
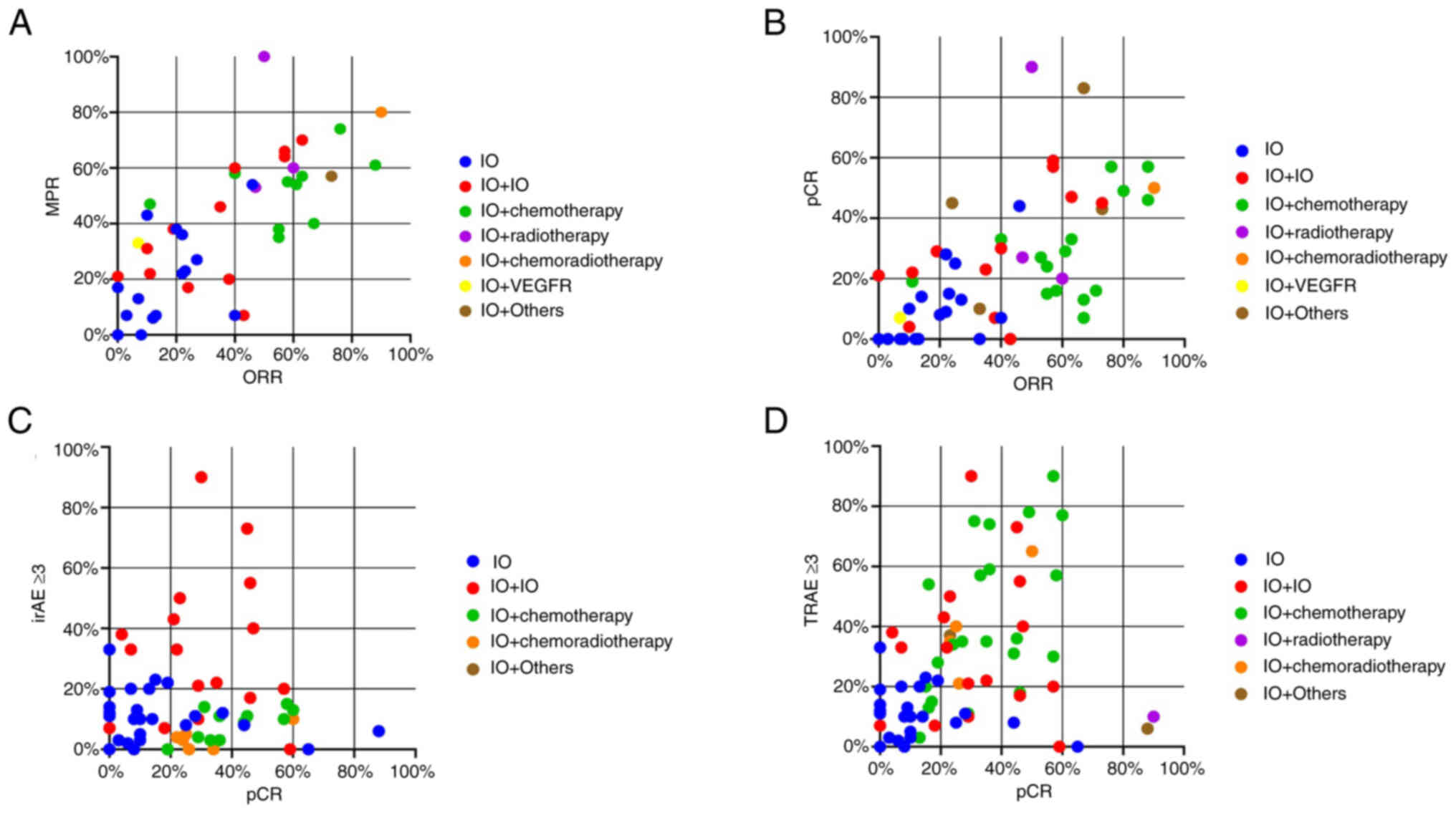 | Figure 4ORR, MPR, pCR and AEs (including
TRAEs and irAEs) of neoadjuvant immunotherapy. (A) ORR and MPR. (B)
ORR and pCR. (C) pCR and grade ≥3 irAEs. (D) pCR and grade ≥3
TRAEs. IO, immune checkpoint inhibitor alone (PD-1 inhibitor or
PD-L1 inhibitor alone); IO+IO, combination of PD-1/PD-L1 inhibitor
and cytotoxic T-lymphocyte associated protein 4 inhibitor or
lymphocyte activation gene-3 inhibitor; IO + chemotherapy,
combination of PD-1/PD-L1 inhibitor and chemotherapy; IO +
radiotherapy, combination of PD-1/PD-L1 inhibitor and radiotherapy;
IO + chemoradiotherapy, combination of PD-1/PD-L1 inhibitor and
chemotherapy plus radiotherapy; IO+VEGFR, combination of PD-1/PD-L1
inhibitor and angiogenesis inhibitor; IO + Others, combination of
PD-1/PD-L1 inhibitor and special drugs; irAE, immune-related
adverse event; MPR, major pathologic response; ORR, objective
response rate; pCR, pathologic complete response; PD-1, programmed
cell death protein 1; PD-L1, programmed death-ligand 1; TRAE,
treatment-related adverse event. |
As can be seen in Fig.
4C and D, in the neoadjuvant immune dual-drug regimen,
NCT02519322 (107) demonstrated
that when relatlimab [anti-lymphocyte activating 3 (LAG3)] was
combined with nivolumab, it resulted in more substantial antitumor
effects than the traditional dual-immunity regimen (nivolumab +
ipilimumab). Furthermore, no serious irAEs were observed in
melanoma. Since anti-LAG3 drugs produced higher efficacy with lower
side effects, they may be an improved PD-1 combination compared
with anti-CTLA4 drugs (107).
More prospective clinical trials are required to validate this
regimen. Additionally, it is hoped that the program will be
extended to other cancer types in the future. The PICC clinical
trial, which used toripalimab for CC with dMMR/MSI-H status in the
neoadjuvant setting, indicated strong antitumor efficacy and no
serious irAEs. The dMMR/MSI-H may be a useful immunotherapy
biomarker since cancer types with dMMR/MSI-H exhibit high efficacy
and low irAEs during immune monotherapy. Therefore, more beneficial
biomarkers should be investigated. By contrast, IO did not produce
antitumor effects for HPV-infected HNSCC (28), but when combined with chemotherapy
or radiotherapy, it produced a strong antitumor effect with mild
side effects (11,24), suggesting that, for some tumors
with specific biomarkers, combination immunotherapy is more
appropriate.
4. Adverse effects of neoadjuvant
immunotherapy
Although neoadjuvant immunotherapy leads to
improved tumor regression rates, its side effects should not be
ignored (Fig. S1). Patients with
bladder cancer and melanoma showed a higher rate of irAEs than
those with other cancer types; however, most irAEs were classified
as grade 1-2 irAEs. In addition, irAEs were more frequent for IO or
IO + IO than for IO + chemotherapy (Fig. S2). As far as the cancer type is
concerned, as shown in Fig. 2D,
patients with HCC, renal cancer and melanoma exhibited a higher
rate of grade ≥3 irAEs than those with breast cancer, esophageal
cancer and GEJ/GC. The reason for this discrepancy is that a
combined therapy (IO and a method that can directly kill tumor
cells) could lessen the severity of irAEs more than IO or IO + IO
(Fig. 3E). Patients with breast
and digestive tract cancer are typically treated with IO combined
therapy (5,54,68),
while patients with HCC, renal cancer and melanoma receive IO or IO
+ IO (72,105). We hypothesized that chemotherapy
not only kills tumor cells but also kills immune cells, resulting
in a reduction in irAEs. The NCT03985670 trial showed that a
delayed administration of toripalimab after NACT might generate a
higher pCR rate than a simultaneous injection (59). Although there was no significant
difference in TRAEs between the two groups, there may have been
some differences in irAEs. The question of whether the higher
efficacy of immunotherapy is directly related to higher incidence
of irAEs deserves further exploration. Chemotherapy plus
immunotherapy was associated with fewer irAEs than immunologic
monotherapy and binary immunotherapy for the same efficacy
(Fig. 4C). Treatment with IO plus
chemotherapy did not result in a higher incidence of TRAEs than
when chemotherapy was used alone (31,53).
Therefore, IO + chemotherapy improved the effect index (ORR, MPR
and pCR) and reduced the incidence of irAEs. Furthermore, the
overall TRAEs and serious TRAEs of IO + chemotherapy were not
significantly different from chemotherapy alone (6). This regimen's specific dose, sequence
and cycle should be explored further.
5. Comparison between anti-PD-1 and
anti-PD-L1 inhibitors in the neoadjuvant settings
It was attempted to discern whether PD-1 inhibitors
or PD-L1 inhibitors are more suitable for neoadjuvant
immunotherapy. In most solid cancer types, the efficacy of PD-1 and
PD-L1 monotherapy was not significantly different in terms of drug
efficacy; however, the incidence of irAEs caused by PD-L1 was
considerably lower than that of PD-1 (Fig. S3F). Notably, PD-L1 combination
chemotherapy achieved a higher pCR than PD-1 combination
chemotherapy and caused more TRAEs (Fig. S3). In lung cancer, PD-1
monotherapy had improved efficacy compared with PD-L1 monotherapy,
while there was no marked difference in the incidence of irAEs
between the two groups. This indicates that PD-1 immune monotherapy
was more beneficial in NAT for lung cancer. Similarly, PD-1
inhibitors combined with chemotherapy has a higher efficacy than
PD-L1 plus chemotherapy and resulted in fewer grade ≥3 TRAEs than
PD-L1 plus chemotherapy (Fig.
S4). Further prospective clinical trials are required to
provide corresponding evidence.
6. Perioperative complications of
neoadjuvant immunotherapy
In addition to the efficacy and side effects of
neoadjuvant immunotherapy, the present review also analyzed
surgical operations and perioperative complications, an aspect
critical for surgeons. As shown in Table II, treatment-related surgery
delays were not reported in most trials. Furthermore, some patients
did not undergo surgery after neoadjuvant immunotherapy due to
disease progression, severe TRAEs or high surgical risks, or
declined surgery (23,45). In terms of perioperative
complications, it is not easy to evaluate whether they are due to
immunotherapy or surgery. Taking lung cancer as an example, an air
leak, which is the most common perioperative complication after
neoadjuvant immunotherapy, is also a major postoperative
complication after lung surgery (50). Additionally, PD-1/PD-L1 inhibitor
in combination with a CTLA4 inhibitor as NAT may increase the rate
of postoperative complications compared with IO, as shown in the
NEOSTAR study (50). However,
because the difficulty of intraoperative resection is subjective
and there is a lack of quantitative indicators, it was not possible
to obtain this indicator in the literature. Previous studies on
lung cancer have demonstrated that immunotherapy may induce severe
tissue reactions, which can lead to dense fibrosis, thus becoming a
technical challenge for surgeons (123,124). However, this phenomenon was not
observed in two recent trials on esophageal cancer (58,64).
This discrepancy suggests that the immunotherapy may produce
different responses depending on which tissue the tumor is located
in.
 | Table IIPerioperative outcomes of the
clinical trials. |
Table II
Perioperative outcomes of the
clinical trials.
| First author/s,
year | Study name | Tumor | Type | Treatment-related
surgical delay, n | Postoperative
complications, n | Not radical
resection, n | No surgery, n | (Refs.) |
|---|
| Schoenfeld et
al, 2020 | NCT02919683-1 | OCSCC | IO | 0 | 5 | NA | 0 | (20) |
| Ferrarotto et
al, 2020 | CIAO-1 | OPSCC | IO | NA | NA | NA | 0 | (21) |
| Uppaluri et
al, 2020 | NCT02296684-1 | HNSCC | IO | 0 | NA | NA | 0 | (22) |
| Uppaluri et
al, 2021 | NCT02296684-2 | HNSCC | IO | NA | NA | NA | 1 | (23) |
| Vos et al,
2021 | IMCISION-1 | HNSCC | IO | 0 | 6 | 0 | 0 | (25) |
| Knochelmann et
al, 2021 | NCT03021993 | OCSCC | IO | 0 | NA | 0 | 0 | (26) |
| Wise-Draper et
al, 2021 | NCT02641093 | HNSCC | IO | NA | NA | NA | NA | (27) |
| Ferris et
al, 2021 | CheckMate358 | HNSCC (HPV+) | IO | 0 | NA | NA | 8 | (28) |
| Ferris et
al, 2021 | | HNSCC (HPV-) | | 0 | NA | NA | 7 | |
| Reuss et al,
2020 |
checkmate-159-1 | NSCLC | IO | 0 | 10 | 1 | 0 | (40) |
| Bar et al,
2019 | MK3475-223 | NSCLC | IO | NA | NA | NA | NA | (42) |
| Gao et al,
2020 |
ChiCTR-OIC-17013726 | NSCLC | IO | 2 | NA | 1 | 3 | (45) |
| Besse et al,
2020 | PRINCEPS | NSCLC | IO | 0 | 3 | 1 | 0 | (46) |
| Lee et al,
2021 | LCMC3 | NSCLC | IO | NA | NA | 14 | 22 | (47) |
| Eichhorn et
al, 2021 | NEOMUN | NSCLC | IO | 1 | 1 | 0 | 0 | (49) |
| Cascone et
al, 2021 | NEOSTAR-1 | NSCLC | IO | NA | NA | 0 | 2 | (50) |
| Altorki et
al, 2021 | NCT02904954-1 | NSCLC | IO | 1 | NA | 3 | 4 | (51) |
| Tong et al,
2022 | TOP 1501 | NSCLC | IO | 1 | 12 | 3 | 5 | (52) |
| Kaseb et al,
2022 | NCT03222076-1 | HCC | IO | 0 | NA | NA | 4 | (73) |
| Marron et
al, 2022 | NCT03916627 | HCC | IO | 1 | NA | NA | 1 | (74) |
| Gao et al,
2019 | NCT02210117-1 | RCC | IO | NA | NA | NA | NA | (75) |
| Gorin et al,
2022 | NCT02575222 | RCC | IO | 0 | NA | NA | 0 | (77) |
| Carlo et al,
2022 | NCT02595918 | RCC | IO | 0 | 4 | NA | 0 | (79) |
| Avallone et
al, 2020 | NICOLE | CC | IO | 0 | 0 | NA | 0 | (82) |
| Hu et al,
2022 | PICC-1 | CC
(dMMR/MSI-H) | IO | 0 | 3 | 0 | 0 | (88) |
| Necchi et
al, 2020 | PURE-01 | MIBC (VH) | IO | NA | NA | NA | 2 | (89) |
| Necchi et
al, 2020 | | MIBC | | NA | NA | NA | | |
| Powles et
al, 2019 and Szabados et al, 2021 | ABACUS | MIBC | IO | 0 | 53 | NA | 8 | (90,91) |
| Wei et al,
2020 | BLASST-2 | MIBC | IO | NA | NA | NA | 2 | (93) |
Natesan et
al, 2021
Natesan et al, 2021
Natesan et al, 2021 | NCT02451423 | MIBC (Cis-) | IO | 0 | NA | NA | 0 | (100) |
| Grivas et
al, 2021 | PrE0807 | MIBC (Cis-) | IO | 0 | NA | NA | 1 | (101) |
| Amaria et
al, 2018 | NCT02519322-1 | Melanoma | IO | NA | NA | NA | NA | (106) |
| Huang et al,
2018; Huang et al, 2019 | NCT02434354 | Melanoma | IO | 0 | NA | NA | 0 | (109,110) |
| Topalian et
al, 2020 | CheckMate358 | MCC | IO | 1 | NA | NA | 3 | (115) |
| Schoenfeld et
al, 2020 | NCT02919683-2 | OCSCC | IO+IO | 0 | 9 | NA | 0 | (20) |
| Ferrarotto et
al, 2020 | CIAO-2 | OPSCC | IO+IO | NA | NA | NA | 0 | (21) |
| Vos et al,
2021 | IMCISION-2 | HNSCC | IO+IO | 0 | 24 | 1 | 3 | (25) |
| Reuss et al,
2020 |
checkmate-159-2 | NSCLC | IO+IO | 0 | NA | NA | 3 | (41) |
| Cascone et
al, 2021 | NEOSTAR-2 | NSCLC | IO+IO | NA | NA | 0 | 5 | (50) |
| Su et al,
2021 | NCT03510871 | HCC | IO+IO | NA | NA | NA | 14 | (72) |
| Kaseb et al,
2022 | NCT03222076-2 | HCC | IO+IO | 0 | NA | NA | 3 | (73) |
| Gao et al,
2019 | NCT02210117-3 | RCC | IO+IO | NA | NA | NA | NA | (75) |
| Chalabi et
al, 2020 | NICHE-1 | CC (dMMR) | IO+IO | 0 | NA | 0 | 0 | (80) |
| Chalabi et
al, 2020 | NICHE-2 | CC (pMMR) | IO+IO | 0 | NA | 0 | 0 | |
| Gao et al,
2020 | NCT02812420 | MIBC (Cis-) | IO+IO | 2 | NA | NA | 4 | (92) |
| Grande et
al, 2020 | DUTRENEO | MIBC (Cis+) | IO+IO | NA | NA | NA | 3 | (94) |
| van Dijk et
al, 2020 | NABUCCO | MIBC | IO+IO | 1 | NA | NA | 0 | (98) |
| Van Dorp et
al, 2021 | | | | 2 | NA | NA | 1 | (99) |
| Grivas et
al, 2021 | PrE0807 | MIBC (Cis-) | IO+IO | 0 | NA | NA | 0 | (101) |
| Blank et al,
2018 | OpACIN | Melanoma | IO+IO | 0 | NA | NA | 0 | (105) |
| Amaria et
al, 2018 | NCT02519322-2 | Melanoma | IO+IO | NA | NA | NA | NA | (106) |
| Amaria et
al, 2021 | NCT02519322-3 | Melanoma | IO+IO | NA | NA | NA | 1 | (107) |
| Rozeman et
al, 2019 | OpACIN-neo | Melanoma | IO+IO | 1 | 21 | NA | 0 | (108) |
| Rozeman et
al, 2019 | | | | 0 | 19 | | 0 | |
| Rozeman et
al, 2019 | | | | 2 | 18 | | 1 | |
| Cocorocchio et
al, 2021 | EudraCT
2018-002172-40 | Melanoma | IO+IO | NA | NA | NA | 3 | (113) |
| Zinner et
al, 2020 | NCT03342911 | HNSCC |
IO+Chemotherapy | NA | NA | 0 | 0 | (11) |
| Loibl et al,
2019 | GeparNuevo | TNBC |
IO+Chemotherapy | NA | NA | NA | NA | (12) |
| Schmid et
al, 2020 | KEYNOTE-173 | TNBC |
IO+Chemotherapy | NA | NA | NA | NA | (29) |
| Foldi et al,
2021 | NCT02489448 | TNBC |
IO+Chemotherapy | NA | 0 | NA | NA | (30) |
| Mittendorf et
al, 2020 | IMpassion031 | TNBC |
IO+Chemotherapy | 1 | NA | NA | 11 | (31) |
| Schmid et
al, 2020 | KEYNOTE-522 | TNBC |
IO+Chemotherapy | NA | NA | NA | NA | (32) |
| Nanda et al,
2020 | I-SPY-2 -1 | BC (HER2-) |
IO+Chemotherapy | NA | NA | NA | 2 | (33) |
| Yam et al,
2021 | NCT02530489 | TNBC |
IO+Chemotherapy | NA | NA | NA | NA | (35) |
| Gianni et
al, 2022 | NeoTRIPaPDL1 | TNBC |
IO+Chemotherapy | NA | NA | NA | 5 | (37) |
| Dieci et al,
2022 | GIADA | BC |
IO+Chemotherapy | NA | NA | NA | 0 | (38) |
| Shu et al,
2020 | NCT02716038 | NSCLC |
IO+Chemotherapy | 0 | 0 | 3 | 1 | (43) |
| Provencio et
al, 2020 | NADIM | NSCLC |
IO+Chemotherapy | 0 | 12 | 0 | 5 | (44) |
| Rothschild et
al, 2021 | SAKK 16/14 | NSCLC |
IO+Chemotherapy | NA | NA | 4 | 7 | (13) |
| Zhao et al,
2021 | NeoTAP01 | NSCLC |
IO+Chemotherapy | 0 | NA | 1 | 3 | (48) |
| Forde et al,
2022 | CheckMate 816 | NSCLC |
IO+Chemotherapy | 6 | 62 | 25 | 30 | (53) |
| Gu et al,
2020 | KEEP-G 03 | ESCC |
IO+Chemotherapy | 0 | NA | 0 | 0 | (54) |
| Zhang et al,
2021 | ESONICT-1 | ESCC |
IO+Chemotherapy | 0 | NA | 0 | 7 | (58) |
| Xing et al,
2021 | NCT03985670 | ESCC |
IO+Chemotherapy | NA | NA | 0 | 4 | (59) |
| Xing et al,
2021 | | | | NA | NA | 0 | 2 | |
| Xu et al,
2022 | NCT04506138 | ESCC |
IO+Chemotherapy | NA | NA | 0 | 9 | (61) |
| Liu et al,
2022 | NIC-ESCC2019 | ESCC |
IO+Chemotherapy | NA | 14 | 0 | 5 | (62) |
| He et al,
2022 | NCT04177797 | ESCC |
IO+Chemotherapy | 4 | NA | 2 | 0 | (63) |
| Liu et al,
2022 |
ChiCTR1900026240 | ESCC |
IO+Chemotherapy | 8 | 24 | 1 | 9 | (60) |
| Liu et al,
2020 | NCT03939962 | G/GEJC |
IO+Chemotherapy | 0 | NA | 2 | 1 | (66) |
| Sun et al,
2022 | NCT03488667 | G/GEJC |
IO+Chemotherapy | NA | NA | 0 | 1 | (68) |
| Tao et al,
2022 | NCT04890392 | G/GEJC |
IO+Chemotherapy | NA | NA | NA | NA | (69) |
| Jiang et al,
2022 | NCT04065282 | G/GEJC |
IO+Chemotherapy | 4 | NA | 1 | 0 | (70) |
| Cathomas et
al, 2021 | SAKK 06/17 | MIBC |
IO+Chemotherapy | NA | NA | 1 | 5 | (102) |
| Rose et al,
2021 | LCCC 1520 | MIBC |
IO+Chemotherapy | 1 | NA | 4 | 1 | (103) |
| Funt et al,
2022 | NCT02989584 | MIBC |
IO+Chemotherapy | 0 | 32 | NA | 8 | (104) |
| Hoimes et
al, 2020 | GU14-188 | MIBC (Cis-) |
IO+Chemotherapy | NA | NA | NA | 4 | (96) |
| Kaimakliotis et
al, 2020 | | MIBC (Cis-) |
IO+Chemotherapy | NA | NA | NA | 3 | (97) |
| Leidner et
al, 2021 | NIRT-HNC | HNSCC (HPV+) | IO+Radio | 0 | NA | NA | 0 | (24) |
| Leidner et
al, 2021 | | HNSCC (HPV-) | | 0 | | | 0 | |
| Altorki et
al, 2021 | NCT02904954-2 | NSCLC | IO+Radio | 1 | NA | 1 | 4 | (51) |
| McArthur et
al, 2021 | NCT03366844 | TNBC | IO+CRT | 0 | NA | NA | 0 | (36) |
| Li et al,
2020 | NCT03604991 | ESCC | IO+CRT | 0 | NA | NA | 2 | (55) |
| Li et al,
2021 | PALACE-1 | ESCC | IO+CRT | 0 | NA | 1 | 2 | (56) |
| van den Ende et
al, 2021 | PERFECT | EA | IO+CRT | 0 | NA | 0 | 7 | (57) |
| Kelly et al,
2019 | NCT03044613 | E/GEJC | IO+CRT | 0 | NA | NA | 0 | (65) |
| Shah et al,
2021 | NCT02998268 | E/GEJC | IO+CRT | NA | NA | NA | NA | (67) |
| Yuki et al,
2020 | EPOC1504 | RC (MSS) | IO+CRT | NA | NA | NA | NA | (81) |
| Yuki et al,
2020 | | RC (MSI-H) | | | | | | |
| Rahma et al,
2021 | NRG-GI002 | RC | IO+CRT | NA | NA | 4 | 21 | (83) |
| Salvatore et
al, 2021 | AVANA | RC | IO+CRT | NA | NA | NA | NA | (84) |
| Tamberi et
al, 2021 | PANDORA | RC | IO+CRT | NA | NA | NA | 1 | (85) |
| Shamseddine et
al, 2021 | Averectal | RC | IO+CRT | NA | NA | NA | 4 | (86) |
| Carrasco et
al, 2021 | R-IMMUNE | RC | IO+CRT | NA | NA | NA | NA | (87) |
| Gao et al,
2019 | NCT02210117-2 | RCC | IO+VEGFR | NA | NA | NA | NA | (75) |
| Karam et al,
2021 | NCT03680521 | RCC | IO+VEGFR | 1 | NA | NA | NA | (76) |
| Bex et al,
2022 | NeoAvAx | RCC | IO+VEGFR | 0 | NA | NA | NA | (78) |
| Ho et al,
2021 | NCT03299946 | HCC | IO+VEGFR | 0 | NA | 0 | 3 | (71) |
| Hu et al,
2022 | PICC-2 | CC
(dMMR/MSI-H) | IO+COX | 0 | 2 | 0 | 0 | (88) |
| Rodriguez-Moreno
et al, 2020 | NEODURVARIB | MIBC | IO+PARP | NA | NA | NA | 3 | (95) |
| Wang et al,
2021 |
OrienX010-II-12 | Melanoma | IO+virus | NA | NA | NA | 3 | (111) |
| Hyngstrom et
al, 2022 | HCI102346 | Melanoma | IO+virus | NA | NA | 1 | 0 | (112) |
| Najjar et
al, 2021 | NCT02339324 | Melanoma | IO+IFN | 0 | NA | NA | 0 | (114) |
| Pusztai et
al, 2021 | I-SPY-2 -2 | BC (HER2-) |
IO+PARP+Chemo | NA | NA | NA | 1 | (34) |
| Chalabi et
al, 2020 | NICHE-3 | CC (pMMR) | IO+IO+COX | 0 | NA | 0 | 0 | (80) |
| Wang et
al, 2021 |
ChiCTR1900023880 | ESCC | IO+Chemotherapy+
VEGFR | 5 | NA | NA | 1 | (60) |
7. Other concerns regarding neoadjuvant
immunotherapy
Other concerns remain regarding the concept of
neoadjuvant immunotherapy. First, because draining lymph nodes are
critical for T cell priming and antigen presentation (125), there is a discussion about
whether to use lymph node resection as the standard criteria
(126). Second, the dose/regiment
of immunotherapy should be recommended before surgery, and it
should consider antitumor efficacy and tolerability (22,23,127). The OpACIN-neo study can explain
this problem to some extent. In this study, in group B, a tolerable
neoadjuvant dosing regimen (two cycles of 1 mg/kg ipilimumab plus 3
mg/kg nivolumab) that might be suitable for broader clinical use
was identified (108). However,
the NABUCCO study suggested that ipilimumab (3 mg/kg) + nivolumab
(1 mg/kg) is more effective than ipilimumab (1 mg/kg) + nivolumab
(3 mg/kg) as a neoadjuvant treatment for stage III UC (99). Another clinical trial (NCT02296684)
demonstrated that two cycles of pembrolizumab caused a stronger
pathological response than one cycle (23). Thirdly, an important question
remaining is if adjuvant immunotherapy is still necessary if
neoadjuvant immunotherapy has been applied.
Although immunotherapy has a limited efficacy in
advanced NSCLC with driver gene mutation (128,129), the clinical value of neoadjuvant
immunotherapy in these patients warrants further discussion. Most
clinical trials of neoadjuvant immunotherapy exclude driver
gene-positive patients (45,68),
since immunotherapy is less effective than corresponding targeted
therapy in advanced driver gene-positive NSCLC (130). Therefore, it was not possible to
obtain large-scale clinical data to explore the link between
neoadjuvant immunotherapy and driver gene-positivity. However, two
exploratory studies (43,44) demonstrated that neoadjuvant
immunotherapy plus chemotherapy could obtain a good pathological
response rate for patients with EGFR mutations, although the sample
size was small, providing evidence for the use of neoadjuvant
immunotherapy plus chemotherapy in patients with driver
gene-positive NSCLC. Furthermore, a retrospective multi-center
study (131) suggested that 39 of
40 patients with NSCLC with driver gene mutation achieved a R0
resection rate of 97.4% after neoadjuvant immunotherapy. The MPR
and pCR rates were 37.5 and 12.5%, respectively, which was superior
to those of patients with EGFR-mutant NSCLC receiving the EGFR
tyrosine kinase inhibitor erlotinib in the CTONG1103 study. This
study (131) indicated the
potential clinical feasibility of neoadjuvant immunotherapy for
resectable oncogene-mutant NSCLC, especially for EGFR-mutant
NSCLC.
Furthermore, a few of the neoadjuvant immunotherapy
studies selected patients based on biomarkers. The CM-159 study
showed that patients with NSCLC with a higher tumor mutation burden
(TMB) exhibited a higher rate of MPR (39), indicating that TMB might be a
potential predictor of a higher pathological response. The PICC
study demonstrated that patients with CC with dMMR or MSI-H had a
100% rate of MPR in the toripalimab monotherapy group. Therefore,
it is critical to investigate further biomarkers related to
neoadjuvant immunotherapy in selected patient cohorts (e.g., TMB,
MSI-H, PD-L1 or CD8). Considering the long follow-up time for
clinical trials of NAT and since MPR indicates an improved DFS time
and OS time in some retrospective analyses (17,18),
it should be clarified whether MPR could replace DFS or OS as the
primary study endpoint. Although current preclinical studies do not
fully reflect the situation in humans (15), they may help explore the optimal
treatment regimens and strategies for neoadjuvant immunotherapy.
Furthermore, in a mouse model of mammary cancer, four additional
adjuvant doses of immunotherapy did not significantly improve the
overall tumor-free survival if neoadjuvant immunotherapy was
administered prior to surgery (132). The NCT03985670 study showed that
the efficiency of chemotherapy and toripalimab varied depending on
the sequence of drug use that was applied during NAT (59). These studies demonstrated that
neoadjuvant immunotherapy was of value and emphasized that the
timing of neoadjuvant immunotherapy for surgery is crucial to the
outcome.
8. Conclusions
In conclusion, neoadjuvant immunotherapy is
effective, safe and feasible for patients harboring resectable
tumors (28,41,100). Compared with the traditional
neoadjuvant chemo- and radiotherapy, neoadjuvant immunotherapy
appears to lead to a higher pathological remission rate, especially
for IO combination regimens, and is well tolerated with an
acceptable rate of perioperative complications. However, increased
vigilance is still necessary concerning the severe TRAEs, as well
as increased surgical risk and difficulty after neoadjuvant
immunotherapy. Neoadjuvant immunotherapy alone or in combination
prolonged PFS compared with NACT alone, and although not
significant in some studies (27,105), we hypothesized that this is a
good trend and that the benefit of PFS will eventually translate
into OS benefits. Neoadjuvant immunotherapy not only prolongs the
duration of tumor recurrence, but also reduces tumor burden, and
reduces the extent of surgical resection. Thus, neoadjuvant
immunotherapy can transform inoperable patients into operable
patients. Based on the current data, the neoadjuvant immunotherapy
regimen is not yet mature; however, CheckMate816 (53) has been used as the only approved
neoadjuvant immunotherapy regimen for NSCLC, and more updated
research results to support this result and expand this to other
cancer types are anticipated. In the novel era of immunotherapy,
exploring the best NAT strategies to balance toxicity and efficacy
is necessary. Further studies are required to evaluate the
long-term survival benefit of neoadjuvant immunotherapy.
Supplementary Data
Availability of data and materials
Not applicable.
Authors' contributions
LP and SX were responsible for the study conception
and design. Administrative support and study design were provided
by ZS and JC. QT, SZ and NZ collected and assembled the data, and
QT, SZ and NZ were responsible for data analysis and
interpretation. JH, LZ and TL contributed to the revision of the
review. All authors helped to write the manuscript. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (82172776), Tianjin Science and Technology Plan
Project (19ZXDBSY00060 and 303078100412), Tianjin Key Medical
Discipline (Specialty) Construction Project (TJYXZDXK-061B) and
Diversified Input Project of Tianjin National Natural Science
Foundation (21JCYBJC01770).
Abbreviations:
|
ORR
|
objective response rate
|
|
MPR
|
major pathologic response
|
|
pCR
|
pathologic complete response
|
|
TRAE
|
treatment-related adverse event
|
|
irAE
|
immune-related adverse event
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
NACT
|
neoadjuvant chemotherapy
|
|
ITT
|
intention-to-treat
|
|
dMMR
|
mismatch repair-deficient
|
|
pMMR
|
mismatch repair-proficient
|
|
MSI-H
|
microsatellite instability-high
|
|
MSS
|
microsatellite stability
|
|
TMB
|
tumor mutation burden
|
|
HR
|
hormone receptor
|
|
SOC
|
standard-of-care treatment
|
|
DLT
|
dose-limiting toxicities
|
|
NSCLC
|
non-small cell lung cancer
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
OCSCC
|
oral cavity squamous cell
carcinoma
|
|
TNBC
|
triple-negative breast cancer
|
|
ESCC
|
esophageal squamous cell
carcinoma
|
|
EAC
|
esophageal adenocarcinoma
|
|
HCC
|
hepatocellular carcinoma
|
|
RCC
|
renal cell carcinoma
|
|
CC
|
colorectal cancer
|
|
MIBC
|
muscle-invasive bladder carcinoma
|
|
UC
|
urothelial cancer MCC, Merkel cell
carcinoma
|
References
|
1
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality Worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garon EB, Hellmann MD, Rizvi NA, Carcereny
E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L,
et al: Five-Year overall survival for patients with advanced
non-small-cell lung cancer treated with pembrolizumab: Results from
the phase I KEYNOTE-001 Study. J Clin Oncol. 37:2518–2527. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar
|
|
5
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Licitra L and Vermorken JB: Is there still
a role for neoadjuvant chemotherapy in head and neck cancer? Ann
Oncol. 15:7–11. 2004. View Article : Google Scholar
|
|
7
|
Wang H and Mao X: Evaluation of the
efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des
Devel Ther. 14:2423–2433. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salvà F and Felip E: Neoadjuvant
chemotherapy in early-stage non-small cell lung cancer. Transl Lung
Cancer Res. 2:398–402. 2013.PubMed/NCBI
|
|
9
|
Peyton CC, Tang D, Reich RR, Azizi M,
Chipollini J, Pow-Sang JM, Manley B, Spiess PE, Poch MA, Sexton WJ,
et al: Downstaging and survival outcomes associated with
neoadjuvant chemotherapy regimens among patients treated with
cystectomy for muscle-invasive bladder cancer. JAMA Oncol.
4:1535–1542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poggio F, Bruzzone M, Ceppi M, Pondé NF,
La Valle G, Del Mastro L, de Azambuja E and Lambertini M:
Platinum-based neoadjuvant chemotherapy in triple-negative breast
cancer: A systematic review and meta-analysis. Ann Oncol.
29:1497–1508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zinner R, Johnson JM, Tuluc M, Curry JM,
Luginbuhl A, Fundakowski CC, Yampolsky A, Goldman RR, Solomides CC,
Mardekian S, et al: Neoadjuvant nivolumab (N) plus weekly
carboplatin (C) and paclitaxel (P) outcomes in HPV(-) resectable
locally advanced head and neck cancer. Ann Oncol. 31(Suppl 4):
S6822020. View Article : Google Scholar
|
|
12
|
Loibl S, Untch M, Burchardi N, Huober J,
Sinn BV, Blohmer JU, Grischke EM, Furlanetto J, Tesch H, Hanusch C,
et al: A randomised phase II study investigating durvalumab in
addition to an anthracycline taxane-based neoadjuvant therapy in
early triple-negative breast cancer: Clinical results and biomarker
analysis of GeparNuevo study. Ann Oncol. 30:1279–1288. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rothschild SI, Zippelius A, Eboulet EI,
Savic Prince S, Betticher D, Bettini A, Früh M, Joerger M,
Lardinois D, Gelpke H, et al: SAKK 16/14: Durvalumab in addition to
neoadjuvant chemotherapy in patients with Stage IIIA(N2)
Non-small-cell lung cancer-a multicenter single-arm phase II trial.
J Clin Oncol. 39:2872–2880. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pauken KE, Sammons MA, Odorizzi PM, Manne
S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al:
Epigenetic stability of exhausted T cells limits durability of
reinvigoration by PD-1 blockade. Science. 354:1160–1165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Blake SJ, Yong MC, Harjunpää H,
Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ and
Teng MW: Improved efficacy of neoadjuvant compared to adjuvant
immunotherapy to eradicate metastatic disease. Cancer Discov.
6:1382–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
17
|
Hellmann MD, Chaft JE, William WN Jr,
Rusch V, Pisters KM, Kalhor N, Pataer A, Travis WD, Swisher SG and
Kris MG: Pathological response after neoadjuvant chemotherapy in
resectable non-small-cell lung cancers: Proposal for the use of
major pathological response as a surrogate endpoint. Lancet Oncol.
15:e42–e50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Dacic S, Wistuba I, Sholl L,
Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, et
al: IASLC Multidisciplinary recommendations for pathologic
assessment of lung cancer resection specimens after neoadjuvant
therapy. J Thorac Oncol. 15:709–740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pataer A, Kalhor N, Correa AM, Raso MG,
Erasmus JJ, Kim ES, Behrens C, Lee JJ, Roth JA, Stewart DJ, et al:
Histopathologic response criteria predict survival of patients with
resected lung cancer after neoadjuvant chemotherapy. J Thorac
Oncol. 7:825–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schoenfeld JD, Hanna GJ, Jo VY, Rawal B,
Chen YH, Catalano PS, Lako A, Ciantra Z, Weirather JL, Criscitiello
S, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab in
untreated oral cavity squamous cell carcinoma: A phase 2 open-label
randomized clinical trial. JAMA Oncol. 6:1563–1570. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrarotto R, Bell D, Rubin ML, Hutcheson
KA, Johnson JM, Goepfert RP, Phan J, Elamin YY, Torman DK, Warneke
CL, et al: Impact of Neoadjuvant durvalumab with or without
tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy
in patients with oropharynx cancer: CIAO trial results. Clin Cancer
Res. 26:3211–3219. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uppaluri R, Campbell KM, Egloff AM,
Zolkind P, Skidmore ZL, Nussenbaum B, Paniello RC, Rich JT, Jackson
R, Pipkorn P, et al: Neoadjuvant and adjuvant pembrolizumab in
resectable locally advanced, human papillomavirus-unrelated head
and neck cancer: A multicenter, Phase II trial. Clin Cancer Res.
26:5140–5152. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uppaluri R, Chernock R, Mansour M, Jackson
R, Rich J, Pipkorn P, Paniello RC, Puram S, Zevallos JP, Annino DJ,
et al: Enhanced pathologic tumor response with two cycles of
neoadjuvant pembrolizumab in surgically resectable, locally
advanced HPV-negative head and neck squamous cell carcinoma
(HNSCC). J Clinical Oncol. 39:60082021. View Article : Google Scholar
|
|
24
|
Leidner R, Crittenden M, Young K, Xiao H,
Wu Y, Couey MA, Patel AA, Cheng AC, Watters AL, Bifulco C, et al:
Neoadjuvant immunoradiotherapy results in high rate of complete
pathological response and clinical to pathological downstaging in
locally advanced head and neck squamous cell carcinoma. J
Immunother Cancer. 9:e0024852021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vos JL, Elbers JBW, Krijgsman O, Traets
JH, Qiao X, Leun AMV, Lubeck Y, Seignette IM, Smit LA, Willems SM,
et al: Neoadjuvant immunotherapy with nivolumab and ipilimumab
induces major pathological responses in patients with head and neck
squamous cell carcinoma. Nature Commun. 12:73482021. View Article : Google Scholar
|
|
26
|
Knochelmann HM, Horton JD, Liu S, Armeson
K, Kaczmar JM, Wyatt MM, Richardson MS, Lomeli SH, Xiong Y,
Graboyes EM, et al: Neoadjuvant presurgical PD-1 inhibition in oral
cavity squamous cell carcinoma. Cell Rep Medicine. 2:1004262021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wise-Draper TM, Takiar V, Mierzwa ML,
Casper K, Palackdharry S, Worden FP, Old MO, Dunlap NE, Kaczmar JM,
Patil Y, et al: Association of pathological response to neoadjuvant
pembrolizumab with tumor PD-L1 expression and high disease-free
survival (DFS) in patients with resectable, local-regionally
advanced, head and neck squamous cell carcinoma (HNSCC). J Clinical
Oncol. 39:60062021. View Article : Google Scholar
|
|
28
|
Ferris RL, Spanos WC, Leidner R, Gonçalves
A, Martens UM, Kyi C, Sharfman W, Chung CH, Devriese LA, Gauthier
H, et al: Neoadjuvant nivolumab for patients with resectable
HPV-positive and HPV-negative squamous cell carcinomas of the head
and neck in the CheckMate 358 trial. J Immunother Cancer.
9:e0025682021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid P, Salgado R, Park YH,
Muñoz-Couselo E, Kim SB, Sohn J, Im SA, Foukakis T, Kuemmel S, Dent
R, et al: Pembrolizumab plus chemotherapy as neoadjuvant treatment
of high-risk, early-stage triple-negative breast cancer: Results
from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann
Oncol. 31:569–581. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foldi J, Silber A, Reisenbichler E, Singh
K, Fischbach N, Persico J, Adelson K, Katoch A, Horowitz N, Lannin
D, et al: Neoadjuvant durvalumab plus weekly nab-paclitaxel and
dose-dense doxorubicin/cyclophosphamide in triple-negative breast
cancer. NPJ Breast Cancer. 7:92021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early-stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai
L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al:
Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic
complete response in women with early-stage breast cancer: An
analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial.
JAMA Oncol. 6:676–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pusztai L, Yau C, Wolf DM, Han HS, Du L,
Wallace AM, String-Reasor E, Boughey JC, Chien AJ, Elias AD, et al:
Durvalumab with olaparib and paclitaxel for high-risk HER2-negative
stage II/III breast cancer: Results from the adaptively randomized
I-SPY2 trial. Cancer cell. 39:989–998.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yam C, Mittendorf EA, Sun R, Huo L,
Damodaran S, Rauch GM, Candelaria RP, Adrada BE, Seth S, Symmans
WF, et al: Neoadjuvant atezolizumab (atezo) and nab-paclitaxel
(nab-p) in patients (pts) with triple-negative breast cancer (TNBC)
with suboptimal clinical response to doxorubicin and
cyclophosphamide (AC). J Clin Oncol. 39:5922021. View Article : Google Scholar
|
|
36
|
McArthur H, Shiao S, Karlan S, Amersi F,
Arnold B, Basho R, Burnison M, Chung A, Chung C, Dang C, et al:
Abstract P3-09-09: Pre-operative pembrolizumab (Pembro) with
radiation therapy (RT) in patients with operable triple-negative
breast cancer (TNBC). Cancer Res. 81:P3-09-092021. View Article : Google Scholar
|
|
37
|
Gianni L, Huang CS, Egle D, Bermejo B,
Zamagni C, Thill M, Anton A, Zambelli S, Bianchini G, Russo S, et
al: Pathologic complete response (pCR) to neoadjuvant treatment
with or without atezolizumab in triple-negative, early high-risk
and locally advanced breast cancer: NeoTRIP Michelangelo randomized
study. Ann Oncol. 33:534–543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dieci MV, Guarneri V, Tosi A, Bisagni G,
Musolino A, Spazzapan S, Moretti G, Vernaci GM, Griguolo G,
Giarratano T, et al: Neoadjuvant chemotherapy and immunotherapy in
luminal b-like breast cancer: Results of the phase II GIADA trial.
Clin Cancer Res. 28:308–317. 2022. View Article : Google Scholar
|
|
39
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bott MJ, Yang SC, Park BJ, Adusumilli PS,
Rusch VW, Isbell JM, Downey RJ, Brahmer JR, Battafarano R, Bush E,
et al: Initial results of pulmonary resection after neoadjuvant
nivolumab in patients with resectable non-small cell lung cancer. J
Thorac Cardiovasc Surg. 158:269–276. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reuss JE, Anagnostou V, Cottrell TR, Smith
KN, Verde F, Zahurak M, Lanis M, Murray JC, Chan HY, McCarthy C, et
al: Neoadjuvant nivolumab plus ipilimumab in resectable non-small
cell lung cancer. J Immunother Cancer. 8:e0012822020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bar J, Urban D, Ofek E, Ackerstein A,
Redinsky I, Golan N, Kamer I, Simansky D, Onn A, Raskin S, et al:
Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell
lung cancer (NSCLC): Updated report of a phase i study, MK3475-223.
J Clin Oncol. 37:8534. 2019. View Article : Google Scholar
|
|
43
|
Shu CA, Gainor JF, Awad MM, Chiuzan C,
Grigg CM, Pabani A, Garofano RF, Stoopler MB, Cheng SK, White A, et
al: Neoadjuvant atezolizumab and chemotherapy in patients with
resectable non-small-cell lung cancer: An open-label, multicentre,
single-arm, phase 2 trial. Lancet Oncol. 21:786–795. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro, Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao S, Li N, Gao S, Xue Q, Ying J, Wang S,
Tao X, Zhao J, Mao Y, Wang B, et al: Neoadjuvant PD-1 inhibitor
(Sintilimab) in NSCLC. J Thorac Oncol. 15:816–826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Besse B, Adam J, Cozic N, Chaput N,
Planchard D, Mezquita L, Mezquita JR, Mezquita P, Mezquita C,
Mezquita A, et al: 1215O-SC Neoadjuvant atezolizumab (A) for
resectable non-small cell lung cancer (NSCLC): Results from the
phase II PRINCEPS trial. Ann Oncol. 31(Suppl): S794–S795. 2020.
View Article : Google Scholar
|
|
47
|
Lee J, Chaft J, Nicholas A, Patterson A,
Waqar S, Toloza E, Haura E, Raz D, Reckamp K, Merritt R, et al:
PS01.05 Surgical and clinical outcomes with neoadjuvant
Atezolizumab in resectable stage IB-IIIB NSCLC: LCMC3 trial primary
analysis. J Thorac Oncol. 16(Suppl): S59–S61. 2021. View Article : Google Scholar
|
|
48
|
Zhao ZR, Yang CP, Chen S, Yu H, Lin YB,
Lin YB, Qi H, Jin JT, Lian SS, Wang YZ, et al: Phase 2 trial of
neoadjuvant toripalimab with chemotherapy for resectable stage III
non-small-cell lung cancer. Oncoimmunology. 10:19960002021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eichhorn F, Klotz LV, Kriegsmann M,
Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U,
Heussel CP, Savai R, et al: Neoadjuvant anti-programmed death-1
immunotherapy by pembrolizumab in resectable non-small cell lung
cancer: First clinical experience. Lung Cancer. 153:150–157. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Altorki NK, McGraw TE, Borczuk AC, Saxena
A, Port JL, Stiles BM, Lee BE, Sanfilippo NJ, Scheff RJ, Pua BB, et
al: Neoadjuvant durvalumab with or without stereotactic body
radiotherapy in patients with early-stage non-small-cell lung
cancer: A single-centre, randomised phase 2 trial. Lancet Oncol.
22:824–835. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tong BC, Gu L, Wang X, Wigle DA, Phillips
JD, Harpole DH Jr, Klapper JA, Sporn T, Ready NE, D'Amico TA, et
al: Perioperative outcomes of pulmonary resection after neoadjuvant
pembrolizumab in patients with non-small cell lung cancer. J Thorac
Cardiovasc Surg. 163:427–436. 2022. View Article : Google Scholar
|
|
53
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gu Y, Chen X, Wang D, Ding M, Xue L, Zhen
F, Xu J, Wang M, Li Y, Sun N, et al: 175P A study of neoadjuvant
sintilimab combined with triplet chemotherapy of lipo-paclitaxel,
cisplatin, and S-1 for resectable esophageal squamous cell
carcinoma (ESCC). Ann Oncol. 31(Suppl 6): S1307–S1308. 2020.
View Article : Google Scholar
|
|
55
|
Li H, Li C, Zheng Y, Zhao S, Chen X, Han
Y, Xiang J and Wu Y: Preoperative combination of pembrolizumab with
chemoradiation for patients with locally advanced esophageal
squamous cell carcinoma: Mid-term results of NCT03604991. Dis
Esophagus. 33:2020 View Article : Google Scholar
|
|
56
|
Li C, Zhao S, Zheng Y, Han Y, Chen X,
Cheng Z, Wu Y, Feng X, Qi W, Chen K, et al: Preoperative
pembrolizumab combined with chemoradiotherapy for oesophageal
squamous cell carcinoma (PALACE-1). Eur J Cancer. 144:232–241.
2021. View Article : Google Scholar
|
|
57
|
van den Ende T, de Clercq NC, van Berge
Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, Meijer SL,
Schokker S, Dings MPG, Bergman JJGHM, et al: Neoadjuvant
chemoradiotherapy combined with atezolizumab for resectable
esophageal adenocarcinoma: A single-arm phase ii feasibility trial
(PERFECT). Clin Cancer Res. 27:3351–3359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu
J, Yang X, Lin Z, Lin J and Kang M: Neoadjuvant sintilimab plus
chemotherapy for locally advanced esophageal squamous cell
carcinoma: A single-arm, single-center, phase 2 trial (ESONICT-1).
Ann Transl Med. 9:16232021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li
T, Zhang Y, Ma B, Yang Y, Shang Y, et al: The Sequence of
chemotherapy and toripalimab might influence the efficacy of
neoadjuvant chemoimmunotherapy in locally advanced esophageal
squamous cell Cancer-A phase ii study. Front Immunol.
12:7724502021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang Z: Neoadjuvant camrelizumab combined
with chemotherapy and apatinib for locally advanced thoracic
esophageal squamous cell carcinoma (ESCC): A single-arm,
open-label, phase Ib study. J Clin Oncol. 39:4047. 2021. View Article : Google Scholar
|
|
61
|
Xu W, Jiang Y, Wang C, Wu J, Li J, Hu Y,
Lu W, Shen D, Wang Y and Chen Q: The efficacy and safety of
neoadjuvant camrelizumab and chemotherapy for locally advanced
thoracic esophageal squamous cell carcinoma. J Clin Oncol. 40(Suppl
4): S2782022. View Article : Google Scholar
|
|
62
|
Liu J, Li J, Lin W, Shao D, Depypere L,
Zhang Z, Li Z, Cui F, Du Z, Zeng Y, et al: Neoadjuvant camrelizumab
plus chemotherapy for resectable, locally advanced esophageal
squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2
study. Int J Cancer. 151:128–137. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
He W, Leng X, Mao T, Luo X, Zhou L, Yan J,
Peng L, Fang Q, Liu G, Wei X, et al: Toripalimab plus paclitaxel
and carboplatin as neoadjuvant therapy in locally advanced
resectable esophageal squamous cell carcinoma. Oncologist.
27:e18–e28. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H,
Zhu L, Shen Y, Zhang H, Sun Y, et al: Multicenter, single-arm,
phase II trial of camrelizumab and chemotherapy as neoadjuvant
treatment for locally advanced esophageal squamous cell carcinoma.
J Immunother Cancer. 10:e0042912022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kelly RJ, Smith KN, Anagnostou V, Thompson
E, Hales RK, James Battafarano RJ, Voong KR, Yang SC, Feliciano JL,
Shin EJ, et al: Neoadjuvant nivolumab plus concurrent
chemoradiation in stage II/III esophageal/gastroesophageal junction
cancer. J Clin Oncol. 37:1422019. View Article : Google Scholar
|
|
66
|
Liu Y, Han G, Li H, Zhao Y, Zhuang J, Wang
G, Li B, Wang J, Li Z, Xia Q, et al: Camrelizumab combined with
FOLFOX as neoadjuvant therapy for resectable locally advanced
gastric and gastroesophageal junction adenocarcinoma. J Clin Oncol.
38:45362020. View Article : Google Scholar
|
|
67
|
Shah MA, Almhanna K, Iqbal S, Thakkar P,
Schneider BJ, Yantiss R, Wu Y, Futamura E, Port JL, Spinelli C, et
al: Multicenter, randomized phase II study of neoadjuvant
pembrolizumab plus chemotherapy and chemoradiotherapy in esophageal
adenocarcinoma (EAC). J Clin Oncol. 39:40052021. View Article : Google Scholar
|
|
68
|
Sun W, Saeed A, Al-Rajabi RMT, Kasi A,
Veeramachaneni NK, Al-Kasspooles MM, Baranda JC, Phadnis M, Godwin
AK, Olyaee M, et al: A phase II study of perioperative mFOLFOX
chemotherapy plus pembrolizumab combination in patients with
potentially resectable adenocarcinoma of the esophageal,
gastroesophageal junction (GEJ), and stomach. J Clin Oncol.
40:3292022. View Article : Google Scholar
|
|
69
|
Tao K, Yin Y, Lin Y, Li W, Li R, Liu W,
Xiong Z, Zeng X, Cai M, Wu C and Zhang P: Neoadjuvant PD-1
inhibitor tislelizumab combined with s-1 plus oxaliplatin in
patients with local advanced gastric cancer or gastroesophageal
junction adenocarcinoma: Interim results of a single-arm, phase II
trial. J Clin Oncol. 40:3002022. View Article : Google Scholar
|
|
70
|
Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D,
Wang W, Wang H, Wang H, He K, et al: Efficacy and safety of
neoadjuvant sintilimab, oxaliplatin and capecitabine in patients
with locally advanced, resectable gastric or gastroesophageal
junction adenocarcinoma: Early results of a phase 2 study. J
Immunother Cancer. 10:e0036352022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ho WJ, Zhu Q, Durham J, Popovic A, Xavier
S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, et al:
Neoadjuvant cabozantinib and nivolumab converts locally advanced
HCC into resectable disease with enhanced antitumor immunity. Nat
Cancer. 2:891–903. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Su Y, Lin Y, Hsiao C, Ou D, Chen S, Wu Y,
Lee W, Lin J, Hsu C, Ho M, et al: P-124 Nivolumab plus ipilimumab
as neoadjuvant therapy for potentially resectable hepatocellular
carcinoma. Ann Oncol. 32(Suppl): S1412021. View Article : Google Scholar
|
|
73
|
Kaseb AO, Hasanov E, Cao HST, Xiao L,
Vauthey JN, Lee SS, Yavuz BG, Mohamed YI, Qayyum A, Jindal S, et
al: Perioperative nivolumab monotherapy versus nivolumab plus
ipilimumab in resectable hepatocellular carcinoma: A randomised,
open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 7:208–218.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Marron TU, Fiel MI, Hamon P, Fiaschi N,
Kim E, Ward SC, Zhao Z, Kim J, Kennedy P, Gunasekaran G, et al:
Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: A
single-arm, open-label, phase 2 trial. Lancet Gastroenterol
Hepatol. 7:219–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gao J, Karam JA, Tannir NM, Campbell MT,
Tidwell RS, Ahrar K, Rao P, Ng CS, Jonasch E, Matin SF, et al: A
pilot randomized study evaluating nivolumab (nivo) or nivo +
bevacizumab (bev) or nivo + ipilimumab (ipi) in patients with
metastatic renal cell carcinoma (MRCC) eligible for cytoreductive
nephrectomy, metastasectomy or post-treatment biopsy (Bx). J Clin
Oncol. 37:45012019. View Article : Google Scholar
|
|
76
|
Karam JA, Msaouel P, Matin SF, Campbell
MT, Zurita AJ, Shah AY, Wistuba II, Haymaker CL, Marmonti E, Duose
DY, et al: A phase II study of sitravatinib (Sitra) in combination
with nivolumab (Nivo) in patients (Pts) undergoing nephrectomy for
locally-advanced clear cell renal cell carcinoma (accRCC). J Clin
Oncol. 39:3122021. View Article : Google Scholar
|
|
77
|
Gorin MA, Patel HD, Rowe SP, Hahn NM,
Hammers HJ, Pons A, Trock BJ, Pierorazio PM, Nirschl TR, Salles DC,
et al: Neoadjuvant nivolumab in patients with high-risk
nonmetastatic renal cell carcinoma. Eur Urol Oncol. 5:113–117.
2022. View Article : Google Scholar :
|
|
78
|
Bex A, Abu-Ghanem Y, Thienen JVV,
Graafland N, Lagerveld B, Zondervan P, Beerlage H, van Moorselaar
J, Kockx M, Van Dam PJ, et al: Efficacy, safety, and biomarker
analysis of neoadjuvant avelumab/axitinib in patients (pts) with
localized renal cell carcinoma (RCC) who are at high risk of
relapse after nephrectomy (NeoAvAx). J Clin Oncol. 40:2892022.
View Article : Google Scholar
|
|
79
|
Carlo MI, Attalla K, Mazaheri Y, Gupta S,
Yildirim O, Murray SJ, Coskey DT, Kotecha R, Lee CH, Feldman DR, et
al: Phase II study of neoadjuvant nivolumab in patients with
locally advanced clear cell renal cell carcinoma undergoing
nephrectomy. Eur Urology. 81:570–573. 2022. View Article : Google Scholar
|
|
80
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yuki S, Bando H, Tsukada Y, Inamori K,
Komatsu Y, Homma S, Uemura M, Kato T, Kotani D, Fukuoka F, et al:
SO-37 Short-term results of VOLTAGE-A: Nivolumab monotherapy and
subsequent radical surgery following preoperative chemoradiotherapy
in patients with microsatellite stability and microsatellite
instability-high, locally advanced rectal cancer (EPOC 1504). Ann
Oncol. 31:S230–S231. 2020. View Article : Google Scholar
|
|
82
|
Avallone A, De Stefano A, Pace U, Catteau
A, Di Gennaro E, Tatangelo F, Boquet I, Cassata A, Costantini S, De
Franciscis S, et al: 491P Neoadjuvant nivolumab in early stage
colorectal cancer. Ann Oncol. 31(Suppl 4): S4492020. View Article : Google Scholar
|
|
83
|
Rahma OE, Yothers G, Hong TS, Russell MM,
You YN, Parker W, Jacobs SA, Colangelo LH, Lucas PC, Gollub MJ, et
al: Use of total neoadjuvant therapy for locally advanced rectal
cancer: Initial results from the pembrolizumab arm of a phase 2
randomized clinical trial. JAMA Oncol. 7:1225–1230. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Salvatore L, Bensi M, Corallo S, Bergamo
F, Pellegrini I, Rasola C, Borelli B, Tamburini E, Randon G,
Galuppo S, et al: O-12 Phase II study of preoperative
chemoradiotherapy plus avelumab in patients with locally advanced
rectal cancer: The AVANA study. Ann Oncol. 32(Suppl 3): S2232021.
View Article : Google Scholar
|
|
85
|
Tamberi S, Grassi E, Corbelli J, Papiani
G, Barbera MA, Zingaretti C, Moretti CC, Montroni I, Petracci E,
Caruso D, et al: A phase II study of capecitabine plus concomitant
radiation therapy followed by durvalumab (MEDI4736) as preoperative
treatment in rectal cancer: PANDORA study first-stage
(NCT04083365). Tumori. 107:92–93. 2021.
|
|
86
|
Shamseddine A, Zeidan Y, Bouferraa Y,
Turfa R, Kattan J, Mukherji D, Temraz S, Alqasem K, Amarin R, Al
Awabdeh T, et al: Efficacy and safety of neoadjuvant short-course
radiation followed by mFOLFOX-6 plus avelumab for locally-advanced
rectal adenocarcinoma: Averectal study. Ann Oncol. 32(Suppl):
S2152021. View Article : Google Scholar
|
|
87
|
Carrasco J, Schröder D, Sinapi I, De
Cuyper A, Beniuga G, Delmarcelle S, van Laethem JL, Huyghe N, Bar
I, Hendlisz A, et al: 397P R-IMMUNE interim analysis: A phase Ib/II
study to evaluate safety and efficacy of atezolizumab combined with
radio-chemotherapy in a preoperative setting for patients with
localized rectal cancer. Ann Oncol. 32(Suppl 5): S5372021.
View Article : Google Scholar
|
|
88
|
Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang
M, Lan P, Wu X, Wang C, Cao W, et al: Neoadjuvant PD-1 blockade
with toripalimab, with or without celecoxib, in mismatch
repair-deficient or microsatellite instability-high, locally
advanced, colorectal cancer (PICC): A single-centre,
parallel-group, non-comparative, randomised, phase 2 trial. Lancet
Gastroenterol Hepatol. 7:38–48. 2022. View Article : Google Scholar
|
|
89
|
Necchi A, Raggi D, Gallina A, Madison R,
Colecchia M, Lucianò R, Montironi R, Giannatempo P, Farè E,
Pederzoli F, et al: Updated results of PURE-01 with preliminary
activity of neoadjuvant pembrolizumab in patients with
Muscle-invasive bladder carcinoma with variant histologies. Eur
Urol. 77:439–446. 2020. View Article : Google Scholar
|
|
90
|
Powles T, Kockx M, Rodriguez-Vida A, Duran
I, Crabb SJ, Van Der Heijden MS, Szabados B, Pous AF, Gravis G,
Herranz UA, et al: Clinical efficacy and biomarker analysis of
neoadjuvant atezolizumab in operable urothelial carcinoma in the
ABACUS trial. Nat Med. 25:1706–1714. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Szabados B, Rodriguez-Vida A, Durá I,
Crabb SJ, Van Der Heijden MS, Pous AF, Gravis G, Herranz UA,
Protheroe A, Ravaud A, et al: Toxicity and surgical complication
rates of neoadjuvant atezolizumab in patients with Muscle-invasive
bladder cancer undergoing radical cystectomy: Updated safety
results from the ABACUS trial. Eur Urol Oncol. 4:456–463. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gao J, Navai N, Alhalabi O, Siefker-Radtke
A, Campbell MT, Tidwell RS, Guo CC, Kamat AM, Matin SF, Araujo JC,
et al: Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with
cisplatin-ineligible operable high-risk urothelial carcinoma. Nat
Med. 26:1845–1851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wei XX, McGregor BA, Lee RJ, Gao X,
Kilbridge KL, Preston MA, Mossanen M, Ingham MD, Steele GS, Klein
A, et al: Durvalumab as neoadjuvant therapy for muscle-invasive
bladder cancer: Preliminary results from the Bladder Cancer Signal
Seeking Trial (BLASST)-2. J Clin Oncol. 38:507. 2020. View Article : Google Scholar
|
|
94
|
Grande E, Guerrero F, Puente J, Galante I,
Duran I, Dominguez M, Gordoa TA, Burgos J, Font A, Pinto A, et al:
DUTRENEO Trial: A randomized phase II trial of DUrvalumab and
TREmelimumab versus chemotherapy as a NEOadjuvant approach to
muscle-invasive urothelial bladder cancer (MIBC) patients (pts)
prospectively selected by an interferon (INF)-gamma immune
signature. J Clin Oncol. 38:50122020. View Article : Google Scholar
|
|
95
|
Rodriguez-Moreno JF, de Velasco G,
Alvarez-Fernandez C, Collado R, Fernandez-Rodriguez R, Vazquez
Estevez S, Virizuela Echaburu JA, Gajate Borau P, Font A, Lainez N,
et al: 761P Impact of the combination of durvalumab (MEDI4736) plus
olaparib (AZD2281) administered prior to surgery in the molecular
profile of resectable urothelial bladder cancer. NEODURVARIB trial.
Ann Oncol. 31(Suppl 4): S5892020. View Article : Google Scholar
|
|
96
|
Hoimes CJ, Adra N, Fleming MT,
Kaimakliotis HZ, Picus J, Smith ZL, Walling R, Alter RS, Kassar M,
Wang J, et al: Phase Ib/II neoadjuvant (N-) pembrolizumab (P) and
chemotherapy for locally advanced urothelial cancer (laUC): Final
results from the cisplatin (C)-eligible cohort of HCRN GU14-188. J
Clin Oncol. 38:50472020. View Article : Google Scholar
|
|
97
|
Kaimakliotis HZ, Adra N, Kelly WK,
Trabulsi EJ, Lauer RC, Picus J, Smith ZL, Walling R, Masterson TA,
Adra N, et al: Phase II neoadjuvant (N-) gemcitabine (G) and
pembrolizumab (P) for locally advanced urothelial cancer (laUC):
Interim results from the cisplatin (C)-ineligible cohort of.
GU14–188. J Clin Oncol. 38:50192020. View Article : Google Scholar
|
|
98
|
van Dijk N, Gil-Jimenez A, Silina K,
Hendricksen K, Smit LA, de Feijter JM, van Montfoort ML, van
Rooijen C, Peters D, Broeks A, et al: Preoperative ipilimumab plus
nivolumab in locoregionally advanced urothelial cancer: The NABUCCO
trial. Nat Med. 26:1839–1844. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Van Dorp J, Suelmann BBM, Mehra N, Van
Montfoort M, Van Dijk N, Hendricksen K, de Feijter J, Boellaard T,
Daletzakis A, Blank CU, et al: LBA31 High- vs low-dose
pre-operative ipilimumab and nivolumab in locoregionally advanced
urothelial cancer (NABUCCO cohort 2). Ann Oncol. 32(Suppl 5):
S1305–S1306. 2021. View Article : Google Scholar
|
|
100
|
Natesan DV, Zhang L, Oh DY, Porten SP,
Meng M, Pruthi R, Cooperberg MR, Carroll P, Chou J, Borno H, et al:
Updated results of phase II trial using escalating doses of
neoadjuvant atezolizumab for cisplatin-ineligible patients with
nonmetastatic urothelial cancer (NCT02451423). J Clin Oncol.
39:e165102021. View Article : Google Scholar
|
|
101
|
Grivas P, Yin J, Koshkin VS, Cole S, Jain
RK, Dreicer R, Cetnar JP, Sundi D, Gartrell BA, Galsky MD, et al:
PrE0807: A phase Ib feasibility trial of neoadjuvant nivolumab (N)
without or with lirilumab (L) in cisplatin-ineligible patients
(pts) with muscle-invasive bladder cancer (MIBC). J Clin Oncol.
39:45182021. View Article : Google Scholar
|
|
102
|
Cathomas R, Rothschild S, Hayoz S, Spahn
M, Schardt J, Seiler R, Erdmann A, Aeppli S, Mach N, Strebel R, et
al: Safety and efficacy of perioperative cisplatin/gemcitabine
(cis/gem) and durvalumab (durva) for operable muscle-invasive
urothelial carcinoma (MIUC): SAKK 06/17. J Clin Oncol. 39:4302021.
View Article : Google Scholar
|
|
103
|
Rose TL, Harrison MR, Deal AM, Ramalingam
S, Whang YE, Brower B, Dunn M, Osterman CK, Heiling HM, Bjurlin MA,
et al: Phase II study of gemcitabine and Split-Dose cisplatin plus
pembrolizumab as neoadjuvant therapy before radical cystectomy in
patients with muscle-invasive bladder cancer. J Clin Oncol.
39:3140–3148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Funt SA, Lattanzi M, Whiting K, Al-Ahmadie
H, Quinlan C, Teo MY, Lee CH, Aggen D, Zimmerman D, McHugh D, et
al: Neoadjuvant atezolizumab with gemcitabine and cisplatin in
patients with muscle-invasive bladder cancer: A multicenter,
Single-Arm, phase II trial. J Clin Oncol. 40:1312–1322. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Blank CU, Rozeman EA, Fanchi LF, Sikorska
K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M,
Philips D, Broeks A, et al: Neoadjuvant versus adjuvant ipilimumab
plus nivolumab in macroscopic stage III melanoma. Nat Med.
24:1655–1661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Amaria RN, Reddy SM, Tawbi HA, Davies MA,
Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, et al:
Neoadjuvant immune checkpoint blockade in high-risk resectable
melanoma. Nat Med. 24:1649–1654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Amaria RN, Postow MA, Tetzlaff MT, Ross
MI, Glitza IC, McQuade JL, Wong MKK, Gershenwald JE, Goepfert R,
Keung EJY, et al: Neoadjuvant and adjuvant nivolumab (nivo) with
anti-LAG3 antibody relatlimab (rela) for patients (pts) with
resectable clinical stage III melanoma. J Clin Oncol. 39:9502.
2021. View Article : Google Scholar
|
|
108
|
Rozeman EA, Menzies AM, van Akkooi ACJ,
Adhikari C, Bierman C, van de Wiel BA, Scolyer RA, Krijgsman O,
Sikorska K, Eriksson H, et al: Identification of the optimal
combination dosing schedule of neoadjuvant ipilimumab plus
nivolumab in macroscopic stage III melanoma (OpACIN-neo): A
multicentre, phase 2, randomised, controlled trial. Lancet Oncol.
20:948–960. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang AC, Xu X, Orlowski RJ, George SM,
Chilukuri L, Kozlov A, Carberry M, Giles L, McGettigan S, Kreider
K, et al: Abstract CT181: Safety, activity, and biomarkers for
neoadjuvant anti- PD-1 therapy in melanoma. Cancer Res. 78:CT181.
2018. View Article : Google Scholar
|
|
110
|
Huang AC, Orlowski RJ, Xu X, Mick R,
George SM, Yan PK, Manne S, Kraya AA, Wubbenhorst B, Dorfman L, et
al: A single dose of neoadjuvant PD-1 blockade predicts clinical
outcomes in resectable melanoma. Nat Med. 25:454–461. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang X, Cui C, Si L, Li C, Dai J, Mao L,
Bai X, Chi J, Sheng X, Kong Y, et al: A phase Ib clinical trial of
neoadjuvant OrienX010, an oncolytic virus, in combination with
toripalimab in patients with resectable stage IIIb to stage IVM1a
acral melanoma. J Clin Oncol. 39:9570. 2021. View Article : Google Scholar
|
|
112
|
Hyngstrom JR, Holmen S, Andtbacka RH,
William B, Tanaka M, Gravley W, David B, Hu-Lieskovan S and Van
Brocklin M: Neoadjuvant Canerpaturev (C-REV) Oncolytic
Immunotherapy in Combination with Nivolumab (Nivo) in Resectable
Advanced Melanoma. Pigment. Cell Melanoma Res. 35:1802022.
|
|
113
|
Cocorocchio E, Nezi L, Gandini S, Manzo T,
Mazzarella L, Lotti F, Pala L, Gnagnarella P, Conforti F,
Pennacchioli E, et al: 1072P Primary ipilimumab/nivolumab
immunotherapy followed by adjuvant nivolumab in patients with
locally advanced or oligometastatic melanoma: Update on outcome.
Ann Oncol. 32:S889–S890. 2021. View Article : Google Scholar
|
|
114
|
Najjar YG, McCurry D, Lin H, Lin Y, Zang
Y, Davar D, Karunamurthy A, Drabick JJ, Neves RI, Butterfield LH,
et al: Neoadjuvant pembrolizumab and High-dose IFNα-2b in
resectable regionally advanced melanoma. Clin Cancer Res.
27:4195–4204. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Topalian SL, Bhatia S, Amin A, Kudchadkar
RR, Sharfman WH, Lebbé C, Delord JP, Dunn LA, Shinohara MM,
Kulikauskas R, et al: Neoadjuvant nivolumab for patients with
resectable merkel cell carcinoma in the CheckMate 358 trial. J Clin
Oncol. 38:2476–2487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fisher B, Gunduz N, Coyle J, Rudock C and
Saffer E: Presence of a growth-stimulating factor in serum
following primary tumor removal in mice. Cancer Res. 49:1996–2001.
1989.PubMed/NCBI
|
|
117
|
Fisher B, Saffer E, Rudock C, Coyle J and
Gunduz N: Effect of local or systemic treatment prior to primary
tumor removal on the production and response to a serum
growth-stimulating factor in mice. Cancer Res. 49:2002–2004.
1989.PubMed/NCBI
|
|
118
|
Bracci L, Schiavoni G, Sistigu A and
Belardelli F: Immune-based mechanisms of cytotoxic chemotherapy:
implications for the design of novel and rationale-based combined
treatments against cancer. Cell Death Differ. 21:15–25. 2014.
View Article : Google Scholar
|
|
119
|
Wei SC, Duffy CR and Allison JP:
Fundamental mechanisms of immune checkpoint blockade therapy.
Cancer Discov. 8:1069–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Waser NA, Adam A, Schweikert B, Vo L,
McKenna M, Breckenridge M, Penrod JR and Goring S: Pathologic
response as early endpoint for survival following neoadjuvant
therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC):
Systematic literature review and meta-analysis. Ann Oncol. 31(Suppl
4): S744–S753. 2020. View Article : Google Scholar
|
|
121
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Menzies AM, Amaria RN, Rozeman EA, Huang
AC, Tetzlaff MT, van de Wiel BA, Lo S, Tarhini AA, Burton EM,
Pennington TE, et al: Pathological response and survival with
neoadjuvant therapy in melanoma: A pooled analysis from the
International Neoadjuvant Melanoma Consortium (INMC). Nat Med.
27:301–309. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chaft JE, Hellmann MD, Velez MJ, Travis WD
and Rusch VW: Initial experience with lung cancer resection after
treatment with T-cell checkpoint inhibitors. Ann Thorac Surg.
104:e217–e218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bott MJ, Cools-Lartigue J, Tan KS, Dycoco
J, Bains MS, Downey RJ, Huang J, Isbell JM, Molena D, Park BJ, et
al: Safety and feasibility of lung resection after immunotherapy
for metastatic or unresectable tumors. Ann Thorac Surg.
106:178–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
du Bois H, Heim TA and Lund AW:
Tumor-draining lymph nodes: At the crossroads of metastasis and
immunity. Sci Immunol. 6. pp. eabg35512021, View Article : Google Scholar
|
|
126
|
Fear VS, Forbes CA, Neeve SA, Fisher SA,
Chee J, Waithman J, Ma SK, Lake R, Nowak AK, Creaney J, et al:
Tumour draining lymph node-generated CD8 T cells play a role in
controlling lung metastases after a primary tumour is removed but
not when adjuvant immunotherapy is used. Cancer Immunol Immunother.
70:3249–3258. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Fransen MF, Arens R and Melief CJM: Local
targets for immune therapy to cancer: Tumor draining lymph nodes
and tumor microenvironment. Int J Cancer. 132:1971–1976. 2013.
View Article : Google Scholar
|
|
128
|
Mazieres J, Drilon A, Lusque A, Mhanna L,
Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et
al: Immune checkpoint inhibitors for patients with advanced lung
cancer and oncogenic driver alterations: Results from the
IMMUNOTARGET registry. Ann Oncol. 30:1321–1328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lee CK, Man J, Lord S, Links M, Gebski V,
Mok T and Yang JC: Checkpoint inhibitors in metastatic EGFR-mutated
non-small cell lung cancer-a meta-analysis. J Thorac Oncol.
12:403–407. 2017. View Article : Google Scholar
|
|
130
|
To KKW, Fong W and Cho WCS: Immunotherapy
in treating EGFR-mutant lung cancer: Current challenges and new
strategies. Front Oncol. 11:6350072021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang C, Chen HF, Yan S, Wu L, Yan LX, Yan
XL, Yue DS, Xu CW, Zheng M, Li JS, et al: Induction
immune-checkpoint inhibitors for resectable oncogene-mutant NSCLC:
A multi-center pooled analysis. NPJ Precis Oncol. 6:662022.
View Article : Google Scholar
|
|
132
|
Liu J, O'Donnell JS, Yan J, Madore J,
Allen S, Smyth MJ and Teng MWL: Timing of neoadjuvant immunotherapy
in relation to surgery is crucial for outcome. Oncoimmunology.
8:e15815302019. View Article : Google Scholar : PubMed/NCBI
|