1. Introduction
Epidemiological studies have shown that cancer is
the second cause of mortality worldwide following ischemic heart
disease. However, cancer is considered the most important clinical,
social and economic burden as regards cause-specific
disability-adjusted life years among all human pathologies
(1). Despite the fact that its
existence was recognized >2,000 years ago by the ancient Greeks,
the underlying causes leading to the uncontrolled growth of cells
became a matter of research during the mid-20th century. Since
then, tremendous advancements, not only in biology, but also in
biochemistry and bioengineering, have made it possible to unveil
some of the mechanisms of carcinogenesis (2). However, given the fact that cancer is
not a single pathology, but rather a cluster of relative
pathological entities, it is expected that certain mechanisms may
have a different impact on different types of cancer. This is the
case with human telomeres (and telomerase). Even though the
connection between the telomere status and carcinogenesis has been
identified, the absence of a universal or a cancer-specific trend
complicates the thorough understanding to a great extent. It is
indicative that both short and long telomere lengths have been
associated with a high risk of cancer incidence. When evaluating
risk associations between cancer and telomere length, a disparity
appears to emerge. Even though shorter telomeres have been adopted
as a marker of a poor health status and an older biological age,
longer telomeres due to increased cell growth potential have been
shown to be associated with cancer-initiating somatic mutations
(3). Therefore, the aim of the
present review was to comprehensively present the most recent
information regarding the implication of telomeres in different
types of cancer.
Telomeres are specific DNA structures positioned at
the end of chromosomes and consist of repetitive nucleotide
sequences (5′-TTAGGG-3′) (4).
These functional non-coding sequences, with the contribution of
shelterin proteins, facilitate the maintenance of chromosome
stability and protect them from degradation and damage (4). Shelterin is a six-subunit protein
complex that consists of a telomere repeat-binding factor (TRF)1
and TRF2, a nuclear protein 2 (TIN2), a repressor activator protein
1, a tripeptidyl-peptidase 1 (TPP1) and a protection of telomeres 1
(POT1) protein (5). Telomeres and
shelterins form structures known as T-loops that prevent DNA repair
mechanisms from processing telomeres and recognizing them as
double-stranded DNA breaks (5).
TRF2 depends on the DNA damage response (DDR) inhibition via T-loop
structure formation. T-loops are created by the invasion of the
long 3′overhang strand at the telomere end into the double-stranded
telomeric DNA (3). Specifically,
the 3′overhang is formed upon DNA replication and involves the
exonucleolytic degradation of the telomeres' 5′ ends. The result of
the respective processing and the concurrent inability of DNA
polymerases to replicate the lagging ends of linear DNA molecules
leads to the shortening of human telomeres by ~50 bp per cell
division. This telomere is restrained by the action of telomerase
reverse transcriptase (TERT), which places GGTTAG repeats to the
chromosomal 3′DNA terminus at the end of the chromosome. The
TERT gene is situated at chromosome 5p15.33 in humans, and
is an integral and essential part of the telomerase holoenzyme. The
TERT gene is 42 kb in length and consists of 15 introns and
16 exons, with a 260-bp promoter core (6). The reverse transcriptase domain is
encoded by 5-9 exons. The TERT transcript can be spliced into 22
isoforms (7). While the
transcriptional regulation of TERT has been studied in depth,
recent research has evaluated the role of alternate splicing of
mRNA transcripts. TERT can be translated from multiple differently
spliced transcripts, with only the longest variant having reverse
transcriptase enzymatic activity (8). Breast cancer cell lines with the
overexpression of transcripts without catalytic function have been
shown to exhibit a reduced apoptosis, conferring a survival
advantage (9). This suggests novel
functions of TERT beyond telomere extension TERT promoter (TERTp)
region contains GC boxes that bind the zinc finger transcription
factor Sp1, which increases TERT transcription, and E-boxes that
bind both transcriptional enhancers and repressors. TERTp lacks a
TATA box, but it contains binding sites for a variety of
transcription factors (10).
However, DNA polymerases cannot fully replicate the lagging strand
of telomere DNA at the chromosome terminus during each mitotic cell
division (4). As a result, there
is an annual rate of telomere shortening of ~20-40 bp, causing cell
proliferation arrest and cell senescence (4,11,12).
Telomerase can prevent telomere shortening. The
activity of this reverse-transcriptase enzyme, using an RNA
template, results in the telomeric DNA repeat synthesis (4,13).
Telomerase consists of the reverse transcriptase (TERT), the
telomerase RNA component, as well as proteins that are necessary
for DNA synthesis, such as dyskerin, nucleolar protein 10,
non-histone protein 2, GAR1 and telomerase Cajal body protein 1
(3) (Fig. 1). For cells not to replicate
indefinitely, TERT is silenced and cells undergo apoptosis or cell
senescence. However, cancer cells manage to overcome cell cycle
arrest and activate telomerase, resulting in cells acquiring
proliferative ability and developing mutations (Fig. 1). Therefore, telomere length can
serve as a marker for biological aging (14).
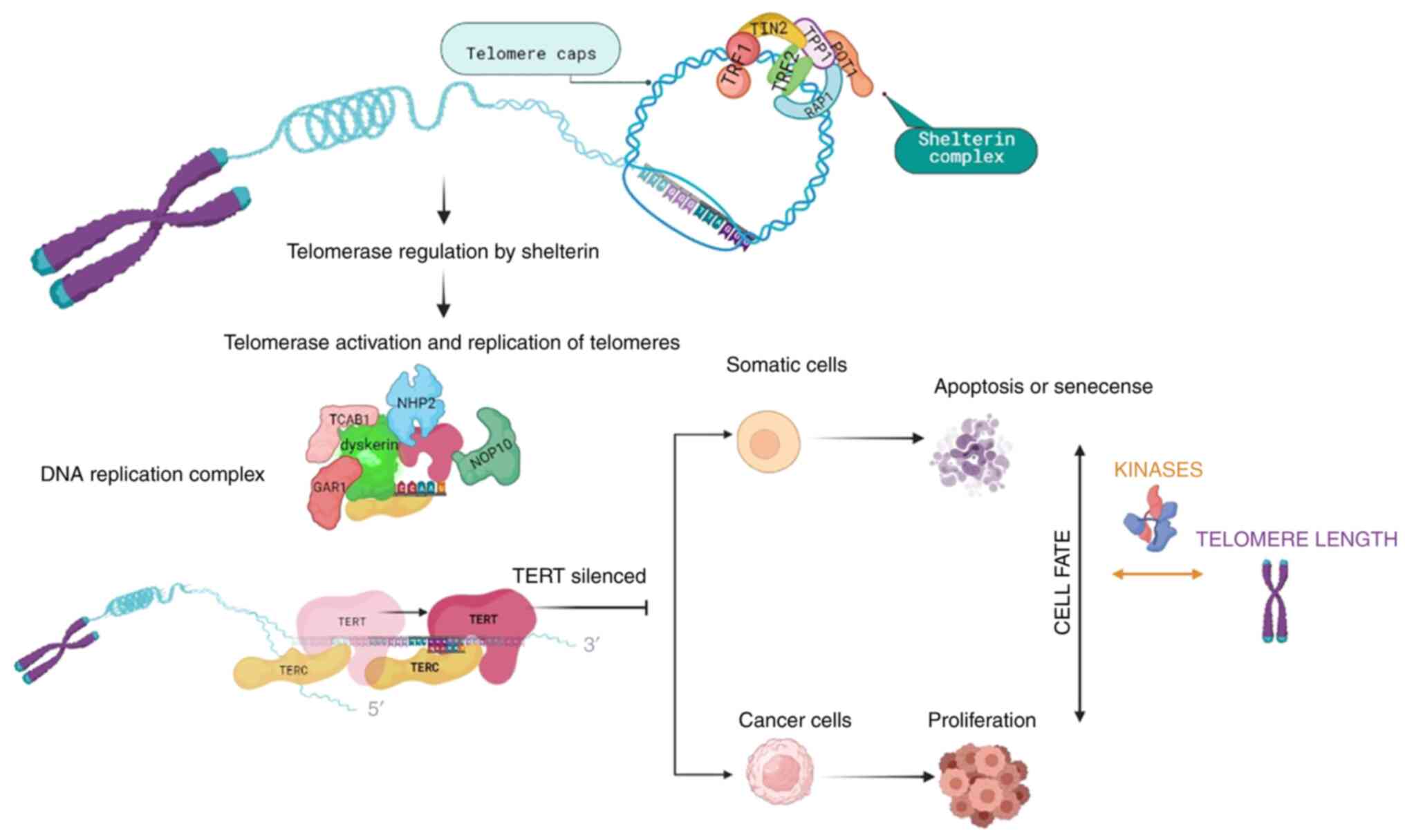 | Figure 1Overview of telomere length
regulatory mechanisms and cell fate. Telomeres are protected by the
shelterin complex and when telomeres need to be replicated, the
activated telomerase consisting of TERT, the telomerase RNA
component and proteins necessary for DNA synthesis (dyskerin,
NOP10, NHP2, GAR1 and TCAB1), replicates the telomere sequences. As
a prevention mechanism for continuous replication, TERT is
silenced, allowing cells to undergo apoptosis or cell senescence.
Cancer cells are able to overcome cell cycle arrest and activate
telomerase, resulting in cells acquiring proliferation ability and
mutations. Protein kinases, being part of the signaling regulating
cell cycle checkpoints, can affect the telomere length dependent
cell fate, by inhibiting DNA replication until damaged DNA is
repaired, or by restoring cell-cycle progression into the S phase
in senescent cells, when kinases are inhibited. TERT, telomerase
reverse transcriptase; NOP10: nucleolar protein 10; NHP2,
non-histone protein 2; TCAB1, telomerase Cajal body protein 1;
TIN2, nuclear protein 2; Rap1, repressor activator protein 1; TRF,
telomere repeat-binding factor; POT1, protection of telomeres
1. |
A number of protein kinases participate in the
signaling regulating DDR-activated cell cycle checkpoints, thus
inhibiting DNA replication until damaged DNA is repaired (15). Therefore, protein kinases regulate
the association between cell fate and telomere length. On the other
hand, inhibiting protein kinases regulating specific damage
checkpoints can restore cell cycle progression into the S phase in
senescent cells. Thus, dysfunctional telomeres induce a DNA damage
checkpoint response that initiates senescence.
Shorter telomeres and an attenuated telomerase
activity contribute to the pathobiology of human disease (16). They have also been shown to be
associated with a numbe rof age-related diseases, such as cancer,
coronary heart (cardiovascular) disease, type 2 diabetes, stroke,
arthritis, osteoporosis, hypertension, chronic obstructive
pulmonary disease and dementia (17). Researchers have also presented a
link between telomere length and stress, drug abuse, Alzheimer's
disease and mental disorders, including depression and
schizophrenia (13).
Telomere length is regulated by a myriad of factors,
including genetic background, as short telomeres can be a
hereditary trait passed by specific factors in parental gametes
(4). In addition, there is evident
sex dependence, as females have been shown to have longer telomeres
than males, associated with a lower biological age (18). Moreover, environmental factors may
also affect telomere lengths, such as physical activity, body mass
index, hormone replacement therapy, smoking, chronic inflammation,
oxidative stress, dietary antioxidants and vitamin intake (19). For instance, vitamin B12, C and E
deficiency may result in genomic instability and telomere
shortening (6). On the contrary,
in vitro experiments have indicated that omega-3
polyunsaturated fatty acids, ascorbic acid and its derivatives, as
well as α-tocopherol, can delay telomere shortening and protect
telomeres against degradation. Thus, more studies must be conducted
to better understand the correlation between supplement intake and
telomere protection.
A less known mechanism that regulates telomere
length is known as the alternative lengthening of telomeres (ALT).
ALT is a telomerase-independent mechanism and is somewhat dependent
on homologous recombination. The homologous recombination-mediated
copying of one telomere by another is the simplest explanation for
the spread of a DNA tag from one telomere to others. However, other
types of elongation events may also occur, as it is observed in the
telomerase null Type II survivors from the budding yeast species
Saccharomyces cerevisiae and Kluyveromyces lactis
(20,21). Even though the telomerase-dependent
pathway appears to be the predominant mechanism of telomere
elongation (85-90% of cases), there is a certain number of cancers,
including some with particularly poor outcomes, that use the ALT
pathway (roughly accounting for 10-15% of cases) (22). Notably, cells of mesenchymal origin
appear to rely more on ALT for telomere elongation than on
telomerase (23). In fact, in
certain types of cancer, including osteosarcomas and cancers of the
central nervous system, the rates of ALT positivity are approaching
90%, which escapes from possible mechanistic reasons for ALT
development (24). The
distribution is explained by the fact that cells of mesenchymal
origin are more likely to have more a stringently regulated
telomerase expression (25).
Cancers with ALT difficult to treat, partially due to their
distribution, the unique mechanism of maintenance and the early
resection that is precluded, rendering them unaffected by therapies
that are telomerase-targeted. ALT-positive cells have several
uncommon features, such as extrachromosomal telomeric DNA which is
separated from chromosome ends and it may be linear or circular
(22). It appears that the optimal
markers for ALT are partially single-stranded circles of telomeric
DNA in which the C-rich (AATCCC)n strand is essentially intact and
the G-rich (TTAGGG)n strand is gapped. This 'C-circle' DNA is
associated with the amount of ALT activity. Promyelocytic leukemia
(PML) bodies that have telomeric DNA are typical of ALT cells and
are introduced as ALT-associated PML bodies (APBs). Large APBs have
been shown to be associated with the senescence of ALT cells and
the sequestration of extrachromosomal DNA, although it is
considered that smaller APBs are sites where telomere lengthening
can occur (22).
Of note, it is essential to state that telomeres can
be measured in all nucleated cells. However, relative telomere
length may vary from one cell population to another, even when only
one disease is present (25). This
is critical because, as it will become evident from the following
description, there is no uniform trend in telomere length even in
the same type of cancer. Therefore, where possible, adequate
information regarding the cell population that was studied will be
provided in the sections below.
2. Cancer burden
Based on the International Agency for Research on
Cancer (IARC), in 2020, the cancer burden was increased to 19.3
million cases, while deaths related to cancer are estimated at 10
million. However, incidence rates differ depending on sex, cancer
site and human development index (HDI). HDI is a statistical index
that has been developed by the United Nations for the measurement
of social and economic development levels in various countries. It
consists of four parameters: The mean years of schooling, expected
years of education, life expectancy at birth and gross national
income per capita. HDI is used to follow changes in developmental
levels over time and to make comparisons among different countries.
The IARC provides statistics for the most common types of cancer
according to sex and HDI, that are presented in the tables below.
For example, HDI is inversely associated with the risk of prostate
cancer, suggesting that socioeconomic parameters related to
telomere status significantly affect cancer risk (26).
3. Modulation of human TERT in cancer
Over the past decades, studies have focused on the
regulation of TERT in humans (hTERT) in cancer. As a result,
several mechanisms of action for altering hTERT gene
expression have been described. Of note, a previous study
demonstrated how the hTERT promoter crucially regulates its
transcription (27). The
expression of hTERT has been shown to be induced by multiple
genetic and epigenetic mechanisms, in tumors. More specifically,
the mechanisms described include hTERT amplifications, structural
variants, promoter mutations and promoter methylation (epigenetic
modification) (28).
Amplification of hTERT
In cancer cells, the overexpression of amplified
genes leads to the gain or loss of genetic material. Telomere
dysfunctions, DNA copying errors and the presence of chromosomal
fragile sites have been described as mechanisms that initiate gene
amplification (29). In the case
of hTERT, the proposed modes are telomere dysfunction, in addition
to breakage at fragile sites and formation of chromosomal fusions
(30).
Genomic rearrangements of hTERT
The overexpression of hTERT in cancer can also
result by genomic rearrangements modulating the gene locus of
hTERT (5p15.33). Genomic rearrangements lead to the
increased proximity of active enhancers and the hTERT gene.
The latter results in the interaction between promoter and the
newly introduced enhancers, enhancing hTERT expression (31).
hTERT promoter mutation
hTERT promoter mutations are a common, yet distinct
genetic modification that regulates hTERT telomerase activation and
expression. The hTERT core promoter contains 260 base pairs and
different transcription factor binding sites that modulate gene
transcription and telomerase initiation (32). Different mutation loci in the
promoter generate added E-twenty-six transcription factor family
binding sites, therefore generating new possible sites of genetic
regulation in cancer (33). hTERT
promoter mutations mostly exist in low rate self-renewal cancers,
such as brain tumors, liver tumors, melanocytes and also low-grade
cancers, such as bladder cancers, proposing a triggering telomerase
activation role (34).
hTERT epigenetic modifications: hTERT
promoter methylations
DNA methylations exist genome-wide at CpG positions,
located in non-coding gene sections. Approximately 70% of the human
gene promoters enclose CpG sites; thus, DNA methylation is
considered a crucial player in gene expression and regulation
(35). Gene silencing and
activation are both associated with the methylation of specific
hTERT promoter sites, particularly upstream of the hTERT core
promoter (36). Different
mechanisms of hTERT promoter methylation have been described for
hTERT stimulation. Castelo-Branco et al (37) indicated that DNA methylation
prevents the binding of repressive elements. In addition, a more
complex mechanism links DNA methylation and chromosome structural
modifications (38). Finally, DNA
methylation contributes to alterations in chromatin conformation,
altering gene expression through differential transcription factor
binding (39).
Effects of microRNAs (miRs/miRNAs) on
hTERT modulation
In various types of cancer, several miRNAs have been
identified as key modulators of hTERT. Such miRNAs have been found
to negatively regulate hTERT expression, preventing carcinogenesis
(40). miRNAs can act towards
hTERT directly or indirectly. Direct binding is presented to the
hTERT 3′untranslated region (3′UTR), that interferes with hTERT
protein expression in cancer cells (41). In thyroid carcinoma, the inhibition
of miR-138 has been found to be associated with an increased
expression of hTERT and the imposed overexpression of miR-138 was
found to attenuate hTERT expression through the association with
the hTERT 3′UTR (41). Indirectly,
miRNAs may modulate transcription factors known to regulate hTERT
(33). Examples include miR-494
and miR-1294, that were found to downregulate c-Myc, a well-known
transcriptional activator of hTERT, in pancreatic and esophageal
squamous cell carcinomas (33)
4. Respiratory system
An altered telomere length has been well-identified
to participate in lung cancer formation. Although several studies
have reported this abnormality, a consensus has yet to be reached
(42-46). Indeed, both short and long telomere
lengths have been shown to be associated with a high risk of lung
cancer formation (47).
Various epidemiological factors have been shown to
affect the association between telomere length and lung cancer
pathogenesis. A study on patients with small cell lung cancer
(SCLC) with a history of heavy smoking demonstrated an association
between a shorter telomere length and higher risk of mortality,
particularly for those classified as having stage III/IV SCLC
(42). Moreover, a stronger
association for women >65 was indicated. Furthermore, Kachuri
et al (42) determined an
association between mortality and shorter telomere length in
no-smoker cohorts of patients with non-small cell lung cancer
(NSCLC) and adenocarcinoma. In a Chinese region characterized by
high indoor pollution, a shorter telomere length was detected in
the peripheral blood leukocytes of patients with lung cancer and
chronic obstructive pulmonary disease (43). The association was attributed to
the high levels of oxidative stress and inflammation in the airways
and blood of patients (43). A
recent study by Steiner et al (44) revealed that coffee was not
associated with telomere length of cancers related to coffee
intake, such as lung cancer. Age is also a putative factor that
could affect such associations. Sun et al (45) concluded that age may influence the
association of telomere length with cancer incidence, since younger
patients with a shorter telomere length and an increased telomere
length variation across all chromosome ends exhibited a higher risk
of lung cancer presentation.
Jang et al (46), using peripheral blood lymphocytes,
found that shorter telomeres indicated a higher risk of developing
small cell carcinoma than squamous cell carcinoma and lung
adenocarcinoma. On the contrary, Sanchez-Espiridion et al
(47), also using peripheral blood
leukocytes, presented a higher risk of lung squamous cell carcinoma
for patients with shorter telomeres. In fact, they suggested that
longer telomeres attenuated the development of squamous cell
carcinoma, particularly in males. Of note, the same debate applies
to telomerase activity as well. Jeon et al (48), using peripheral blood mononuclear
cells, found that low telomerase activity was significantly linked
to increased risk of lung cancer (adjusted odds ratio, 3.05; 95%
confidence interval, 1.60-5.82; P=7×10−4). Dobija-Kubica
et al (49) evaluated
telomerase activity in 47 tissue specimens obtained from patients
with NSCLC. According to their findings, 66.7% of healthy pulmonary
parenchyma samples had a high telomerase activity, while a variable
level of telomerase activity was reported in cancer cells. In
detail, even though no association was found between the level of
telomerase activity in NSCLC specimens and the 2-year survival rate
of patients, there were significantly higher levels of telomerase
activity in poorly differentiated (high grade) NSCLC tumors (grade
3), as compared to moderately differentiated (intermediate grade)
tumors (grade 2) (49).
Genetic factors are implicated in telomere biology
participation, as shown in a study in which patients with lung
adenocarcinoma were treated with epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitors (gefitinib) (50). Shorter telomeres were associated
with a poor prognosis following such a treatment and with a shorter
overall survival of lung cancer patients. Moreover, a short
telomere length indicated an elevated risk of resistance regarding
EGFR mutations (48). Therefore,
short telomeres could act as a marker for this therapeutic response
and the development of chromosomal instability (46). Furthermore, shorter telomeres may
cause damage to immune cell function and promote immune
senescence.
A longer telomere length has also been shown to be
associated with a high risk of developing lung cancer, as indicated
by a systematic review concluding that longer telomere length was
associated with a higher risk of developing lung cancer (51). A previous study conducted in an
East Asian region also demonstrated that longer telomere length was
positively associated with the risk of developing lung cancer
(52). Machiela et al
(53) indicated that non-smoking
women in Asia with a longer telomere length had an increased risk
of developing lung cancer. Sanchez-Espiridion et al
(47) suggested that patients with
a longer telomere length had a higher risk of developing lung
adenocarcinoma, particularly for women, individuals <60 years of
age and light smokers. The findings in the study by Yuan et
al (54) are in agrement with
those of the study by results of Sanchez-Espiridion et al
(47), where longer telomeres were
associsated with an elevated risk of developing lung
adenocarcinoma, but not squamous cell carcinoma. The aforementioned
association may could be due to different mechanisms of
tumorigenesis and may be associated with a specific type of cancer
(47).
Indeed, since longer telomeres bestow an increased
rate of proliferation to cells (54), the accumulation of somatic
mutations in carcinogenesis is possible, leading to malignant
transformations (52).
Specifically, cells with longer telomeres have an increased
telomerase activity and this may result in uncontrollable cellular
and tumor development (53).
Notably, de-Torres et al (55) also suggested that long telomeres
exhibited a high risk of lung cancer development, regardless of the
presence of chronic obstructive pulmonary disease and/or emphysema.
These authors suggested the existence of a potential mechanism
termed the 'long telomere syndrome' that is associated with
mutations in telomerase and shelterin genes (55). Consequently, both short and long
telomere lengths may indicate telomere dysfunction (26). Indeed, telomere length may be used
as a prognostic and therapeutic tool for specific cohorts of
patients with lung cancer, bestowing sensitivity regarding
therapeutic approaches and disease monitoring (56). Furthermore, the identification of
more drivers could increase the specificity of these markers
(57). However, a standing
limitation concerning these studies is that different methods of
measuring telomere length are used, which decreases the sensitivity
of comparison (58) (Table I).
 | Table ITelomere length and cancer risk. |
Table I
Telomere length and cancer risk.
| Cancer type | Sample type | Origin of study
population | TL (mean ± SD) | TL and cancer
risk | Clinical
significance |
Authors/(Refs.) |
|---|
| Lung
adenocarcinoma | | European | | Longer telomere
length/higher risk | Risk
prediction/intervention target for disease progression | Haycock et
al (56) |
| East Asian | | Longer telomere
length/higher risk | Longer telomere
length may play a role in carcinogenesis | Cao et al
(52) |
| Peripheral blood
leukocytes | Caucasian | 1.23±0.38 | Longer telomere
length/higher risk | According to the
histology of the tumor, cancer risk differs | Sanchez-Espiridion
et al (47) |
| Peripheral blood
leukocytes | Asian
(Chinese) | | Longer telomere
length/higher risk | Dose-dependent
association for telomere length in peripheral blood leukocytes at
baseline with an increased risk of lung adenocarcinoma | Yuan et al
(54) |
| Interstitial lung
disease | | European | | longer telomere
length/lower risk | risk prediction/
intervention target for disease progression | Haycock et
al (56) |
| Non-small cell lung
cancer | Blood
lymphocytes | | | In the younger age
group, short telomere length and high TLV in blood lymphocytes
jointly increased the risk of lung cancer by 8-fold compared with
individuals who had long telomere length and low TLV | Age may be critical
in establishing cancer risk | Sun et al
(45) |
| Squamous cell
carcinoma | | East Asian | | Marginal nonlinear
association | | Cao et al
(52) |
| Peripheral blood
leukocytes | Caucasian | 1.10±0.44 | Shorter telomere
length/lower risk | | Sanchez-Espiridion
et al (47) |
| Lung cancer (all
types) | Peripheral blood
lymphocytes | Asian (Korean) | 1.59±0.75 | Shorter telomere
length/higher risk (more pronounced in patients with small cell
carcinoma than in those with squamous cell carcinoma and
adenocarcinoma) | Short
telomere-associated with risk of cancer development | Jang et al
(46) |
| Peripheral white
blood cell | Asian | | Longer telomere
length/higher risk | | Machiela et
al (53) |
| Peripheral blood
leukocytes | Asian
(Chinese) | 0.76±0.35 | Short telomere/
higher risk | 3.90- and 4.54-fold
increased risk | Xue et al
(43) |
5. Laryngeal cancer
A relatively limited number of studies have examined
the putative association between laryngeal cancer and telomere
length. It has been suggested that telomeres are shorter in
patients with laryngeal squamous cell carcinoma in the tumor
differentiation grade 3 group than in the grade 1 and grade 2
groups. The grade 3 subgroup had the worst prognosis, with the
highest mortality rate (59).
Genetic factors that affect telomere biology in
laryngeal cancer pathogenesis have been identified. It has been
well-documented that mutations within the OBFC1 gene result
in a shorter telomere length in the cancer cells of patients with
these mutations (60). The OBFC1
gene is associated with the replication and capping of telomeres.
Therefore, it can be concluded that silencing such genes may reduce
the risk of cancer and may exert a protective effect against
tumorigenesis (60). Furthermore,
it has been indicated that the hPOT1 gene is associated with
telomere length and that mutations in this gene result in telomere
dysfunction, telomere shortening, apoptosis and laryngeal cancer
cell senescence (61). Lastly, it
has been shown that the anti-telomerase treatment of laryngeal
cancer cells is likely to activate the mechanisms of the
alternative lengthening of telomeres monitored with the detection
of ALT-specific promyelocytic leukemia bodies. Moreover, an
enhanced exchange between telomeric sister-chromatids is evident,
as well as the differential expression of telomere biology-related
genes (62). Specifically, such
cells exhibit a longer telomere length, an attenuated
proliferation, and the development of a less invasive and
tumorigenic phenotype (62). These
data demonstrate the existence of two mechanisms maintaining
telomere homeostasis, whose clarification might provide therapeutic
targets for cancer.
6. Urinary/renal system
Bladder cancer
Epidemiological factors, such as age, sex,
physiological status, genetic predisposition, or smoking have been
associated with the development of bladder cancer. Notably,
associations between epidemiological parameters and telomere length
have been identified. Thus, smokers with shorter telomeres have
been shown to have an increased risk of developing bladder cancer
(63). Furthermore, it has been
shown that patients who smoked present shorter telomeres than
non-smokers (64). In addition,
older patients with shorter telomeres exhibit a poorer prognosis
(65). Notably, female patients
have been found to have longer telomere lengths than males
(66). Lin et al (67) demonstrated that depression could
increase the risk of mortality in patients with a shorter telomere
length compared to those with a longer telomere length, no signs of
depression, and shorter cancer-free survival time. Specifically,
they concluded that shorter telomeres could elevate the risk of
mortality in depressed patients since the same neuroendocrine and
immunological pathways are linked with depression and telomere
length, and thus result in tumor progression and growth (67).
Specifically, genetic factors appear to be closely
associated with telomere length during the process of
tumorigenesis. Thus, patients with both short telomeres and GSTM1
homozygous deletions exhibit an increased risk of developing
bladder cancer (68). Hosen et
al (69) studied tumors with
TERT promoter and fibroblast growth factor receptor 3 (FGFR3)
mutations. More specifically, tumors solely with FGFR3 mutations
(mainly in papillary carcinomas) had the shortest telomere length,
followed by tumors with both mutations, then with TERT promoter
mutations (found in both muscle-invasive and invasive tumors), and
lastly by tumors not harboring the specific mutations (69).
The majority of studies concur that this type of
cancer is associated with shorter telomeres. Indeed, short
telomeres lead to chromosome instability in bladder cancer tissue
(68). Moreover, telomere length
appears to be associated with disease progression. Patients with
muscle-invasive bladder cancer have been shown to have a shorter
telomere length than those with non-muscle invasive bladder cancer,
suggesting that telomere length is associated with cancer stage
(64,68). In addition, shorter telomeres have
been shown to be associated with a reduced survival rate, possibly
due to poorer tolerance and higher chemotoxicity of therapy.
Therefore, telomere length may be used as a marker of an optimal
therapeutic strategy in bladder cancer (68).
However, some studies have found an association
between bladder cancer and a longer telomere length.
Fernandez-Gomez et al (70), using flow cytometry-based
fluorescence in situ hybridization (FISH), observed a longer
telomere length in more aggressive and aneuploid tumors compared to
diploid ones. A separate study by Wang et al (71) indicated that a longer telomere and
a higher telomere length variation could increase the risk of
developing bladder cancer by 14-fold. Moreover, telomere length
variation was increased in patients with bladder cancer, indicating
severe telomere dysfunction (71).
Furthermore, in another study, a specific genetic locus (rs398652on
14q21) was found to be associated with a longer telomere length, as
well as a reduced risk of bladder cancer (72). This single nucleotide polymorphism
(SNP) is associated with the PELI2 protein, which participates in
the inflammatory response and cytokine production, protecting cells
against chronic inflammation, closely associated with the process
of cancerogenesis (72).
Even though short telomeres appear to be directly
associated with the risk of developing bladder cancer, extreme
telomere variation, including longer telomeres, has been associated
with aggressive tumors.
Renal cancer
Even early reports identified an association between
telomere length and kidney cancer development. Thus, in 1993,
Holzmann et al (73)
indicated that renal tumors were characterized by telomeric
shortening, a process that could participate in tumor pathogenesis.
The most common type of kidney cancer is renal cell carcinoma
(RCC). Patients with RCC exhibit a shorter telomere length
(74-85). However, Dahse et al
(75) observed that telomere
shortening occurred in distinct tumor cell populations, thus
suggesting the heterogeneity of RCC. High-grade tumors exhibit
shorter telomeres than low-grade tumors, associated with a high
proliferation rate (76). In
addition, shorter telomeres indicate a poorer disease-specific
survival, since telomere shortening may facilitate tumor
development and acceleration of immune cell senescence (77).
The examination of telomere length in cells in the
blood of patients with RCC, however, has yielded somewhat
contradictory results. Hoffman et al (78) did not find an association between
pre-diagnostic leukocyte telomere length and the risk of developing
RCC. Moreover, another study by Hofmann et al (79) did not find any association between
blood cell telomere length and the risk of developing RCC. However,
the study by Svenson et al (80) indicated that patients with a longer
blood cell telomere length had a poorer prognosis than patients
with a shorter one. In addition, patients with longer leukocyte
telomeres and without any distant metastasis or capsule
involvement, and patients with nuclear tumors of grade 1 to 3 had
more unsatisfactory outcome (80).
However, Morais et al (81) hypothesized that telomeres may play
a dual role: During early stages, shorter telomeres increase the
risk of developing RCC due to the genetic instability that occurs
during late carcinogenesis, while longer telomeres induce tumor
progression. Genetically inferred telomere length, predictive of
leukocyte telomere length, was established from the genotypes of
nine telomere length-associated variants performed in six
genome-wide association studies of RC (81). This approach suggested that
individuals with an inherited predisposition exhibit more extended
telomere length and harbor a higher risk of developing RCC
(82). Notably, histologically
different renal cancers exhibit a similar positive association with
longer genetically inferred telomere length (82). On the other hand, it was
demonstrated that the hTERT gene variantRs2736098 increased
telomere length with each G allele added. Specifically, this G
allele may enhance hTERT expression, thus increasing telomerase
activity, elongating telomere length and reducing the risk of
developing RCC (83).
As with other cancer types, an association between
telomere length and cancer immune response was identified in renal
cancer. Whole blood cell relative telomere length was positively
associated with regulatory T-cells (Tregs), since they contribute
to tumor angiogenesis and may promote tumor progression (84). Moreover, Svenson et al
(84) indicated an association
between cancer cell telomere length and serum levels of interleukin
(IL)-7, -8 and -10 in RCC. These cytokines are critical
immunological parameters. Specifically, IL-7 is associated with a
poor survival, since it is imperative for the regulation of T- and
B-cell development and T-cell homeostasis; IL-8 is a chemokine
involved in tumor growth and development, and IL-10 induces immune
suppression (84). Notably,
patients with higher Treg levels exhibit longer T-cell telomeres.
This association may indicate a suppressed immune system with
attenuated cell division and subsequent lower telomere shortening
(84).
It is noteworthy that, as previously demonstrated,
after kidney transplants, pediatric cancer patients exhibited a
shorter blood cell telomere length compared to the controls, but
presented with elevated gene expression levels of telomere
length-preserving proteins (85).
Therefore, also in renal cancer, a significant association between
the variation of telomere length and cancer risk has been
established.
7. Hematogenous malignancies
Non-Hodgkin's lymphoma
Telomere length variations are strongly implicated
in the pathogenesis of hematogenous malignancies. Thus, patients
with non-Hodgkin's lymphoma were initially shown to have shorter
telomeres length than the controls (86,87).
Notably, patients with secondary diffuse large B-cell lymphoma were
shown to have shorter telomeres than those with follicular
lymphoma, indicating that telomere reduction induces disease
progression (86). Furthermore,
Widmann et al (87)
demonstrated that patients had shorter telomeres in the myeloid
subpopulations than the lymphoid ones.
On the other hand, Lan et al (88) were the first to associate longer
telomere length with an elevated risk of developing non-Hodgkin's
lymphoma. Specifically, it is suggested that longer telomeres
create delayed senescence; thus, the cell can accumulate more
mutations and increase the risk of transformation (88). Machiela et al (89) concurred with the aforementioned
statement, indicating that longer telomeres bestow more significant
replicative potential to hematogenous cancer cells.
It is essential to mention that patients undergoing
chemotherapy have been shown to exhibit shorter telomeres, perhaps
due to the proliferative stress of the high dose therapy in
hematopoietic reconstruction (90). Notably, patients that relapsed
exhibit shorter, longer as well as unaltered telomere lengths
(91). The variations mentioned
above may result from the selective loss of cells due to the
therapy received or the surviving subclones having a specific
telomere length constitution present in the tumor cell population
(91).
Acute lymphocytic leukemia
The majority of studies focusing on leukemia
progression and telomere biology have revealed an association with
a shorter telomere length. Thus, patients with acute lymphocytic
leukemia (ALL) are characterized by telomere shortening in their
blood cells, a process that affects the pathogenesis of the disease
(92). In a separate study,
telomere lengths estimated from bone marrow samples were shorter
than ones from peripheral blood of patients with ALL (93). However, upon chemotherapy, the mean
telomere length increased, although it was later reduced due to the
consolidation and maintenance of chemotherapy (93). The study by Borssén et al
(94) concurred that the telomere
length in blood cells of patients with ALL at the time of the
diagnosis of lymphocytic leukemia was shorter than the telomere
length measured at the end of therapy (94). Notably, a separate study
demonstrated that the shortest telomeres were determined in the
blood cells of relapsed patients, followed by newly diagnosed
patients, and then by the complete remission group (95). Another study demonstrated that
patients with late-stage ALL had a shorter telomere length and
higher telomerase activity, associated with disease progression and
more unsatisfactory outcomes; a short telomere length increased the
risk of developing ALL, but was not associated with the TERT
gene polymorphism (96). However,
a separate study indicated that the rs16847897 CG genotype
increased the risk of developing ALL by 29% compared to the CC
genotype (97). Longer telomeres
in low-risk B-cell precursor ALL indicated inferior outcomes
compared with short telomeres (94). Considering these data, one can
conclude that the effect of telomere variation in leukemia is
subtype-dependent.
Acute myelogenous leukemia
An early study by Takauchi et al (98) indicated that patients with acute
myelogenous leukemia (AML) had shorter telomere lengths.
Furthermore, shorter telomere lengths were shown to be indicative
of conversion from myelodysplastic syndrome to AML. The conversion
was attributed either to heterogeneity or telomere shortening
(99). However, telomere
shortening is not an indication of cells undergoing a 'telomere
crisis' (100). This may be due
to the upregulation of telomerase activity in AML stem cells or the
extensive replicative potential of normal blood-forming stem cells
(100). Moreover, an inverse
association between age and telomere length in AML has been shown
(101).
Chromosomal aberrations are strongly associated
with AML pathogenesis. Indeed, patients with AML with the loss or
gain of chromosome fractions carry critically short telomeres,
resulting in telomere dysfunction (102). Furthermore, patients with shorter
telomeres are prone to jumping translocations (103), while FMS-like tyrosine kinase 3
(FLT3) and internal tandem duplication (ITD) mutations have also
been shown to be associated with shorter telomeres (104). On the other hand, isocitrate
dehydrogenase (IDH)1 and IDH2 mutations have been shown to be
associated with longer telomeres and improved outcomes in patients
with AML, possibly due to higher sensitivity to chemotherapy, the
duration of aplasia, or other diseases/host factors (104).
A previous study on long-term
granulocyte-colony-stimulating factor treatment demonstrated an
elevated risk of developing AML due to bone marrow stress from
telomere shortening. Indeed, Li et al (105) suggested that this process may be
associated with the early stages of leukemogenesis.
As regards pediatric AML, Aalbers et al
indicated that these patients exhibited very short telomeres and an
increased risk of FLT3/ITD molecular aberrations FLT3/ITD. However,
no association was identified with the number of cytogenetic
abnormalities, contrary to adult AML (106).
Chronic lymphocytic leukemia (CLL)
Short-length telomeres are a prominent
characteristic of CLL. Notably, a shorter telomere length in CLL
has been found to be associated with reduced hemoglobin levels and
an adverse survival, particularly in patients with biallelic ATM
defects (107). Moreover, ATM
defects, as well as TP53 defects, have been shown to be associated
with telomere shortening and the poor survival of patients with CLL
(108). In addition, short
telomeres and TP53 mutations increase chromosome instability since,
with every cell cycle, the ability of telomeres to protect
chromosome ends weakens, thus facilitating the creation of complex
aberrations (109,110). Notably, an elevated risk of
disease progression has also been found to be associated with TP53
abnormalities (111).
The association of specific mutations with telomere
length was highlighted by Jebaraj et al (108), who demonstrated that individuals
carrying 17p- and 11q-associated with TP53 and ATM loss had the
shortest telomeres even when the abnormalities were minor.
Furthermore, it was indicated that patients with two or more
genetic abnormalities had shorter telomeres compared with
individuals carrying a smaller number of congenital anomalies.
Therefore, the authors suggested that telomere shortening was
associated with genetic complexity (112).
Some exceptions are evident as patients with normal
immunoglobulin variable heavy chain (IGHV) genes have shorter
telomere lengths than those with mutated ones (113,114). On the other hand, Roos et
al (115) observed an inverse
correlation between telomere length and IGHV homology, further
adding that shorter telomeres create genetic complexity by
increasing the number and occurrence of unwanted chromosomal
abnormalities.
Notably, the study by Lin et al (116) indicated that short telomeres were
also prone to fusions. The prevalence mentioned above may lead to
tumorigenic genomic rearrangements, particularly in patients with
early-stage disease. Moreover, it was concluded that shorter
telomeres were associated with more aggressive disease due to the
high telomere attrition rate in highly proliferative tumors
(117). Furthermore, patients
with less advanced stages of CLL were shown to exhibit longer
telomeres (118). However, both
studies suggested that longer telomeres were associated with
mutations in TERC, TERT and OBFC1, variants as well
as with a higher risk of developing CLL (117,118).
Notably, Furtado et al (119) suggested that telomere shortening
was an early event regarding leukemogenesis, since short telomeres
are already present in small abnormal B-cell clones of high-count
monoclonal B-cell lymphocytosis. This disease precedes CLL.
As regards methodology, both monochrome multiplex
quantitative PCR and single telomere length analysis can provide
clinically relevant information (111). However, Yang et al
(120) suggested that telomere
length should not be estimated from buccal samples, as telomere
length in buccal and leukemic cells is not associated with patient
survival or has any prognostic relevance.
In summary, it is suggested that telomere length
can act as a potential prognostic factor, as it may improve risk
stratification in patients with CLL for the early initiation of
therapy (111,121).
Chronic myelogenous leukemia (CML)
Early studies on CML regarding telomere length
demonstrated that patients with CML had shorter telomeres than
healthy individuals (122,123).
In continuation, it was indicated that more rapid telomere
shortening occurs in leukemic rather than non-leukemic
hematopoietic stem cells. This accelerated shortening has been
shown to be positively associated with the leukemic clone size in
the hematopoietic stem cell compartment (124). In addition, studies have
indicated that patients with CML in the accelerated or blast phase
have shorter telomeres than those in the chronic phase or cytogenic
remission (123,125,126). Moreover, telomere shortening is
more prominent in high-risk patients than in low-risk ones
(126). Specifically, such
shortening has been shown to be associated with disease
progression/stage, indicating increased genetic instability and a
high ability to accumulate secondary genetic events that may induce
disease evolution (127).
Indeed, it was hypothesized that a high-risk
subgroup of patients with CML who lack telomere maintenance
mechanisms enter the accelerated phase of CML early (128). On the other hand, it was observed
that patients with treatment-free remission (TFR) had shorter
telomeres than those who relapsed (129). This may be attributed to the fact
that the longer telomere-carrying CML cells can escape senescence
and can divide following hte discontinuation of therapy (129).
Notably, Samassekou et al (130), examining telomere length at both
ends of chromosomes, observed that p-ends carried longer telomeres
than q-ends and that q-ends presented a higher shortening rate than
p-ends). Furthermore, patients with CML in the chronic phase
harbored specific telomere length changes of the longest individual
telomeres on chromosomes 18p and Xp and the shortest individual
telomeres on chromosomes 21p and 21q (130).
8. Integumentary system
Melanoma of the skin
Associations between telomere length and the
presentation of cutaneous melanoma are heterogeneous, with the
majority of studies concluding that shorter telomere lengths are
associated with a decreased risk of developing skin melanoma. By
contrast, longer telomeres exhibit a positive association (131-139). In the case of melanoma, shorter
telomeres exhibit a protective function against the malignant
transformation of melanocytes, since these cells have a limited
proliferative ability and capability of undergoing apoptosis
(132,136). Indeed, melanocytes carrying
longer telomeres do not go through senescence or apoptosis; thus,
there is increased melanocyte proliferation, as well as a
propensity for nevi and melanoma development (138). Indeed, Viceconte et al
(140) suggested that metastatic
cutaneous melanoma cells carried longer telomeres, which provides
these cells with sufficient replicative potential without
activating a telomere maintenance mechanism, and finally
contributing to tumor development. On the other hand, shorter
telomeres have also been associated with an inferior survival,
since critically short telomeres can trigger events that create
genetic instability and tumorigenesis (139).
Notably, shorter telomeres have also been found to
be associated with a lower number of skin moles (135), while longer telomeres are
positively associated with a higher number of skin moles (133). Indeed, some authors have
suggested that melanomas may develop from existing moles whose
cells continue to proliferate because of delayed replicative
senescence (133). Anic et
al (133) also identified an
association between longer telomeres and an elevated risk of
developing melanoma in females, although no association was
indicated for males.
However, the association between telomere length
and the incidence of melanoma appears to differ between sporadic
and familial melanoma. Thus, Menin et al (141) demonstrated that patients with
sporadic melanoma exhibited a shorter telomere length than patients
with familial melanoma. Indeed, even though shorter telomeres
decreased the risk of developing familial melanoma, they tripled
the risk of developing single sporadic melanoma (141). These data correlate well with the
characterization of melanoma as a complex disease with a
multifaceted etiology, and indicate that telomere length may affect
each type of melanoma in a discrete manner (141). Undoubtedly, telomere-related
genes are also related to the susceptibility of melanoma (134). However, further extensive studies
need to be conducted to comprehend the role of telomeres in
melanoma.
9. Endocrine system
Thyroid cancer
In 2000, Kammori et al (142) indicated that telomere length was
reduced in thyroid cancer tissues and follicular adenomas, compared
to normal tissues. However, it was shown that follicular adenomas
and papillary carcinomas had elevated mean terminal restriction
fragment values compared to the controls. Moreover, the mean
terminal restriction fragment values were significantly shorter in
telomerase-positive samples than in telomerase-negative ones in
both follicular and papillary carcinomas (143).
Moreover, efforts were made to identify potential
differences in telomere length among familial and sporadic thyroid
cancer patients. This distinction may be critical as thyroid cancer
exhibits the highest genetic predisposition among other cancer
types (144), even though
Jendrzejewski et al (145)
did not detect any differences between telomere length in blood
samples of familial papillary thyroid cancer (fPTC) and sporadic
papillary thyroid cancer (sPTC) cases of papillary thyroid cancer.
Capezzone et al (146)
identified shorter telomeres in fPTC than in sPTC blood samples, as
demonstrated using both quantitative PCR and FISH. Notably, a
shorter telomere length was detected in all tissues of patients
with fPTC in contrast to those with sPTC, indicating that the
differences in telomere length were not restricted to tumor sites
(147). These authors
hypothesized that the shorter telomeres may have been inherited
from parents (147). Indeed, it
had been demonstrated that the relative telomere length in patients
with second-generation fPTC was similar or even shorter to that of
parents and unaffected siblings, suggesting that telomere length is
partly transmitted to offspring (146).
On the other hand, patients with familial
non-medullary thyroid cancer had shorter telomeres than the
controls (148).
As regards cancer risk, no association between
telomere length and the risk of thyroid subsequent malignant
neoplasm was detected in childhood cancer survivors (149). Nonetheless, Li et al
(150) demonstrated that telomere
length was associated with the risk of papillary thyroid cancer.
Specifically, a reverse U-shaped association between telomere
length and the risk of cancer was identified, particularly in
younger subjects, indicating that both short and long telomeres can
be correlated with the risk of cancer development (150).
Therefore, a complex pattern between the risk of
developing thyroid cancer and telomere length variation is
emerging, and this warrants further analysis.
10. Reproductive system
Prostate cancer
Prostate cancer is characterized by significant
telomere shortening, which results in genomic instability and even
chromothripsis identified in >50% of prostate cancer precursor
lesions (151). Indeed, short
telomeres have been shown to be associated with an increased risk
of developing prostate cancer, the risk of recurrence, and a worse
prognosis due to the accelerated senescence of immune cells
(152). Thus, more aggressive
types of prostate cancer presented shorter telomeres (152). Tsai et al (153) also concurred with these results
in a study conducted on African-American males. However, a separate
study did not detect an association between telomere length and
recurrence and prostate cancer-specific mortality. However, shorter
telomeres detected in the stroma and epithelial cells were
associated with metastasis (154). In another study telomere length
was assessed in a cohort of 15 patients with prostate cancer who
underwent radiotherapy utilizing telomere FISH (155). Length data were implemented in a
machine learning model, XGBoost, trained on pre-irradiation
(baseline) and in vitro exposed (4 Gy γ-rays) telomere length
measurements, to predict post-irradiation telomeric outcomes. The
authors of that study demonstrated that a machine learning model
with individual telomere length data for the prediction of
post-radiotherapy telomeric outcomes can provide an improved
predictive power and novel insight into individual patient
radiosensitivity and the risk of radiation-late toxicity. It could
be used regardless of cancer type, radiation method, or genetic
susceptibilities (155).
Genetic factors also appear to play a role. It was
previously demonstrated that individuals carrying the RTEL1
rs2297441 variant AA had shorter telomeres and an increased risk of
prostate cancer (156). Hurwitz
et al (157) did not
observe an association between leukocyte telomere length and
prostate cancer in males from hereditary prostate cancer families.
Still, they hypothesized that shorter telomeres may be associated
with an elevated risk of developing prostate cancer in a subset of
genetic diseases (157).
On the other hand, longer telomeres have also been
shown to be associated with the risk of developing prostate cancer.
The study by Julin et al (158) revealed a moderate association
between longer telomeres and an increased risk of developing
prostate cancer, particularly in males with a family history of the
disease. In addition, longer telomeres increased overall mortality
due to a suppressed immune system (158). In another study, longer telomeres
were associated with a worse prostate cancer-specific and
metastasis-free survival compared to shorter ones (160). Of note, Wulaningsih et al
(161) first indicated that
increased levels of total prostate-specific antigen were associated
with longer telomeres.
In a separate study, the telomere lengths of
prostatic small cell neuroendocrine carcinoma (SCNC) and prostatic
adenocarcinoma (AdCa) were compared (162). Both cell types exhibited
relatively similar telomere lengths, indicating their common
origin, although longer telomeres were more common in SCNC
(162). Furthermore, longer
telomeres in AdCa were associated with more aggressive tumors of
aggressive pathological and molecular characteristics (162).
Smoking has also been found to be associated with
the development of prostate cancer. Notably, Mirabello et al
(163) indicated that,
particularly in the case of heavy smokers of the male sex without a
family history of the disease, shorter telomeres were associated
with a reduced risk of developing prostate cancer. However, another
study did not detect any difference concerning telomere length,
smoking and prostate cancer. Indeed, it was shown that recent
smokers had an elevated variability in telomere length in prostate
stromal and cancer cells than long-term smokers (164). Moreover, it was indicated that
males of African origin with higher-grade disease had a higher
variability in telomere length than Caucasian males with the same
disease classification (165).
Breast cancer
Numerous studies have focused on the association
between breast cancer risk and telomere length. Thus, longer, as
well as shorter telomeres have been found to be associated with an
increased risk of developing breast cancer. Indeed, it has been
well-established that longer telomeres are associated with an
enhanced telomerase activity and may facilitate the incidence of
genetic mutations (166). In a
previous study, longer telomeres were detected in patients with
breast cancer compared with the controls (167). That study was performed on blood
cells collected from 611 patients with breast cancer and 154
healthy women in Prague between 2002 and 2010 (167). A similar association on blood
cell telomere length was determined in a Chinese female population
(168), as well as in Indigenous
American women (169).
However, shorter telomeres have also been shown to
be associated with an increased risk of developing breast cancer,
initially in older, premenopausal or postmenopausal women (170,171). Indeed, estrogen levels have been
previously linked with telomere length; thus, the menopausal status
could influence telomere length and its connection to insulin
resistance and inflammation (171). However, no association between
telomere length and the risk of hereditary breast cancer has been
observed (172).
Varying results were also obtained when the
putative association of telomere length with breast cancer
progression was examined. For example, measuring peripheral
leukocyte telomere length at baseline and 30 months post-diagnosis
in a cohort of breast cancer survivors did not detect an
association with either all-cause or breast cancer-specific
mortality. However, participants whose telomeres exhibited
shortening between baseline and 30 months exhibited a higher risk
of breast cancer-specific and all-cause mortality (173). These authors hypothesized that
longer telomeres may protect cells from entering into
breakage-fusion-bridge cycles, especially those that induce cell
senescence (173).
When telomere length and telomerase activity were
examined in breast cancer cell lines with various levels of
invasiveness, a paradoxical concurrence of enhanced telomerase
activity and short telomeres was detected in the most aggressive
cell lines. Furthermore, the intracellular localization of hTERT
intracellular localization was associated with its activity levels
(174). Indeed, it was suggest
that telomere length and telomerase activity may be utilized as
biomarkers for assessing the aggressiveness of breast cancer cells
(174).
A clinical study examining a total of 44 breast
cancer tissues, including 15 papillotubular, 17 scirrhous and 12
solid-tubular carcinomas, determined that telomeres measured using
quantitative FISH were shorter in cancer cells compared to normal
epithelial cells (175). In
another clinical study, blood leukocyte telomere length was
measured in 52 cancer patients and matching control subjects
utilizing quantitative PCR. This approach demonstrated that the
average telomere length of patients with advanced-stage disease was
shorter compared to those with early-stage disease. Notably,
patients with human epidermal growth factor receptor 2
(HER2)+ breast cancer had significantly longer telomeres
than HER2− patients (176). HER2 is a biological marker for
disease prognosis and disease aggressiveness, and its association
with telomere length may provide insight into disease progression
and malignancy (177). These data
indicate the complexity of the roles of telomeres in breast cancer
pathogenesis. Indeed, the association of telomeres with breast
cancer progression appears to depend on disease stage, patient age
and hormone receptor status.
A number of studies have confirmed the complex
pattern of putative associations where genetic factors play a role.
For example, it was previously demonstrated that patients
homozygous for the variant allele (CC) of hTERC rs16847897
presented longer telomeres (167), while patients with the AA allele
of rs2853677 had longer telomeres than those with AG (170). Other examples are BRCA1
and BRCA2 gene mutations concerning telomere length and
breast cancer susceptibility in women with a high hereditary risk
of developing breast cancer. Thus, Eyüboǧlu et al (177) indicated that patients with BRCA1
and/or BRCA2 mutations had a 12% telomere attrition compared with
women with no BRCA1 and/or BRCA2 mutations. Notably, BRCA2
mutations have been shown to be associated with the maintenance of
telomere length (178).
Thorvaldsdottir et al (179) also concurred with the latter
result and indicated that patients with breast cancer had shorter
telomeres compared with healthy women in the case of both
BRCA2 mutation carriers and noncarriers. Moreover,
BRCA2 mutation carriers with shorter telomeres exhibited an
increased risk of developing increased breast cancer, which was not
evidenced in non-carriers. Other factors, however, affect the
connection to telomere biology. Shorter telomeres in patients with
breast cancer have also been shown to be associated with low levels
of physical activity (180,181). Indeed, physical activity may
hinder cellular aging and protect individuals from age-related
diseases (181).
Moreover, telomere length is associated with
psychoneurological symptoms (PNS) in breast cancer survivors
(182). Specifically, increased
levels of pain and lower scores in the visual memory domain have
been shown to be associated with shorter telomeres (182). Chemotherapy perhaps induces
telomere breakage and chromosome instability, triggering immune
surveillance pathways and causing inflammation (182). This may compromise tissue
homeostasis and create genetic alteration, leading to the
acquisition or persistence of PNS. To summarize, further studies
are required in order to better understand the mechanistic aspects
of telomere involvement in breast cancer development and
progression and enhance telomere biology application in disease
evaluation (Table II).
 | Table IITelomere length and breast
cancer. |
Table II
Telomere length and breast
cancer.
| Cancer type | Sample type | Origin of study
population | Telomere length
(mean ± SD) | Telomere
length/cancer risk | Clinical
significance |
Authors/(Refs.) |
|---|
| Breast cancer | Blood sample | Turkish | | Shorter
telomere/higher risk | BRCA1/BRCA2
mutations are associated with shorter telomere length in women with
a high hereditary risk of developing breast cancer | Eyüboğlu et
al (177) |
| Breast cancer | Peripheral blood
leukocytes | Chinese | 1.07±0.22 | Longer
telomere/higher risk | Longer telomeres
may be a risk factor and act as a cancer risk predictor | Samavat et
al (168) |
| Breast cancer | Leukocyte telomere
length | Chinese | | Shorter
telomere/lower risk | Telomere length
associated with breast cancer susceptibility | Luu et al
(229) |
| Breast cancer | Peripheral blood
leukocytes | | | No correlation | | Pavanello et
al (172) |
| Breast cancer | Whole blood or
mouthwash samples | US/Hispanic | | Longer
telomere/higher risk | Risk assessment
appears to be modeled by genetic ancestry, specifically Indigenous
American | Pellatt et
al (169) |
| Breast cancer | White blood
cell | | 0.70±0.33 | Shorter
telomere/higher risk | More pronounced in
pre-menopausal women | Shen et al
(171) |
| Breast cancer | Whole blood | | | Shorter
telomere/higher risk | Such an association
was observed in patients with BRCA2 mutations, not in
non-carriers | Thorvaldsdottir
et al (179) |
| Breast cancer | Peripheral
blood | Chinese/Han | | Shorter
telomere/higher risk | This association
applies to all subject groups (age >40 years, BMI ≤24
kg/m2 and post-menopausal women) | Wang et al
(218) |
Ovarian cancer
Ovarian cancer is another hormone-responsive cancer
whose pathogenesis is closely associated with fluctuations in sex
hormones and discrete receptor expression. Initially, it was shown
that the peripheral blood leukocytes of patients with ovarian
cancer have shorter telomeres compared to those of age-matched
healthy women (183). Moreover,
it was determined that the strength of the association was
inversely related to the telomere length of more aggressive types
of tumors (183). That study was
in agreement with the findings of the study by Kuhn et al
(184), demonstrating that
telomere length changes depending on the ovarian tumor histological
type. Specifically, shorter telomeres were detected in high- and
low-grade serous carcinomas and low-grade endometrioid carcinomas
of the ovaries than clear cell ovarian carcinoma (184). However, these authors did not
find an association between overall mortality and telomere length
in these main ovarian cancer types (185). The exception was clear cell
carcinoma of the ovaries, where the death hazard ratio among
females with a telomere index >1 was higher when compared with
those with a telomere index ≤1. The telomere index was defined as
the mean telomere length of cancer cells relative that of to
stromal cells (184).
Martinez-Delgado et al (186) demonstrated that sporadic, as well
as familial cases of ovarian cancer had shorter telomeres than the
controls when age-adjusted. Furthermore, these authors suggested
that shorter telomeres were associated with an increased risk of
developing ovarian cancer, particularly in younger females, with
the risk progressively decreasing with age (186). In separate studies, shorter
telomeres were associated with worse outcomes, as well as unplanned
hospital admissions (187), while
longer telomeres were associated with a reduced risk of non-severe
and rapidly fatal cases (188).
On the other hand, it was shown that the minor allele at the peak 2
SNP rs7705526 was associated with longer telomeres and an increased
risk of developing low-malignant-potential ovarian cancer (the
change in relative telomere length being 1.020-fold per allele)
(189).
However, Terry et al (190) did not observe any difference
between the telomere length of ovarian cancer cases and the
controls, although they suggested that a genetic variation in the
TERT gene could affect the risk for this malignancy. In
addition, the study by Kotsopoulos et al (191) did not find any association
between telomere length and ovarian cancer-specific mortality,
suggesting that telomere length cannot predict outcome following
diagnosis.
Several associations between telomere length and
treatment strategies have been identified for patients with ovarian
cancer. As regards therapies against ovarian carcinoma, some women
are treated with glucose restriction combined with chemotherapy
(192). Notably, telomerase is
overexpressed in >80% of human cancers (193). It was previously shown that the
administration of platinum-taxane chemotherapy, under fasting
glucose conditions, significantly decreased telomerase expression,
resulting in a 30% decrease in telomere length and in the
attenuation of ovarian cancer cell immortalization (192). Notably, ovarian tissue
cryopreservation is a process through which patients with ovarian
cancer manage to preserve fertility (194). However, the mean telomere length
is reduced following cryopreservation, inducing cellular senescence
and DNA damage (194).
Therefore, these collective data indicate that the
association between telomere length and ovarian cancer pathogenesis
is influenced by the patients' age and the ovarian tumor
histological type. These factors need to be taken into
considerations before consensus can be reached.
Cervical cancer
The pattern of discrete associations between cancer
incidence and telomere length is repeated in cervical cancer. Zhang
et al (195) initially
observed both the shortening and elongation of telomeres in
patients with cervical cancer. However, in another study, telomere
FISH assays revealed that early-stage cervical intraepithelial
neoplasias (CINs), particularly CIN2, exhibited shorter telomeres
compared to neighboring normal squamous epithelia. This was
strongly associated with increased rates of chromosomal arm
loss/gain (196). Moreover,
cervical cancer tissue presented more significant heterogeneity as
regards telomere length, suggesting that the progressive shortening
of telomeres may facilitate the transformation of CIN to cervical
cancer. On the other hand, no significant differences in the
telomere length of the normal endometrium and endometrial
hyperplasia and cancer were detected (196).
High-risk human papillomavirus (HR-HPV) can cause
cervical cancer; however, a shortened telomere length in cervical
exfoliated cells has been shown to be associated with a lower risk
of developing cervical cancer among HR-HPV-positive women. Thus, it
has been suggest that shorter telomeres may decrease the risk of
developing cervical cancer in HR-HPV-positive patients (197). Indeed, in this case, the shorter
telomeres may act as a suppressor and hinder proliferation
(197).
It is noteworthy that telomeres are transcribed
into heterogeneous long non-coding RNA, known as telomeric
repeat-containing RNA (TERRA) (198). Of note, TERRA, which usually have
a short half-life, tend to accumulate in rapidly-growing cancer
cells, with the result that high TERRA levels are detected in
various human cancer types (199). Thus, even though TERRA abundance
was not found to be associated with telomere length in six cervical
cancer cell lines, its abundance was found to be associated with
RNA stability, and possibly, telomeres (200). Another example of genetic
influence is the participation of homeobox containing 1 (HMBOX1) on
telomere length. HMBOX1 was initially attributed to the properties
of binding to double-stranded DNA (201). Moreover, this protein was
identified as a positive regulator of telomere length (202). Furthermore, it was indicated that
HMBOX1 knockdown induced radiosensitivity in cervical cancer cells
and led to shorter telomeres, enhanced DNA damage response, and
increased levels of apoptosis (203).
In summary, shorter telomeres appear to be
associated with CIN transformation to cervical cancer, but not in
HPV-positive patients, whereas the asscoiation with genetic factors
may play a significant role (Table
III).
 | Table IIITelomere length and HPV
infection. |
Table III
Telomere length and HPV
infection.
| Sample type | Measurement
method |
Association/significance |
Authors/(Refs.) |
|---|
| Tissue sample | PCR-based TRAP
assay | No correlation
between HPV infection/telomere length | Zhang et al
(233) |
| Cervical exfoliated
cells | PCR | Shorter telomere
length/lower risk of cancer in HPV-positive women; telomere length
may function as a biomarker to detect high-risk individuals during
screening | Chen et al
(197) |
11. Digestive system
Esophageal cancer
A complex pattern emerges when analyzing data on
the association between telomere length and esophageal cancer. Both
short and long telomeres are implicated with a U-shaped
association. As with the other types of cancer, the findings of
research studies vary considerably, depending on the clinical
outcomes and the parameters under investigation. To begin with,
multiple studies using esophageal squamous cell carcinoma (ESCC)
cells have proven that these cells possess shorter telomeres than
the controls (204-206). Moreover, it has also been shown
that telomere alterations not only affect the esophageal
epithelium, but also stromal cells. This is crucial, as, in this
case, stromal cells of cancer lesions have been identified to have
longer telomeres resulting in chromosome 4q, 13q and 15q
instability (207). Notably, Xing
et al (205) indicated
that shorter telomeres were detected, particularly on chromosomes
17p and 12q, but not 11q and 2p of ESCC cells. This may occur since
p53 and other tumor suppressor genes are located on 17p (205). On the other hand, Du et al
(204) observed a U-shaped
association between telomere length and ESCC risk, indicating that
both extremely short and long telomeres may affect tumor
progression. However, Lin et al (207) did not find any association
between telomere length and ESCC precursor lesions. Furthermore,
genotyping studies have identified several SNPs related to telomere
length that are associated with the susceptibility to ESCC
(208-211). Specifically, CXCR4
rs6430612, TERT rs13172201 and OBFC1 rs4387287 in
short telomeres were found to increase the risk of developing ESCC
(208). At the same time, the A
allele of telomere-related SNP rs2736108 was associated with longer
telomeres, as well as a more prolonged survival (209) suggesting an underlying protective
mechanism against ESCC (210). It
was shown that the rs621559 AA genotype decreased the risk of
developing ESCC, compared to the GG genotype, while the 14q21
rs398652 G allele exhibited an increased cancer risk (210). These associations were
sex-dependent, with stronger associations detected in males
(210). Lastly, Hao et al
(211) indicated that patients
with p53 somatic mutations had shorter telomeres, inducing
increased proliferation and susceptibility to tumor development.
Moreover, the rs12951053 CC genotype and the rs1042522 GG genotype
were shown to be associated with shorter telomeres (211). Yu et al (212) demonstrated that short telomeres
in combination with Arg/Pro or Arg/Arg genotypes and HPV-16
seropositivity increased the risk of ESCC 12.08-fold. On the other
hand, in another study, no association between leukocyte telomere
length and disease was detected in esophageal adenocarcinoma
(213). Of note, Pan et al
(214) demonstrated that when
short telomeres were combined with epidemiological factors, such as
smoking and excessive alcohol intake, there was a 16.82-fold
increase in the risk of developing ESCC.
Gastric cancer
Even though gastric cancer is a rather complex
entity where genetic, environmental and microbial parameters appear
to be involved (215). Tahara
et al (215) demonstrated
that patients with gastric cancer exhibited shorter leukocyte
telomeres than the healthy controls, this observation is in
agreement with the results from previous and later studies
(216,217). When an analysis of telomere
length in patients with gastric cancer and age- and sex-matched
controls was performed, an association with aging, a history of
smoking, a decreased fruit intake and Helicobacter pylori
positivity with short telomeres was established (216). Shorter leukocyte telomeres were
also shown to be associated with a worse overall survival and
progression-free survival in patients with advanced-stage disease
(215). An association between
peripheral blood leukocyte telomere length and the risk of gastric
cancer was prospectively assessed in a cohort of 26,540 middle-aged
or older Chinese patients. This strategy identified a significantly
higher risk of developing gastric cancer with the lowest or the
highest quintile of telomere length, most apparent in males and
younger individuals (218). On
the other hand, a prospective study encompassing a cohort of 40,000
European participants did not identify an association between the
risk of gastric cancer and telomere length (219). These data collectively suggest
that the effect of telomere length may differ depending on the
disease stage, sex, or even racial origin.
Furthermore, a reduced immune response and a higher
percentage of CD4+ T-cells and CD19+
IL-10+ Breg percentage in B-cells and plasma IL-10
concentration were shown to be associated with shorter telomeres in
cancer patients (217). Shorter
telomeres were also linked associated with Helicobacter
pylori-positive patients, smokers with a low fruit/vegetable
intake, moderate or severe gastritis and intestinal metaplasia
(215,216). In separate studies, smoking was
also found to be associated with the risk of developing gastric
cancer and a shorter telomere length (220). Moreover, leukocyte telomere
length was found to strongly contribute to the predisposition to
gastric cardia carcinoma in a cohort of the Chinese Han population.
Notably, the combination of shorter telomere length and smoking
enhanced the development of this cancer type (221). Thus, Hou et al (216) concluded that shorter telomeres
increased the risk of developing gastric cancer, perhaps through
the impairment of cellular functions, creating chromosome
instability.
The known association between Helicobacter
pylori and gastric cancer has also been linked to telomere
length. Thus, patients infected with Helicobacter pylori
infection have shorter telomeres, perhaps due to related
inflammatory cytokine release (222). Furthermore, the
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (PIK3CA) amplification could possibly be an integral part of
carcinogenesis (222,223). Therefore, Tahara et al
(222) suggested that
Helicobacter pylori eradication could significantly decrease
the risk and mortality from gastric cancer.
Genotypic factors associated with telomere length,
such as different TERT variants, can increase the risk of
developing gastric cancer (224).
For instance, TERT variants such as rs10069690, rs2242652 and
rs2853676, and TN1P1 variants such as rs7708392 and rs10036748 have
been shown to be associated with an increased risk of gastric
cancer (224). In addition, Du
et al (225) indicated
that the G allele of rs2736100 in TERT at 5p15.33 was
associated with longer telomeres; Choi et al (226) indicated that in the same variant,
the CC genotype had longer telomeres than the AA. Notably, Du et
al (225) also identified a
U-shaped association between telomere length and the risk of
developing gastric cancer.
Pancreatic cancer
Multiple studies have suggested that short
telomeres increase the risk of developing pancreatic cancer.
Telomere shortening is one of the earliest events in tumorigenesis;
thus, shorter telomeres may increase the risk of developing
pancreatic cancer and also with its progression (227,228). On the other hand, some studies
have demonstrated that longer telomeres increase the risk of
developing pancreatic cancer, as they bestow an elevated ability to
proliferate to transformed cells (229,230). According to Mormile (231), long telomeres result from high
telomerase activity, set off by surviving overexpression through
transcriptional activation of hTERT. However, the results of
extensive epidemiological research are inconclusive regarding the
association between the risk of pancreatic ductal adenocarcinoma
(PDAC) and telomere length. This is attributed to various reasons,
including the study design and method of telomere measurement, as
discussed by Duell (228).
Other studies, however, have indicated that both
extremely long and short telomeres increase the risk of developing
this type of cancer. Skinner et al (232) demonstrated a skewed U-shape
association between telomere length and pancreatic cancer risk,
whereas Zhang et al (233)
concurred with these results for an Asian population cohort.
In a previous study, polymorphisms previously
associated with variations in telomere length were genotyped to
assess the association of genetically predicted short telomere
length with the risk of developing PDAC in light of the conflicting
findings. This approach revealed that genetically predicted short
telomere length was not associated with the risk of developing PDAC
(234). Indeed, since genetically
predicted short telomere length is not associated with the risk of
developing PDAC, it is suggested that telomere length may be a
marker of long-term exposure to various risk factors, such as
obesity, smoking and diabetes (233).
In continuation, it was determined that
treatment-naïve short leukocyte short telomere length was
associated with a higher risk of developing PDAC. The association
was not affected by the germline variation of the genotyped SNPs
(235). Furthermore,
treatment-naïve short leukocyte short telomere length was
associated with the poorer overall survival of patients with PDAC
(235). The association may
partially be attributed to the fact that shorter telomeres create
chromosome instability and can enhance the progression rapidly from
precursor lesions to invasive ductal carcinoma (236). Notably, the minor TERT allele
rs401681 was also found to be associated with short telomeres,
resulting in an increased risk of developing pancreatic cancer
(237).
The study by Posch et al (238) indicated that patients with
sporadic pancreatic neuroendocrine neoplasms with promoter
mutations had longer telomeres than those with wild-type ones. By
contrast, another study demonstrated that IL-6 cytokine production
was reduced in individuals with longer telomeres (239). It was hypothesized that shorter
telomeres were a consequence and not a result, since they may
indicate a model of pancreatic tumorigenesis with increased levels
of IL-6 accounting for the strong STAT3 (major pro-tumorigenic IL-6
effector and can influence KRAS-induced pancreatic tumorigenesis)
activation implicated in KRAS-driven pancreatic cancer (239). Both epidemiological and
environmental factors appear to affect the association of telomere
length with the risk of developing pancreatic cancer.
Colorectal cancer
The latest advances regarding colorectal cancer
suggest that telomere length in tumor tissues is shorter than in
the adjacent mucosa (240,241).
Furthermore, tumors with a higher number of somatic mutations
present shorter telomeres (241).
Moreover, an association between tumor stage and telomere length
was previously identified, as lower-stage tumor tissues exhibited
shorter telomeres than advanced-stage and metastatic tumors
(240). These studies
characterize colorectal cancer tissue with both chromosomal or
microsatellite instability (240,241). Moreover, Piñol-Felis et al
(242) concluded that telomere
length could be used as a reliable prognostic factor, since
telomere shortening was, in their study, associated with an early
stage of tumorigenesis.
However, some studies have identified an
association between the risk of developing colorectal cancer and
longer telomeres (240,243). The two different associations
detected in patients with colon cancer are suggested to reflect
alternative mechanisms of tumorigenesis and specific disease stages
(240,243). Likewise, the studies by Luu et
al (244) and Peacock et
al (245) demonstrated an
association between longer telomeres and an increased risk of
colorectal cancer. Specifically, longer telomeres exhibited an
elevated risk of accumulating mutations that could lead to
transformation and cancer progression (244,245). However, a meta-analysis study
indicated no association between telomere length and the risk of
colorectal cancer (246).
Furthermore, in another study, the analysis of
telomere-related protein expression in colorectal cancer tissues
revealed differences relative to the adjacent mucosa. A positive
association between hTERT expression and patient age in a Saudi
Arabian cohort was identified and was associated with the patients'
clinicopathological characteristics (247).
Genetic factors affect the association between the
risk of developing colorectal cancer and telomere length. Park
et al (248) identified
that telomere shortening in cases of tubular adenomas was mainly
caused by the PIK3CA amplification. Bu contrast, telomere
shortening in serrated polyps was attributed to BRAF mutations
(248). Furthermore, these
authors suggested that tumor genotyping may be a helpful tool to
monitor tumor progression (248).
Moreover, a rare P507L variant in TPP1 may increase the risk of
developing colorectal cancer by interrupting the TPP1-TIN2
interaction, thus impairing telomerase activity and decreasing
telomeres (249).
Epidemiological factors appear to be involved, as a
study detected shorter telomeres in depressed individuals and
identified education and social support as factors towards
alterations in telomere length (250). However, a separate study failed
to detect an association between religiosity and telomere length
(251).
A complex pattern on the association between colon
cancer risk and telomere length is emerging, which warrants further
validation through studies performed with a larger number of
patients (Table IV).
 | Table IVTelomere length and colorectal
cancer. |
Table IV
Telomere length and colorectal
cancer.
| Cancer type | Sample type | Origin of study
population | Telomere
length/cancer risk | Clinical
significance |
Authors/(Refs.) |
|---|
| Colorectal
adenoma | Colonic tissue
samples | | Longer
telomere/higher risk | | Peacock et
al (245) |
| Colorectal
cancer | | Chinese | Shorter
telomere/higher risk | A rare variant,
P507L in TPP1 increases the risk of colorectal cancer through
telomere shortening | Li et al
(150) |
| Colorectal
cancer | White blood
cells | Chinese | Longer
telomere/higher risk | Particularly for
rectal cancer, longer telomeres could play a role in cancer
pathogenesis, thus acting as a biomarker | Luu et al
(244) |
| Colorectal
cancer | Peripheral blood
leucocytes | | Inconclusive
results (metanalysis of seven studies) | The complex
relationship between telomere length and cancer risk | Naing et al
(246) |
Liver cancer
The association between the incidence of
hepatocellular cancer (HCC) and telomere length is dependent on
cancer stage. Patients with HCC have shorter telomeres compared to
healthy controls, although longer telomere lengths have been
detected in patients with advanced-stage disease (252-255). Furthermore, shorter telomeres
have been shown to be associated with a decreased survival,
increased recurrence and numerous TERT promoter mutations (253,255). On the other hand, longer
telomeres have been found to be associated with more aggressive
types of tumors and a poor prognosis (252,254). In addition, it is suggested that
longer telomeres may prevent telomere attrition by suppressing
reactive oxygen species or phosphorylated AKT levels (252).
Notably, longer telomeres, in combination with
hepatitis B virus (HBV) or hepatitis C virus (HCV) infections,
increased the risk of developing HCC (256,257). However, Cheng et al
(257) detected a U-shaped
association between telomere length and the risk of developing HCC.
At the same time, Zeng et al (256) indicated that 5 years prior to
diagnosis, shorter telomeres were associated with an increased risk
of the disease. On the contrary, longer telomere length detected 10
years prior to diagnosis contributed to the risk of developing HCC
(256). Finally, Feng et
al (258) indicated that
peripheral blood samples could be used to measure telomere length
in HBV or HCV-infected patients, but not in non-infected ones. The
viral genome may affect telomerase activity, thus affecting disease
development and persistence (258).
12. Conclusions and future perspectives
Telomeres, chromosome-end DNA-protein structures,
are known to progressively shorten over time in the majority of
somatic cells. These genome-protecting structures are markers of
aging. Notably, short telomeres have been associated with an older
age and chronic diseases. An association between cancer and
telomere length has been suggested with related uncertainties due
to objective difficulties in designing studies of sufficient
robustness. A disparity appears to emerge when evaluating risk
associations between cancer and telomere length. Even though
shorter telomeres have been adopted as a marker of a poorer health
status and an older biological age, longer telomeres due to
increased growth potential are associated with acquiring
cancer-initiating somatic mutations (56). Indeed, the majority of
retrospective studies report an increased risk of cancer in
individuals carrying shorter telomeres (27,134,145,163,209) whereas prospective observational
studies have detected a weak positive association between longer
leukocyte telomeres with the risk of cancer (51,88,123,136,245,256). This association pattern may be
partly accounted for by the varying ability of somatic cells to
grow; thus, telomere length could exert discrete potential effects.
Moreover, a U-shaped curve of telomere length effects has been
detected in various types of cancer (150,156,216,237,256). This suggests that the association
with cancer risk would vary among telomere length distribution and
would not be linear. The pivotal clinical relevance of this
knowledge stands on the fact that oncologists who treat these
malignancies may seek for another tool in their effort to tackle
cancer. Moreover, pathologists may use telomeres, telomerase, hTERT
or any of the implicated proteins as potential biomarkers for
cancer prognosis (259,260). Furthermore, the
cancer-type-specific association is influenced by co-factors, e.g.,
virus load, inflammatory status, or SNP-disease association.
Moreover, the effect of telomere length may differ, depending on
the disease stage, sex, or even racial belonging. This is critical,
particularly for treatment strategies targeting telomerase, since
tumors that present long telomeres may be affected, but others that
exhibit short telomeres may not have the same response (261). Therefore, telomere length data
may provide valuable input on cancer development and progression
for certain types of cancers; however, further studies are required
for more generalized conclusions.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (AT, TO, TKN, EV, MTz, MF, ER, PF, EI,
MB, VK, IK, FK, EH, MTo, AAS, DAS, DN, JT and AB) contributed to
the conception and design of the study. TO, TKN, EV, MF, ER, PF,
EI, MB, VK, IK and FK searched the literature for studies to be
included in the review; the selected literature was then examined
and reviewed by EH, DAS, JT, DN, AAS, EV, AB and MTz drafted and
wrote the manuscript. AT, AAS, AB and DAS provided advice and
critically revised the manuscript. All authors have read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Abbreviations:
|
HDI
|
human development index
|
|
TRF
|
telomere repeat-binding factor
|
|
TIN2
|
nuclear protein 2
|
|
TPP1
|
tripeptidyl-peptidase 1
|
|
POT1
|
protection of telomeres 1
|
|
DDR
|
DNA damage response
|
|
TERT
|
telomerase reverse transcriptase
|
|
ALT
|
alternative lengthening of
telomeres
|
|
PML
|
promyelocytic leukemia
|
|
APBs
|
ALT-associated PML bodies
|
|
IARC
|
International Agency for Research on
Cancer
|
|
SCLC
|
small cell lung cancer
|
|
NSCLC
|
non-small cell lung cancer
|
|
RCC
|
renal cell carcinoma
|
|
ALL
|
acute lymphocytic leukemia
|
|
AML
|
acute myelogenous leukemia
|
|
CLL
|
chronic lymphocytic leukemia
|
|
CML
|
chronic myelogenous leukemia
|
|
fPTC
|
familial papillary thyroid cancer
|
|
sPTC
|
sporadic papillary thyroid cancer
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
SCNC
|
small cell neuroendocrine
carcinoma
|
|
AdCa
|
prostatic adenocarcinoma
|
|
CINs
|
cervical intraepithelial
neoplasias
|
|
HR-HPV
|
high-risk human papillomavirus
|
|
HMBOX1
|
homeobox containing 1
|
|
ESCC
|
esophageal squamous cell
carcinoma
|
|
HCC
|
hepatocellular carcinoma
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tzanakakis G, Giatagana EM, Kuskov A,
Berdiaki A, Tsatsakis A, Neagu M and Nikitovic D: Proteoglycans in
the pathogenesis of hormone-dependent cancers: Mediators and
effectors. Cancers (Basel). 12:24012020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maciejowski J and de Lange T: Telomeres in
cancer: Tumor suppression and genome instability. Nat Rev Mol Cell
Biol. 18:175–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shay JW and Wright WE: Telomeres and
telomerase: Three decades of progress. Nat Rev Genet. 20:299–309.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim CJ and Cech TR: Shaping human
telomeres: From shelterin and CST complexes to telomeric chromatin
organization. Nat Rev Mol Cell Biol. 22:283–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dratwa M, Wysoczańska B, Łacina P, Kubik T
and Bogunia-Kubik K: TERT-Regulation and roles in cancer formation.
Front Immunol. 11:29302020. View Article : Google Scholar
|
|
7
|
Hrdličková R, Nehyba J and Bose HR:
Alternatively spliced telomerase reverse transcriptase variants
lacking telomerase activity stimulate cell proliferation. Mol Cell
Biol. 32:4283–4296. 2012. View Article : Google Scholar
|
|
8
|
Sæbøe-Larssen S, Fossberg E and Gaudernack
G: Characterization of novel alternative splicing sites in human
telomerase reverse transcriptase (hTERT): Analysis of expression
and mutual correlation in mRNA isoforms from normal and tumour
tissues. BMC Mol Biol. 7:262006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Listerman I, Sun J, Gazzaniga F, Lukas JL
and Blackburn EH: The major reverse transcriptase-incompetent
splice variant of the human telomerase protein inhibits telomerase
activity but protects from apoptosis. Cancer Res. 73:2817–2828.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takakura M, Kyo S, Kanaya T, Hirano H,
Takeda J, Yutsudo M and Inoue M: Cloning of human telomerase
catalytic subunit (hTERT) gene promoter and identification of
proximal core promoter sequences essential for transcriptional
activation in immortalized and cancer cells. Cancer Res.
59:551–557. 1999.PubMed/NCBI
|
|
11
|
Tsoukalas D, Fragkiadaki P, Docea AO,
Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic
D, Kovatsi L, et al: Association of nutraceutical supplements with
longer telomere length. Int J Mol Med. 44:218–226. 2019.PubMed/NCBI
|
|
12
|
Vasilopoulos E, Fragkiadaki P, Kalliora C,
Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM,
Georgiadis G, et al: The association of female and male infertility
with telomere length (Review). Int J Mol Med. 44:375–389.
2019.PubMed/NCBI
|
|
13
|
Vakonaki E, Tsiminikaki K, Plaitis S,
Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN,
Spandidos DA and Tsatsakis AM: Common mental disorders and
association with telomere length. Biomed Rep. 8:111–116.
2018.PubMed/NCBI
|
|
14
|
Razgonova MP, Zakharenko AM, Golokhvast
KS, Thanasoula M, Sarandi E, Nikolouzakis K, Fragkiadaki P,
Tsoukalas D, Spandidos DA and Tsatsakis A: Telomerase and telomeres
in aging theory and chronographic aging theory (Review). Mol Med
Rep. 22:1679–1694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sfeir A and de Lange T: Removal of
shelterin reveals the telomere end-protection problem. Science.
336:593–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blasco MA: Telomeres and human disease:
Ageing, cancer and beyond. Nat Rev Genet. 6:611–622. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jäger K and Walter M: Therapeutic
targeting of telomerase. Genes (Basel). 7:392016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsatsakis A, Tsoukalas D, Fragkiadaki P,
Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G and
Alegakis AK: Developing BIOTEL: A Semi-Automated spreadsheet for
estimating telomere length and biological age. Front Genet.
10:842019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dunham MA, Neumann AA, Fasching CL and
Reddel RR: Telomere maintenance by recombination in human cells.
Nat Genet. 26:447–450. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teng SC and Zakian VA: Telomere-telomere
recombination is an efficient bypass pathway for telomere
maintenance in Saccharomyces cerevisiae. Mol Cell Biol.
19:8083–8093. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cesare A and Reddel R: Alternative
lengthening of telomeres: Models mechanisms and implications. Nat
Rev Genet. 11:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henson J, Neumann AA, Yeager TR and Reddel
RR: Alternative lengthening of telomeres in mammalian cells.
Oncogene. 21:598–610. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heaphy CM, Subhawong AP, Hong SM, Goggins
MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL,
Westra WH, et al: Prevalence of the alternative lengthening of
telomeres telomere maintenance mechanism in human cancer subtypes.
Am J Pathol. 179:1608–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demanelis K, Jasmine F, Chen LS, Chernoff
M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, et
al: Determinants of telomere length across human tissues. Science.
369:eaaz68762020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peleteiro B, Padrão P, Castro C, Ferro A,
Morais S and Lunet N: Worldwide burden of gastric cancer in 2012
that could have been prevented by increasing fruit and vegetable
intake and predictions for 2025. Br J Nutr. 115:851–859. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doherty JA, Grieshober L, Houck JR,
Barnett MJ, Tapsoba JD, Thornquist M, Wang CY, Goodman GE and Chen
C: Telomere length and lung cancer mortality among heavy smokers.
Cancer Epidemiol Biomarkers Prev. 27:829–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leão R, Apolónio JD, Lee D, Figueiredo A,
Tabori U and Castelo-Branco P: Mechanisms of human telomerase
reverse transcriptase (hTERT) regulation: Clinical impacts in
cancer. J Biomed Sci. 251:222018. View Article : Google Scholar
|
|
28
|
Barthel FP, Wei W, Tang M,
Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang
Q, et al: Systematic analysis of telomere length and somatic
alterations in 31 cancer types. Nat Genet. 49:349–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albertson DG: Gene amplification in
cancer. Trends Genet. 22:447–455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McClintock B: The fusion of broken ends of
chromosomes following nuclear fusion. Proc Natl Acad Sci.
28:458–463. 1942. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valentijn L, Koster J, Zwijnenburg D,
Hasselt NE, van Sluis P, Volckmann R, van Noesel MM, George RE,
Tytgat GA, Molenaar JJ and Versteeg R: NH-N and 2015 undefined:
TERT rearrangements are frequent in neuroblastoma and identify
aggressive tumors. Nat Genet. 47:1411–1414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Wiley Online Libr.
99:1528–1538. 2008.
|
|
33
|
Lewis KA and Tollefsbol TO: Regulation of
the telomerase reverse transcriptase subunit through epigenetic
mechanisms. Front Genet. 7:832016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng L, Montironi R and Lopez-Beltran A:
TERT promoter mutations occur frequently in urothelial papilloma
and papillary urothelial neoplasm of low malignant potential. Eur
Urol. 71:497–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deaton AM and Bird A: CpG islands and the
regulation of transcription. Genes Dev. 25:1010–1022. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castelo-Branco P, Choufani S, Mack S,
Gallagher D, Zhang C, Lipman T, Zhukova N, Walker EJ, Martin D,
Merino D, et al: Methylation of the TERT promoter and risk
stratification of childhood brain tumours: An integrative genomic
and molecular study. Lancet Oncol. 14:534–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castelo-Branco P, Leão R, Lipman T,
Campbell B, Lee D, Price A, Zhang C, Heidari A, Stephens D, Boerno
S, et al: A cancer specific hypermethylation signature of the TERT
promoter predicts biochemical relapse in prostate cancer: A
retrospective cohort study. Oncotarget. 7:57726–57736. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sepehri Z, Beacon TH, Osman FDS, Jahan S
and Davie JR: DNA methylation and chromatin modifications.
Nutritional Epigenomics. 13–36. 2019. View Article : Google Scholar
|
|
39
|
Bert SA, Robinson MD, Strbenac D, Statham
AL, Song JZ, Hulf T, Sutherland RL, Coolen MW, Stirzaker C and
Clark SJ: Regional Activation of the cancer genome by long-range
epigenetic remodeling. Cancer Cell. 23:9–22. 2013. View Article : Google Scholar
|
|
40
|
Hrdličková R, Nehyba J, Bargmann W and
Bose HR: Multiple tumor suppressor microRNAs regulate telomerase
and TCF7 an important transcriptional regulator of the Wnt pathway.
PLoS One. 9:e869902014. View Article : Google Scholar
|
|
41
|
Mitomo S, Maesawa C, Ogasawara S, Iwaya T,
Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu
N, et al: Downregulation of miR-138 is associated with
overexpression of human telomerase reverse transcriptase protein in
human anaplastic thyroid carcinoma cell lines. Cancer Sci.
99:280–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kachuri L, Helby J, Bojesen SE, Christiani
DC, Su L, Wu X, Tardón A, Fernández-Tardón G, Field JK, Davies MP,
et al: Investigation of leukocyte telomere length and genetic
variants in chromosome 5p15.33 as prognostic markers in lung
cancer. Cancer Epidemiol Biomarkers Prev. 28:1228–1237. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue Y, Guo X, Huang X, Zhu Z, Chen M, Chu
J, Yang G, Wang Q and Kong X: Shortened telomere length in
peripheral blood leukocytes of patients with lung cancer, chronic
obstructive pulmonary disease in a high indoor air pollution region
in China. Mutat Res. 858-860:5032502020. View Article : Google Scholar
|
|
44
|
Steiner B, Ferrucci LM, Mirabello L, Lan
Q, Hu W, Liao LM, Savage SA, De Vivo I, Hayes RB, Rajaraman P, et
al: Association between coffee drinking and telomere length in the
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
PLoS One. 15:e02269722020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun B, Wang Y, Kota K, Shi Y, Motlak S,
Makambi K, Loffredo CA, Shields PG, Yang Q, Harris CC and Zheng YL:
Telomere length variation: A potential new telomere biomarker for
lung cancer risk. Lung Cancer. 88:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jang JS, Choi YY, Lee WK, Choi JE, Cha SI,
Kim YJ, Kim CH, Kam S, Jung TH and Park JY: Telomere length and the
risk of lung cancer. Cancer Sci. 99:385–1389. 2008. View Article : Google Scholar
|
|
47
|
Sanchez-Espiridion B, Chen M, Chang JY, Lu
C, Chang DW, Roth JA, Wu X and Gu J: Telomere length in peripheral
blood leukocytes and lung cancer risk: A large case-control study
in Caucasians. Cancer Res. 74:2476–2486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jeon HS, Choi JE, Jung DK, Choi YY, Kang
HG, Lee WK, Yoo SS, Lim JO and Park JY: Telomerase activity and the
risk of lung cancer. J Korean Med Sci. 27:141–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dobija-Kubica K, Zalewska-Ziob M,
Bruliński K, Rogoziński P, Wiczkowski A, Gawrychowska A and
Gawrychowski J: Telomerase activity in non-small cell lung cancer.
Kardiochir Torakochirurgia Pol. 13:15–20. 2016.PubMed/NCBI
|
|
50
|
Li J, Zhang L, Zhu H, Pan W, Zhang N, Li Y
and Yang M: Leukocyte telomere length and clinical outcomes of
advanced lung adenocarcinoma patients with epidermal growth factor
receptor tyrosine kinase inhibitors treatment. DNA Cell Biol.
37:903–908. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Zhao Q, Zhu W, Liu T, Xie SH,
Zhong LX, Cai YY, Li XN, Liang M, Chen W, et al: The association of
telomere length in peripheral blood cells with cancer risk: A
systematic review and meta-analysis of prospective studies. Cancer
Epidemiol Biomarkers Prev. 26:1381–1390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cao X, Huang M, Zhu M, Fang R, Ma Z, Jiang
T, Dai J, Ma H, Jin G, Shen H, et al: Mendelian randomization study
of telomere length and lung cancer risk in East Asian population.
Cancer Med. 8:7469–7476. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Machiela MJ, Hsiung CA, Shu XO, Seow WJ,
Wang Z, Matsuo K, Hong YC, Seow A, Wu C, Hosgood HD, et al: Genetic
variants associated with longer telomere length are associated with
increased lung cancer risk among never-smoking women in Asia: A
report from the female lung cancer consortium in Asia. Int J
Cancer. 137:311–319. 2015. View Article : Google Scholar
|
|
54
|
Yuan JM, Beckman KB, Wang R, Bull C,
Adams-Haduch J, Huang JY, Jin A, Opresko P, Newman AB, Zheng YL, et
al: Leukocyte telomere length in relation to risk of lung
adenocarcinoma incidence: Findings from the Singapore Chinese
Health Study. Int J Cancer. 142:2234–2243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
de-Torres JP, Sanchez-Salcedo P,
Bastarrika G, Alcaide AB, Pío R, Pajares MJ, Campo A, Berto J,
Montuenga L, Del Mar Ocon M, et al: Telomere length, COPD and
emphysema as risk factors for lung cancer. Eur Respir J.
49:16015212017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Telomeres Mendelian Randomization
Collaboration; Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN,
Bowden J, Wade KH, Timpson NJ, Evans DM, et al: Association between
telomere length and risk of cancer and non-neoplastic diseases: A
mendelian randomization study. JAMA Oncol. 3:636–651. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goh F, Yang IA, Bowman RV and Fong KM:
Subtype variation and actionability of telomere length abnormality
in lung cancer. Transl Lung Cancer Res. 7(Suppl 3): S251–S253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kachuri L, Latifovic L, Liu G and Hung RJ:
Systematic review of genetic variation in chromosome 5p15.33 and
telomere length as predictive and prognostic biomarkers for lung
cancer. Cancer Epidemiol Biomarkers Prev. 25:1537–1549. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vaiciulis P, Liutkeviciene R, Liutkevicius
V, Vilkeviciute A, Gedvilaite G and Uloza V: Association of
relative leucocyte telomere length and gene single nucleotide
polymorphisms (TERT, TRF1, TNKS2) in laryngeal squamous cell
carcinoma. Cancer Genomics Proteomics. 17:431–439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Han P, Dang Z, Shen Z, Dai H, Bai Y, Li B
and Shao Y: Association of SNPs in the OBFC1 gene and laryngeal
carcinoma in Chinese Han male population. Int J Clin Oncol.
24:1042–1048. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lei H, Feng D, Zhou F, Xu H, Tang T, Yu H,
Xie C and Zhou Y: Expression of human protection of telomere 1
correlates with telomere length and radiosensitivity in the human
laryngeal cancer Hep-2 cell line. Oncol Lett. 10:1149–1154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chen W, Xiao BK, Liu JP, Chen SM and Tao
ZZ: Alternative lengthening of telomeres in hTERT-inhibited
laryngeal cancer cells. Cancer Sci. 101:1769–1776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Broberg K, Björk J, Paulsson K, Höglund M
and Albin M: Constitutional short telomeres are strong genetic
susceptibility markers for bladder cancer. Carcinogenesis.
26:1263–1271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen M, Xu Y, Xu J, Chancoco H and Gu J:
Leukocyte telomere length and bladder cancer risk: A large
case-control study and mendelian randomization analysis. Cancer
Epidemiol Biomarkers Prev. 30:203–209. 2021. View Article : Google Scholar
|
|
65
|
Pavanello S, Carta A, Mastrangelo G,
Campisi M, Arici C and Porru S: Relationship between telomere
length, genetic traits and Environmental/Occupational exposures in
bladder cancer risk by structural equation modelling. Int J Environ
Res Public Health. 15:52017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
McGrath M, Wong JY, Michaud D, Hunter DJ
and De Vivo I: Telomere length, cigarette smoking, and bladder
cancer risk in men and women. Cancer Epidemiol Biomarkers Prev.
16:815–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lin J, Blalock JA, Chen M, Ye Y, Gu J,
Cohen L, Cinciripini PM and Wu X: Depressive symptoms and short
telomere length are associated with increased mortality in bladder
cancer patients. Cancer Epidemiol Biomarkers Prev. 24:336–343.
2015. View Article : Google Scholar :
|
|
68
|
Yu C, Hequn C, Longfei L, Long W, Zhi C,
Feng Z, Jinbo C, Chao L and Xiongbing Z: GSTM1 and GSTT1
polymorphisms are associated with increased bladder cancer risk:
Evidence from updated meta-analysis. Oncotarget. 8:3246–3258. 2017.
View Article : Google Scholar :
|
|
69
|
Hosen I, Rachakonda PS, Heidenreich B, de
Verdier PJ, Ryk C, Steineck G, Hemminki K and Kumar R: Mutations in
TERT promoter and FGFR3 and telomere length in bladder cancer. Int
J Cancer. 137:1621–1629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fernandez-Gomez J, Escaf Barmadah S,
Gosalbez D, Rodriguez-Faba O, Jalon A, Gonzalez R, Garcia Miralles
T and Calas A: Telomere length on bladder washing samples from
patients with bladder cancer correlates with tumor characteristics
flow cytometry method for quantitative fluorescence in situ
hybridization (flow-FISH technique). Eur Urol. 48:432–437. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang H, Wang Y, Kota KK, Kallakury B,
Mikhail NN, Sayed D, Mokhtar A, Maximous D, Yassin EH, Gouda I, et
al: Strong association between long and heterogeneous telomere
length in blood lymphocytes and bladder cancer risk in Egyptian.
Carcinogenesis. 36:1284–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gu J, Chen M, Shete S, Amos CI, Kamat A,
Ye Y, Lin J, Dinney CP and Wu X: A genome-wide association study
identifies a locus on chromosome 14q21 as a predictor of leukocyte
telomere length and as a marker of susceptibility for bladder
cancer. Cancer Prev Res (Phila). 4:514–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Holzmann K, Blin N, Welter C, Zang KD,
Seitz G and Henn W: Telomeric associations and loss of telomeric
DNA repeats in renal tumors. Genes Chromosomes Cancer. 6:178–181.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mehle C, Ljungberg B and Roos G: Telomere
shortening in renal cell carcinoma. Cancer Res. 54:236–241.
1994.PubMed/NCBI
|
|
75
|
Dahse R, Fiedler W, Junker K, Schlichter
A, Schubert J and Claussen U: Telomerase activity and telomere
lengths: Alterations in renal cell carcinomas. Kidney Int.
56:1289–1290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pal D, Sharma U, Khajuria R, Singh SK,
Kakkar N and Prasad R: Augmented telomerase activity, reduced
telomere length and the presence of alternative lengthening of
telomere in renal cell carcinoma: Plausible predictive and
diagnostic markers. Gene. 562:145–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Callahan CL, Schwartz K, Ruterbusch JJ,
Shuch B, Graubard BI, Lan Q, Cawthon R, Baccarelli AA, Chow WH,
Rothman N, et al: Leukocyte telomere length and renal cell
carcinoma survival in two studies. Br J Cancer. 117:752–755. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hofmann JN, Lan Q, Cawthon R, Hosgood HD
III, Shuch B, Moore LE, Rothman N, Chow WH and Purdue MP: A
prospective study of leukocyte telomere length and risk of renal
cell carcinoma. Cancer Epidemiol Biomarkers Prev. 22:997–1000.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hofmann JN, Baccarelli A, Schwartz K,
Davis FG, Ruterbusch JJ, Hoxha M, McCarthy BJ, Savage SA, Wacholder
S, Rothman N, et al: Risk of renal cell carcinoma in relation to
blood telomere length in a population-based case-control study. Br
J Cancer. 105:1772–1775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Svenson U, Ljungberg B and Roos G:
Telomere length in peripheral blood predicts survival in clear cell
renal cell carcinoma. Cancer Res. 69:2896–2901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Morais M, Dias F, Teixeira AL and Medeiros
R: Telomere length in renal cell carcinoma: The Jekyll and Hyde
biomarker of ageing of the kidney. Cancer Manag Res. 12:1669–1679.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Machiela MJ, Hofmann JN, Carreras-Torres
R, Brown KM, Johansson M, Wang Z, Foll M, Li P, Rothman N, Savage
SA, et al: Genetic variants related to longer telomere length are
associated with increased risk of renal cell carcinoma. Eur Urol.
72:747–754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
de Martino M, Taus C, Lucca I, Hofbauer
SL, Haitel A, Shariat SF and Klatte T: Association of human
telomerase reverse transcriptase gene polymorphisms, serum levels,
and telomere length with renal cell carcinoma risk and pathology.
Mol Carcinog. 55:1458–1466. 2016. View Article : Google Scholar
|
|
84
|
Svenson U, Grönlund E, Söderström I,
Sitaram RT, Ljungberg B and Roos G: Telomere length in relation to
immunological parameters in patients with renal cell carcinoma.
PLoS One. 8:e555432013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Endén K, Tainio J, Hou M, Suominen A,
Pakarinen M, Huang T, Söder O, Jalanko H, Jahnukainen K and
Jahnukainen T: Telomere length regulators are activated in young
men after pediatric kidney transplantation compared to healthy
controls and survivors of childhood cancer-A cross-sectional study.
Pediatr Transplant. 23:e135502019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
HaydeéCottliar AS, Noriega MF, Narbaitz M,
Rodríguez A and Slavutsky IR: Association between telomere length
and BCL2 gene rearrangements in low- and high-grade non-Hodgkin
lymphomas. Cancer Genet Cytogenet. 171:1–8. 2006. View Article : Google Scholar
|
|
87
|
Widmann TA, Herrmann M, Taha N, König J
and Pfreundschuh M: Short telomeres in aggressive non-Hodgkin's
lymphoma as a risk factor in lymphomagenesis. Exp Hematol.
35:939–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lan Q, Cawthon R, Shen M, Weinstein SJ,
Virtamo J, Lim U, Hosgood HD III, Albanes D and Rothman N: A
prospective study of telomere length measured by monochrome
multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin
Cancer Res. 15:7429–7433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Machiela MJ, Lan Q, Slager SL, Vermeulen
RC, Teras LR, Camp NJ, Cerhan JR, Spinelli JJ, Wang SS, Nieters A,
et al: Genetically predicted longer telomere length is associated
with increased risk of B-cell lymphoma subtypes. Hum Mol Genet.
25:1663–1676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lee JJ, Nam CE, Cho SH, Park KS, Chung IJ
and Kim HJ: Telomere length shortening in non-Hodgkin's lymphoma
patients undergoing chemotherapy. Ann Hematol. 82:492–495. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Remes K, Norrback KF, Rosenquist R, Mehle
C, Lindh J and Roos G: Telomere length and telomerase activity in
malignant lymphomas at diagnosis and relapse. Br J Cancer.
82:601–607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Adamson DJ, King DJ and Haites NE:
Significant telomere shortening in childhood leukemia. Cancer Genet
Cytogenet. 61:204–206. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Engelhardt M, Ozkaynak MF, Drullinsky P,
Sandoval C, Tugal O, Jayabose S and Moore MA: Telomerase activity
and telomere length in pediatric patients with malignancies
undergoing chemotherapy. Leukemia. 12:13–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Borssén M, Cullman I, Norén-Nyström U,
Sundström C, Porwit A, Forestier E and Roos G: hTERT promoter
methylation and telomere length in childhood acute lymphoblastic
leukemia: Associations with immunophenotype and cytogenetic
subgroup. Exp Hematol. 39:1144–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang Y, Fang M, Sun X and Sun J:
Telomerase activity and telomere length in acute leukemia:
Correlations with disease progression, subtypes and overall
survival. Int J Lab Hematol. 32:230–238. 2010. View Article : Google Scholar
|
|
96
|
Eskandari E, Hashemi M, Naderi M, Bahari
G, Safdari V and Taheri M: Leukocyte telomere length shortening,
hTERT genetic polymorphisms and risk of childhood acute
lymphoblastic leukemia. Asian Pac J Cancer Prev. 19:1515–1521.
2018.PubMed/NCBI
|
|
97
|
Sheng X, Zhang L, Luo D, Tong N, Wang M,
Fang Y, Li J and Zhang Z: A common variant near TERC and telomere
length are associated with susceptibility to childhood acute
lymphoblastic leukemia in Chinese. Leuk Lymphoma. 53:1688–1692.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Takauchi K, Tashiro S, Ohtaki M and Kamada
N: Telomere reduction of specific chromosome translocation in acute
myelocytic leukemia. Jpn J Cancer Res. 85:127–130. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sieglová Z, Zilovcová S, Cermák J, Ríhová
H, Brezinová D, Dvoráková R, Marková M, Maaloufová J, Sajdová J,
Brezinová J, et al: Dynamics of telomere erosion and its
association with genome instability in myelodysplastic syndromes
(MDS) and acute myelogenous leukemia arising from MDS: A marker of
disease prognosis? Leuk Res. 28:1013–1021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lansdorp PM: Maintenance of telomere
length in AML. Blood Adv. 1:2467–2472. 2017. View Article : Google Scholar
|
|
101
|
Wang Y, Wang T, Dagnall C, Haagenson M,
Spellman SR, Hicks B, Jones K, Lee SJ, Savage SA and Gadalla SM:
Relative telomere length before hematopoietic cell transplantation
and outcome after unrelated donor hematopoietic cell
transplantation for acute leukemia. Biol Blood Marrow Transplant.
23:1054–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Swiggers SJ, Kuijpers MA, de Cort MJ,
Beverloo HB and Zijlmans JM: Critically short telomeres in acute
myeloid leukemia with loss or gain of parts of chromosomes. Genes
Chromosomes Cancer. 45:247–256. 2006. View Article : Google Scholar
|
|
103
|
Behrens YL, Thomay K, Hagedorn M, Ebersold
J, Schmidt G, Lentes J, Davenport C, Schlegelberger B and Göhring
G: Jumping translocations: Short telomeres or pathogenic TP53
variants as underlying mechanism in acute myeloid leukemia and
myelodysplastic syndrome? Genes Chromosomes Cancer. 58:139–148.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Watts JM, Dumitriu B, Hilden P, Kishtagari
A, Rapaport F, Chen C, Ahn J, Devlin SM, Stein EM, Rampal R, et al:
Telomere length and associations with somatic mutations and
clinical outcomes in acute myeloid leukemia. Leuk Res. 49:62–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li AM, Hyagu S, Maze D, Schreiber R, Sirrs
S, Stockler-Ipsiroglu S, Sutherland H, Vercauteren S and Schultz
KR: Prolonged granulocyte colony stimulating factor use in glycogen
storage disease type 1b associated with acute myeloid leukemia and
with shortened telomere length. Pediatr Hematol Oncol. 35:45–51.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Aalbers AM, Calado RT, Young NS, Zwaan CM,
Wu C, Kajigaya S, Coenen EA, Baruchel A, Geleijns K, de Haas V, et
al: Telomere length and telomerase complex mutations in pediatric
acute myeloid leukemia. Leukemia. 27:1786–1789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Song DY, Kim JA, Jeong D, Yun J, Kim SM,
Lim K, Park SN, Im K, Choi S, Yoon SS and Lee DS: Telomere length
and its correlation with gene mutations in chronic lymphocytic
leukemia in a Korean population. PLoS One. 14:e02201772019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jebaraj BMC, Tausch E, Landau DA, Bahlo J,
Robrecht S, Taylor-Weiner AN, Bloehdorn J, Scheffold A, Mertens D,
Böttcher S, et al: Short telomeres are associated with inferior
outcome, genomic complexity, and clonal evolution in chronic
lymphocytic leukemia. Leukemia. 33:2183–2194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Thomay K, Fedder C, Hofmann W, Kreipe H,
Stadler M, Titgemeyer J, Zander I, Schlegelberger B and Göhring G:
Telomere shortening, TP53 mutations and deletions in chronic
lymphocytic leukemia result in increased chromosomal instability
and breakpoint clustering in heterochromatic regions. Ann Hematol.
96:1493–1500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Steinbrecher D, Jebaraj BMC, Schneider C,
Edelmann J, Cymbalista F, Leblond V, Delmer A, Ibach S, Tausch E,
Scheffold A, et al: Telomere length in poor-risk chronic
lymphocytic leukemia: Associations with disease characteristics and
outcome. Leuk Lymphoma. 59:1614–1623. 2018. View Article : Google Scholar
|
|
111
|
Strefford JC, Kadalayil L, Forster J,
Rose-Zerilli MJ, Parker A, Lin TT, Heppel N, Norris K, Gardiner A,
Davies Z, et al: Telomere length predicts progression and overall
survival in chronic lymphocytic leukemia: Data from the UK LRF CLL4
trial. Leukemia. 29:2411–2414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dos Santos P, Panero J, Palau Nagore V,
Stanganelli C, Bezares RF and Slavutsky I: Telomere shortening
associated with increased genomic complexity in chronic lymphocytic
leukemia. Tumour Biol. 36:8317–8324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Palma M, Parker A, Hojjat-Farsangi M,
Forster J, Kokhaei P, Hansson L, Osterborg A and Mellstedt H:
Telomere length and expression of human telomerase reverse
transcriptase splice variants in chronic lymphocytic leukemia. Exp
Hematol. 41:615–626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sellmann L, de Beer D, Bartels M, Opalka
B, Nückel H, Dührsen U, Dürig J, Seifert M, Siemer D, Küppers R, et
al: Telomeres and prognosis in patients with chronic lymphocytic
leukaemia. Int J Hematol. 93:74–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Roos G, Kröber A, Grabowski P, Kienle D,
Bühler A, Döhner H, Rosenquist R and Stilgenbauer S: Short
telomeres are associated with genetic complexity, high-risk genomic
aberrations, and short survival in chronic lymphocytic leukemia.
Blood. 111:2246–2252. 2008. View Article : Google Scholar
|
|
116
|
Lin TT, Letsolo BT, Jones RE, Rowson J,
Pratt G, Hewamana S, Fegan C, Pepper C and Baird DM: Telomere
dysfunction and fusion during the progression of chronic
lymphocytic leukemia: Evidence for a telomere crisis. Blood.
116:1899–1907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ojha J, Codd V, Nelson CP, Samani NJ,
Smirnov IV, Madsen NR, Hansen HM, de Smith AJ, Bracci PM, Wiencke
JK, et al: ENGAGE Consortium Telomere Group. Genetic variation
associated with longer telomere length increases risk of chronic
lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev.
25:1043–1049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wysoczanska B, Dratwa M, Gebura K, Mizgala
J, Mazur G, Wrobel T and Bogunia-Kubik K: Variability within the
human TERT gene, telomere length and predisposition to chronic
lymphocytic leukemia. Onco Targets Ther. 12:4309–4320. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Furtado FM, Scheucher PS, Santana BA,
Scatena NF, Calado RT, Rego EM, Matos DM and Falcão RP: Telomere
length analysis in monoclonal B-cell lymphocytosis and chronic
lymphocytic leukemia Binet A. Braz J Med Biol Res. 50:e60192017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang L, Kost SEF, Beiggi S, Zhang Y,
Schmidt R, Nugent Z, Marshall A, Banerji V, Gibson SB and Johnston
JB: Buccal cell telomere length is not a useful marker for
comorbidities in chronic lymphocytic leukemia. Leuk Res.
86:1062202019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kaifie A, Schikowsky C, Vasko T, Kraus T,
Brümmendorf TH and Ziegler P: Additional benefits of telomere
length (TL) measurements in chronic lymphocytic leukemia. Leuk
Lymphoma. 60:541–543. 2019. View Article : Google Scholar
|
|
122
|
Iwama H, Ohyashiki K, Ohyashiki JH,
Hayashi S, Kawakubo K, Shay JW and Toyama K: The relationship
between telomere length and therapy-associated cytogenetic
responses in patients with chronic myeloid leukemia. Cancer.
79:1552–1560. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Brümmendorf TH, Holyoake TL, Rufer N,
Barnett MJ, Schulzer M, Eaves CJ, Eaves AC and Lansdorp PM:
Prognostic implications of differences in telomere length between
normal and malignant cells from patients with chronic myeloid
leukemia measured by flow cytometry. Blood. 95:1883–1890. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bouillon AS, Ventura Ferreira MS, Awad SA,
Richter J, Hochhaus A, Kunzmann V, Dengler J, Janssen J,
Ossenkoppele G, Westerweel PE, et al: Telomere shortening
correlates with leukemic stem cell burden at diagnosis of chronic
myeloid leukemia. Blood Adv. 2:1572–1579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Boultwood J, Fidler C, Shepherd P, Watkins
F, Snowball J, Haynes S, Kusec R, Gaiger A, Littlewood TJ, Peniket
AJ and Wainscoat JS: Telomere length shortening is associated with
disease evolution in chronic myelogenous leukemia. Am J Hematol.
61:5–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Drummond M, Lennard A, Brûmmendorf T and
Holyoake T: Telomere shortening correlates with prognostic score at
diagnosis and proceeds rapidly during progression of chronic
myeloid leukemia. Leuk Lymphoma. 45:1775–1781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Keller G, Brassat U, Braig M, Heim D, Wege
H and Brümmendorf TH: Telomeres and telomerase in chronic myeloid
leukaemia: Impact for pathogenesis, disease progression and
targeted therapy. Hematol Oncol. 27:123–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Boultwood J, Peniket A, Watkins F,
Shepherd P, McGale P, Richards S, Fidler C, Littlewood TJ and
Wainscoat JS: Telomere length shortening in chronic myelogenous
leukemia is associated with reduced time to accelerated phase.
Blood. 96:358–361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Caocci G, Greco M, Delogu G, Secchi C,
Martino B, Labate C, Abruzzese E, Trawinska MM, Galimberti S, Orru
F, et al: Telomere length shortening is associated with
treatment-free remission in chronic myeloid leukemia patients. J
Hematol Oncol. 9:632016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Samassekou O, Ntwari A, Hébert J and Yan
J: Individual telomere lengths in chronic myeloid leukemia.
Neoplasia. 11:1146–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nikolouzakis TK, Falzone L, Lasithiotakis
K, Krüger-Krasagakis S, Kalogeraki A, Sifaki M, Spandidos DA,
Chrysos E, Tsatsakis A and Tsiaoussis J: Current and future trends
in molecular biomarkers for diagnostic, prognostic, and predictive
purposes in non-melanoma skin cancer. J Clin Med. 9:28682020.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Llorca-Cardeñosa MJ, Peña-Chilet M, Mayor
M, Gomez-Fernandez C, Casado B, Martin-Gonzalez M, Carretero G,
Lluch A, Martinez-Cadenas C, Ibarrola-Villava M and Ribas G: Long
telomere length and a TERT-CLPTM1 locus polymorphism association
with melanoma risk. Eur J Cancer. 50:3168–3177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Anic GM, Sondak VK, Messina JL, Fenske NA,
Zager JS, Cherpelis BS, Lee JH, Fulp WJ, Epling-Burnette PK, Park
JY and Rollison DE: Telomere length and risk of melanoma, squamous
cell carcinoma, and basal cell carcinoma. Cancer Epidemiol.
37:434–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bodelon C, Pfeiffer RM, Bollati V,
Debbache J, Calista D, Ghiorzo P, Fargnoli MC, Bianchi-Scarra G,
Peris K, Hoxha M, et al: On the interplay of telomeres, nevi and
the risk of melanoma. PLoS One. 7:e524662012. View Article : Google Scholar
|
|
135
|
Han J, Qureshi AA, Prescott J, Guo Q, Ye
L, Hunter DJ and De Vivo I: A prospective study of telomere length
and the risk of skin cancer. J Invest Dermatol. 129:415–421. 2009.
View Article : Google Scholar :
|
|
136
|
Nan H, Du M, De Vivo I, Manson JE, Liu S,
McTiernan A, Curb JD, Lessin LS, Bonner MR, Guo Q, et al: Shorter
telomeres associate with a reduced risk of melanoma development.
Cancer Res. 71:6758–6763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Burke LS, Hyland PL, Pfeiffer RM, Prescott
J, Wheeler W, Mirabello L, Savage SA, Burdette L, Yeager M, Chanock
S, et al: Telomere length and the risk of cutaneous malignant
melanoma in melanoma-prone families with and without CDKN2A
mutations. PLoS One. 8:711212013. View Article : Google Scholar
|
|
138
|
Caini S, Raimondi S, Johansson H, De
Giorgi V, Zanna I, Palli D and Gandini S: Telomere length and the
risk of cutaneous melanoma and non-melanoma skin cancer: A review
of the literature and meta-analysis. J Dermatol Sci. 80:168–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Rachakonda S, Srinivas N, Mahmoudpour SH,
Garcia-Casado Z, Requena C, Traves V, Soriano V, Cardelli M,
Pjanova D, Molven A, et al: Telomere length and survival in primary
cutaneous melanoma patients. Sci Rep. 8:109472018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Viceconte N, Dheur MS, Majerova E,
Pierreux CE, Baurain JF, van Baren N and Decottignies A: Highly
aggressive metastatic melanoma cells unable to maintain telomere
length. Cell Rep. 19:2529–2543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Menin C, Bojnik E, Del Bianco P, Elefanti
L, Gianesin K, Keppel S, Stagni C, Mocellin S, Vecchiato A and De
Rossi A: Differences in telomere length between sporadic and
familial cutaneous melanoma. Br J Dermatol. 175:937–943. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Kammori M, Takubo K, Nakamura K, Furugouri
E, Endo H, Kanauchi H, Mimura Y and Kaminishi M: Telomerase
activity and telomere length in benign and malignant human thyroid
tissues. Cancer Lett. 159:175–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Matthews P, Jones CJ, Skinner J, Haughton
M, de Micco C and Wynford-Thomas D: Telomerase activity and
telomere length in thyroid neoplasia: Biological and clinical
implications. J Pathol. 194:183–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Dong C and Hemminki K: Modification of
cancer risks in offspring by sibling and parental cancers from
2,112,616 nuclear families. Int J Cancer. 92:144–150. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jendrzejewski J, Tomsic J, Lozanski G,
Labanowska J, He H, Liyanarachchi S, Nagy R, Ringel MD, Kloos RT,
Heerema NA and de la Chapelle A: Telomere length and telomerase
reverse transcriptase gene copy number in patients with papillary
thyroid carcinoma. J Clin Endocrinol Metab. 96:1876–1880. 2011.
View Article : Google Scholar
|
|
146
|
Capezzone M, Cantara S, Marchisotta S,
Filetti S, De Santi MM, Rossi B, Ronga G, Durante C and Pacini F:
Short telomeres, telomerase reverse transcriptase gene
amplification, and increased telomerase activity in the blood of
familial papillary thyroid cancer patients. J Clin Endocrinol
Metab. 93:3950–3957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Capezzone M, Cantara S, Marchisotta S,
Busonero G, Formichi C, Benigni M, Capuano S, Toti P,
Pazaitou-Panayiotou K, Caruso G, et al: Telomere length in
neoplastic and nonneoplastic tissues of patients with familial and
sporadic papillary thyroid cancer. J Clin Endocrinol Metab.
96:1852–1856. 2011. View Article : Google Scholar
|
|
148
|
He M, Bian B, Gesuwan K, Gulati N, Zhang
L, Nilubol N and Kebebew E: Telomere length is shorter in affected
members of families with familial nonmedullary thyroid cancer.
Thyroid. 23:301–307. 2013. View Article : Google Scholar :
|
|
149
|
Gramatges MM, Morton LM, Yasui Y, Arnold
MA, Neglia JP, Leisenring WM, Machiela MJ, Dagnall CL, Chanock SJ,
Armstrong GT, et al: Telomere length-associated genetic variants
and the risk of thyroid cancer in survivors of childhood cancer: A
report from the childhood cancer survivor study (CCSS). Cancer
Epidemiol Biomarkers Prev. 28:417–419. 2019. View Article : Google Scholar :
|
|
150
|
Li J, An C, Zheng H, Lei T, Zhang N, Zheng
Y and Yang M: Leukocyte telomere length and risk of papillary
thyroid carcinoma. J Clin Endocrinol Metab. 104:2712–2718. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Graham MK and Meeker A: Telomeres and
telomerase in prostate cancer development and therapy. Nat Rev
Urol. 14:607–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Xu J, Chang WS, Tsai CW, Bau DT, Xu Y,
Davis JW, Thompson TC, Logothetis CJ and Gu J: Leukocyte telomere
length is associated with aggressive prostate cancer in localized
prostate cancer patients. EBioMedicine. 52:1026162020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Tsai CW, Chang WS, Xu J, Xu Y, Huang M,
Pettaway C, Bau DT and Gu J: Leukocyte telomere length is
associated with aggressive prostate cancer in localized African
American prostate cancer patients. Carcinogenesis. 41:1213–1218.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hu R, Hua XG and Jiang QC: Associations of
telomere length in risk and recurrence of prostate cancer: A
meta-analysis. Andrologia. 51:e133042019. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Luxton JJ, McKenna MJ, Lewis AM, Taylor
LE, Jhavar SG, Swanson GP and Bailey SM: Telomere length dynamics
and chromosomal instability for predicting individual
radiosensitivity and risk via machine learning. J Pers Med.
11:1882021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Gu CY, Jin SM, Qin XJ, Zhu Y, Bo D, Lin
GW, Shi GH and Ye DW: Genetic variants in RTEL1 influencing
telomere length are associated with prostate cancer risk. J Cancer.
10:6170–6174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hurwitz LM, Heaphy CM, Joshu CE, Isaacs
WB, Konishi Y, De Marzo AM, Isaacs SD, Wiley KE, Platz EA and
Meeker AK: Telomere length as a risk factor for hereditary prostate
cancer. Prostate. 74:359–364. 2014. View Article : Google Scholar
|
|
158
|
Julin B, Shui I, Heaphy CM, Joshu CE,
Meeker AK, Giovannucci E, De Vivo I and Platz EA: Circulating
leukocyte telomere length and risk of overall and aggressive
prostate cancer. Br J Cancer. 112:769–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Renner W, Krenn-Pilko S, Gruber HJ,
Herrmann M and Langsenlehner T: Relative telomere length and
prostate cancer mortality. Prostate Cancer Prostatic Dis.
21:579–583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Svenson U, Roos G and Wikström P: Long
leukocyte telomere length in prostate cancer patients at diagnosis
is associated with poor metastasis-free and cancer-specific
survival. Tumour Biol. 39:10104283176922362017. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wulaningsih W, Astuti Y, Matsuguchi T,
Anggrandariyanny P and Watkins J; PILAR Research Network:
Circulating Prostate-specific antigen and telomere length in a
nationally representative sample of men without history of prostate
cancer. Prostate. 77:22–32. 2017. View Article : Google Scholar
|
|
162
|
Heaphy CM, Haffner MC, Graham MK, Lim D,
Davis C, Corey E, Epstein JI, Eisenberger MA, Wang H, De Marzo AM,
et al: Telomere lengths differ significantly between small-cell
neuroendocrine prostate carcinoma and adenocarcinoma of the
prostate. Hum Pathol. 101:70–79. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Mirabello L, Huang WY, Wong JY, Chatterjee
N, Reding D, Crawford ED, De Vivo I, Hayes RB and Savage SA: The
association between leukocyte telomere length and cigarette
smoking, dietary and physical variables, and risk of prostate
cancer. Aging Cell. 8:405–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Joshu CE, Peskoe SB, Heaphy CM, Kenfield
SA, Mucci LA, Giovannucci EL, Stampfer MJ, Yoon G, Lee TK, Hicks
JL, et al: Current or recent smoking is associated with more
variable telomere length in prostate stromal cells and prostate
cancer cells. Prostate. 78:233–238. 2018. View Article : Google Scholar :
|
|
165
|
Heaphy CM, Joshu CE, Barber JR, Davis C,
Zarinshenas R, De Marzo AM, Lotan TL, Sfanos KS, Meeker AK, Platz
EA, et al: Racial difference in prostate cancer cell telomere
lengths in men with higher grade prostate cancer: A clue to the
racial disparity in prostate cancer outcomes. Cancer Epidemiol
Biomarkers Prev. 29:676–680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Luu HN, Long J, Wen W, Zheng Y, Cai Q, Gao
YT, Zheng W and Shu XO: Association between genetic risk score for
telomere length and risk of breast cancer. Cancer Causes Control.
27:1219–1228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Kroupa M, Rachakonda S, Vymetalkova V,
Tomasova K, Liska V, Vodenkova S, Cumova A, Rossnerova A, Vodickova
L, Hemminki K, et al: Telomere length in peripheral blood
lymphocytes related to genetic variation in telomerase, prognosis
and clinicopathological features in breast cancer patients.
Mutagenesis. 35:491–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Samavat H, Xun X, Jin A, Wang R, Koh WP
and Yuan JM: Association between prediagnostic leukocyte telomere
length and breast cancer risk: The Singapore Chinese Health Study.
Breast Cancer Res. 21:502019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Pellatt AJ, Wolff RK, Torres-Mejia G, John
EM, Herrick JS, Lundgreen A, Baumgartner KB, Giuliano AR, Hines LM,
Fejerman L, et al: Telomere length, telomere-related genes, and
breast cancer risk: The breast cancer health disparities study.
Genes Chromosomes Cancer. 52:595–609. 2013.PubMed/NCBI
|
|
170
|
Wang Z, Zhang Z, Guo Y, Shui H, Liu G, Jin
T and Wang H: Shorter telomere length is associated with increased
breast cancer risk in a Chinese Han population: A Case-Control
analysis. J Breast Cancer. 21:391–398. 2018. View Article : Google Scholar
|
|
171
|
Shen J, Terry MB, Gurvich I, Liao Y, Senie
RT and Santella RM: Short telomere length and breast cancer risk: A
study in sister sets. Cancer Res. 67:5538–5544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Pavanello S, Varesco L, Gismondi V, Bruzzi
P and Bolognesi C: Leucocytes telomere length and breast cancer
risk/susceptibility: A case-control study. PLoS One.
13:e01975222018. View Article : Google Scholar
|
|
173
|
Duggan C, Risques R, Alfano C, Prunkard D,
Imayama I, Holte S, Baumgartner K, Baumgartner R, Bernstein L,
Ballard-Barbash R, et al: Change in peripheral blood leukocyte
telomere length and mortality in breast cancer survivors. J Natl
Cancer Inst. 106:dju0352014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Ceja-Rangel HA, Sánchez-Suárez P,
Castellanos-Juárez E, Peñaroja-Flores R, Arenas-Aranda DJ, Gariglio
P and Benítez-Bribiesca L: Shorter telomeres and high telomerase
activity correlate with a highly aggressive phenotype in breast
cancer cell lines. Tumour Biol. 37:11917–11926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kammori M, Sugishita Y, Okamoto T,
Kobayashi M, Yamazaki K, Yamada E and Yamada T: Telomere shortening
in breast cancer correlates with the pathological features of tumor
progression. Oncol Rep. 34:627–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Barczak W, Rozwadowska N, Romaniuk A,
Lipińska N, Lisiak N, Grodecka-Gazdecka S, Książek K and Rubiś B:
Telomere length assessment in leukocytes presents potential
diagnostic value in patients with breast cancer. Oncol Lett.
11:2305–2309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Eyüboğlu İP, Yenmiş G, Bingöl EN, Yüksel
Ş, Tokat F, Özbek P, Güllü Amuran G, Yakıcıer C and Akkiprik M:
Next-generation sequencing identifies BRCA1 and/or BRCA2 mutations
in Women at high hereditary risk for breast cancer with shorter
telomere length. OMICS. 24:5–15. 2020. View Article : Google Scholar
|
|
178
|
Badie S, Escandell JM, Bouwman P, Carlos
AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig
U, et al: BRCA2 acts as a RAD51 loader to facilitate telomere
replication and capping. Nat Struct Mol Biol. 17:1461–1469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Thorvaldsdottir B, Aradottir M, Stefansson
OA, Bodvarsdottir SK and Eyfjörd JE: Telomere length is predictive
of breast cancer risk in BRCA2 mutation carriers. Cancer Epidemiol
Biomarkers Prev. 26:1248–1254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ennour-Idrissi K, Maunsell E and Diorio C:
Telomere length and breast cancer prognosis: A systematic review.
Cancer Epidemiol Biomarkers Prev. 26:3–10. 2017. View Article : Google Scholar
|
|
181
|
Garland SN, Johnson B, Palmer C, Speck RM,
Donelson M, Xie SX, DeMichele A and Mao JJ: Physical activity and
telomere length in early stage breast cancer survivors. Breast
Cancer Res. 16:4132014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Alhareeri AA, Archer KJ, Fu H, Lyon DE,
Elswick RK Jr, Kelly DL, Starkweather AR, Elmore LW, Bokhari YA and
Jackson-Cook CK: Telomere lengths in women treated for breast
cancer show associations with chemotherapy, pain symptoms, and
cognitive domain measures: A longitudinal study. Breast Cancer Res.
22:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Mirabello L, Garcia-Closas M, Cawthon R,
Lissowska J, Brinton LA, Pepłońska B, Sherman ME and Savage SA:
Leukocyte telomere length in a population-based case-control study
of ovarian cancer: A pilot study. Cancer Causes Control. 21:77–82.
2010. View Article : Google Scholar
|
|
184
|
Kuhn E, Meeker AK, Visvanathan K, Gross
AL, Wang TL, Kurman RJ and Shih IeM: Telomere length in different
histologic types of ovarian carcinoma with emphasis on clear cell
carcinoma. Mod Pathol. 24:1139–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Vajpeyi R: WHO Classification of Tumours:
Pathology and genetics of tumours of the breast and female genital
organs. J Clin Pathol. 58:671–672. 2005.
|
|
186
|
Martinez-Delgado B, Anowsky K,
Inglada-Perez L, de la Hoya M, Caldes T, Vega A, Blanco A, Martin
T, Gonzalez-Sarmiento R, Blasco M, et al: Shorter telomere length
is associated with increased ovarian cancer risk in both familial
and sporadic cases. J Med Genet. 49:341–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Falandry C, Horard B, Bruyas A, Legouffe
E, Cretin J, Meunier J, Alexandre J, Delecroix V, Fabbro M, Certain
MN, et al: Telomere length is a prognostic biomarker in elderly
advanced ovarian cancer patients: A multicenter GINECO study. Aging
(Albany NY). 7:1066–1076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Yang M, Prescott J, Poole EM, Rice MS,
Kubzansky LD, Idahl A, Lundin E, De Vivo I and Tworoger SS:
Prediagnosis leukocyte telomere length and risk of ovarian cancer.
Cancer Epidemiol Biomarkers Prev. 26:339–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Bojesen SE, Pooley KA, Johnatty SE,
Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen
HC, Smart CE, et al: Multiple independent variants at the TERT
locus are associated with telomere length and risks of breast and
ovarian cancer. Nat Genet. 45:371–384. 384e1–2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Terry KL, Tworoger SS, Vitonis AF, Wong J,
Titus-Ernstoff L, De Vivo I and Cramer DW: Telomere length and
genetic variation in telomere maintenance genes in relation to
ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 21:504–512.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kotsopoulos J, Prescott J, De Vivo I, Fan
I, Mclaughlin J, Rosen B, Risch H, Sun P and Narod SA: Telomere
length and mortality following a diagnosis of ovarian cancer.
Cancer Epidemiol Biomarkers Prev. 23:2603–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Antoun S, Atallah D, Tahtouh R, Assaf MD,
Moubarak M, Ayoub EN, Chahine G and Hilal G: Glucose restriction
combined with chemotherapy decreases telomere length and cancer
antigen-125 secretion in ovarian carcinoma. Oncol Lett.
19:1338–1350. 2020.PubMed/NCBI
|
|
193
|
Victorelli S and Passos JF: Telomeres and
cell senescence-size matters not. EBioMedicine. 21:14–20. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Kim B, Ryu KJ, Lee S and Kim T: Changes in
telomere length and senescence markers during human ovarian tissue
cryopreservation. Sci Rep. 11:22382021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Zhang DK, Ngan HY, Cheng RY, Cheung AN,
Liu SS and Tsao SW: Clinical significance of telomerase activation
and telomeric restriction fragment (TRF) in cervical cancer. Eur J
Cancer. 35:154–160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Maida Y, Kyo S, Forsyth NR, Takakura M,
Sakaguchi J, Mizumoto Y, Hashimoto M, Nakamura M, Nakao S and Inoue
M: Distinct telomere length regulation in premalignant cervical and
endometrial lesions: Implications for the roles of telomeres in
uterine carcinogenesis. J Pathol. 210:214–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Chen X, Wei S, Ma H, Jin G, Hu Z, Suping
H, Li D, Hang D, Wu X and Li N: Telomere length in cervical
exfoliated cells, interaction with HPV genotype, and cervical
cancer occurrence among high-risk HPV-positive women. Cancer Me.
8:4845–4851. 2019. View Article : Google Scholar
|
|
198
|
Azzalin CM, Reichenbach P, Khoriauli L,
Giulotto E and Lingner J: Telomeric repeat containing RNA and RNA
surveillance factors at mammalian chromosome ends. Science.
318:798–801. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Deng Z, Wang Z, Xiang C, Molczan A, Baubet
V, Conejo-Garcia J, Xu X, Lieberman PM and Dahmane N: Formation of
telomeric repeat-containing RNA (TERRA) foci in highly
proliferating mouse cerebellar neuronal progenitors and
medulloblastoma. J Cell Sci. 125:4383–4394. 2012.PubMed/NCBI
|
|
200
|
Oh BK, Keo P, Bae J, Ko JH and Choi JS:
Variable TERRA abundance and stability in cervical cancer cells.
Int J Mol Med. 39:1597–1604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Déjardin J and Kingston RE: Purification
of proteins associated with specific genomic Loci. Cell.
136:175–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Kappei D, Butter F, Benda C, Scheibe M,
Draškovič I, Stevense M, Novo CL, Basquin C, Araki M, Krastev DB,
et al: HOT1 is a mammalian direct telomere repeat-binding protein
contributing to telomerase recruitment. EMBO J. 32:1681–1701. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Zhou S, Xiao Y, Zhuang Y, Liu Y, Zhao H,
Yang H, Xie C, Zhou F and Zhou Y: Knockdown of homeobox containing
1 increases the radiosensitivity of cervical cancer cells through
telomere shortening. Oncol Rep. 38:515–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Du J, Xue W, Ji Y, Zhu X, Gu Y, Zhu M,
Wang C, Gao Y, Dai J, Ma H, et al: U-shaped association between
telomere length and esophageal squamous cell carcinoma risk: A
case-control study in Chinese population. Front Med. 9:478–486.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Xing J, Ajani JA, Chen M, Izzo J, Lin J,
Chen Z, Gu J and Wu X: Constitutive short telomere length of
chromosome 17p and 12q but not 11q and 2p is associated with an
increased risk for esophageal cancer. Cancer Prev Res (Phila).
2:459–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Zheng YL, Hu N, Sun Q, Wang C and Taylor
PR: Telomere attrition in cancer cells and telomere length in tumor
stroma cells predict chromosome instability in esophagealsquamous
cell carcinoma: A genome-wide analysis. Cancer Res. 69:1604–1614.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Lin SW, Abnet CC, Freedman ND, Murphy G,
Risques R, Prunkard D, Rabinovitch P, Pan QJ, Roth MJ, Wang GQ, et
al: Measuring telomere length for the early detection of precursor
lesions of esophageal squamous cell carcinoma. BMC Cancer.
13:5782013. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Li Z, Song Y, Xu Y, Shen Y, Zhang N, Yang
M and Yu D: Identification of Leukocyte telomere length-related
genetic variants contributing to predisposition of Esophageal
Squamous Cell Carcinoma. J Cancer. 11:5025–5031. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Lu Y, Yan C, Du J, Ji Y, Gao Y, Zhu X, Yu
F, Huang T, Dai J, Ma H, et al: Genetic variants affecting telomere
length are associated with the prognosis of esophageal squamous
cell carcinoma in a Chinese population. Mol Carcinog. 56:1021–1029.
2017. View Article : Google Scholar
|
|
210
|
Shi J, Sun F, Peng L, Li B, Liu L, Zhou C,
Han J, Zhang L, Zhou L, Zhang X, et al: Leukocyte telomere
length-related genetic variants in 1p34.2 and 14q21 loci contribute
to the risk of esophageal squamous cell carcinoma. Int J Cancer.
132:2799–2807. 2013. View Article : Google Scholar
|
|
211
|
Hao XD, Yang Y, Song X, Zhao XK, Wang LD,
He JD, Kong QP, Tang NL and Zhang YP: Correlation of telomere
length shortening with TP53 somatic mutations, polymorphisms and
allelic loss in breast tumors and esophageal cancer. Oncol Rep.
29:226–236. 2013. View Article : Google Scholar
|
|
212
|
Yu Q, Yang J, Liu B, Li W, Hu G, Qiu H,
Huang L, Xiong H and Yuan X: Combined effects of leukocyte telomere
length, p53 polymorphism and human papillomavirus infection on
esophageal squamous cell carcinoma in a Han Chinese population.
Cancer Epidemiol. 38:569–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wennerström EC, Risques RA, Prunkard D,
Giffen C, Corley DA, Murray LJ, Whiteman DC, Wu AH, Bernstein L, Ye
W, et al: Leukocyte telomere length in relation to the risk of
Barrett's esophagus and esophageal adenocarcinoma. Cancer Med.
5:2657–2665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Pan W, Du J, Shi M, Jin G and Yang M:
Short leukocyte telomere length, alone and in combination with
smoking, contributes to increased risk of gastric cancer or
esophageal squamous cell carcinoma. Carcinogenesis. 38:12–18. 2017.
View Article : Google Scholar
|
|
215
|
Tahara T, Tahara S, Horiguchi N, Kawamura
T, Okubo M, Ishizuka T, Yamada H, Yoshida D, Ohmori T, Maeda K, et
al: Telomere length in leukocyte DNA in gastric cancer patients and
its association with Clinicopathological features and prognosis.
Anticancer Res. 37:1997–2001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Hou L, Savage SA, Blaser MJ, Perez-Perez
G, Hoxha M, Dioni L, Pegoraro V, Dong LM, Zatonski W, Lissowska J,
et al: Telomere length in peripheral leukocyte DNA and gastric
cancer risk. Cancer Epidemiol Biomarkers Prev. 18:3103–3109. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Qu F, Li R, He X, Li Q, Xie S, Gong L, Ji
G, Lu J and Bao G: Short telomere length in peripheral blood
leukocytes predicts poor prognosis and indicates an
immunosuppressive phenotypegastric cancer patients. Mol Oncol.
9:727–739. 2015. View Article : Google Scholar
|
|
218
|
Wang Z, Koh WP, Jin A, Wang R and Yuan JM:
Telomere length and risk of developing gastric adenocarcinoma: The
Singapore Chinese Health Study. Gastric Cancer. 2:598–605. 2018.
View Article : Google Scholar
|
|
219
|
Weischer M, Nordestgaard BG, Cawthon RM,
Freiberg JJ, Tybjærg-Hansen A and Bojesen SE: Short telomere
length, cancer survival, and cancer risk in 47102 individuals. J
Natl Cancer Inst. 105:459–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Liu X, Bao G, Huo T, Wang Z, He X and Dong
G: Constitutive telomere length and gastric cancer risk:
Case-control analysis in Chinese Han population. Cancer Sci.
100:1300–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Liu Y, Lei T, Zhang N, Zheng Y, Kou P,
Shang S and Yang M: Leukocyte telomere length and risk of gastric
cardia adenocarcinoma. Sci Rep. 8:145842018. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Tahara T, Shibata T, Kawamura T, Horiguchi
N, Okubo M, Nakano N, Ishizuka T, Nagasaka M, Nakagawa Y and Ohmiya
N: Telomere length shortening in gastric mucosa is a field effect
associated with increased risk of gastric cancer. Virchows Arch.
469:19–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Heo YR and Lee JH: Association between
telomere length and PIK3CA amplification in gastric cancer. Clin
Exp Med. 18:133–134. 2018. View Article : Google Scholar
|
|
224
|
Lili M, Yuxiang F, Zhongcheng H, Ying S,
Ru C, Rong X and Jiang L: Genetic variations associated with
telomere length affect the risk of gastric carcinoma. Medicine
(Baltimore). 99:e205512020. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Du J, Zhu X, Xie C, Dai N, Gu Y, Zhu M,
Wang C, Gao Y, Pan F, Ren C, et al: Telomere length, genetic
variants and gastric cancer risk in a Chinese population.
Carcinogenesis. 36:963–970. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Choi BJ, Yoon JH, Kim O, Choi WS, Nam SW,
Lee JY and Park WS: Influence of the hTERT rs2736100 polymorphism
on telomere length in gastric cancer. World J Gastroenterol.
21:9328–9336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Campa D, Matarazzi M, Greenhalf W, Bijlsma
M, Saum KU, Pasquali C, van Laarhoven H, Szentesi A, Federici F,
Vodicka P, et al: Genetic determinants of telomere length and risk
of pancreatic cancer: A PANDoRA study. Int J Cancer. 144:1275–1283.
2019. View Article : Google Scholar
|
|
228
|
Duell EJ: Telomere length and pancreatic
cancer risk: Breaking down the evidence. Gut. 66:12017. View Article : Google Scholar
|
|
229
|
Luu HN, Huang JY, Wang R, Adams-Haduch J,
Jin A, Koh WP and Yuan JM: Association between leukocyte telomere
length and the risk of pancreatic cancer: Findings from a
prospective study. PLoS One. 14:e02216972019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Campa D, Mergarten B, De Vivo I,
Boutron-Ruault MC, Racine A, Severi G, Nieters A, Katzke VA,
Trichopoulou A, Yiannakouris N, et al: Leukocyte telomere length in
relation to pancreatic cancer risk: A prospective study. Cancer
Epidemiol Biomarkers Prev. 23:2447–2454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Mormile R: Telomere length and pancreatic
cancer risk-letter. Cancer Epidemiol Biomarkers Prev. 26:11572017.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Skinner HG, Gangnon RE, Litzelman K,
Johnson RA, Chari ST, Petersen GM and Boardman LA: Telomere length
and pancreatic cancer: A case-control study. Cancer Epidemiol
Biomarkers Prev. 21:2095–2100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Zhang R, Zhao J, Xu J and Liu F:
Association of peripheral leukocyte telomere length and its
variation with pancreatic cancer and colorectal cancer risk in
Chinese population. Oncotarget. 7:38579–38585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Antwi SO, Bamlet WR, Broderick BT, Chaffee
KG, Oberg A, Jatoi A, Boardman LA and Petersen GM: Genetically
predicted telomere length is not associated with pancreatic cancer
risk. Cancer Epidemiol Biomarkers Prev. 26:971–974. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Antwi SO, Bamlet WR, Rabe KG, Cawthon RM,
Umudi I, Druliner BR, Sicotte H, Oberg AL, Jatoi A, Boardman LA and
Petersen GM: Leukocyte telomere length and its interaction with
germline variation in Telomere-Related genes in relation to
pancreatic adenocarcinoma risk. Cancer Epidemiol Biomarkers Prev.
29:1492–1500. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Hamada T, Yuan C, Bao Y, Zhang M, Khalaf
N, Babic A, Morales-Oyarvide V, Cochrane BB, Gaziano JM,
Giovannucci EL, et al: Prediagnostic leukocyte telomere length and
pancreatic cancer survival. Cancer Epidemiol Biomarkers Prev.
28:1868–1875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Bao Y, Prescott J, Yuan C, Zhang M, Kraft
P, Babic A, Morales-Oyarvide V, Qian ZR, Buring JE, Cochrane BB, et
al: Leucocyte telomere length genetic variants at the TERT gene
region and risk of pancreatic cancer. Gut. 66:1116–1122. 2017.
View Article : Google Scholar
|
|
238
|
Posch A, Hofer-Zeni S, Klieser E,
Primavesi F, Naderlinger E, Brandstetter A, Filipits M, Urbas R,
Swiercynski S, Jäger T, et al: Hot Spot TERT promoter mutations are
rare in sporadic pancreatic neuroendocrine Neoplasms and associated
with telomere length and epigenetic expression patterns. Cancers
(Basel). 12:16252020. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Mormile R: Leukocyte telomere length and
pancreatic cancer survival: A consequence of activation of IL-6
signaling pathway in the carcinogenic process? J Gastrointest
Cancer. 51:720–721. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Kroupa M, Rachakonda SK, Liska V, Srinivas
N, Urbanova M, Jiraskova K, Schneiderova M, Vycital O, Vymetalkova
V, Vodickova L, et al: Relationship of telomere length in
colorectal cancer patients with cancer phenotype and patient
prognosis Relationship of telomere length in colorectal cancer
patients with cancer phenotype and patient prognosis. Br J Cance.
121:344–350. 2019. View Article : Google Scholar
|
|
241
|
Lopez-Doriga A, Valle L, Alonso MH, Aussó
S, Closa A, Sanjuan X, Barquero D, Rodríguez-Moranta F,
Sanz-Pamplona R and Moreno V: Telomere length alterations in
microsatellite stable colorectal cancer and association with the
immune response. Biochim Biophys Acta Mol Basis Dis.
1864:2992–3000. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Piñol-Felis C, Fernández-Marcelo T,
Viñas-Salas J and Valls-Bautista C: Telomeres and telomerase in the
clinical management of colorectal cancer. Clin Transl Oncol.
19:399–408. 2017. View Article : Google Scholar
|
|
243
|
Balc'h EL, Grandin N, Demattei MV,
Guyétant S, Tallet A, Pagès JC, Ouaissi M, Lecomte and Charbonneau
M: Measurement of telomere length in colorectal cancers for
improved molecular diagnosis. Int J Mol Sci. 18:18712017.
View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Luu HN, Qi M, Wang R, Adams-Haduch J,
Miljkovic I, Opresko PL, Jin A, Koh WP and Yuan JM: Association
between leukocyte telomere length and colorectal cancer risk in the
Singapore Chinese Health Study. Clin Transl Gastroenterol. 10:1–9.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Peacock SD, Massey TE, Vanner SJ and King
WD: Telomere length in the colon is related to colorectal adenoma
prevalence. PLoS One. 13:e02056972018. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Naing C, Aung K, Lai PK and Mak JW:
Association between telomere length and the risk of colorectal
cancer: A meta-analysis of observational studies. BMC Cancer.
17:242017. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Aljarbou F, Almobarak A, Binrayes A and
Alamri HM: The expression of telomere-related proteins and DNA
damage response and their association with telomere length in
colorectal cancer in Saudi patients. PLoS One. 13:e01971542018.
View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Park WJ, Bae SU, Heo YR, Jung SJ and Lee
JH: Telomere shortening in non-tumorous and tumor mucosa is
independently related to colorectal carcinogenesis in precancerous
lesions. Int J Mol Epidemiol Genet. 8:53–58. 2017.PubMed/NCBI
|
|
249
|
Li J, Chang J, Tian J, Ke J, Zhu Y, Yang
Y, Gong Y, Zou D, Peng X, Yang N, et al: A Rare Variant P507L in
TPP1 interrupts TPP1-TIN2 interaction, influences telomere length,
and confers colorectal cancer risk in Chinese population. Cancer
Epidemiol Biomarkers Prev. 27:1029–1035. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Ridout KK, Ridout SJ, Price LH, Sen S and
Tyrka AR: Depression and telomere length: A meta-analysis. J Affect
Disord. 191:237–247. 2016. View Article : Google Scholar :
|
|
251
|
AlAhwal MS, Zaben FA, Sehlo MG, Khalifa
DA, Al-Aama JY, Edris S, Ashy JA and Koenig HG: Depression and
telomere length in colorectal cancer patients in Saudi Arabia.
Asian J Psychiatr. 40:130–131. 2019. View Article : Google Scholar
|
|
252
|
Ko E, Seo HW and Jung G: Telomere length
and reactive oxygen species levels are positively associated with a
high risk of mortality and recurrence in hepatocellular carcinoma.
Hepatology. 67:1378–1391. 2018. View Article : Google Scholar
|
|
253
|
Ma LJ, Wang XY, Duan M, Liu LZ, Shi JY,
Dong LQ, Yang LX, Wang ZC, Ding ZB and Ke AW: Telomere length
variation in tumor cells and cancer-associated fibroblasts:
Potential biomarker for hepatocellular carcinoma. J Pathol.
243:407–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Lee HW, Park TI, Jang SY, Park SY, Park
WJ, Jung SJ and Lee JH: Clinicopathological characteristics of TERT
promoter mutation and telomere length in hepatocellular carcinoma.
Medicine (Baltimore). 96:e57662017. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Ningarhari M, Caruso S, Hirsch TZ, Bayard
Q, Franconi A, Védie AL, Noblet B, Blanc JF, Amaddeo G, Ganne N, et
al: Telomere length is key to hepatocellular carcinoma diversity
and telomerase addiction is an actionable therapeutic target. J
Hepatol. 74:1155–1166. 2021. View Article : Google Scholar
|
|
256
|
Zeng H, Wu HC, Wang Q, Yang HI, Chen CJ,
Santella RM and Shen J: Telomere length and risk of hepatocellular
carcinoma: A nested Case-control study in Taiwan cancer screening
program cohort. Anticancer Res. 37:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Cheng Y, Yu C, Huang M, Du F, Song C, Ma
Z, Zhai X, Yang Y, Liu J, Bei JX, et al: Genetic association of
telomere length with hepatocellular carcinoma risk: A Mendelian
randomization analysis. Cancer Epidemiol. 50:39–45. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Feng W, Yu D, Li B, Luo OY, Xu T, Cao Y
and Ding Y: Paired assessment of liver telomere lengths in
hepatocellular cancer is a reliable predictor of disease
persistence. Biosci Rep. 37:BSR201606212017. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Nikolouzakis TK, Stivaktakis PD, Apalaki
P, Kalliantasi K, Sapsakos TM, Spandidos DA, Tsatsakis A, Souglakos
J and Tsiaoussis J: Effect of systemic treatment on the micronuclei
frequency in the peripheral blood of patients with metastatic
colorectal cancer. Oncol Lett. 17:2703–2712. 2019.PubMed/NCBI
|
|
260
|
Nikolouzakis TK, Vakonaki E, Stivaktakis
PD, Alegakis A, Berdiaki A, Razos N, Souglakos J, Tsatsakis A and
Tsiaoussis J: Novel prognostic biomarkers in metastatic and locally
advanced colorectal cancer: Micronuclei frequency and telomerase
activity in peripheral blood lymphocytes. Front Oncol.
11:6836052021. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Guterres AN and Villanueva J: Targeting
telomerase for cancer therapy. Oncogene. 39:5811–5824. 2020.
View Article : Google Scholar : PubMed/NCBI
|















