MicroRNA (miRNAs/miRs) are small non-coding (nc)
RNAs with the size of 17-25 nucleotides. The first miRNA was
identified in 1993 when a small ncRNA was discovered in
Caenorhabditis elegans heterochronic gene lin-4 (1). Subsequently, other small RNAs were
found in Caenorhabditis elegans, Drosophila and
humans (2-4). Later, researchers realized that small
ncRNAs are functional products that have an impact on development
outside of translating proteins (5-7). The
discovery of miRNAs shed light on post-transcriptional regulation
of gene expression. Briefly, miRNA genes are transcribed into
primary miRNAs and processed into mature miRNA duplexes by pol-II,
Drosha and Dicer. Then, the miRNA duplex is loaded into argonaute
protein to form the RNA-induced silencing complex, which navigates
the mature miRNA to the 3'UTR of their targeted mRNA through base
pairing, resulting in mRNA transcriptional inhibition or mRNA
degradation (8). In the past few
years, studies on miRNAs have increased, and as of June 2023,
38,589 miRNAs have been annotated in the miRBase miRNA database
(https://www.mirbase.org/). Abundant studies have
substantiated the intricate association between dysregulated miRNAs
and a multitude of diseases, notably carcinogenesis (9,10).
These miRNAs are powerful regulators of various cellular processes
including cell proliferation, differentiation, development and
apoptosis (11,12). As a pivotal constituent of the
ncRNA network, miRNAs possess the ability to occupy numerous nodes
owing to their capacity to target a considerable number of mRNAs
(13). With the development of
computational and sequencing technology, researchers can easily
predict the target genes of a miRNA through its sequence for target
recognition called the 'seed sequence', which is the nucleotides
2-8 of a miRNA (13,14). In recent years, studies have
deciphered the biological function of miRNAs extensively, but
understanding of the role of miRNAs still requires a tremendous
amount of work.
MiR-181a-5p has been extensively studied as a
regulatory miRNA with altered expression in various diseases. For
instance, studies have revealed that miR-181a-5p alleviates
vascular inflammation, atherosclerosis and inflammatory response in
monocrotaline-induced pulmonary arterial hypertension (15,16).
In addition, it is associated with obesity and insulin resistance
(17). Currently, numerous
research has identified upregulated or downregulated expression
levels of miR-181a-5p in different tumors, highlighting its role in
regulating tumorigenesis through post-transcriptional suppression
of its targeted genes (18,19).
The present review summarizes the recent studies on miR-181a-5p,
explain its role in cancer and chemotherapy and outlines its
potential as a biomarker.
MiR-181a-5p is a conserved miRNA belonging to the
miR-181 family, which comprises four mature miRNAs. These mature
miRNAs, namely miR-181a, miR-181b, miR-181c and miR-181d, all share
the identical 'seed' sequence 'ACAUUCA'. In humans, miR-181a is
located in chromosome 1. MiR-181a-5p is a mature single strand of
miR-181a with the sequence 'AACAUUCAACGCUGUCGGUGAGU', while
miR-181a-3p is a passenger strand (15,20).
Numerous studies have suggested that dysregulation
of miR-181a-5p in tumors is regulated the following factors:
The growth of cancer is associated with its
malignant cell hallmarks, which include the capabilities for
sustaining proliferative signaling, evading growth suppressors,
resisting cell death, enabling replicative immortality,
inducing/accessing vasculature, activating invasion and metastasis,
reprogramming cellular metabolism and avoiding immune destruction
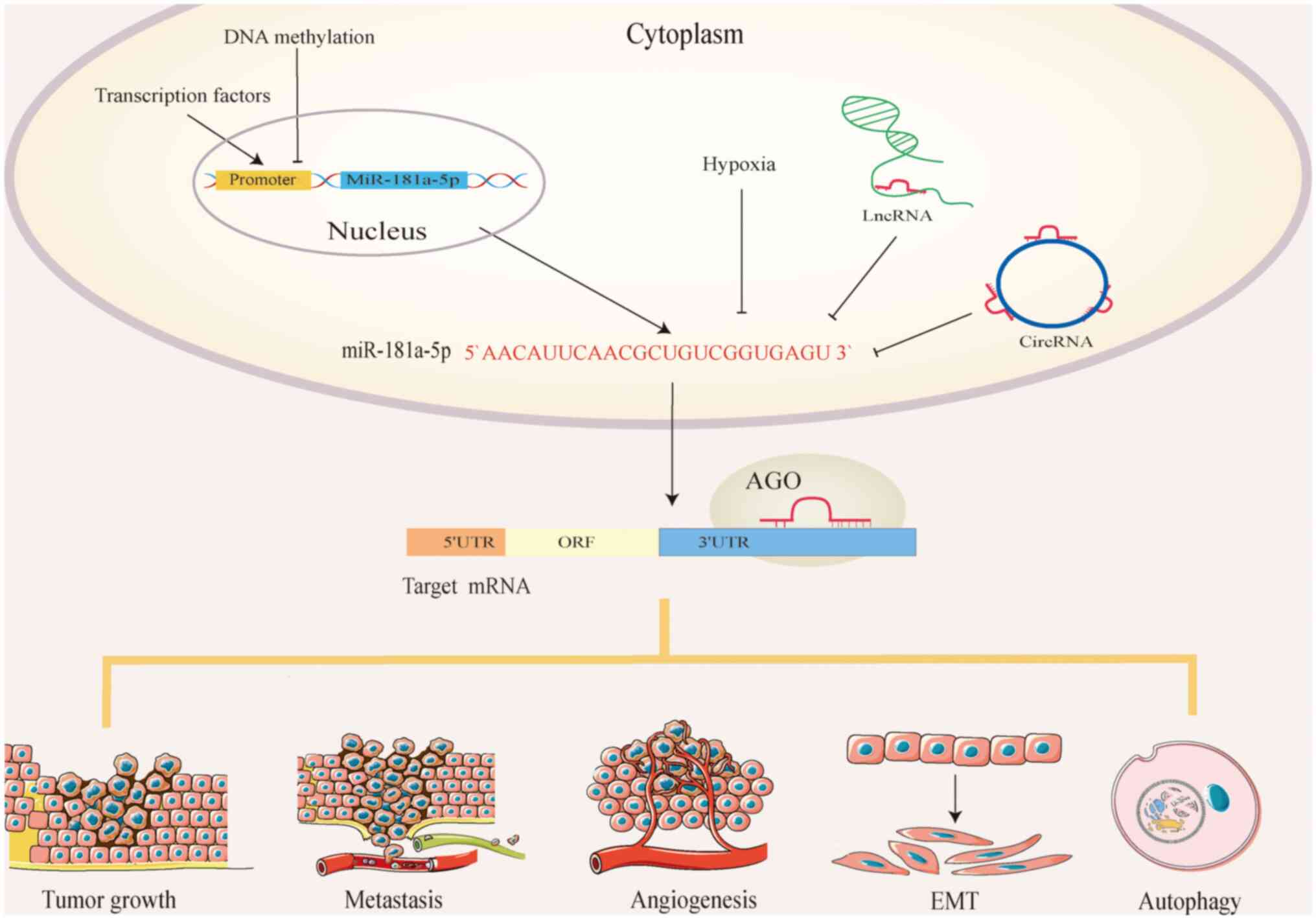
(31). MiR-181a-5p exerts its
influence on various tumor properties, including cell
proliferation, metasitasis, angiogenesis, epithelial-mesenchymal
transition (EMT) and autophagy (Fig.
1). It is important to note that the expression of miR-181a-5p
is specific to certain tissues and it can simultaneously target
multiple genes, potentially playing dual roles. The function of
miR-181a-5p is not reliant on a specific target, but rather on the
collective impact of its targets, which may encompass both tumor
suppressor genes and oncogenes (32). The present study summarizes the
existing studies on miR-181a-5p in different tumors and has
described them in various systems. The summary of results is
provided in (Table I).
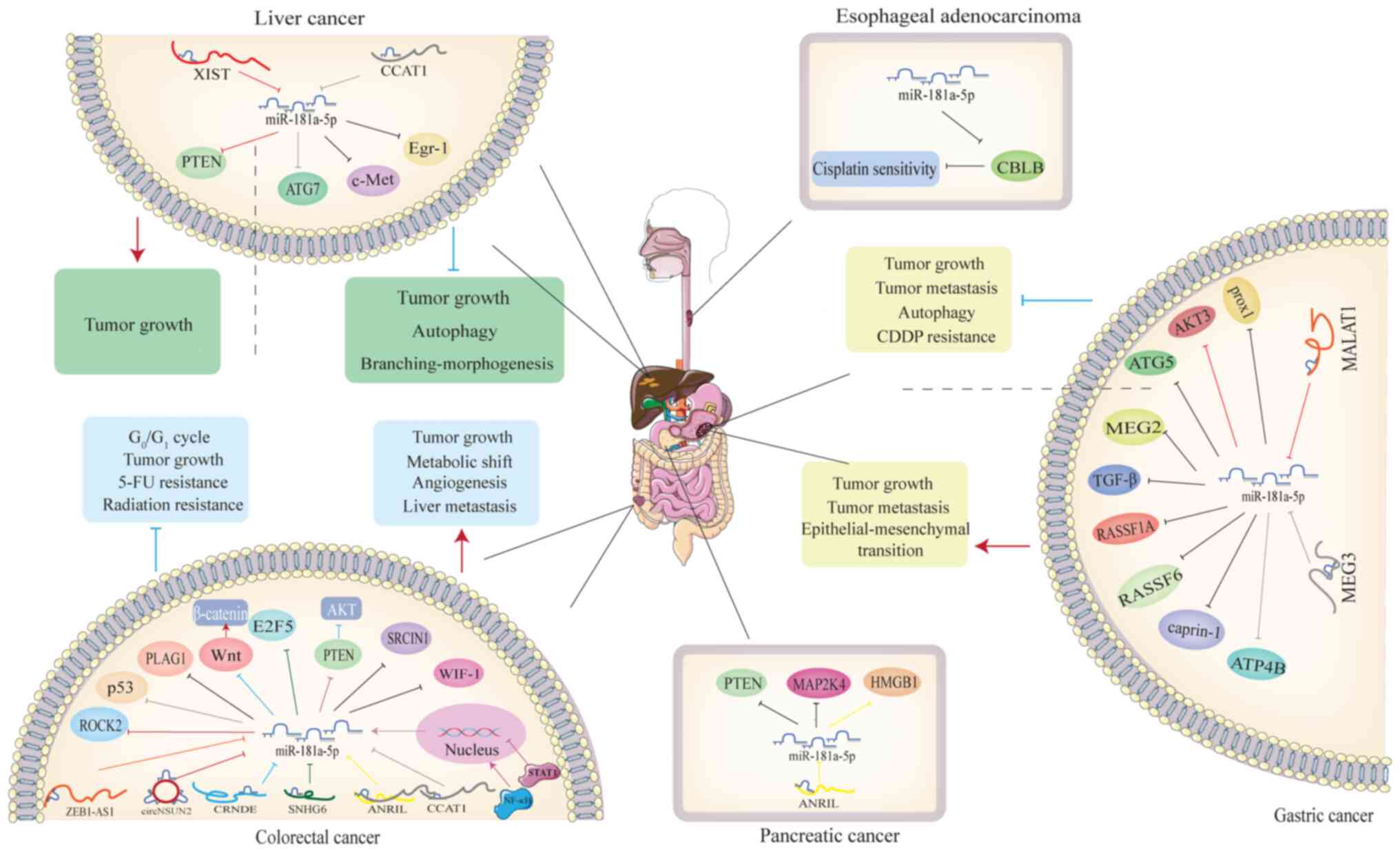
Colorectal cancer (CRC) is the third most common
cancer worldwide and also the most frequent tumor of the digestive
tract. The high migratory and invasive properties of CRC cells
promote the progression of CRC and lead to poor prognosis of
patients with CRC (33).
In CRC, results indicate that miR-181a-5p suppresses
tumor growth by regulating the Wnt/β-catenin signaling pathway. It
has been observed to inhibit cell proliferation, 5-FU sensitivity
and promote apoptosis (34,35).
The inhibitory effect of miR-181a-5p has also been confirmed in
microsatellite-instable CRC, where its expression of miR-181a-5p is
reduced and miR-181a-5p directly binds to the 3'UTR of pleomorphic
adenoma gene 1 (PLAG1) (28).
Another well-established target of miR-181a-5p is p53. Upregulation
of miR-181a-5p promotes apoptosis by modulating Bax and Bcl-2
(24). In addition, a previous
study revealed that lncRNA-ANRIL can sponge miR-181a-5p, inhibiting
apoptosis and radiosensitivity in colon cancer cells (36).
The lncRNA-SNHG6 has been identified to be
positively correlated with tumor progression and distant metastasis
(37). MiR-181a-5p is the direct
target of both SNHG6 and E2F5. By inhibiting E2F5, miR-181a-5p
induces G0/G1 arrest and suppresses CRC cell
migration and invasion (38).
Additionally, a study revealed that miR-181a-5p reverses the
effects of circRNA-NSUN2 on promoting cell proliferation and
migration by binding to the 3'UTR of Rho-associated
coiled-coil-containing protein kinase 2 (ROCK2) (39).
MiR-181a-5p have been demonstrated to be associated
with liver metastasis of CRC. This may be attributed to the
enrichment of miR-181a-5p in extracellular vesicles of CRC, which
alters the tumor environment (TME) (40). MiR-181a-5p promotes motility,
invasion and tumor growth by directly targeting Wnt inhibitory
factor 1 (WIF-1). Notably, it participates in the regulation of
EMT, which is considered a crucial process in cancer metastasis
(41). MiR-181a-5p also promotes
metastasis and cell proliferation in CRC by inhibiting PTEN, a
tumor suppressor gene (42).
Multiple studies have showed that miR-181a-5p binds to the 3'UTR of
PTEN mRNA, leading to reduced PTEN expression and subsequent
activation of the phosphorylated (p)-AKT pathway (27,43).
In these cases, the expression of miR-181a-5p is upregulated by
IL-1β/NF-kb signaling (26), while
STAT1 acts as an inhibitor of miR-181a-5p (27). Furthermore, miR-181a-5p induces
metabolic shifts in CRC, favoring glycolysis over oxidative
pathways and resulting in increased lactic acid release (43). Finally, angiogenesis is an
important feature for tumor growth and metastasis. The
pro-angiogenic ability of miR-181a-5p has been demonstrated, and it
inhibits the expression of SRC kinase signaling inhibitor 1
(SRCIN1) and reversion-inducing cysteine-rich protein with Kazal
motifs (RECK) to promote angiogenesis (Fig. 2) (44,45).
Gastric cancer (GC) is a major health burden
worldwide. It is the second cause of cancer-related mortalities
after lung cancer (46).
A previous study has reported an elevation of
miR-181a-5p in GC tissues, which is corelated with tumor
progression (47). Meanwhile,
TGF-β level is decreased in GC tissues compared with normal
tissues. Experimental results indicate that miR-181a-5p directly
interacts with TGF-β, thereby facilitating tumor cell proliferation
in vivo and in vitro (48). The Ras association domain family
(RASSF) is a crucial contributor in the formation of tumors.
Another study has demonstrated that miR-181a-5p promotes GC cell
proliferation and G1/S transition, and suppresses
apoptosis by inhibiting RASSF1A (49). MiR-181a-5p also inhibits ATP4B and
tyrosine-protein phosphatase megakaryocyte 2 to promote GC tumor
growth (50,51).
MiR-181a-5p modulates the RASSF6/MAPK pathway,
promoting proliferation, migration, invasion, metastasis and
inducing EMT in GC (52). The role
of miR-181a-5p in promoting metastasis in GC is further confirmed
by evidence showing that it inhibits caprin-1 to promote cell
proliferation, invasion and migration, while reducing apoptosis
in vitro and in vivo (53).
MiR-181a-5p has been reported to negatively regulate
autophagy of cisplatin resistant cells. MiR-181a-5p increases
sensitivity of drug-resistant cells to cisplatin and the tumor
volume of nude mice. In this context, ATG5 is a potential target of
miR-181a-5p (54). Lin et
al also revealed that miR-181a-5p blocks GC cell proliferation,
migration and invasion (55).
Oncogenic factor, Prox1, was considered to be a downstream target
of miR-181a-5p (55). In gastric
adenocarcinoma, miR-181a-5p has been indicated to inhibit cell
proliferation and increase apoptosis by modulating the AKT pathway
(Fig. 2) (56).
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer, accounting for 75-85% of all types of liver
cancer, which caused ~830,000 mortalities worldwide as of 2020
(46). Several experimental
results indicate that miR-181a-5p may plays its role as a tumor
inhibitor in HCC (Fig. 2).
MiR-181a-5p inhibits c-Met to promote
branching-morphogenesis and invasion of HCC cells (18). Additionally, it induces glucose
metabolism reprogramming, which is associated with the progression
and early lung metastasis of HCC. Mechanistically, miR-181a-5p
reduces the expression of mitochondrially encoded (mt)-Cytochrome B
and mt-Cytochrome C oxidase subunit 2 proteins, thus decreasing the
electron transport chain (ETC). This reduction in ETC activity
results in an increase in hexokinase 2 (HK2) and glucose
transporter 1, enhancing glucose uptake, lactic acid release and
LDH activity (57).
Early growth response factor1 (Egr1) plays a crucial
role in cancer progression by activating the TGF-β/Smad pathway.
Evidence has demonstrated that miR-181a-5p inhibits Egr1 by binding
to its 3'UTR, resulting in tumor proliferation suppression in HCC
(58). Assays have showed that
miR-181a-5p inhibits HCC cell autophagy by targeting ATG7, which is
positively correlated with autophagy (59).
However, a previous report suggested that
lncRNA-XIST increases the cancer suppressor gene PTEN through the
inhibition of miR-181a-5p. Restored miR-181a-5p expression promotes
the HCC cell proliferation and invasion (60).
MiR-181a-5p inhibits cisplatin resistance in
esophageal adenocarcinoma. In comparison with constructed
cisplatin-resistant EAC cells, the miR-181a-5p expression is
significantly higher in normal EAC cells. Furthermore, miR-181a-5p
exhibits stronger cisplatin-induced inhibition of proliferation and
promotion of apoptosis. In addition, CBLB, which is involved in
ubiquitination to aggravate cisplatin resistance, is identified as
a direct target of miR-181a-5p (Fig.
2) (61).
Pancreatic cancer (PC) is the most malignant tumor
of the digestive system, which is extremely aggressive (62). Although early findings have
revealed that miR-181a-5p, which indirectly inhibits the PTEN and
MAP2K4, enhances the invasion capability of PC (63), another report after 8 years
revealed that miR-181a-5p inhibits PC by targeting high mobility
group box 1 (HMGB1). It inhibits PC cell proliferation, invasion,
migration and resistance of gemcitabine, while reducing the
expression of miR-181a-5p attenuates these effects. In addition,
lncRNA-ANRIL decreases HMGB1 by sponging miR-181a-5p to activate
cell autophagy (Fig. 2) (64).
Lung cancer is the most commonly diagnosed cancer in
the world and is characterized by a high rate of metastasis and
delayed diagnosis (65). NSCLC
accounts for 80-85% of all types of lung cancer (66). The following studies indicate that
miR-181a-5p is a tumor inhibitor in NSCLC. It can inhibit tumor
growth and metastasis by targeting multiple pro-tumorigenic
factors. MiR-181a-5p targets the recognized oncogene Kras by
binding to its 3'UTR, thereby slowing cell proliferation and
migration (67). It has also been
found to target CDK1 and E2F7, which regulate the cell cycle and
promote tumor proliferation (68,69).
Additionally, miR-181a-5p has been demonstrated to target HMGB2 to
inhibit NSCLC cell migration and invasion (25). It is worth mentioning that NF-κB
has been indicated to promote miR-181a-5p expression in colorectal
cancer (26). However, a report
has demonstrated that IL-17 inhibits miR-181a-5p by activating
NF-κB (70). Furthermore, vascular
cell adhesion molecule 1 has been demonstrated to be a direct
target of miR-181a-5p. This laterally proves that miR-181a-5p can
reduce vascular oxygen supply (70). In these cases, lncRNA NEAT1 and
SNHG7 have been demonstrated to be up-stream regulators of
miR-181a-5p as a miRNA sponge (25,69,71).
Notably, miR-181a-5p can alleviate immunosuppression and anti-PD-1
resistance of NSCLC, providing a novel strategy for enhancing the
efficacy of immunotherapy (72).
MiR-181a-5p has a tumor suppressor property in
laryngeal cancer. Myc target protein 1 (MYCT1) is known to regulate
cell apoptosis (73). Recently,
Wang et al revealed that MYCT1 collaborates with
MYC-associated protein X to enhance the promoter of miR-181a-5p,
which binds to the 3'UTR of nucleophosmin 1 (NPM1), thus inhibiting
laryngeal cancerous cell viability, colony formation and promoting
apoptosis (74). Another study has
demonstrated that miR-181a-5p inhibits EMT of laryngeal cancer by
targeting Snai2 (75).
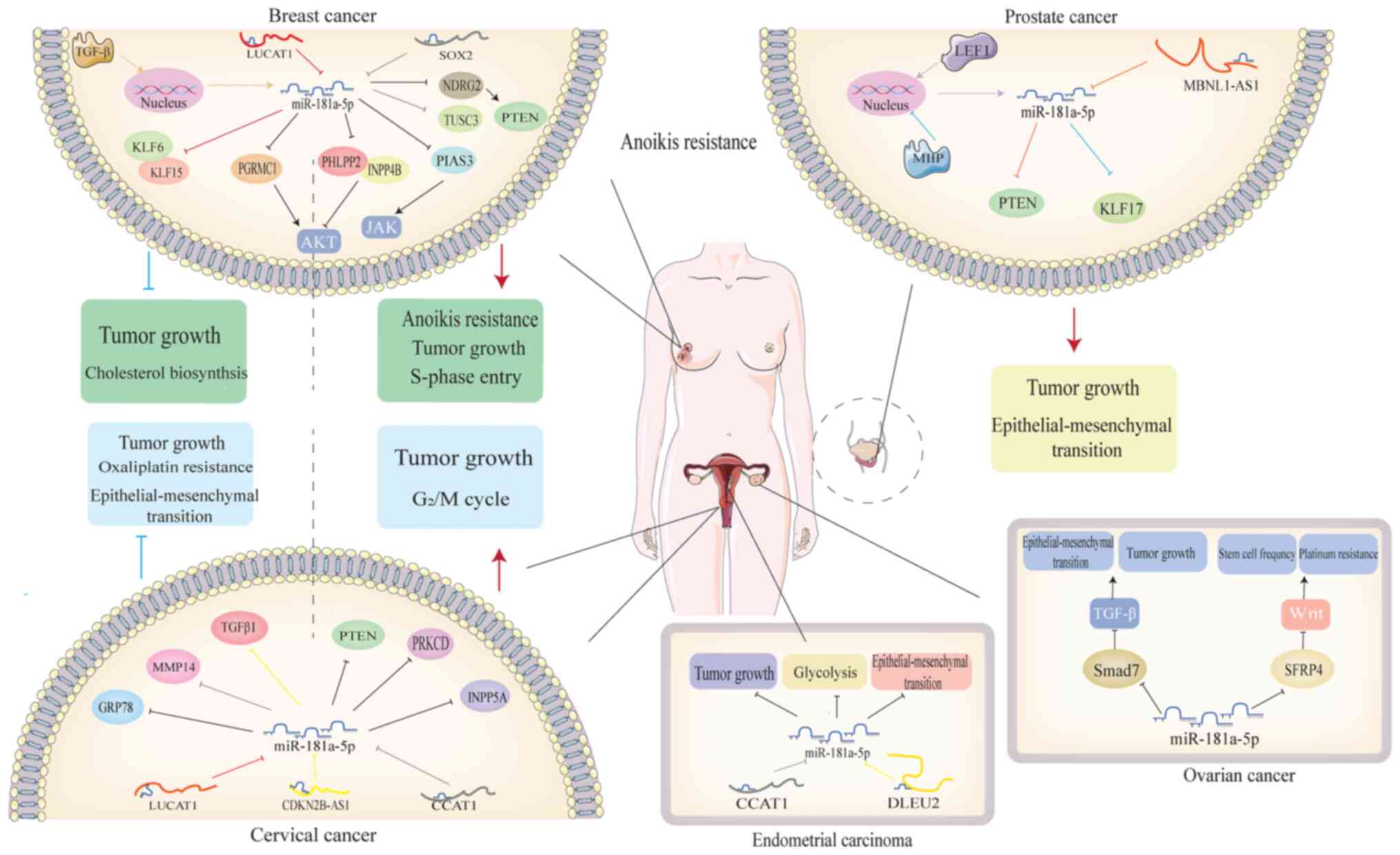
Exosome-derived miR-181a-5p is upregulated in BC. By
targeting PIAS3, it promotes the expansion of early-stage
myeloid-derived suppressor cells, which in turn exacerbate cell
proliferation, induce tumor growth and evade immune destruction
(19). In addition, miR-181a-5p
increases the level of p-AKT by co-targeting PH-domain leucine-rich
repeat-containing protein phosphatase 2 and Inositol polyphosphate
4-phosphatase type II phosphatases in luminal breast cancer,
resulting in cell proliferation and S phase entry (77). TGF-β has been demonstrated to
upregulate the expression of miR-181a-5p through mediation of its
transcription. Increased miR-181a-5p reduces apoptosis and
sensitivity to anoikis (78).
It has been reported that lncRNA-SOX2 is
downregulated and correlates with poor survival of BC. A
tumorigenesis experiment conducted on nude mice has demonstrated
that SOX2 suppresses tumor development and metastasis by sponging
miR-181a-5p. In this case, miR-181a-5p promotes BC metastasis by
inhibiting Tumor suppressor candidate 3 (79). MiR-181a-5p also targets the NDRG2
to reduce activation of PTEN, thus facilitating proliferation,
invasion and glycolysis of BC (80).
MiR-181a-5p is upregulated in TNBC tissues and
cells. This may be associated with the suppressive effect of ER-β,
which modulates the expression of miRNA to inhibit TNBC.
Upregulation of miR-181a-5p is an auxiliary mechanism of
ER-β-induced cholesterol biosynthesis inhibition (81). In addition, miR-181a-5p has been
revealed to be positively correlated with Bax and Caspase-9, which
promote cell apoptosis (82,83).
Functional experiment has revealed that miR-181a-5p inhibits cell
proliferation, migration and invasion by targeting oncogenes
Kruppel-like factor (KLF) 6, KLF15 and progesterone receptor
membrane component 1 (Fig. 3)
(84).
CC is the fourth leading cause of cancer-associated
mortality among women, accounting for >2.6 million deaths
worldwide every year (46,85).
MiR-181a-5p expression is elevated in CC tissues. It
negatively targets INPP5A to promote CC cell proliferation and
invasion while inhibiting apoptosis (86). MiR-181a-5p also
post-transcriptionally inhibits PTEN in CC. Inhibition of
miR-181a-5p can impede cell cycle progression by increasing P21,
P27, Bax and decreasing Bcl-2 (87). Moreover, miR-181a-5p is upregulated
in human CC specimens and cell lines that are not responsive to
radiation therapy. It suppresses radiation-induced apoptosis and
G2/M cell cycle arrest by inhibiting protein kinase C
delta type (PRKCD) (88).
A study revealed that miR-181a-5p is aberrantly
reduced in CC and inhibits proliferation and resistance of
oxaliplatin. In this case, GRP78 is identified as the direct target
of miR-181a-5p (89).
MiR-181a-5p has direct binding sites with TGFβ1,
promoting the expression of TGFβ1 to inhibit CC proliferation,
migration and invasion (90).
Another group showed that miR-181a-5p inhibits invasion, migration
and EMT of CC (91). LncRNA-CCAT1
is located on chromosome 8q24, where human papillomavirus
integration usually occurs (92).
Overexpression of CCAT1 promotes CC cell proliferation and
invasion. MiR-181a-5p is identified as a downstream target of
CCAT1and decreases the expression of MMP14. CCAT1 indirectly
upregulates the MMP14 by suppressing miR-181a-5p to promote CC
progression (Fig. 3) (23).
EC is one of the most common malignancies in women
and a leading cause of cancer-associated mortalities worldwide
(93).
A preliminary experiment revealed that the PTEN is
decreased and miR-181a-5p is increased in non-obese patients with
EC, suggesting that PTEN is negatively correlated with miR-181a-5p
(94). More focused work revealed
that miR-181a-5p inhibits EC cell proliferation and migration,
while miR-181a-5p inhibitor can neutralize these effects (95). Accumulation of HK2 in EC promotes
EMT and glycolysis. MiR-181a-5p is an inhibitor of HK2. An
experiment has confirmed that DLEU2 interacts with enhancer of
zeste homolog 2 to silence miR-181a-5p, thus inducing EMT and
glycolysis (96) (Fig. 3).
Ovarian cancer is a common tumor of the
gynecological malignancy. Clinical data has demonstrated that
miR-181a-5p is increased in advanced epithelial ovarian cancer and
promotes tumor development (97).
In an in vivo and vitro experiment, miR-181a-5p has
been further indicated to promote cell proliferation, migration,
invasion and EMT. SMAD family member 7 (Smd7), an inhibitor of TGF,
has been identified as a direct target of miR-181a-5p (97). In high-grade serous ovarian cancer,
miR-181a-5p increases stem-cell frequency and resistance of
cisplatin by activating the Wnt/β-catenin signaling pathway. This
activation is achieved by directly targeting Secreted
frizzled-related protein 4 (SFRP4), an inhibitor of the
Wnt/β-catenin pathway (Fig. 3)
(98).
PCa is the second most frequently diagnosed cancer
and the sixth leading cause of cancer-associated mortality among
men worldwide (99).
The inhibitory effect of miR-181a-5p on PTEN has
been observed in PCa. In this context, miR-181a-5p enhances cell
proliferation, migration and invasion (100). To the best of our knowledge, two
studies have investigated the effect of miR-181a-5p on EMT. The
results revealed that miR-181a-5p promotes EMT with high E-cadherin
expression by inhibiting the EMT negative regulator, KLF17.
Notably, lymphoid enhancer-binding factor 1 and migration and
invasion-inhibitory protein have been identified to downregulate
the expression of miR-181a-5p in PCa (101,102).
To the best of our knowledge, there is only one
report focused on the role of miR-181a-5p in BCa. MiR-181a-5p is a
tumor inhibitor in BCa. A group demonstrated that expression of
circRNA-0068871 is increased, while miR-181a-5p is expressed at a
low level in BCa. circRNA-0068871 intensifies cell proliferation,
migration and suppressed apoptosis in vivo and vitro.
Mechanistically, circRNA-0068871 acts as a sponge for miR-181a-5p,
which directly targets EGFR3, indicating that miR-181a-5p is a
tumor inhibitor in BCa (103).
A preliminary experiment indicated that miR-181a-5p
may play a role as an oncomiR in renal cancer. Compared with normal
tissues and cells, miR-181a-5p is upregulated in both renal cancer
tissues and cell lines. In vitro, miR-181a-5p inhibits
apoptosis and promotes proliferation, invasion and migration of
786-O and ACHN cell lines (104).
In addition, miR-181a-5p has been identified to be associated with
tumor size and TNM stages in clear cell renal cell carcinoma.
MiR-181a-5p directly binds to KLF6, which induces apoptosis,
promoting renal cancer progression and metastasis (105).
According to 2020 statistics, TC is the most common
endocrine cancer. Papillary thyroid cancer (PTC) is the most common
type of thyroid cancer, accounting for ~85% of thyroid cancer
worldwide (106). The following
experiments suggest that miR-181a-5p promotes the progression of TC
by modulating multiple target genes. In vivo and
vitro, a group demonstrated that miR-181a-5p inhibits
papillary demethylase and lysine-specific demethylase 5C (KDM5C) to
induce cell proliferation and migration, thus promoting the tumor
growth (107). In addition, it is
widely acknowledged that angiogenesis is a key factor in PTC
recurrence and metastasis. By inhibiting MIL3, exosomal miR-181a-5p
promotes tumor angiogenesis and growth. In this case, it decreases
DACT2 and increases VEGF and YAP (29). Other reports further validated that
miR-181a-5p promotes metastasis of PTC. MiR-181a-5p promotes cell
proliferation, invasion and EMT to aggravate PCT metastasis, while
its downstream target KLF15 and suppressor of cytokine signaling 4
can counteract these effects (108,109). In addition, miR-181a-5p reduces
the efficacy of radioactive iodine treatment by suppressing sodium
iodide symporter (NIS), which is a potent iodine transporter. A
report has indicated that miR-181a-5p directly inhibits
sodium/iodide cotransporter (SLC5A5) to regulate NIS (110).
To the best of our knowledge, one study explored the
role of miR-181a-5p in salivary adenoid cystic carcinoma (SACC)
with lung metastasis. Compared with SACC-83 cells (cells from
patients with SACC), Ju et al revealed that miR-181a-5p is
decreased in SACC-LM cells (SACC patients with lung metastasis).
Furthermore, miR-181a-5p is sponged by circRNA-001982, resulting in
stronger ability of migration and invasion (111).
Leukemia, the most common circulatory system tumor,
with >470,000 new cases worldwide in 2020 (46). It can be divided into myeloid
leukemia and lymphocyte leukemia according to the pathological
cells. Targeting miRNAs associated with leukemia may be an
effective approach to treat leukemia. MiR-181a-5p has been
identified to be associated with acute myeloid leukemia (AML).
Clinical data has demonstrated that miR-181a-5p is decreased in
children with AML, along with a decrease in TGF-β and an increase
in Smad7 (112). However, there
is a conflicting study that suggests miR-181a-5p promotes AML cell
proliferation and G1/S transition by targeting ataxia
telangiectasia mutated (113). In
addition, miR-181a-5p has also been found to promote the
progression of acute lymphoblastic leukemia (ALL) and lymphocyte
leukemia by inhibiting WIF1 and STAT3 (114,115). However, another study revealed
that miR-181a-5p has an inhibitory effect on myelogenous leukemia.
MiR-181a-5p directly binds to the 3'UTR of Ra1A, thus inhibiting
proliferation and promoting G2 cell cycle arrest and
apoptosis (116).
Multiple myeloma (MM) is the second most common
hematologic tumor with 176,404 new cases worldwide in 2020
(46). MM is a hematological tumor
characterized by abnormal proliferation of plasma cells.
MiR-181a-5p appears to be a potential therapeutic target for MM.
In vitro experiments, miR-181a-5p is associated with lower
CDK2, Cyclin E1 and Bcl2 and higher p21, Bax and caspase 3 to
regulate proliferation and apoptosis of MM cells by inhibiting the
Hippo/YAP axis (117). In
addition, miR-181a-5p induces cell cycle to
G0/G1 phase arrest, and directly targets
homeobox transcription factor A1 (HOXA1), which has been
demonstrated to promote cell growth and tumor progression (118). These findings suggest that
miR-181a-5p plays a suppressive role in MM.
Lymphoma is a malignant tumor originating in the
lymphatic hematopoietic system. Diffuse large B-cell lymphoma
(DLBCL) is the most frequent subtype of non-Hodgkin lymphoma,
accounting for 31% in Europe and the USA (119). Common standard treatments cure
only about half of patients. MiR-181a-5p suppresses the
proliferation and survival of DLBCL, and it modulates NF-kB by
directly targeting NF-kB regulatory factors caspase recruitment
domain-containing protein 11, encoding nuclear factor of κ-light
polypeptide gene enhancer in B-cells inhibitor-α, p50, p65 and
c-Rel. Study using xenograft models revealed that miR-181a-5p
prevents tumor growth rate and prolongs the animal survival in
NF-kB-dependent DLBCL (120).
Glioma is a highly malignant tumor in the central
nervous system, which develops rapidly and is prone to metastasis
from early stage (121). The
clinical data reveal a decrease in miR-181a-5p in glioma tissues
and cell lines. It has been determined that circRNA 0076248 acts as
an upstream regulator of miR-181a-5p, indirectly increasing the
expression of Sirtuin 1 (SIRT1) (122). Overexpression of miR-181a-5p
inhibits cell proliferation, invasion and sensitizes cells to
Temozolomide (TMZ) (122). An
investigation has also demonstrated that miR-181a-5p acts as a
suppressive regulator of glioblastoma multiform (GBM), the most
malignant glioma (123). In a
study involving patients with GBM treated with carmustine,
miR-181a-5p has been shown to enhance G1 cell cycle
arrest and apoptosis by regulating caspase-9, Bcl-2 and SIRT1.
Mechanistically, miR-181a-5p inhibits the PI3K/AKT signaling
pathway to promote GBM cell apoptosis and carmustine sensitivity
(124). MiR-181a-5p also
decreases glioblastoma stem-like cells formation and Osteopontin
production of GBM, thus inhibiting the tumor development and
progression (125,126). In addition, miR-181a-5p has been
demonstrated to increase the permeability of the blood-tumor
barrier (BTB), thereby improving the delivery of therapeutic drugs
(127). However, a study
conducted by Liao and coworkers demonstrated that increased
expression of miR-181a-5p promotes cell proliferation and TMZ
sensitivity through the regulation of the PTEN/AKT signaling
pathway (128).
Neuroblastoma is the most frequent extracranial
solid tumor in infants worldwide, with 25-50 cases per million
individuals. More than 50% of patients already have distant
metastases by the time they are diagnosed (129). In a study, researchers attributed
the oncogenic role of miR-181a-5p to inhibit ABI1 mRNA. In
vitro experiments, it promotes cell proliferation, migration
and invasion. Furthermore, a nude mice xenograft model provided
further evidence that consolidates the pro-tumorigenic effect of
miR-181a-5p (130). MB is an
aggressive cerebral tumor, divided into four molecular subtypes: i)
WNT; ii) SHH; iii) 3 group; and iv) 4 group. Among them, 3 group
has the worst prognosis and the majority of patients have
metastasized at the time of diagnosis (131). A previous report provides novel
treatment strategies for 3 group-MB. Experimental evidence
indicates that miR-181a-5p expression is increased in 3 group-MB
cells compared with SHH-MB cells. SHH-MB cells treated with 3
group-MB exosomal miR-181a-5p demonstrate increased aggressiveness
and mobility. The tumor-promoting effects of exosomal miR-181a-5p
are attributed to activation of the RAS/MAPK signaling pathway
(132).
Melanoma is an aggressive cancer of the skin.
MiR-181a-5p promotes melanoma cell proliferation and invasion,
suggesting that miR-181a-5p has a role of tumor inhibitor. Then,
miR-181a-5p is found to directly bind to the 3'UTR of Plexin C1 and
is sponged by lncRNA-CASC2 (133). However, another study provided
evidence that miR-181a-5p reduces the expression of Bcl2 and
induces apoptosis of melanoma stem cells (134).
Cutaneous squamous cell carcinoma (cuSCC) is the
second most commonly diagnosed malignant cancer of the skin after
melanoma, accounting for 20% of skin cancer worldwide (135). Two reports came to opposite
conclusions on the role of miR-181a-5p in CSCC. In normal epidermal
keratinocytes (HaCaT), a group uncovered that expression of
miR-181a-5p increases in cuSCC tissues and inhibits apoptosis of UV
induced HaCaT cells. In addition, miR-181a-5p suppresses TGF R3 to
increase the expression of TGF, which promotes multiple tumorigenic
functions (136). The other study
demonstrated that miR-181a-5p blocks the Kras/MAPK pathway to slow
SCC13 cell proliferation (137).
Notably, the two reports treated the cells in different ways and
utilized different cell lines for in vitro experiments.
In OS, miR-181a-5p has been observed to target
RASSF6 to promote cell proliferation and invasion. TUSC7 and CASC2
were established as upstream regulators of miR-181a-5p (139,140). Chondrosarcoma is a primary
osteosarcoma in which mortality is usually due to lung metastasis.
The expression of miR-181a-5p is upregulated significantly in
chondrosarcoma (141). By
regulating VEGF and G-protein signaling 16, miR-181a-5p has been
shown to promote angiogenesis, which is critical for the
progression and metastasis of OS (22,142).
With the development of technology, miR-181a-5p can
be accurately and conveniently quantitatively detected (143), which makes it a potential
biomarker. The following studies showed the potential of using
miR-181a-5p as one of the biomarkers for diagnosis, prognosis and
assessment of chemotherapy response. These studies are summarized
in Table II.
Although tissue biopsies remain the gold standard
for cancer diagnosis, there is evidence that miR-181a-5p can be
used as a biomarker for early diagnosis. For example, serum
miR-181a-5p level is decreased in patients with BC compared with
normal subjects, and the sensitivity of miR-181a-5p level in early
diagnosis of BC is higher compared with that of conventional tumor
markers CA153 and carcinoembryonic antigen (144). Another study found that plasma
exosomal miR-181a-5p is significantly increased in patients with
CRC, suggesting that it has the potential as a marker for
diagnosing CRC (145). In
addition, miR-181a-5p can also be used to determine the subtype or
stage of a certain cancer. Researchers found that compared with
controls, patients with early esophageal cancer have significantly
lower level of miR-181a-5p, which can be used as a novel biomarker
for early diagnosis of esophageal cancer (146). In endometrial carcinoma (EC), it
is identified to be increased in type I EC and type II EC, while
the increase in type II EC was significantly higher compared with
that in type I (147). In
addition, miR-181a-5p level is elevated in the tissues of Chinese
males with lung squamous cell carcinoma (148).
Numerous studies have found that the level of
miR-181a-5p may predict the risk of progression and survival of
multiple types of cancer. In the majority of reports, increased
miR-181a-5p in tumor tissue or serum is correlated with poor
outcome and shorter overall survival (149-152). For example, in pediatric acute
lymphoblastic leukemia, miR-181a-5p increases the risk of central
nervous system of leukemia. The expression of miR-181a-5p provides
a novel marker for the course of pediatric ALL. In addition, its
expression in bone marrow and peripheral blood samples is
significantly decreased to the 33rd day of treatment (153). But in NSCLC (154,155) and AML (156), miR-181a-5p is positively
associated with an improved prognosis. Moreover, extracellular
vesicle-delivered miR-181a-5p may indicate the risk of tumor
metastasis. For example, miR-181a-5p is significantly upregulated
in patients with bone metastatic prostate cancer (157), while in rectal cancer with lymph
node metastasis, it is decreased (158).
Overall, miR-181a-5p may be an effective tool for
predicting cancer prognosis. However, more studies are warranted to
provide evidence for clinical application.
The level of miR-181a-5p expression can predict the
treatment response of patients with cancer, which can improve the
reference for the selection of clinical treatment strategy. For
example, EGFR-tyrosine kinase inhibitors (TKIs), such as gefitinib,
are the first-line treatment of advanced NSCLC in the presence of
allergenic mutations. Circulating miR-181a-5p was quantified in
plasma samples of 39 patients with advanced EGFR-mutated NSCLC
treated with EGFR-TKIs, and the results showed that patients with
partial/complete response (PR/CR) had higher baseline miR-181-5p
compared with patients with stable/progressive disease (SD/PD)
(159). This trend is similar in
patients with colorectal cancer treated with EGFR-TKI. A low level
of miR-181a-5p indicates poor progression-free survival (PFS)
(160). Uniformly, the level of
miR-181a-5p is positively associated with the outcomes of
anti-tumor treatment, such as EOX
(epirubicin/capecitabine/oxaliplatin) regimen, bortezomib,
sorafenib or stem cell transplantation. In these cases, serum
miR-181a-5p in PR patients may be significantly higher than that in
PD patients (161-164). However, in advanced unresectable
epithelial ovarian cancer, patients with higher miR-181a-5p
expression have shorter overall survival and PFS accompanied by
elevated smad2. Combined analysis of p-smad2 and miR-181a-5p may
potentially identify those patients with ovarian cancer with a
lower chance of responding to platinum-based neoadjuvant
chemotherapy (165).
Chemotherapy is one of the main therapeutic methods
used against cancer, and the response of patients with cancer to
chemotherapy is influenced by various factors, such as PTEN and the
Wnt/β-catenin pathway (166,167). Numerous genes have been
identified as being involved in chemotherapy. Therefore, miRNA can
impact cancer chemotherapy by binding to chemotherapy-related
targets (168,169). Recent research has focused on the
role of miR-181a-5p in chemotherapy. These findings provide a
foundation for the clinical use of miR-181a-5p mimics or inhibitors
to enhance the sensitivity of chemotherapeutics. In this section,
the present study discusses the interaction between miR-181a-5p and
platinum, as well as other chemotherapeutic agents (Table III).
Platinum drugs, such as cisplatin, carboplatin and
oxaliplatin, are one of the most commonly used drugs in
chemotherapy and are often used in combination with other
chemotherapeutic drugs (170). A
recent study has shown that miR-181a-5p can enhance the sensitivity
of platinum drugs by targeting some oncogenes in certain types of
cancer. It has been observed that in cisplatin resistant cells, the
level of miR-181a-5p is reduced. Conversely, CUGBP Elav-like family
member 1, which is inhibited by miR-181a-5p, increases in cisplatin
resistant cells in lung squamous cell carcinoma and as an oncogene.
MiR-181a-5p can decrease the IC50 of cells and
effectually recover the cisplatin resistance (171).
Overall, the studies indicated that miR-181a-5p can
increase the sensitivity to platinum except in ovarian cancer.
Using miR-181a-5p mimics to increase the miR-181a-5p expression may
be a potential strategy for platinum resistance patients.
MiR-181a-5p showed different effects on various
chemotherapeutic agents. For example, it inhibits resistance of
gemcitabine (64) and Ara-c
(177), but promotes gefitinib
resistance (178). Wnt/β-catenin
pathway promotes resistance to 5-FU in colorectal cancer.
MiR-181a-5p has been identified as an inhibitor of Wnt and PLAG1,
and increases the 5-FU sensitivity of colorectal cancer cells
(28,34). In melanoma, miR-181a-5p decreases
in BRAF inhibitor (dabrafenib) resistant patients. MiR-181a-5p
mimics inhibit mitochondrial transcription factor A and reverses
dabrafenib resistance (179). In
addition, promoting sensitivity of chemotherapy by using
miR-181a-5p has been applied in a rat-seeded retinoblastoma model,
which demonstrated that using lipid nanoparticles to co-deliver
miR-181a-5p and melphalan can enhance the efficacy while reducing
cytotoxic side effects by inhibiting BCL-2, MAPK1 and promoting Bax
(180). In addition, Carmustine
and TMZ are alkylating agents for glioma, and miR-181a-5p can
promote sensitivity of carmustine (124); however, results of the effect of
miR-181a-5p on TMZ was opposite in two independent studies
(122,128).
The present study elucidates the role of
miR-181a-5p in cancers of different systems. These studies helped
to understand the effects of miR-181a-5p on tumor progression,
chemotherapy and revealed that it has the potential to be a
biomarker. However, as a kind of miRNAs, miR-181a-5p is
environment-dependent. Since miR-181a-5p can simultaneously bind to
multiple targets (possibly oncogenes or tumor suppressor genes),
and these targets express differently in different types of cancer,
scientists often get conflicting results in the study of
miR-181a-5p. Due to this uncertainty, it is difficult to actually
put miR-181a-5p into clinical use. More research and clinical
trials are needed to provide a further understanding of
miR-181a-5p.
The present review summarizes some interesting
studies on miR-181a-5p for its role in different systems of cancer.
The dysregulation of miR-181a-5p has been implicated in various
types of cancer and functions as an oncomiR or tumor inhibitor.
Mechanistically, miR-181a-5p targets multiple mRNAs to regulate
intricate and diverse signaling pathways. Additionally, numerous
factors, such as ncRNAs and TFs, serve as upstream regulators that
modulate the expression of miR-181a-5p. MiR-181a-5p is capable of
mediating cellular processes, such as proliferation, apoptosis,
autophagy, angiogenesis and the regulation of tumor growth in
xenograft models. It can also either promote or suppress the cell
migration, invasion, EMT and tumor metastasis. In addition,
miR-181a-5p shows promise as a potential biomarker and target to
increase the sensitivity for chemotherapy. These findings may
provide implications for oncological research and treatment
strategies of cancer.
Not applicable.
JL wrote the major parts of the manuscript and
prepared the figures and tables. JS and YZ revised the manuscript.
FD, ML, XW and YC prepared the manuscript. SW and ZX oversaw the
process and wrote the manuscript. ZW conceptualized the study and
oversaw the process. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
Authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Project of Science and Technology
Department of Sichuan Province (grant no. 2021YJ0445).
|
1
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cable J, Heard E, Hirose T, Prasanth KV,
Chen LL, Henninger JE, Quinodoz SA, Spector DL, Diermeier SD,
Porman AM, et al: Noncoding RNAs: Biology and applications-a
keystone symposia report. Ann N Y Acad Sci. 1506:118–141. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bejerano G, Pheasant M, Makunin I, Stephen
S, Kent WJ, Mattick JS and Haussler D: Ultraconserved elements in
the human genome. Science. 304:1321–1325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rivera-Barahona A, Pérez B, Richard E and
Desviat LR: Role of miRNAs in human disease and inborn errors of
metabolism. J Inherit Metab Dis. 40:471–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeffries J, Zhou W, Hsu AY and Deng Q:
miRNA-223 at the crossroads of inflammation and cancer. Cancer
Lett. 451:136–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Liao Y and Tang L: MicroRNA-34
family: A potential tumor suppressor and therapeutic candidate in
cancer. J Exp Clin Cancer Res. 38:532019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rani V and Sengar RS: Biogenesis and
mechanisms of microRNA-mediated gene regulation. Biotechnol Bioeng.
119:685–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su Y, Yuan J, Zhang F, Lei Q, Zhang T, Li
K, Guo J, Hong Y, Bu G, Lv X, et al: MicroRNA-181a-5p and
microRNA-181a-3p cooperatively restrict vascular inflammation and
atherosclerosis. Cell Death Dis. 10:3652019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Guo Y, Sun Y, Zhang N and Wang X:
miR-181a/b-5p ameliorates inflammatory response in
monocrotaline-induced pulmonary arterial hypertension by targeting
endocan. J Cell Physiol. 235:4422–4433. 2020. View Article : Google Scholar
|
|
17
|
Lozano-Bartolomé J, Llauradó G,
Portero-Otin M, Altuna-Coy A, Rojo-Martínez G, Vendrell J, Jorba R,
Rodríguez-Gallego E and Chacón MR: Altered expression of
miR-181a-5p and miR-23a-3p Is associated with obesity and
TNFα-induced insulin resistance. J Clin Endocrinol Metab.
103:1447–1458. 2018. View Article : Google Scholar
|
|
18
|
Korhan P, Erdal E and Atabey N:
MiR-181a-5p is downregulated in hepatocellular carcinoma and
suppresses motility, invasion and branching-morphogenesis by
directly targeting c-Met. Biochem Biophys Res Commun.
450:1304–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu
W, Guo X and Yu J: Cancer exosome-derived miR-9 and miR-181a
promote the development of early-stage MDSCs via interfering with
SOCS3 and PIAS3 respectively in breast cancer. Oncogene.
39:4681–4694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Wan X, Gu Z, Zhang H, Yang X, He
L, Miao R, Zhong Y and Zhao H: Evolution of the mir-181 microRNA
family. Comput Biol Med. 52:82–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smillie CL, Sirey T and Ponting CP:
Complexities of post-transcriptional regulation and the modeling of
ceRNA crosstalk. Crit Rev Biochem Mol Biol. 53:231–245. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen H, Wang L, Xiong J, Ren C, Gao C,
Ding W, Zhu D, Ma D and Wang H: Long non-coding RNA CCAT1 promotes
cervical cancer cell proliferation and invasion by regulating the
miR-181a-5p/MMP14 axis. Cell Cycle. 18:1110–1121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shang A, Wang W, Gu C, Chen W, Lu W, Sun Z
and Li D: Long non-coding RNA CCAT1 promotes colorectal cancer
progression by regulating miR-181a-5p expression. Aging (Albany
NY). 12:8301–8320. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Yang J, Xia Y, Fan Q and Yang KP:
Long noncoding RNA NEAT1 promotes proliferation and invasion via
targeting miR-181a-5p in non-small cell lung cancer. Oncol Res.
26:289–296. 2018. View Article : Google Scholar
|
|
26
|
Hai Ping P, Feng Bo T, Li L, Nan Hui Y and
Hong Z: IL-1β/NF-kb signaling promotes colorectal cancer cell
growth through miR-181a/PTEN axis. Arch Biochem Biophys. 604:20–26.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Li X, Tan F, Yu N and Pei H:
STAT1 inhibits MiR-181a expression to suppress colorectal cancer
cell proliferation through PTEN/Akt. J Cell Biochem. 118:3435–3443.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi L, Li X, Wu Z, Li X, Nie J, Guo M, Mei
Q and Han W: DNA methylation-mediated repression of
miR-181a/135a/302c expression promotes the microsatellite-unstable
colorectal cancer development and 5-FU resistance via targeting
PLAG1. J Genet Genomics. 45:205–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Cen A, Yang Y, Ye H, Li J, Liu S
and Zhao L: miR-181a, delivered by hypoxic PTC-secreted exosomes,
inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated
angiogenesis. Mol Ther Nucleic Acids. 24:610–621. 2021. View Article : Google Scholar :
|
|
30
|
Sun X, Wei L, Chen Q and Terek RM:
MicroRNA regulates vascular endothelial growth factor expression in
chondrosarcoma cells. Clin Orthop Relat Res. 473:907–913. 2015.
View Article : Google Scholar :
|
|
31
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of MicroRNAs in cancer.
Cancer Res. 76:3666–3670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar
|
|
34
|
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu
XY, Yu ZW, Jia YH, Bai XF, Li L, et al: The lncRNA CRNDE promotes
colorectal cancer cell proliferation and chemoresistance via
miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol
Cancer. 16:92017. View Article : Google Scholar
|
|
35
|
Lv SY, Shan TD, Pan XT, Tian ZB, Liu XS,
Liu FG, Sun XG, Xue HG, Li XH, Han Y, et al: The lncRNA ZEB1-AS1
sponges miR-181a-5p to promote colorectal cancer cell proliferation
by regulating Wnt/β-catenin signaling. Cell Cycle. 17:1245–1254.
2018. View Article : Google Scholar :
|
|
36
|
Sun C, Shen C, Zhang Y and Hu C: LncRNA
ANRIL negatively regulated chitooligosaccharide-induced
radiosensitivity in colon cancer cells by sponging miR-181a-5p. Adv
Clin Exp Med. 30:55–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang L, Yuan Y, Li C, Guo T, Qi H, Xiao
Y, Dong X, Liu Z and Liu Q: Upregulation of SNHG6 regulates ZEB1
expression by competitively binding miR-101-3p and interacting with
UPF1 in hepatocellular carcinoma. Cancer Lett. 383:183–194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu C, Sun J, Leng X and Yang J: Long
noncoding RNA SNHG6 functions as a competing endogenous RNA by
sponging miR-181a-5p to regulate E2F5 expression in colorectal
cancer. Cancer Manag Res. 11:611–624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chi J, Liu S, Wu Z, Shi Y, Shi C, Zhang T,
Xiong B, Zeng Y and Dong X: circNSUN2 promotes the malignant
biological behavior of colorectal cancer cells via the
miR-181a-5p/ROCK2 axis. Oncol Rep. 46:1422021. View Article : Google Scholar :
|
|
40
|
Zhao S, Mi Y, Zheng B, Wei P, Gu Y, Zhang
Z, Xu Y, Cai S, Li X and Li D: Highly-metastatic colorectal cancer
cell released miR-181a-5p-rich extracellular vesicles promote liver
metastasis by activating hepatic stellate cells and remodelling the
tumour microenvironment. J Extracell Vesicles. 11:e121862022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji D, Chen Z, Li M, Zhan T, Yao Y, Zhang
Z, Xi J, Yan L and Gu J: MicroRNA-181a promotes tumor growth and
liver metastasis in colorectal cancer by targeting the tumor
suppressor WIF-1. Mol Cancer. 13:862014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Z, Wang H, Xu Z, Sun Y and Han J:
Expression and mechanism of microRNA-181A on incidence and survival
in late liver metastases of colorectal cancer. Oncol Rep.
35:1403–1408. 2016. View Article : Google Scholar
|
|
43
|
Wei Z, Cui L, Mei Z, Liu M and Zhang D:
miR-181a mediates metabolic shift in colon cancer cells via the
PTEN/AKT pathway. FEBS Lett. 588:1773–1779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun W, Wang X, Li J, You C, Lu P, Feng H,
Kong Y, Zhang H, Liu Y, Jiao R, et al: MicroRNA-181a promotes
angiogenesis in colorectal cancer by targeting SRCIN1 to promote
the SRC/VEGF signaling pathway. Cell Death Dis. 9:4382018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Q, Wang C, Li R, Liu J, Wang J, Wang
T and Wang B: The BAP31/miR-181a-5p/RECK axis promotes angiogenesis
in colorectal cancer via fibroblast activation. Front Oncol.
13:10569032023. View Article : Google Scholar :
|
|
46
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen G, Shen ZL, Wang L, Lv CY, Huang XE
and Zhou RP: Hsa-miR-181a-5p expression and effects on cell
proliferation in gastric cancer. Asian Pac J Cancer Prev.
14:3871–3875. 2013. View Article : Google Scholar
|
|
48
|
Ge S, Zhang H, Deng T, Sun W, Ning T, Fan
Q, Wang Y, Wang X, Zhang Q, Zhou Z, et al: MiR-181a, a new
regulator of TGF-β signaling, can promote cell migration and
proliferation in gastric cancer. Invest New Drugs. 37:923–934.
2019. View Article : Google Scholar
|
|
49
|
Yu J, Qi J, Sun X, Wang W, Wei G, Wu Y,
Gao Q and Zheng J: MicroRNA-181a promotes cell proliferation and
inhibits apoptosis in gastric cancer by targeting RASSF1A. Oncol
Rep. 40:1959–1970. 2018.
|
|
50
|
Ding L, Tian Y, Wang L, Bi M, Teng D and
Hong S: Hypermethylated long noncoding RNA MEG3 promotes the
progression of gastric cancer. Aging (Albany NY). 11:8139–8155.
2019. View Article : Google Scholar
|
|
51
|
Liu Z, Sun F, Hong Y, Liu Y, Fen M, Yin K,
Ge X, Wang F, Chen X and Guan W: MEG2 is regulated by miR-181a-5p
and functions as a tumour suppressor gene to suppress the
proliferation and migration of gastric cancer cells. Mol Cancer.
16:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mi Y, Zhang D, Jiang W, Weng J, Zhou C,
Huang K, Tang H, Yu Y, Liu X, Cui W, et al: miR-181a-5p promotes
the progression of gastric cancer via RASSF6-mediated MAPK
signalling activation. Cancer Lett. 389:11–22. 2017. View Article : Google Scholar
|
|
53
|
Lu Q, Chen Y, Sun D, Wang S, Ding K, Liu
M, Zhang Y, Miao Y, Liu H and Zhou F: MicroRNA-181a functions as an
oncogene in gastric cancer by targeting caprin-1. Front Pharmacol.
9:15652019. View Article : Google Scholar :
|
|
54
|
Zhao J, Nie Y, Wang H and Lin Y: MiR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar
|
|
55
|
Lin F, Li Y, Yan S, Liu S, Qian W, Shen D,
Lin Q and Mao W: MicroRNA-181a inhibits tumor proliferation,
invasiveness, and metastasis and is downregulated in gastric
cancer. Oncol Res. 22:75–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu Z, Luo T, Pang T, Du Z, Yin X, Cui H,
Fang G and Xue X: MALAT1 promotes gastric adenocarcinoma through
the MALAT1/miR-181a-5p/AKT3 axis. Open Biol. 9:1900952019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhuang X, Chen Y, Wu Z, Xu Q, Chen M, Shao
M, Cao X, Zhou Y, Xie M, Shi Y, et al: Mitochondrial miR-181a-5p
promotes glucose metabolism reprogramming in liver cancer by
regulating the electron transport chain. Carcinogenesis.
41:972–983. 2020. View Article : Google Scholar
|
|
58
|
Bi JG, Zheng JF, Li Q, Bao SY, Yu XF, Xu P
and Liao CX: MicroRNA-181a-5p suppresses cell proliferation by
targeting Egr1 and inhibiting Egr1/TGF-β/Smad pathway in
hepatocellular carcinoma. Int J Biochem Cell Biol. 106:107–116.
2019. View Article : Google Scholar
|
|
59
|
Guo J, Ma Y, Peng X, Jin H and Liu J:
LncRNA CCAT1 promotes autophagy via regulating ATG7 by sponging
miR-181 in hepatocellular carcinoma. J Cell Biochem.
120:17975–17983. 2019. View Article : Google Scholar
|
|
60
|
Chang S, Chen B, Wang X, Wu K and Sun Y:
Long non-coding RNA XIST regulates PTEN expression by sponging
miR-181a and promotes hepatocellular carcinoma progression. BMC
Cancer. 17:2482017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang S, Wang P, Wang S, Cong A, Zhang Q,
Shen W, Li X, Zhang W and Han G: miRNA-181a-5p enhances the
sensitivity of cells to cisplatin in esophageal adenocarcinoma by
targeting CBLB. Cancer Manag Res. 12:4981–4990. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu J, Xu D, Wang Q, Zheng D, Jiang X and
Xu L: LPS induced miR-181a promotes pancreatic cancer cell
migration via targeting PTEN and MAP2K4. Dig Dis Sci. 59:1452–1460.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang L, Bi R, Li L, Zhou K and Yin H:
lncRNA ANRIL aggravates the chemoresistance of pancreatic cancer
cells to gemcitabine by targeting inhibition of miR-181a and
targeting HMGB1-induced autophagy. Aging (Albany NY).
13:19272–19281. 2021. View Article : Google Scholar
|
|
65
|
Harðardottir H, Jonsson S, Gunnarsson O,
Hilmarsdottir B, Asmundsson J, Gudmundsdottir I, Saevarsdottir VY,
Hansdottir S, Hannesson P and Gudbjartsson T: Advances in lung
cancer diagnosis and treatment-a review. Laeknabladid. 108:17–29.
2022.In Icelandic.
|
|
66
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma Z, Qiu X, Wang D, Li Y, Zhang B, Yuan
T, Wei J, Zhao B, Zhao X, Lou J, et al: MiR-181a-5p inhibits cell
proliferation and migration by targeting Kras in non-small cell
lung cancer A549 cells. Acta Biochim Biophys Sin (Shanghai).
47:630–638. 2015. View Article : Google Scholar
|
|
68
|
Shi Q, Zhou Z, Ye N, Chen Q, Zheng X and
Fang M: MiR-181a inhibits non-small cell lung cancer cell
proliferation by targeting CDK1. Cancer Biomark. 20:539–546. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang L, Zhang L and Wang L: SNHG7
contributes to the progression of non-small-cell lung cancer via
the SNHG7/miR-181a-5p/E2F7 axis. Cancer Manag Res. 12:3211–3222.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cao Y, Zhao D, Li P, Wang L, Qiao B, Qin
X, Li L and Wang Y: MicroRNA-181a-5p impedes IL-17-induced nonsmall
cell lung cancer proliferation and migration through targeting
VCAM-1. Cell Physiol Biochem. 42:346–356. 2017. View Article : Google Scholar
|
|
71
|
Li L, Ye D, Liu L, Li X, Liu J, Su S, Lu W
and Yu Z: Long noncoding RNA SNHG7 accelerates proliferation,
migration and invasion of non-small cell lung cancer cells by
suppressing miR-181a-5p through AKT/mTOR signaling pathway. Cancer
Manag Res. 12:8303–8312. 2020. View Article : Google Scholar :
|
|
72
|
Zhang LX, Gao J, Long X, Zhang PF, Yang X,
Zhu SQ, Pei X, Qiu BQ, Chen SW, Lu F, et al: The circular RNA
circHMGB2 drives immunosuppression and anti-PD-1 resistance in lung
adenocarcinomas and squamous cell carcinomas via the
miR-181a-5p/CARM1 axis. Mol Cancer. 21:1102022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fu S, Fu Y, Chen F, Hu Y, Quan B and Zhang
J: Overexpression of MYCT1 inhibits proliferation and induces
apoptosis in human acute myeloid leukemia HL-60 and KG-1a cells in
vitro and in vivo. Front Pharmacol. 9:10452018. View Article : Google Scholar :
|
|
74
|
Wang HT, Tong X, Zhang ZX, Sun YY, Yan W,
Xu ZM and Fu WN: MYCT1 represses apoptosis of laryngeal cancerous
cells through the MAX/miR-181a/NPM1 pathway. FEBS J. 286:3892–3908.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hao YR, Zhang DJ, Fu ZM, Guo YY and Guan
GF: Long non-coding RNA ANRIL promotes proliferation,
clonogenicity, invasion and migration of laryngeal squamous cell
carcinoma by regulating miR-181a/Snai2 axis. Regen Ther.
11:282–289. 2019. View Article : Google Scholar :
|
|
76
|
Wilkinson L and Gathani T: Understanding
breast cancer as a global health concern. Br J Radiol.
95:202110332022. View Article : Google Scholar :
|
|
77
|
Strotbek M, Schmid S, Sánchez-González I,
Boerries M, Busch H and Olayioye MA: miR-181 elevates Akt signaling
by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast
cancer. Int J Cancer. 140:2310–2320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Taylor MA, Sossey-Alaoui K, Thompson CL,
Danielpour D and Schiemann WP: TGF-β upregulates miR-181a
expression to promote breast cancer metastasis. J Clin Invest.
123:150–163. 2013. View Article : Google Scholar
|
|
79
|
Liu K, Xie F, Gao A, Zhang R, Zhang L,
Xiao Z, Hu Q, Huang W, Huang Q, Lin B, et al: SOX2 regulates
multiple malignant processes of breast cancer development through
the SOX2/miR-181a-5p, miR-30e-5p/TUSC3 axis. Mol Cancer. 16:622017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhai Z, Mu T, Zhao L, Li Y, Zhu D and Pan
Y: MiR-181a-5p facilitates proliferation, invasion, and glycolysis
of breast cancer through NDRG2-mediated activation of PTEN/AKT
pathway. Bioengineered. 13:83–95. 2022. View Article : Google Scholar :
|
|
81
|
Alexandrova E, Lamberti J, Saggese P,
Pecoraro G, Memoli D, Cappa VM, Ravo M, Iorio R, Tarallo R, Rizzo
F, et al: Small non-coding RNA profiling identifies miR-181a-5p as
a mediator of estrogen receptor beta-induced inhibition of
cholesterol biosynthesis in triple-negative breast cancer. Cells.
9:8742020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gu M, Wang L, Yang C, Li X, Jia C, Croteau
S, Ruan X and Hardy P: Micro-RNA-181a suppresses progestin-promoted
breast cancer cell growth. Maturitas. 114:60–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cai G, Wang Y, Houda T, Yang C, Wang L, Gu
M, Mueck A, Croteau S, Ruan X and Hardy P: MicroRNA-181a suppresses
norethisterone-promoted tumorigenesis of breast epithelial MCF10A
cells through the PGRMC1/EGFR-PI3K/Akt/mTOR signaling pathway.
Transl Oncol. 14:1010682021. View Article : Google Scholar
|
|
84
|
Liu Y, Cheng T, Du Y, Hu X and Xia W:
LncRNA LUCAT1/miR-181a-5p axis promotes proliferation and invasion
of breast cancer via targeting KLF6 and KLF15. BMC Mol Cell Biol.
21:692020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tsu V and Jerónimo J: Saving the world's
women from cervical cancer. N Engl J Med. 374:2509–2511. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang M, Zhai X, Ge T, Yang C and Lou G:
miR-181a-5p promotes proliferation and invasion and inhibits
apoptosis of cervical cancer cells via regulating inositol
polyphosphate-5-phosphatase A (INPP5A). Oncol Res. 26:703–712.
2018. View Article : Google Scholar
|
|
87
|
Xu H, Zhu J, Hu C, Song H and Li Y:
Inhibition of microRNA-181a may suppress proliferation and invasion
and promote apoptosis of cervical cancer cells through the
PTEN/Akt/FOXO1 pathway. J Physiol Biochem. 72:721–732. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ke G, Liang L, Yang JM, Huang X, Han D,
Huang S, Zhao Y, Zha R, He X and Wu X: MiR-181a confers resistance
of cervical cancer to radiation therapy through targeting the
pro-apoptotic PRKCD gene. Oncogene. 32:3019–3027. 2013. View Article : Google Scholar
|
|
89
|
Luo C and Qiu J: miR-181a inhibits
cervical cancer development via downregulating GRP78. Oncol Res.
25:1341–1348. 2017. View Article : Google Scholar
|
|
90
|
Zhu L, Zhang Q, Li S, Jiang S, Cui J and
Dang G: Interference of the long noncoding RNA CDKN2B-AS1
upregulates miR-181a-5p/TGFβI axis to restrain the metastasis and
promote apoptosis and senescence of cervical cancer cells. Cancer
Med. 8:1721–1730. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang L, Liu SK, Song L and Yao HR:
SP1-induced up-regulation of lncRNA LUCAT1 promotes proliferation,
migration and invasion of cervical cancer by sponging miR-181a.
Artif Cells Nanomed Biotechnol. 47:555–564. 2019. View Article : Google Scholar
|
|
92
|
Hu Z, Zhu D, Wang W, Li W, Jia W, Zeng X,
Ding W, Yu L, Wang X, Wang L, et al: Genome-wide profiling of HPV
integration in cervical cancer identifies clustered genomic hot
spots and a potential microhomology-mediated integration mechanism.
Nat Genet. 47:158–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Felix AS, Scott McMeekin D, Mutch D,
Walker JL, Creasman WT, Cohn DE, Ali S, Moore RG, Downs LS, Ioffe
OB, et al: Associations between etiologic factors and mortality
after endometrial cancer diagnosis: The NRG oncology/gynecologic
oncology group 210 trial. Gynecol Oncol. 139:70–76. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Geletina NS, Kobelev VS, Babayants EV,
Feng L, Pustylnyak VO and Gulyaeva LF: PTEN negative correlates
with miR-181a in tumour tissues of non-obese endometrial cancer
patients. Gene. 655:20–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yu J, Jiang L, Gao Y, Sun Q, Liu B, Hu Y
and Han X: LncRNA CCAT1 negatively regulates miR-181a-5p to promote
endometrial carcinoma cell proliferation and migration. Exp Ther
Med. 17:4259–4266. 2019.PubMed/NCBI
|
|
96
|
Dong P, Xiong Y, Konno Y, Ihira K,
Kobayashi N, Yue J and Watari H: Long non-coding RNA DLEU2 drives
EMT and glycolysis in endometrial cancer through HK2 by
competitively binding with miR-455 and by modulating the
EZH2/miR-181a pathway. J Exp Clin Cancer Res. 40:2162021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Parikh A, Lee C, Joseph P, Marchini S,
Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, et al:
microRNA-181a has a critical role in ovarian cancer progression
through the regulation of the epithelial-mesenchymal transition.
Nat Commun. 5:29772014. View Article : Google Scholar
|
|
98
|
Belur Nagaraj A, Knarr M, Sekhar S, Connor
RS, Joseph P, Kovalenko O, Fleming A, Surti A, Nurmemmedov E,
Beltrame L, et al: The miR-181a-SFRP4 axis regulates Wnt activation
to drive stemness and platinum resistance in ovarian cancer. Cancer
Res. 81:2044–2055. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhou CK, Check DP, Lortet-Tieulent J,
Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB and Devesa SS:
Prostate cancer incidence in 43 populations worldwide: An analysis
of time trends overall and by age group. Int J Cancer.
138:1388–1400. 2016. View Article : Google Scholar :
|
|
100
|
Ding X, Xu X, He XF, Yuan Y, Chen C, Shen
XY, Su S, Chen Z, Xu ST and Huang YH: Muscleblind-like 1 antisense
RNA 1 inhibits cell proliferation, invasion, and migration of
prostate cancer by sponging miR-181a-5p and regulating
PTEN/PI3K/AKT/mTOR signaling. Bioengineered. 12:803–814. 2021.
View Article : Google Scholar
|
|
101
|
Liang J, Li X, Li Y, Wei J, Daniels G,
Zhong X, Wang J, Sfanos K, Melamed J, Zhao J and Lee P: LEF1
targeting EMT in prostate cancer invasion is mediated by miR-181a.
Am J Cancer Res. 5:1124–1132. 2015.
|
|
102
|
Hu W, Yan F, Ru Y, Xia M, Yan G, Zhang M,
Wang H, Wu G, Yao L, Shen L, et al: MIIP inhibits EMT and cell
invasion in prostate cancer through miR-181a/b-5p-KLF17 axis. Am J
Cancer Res. 10:630–647. 2020.PubMed/NCBI
|
|
103
|
Mao W, Huang X, Wang L, Zhang Z, Liu M, Li
Y, Luo M, Yao X, Fan J and Geng J: Circular RNA hsa_circ_0068871
regulates FGFR3 expression and activates STAT3 by targeting
miR-181a-5p to promote bladder cancer progression. J Exp Clin
Cancer Res. 38:1692019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lai Y, Zhao L, Hu J, Quan J, Chen P, Xu J,
Guan X, Lai Y and Ni L: microRNA-181a-5p functions as an oncogene
in renal cell carcinoma. Mol Med Rep. 17:8510–8517. 2018.PubMed/NCBI
|
|
105
|
Lei Z, Ma X, Li H, Zhang Y, Gao Y, Fan Y,
Li X, Chen L, Xie Y, Chen J, et al: Up-regulation of miR-181a in
clear cell renal cell carcinoma is associated with lower KLF6
expression, enhanced cell proliferation, accelerated cell cycle
transition, and diminished apoptosis. Urol Oncol. 36:93.e23–93.e37.
2018. View Article : Google Scholar
|
|
106
|
Coca-Pelaz A, Shah JP, Hernandez-Prera JC,
Ghossein RA, Rodrigo JP, Hartl DM, Olsen KD, Shaha AR, Zafereo M,
Suarez C, et al: Papillary thyroid cancer-aggressive variants and
impact on management: A narrative review. Adv Ther. 37:3112–3128.
2020. View Article : Google Scholar :
|
|
107
|
Wang Y, Ye H, Yang Y, Li J, Cen A and Zhao
L: microRNA-181a promotes the oncogene S100A2 and enhances
papillary thyroid carcinoma growth by mediating the expression of
histone demethylase KDM5C. J Endocrinol Invest. 45:17–28. 2022.
View Article : Google Scholar
|
|
108
|
Sun CX, Liu BJ, Su Y, Shi GW, Wang Y and
Chi JF: MiR-181a promotes cell proliferation and migration through
targeting KLF15 in papillary thyroid cancer. Clin Transl Oncol.
24:66–75. 2022. View Article : Google Scholar
|
|
109
|
Le F, Li HM, Lv QL, Chen JJ, Lin QX, Ji YL
and Yi B: lncRNA ZNF674-AS1 inhibits the migration, invasion and
epithelial-mesenchymal transition of thyroid cancer cells by
modulating the miR-181a/SOCS4 axis. Mol Cell Endocrinol.
544:1115512022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gierlikowski W, Broniarek K, Cheda Ł,
Rogulski Z and Kotlarek-Łysakowska M: MiR-181a-5p regulates NIS
expression in papillary thyroid carcinoma. Int J Mol Sci.
22:60672021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ju R, Huang Y, Guo Z, Han L, Ji S, Zhao L
and Long J: The circular RNAs differential expression profiles in
the metastasis of salivary adenoid cystic carcinoma cells. Mol Cell
Biochem. 476:1269–1282. 2021. View Article : Google Scholar
|
|
112
|
Nabhan M, Louka ML, Khairy E, Tash F,
Ali-Labib R and El-Habashy S: MicroRNA-181a and its target Smad 7
as potential biomarkers for tracking child acute lymphoblastic
leukemia. Gene. 628:253–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Liu X, Liao W, Peng H, Luo X, Luo Z, Jiang
H and Xu L: miR-181a promotes G1/S transition and cell
proliferation in pediatric acute myeloid leukemia by targeting ATM.
J Cancer Res Clin Oncol. 142:77–87. 2016. View Article : Google Scholar
|
|
114
|
Lyu X, Li J, Yun X, Huang R, Deng X, Wang
Y, Chen Y and Xiao G: miR-181a-5p, an inducer of Wnt-signaling,
facilitates cell proliferation in acute lymphoblastic leukemia.
Oncol Rep. 37:1469–1476. 2017. View Article : Google Scholar
|
|
115
|
Assmann JLJC, Leon LG, Stavast CJ, van den
Bogaerdt SE, Schilperoord-Vermeulen J, Sandberg Y, Bellido M,
Erkeland SJ, Feith DJ, Loughran TP Jr and Langerak AW: miR-181a is
a novel player in the STAT3-mediated survival network of TCRαβ+
CD8+ T large granular lymphocyte leukemia. Leukemia. 36:983–993.
2022. View Article : Google Scholar
|
|
116
|
Fei J, Li Y, Zhu X and Luo X: miR-181a
post-transcriptionally downregulates oncogenic RalA and contributes
to growth inhibition and apoptosis in chronic myelogenous leukemia
(CML). PLoS One. 7:e328342012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sun Y, Jiang T, Jia Y, Zou J, Wang X and
Gu W: LncRNA MALAT1/miR-181a-5p affects the proliferation and
adhesion of myeloma cells via regulation of Hippo-YAP signaling
pathway. Cell Cycle. 18:2509–2523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen L, Hu N, Wang C, Zhao H and Gu Y:
Long non-coding RNA CCAT1 promotes multiple myeloma progression by
acting as a molecular sponge of miR-181a-5p to modulate HOXA1
expression. Cell Cycle. 17:319–329. 2018. View Article : Google Scholar :
|
|
119
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar
|
|
120
|
Kozloski GA, Jiang X, Bhatt S, Ruiz J,
Vega F, Shaknovich R, Melnick A and Lossos IS: miR-181a negatively
regulates NF-κB signaling and affects activated B-cell-like diffuse
large B-cell lymphoma pathogenesis. Blood. 127:2856–2866. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bao Z, Wang Y, Wang Q, Fang S, Shan X,
Wang J and Jiang T: Intratumor heterogeneity, microenvironment, and
mechanisms of drug resistance in glioma recurrence and evolution.
Front Med. 15:551–561. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lei B, Huang Y, Zhou Z, Zhao Y, Thapa AJ,
Li W, Cai W and Deng Y: Circular RNA hsa_circ_0076248 promotes
oncogenesis of glioma by sponging miR-181a to modulate SIRT1
expression. J Cell Biochem. 120:6698–6708. 2019. View Article : Google Scholar
|
|
123
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee SU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18:3–9. 2017.PubMed/NCBI
|
|
124
|
Rezaei T, Hejazi M, Mansoori B, Mohammadi
A, Amini M, Mosafer J, Rezaei S, Mokhtarzadeh A and Baradaran B:
microRNA-181a mediates the chemo-sensitivity of glioblastoma to
carmustine and regulates cell proliferation, migration, and
apoptosis. Eur J Pharmacol. 888:1734832020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Huang SX, Zhao ZY, Weng GH, He XY, Wu CJ,
Fu CY, Sui ZY, Ma YS and Liu T: Upregulation of miR-181a suppresses
the formation of glioblastoma stem cells by targeting the Notch2
oncogene and correlates with good prognosis in patients with
glioblastoma multiforme. Biochem Biophys Res Commun. 486:1129–1136.
2017. View Article : Google Scholar
|
|
126
|
Marisetty A, Wei J, Kong LY, Ott M, Fang
D, Sabbagh A and Heimberger AB: MiR-181 family modulates
osteopontin in glioblastoma multiforme. Cancers (Basel).
12:38132020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ma J, Yao Y, Wang P, Liu Y, Zhao L, Li Z,
Li Z and Xue Y: MiR-181a regulates blood-tumor barrier permeability
by targeting Krüppel-like factor 6. J Cereb Blood Flow Metab.
34:1826–1836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: LncRNA CASC2 interacts with miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar
|
|
129
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Primers. 2:160782016. View Article : Google Scholar
|
|
130
|
Liu X, Peng H, Liao W, Luo A, Cai M, He J,
Zhang X, Luo Z, Jiang H and Xu L: MiR-181a/b induce the growth,
invasion, and metastasis of neuroblastoma cells through targeting
ABI1. Mol Carcinog. 57:1237–1250. 2018. View Article : Google Scholar
|
|
131
|
Orr BA: Pathology, diagnostics, and
classification of medulloblastoma. Brain Pathol. 30:664–678. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhu LY, Wu XY, Liu XD, Zheng DF, Li HS,
Yang B, Zhang J and Chang Q: Aggressive medulloblastoma-derived
exosomal miRNAs promote in vitro invasion and migration of tumor
cells via Ras/MAPK pathway. J Neuropathol Exp Neurol. 79:734–745.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang Z, Wang X, Zhou H, Dan X, Jiang L and
Wu Y: Long non-coding RNA CASC2 inhibits tumorigenesis via the
miR-181a/PLXNC1 axis in melanoma. Acta Biochim Biophys Sin
(Shanghai). 50:263–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Zhang S, Wan H and Zhang X: LncRNA
LHFPL3-AS1 contributes to tumorigenesis of melanoma stem cells via
the miR-181a-5p/BCL2 pathway. Cell Death Dis. 11:9502020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Waldman A and Schmults C: Cutaneous
squamous cell carcinoma. Hematol Oncol Clin North Am. 33:1–12.
2019. View Article : Google Scholar
|
|
136
|
Chitsazzadeh V, Nguyen TN, de Mingo Pulido
A, Bittencourt BB, Du L, Adelmann CH, Ortiz Rivera I, Nguyen KA,
Guerra LD, Davis A, et al: miR-181a promotes multiple
protumorigenic functions by targeting TGFβR3. J Invest Dermatol.
142:1956–1965.e2. 2022. View Article : Google Scholar
|
|
137
|
Neu J, Dziunycz PJ, Dzung A, Lefort K,
Falke M, Denzler R, Freiberger SN, Iotzova-Weiss G, Kuzmanov A,
Levesque MP, et al: miR-181a decelerates proliferation in cutaneous
squamous cell carcinoma by targeting the proto-oncogene KRAS. PLoS
One. 12:e01850282017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ba Z, Gu L, Hao S, Wang X, Cheng Z and Nie
G: Downregulation of lncRNA CASC2 facilitates osteosarcoma growth
and invasion through miR-181a. Cell Prolif. 51:e124092018.
View Article : Google Scholar
|
|
140
|
Zhao A, Liu W, Cui X, Wang N, Wang Y, Sun
L, Xue H, Wu L, Cui S, Yang Y and Bai R: lncRNA TUSC7 inhibits
osteosarcoma progression through the miR-181a/RASSF6 axis. Int J
Mol Med. 47:583–594. 2021. View Article : Google Scholar :
|
|
141
|
Mutlu S, Mutlu H, Kirkbes S, Eroglu S,
Kabukcuoglu YS, Kabukcuoglu F, Duymus TM, ISık M and Ulasli M: The
expression of miR-181a-5p and miR-371b-5p in chondrosarcoma. Eur
Rev Med Pharmacol Sci. 19:2384–2388. 2015.PubMed/NCBI
|
|
142
|
Sun X, Charbonneau C, Wei L, Chen Q and
Terek RM: miR-181a targets RGS16 to promote chondrosarcoma growth,
angiogenesis, and metastasis. Mol Cancer Res. 13:1347–1357. 2015.
View Article : Google Scholar :
|
|
143
|
Ho PTB, Clark IM and Le LTT:
MicroRNA-based diagnosis and therapy. Int J Mol Sci. 23:71672022.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Guo LJ and Zhang QY: Decreased serum
miR-181a is a potential new tool for breast cancer screening. Int J
Mol Med. 30:680–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang H, Zhu M, Shan X, Zhou X, Wang T,
Zhang J, Tao J, Cheng W, Chen G, Li J, et al: A panel of
seven-miRNA signature in plasma as potential biomarker for
colorectal cancer diagnosis. Gene. 687:246–254. 2019. View Article : Google Scholar
|
|
146
|
Lin Z, Chen Y, Lin Y, Lin H, Li H, Su X,
Fang Z, Wang J, Wei Q, Teng J and Zhang Z: Potential miRNA
biomarkers for the diagnosis and prognosis of esophageal cancer
detected by a novel absolute quantitative RT-qPCR method. Sci Rep.
10:200652020. View Article : Google Scholar :
|
|
147
|
He S, Zeng S, Zhou ZW, He ZX and Zhou SF:
Hsa-microRNA-181a is a regulator of a number of cancer genes and a
biomarker for endometrial carcinoma in patients: A bioinformatic
and clinical study and the therapeutic implication. Drug Des Devel
Ther. 9:1103–1175. 2015.PubMed/NCBI
|
|
148
|
Shan X, Zhang H, Zhang L, Zhou X, Wang T,
Zhang J, Shu Y, Zhu W, Wen W and Liu P: Identification of four
plasma microRNAs as potential biomarkers in the diagnosis of male
lung squamous cell carcinoma patients in China. Cancer Med.
7:2370–2381. 2018. View Article : Google Scholar :
|
|
149
|
Nishimura J, Handa R, Yamamoto H, Tanaka
F, Shibata K, Mimori K, Takemasa I, Mizushima T, Ikeda M, Sekimoto
M, et al: microRNA-181a is associated with poor prognosis of
colorectal cancer. Oncol Rep. 28:2221–2226. 2012. View Article : Google Scholar
|
|
150
|
Panoutsopoulou K, Avgeris M, Magkou P,
Mavridis K, Dreyer T, Dorn J, Obermayr E, Reinthaller A,
Michaelidou K, Mahner S, et al: miR-181a overexpression predicts
the poor treatment response and early-progression of serous ovarian
cancer patients. Int J Cancer. 147:3560–3573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Papadimitriou MA, Papanota AM, Adamopoulos
PG, Pilala KM, Liacos CI, Malandrakis P, Mavrianou-Koutsoukou N,
Patseas D, Eleutherakis-Papaiakovou E, Gavriatopoulou M, et al
miRNA-seq and clinical evaluation in multiple myeloma: miR-181a
overexpression predicts short-term disease progression and poor
post-treatment outcome. Br J Cancer. 126:79–90. 2022. View Article : Google Scholar
|
|
152
|
Meijer LL, Garajová I, Caparello C, Le
Large TYS, Frampton AE, Vasile E, Funel N, Kazemier G and
Giovannetti E: Plasma miR-181a-5p downregulation predicts response
and improved survival after FOLFIRINOX in pancreatic ductal
adenocarcinoma. Ann Surg. 271:1137–1147. 2020. View Article : Google Scholar
|
|
153
|
Egyed B, Kutszegi N, Sági JC, Gézsi A,
Rzepiel A, Visnovitz T, Lőrincz P, Müller J, Zombori M, Szalai C,
et al: MicroRNA-181a as novel liquid biopsy marker of central
nervous system involvement in pediatric acute lymphoblastic
leukemia. J Transl Med. 18:2502020. View Article : Google Scholar :
|
|
154
|
Xue WX, Zhang MY, Rui Li, Liu X, Yin YH
and Qu YQ: Serum miR-1228-3p and miR-181a-5p as noninvasive
biomarkers for non-small cell lung cancer diagnosis and prognosis.
Biomed Res Int. 2020:96018762020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Gao W, Yu Y, Cao H, Shen H, Li X, Pan S
and Shu Y: Deregulated expression of miR-21, miR-143 and miR-181a
in non small cell lung cancer is related to clinicopathologic
characteristics or patient prognosis. Biomed Pharmacother.
64:399–408. 2010. View Article : Google Scholar
|
|
156
|
Schwind S, Maharry K, Radmacher MD, Mrózek
K, Holland KB, Margeson D, Whitman SP, Hickey C, Becker H, Metzeler
KH, et al: Prognostic significance of expression of a single
microRNA, miR-181a, in cytogenetically normal acute myeloid
leukemia: A cancer and leukemia group B study. J Clin Oncol.
28:5257–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang Y, Fang YX, Dong B, Du X, Wang J,
Wang X, Gao WQ and Xue W: Discovery of extracellular vesicles
derived miR-181a-5p in patient's serum as an indicator for
bone-metastatic prostate cancer. Theranostics. 11:878–892. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Bjørnetrø T, Redalen KR, Meltzer S,
Thusyanthan NS, Samiappan R, Jegerschöld C, Handeland KR and Ree
AH: An experimental strategy unveiling exosomal microRNAs 486-5p,
181a-5p and 30d-5p from hypoxic tumour cells as circulating
indicators of high-risk rectal cancer. J Extracell Vesicles.
8:15672192019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Leonetti A, Capula M, Minari R, Mazzaschi
G, Gregori A, El Hassouni B, Papini F, Bordi P, Verzè M, Avan A, et
al: Dynamic evaluation of circulating miRNA profile in EGFR-mutated
NSCLC patients treated with EGFR-TKIs. Cells. 10:15202021.
View Article : Google Scholar :
|
|
160
|
Pichler M, Winter E, Ress AL, Bauernhofer
T, Gerger A, Kiesslich T, Lax S, Samonigg H and Hoefler G: miR-181a
is associated with poor clinical outcome in patients with
colorectal cancer treated with EGFR inhibitor. J Clin Pathol.
67:198–203. 2014. View Article : Google Scholar
|
|
161
|
Robak P, Dróżdż I, Jarych D, Mikulski D,
Węgłowska E, Siemieniuk-Ryś M, Misiewicz M, Stawiski K, Fendler W,
Szemraj J, et al: The value of serum MicroRNA expression signature
in predicting refractoriness to bortezomib-based therapy in
multiple myeloma patients. Cancers (Basel). 12:25692020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Nishida N, Arizumi T, Hagiwara S, Ida H,
Sakurai T and Kudo M: MicroRNAs for the prediction of early
response to sorafenib treatment in human hepatocellular carcinoma.
Liver Cancer. 6:113–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Seipel K, Messerli C, Wiedemann G, Bacher
U and Pabst T: MN1, FOXP1 and hsa-miR-181a-5p as prognostic markers
in acute myeloid leukemia patients treated with intensive induction
chemotherapy and autologous stem cell transplantation. Leuk Res.
89:1062962020. View Article : Google Scholar
|
|
164
|
Danza K, Silvestris N, Simone G, Signorile
M, Saragoni L, Brunetti O, Monti M, Mazzotta A, De Summa S, Mangia
A and Tommasi S: Role of miR-27a, miR-181a and miR-20b in gastric
cancer hypoxia-induced chemoresistance. Cancer Biol Ther.
17:400–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Petrillo M, Zannoni GF, Beltrame L,
Martinelli E, DiFeo A, Paracchini L, Craparotta I, Mannarino L,
Vizzielli G, Scambia G, et al: Identification of high-grade serous
ovarian cancer miRNA species associated with survival and drug
response in patients receiving neoadjuvant chemotherapy: A
retrospective longitudinal analysis using matched tumor biopsies.
Ann Oncol. 27:625–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Shi L, Zhu W, Huang Y, Zhuo L, Wang S,
Chen S, Zhang B and Ke B: Cancer-associated fibroblast-derived
exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to
promote the progression and chemoresistance of non-small cell lung
cancer. Clin Transl Med. 12:e9892022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Novoa Díaz MB, Martín MJ and Gentili C:
Tumor microenvironment involvement in colorectal cancer progression
via Wnt/β-catenin pathway: Providing understanding of the complex
mechanisms of chemoresistance. World J Gastroenterol. 28:3027–3046.
2022. View Article : Google Scholar
|
|
168
|
Kousar K, Ahmad T, Abduh MS, Kanwal B,
Shah SS, Naseer F and Anjum S: miRNAs in regulation of tumor
microenvironment, chemotherapy resistance, immunotherapy modulation
and miRNA therapeutics in cancer. Int J Mol Sci. 23:138222022.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: microRNA therapeutics in cancer-an
emerging concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar
|
|
171
|
Zhao X, Wang J, Zhu R, Zhang J and Zhang
Y: DLX6-AS1 activated by H3K4me1 enhanced secondary cisplatin
resistance of lung squamous cell carcinoma through modulating
miR-181a-5p/miR-382-5p/CELF1 axis. Sci Rep. 11:210142021.
View Article : Google Scholar :
|
|
172
|
Sun J: VDR/vitamin D receptor regulates
autophagic activity through ATG16L1. Autophagy. 12:1057–1058. 2016.
View Article : Google Scholar
|
|
173
|
Lin J, Chen X, Sun M, Qu X, Wang Y, Li C,
Li X, Zhao L, Su Z and Ye H: Upregulation of microRNA-181a-5p
increases the sensitivity of HS578T breast cancer cells to
cisplatin by inducing vitamin D receptor-mediated cell autophagy.
Oncol Lett. 21:2472021. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Galluzzi L, Morselli E, Vitale I, Kepp O,
Senovilla L, Criollo A, Servant N, Paccard C, Hupé P, Robert T, et
al: miR-181a and miR-630 regulate cisplatin-induced cancer cell
death. Cancer Res. 70:1793–1803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Hu X, Lou T, Yuan C, Wang Y, Tu X, Wang Y
and Zhang T: Effects of lncRNA ANRIL-knockdown on the
proliferation, apoptosis and cell cycle of gastric cancer cells.
Oncol Lett. 22:6212021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Wu J, Ma C, Tang X, Shi Y, Liu Z, Chai X,
Tang Q, Li L and Hann SS: The regulation and interaction of PVT1
and miR181a-5p contributes to the repression of SP1 expression by
the combination of XJD decoction and cisplatin in human lung cancer
cells. Biomed Pharmacother. 121:1096322020. View Article : Google Scholar
|
|
177
|
Bai H, Cao Z, Deng C, Zhou L and Wang C:
miR-181a sensitizes resistant leukaemia HL-60/Ara-C cells to Ara-C
by inducing apoptosis. J Cancer Res Clin Oncol. 138:595–602. 2012.
View Article : Google Scholar
|
|
178
|
Ping W, Gao Y, Fan X, Li W, Deng Y and Fu
X: MiR-181a contributes gefitinib resistance in non-small cell lung
cancer cells by targeting GAS7. Biochem Biophys Res Commun.
495:2482–2489. 2018. View Article : Google Scholar
|
|
179
|
Barbato A, Iuliano A, Volpe M, D'Alterio
R, Brillante S, Massa F, De Cegli R, Carrella S, Salati M, Russo A,
et al: Integrated genomics identifies miR-181/TFAM pathway as a
critical driver of drug resistance in melanoma. Int J Mol Sci.
22:18012021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Tabatabaei SN, Derbali RM, Yang C,
Superstein R, Hamel P, Chain JL and Hardy P: Co-delivery of
miR-181a and melphalan by lipid nanoparticles for treatment of
seeded retinoblastoma. J Control Release. 298:177–185. 2019.
View Article : Google Scholar : PubMed/NCBI
|