1. Introduction
Salt-inducible kinases (SIKs) are members of the
AMP-activated protein kinase (AMPK) family, including three
subtypes: SIK1, SIK2 and SIK3 (1).
After being identified first in the adrenal glands of high-salt
diet-fed rats in 1999 (2), this
special kinase was named SIK1 and was initially described as a
novel serine/threonine protein kinase (3). Subsequent studies identified the
other two subtypes, SIK2 and SIK3 (4,5). In
humans, SIK1 is abundantly expressed in the adrenal cortex, adipose
and neural tissues (4,6,7),
exhibiting the function of self-phosphorylation and regulating
adrenocortical function under the stimulation of high salt or
adrenocorticotropic hormone (ACTH) (2). SIK2 and SIK3 are constitutively
expressed in tissues and are ubiquitous in humans. Among them, SIK2
is highly expressed in adipose tissues and is involved in the
regulation of cell metabolism, including the control of insulin
signaling (4,8) and gluconeogenesis (9), while the highest expression place of
SIK3 is brain (10); it
coordinates with the mTOR complex (11,12)
and can be activated by inflammatory cytokines under stress,
exerting a cancer-promoting effect (13).
The dysregulation of SIKs has been identified in
various types of cancer, such as lung cancer, ovarian cancer,
breast cancer, prostate cancer, gastric cancer and hepatocellular
carcinoma (HCC) (14-17), which might be associated with
tumorigenesis and tumor progression. A number of signaling
molecules involved in cancer progression have been reported to
regulate SIKs, including liver kinase B1 (LKB1) and protein kinase
A (PKA). Additionally, downstream molecules such as cAMP response
element-binding protein (CREB), hippo and β-catenin may also be
regulated by multiple types of SIKs. Therefore, SIKs serve as
intermediate links in the molecular signaling pathways involved in
cancer development.
Existing studies indicate that SIKs play intricate
roles in tumor progression. In most types of cancer, SIK1 is
regarded as a tumor suppressor, whose expression is downregulated
in malignant tumors (18-21). By contrast, SIK2 and SIK3 are
considered candidate oncogenes endowing survival advantages to
cancer cells for growth and correlating with the
clinicopathological results of patients suffering from tumor
(15), especially in breast cancer
and ovarian cancer (6,13,22,23).
However, the exact roles of SIKs in cancer development are still
not well-characterized. The purpose of the present review was to
comprehensively summaries the roles of SIKs in the progression of
different types of cancer, fully elucidate their clinical value and
explore potential strategies for targeting SIKs for cancer therapy
in clinical use.
2. The structure and regulatory molecules of
SIKs
Structure and phosphorylation of
SIKs
In humans, the SIK1 gene is located on chromosome
21, while genes encoding SIK2 and SIK3 are on chromosome 11
(24,25). All SIKs contain an N-terminal
protein kinase domain, followed by a ubiquitin-associated (UBA)
domain located inside a central sucrose non fermenting (SNF-1)
homology (SNH) domain and a long C-terminal tail (5,26)
(Fig. 1). The catalytic activity
of SIKs relies on the phosphorylation of their threonine residues
in the activation loop (T-loop, especially the binding sites of
Thr182 in SIK1, Thr175 in SIK2 and Thr221 in SIK3) (25,27),
which could be achieved by the kinase activity of LKB1 (27). Notably, the mutation of threonine
to alanine could induce SIK inactivation (27). The phosphorylation threonine site
of LKB1 is relatively conserved, located in the N-terminal protein
kinase domain of SIK family. The SNH domain is distinct among SIKs:
SIK2 and SIK3 share 70 and 37% similar sequences with SIK1,
respectively (1). The C-terminal
domain is highly conserved between SIK1 and SIK2, with multiple PKA
phosphorylation sites. The two serine residues in SIK1, four in
SIK2 and three in SIK3 can be phosphorylated by PKA to elevate
intracellular cyclic AMP level (28). The elevated cyclic AMP induces the
dephosphorylation of physiological substrates of the SIKs,
indicating that the catalytic activity of SIKs could be inhibited
by PKA phosphorylation (29-32).
Another effect of PKA phosphorylation is to promote the nucleus
translocation of SIK1 (28,32)
to reduce its phosphorylation by LKB1, while SIK2 and SIK3 are
localized predominantly within the cytoplasm (25). In addition, SIKs contain multiple
motifs harboring PKA phosphorylation and 14-3-3 binding sites
(10,31). Blocking these potential
phosphorylation residues largely eliminates the binding of SIKs
with 14-3-3, indicating that the combination of PKA phosphorylation
and 14-3-3 protein binding is necessary for the inactivation of
SIKs (11,25). In addition, the UBA domain is
defined within the SNH domain (33) and mutations in this domain can
interfere with the interaction between SIK and 14-3-3 adaptor
protein and promote SIK nuclear transport, leading to the reduction
in LKB1-mediated signaling pathways (33,34).
Calcium-dependent protein kinase (CaMK) is another kinase for the
activation of SIKs, which phosphorylates Thr322 residue in the SNH
domain of SIK1 (26,35). However, in SIK2, the activity of
CaMK is associated with its degradation (36). In addition, SIKs can also be
activated by autophosphorylation. In the T-loop of SIK1 and SIK2,
autophosphorylation sites exist in Ser186 and Ser179, respectively
(37). The hypothesis for SIK
autophosphorylation process was considered as follows: The
autophosphorylation sites are located at the four amino acid
C-terminal of the activated phosphor-threonine residue, creating a
consensus motif for glycogen synthase kinase 3 (GSK3)
phosphorylation (38). The
formation of this motif allows the phosphorylation of SIK1 and SIK2
by GSK3 at Thr182 and Thr175, respectively, forming a positive
feedback regulation on SIK activation.
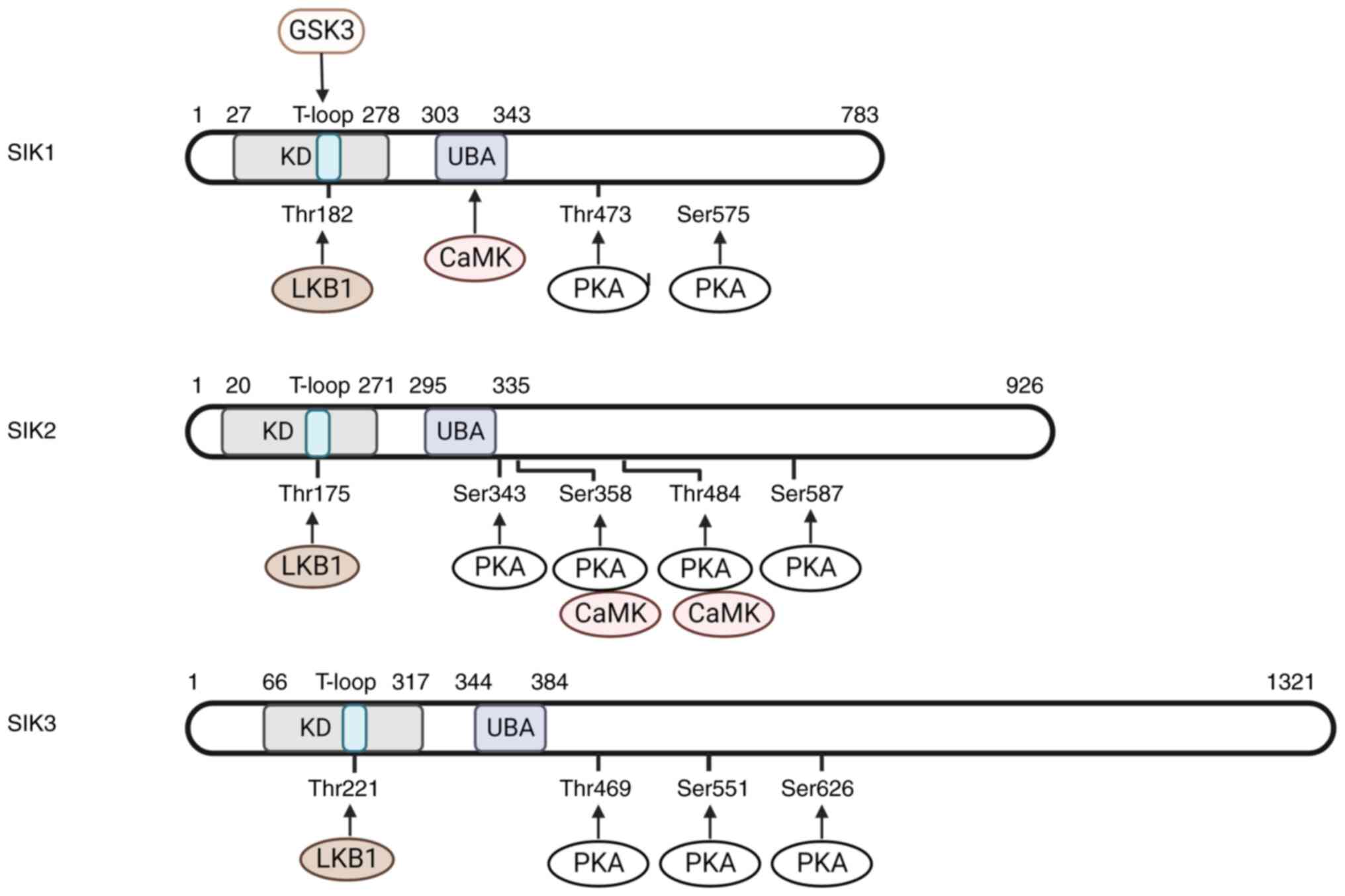 | Figure 1Structure and phosphorylation sites
of SIKs. SIKs could be divided into three domains include KD, SNH
domain and C-terminal domain. LKB1 phosphorylation sites are in KD,
UBA domain is inside SNH, which was not shown in the figure.
C-terminal domain contains multiple PKA phosphorylation sites.
SIKs, salt inducible kinases; KD, kinase domain; SNH, sucrose non
fermenting homology; LKB1, liver kinase B1; UBA,
ubiquitin-associated; PKA, protein kinase A; CaMK,
calcium-dependent protein kinase. |
Regulatory molecules of SIKs
In addition to the direct phosphorylation of binding
sites, the expression of SIKs is also under the control of other
extracellular signals and non-coding RNAs. SIK1 could be
upregulated by high salt dietary intake (10), ACTH signaling (3), glucagon signaling (39), excitable cell depolarization
(40) and circadian rhythms
(41). Similarly, the synergistic
effect of high salt and cytokine IL-17 also plays a role in
stimulating SIK3 expression (13,42).
Non-coding RNAs constitute most of the human RNA,
including microRNA, long non-coding RNA (lncRNA), circular RNA
(circRNA) and enhancer RNA (43).
They modulate cell physiology and functions, from epigenetic gene
silencing to post-transcriptional regulation of mRNA stability
(43). The expression level of
SIK1 and SIK2 can be regulated by different non-coding RNAs. Based
on existing studies, five microRNAs inside tumor cells promote
tumor proliferation, migration and metastasis by suppressing the
activity of SIK1: miR-17 affects the proliferation and migration
process of human colorectal cancer (44), miR-203 plays a role in the
progression of pancreatic cancer (45), miR-141 promote ovarian cancer
proliferation (46) and the
overexpression of miRNA-373 is associated with the migration of
melanoma cells (47) (Fig. 2A). Only miR-103b-3p, as an exosomal
RNA, affects SIK1 expression and shows a distinct role in tumor
development (48). A total of six
other miRNAs (miR-149-5p, miR-103a-3p, miR-526b, miR203,
miR-654-5p, miR-874-3p and miR-874-5p) inhibit tumor development by
repressing the expression of SIK2 (49-53)
(Fig. 2B).
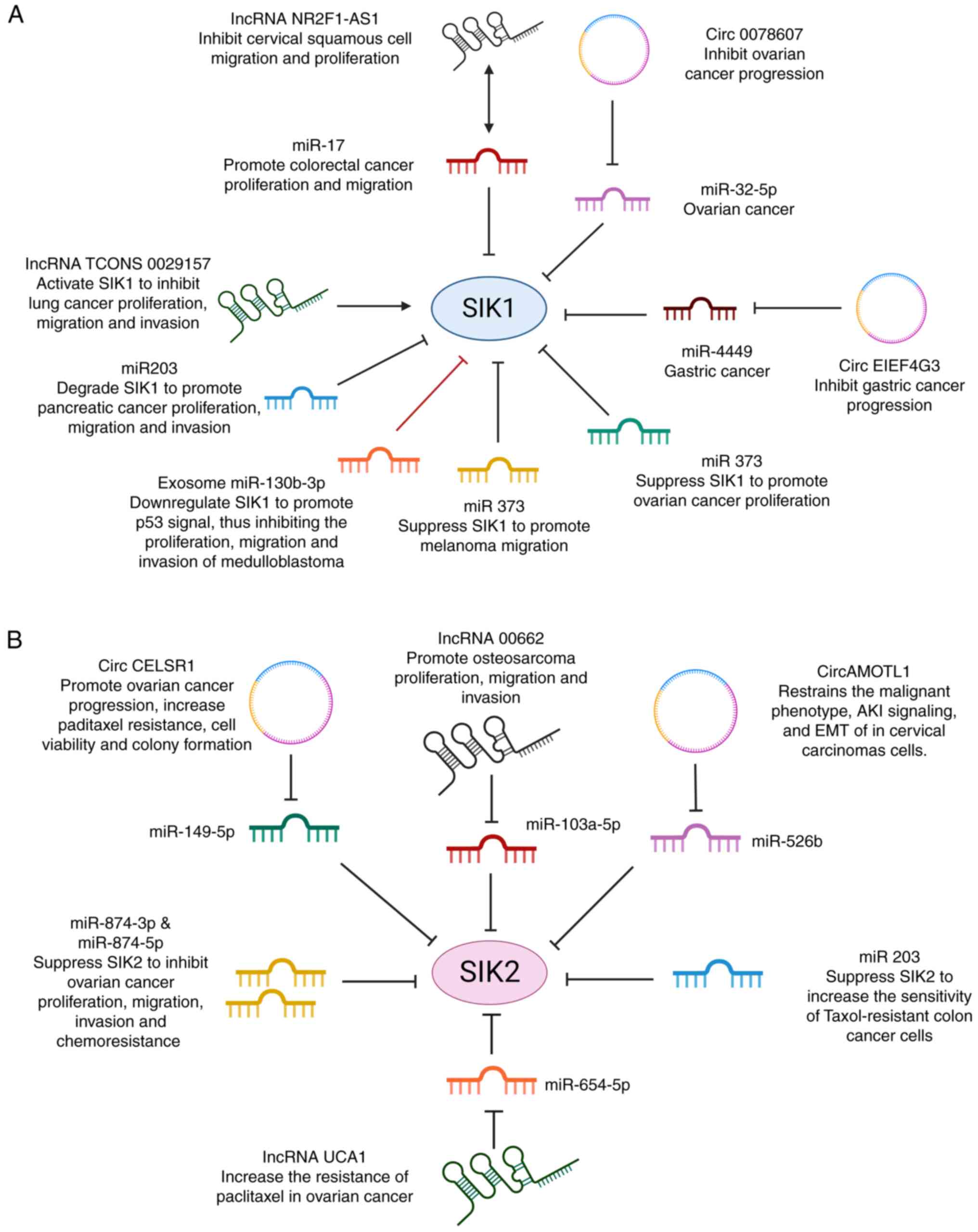 | Figure 2Non-coding RNAs regulate the
expression of SIK1 and SIK2. (A) The activity of SIK1 could be
regulated by six miRNAs, two lncRNAs and one circRNA. Among them,
miR-17, miR-203, miR-141 miR-32-5p and miRNA-373 promote
tumorigenesis by directly suppressing SIK1. LncRNA TCONS 0029157
activates SIK1 directly thereby inhibiting lung cancer progression.
NR2F-AS1 and Circ 0078607 inhibit tumor development by inactivating
the function of miRNA. Double arrow: NR2F-AS1 could interact with
miR-17 to suppress tumor progression, but it cannot inhibit the
expression of miR17. (B) The activity of SIK2 could be regulated by
six miRNAs, two lncRNAs and two circRNAs. miRNAs inhibit tumor
invasion by suppressing the activity of SIK2, while circRNAs and
lncRNAs are associated with chemotherapeutic resistance by
affecting SIK2 expression. SIKs, salt inducible kinases;
miRNAs/miRs, microRNAs; lncRNA, long non-coding RNA; circRNA,
circular RNA. |
Other types of non-coding RNAs, including lncRNAs
and circRNAs, indirectly control the activity of SIKs by
interacting with microRNAs. LncRNA NR2F1-AS1 and TCONS 0029157
regulate SIK1-mediated tumor proliferation and migration.
SIK1-adjusted tumor proliferation and migration are under the
control of lnc RNA NR2F1-AS1 and TCONS 0029157 (54,55).
Among them, TCONS 0029157 inhibits the progression of lung cancer,
while lncNR2F1-AS1 prevents the development of cervical squamous
cancer by sponging the suppressive effect of miR-17 on SIK1. SIK2
is controlled by lncRNA 00662 and UCA1, promoting the migration of
various tumors (53,56). Single-stranded, covalently closed
circRNAs frequently function as transcriptional regulators, miRNA
sponges and protein templates (57). Circ 0078607 inhibits the
progression of ovarian cancer by regulating miR-35-5p/SIK1 axis
(58) and a similar mechanism was
also detected in circEIF4G3 and miR-4449 in gastric cancer
(59); while circAMOTL1 and
circCELSR1 are regulators of SIK2 (49,50),
playing a role in regulating cervical carcinoma and ovarian cancer,
respectively. It is worth noting that the status of drug resistance
could also be influenced by the effect of non-coding RNAs on SIK2
expression level. Studies focusing on ovarian and colon cancer have
shown that SIK2 inhibition by miRNAs can effectively restore
sensitivity in paclitaxel-resistant tumors, while lncRNAs and
circRNAs that increase the activity of SIK2 can amplify tumor taxol
resistance (49,51-53).
Substrates of SIKs
CREB-regulated transcriptional coactivators (CRTC),
including CRTC1, CRTC2 and CRTC3, as well as Class 2a histone
deacetylases (HDAC4, HDAC5, HDAC7 and HDAC9), have been identified
as substrates of SIKs (25). The
phosphorylation of CRTCs by SIKs induces them to bind with 14-3-3
proteins in the cytosol, depriving their ability to activate
nuclear transcription factor CREB (5,28,31).
Conversely, SIK inhibition and CRTC dephosphorylation can activate
CREB-dependent gene transcription59-63 (60-64).
Phosphorylation of Class 2a HDACs by SIKs leads to their binding to
14-3-3 proteins and retention in the cytosol. When SIKs are
inactivated, these proteins can enter the nucleus and bind to
myocyte enhancer factor 2 (MEF2), repressing its target gene
transcription (32,63,65,66).
3. Distinct roles of SIKs in cancer
development
Roles of SIK1 in cancer progression
LKB1-SIK1 axis inhibits cancer
progression
LKB1 has been identified as a critical barrier of
cancer initiation and metastasis (27,67).
As it widely regulates the AMPK family, the LKB1-SIK1 axis is a
crucial pathway for LKB1 to suppress SIK-related cancers. Multiple
tumor suppressors are under the control of this axis, including
CRTC, HDAC, p53, ZEB1 (Fig. 3). In
gastric adenocarcinoma, the LKB1-SIK1 axis could be activated by
gastrin, inhibiting tumor metastasis by phosphorylating HDAC4 and
enhancing the gastrin-induced transcription of c-fos and CRE-,
SRE-, AP1- and NF-kB (21). In
human breast cancer, SIK1 is required for the activation of p53 to
promote tumor cell anoikis and loss of the function of either LKB1
or SIK1 is closely associated with tumor metastasis (19). Additionally, it has been found that
the enhancement of aerobic glycolysis in breast cancer is dependent
on p53 suppression induced by the lack of SIK1 (68). This is achieved by inhibiting
glucose intake control gene Glut1 (69) and blunting the expression of LDHA
to alleviate pyruvate-to-lactate conversion (68).
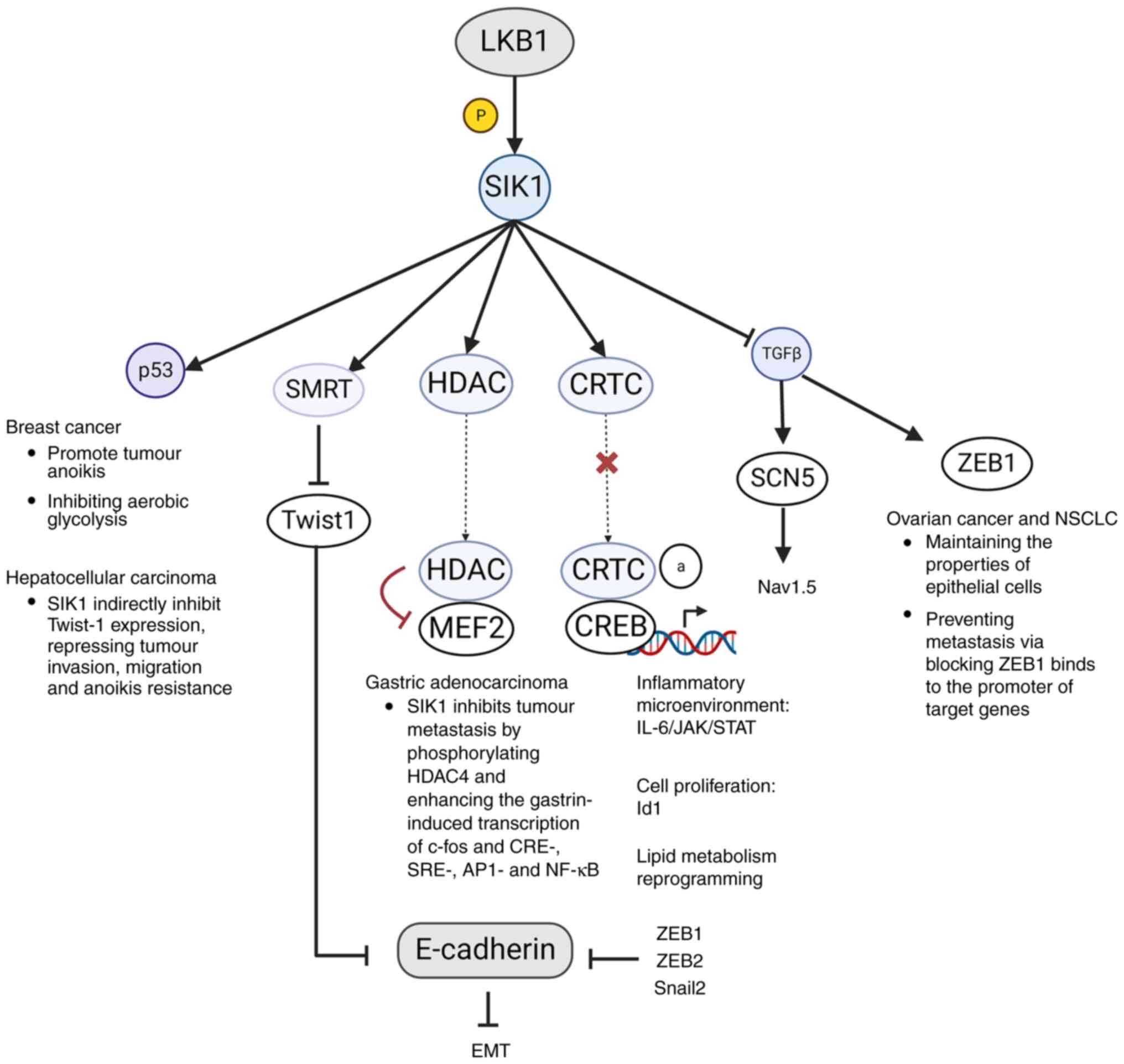 | Figure 3Roles of SIK1 in cancer progression.
SIKs, salt inducible kinases; LKB1, liver kinase B1; HDAC, histone
deacetylase; SMRT, silencing mediator of retinoic acid and thyroid
hormone receptor; CRTC, CREB-regulated transcriptional
co-activators; MEF2, myocyte enhancer factor 2; CREB, cAMP response
element-binding protein; ZEB1, zinc finger E-box-binding homeobox
1; SCN5, sodium channel protein type 5; NSCLC, non-small cell lung
cancer; EMT, epithelial-mesenchymal transition; Snail2, snail
family zinc finger 2. |
Another substrate of LKB1-SIK1 axis is TGFβ
(70), which controls tumor
development via positively stimulating the expression of two genes
Zinc finger E-box-binding homeobox 1 (ZEB1) and SCN5. In ovarian
carcinoma and non-small cell lung cancer (NSCLC) (18,71),
ZEB1 can decrease the properties of epithelial cells and promote
the expression of genes responsible for tumor metastasis (72,73).
As for SCN5, its product voltage-gated sodium channel
(NaV)1.5 could be regulated by both SIK1 and TGFβ
(74). Previous studies have shown
that tumor cells are more permeable to Na+ compared with
normal cells (75). In breast
cancer cells with significantly downregulated SIK1 levels, Nav1.5
overexpression has been observed, promoting Na+-mediated
invasiveness (76-78).
SIK1 blocks tumor
epithelial-mesenchymal transition (EMT) via regulating the
Wnt/β-catenin signaling pathway
Epithelial to mesenchymal transition is also a
crucial process of tumor metastasis controlled by SIK1. It is
characterized by the loss of epithelial markers including
E-cadherin and γ-catenin and increased expression of mesenchymal
markers such as N-cadherin, vimentin, Snail, Twist and ZEB
(79,80). SIK1 regulates the EMT process by
interacting with the Wnt/β-catenin signaling pathway (81) (Fig.
4). In normal cells, the silencing mediator of retinoic acid
and thyroid hormone receptor (SMRT) could be phosphorylated by SIK
at threonine 1391. The activated SMRT is translocated into the
nucleus and recruits transducin β-like protein 1
(TBL1)/TBL1-related protein (TBLR1) and NCoR/HDAC3 to β-catenin
target gene Twist1 promoter region, thereby inhibiting the
expression of Twist1 and intercepting the subsequent effects of
β-catenin signal (81). In HCC
cells, SIK1 is suppressed by its E3 ligase RNF2 (82), restoring β-catenin activity. The
enhanced Twist-1 expression increases tumor invasion, migration and
anoikis resistance (83,84), also binds to the E-box motif of the
SIK1 promoter, relieving the restriction of SIK1 and SMRT on
β-catenin signaling pathway (81).
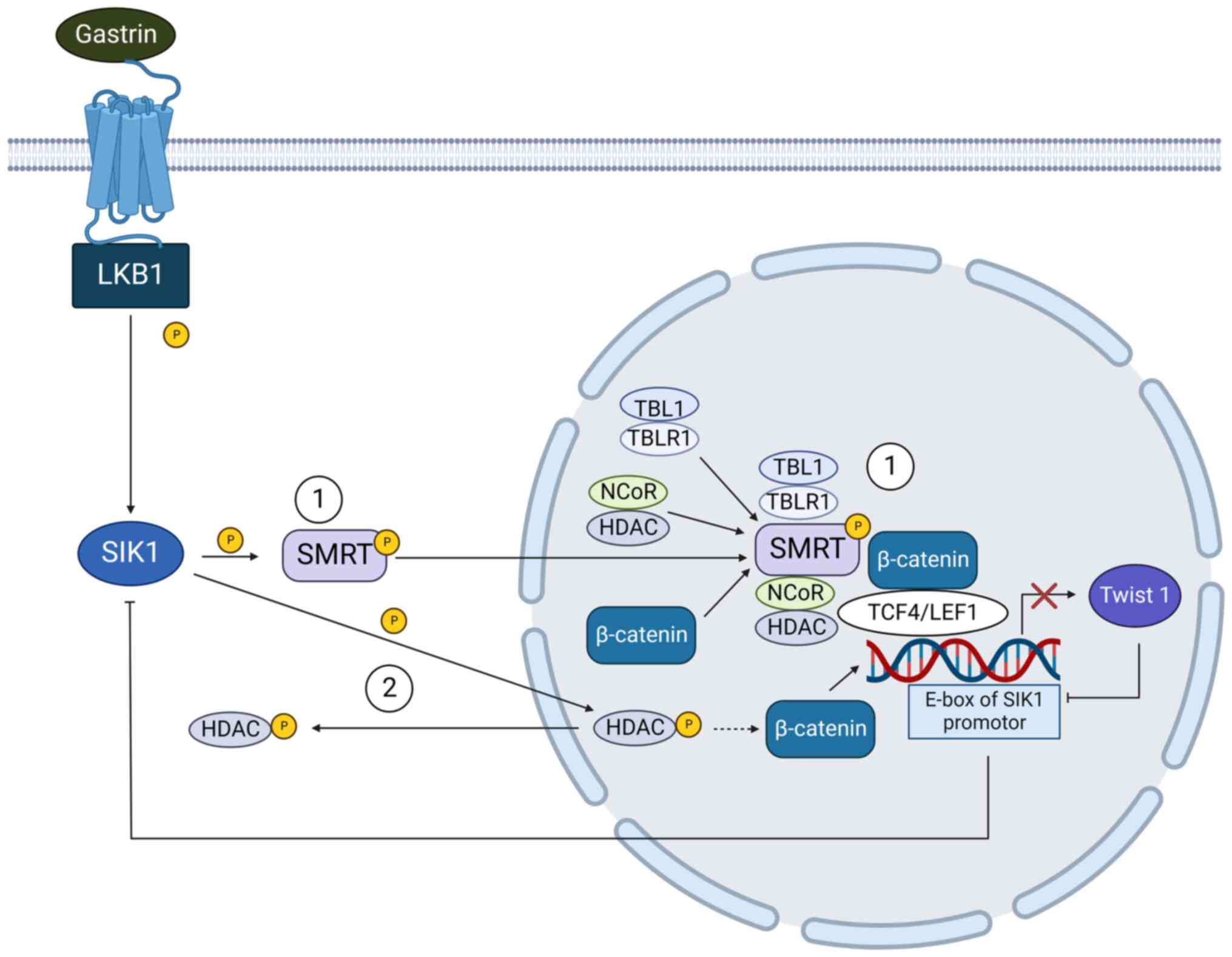 | Figure 4SIK1 inhibits tumor cell EMT by
suppressing β-catenin signaling pathway and the expression of Twist
1. (1) In hepatocellular
carcinoma, SIK1 phosphorylates SMRT, which forms complex
withβ-catenin thereby inhibiting Twist1-associated EMT. Twist 1
could also negatively control the expression of SIK1 by binding to
the E-box motif of the SIK1 promoter. (2) In gastric adenocarcinoma, SIK1
prevents tumor cell EMT by inducing the cytosolic translocation of
HDAC, which in turn reduces the activity of β-catenin signaling
pathway and blocks the tumor metastasis. Dashed arrow: This process
was identified in human Uterine Fibroid, which is waiting for
further verification in gastric adenocarcinoma cells. SIKs, salt
inducible kinases; EMT, epithelial-mesenchymal transition; LKB1,
liver kinase B1; SMRT, silencing mediator of retinoic acid and
thyroid hormone receptor; HDAC, histone deacetylase; TBL1,
transducing β-like protein 1; TBLR1, TBL1-related protein; NCoR,
nuclear receptor corepressor. |
Could SIK1 be identified directly as a
tumor suppressor gene?
As described above, most studies indicate that the
effects of SIK1 on tumor cells are close to a tumor suppressor.
Upon SIK1 activation by LKB1, it inhibits tumorigenesis and the EMT
process, reducing cancer metastasis and promoting cancer apoptosis
(1,25,85).
However, controversy remains over SIK1: In medulloblastoma (MB), an
uncovered novel effect of miR-130b-3p on SIK1 indicates that SIK1
might also be a tumor promoting protein (48). In 2020, Huang et al proposed
that although miR-130b-3p is suppressed in MB cells, it is
upregulated in the tumor-secreted exosomes in the plasma of MB
patients and can be transferred to tumor cells (48). Of note, the inhibition of exosomal
miR-130b-3p on SIK1 in transferred tumor cells produces anti-tumor
effects, suggesting the potential oncogenic role of SIK1 (48). By contrast, in HCC the role of
exosomal miRNA induced SIK1 inhibition remains to promote tumor
progression (86). Additionally,
the emerging oncogenic role of SIK1 has also been shown in the
development of Desmoplastic small round cell tumor (DSRCT)
(87): SIK1 can be activated by
oncogenic transcription factor EWSR1, affecting DNA replication
through regulating MCM DNA helicase (88). Consistently, the depletion of SIK1
leads to rapid growth arrest of DSRCT cells at the G1/S
phase, exhibiting a strong tumor repression effect (87). The results of these investigations
suggest that the function of SIK1 may not be limited to a tumor
suppressor, it could also exhibit stimulative function in some
tumors. Further studies are required to validate the roles of SIK1
in distinct tumors.
Roles of SIK2 in cancer progression
SIK2 modulates tumor cell
proliferation by regulating cell cycle
As aforementioned, uncontrolled mitosis is a
hallmark of cancer cells. Therefore, anti-mitotic drugs such as the
tubulin inhibitor paclitaxel have been developed for anticancer use
(89). SIK2 is a centrosome kinase
required for the initiation of mitosis and its inhibition induces
the altered position of the mitotic spindle (90). Long-lasting suppression of SIK2 can
lead to chromosomal instability (90). To accurately establish cell
division plane, SIK2 orchestrates the centrosome alignment and
spindle position during the cell division, maintaining the
stability of chromosome (90). In
ovarian cancer, the depletion of SIK2 induces decreased AKT
phosphorylation and delayed G1/S transition (89). The consequence of SIK2 induced
PI3K/AKT activation is the upregulated expression of cell division
regulator survivin (91), which
affects microtubule dynamics, stability and mitotic progression
(92,93). During mitosis, it serves as an
interface between the centromere/central spindle and the
chromosomal passenger complex (94). Survivin is overexpressed in
multiple malignancies, inducing cell-cycle checkpoint bypasses and
uncontrolled aberrant progression of transformed cells (95). Likewise, Bon et al (96) indicated that SIK2 knockdown could
significantly reduce the growth rate of prostate tumor cells. This
was accompanied by the arrest of G1 cell cycle via up
regulating p21 and p27 and downregulating Cyclin D1. Using SIK2
inhibitors on SIK2-overexpressed cancer cells could reduce the
expression of survivin to a certain extent, providing evidence for
the development of anticancer drugs.
Similar to SIK1, wild-type SIK2 could also impact
tumorigenesis by phosphorylating CRTC1 and CRTC2 (96,97),
preventing their translocation and thus inhibiting the activation
of CREB1. Theoretically, this effect is associated with tumor
suppression. However, high levels of auto-antibodies against SIK2
were found in the plasma of patients with prostate cancer (96), transforming SIK2/CREB interaction
into a tumor-promoting effect. Under the attack of these
auto-antibodies, the kinase activity of SIK2 is lost, making it
forms a complex with CRTC1 (96).
This complex can be translocated into the nucleus, acting as a CREB
trans-activator to trigger the activation of other transcription
factors such as HSF, IRF and NFκB, resulting in endoplasmic
reticulum stress response and cell apoptosis (96). These results indicated that wild
type SIK2 remains a tumor promotor in prostate cancer, while loss
of its kinase activity will accelerate tumor cell death (96). Therefore, it is reasonable to
hypothesize that CREB exhibits diverse roles in prostate cancer; it
serves as a tumor suppressor gene in Hodgkin's lymphoma and
melanoma, directly binding to the promoter regions of cyclin D1,
cyclin E1, CDK2 and CDK4 to disturb tumor proliferation (98,99).
SIK2-induced downregulation promotes G1/S phase
transition, thereby promoting tumor cell cycle progression.
SIK2 is also an antagonist of the hippo signaling
pathway, which is highly conserved from Drosophila to humans
(100). Dysregulation of this
signaling pathway has been detected in a wide variety of types of
cancer. In humans, SIK2 dampens the Hippo signal by directly
binding to and phosphorylating its partner, Sav, at Ser413
(85). This disrupts the
interactions between mammalian STe20-like kinases (MST) 1/2 and
large tumor suppressor homolog (LATS) 1/2 (homologous to hpo-warts
in Drosophila), leading to increased expression of Yes
kinase-associated protein (YAP) and its target genes, which confer
growth advantages to cells. Thus, upon SIK2 activation, the Hpo
signaling dependent-cell cycle exit and cell apoptosis are
inhibited, resulting in tissue overgrowth. Notably, the effect of
SIK2 inhibitors may enhance the Hpo pathway in ovarian tumor cells
and this strategy might be less effective in tumors that are
inherently rich in YAP expression (101) (Fig.
5).
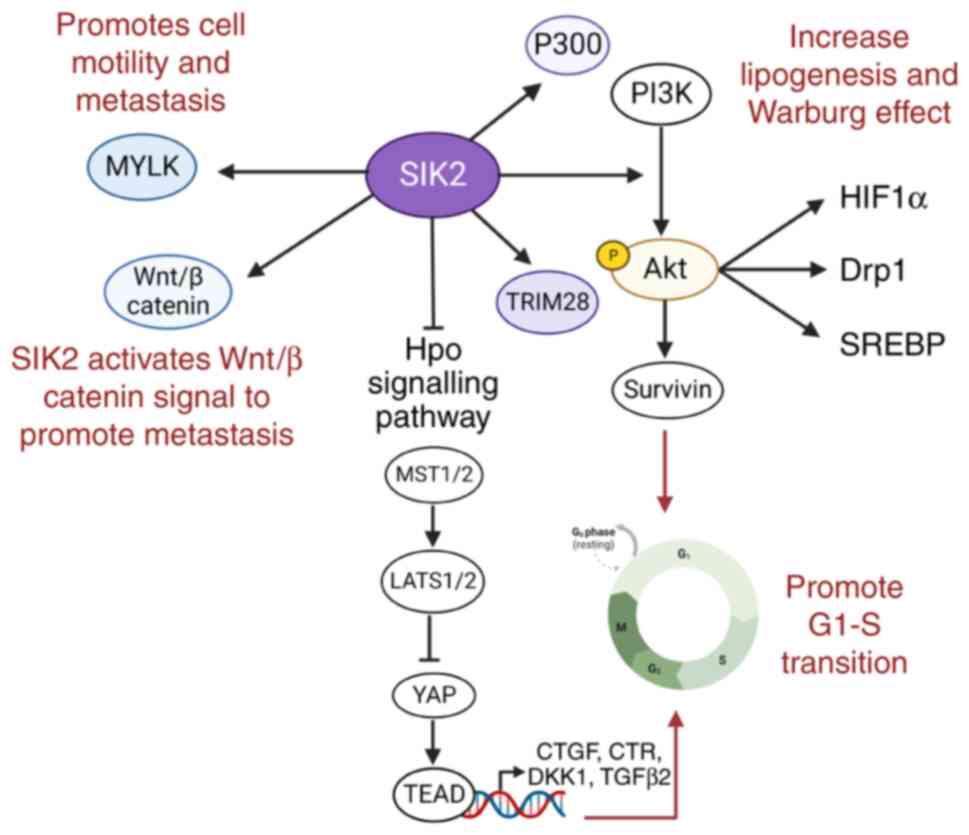 | Figure 5Roles of SIK2 in cancer development.
SIKs, salt inducible kinases; MYLK, myosin light chain kinase;
TRIM28, activating tripartite motif 28; MST, macrophage-stimulating
protein; LATS1, large tumor suppressor homolog 1; YAP, Yes
kinase-associated protein; TEAD, transcriptional enhanced associate
domain; HIF1α, hypoxia-inducible factor-1α; Drp1, Dynamin-related
protein 1; SREPB, sterol regulatory element-binding protein. |
SIK2 regulates tumor cell metabolic
reprogramming
Metabolic reprogramming is an emerging hallmark of
cancer, as cancer cells are defined as a 'metabolically abnormal
system' (102). Cancer cell
metabolism relies on oxidative glycolysis known as the Warburg
effect. Hyperactive glycolysis is associated with the faster
generation of ATP in malignancies, inducing the formation of
metabolic intermediates macromolecules such as lipids and amino
acids in rapidly dividing tumor cells (103). The rapid synthesis of these
molecules significantly promotes the tumorigenesis process
(103). In addition, the
dysregulation of fatty acid metabolism also takes part in the
malignant transformation in a number of different cancers (104,105). In most cases, oncogenic molecules
trigger tumorigenesis by stimulating abnormal metabolism and SIK2
is a vital metabolic regulator. In ovarian cancer, it boosts the
Warburg effect and tumor lipogenesis by activating
PI3K/AKT-hypoxia-inducible factor-1α signaling pathway (103). SIK2 also inhibits oxidative
phosphorylation by activating Drp-1 to promote mitochondria
fission, then tumor cells rely on aerobic glycolysis for energy
supply (103). In colorectal
cancer, SIK2 enhances glycolysis by activating tripartite motif 28
(TRIM28) (106), whose expression
level is positively associated with poor overall survival and
progression-free survival (106,107). The silencing state of SIK2 could
be reversed by TRIM28 overexpression on tumor proliferation,
migration, invasion and glycolysis, enhancing the tumorigenesis
process.
As for the process of lipogenesis, AKT is a crucial
molecule regulated by SIK2 in various types of cancer. In ovarian
cancer, SIK2 enhances AMPK-induced phosphorylation of acetyl-CoA
carboxylase, activating the PI3K/AKT pathway through p85a-S154
phosphorylation to promote tumor proliferation, survival and
omental metastasis (108). Thus,
upon SIK2 activation, the Hpo signaling dependent-cell cycle exit
and cell apoptosis are inhibited, resulting in tissue overgrowth
(109). The activation of AKT by
SIK2 was also detected in the generation process of pancreatic
cancer: SIK2 acts upstream in mTORC2/AKT signaling, regulating
insulin-induced UPP-1 gene expression in brown adipocytes, thereby
enhancing the metabolism of adipose tissue (110,111). Additionally, SIK2 also
accelerates lipogenesis through other approaches. In the liver,
SIK2 activates carbohydrate-response element-binding protein
(ChREBP) by regulating histone acetyltransferase coactivator p300
(9), promoting lipogenesis and
hepatic steatosis. ChREBP was identified as possessing the function
of activating target genes favoring downstream tumorigenic pathways
(112). Theoretically, steatosis
accumulation and ChREBP activation significantly increase the risk
of hepatocellular carcinoma. However, SIK2 is also reported to
repress HCC by inhibiting the Wnt/β-catenin signaling pathway
(14) (Fig. 6).
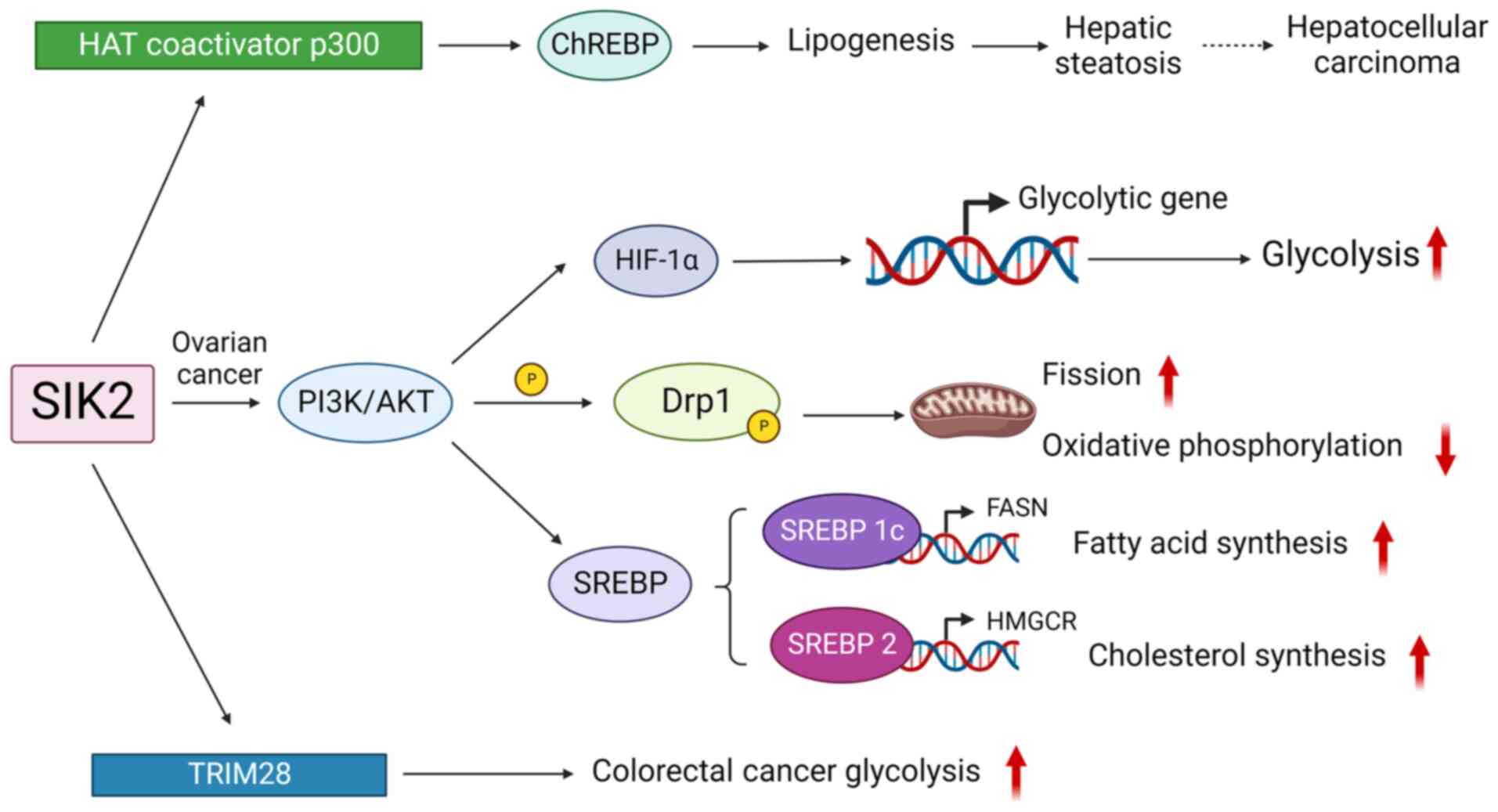 | Figure 6SIK2 promotes tumorigenesis by
regulating cell metabolism. SIKs, salt inducible kinases; HAT,
histone acetyltransferase; ChREBP, carbohydrate-response
element-binding protein; TRIM, tripartite motif; SREBP, sterol
regulatory element binding protein; FASN, fatty acid synthase;
HMGCR, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase; HIF1α,
hypoxia-inducible factor-1α; Drp1, Dynamin-related protein 1;
TRIM28, activating tripartite motif 28. |
SIK2 regulates tumor metastasis
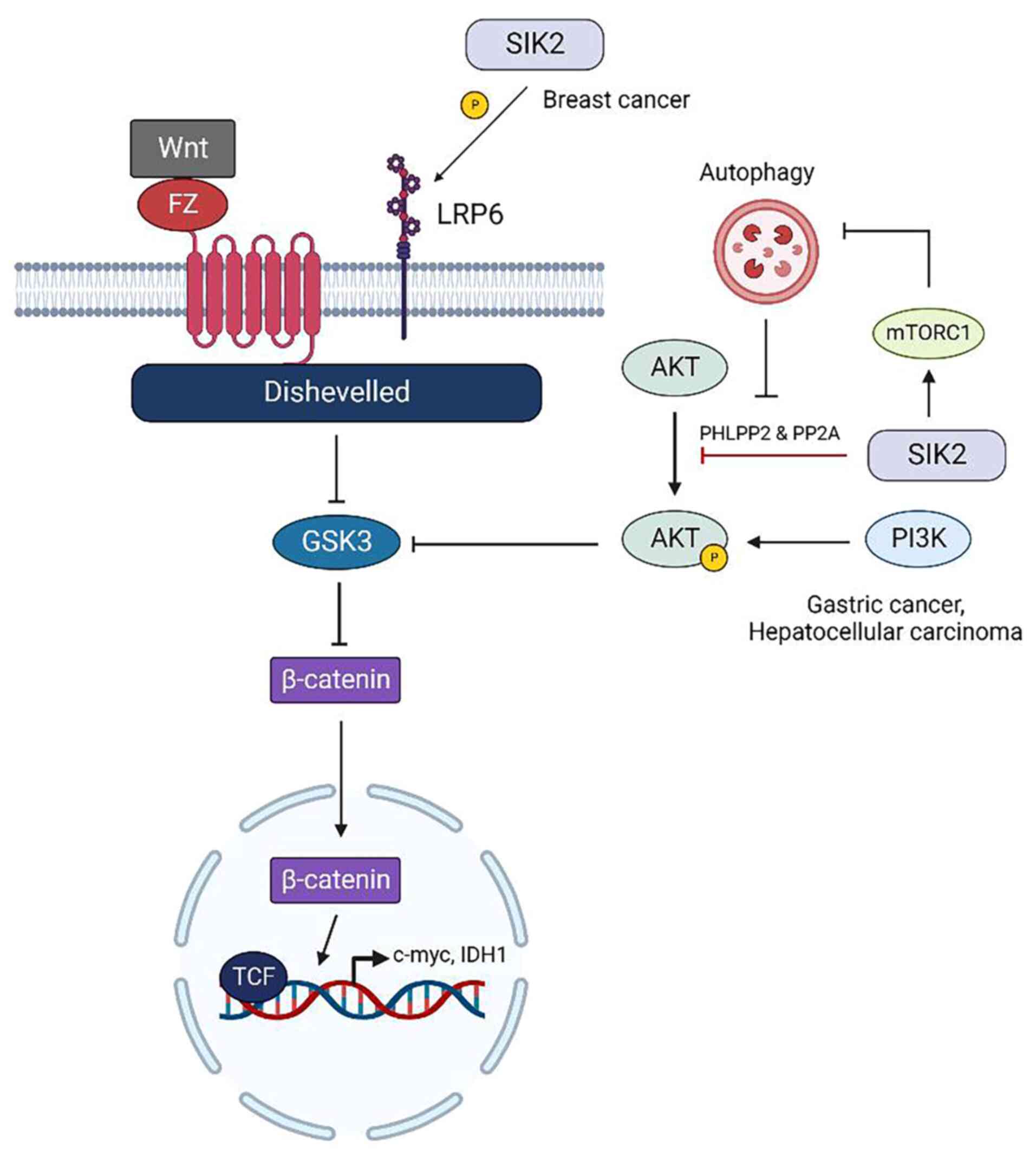
SIK2 and Wnt/β-catenin signaling
pathway
In contrast to SIK1, which exclusively inhibits
Wnt/β-catenin signaling pathway by activating transcriptional
co-repressor proteins to prevent the binding of β-catenin to
cellular DNA (81), the effects of
SIK2 on β-catenin are different in distinct types of cancer. In
gastric cancer and HCC, SIK2 promotes the activity of glycogen
synthase kinase 3 (GSK3) by dephosphorylating AKT through the
protein phosphatases PHLPP2 and PP2A (14,113). GSK3 effectively induces the
degradation of β-catenin, enhancing Wnt/β-catenin transcription and
tumor cell metastasis could be blocked (14,113). However, in breast cancer SIK2
acts as an oncogene to activate LPR6 receptor and enhance the
Wnt/β-catenin signaling pathway, which contributes to maintaining
the stemness of breast cancer stem cells (114). Cancer stem cells primarily drive
tumor heterogeneity, contributing to breast cancer recurrence,
metastasis and therapeutic resistance (115). By activating low density
lipoprotein receptor-related protein 6, which is overexpressed in
20-36% of patients with breast cancer (116), SIK2 efficiently promotes the
maintenance of stemness features of breast cancer stem cells
(117,118). In addition, SIK2 could also
restrict tumor autophagy to support the survival of triple-negative
breast cancer (119) (Fig. 7).
SIK2 and myosin light chain kinase
(MYLK)
Tumor metastasis typically depends on lymphatic
circulation and blood pathways, which are driven by increased cell
motility involving cycles of actin polymerization, cell adhesion
and actomyosin contraction (120,121). In addition to regulating
intracellular signaling pathways, SIK2 can directly phosphorylate
MYLK on Ser343 to further activate myosin light chain 2, which then
facilitates cell contraction and motility, inducing alterations in
the actin cytoskeleton (122).
Rapid and dynamic changes in the cytoskeleton are required for
cancer cell invasion and metastasis (123). This pathway is activated by
omentum-derived adipocytes, which induce calcium-dependent
activation and autophosphorylation of SIK2 (22).
Roles of SIK3 in cancer progression
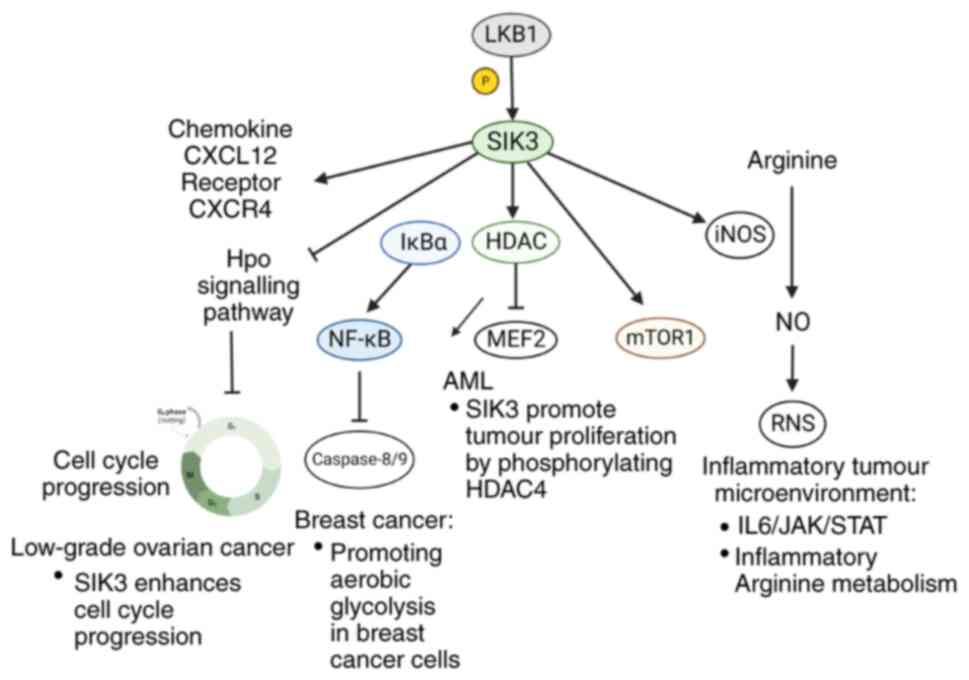
SIK3 in tumorigenesis
As a cell cycle regulator, SIK3 controls tumor cell
proliferation. Its activity significantly suppresses the Hippo
signaling pathway, promoting the continuous progression of the cell
cycle and preventing tumor apoptosis (100). In response to high salt
stimulation, upregulated SIK3 enhances cell cycle progression by
releasing G1/S arrest, thereby bestowing growth
advantages to breast cancers (13). More specifically, SIK3 upregulates
the cyclin D and E and G1/S-promoting CDK2 activity.
These cell cycle arresting and apoptosis promotion effects were
achieved by the interactions between SIK3 and Akt signaling pathway
(124). Likewise,
Charoenfuprasert et al (15) demonstrated that SIK3 enhances cell
cycle progression in low-grade ovarian cancer through attenuating
p21 Waf/Cip1 and p27 Kip activity, which are key effectors
underlying the SIK3-mediated cell cycle regulation (125). Among them, c-Scr is the major
signaling component responsible for SIK3-mediated downregulation of
p21 in ovarian cancer, establishing a linkage between SIK3-Scr
activation and p21 Waf/Cip1 gene regulation (125).
In addition, SIK3 can modulate tumor resistance to
apoptosis by TNF-NFκB axis (23).
It renders tumor cells susceptible to TNF secreted by
tumor-activated cytotoxic T cells. Following TNF stimulation, SIK3
promotes nuclear translocation of NF-κB via the phosphorylation of
IκBα. The accumulated nuclear NF-κB inhibits caspase-8/9 (23). Chromatin accessibility and
transcriptome analyses from Sorrentino et al (23) indicated that SIK3 knockdown could
disrupt the expression of pro-survival genes under the TNF-NF-κB
axis, which exhibited the effect of SIK3 in regulating tumor cell
survival. In addition, the phosphorylation of mTOR complex 1
(mTORC1) signaling also relies on SIK3 activity, conferring growth
advantages to breast cancer cells by promoting aerobic glycolysis
(126). After using clustered
regularly interspaced short palindromic repeats (CRISPR) to
knockout SIK3, the phosphorylation levels of mTOR1 targeted
molecules were decreased (124).
In acute myeloid leukemia (AML), SIK3 regulates tumor proliferation
under the control of LKB1 and HDAC4 is the downstream molecule
participating in this process. When the catalytic activity of SIK3
is normal, HDAC4 is limited to the cytosol and MEF2-induced
transcription maintains AML proliferation (127). The blockade of SIK3 releases
HDAC4 into nucleus, thereby inhibiting the activity of MEF2 and
suppressing AML development (128) (Fig.
8).
SIK3 affects tumor microenvironment
via inducing inflammation
The activity of SIK3 is closely associated with
chronic inflammation, tumor formation and proliferation (129). Unlike acute inflammation, which
effectively eliminates pathogen or disease, chronic inflammation is
the initiation of several molecular cascades, such as reactive
nitrogen and oxygen species (RNS/ROS), resulting in DNA damage and
tumor formation (13).
Simultaneously, chronic inflammation can activate a range of
signaling transcription factors, contributing to uncontrolled cell
growth and tumor progression (13). In addition, cell stress is also
related to inflammation, which then promotes the release of growth
factors to increase tumor angiogenesis. These newly formed blood
vessels provide access for tumor cells to metastasize to various
parts of human body (130).
The roles of SIK3 in inflammation have been
established: It induces pro-inflammatory arginine metabolism and
RNS release. In breast cancer cells treated with high salt and
IL-17, the formation of RNS, nitric oxide and citrulline were
significantly higher than basal control conditions and the
expression of pro-inflammatory inducible nitric oxide synthetase
(iNOS) and arginosuccinate synthetase (ASS-1) was also enhanced
(13). As an important enzyme for
converting arginine into nitric dioxide (NO), the expression level
of iNOS could directly affect RNS level in the tumor
microenvironment (13).
Noticeably, the downregulation of anti-inflammatory arginase-1 and
ornithine decarboxylase was also detected in the experiments,
indicating that the roles of tumor promotion in SIK3 are closely
associated with inflammatory reactions (13).
The mediators and cellular effectors of
inflammation are important constituents of the tumor environment.
In some types of tumors, inflammation can be considered a precursor
to the occurrence of malignancy (131-133). By contrast, the oncogenic change
could also induce an inflammatory microenvironment to promote tumor
development (134). Regardless of
its origin, the consistent existence of inflammation in tumor
microenvironment contributes to the development of malignancies,
promoting angiogenesis and metastasis, subverting adaptive immune
responses and altering responses to hormones and chemotherapeutic
agents (134).
The tumorigenic potency of SIK3 is also reflected
in the metastatic hallmark of breast cancer. Several lines of
evidence have reported that chemokine CXCL12 and its specific
receptor CXCR4 expressed on cancer cells contribute to the
metastatic property of malignant tumors (135). Amara et al (13) also showed that SIK3 can induce a
pronounced increase in these metastatic markers in breast cancer.
Consistently, SIK3 inhibition by prostratin also exerts anti-cancer
effects partially through attenuating the expression of CXCR4 on
breast cancer as anticipated (42).
Interactions between SIKs in cancer
development
As aforementioned, most of the current studies
indicate that SIK1 and SIK2 exhibit antagonistic effects in the
process of tumorigenesis progression. However, although the
research on SIK3 is still very limited, the function of SIK3 has
shown synergistic effects with both SIK1 and SIK2 and occasionally
three subtypes of SIKs can exhibit similar effects.
SIK1 and SIK3 show synergistic effects
in tumor inhibition and inflammation
As the substrates of LKB1, SIK1 and SIK3 have
exhibited synergistic roles in KRAS-driven tumors (17). By using CRISPR, Hollstein et
al (17) indicated that the
tumorigenesis process is accelerated in KRAS mutated lung cancer
with concomitant loss of SIK1 and SIK3. This tumor growth promoted
ability is comparable to those loss of LKB1 expression. The
tumorigenesis process is closely associated with the effect of SIKs
on pro-inflammatory cytokines, which might be achieved by two
pathways including the direct phosphorylation of the substrates of
SIKs and the indirect influence on Toll-like receptor 4 (TLR4)
mediated cytokine production. It has been reported that the
overexpressed SIK1 and SIK3 repress the expression of NF-κβ which
is one of the downstream signals of TLR4 (136). Under the tumor microenvironment,
NF-κβ mediates the expression of a number of pro-inflammatory
cytokines including TNF-α, IL-1β and IL-6 (137-139), which can be enhanced when SIK1
and SIK3 are suppressed in tumor cells. Simultaneously, after the
phosphorylation level of CRTC2 is reduced, IL-6 signal is
upregulated in the tumor microenvironment, which increases tumor
proliferation via activating Ras/Raf/MEK/MAPK, PI3K/AKT and
JAK/STAT signaling pathways (140). IL-6 has the function of
facilitating the repair and induction of countersignaling pathways,
including antioxidant and anti-apoptotic/pro-survival signaling and
protecting cancer cells from therapy-induced DNA damage, oxidative
stress and apoptosis (140). In
addition, it has been reported that SIK inhibitor elevates IL-10
production by inducing the dephosphorylation of CRTC3 (60). Although IL-10 has been identified
as an immunosuppressive cytokine, it has been thought to promote
tumor immune escape by diminishing anti-tumor immune response in
the tumor microenvironment (141). Conversely, the recovery of either
LKB1 or SIK1/3 function can significantly reduce the expression
level of cytokines (17),
supporting the synergistic roles of SIK1 and SIK3 in inflammatory
regulation.
SIK2 and SIK3 show synergistic effect
in regulating metabolism and T cell activity
With respect to cancer development, studies on the
synergistic effect of SIK2 and SIK3 are relatively limited.
However, their similar roles in other aspects have been reported.
It is well-established that SIK2 is the major type of SIK in human
adipose tissue (142) and
stimulates tumorigenesis by upregulating the abnormal synthesis of
metabolites. The expression levels of SIK2 and SIK3 in adipose
tissue are consistent and can be downregulated by TNFα in patients
with insulin resistance (142),
indicating that these two types of SIKs might act synergistically
in regulating metabolism. In addition, T cell dysregulation is also
a crucial feature of tumorigenesis (143). Knockout of SIK3 is associated
with the reduced formation of peripheral T cells and the
constitutive knockout of SIK2 and SIK3 in the haemopoietic cells
can accelerate this reduction (144). Although the synergistic effects
of SIK2 and SIK3 in cancer formation and development have not been
extensively reported, their combined roles in processes related to
tumorigenesis have been found.
Three subtypes of SIKs exhibits
synergistic roles in regulating macrophage phenotype
Tumor-associated macrophages (TAM) are a part of
the tumor microenvironment and are usually controlled by tumor
cells to promote their growth, immune escape, angiogenesis and
metastasis (145). The roles of
M2 macrophage are similar to TAM and their polarization is under
the control of diverse cytokines in the tumor microenvironment
(145). SIK2 is the major
contributor of overall SIK activity in macrophages. Its knockout in
mice model is associated with the upregulation of
macrophage-secreted IL-10 in the tumor microenvironment and
macrophages are more prone to polarize into the M2 phenotype
(146). This process is mediated
by the activity of CREB target gene Nur77. However, the use of SIK2
inhibitors alone is insufficient to fully convert macrophages to
the M2 phenotype. Only the simultaneous blockade of SIK1, SIK2 and
SIK3 can induce mouse macrophages to be polarized into stable
anti-inflammatory phenotype and simultaneous knockout of SIK2 and
SIK3 showed a significantly stronger effect on IL-10 expression
stimulation than single knockout, indicating that three subtypes of
SIKs represent synergistic effects on the determination of
macrophage phenotype (146).
4. SIKs as the target of anti-cancer
agents
Potential applications of SIK1
activator
As SIK1 can be identified as a tumor suppressor in
most types of cancers, it is reasonable to consider that its
activator with the potential of becoming new agents for cancer
treatment. Although current studies suggest that LKB1 is the
natural SIK1 activator (1,25,85),
extra SIK1 activators can remain to be developed to inhibit tumor
development and metastasis. Based on existing investigations
(1,25,85),
it is reasonable to consider that exogenous SIK1 activators can
take a variety of forms, including the direct activation of SIK1,
enhancing the function of LKB1, or lncRNA or CircRNA that reduce
the degree of miRNA inhibition of SIK1 in tumor cells. However,
there are currently no cellular experiments or preclinical studies
focusing on additional SIK1 activators, indicating that this could
be the direction of future research on the relationship between SIK
and cancer.
Potential applications of SIK2
inhibitor
As aforementioned, SIK2 is an important cell cycle
regulator affecting tumor proliferation and metastasis (89,147). Using SIK2 inhibitors to block its
downstream signal is a theoretically feasible cancer treatment
strategy. Several SIK2 inhibitors with sharing mechanisms have been
already investigated in preclinical studies. Among them, the
effects of MRIA9 are being tested in cell lines; the single use of
HG-9-91-01, ARN3236, ARN3261, as well as their combination with
traditional chemotherapeutics are undergoing pre-clinical trials in
animal models. Additionally, the roles of combining ARN3261
(GRN300) with paclitaxel for cancer treatment are evaluated in
clinical trials (Tables I and
II). The updating progress or
promising strategies of these drugs are described following:
 | Table IPre-clinical data of SIKs inhibitors
in cell and animal models. |
Table I
Pre-clinical data of SIKs inhibitors
in cell and animal models.
| Agent | Type | Targeted
cancers | Stage | Effects |
|---|
| ARN-3236 | SIK2 inhibitor | Serous ovarian
cancer; breast cancer | Pre-clinical trials
in animal models (mice) | ARN-3236 inhibits
tumor growth and boosts the sensitivity of ovarian cancer cells to
paclitaxel; ARN-3236 enhances the olaparib-mediated inactivation of
PARP enzyme, sensitizing breast and ovarian cancer cells. |
| ARN-3261 | SIK2 inhibitor | Ovarian cancer;
breast cancer | Phase I clinical
trial | ARN-3261 inhibits
tumorigenesis and sensitizes ovarian cancer cells to carboplatin;
ARN-3261 increases the sensitivity of ovarian and breast cancer to
PARP inhibitors. |
| HG-9-91-01 | SIK2 inhibitor | Ovarian cancer | Pre-clinical trials
in animal models (mice) | HG inhibits ovarian
tumor growth and metastasis; Nap-S+HG is a SIK2-responsive compound
with less systemic toxicity. |
| MRIA9 | SIK2 inhibitor | Ovarian cancer | Pre-clinical trials
in cell lines | MRIA9 induces cell
apoptosis and enhances paclitaxel sensitivity in ovarian cancer
cells. |
| Berberine and
Emodin | SIK3 inhibitor | Breast cancer | Pre-clinical trials
in cell lines | Berberine and
Emodin exert synergistic cytotoxic potential against breast cancer
cells via SIK3 kinase. |
| OMX-0370 | SIK3 inhibitor | Colorectal cancer,
breast cancer, renal carcinoma, pancreatic carcinoma | Pre-clinical trials
incell lines | Abating the
TNF-driven NF-κB activity in tumors and enhancing the sensitivity
to TNF-induced cell death. |
| OMX-0407 | SIK3 inhibitor | Colorectal cancer,
breast cancer; lung cancer | Phase I clinical
trial | OMX-0407 blunts
TNF-mediated HDAC4/NF-κB activity in a dose-dependent manner. |
| Prostratin | SIK3 inhibitor | Breast cancer | Pre-clinical trials
in cell lines | Prostratin exerts
its anti-tumor effect by inhibiting SIK3/HDAC4-mediated cell
proliferation. |
| YKL-05-099 | SIK3 inhibitor | Acute myeloid
leukemia | Pre-clinical trials
in animal models (mice) | YKL-05-099
treatment abrogates AML progression and extends survival in two
mouse models of MLL-AF9 AML. |
 | Table IICurrently ongoing clinical trials of
oncolytic adenovirus for the treatment of prostate cancer. |
Table II
Currently ongoing clinical trials of
oncolytic adenovirus for the treatment of prostate cancer.
| Responsible Party,
year | Study title | Official title | Clinical trials
ID | Intervention | Study
description | Phase | Status | (Refs.) |
|---|
| Green3Bio, Inc,
2020 | First-in-Human
Evaluation of GRN-300 in Subjects with Recurrent Ovarian, Primary
Peritoneal and Fallopian Tube Cancers. | Ph 1/1B Evaluation
of the Safety, Pharmacokinetics and Efficacy of GRN-300, a
Salt-inducible Kinase Inhibitor, Alone and in Combination With
Paclitaxel, in Recurrent Ovarian, Primary Peritoneal and Fallopian
Tube Cancers. | NCT04711161 | GRN-300 and
Paclitaxel | This study is
divided into two parts; In Part 1, the tolerability of continuous
twice-daily oral GRN-300 will be assessed with each cycle
consisting of 28 days of treatment. Monitor the tolerability at
each dose level and incidence of dose-limiting toxicities to adjust
the number of dosing cycles.
Part 2 will test the tolerability of continuous 28-day cycles of
GRN-300 in combination with weekly paclitaxel given 3 of 4 weeks
per month (x3). | I | Active | (153) |
iOmx
Therapeutics
AG, 2023 | A Study of OMX-0407
in Patients With Previously Treated Solid Tumors That Can't be
Removed Surgically | A Phase I Dose
Escalation Study of OMX-0407 a Salt-inducible Kinase Inhibitor in
Patients With Previously Treated Unresectable Solid Tumors | NCT05826600 | OMX-0407 | Identify the
maximum tolerated dose and recommended dose for Phase II based on
adverse events of each dose level; Assess the safety and
tolerability of OMX-0407: Occurrence and severity of toxicities at
each dose level; Pharmacokinetics: Maximum observed plasma
concentration; Time of maximum observed plasma concentration; Area
under the plasma concentration-time curve from time of dosing to
the last quantifiable timepoint; Area under the plasma
concentration-time curve from time of dosing to infinity and its
percentage; Terminal elimination half-life | I | Recruiting | (159) |
MRIA9
MRIA9 is a potent pan-SIK inhibitor with a high
selectivity against SIK2 at the concentration of 1 μM
(148). MRIA9-induced SIK2
inhibition interferes with the complete separation of centrosome in
ovarian cancer cells, leading to malfunctioning mitotic spindle
assembly and G2-M transition block (90). In line with this, the mitotic
indices are significantly reduced in SKOV-3 cells treated with
MRIA9 of 1 μM compared with the control group (8 vs. 37.7%)
(90). Additionally, after a
three-week continuous treatment of low dose MRIA9 (0.5 μM),
the mean number of chromosomes was observed to increase from
47.54-76.86% in SKOV-3 cells and 58.83-71.26% in OVCAR3 cells
(90). This suggests that
MRIA9-dependent long-lasting SIK2 inactivation can also enhance the
chromosomal instability, which might be attributed to failure in
accurate positioning and aberrant transmission of genomic materials
(90). All these findings indicate
that MRIA9 has shown favorable anti-tumor mechanisms in preclinical
studies and has a promising future prospect.
ARN3236 and ARN3261
ARN-3236 and ARN 3261 are newly developed SIK2
inhibitors with similar tumor-suppressive mechanisms summarized as
follows: i) promoting centrosome uncoupling from nucleus; ii)
inhibiting centrosome splitting in cells undergoing mitosis; iii)
inducing cell cycle arrest, apoptosis and the formation of
tetraploid; and iv) attenuating SIK2/AKT/survivin pathway (149,150). These anti-tumor effects have been
validated in ovarian and breast cancer cell lines, as well as in
female athymic nude mice models, yet transposing into clinical
practice remains a challenge.
NaP-S+HG
HG-9-91-01 is a SIK2 inhibitor with a remarkable
therapeutic effect on ovarian cancer in preclinical trials.
However, its significant off-target effects limit the clinical
utility. To deal with this, an emerging compound Nap-S+HG with SIK2
responsiveness was rationally designed as a vector for HG-9-91-01
and is undergoing pre-clinical trials in animal models. Upon the
activation of SIK2, Nap-S is phosphorylated and disassembled from
HG. Then, the SIK2-responsive release of HG in turn downregulates
the overactivation of SIK2, exhibiting a stronger anti-tumor effect
in Balb/c nude mice intraperitoneally injected with SKOv3-SIK2
ovarian cancer cells (151).
Consistently, the tumor weight and ascites volume are significantly
decreased in the Nap-S+HG mice at day 8 compared with the other
three groups treated with PBS, Nap-S and HG, respectively (151). These encouraging results
indicated that NaP-S+HG with the potential of maximizing the
therapeutic effects of SIK2 inhibitor and deserves to transpose
into practice in future.
Combination therapy of SIK2
inhibitors
In breast cancer and ovarian cancer, Poly
ADP-ribose polymerase (PARP) inhibitors, paclitaxel and platinum
are the most common agents. All of them kill tumor cells by
disturbing tumor DNA structure and mitosis stabilization. Direct
using chemotherapeutic drugs in cancer cells can induce a number of
lesions including bulky platinum-DNA adducts and DNA double-strand
breaks (DSBs) (152). Currently,
an increasing number of studies place a high premium on
combinational strategy of SIK2 inhibitors and have preliminarily
demonstrated that the combination treatment is viable and effective
in preclinical settings.
MRIA9, ARN-3261 and ARN-3236 have been investigated
to increase paclitaxel sensitivity in ovarian cancer cell lines by
interfering with mitotic progression (90,149,150). Of these, ARN-3261 (GRN300) is
currently being assessed in a clinical phase I trial to find its
maximum tolerated dose or the effects when combining with
paclitaxel (153) (Table II).
ARN-3261 and ARN-3236 can boost the sensitivity of
ovarian cancer to carboplatin treatment by enhancing
carboplatin-mediated DNA damage (150). In addition, these two drugs
enhance the PARP inhibitor (Olaparib) synergistically in ovarian
cancer and triple-negative breast cancer in mice models (154). The inactivation of Class-IIa
HDAC/MEF2D pathway appears to be a key event in the synergistic
effect observed between SIK inhibitors and Olaparib: ARN-3261 and
ARN-3236 reduce the phosphorylation of Class-IIa HDACs and promote
the activity of MEF2 transcription factors, repressing the
transcription of genes involved in DNA DSB repair (150,154). Consequently, this malfunction of
DNA repair machinery contributes to chromosomal instability and
form 'synthetic lethality' with Olaparib (150).
Taken together, combination strategy is of
considerate interest for maximizing the therapeutic benefits and
further assessing the combination of SIK2 inhibitors with these
therapies should be prioritized to optimize the clinical utility of
these drugs in the near future.
Potential applications of SIK3
inhibitors
As the role of SIK3 in cancer is being continuously
clarified, insights from emerging evidence contribute to the
development of SIK3 inhibitors. Several drugs have been tested in
pre-clinical and clinical trials. The single use of prostratin,
photochemicals and OMX-0370 are tested in tumor cell lines;
YKL-05-099 and the combination of OMX-0407 with immunotherapeutic
agents are undergoing pre-clinical trials in animal models; while
the roles of OMX-0407 are being examined in a clinical trial
(Tables I and II). These trials will further provide a
rationale for advanced clinical validations and studies in
patients.
Prostratin
Prostratin, a phorbol ester natural plant compound,
was identified with the function of suppressing tumor metastasis by
targeting SIK3 (42). In a
pre-clinical study, it suppressed SIK3, HDAC4 and CXCL4
simultaneously, completely blocking the SIK3 signaling pathway in
breast cancer cell lines (42). It
has been established that SIK3 can upregulate CXCR4 to promote
tumor invasion and metastasis (155). Investigation from Alotaibi et
al (42) also confirmed this
role, in which prostratin showed higher cytotoxicity in
highly-metastatic breast cancer cell lines. Therefore, the SIK3
inhibitor prostratin could be considered a promising anti-cancer
chemotherapeutic regimen to restrain tumor metastasis.
Photochemicals
Photochemicals are also identified as anticancer
agents. They modulate deregulated signaling pathways involving
various cellular events including cell growth, metabolism and death
(156). Berberine and Emodinare
are two photochemicals targeting SIK3 in breast cancer cells. Their
combination effectively downregulates the mTOR signaling pathway
and Akt signaling pathway, blocking aerobic glycolysis and cell
cycle progression; therefore, it is considered a promising
anti-breast cancer regimen (124).
OMX-0407 and OMX-0370
OMX-0407 and OMX-0370 as first-in-class SIK3
inhibitors, has exhibited similar tumor-suppressive effects mainly
by perturbing the SIK3-HDAC4/5-NF-κB axis, which has been validated
in tumor cell lines MC38, MC38 NF-κB-luc lines, RENCA, EMT-6 and
human PANC1 (157,158). As aforementioned, the loss of
SIK3 function results in decreased phosphorylation of HDACs,
preventing its nuclear retention and abating NF-κB mediated
pro-survival gene transcription in response to TNF. Besides the
inhibitory effect on TNF-driven pro-tumorigenic NF-κB activity in
MC38 NF-κB-luc lines, SIK3 inhibitors can also re-sensitize MC38
and PANC1 tumor cells to TNF-mediated caspase activation and
apoptosis. Notably, it has been demonstrated that the tumor
suppressive capacity of OMX-0307 could be superior to anti-PD-1
antibody therapy in RENCA and EMT-6 cell lines (158). Additionally, both OMX-0407 and
-0370 can remodel the tumor microenvironment (TME) from an
immunosuppressed to a pro-inflammatory setting by reducing
regulatory T cells (T-regs) and increasing activated cytotoxic T
lymphocytes (157,158). This suggests that SIK3 inhibitors
harbor tremendous clinical potentials for monotherapy. A phase I
clinical trial of OMX-0407 is ongoing to identify the maximum
tolerated dose and profile its pharmacokinetics (159) (Table II).
YKL-05-099
YKL-05-099 is a chemosynthetic pan-SIK inhibitor
with in vitro 50% inhibitory dose of SIK3 being 30 nM
(160). Currently, the
therapeutic effects of YKL-05-099 are being tested in mice:
YKL-05-099-dependent SIK3 inhibition suppresses the AML progression
by abolishing SIK3/HDAC4/MEF2C signaling pathways (128). In addition, both
YKL-05-099-treated animals and SIK3-knockdown mice showed favorable
viability and limited toxic responses, which might be attributed to
the minimal on-target effects on the growth of normal tissues. All
these findings indicate that pharmacological inhibition of SIK3
could harbor great clinical significance in AML treatment. However,
to achieve its therapeutic significance, two imperative problems
remain to address: i) Off-target activity of YKL-05-099 for other
important cellular kinases obscures the correlation between MEF2C
addiction and the sensitivity to YKL-05-099; ii) Considering a
tumor-suppressive role of SIK3 in the context of lung cancer,
whether sustained SIK3 inhibition may play a tumorigenic role in
non-hematopoietic tissues yet requires further investigations.
Combination therapy of SIK3 inhibitors
with immunotherapy
Although the potent anti-cancer effects of OMX-0407
as a monotherapy regimen have been demonstrated, the
coadministration of these drugs with other therapies also has a
promising future. A recent study has suggested that OMX-0407 can
act synergistically in combination with anti-PD/PD-L1 immunotherapy
by sensitizing tumor cells to apoptosis and reshaping the
immunosuppressive TME in immune checkpoint inhibitor-resistant
breast cancer and lung cancer animal models (157). These two models were established
by implanting EMT6 tumor cells into the mammary fat pad of BALB/c
mice and subcutaneously injecting KLN205 tumor cells into DBA/2
mice (157). This combinational
strategy might particularly benefit patients with resistance to
currently available immune checkpoint inhibitors, thereby
possessing great clinical significance.
Combination therapy of SIK3 inhibitors
with antimitotic drugs
A previous study has shown that the depletion of
SIK3 by using siRNA exhibited the effect of prolonging mitotic
duration (100). Therefore, it is
reasonable to consider that specific SIK3 inhibitors might act
synergistically with conventional antimitotic drugs. If the
inactivation of SIK3 allows the lower doses of antimitotic drugs to
be prescribed, patients will suffer fewer side effects of the
drugs. Chen et al (161)
conducted SIK3 depletion in HELA cells and indicated that the
downregulation of SIK3 could enhance the mitotic arrest and cell
apoptosis effects of spindle poisons, including nocodazole and
Taxol. In addition, the depletion of SIK3 promotes mitotic arrest
induced by emerging types of antimitotic drugs, including those
targeting AURKA, AURKB, PLK1 and Eg5 (161).
However, SIK3 inhibitor is not suitable for all
cancers with high levels of SIK3 expression. Although the
preferential expression of SIK3 has been found in ovarian cancer
(15), trying to repress its
activity has been shown to be associated with a poor prognosis in
patients with advanced ovarian cancer: Liang et al (162) reported that stage III/IV
epithelial ovarian cancer patients with high SIK3 levels benefit
more from chemotherapy than those with lower expression levels.
This was specifically related to the upregulation of ATP-binding
cassette protein ABCG2. In addition, in NSCLC, the simultaneous
downregulation of SIK1 and SIK3 in KRAS mutant tumors accelerates
lung tumorigenesis comparably to those with loss of LKB1 (17). The most appropriate conditions for
its use remain to be explored.
As the role of SIK in cancer is being continuously
clarified, insights from emerging evidence contribute to the
development of more potent drugs. Nevertheless, most of the present
research on SIK-targeted drugs is still in the pre-clinical trial
stage and their effects have been initially investigated, which
means further studies for SIK-targeted agents are required.
5. Conclusion and future directions
The roles of SIKs are distinct in the context of
different cancers. Under most circumstances, SIK1 acts as a tumor
suppressor, inhibiting tumorigenesis and the EMT process, thereby
reducing cancer metastasis and promoting cancer apoptosis. SIK2
serves as an oncogene, promoting tumorigenesis by regulating cell
cycle and metabolism of tumor cells and enhancing the Warburg
effect. Upregulated SIK3 is mainly detected in breast cancer and
ovarian cancer and accelerates tumorigenesis by preventing cell
cycle arrest and increasing inflammatory response. However, with
the continuous in-depth exploration of SIKs, their roles in certain
tumors are in contrast to their traditional effects, indicating
that SIKs cannot be simply defined as tumor suppressors or
oncogenes. Despite the fact that the roles of SIKs are distinct in
cancer regulation, their upstream and downstream signaling
molecules have shown strong associations, suggesting that SIKs are
not the initial regulators of tumor metastasis but are located in
the central of this signaling pathway. Their targeted agents might
be a feasible option to inhibit tumor metastasis. Currently, the
inhibitors of SIK2 and SIK3 are still in the preclinical stage.
Considering their synergistic effects with chemotherapy drugs,
adding SIK inhibitors into chemotherapeutic regimens is a promosing
strategy to improve the therapeutic effect.
However, the questions about the roles of SIKs in
cancer development are not fully resolved. First, although most
studies indicated that SIK1 is a tumor suppressor, its tumor
promoting effects were also reported in MB and DSRCT (48,87).
This might be related to the difference in the occurrence and
progression of these two tumors and other cancers. The roles of
SIK1 in other neuroendocrine neoplasms similar to MB needs to be
further explored, which may improve the definition of the function
of SIK1 in tumor development. Second, SIK2 has exhibited
controversial effects in the same cancer by interacting with
different molecules. Its ultimate effect on tumor cells requires
further determination and developing new drugs to amplify its tumor
suppressive effects is a possible strategy for cancer treatment.
Third, p300 has been identified as a target of SIKs (163). Its effects on inhibiting tumor
progression and enhancing chemotherapeutic drugs have been
discussed (164,165). However, p300 was only reported as
a downstream molecule of SIK2 for metabolic regulation. Whether
SIKs can regulate cancer progression by affecting the activity of
p300 requires further investigation. Last, although the clinical
use of SIK inhibitors is theoretically possible in cancer
treatment, their defects, such as low bioavailablity, remain
unresolved and warrant further study.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Author contributions
SF, DH and LL were responsible for conceiving and
designing the present study; SF and FW drafted the manuscript. SF,
FW, HS, SC, and BW revised the manuscript critically for important
intellectual content. SF, FW, HS, SC, BW, DH and LL gave final
approval of the version to be published. Each author participated
sufficiently in the work to take public responsibility for
appropriate portions of the content; and agreed to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of any part of the work are appropriately
investigated and resolved. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Nature Science
Foundation of China (grant nos. 82360517 and 82060450), Nature
Science Foundation of Jiangxi province of China (grant nos.
20192BAB205072, 20203BBGL73206 and 20232BAB206086).
References
|
1
|
Sun Z, Jiang Q, Li J and Guo J: The potent
roles of salt-inducible kinases (SIKs) in metabolic homeostasis and
tumorigenesis. Signal Transduct Target Ther. 5:1502020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Z, Takemori H, Halder SK, Nonaka Y
and Okamoto M: Cloning of a novel kinase (SIK) of the SNF1/AMPK
family from high salt diet-treated rat adrenal. FEBS Lett.
453:135–1339. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin X, Takemori H, Katoh Y, Doi J, Horike
N, Makino A, Nonaka Y and Okamoto M: Salt-inducible kinase is
involved in the ACTH/cAMP-dependent protein kinase signaling in Y1
mouse adrenocortical tumor cells. Mol Endocrinol. 15:1264–1276.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horike N, Takemori H, Katoh Y, Doi J, Min
L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, et al:
Adipose-specific expression, phosphorylation of Ser794 in insulin
receptor substrate-1, and activation in diabetic animals of
salt-inducible kinase-2. J Biol Chem. 278:18440–1847. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoh Y, Takemori H, Horike N, Doi J,
Muraoka M, Min L and Okamoto M: Salt-inducible kinase (SIK)
isoforms: Their involvement in steroidogenesis and adipogenesis.
Mol Cell Endocrinol. 217:109–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen F, Chen L, Qin Q and Sun X:
Salt-inducible Kinase 2: An oncogenic signal transmitter and
potential target for cancer therapy. Front Oncol. 9:182019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feldman JD, Vician L, Crispino M, Hoe W,
Baudry M and Herschman HR: The salt-inducible kinase, SIK, is
induced by depolarization in brain. J Neurochem. 74:2227–2238.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Küser-Abali G, Ozcan F, Ugurlu A, Uysal A,
Fuss SH and Bugra-Bilge K: SIK2 is involved in the negative
modulation of insulin-dependent muller cell survival and implicated
in hyperglycemia-induced cell death. Invest Ophthalmol Vis Sci.
54:3526–3537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bricambert J, Miranda J, Benhamed F,
Girard J, Postic C and Dentin R: Salt-inducible kinase 2 links
transcriptional coactivator p300 phosphorylation to the prevention
of ChREBP-dependent hepatic steatosis in mice. J Clin Invest.
120:4316–4331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wein MN, Foretz M, Fisher DE, Xavier RJ
and Kronenberg HM: Salt-inducible kinases: Physiology, regulation
by cAMP, and therapeutic potential. Trends Endocrinol Metab.
29:723–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berggreen C, Henriksson E, Jones HA,
Morrice N and Göransson O: cAMP-elevation mediated by β-adrenergic
stimulation inhibits salt-inducible kinase (SIK) 3 activity in
adipocytes. Cell Signal. 24:1863–1871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Itoh Y, Sanosaka M, Fuchino H, Yahara Y,
Kumagai A, Takemoto D, Kagawa M, Doi J, Ohta M, Tsumaki N, et al:
Salt-inducible Kinase 3 signaling is important for the
gluconeogenic programs in mouse hepatocytes. J Biol Chem.
290:17879–1793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amara S, Majors C, Roy B, Hill S, Rose KL,
Myles EL and Tiriveedhi V: Critical role of SIK3 in mediating high
salt and IL-17 synergy leading to breast cancer cell proliferation.
PLoS One. 12:e01800972017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Yu J, Jia M, Ma P and Dong C:
Salt-inducible kinase 2 functions as a tumor suppressor in
hepatocellular carcinoma. Environ Toxicol. 36:2530–2540. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charoenfuprasert S, Yang YY, Lee YC, Chao
KC, Chu PY, Lai CR, Hsu KF, Chang KC, Chen YC, Chen LT, et al:
Identification of salt-inducible kinase 3 as a novel tumor antigen
associated with tumorigenesis of ovarian cancer. Oncogene.
30:3570–3584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xin L, Liu C, Liu Y, Mansel RE, Ruge F,
Davies E, Jiang WG and Martin TA: SIKs suppress tumor function and
regulate drug resistance in breast cancer. Am J Cancer Res.
11:3537–3557. 2021.PubMed/NCBI
|
|
17
|
Hollstein PE, Eichner LJ, Brun SN,
Kamireddy A, Svensson RU, Vera LI, Ross DS, Rymoff TJ, Hutchins A,
Galvez HM, et al: The AMPK-related Kinases SIK1 and SIK3 mediate
key tumor-suppressive effects of LKB1 in NSCLC. Cancer Discov.
9:1606–1627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong B, Zhang J and Yang W: Activation of
the LKB1-SIK1 signaling pathway inhibits the TGF-β-mediated
epithelial-mesenchymal transition and apoptosis resistance of
ovarian carcinoma cells. Mol Med Rep. 17:2837–2844. 2018.
|
|
19
|
Cheng H, Liu P, Wang ZC, Zou L, Santiago
S, Garbitt V, Gjoerup OV, Iglehart JD, Miron A, Richardson AL, et
al: SIK1 couples LKB1 to p53-dependent anoikis and suppresses
metastasis. Sci Signal. 2:ra352009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Xie N, Huang J, Huang H, Xu S,
Wang Z and Cai J: SIK1-LNC represses the proliferative, migrative,
and invasive abilities of lung cancer cells. Onco Targets Ther.
11:4197–4206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selvik LK, Rao S, Steigedal TS, Haltbakk
I, Misund K, Bruland T, Prestvik WS, Lægreid A and Thommesen L:
Salt-inducible kinase 1 (SIK1) is induced by gastrin and inhibits
migration of gastric adenocarcinoma cells. PLoS One. 9:e1124852014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi X, Yu X, Wang J, Bian S, Li Q, Fu F,
Zou X, Zhang L, Bast RC Jr, Lu Z, et al: SIK2 promotes ovarian
cancer cell motility and metastasis by phosphorylating MYLK. Mol
Oncol. 16:2558–2574. 2022. View Article : Google Scholar :
|
|
23
|
Sorrentino A, Menevse AN, Michels T,
Volpin V, Durst FC, Sax J, Xydia M, Hussein A, Stamova S, Spoerl S,
et al: Salt-inducible kinase 3 protects tumor cells from cytotoxic
T-cell attack by promoting TNF-induced NF-κB activation. J
Immunother Cancer. 10:e0042582022. View Article : Google Scholar
|
|
24
|
Taub M, Springate JE and Cutuli F:
Targeting of renal proximal tubule Na,K-ATPase by salt-inducible
kinase. Biochem Biophys Res Commun. 393:339–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darling NJ and Cohen P: Nuts and bolts of
the salt-inducible kinases (SIKs). Biochem J. 478:1377–1397. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakamoto K, Bultot L and Göransson O: The
Salt-inducible kinases: Emerging metabolic regulators. Trends
Endocrinol Metab. 29:827–840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lizcano JM, Göransson O, Toth R, Deak M,
Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG and
Alessi DR: LKB1 is a master kinase that activates 13 kinases of the
AMPK subfamily, including MARK/PAR-1. EMBO J. 23:833–843. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takemori H, Katoh Y, Horike N, Doi J and
Okamoto M: ACTH-induced nucleocytoplasmic translocation of
salt-inducible kinase. Implication in the protein kinase
A-activated gene transcription in mouse adrenocortical tumor cells.
J Biol Chem. 277:42334–42343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel K, Foretz M, Marion A, Campbell DG,
Gourlay R, Boudaba N, Tournier E, Titchenell P, Peggie M, Deak M,
et al: The LKB1-salt-inducible kinase pathway functions as a key
gluconeogenic suppressor in the liver. Nat Commu. 5:45352014.
View Article : Google Scholar
|
|
30
|
MacKenzie KF, Clark K, Naqvi S, McGuire
VA, Nöehren G, Kristariyanto Y, van den Bosch M, Mudaliar M,
McCarthy PC, Pattison MJ, et al: PGE(2) induces macrophage IL-10
production and a regulatory-like phenotype via a protein kinase
A-SIK-CRTC3 pathway. J Immunol. 190:565–577. 2013. View Article : Google Scholar :
|
|
31
|
Sonntag T, Vaughan JM and Montminy M:
14-3-3 proteins mediate inhibitory effects of cAMP on
salt-inducible kinases (SIKs). FEBS J. 285:467–480. 2018.
View Article : Google Scholar :
|
|
32
|
Berdeaux R, Goebel N, Banaszynski L,
Takemori H, Wandless T, Shelton GD and Montminy M: SIK1 is a class
II HDAC kinase that promotes survival of skeletal myocytes. Nat
Med. 13:597–603. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaleel M, Villa F, Deak M, Toth R,
Prescott AR, Van Aalten DM and Alessi DR: The ubiquitin-associated
domain of AMPK-related kinases regulates conformation and
LKB1-mediated phosphorylation and activation. Biochem J.
394:545–555. 2006. View Article : Google Scholar :
|
|
34
|
Al-Hakim AK, Göransson O, Deak M, Toth R,
Campbell DG, Morrice NA, Prescott AR and Alessi DR: 14-3-3
cooperates with LKB1 to regulate the activity and localization of
QSK and SIK. J Cell Sci. 118:5661–5673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bertorello AM and Zhu JK: SIK1/SOS2
networks: Decoding sodium signals via calcium-responsive protein
kinase pathways. Pflugers Arch. 458:613–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasaki T, Takemori H, Yagita Y, Terasaki
Y, Uebi T, Horike N, Takagi H, Susumu T, Teraoka H, Kusano K, et
al: SIK2 is a key regulator for neuronal survival after ischemia
via TORC1-CREB. Neuron. 69:106–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashimoto YK, Satoh T, Okamoto M and
Takemori H: Importance of autophosphorylation at Ser186 in the
A-loop of salt inducible kinase 1 for its sustained kinase
activity. J Cell Biochem. 104:1724–1739. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW
and Roach PJ: Formation of protein kinase recognition sites by
covalent modification of the substrate. Molecular mechanism for the
synergistic action of casein Kinase II and glycogen synthase kinase
3. J Biol Chem. 262:14042–14048. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koo SH, Flechner L, Qi L, Zhang X,
Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P,
et al: The CREB coactivator TORC2 is a key regulator of fasting
glucose metabolism. Nature. 437:1109–1111. 2005. View Article : Google Scholar
|
|
40
|
Liu W, Feldman JD, Machado HB, Vician LJ
and Herschman HR: Expression of depolarization-induced immediate
early gene proteins in PC12 cells. J Eur Res. 72:670–678. 2003.
|
|
41
|
Jagannath A, Butler R, Godinho SIH, Couch
Y, Brown LA, Vasudevan SR, Flanagan KC, Anthony D, Churchill GC,
Wood MJA, et al: The CRTC1-SIK1 pathway regulates entrainment of
the circadian clock. Cell. 154:1100–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alotaibi D, Amara S, Johnson TL and
Tiriveedhi V: Potential anticancer effect of prostratin through
SIK3 inhibition. Oncol Lett. 15:3252–3258. 2018.PubMed/NCBI
|
|
43
|
Panni S, Lovering RC, Porras P and Orchard
S: Non-coding RNA regulatory networks. Biochim Biophys Acta Gene
Regul Mech. 1863:1944172020. View Article : Google Scholar
|
|
44
|
Huang C, Liu J, Xu L, Hu W, Wang J, Wang M
and Yao X: MicroRNA-17 promotes cell proliferation and migration in
human colorectal cancer by downregulating SIK1. Cancer Manag Res.
11:3521–3534. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ren ZG, Dong SX, Han P and Qi J: miR-203
promotes proliferation, migration and invasion by degrading SIK1 in
pancreatic cancer. Oncol Rep. 35:1365–1374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen JL, Chen F, Zhang TT and Liu NF:
Suppression of SIK1 by miR-141 in human ovarian cancer cell lines
and tissues. Int J Mol Med. 37:1601–1610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bai X, Yang M and Xu Y: MicroRNA-373
promotes cell migration via targeting salt-inducible kinase 1
expression in melanoma. Exp Ther Med. 16:4759–4764. 2018.PubMed/NCBI
|
|
48
|
Huang S, Xue P, Han X, Zhang C, Yang L,
Liu L, Wang X, Li H, Fu J and Zhou Y: Exosomal miR-130b-3p targets
SIK1 to inhibit medulloblastoma tumorigenesis. Cell Death Dis.
11:4082020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wei S, Qi L and Wang L: Overexpression of
circ_CELSR1 facilitates paclitaxel resistance of ovarian cancer by
regulating miR-149-5p/SIK2 axis. Anticancer Drugs. 32:496–507.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun Z, Niu S, Xu F, Zhao W, Ma R and Chen
M: CircAMOTL1 promotes tumorigenesis through miR-526b/SIK2 axis in
cervical cancer. Front Cell Dev Biol. 8:5681902020. View Article : Google Scholar :
|
|
51
|
Liu Y, Gao S, Chen X, Liu M, Mao C and
Fang X: Overexpression of miR-203 sensitizes paclitaxel
(Taxol)-resistant colorectal cancer cells through targeting the
salt-inducible kinase 2 (SIK2). Tumor Biol. 37:12231–12239. 2016.
View Article : Google Scholar
|
|
52
|
Xia B, Lin M, Dong W, Chen H, Li B, Zhang
X, Hou Y and Lou G: Upregulation of miR-874-3p and miR-874-5p
inhibits epithelial ovarian cancer malignancy via SIK2. J Biochem
Mol Toxicol. 32:e221682018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li ZY, Wang XL, Dang Y, Zhu XZ, Zhang YH,
Cai BX and Zheng L: Long non-coding RNA UCA1 promotes the
progression of paclitaxel resistance in ovarian cancer by
regulating the miR-654-5p/SIK2 axis. Eur Rev Med Pharmacol Sci.
24:591–603. 2020.PubMed/NCBI
|
|
54
|
Peng J, Hou F, Zhu W, Li J and Teng Z:
lncRNA NR2F1-AS1 Regulates miR-17/SIK1 axis to suppress the
invasion and migration of cervical squamous cell carcinoma cells.
Reprod Sci. 27:1534–1539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bawa P, Zackaria S, Verma M, Gupta S,
Srivatsan R, Chaudhary B and Srinivasan S: Integrative analysis of
normal long intergenic Non-Coding RNAs in prostate cancer. PLoS
One. 10:e01221432015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang J, Lin F, Xu C and Xu Y: LINC00662
facilitates osteosarcoma progression via sponging miR-103a-3p and
regulating SIK2 expression. J Tissue Eng Regen Med. 15:1082–1091.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ and
Xu RH: Circular RNA: Metabolism, functions and interactions with
proteins. Mol Cancer. 19:1722020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jin Y and Wang H: Circ_0078607 inhibits
the progression of ovarian cancer via regulating the miR-32-5p/SIK1
network. J Ovarian Res. 15:32022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zang X, Jiang J, Gu J, Chen Y, Wang M,
Zhang Y, Fu M, Shi H, Cai H, Qian H, et al: Circular RNA EIF4G3
suppresses gastric cancer progression through inhibition of
β-catenin by promoting δ-catenin ubiquitin degradation and
upregulating SIK1. Mol Cancer. 21:1412022. View Article : Google Scholar
|
|
60
|
Clark K, MacKenzie KF, Petkevicius K,
Kristariyanto Y, Zhang J, Choi HG, Peggie M, Plater L, Pedrioli PG,
McIver E, et al: Phosphorylation of CRTC3 by the salt-inducible
kinases controls the interconversion of classically activated and
regulatory macrophages. Proc Natl Acad Sci USA. 109:16986–16991.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Screaton RA, Conkright MD, Katoh Y, Best
JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR III,
Takemori H, et al: The CREB coactivator TORC2 functions as a
calcium- and cAMP-sensitive coincidence detector. Cell. 119:61–74.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Altarejos JY and Montminy M: CREB and the
CRTC co-activators: Sensors for hormonal and metabolic signals.
Natu Rev Mol Cell Biol. 12:141–151. 2011. View Article : Google Scholar
|
|
63
|
Henriksson E, Jones HA, Patel K, Peggie M,
Morrice N, Sakamoto K and Göransson O: The AMPK-related kinase SIK2
is regulated by cAMP via phosphorylation at Ser358 in adipocytes.
Biochemical J. 444:503–514. 2012. View Article : Google Scholar
|
|
64
|
Luo Q, Viste K, Urday-Zaa JC, Senthil
Kumar G, Tsai WW, Talai A, Mayo KE, Montminy M and Radhakrishnan I:
Mechanism of CREB recognition and coactivation by the
CREB-regulated transcriptional coactivator CRTC2. Proc Natl Acad
Sci USA. 109:20865–20870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
van der Linden AM, Nolan KM and Sengupta
P: KIN-29 SIK regulates chemoreceptor gene expression via an MEF2
transcription factor and a class II HDAC. EMBO J. 26:358–370. 2007.
View Article : Google Scholar
|
|
66
|
Chan JK, Sun L, Yang XJ, Zhu G and Wu Z:
Functional characterization of an amino-terminal region of HDAC4
that possesses MEF2 binding and transcriptional repressive
activity. J Biol Chem. 278:23515–23521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Alessi DR, Sakamoto K and Bayascas JR:
LKB1-dependent signaling pathways. Ann Rev Biochemistry.
75:137–163. 2006. View Article : Google Scholar
|
|
68
|
Ponnusamy L and Manoharan R: Distinctive
role of SIK1 and SIK3 isoforms in aerobic glycolysis and cell
growth of breast cancer through the regulation of p53 and mTOR
signaling pathways. Biochim Biophys Acta Mol Cell Res.
1868:1189752021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Schwartzenberg-Bar-Yoseph F, Armoni M and
Karnieli E: The tumor suppressor p53 down-regulates glucose
transporters GLUT1 and GLUT4 gene expression. Cancer Res.
64:2627–2633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kowanetz M, Lönn P, Vanlandewijck M,
Kowanetz K, Heldin CH and Moustakas A: TGFbeta induces SIK to
negatively regulate type I receptor kinase signaling. J Cell Biol.
182:655–662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yao YH, Cui Y, Qiu XN, Zhang LZ, Zhang W,
Li H and Yu JM: Attenuated LKB1-SIK1 signaling promotes
epithelial-mesenchymal transition and radioresistance of non-small
cell lung cancer cells. Chin J Cancer. 35:502016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sánchez-Tilló E, Siles L, de Barrios O,
Cuatrecasas M, Vaquero EC, Castells A and Postigo A: Expanding
roles of ZEB factors in tumorigenesis and tumor progression. Am J
Cancer Res. 1:897–912. 2011.PubMed/NCBI
|
|
73
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gradek F, Lopez-Charcas O, Chadet S,
Poisson L, Ouldamer L, Goupille C, Jourdan ML, Chevalier S,
Moussata D, Besson P and Roger S: Sodium channel Nav1.5
controls epithelial-to-mesenchymal transition and invasiveness in
breast cancer cells through its regulation by the Salt-inducible
Kinase-1. Sci Rep. 9:186522019. View Article : Google Scholar
|
|
75
|
Cameron IL, Smith NK, Pool TB and Sparks
RL: Intracellular concentration of sodium and other elements as
related to mitogenesis and oncogenesis in vivo. Cancer Res.
40:1493–1500. 1980.PubMed/NCBI
|
|
76
|
Yang M, Kozminski DJ, Wold LA, Modak R,
Calhoun JD, Isom LL and Brackenbury WJ: Therapeutic potential for
phenytoin: Targeting Na(v)1.5 sodium channels to reduce migration
and invasion in metastatic breast cancer. Breast Cancer Res Treat.
134:603–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fraser SP, Diss JK, Chioni AM, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–5389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nelson M, Yang M, Millican-Slater R and
Brackenbury WJ: Nav1.5 regulates breast tumor growth and metastatic
dissemination in vivo. Oncotarget. 6:32914–32929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lei Y, Chen L, Zhang G, Shan A, Ye C,
Liang B, Sun J, Liao X, Zhu C, Chen Y, et al: MicroRNAs target the
Wnt/β-catenin signaling pathway to regulate epithelial-mesenchymal
transition in cancer (Review). Oncol Rep. 44:1299–1313.
2020.PubMed/NCBI
|
|
80
|
Chen L, Mai W, Chen M, Hu J, Zhuo Z, Lei
X, Deng L, Liu J, Yao N, Huang M, et al: Arenobufagin inhibits
prostate cancer epithelial-mesenchymal transition and metastasis by
down-regulating β-catenin. Pharmacol Res. 123:130–142. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Qu C, He D, Lu X, Dong L, Zhu Y, Zhao Q,
Jiang X, Chang P, Jiang X, Wang L, et al: Salt-inducible Kinase
(SIK1) regulates HCC progression and WNT/beta-catenin activation. J
Hepatol. 64:1076–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Qu C and Qu Y: Down-regulation of
salt-inducible kinase 1 (SIK1) is mediated by RNF2 in
hepatocarcinogenesis. Oncotarget. 8:3144–3155. 2017. View Article : Google Scholar :
|
|
83
|
Gajula RP, Chettiar ST, Williams RD,
Thiyagarajan S, Kato Y, Aziz K, Wang R, Gandhi N, Wild AT, Vesuna
F, et al: The twist box domain is required for Twist1-induced
prostate cancer metastasis. Mol Cancer Res. 11:1387–1400. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhu QQ, Ma C, Wang Q, Song Y and Lv T: The
role of TWIST1 in epithelial-mesenchymal transition and cancers.
Tumor Biol. 37:185–197. 2016. View Article : Google Scholar
|
|
85
|
Du WQ, Zheng JN and Pei DS: The diverse
oncogenic and tumor suppressor roles of salt-inducible kinase (SIK)
in cancer. Expert Opin Ther Targets. 20:477–485. 2016. View Article : Google Scholar
|
|
86
|
Fu X, Tang Y, Wu W, Ouyang Y, Tan D and
Huang Y: Exosomal microRNA-25 released from cancer cells targets
SIK1 to promote hepatocellular carcinoma tumorigenesis. Dig Liver
Dis. 54:954–963. 2022. View Article : Google Scholar
|
|
87
|
Hartono AB, Kang HJ, Shi L, Phipps W,
Ungerleider N, Giardina A, Chen W, Spraggon L, Somwar R, Moroz K,
et al: Salt-Inducible Kinase 1 is a potential therapeutic target in
desmoplastic small round cell tumor. Oncogenesis. 11:182022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Joshi K, Shah VJ and Maddika S: GINS
complex protein Sld5 recruits SIK1 to activate MCM helicase during
DNA replication. Cell Signal. 28:1852–1862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ahmed AA, Lu Z, Jennings NB,
Etemadmoghadam D, Capalbo L, Jacamo RO, Barbosa-Morais N, Le XF;
Australian Ovarian Cancer Study Group; Vivas-Mejia P, et al: SIK2
is a centrosome kinase required for bipolar mitotic spindle
formation that provides a potential target for therapy in ovarian
cancer. Cancer Cell. 18:109–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Raab M, Rak M, Tesch R, Gasimli K, Becker
S, Knapp S, Strebhardt K and Sanhaji M: The small-molecule
inhibitor MRIA9 reveals novel insights into the cell cycle roles of
SIK2 in ovarian cancer cells. Cancers (Basel). 13:36582021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun XP, Dong X, Lin L, Jiang X, Wei Z,
Zhai B, Sun B, Zhang Q, Wang X, Jiang H, et al: Up-regulation of
survivin by AKT and hypoxia-inducible factor 1α contributes to
cisplatin resistance in gastric cancer. FEBS J. 281:115–128. 2014.
View Article : Google Scholar
|
|
92
|
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ,
Tognin S, Marchisio PC, Symons M and Altieri DC: Regulation of
microtubule stability and mitotic progression by survivin. Cancer
Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
93
|
Shojaei F, Yazdani-Nafchi F,
Banitalebi-Dehkordi M, Chehelgerdi M and Khorramian-Ghahfarokhi M:
Trace of survivin in cancer. Eur J Cancer Prevention. 28:365–372.
2019. View Article : Google Scholar
|
|
94
|
Vader G, Kauw JJ, Medema RH and Lens SM:
Survivin mediates targeting of the chromosomal passenger complex to
the centromere and midbody. EMBO Rep. 7:85–92. 2006. View Article : Google Scholar
|
|
95
|
Ryan BM, O'Donovan N and Duffy MJ:
Survivin: A new target for anti-cancer therapy. Cancer Treatment
Rev. 35:553–562. 2009. View Article : Google Scholar
|
|
96
|
Bon H, Wadhwa K, Schreiner A, Osborne M,
Carroll T, Ramos-Montoya A, Ross-Adams H, Visser M, Hoffmann R,
Ahmed AA, et al: Salt-inducible kinase 2 regulates mitotic
progression and transcription in prostate cancer. Mol Cancer Res.
13:620–635. 2015. View Article : Google Scholar :
|
|
97
|
Nagel S, Leich E, Quentmeier H, Meyer C,
Kaufmann M, Zaborski M, Rosenwald A, Drexler HG and Macleod RA:
Amplification at 11q23 targets protein kinase SIK2 in diffuse large
B-cell lymphoma. Leuk Lymphoma. 51:881–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lu F, Zheng Y, Donkor PO, Zou P and Mu P:
Downregulation of CREB promotes cell proliferation by mediating
G1/S phase transition in hodgkin lymphoma. Oncol Res. 24:171–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Melnikova VO, Dobroff AS, Zigler M,
Villares GJ, Braeuer RR, Wang H, Huang L and Bar-Eli M: CREB
inhibits AP-2alpha expression to regulate the malignant phenotype
of melanoma. PLoS One. 5:e124522010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wehr MC, Holder MV, Gailite I, Saunders
RE, Maile TM, Ciirdaeva E, Instrell R, Jiang M, Howell M, Rossner
MJ and Tapon N: Salt-inducible kinases regulate growth through the
Hippo signaling pathway in Drosophila. Nat Cell Biol. 15:61–71.
2013. View Article : Google Scholar :
|
|
101
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Brugge JS and Haber DA: Transforming properties of
YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc
Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fukushi A, Kim HD, Chang YC and Kim CH:
Revisited metabolic control and reprogramming cancers by means of
the warburg effect in tumor cells. Int J Mol Sci. 23:100372022.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gao T, Zhang X, Zhao J, Zhou F, Wang Y,
Zhao Z, Xing J, Chen B, Li J and Liu S: SIK2 promotes reprogramming
of glucose metabolism through PI3K/AKT/HIF-1alpha pathway and
Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett.
469:89–101. 2020. View Article : Google Scholar
|
|
104
|
Röhrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Corbet C and Feron O: Emerging roles of
lipid metabolism in cancer progression. Curr Opin Clin Nutr Metab
Care. 20:254–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ni X, Feng Y and Fu X: Role of
saltinducible kinase 2 in the malignant behavior and glycolysis of
colorectal cancer cells. Mol Med Rep. 24:8222021. View Article : Google Scholar
|
|
107
|
Qi ZX, Cai JJ, Chen LC, Yue Q, Gong Y, Yao
Y and Mao Y: TRIM28 as an independent prognostic marker plays
critical roles in glioma progression. J Neurooncol. 126:19–26.
2016. View Article : Google Scholar
|
|
108
|
Miranda F, Mannion D, Liu S, Zheng Y,
Mangala LS, Redondo C, Herrero-Gonzalez S, Xu R, Taylor C, Chedom
DF, et al: Salt-inducible kinase 2 couples ovarian cancer cell
metabolism with survival at the adipocyte-rich metastatic niche.
Cancer Cell. 30:273–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhao J, Zhang X, Gao T, Wang S, Hou Y,
Yuan P, Yang Y, Yang T, Xing J, Li J and Liu S: SIK2 enhances
synthesis of fatty acid and cholesterol in ovarian cancer cells and
tumor growth through PI3K/Akt signaling pathway. Cell Death Dis.
11:252020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Martinez Calejman C, Trefely S, Entwisle
SW, Luciano A, Jung SM, Hsiao W, Torres A, Hung CM, Li H, Snyder
NW, et al: mTORC2-AKT signaling to ATP-citrate lyase drives brown
adipogenesis and de novo lipogenesis. Nat Commun. 11:5752020.
View Article : Google Scholar :
|
|
111
|
Zhang MX, Wang H and Sun GP:
Tumor-suppressor Fbxw7 targets SIK2 for degradation to interfere
with TORC2-AKT signaling in pancreatic cancer. Cell Biol Int.
44:1900–1910. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Airley RE, McHugh P, Evans AR, Harris B,
Winchester L, Buffa FM, Al-Tameemi W, Leek R and Harris AL: Role of
carbohydrate response element-binding protein (ChREBP) in
generating an aerobic metabolic phenotype and in breast cancer
progression. Br J Cancer. 110:715–723. 2014. View Article : Google Scholar :
|
|
113
|
Dai XM, Zhang YH, Lin XH, Huang XX, Zhang
Y, Xue CR, Chen WN, Ye JX, Lin XJ and Lin X: SIK2 represses
AKT/GSK3β/β-catenin signaling and suppresses gastric cancer by
inhibiting autophagic degradation of protein phosphatases. Mol
Oncol. 15:228–245. 2021. View Article : Google Scholar
|
|
114
|
Rong Z, Zhang L, Li Z, Xiao Z, Duan Y, Ren
X, Zi Y, Gao J, Mu Y, Guan Y, et al: SIK2 maintains breast cancer
stemness by phosphorylating LRP6 and activating Wnt/β-catenin
signaling. Oncogene. 41:2390–2403. 2022. View Article : Google Scholar
|
|
115
|
Dittmer J: Breast cancer stem cells:
Features, key drivers and treatment options. Semin Cancer Biol.
53:59–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Raisch J, Côté-Biron A and Rivard N: A
Role for the WNT Co-receptor LRP6 in pathogenesis and therapy of
epithelial cancers. Cancers (Basel). 11:11622019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jackson HW, Fischer JR, Zanotelli VRT, Ali
HR, Mechera R, Soysal SD, Moch H, Muenst S, Varga Z, Weber WP and
Bodenmiller B: The single-cell pathology landscape of breast
cancer. Nature. 578:615–620. 2020. View Article : Google Scholar
|
|
119
|
Maxfield KE, Macion J, Vankayalapati H and
Whitehurst AW: SIK2 Restricts autophagic flux to support
triple-negative breast cancer survival. Mol Cell Biol.
36:3048–3057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Pradeep S, Kim SW, Wu SY, Nishimura M,
Chaluvally-Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ,
Bischoff FZ, et al: Hematogenous metastasis of ovarian cancer:
Rethinking mode of spread. Cancer Cell. 26:77–91. 2014. View Article : Google Scholar :
|
|
121
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the Theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou Q, Gensch C and Liao JK:
Rho-associated coiled-coil-forming kinases (ROCKs): Potential
targets for the treatment of atherosclerosis and vascular disease.
Trends Pharmacol Sci. 32:167–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Madsen CD, Hooper S, Tozluoglu M,
Bruckbauer A, Fletcher G, Erler JT, Bates PA, Thompson B and Sahai
E: STRIPAK components determine mode of cancer cell migration and
metastasis. Nat Cell Biol. 17:68–80. 2015. View Article : Google Scholar
|
|
124
|
Ponnusamy L, Kothandan G and Manoharan R:
Berberine and Emodin abrogates breast cancer growth and facilitates
apoptosis through inactivation of SIK3-induced mTOR and Akt
signaling pathway. Biochim Biophys Acta Mol Basis Dis.
1866:1658972020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH,
Kong HK, Park EY, Kim HK, Han J, Chang M and Park JH: Inhibition of
aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores
tamoxifen sensitivity in antiestrogen-resistant breast cancer
cells. PLoS One. 10:e01322852015. View Article : Google Scholar
|
|
127
|
Tarumoto Y, Lu B, Somerville TDD, Huang
YH, Milazzo JP, Wu XS, Klingbeil O, El Demerdash O, Shi J and Vakoc
CR: LKB1, Salt-inducible kinases, and MEF2C are linked dependencies
in acute myeloid leukemia. Mol Cell. 69:1017–1027.e6. 2018.
View Article : Google Scholar :
|
|
128
|
Tarumoto Y, Lin S, Wang J, Milazzo JP, Xu
Y, Lu B, Yang Z, Wei Y, Polyanskaya S, Wunderlich M, et al:
Salt-inducible kinase inhibition suppresses acute myeloid leukemia
progression in vivo. Blood. 135:56–70. 2020. View Article : Google Scholar :
|
|
129
|
Crusz SM and Balkwill FR: Inflammation and
cancer: Advances and new agents. Nat Rev Clin Oncol. 12:584–596.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Stubbins RJ, Platzbecker U and Karsan A:
Inflammation and myeloid malignancy: Quenching the flame. Blood.
140:1067–1074. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Diakos CI, Charles KA, McMillan DC and
Clarke SJ: Cancer-related inflammation and treatment effectiveness.
Lancet Oncol. 15:e493–e503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Candido J and Hagemann T: Cancer-related
inflammation. J Clin Immunol. 33(Suppl 1): S79–S84. 2013.
View Article : Google Scholar
|
|
134
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S,
Jin Q, Li B, Yao F and Jin F: CXCL12-CXCR4 axis promotes the
natural selection of breast cancer cell metastasis. Tumor Biol.
35:7765–7773. 2014. View Article : Google Scholar
|
|
136
|
Yong Kim S, Jeong S, Chah KH, Jung E, Baek
KH, Kim ST, Shim JH, Chun E and Lee KY: Salt-inducible kinases 1
and 3 negatively regulate Toll-like receptor 4-mediated signal. Mol
Endocrinol. 27:1958–1968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Janssens S and Beyaert R: Role of
Toll-like receptors in pathogen recognition. Clin Microbiol Rev.
16:637–646. 2003. View Article : Google Scholar :
|
|
138
|
Kim SY, Jeong S, Jung E, Baik KH, Chang
MH, Kim SA, Shim JH, Chun E and Lee KY: AMP-activated protein
kinase-α1 as an activating kinase of TGF-β-activated kinase 1 has a
key role in inflammatory signals. Cell Death Dis. 3:e3572012.
View Article : Google Scholar
|
|
139
|
West AP, Koblansky AA and Ghosh S:
Recognition and signaling by toll-like receptors. Ann Rev Cell Dev
Biol. 22:409–437. 2006. View Article : Google Scholar
|
|
140
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumor Biol. 37:11553–11572. 2016. View Article : Google Scholar
|
|
141
|
Mannino MH, Zhu Z, Xiao H, Bai Q,
Wakefield MR and Fang Y: The paradoxical role of IL-10 in immunity
and cancer. Cancer Lett. 367:103–107. 2015. View Article : Google Scholar
|
|
142
|
Sall J, Pettersson AM, Bjork C, Henriksson
E, Wasserstrom S, Linder W, Zhou Y, Hansson O, Andersson DP,
Ekelund M, et al: Salt-inducible kinase 2 and -3 are downregulated
in adipose tissue from obese or insulin-resistant individuals:
Implications for insulin signaling and glucose uptake in human
adipocytes. Diabetologia. 60:314–323. 2017. View Article : Google Scholar
|
|
143
|
Thommen DS and Schumacher TN: T cell
dysfunction in cancer. Cancer Cell. 33:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Nefla M, Darling NJ, van Gijsel Bonnello
M, Cohen P and Arthur JSC: Salt inducible kinases 2 and 3 are
required for thymic T cell development. Sci Rep. 11:215502021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020.
View Article : Google Scholar
|
|
146
|
Darling NJ, Toth R, Arthur JS and Clark K:
Inhibition of SIK2 and SIK3 during differentiation enhances the
anti-inflammatory phenotype of macrophages. Biochem J. 474:521–537.
2017. View Article : Google Scholar
|
|
147
|
Di Giorgio E and Brancolini C: Regulation
of class IIa HDAC activities: It is not only matter of subcellular
localization. Epigenomics. 8:251–269. 2016. View Article : Google Scholar
|
|
148
|
Tesch R, Rak M, Raab M, Berger LM,
Kronenberger T, Joerger AC, Berger BT, Abdi I, Hanke T, Poso A, et
al: Structure-based design of selective salt-inducible kinase
inhibitors. J Med Chem. 64:8142–8160. 2021. View Article : Google Scholar
|
|
149
|
Zhou J, Alfraidi A, Zhang S,
Santiago-O'Farrill JM, Yerramreddy Reddy VK, Alsaadi A, Ahmed AA,
Yang H, Liu J, Mao W, et al: A novel compound ARN-3236 inhibits
salt-inducible Kinase 2 and sensitizes ovarian cancer cell lines
and xenografts to paclitaxel. Clin Cancer Res. 23:1945–1954. 2017.
View Article : Google Scholar
|
|
150
|
Fan D, Yang H, Mao W, Rask PJ, Pang L, Xu
C, Vankayalapat H, Ahmed AA, Bast RC Jr and Lu Z: A novel salt
inducible kinase 2 inhibitor, ARN-3261, sensitizes ovarian cancer
cell lines and xenografts to carboplatin. Cancers (Basel).
13:4462021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hua Y, Yin H, Liu X, Xie J, Zhan W, Liang
G and Shen Y: Salt-inducible kinase 2-triggered release of its
inhibitor from hydrogel to suppress ovarian cancer metastasis. Adv
Sci (Weinh). 9:e22022602022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Dungl DA, Maginn EN and Stronach EA:
Preventing damage limitation: Targeting DNA-PKcs and DNA
double-strand break repair pathways for ovarian cancer therapy.
Front Oncol. 5:2402015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Green3Bio I: First-in-Human Evaluation of
GRN-300 in Subjects With Recurrent Ovarian, Primary Peritoneal, and
Fallopian Tube Cancers. Available from: https://clinicaltrials.gov/study/NCT04711161.
|
|
154
|
Lu Z, Mao W, Yang H, Santiago-O'Farrill
JM, Rask PJ, Mondal J, Chen H, Ivan C, Liu X, Liu CG, et al: SIK2
inhibition enhances PARP inhibitor activity synergistically in
ovarian and triple-negative breast cancers. J Clin Invest.
132:e1464712022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Xu C, Zhao H, Chen H and Yao Q: CXCR4 in
breast cancer: Oncogenic role and therapeutic targeting. Drug
Design Dev Ther. 9:4953–4964. 2015.
|
|
156
|
Ponnusamy L, Natarajan SR, Thangaraj K and
Manoharan R: Therapeutic aspects of AMPK in breast cancer:
Progress, challenges, and future directions. Biochim Biophys Acta
Rev Cancer. 1874:1883792020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hartl C, Maser IP, Michels T, Milde R,
Klein V, Beckhove P, Khandelwal N, Loferer H and Bissinger S:
Abstract 3708: OMX-0407, a highly potent SIK3 inhibitor, sensitizes
tumor cells to cell death and eradicates tumors in combination with
PD-1 inhibition. Cancer Res. 82(12_Suppl): S37082022. View Article : Google Scholar
|
|
158
|
Michels T, Bissinger S, Sennhenn P,
Loferer H, Freire CM, Reidell O, Meier-Ewert S, Papadimitriou A,
Beckhove P and Khandelwal N: Abstract 6698: A first-in-class SIK3
inhibitor, OMX-0370, effectively inhibits tumor growth in syngeneic
tumor models, as single agent, by abolishing tumor resistance to
immune-derived TNF. Cancer Res. 80(16_Suppl): S66982020. View Article : Google Scholar
|
|
159
|
AG iT: A Study of OMX-0407 in patients
with Previously Treated Solid Tumors That Can't be Removed
Surgically. Available from: https://clinicaltrials.gov/study/NCT05826600.
|
|
160
|
Sundberg TB, Liang Y, Wu H, Choi HG, Kim
ND, Sim T, Johannessen L, Petrone A, Khor B, Graham DB, et al:
Development of chemical probes for investigation of salt-inducible
kinase function in vivo. ACS Chem Biol. 11:2105–2111. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen H, Huang S, Han X, Zhang J, Shan C,
Tsang YH, Ma HT and Poon RY: Salt-inducible kinase 3 is a novel
mitotic regulator and a target for enhancing antimitotic
therapeutic-mediated cell death. Cell Death Dis. 5:e11772014.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Liang YL, Wu CH, Kang CY, Lin CN, Shih NY,
Lin SH, Chen YC and Hsu KF: Downregulated Salt-inducible Kinase 3
expression promotes chemoresistance in serous ovarian cancer via
the ATP-binding cassette protein ABCG2. J Cancer. 10:6025–6036.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumor suppression. Nat
Rev Cancer. 9:563–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Cai LY, Chen SJ, Xiao SH, Sun QJ, Ding CH,
Zheng BN, Zhu XY, Liu SQ, Yang F, Yang YX, et al: Targeting
p300/CBP attenuates hepatocellular carcinoma progression through
epigenetic regulation of metabolism. Cancer Res. 81:860–872. 2021.
View Article : Google Scholar
|
|
165
|
Ono H, Basson MD and Ito H: P300
inhibition enhances gemcitabine-induced apoptosis of pancreatic
cancer. Oncotarget. 7:51301–51310. 2016. View Article : Google Scholar : PubMed/NCBI
|