Introduction
Numerous antimicrobial agents in consumer and
personal care products, including triclosan, triclocarban,
benzalkonium chloride, benzethonium chloride and chloroxylenol,
have become ubiquitous contaminants in the environment (1,2).
Human exposure to these contaminants has gained public attention
due to their biological effects such as inflammatory responses and
genotoxic effects on mammals (3).
However, the potential health risks and biological activities of
these compounds to humans and animals remain largely unknown.
Evaluation of the biological features and environmental
implications of these contaminants is necessary (4,5).
Colorectal cancer (CRC) is the third most commonly
diagnosed malignancy and the fourth leading cause of cancer death
worldwide, with an estimated 52,550 deaths caused by colorectal
cancer in 2023 (6). Although
clinical diagnosis and comprehensive treatment including
colonoscopy screening and targeted therapeutics in combination with
chemotherapy (7,8) have greatly improved, the prognosis of
patients with metastasis remains poor, with a five-year survival
rate only 14% (9). Accumulating
evidence suggests that cancer stem cells (CSCs) serve an important
role in tumorigenesis, drug resistance, metastasis and recurrence
through internal molecular mechanisms (10-13).
CSCs are a subset of cancer cells with self-renewal ability and
pose a preferentially high risk of drug resistance and recurrence
(10,14). Colorectal CSCs are defined by
certain specific markers such as Leucine-rich G protein-coupled
receptor-5 (LGR5), CD133, sex determining region Y-box 2 (SOX2) and
CD44 (15,16). Therefore, drugs targeting these
CSCs might effectively improve the clinical outcome of patients
(17). The canonical Wnt/β-catenin
signaling pathway is required for the occurrence of CRC and the
maintenance of colorectal CSCs (18).
The evolutionarily conserved Wnt/β-catenin signaling
pathway serves a fundamental role in determining cell
differentiation, proliferation and death, and participates in the
regulation of embryonic development and tissue homeostasis
(19). Dysregulation of the Wnt
signaling cascade has been linked to the pathogenesis of certain
diseases, such as human birth defects and cancers such as CRC,
melanoma and ovarian cancer (20,21).
Research on genetic mutations has indicated that ~93% of patients
with CRC carry somatic mutations in the genes encoding Wnt
signaling components, including biallelic inactivation of
adenomatous polyposis coli (APC), activating mutations in CTNNB1
and inactivating mutations in RNF43. Moreover, overexpression of
the Wnt receptors Frizzled (FZD) and low-density lipoprotein
receptor-related protein 5/6 (LRP5/6) is reported in CRC patients
(22-24). Due to the high mutation rate of Wnt
signaling pathway components and the pivotal role of Wnt signaling
to support the CSC niche and plasticity in human CRCs, the
development of small molecular inhibitors targeting the
Wnt/β-catenin signaling pathway may have potential therapeutic
effects in CRC (25).
In the Wnt/β-catenin signaling cascade, the
transcriptional coactivator β-catenin is a kernel component of the
pathway with its protein level and activity closely controlled by a
cytoplasmic destruction complex consisting of APC, Axin, casein
kinase 1 (CK1) and glycogen synthase kinase 3β (20). Binding of Wnt ligands to the FZD
receptors and coreceptor LRP5/6 is deemed to be the initiation of
the signal transduction pathway (26). This binding triggers the
phosphorylation and recruitment of Disheveled (DVL) by activated
CK1. DVL in turn inactivates the β-catenin destruction complex
(27). Subsequently,
depolymerization of the destruction complex renders it incapable of
phosphorylating β-catenin, which leads to cytoplasmic β-catenin no
longer being ubiquitinated and degraded through the
ubiquitin-proteasome pathway (28). Unphosphorylated active β-catenin
accumulates in the cytosol and translocates into the nucleus,
interacting with transcription factors T cell-specific
factor/lymphoid enhancer-binding factor (TCF/LEF) to activate
transcription of downstream Wnt target genes such as c-Myc, cyclin
D1, Survivin, LGR5 and Axin2 (29,30).
Chloroxylenol (para-chloro-meta-xylenol, PCMX) is a
halogenated phenol widely used in antiseptic, disinfectant and
cosmetic products (1). As the
principal active ingredient of commonly used antiseptics,
chloroxylenol exhibits its bactericidal activity primarily against
Gram positive bacteria, which could be associated with its ability
to decrease membrane fluidity and to interfere with the function of
membrane proteins (31,32). Previous studies have reported that
chloroxylenol could induce phase-transition of the cell membrane,
changing it from a liquid-crystalline to a liquid-ordered phase,
with increased susceptibility for bacterial membranes (31,33).
Nevertheless, the anticancer effect of chloroxylenol and its
underlying mechanisms are still unknown. In the present study, it
was demonstrated that chloroxylenol could suppress the
Wnt/β-catenin signaling pathway by inhibiting the nuclear
translocation of β-catenin and blocking of the β-catenin/T-cell
factor 4 interaction, thus exerting anticancer activity in CRC
cells. The results demonstrated that chloroxylenol might be a novel
Wnt/β-catenin signaling inhibitor with anticancer efficacy.
Materials and methods
Reagents and plasmids
Chloroxylenol was purchased from Sigma-Aldrich
(Merck KGaA) and dissolved in dimethyl sulfoxide (DMSO). The
SuperTOPFlash reporter vector was donated by Dr Karl Willert
(University of California at San Diego, USA). Activator protein
1-Luc (AP1-Luc) and nuclear factor of activated T cells-Luc
(NFAT-Luc) reporters were purchased from BD Biosciences. The
expression plasmids encoding β-catenin, β-catenin 4A, TCF4E and
β-galactosidase (β-gal) and pDKK4-Luc reporter have been described
previously (34).
Cell culture
The human embryonic kidney 293T cells, colorectal
cancer HCT116 and SW480 cell lines, and mouse colon cancer MC38
cells were purchased from the American Type Culture Collection.
Cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM;
Thermo Fisher Scientific, Inc.) supplemented with 1%
penicillin-streptomycin and 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C
with 5% CO2. The cultured cells were tested for
mycoplasma every month to ensure that they are not contaminated
using a luciferase mycoplasma detection kit (cat. no. FM301-02-V2,
Transgen, Inc.). The authenticity of the cell lines was confirmed
using STR profiling.
Transfection and luciferase reporter gene
assays
For the luciferase reporter assays, 293T cells were
transferred to 24-well plates pre-coated with 1% poly-D-lysine at
37°C for 24 h, prior to use for the SuperTopFlash or DKK4-Luc
assays. Colorectal cancer cells (HCT116 and SW480) were transfected
with 0.25 μg SuperTOPFlash or SuperFOPFlash (negative
control) reporters along with control plasmid pCMXβgal (0.05
μg) in three replicates. 293T cells were transiently
transfected with SuperTOPFlash reporter (0.25 μg) or
pDKK4-Luc (0.25 μg) reporter along with transfection control
plasmid pCMXβgal (0.05 μg), and β-catenin, β-catenin 4A,
β-catenin 4A/TCF4E or pcDNA3.1 (0.15 μg) expression
plasmids. The promoter region of SuperTOPflash reporter gene
contained 8 duplicate TCF/LEF transcription factor binding sites
and the expression level of luciferase represented the activation
level of the Wnt signaling pathway. SuperFOPFlash is a negative
control reporter gene of SuperTOPFlash. It was constructed by
inserting 8 duplicate mutant TCF/LEF binding site sequences into
its polyclonal sites. Cell lysates were used for SuperTOPFlash or
SuperFOPFlash luciferase activity assessment after 24 h drug
treatment using a Luciferase assay kit (cat. no. E1501; Promega
Corporation). The luciferase values were normalized to
β-galactosidase (β-gal) activity following detection using a
Gal-Screen kit (cat. no. T1028; Thermo Fisher Scientific, Inc.)
according to the manufacture's protocol. For immunoprecipitation
experiments with 293T cells, transfections were performed in 10 cm
dishes using 1 μg of pcDNA or expression plasmids encoding
β-catenin and TCF4E. Following chloroxylenol treatment, protein
collection was performed. All transfections were performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols at 37°C
for 24 h. Twenty four hours after transfection, cells were treated
with a range of concentrations of antimicrobial agents and assessed
with subsequent assays.
Immunoblotting
Colorectal cancer HCT116 and SW480 cells were
initially collected with a scraper and then centrifuged at 150 × g
at 4°C for 5 min and lysed in lysis buffer consisting of 20 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 2.5 mM sodium pyrophosphate, 1 mM
EDTA, 1 mM EGTA, 1 mM PMSF, 1% Triton X-100, 1 mM β-glycerol
phosphate, 1 mM sodium orthovanadate and 2 μg/ml leupeptin,
followed by sonication on ice, with a power of 15% and a lysis time
of 1 min (sonication for 2 sec, rest for 4 sec). A BCA protein
assay kit (Cell Signaling Technology, Inc.) was then used to assess
the concentration of the protein. Equal amounts of 20-40 μg
per lane proteins were then separated using SDS-PAGE on an 8%
SDS-polyacrylamide gel and transferred to PVDF (MilliporeSigma).
Western blotting was performed at 4°C overnight with primary
antibodies as follows: Anti-V5 (1:1,000; cat. no. 13202S; Cell
Signaling Technology, Inc.), anti-Flag (1:1,000; cat. no. 14793S;
Cell Signaling Technology, Inc.), anti-non-phospho active β-catenin
(1:2,000; cat. no. 8814; Cell Signaling Technology, Inc.),
anti-TCF4 (1:2,000; cat. no. 2569; Cell Signaling Technology,
Inc.), anti-LGR5 (1:1,000; cat. no. ab273092; Abcam), anti-SOX2
(1:1,000; cat. no. 23064S; Cell Signaling Technology, Inc.),
anti-β-catenin (1:2,000; cat. no. Sc-7963; Santa Cruz
Biotechnology, Inc.), anti-mLGR5 (1:1,000; cat. no. DF2816;
Affinity Biosciences, Ltd.), anti-GAPDH (1:5,000; cat. no.
60004-1-Ig; Proteintech Group, Inc.) and anti-β-actin (1:5,000;
cat. no. HC201-01; TransGen Biotech Co., Ltd.). Blocking was
performed before incubation with primary antibody using 5% non-fat
powdered milk (Sangon Biotech Co., Ltd.) at room temperature for 1
h. The washing solution was prepared by adding 0.1% Tween 20 to TBS
solution. The PVDF membranes were then incubated for 1 h at room
temperature with relevant HRP-conjugated goat anti-mouse (1:10,000;
cat. no. A16066; Thermo Fisher Scientific, Inc.) or HRP-conjugated
goat anti-rabbit (1:10,000; cat. no. A16096; Thermo Fisher
Scientific, Inc.) IgG secondary antibodies. The blots were
visualized using Tanon 5200 Chemiluminescent Imaging System (Tanon
Science and Technology Co., Ltd.,) or X-ray film (Kodak) after
incubation with ECL Plus Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). The intensity of the was semi-quantified using
ImageJ 1.8.0 (National Institutes of Health).
Immunoprecipitation
Total protein was extracted from 293T or HCT116
cells using 500 μl of RIPA buffer which contained a cocktail
of protease inhibitors and phosphatase inhibitors. After
quantification of the lysate protein concentration using a BCA
protein assay kit (Cell Signaling Technology, Inc.), proteins were
incubated with 2 μg anti-β-catenin (1:250; cat. no. Sc-7963;
Santa Cruz Biotechnology, Inc.) or anti-Flag (1:250; cat. no.
14793S; Cell Signaling Technology, Inc.) antibodies and protein A/G
agarose beads overnight at 4°C. Beads were washed four times with
700 μl RIPA buffer, heat denatured at 95°C for 5 min
followed by gel electrophoresis on a 9% SDS-PAGE gel. For
subcellular separation, a Subcellular Protein Fractionation Kit
(Thermo Fisher Scientific, Inc.) was used to separate the
cytoplasmic and nuclear parts according to the manufacturer's
instructions. GAPDH and Histone H3 were used as the cytoplasmic and
nuclear markers, respectively. The expression of GAPDH and Histone
H3 was detected by immunoblotting with anti-GAPDH (1:5,000; cat.
no. 60004-1-Ig; Proteintech Group, Inc.) and anti-Histone H3
antibodies (1:5,000; cat. no. 9715; Cell Signaling Technology,
Inc.). The PVDF membranes were incubated for 1 h at room
temperature with the aforementioned HRP-conjugated secondary
antibodies.
Immunofluorescence staining
Cells were plated in 12-well plates at a density of
70-80% and allowed to grow for 24 h in the cell incubator at 37°C.
After treatment with chloroxylenol at 37°C for 24 h, the cells were
fixed with 4% paraformaldehyde at room temperature for 15 min and
permeabilized with 0.4% TritonX-100. After blocking with blocking
buffer (5% non-fat powdered milk and 0.1% Tween20 dissolved in TBS)
at room temperature for 2 h, the cells were incubated with
anti-β-catenin antibodies (1:200; cat. no. Sc-7963 Santa Cruz
Biotechnology, inc.) for 2 h at room temperature. Alexa
Fluor488-conjugated goat anti-mouse IgG antibodies (1:200; cat. no.
Z-25402; Molecular Probes; Thermo Fisher Scientific, Inc.) were
used as a secondary antibody and incubated at 37°C for 1 h and DAPI
was used to visualize the nucleus with an incubation time of 5 min
at room temperature. The slides were imaged using a Leica TCSSP5II
laser scanning confocal microscope (Leica Microsystems GmbH).
Acquisition settings were as follows: DAPI, excitation/emission at
358/461 nm and β-catenin, excitation/emission at 495/519 nm. Images
were analyzed using Leica LASA Flite software (Leica Application
Suite X; version 2.6.0; Leica Microsystems GmbH).
Reverse transcription-quantitative PCR
(RT-qPCR) analyses
RNAiso Plus (Takara Bio, Inc.) was used to isolate
total RNA, which then reverse transcribed into cDNA according to
the manufacturer's protocol (37°C for 15 min, 85°C for 5 sec) using
the Primescript RT reagent kit (Takara Bio, Inc.). Quantitative PCR
analyses (95°C for 5 min; 95°C for 15 sec, 60°C for 1 min) on the
prepared cDNA were then performed using FastStart Universal
SYBR-Green Master (Selleck Chemicals). The comparative
2-ΔΔCq method was used to evaluate the relative
expression of the genes (35). The
primer sequences used were as follows: Axin2 sense (S), 5′-TAC ACT
CCT TAT TGG GCG ATC A-3′ and antisense (AS), 5′-TTG GCT ACT CGT AAA
GTT TTG GT-3′; Survivin S, 5′-AGG ACC ACC GCA TCT CTA CAT-3′ and
AS, 5′-AAG TCT GGC TCG TTC TCA GTG-3′; LGR5 S, 5′-CTC CCA GGT CTG
GTG TGT TG-3′ and AS, 5′-GAG GTC TAG GTA GGA GGT GAA G-3′; GAPDH S;
5′-CCA GAA CAT CAT CCC TGC CTC TAC T-3′ and AS, 5′-GGT TTT TCT AGA
CGG CAG GTC AGG T-3′; mouse (m)Axin2 S, 5′-ATG AGT AGC GCC GTG TTA
GTG-3′ and AS, 5′-GGG CAT AGG TTT GGT GGA CT-3′, mSOX2 S, 5′-GCG
GAG TGG AAA CTT TTG TCC-3′ and AS, 5′-GGG AAG CGT GTA CTT ATC CTT
CT-3′; mLGR5 S, 5′-ACA TTC CCA AGG GAG CGT TC-3′ and AS, 5′-ATG TGG
TTG GCA TCT AGG CG-3′, mSurvivin S, 5′-GAG GCT GGC TTC ATC CAC
TG-3′; AS, 5′-ATG CTC CTC TAT CGG GTT GTC-3′; and mGAPDH S, 5′-AGG
TCG GTG TGA ACG GAT TTG-3 and AS, 5′-GGG GTC GTT GAT GGC AAC
A-3′.
Chromatin immunoprecipitation (ChIP)
assays
A ChIP-IT Express Enzymatic Chromatin
Immunoprecipitation Kit (Active Motif, Inc.) was used for ChIP
assays according to the manufacturer's protocols. Briefly, HCT116
and SW480 cells were treated with chloroxylenol for 24 h at 37°C.
After crosslinking with 1% formaldehyde for 10 min at room
temperature and termination with 125 mM glycine, the cells were
washed, lysed with RIPA buffer at 4°C for 10 min and sonicated to
reduce the DNA fragment length to 300-600 bp. The chromatin
complexes were incubated with 2 μg mouse antibodies against
β-catenin (1:50; cat. no. Sc-7963; Santa Cruz Biotechnology, Inc.)
or mouse IgG (1:50; cat. no. Sc-2025; Santa Cruz Biotechnology,
Inc.) and protein A/G agarose at 4°C overnight. The complexes were
then precipitated by centrifugation at 4°C, 7,000 × g for 1 min,
eluted and treated with Protease K. The DNA in the precipitated
complex was extracted with Chelex100 (Bio-Rad Laboratories, Inc.)
and then detected using qPCR. qPCR analyses (95°C for 5 min; 95°C
for 15 sec, 60°C for 1 min) on the prepared DNA were then performed
using FastStart Universal SYBR-Green Master (Selleck Chemicals).
The comparative 2-ΔΔCq method was used to evaluate the
relative expression of the genes (35). The following primers for the
putative β-catenin binding site in Survivin, Axin2, Sox2 and LGR5
promoter regions, which had been confirmed in our previous study
(36), were used for
amplification. The primer sequences used were as follows: Survivin
S, 5′-GCG TTC TTT GAA AGC AGT-3′ and AS, 5′-ATC TGG CGG TTA ATG
GCG-3′; Axin2 S, 5′-CGG TTG GCG AAA GTT TGC-3′ and AS, 5′-GGA CTC
GGG AGC CTA AAG GT-3′; LGR5 S, 5′-ACC ACC TCT TTCAGC AGC TC-3′ and
AS, 5′-GAA CTG AGA GCA GTC CCA CC-3′; and SOX2 S, 5′-AAT ACG AGT
TGG ACA GCC GC-3′ and AS, 5′-TTT GTA TCC CCT CTC GCA GC-3′.
Cell viability and proliferation
assays
Cells were plated in 96-well plates at a density of
2×104 cells per well and then treated with the indicated
concentrations of chloroxylenol for 24 h. For the viability assay,
the cells were cultured for another 4 h with fresh medium
containing 0.5 mg/ml MTT at 37°C. After the medium was removed,
formazan crystals were dissolved in DMSO and the absorbance of
formazan solution was quantified at 490 nm with 570 nm used as a
reference. For cell proliferation evaluation, the bromodeoxyuridine
(BrdU) incorporation assay was performed using the Cell
Proliferation ELISA BrdU Chemiluminescent Kit (Roche Diagnostics)
according to the manufacturer's protocols.
Colony formation assays
Cells were added to six-well plates at a cell
density of 1×103 cells per well, and then incubated in
medium containing 10% FBS with the indicated concentrations of
chloroxylenol at 37°C in a humidified incubator with 5%
CO2. After 10 days of incubation, cells were fixed with
4% paraformaldehyde at room temperature for 15 min, stained with
0.1% crystal violet at room temperature for 20 min and imaged using
a DP74 light microscope (Olympus Corporation). Colonies were
counted and quantified manually to support subsequent statistical
analysis.
Transwell assays
Transwell assays were utilized to perform in
vitro cell migration and invasion assessments as previously
described (37). Briefly,
2×105 cells were suspended in 100 μl serum-free
medium containing the indicated concentrations of chloroxylenol and
then seeded into 24-well Transwell chambers with 8-μm pore
size membranes. The lower chamber medium containing 20% FBS acted
as a chemoattractant, while the upper chamber contained DMEM medium
without FBS. After incubation at 37°C for 12 h, the cells on the
upper side membrane that had not migrated were removed. The
migrated cells were stained with 0.1% crystal violet for 15 min at
room temperature and then imaged using a DP74 light microscope
(Olympus Corporation). With regard to invasion assay, the procedure
was the same as for the cell migration assay except that the
Transwell chambers with 8 μm pore size membranes were
precoated with Matrigel (Corning Life Sciences) at 4°C and
incubated at 37°C for 3 h. Stained cells were rinsed with 33%
acetic acid at room temperature for 10 min and then the absorbance
of the resulting solution was assessed at 570 nm for quantitative
analysis.
Flow cytometric analyses
After treatment with chloroxylenol (62.5-250
μM) at 37°C for 24 h or 48 h, HCT116 and SW480 cells were
collected. Quantitative fluorescence sorting was performed using a
FACSCalibur™ (BD Biosciences) fluorescence-activated cell sorting
instrument and FlowJo v10.0.8 (Tree Star, Inc.) for subsequent
analyses. For the apoptosis assay, cells were treated with Annexin
V-fluorescein isothiocyanate and propidium iodide solution
(TransGen Biotech Co., Ltd.) according to the manufacturer's
protocols. To test the stemness of CRC cells, SW480 cells were
stained with PE-conjugated anti-human LGR5 antibodies (1:100; cat.
no. 563470, BD Biosciences) at room temperature for 45 min.
Sphere formation assays
HCT116 cells were seeded at 250 cells per well into
the sphere culture medium MammCult (Stemcell Technologies, Inc.)
with the indicated concentrations of chloroxylenol in a 24-well
plate. After 10 days of incubation at 37°C, spheres with a diameter
>50 μm were tallied manually and microphotographed using
a DP74 light microscope (Olympus Corporation).
Human tissue samples and organoid
culture
The present study was approved by The Research
Ethics Committee of Shenzhen University (approval no. PN-2022-001)
and the patient agreed and signed written informed consent. The
tissues of a CRC patient in the First Affiliated Tumor Hospital of
Guangxi Medical University confirmed by colonoscopy biopsy were
included in the present study. The patient had not received any
anti-cancer therapy before CRC biopsies were taken. Human CRC
biopsies were washed with PBS and incubated on ice in a mixture of
antibiotics for 30 min and 2 mM EDTA/PBS for 60 min to remove
normal epithelial cells. Tissues were cut into small pieces and
digested enzymatically in digestion buffer (2.5% FBS, 200 U/ml type
IV collagenase, 125 μg/ml type II dispersase) at 37°C for 1
h with continuous shaking in a water bath. The tumor samples were
then embedded in Matrigel on ice and seeded in a preheated 24-well
plate. After polymerization at 37°C for 15 min, 500 μl
advanced DMEM/F12 containing 50 ng/ml EGF, 50 ng/ml noggin, 500
ng/ml R-spondin 1, 100 ng/ml Wnt3a, 10 mM nicotinamide, 1 mM
N-acetylcysteine, and 10 μM Y-27631 was added and replaced
every two to three days.
Animal model study
All animal experimental protocols were approved by
the Animal Ethics Committee of Shenzhen University (approval no.
AEWC-2021006). Male C57BL/6 mice were purchased from the Guangdong
Medical Laboratory Animal Center. The animals were acclimatized to
the laboratory for at least 1 week prior to the start of the
experiments. All mice were housed in a specific-pathogen-free
facility at the Animal Research Center of Shenzhen University, with
five mice per cage under a 12:12 h light/dark cycle at a constant
temperature of 24°C with 50-60% relative humidity and fed a
standard rodent diet with free access to sterile water, which was
replaced every day. Ten mice were included in the present study and
the mice were anesthetized with isoflurane inhalation at a
concentration of 3-5% in oxygen and maintained at 1-2% during the
surgery. At the end of the experiments, euthanasia was performed by
CO2 asphyxiation with CO2 displacement rate
at 60% of the container volume per min. Death was confirmed by
checking for the cessation of heartbeat and respiration. Any animal
which showed maximum tumor volume >750 mm3 or rapid
loss of 15-20% of original body weight or signs of cachexia and
persistent muscle wasting even without weight loss, was euthanized.
In animal studies, both the carer of the animals and the assessor
of the results were blinded.
For the colorectal cancer xenograft mouse model,
mouse colon cancer MC38 cells were subcutaneously implanted into
the right flank of 7-week-old male C57BL/6 mice at 2×106
cells/100 μl of PBS per mouse. Tumor growth was monitored
daily and measured on alternate days after implantation. When the
tumor size reached ~50 mm3, ten mice were randomly
divided into two groups (5 mice/group) and treated with vehicle (8%
ethanol/12% polyethylene glycol in saline) or 5 mg/kg chloroxylenol
in the vehicle via intraperitoneal injection every other day. Tumor
volume was measured using calipers on alternate days and calculated
using the formula: 0.528 × (length/2) × (width/2)2.
After two weeks of treatment, the mice were sacrificed, and the
tumors were excised and weighed. For RNA and protein analyses,
tumor tissues were homogenized, and RNA or protein was extracted.
RT-qPCR and western blot assays were performed according to the
aforementioned methods. For histological analysis, tumors were
fixed in formalin at room temperature for 24 h, embedded in
paraffin and sectioned to 5 μm slices. Hematoxylin and eosin
(H&E) staining and immunohistochemical analysis were performed
as previously described (37).
Data and statistical analyses
Data were presented as mean ± SD. GraphPad Prism
software (v8.0; GraphPad software; Dotmatics) was used for
statistical analysis. On the basis of their distribution, data were
analyzed by one-way analysis of variance followed by Dunnett's
t-test or Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of Wnt/β-catenin signaling by
chloroxylenol
To identify novel small molecules that modulated the
Wnt/β-catenin signaling pathway, a cell-based SuperTOPFlash
reporter system was used to screen a known-compound library.
Preliminary screening results demonstrated that chloroxylenol could
inhibit β-catenin-mediated Wnt signaling in 293T cells. As
chloroxylenol is a frequently used antimicrobial compound, the
effects of several common antimicrobial agents, in health care
products, on the Wnt/β-catenin signaling pathway in 293T cells were
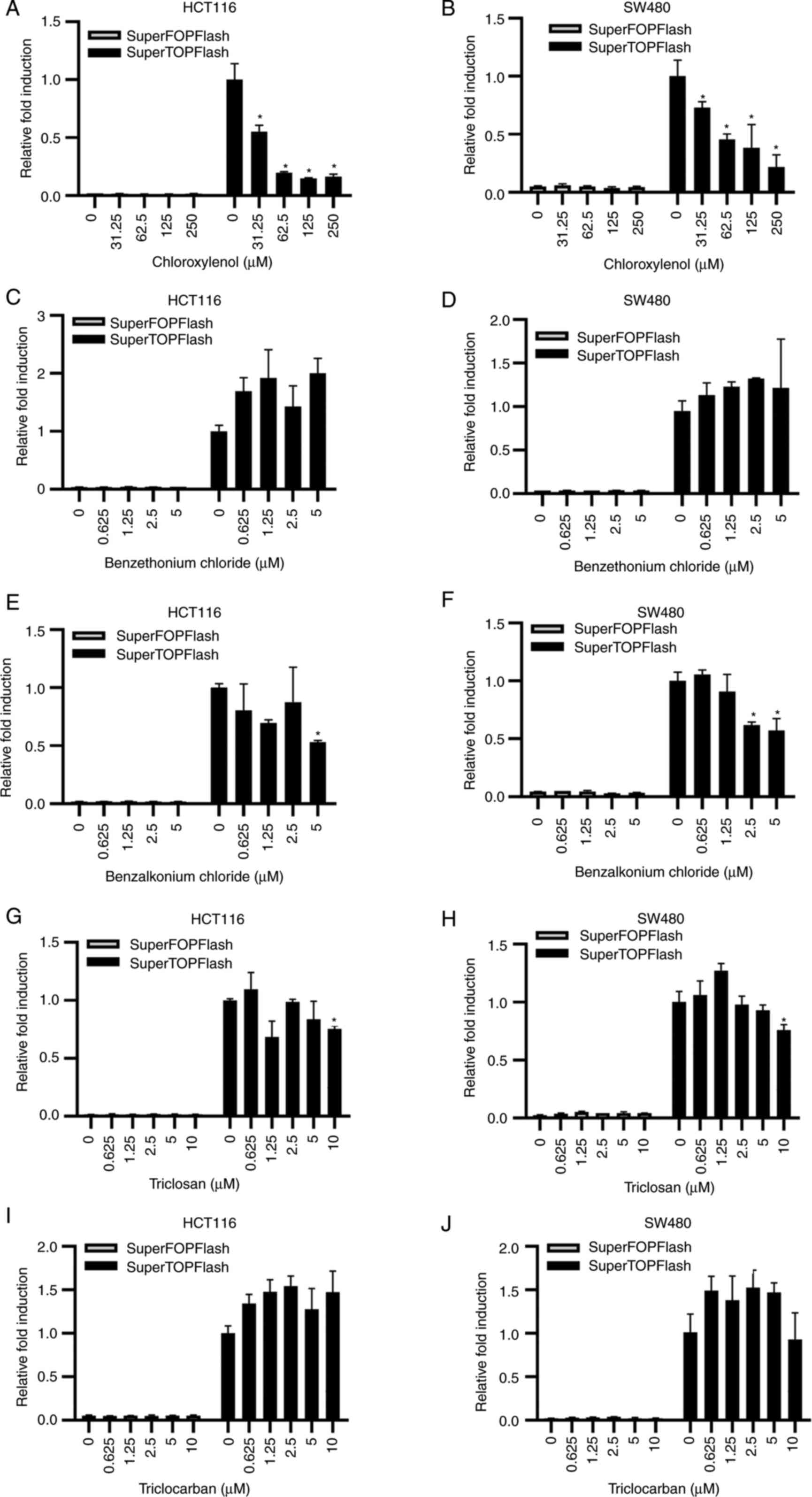
assessed in the present study. Only chloroxylenol demonstrated a
dose-dependent inhibitory effect on the activity of the
SuperTOPFlash reporter activated by Wnt1 and LPR6 (Fig. S1A and B), while benzethonium
chloride (Fig. S1C and D),
benzalkonium chloride (Fig. S1E and
F), triclosan (Fig. S1G and
H) and triclocarban (Fig. S1I and
J) demonstrated not significant effect on the SuperTOPFlash
reporter in 293T cells, even at toxic doses. In control
experiments, Wnt inhibiting concentrations of chloroxylenol had no
effect on the luciferase activity of an activator protein 1 (AP-1)
reporter gene (Fig. S2A) or a
nuclear factor of activated T cells (NFAT) reporter gene (Fig. S2B).
As aberrant activation of the Wnt/β-catenin
signaling pathway is common in CRC (38), the effect of chloroxylenol on
Wnt/β-catenin signaling in CRC cells was further assessed. SW480
and HCT116 cells were transfected with the SuperTOPFlash reporter.
Treatment with 31.25-250 μM chloroxylenol in HCT116 and
SW480 cells significantly inhibited the transcriptional activity of
SuperTOPFlash reporter, but had little effect on the activity of
SuperFOPFlash reporter (Fig. 1A and
B). In HCT116 cells, the activity of SuperTOPFlash reporter
showed a downward trend with the treatment of 31.25-125 μM
chloroxylenol. In SW480 cells, although the downward trend was not
significant, treatment with chloroxylenol significantly reduced the
SuperTOPflash activity compared with the untreated group. At doses
of toxic concentration, benzalkonium chloride (2.5-5 μM;
Fig. 1E and F) and triclosan (10
μM; Fig. 1G and H) also
demonstrated a significant decrease in SuperTOPFlash activity.
However, at non-toxic concentrations, benzethonium chloride (0-1.25
μM; Fig. 1C and D),
benzalkonium chloride (0-1.25 μM; Fig. 1E and F), triclosan (0-5 μM;
Fig. 1G and H) and triclocarban
(0-5 μM; Fig. 1I and J) did
not significantly affect the transcription of the SuperTOPFlash
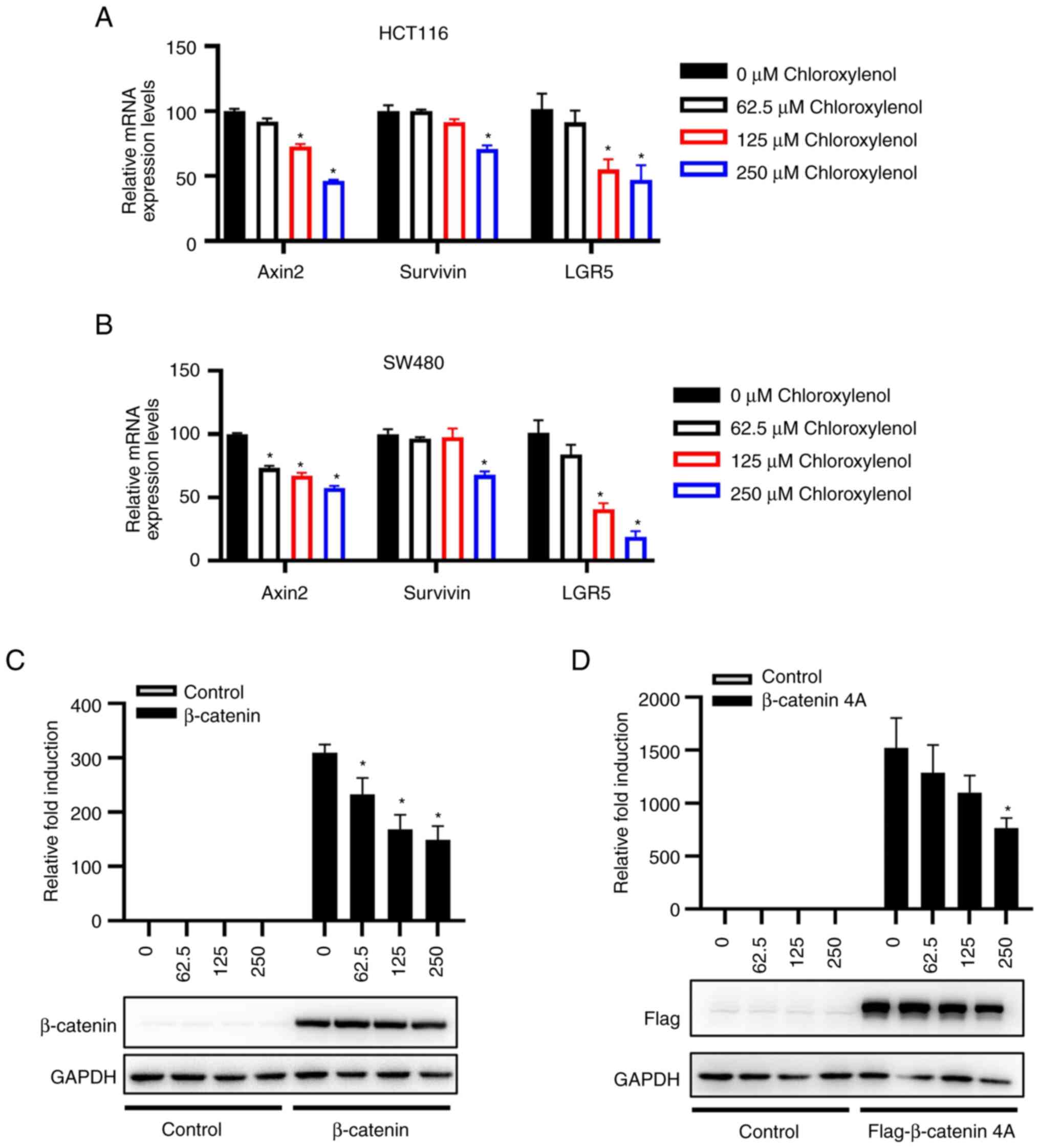
reporter in CRC cells. Moreover, the mRNA expression levels of
certain Wnt target genes in HCT116 and SW480 cells were quantified
using RT-qPCR assays to further determine the effect of
chloroxylenol on β-catenin-mediated transcription in CRC cells. In
HCT116 cells, concentrations of 125-250 μM chloroxylenol
could exert a significant decrease in mRNA levels of Axin2 and
LGR5, while for Survivin, there was only a significant decrease at
250 μM. In SW480 cells, Axin2 demonstrated a significant
decrease within the concentration range of 62.5-250 μM,
while LGR5 and Survivin decreased at 125 and 250 μM
treatment, respectively (Fig. 2A and
B). These results demonstrated the antagonistic effect of
chloroxylenol on the Wnt/β-catenin signaling cascade in CRC
cells.
HCT116 cells carry an S45 mutation in β-catenin and
SW480 cells harbor an APC deletion at the C terminus at residue
1338, this suggested that chloroxylenol might exert its
antagonistic effect via targeting downstream of APC and β-catenin
in the Wnt signaling pathway in CRC cells. To evaluate this
hypothesis, β-catenin and its N-terminal mutant β-catenin 4A along
with SuperTOPFlash reporter were transfected into 293T cells. The
expression plasmid of β-catenin 4A was previously constructed by
mutating four serine and threonine residues (Ser-33, Ser-37, Thr-41
and Ser-45) in the N-terminal of β-catenin to alanine, which
indicated the persistent activation of the Wnt signaling cascade
(36,39). The results demonstrated that
chloroxylenol significantly inhibited the transcriptional activity
mediated by both wild type β-catenin and β-catenin 4A, which
suggested that chloroxylenol may act downstream of the Wnt
signaling pathway (Fig. 2C and
D).
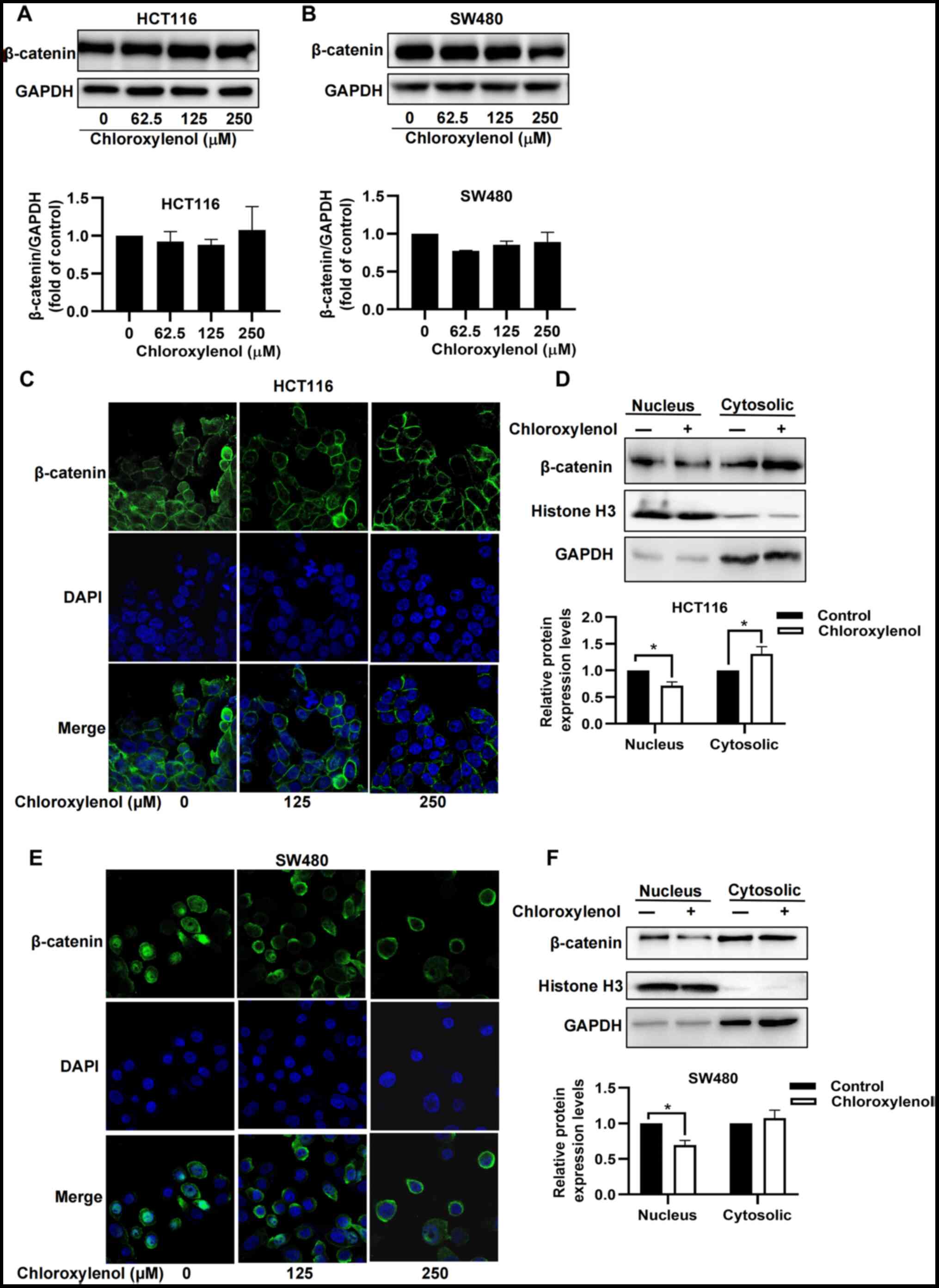
Chloroxylenol prevents nuclear
translocation of β-catenin in CRC cells
β-catenin, a key component of the Wnt signaling
pathway, is tightly regulated at three hierarchical levels; protein
stability, subcellular localization and transcriptional activity
(40). To elucidate the mechanism
underlying the inhibitory effect of chloroxylenol on Wnt signaling,
its effect on the protein expression level of β-catenin was
assessed. Quantitative analysis of the β-catenin protein expression
level demonstrated that chloroxylenol had no significant effect on
the protein expression level of β-catenin in HCT116 and SW480 cells
(Fig. 3A and B). Furthermore, an
immunofluorescence staining assay was performed to determine the
effect of chloroxylenol on the subcellular localization of
β-catenin. Chloroxylenol markedly decreased nuclear localization of
β-catenin in HCT116 and SW480 cells (Fig. 3C and D). Subcellular localization
analysis further demonstrated that chloroxylenol treatment could
significantly decrease the accumulation of β-catenin in the
nucleus, accompanied by a marked increase in the levels of
cytoplasmic β-catenin in SW480 cells and a significant increase in
the levels of cytoplasmic β-catenin in HCT116 cells (Fig. 3E and F).
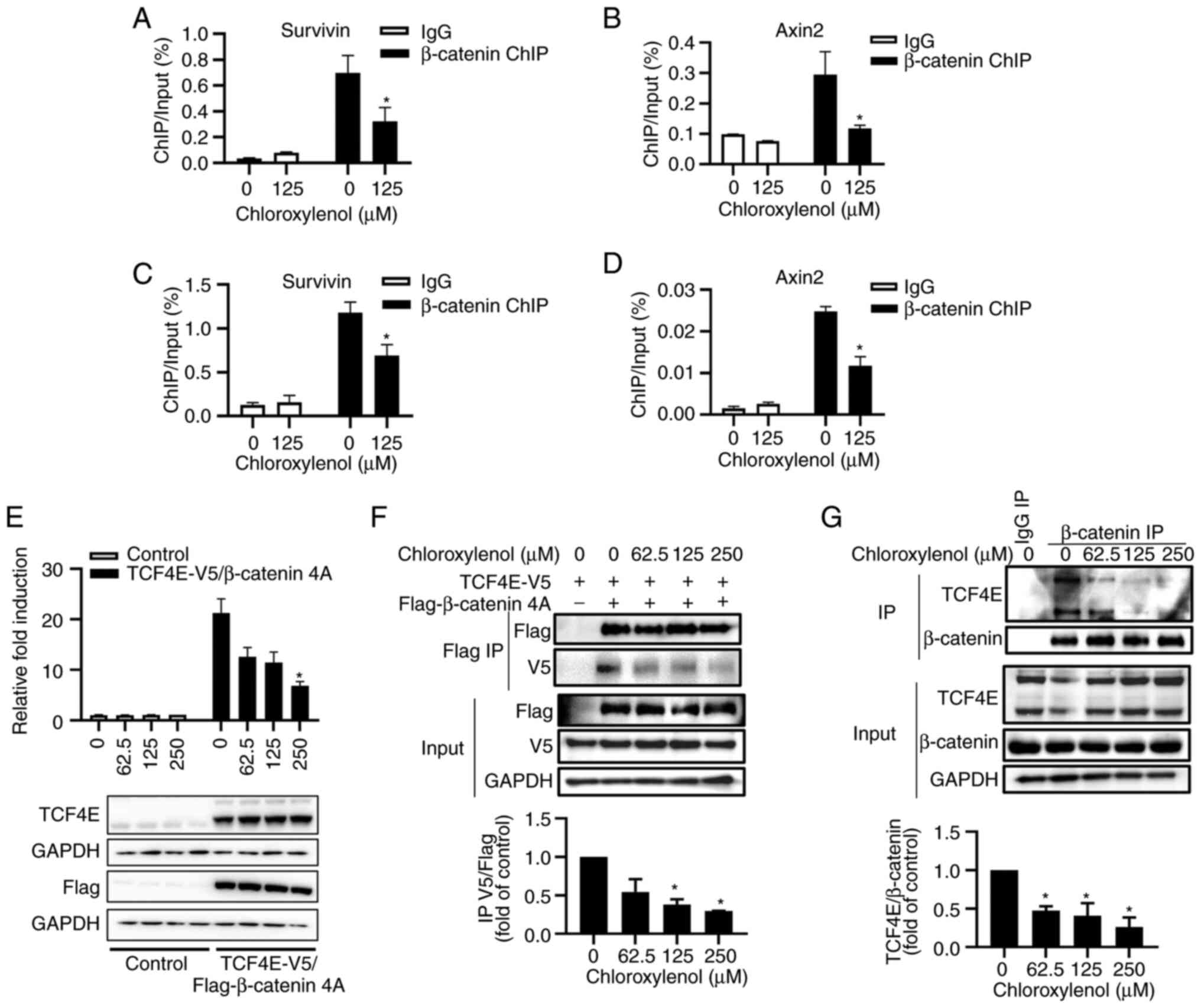
Chloroxylenol inhibits β-catenin/TCF4
mediated transcriptional activity and interferes with the
interaction of β-catenin with TCF4E
The transcription factor TCF/LEF recruits β-catenin
to the promoter region of Wnt target genes to activate gene
transcription. ChIP assays were performed to determine the effect
of chloroxylenol on the binding of β-catenin to the promoter region
of Wnt downstream target genes Axin2 and Survivin. The results
demonstrated that chloroxylenol treatment significantly reduced
β-catenin binding to Axin2 and Survivin promoter region in both
HCT116 (Fig. 4A and B) and SW480
cells (Fig. 4C and D).
To assess the effect of chloroxylenol on
β-catenin-mediated transcriptional activity, the pDKK4-Luc reporter
was constructed by cloning the DKK4 promoter with five putative
TCF-binding sites into a luciferase reporter vector as previously
described (41). The simultaneous
presence of β-catenin and TCF4 is required for the activation of
the pDKK4-Luc reporter (42). 293T
cells were transfected with pDKK4-Luc reporter and expression
vectors encoding β-catenin 4A, TCF4E and β-catenin 4A/TCF4E,
respectively. The results illustrated that the expression of
β-catenin 4A/TCF4E activated the transcriptional activity of the
pDKK4-Luc reporter, and chloroxylenol treatment reduced β-catenin
4A/TCF4E-mediated reporter activity in a dose dependent manner
(Fig. 4E).
Next, the effect of chloroxylenol on the interaction
between β-catenin and TCF4E was evaluated. The expression plasmids
encoding Flag-β-catenin 4A and TCF4E-V5 were transfected into 293T
cells, and Flag-β-catenin 4A was co-immunoprecipitated with an
anti-Flag agarose, followed by immunoblotting analyses. The results
demonstrated that Flag-β-catenin 4A was specifically coprecipitated
with TCF4E-V5, and the interaction was significantly reduced upon
chloroxylenol treatment (Fig. 4F).
The effect of chloroxylenol on the interaction between β-catenin
and TCF4E was further assessed in HCT116 cells. The results
demonstrated that endogenous β-catenin and TCF4E were specifically
co-immunoprecipitated by anti-β-catenin antibodies and
chloroxylenol treatment decreased the β-catenin/TCF4E interaction
in a dose-dependent manner (Fig.
4G).
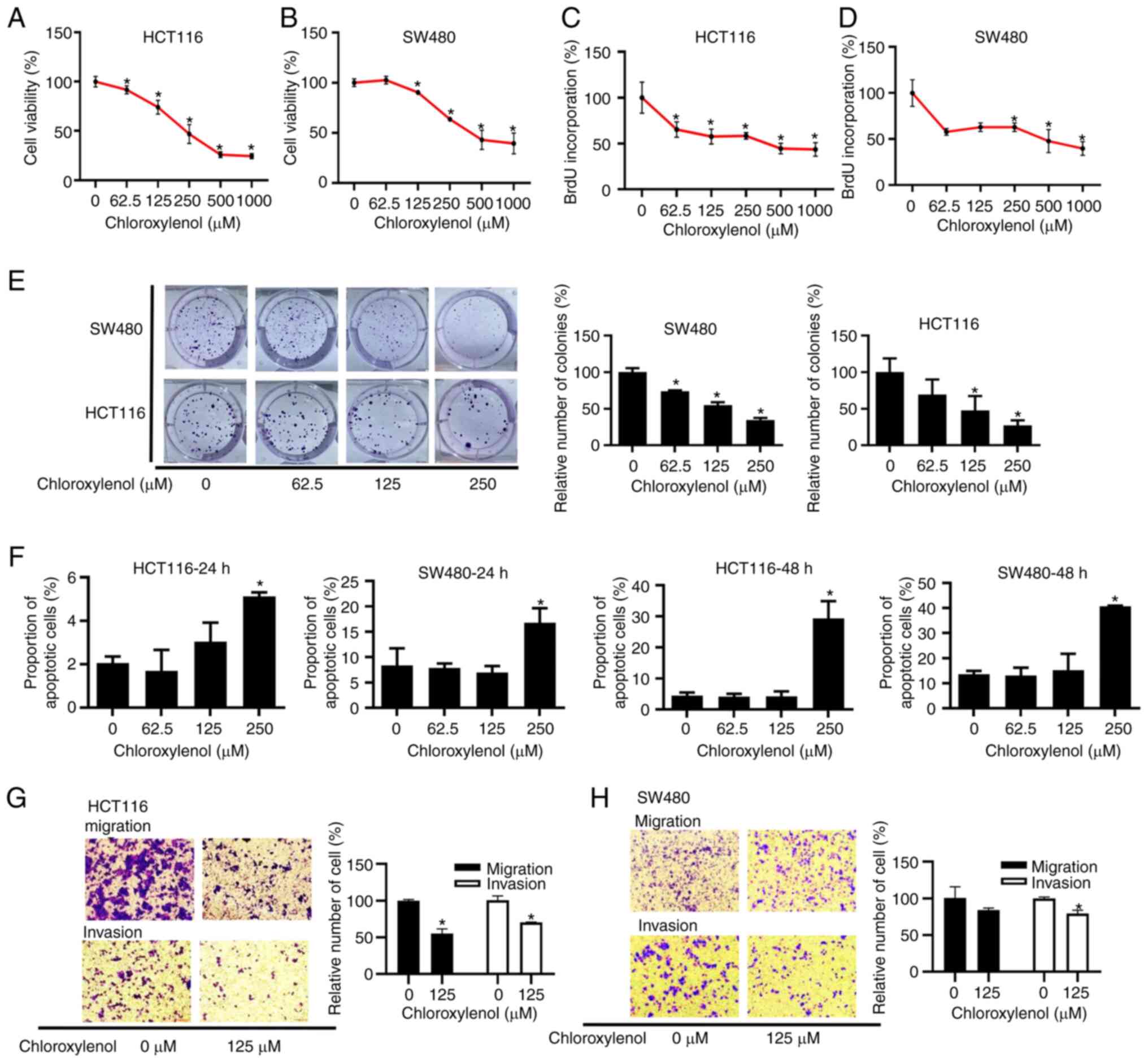
Chloroxylenol represses the viability,
proliferation, migration and invasion of CRC cells, and induces
apoptosis in CRC cells
To assess the cytotoxicity of chloroxylenol against
CRC cells, an MTT assay was performed. HCT116 and SW480 cells were
treated with 62.5-1,000 μM chloroxylenol for 48 h. After 4 h
of incubation with MTT, formazan solution was measured at 490 nm to
evaluate the relative cell viability. The experimental results
indicated that chloroxylenol effectively reduced the viability of
CRC cells, with IC50 values of 280.8 μM in HCT116
and 378.5 μM in SW480 cells (Fig. 5A and B). A BrdU cell proliferation
assay was used to assess the proliferation of CRC cells.
Chloroxylenol treatment significantly repressed the proliferation
of HCT116 and SW480 cells (Fig. 5C and
D). Furthermore, colony formation assays were performed which
demonstrated that chloroxylenol significantly decreased the number
of colonies formed by HCT116 and SW480 cells (Fig. 5E), which illustrated that
chloroxylenol could effectively block the colony formation of CRC
cells. The effect of chloroxylenol on apoptosis in HCT116 and SW480
cells was also assessed. Apoptotic cells were detected using flow
cytometry after treatment with different concentrations of
chloroxylenol ranging from 62.5-250 μM for 24 h or 48 h. The
results demonstrated that 250 μM chloroxylenol significantly
induced apoptosis in CRC cells (Figs.
5F and S3).
Considering the importance of Wnt/β-catenin
signaling in the migration and invasion of cancer cells, Transwell
assays were used to assess the in vitro migration and
invasion ability of CRC cells in response to a range of
concentrations of chloroxylenol. In the presence of 20% FBS as a
chemoattractant, migration was evaluated by counting those cells
that had migrated. For invasion assays, Matrigel-coated chambers
were used. Chloroxylenol at 125 μM exerted a significant
inhibitory effect on the migration and invasion of HCT116 cells
(Fig. 5G). In SW480 cells,
chloroxylenol also significantly suppressed invasion, but the
inhibition of migration was not significant (Fig. 5H).
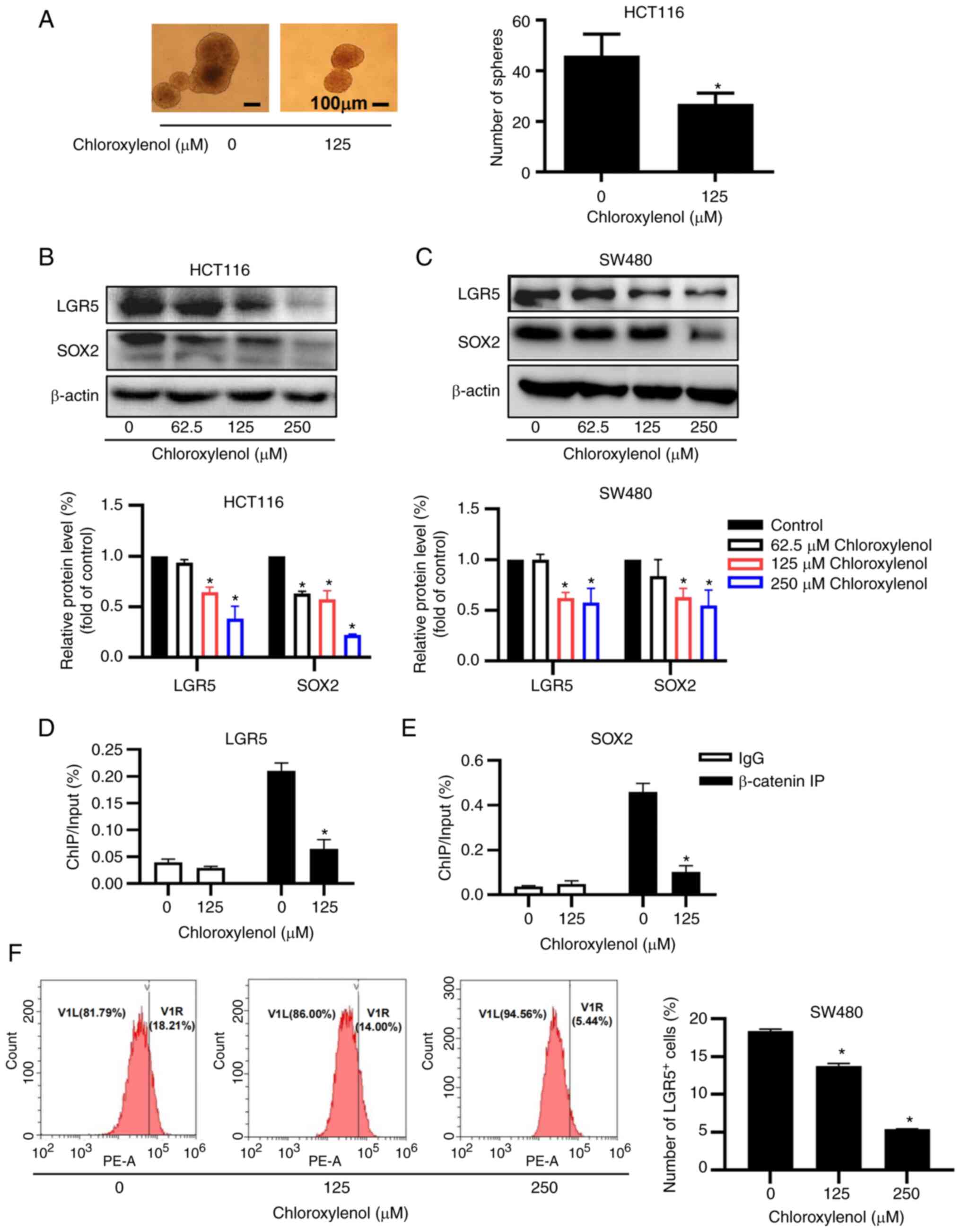
Suppression of stemness by chloroxylenol
in CRC cells
The Wnt/β-catenin signaling cascade serves a fatal
role in the maintenance of cancer cell stemness. A sphere formation
assay was performed to analyze the effect of chloroxylenol on the
stemness of HCT116 cells. The number and size of the spheres was
remarkably reduced after 10 days of incubation with 125 μM
chloroxylenol (Fig. 6A). SOX2 and
LGR5 are well-known CRC stem cell markers (15,43).
The protein expression levels of SOX2 and LGR5 were significantly
decreased after chloroxylenol treatment in HCT116 and SW480 cells
(Fig. 6B and C). Furthermore, ChIP
assays were performed to test whether chloroxylenol has any effect
on the binding of β-catenin to the promoter regions of LGR5 and
SOX2. The results demonstrated that chloroxylenol treatment
decreased β-catenin binding to the promoters of LGR5 or SOX2 in
HCT116 cells (Fig. 6D and E),
which suggested that chloroxylenol-mediated β-catenin nuclear
translocation may lead to reduced expression of LGR5 and SOX2.
Human colon cancer cells with LGR5-positive have been reported to
serve as CSCs in growing cancer tissues (16). Flow cytometric analysis
demonstrated that the number of LGR5-positive cells was
significantly decreased when SW480 cells were treated with
chloroxylenol (Fig. 6F). Taken
together, these results demonstrated that chloroxylenol effectively
blocked sphere formation of CRC cells and decreased protein
expression levels of the stemness markers LGR5 and SOX2, which
indicated the suppressive effect of chloroxylenol on CRC
stemness.
In vivo inhibition of tumor growth by
chloroxylenol in an MC38 cell xenograft model
To further evaluate the in vivo anti-tumor
effects of chloroxylenol, an MC38 mouse colon cancer xenograft
model was used to assess its anti-cancer activity. MC38 cells were
subcutaneously injected into C57BL/6 mice and when the tumor volume
reached ~50 mm3, mice were administered either a control
solvent or chloroxylenol at 5 mg/kg via intraperitoneal injection
on days 0, 2, 4, 6, 8 and 10. Mice were euthanized on day 14 after
six treatments, and the tumor volume and weight were measured.
Total RNA and protein were extracted from the xenografts and tumor
histology was studied. Chloroxylenol exhibited marked inhibitory
effects on tumor growth (Fig. 7A).
During chloroxylenol treatment process, there were no significant
changes in mouse body weight compared with the control group
(Fig. 7B). Tumor volume (Fig. 7C) and weight (Fig. 7D) were significantly decreased
after chloroxylenol treatment. Histological H&E staining
indicated that chloroxylenol treatment markedly reduced tumor cell
density (Fig. 7E) compared with
the vehicle control. Furthermore, immunohistochemical staining
results demonstrated that chloroxylenol markedly suppressed the
expression of cancer stemness marker LGR5 in the tumor tissue of
the xenografts (Fig. 7F). RT-qPCR
analysis further demonstrated that the inhibitory effect of
chloroxylenol on Wnt signaling, demonstrated a significant decrease
in the mRNA expression levels of Wnt target genes (Fig. 7G). Moreover, chloroxylenol
treatment significantly reduced the protein expression levels of
LGR5 and SOX2 (Fig. 7H).
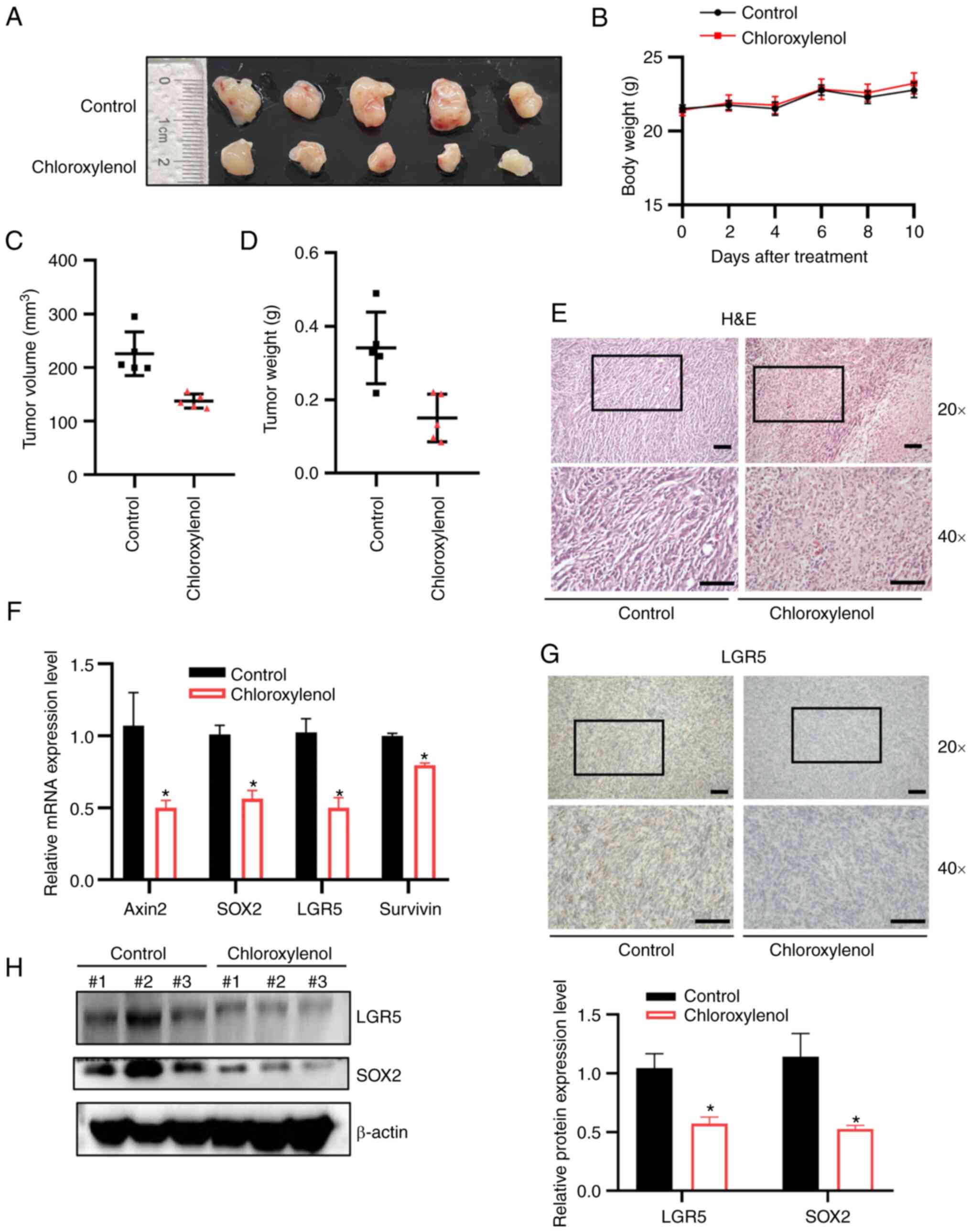 | Figure 7Chloroxylenol inhibits tumor growth
in an MC38 mouse colon cancer xenograft model. MC38 xenografts were
treated with control solvent or chloroxylenol at 5 mg/kg on days 0,
2, 4, 6, 8 and 10 by intraperitoneally injection. After 6 rounds of
treatment, mice were sacrificed on the fourteenth day, and tumors
were excised and weighed. (A) Images of tumors from control group
and treatment group. (B) Weight of mice after being treated with
control solvent or chloroxylenol. (C) Mean tumor volumes. (D) Mean
tumor weight. (E) H&E staining of tumor section; scale bar, 200
μm. (G) Immunohistochemical staining of LGR5; scale bar, 200
μm. (F) The mRNA expression levels of the Wnt target genes
Axin2, LGR5, SOX2 and Survivin were quantitated using reverse
transcription-quantitative PCR. Data are presented as mean ± SD.
(H) The protein expression levels of LGR5 and SOX2 in tumor samples
were assessed using western blotting. n=5. *P<0.05
vs. control (0 μM) group. |
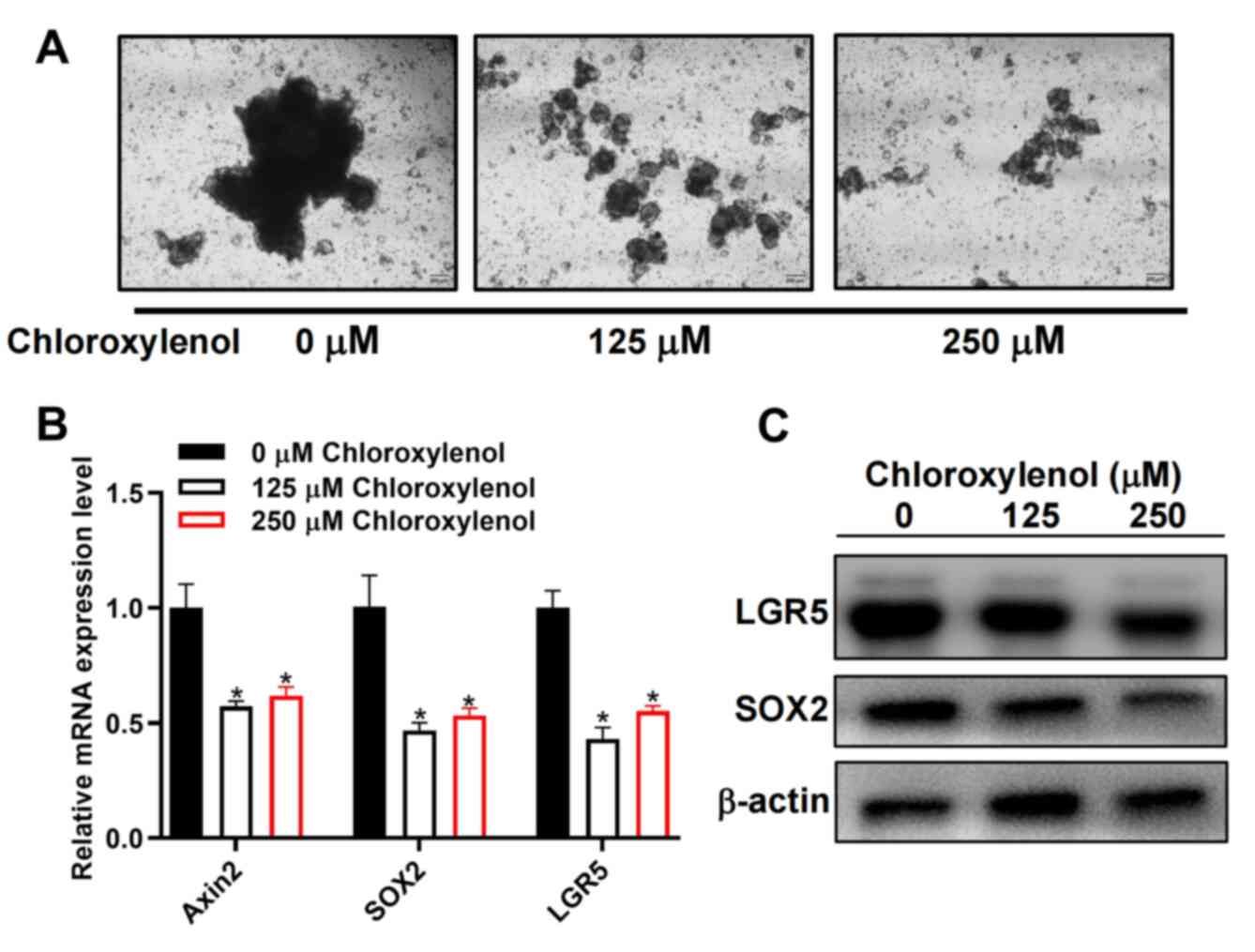
Reduction of organoid formation by
chloroxylenol in human CRC tissue samples
To further appraise the effect of chloroxylenol on
human colorectal CSCs and the therapeutic benefits for patients
with CRC, organoids derived from the CRC patient were developed and
used to analyze the effect of chloroxylenol on their growth. The
organoids were treated with 125 and 250 μM chloroxylenol.
Chloroxylenol treatment inhibited the growth of organoids (Fig. 8A) and markedly reduced the mRNA and
protein expression levels of CSC related proteins LGR5 and SOX2
(Fig. 8B and C). These results
indicated that chloroxylenol could significantly inhibit the growth
of CRC organoids.
Discussion
As a widely used antibacterial and antifungal agent,
chloroxylenol has been reported to have antiviral activity in
recent years (44). Compared with
other antimicrobial ingredients in consumer products, including
triclosan, triclocarban, benzalkonium chloride and benzethonium
chloride, chloroxylenol has been previously reported to exhibit low
acute toxicity, low systemic toxicity, and a lack of genotoxicity
and carcinogenicity (45). Yang
et al (46) reported that
triclosan could increase the severity of colitis symptoms and
exacerbated colitis-associated colon cancer cell growth via gut
microbiota- and TLR4-dependent mechanisms. Triclocarban has been
reported to increase dextran sodium sulfate (DSS) and IL-10
knockout-induced colitis, and to have exaggerated azoxymethane
(AOM)/DSS-induced colon tumorigenesis in mice (47). Moreover, exposure to benzalkonium
chloride and benzethonium chloride also exaggerated DSS-induced
colonic inflammation and AOM/DSS-induced colitis-associated colon
tumorigenesis in mice (48).
However, compared with benzalkonium chloride and benzethonium
chloride, chloroxylenol was reported to have had little effect on
DSS-induced colonic inflammation and AOM/DSS-induced
colitis-associated colon tumorigenesis in mice (49). It remains unclear why chloroxylenol
exerts a different effect on colonic inflammation and
colitis-associated colon tumorigenesis compared with other
antimicrobial agents.
Inflammatory bowel disease (IBD) is a chronic
gastrointestinal inflammatory disorder with an increasing incidence
and includes two major forms, Crohn's disease and ulcerative
colitis (49). In patients with
IBD, the most serious complication is the development of
colitis-associated colon cancer (50,51).
It has been previously reported that the constitutive activation of
canonical Wnt/β-catenin signaling is critical in the initiation and
progression of CRC (38). The
Wnt/β-catenin signaling pathway is also essential for gut
development and homeostasis (52).
Significant differences in cell-surface components of the Wnt
pathway have been reported in the colonic mucosa of patients with
IBD compared with non-IBD patients (53). Moreover, conditional knockout mice
that specifically lack the Wnt co-receptor LRP5/6 or β-catenin in
CD11c+ antigen-presenting cells developed more severe
acute colitis (54). Concerning
the importance of Wnt/β-catenin signaling in chronic intestinal
inflammation and CRC, the Wnt antagonistic actions of chloroxylenol
may at least partially counteract its effect on colonic
inflammation and colitis-associated colon tumorigenesis. The
present study provided novel insight into the effect of
chloroxylenol on colonic inflammation and colitis-associated colon
tumorigenesis.
CSCs, also known as tumor-initiating cells, are a
subgroup of cancer cells with the ability to self-renew and
differentiate into heterogeneous cancer cell lineages and are
considered to be responsible for drug resistance and cancer
recurrence in multiple forms of cancer including CRC (55,56).
Constitutive activation of Wnt signaling has been reported to serve
an important role in the growth and maintenance of colorectal CSCs.
Colorectal CSCs have also been reported to exert a Wnt/β-catenin
signaling high activity (57). In
the present study, the antagonistic effect of chloroxylenol on the
Wnt/β-catenin signaling pathway was demonstrated. Chloroxylenol
inhibited β-catenin nuclear translocation and β-catenin-mediated
transcriptional activity and downregulated the expression of Wnt
target genes. The inhibitory effect of chloroxylenol on Wnt
signaling was demonstrated at concentration comparable to that
which demonstrated an inhibitory effect on CRC cells, which
indicated that the Wnt inhibitory effect of chloroxylenol was
associated with its anti-tumor activity in CRC. Furthermore, the
results of the present study demonstrated that chloroxylenol could
inhibit colorectal cancer xenograft growth, the sphere-forming
ability and organoid formation in CRC, and result in a significant
decrease in the expression of the stemness marker genes LGR5 and
SOX2. These results indicated that chloroxylenol could selectively
inhibit colorectal CSCs through targeting of the Wnt/β-catenin
signaling pathway. Further studies are needed to develop
chloroxylenol derivatives with lower toxicity and more potent
inhibitory effects on Wnt signaling for CRC treatment.
There were certain limitations of the present study
due to the utilization of a relatively high concentration range of
chloroxylenol to elicit its anticancer effects. This effective
dosage, spanning 62.5-250 μM, may engender challenges
concerning its translational implementation and further advancement
in therapeutic development. Consequently, exploration of the
synergistic effects of chloroxylenol in combination with other
anticancer agents should be further evaluated to fully exploit the
potential of this small molecule targeting the Wnt/β-catenin
signaling pathway.
Chloroxylenol is an antimicrobial chemical agent
with a long history of safe use in topical antiseptic drug products
for over-the-counter human use. The toxicological data for
chloroxylenol indicate minimal systemic toxicity, and a lack of
genotoxicity and carcinogenicity (45). In the present study, it was
demonstrated that chloroxylenol could downregulate the
Wnt/β-catenin signaling pathway and inhibit the stemness of CRC
cells. Concerning the crucial role of Wnt/β-catenin signaling in
development and somatic stem cell biology (58), further research should investigate
whether long-term exposure to environmental doses of chloroxylenol
has any adverse effects on embryonic development and tissue stem
cell behavior in organ systems, including the gut, the
hematopoietic system and the nervous system.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QS, GY and DL developed the concept and designed the
present study. QS, BL, QL, ZS, QF, LW, CL and YD performed the
experiments and data acquisition. QS, BL, VWX, SL, XC and DL
performed data analysis. QS and DL edited and revised the
manuscript. DL supervised this study. All authors read and approved
this manuscript.
Ethics approval and consent to
participate
The ethics committee of Shenzhen University approved
the research (approval nos. AEWC-2021006 and PN-2022-001).
Patient consent for publication
All patients involved in this study gave consent
for the publication of the data.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
APC
|
adenomatous polyposis coli
|
|
LRP6
|
low density lipoprotein
receptor-related protein 6
|
|
DVL
|
Disheveled
|
|
FZD
|
Frizzled
|
|
CK1
|
casein kinase 1
|
|
TCF
|
T cell factor
|
|
LEF
|
Lymphoid-enhancing factor
|
|
CSCs
|
cancer stem cells
|
Acknowledgments
Not applicable.
Funding
This work was supported by The National Natural Science
Foundation of China (grant nos. 31970739 and 82273485), The Natural
Science Foundation of Guangdong Province (grant nos.
2020A1515010340 and 2022A1515010598), The Shenzhen Key Basic
Research Program (grant no. JCYJ20200109105001821) and The Shenzhen
Natural Science Fund (the Stable Support Plan Program) (grant no.
20200826134656001).
References
|
1
|
Rundle CW, Hu S, Presley CL and Dunnick
CA: Triclosen and its alternatives in antibacterial soaps.
Dermatitis. 30:352–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sreevidya VS, Lenz KA, Svoboda KR and Ma
H: Benzalkonium chloride, benzethonium chloride, and
chloroxylenol-three replacement antimicrobials are more toxic than
triclosan and triclocarban in two model organisms. Environ Pollut.
235:814–824. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antunes SC, Nunes B, Rodrigues S, Nunes R,
Fernandes J and Correia AT: Effects of chronic exposure to
benzalkonium chloride in Oncorhynchus mykiss: Cholinergic
neurotoxicity, oxidative stress, peroxidative damage and
genotoxicity. Environ Toxicol Pharmacol. 45:115–122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Food and Drug Adminnistration, HHS: Safety
and effectiveness of consumer antiseptics; topical antimicrobial
drug products for over-the-counter human use. Final rule. Fed
Regist. 81:61106–61130. 2016.PubMed/NCBI
|
|
5
|
Wang J, Shan S, Li D, Zhang Z and Ma Q:
Long-term influence of chloroxylenol on anaerobic microbial
community: Performance, microbial interaction, and antibiotic
resistance gene behaviors. Sci Total Environ. 897:1653302023.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Wagle NS, Cercek A, Smith RA
and Jemal A: Colorectal cancer statistics, 2023. CA Cancer J Clin.
73:233–254. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biller LH and Schrag D: Diagnosis and
treatment of metastatic colorectal cancer: A review. JAMA.
325:669–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doubeni CA, Corley DA, Quinn VP, Jensen
CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao
WK, et al: Effectiveness of screening colonoscopy in reducing the
risk of death from right and left colon cancer: A large
community-based study. Gut. 67:291–298. 2018. View Article : Google Scholar
|
|
9
|
Song M, Garrett WS and Chan AT: Nutrients,
foods, and colorectal cancer prevention. Gastroenterology.
148:1244–1260.e16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lytle NK, Barber AG and Reya T: Stem cell
fate in cancer growth, progression and therapy resistance. Nat Rev
Cancer. 18:669–680. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medema JP: Targeting the colorectal cancer
stem cell. N Engl J Med. 377:888–890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimokawa M, Ohta Y, Nishikori S, Matano
M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T and Sato T:
Visualization and targeting of LGR5+ human colon cancer
stem cells. Nature. 545:187–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frank MH, Wilson BJ, Gold JS and Frank NY:
Clinical implications of colorectal cancer stem cells in the age of
single-cell omics and targeted therapies. Gastroenterology.
160:1947–1960. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Genome Atlas Network: Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wood LD, Parsons DW, Jones S, Lin J,
Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al: The
genomic landscapes of human breast and colorectal cancers. Science.
318:1108–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L and Shay JW: Multiple roles of APC
and its therapeutic implications in colorectal cancer. J Natl
Cancer Inst. 109:djw3322017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar
|
|
26
|
Bilic J, Huang YL, Davidson G, Zimmermann
T, Cruciat CM, Bienz M and Niehrs C: Wnt induces LRP6 signalosomes
and promotes dishevelled-dependent LRP6 phosphorylation. Science.
316:1619–1622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar
|
|
28
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. EMBO J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Behrens J, von Kries JP, Kühl M, Bruhn L,
Wedlich D, Grosschedl R and Birchmeier W: Functional interaction of
beta-catenin with the transcription factor LEF-1. Nature.
382:638–642. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Doumpas N, Lampart F, Robinson MD, Lentini
A, Nestor CE, Cantù C and Basler K: TCF/LEF dependent and
independent transcriptional regulation of Wnt/β-catenin target
genes. EMBO J. 38:e988732019. View Article : Google Scholar
|
|
31
|
Poger D and Mark AE: Effect of triclosan
and chloroxylenol on bacterial membranes. J Phys Chem B.
123:5291–5301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cutts TA, Ijaz MK, Nims RW, Rubino JR and
Theriault SS: Effectiveness of dettol antiseptic liquid for
inactivation of Ebola virus in suspension. Sci Rep. 9:65902019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nowak M, Zawadzka K, Szemraj J,
Góralczyk-Bińkowska A and Lisowska K: Biodegradation of
chloroxylenol by cunninghamella elegans IM 1785/21GP and trametes
versicolor IM 373: Insight into ecotoxicity and metabolic pathways.
Int J Mol Sci. 22:43602021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Zhou L, Xiong Y, Yu S, Li H, Fan
J, Li F, Su Z, Song J, Sun Q, et al: Salinomycin exerts
anti-colorectal cancer activity by targeting the β-catenin/T-cell
factor complex. Br J Pharmacol. 176:3390–3406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Wang L, Deng K, Gong L, Zhou L, Sayed S,
Li H, Sun Q, Su Z, Wang Z, Liu S, et al: Chlorquinaldol targets the
β-catenin and T-cell factor 4 complex and exerts anti-colorectal
cancer activity. Pharmacol Res. 159:1049552020. View Article : Google Scholar
|
|
37
|
Sun Q, Wang Y, Fu Q, Ouyang A, Liu S, Wang
Z, Su Z, Song J, Zhang Q, Zhang P and Lu D: Sulfur-coordinated
organoiridium(III) complexes exert breast anticancer activity via
inhibition of Wnt/β-catenin signaling. Angew Chem Int Ed Engl.
60:4841–4848. 2021. View Article : Google Scholar
|
|
38
|
Zhao H, Ming T, Tang S, Ren S, Yang H, Liu
M, Tao Q and Xu H: Wnt signaling in colorectal cancer: Pathogenic
role and therapeutic target. Mol Cancer. 21:1442022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hagen T, Di Daniel E, Culbert AA and Reith
AD: Expression and characterization of GSK-3 mutants and their
effect on beta-catenin phosphorylation in intact cells. J Biol
Chem. 277:23330–23335. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu C, Xu Z, Zhang Y, Evert M, Calvisi DF
and Chen X: β-Catenin signaling in hepatocellular carcinoma. J Clin
Invest. 132:e1545152022. View Article : Google Scholar
|
|
41
|
Su Z, Song J, Wang Z, Zhou L, Xia Y, Yu S,
Sun Q, Liu SS, Zhao L, Li S, et al: Tumor promoter TPA activates
Wnt/β-catenin signaling in a casein kinase 1-dependent manner. Proc
Natl Acad Sci USA. 115:E7522–E7531. 2018. View Article : Google Scholar
|
|
42
|
Bazzi H, Fantauzzo KA, Richardson GD,
Jahoda CA and Christiano AM: The Wnt inhibitor, Dickkopf 4, is
induced by canonical Wnt signaling during ectodermal appendage
morphogenesis. Dev Biol. 305:498–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lundberg IV, Edin S, Eklöf V, Öberg Å,
Palmqvist R and Wikberg ML: SOX2 expression is associated with a
cancer stem cell state and down-regulation of CDX2 in colorectal
cancer. BMC Cancer. 16:4712016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dellanno C, Vega Q and Boesenberg D: The
antiviral action of common household disinfectants and antiseptics
against murine hepatitis virus, a potential surrogate for SARS
coronavirus. Am J Infect Control. 37:649–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yost LJ, Rodricks JD, Turnbull D, DeLeo
PC, Nash JF, Quiñones-Rivera A and Carlson PA: Human health risk
assessment of chloroxylenol in liquid hand soap and dishwashing
soap used by consumers and health-care professionals. Regul Toxicol
Pharmacol. 80:116–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang H, Wang W, Romano KA, Gu M, Sanidad
KZ, Kim D, Yang J, Schmidt B, Panigrahy D, Pei R, et al: A common
antimicrobial additive increases colonic inflammation and
colitis-associated colon tumorigenesis in mice. Sci Transl Med.
10:eaan41162018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang H, Sanidad KZ, Wang W, Xie M, Gu M,
Cao X, Xiao H and Zhang G: Triclocarban exposure exaggerates
colitis and colon tumorigenesis: Roles of gut microbiota involved.
Gut Microbes. 12:16903642020. View Article : Google Scholar :
|
|
48
|
Sanidad KZ, Yang H, Wang W, Ozay EI, Yang
J, Gu M, Karner E, Zhang J, Kim D, Minter LM, et al: Effects of
consumer antimicrobials benzalkonium chloride, benzethonium
chloride, and chloroxylenol on colonic inflammation and
colitis-associated colon tumorigenesis in mice. Toxicol Sci.
163:490–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaplan GG: The global burden of IBD: From
2015 to 2025. Nat Rev Gastroenterol Hepatol. 12:720–727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rogler G: Chronic ulcerative colitis and
colorectal cancer. Cancer Lett. 345:235–241. 2014. View Article : Google Scholar
|
|
51
|
Rubin DC, Shaker A and Levin MS: Chronic
intestinal inflammation: Inflammatory bowel disease and
colitis-associated colon cancer. Front Immunol. 3:1072012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tian A, Benchabane H and Ahmed Y:
Wingless/Wnt signaling in intestinal development, homeostasis,
regeneration and tumorigenesis: A drosophila perspective. J Dev
Biol. 6:82018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
You J, Nguyen AV, Albers CG, Lin F and
Holcombe RF: Wnt pathway-related gene expression in inflammatory
bowel disease. Dig Dis Sci. 53:1013–1019. 2008. View Article : Google Scholar
|
|
54
|
Swafford D, Shanmugam A, Ranganathan P,
Hussein MS, Koni PA, Prasad PD, Thangaraju M and Manicassamy S:
Canonical Wnt signaling in CD11c+ APCs regulates
microbiota-induced inflammation and immune cell homeostasis in the
colon. J Immunol. 200:3259–3268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shlush LI, Mitchell A, Heisler L, Abelson
S, Ng SWK, Trotman-Grant A, Medeiros JJF, Rao-Bhatia A,
Jaciw-Zurakowsky I, Marke R, et al: Tracing the origins of relapse
in acute myeloid leukaemia to stem cells. Nature. 547:104–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Phi LTH, Sari IN, Yang YG, Lee SH, Jun N,
Kim KS, Lee YK and Kwon HY: Cancer stem cells (CSCs) in drug
resistance and their therapeutic implications in cancer treatment.
Stem Cells Int. 2018:54169232018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ring A, Kim YM and Kahn M: Wnt/catenin
signaling in adult stem cell physiology and disease. Stem Cell Rev
Rep. 10:512–525. 2014. View Article : Google Scholar : PubMed/NCBI
|