|
1
|
National Cancer Institute: What is cancer?
2021, https://www.cancer.gov/about-cancer/understanding/what-is-cancer.
|
|
2
|
Blackadar CB: Historical review of the
causes of cancer. World J Clin Oncol. 7:54–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Debela DT, Muzazu SG, Heraro KD, Ndalama
MT, Mesele BW, Haile DC, Kitui SK and Manyazewal T: New approaches
and procedures for cancer treatment: Current perspectives. SAGE
Open Med. 9:205031212110343662021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Decker WK and Safdar A: Bioimmunoadjuvants
for the treatment of neoplastic and infectious disease: Coley's
legacy revisited. Cytokine Growth Factor Rev. 20:271–281. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valent P, Groner B, Schumacher U,
Superti-Furga G, Busslinger M, Kralovics R, Zielinski C, Penninger
JM, Kerjaschki D, Stingl G, et al: Paul Ehrlich (1854-1915) and his
contributions to the foundation and birth of translational
medicine. J Innate Immun. 8:111–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenberg SA: IL-2: The first effective
immunotherapy for human cancer. J Immunol. 192:5451–5458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pierpont TM, Limper CB and Richards KL:
Past, present, and future of rituximab-the world's first oncology
monoclonal antibody therapy. Front Oncol. 8:163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
June CH, O'Connor RS, Kawalekar OU,
Ghassemi S and Milone MC: CAR T cell immunotherapy for human
cancer. Science. 359:1361–1365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gross G, Waks T and Eshhar Z: Expression
of immunoglobulin-T-cell receptor chimeric molecules as functional
receptors with antibody-type specificity. Proc Natl Acad Sci USA.
86:10024–10028. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graham C, Hewitson R, Pagliuca A and
Benjamin R: Cancer immunotherapy with CAR-T cells-behold the
future. Clin Med (Lond). 18:324–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maus MV: A decade of CAR T cell evolution.
Nat Cancer. 3:270–271. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cameron BJ, Gerry AB, Dukes J, Harper JV,
Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, et al:
Identification of a Titin-derived HLA-A1-presented peptide as a
cross-reactive target for engineered MAGE A3-directed T cells. Sci
Transl Med. 5:197ra1032013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
June CH, Riddell SR and Schumacher TN:
Adoptive cellular therapy: A race to the finish line. Sci Transl
Med. 7:280ps72015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YH and Kim CH: Evolution of chimeric
antigen receptor (CAR) T cell therapy: current status and future
perspectives. Arch Pharm Res. 42:607–616. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
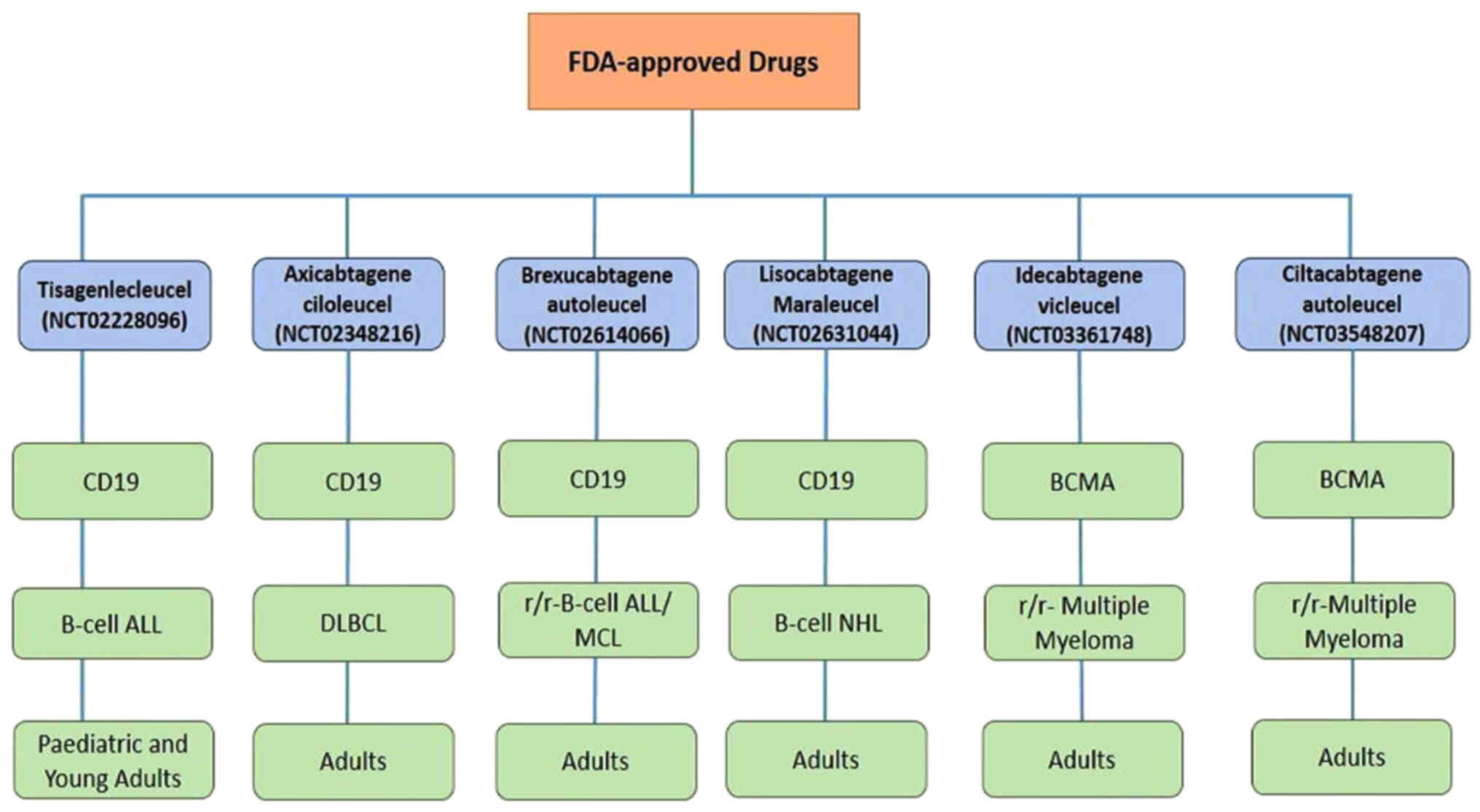
16
|
Titov A, Valiullina A, Zmievskaya E,
Zaikova E, Petukhov A, Miftakhova R, Bulatov E and Rizvanov A:
Advancing CAR T-cell therapy for solid tumors: Lessons learned from
lymphoma treatment. Cancers (Basel). 12:1252020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jayaraman J, Mellody MP, Hou AJ, Desai RP,
Fung AW, Pham AHT, Chen YY and Zhao W: CAR-T design: Elements and
their synergistic function. EBioMedicine. 58:1029312020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie YJ, Dougan M, Jailkhani N, Ingram J,
Fang T, Kummer L, Momin N, Pishesha N, Rickelt S, Hynes RO and
Ploegh H: Nanobody-based CAR T cells that target the tumor
microenvironment inhibit the growth of solid tumors in
immunocompetent mice. Proc Natl Acad Sci USA. 116:7624–7631. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barber A, Rynda A and Sentman CL: Chimeric
NKG2D expressing T cells eliminate immunosuppression and activate
immunity within the ovarian tumor microenvironment. J Immunol.
183:6939–6947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynn RC, Feng Y, Schutsky K, Poussin M,
Kalota A, Dimitrov DS and Powell DJ Jr: High-affinity FRβ-specific
CAR T cells eradicate AML and normal myeloid lineage without HSC
toxicity. Leukemia. 30:1355–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guest RD, Hawkins RE, Kirillova N, Cheadle
EJ, Arnold J, O'Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM,
et al: The role of extracellular spacer regions in the optimal
design of chimeric immune receptors: Evaluation of four different
scFvs and antigens. J Immunother. 28:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hudecek M, Sommermeyer D, Kosasih PL,
Silva-Benedict A, Liu L, Rader C, Jensen MC and Riddell SR: The
nonsignaling extracellular spacer domain of chimeric antigen
receptors is decisive for in vivo antitumor activity. Cancer
Immunol Res. 3:125–135. 2015. View Article : Google Scholar
|
|
23
|
Zhang C, Liu J, Zhong JF and Zhang X:
Engineering CAR-T cells. Biomark Res. 5:222017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11:692021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohanty R, Chowdhury CR, Arega S, Sen P,
Ganguly P and Ganguly N: CAR T cell therapy: A new era for cancer
treatment (Review). Oncol Rep. 42:2183–2195. 2019.PubMed/NCBI
|
|
26
|
Zhang Q, Ping J, Huang Z, Zhang X, Zhou J,
Wang G, Liu S and Ma J: CAR-T cell therapy in cancer: Tribulations
and road ahead. J Immunol Res. 2020:19243792020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duong CP, Yong CS, Kershaw MH, Slaney CY
and Darcy PK: Cancer immunotherapy utilizing gene-modified T cells:
From the bench to the clinic. Mol Immunol. 67:46–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian L, Li D, Ma L, He T, Qi F, Shen J and
Lu XA: The novel anti-CD19 chimeric antigen receptors with
humanized scFv (single-chain variable fragment) trigger leukemia
cell killing. Cell Immunol. 304:49–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lock D, Mockel-Tenbrinck N, Drechsel K,
Barth C, Mauer D, Schaser T, Kolbe C, Al Rawashdeh W, Brauner J,
Hardt O, et al: Automated manufacturing of potent CD20-directed
chimeric antigen receptor t cells for clinical use. Hum Gene Ther.
28:914–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Z, Condomines M, van der Stegen SJC,
Perna F, Kloss CC, Gunset G, Plotkin J and Sadelain M: Structural
design of engineered costimulation determines tumor rejection
kinetics and persistence of CAR T cells. Cancer Cell. 28:415–428.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hombach A, Hombach AA and Abken H:
Adoptive immunotherapy with genetically engineered T cells:
Modification of the IgG1 Fc 'spacer' domain in the extracellular
moiety of chimeric antigen receptors avoids 'off-target'activation
and unintended initiation of an innate immune response. Gene Ther.
17:1206–1213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong XS, Matsushita M, Plotkin J, Riviere
I and Sadelain M: Chimeric antigen receptors combining 4-1BB and
CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and
CD8+ T cell-mediated tumor eradication. Mol Ther. 18:413–420. 2010.
View Article : Google Scholar
|
|
34
|
Quintarelli C, Orlando D, Boffa I, Guercio
M, Polito VA, Petretto A, Lavarello C, Sinibaldi M, Weber G, Del
Bufalo F, et al: Choice of costimulatory domains and of cytokines
determines CAR T-cell activity in neuroblastoma. Oncoimmunology.
7:e14335182018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abate-Daga D, Lagisetty KH, Tran E, Zheng
Z, Gattinoni L, Yu Z, Burns WR, Miermont AM, Teper Y, Rudloff U, et
al: A novel chimeric antigen receptor against prostate stem cell
antigen mediates tumor destruction in a humanized mouse model of
pancreatic cancer. Hum Gene Ther. 25:1003–1012. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pulè MA, Straathof KC, Dotti G, Heslop HE,
Rooney CM and Brenner MK: A chimeric T cell antigen receptor that
augments cytokine release and supports clonal expansion of primary
human T cells. Mol Ther. 12:933–941. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beatty GL and Moon EK: Chimeric antigen
receptor T cells are vulnerable to immunosuppressive mechanisms
present within the tumor microenvironment. Oncoimmunology.
3:e9700272014. View Article : Google Scholar
|
|
38
|
Chmielewski M and Abken H: TRUCKs: The
fourth generation of CARs. Expert Opin Biol Ther. 15:1145–1154.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kueberuwa G, Kalaitsidou M, Cheadle E,
Hawkins RE and Gilham DE: CD19 CAR T cells expressing IL-12
eradicate lymphoma in fully lymphoreplete mice through induction of
host immunity. Mol Ther Oncolytics. 8:41–51. 2017. View Article : Google Scholar
|
|
40
|
John LB, Devaud C, Duong CP, Yong CS,
Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH and Darcy PK:
Anti-PD-1 antibody therapy potently enhances the eradication of
established tumors by gene-modified T cells. Clin Cancer Res.
19:5636–5646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim DW and Cho JY: Recent advances in
allogeneic CAR-T cells. Biomolecules. 10:2632020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kagoya Y, Tanaka S, Guo T, Anczurowski M,
Wang CH, Saso K, Butler MO, Minden MD and Hirano N: A novel
chimeric antigen receptor containing a JAK-STAT signaling domain
mediates superior antitumor effects. Nat Med. 24:352–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai H, Wang Y, Lu X and Han W: Chimeric
antigen receptors modified T-cells for cancer therapy. J Natl
Cancer Inst. 108:djv4392016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li D, Li X, Zhou WL, Huang Y, Liang X,
Jiang L, Yang X, Sun J, Li Z, Han WD and Wang W: Genetically
engineered T cells for cancer immunotherapy. Signal Transduct
Target Ther. 4:352019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Levine BL, Miskin J, Wonnacott K and Keir
C: Global manufacturing of CAR T cell therapy. Mol Ther Methods
Clin Dev. 4:92–101. 2016. View Article : Google Scholar
|
|
46
|
Benmebarek MR, Karches CH, Cadilha BL,
Lesch S, Endres S and Kobold S: Killing mechanisms of chimeric
antigen receptor (CAR) T cells. Int J Mol Sci. 20:12832019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo S and Deng CX: Effect of stromal cells
in tumor microenvironment on metastasis initiation. Int J Biol Sci.
14:2083–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morton JJ, Bird G, Keysar SB, Astling DP,
Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, et
al: XactMice: Humanizing mouse bone marrow enables microenvironment
reconstitution in a patient-derived xenograft model of head and
neck cancer. Oncogene. 35:290–300. 2016. View Article : Google Scholar
|
|
49
|
Najima Y, Tomizawa-Murasawa M, Saito Y,
Watanabe T, Ono R, Ochi T, Suzuki N, Fujiwara H, Ohara O, Shultz
LD, et al: Induction of WT1-specific human CD8+ T cells from human
HSCs in HLA class I Tg NOD/SCID/IL2rgKO mice. Blood. 127:722–734.
2016. View Article : Google Scholar :
|
|
50
|
Yin L and Wang XJ, Chen DX, Liu XN and
Wang XJ: Humanized mouse model: A review on preclinical
applications for cancer immunotherapy. Am J Cancer Res.
10:4568–4584. 2020.
|
|
51
|
Zitvogel L, Pitt JM, Daillère R, Smyth MJ
and Kroemer G: Mouse models in on immunology. Nat Rev Cancer.
16:759–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Slabik C, Kalbarczyk M, Danisch S, Zeidler
R, Klawonn F, Volk V, Krönke N, Feuerhake F, Ferreira de Figueiredo
C, Blasczyk R, et al: CAR-T cells targeting Epstein-Barr virus
gp350 validated in a humanized mouse model of EBV infection and
lymphoproliferative disease. Mol Ther Oncolytics. 18:504–524. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin CH, Xia J, Rafiq S, Huang X, Hu Z,
Zhou X, Brentjens RJ and Yang YG: Modeling anti-CD19 CAR T cell
therapy in humanized mice with human immunity and autologous
leukemia. EBioMedicine. 39:173–181. 2019. View Article : Google Scholar :
|
|
54
|
Gulati P, Rühl J, Kannan A, Pircher M,
Schuberth P, Nytko KJ, Pruschy M, Sulser S, Haefner M, Jensen S, et
al: Aberrant Lck signal via CD28 costimulation augments
antigen-specific functionality and tumor control by redirected T
cells with PD-1 blockade in humanized mice. Clin Cancer Res.
24:3981–3993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar
|
|
56
|
Szöőr Á, Tóth G, Zsebik B, Szabó V, Eshhar
Z, Abken H and Vereb G: Trastuzumab derived HER2-specific CARs for
the treatment of trastuzumab-resistant breast cancer: CAR T cells
penetrate and eradicate tumors that are not accessible to
antibodies. Cancer Lett. 484:1–8. 2020. View Article : Google Scholar
|
|
57
|
Liu Y, Zhou Y, Huang KH, Li Y, Fang X, An
L, Wang F, Chen Q, Zhang Y, Shi A, et al: EGFR-specific CAR-T cells
trigger cell lysis in EGFR-positive TNBC. Aging (Albany NY).
11:11054–11072. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Corti C, Venetis K, Sajjadi E, Zattoni L,
Curigliano G and Fusco N: CAR-T cell therapy for triple-negative
breast cancer and other solid tumors: Preclinical and clinical
progress. Expert Opin Investig Drugs. 31:593–605. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wei J, Sun H, Zhang A, Wu X, Li Y, Liu J,
Duan Y, Xiao F, Wang H, Lv M, et al: A novel AXL chimeric antigen
receptor endows T cells with anti-tumor effects against triple
negative breast cancers. Cell Immunol. 331:49–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wallstabe L, Mades A, Frenz S, Einsele H,
Rader C and Hudecek M: CAR T cells targeting
αvβ3 integrin are effective against advanced
cancer in preclinical models. Adv Cell Gene Ther. 1:e112018.
View Article : Google Scholar
|
|
61
|
Zhao X, Qu J, Hui Y, Zhang H, Sun Y, Liu
X, Zhao X, Zhao Z, Yang Q, Wang F and Zhang S: Clinicopathological
and prognostic significance of c-Met overexpression in breast
cancer. Oncotarget. 8:56758–56767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Han Y, Xie W, Song DG and Powell DJ Jr:
Control of triple-negative breast cancer using ex vivo
self-enriched, costimulated NKG2D CAR T cells. J Hematol Oncol.
11:922018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou R, Yazdanifar M, Roy LD, Whilding LM,
Gavrill A, Maher J and Mukherjee P: CAR T cells targeting the tumor
MUC1 glycoprotein reduce triple-negative breast cancer growth.
Front Immunol. 10:11492019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wallstabe L, Göttlich C, Nelke LC,
Kühnemundt J, Schwarz T, Nerreter T, Einsele H, Walles H, Dandekar
G, Nietzer SL and Hudecek M: ROR1-CAR T cells are effective against
lung and breast cancer in advanced microphysiologic 3D tumor
models. JCI Insight. 4:e1263452019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao Z, Li Y, Liu W and Li X: Engineered
IL-7 receptor enhances the therapeutic effect of AXL-CAR-T cells on
triple-negative breast cancer. Biomed Res Int.
2020:47951712020.PubMed/NCBI
|
|
66
|
Caratelli S, Arriga R, Sconocchia T,
Ottaviani A, Lanzilli G, Pastore D, Cenciarelli C, Venditti A, Del
Principe MI, Lauro D, et al: In vitro elimination of epidermal
growth factor receptor-overexpressing cancer cells by
CD32A-chimeric receptor T cells in combination with cetuximab or
panitumumab. Int J Cancer. 146:236–247. 2020. View Article : Google Scholar
|
|
67
|
Song DG, Ye Q, Poussin M, Chacon JA,
Figini M and Powell DJ Jr: Effective adoptive immunotherapy of
triple-negative breast cancer by folate receptor-alpha redirected
CAR T cells is influenced by surface antigen expression level. J
Hematol Oncol. 9:562016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Seitz CM, Schroeder S, Knopf P, Krahl AC,
Hau J, Schleicher S, Martella M, Quintanilla-Martinez L, Kneilling
M, Pichler B, et al: GD2-targeted chimeric antigen receptor T cells
prevent metastasis formation by elimination of breast cancer
stem-like cells. Oncoimmunology. 9:16833452019. View Article : Google Scholar
|
|
69
|
Yang Y, Vedvyas Y, McCloskey JE, Min IM
and Jin MM: ICAM-1 targeting CAR T cell therapy for triple negative
breast cancer. Cancer Res. 79(13 Suppl): S23222019. View Article : Google Scholar
|
|
70
|
Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma
X and Wei F: CRISPR/Cas9-mediated PD-1 disruption enhances human
mesothelin-targeted CAR T cell effector functions. Cancer Immunol
Immunother. 68:365–377. 2019. View Article : Google Scholar
|
|
71
|
Petrovic K, Robinson J, Whitworth K, Jinks
E, Shaaban A and Lee SP: TEM8/ANTXR1-specific CAR T cells mediate
toxicity in vivo. PLoS One. 14:e02240152019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Byrd TT, Fousek K, Pignata A, Szot C,
Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K,
et al: TEM8/ANTXR1-specific CAR T cells as a targeted therapy for
triple-negative breast cancer. Cancer Res. 78:489–500. 2018.
View Article : Google Scholar :
|
|
73
|
Bedoya DM, King T and Posey AD: Generation
of CART cells targeting oncogenic TROP2 for the elimination of
epithelial malignancies. Cytotherapy. 21(Suppl): S11–S12. 2019.
View Article : Google Scholar
|
|
74
|
National Library of Medicine (NLM):
Genetically Modified T-cells in Treating Patients With Recurrent or
Refractory Malignant Glioma. ClinicalTrials.gov ID, NCT02208362.
NLM; Bethesda, MD: 2015, https://clinicaltrials.gov/study/NCT02208362.
|
|
75
|
National Library of Medicine (NLM):
CART-EGFRvIII + Pembrolizumab in GBM. ClinicalTrials.gov ID,
NCT03726515. NLM; Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT03726515.
|
|
76
|
National Library of Medicine (NLM):
GPC3-CAR-T Cells for Immunotherapy of Cancer With GPC3 Expression.
ClinicalTrials.gov ID, NCT03198546. NLM; Bethesda, MD: 2017,
https://clinicaltrials.gov/ct2/show/NCT03198546.
|
|
77
|
National Library of Medicine (NLM): Her2
Chimeric Antigen Receptor Expressing T Cells in Advanced Sarcoma.
ClinicalTrials.gov ID, NCT00902044. NLM; Bethesda, MD: 2010,
https://clinicaltrials.gov/ct2/show/NCT00902044.
|
|
78
|
National Library of Medicine (NLM):
CEA-Expressing Liver Metastases Safety Study of Intrahepatic
Infusions of Anti-CEA Designer T Cells (HITM). ClinicalTrials.gov
ID, NCT01373047. NLM; Bethesda, MD: 2011, https://clinicaltrials.gov/ct2/show/NCT01373047.
|
|
79
|
National Library of Medicine (NLM):
Autologous Redirected RNA Meso CAR T Cells for Pancreatic Cancer.
ClinicalTrials.gov ID, NCT01897415. NLM; Bethesda, MD: 2013,
https://clinicaltrials.gov/ct2/show/NCT01897415.
|
|
80
|
National Library of Medicine (NLM): CAR T
Cell Immunotherapy for Pancreatic Cancer. ClinicalTrials.gov ID,
NCT03323944. NLM; Bethesda, MD: 2017, https://clinicaltrials.gov/ct2/show/NCT03323944.
|
|
81
|
National Library of Medicine (NLM):
Clinical Study of CAR-CLD18 T Cells in Patients With Advanced
Gastric Adenocarcinoma and Pancreatic Adenocarcinoma.
ClinicalTrials.gov ID, NCT03159819. NLM; Bethesda, MD: 2017,
https://clinicaltrials.gov/ct2/show/NCT03159819.
|
|
82
|
National Library of Medicine (NLM): Safety
and Efficacy of CCT301 CAR-T in Adult Subjects With Recurrent or
Refractory Stage IV Renal Cell Carcinoma. ClinicalTrials.gov ID,
NCT03393936. NLM; Bethesda, MD: 2018, https://clinicaltrials.gov/ct2/show/NCT03393936.
|
|
83
|
National Library of Medicine (NLM):
PSCA-CAR T Cells in Treating Patients With PSCA+ Metastatic
Castration Resistant Prostate Cancer. ClinicalTrials.gov ID,
NCT03873805. NLM; Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT03873805.
|
|
84
|
National Library of Medicine (NLM):
CART-PSMA-TGFβRDN Cells for Castrate-Resistant Prostate Cancer.
ClinicalTrials.gov ID, NCT03089203. NLM; Bethesda, MD: 2017,
https://clinicaltrials.gov/ct2/show/NCT03089203.
|
|
85
|
National Library of Medicine (NLM):
Autologous huMNC2-CAR44 or huMNC2-CAR22 T Cells for Breast Cancer
Targeting Cleaved Form of MUC1 (MUC1*). ClinicalTrials.gov ID,
NCT04020575. NLM; Bethesda, MD: 2020, https://clinicaltrials.gov/ct2/show/NCT04020575.
|
|
86
|
National Library of Medicine (NLM):
MOv19-BBz CAR T Cells in aFR Expressing Recurrent High Grade Serous
Ovarian, Fallopian Tube, or Primary Peritoneal Cancer.
ClinicalTrials.gov ID, NCT03585764. NLM; Bethesda, MD: 2018,
https://clinicaltrials.gov/ct2/show/NCT03585764.
|
|
87
|
National Library of Medicine (NLM):
Cyclophosphamide Followed by Intravenous and Intraperitoneal
Infusion of Autologous T Cells Genetically Engineered to Secrete
IL-12 and to Target the MUC16ecto Antigen in Patients With
Recurrent MUC16ecto+ Solid Tumors. ClinicalTrials.gov ID,
NCT02498912. NLM; Bethesda, MD: 2015, https://clinicaltrials.gov/ct2/show/NCT02498912.
|
|
88
|
National Library of Medicine (NLM): T-Cell
Therapy for Advanced Breast Cancer. ClinicalTrials.gov ID,
NCT02792114. NLM; Bethesda, MD: 2016, https://clinicaltrials.gov/ct2/show/NCT02792114.
|
|
89
|
National Library of Medicine (NLM): T
Cells Expressing HER2-specific Chimeric Antigen Receptors(CAR) for
Patients With HER2-Positive CNS Tumors (iCAR). ClinicalTrials.gov
ID, NCT02442297. NLM; Bethesda, MD: 2016, https://clinicaltrials.gov/ct2/show/NCT02442297.
|
|
90
|
National Library of Medicine (NLM):
HER2-CAR T Cells in Treating Patients With Recurrent Brain or
Leptomeningeal Metastases. ClinicalTrials.gov ID, NCT03696030. NLM;
Bethesda, MD: 2018, https://clinicaltrials.gov/ct2/show/NCT03696030.
|
|
91
|
National Library of Medicine (NLM):
Malignant Pleural Disease Treated With Autologous T Cells
Genetically Engineered to Target the Cancer-Cell Surface Antigen
Mesothelin. ClinicalTrials.gov ID, NCT02414269. NLM; Bethesda, MD:
2015, https://clinicaltrials.gov/ct2/show/NCT02414269.
|
|
92
|
National Library of Medicine (NLM):
Precursor B Cell Acute Lymphoblastic Leukemia (B-ALL) Treated With
Autologous T Cells Genetically Targeted to the B Cell Specific
Antigen CD19. ClinicalTrials.gov ID, NCT01044069. NLM; Bethesda,
MD: 2010, https://clinicaltrials.gov/ct2/show/NCT01044069.
|
|
93
|
National Library of Medicine (NLM):
Treatment of Relapsed or Chemotherapy Refractory Chronic
Lymphocytic Leukemia or Indolent B Cell Lymphoma Using Autologous T
Cells Genetically Targeted to the B Cell Specific Antigen CD19.
ClinicalTrials.gov ID, NCT00466531. NLM; Bethesda, MD: 2007,
https://clinicaltrials.gov/ct2/show/NCT00466531.
|
|
94
|
National Library of Medicine (NLM): CD19
Chimeric Receptor Expressing T Lymphocytes In B-Cell Non Hodgkin's
Lymphoma, ALL & CLL (CRETI-NH). ClinicalTrials.gov ID,
NCT00586391. NLM; Bethesda, MD: 2009, https://clinicaltrials.gov/ct2/show/NCT00586391.
|
|
95
|
National Library of Medicine (NLM): CD19
Chimeric Receptor Expressing T Lymphocytes In B-Cell Non Hodgkin's
Lymphoma, ALL & CLL (CRETI-NH). ClinicalTrials.gov ID,
NCT00608270. NLM; Bethesda, MD: 2009, https://clinicaltrials.gov/ct2/show/NCT00608270.
|
|
96
|
National Library of Medicine (NLM):
Anti-CD22 Chimeric Receptor T Cells in Pediatric and Young Adults
With Recurrent or Refractory CD22-expressing B Cell Malignancies.
ClinicalTrials.gov ID, NCT02315612. NLM; Bethesda, MD: 2014,
https://clinicaltrials.gov/ct2/show/NCT02315612.
|
|
97
|
National Library of Medicine (NLM):
Re-directed T Cells for the Treatment (FAP)-Positive Malignant
Pleural Mesothelioma. ClinicalTrials.gov ID, NCT01722149. NLM;
Bethesda, MD: 2015, https://clinicaltrials.gov/ct2/show/NCT01722149.
|
|
98
|
National Library of Medicine (NLM):
Engineered Neuroblastoma Cellular Immunotherapy (ENCIT)-01.
ClinicalTrials.gov ID, NCT02311621. NLM; Bethesda, MD: 2014,
https://clinicaltrials.gov/ct2/show/NCT02311621.
|
|
99
|
National Library of Medicine (NLM): Study
of bb21217 in Multiple Myeloma. ClinicalTrials.gov ID, NCT03274219.
NLM; Bethesda, MD: 2017, https://clinicaltrials.gov/ct2/show/NCT03274219.
|
|
100
|
National Library of Medicine (NLM):
Kappa-CD28 T Lymphocytes, Chronic Lymphocytic Leukemia, B-cell
Lymphoma or Multiple Myeloma, CHARKALL (CHARKALL).
ClinicalTrials.gov ID, NCT00881920. NLM; Bethesda, MD: 2009,
https://clinicaltrials.gov/ct2/show/NCT00881920.
|
|
101
|
National Library of Medicine (NLM):
KSafety and Efficacy of ALLO-501 Anti-CD19 Allogeneic CAR T Cells
in Adults With Relapsed/Refractory Large B Cell or Follicular
Lymphoma (ALPHA). ClinicalTrials.gov ID, NCT03939026. NLM;
Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT03939026.
|
|
102
|
National Library of Medicine (NLM):
Dose-escalation, Dose-expansion Study of Safety of PBCAR0191 in
Patients With r/r NHL and r/r B-cell ALL. ClinicalTrials.gov ID,
NCT03666000. NLM; Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT03666000.
|
|
103
|
National Library of Medicine (NLM): A
Safety and Efficacy Study Evaluating CTX110 in Subjects With
Relapsed or Refractory B-Cell Malignancies (CARBON).
ClinicalTrials.gov ID, NCT04035434. NLM; Bethesda, MD: 2019,
https://clinicaltrials.gov/ct2/show/NCT04035434.
|
|
104
|
National Library of Medicine (NLM): Study
Evaluating Safety and Efficacy of UCART123 in Patients With
Relapsed/Refractory Acute Myeloid Leukemia (AMELI-01).
ClinicalTrials.gov ID, NCT03190278. NLM; Bethesda, MD: 2017,
https://clinicaltrials.gov/ct2/show/NCT03190278.
|
|
105
|
National Library of Medicine (NLM): Safety
and Efficacy of ALLO-715 BCMA Allogenic CAR T Cells in in Adults
With Relapsed or Refractory Multiple Myeloma (UNIVERSAL)
(UNIVERSAL). ClinicalTrials.gov ID, NCT04093596. NLM; Bethesda, MD:
2019, https://clinicaltrials.gov/ct2/show/NCT04093596.
|
|
106
|
National Library of Medicine (NLM):
SaalloSHRINK - Standard cHemotherapy Regimen and Immunotherapy With
Allogeneic NKG2D-based CYAD-101 Chimeric Antigen Receptor T-cells
(alloSHRINK). ClinicalTrials.gov ID, NCT03692429. NLM; Bethesda,
MD: 2018, https://clinicaltrials.gov/ct2/show/NCT03692429.
|
|
107
|
National Library of Medicine (NLM):
Adoptive Transfer of Autologous T Cells Targeted to Prostate
Specific Membrane Antigen (PSMA) for the Treatment of Castrate
Metastatic Prostate Cancer (CMPC). ClinicalTrials.gov ID,
NCT01140373. NLM; Bethesda, MD: 2010, https://clinicaltrials.gov/ct2/show/NCT01140373.
|
|
108
|
National Library of Medicine (NLM): 3rd
Generation GD-2 Chimeric Antigen Receptor and iCaspase Suicide
Safety Switch, Neuroblastoma, GRAIN (GRAIN). ClinicalTrials.gov ID,
NCT01822652. NLM; Bethesda, MD: 2013, https://clinicaltrials.gov/ct2/show/NCT01822652.
|
|
109
|
National Library of Medicine (NLM):
iC9-GD2-CARVZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma
and Neuroblastoma (VEGAS). ClinicalTrials.gov ID, NCT01953900. NLM;
Bethesda, MD: 2014, https://clinicaltrials.gov/ct2/show/NCT01953900.
|
|
110
|
Almond LM, Charalampakis M, Ford SJ,
Gourevitch D and Desai A: Myeloid sarcoma: Presentation, diagnosis,
and treatment. Clin Lymphoma Myeloma Leuk. 17:263–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Locke FL, Neelapu SS, Bartlett NL, Siddiqi
T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, et
al: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19
anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol
Ther. 25:285–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jain MD, Bachmeier CA, Phuoc VH and Chavez
JC: Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy
for the treatment of relapsed/refractory aggressive B-cell
non-Hodgkin's lymphoma. Ther Clin Risk Manag. 14:1007–1017. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Abramson JS, Palomba ML, Gordon LI,
Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG,
Andreadis C, et al: Lisocabtagene maraleucel for patients with
relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001):
A multicentre seamless design study. Lancet. 396:839–852. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Abbott RC, Cross RS and Jenkins MR:
Finding the keys to the CAR: Identifying novel target antigens for
T cell redirection immunotherapies. Int J Mol Sci. 21:5152020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Posey AD Jr, Schwab RD, Boesteanu AC,
Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K,
Haines KM, et al: Engineered CAR T cells targeting the
cancer-associated Tn-glycoform of the membrane mucin MUC1 control
adenocarcinoma. Immunity. 44:1444–1454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Li X, Ding Y, Zi M, Sun L, Zhang W, Chen S
and Xu Y: CD19, from bench to bedside. Immunol Lett. 183:86–95.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ahmed N, Brawley VS, Hegde M, Robertson C,
Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al:
Human epidermal growth factor receptor 2 (HER2)-specific chimeric
antigen receptor-modified T cells for the immunotherapy of
HER2-positive sarcoma. J Clin Oncol. 33:16882015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cortesi L, Rugo HS and Jackisch C: An
overview of PARP inhibitors for the treatment of breast cancer.
Target Oncol. 16:255–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ye F, Dewanjee S, Li Y, Jha NK, Chen ZS,
Kumar A, Vishakha, Behl T, Jha SK and Tang H: Advancements in
clinical aspects of targeted therapy and immunotherapy in breast
cancer. Mol Cancer. 22:1052023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schoninger SF and Blain SW: The ongoing
search for biomarkers of CDK4/6 inhibitor responsiveness in breast
cancer. Mol Cancer Ther. 19:3–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Martorana F, Motta G, Pavone G, Motta L,
Stella S, Vitale SR, Manzella L and Vigneri P: AKT inhibitors: New
weapons in the fight against breast cancer? Front Pharmacol.
12:6622322021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Goutsouliak K, Veeraraghavan J, Sethunath
V, De Angelis C, Osborne CK, Rimawi MF and Schiff R: Towards
personalized treatment for early stage HER2-positive breast cancer.
Nat Rev Clin Oncol. 17:233–250. 2020. View Article : Google Scholar
|
|
123
|
Tóth G, Szöllősi J, Abken H, Vereb G and
Szöőr Á: A small number of HER2 redirected CAR T cells
significantly improves immune response of adoptively transferred
mouse lymphocytes against human breast cancer xenografts. Int J Mol
Sci. 21:10392020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Toulouie S, Johanning G and Shi Y:
Chimeric antigen receptor T-cell immunotherapy in breast cancer:
Development and challenges. J Cancer. 12:1212–1219. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Carter P, Presta L, Gorman CM, Ridgway JB,
Henner D, Wong WL, Rowland AM, Kotts C, Carver ME and Shepard HM:
Humanization of an anti-p185HER2 antibody for human cancer therapy.
Proc Natl Acad Sci USA. 89:4285–4289. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bou-Dargham MJ, Draughon S, Cantrell V,
Khamis ZI and Sang QA: Advancements in human breast cancer targeted
therapy and immunotherapy. J Cancer. 12:6949–6963. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Rivas SR, Valdez MJM, Govindarajan V,
Seetharam D, Doucet-O'Hare TT, Heiss JD and Shah AH: The role of
HERV-K in cancer stemness. Viruses. 14:20192022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Zhao J, Rycaj K, Geng S, Li M, Plummer JB,
Yin B, Liu H, Xu X, Zhang Y, Yan Y, et al: Expression of human
endogenous retrovirus type K envelope protein is a novel candidate
prognostic marker for human breast cancer. Genes Cancer. 2:914–922.
2011. View Article : Google Scholar
|
|
130
|
Pegram M and Slamon D: Biological
rationale for HER2/neu (c-erbB2) as a target for monoclonal
antibody therapy. Semin Oncol. 27(Suppl 9): S13–S19. 2000.
|
|
131
|
Walsh EM, Keane MM, Wink DA, Callagy G and
Glynn SA: Review of triple negative breast cancer and the impact of
inducible nitric oxide synthase on tumor biology and patient
outcomes. Crit Rev Oncog. 21:333–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tsutsui S, Ohno S, Murakami S, Hachitanda
Y and Oda S: Prognostic value of epidermal growth factor receptor
(EGFR) and its relationship to the estrogen receptor status in 1029
patients with breast cancer. Breast Cancer Res Treat. 71:67–75.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Nasiri F, Kazemi M, Mirarefin SMJ,
Mahboubi Kancha M, Ahmadi Najafabadi M, Salem F, Dashti Shokoohi S,
Evazi Bakhshi S and Safarzadeh Kozani P and Safarzadeh Kozani P:
CAR-T cell therapy in triple-negative breast cancer: Hunting the
invisible devil. Front Immunol. 13:10187862022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Pantelidou C, Sonzogni O, De Oliveria
Taveira M, Mehta AK, Kothari A, Wang D, Visal T, Li MK, Pinto J,
Castrillon JA, et al: PARP inhibitor efficacy depends on
CD8+ T-cell recruitment via intratumoral sting pathway
activation in BRCA-deficient models of triple-negative breast
cancer. Cancer Discov. 9:722–737. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ternette N, Olde Nordkamp MJM, Müller J,
Anderson AP, Nicastri A, Hill AVS, Kessler BM and Li D:
Immunopeptidomic profiling of HLA-A2-positive triple negative
breast cancer identifies potential immunotherapy target antigens.
Proteomics. 18:17004652018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
National Library of Medicine (NLM):
Clinical Study of Recombinant Anti-HER2 Humanized Monoclonal
Antibody (GB221) for Injection. ClinicalTrials.gov ID, NCT04164615.
NLM; Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT04164615.
|
|
137
|
National Library of Medicine (NLM):
Concurrent WOKVAC Vaccination, Chemotherapy, and HER2-Targeted
Monoclonal Antibody Therapy Before Surgery for the Treatment of
Patients With Breast Cancer. ClinicalTrials.gov ID, NCT04329065.
NLM; Bethesda, MD: 2020, https://clinicaltrials.gov/ct2/show/NCT04329065.
|
|
138
|
National Library of Medicine (NLM):
Anti-HER2 Bispecific Antibody ZW25 Activity in Combination With
Chemotherapy With/Without Tislelizumab. ClinicalTrials.gov ID,
NCT04276493. NLM; Bethesda, MD: 2020, https://clinicaltrials.gov/ct2/show/NCT04276493.
|
|
139
|
National Library of Medicine (NLM):
Clinical Study of Recombinant Anti-HER2 Humanized Monoclonal
Antibody for Injection. ClinicalTrials.gov ID, NCT04170595. NLM;
Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT04170595.
|
|
140
|
National Library of Medicine (NLM): A
Study of RC48-ADC Administered Intravenously to Patients With
HER2-Positive Metastatic Breast Cancer With or Without Liver
Metastases. ClinicalTrials.gov ID, NCT03500380. NLM; Bethesda, MD:
2018, https://clinicaltrials.gov/ct2/show/NCT03500380.
|
|
141
|
National Library of Medicine (NLM):
Monoclonal Antibody Plus Chemotherapy in Treating Patients With
Metastatic Breast Cancer That Overexpresses HER2.
ClinicalTrials.gov ID, NCT00019812. NLM; Bethesda, MD: 2003,
https://clinicaltrials.gov/ct2/show/NCT00019812.
|
|
142
|
National Library of Medicine (NLM):
Paclitaxel Plus Monoclonal Antibody Therapy in Treating Women With
Recurrent or Metastatic Breast Cancer. ClinicalTrials.gov ID,
NCT00003539. NLM; Bethesda, MD: 2004, https://clinicaltrials.gov/ct2/show/NCT00003539.
|
|
143
|
National Library of Medicine (NLM):
Trastuzumab and Interleukin-2 in Treating Patients With Metastatic
Breast Cancer. ClinicalTrials.gov ID, NCT00006228. NLM; Bethesda,
MD: 2003, https://clinicaltrials.gov/ct2/show/NCT00006228.
|
|
144
|
National Library of Medicine (NLM):
Chemotherapy Plus Monoclonal Antibody Therapy in Treating Women
With Stage II or Stage IIIA Breast Cancer That Overexpresses HER2.
ClinicalTrials.gov ID, NCT00003992. NLM; Bethesda, MD: 2004,
https://clinicaltrials.gov/ct2/show/NCT00003992.
|
|
145
|
National Library of Medicine (NLM): Impact
of Pegfilgrastim on Trastuzumab Anti-tumor Effect and ADCC in
Operable HER2+ Breast Cancer Breast Cancer (BREASTIMMU02).
ClinicalTrials.gov ID, NCT03571633. NLM; Bethesda, MD: 2018,
https://clinicaltrials.gov/ct2/show/NCT03571633.
|
|
146
|
National Library of Medicine (NLM): A
Study of Pertuzumab in Participants With Metastatic Breast Cancer.
ClinicalTrials.gov ID, NCT02491892. NLM; Bethesda, MD: 2015,
https://clinicaltrials.gov/ct2/show/NCT02491892.
|
|
147
|
National Library of Medicine (NLM):
Trastuzumab and Pertuzumab in Treating Patients With Unresectable
Locally Advanced or Metastatic Breast Cancer That Did Not Respond
to Previous Trastuzumab. ClinicalTrials.gov ID, NCT00301899. NLM;
Bethesda, MD: 2006, https://clinicaltrials.gov/ct2/show/NCT00301899.
|
|
148
|
National Library of Medicine (NLM): A
Study of MRG002 in the Treatment of Patients With HER2-positive
Unresectable Locally Advanced or Metastatic Breast Cancer.
ClinicalTrials.gov ID, NCT04924699. NLM; Bethesda, MD: 2021,
https://clinicaltrials.gov/ct2/show/NCT04924699.
|
|
149
|
National Library of Medicine (NLM): ARX788
in HER2-positive, Metastatic Breast Cancer Subjects
(ACE-Breast-03). ClinicalTrials.gov ID, NCT04829604. NLM; Bethesda,
MD: 2021, https://clinicaltrials.gov/ct2/show/NCT04829604.
|
|
150
|
National Library of Medicine (NLM): Haplo
/ Allogeneic NKG2DL-targeting Chimeric Antigen Receptor-grafted γδ
T Cells for Relapsed or Refractory Solid Tumour. ClinicalTrials.gov
ID, NCT04107142. NLM; Bethesda, MD: 2019, https://clinicaltrials.gov/ct2/show/NCT04107142.
|
|
151
|
National Library of Medicine (NLM): A
Study to Investigate LYL797 in Adults With Solid Tumors.
ClinicalTrials.gov ID, NCT05274451. NLM; Bethesda, MD: 2022,
https://clinicaltrials.gov/ct2/show/NCT05274451.
|
|
152
|
National Library of Medicine (NLM): A
Biomarker Screening Protocol for Participants With Solid Tumors
(START). ClinicalTrials.gov ID, NCT05891197. NLM; Bethesda, MD:
2023, https://clinicaltrials.gov/ct2/show/NCT05891197.
|
|
153
|
National Library of Medicine (NLM): cMet
CAR RNA T Cells Targeting Breast Cancer. ClinicalTrials.gov ID,
NCT01837602. NLM; Bethesda, MD: 2013, https://clinicaltrials.gov/ct2/show/NCT01837602.
|
|
154
|
National Library of Medicine (NLM):
Treatment of Relapsed and/or Chemotherapy Refractory Advanced
Malignancies by CART-meso. ClinicalTrials.gov ID, NCT02580747. NLM;
Bethesda, MD: 2015, https://clinicaltrials.gov/ct2/show/NCT02580747.
|
|
155
|
National Library of Medicine (NLM): Phase
I/II Study of Anti-Mucin1 (MUC1) CAR T Cells for Patients With
MUC1+ Advanced Refractory Solid Tumor. ClinicalTrials.gov ID,
NCT02587689. NLM; Bethesda, MD: 2015, https://clinicaltrials.gov/ct2/show/NCT02587689.
|
|
156
|
National Library of Medicine (NLM):
Multi-4SCAR-T Therapy Targeting Breast Cancer. ClinicalTrials.gov
ID, NCT04430595. NLM; Bethesda, MD: 2020, https://clinicaltrials.gov/ct2/show/NCT04430595.
|
|
157
|
National Library of Medicine (NLM): EpCAM
CAR-T for Treatment of Advanced Solid Tumors. ClinicalTrials.gov
ID, NCT02915445. NLM; Bethesda, MD: 2016, https://clinicaltrials.gov/ct2/show/NCT02915445.
|
|
158
|
National Library of Medicine (NLM):
C7R-GD2.CART Cells for Patients With Relapsed or Refractory
Neuroblastoma and Other GD2 Positive Cancers (GAIL-N).
ClinicalTrials.gov ID, NCT03635632. NLM; Bethesda, MD: 2018,
https://clinicaltrials.gov/ct2/show/NCT03635632.
|
|
159
|
Bonifant CL, Jackson HJ, Brentjens RJ and
Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther
Oncolytics. 3:160112016. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Almåsbak H, Aarvak T and Vemuri MC: CAR T
cell therapy: A game changer in cancer treatment. J Immunol Res.
2016:54746022016. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Abreu TR, Fonseca NA, Gonçalves N and
Moreira JN: Current challenges and emerging opportunities of CAR-T
cell therapies. J Control Release. 319:246–261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Hege KM, Bergsland EK, Fisher GA,
Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH
and Sherwin SA: Safety, tumor trafficking and immunogenicity of
chimeric antigen receptor (CAR)-T cells specific for TAG-72 in
colorectal cancer. J Immunother Cancer. 5:222017. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Chen H, Wang F, Zhang P, Zhang Y, Chen Y,
Fan X, Cao X, Liu J, Yang Y, Wang B, et al: Management of cytokine
release syndrome related to CAR-T cell therapy. Front Med.
13:610–617. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
124:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Teachey DT, Rheingold SR, Maude SL,
Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M,
Berg RA, et al: Cytokine release syndrome after blinatumomab
treatment related to abnormal macrophage activation and ameliorated
with cytokine-directed therapy. Blood. 121:5154–5157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar
|
|
167
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar :
|
|
168
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Lamers CH, Sleijfer S, Vulto AG, Kruit WH,
Kliffen M, Debets R, Gratama JW, Stoter G and Oosterwijk E:
Treatment of metastatic renal cell carcinoma with autologous
T-lymphocytes genetically retargeted against carbonic anhydrase IX:
First clinical experience. J Clin Oncol. 24:e20–e22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Marin V, Cribioli E, Philip B, Tettamanti
S, Pizzitola I, Biondi A, Biagi E and Pule M: Comparison of
different suicide-gene strategies for the safety improvement of
genetically manipulated T cells. Hum Gene Ther Methods. 23:376–386.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Rubin DB, Danish HH, Ali AB, Li K, LaRose
S, Monk AD, Cote DJ, Spendley L, Kim AH, Robertson MS, et al:
Neurological toxicities associated with chimeric antigen receptor
T-cell therapy. Brain. 142:1334–1348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Miao L, Zhang Z, Ren Z and Li Y: Reactions
related to CAR-T cell therapy. Front Immunol. 12:6632012021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Maus MV, Haas AR, Beatty GL, Albelda SM,
Levine BL, Liu X, Zhao Y, Kalos M and June CH: T cells expressing
chimeric antigen receptors can cause anaphylaxis in humans. Cancer
Immunol Res. 1:26–31. 2013. View Article : Google Scholar
|
|
177
|
Zhao Y, Moon E, Carpenito C, Paulos CM,
Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, et
al: Multiple injections of electroporated autologous T cells
expressing a chimeric antigen receptor mediate regression of human
disseminated tumor. Cancer Res. 70:9053–9061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Barrett DM, Zhao Y, Liu X, Jiang S,
Carpenito C, Kalos M, Carroll RG, June CH and Grupp SA: Treatment
of advanced leukemia in mice with mRNA engineered T cells. Hum Gene
Ther. 22:1575–1586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA.
93:136–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar :
|
|
181
|
Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA and
Kang CY: Immunosuppressive myeloid-derived suppressor cells can be
converted into immunogenic APCs with the help of activated NKT
cells: An alternative cell-based antitumor vaccine. J Immunol.
182:1818–1828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Davis RJ, Van Waes C and Allen CT:
Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and
Tregs as mediators of the immunosuppressive microenvironment in
head and neck cancer. Oral Oncol. 58:59–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Fedorov VD, Themeli M and Sadelain M:
PD-1- and CTLA-4-based inhibitory chimeric antigen receptors
(iCARs) divert off-target immunotherapy responses. Sci Transl Med.
5:215ra1722013. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Chmielewski M, Kopecky C, Hombach AA and
Abken H: IL-12 release by engineered T cells expressing chimeric
antigen receptors can effectively Muster an antigen-independent
macrophage response on tumor cells that have shut down tumor
antigen expression. Cancer Res. 71:5697–5706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Pegram HJ, Lee JC, Hayman EG, Imperato GH,
Tedder TF, Sadelain M and Brentjens RJ: Tumor-targeted T cells
modified to secrete IL-12 eradicate systemic tumors without need
for prior conditioning. Blood. 119:4133–4141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Patel U, Abernathy J, Savani BN, Oluwole
O, Sengsayadeth S and Dholaria B: CAR T cell therapy in solid
tumors: A review of current clinical trials. EJHaem. 3(Suppl 1):
S24–S31. 2022. View Article : Google Scholar
|
|
187
|
Włodarczyk M and Pyrzynska B: CAR-NK as a
rapidly developed and efficient immunotherapeutic strategy against
cancer. Cancers (Basel). 15:1172022. View Article : Google Scholar
|
|
188
|
Hawkins ER, D'Souza RR and Klampatsa A:
Armored CAR T-cells: The next chapter in T-cell cancer
immunotherapy. Biologics. 15:95–105. 2021.PubMed/NCBI
|
|
189
|
Rafiq S, Hackett CS and Brentjens RJ:
Engineering strategies to overcome the current roadblocks in CAR T
cell therapy. Nat Rev Clin Oncol. 17:147–167. 2020. View Article : Google Scholar :
|
|
190
|
Xia L, Zheng Z, Liu JY, Chen YJ, Ding J,
Hu GS, Hu YH, Liu S, Luo WX, Xia NS and Liu W: Targeting
triple-negative breast cancer with combination therapy of EGFR CAR
T cells and CDK7 inhibition. Cancer Immunol Res. 9:707–722. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang H, Qin C, An C, Zheng X, Wen S, Chen
W, Liu X, Lv Z, Yang P, Xu W, et al: Application of the
CRISPR/Cas9-based gene editing technique in basic research,
diagnosis, and therapy of cancer. Mol Cancer. 20:1262021.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Yan T, Zhu L and Chen J: Current advances
and challenges in CAR T-Cell therapy for solid tumors:
Tumor-associated antigens and the tumor microenvironment. Exp
Hematol Oncol. 12:142023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Collins DC, Sundar R, Lim JSJ and Yap TA:
Towards precision medicine in the clinic: From biomarker discovery
to novel therapeutics. Trends Pharmacol Sci. 38:25–40. 2017.
View Article : Google Scholar
|