Cholangiocarcinoma (CCA) originates from the bile
duct epithelium and is the second most common malignant tumor of
the hepatobiliary system, which accounts for ~3% of all digestive
tract tumors. CCA is characterized by a specific anatomical
position, insidious clinical symptoms and an early tendency for
neural-vascular invasion and lymph node metastases. Therefore, the
majority of patients are diagnosed at an advanced stage with either
locally advanced tumors or distant metastases, precluding them from
undergoing surgical intervention. While a minority of patients may
qualify for surgical resection, the disease often exhibits a
propensity for recurrence and metastasis even after radical
surgery. Furthermore, the 5-year survival rate of patients remains
dismally low, at <10%, accompanied by a staggering 1-year
recurrence rate of ~60% (1-3).
Additionally, CCA has exhibited resistance to systemic therapies,
such as chemotherapy and targeted treatments. Consequently, there
is an urgent need for the development of innovative treatment
approaches.
The progress in cancer immunology holds significant
promise for the development of novel treatment approaches for CCA.
Recent studies have revealed a close association between the
majority of CCA cases and the biliary system, which is
characterized by persistent, long-term chronic inflammation. The
tumor immune microenvironment (TIME) refers to the spatial
organization and abundance of immune cells, which play a pivotal
role in tumorigenesis and development. The TIME of CCA is
characterized by significant interstitial fibrosis and infiltration
of abundant cancer-associated fibroblasts (CAFs), as well as
pro-cancer and pro-inflammatory immune cells, such as
tumor-associated macrophages (TAMs), tumor-associated neutrophils
(TANs) and tumor-infiltrating lymphocytes (TILs). Through
interactions with tumor cells, these immune cells play a crucial
role in regulating specific biological behaviors of CCA, including
tumor growth, angiogenesis, lymphangiogenesis, invasion and
metastasis (4). Additionally,
these immune cells have the potential to serve as prognostic
factors associated with the clinical outcomes of patients with CCA
(5,6).
Cancer immunoediting is founded on the concept that
the immune system has the dual capacity to suppress tumor growth
and alter tumor immunogenicity. This concept encapsulates the
dynamic interactions between the immune system and tumors
throughout various stages, which can be delineated into three
successive phases: Elimination, equilibrium and escape (7,8).
In the elimination phase, the innate and adaptive immune systems
cooperate to eradicate tumor cells, rendering the tumor
undetectable. However, in the event that certain subclonal tumor
cells succeed in evading the cytotoxic effects of the immune
system, they progress to the following phase. The equilibrium phase
represents the efforts of the adaptive immune system to modify the
immunogenicity of tumor cells, allowing them to evade immune
surveillance and avoid destruction. It is important to note that at
this stage, the growth of tumor cells is restricted or may even
come to a halt. These immunogenically reduced (immuno-edited) tumor
cells then progress to the escape phase, where they exhibit typical
tumor characteristics, such as unlimited growth and can be detected
through clinical means. Cancer immunoediting is characterized by
the recognition of antigens expressed by tumor cells by T-cells,
which can lead to either the death of tumor cells or a reduction in
their immunogenicity. As a result, T-cells play a predominant role
in cancer immunoediting (9).
During all stages of cancer immunoediting, the components of the
TIME interact with each other, which may impact or inhibit the
activation and/or function of T-cells and eventually influence
their antitumor effects. The TIME plays a crucial role in the
cancer immunoediting process, with its components actively
participating in all stages of cancer immunoediting.
With the application of immunotherapy in solid
tumors, immune-mediated primary or adjuvant therapy is increasingly
recognized as having immense potential in CCA, which functions by
enhancing the immune response against tumors, involving both innate
and adaptive immune cells. The inhibition of signaling pathways
mediated by immune checkpoints has been proposed as a potential
therapeutic strategy for CCA. Based on this premise, immune
checkpoint inhibitors (ICIs) have been widely used in patients with
CCA and have shown promising results for the treatment of CCA
(10-12). However, the response rate to
immunotherapy is relatively low, and only a small portion of
patients can benefit from it. Mounting evidence has indicated a
connection between the TIME and the response to immunotherapy, and
the failure of immunotherapy may be partially attributed to the
high heterogeneity and intricate TIME of CCA.
The present review aimed to provide insight into the
compositional characteristics and regulatory factors governing the
TIME of CCA, shedding light on possible immune escape mechanisms.
Furthermore, the recent advances in immunotherapy for CCA are
summarized. The aim of the present review was to provide a valuable
reference point for the development of innovative immunotherapeutic
strategies tailored to the unique challenges posed by CCA.
CAFs are activated myofibroblasts and are
characterized by the expression of α-smooth muscle (α-SMA) actin
and Tenascin C protein (13,14). They constitute the primary cell
population responsible for the fibrotic stroma in CCA. Current
evidence suggests that CAFs are a heterogeneous group of cells
derived from various lineages, including pericytes, mesenchymal
stem cells, adipocytes, liver resident hepatic stellate cells
(HSCs), portal fibroblasts and bone marrow-derived precursor cells
(15-17). In the study by Affo et al
(18), it was demonstrated that
HSCs are a major source of CAFs, and among the CAF subpopulations,
HSC-derived CAFs engage in the most significant ligand-receptor
interactions with CCA cells. CAFs influence tumor progression by
tumor extracellular matrix (ECM) remodulation, and by interacting
with tumor cells and immune cells. In previous study using a
syngeneic orthotopic rat model of CCA, the induction of CAF
apoptosis using the BH3 mimetic navitoclax resulted in reduced
primary tumor growth, as well as in the inhibition of tumor
lymphatic vascularization, regional lymph node metastases and
peritoneum metastases (19).
HSC-derived CAFs can trigger the secretion of hepatocyte growth
factor (HGF) from inflammatory CAFs. This process occurs through a
direct interaction involving the HSC-CAF-tumor pathway, which
subsequently promotes the proliferation of intrahepatic CCA (iCCA)
cells by means of mesenchymal-epithelial transition (MET) factor
expressed by the tumor (18).
Furthermore, it has been reported that high expression of α-SMA is
associated with poor survival outcomes in patients with CCA
(20,21).
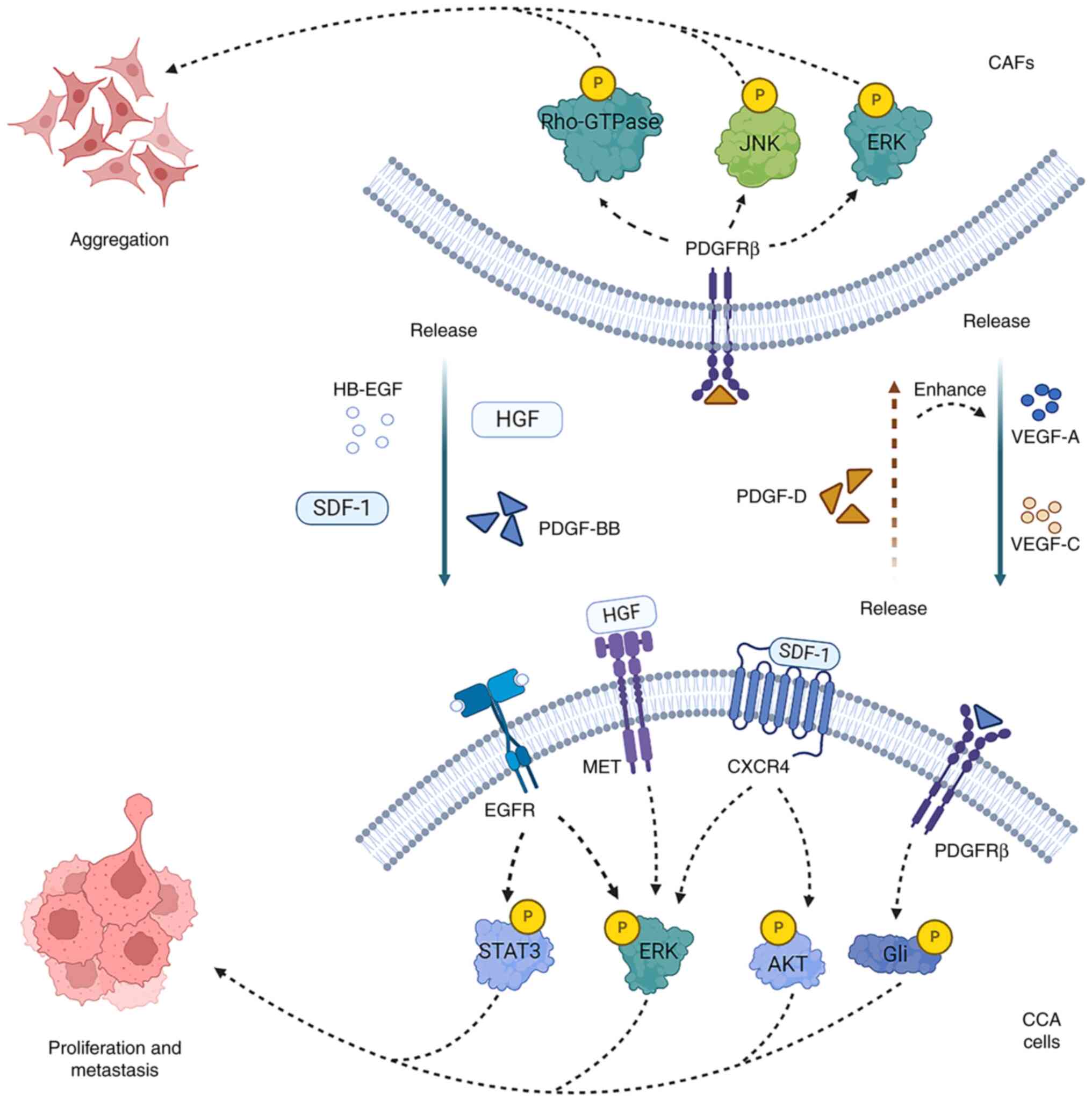
CAFs secrete various soluble cytokines, including
HGF, transforming growth factor β1 (TGF-β1), epidermal growth
factor (EGF), connective tissue growth factor and stromal
cell-derived factor-1 (SDF-1), which can enhance the malignant
phenotype of CCA cells (22).
Heparin-binding EGF, released by CAFs, interacts with EGF receptor
(EGFR) on the plasma membrane of CCA cells to activate EGFR. This
activation, in turn, stimulates ERK1/2 and STAT3, leading to the
nuclear translocation of β-catenin and disruption of the adherens
junction complexes with E-cadherin internalization. The nuclear
translocation of β-catenin triggers a transcriptional program that
promotes tumor progression (23).
Additionally, it has been demonstrated that the disruption of
E-cadherin-mediated adherens junctions leads to
epithelial-to-mesenchymal transition (EMT), a cellular process
strongly associated with cancer progression (24). A previous study proved that SDF-1
(also known as CXCL12) released by WI-38 fibroblasts promoted iCCA
cell migration (25). CAF-derived
SDF-1 binds to the C-X-C chemokine receptor (CXCR)4 on CCA cells in
a paracrine manner, stimulating ERK1/2 and AKT signaling to
increase the invasive ability of CCA (26). A recent study divided CAFs into
inflammatory and growth factor-enriched and myofibroblastic (myCAF)
subpopulations, which exhibited different ligand-receptor
interactions (18). myCAFs
synthesize and secrete hyaluronan synthase 2 (Has2) after
interacting with CCA cells. Has2 exerts pro-tumorigenic effects via
binding to non-tumor cells or receptors other than CD44. Therefore,
myCAFs promote tumor growth through Has2, but not type I collagen
(18). In addition, hyaluronan
(HA) is associated with tumor promotion, treatment resistance and a
poor prognosis in pancreatic, head and neck, colorectal, gastric
and liver cancer (27). The
molecular size and degradation of HA are also factors in its
bioactivity; high-molecular-weight HA is considered to be
antitumorigenic, whereas low-molecular-weight HA is
pro-inflammatory and tumor-promoting (28,29). Furthermore, vascular CAFs express
high levels of interleukin (IL)-6, leading to notable changes in
the epigenetics of iCCA cells. These alterations notably include
the increased expression of enhancer of zeste homolog 2,
consequently intensifying the malignancy of the tumor cells
(30).
CCA cells recruit and activate fibroblasts or
precursor cells of myofibroblasts via platelet-derived growth
factor (PDGF)-D and TGF-β1. PDGF-D released by CCA cells
contributes to fibroblast aggregation (31). In turn, the binding of
CAF-secreted PDGF-BB to PDGFRβ in CCA cells decreases the
susceptibility of CCA cells to tumor necrosis factor
(TNF)-α-related apoptosis-inducing ligand, inducing tumor growth
and metastasis (32). In
addition, PDGF-D can stimulate fibroblast to secrete vascular
endothelial growth factor (VEGF)-A and VEGF-C that increase the
markedly generation of tumor lymphangiogenesis and lead to the
invasion of tumor cells in lymphatic vessels (33). Therefore, interacting paracrine
loops exist between CAFs and CCA cells, establishing a
bidirectional reinforcing relationship (Fig. 1).
CAFs can secrete major ECM components, such as
periostin (PN) and various matrix metalloproteinases (MMPs)
(22). PN can interact with
components, such as collagen type I, Tenascin C and integrins
(34). Previous studies have
highlighted that when PN combines with integrins, it activates
various signaling pathways, influencing downstream molecules, and
thereby contributing to tumor progression (35-38). In the context of CCA, PN has been
shown to enhance invasion through the ITGα5β1/PI3K/AKT pathway
(39). Furthermore, it induces
EMT, promoting CCA migration, primarily through the integrin
α5β1/TWIST-2 axis (40). MMPs
play a crucial role in degrading and remodeling the ECM, thereby
contributing to tumor progression. Among these MMPs, CAFs have been
shown to produce MMP1, MMP2, MMP3 and MMP9, all of which
collectively increase tumor aggressiveness (41).
Macrophages play a pivotal role in regulating tumor
cell proliferation and progression by releasing various
inflammatory factors and cytokines. Notably, they are the most
commonly encountered immune infiltrating cells within the tumor
microenvironment. Of particular concern in CCA is the presence of
high levels of M2 macrophages, which have been shown to be strongly
associated with carcinogenesis and poor outcomes in with CCA, as
evidenced by prior studies (42-44). TAMs, a subtype of M2 macrophages,
exert potent pro-tumor effects. The shift of macrophages toward
this alternative M2 phenotype is primarily orchestrated by the
actions of specific signaling pathways, notably involving IL6/STAT3
and PCAT6/miR-326/RohA pathway (43,45). Notably, Kitano et al
demonstrated an association between TAM infiltration, increased
levels of Tregs and TANs, and a poor recurrence-free survival (RFS)
(46). TAMs participate in
remodeling the ECM via secretion of MMPs and the release IL-4,
IL-8, IL-10, chemokine ligand (CCL)2, CCL22 and CCL17 to recruit
immunosuppressive cells, such as TANs, myeloid-derived suppressor
cells (MDSCs) and regulatory T-cells (Tregs), to form the
suppressive immune microenvironment (47).
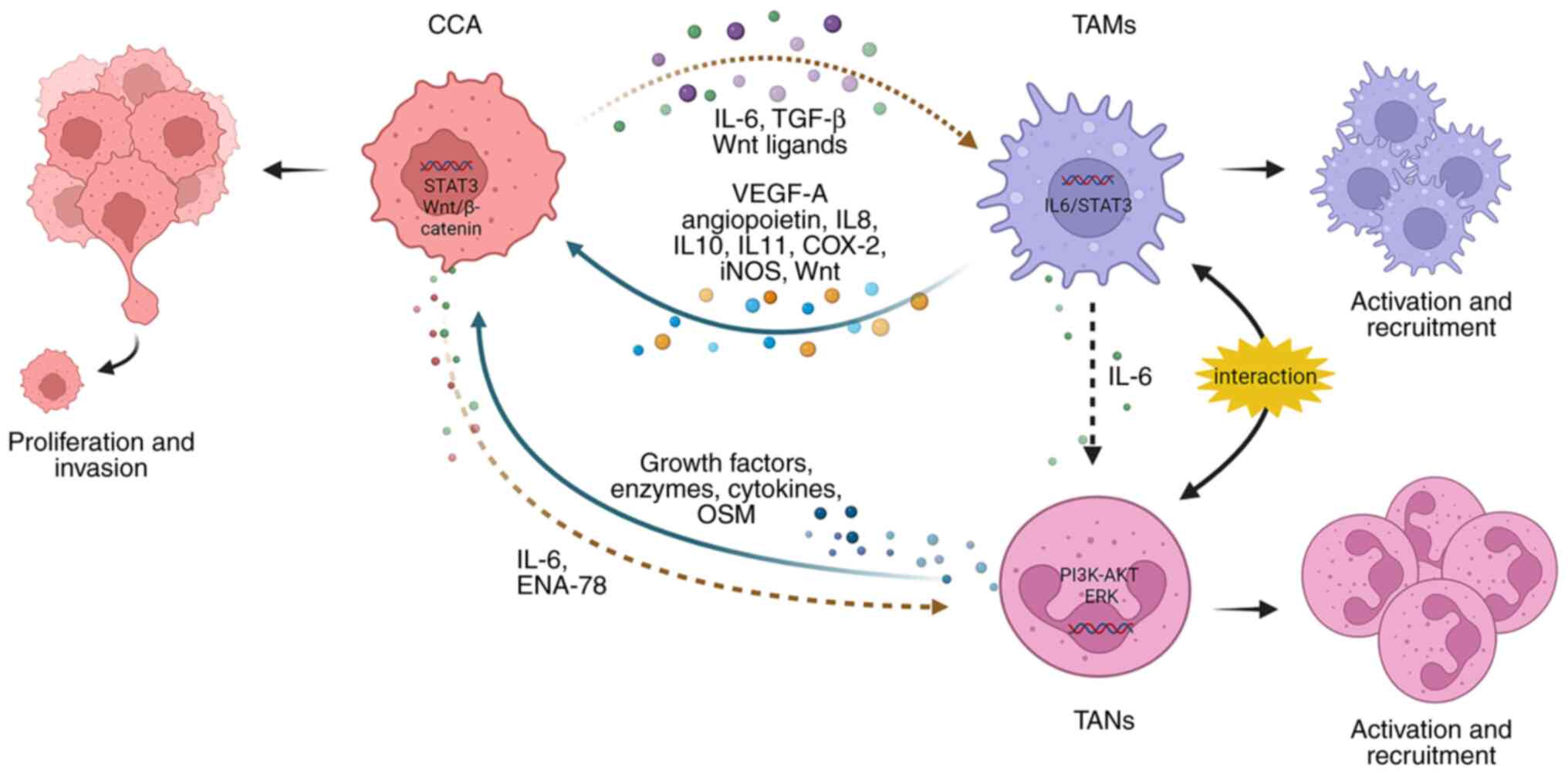
TAMs play a multifaceted role in promoting
tumorigenesis and can exert their tumor-promoting effects by
interacting with CCA cells and TANs (Fig. 2). CCA cells can produce IL-6 and
TGF-β, which are involved in the activation of TAMs. In a
reciprocal interaction, TAMs release significant amounts of IL-10,
which, can activate the STAT3 pathway in CCA cells. This
activation, in turn, enhances the migration and invasion of tumor
cells, largely through the process of EMT (48). Additionally, activated TAMs are
capable of producing molecules, including VEGF-A, angiopoietin,
IL-8, cyclooxygenase-2 and inducible nitric oxide synthase, to
promote tumor angiogenesis (43,49). Furthermore, CCA cells express
certain Wnt ligands, including Wnt3, Wnt5a, and Wnt7b, which have
the capability to recruit and activate TAMs. Subsequently, TAMs
release Wnt, which in turn stimulates the Wnt/β-catenin pathway,
leading to increased tumor cell proliferation (50,51). Moreover, in vitro
experiments involving the inhibition of Wnt signaling using a Wnt
inhibitor have revealed a significant reduction in CCA
proliferation and an increase in apoptosis. These effects have been
observed in mouse and rat models of CCA, ultimately resulting in
tumor regression (52). TAMs
secret IL-6 to promote the activation of TANs and CCA cells can
express epithelial-derived neutrophil-activating peptide-78
(ENA-78) to recruit TANs mediated by the PI3K-AKT and ERK1/2
signaling pathways (53). The
interaction between TANs and TAMs leads to the production of
oncostatin M (OSM) and IL-11 by TANs and TAMs, respectively. Both
OSM and IL-11 have been found to stimulate the STAT3 pathway in CCA
cells, resulting in increased tumor cell proliferation and
invasion. Of note, when STAT3 is knocked down, it mitigates the
pro-tumor effects of TANs and TAMs in iCCA (54).
The role of TANs in tumorigenesis and development is
still under investigation. TANs are likely to assume the N2
subtype, which is distinct from N1 neutrophils that are activated
in normal tissue (55,56). The activation of the N2 subtype
neutrophils is mainly induced by TGF-β and granulocyte
colony-stimulating factor (CSF) (57). Activated N2 neutrophils can
release various growth factors, enzymes and cytokines to promote
tumor growth, shape the TIME and stimulate angiogenesis (58). The high level of TAN infiltration
tends to be associated with a poor prognosis, as it is related to
decreased overall survival (OS) and RFS of patients with CCA
(46,59). However, a recent study proposed a
contrasting view, suggesting that patients with biliary tract
cancer (BTC) with a higher neutrophil infiltration exhibit an
improved prognosis (60). Owing
to the limited amount of evidence and the conflicting findings, it
remains challenging to arrive at a definitive conclusion regarding
the prognostic significance of TAN infiltration. High levels of TAN
infiltration have been shown to promote the growth and invasion of
tumors in vivo, although they do not appear to alter the
in vitro proliferative and invasive abilities of iCCA cells
(53).
MDSCs are a group of heterogeneous immune cells
which exert potent immunosuppressive effects that can inhibit
various immune cell activities. Chronic inflammation functions as a
stimulus for MDSCs, prompting them to synthesize molecules such as
arginase, reactive oxygen species, inducible nitric oxide synthase
and indoleamine 2,3-dioxygenase. Furthermore, MDSCs release
immunosuppressive factors, such as TGF-β and IL, which act to
curtail the function of cytotoxic T-lymphocytes, natural killer
(NK) cells and their respective subpopulations, thereby achieving a
state of immunosuppression (61).
The accumulation of MDSCs in the TIME has been linked to heightened
immune evasion and increased resistance to immunotherapy in various
types of cancers (62,63). Studies on primary hepatocellular
carcinoma (HCC) have shown that MDSCs play a role in promoting the
development of Tregs, the inactivation of CD8+ T-cells,
and the suppression of the cytotoxic activity of NK cells.
Furthermore, elevated levels of mononuclear MDSCs in the peripheral
blood have been shown to be associated with the poor OS of patients
with HCC (64). Nevertheless, the
precise functions of MDSCs in the context of CCA remain
incompletely understood. Recent research has indicated that the gut
microbiome can induce the accumulation of CXCR2+
polymorphonuclear MDSCs through TLR4-dependent CXCL1 production,
thus facilitating the establishment of an immunosuppressive
environment that promotes CCA progression (65). The level of CD33+ MDSCs
in the blood and tumor tissues of patients with iCCA has been found
to be increased and to be associated with a poor clinical outcome
(66). The relevance between CAFs
and MDSCs has been brought to light. Specifically, CAFs have been
found to secrete IL-6 and IL-33, which in turn stimulate MDSCs to
upregulate the expression of 5-lipoxygenase (5-LO). Notably, one of
the metabolites of 5-LO, known as LTB4, has been shown to activate
the PI3K/Akt-mTORC1 signaling pathway in iCCA cells through the
interaction with BLT2. This activation ultimately contributes to
the promotion of cancer stemness in iCCA (66). Additionally, Loeuillard et
al (67) highlighted the
significance of the interaction between TAMs and MDSCs. They
identified an abundance of programmed death ligand 1
(PD-L1)-positive TAMs in both human CCA samples and CCA mouse
models. Elevated levels of PD-L1+ TAMs were associated
with an enhanced cancer progression. However, attempts to block
TAMs alone have not effectively reduced CCA tumor burden (67). This lack of a response may be
attributed to the compensatory accumulation of granulocyte MDSCs
(G-MDSCs), which impair the T-cell response and induce immune
evasion. Notably, the simultaneous inhibition of G-MDSCs and TAMs
has shown promise in enhancing the efficacy of anti-PD-1 therapy
for CCA (67). Collectively, that
study underscored the role of MDSCs as mediators that collaborate
with other components within the CCA TIME to promote tumor
progression (67).
NK cells are known for their potent antitumor
activity, as they possess the ability to induce tumor cell
apoptosis directly by releasing molecules, such as perforin,
cytotoxic factors and TNF (68).
Previous findings have suggested that enhancing NK cell function or
increasing the numbers of NK cells delays CCA progression, which
may be a potential therapeutic target for CCA. Anti-Globo H
antibody VK9 can enhance the activation and increase the presence
of NK cells in the TIME, resulting in the inhibition of iCCA rat
tumor growth (69). In
vitro studies have demonstrated that the cytotoxic effect of
activated NK cells on CCA cell lines may be augmented by cetuximab
and cordycepin, effectively restraining CCA cell growth (70,71). Additionally, in a xenograft mouse
model of CCA, the transplantation of ex vivo-expanded human
NK cells has been found to inhibit tumor growth (72). Fukuda et al (73) observed that low numbers of
tumor-infiltrating NK, cells regulated by endogenous CXCL9, were
associated with a large tumor size and the poor survival of iCCA
(73). The activating receptor
natural-killer group 2D (NKG2D), predominantly expressed on NK
cells, holds promise as a therapeutic target. A high expression of
the NKG2D receptor in patients with CCA has been linked to an
improved prognosis (74).
Moreover, it is worth noting that variations in NKG2D have been
found to be associated with bile duct tumorigenesis in patients
with primary sclerosing cholangitis (75).
The adaptive immune system is the predominant
defense system against tumors. The major components of the adaptive
immune system in the CCA TIME are TILs, including B-lymphocytes,
CD4+ helper T-lymphocytes, CD8+ cytotoxic
T-cells and Tregs. While various studies have examined the spatial
distribution of TILs in CCA tissue (49,76,77), it remains a topic of debate and
discussion. According to the current literature, it appears that
CD3+, CD4+ and CD8+ T-cells
predominantly reside in the peritumoral region, regardless of the
CCA subtype. However, as for Foxp3+ T-cells and B-cells,
the exact distribution location has not been definitively
determined (6). Recent studies
have demonstrated that Foxp3+ T-cells have been observed
to accumulate in the tumor border area (76,77). Another previous study indicated
that Foxp3+ T-cells were distributed in the intratumoral
area (78), while another study
failed to found the distribution difference of Foxp3+
T-cells (79). In the case of
B-cells, two separate studies have reported their presence in the
peritumoral area (78,80). These varying observations
regarding the distribution of Foxp3+ T-cells and B-cells
may be attributed to distinct contexts or factors, warranting
further investigation for a comprehensive understanding. The
potential association between TILs and various signaling pathways
in tumor promotion has been revealed by numerous studies. Recent
research has demonstrated that a low abundance of tissue-resident
memory T-cells is associated with a significantly increased
expression of genes related to the Wnt/β-catenin and TGF-β
signaling pathways (81).
Carnevale et al (82) made
an intriguing discovery where the expression of cellular FADD-like
IL-1β-converting enzyme-inhibitory protein in iCCA cells
significantly increased following co-culture with human peripheral
blood mononuclear cells. This led to the activation of the Fas/FasL
pathway, inducing the apoptosis of T-cells and NK cells, ultimately
resulting in tumor immune escape (82). Another study highlighted the
significance of the B7-H1/PD-1 signaling pathways in the induction
of CD8+ TIL apoptosis and the inhibition of antitumor
immune responses (83).
Isocitrate dehydrogenase 1 mutations drive the activation of
interferon γ (IFN-γ)-responsive genes in iCCA cells through a
TET2-dependent mechanism. This occurs by impeding the recruitment
of activated CD8+ T-cells and the expression of IFN-γ
(84).
It has been proven that the number or density of
TILs in the TIME of CCA affects prognosis. High levels of
CD8+ T-cells have consistently been associated with an
improved survival and reduced invasion into surrounding tissues in
several studies (76,78,85,86). A substantial infiltration of
CD4+ T-cells has similarly been linked to a favorable OS
and RFS (77,78). Foxp3+ T cell
infiltration, however, has generated mixed results in terms of
prognosis. While the majority of studies suggest that it is
associated with a poor prognosis of patients with CCA (49,76,87), Goeppert et al (78) indicated that patients with
Foxp3+ T cell infiltration experience improved outcomes.
Thus, the prognostic value of Foxp3+ T-cells remains
uncertain, necessitating further studies to clarify their specific
impact on long-term results. Current studies have revealed that
patients with CCA benefited from high levels of B-cell infiltration
(78,88). Nevertheless, due to limited
available evidence, the association between B-cells and the
prognosis of patients with CCA also warrants further investigation
through additional research.
DCs are essential components of the immune system,
functioning as professional antigen-presenting cells (APCs) that
bridge innate and adaptive immune responses. Their presence in
tumor tissues can trigger robust antitumor immune responses,
potentially improving cancer patient outcomes. The abundance of DCs
in the peripheral blood of patients is significantly decreased
compared to healthy individuals (96). It has been shown that patients
with a higher number of DCs infiltrating the tumor margin
experience a lower rate of lymph node metastasis and better
prognosis (97). DCs may affect
the number of TILs within the TIME. Junking et al (98) found that DCs pulsed by CCA cells
induced the differentiation of PBMCs into DCs. This process
increased and activated CD3+ CD8+ T-cells,
empowering them to induce tumor cell apoptosis (98). DCs generated by the stimulation of
CCA cells can utilize activated lymphocytes as anti-CCA effector
cells. IL-12 and TGF-β in TIME can impair the functions of DCs. The
application of specific neutralizing antibodies that block the
IL-10 and TGF-β receptors on DCs, or the knockdown of TGF-βRII and
IL-10RA mRNA, has been shown to enhance the performance of effector
T-cells (99,100). Sung et al (101) discovered that ABL501, a
bispecific antibody targeting lymphocyte-activation gene 3 (LAG-3)
and PD-L1, induced CD8+ T-cell activation by promoting
DC maturation. This activation ultimately amplifies the cytotoxic
effects of CD8+ T-cells against tumor cells (101). CTLA-4 is a transmembrane protein
encoded by the CTLA-4 gene as well as a homologous protein of CD28,
which is expressed in activated CD4+ and CD8+
T-cells (102), which presents
with higher affinity competes with CD28 for binding to B7 (CD80/86)
to impair T-cells. Mature DCs have been demonstrated to
significantly release CTLA-4 into the extracellular compartment by
vesicular transport, which can inhibit the antibody binding of B7,
eventually resulting in the dysfunction of T-cells (103). In addition, the activation of
the CD40/CD40L pathway can enhance the function of DCs and induce
cytotoxic effects to CCA cell (104). CD40/CD40L is significantly
involved in the maturation, proliferation and survival of DCs,
which were triggered by activating p38 MAPK, PI3K-Akt and NF-κB
pathways (105). In addition,
CD40 signaling triggered the expression of Bcl2l1 to reduce the
caspase activation and apoptosis, eventually contributing to
maintaining classical type 1 DCs survival during the initiation of
anti-tumor immune responses (106). The signaling pathways associated
with DCs exerting antitumor effects are summarized in Table SI. In summary, these findings
underscore the potential of DC-based immunotherapies as promising
approaches for improving outcomes in the context of CCA
treatment.
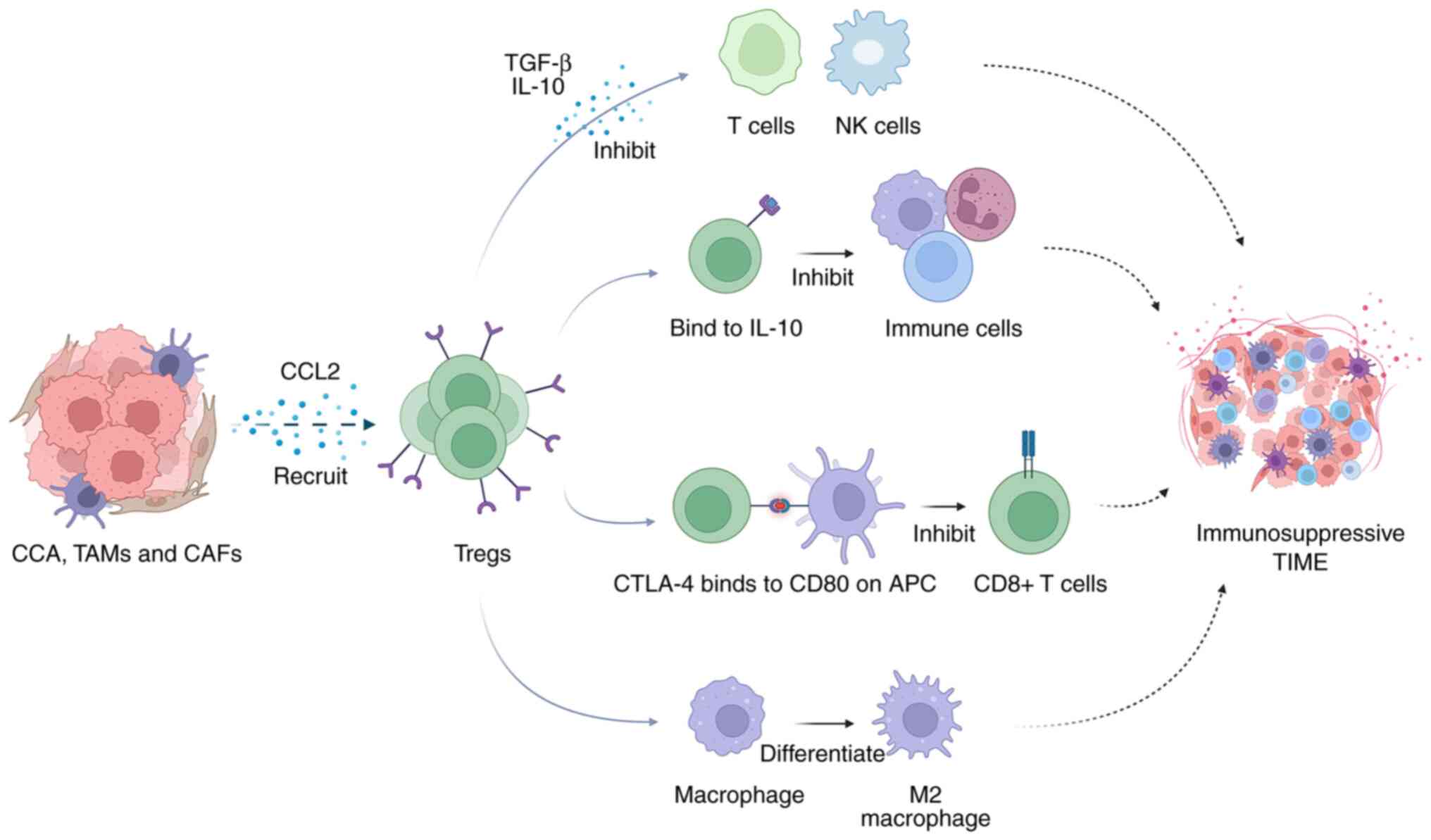
Evading immune surveillance is one of the features
of tumor cells. Although CCA cells express immunogenic
tumor-associated antigens (TAAs), the body generates
immunosuppressive signals that effectively neutralize the
tumor-killing effect. CCA cells employ a strategy of recruiting
immunosuppressive cells by releasing factors, such as TGF-β and
IL-10. These recruited cells not only enhance tumor activity, but
also contribute to the formation of an immunosuppressive TIME,
effectively hindering the antitumor immune response. To counter
this immunosuppression, inhibiting IL-10 and TGF-β or
downregulating their expression could significantly amplify the
cytolytic activity of effector T-cells, potentially reinvigorating
the immune response against the tumor (99,100). The intricate interaction between
these immunosuppressive cells grants CCA the ability to acquire and
deploy immunosuppressive mechanisms (107). Consequently, blocking the
recruitment of these immunosuppressive cells emerges as a promising
avenue for CCA immunotherapy. Notably, the simultaneous inhibition
of TAMs and G-MDSCs has demonstrated the potential to enhance the
effectiveness of anti-PD-1 treatment, while suppressing tumor
growth (67). Moreover, blocking
granulocyte macrophage-CSF signaling has been shown to reduce the
accumulation of bone marrow-derived monocytes, impair TAM viability
and promote the repolarization of both TAMs and MDSCs. This
concerted action leads to an increased infiltration and activation
of cytotoxic T-lymphocytes, further strengthening the antitumor
immune response (108).
In the usual course, the acquisition of adaptive
immunity hinges on activating DCs and macrophages as APCs.
Consequently, a TIME deficient in APCs renders T-cells ineffective
(109). In CCA, M2 macrophages
play a significant role in immune evasion by hampering DC
maturation and impairing the function of T-cell effectors.
Furthermore, Tregs also inhibit APCs, which disrupt metabolic
pathways directly through their cytotoxic effects, leading to the
suppression of immune responses (110). Tumor cells employ various
strategies to evade the immunosurveillance and innate immune system
elimination. Tumor cells upregulate the expression of CD47, which
interacts with signal regulatory protein α (SIRPα) on macrophages,
facilitating the escape of tumors from phagocytosis (111). Targeting CD47 and disrupting the
CD47-SIRPα interaction can enhance macrophage phagocytosis in all
macrophage subtypes, effectively suppressing CCA growth and
metastasis (112). Tumor cells
block the antitumor effects of NK cells by preserving major
histocompatibility complex class I molecules, downregulating NKG2D
ligands on tumor cells and releasing immunoregulatory factors (such
as TGF-β, prostaglandin E2 and indoleamine 2,3-dioxygenase) that
compromise NK cell activity (113). An example of an approach to
counteract this is the use of the monoclonal antibody 7C6, which
can inhibit the cleavage of major histocompatibility complex-class
I chain related proteins A and B and subsequently lead to
NKG2D-dependent activation of NK cells (114).
CCA cells employ immune checkpoint manipulation to
achieve immune evasion, notably targeting immune checkpoints, such
as PD-1 and CTLA-4. PD-1 is expressed on activated T-cells. When
PD-1 binds to its ligand PD-L1, it assembles protein tyrosine
phosphatase, which inhibits the downstream PI3K-Akt-mTOR and
Ras-MEK-ERK signaling pathways. This leads to an altered
metabolism, the exhaustion of peripheral T-cells, the suppression
of the tumoricidal immune response, and ultimately, to tumor
progression (115,116). Additionally, PD-L1 has been
found to prevent tumor cells from cytotoxic T-lymphocyte-induced
apoptosis and interfere with interferon-mediated cytotoxicity
(117,118). CTLA-4 mediates inhibitory
signaling in several ways to block the proliferation and activation
of T cells (119). These
mechanisms include the following: i) Inducing the production of
indoleamine 2,3-dioxygenase; ii) hindering the establishment of a
zeta-associated protein of 70 kDa; iii) increasing the expression
of Casitas-B-lineage lymphoma-b protein; and iv) repressing the
NF-κB and PI3K/Akt pathways, CDK4/CDK6 and cyclin D3.
CCA exhibits regional variation, leading to
differences in the positive rate of PD-L1 expression among
patients. In the Western world, the positive rate is ~11.6%
(120), whereas in the East, it
ranges from 28 to 45% (79,121-123). In addition, PD-L1 expression is
significantly higher in tumor tissues than in paraneoplastic
tissues. An elevated expression of PD-L1 has been linked to tumor
progression and a poorer prognosis. CCA tumors with a high PD-L1
expression tend to display more aggressive features and shorter
survival times (124,125). CTLA-4 has also been observed to
be upregulated in CCA, and of note, a significant positive
correlation exists between the expression levels of PD-1 and CTLA-4
(126). An increased expression
of CTLA-4 in TILs has been shown to be associated with malignant
characteristics and poor survival outcomes in iCCA. However, a high
expression of CTLA-4 in CCA cells does not appear to predict a poor
patient prognosis (127).
Tumor immunotherapy mainly uses monoclonal
antibodies to enhance endogenous antitumor activity. These
monoclonal antibodies predominantly focus on immune checkpoint
regulators, collectively known as ICIs (128). CTLA-4 and PD-1 represent the
most classical T-cell immune checkpoints and are the most
extensively studied targets for ICIs. Moreover, ongoing research is
exploring ICIs that target additional immune checkpoints, such as
LAG-3, TIM-3, TIGIT and B7-H3 (129). Favorable therapeutic responses
to ICIs have been documented in several types of solid tumors
(130-132). Presently, pembrolizumab and
nivolumab have received approval from the Food and Drug
Administration (FDA) for the treatment of advanced malignancies
(133,134).
The response of tumor cells to ICIs appears to be
closely related to the extent of CD8+ T-cell
infiltration and the expression of immune checkpoint molecules
within the tumor (135). Tumors
characterized by a high presence of CD8+ T-cell
infiltration and elevated immune checkpoint molecule expression are
often termed immunologically 'hot' tumors and exhibit high response
rates to ICIs. However, almost half of all patients with CCA have
immunologically 'cold' tumors and have low response rates to
treatment with ICIs (136).
Genetic abnormalities in tumor cells, such as defective DNA
mismatch repair (dMMR) and microsatellite instability-high (MSI-H),
can also influence the responsiveness of tumors to ICIs (137-139). Studies have suggested that
patients with solid tumors that feature abundant CD8+ T
cells, significant PD-L1 expression, MSI-H, high levels of dMMR and
a high tumor mutation burden (TMB) may exhibit sensitivity to
immunotherapy (140,141). Consequently, CD8+
T-cell infiltration, PD-L1 expression, MSI and TMB are employed as
biomarkers to predict immunotherapy response rates. Nevertheless,
the accuracy of these biomarkers still requires refinement. There
is a pressing need for more precise biomarkers and improved
protocols for personalized treatment.
The clinical trial NCT01876511 exhibited that 86
(including CCA) patients with dMMR or MSI-H were treated with
pembrolizumab and achieved satisfactory treatment outcomes
(139). In the phase 1b trial
KEYNOTE-028, 20 patients with advanced-stage CCA and 4 patients
with advanced-stage gallbladder cancer (GBC), all of whom tested
positive for PD-L1, received pembrolizumab monotherapy (142). A total of 3 patients with CCA
and 1 patient with GBC achieved stable disease (SD). Grade 3
toxicities were observed in 17% of cases, with no grade 4 events
reported. The objective response rate (ORR) was 17%, and the median
progression-free survival (mPFS) and median OS (mOS) were 1.8 and
6.2 months, respectively. Notably, that study confirmed that
pembrolizumab was well-tolerated, displayed excellent antitumor
activity, and exhibited manageable safety and effectiveness
(142).
In a phase 2 study involving 54 patients with BTC
pre-treated with at least one line, but no more than three lines of
systemic therapy, the anticancer activity of nivolumab in advanced
refractory BTC was evaluated (143). More than half of the patients
had well-controlled conditions, resulting in a mOS of 14.22 months
and a mPFS of 3.68 months. However, Ueno et al (144) suggested a poor response rate to
nivolumab monotherapy. In summary, the effectiveness of nivolumab
monotherapy for CCA remains uncertain due to the cohort size, and
further research is required to provide a clearer picture.
Durvalumab, atezolizumab and avelumab are also approved by the FDA
for the treatment for various solid tumors. However, studies have
indicated that these monoclonal antibodies have limited efficacy
when used as monotherapy for CCA. For instance, Doki et al
(145) reported that among 42 patients with BTC treated with
durvalumab alone, the median OS was 1.5 months, the mPFS was 8.1
months and the ORR was 4.8%.
Apart from ICIs targeting a single immune
checkpoint, there is growing interest in ICIs that can
simultaneously act on two immune checkpoints. A promising example
is ABL501, which can inhibit both LAG-3 and PD-L1 concurrently,
demonstrating higher antitumor activity compared to a combination
of anti-LAG-3 and anti-PD-L1 treatments (101). ABL501 has emerged as a promising
candidate in cancer immunotherapy and is currently undergoing its
initial human trial (NCT05101109). Another innovative approach is
represented by M7824, a novel bifunctional fusion protein. M7824
comprises a monoclonal antibody against PD-L1 fused to the
extracellular domain of human TGF-β receptor II. This design allows
M7824 to serve a dual function by blocking PD-L1 and sequestering
TGF-β molecules (146). Research
has explored the response of M7824 in Asian patients with CCA,
revealing an ORR of 23%. However, it is essential to note that
treatment-related adverse events (TRAEs) have been observed in 63%
of patients (147). Further
investigations are warranted to assess the overall safety and
efficacy of this approach in CCA immunotherapy.
Given the limited effectiveness of ICI monotherapy
for CCA, there is a growing focus on exploring combination
immunotherapies to enhance treatment responses and overcome immune
tolerance. Combination therapy involving two ICIs has demonstrated
promising outcomes in various solid tumors. In a phase 2 study
evaluating the combination of nivolumab and ipilimumab in 39
patients with advanced-stage BTC (148), the trial reported an ORR of 23%
and a disease control rate (DCR) of 44%, highlighting the potential
superiority of dual ICI combination therapy compared to
monotherapy. A phase 1 study investigated the combination of
durvalumab and tremelimumab in advanced-stage BTC (145), revealing an ORR of 10.8% in a
cohort of 65 BTC patients. The study reported a mPFS of 1.6 months
and a mOS of 10.1 months. However, grade 3 or higher TRAEs were
observed in 23.1% of patients. Nevertheless, it is worth mentioning
that durvalumab plus tremelimumab combination therapy, when tested
in Japanese patients with HCC and BTC, yielded less encouraging
response rates and survival outcomes (149). Considering the mixed results,
the efficacy of durvalumab and tremelimumab combination therapy in
patients with CCA remains unclear, and the safety profile of this
approach warrants further refinement. It is important to exercise
caution when interpreting these conclusions due to the limited
sample sizes in these studies. Therefore, while combination
immunotherapies hold promise, particularly in the context of dual
immune checkpoint inhibition, continued research with larger and
more diverse patient cohorts is essential to establish the true
effectiveness and safety of these approaches in the treatment of
CCA.
The trial NCT03046862 classified 124 patients with
advanced-stage BTC into three treatment cohorts and revealed that
ICIs combined with gemcitabine and cisplatin (GS) hold promise as
an effective treatment for advanced BTC (150). Another study supported the
strategy of GS plus immunotherapy for BTC. The TOPAZ-1 trial
represents a significant advancement in the field, being the first
phase 3 study designed to investigate PD-L1 inhibitors in
combination with chemotherapy for progressive CCA (151). In that trial, 685 patients were
randomly assigned to either the durvalumab + GS group or the
placebo + GS group. The interim analysis revealed encouraging
results, with a mOS of 12.8 months and a mPFS of 7.2 months in the
durvalumab + GS group, compared to 11.5 and 5.7 months in the
placebo + GS group, respectively. Notably, while TRAEs were
observed in 62.7% of patients in the treatment group and 64.9% of
patients in the control group, the combination of chemotherapy
regimens for advanced-stage BTC with ICIs appeared to enhance
survival and other efficacy outcomes without significantly
increasing the risk of treatment toxicity. This suggests a
promising avenue for improving treatment outcomes in advanced-stage
BTC.
In addition, several trials have evaluated the
therapeutic efficacy and safety of GS in combination with nivolumab
(11,144,152), pembrolizumab plus capecitabine
and oxaliplatin (CAPOX) (12) and
paclitaxel with durvalumab and tremelimumab (153) in BTC (Table I). Apart from the trial
NCT03704480, the results from these studies have been consistently
positive. This collective body of evidence suggests a promising
treatment approach that combines chemotherapy with ICIs in patients
with BTC, offering not only enhanced efficacy, but also an
acceptable safety profile.
The combination of molecular targeted therapy and
immunotherapy has demonstrated synergistic effects. The capacity of
immunotherapy to eliminate immunosuppression can extend the
remission effect induced by molecular targeted therapy, thereby
enhancing the overall effectiveness of targeted therapy.
The trial NCT02443324 assessed the benefits of
pembrolizumab in combination with ramucirumab (a VEGFR-2 monoclonal
antibody) in 26 patients with locally advanced, unresectable or
metastatic BTC (154). However,
the results were quite disappointing, with only one patient
achieving a partial response, and a mOS of 6.4 months. The trial
NCT03201458 compared the efficacy of atezolizumab monotherapy with
atezolizumab in combination with cobimetinib (MEK inhibitor) in
patients with BTC who had failed first/second line therapy
(155). While the combined
treatment group exhibited a slightly better mPFS, both groups had
extremely low ORRs. Similarly, a single-arm study investigated the
efficacy and tolerability of pembrolizumab + lenvatinib in the
treatment of patients with BTC who had failed prior systemic
treatments (156). That study
reported that 25% of patients responded to treatment and the DCR
was 78.1%, with a clinical benefit rate of 40.5% (156). Additional studies exploring the
combination of ICIs with targeted therapies are listed in Table I.
Local treatment may be an effective option for
patients with BTC who are not eligible for surgery or have advanced
lesions. Techniques such as local ablative therapy and radiation
therapy can effectively kill tumor cells, boost the production of
tumor neoantigens and enhance the immune recognition response.
Consequently, there is a theoretical synergy between local therapy
and immunotherapy. The trial NCT01853618 revealed that in the
evaluable patients who received combination therapy with
tremeliumab + microwave ablation, 12.5% achieved a PR, 31.2%
achieved SD, with a mPFS of 3.4 months, and a mOS of 6 months
(157). Furthermore, studies
have suggested that the immune response triggered by antigens
released from dead tumor cells following radiation therapy can
extend to distant metastatic lesions (140). This phenomenon underscores the
potential of combining local therapies with immunotherapy for more
comprehensive cancer treatment.
The dense fibrotic matrix found in CCA tissue can
impede the effectiveness of antitumor drugs and immune cell
infiltration. Therefore, reducing stromal fibrosis in CCA may
enhance the response to therapy. The trial NCT03267940 is currently
investigating the effectiveness of hyaluronidase when combined with
ICIs and chemotherapy for progressive BTC. As aforementioned,
increasing the number of NK cells can inhibit tumor growth. A phase
1/2a trial explored the safety and efficacy of pembrolizumab
combining allogeneic NK cells in chemotherapy-refractory BTC
patients (158). That study
reported an overall ORR and DCR of 17.4 and 30.4%, all without
severe TRAEs. That study demonstrated that pembrolizumab plus
allogeneic NK cells represents a promising therapeutic approach,
exhibiting an improved efficacy and a favorable safety profile
(158).
Chimeric antigen receptor (CAR) T-cells, derived
from peripheral blood and modified in vitro, express CARs
formed by merging antigen recognition sites from tumor-specific
antibodies with costimulatory molecules, such as CD28. These CAR
T-cells can selectively target tumor antigens and activate
antitumor responses. While CAR T-cell therapy has achieved success
in hematological malignancies, particularly gaining FDA approval
for B-lymphoblastic leukemia in 2017 (159), there is growing interest in its
application against solid tumors. Capitalizing on the
overexpression of EGFR and CD133 in CCA, studies have devised
treatment strategies involving EGFR or CD133 CAR T-cells. In
vitro experiments with anti-CD133 CAR T-cells demonstrated
significant and potent cytolytic activity against CCA cells
(160). However, a phase 1
clinical study evaluating patients with EGFR-positive metastatic or
recurrent BTC found insignificant benefits (161). Another study investigated the
effectiveness of EGFR-targeted and CD133-targeted CAR T-cell
sequential therapy in a patient with advanced-stage CCA, yielding a
partial response of 8.5 and 4.5 months after CAR T-cell EGFR and
CAR T-cell CD133 treatments, respectively (162). In addition to CAR T-cell
therapy, the efficacy of adoptive cellular transfer of TILs in CCA
has been substantiated by various studies. Case reports have
revealed that immune cell adoptive transfer exhibits encouraging
efficacy in patients with CCA, which a reduced tumor load and
prolonged survival (163,164).
Moreover, the effectiveness and safety of immune cell adoptive
transfer therapy in combination with other treatments have been
investigated. Zhang et al (165) combined local treatment with the
adoptive transfer of allogeneic γδ T-cells for CCA and found no
significant survival benefit for patients receiving the combination
therapy, despite a favorable safety profile. The combination of the
DC vaccine and activated T-cell transfer was proven to be an
adjuvant immunotherapy that significantly prolonged the survival of
patients with iCCA and HCC undergoing surgery (166,167). Although numerous studies have
shown that immune cell adoptive transfer therapy is a potential
treatment modality for BTC, the use of this therapy in BTC is still
in its infancy and needs to be validated in more high-quality
clinical trials.
CD40, a member of the TNF receptor superfamily,
plays a pivotal role in the immune response. Upon interaction with
its ligands, CD40 can stimulate DCs to initiate T-cell-dependent
antitumor responses and induce the macrophage-mediated destruction
of the tumor stroma. CD40 agonists hold the potential to transform
'cold' tumors into 'hot' tumors, rendering them more responsive to
immunotherapy. In vitro research has illustrated that CD40
agonists can activate DCs, leading to tumor cell killing (168). Furthermore, combining CD40
agonists with immunotherapy has been shown to increase the number
of DCs and restore their function, enhancing the antitumor response
of T-cells in vivo (169). The combination of CD40 agonists
with ICIs has shown significant promise in treating various solid
tumors (170-173). However, there has been limited
exploration of CD40 agonists in CCA. Humphreys et al
(174) demonstrated the
effectiveness of CD40 agonists in inducing the apoptosis of CCA
cells (174). A recent study
provided compelling evidence that combining CD40 agonists with
anti-PD-1 therapy yielded robust antitumor activity in iCCA mouse
models and significantly improved OS with good tolerability
(175). The findings of that
study suggest that the triple combination of CD40 agonists, ICIs
and chemotherapy holds promise as an effective therapy for CCA.
This triple combination therapy was tested in pancreatic cancer.
Notably, a similar triple combination therapy was evaluated in
pancreatic cancer, demonstrating exciting therapeutic effects in a
phase 1b clinical trial. That trial investigated
gemcitabine/nab-paclitaxel combined with a CD40 agonist (APX005M),
with or without nivolumab, for the treatment of untreated
metastatic pancreatic cancer (176).
Vaccine therapy represents a promising avenue for
enhancing the immune microenvironment in cancer treatment. It
functions by eliciting a pre-existing immune response against
target antigens within the body, ultimately triggering potent,
specific cellular immunity. CCA poses a challenge for immunotherapy
due to its low tumor mutation burden and limited expression of
neoantigens (177). Therefore,
vaccine therapy holds particular appeal as a treatment option for
CCA. These cancer vaccines can be broadly categorized into three
groups: Cancer antigen peptide or protein vaccines, cellular
vaccines and tumor antigen gene vaccines (178).
Peptide or protein-based vaccines commonly
incorporate antigens that are overexpressed to enhance
immunogenicity. For CCA, Wilm's tumor protein 1 (WT1) and MUC1 are
widely expressed (179). Many single peptide-based cancer vaccines
for CCA have targeted WT1 or MUC1. Despite being well-tolerated,
these tumor vaccines often exhibit limited effectiveness when used
as monotherapy (180-182).
The efficacy of single peptide-based vaccines is
constrained by the heterogeneity of CCA, stemming from the uneven
distribution of TAAs. The response of the immune system interacting
with TAAs varies widely from one patient to another. The immune
system's response to TAAs can vary significantly from one patient
to another. Immune cells induced by individual peptide vaccines
specifically target tumor cells expressing that single peptide
(protein). However, when these tumor cells downregulate or silence
the targeted peptide (protein), the single peptide vaccines tend to
lose their efficacy. By contrast, vaccines designed to target
multiple antigenic peptides have the potential to address these
limitations. Aruga et al (183) conducted investigations into the
safety, immune responses and antitumor effects of a four-peptide
vaccination in patients with advanced-stage refractory BTC. The
results of their study revealed detectable peptide-specific T-cell
immune responses in 7 out of 9 vaccinated patients, indicating that
the four-peptide vaccine was both safe and associated with a
survival benefit (183).
Subsequently, the same cohort was treated with a three-peptide
vaccine, which yielded similar results in terms of safety and
effective immune responses (184). Additionally, a phase 2 trial
identified four peptides for the development of personalized
multiple-peptide vaccines based on patients' immunological profiles
(185). These personalized
vaccines were found to induce robust immune responses with
favorable tolerability. While these studies demonstrate the
feasibility and safety of multi-peptide approaches for refractory
BTC, their impact on survival warrants further investigation in
larger prospective studies. However, it is worth noting that in the
limited immune space, immune cells compete with each other. In
cases where an inappropriate peptide vaccine induces an immune
response, it may inadvertently suppress the function of
pre-existing memory immune cells. This phenomenon may contribute to
accelerated disease progression and even premature mortality among
patients.
Cellular vaccines are designed to expose the immune
system to antigens, thereby stimulating the generation of memory
lymphocytes and facilitating a robust immune response against
tumors. DCs, modified autologous cancer cells and allogeneic tumor
cell lines are commonly used cell types in cell-based tumor
vaccines (178). DC-based tumor
vaccines loaded with TAAs have demonstrated favorable tolerability
and potential efficacy in CCA. In a phase 1/2 study, 12 patients
with CCA or pancreatic cancer received a DC vaccine loaded with
MUC1 following primary tumor resection, resulting in a mOS of 26
months with good tolerability (186). Another study involving 65
patients with unresectable or recurrent BTC utilized DC vaccines
pulsed with WT1 and/or MUC1, which proved to be well-tolerated,
with 15% of patients experiencing an attenuated disease progression
(187). A recent study employed
Listeria monocytogenes expressing antigen of interest (LmAIO) for
prophylactic vaccination in a mouse model of CCA (188). This approach successfully
induced potent tumor-specific Th1 immunity, leading to reduced
tumor burden, delayed disease progression, and prolonged survival
(188). That study employed an
attenuated strain of Listeria monocytogenes as a TAA presentation
vehicle, shedding new light on the development of cell-based tumor
vaccines.
Nucleic acid-based cancer vaccines, which leverage
genetic material, have emerged as a focal point in tumor
immunotherapy. These vaccines offer several advantages compared to
other types. They can concurrently deliver multiple TAAs,
mitigating the risk of resistance, and encode full-length TAAs to
stimulate broader T-cell responses. Moreover, these vaccines have
demonstrated good tolerability and safety profiles across various
digestive system tumors (189).
Huang et al (190)
applied bioinformatics techniques to identify three potential TAAs
for CCA mRNA vaccines. They further stratified patients with CCA
based on immunophenotyping and suggested that those with an
'immune-cold' phenotype may derive more substantial benefits from
mRNA vaccine therapy (190).
However, the application of genetic vaccines in CCA remains at a
preliminary stage, necessitating further research to establish
their safety and true efficacy.
CCA is a highly malignant tumor and characterized
by a poor prognosis. Risk factors for CCA include hepatitis viral
infection, parasitic infection, cirrhosis, primary sclerosing
cholangitis and cholelithiasis (191). Furthermore, studies using
animals have suggested that exposure to dioxin-like compounds can
increase the incidence of CCA in mice (192,193); however, this association has not
been definitively confirmed in humans. At present, the main
treatment option for CCA is radical surgery, supplemented by
chemotherapy and radiotherapy. However, even with these
interventions, the survival rates of patients with CCA remain
disoncertingly low. The emergence of immunotherapy as a treatment
modality for solid tumors has garnered increasing attention,
holding significant promise for CCA. Nevertheless, the progress of
immunotherapy for CCA remains in its infancy, a circumstance that
may be attributed to several factors. Firstly, the majority of CCA
cases are immunologically 'cold' tumors with a suppressive TIME and
present with a low response rate to immunotherapies. Secondly, CCA
is a heterogenous disease and the molecular characteristics of CCA
derived from different/same regions of bile duct differ from each
other. Thirdly, for unselected patients with CCA, single ICI
therapy is less effective and patients are more likely to exhibit
resistance to therapy. These multifaceted challenges underscore the
need for a more in-depth understanding of the immune landscape in
CCA and the development of tailored, combinatorial
immunotherapeutic approaches that can overcome the complexities
posed by this aggressive malignancy.
The TIME of CCA is highly intricate and dynamic,
associated with CCA progression, metastasis and treatment failure.
Within the TIME of CCA lies a wealth of potential drug targets for
the development of innovative immune-based therapies. To pave the
way for more precise and efficacious treatments for CCA, future
research endeavors should harness cutting-edge techniques, such as
single-cell sequencing, transcriptomics, proteomics and
metabolomics. These approaches will enable a comprehensive
exploration of the intricate mechanisms governing the interplay
between CCA and its TIME. By elucidating the TIME landscape of CCA
in its entirety, invaluable insight can be obtained into novel
therapeutic avenues targeting specific components of the TIME,
heralding a new era in CCA treatment.
CCA can be classified into distinct subtypes based
on its anatomical origin, primarily as iCCA and extrahepatic CCA
(eCCA), which further includes perihilar and distal CCA. Notably,
the molecular profile of cancerous tissues varies significantly
across different biliary system sites. For instance, mutations in
genes such as IDH1, BAP1 and PBRM1 are prevalent among patients
with iCCA, whereas KRAS, CDKN2A and BRCA1 mutations are more
commonly observed in eCCA cases (194,195). Even within the same anatomical
region, CCAs with distinct histological features may exhibit
differing gene mutations (196).
This inherent heterogeneity poses a challenge in predicting
responses to immunotherapies. Studies have indicated that ICI
treatments tend to be more effective for iCCA compared to eCCA
(195). Therefore, conducting
comprehensive multi-omics investigations of CCA using genomic,
proteomic, metabolomic and colonyomic technologies holds the
promise of providing detailed insight into the characteristics
relevant to CCA immunotherapies. This approach may shift the
classification of CCA from anatomical and morphological criteria to
molecular typing, offering a more accurate reflection of the
tumor's biological essence. Ultimately, this may enable a more
precise diagnosis and may lead to the development of treatment
strategies tailored to the specific molecular profile of CCA.
Combination therapy focused on ICIs is a promising
and valuable first-line or translational treatment approach for
intractable biliary tract malignancies. Dual ICI treatment
targeting different immune checkpoints has also shown prospective
synergistic therapeutic effects. However, there remain several
caveats for ICI combination therapy in clinical practice. Notably,
the majority of patients are insensitive to ICI combination
therapy, and the overall ORR is relatively low. The ICI combination
therapy has a lower overall ORR and risks of therapeutic
resistance. The therapeutic resistance phenomenon is also observed.
Therefore, future prospective precision immunotherapy should focus
on developing more well-established and definite personalized
treatment for patients with CCA with different subtypes. With the
greater understanding of the molecular features of CCA, the issue
of identifying more accurate and reliable biomarkers of
immunotherapy effects needs to be solved imminently. Although the
favorable safety profile of ICI combination therapy has been proven
in several clinical trials, it is necessary and crucial to evaluate
the treatment-related adverse effects. On the one hand, the liver
function of the majority of patients with CCA is impaired,
affecting metabolic detoxification. On the other hand, the sample
volume of existing studies is minimal, which is likely to lead to
bias in conclusions. Further large-volume, high-quality,
prospective and randomized controlled trials are required to
identify the safety, as well as the therapeutic effects of
different immune combination regimens.
CCA stands out as an exceptionally malignant tumor,
marked by its often-grim prognosis. In the quest for precision
immunotherapy, future efforts should harness advanced techniques to
delve deeper into the intricate mechanisms governing the interplay
between CCA and the TIME. Such endeavors should ultimately yield a
comprehensive TIME landscape specific to CCA. This knowledge may
serve as the foundation for developing more robust and tailored
personalized treatments, accounting for the diversity of CCA
subtypes. One particularly promising avenue is the exploration of
combination therapy, with a specific focus on ICIs. This approach
holds substantial potential as a first-line or translational
treatment strategy, particularly for the challenging realm of
intractable biliary tract malignancies.
Not applicable.
SY contributed to data acquisition and drafted the
manuscript. YH, RZ and YD contributed to data acquisition. FL and
HH contributed to the study design and the revision of the
manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the 1.3.5 project for
disciplines of excellence, West China Hospital, Sichuan University
(grant no. ZYJC21046); the 1.3.5 project for disciplines of
excellence-Clinical Research Incubation Project, West China
Hospital, Sichuan University (grant no. 2021HXFH001); the Natural
Science Foundation of Sichuan Province (grant no. 2022NSFSC0806);
the National Natural Science Foundation of China for Young
Scientists Fund (grant no. 82203782), Sichuan Science and
Technology Program (grant nos. 2021YJ0132 and 2021YFS0100); the
fellowship of China Postdoctoral Science Foundation (grant no.
2021M692277); the Sichuan University-Zigong School-local
Cooperation project (grant no. 2021CDZG-23); the Science and
Technology project of the Health planning committee of Sichuan
(21PJ046); and the Post-Doctor Research Project, West China
Hospital, Sichuan University (grant no. 2021HXBH127).
|
1
|
Banales JM, Cardinale V, Carpino G,
Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes
SJ, Fouassier L, et al: Expert consensus document:
Cholangiocarcinoma: Current knowledge and future perspectives
consensus statement from the European network for the study of
cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol.
13:261–280. 2016.
|
|
2
|
Rizzo A, Carloni R, Frega G, Palloni A, Di
Federico A, Ricci AD, De Luca R, Tavolari S and Brandi G: Intensive
follow-up program and oncological outcomes of biliary tract cancer
patients after curative-intent surgery: A twenty-year experience in
a single tertiary medical center. Curr Oncol. 29:5084–5090.
2022.
|
|
3
|
Cai Y, Cheng N, Ye H, Li F, Song P and
Tang W: The current management of cholangiocarcinoma: A comparison
of current guidelines. Biosci Trends. 10:92–102. 2016.
|
|
4
|
Fabris L, Perugorria MJ, Mertens J,
Björkström NK, Cramer T, Lleo A, Solinas A, Sänger H, Lukacs-Kornek
V, Moncsek A, et al: The tumour microenvironment and immune milieu
of cholangiocarcinoma. Liver Int. 39(Suppl 1): S63–S78. 2019.
|
|
5
|
Xia T, Li K, Niu N, Shao Y, Ding D, Thomas
DL, Jing H, Fujiwara K, Hu H, Osipov A, et al: Immune cell atlas of
cholangiocarcinomas reveals distinct tumor microenvironments and
associated prognoses. J Hematol Oncol. 15:372022.
|
|
6
|
Liu D, Heij LR, Czigany Z, Dahl E, Lang
SA, Ulmer TF, Luedde T, Neumann UP and Bednarsch J: The role of
tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin
Cancer Res. 41:1272022.
|
|
7
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014.
|
|
8
|
O'Donnell JS, Teng MWL and Smyth MJ:
Cancer immunoediting and resistance to T cell-based immunotherapy.
Nat Rev Clin Oncol. 16:151–167. 2019.
|
|
9
|
Gubin MM and Vesely MD: Cancer
immunoediting in the era of immuno-oncology. Clin Cancer Res.
28:3917–3928. 2022.
|
|
10
|
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse
J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, et al: Pembrolizumab
in combination with gemcitabine and cisplatin compared with
gemcitabine and cisplatin alone for patients with advanced biliary
tract cancer (KEYNOTE-966): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet. 401:1853–1865. 2023.
|
|
11
|
Sahai V, Griffith KA, Beg MS, Shaib WL,
Mahalingam D, Zhen DB, Deming DA and Zalupski MM: A randomized
phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab
and ipilimumab in previously untreated advanced biliary cancer:
BilT-01. Cancer. 128:3523–3530. 2022.
|
|
12
|
Monge C, Pehrsson EC, Xie C, Duffy AG,
Mabry D, Wood BJ, Kleiner DE, Steinberg SM, Figg WD, Redd B, et al:
A phase II study of pembrolizumab in combination with capecitabine
and oxaliplatin with molecular profiling in patients with advanced
biliary tract carcinoma. Oncologist. 27:e273–e285. 2022.
|
|
13
|
Sirica AE and Gores GJ: Desmoplastic
stroma and cholangiocarcinoma: Clinical implications and
therapeutic targeting. Hepatology. 59:2397–2402. 2014.
|
|
14
|
Montori M, Scorzoni C, Argenziano ME,
Balducci D, De Blasio F, Martini F, Buono T, Benedetti A, Marzioni
M and Maroni L: Cancer-associated fibroblasts in
cholangiocarcinoma: Current knowledge and possible implications for
therapy. J Clin Med. 11:64982022.
|
|
15
|
Okabe H, Beppu T, Hayashi H, Horino K,
Masuda T, Komori H, Ishikawa S, Watanabe M, Takamori H, Iyama K and
Baba H: Hepatic stellate cells may relate to progression of
intrahepatic cholangiocarcinoma. Ann Surg Oncol. 16:2555–2564.
2009.
|
|
16
|
Dranoff JA and Wells RG: Portal
fibroblasts: Underappreciated mediators of biliary fibrosis.
Hepatology. 51:1438–1444. 2010.
|
|
17
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272.
2011.
|
|
18
|
Affo S, Nair A, Brundu F, Ravichandra A,
Bhattacharjee S, Matsuda M, Chin L, Filliol A, Wen W, Song X, et
al: Promotion of cholangiocarcinoma growth by diverse
cancer-associated fibroblast subpopulations. Cancer Cell.
39:866–882. 2021.
|
|
19
|
Mertens JC, Fingas CD, Christensen JD,
Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts
LR, Sirica AE and Gores GJ: Therapeutic effects of deleting
cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res.
73:897–907. 2013.
|
|
20
|
Zhang XF, Dong M, Pan YH, Chen JN, Huang
XQ, Jin Y and Shao CK: Expression pattern of cancer-associated
fibroblast and its clinical relevance in intrahepatic
cholangiocarcinoma. Hum Pathol. 65:92–100. 2017.
|
|
21
|
Itou RA, Uyama N, Hirota S, Kawada N, Wu
S, Miyashita S, Nakamura I, Suzumura K, Sueoka H, Okada T, et al:
Immunohistochemical characterization of cancer-associated
fibroblasts at the primary sites and in the metastatic lymph nodes
of human intrahepatic cholangiocarcinoma. Hum Pathol. 83:77–89.
2019.
|
|
22
|
Sirica AE: The role of cancer-associated
myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev
Gastroenterol Hepatol. 9:44–54. 2011.
|
|
23
|
Clapéron A, Mergey M, Aoudjehane L,
Ho-Bouldoires TH, Wendum D, Prignon A, Merabtene F, Firrincieli D,
Desbois-Mouthon C, Scatton O, et al: Hepatic myofibroblasts promote
the progression of human cholangiocarcinoma through activation of
epidermal growth factor receptor. Hepatology. 58:2001–2011.
2013.
|
|
24
|
Clapéron A, Mergey M, Nguyen Ho-Bouldoires
TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A,
Paradis V, Housset C, et al: EGF/EGFR axis contributes to the
progression of cholangiocarcinoma through the induction of an
epithelial-mesenchymal transition. J Hepatol. 61:325–332. 2014.
|
|
25
|
Ohira S, Sasaki M, Harada K, Sato Y, Zen
Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y and Nakanuma Y:
Possible regulation of migration of intrahepatic cholangiocarcinoma
cells by interaction of CXCR4 expressed in carcinoma cells with
tumor necrosis factor-alpha and stromal-derived factor-1 released
in stroma. Am J Pathol. 168:1155–1168. 2006.
|
|
26
|
Gentilini A, Rombouts K, Galastri S,
Caligiuri A, Mingarelli E, Mello T, Marra F, Mantero S, Roncalli M,
Invernizzi P and Pinzani M: Role of the stromal-derived factor-1
(SDF-1)-CXCR4 axis in the interaction between hepatic stellate
cells and cholangiocarcinoma. J Hepatol. 57:813–820. 2012.
|
|
27
|
McCarthy JB, El-Ashry D and Turley EA:
Hyaluronan, cancer-associated fibroblasts and the tumor
microenvironment in malignant progression. Front Cell Dev Biol.
6:482018.
|
|
28
|
Cyphert JM, Trempus CS and Garantziotis S:
Size matters: Molecular weight specificity of hyaluronan effects in
cell biology. Int J Cell Biol. 2015:5638182015.
|
|
29
|
Tian X, Azpurua J, Hine C, Vaidya A,
Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V and
Seluanov A: High-molecular-mass hyaluronan mediates the cancer
resistance of the naked mole rat. Nature. 499:346–349. 2013.
|
|
30
|
Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge
C, Liu Y, Hao Y, Zhang D, Shi G, et al: Single-cell transcriptomic
architecture and intercellular crosstalk of human intrahepatic
cholangiocarcinoma. J Hepatol. 73:1118–1130. 2020.
|
|
31
|
Cadamuro M, Nardo G, Indraccolo S,
Dall'olmo L, Sambado L, Moserle L, Franceschet I, Colledan M,
Massani M, Stecca T, et al: Platelet-derived growth factor-D and
Rho GTPases regulate recruitment of cancer-associated fibroblasts
in cholangiocarcinoma. Hepatology. 58:1042–1053. 2013.
|
|
32
|
Fingas CD, Bronk SF, Werneburg NW, Mott
JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE and Gores GJ:
Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling
in cholangiocarcinoma cells. Hepatology. 54:2076–2088. 2011.
|
|
33
|
Cadamuro M, Brivio S, Mertens J, Vismara
M, Moncsek A, Milani C, Fingas C, Cristina Malerba M, Nardo G,
Dall'Olmo L, et al: Platelet-derived growth factor-D enables liver
myofibroblasts to promote tumor lymphangiogenesis in
cholangiocarcinoma. J Hepatol. 70:700–709. 2019.
|
|
34
|
Wang Z, An J, Zhu D, Chen H, Lin A, Kang
J, Liu W and Kang X: Periostin: An emerging activator of multiple
signaling pathways. J Cell Commun Signal. 16:515–530. 2022.
|
|
35
|
Yue H, Li W, Chen R, Wang J, Lu X and Li
J: Stromal POSTN induced by TGF-β1 facilitates the migration and
invasion of ovarian cancer. Gynecol Oncol. 160:530–538. 2021.
|
|
36
|
Chen G, Wang Y, Zhao X, Xie XZ, Zhao JG,
Deng T, Chen ZY, Chen HB, Tong YF, Yang Z, et al: A positive
feedback loop between periostin and TGFβ1 induces and maintains the
stemness of hepatocellular carcinoma cells via AP-2α activation. J
Exp Clin Cancer Res. 40:2182021.
|
|
37
|
Yu B, Wu K, Wang X, Zhang J, Wang L, Jiang
Y, Zhu X, Chen W and Yan M: Periostin secreted by cancer-associated
fibroblasts promotes cancer stemness in head and neck cancer by
activating protein tyrosine kinase 7. Cell Death Dis.
9:10822018.
|
|
38
|
Ma H, Wang J, Zhao X, Wu T, Huang Z, Chen
D, Liu Y and Ouyang G: Periostin promotes colorectal tumorigenesis
through integrin-FAK-Src pathway-mediated YAP/TAZ activation. Cell
Rep. 30:793–806.e6. 2020.
|
|
39
|
Utispan K, Sonongbua J, Thuwajit P,
Chau-In S, Pairojkul C, Wongkham S and Thuwajit C: Periostin
activates integrin α5β1 through a PI3K/AKT-dependent pathway in
invasion of cholangiocarcinoma. Int J Oncol. 41:1110–1118.
2012.
|
|
40
|
Sonongbua J, Siritungyong S, Thongchot S,
Kamolhan T, Utispan K, Thuwajit P, Pongpaibul A, Wongkham S and
Thuwajit C: Periostin induces epithelial-to-mesenchymal transition
via the integrin α5β1/TWIST-2 axis in cholangiocarcinoma. Oncol
Rep. 43:1147–1158. 2020.
|
|
41
|
Peng H, Zhu E and Zhang Y: Advances of
cancer-associated fibroblasts in liver cancer. Biomark Res.
10:592022.
|
|
42
|
Kunk PR, Dougherty SC, Lynch K, Whitehair
R, Meneveau M, Obeid JM, Winters K, Ju JY, Stelow EB, Bauer TW, et
al: Myeloid cell infiltration correlates with prognosis in
cholangiocarcinoma and varies based on tumor location. J
Immunother. 44:254–263. 2021.
|
|
43
|
Hasita H, Komohara Y, Okabe H, Masuda T,
Ohnishi K, Lei XF, Beppu T, Baba H and Takeya M: Significance of
alternatively activated macrophages in patients with intrahepatic
cholangiocarcinoma. Cancer Sci. 101:1913–1919. 2010.
|
|
44
|
Charbel A, Tavernar L, Albrecht T,
Brinkmann F, Verheij J, Roos E, Vogel MN, Köhler B, Springfeld C,
Brobeil A, et al: Spatiotemporal analysis of tumour-infiltrating
immune cells in biliary carcinogenesis. Br J Cancer. 127:1603–1614.
2022.
|
|
45
|
Tu J, Wu F, Chen L, Zheng L, Yang Y, Ying
X, Song J, Chen C, Hu X, Zhao Z and Ji J: Long non-coding RNA PCAT6
induces M2 polarization of macrophages in cholangiocarcinoma via
modulating miR-326 and RhoA-ROCK signaling pathway. Front Oncol.
10:6058772021.
|
|
46
|
Kitano Y, Okabe H, Yamashita YI, Nakagawa
S, Saito Y, Umezaki N, Tsukamoto M, Yamao T, Yamamura K, Arima K,
et al: Tumour-infiltrating inflammatory and immune cells in
patients with extrahepatic cholangiocarcinoma. Br J Cancer.
118:171–180. 2018.
|
|
47
|
Paillet J, Kroemer G and Pol JG: Immune
contexture of cholangiocarcinoma. Curr Opin Gastroenterol.
36:70–76. 2020.
|
|
48
|
Yuan H, Lin Z, Liu Y, Jiang Y, Liu K, Tu
M, Yao N, Qu C and Hong J: Intrahepatic cholangiocarcinoma induced
M2-polarized tumor-associated macrophages facilitate tumor growth
and invasiveness. Cancer Cell Int. 20:5862020.
|
|
49
|
Bai R, Li Y, Jian L, Yang Y, Zhao L and
Wei M: The hypoxia-driven crosstalk between tumor and
tumor-associated macrophages: Mechanisms and clinical treatment
strategies. Mol Cancer. 21:1772022.
|
|
50
|
Loilome W, Bungkanjana P, Techasen A,
Namwat N, Yongvanit P, Puapairoj A, Khuntikeo N and Riggins GJ:
Activated macrophages promote Wnt/β-catenin signaling in
cholangiocarcinoma cells. Tumour Biol. 35:5357–5367. 2014.
|
|
51
|
Cheng H and Li Q: Sevoflurane inhibits
cholangiocarcinoma via Wnt/β-catenin signaling pathway. BMC
Gastroenterol. 23:2792023.
|
|
52
|
Boulter L, Guest RV, Kendall TJ, Wilson
DH, Wojtacha D, Robson AJ, Ridgway RA, Samuel K, Van Rooijen N,
Barry ST, et al: WNT signaling drives cholangiocarcinoma growth and
can be pharmacologically inhibited. J Clin Invest. 125:1269–1285.
2015.
|
|
53
|
Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z,
Xiao YS, Hu ZQ, Huang XY, Yang GH, Shi YH, et al: CXCL5 contributes
to tumor metastasis and recurrence of intrahepatic
cholangiocarcinoma by recruiting infiltrative intratumoral
neutrophils. Carcinogenesis. 35:597–605. 2014.
|
|
54
|
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H,
Luo C, Zhou J, Fan J and Zhou S: Tumor-associated neutrophils and
macrophages interaction contributes to intrahepatic
cholangiocarcinoma progression by activating STAT3. J Immunother
Cancer. 9:e0019462021.
|
|
55
|
Pandey G: Tumor-associated macrophages in
solid tumor: Friend or foe. Ann Transl Med. 8:10272020.
|
|
56
|
Brandau S, Dumitru CA and Lang S: Protumor
and antitumor functions of neutrophil granulocytes. Semin
Immunopathol. 35:163–176. 2013.
|
|
57
|
Ohms M, Möller S and Laskay T: An attempt
to polarize human neutrophils toward N1 and N2 phenotypes in vitro.
Front Immunol. 11:5322020.
|
|
58
|
Jaillon S, Ponzetta A, Di Mitri D, Santoni
A, Bonecchi R and Mantovani A: Neutrophil diversity and plasticity
in tumour progression and therapy. Nat Rev Cancer. 20:485–503.
2020.
|
|
59
|
Mao ZY, Zhu GQ, Xiong M, Ren L and Bai L:
Prognostic value of neutrophil distribution in cholangiocarcinoma.
World J Gastroenterol. 21:4961–4968. 2015.
|
|
60
|
Branchi V, Jürgensen B, Esser L,
Gonzalez-Carmona M, Weismüller TJ, Strassburg CP, Henn J, Semaan A,
Lingohr P, Manekeller S, et al: Tumor infiltrating neutrophils are
frequently found in adenocarcinomas of the biliary tract and their
precursor lesions with possible impact on prognosis. J Pers Med.
11:2332021.
|
|
61
|
Parker KH, Beury DW and Ostrand-Rosenberg
S: Myeloid-derived suppressor cells: Critical cells driving immune
suppression in the tumor microenvironment. Adv Cancer Res.
128:95–139. 2015.
|
|
62
|
Desai R, Coxon AT and Dunn GP: Therapeutic
applications of the cancer immunoediting hypothesis. Semin Cancer
Biol. 78:63–77. 2022.
|
|
63
|
Qin G, Liu S, Liu J, Hu H, Yang L, Zhao Q,
Li C, Zhang B and Zhang Y: Overcoming resistance to immunotherapy
by targeting GPR84 in myeloid-derived suppressor cells. Signal
Transduct Target Ther. 8:1642023.
|
|
64
|
Kalathil S, Lugade AA, Miller A, Iyer R
and Thanavala Y: Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T
regulatory cells and myeloid-derived suppressor cells in
hepatocellular carcinoma patients are associated with impaired
T-cell functionality. Cancer Res. 73:2435–2444. 2013.
|
|
65
|
Zhang Q, Ma C, Duan Y, Heinrich B, Rosato
U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ, et al: Gut microbiome
directs hepatocytes to recruit MDSCs and promote
cholangiocarcinoma. Cancer Discov. 11:1248–1267. 2021.
|
|
66
|
Lin Y, Cai Q, Chen Y, Shi T, Liu W, Mao L,
Deng B, Ying Z, Gao Y, Luo H, et al: CAFs shape myeloid-derived
suppressor cells to promote stemness of intrahepatic
cholangiocarcinoma through 5-lipoxygenase. Hepatology. 75:28–42.
2022.
|
|
67
|
Loeuillard E, Yang J, Buckarma E, Wang J,
Liu Y, Conboy C, Pavelko KD, Li Y, O'Brien D, Wang C, et al:
Targeting tumor-associated macrophages and granulocytic
myeloid-derived suppressor cells augments PD-1 blockade in
cholangiocarcinoma. J Clin Invest. 130:5380–5396. 2020.
|
|
68
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018.
|
|
69
|
Hung TH, Hung JT, Wu CE, Huang Y, Lee CW,
Yeh CT, Chung YH, Lo FY, Lai LC, Tung JK, et al: Globo H is a
promising theranostic marker for intrahepatic cholangiocarcinoma.
Hepatol Commun. 6:194–208. 2022.
|
|
70
|
Morisaki T, Umebayashi M, Kiyota A, Koya
N, Tanaka H, Onishi H and Katano M: Combining cetuximab with killer
lymphocytes synergistically inhibits human cholangiocarcinoma cells
in vitro. Anticancer Res. 32:2249–2256. 2012.
|
|
71
|
Panwong S, Wathikthinnakon M, Kaewkod T,
Sawasdee N, Tragoolpua Y, Yenchitsomanus PT and Panya A: Cordycepin
sensitizes cholangiocarcinoma cells to be killed by natural
killer-92 (NK-92) cells. Molecules. 26:59732021.
|
|
72
|
Jung IH, Kim DH, Yoo DK, Baek SY, Jeong
SH, Jung DE, Park SW and Chung YY: In Vivo study of natural killer
(NK) cell cytotoxicity against cholangiocarcinoma in a nude mouse
model. In Vivo. 32:771–781. 2018.
|
|
73
|
Fukuda Y, Asaoka T, Eguchi H, Yokota Y,
Kubo M, Kinoshita M, Urakawa S, Iwagami Y, Tomimaru Y, Akita H, et
al: Endogenous CXCL9 affects prognosis by regulating
tumor-infiltrating natural killer cells in intrahepatic
cholangiocarcinoma. Cancer Sci. 111:323–333. 2020.
|
|
74
|
Tsukagoshi M, Wada S, Yokobori T, Altan B,
Ishii N, Watanabe A, Kubo N, Saito F, Araki K, Suzuki H, et al:
Overexpression of natural killer group 2 member D ligands predicts
favorable prognosis in cholangiocarcinoma. Cancer Sci. 107:116–122.
2016.
|
|
75
|
Melum E, Karlsen TH, Schrumpf E, Bergquist
A, Thorsby E, Boberg KM and Lie BA: Cholangiocarcinoma in primary
sclerosing cholangitis is associated with NKG2D polymorphisms.
Hepatology. 47:90–96. 2008.
|
|
76
|
Asahi Y, Hatanaka KC, Hatanaka Y, Kamiyama
T, Orimo T, Shimada S, Nagatsu A, Sakamoto Y, Kamachi H, Kobayashi
N, et al: Prognostic impact of CD8+ T cell distribution and its
association with the HLA class I expression in intrahepatic
cholangiocarcinoma. Surg Today. 50:931–940. 2020.
|
|
77
|
Kim HD, Kim JH, Ryu YM, Kim D, Lee S, Shin
J, Hong SM, Kim KH, Jung DH, Song GW, et al: Spatial distribution
and prognostic implications of tumor-infiltrating FoxP3-CD4+ T
cells in biliary tract cancer. Cancer Res Treat. 53:162–171.
2021.
|
|
78
|
Goeppert B, Frauenschuh L, Zucknick M,
Stenzinger A, Andrulis M, Klauschen F, Joehrens K, Warth A, Renner
M, Mehrabi A, et al: Prognostic impact of tumour-infiltrating
immune cells on biliary tract cancer. Br J Cancer. 109:2665–2674.
2013.
|
|
79
|
Ueno T, Tsuchikawa T, Hatanaka KC,
Hatanaka Y, Mitsuhashi T, Nakanishi Y, Noji T, Nakamura T, Okamura
K, Matsuno Y and Hirano S: Prognostic impact of programmed cell
death ligand 1 (PD-L1) expression and its association with
epithelial-mesenchymal transition in extrahepatic
cholangiocarcinoma. Oncotarget. 9:20034–20047. 2018.
|
|
80
|
Kasper HU, Drebber U, Stippel DL, Dienes
HP and Gillessen A: Liver tumor infiltrating lymphocytes:
Comparison of hepatocellular and cholangiolar carcinoma. World J
Gastroenterol. 15:5053–5057. 2009.
|
|
81
|
Kim HD, Jeong S, Park S, Lee YJ, Ju YS,
Kim D, Song GW, Lee JH, Kim SY, Shin J, et al: Implication of
CD69+ CD103+ tissue-resident-like
CD8+ T cells as a potential immunotherapeutic target for
cholangiocarcinoma. Liver Int. 41:764–776. 2021.
|
|
82
|
Carnevale G, Carpino G, Cardinale V,
Pisciotta A, Riccio M, Bertoni L, Gibellini L, De Biasi S, Nevi L,
Costantini D, et al: Activation of Fas/FasL pathway and the role of
c-FLIP in primary culture of human cholangiocarcinoma cells. Sci
Rep. 7:144192017.
|
|
83
|
Ye Y, Zhou L, Xie X, Jiang G, Xie H and
Zheng S: Interaction of B7-H1 on intrahepatic cholangiocarcinoma
cells with PD-1 on tumor-infiltrating T cells as a mechanism of
immune evasion. J Surg Oncol. 100:500–504. 2009.
|
|
84
|
Wu MJ, Shi L, Dubrot J, Merritt J, Vijay
V, Wei TY, Kessler E, Olander KE, Adil R, Pankaj A, et al: Mutant
IDH inhibits IFNγ-TET2 signaling to promote immunoevasion and tumor
maintenance in cholangiocarcinoma. Cancer Discov. 12:812–835.
2022.
|
|
85
|
Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY,
Zhang PF, Yang X, Peng R, Gao C, Wei CY, et al: Distinct PD-L1/PD1
profiles and clinical implications in intrahepatic
cholangiocarcinoma patients with different risk factors.
Theranostics. 9:4678–4687. 2019.
|
|
86
|
Tian L, Ma J, Ma L, Zheng B, Liu L, Song
D, Wang Y, Zhang Z, Gao Q, Song K and Wang X: PD-1/PD-L1 expression
profiles within intrahepatic cholangiocarcinoma predict clinical
outcome. World J Surg Oncol. 18:3032020.
|
|
87
|
Vigano L, Soldani C, Franceschini B,
Cimino M, Lleo A, Donadon M, Roncalli M, Aghemo A, Di Tommaso L and
Torzilli G: Tumor-infiltrating lymphocytes and macrophages in
intrahepatic cholangiocellular carcinoma. Impact on prognosis after
complete surgery. J Gastrointest Surg. 23:2216–2224. 2019.
|
|
88
|
Goeppert B, Roessler S, Renner M, Singer
S, Mehrabi A, Vogel MN, Pathil A, Czink E, Köhler B, Springfeld C,
et al: Mismatch repair deficiency is a rare but putative
therapeutically relevant finding in non-liver fluke associated
cholangiocarcinoma. Br J Cancer. 120:109–114. 2019.
|
|
89
|
Whiteside TL: What are regulatory T cells
(Treg) regulating in cancer and why? Semin Cancer Biol. 22:327–334.
2012.
|
|
90
|
Tan YS, Sansanaphongpricha K, Xie Y,
Donnelly CR, Luo X, Heath BR, Zhao X, Bellile E, Hu H, Chen H, et
al: Mitigating SOX2-potentiated immune escape of head and neck
squamous cell carcinoma with a STING-inducing nanosatellite
vaccine. Clin Cancer Res. 24:4242–4255. 2018.
|
|
91
|
Ma C, Peng C, Lu X, Ding X, Zhang S, Zou X
and Zhang X: Downregulation of FOXP3 inhibits invasion and immune
escape in cholangiocarcinoma. Biochem Biophys Res Commun.
458:234–239. 2015.
|
|
92
|
Ma K, Sun Z, Li X, Guo J, Wang Q and Teng
M: Forkhead box M1 recruits FoxP3+ Treg cells to induce
immune escape in hilar cholangiocarcinoma. Immun Inflamm Dis.
10:e7272022.
|
|
93
|
Sarkar T, Dhar S and Sa G:
Tumor-infiltrating T-regulatory cells adapt to altered metabolism
to promote tumor-immune escape. Curr Res Immunol. 2:132–141.
2021.
|
|
94
|
Zhang G, Zheng G, Zhang H and Qiu L: MUC1
induces the accumulation of Foxp3+ Treg cells in the
tumor microenvironment to promote the growth and metastasis of
cholangiocarcinoma through the EGFR/PI3K/Akt signaling pathway. Int
Immunopharmacol. 118:1100912023.
|
|
95
|
Wang H, Li C, Jian Z, Ou Y and Ou J:
TGF-β1 reduces miR-29a expression to promote tumorigenicity and
metastasis of cholangiocarcinoma by targeting HDAC4. PLoS One.
10:e01367032015.
|
|
96
|
Martín-Sierra C, Martins R, Laranjeira P,
Abrantes AM, Oliveira RC, Tralhão JG, Botelho MF, Furtado E,
Domingues R and Paiva A: Functional impairment of circulating
FcεRI+ monocytes and myeloid dendritic cells in
hepatocellular carcinoma and cholangiocarcinoma patients. Cytometry
B Clin Cytom. 96:490–495. 2019.
|
|
97
|
Böttcher JP and Reis e Sousa C: The role
of type 1 conventional dendritic cells in cancer immunity. Trends
Cancer. 4:784–792. 2018.
|
|
98
|
Junking M, Grainok J, Thepmalee C,
Wongkham S and Yenchitsomanus PT: Enhanced cytotoxic activity of
effector T-cells against cholangiocarcinoma by dendritic cells
pulsed with pooled mRNA. Tumour Biol. 39:10104283177333672017.
|
|
99
|
Thepmalee C, Panya A, Sujjitjoon J,
Sawasdee N, Poungvarin N, Junking M and Yenchitsomanus PT:
Suppression of TGF-β and IL-10 receptors on self-differentiated
dendritic cells by short-hairpin RNAs enhanced activation of
effector T-cells against cholangiocarcinoma cells. Hum Vaccin
Immunother. 16:2318–2327. 2020.
|
|
100
|
Thepmalee C, Panya A, Junking M,
Chieochansin T and Yenchitsomanus PT: Inhibition of IL-10 and TGF-β
receptors on dendritic cells enhances activation of effector
T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother.
14:1423–1431. 2018.
|
|
101
|
Sung E, Ko M, Won JY, Jo Y, Park E, Kim H,
Choi E, Jung UJ, Jeon J, Kim Y, et al: LAG-3xPD-L1 bispecific
antibody potentiates antitumor responses of T cells through
dendritic cell activation. Mol Ther. 30:2800–2816. 2022.
|
|
102
|
Zeng FL and Chen JF: Application of immune
checkpoint inhibitors in the treatment of cholangiocarcinoma.
Technol Cancer Res Treat. 20:153303382110399522021.
|
|
103
|
Halpert MM, Konduri V, Liang D, Chen Y,
Wing JB, Paust S, Levitt JM and Decker WK: Dendritic cell-secreted
cytotoxic T-lymphocyte-associated protein-4 regulates the T-cell
response by downmodulating bystander surface B7. Stem Cells Dev.
25:774–787. 2016.
|
|
104
|
Sadeghlar F, Vogt A, Mohr RU, Mahn R, van
Beekum K, Kornek M, Weismüller TJ, Branchi V, Matthaei H, Toma M,
et al: Induction of cytotoxic effector cells towards
cholangiocellular, pancreatic, and colorectal tumor cells by
activation of the immune checkpoint CD40/CD40L on dendritic cells.
Cancer Immunol Immunother. 70:1451–1464. 2021.
|
|
105
|
Djureinovic D, Wang M and Kluger HM:
Agonistic CD40 antibodies in cancer treatment. Cancers (Basel).
13:13022021.
|
|
106
|
Wu R, Ohara RA, Jo S, Liu TT, Ferris ST,
Ou F, Kim S, Theisen DJ, Anderson DA III, Wong BW, et al:
Mechanisms of CD40-dependent cDC1 licensing beyond costimulation.
Nat Immunol. 23:1536–1550. 2022.
|
|
107
|
Najafi M, Goradel NH, Farhood B, Salehi E,
Solhjoo S, Toolee H, Kharazinejad E and Mortezaee K: Tumor
microenvironment: Interactions and therapy. J Cell Physiol.
234:5700–5721. 2019.
|
|
108
|
Ruffolo LI, Jackson KM, Kuhlers PC, Dale
BS, Figueroa Guilliani NM, Ullman NA, Burchard PR, Qin SS, Juviler
PG, Keilson JM, et al: GM-CSF drives myelopoiesis, recruitment and
polarisation of tumour-associated macrophages in cholangiocarcinoma
and systemic blockade facilitates antitumour immunity. Gut.
71:1386–1398. 2022.
|
|
109
|
Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX
and Weissman IL: Phagocytosis checkpoints as new targets for cancer
immunotherapy. Nat Rev Cancer. 19:568–586. 2019.
|
|
110
|
Grinberg-Bleyer Y, Oh H, Desrichard A,
Bhatt DM, Caron R, Chan TA, Schmid RM, Klein U, Hayden MS and Ghosh
S: NF-κB c-Rel is crucial for the regulatory T cell immune
checkpoint in cancer. Cell. 170:1096–1108.e13. 2017.
|
|
111
|
Li Z, Li Y, Gao J, Fu Y, Hua P, Jing Y,
Cai M, Wang H and Tong T: The role of CD47-SIRPα immune checkpoint
in tumor immune evasion and innate immunotherapy. Life Sci.
273:1191502021.
|
|
112
|
Vaeteewoottacharn K, Kariya R, Pothipan P,
Fujikawa S, Pairojkul C, Waraasawapati S, Kuwahara K, Wongkham C,
Wongkham S and Okada S: Attenuation of CD47-SIRPα signal in
cholangiocarcinoma potentiates tumor-associated macrophage-mediated
phagocytosis and suppresses intrahepatic metastasis. Transl Oncol.
12:217–225. 2019.
|
|
113
|
Morvan MG and Lanier LL: NK cells and
cancer: You can teach innate cells new tricks. Nature reviews
Cancer. 16:7–19. 2016.
|
|
114
|
Oliviero B, Varchetta S, Mele D, Pessino
G, Maiello R, Falleni M, Tosi D, Donadon M, Soldani C, Franceschini
B, et al: MICA/B-targeted antibody promotes NK cell-driven tumor
immunity in patients with intrahepatic cholangiocarcinoma.
Oncoimmunology. 11:20359192022.
|
|
115
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016.
|
|
116
|
Sharpe AH and Pauken KE: The diverse
functions of the PD1 inhibitory pathway. Nat Rev Immunol.
18:153–167. 2018.
|
|
117
|
Azuma T, Yao S, Zhu G, Flies AS, Flies SJ
and Chen L: B7-H1 is a ubiquitous antiapoptotic receptor on cancer
cells. Blood. 111:3635–3643. 2008.
|
|
118
|
Gato-Cañas M, Zuazo M, Arasanz H,
Ibañez-Vea M, Lorenzo L, Fernandez-Hinojal G, Vera R, Smerdou C,
Martisova E, Arozarena I, et al: PDL1 signals through conserved
sequence motifs to overcome interferon-mediated cytotoxicity. Cell
Rep. 20:1818–1829. 2017.
|
|
119
|
Hosseini A, Gharibi T, Marofi F, Babaloo Z
and Baradaran B: CTLA-4: From mechanism to autoimmune therapy. Int
Immunopharmacol. 80:1062212020.
|
|
120
|
Walter D, Herrmann E, Schnitzbauer AA,
Zeuzem S, Hansmann ML, Peveling-Oberhag J and Hartmann S: PD-L1
expression in extrahepatic cholangiocarcinoma. Histopathology.
71:383–392. 2017.
|
|
121
|
Yu F, Gong L, Mo Z, Wang W, Wu M, Yang J,
Zhang Q, Li L, Yao J and Dong J: Programmed death ligand-1, tumor
infiltrating lymphocytes and HLA expression in Chinese extrahepatic
cholangiocarcinoma patients: Possible immunotherapy implications.
Biosci Trends. 13:58–69. 2019.
|
|
122
|
Ma K, Wei X, Dong D, Wu Y, Geng Q and Li
E: PD-L1 and PD-1 expression correlate with prognosis in
extrahepatic cholangiocarcinoma. Oncol Lett. 14:250–256. 2017.
|
|
123
|
Kim H, Kim J, Byeon S, Jang KT, Hong JY,
Lee J, Park SH, Park JO, Park YS, Lim HY, et al: Programmed death
ligand 1 expression as a prognostic marker in patients with
advanced biliary tract cancer. Oncology. 99:365–372. 2021.
|
|
124
|
Kitano Y, Yamashita YI, Nakao Y, Itoyama
R, Yusa T, Umezaki N, Tsukamoto M, Yamao T, Miyata T, Nakagawa S,
et al: Clinical significance of PD-L1 expression in both cancer and
stroma cells of cholangiocarcinoma patients. Ann Surg Oncol.
27:599–607. 2020.
|
|
125
|
Xian F, Ren D, Bie J and Xu G: Prognostic
value of programmed cell death ligand 1 expression in patients with
intrahepatic cholangiocarcinoma: a meta-analysis. Front Immunol.
14:11191682023.
|
|
126
|
Cai Z, Ang X, Xu Z, Li S, Zhang J, Pei C
and Zhou F: A pan-cancer study of PD-1 and CTLA-4 as therapeutic
targets. Transl Cancer Res. 10:3993–4001. 2021.
|
|
127
|
Guo XJ, Lu JC, Zeng HY, Zhou R, Sun QM,
Yang GH, Pei YZ, Meng XL, Shen YH, Zhang PF, et al: CTLA-4
synergizes with PD1/PD-L1 in the inhibitory tumor microenvironment
of intrahepatic cholangiocarcinoma. Front Immunol.
12:7053782021.
|
|
128
|
Perkhofer L, Beutel AK and Ettrich TJ:
Immunotherapy: Pancreatic cancer and extrahepatic biliary tract
cancer. Visc Med. 35:28–37. 2019.
|
|
129
|
Andrews LP, Yano H and Vignali DAA:
Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4:
breakthroughs or backups. Nat Immunol. 20:1425–1434. 2019.
|
|
130
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330.
2015.
|
|
131
|
Sharma P, Retz M, Siefker-Radtke A, Baron
A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, et
al: Nivolumab in metastatic urothelial carcinoma after platinum
therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial.
Lancet Oncol. 18:312–322. 2017.
|
|
132
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
|
|
133
|
Casak SJ, Marcus L, Fashoyin-Aje L, Mushti
SL, Cheng J, Shen YL, Pierce WF, Her L, Goldberg KB, Theoret MR, et
al: FDA approval summary: Pembrolizumab for the first-line
treatment of patients with MSI-H/dMMR advanced unresectable or
metastatic colorectal carcinoma. Clin Cancer Res. 27:4680–4684.
2021.
|
|
134
|
Nakajima EC, Vellanki PJ, Larkins E,
Chatterjee S, Mishra-Kalyani PS, Bi Y, Qosa H, Liu J, Zhao H,
Biable M, et al: FDA approval summary: Nivolumab in combination
with ipilimumab for the treatment of unresectable malignant pleural
mesothelioma. Clin Cancer Res. 28:446–451. 2022.
|
|
135
|
Jenkins L, Jungwirth U, Avgustinova A,
Iravani M, Mills A, Haider S, Harper J and Isacke CM:
Cancer-associated fibroblasts suppress CD8+ T-cell infiltration and
confer resistance to immune-checkpoint blockade. Cancer Res.
82:2904–2917. 2022.
|
|
136
|
Job S, Rapoud D, Dos Santos A, Gonzalez P,
Desterke C, Pascal G, Elarouci N, Ayadi M, Adam R, Azoulay D, et
al: Identification of four immune subtypes characterized by
distinct composition and functions of tumor microenvironment in
intrahepatic cholangiocarcinoma. Hepatology. 72:965–981. 2020.
|
|
137
|
Ikeda Y, Ono M, Ohmori G, Ameda S, Yamada
M, Abe T, Fujii S, Fujita M and Maeda M: Successful pembrolizumab
treatment of microsatellite instability-high intrahepatic
cholangiocarcinoma: A case report. Clin Case Rep. 9:2259–2263.
2021.
|
|
138
|
Mody K, Jain P, El-Refai SM, Azad NS,
Zabransky DJ, Baretti M, Shroff RT, Kelley RK, El-Khouiery AB,
Hockenberry AJ, et al: Clinical, genomic, and transcriptomic data
profiling of biliary tract cancer reveals subtype-specific immune
signatures. JCO Precis Oncol. 6:e21005102022.
|
|
139
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017.
|
|
140
|
Liu X, Yao J, Song L, Zhang S, Huang T and
Li Y: Local and abscopal responses in advanced intrahepatic
cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1
expression following combined therapy of SBRT with PD-1 blockade. J
Immunother Cancer. 7:2042019.
|
|
141
|
Mou H, Yu L, Liao Q, Hou X, Wu Y, Cui Q,
Yan N, Ma R, Wang L, Yao M and Wang K: Successful response to the
combination of immunotherapy and chemotherapy in cholangiocarcinoma
with high tumour mutational burden and PD-L1 expression: A case
report. BMC Cancer. 18:11052018.
|
|
142
|
Piha-Paul SA, Oh DY, Ueno M, Malka D,
Chung HC, Nagrial A, Kelley RK, Ros W, Italiano A, Nakagawa K, et
al: Efficacy and safety of pembrolizumab for the treatment of
advanced biliary cancer: Results from the KEYNOTE-158 and
KEYNOTE-028 studies. Int J Cancer. 147:2190–2198. 2020.
|
|
143
|
Kim RD, Chung V, Alese OB, El-Rayes BF, Li
D, Al-Toubah TE, Schell MJ, Zhou JM, Mahipal A, Kim BH and Kim DW:
A phase 2 multi-institutional study of nivolumab for patients with
advanced refractory biliary tract cancer. JAMA Oncol. 6:888–894.
2020.
|
|
144
|
Ueno M, Ikeda M, Morizane C, Kobayashi S,
Ohno I, Kondo S, Okano N, Kimura K, Asada S, Namba Y, et al:
Nivolumab alone or in combination with cisplatin plus gemcitabine
in Japanese patients with unresectable or recurrent biliary tract
cancer: A non-randomised, multicentre, open-label, phase 1 study.
Lancet Gastroenterol Hepatol. 4:611–621. 2019.
|
|
145
|
Doki Y, Ueno M, Hsu CH, Oh DY, Park K,
Yamamoto N, Ioka T, Hara H, Hayama M, Nii M, et al: Tolerability
and efficacy of durvalumab, either as monotherapy or in combination
with tremelimumab, in patients from Asia with advanced biliary
tract, esophageal, or head-and-neck cancer. Cancer Med.
11:2550–2560. 2022.
|
|
146
|
Lan Y, Zhang D, Xu C, Hance KW, Marelli B,
Qi J, Yu H, Qin G, Sircar A, Hernández VM, et al: Enhanced
preclinical antitumor activity of M7824, a bifunctional fusion
protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med.
10:eaan54882018.
|
|
147
|
Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M,
Kondo S, Chen LT, Osada M, Helwig C, Dussault I and Ikeda M: Phase
I study of bintrafusp alfa, a bifunctional fusion protein targeting
TGF-β and PD-L1, in patients with pretreated biliary tract cancer.
J Immunother Cancer. 8:e0005642020.
|
|
148
|
Klein O, Kee D, Nagrial A, Markman B,
Underhill C, Michael M, Jackett L, Lum C, Behren A, Palmer J, et
al: Evaluation of combination nivolumab and ipilimumab
immunotherapy in patients with advanced biliary tract cancers:
Subgroup analysis of a phase 2 nonrandomized clinical trial. JAMA
Oncol. 6:1405–1409. 2020.
|
|
149
|
Floudas CS, Xie C, Brar G, Morelli MP,
Fioravanti S, Walker M, Mabry-Hrones D, Wood BJ, Levy EB,
Krishnasamy VP and Greten TF: Combined immune checkpoint inhibition
(ICI) with tremelimumab and durvalumab in patients with advanced
hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC). J
Clin Oncol. 37(4 Suppl): S3362019.
|
|
150
|
Oh DY, Lee KH, Lee DW, Yoon J, Kim TY,
Bang JH, Nam AR, Oh KS, Kim JM, Lee Y, et al: Gemcitabine and
cisplatin plus durvalumab with or without tremelimumab in
chemotherapy-naive patients with advanced biliary tract cancer: An
open-label, single-centre, phase 2 study. Lancet Gastroenterol
Hepatol. 7:522–532. 2022.
|
|
151
|
Oh DY, Lee KH, Lee DW, Kim TY, Bang JH,
Nam AR, Lee Y, Zhang Q, Rebelatto M, Li W and Kim JW: Phase II
study assessing tolerability, efficacy, and biomarkers for
durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin
(GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J
Clin Oncol. 38(15 Suppl): S45202020.
|
|
152
|
Feng K, Liu Y, Zhao Y, Yang Q, Dong L, Liu
J, Li X, Zhao Z, Mei Q and Han W: Efficacy and biomarker analysis
of nivolumab plus gemcitabine and cisplatin in patients with
unresectable or metastatic biliary tract cancers: Results from a
phase II study. J Immunother Cancer. 8:e0003672020.
|
|
153
|
Boilève A, Hilmi M, Gougis P, Cohen R,
Rousseau B, Blanc JF, Ben Abdelghani M, Castanié H, Dahan L,
Tougeron D, et al: Triplet combination of durvalumab, tremelimumab,
and paclitaxel in biliary tract carcinomas: Safety run-in results
of the randomized IMMUNOBIL PRODIGE 57 phase II trial. Eur J
Cancer. 143:55–63. 2021.
|
|
154
|
Arkenau HT, Martin-Liberal J, Calvo E,
Penel N, Krebs MG, Herbst RS, Walgren RA, Widau RC, Mi G, Jin J, et
al: Ramucirumab plus pembrolizumab in patients with previously
treated advanced or metastatic biliary tract cancer: Nonrandomized,
open-label, phase I trial (JVDF). Oncologist. 23:1407–e136.
2018.
|
|
155
|
Yarchoan M, Cope L, Ruggieri AN, Anders
RA, Noonan AM, Goff LW, Goyal L, Lacy J, Li D, Patel AK, et al:
Multicenter randomized phase II trial of atezolizumab with or
without cobimetinib in biliary tract cancers. J Clin Invest.
131:e1526702021.
|
|
156
|
Lin J, Yang X, Long J, Zhao S, Mao J, Wang
D, Bai Y, Bian J, Zhang L, Yang X, et al: Pembrolizumab combined
with lenvatinib as non-first-line therapy in patients with
refractory biliary tract carcinoma. Hepatobiliary Surg Nutr.
9:414–424. 2020.
|
|
157
|
Xie C, Duffy AG, Mabry-Hrones D, Wood B,
Levy E, Krishnasamy V, Khan J, Wei JS, Agdashian D, Tyagi M, et al:
Tremelimumab in combination with microwave ablation in patients
with refractory biliary tract cancer. Hepatology. 69:2048–2060.
2019.
|
|
158
|
Leem G, Jang SI, Cho JH, Jo JH, Lee HS,
Chung MJ, Park JY, Bang S, Yoo DK, Cheon HC, et al: Safety and
efficacy of allogeneic natural killer cells in combination with
pembrolizumab in patients with chemotherapy-refractory biliary
tract cancer: A multicenter open-label phase 1/2a trial. Cancers
(Basel). 14:42292022.
|
|
159
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11:692021.
|
|
160
|
Sangsuwannukul T, Supimon K, Sujjitjoon J,
Phanthaphol N, Chieochansin T, Poungvarin N, Wongkham S, Junking M
and Yenchitsomanus PT: Anti-tumour effect of the fourth-generation
chimeric antigen receptor T cells targeting CD133 against
cholangiocarcinoma cells. Inte Int Immunopharmacol.
89:1070692020.
|
|
161
|
Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q,
Wang Y, Jia H and Han W: Phase I study of chimeric antigen
receptor-modified T cells in patients with EGFR-positive advanced
biliary tract cancers. Clin Cancer Res. 24:1277–1286. 2018.
|
|
162
|
Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv
HY, Huang JH, Yang QM and Han WD: Cocktail treatment with
EGFR-specific and CD133-specific chimeric antigen receptor-modified
T cells in a patient with advanced cholangiocarcinoma. J Hematol
Oncol. 10:42017.
|
|
163
|
Alnaggar M, Xu Y, Li J, He J, Chen J, Li
M, Wu Q, Lin L, Liang Y, Wang X, et al: Allogenic Vγ9Vδ2 T cell as
new potential immunotherapy drug for solid tumor: A case study for
cholangiocarcinoma. J Immunother Cancer. 7:362019.
|
|
164
|
Tran E, Turcotte S, Gros A, Robbins PF, Lu
YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS,
et al: Cancer immunotherapy based on mutation-specific CD4+ T cells
in a patient with epithelial cancer. Science. 344:641–645.
2014.
|
|
165
|
Zhang T, Chen J, Niu L, Liu Y, Ye G, Jiang
M and Qi Z: Clinical safety and efficacy of locoregional therapy
combined with adoptive transfer of allogeneic γδ T cells for
advanced hepatocellular carcinoma and intrahepatic
cholangiocarcinoma. J Vasc Interv Radiol. 33:19–27.e3. 2022.
|
|
166
|
Shimizu K, Kotera Y, Aruga A, Takeshita N,
Takasaki K and Yamamoto M: Clinical utilization of postoperative
dendritic cell vaccine plus activated T-cell transfer in patients
with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci.
19:171–178. 2012.
|
|
167
|
Shimizu K, Kotera Y, Aruga A, Takeshita N,
Katagiri S, Ariizumi S, Takahashi Y, Yoshitoshi K, Takasaki K and
Yamamoto M: Postoperative dendritic cell vaccine plus activated
T-cell transfer improves the survival of patients with invasive
hepatocellular carcinoma. Hum Vaccin Immunother. 10:970–976.
2014.
|
|
168
|
Vonderheide RH: CD40 agonist antibodies in
cancer immunotherapy. Annu Rev Med. 71:47–58. 2020.
|
|
169
|
Hegde S, Krisnawan VE, Herzog BH, Zuo C,
Breden MA, Knolhoff BL, Hogg GD, Tang JP, Baer JM, Mpoy C, et al:
Dendritic cell paucity leads to dysfunctional immune surveillance
in pancreatic cancer. Cancer Cell. 37:289–307.e9. 2020.
|
|
170
|
Zhang J, Li Y, Yang S, Zhang L and Wang W:
Anti-CD40 mAb enhanced efficacy of anti-PD1 against osteosarcoma. J
Bone Oncol. 17:1002452019.
|
|
171
|
Leblond MM, Tillé L, Nassiri S, Gilfillan
CB, Imbratta C, Schmittnaegel M, Ries CH, Speiser DE and Verdeil G:
CD40 agonist restores the antitumor efficacy of anti-PD1 therapy in
muscle-invasive bladder cancer in an IFN I/II-mediated manner.
Cancer Immunol Res. 8:1180–1192. 2020.
|
|
172
|
Ma HS, Poudel B, Torres ER, Sidhom JW,
Robinson TM, Christmas B, Scott B, Cruz K, Woolman S, Wall VZ, et
al: A CD40 agonist and PD-1 antagonist antibody reprogram the
microenvironment of nonimmunogenic tumors to allow T-cell-mediated
anticancer activity. Cancer Immunol Res. 7:428–442. 2019.
|
|
173
|
Moreno V, Perets R, Peretz-Yablonski T,
Fourneau N, Girgis S, Guo Y, Hellemans P, Verona R, Pendás N, Xia
Q, et al: A phase 1 study of intravenous mitazalimab, a CD40
agonistic monoclonal antibody, in patients with advanced solid
tumors. Invest New Drugs. 41:93–104. 2023.
|
|
174
|
Humphreys EH, Williams KT, Adams DH and
Afford SC: Primary and malignant cholangiocytes undergo CD40
mediated Fas dependent apoptosis, but are insensitive to direct
activation with exogenous Fas ligand. PLoS One. 5:e140372010.
|
|
175
|
Diggs LP, Ruf B, Ma C, Heinrich B, Cui L,
Zhang Q, McVey JC, Wabitsch S, Heinrich S, Rosato U, et al:
CD40-mediated immune cell activation enhances response to anti-PD-1
in murine intrahepatic cholangiocarcinoma. J Hepatol. 74:1145–1154.
2021.
|
|
176
|
O'Hara MH, O'Reilly EM, Rosemarie M,
Varadhachary G, Wainberg ZA, Ko A, Fisher GA, Rahma O, Lyman JP,
Cabanski CR, et al: Abstract CT004: A phase Ib study of CD40
agonistic monoclonal antibody APX005M together with gemcitabine
(Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in
untreated metastatic ductal pancreatic adenocarcinoma (PDAC)
patients. Cancer Res. 79(13 Suppl): CT0042019.
|
|
177
|
Lin Y, Peng L, Dong L, Liu D, Ma J, Lin J,
Chen X, Lin P, Song G, Zhang M, et al: Geospatial immune
heterogeneity reflects the diverse tumor-immune interactions in
intrahepatic cholangiocarcinoma. Cancer Discov. 12:2350–2371.
2022.
|
|
178
|
Morse MA, Gwin WR III and Mitchell DA:
Vaccine therapies for cancer: Then and now. Target Oncol.
16:121–152. 2021.
|
|
179
|
Goldstein D, Lemech C and Valle J: New
molecular and immunotherapeutic approaches in biliary cancer. ESMO
Open. 2(Suppl 1): e0001522017.
|
|
180
|
Koido S, Kan S, Yoshida K, Yoshizaki S,
Takakura K, Namiki Y, Tsukinaga S, Odahara S, Kajihara M, Okamoto
M, et al: Immunogenic modulation of cholangiocarcinoma cells by
chemoimmunotherapy. Anticancer Res. 34:6353–6361. 2014.
|
|
181
|
Kaida M, Morita-Hoshi Y, Soeda A, Wakeda
T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, et al:
Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and
gemcitabine combination therapy in patients with advanced
pancreatic or biliary tract cancer. J Immunother. 34:92–99.
2011.
|
|
182
|
Yamamoto K, Ueno T, Kawaoka T, Hazama S,
Fukui M, Suehiro Y, Hamanaka Y, Ikematsu Y, Imai K, Oka M and
Hinoda Y: MUC1 peptide vaccination in patients with advanced
pancreas or biliary tract cancer. Anticancer Res. 25:3575–3579.
2005.
|
|
183
|
Aruga A, Takeshita N, Kotera Y, Okuyama R,
Matsushita N, Ohta T, Takeda K and Yamamoto M: Long-term
vaccination with multiple peptides derived from cancer-testis
antigens can maintain a specific T-cell response and achieve
disease stability in advanced biliary tract cancer. Clin Cancer
Res. 19:2224–2231. 2013.
|
|
184
|
Aruga A, Takeshita N, Kotera Y, Okuyama R,
Matsushita N, Ohta T, Takeda K and Yamamoto M: Phase I clinical
trial of multiple-peptide vaccination for patients with advanced
biliary tract cancer. J Transl Med. 12:612014.
|
|
185
|
Yoshitomi M, Yutani S, Matsueda S, Ioji T,
Komatsu N, Shichijo S, Yamada A, Itoh K, Sasada T and Kinoshita H:
Personalized peptide vaccination for advanced biliary tract cancer:
IL-6, nutritional status and pre-existing antigen-specific immunity
as possible biomarkers for patient prognosis. Exp Ther Med.
3:463–469. 2012.
|
|
186
|
Lepisto AJ, Moser AJ, Zeh H, Lee K,
Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP,
Whiteside T, et al: A phase I/II study of a MUC1 peptide pulsed
autologous dendritic cell vaccine as adjuvant therapy in patients
with resected pancreatic and biliary tumors. Cancer Ther.
6:955–964. 2008.
|
|
187
|
Kobayashi M, Sakabe T, Abe H, Tanii M,
Takahashi H, Chiba A, Yanagida E, Shibamoto Y, Ogasawara M,
Tsujitani S, et al: Dendritic cell-based immunotherapy targeting
synthesized peptides for advanced biliary tract cancer. J
Gastrointest Surg. 17:1609–1617. 2013.
|
|
188
|
Hochnadel I, Hoenicke L, Petriv N, Neubert
L, Reinhard E, Hirsch T, Alfonso JCL, Suo H, Longerich T, Geffers
R, et al: Safety and efficacy of prophylactic and therapeutic
vaccine based on live-attenuated Listeria monocytogenes in
hepatobiliary cancers. Oncogene. 41:2039–2053. 2022.
|
|
189
|
Miao L, Zhang Y and Huang L: mRNA vaccine
for cancer immunotherapy. Mol Cancer. 20:412021.
|
|
190
|
Huang X, Tang T, Zhang G and Liang T:
Identification of tumor antigens and immune subtypes of
cholangiocarcinoma for mRNA vaccine development. Mol Cancer.
20:502021.
|
|
191
|
Izquierdo-Sanchez L, Lamarca A, La Casta
A, Buettner S, Utpatel K, Klümpen HJ, Adeva J, Vogel A, Lleo A,
Fabris L, et al: Cholangiocarcinoma landscape in Europe:
Diagnostic, prognostic and therapeutic insights from the ENSCCA
Registry. J Hepatol. 76:1109–1121. 2022.
|
|
192
|
Walker NJ, Crockett PW, Nyska A, Brix AE,
Jokinen MP, Sells DM, Hailey JR, Easterling M, Haseman JK, Yin M,
et al: Dose-additive carcinogenicity of a defined mixture of
'dioxin-like compounds'. Environ Health Perspect. 113:43–48.
2005.
|
|
193
|
National Toxicology Program: Toxicology
and carcinogenesis studies of 2,3',4,4',5-pentachlorobiphenyl (PCB
118) (CAS No. 31508-00-6) in female harlan Sprague-Dawley rats
(gavage studies). Natl Toxicol Program Tech Rep Ser. 1–174.
2010.
|
|
194
|
Lowery MA, Ptashkin R, Jordan E, Berger
MF, Zehir A, Capanu M, Kemeny NE, O'Reilly EM, El-Dika I, Jarnagin
WR, et al: Comprehensive molecular profiling of intrahepatic and
extrahepatic cholangiocarcinomas: Potential targets for
intervention. Clin Cancer Res. 24:4154–4161. 2018.
|
|
195
|
Weinberg BA, Xiu J, Lindberg MR, Shields
AF, Hwang JJ, Poorman K, Salem ME, Pishvaian MJ, Holcombe RF,
Marshall JL and Morse MA: Molecular profiling of biliary cancers
reveals distinct molecular alterations and potential therapeutic
targets. J Gastrointest Oncol. 10:652–662. 2019.
|
|
196
|
Kendall T, Verheij J, Gaudio E, Evert M,
Guido M, Goeppert B and Carpino G: Anatomical, histomorphological
and molecular classification of cholangiocarcinoma. Liver Int.
39(Suppl 1): S7–S18. 2019.
|