Gastric cancer poses a significant threat to human
health and well-being and results in extensive mortality worldwide
(1). Characterized by its
malignant properties, gastric cancer is the fifth most common
malignancy and fourth leading cause of cancer-related death
globally (2). Prevalence rates of
gastric cancer are higher in East Asian and Eastern European
countries compared to North American and Northern European regions,
which is possibly due to variations in dietary patterns and
lifestyle choices among local populations (3,4).
The primary risk factor associated with gastric cancer is
Helicobacter pylori (H. pylori) infection, which is
widely recognized as the main causative agent (5). H. pylori infection may
accelerate the progression of intestinal metaplasia in the stomach
and results in gastric inflammation, and eventually, the
development of gastric cancer (6). However, other environmental factors,
such as tobacco smoking, alcohol consumption and high consumption
of grilled foods, may increase the risk of developing this disease
(7). Given the seriousness of
this clinical condition, additional research into the aetiology and
pathogenesis of gastric cancer is urgently needed. Furthermore,
efforts must be made to develop appropriate diagnostic approaches
and therapeutic measures to combat this condition.
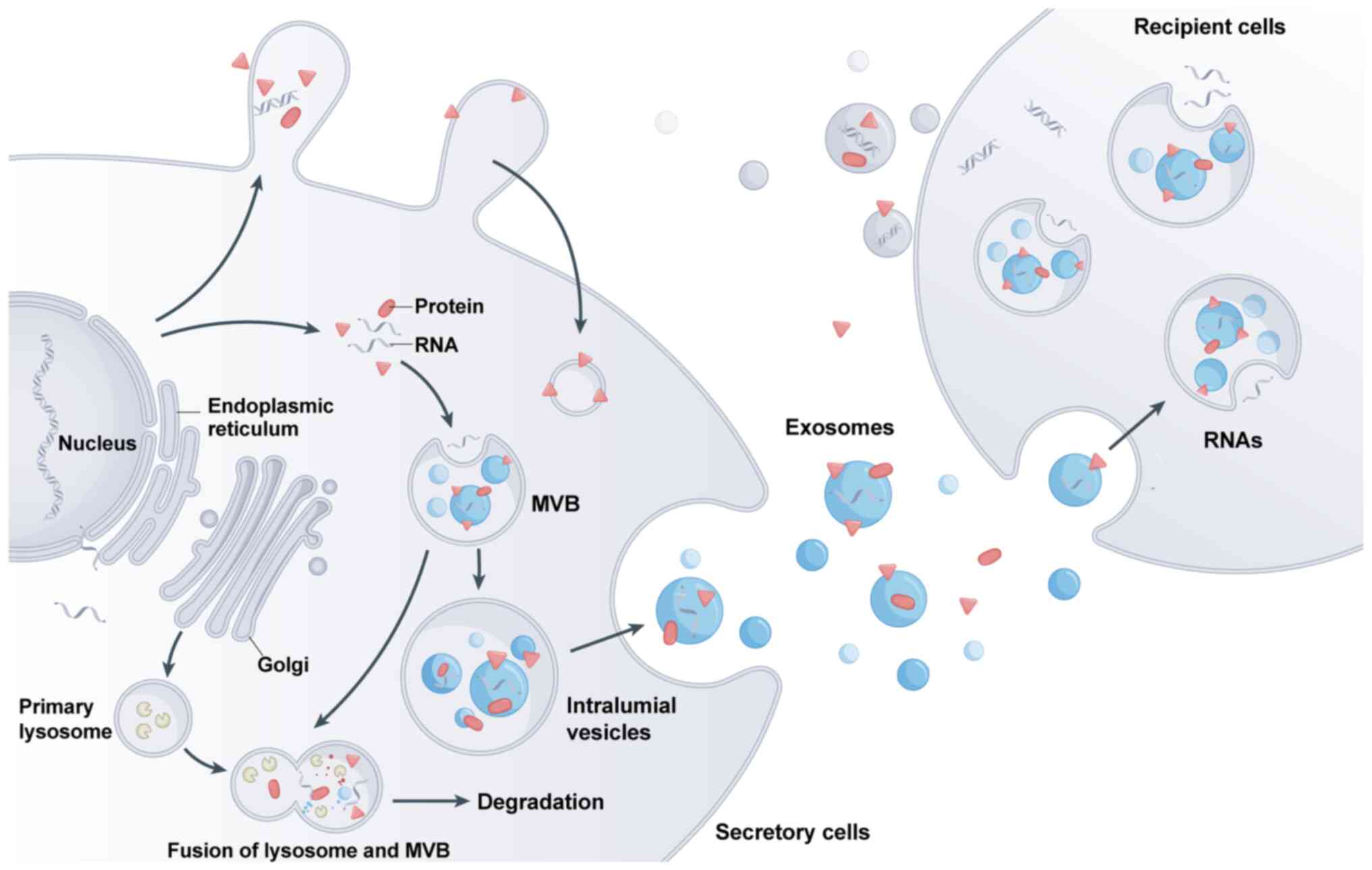
Exosomes are nanosized membrane vesicles that are
released into the extracellular environment and contain a diverse
mixture of essential biomolecules including proteins, lipids,
functional RNA species and other bioactive substances that perform
crucial cellular functions (16,17). Exosomes exhibit significant
heterogeneity and their molecular composition varies based on the
cell type and the fluidic microenvironment (18). Proteins are a crucial component of
exosomes and their profile differs based on the cell type and
extracellular fluid conditions (19). Exosomal proteins regulate cellular
processes in tumor cells and thus promote the metastatic cascade
(20,21). Lipids, which consist of
cholesterol, phosphatidylserine and sphingolipids, are essential
constituents of exosomes and have a critical role in their release.
These proteins serve as anchor sites for membrane proteins and
protect proteins from protease-related damage (22). Exosomes are membrane-bound
vesicles that originate from endocytic pathways and have a crucial
role in intercellular communication. These extracellular vesicles
encapsulate various RNA molecules, including microRNAs (miRNAs),
mRNAs and long noncoding RNAs (lncRNAs), which may be transferred
between cells to influence cellular behaviour and signalling
pathways (23). These RNAs
modulate gene expression and thereby impact various biological
processes, such as cellular proliferation, differentiation and
apoptosis (24). Furthermore, the
multifunctional nucleic acid molecules that are miRNAs indubitably
have a fundamental role in the pathogenesis of cancer, particularly
in its progression and metastatic spread across diverse cancer
types (25,26). In summary, exosomes exhibit
significant heterogeneity, which suggests that their compositional
profiles are viable biomarkers for both the identification and
amelioration of various malignancies. Therefore, exosomes and their
molecular components, including proteins, lipids and RNA species,
are highly important in cancer research and may be the key to
developing novel therapeutic approaches for cancer management.
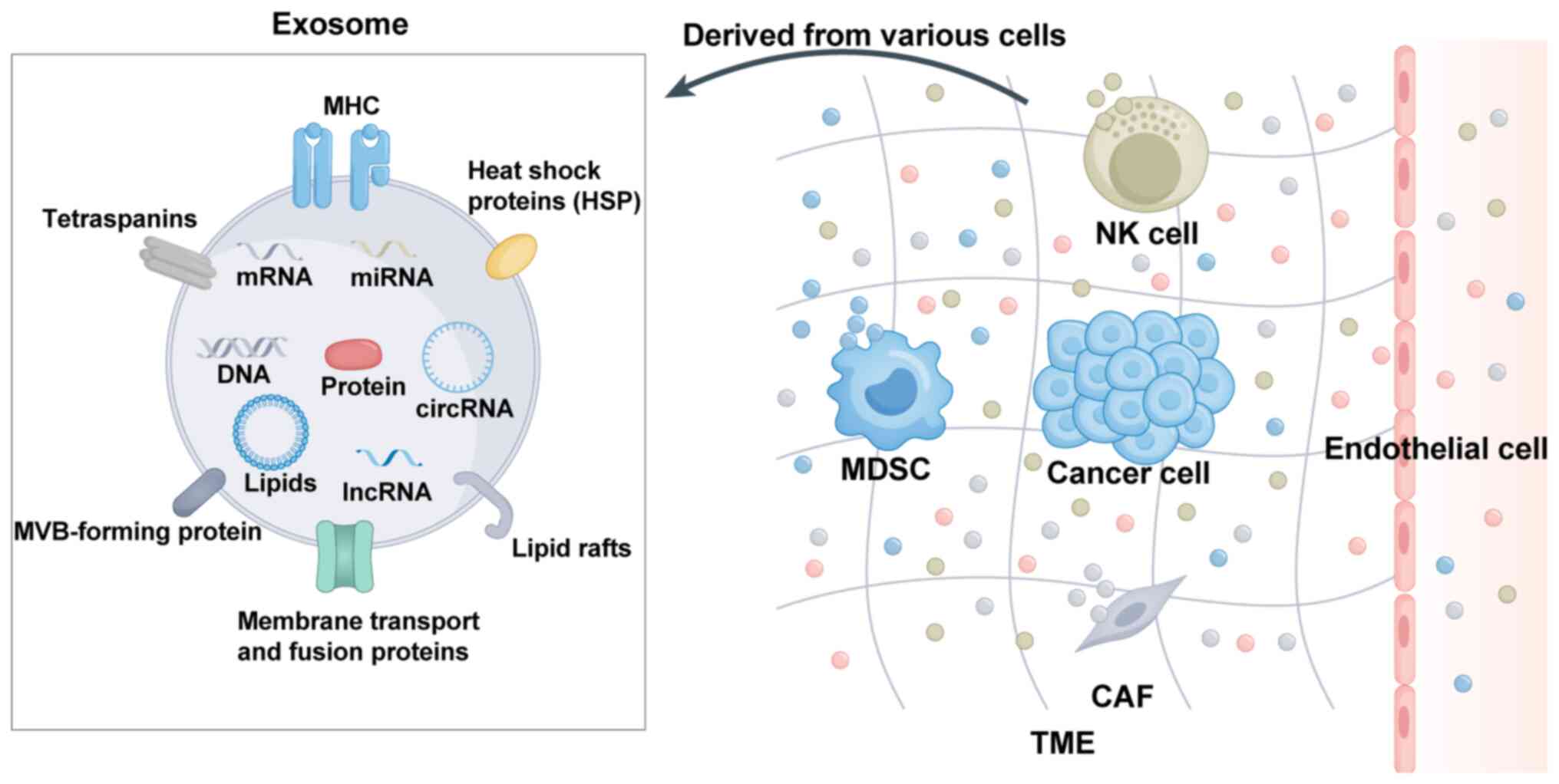
The TME has a pivotal role in tumor metabolism,
development, progression and therapeutic response. The tumorigenic
potential of cancer cells is significantly influenced by
alterations in the TME (32).
This dynamic interplay may be likened to the correlation between
the quality of soil and growth of seeds, where enriched soil
fertility facilitates rapid and robust seedling development
(33). The composition of the TME
varies depending on the type of tumor but hallmark features include
immune cells, nonimmune cells and the extracellular matrix
(34). Both immune and nonimmune
cell populations and other factors influence the role of exosomes
within the TME. As shown in Fig.
2, exosomes released by immune cells and nonimmune cells are
present in the TME and have an important role in regulating
intercellular communication by transmitting signals. In the
chapters below, the impacts of immune and nonimmune cell
populations and other factors were summarized to shed light on the
crucial role of exosomes within the TME. This provides novel
insight and perspectives for further understanding the significant
contributions of these cells to the treatment of tumors.
The role of the immune cell population in the
occurrence and progression of cancer is paramount, and this
population holds promise as a novel approach for cancer
therapeutics (35,36). The discovery of this immune cell
population has propelled the development of immunotherapy and
opened avenues for treating a subset of cancers (37). For instance, strategies aimed at
reversing the suppressive immune milieu and augmenting effector
T-cell function may potentially increase the success rate of
immunotherapy for cervical cancer (38,39). However, immunotherapy regimens do
not uniformly yield positive outcomes in all cancer treatments
(40). Consequently, unravelling
the elusive molecular mechanisms underlying cancer-related immune
responses has emerged as an imperative objective.
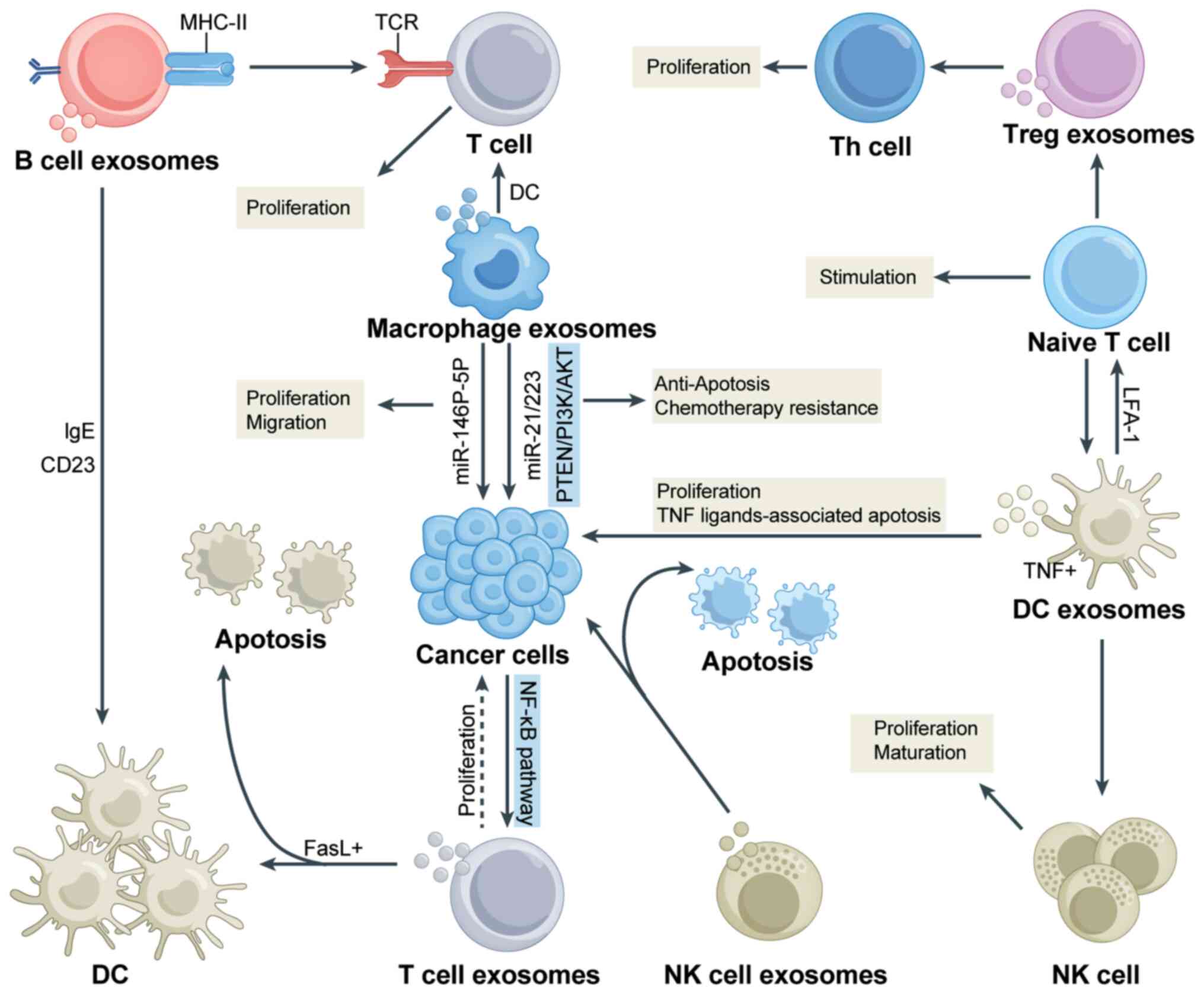
Exosomes have been shown to have a significant role
in the polarization of macrophages towards the M1 and M2 phenotypes
(41). M1 macrophages are
proinflammatory and may promote inflammation and swallow extraneous
substances to provide relative protection to tissues and organs
(41,42). M2 macrophages are
anti-inflammatory agents that may suppress the activity of M1
macrophages through the secretion of anti-inflammatory factors and
have essential effects on processes such as wound healing and
tissue repair (41-43). Pritchard et al (44) demonstrated that lung tumor-derived
exosomes promote M2 macrophage polarization and are a novel
therapeutic target for immunotherapy in lung cancer. Tumor cells
can recruit, aggregate and induce macrophages to transform into
tumor-associated macrophages and facilitate tumor development
within the TME (45-47). Previous studies revealed that
exosome-borne Epstein-Barr virus-encoded small RNA-1 induces
indoleamine 2,3-dioxygenase expression in tumor-infiltrating
macrophages of oral squamous-cell carcinomas and suppresses T-cell
activity by activating the retinoic acid-inducible gene
I/IL-6/TNF-α pathway (48). In
addition, the intracellular signalling mechanisms involved in
exosome uptake and the regulatory payload of the TME in macrophages
have yet to be fully elucidated. Dendritic cells constitute a vital
component of the TME. Previous studies have shown that
tumor-derived exosomes may impinge on dendritic cells through the
heat shock protein (HSP)72/HSP105-Toll-like receptor (TLR)2/TLR4
pathway and thereby promote tumor metastasis and development
(49). Exosomes can stimulate
both the innate and adaptive immune systems by activating dendritic
cells, natural killer (NK) cells and T cells. This activation
enables these immune cells to exert antitumor effects, particularly
in ovarian cancer (50).
Tumor-associated neutrophils mediate protumor immunosuppressive
activity and their infiltration into tumors is associated with
adverse outcomes in a wide array of malignant diseases (51). Of note, tumor-derived exosomes
can, to a certain extent, induce N2 polarization of neutrophils and
promote the migration of gastric cancer cells, leading to further
deterioration of gastric cancer (52). Multiple myeloma-derived exosomes
have been shown to promote apoptosis and inhibit T-lymphocyte
proliferation, which highlights their significant role in
regulating immune cell function (53). The innate ability of NK cells to
recognize and eliminate target cells renders them the first line of
defence in identifying and eliminating infected or transformed
cells (54). Furthermore, there
is growing evidence demonstrating crosstalk between exosomal
biogenesis and autophagy pathways with autophagy-mediated exosomes
acting as immunomodulators of NK cells in the pancreatic cancer
microenvironment. This crosstalk pathway holds promising potential
in advancing the development of pancreatic cancer immunotherapy
(55). In addition, NK
cell-derived exosomes have been shown to enhance antitumor effects
in ovarian cancer by delivering cisplatin and reactivating NK-cell
function, which underscores their therapeutic relevance (56). Of note, polymorphonuclear
myelopoietic suppressor cells are prevalent in the peripheral blood
of cancer patients and these cells can inhibit the antitumor
activity of NK cells, highlighting the complex interplay between
different immune cell populations in cancer progression (57). The interaction between exosomes
derived from immune cells and cancer cells is illustrated in
Fig. 3.
In addition to immune cell populations, the TME is
populated by diverse and significant groups of nonimmune cells.
These nonimmune cells in the TME include cancer-associated
fibroblasts (CAFs) and endothelial cells, among others, which have
pivotal roles in regulating tumor progression, metastasis and
sensitivity to anticancer drugs. CAFs influence the occurrence and
development of gastric cancer by controlling individual cytokine
profiles and direct cell-cell interactions (58). Previous studies demonstrated that
hepatocyte growth factor (HGF), which is derived from CAFs,
promotes angiogenesis in gastric cancer through the PI3K/AKT and
ERK1/2 signalling pathways (59).
CAFs can foster chemotherapy resistance in gastric cancer by
secreting IL-11 to target the JAK/STAT3/Bcl2 signalling pathway
(60). A study conducted by Liu
et al (61) confirmed that
immunosuppressive microfibril associated protein 2 CAFs can lead to
adverse outcomes and therapeutic resistance in gastric cancer.
Furthermore, recent studies have revealed that CAF-derived slit
homolog 2 protein can promote metastasis of gastric cancer cells by
activating NIMA-related kinase 9, which leads to the exacerbation
of gastric cancer (62). The
exosome glucose-regulated protein 78 derived from gastric cancer
cells stimulates the secretion of cytokines by vascular endothelial
cells to induce angiogenesis (63). In gastric cancer exosome
metastasis, Y-box binding protein 1 can also promote angiogenesis
through increased expression of angiogenic factors in vascular
endothelial cells (64). Exosomes
carrying miR-155 can promote angiogenesis in gastric cancer by
targeting the forkhead box O3 protein in endothelial cells
(65). Endothelial cells not only
supply essential nutrients to tumor cells but also engage in
cross-talk with other cells through the secretion of cytokines.
Deciphering these complex interactions within the TME may pave the
way for the identification of novel therapeutic targets in the
treatment of gastric cancer. In general, nonimmune cell populations
have an equally critical and irreplaceable role in the TME.
In addition to the pivotal role of exosomes in cell
populations, other factors within the TME, such as metabolic
processes, inflammatory responses and gene expression, also
influence the function of exosomes (66). Tumor cells exhibit metabolic
profiles distinct from those of normal cells and require an
abundance of energy to support their rapid growth and
dissemination. This distinct metabolic pattern can influence the
function of exosomes. Previous studies demonstrated that certain
tumor cells release substantial amounts of lactic acid, which
results in a correspondingly acidic environment that impacts the
stability of exosomes and the activity of the bioactive molecules
they carry (67). An emerging
study revealed that microbial proteins encoded by exosomal IncRNA
aldo-keto reductase family 1 member C2 can facilitate lymph node
metastasis in gastric cancer by modulating fatty acid metabolism
(68). Exosomes have been
implicated in lipid metabolism and the regulation of multiple
processes in gastric cancer, including communication between
gastric cancer cells, the establishment of the TME and the
biological behaviour of gastric cancer, such as metastasis,
invasion and chemotherapy resistance. Inflammation is a pivotal
initiating step in the formation of the TME and is intricately
linked to the recruitment of various immune cells and activation of
epithelial stem cells or direct progenitors, which serve as the
primary origin of gastrointestinal cancers (69). Therefore, inflammation has an
important role in the complex interplay of events within the TME
and warrants further exploration in the context of gastric cancer
development and progression. Furthermore, gene expression patterns
are frequently altered, which can influence the function of
exosomes in tumors (70). The
incorrect expression of certain key genes can affect the
communication between exosomes and tumor cells, which contributes
to the development and progression of tumor cells.
The relationship between gastric cancer cells and
the TME can be likened to that between a seed and the soil. In this
context, exosomes have emerged as important factors that facilitate
the development of metastatic gastric cancer by serving as crucial
molecular agents of intercellular communication (71). Through their ability to transfer
proteins and other related substances between cells via tissue
fluid, exosomes are capable of regulating various phenotypic
changes within gastric cancer cells while also inducing metastasis
of distant tumor cells (72,73). Of note, exosomes are also capable
of modulating the TME by regulating diverse physiological
processes, such as immunity, angiogenesis and metastasis, and
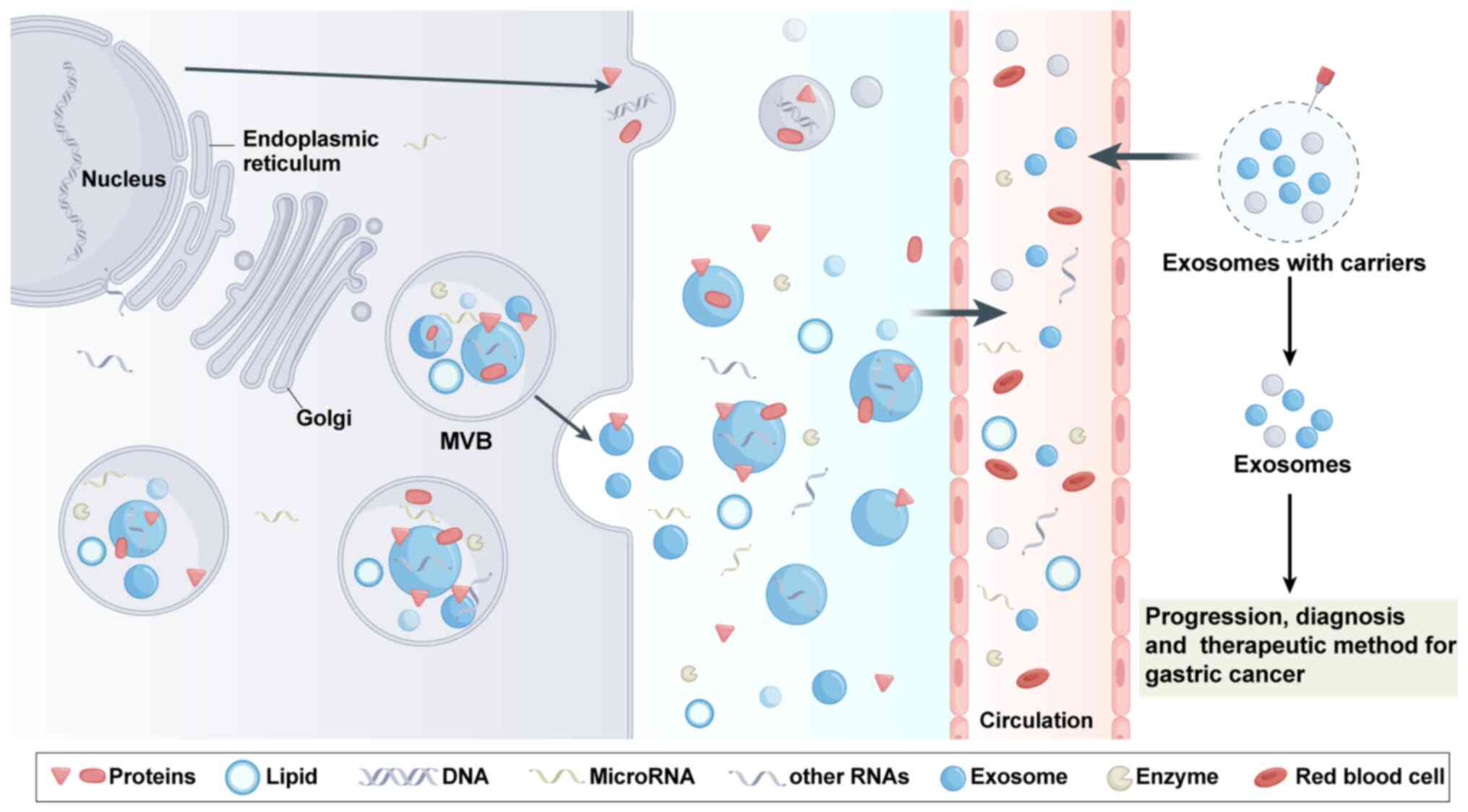
thereby promote the progression of gastric cancer (74,75). Based on the above research, it was
found that exosomes have critical roles in the development of novel
diagnostic and therapeutic strategies for gastric cancer (Fig. 4).
Exosomes have emerged as crucial players in the
carcinogenesis and pathogenesis of gastric cancer and are
intricately involved in multiple tumorigenic events (76,77). Exosomes are involved in the
antiapoptotic, proliferative, metastatic, autophagic and angiogenic
processes of gastric cancer cells and are an important means of
intercellular communication between tumor cells and the surrounding
microenvironment (78). Previous
studies demonstrated that exosomes derived from gastric cancer
cells can impede the processes of senescence and apoptosis in
primary normal gastric epithelial cells. This suggests that gastric
cancer exosomes influence the development of field cancer within
surrounding gastric epithelial cells (79). Exosomes originating from gastric
cancer cells were found to elicit neutrophil-mediated autophagy and
facilitate activation of the same cancer cell type, predominantly
via the high mobility group box 1 protein/TLR4/NF-κB signalling
pathway (52). Liang et al
(80) reported that the circ670
molecule, which is present in exosomes, has an influential role in
the pathogenesis of tobacco-induced gastric cancer. Chang et
al (81) demonstrated that
the ectopic expression of miR-1228 promoted the emergence of
exosomes, which in turn led to a discernible attenuation of
recombinant matrix metalloproteinase (MMP)-14 expression. As a
consequence, the incidence and progression of gastric cancer is
thwarted. The present study revealed that decreased expression of
miR-3184-5p within exosomes from individuals diagnosed with gastric
cancer may potentially augment the overall proliferative capacity
of these malignant cells through the modulation of distinct
molecular signalling pathways, namely, the AKT, STAT3 and
inositol-requiring enzyme 1 pathways (82). Exosomes have emerged as a
promising modality for promoting the development of gastric cancer
cells by serving as transport vehicles. Specifically, exosomes
ferry the lncRNA prostate cancer gene expression marker 1 to
support gastric cancer invasion and metastasis via stabilization of
SNAI1. This fascinating mechanism underscores the crucial role that
exosomes play as functional vectors in orchestrating pathogenic
progression in gastric cancer (83). Exosomes derived from gastric
cancer cells can deliver miR-130a to vascular cells. This transfer
is mediated by targeting the c-MYB gene and ultimately promoting
angiogenesis and enhancing tumor growth (84). Extracellular vesicles,
specifically exosomes originating from mesenchymal stem cells
(MSCs), promote the proliferation and metastasis of gastric
carcinoma cells through the promotion of AKT pathway activation
(85). These findings align with
the current research and demonstrate that exosomes derived from
gastric cancer cells stimulate programmed cell death 1 ligand 1
expression in MSCs via the AKT/C-Myc signalling axis and promote
the metastasis of gastric cancer cells (86). Exosomes have a pivotal role in the
pathogenesis of gastric cancer. Despite numerous strides in
elucidating the functional relevance of these genes, several
aspects of these genes remain poorly understood and necessitate
further investigation.
Numerous studies on exosomes have shown their
potential as biomarkers for various types of cancer. In particular,
the concentration of exosomes has emerged as a promising diagnostic
indicator for gastric cancer. Xia et al (15) demonstrated that patients with
gastric cancer exhibit greater specificity and sensitivity in terms
of exosome expression and reached 65.2 and 73.1%, respectively,
compared to healthy individuals. This was achieved through
characterization of exosome morphology via transmission electron
microscopy and the detection of exosome size and concentration
through nanoparticle tracking analysis (15). Furthermore, exosomal tripartite
motif-containing 3 protein has potential as a diagnostic biomarker
for gastric cancer and has paved the way for a novel approach to
the management of this malignancy (87). In another study conducted by Sun
et al (88) confirmed that
inter-alpha-trypsin inhibitor heavy chain 4 was identified as a
crucial serum biomarker for the detection of early gastric cancer
(EGC) in patients. Specifically, mass spectrometry has validated
its value as a highly valuable diagnostic tool for EGC detection
compared to that of a healthy control group (88,89). Another investigation of patients
with gastric cancer suggests that the serum exosomal membrane type
1-MMP mRNA and the serum exosomal lncRNA pcsk2-2:1 has pivotal
roles in the progression of this disease. These findings underscore
the potential of targeting serum exosomes in a pancancer liquid
biopsy to uncover specific diagnostic biomarkers for gastric cancer
(90,91). Although these studies have
unveiled the potential significance of exosomes in gastric cancer
diagnosis, further elucidation is required regarding the exact
threshold and practical scope of application of these markers.
Given the inherent heterogeneity of gastric cancer, it may be
imperative to employ a combination of multiple markers for enhanced
diagnostic accuracy and reliability. It is worth mentioning that
the precise detection of exosomes was found to be highly important
for the early identification of gastric cancer. However, achieving
this precision in exosome detection remains a challenging task and
demands extensive investigative efforts. Research indicates that
exosomes derived from MSCs possess immense potential for mitigating
disease pathologies and enhancing cognitive function in patients
with conditions such as Alzheimer's disease, Parkinson's disease
and vascular dementia (92).
Prospective studies must further explore the concentration of
exosomes, establish a specific diagnostic threshold and define the
applicable range of pertinent markers to facilitate a more precise
foundation for clinical diagnosis. Concurrently, a surge in
clinical trials is imperative to validate these findings and propel
the practical use of exosomes in gastric cancer diagnosis. It is
envisaged that continued research and scientific scrutiny will pave
the way for the effective utilization of exosomes as diagnostic
biomarkers and revolutionize the management of gastric cancer and
other malignancies.
Exosomes are increasingly assumed to play a critical
role in public health medicine and exhibit potential applications
for treating a wide range of diseases including cancer and central
nervous system disorders (93,94). Due to their low immunogenicity and
efficient ability to cross the blood-brain barrier, exosomes have
the potential to become crucial players in the management of
ischaemic stroke (95). However,
this investigation is limited by a lack of convincing evidence
regarding the safety of exosome therapy. Of note, exosomal
polyphosphates can be stimulated through the mediation of exosomes
derived from cancer cells, which elicit the activation of factor
XII (96). Growing evidence
suggests that exosomes may hold significant potential in the
development of effective therapeutics for gastric cancer.
Specifically, exosomes have been demonstrated to hinder tumor cell
growth and angiogenesis through the transport of HGF small
inhibitory RNA (97).
Furthermore, exosomes serve as ideal nanoparticles for transporting
miR-214 and effectively reversing chemical resistance in gastric
cancer and thus represent a potentially selective treatment option
for cisplatin-resistant gastric cancer (98). However, despite recent progress,
numerous unknowns surround the use of exosomes in clinical
practice. Significant improvements and challenges must be addressed
before exosomes can become a viable therapeutic tool. The field of
exosome-based medicine is in its infancy and further research is
needed to determine its full potential.
Exosomes serve as crucial transporters in promoting
the interaction between gastric cancer cells and the TME. These
nanoscale extracellular vesicles possess several potential
advantages, including biocompatibility, stability and intrinsic
targeting capabilities, which render them promising therapeutic
options for gastric cancer. However, the application of exosomes as
drug delivery systems is still in its infancy and numerous
challenges need to be addressed. Research geared towards clinical
translation should strive to improve the yield, purity, targeting
efficiency and biological activity of exosomes. Overall, it may
take time for clinical success to be achieved regarding exosomes,
but with the wealth of research and clinical trials underway, it is
expected that exosome-related applications will soon benefit a
majority of gastric cancer patients.
Not applicable.
ZZ, SM, SH and MW conceived and designed the
approach. MW, SH, XC and HX wrote and edited the manuscript. MW,
SH, XC, ZJ and NY generated the figures. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
All figures were created with Figdraw.
This research was funded by the National Natural Science
Foundation of China (grant nos. 82060453 and 82103645 and
82260596); the Training Plan for Academic and Technical Young
Leaders of Major Disciplines in Jiangxi Province (grant no.
20204BCJ23021), the Science and Technology Program of Jiangxi
Provincial Health and Family Planning Commission (grant no.
202410246) and the China Postdoctoral Science Foundation (grant no.
2023M741523).
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011.
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer Statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
|
|
3
|
Estevez-Ordonez D, Montalvan-Sanchez EE,
Wong RE, Montalvan-Sanchez DM, Rodriguez-Murillo AA, Dominguez RL
and Morgan DR: Health barriers and patterns of gastric cancer care
in rural central American resource-limited settings. JAMA Oncol.
4:1131–1133. 2018.
|
|
4
|
Yeoh KG and Tan P: Mapping the genomic
diaspora of gastric cancer. Nat Rev Cancer. 22:71–84. 2022.
|
|
5
|
Walker MM, Teare L and McNulty C: Gastric
cancer and Helicobacter pylori: The bug, the host or the
environment? Postgrad Med J. 84:169–170. 2008.
|
|
6
|
Zeyaullah M, AlShahrani AM and Ahmad I:
Association of helicobacter pylori infection and host cytokine gene
polymorphism with gastric cancer. Can J Gastroenterol Hepatol.
2021:88106202021.
|
|
7
|
Zeng Y and Jin RU: Molecular pathogenesis,
targeted therapies, and future perspectives for gastric cancer.
Semin Cancer Biol. 86:566–582. 2021.
|
|
8
|
Trams EG, Lauter CJ, Salem N and Heine U:
Exfoliation of membrane ecto-enzymes in the form of micro-vesicles.
Biochim Biophys Acta. 645:63–70. 1981.
|
|
9
|
Li Y, Tian L, Zhao T and Zhang J: A
nanotherapeutic system for gastric cancer suppression by
synergistic chemotherapy and immunotherapy based on iPSCs and DCs
exosomes. Cancer Immunol Immunother. 72:1673–1683. 2023.
|
|
10
|
Chen BY, Sung CWH, Chen C, Cheng CM, Lin
DP, Huang CT and Hsu MY: Advances in exosomes technology. Clin Chim
Acta. 493:14–19. 2019.
|
|
11
|
Beit-Yannai E, Tabak S and Stamer WD:
Exosomes. Nat Biotechnol. 38:11502020.
|
|
12
|
Ayyar KK and Moss AC: Exosomes in
intestinal inflammation. Front Pharmacol. 12:6585052021.
|
|
13
|
Beit-Yannai E, Tabak S and Stamer WD:
Physical exosome: Exosome interactions. J Cell Mol Med.
22:2001–2006. 2018.
|
|
14
|
Wang J, Liu Y, Sun W, Zhang Q, Gu T and Li
G: Plasma exosomes as novel biomarker for the early diagnosis of
gastric cancer. Cancer Biomark. 21:805–812. 2018.
|
|
15
|
Xia Y, Hu X, Di K, Liu C, Tan T, Lin Y, Xu
H, Xie H, Wang S, Yang Z, et al: Combined detection of exosome
concentration and tumor markers in gastric cancer. J Biomed
Nanotechnol. 16:252–258. 2020.
|
|
16
|
Exosomes. Nat Biotechnol. 38:11502020.
|
|
17
|
Li J, Zhang Y and Luo B: Effects of
exosomal viral components on the tumor microenvironment. Cancers
(Basel). 14:35522022.
|
|
18
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019.
|
|
19
|
Wang X, Huang J, Chen W, Li G, Li Z and
Lei J: The updated role of exosomal proteins in the diagnosis,
prognosis, and treatment of cancer. Exp Mol Med. 54:1390–1400.
2022.
|
|
20
|
Wang M, Zhao X, Huang F, Wang L, Huang J,
Gong Z and Yu W: Exosomal proteins: Key players mediating
pre-metastatic niche formation and clinical implications (Review).
Int J Oncol. 58:42021.
|
|
21
|
Huda MN and Nurunnabi M: Potential
application of exosomes in vaccine development and delivery. Pharm
Res. 39:2635–2671. 2022.
|
|
22
|
Wang W, Zhu N, Yan T, Shi YN, Chen J,
Zhang CJ, Xie XJ, Liao DF and Qin L: The crosstalk: Exosomes and
lipid metabolism. Cell Commun Signal. 18:1192020.
|
|
23
|
Puno MR, Weick E-M, Das M and Lima CD:
SnapShot: The RNA exosome. Cell. 179:282–282.e1. 2019.
|
|
24
|
Kilchert C, Wittmann S and Vasiljeva L:
The regulation and functions of the nuclear RNA exosome complex.
Nat Rev Mol Cell Biol. 17:227–239. 2016.
|
|
25
|
Bach DH, Hong JY, Park HJ and Lee SK: The
role of exosomes and miRNAs in drug-resistance of cancer cells. Int
J Cancer. 141:220–230. 2017.
|
|
26
|
Wu Z, Fang ZX, Hou YY, Wu BX, Deng Y, Wu
HT and Liu J: Exosomes in metastasis of colorectal cancers: Friends
or foes? World J Gastrointest Oncol. 15:731–756. 2023.
|
|
27
|
Patil AA and Rhee WJ: Exosomes:
Biogenesis, composition, functions, and their role in
pre-metastatic niche formation. Biotechnol Bioprocess Engineering.
24:689–701. 2019.
|
|
28
|
Kanemoto S, Nitani R, Murakami T, Kaneko
M, Asada R, Matsuhisa K, Saito A and Imaizumi K: Multivesicular
body formation enhancement and exosome release during endoplasmic
reticulum stress. Biochem Biophys Res Commun. 480:166–172.
2016.
|
|
29
|
Xia Z, Qing B, Wang W, Gu L, Chen H and
Yuan Y: Formation, contents, functions of exosomes and their
potential in lung cancer diagnostics and therapeutics. Thorac
Cancer. 12:3088–3100. 2021.
|
|
30
|
Wang Z, Zhao Z, Gao B and Zhang L: Exosome
mediated biological functions within skeletal microenvironment.
Front Bioeng Biotechnol. 10:9539162022.
|
|
31
|
Zhang Y, Liu Y, Liu H and Tang WH:
Exosomes: Biogenesis, biologic function and clinical potential.
Cell Biosci. 9:192019.
|
|
32
|
Kuang G, Wang Z, Luo C, Luo J and Wang J:
Mechanism of exosomes in the tumor microenvironment in the abscopal
effect (Review). Int J Oncol. 62:22023.
|
|
33
|
Han L, Lam EWF and Sun Y: Extracellular
vesicles in the tumor microenvironment: Old stories, but new tales.
Mol Cancer. 18:592019.
|
|
34
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020.
|
|
35
|
Guerra E, Di Pietro R, Basile M, Trerotola
M and Alberti S: Cancer-Homing CAR-T cells and endogenous immune
population dynamics. Int J Mol Sci. 23:4052021.
|
|
36
|
Kong H and Kim SB: Exosomal communication
between the tumor microenvironment and innate immunity and its
therapeutic application. Immune Netw. 22:e382022.
|
|
37
|
Wang Z, Chen JQ, Liu JL and Tian L:
Exosomes in tumor microenvironment: Novel transporters and
biomarkers. J Transl Med. 14:2972016.
|
|
38
|
Louise F, Ken YL, Richard BSR, Chien-Fu H
and Wu TC: Cervical cancer immunotherapy: Facts and hopes. Clin
Cancer Res. 27:4953–4973. 2021.
|
|
39
|
Li I and Nabet BY: Exosomes in the tumor
microenvironment as mediators of cancer therapy resistance. Mol
Cancer. 18:322019.
|
|
40
|
Hu X, Qiu Y, Zeng X and Wang H: Exosomes
reveal the dual nature of radiotherapy in tumor immunology. Cancer
Sci. 113:1105–1112. 2022.
|
|
41
|
Baig MS, Roy A, Rajpoot S, Liu D, Savai R,
Banerjee S, Kawada M, Faisal SM, Saluja R, Saqib U, et al:
Tumor-derived exosomes in the regulation of macrophage
polarization. Inflamm Res. 69:435–451. 2020.
|
|
42
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020.
|
|
43
|
Xia Y, Rao L, Yao H, Wang Z, Ning P and
Chen X: Engineering macrophages for cancer immunotherapy and drug
delivery. Adv Mater. 32:e20020542020.
|
|
44
|
Pritchard A, Tousif S, Wang Y, Hough K,
Khan S, Strenkowski J, Chacko BK, Darley-Usmar VM and Deshane JS:
Lung tumor Cell-Derived exosomes promote M2 macrophage
polarization. Cells. 9:13032020.
|
|
45
|
Khan FH, Reza MJ, Shao YF, Perwez A, Zahra
H, Dowlati A and Abbas A: Role of exosomes in lung cancer: A
comprehensive insight from immunomodulation to theragnostic
applications. Biochim Biophys Acta Rev Cancer. 1877:1887762022.
|
|
46
|
Guo W, Li Y, Pang W and Shen H: Exosomes:
A potential therapeutic tool targeting communications between tumor
cells and macrophages. Mol Ther. 28:1953–1964. 2020.
|
|
47
|
Bożyk A, Wojas-Krawczyk K, Krawczyk P and
Milanowski J: Tumor Microenvironment-A short review of cellular and
interaction diversity. Biology (Basel). 11:9292022.
|
|
48
|
Burassakarn A, Srisathaporn S, Pientong C,
Wongjampa W, Vatanasapt P, Patarapadungkit N and Ekalaksananan T:
Exosomes-carrying Epstein-Barr virus-encoded small RNA-1 induces
indoleamine 2, 3-dioxygenase expression in tumor-infiltrating
macrophages of oral squamous-cell carcinomas and suppresses T-cell
activity by activating RIG-I/IL-6/TNF-α pathway. Oral Oncol.
117:1052792021.
|
|
49
|
Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang
Y, Wang P, Wang J and Cai Z: Tumor-derived exosomes educate
dendritic cells to promote tumor metastasis via
HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology. 6:e13625272017.
|
|
50
|
Li X, Liu Y, Zheng S, Zhang T, Wu J, Sun
Y, Zhang J and Liu G: Role of exosomes in the immune
microenvironment of ovarian cancer. Oncol Lett. 21:3772021.
|
|
51
|
Rubenich DS, Omizzollo N, Szczepański MJ,
Reichert TE, Whiteside TL, Ludwig N and Braganhol E: Small
extracellular vesicle-mediated bidirectional crosstalk between
neutrophils and tumor cells. Cytokine Growth Factor Rev. 61:16–26.
2021.
|
|
52
|
Zhang X, Shi H, Yuan X, Jiang P, Qian H
and Xu W: Tumor-derived exosomes induce N2 polarization of
neutrophils to promote gastric cancer cell migration. Mol Cancer.
17:1462018.
|
|
53
|
Shao Q, Deng L, Liu H, Liu Z, Chen J,
Jiang F, Yan S and Fu R: Involvement of MM cell-derived exosomes in
T lymphocytes immune responses. Oncol Lett. 20:312020.
|
|
54
|
Ferguson Bennit HR, Gonda A, Kabagwira J,
Oppegard L, Chi D, Licero Campbell J, De Leon M and Wall NR:
Natural killer cell phenotype and functionality affected by
exposure to extracellular survivin and Lymphoma-Derived exosomes.
Int J Mol Sci. 22:12552021.
|
|
55
|
Papademetrio DL, Garcia MN, Grasso D and
Alvarez É: Autophagy-Mediated exosomes as immunomodulators of
natural killer cells in pancreatic cancer microenvironment. Front
Oncol. 10:6229562021.
|
|
56
|
Luo H, Zhou Y, Zhang J, Zhang Y, Long S,
Lin X, Yang A, Duan J, Yang N, Yang Z, et al: NK cell-derived
exosomes enhance the anti-tumor effects against ovarian cancer by
delivering cisplatin and reactivating NK cell functions. Front
Immunol. 13:10876892023.
|
|
57
|
Tumino N, Besi F, Martini S, Di Pace AL,
Munari E, Quatrini L, Pelosi A, Fiore PF, Fiscon G, Paci P, et al:
Polymorphonuclear Myeloid-Derived suppressor cells are abundant in
peripheral blood of cancer patients and suppress natural killer
cell Anti-tumor activity. Front Immunol. 12:8030142022.
|
|
58
|
Yan Y, Wang R, Guan W, Qiao M and Wang L:
Roles of microRNAs in cancer associated fibroblasts of gastric
cancer. Pathol Res Pract. 213:730–736. 2017.
|
|
59
|
Ding X, Xi W, Ji J, Cai Q, Jiang J, Shi M,
Yu Y, Zhu Z and Zhang J: HGF derived from cancer-associated
fibroblasts promotes vascularization in gastric cancer via PI3K/AKT
and ERK1/2 signaling. Oncol Rep. 40:1185–1195. 2018.
|
|
60
|
Ma J, Song X, Xu X and Mou Y:
Cancer-Associated-Fibroblasts promote the chemo-resistance in
gastric cancer through secreting IL-11 targeting JAK/STAT3/Bcl2
pathway. Cancer Res Treat. 51:194–210. 2019.
|
|
61
|
Wei R, Song J and Liu X, Huo S, Liu C and
Liu X: Immunosuppressive MFAP2+ cancer associated fibroblasts
conferred unfavorable prognosis and therapeutic resistance in
gastric cancer. Cell Oncol (Dordr). Aug 4–2023. View Article : Google Scholar : Epub ahead of
print.
|
|
62
|
Lu G, Du R, Dong J, Sun Y, Zhou F, Feng F,
Feng B, Han Y and Shang Y: Cancer associated fibroblast derived
SLIT2 drives gastric cancer cell metastasis by activating NEK9.
Cell Death Dis. 14:4212023.
|
|
63
|
Iha K, Sato A, Tsai HY, Sonoda H, Watabe
S, Yoshimura T, Lin MW and Ito E: Gastric cancer cell-derived
exosomal GRP78 enhances angiogenesis upon stimulation of vascular
endothelial cells. Curr Issues Mol Biol. 44:6145–6157. 2022.
|
|
64
|
Xue X, Huang J, Yu K, Chen X, He Y, Qi D
and Wu Y: YB-1 transferred by gastric cancer exosomes promotes
angiogenesis via enhancing the expression of angiogenic factors in
vascular endothelial cells. BMC Cancer. 20:9962020.
|
|
65
|
Zhou Z, Zhang H, Deng T, Ning T, Liu R,
Liu D, Bai M, Ying G and Ba Y: Exosomes Carrying MicroRNA-155
target forkhead Box O3 of endothelial cells and promote
angiogenesis in gastric cancer. Mol Ther Oncolytics.
25:2622022.
|
|
66
|
Oya Y, Hayakawa Y and Koike K: Tumor
microenvironment in gastric cancers. Cancer Sci. 111:2696–2707.
2020.
|
|
67
|
Hao Y, Zheng C, Wang L, Hu Y, Guo H, Song
Q, Zhang H, Zhang Z and Zhang Y: Covalent self-assembled
nanoparticles with pH-dependent enhanced tumor retention and drug
release for improving tumor therapeutic efficiency. J Mater Chem B.
5:2133–2144. 2017.
|
|
68
|
Zhu KG, Yang J, Zhu Y, Zhu Q, Pan W, Deng
S, He Y, Zuo D, Wang P, Han Y and Zhang HY: The microprotein
encoded by exosomal lncAKR1C2 promotes gastric cancer lymph node
metastasis by regulating fatty acid metabolism. Cell Death Dis.
14:7082023.
|
|
69
|
Wang R, Liang L, Matsumoto M, Iwata K,
Umemura A and He F: Reactive Oxygen species and NRF2 signaling,
friends or foes in cancer? Biomolecules. 13:3532023.
|
|
70
|
Chen H, Sun Q, Zhang C, She J, Cao S, Cao
M, Zhang N, Adiila AV, Zhong J, Yao C, et al: Identification and
Validation of CYBB, CD86, and C3AR1 as the key genes related to
macrophage infiltration of gastric cancer. Front Mol Biosci.
8:7560852021.
|
|
71
|
Zhao LX, Zhang K, Shen BB and Li JN:
Mesenchymal stem cell-derived exosomes for gastrointestinal cancer.
World J Gastrointest Oncol. 13:1981–1996. 2021.
|
|
72
|
Tian X, Shen H, Li Z, Wang T and Wang S:
Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor
microenvironment. J Hematol Oncol. 12:842019.
|
|
73
|
Liu K, Gao X, Kang B, Liu Y, Wang D and
Wang Y: The role of tumor stem cell exosomes in cancer invasion and
metastasis. Front Oncol. 12:8365482022.
|
|
74
|
da Costa VR, Araldi RP, Vigerelli H,
D'Ámelio F, Mendes TB, Gonzaga V, Policíquio B, Colozza-Gama GA,
Valverde CW and Kerkis I: Exosomes in the tumor microenvironment:
From biology to clinical applications. Cells. 10:26172021.
|
|
75
|
Wang T, Nasser MI, Shen J, Qu S, He Q and
Zhao M: Functions of exosomes in the triangular relationship
between the tumor, inflammation, and immunity in the tumor
microenvironment. J Immunol Res. 2019:41978292019.
|
|
76
|
Lopez K, Lai SWT, Lopez Gonzalez EDJ,
Dávila RG and Shuck SC: Extracellular vesicles: A dive into their
role in the tumor microenvironment and cancer progression. Front
Cell Dev Biol. 11:11545762023.
|
|
77
|
Thakur A, Johnson A, Jacobs E, Zhang K,
Chen J, Wei Z, Lian Q and Chen HJ: Energy sources for exosome
communication in a cancer microenvironment. Cancers (Basel).
14:16982022.
|
|
78
|
Yan Y, Fu G, Ye Y and Ming L: Exosomes
participate in the carcinogenesis and the malignant behavior of
gastric cancer. Scand J Gastroenterol. 52:499–504. 2017.
|
|
79
|
Yoon JH, Choi BJ, Nam SW and Park WS:
Gastric cancer exosomes contribute to the field cancerization of
gastric epithelial cells surrounding gastric cancer. Gastric
Cancer. 25:490–502. 2022.
|
|
80
|
Liang ZF, Zhang Y, Guo W, Chen B, Fang S
and Qian H: Gastric cancer stem cell-derived exosomes promoted
tobacco smoke-triggered development of gastric cancer by inducing
the expression of circ670. Med Oncol. 40:242022.
|
|
81
|
Chang L, Gao H, Wang L, Wang N, Zhang S,
Zhou X and Yang H: Exosomes derived from miR-1228 overexpressing
bone marrow-mesenchymal stem cells promote growth of gastric cancer
cells. Aging (Albany NY). 13:11808–11821. 2021.
|
|
82
|
Lin S, Que Y, Que C, Li F, Deng M and Xu
D: Exosome miR-3184-5p inhibits gastric cancer growth by targeting
XBP1 to regulate the AKT, STAT3, and IRE1 signalling pathways. Asia
Pac J Clin Oncol. 19:e27–e38. 2023.
|
|
83
|
Piao HY, Guo S, Wang Y and Zhang J:
Exosome-transmitted lncRNA PCGEM1 promotes invasive and metastasis
in gastric cancer by maintaining the stability of SNAI1. Clin
Transl Oncol. 23:246–256. 2021.
|
|
84
|
Yang H, Zhang H, Ge S, Ning T, Bai M, Li
J, Li S, Sun W, Deng T, Zhang L, et al: Exosome-Derived miR-130a
activates angiogenesis in gastric cancer by targeting C-MYB in
vascular endothelial cells. Mol Ther. 26:2466–2475. 2018.
|
|
85
|
Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian
H, Chen Y, Jiang P and Xu W: Exosomes derived from human
mesenchymal stem cells promote gastric cancer cell growth and
migration via the activation of the Akt pathway. Mol Med Rep.
14:3452–3458. 2016.
|
|
86
|
Gao Q, Cui L, Huang C, Zhou C, Chen B,
Wang Q, Wang M, Shene B, Xu W and Zhu W: Gastric cancer-derived
exosomes induce PD-L1 expression on human bone marrow mesenchymal
stem cells through the AKT-c-Myc signal axis. Front Life Sci.
442–451. 2022.
|
|
87
|
Fu H, Yang H, Zhang X, Wang B, Mao J, Li
X, Wang M, Zhang B, Sun Z, Qian H and Xu W: Exosomal TRIM3 is a
novel marker and therapy target for gastric cancer. J Exp Clin
Cancer Res. 37:1622018.
|
|
88
|
Sun Y, Jin J, Jing H, Lu Y, Zhu Q, Shu C,
Zhang Q and Jing D: ITIH4 is a novel serum biomarker for early
gastric cancer diagnosis. Clin Chim Acta. 523:365–373. 2021.
|
|
89
|
Wang S, He Y, Lu J, Wang Y, Wu X, Yan G,
Fang X and Liu B: All-in-One strategy for downstream molecular
profiling of tumor-derived exosomes. ACS Appl Mater Interfaces.
14:36341–36352. 2022.
|
|
90
|
Dong Z, Sun X, Xu J, Han X, Xing Z, Wang
D, Ge J, Meng L and Xu X: Serum Membrane Type 1-Matrix
Metalloproteinase (MT1-MMP) mRNA Protected by exosomes as a
potential biomarker for gastric cancer. Med Sci Monit.
25:7770–7783. 2019.
|
|
91
|
Cai C, Zhang H, Zhu Y, Zheng P, Xu Y, Sun
J, Zhang M, Lan T, Gu B, Li S and Ma P: Serum exosomal long
noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker
for gastric cancer. OncoTargets Ther. 12:10035–10041. 2019.
|
|
92
|
Joo HS, Jeon HY, Hong EB, Kim HY and Lee
JM: Exosomes for the diagnosis and treatment of dementia. Curr Opin
Psychiatry. 36:119–125. 2023.
|
|
93
|
Aheget H, Mazini L, Martin F, Belqat B,
Marchal JA and Benabdellah K: Exosomes: Their role in pathogenesis,
diagnosis and treatment of diseases. Cancers (Basel).
13:842020.
|
|
94
|
Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J
and Cao P: Exosomes: A novel strategy for treatment and prevention
of diseases. Front Pharmacol. 8:3002017.
|
|
95
|
Jiang L, Chen W, Ye J and Wang Y:
Potential role of exosomes in ischemic stroke treatment.
Biomolecules. 115:122022.
|
|
96
|
You D, Kundu S, Schmaier AH, Khorana AA
and McCrae KR: Exosome polyphosphate mediates the activation of
FXII by cancer cell-derived exosomes. Blood. 132:38002018.
|
|
97
|
Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu
R, Ge S, Li J, Ning T, Deng T, et al: Exosomes serve as
nanoparticles to suppress tumor growth and angiogenesis in gastric
cancer by delivering hepatocyte growth factor siRNA. Cancer Sci.
109:629–641. 2018.
|
|
98
|
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng
T, Liu R, Zhang L, Ying G and Ba Y: Exosomes serve as nanoparticles
to deliver Anti-miR-214 to reverse chemoresistance to cisplatin in
gastric cancer. Mol Ther. 26:774–783. 2018.
|