Immune checkpoint molecules are crucial for
maintaining self-tolerance and modulating immune responses
(4). However, certain cancers
protect themselves from anti-tumor immunity by utilizing this
system. Immune checkpoint molecules are mainly expressed on the
surface of T cells and co-inhibit T-cell activation by binding to
their ligands on antigen-presenting cells and/or cancer cells at
the time of recognition of cancer antigens (4). Recently, cancer immunotherapy
utilizing immune checkpoint inhibitors (ICIs) has demonstrated the
potential of the immune system to eradicate cancer cells, thereby
impressively improving the survival of cancer patients (5,6).
Several kinds of ICIs have been approved for the treatment of a
wide range of tumor types. Among oncology therapeutic products
approved by the US Food and Drug Administration (FDA) in this
century, ICIs are the second most approved drugs despite having
been on the market since 2011 (7).
The successful development of ICIs suggests that ICIs may be a
potentially powerful strategy for the treatment of bone
metastases.
Among the currently available ICIs, monoclonal
antibodies that block programmed cell death 1 (PD-1) and programmed
cell death ligand 1 (PD-L1) are the mainstay of cancer
immunotherapy in the clinic. In the tumor microenvironment, PD-L1
expressed on tumor cells interacts with the PD-1 receptor on
activated T cells, leading to suppression of the immune response
through T-cell exhaustion, anergy and apoptosis, and facilitating
tumor immune evasion and tumor growth (8). Antibodies against PD-1 and PD-L1
block this pathway, increase immune cell proliferation, enhance
anti-tumor activity and thereby suppress tumors. However, clinical
studies have shown that the efficacy of anti-PD-1/PD-L1 antibodies
against bone metastases remains lower than expected. To overcome
this limitation, it is necessary to learn more about the immune
microenvironment of bone metastases and to find ways to better
utilize the antibodies. Therefore, the present review focuses on
the effects of anti-PD-1/PD-L1 antibodies on bone metastases; it i)
summarizes the current understanding of the immune microenvironment
of bone metastases, ii) provides a comprehensive analysis of
clinical reports to date, iii) presents possible candidates for
combination therapies and discusses the shortcomings of the
existing literature to guide future studies.
In addition to their effects on immune cells, immune
checkpoint molecules have been shown to have certain effects on
bone metabolism, particularly bone resorption. Several papers
reported that osteoclast differentiation and bone resorption were
suppressed in PD-1-deficient mice and in mice treated with
anti-PD-1 antibody, whereas little effect was found on bone
formation indices (9,10). Wang et al (11) showed similar results only in
tumor-bearing bone but not in naive bone. These preclinical data
are partially supported by a clinical study showing that ICIs
increased plasma levels of C-terminal telopeptide of type I
collagen, a bone resorption marker, in cancer patients without bone
metastases, while plasma levels of N-terminal propeptide of type I
procollagen, a bone formation marker, showed a trend toward a
decrease but was not statistically significant (12). Although the precise mechanisms of
the suppressive effects of PD-1 inhibition on osteoclastogenesis
are still unclear, Wang et al (11) proposed that, in the bone metastasis
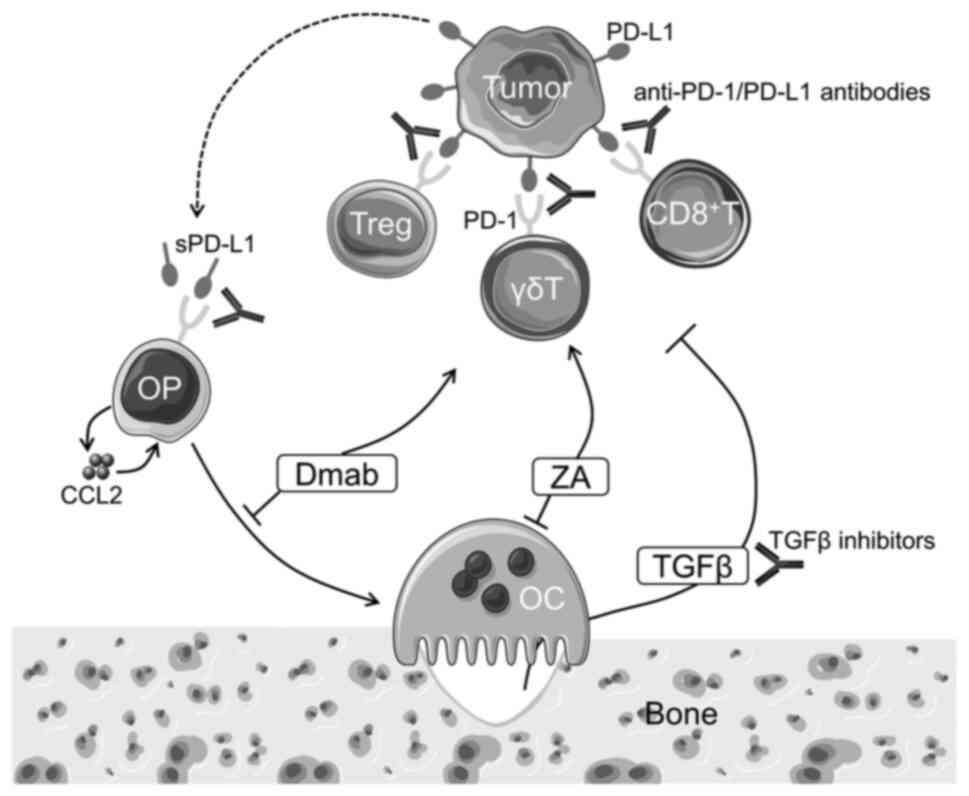
microenvironment, binding of soluble PD-L1 produced by tumor cells
to PD-1 in preosteoclasts causes the release of chemokine C-C motif
ligand 2 (CCL2), thereby promoting osteoclast differentiation.
Therefore, anti-PD-1 treatment prevents osteoclast differentiation
by inhibiting CCL2 production (Fig.
1). Given that osteoclastic bone destruction has critical roles
in the progression of bone metastases, these effects of PD-1
blockade on osteoclasts are expected to act to reduce bone
metastases. In support of this notion, Zuo and Wan (13) reported that anti-PD-L1 antibody
treatment reduced bone metastases of breast cancer and melanoma by
simultaneously suppressing osteoclast differentiation and enhancing
immunity. By contrast, Wang et al (11) showed that PD-1 blockade inhibited
osteoclastogenesis but did not affect tumor burden in bone.
Bone marrow provides a niche that supports
hematopoietic stem cells and has a central role in hematopoiesis
(14). In addition, bone marrow
contains various immune cells and acts as a site of primary or
memory immune response, indicating that bone marrow functions not
only as a primary lymphoid organ but also as a secondary lymphoid
organ (15). Thus, immune cells
residing in bone marrow most likely have an important role in the
development of bone metastases.
Various studies have indicated that immune
checkpoint molecules regulate not only T cells but also a variety
of immune cells, including B cells, natural killer cells and
myeloid cells (4–6). However, currently available ICIs
largely target CD8+ cytotoxic T cells against tumor
cells. High CD8+ cytotoxic T-cell infiltration has been
reported to be associated with response to ICI treatment (16). When CD8+ T cells were
PD-1-deficient in murine colon cancer models, anti-PD-1 antibody
was ineffective (17).
Because most preclinical studies of bone metastasis
have been conducted using immunodeficient mice, the current
knowledge of the role of immunity in bone metastasis is limited.
However, some recent work has shown that CD8+ cytotoxic
T lymphocytes have a key role in antitumor immune responses also in
the formation of bone metastases of melanoma (18,19),
as well as breast (20,21) and prostate (22) cancers. A recent study by our group
using syngeneic immunocompetent mouse models revealed that primary
tumor-primed immune cells inhibit the development of bone
metastases of breast cancer, which is predominantly mediated by
CD8+ cytotoxic T lymphocytes (23). In addition, a study using clinical
specimens showed that the proportion of CD8+ memory T
cells was increased in the bone marrow of patients with breast
cancer compared with that of healthy donors (24), and adoptive transfer of bone marrow
T cells from patients with breast cancer into immunodeficient mice
induced regression of xenografted autologous tumors (25). These results suggest that adequate
control of the function of CD8+ cytotoxic T lymphocytes
allows us to eliminate bone metastases. On the other hand, a study
using clinical specimens of bone metastases of breast cancer showed
that the number of CD8+ tumor-infiltrating lymphocytes
was significantly lower than those in primary breast tumors
(26), suggesting the
immunologically cold microenvironment of bone metastases.
It is crucial to define the biomarkers to predict
the therapeutic effects of ICIs. Among several proposed biomarkers,
PD-L1 expression quantified by immunohistochemistry is currently
the most widely validated, used and accepted biomarker for
selecting patients to receive anti-PD-1 or anti-PD-L1 antibody
treatment (29). However, PD-L1
expression is not always associated with the prognosis of cancer
patients. Several studies have indicated a positive association
across cancers (30,31), while certain others have reported
no association (32,33). One critical reason is that there
are some technical issues with this screening method. Each
pharmaceutical company developed its own diagnostic antibody and
corresponding protocol for PD-L1 staining and interpretation;
however, the use of different antibodies has been shown to affect
PD-L1 positivity in the same tumors (34,35).
PD-L1 expression is assessed on tumor cells and/or
tumor-infiltrating immune cells, including macrophages, dendritic
cells, neutrophils, myeloid-derived suppressor cells, and T cells
and/or B cells (29). However, it
has not been standardized which cells should be evaluated and what
formulas should be used for scoring the threshold. Furthermore, the
concordance between pathologists' scores was particularly poor for
immune cells compared to tumor cells (36). These issues of inter-assay
heterogeneity and inter-observer reproducibility are major
challenges in the development of PD-L1 expression as a universal
predictive biomarker. In addition to PD-L1, the FDA approved two
other markers, tumor mutational burden and deficient mismatch
repair/microsatellite instability-high, both of which still require
refinement to improve validity across cancers (37).
As mentioned above, PD-L1 is a widely used but
imperfect predictive biomarker, particularly for selecting patients
to receive anti-PD-1/PD-L1 antibodies. Recently, the discordant
expression of PD-L1 between primary tumors and paired metastases
has been increasingly demonstrated. According to a meta-analysis by
Zou et al (38), the
percentage of PD-L1 that changed from positive to negative was 41%
and that from negative to positive was 16%, although the conversion
trend differed among cancer types and metastatic sites. Regarding
bone metastases, certain papers reported the differential
expression of PD-L1 in primary tumors and metastatic lesions in
bone and other organs of breast, prostate and renal cell cancer
(RCC) (Table I) (26,39–41).
When compared with primary tumors, the PD-L1-positive rate was
generally lower in bone metastases, regardless of the cancer type
and cells examined. Furthermore, of note, Zhu et al
(42) reported that the PD-L1
expression level in primary non-small cell lung cancer (NSCLC) was
significantly lower in patients with bone metastases than in those
without. These characteristics may contribute to the poor efficacy
of ICIs in bone metastases.
Preclinical studies have shown that treatment with
anti-PD-1/PD-L1 antibodies reduced bone metastases of breast cancer
and melanoma in mice (13,43,44).
The increase in infiltrating CD8+ T cells in metastatic
bone lesions was found in these antibody-treated mice. Consistent
with the findings described in Section 2, studies by Zuo and Wan
(13) and Arellano et al
(21) showed that the activation
of T cells by immunotherapy suppressed osteoclast formation, which
may also contribute to the inhibition of bone metastases. These
results suggest the potential of anti-PD-1/PD-L1 antibodies to
inhibit bone metastases. Clinical studies have reported the effects
of anti-PD-1 antibodies (nivolumab and pembrolizumab) and
anti-PD-L1 antibodies (atezolizumab and durvalumab) on bone
metastases evaluated using the bone metastasis-specific response
classification criteria developed by the University of Texas MD
Anderson Cancer Center (MDA criteria) (45), all of which are data from patients
with NSCLC (Table II) (46–50).
Although responses varied among studies and agents used, the
overall response rates were unsatisfactory compared with the
preclinical studies. This is consistent with the findings that
PD-L1 expression was low in bone metastases in cancer patients
(Table I). It should be noted,
however, that the number of studies and their sample sizes are
limited to date.
Numerous clinical studies have been conducted on the
effects of ICIs on cancer patients with bone metastases. The impact
of bone metastases on the prognosis of cancer patients treated with
anti-PD-1 antibodies (camrelizumab, nivolumab, pembrolizumab,
sintilimab, tislelizumab and toripalimab) and anti-PD-L1 antibodies
(atezolizumab, avelumab and durvalumab) is summarized in Table III (42,49,51–85).
Although these studies did not show the effects on bone metastases
themselves and the results were not always consistent across the
studies, the overall trend suggests that the presence of bone
metastases is a potential predictor of poor progression-free
survival (PFS) and overall survival (OS). The contribution of bone
metastases to the survival rates appears to be similar to that of
liver metastases and more severe than that of other metastatic
sites. These data again suggest a lower efficacy of ICIs in bone
metastases, although it should be noted that most of the studies
are retrospective analyses with limited sample sizes. Most of these
data were reported in patients with NSCLC. Numerous clinical trials
have been completed or are ongoing (86,87);
however, the number of trials conducted on cancers that frequently
metastasize to bone, such as breast and prostate cancer, is
limited.
Due to the limited efficacy of ICIs in the treatment
of cancer, there is a growing interest in the development of
therapeutic combinations with ICIs. With respect to bone
metastases, bone-modifying agents (BMAs), including the
anti-receptor activator of NF-κB ligand (RANKL) antibody Denosumab
(Dmab) and bisphosphonates (BPs), are often combined with ICIs to
reduce skeletal-related events and cancer progression in patients
with bone metastases. In the studies listed in Table II, a significant proportion of
patients were treated with ICIs in combination with BMAs, such as
Dmab or the BP zoledronic acid (ZA), and the combination therapy
showed a trend toward improved response in bone metastases
(46–50). Several studies also evaluated the
prognostic impact of the combination of ICIs and BMAs in patients
with bone metastases (Table
IV)(42,46,48,60,64,75,88–90).
A substantial proportion of studies have shown that the combination
of BMAs confers a survival benefit. The mechanisms of action of
individual BMAs are discussed below. In addition to BMAs,
inhibitors of transforming growth factor β (TGFβ) and angiogenesis
are also potential candidates for combination therapy.
Among several kinds of BPs, ZA is currently the most
widely used for the treatment of bone metastases (91). As a ZA-specific effect, ZA has been
shown to expand γδ T lymphocytes and induce their effector
phenotype in cancer patients (92). Iwasaki et al (93) reported that, although PD-1
expression was rapidly induced on γδ T cells after antigenic
stimulation, the attenuated effector function was reversed by
anti-PD-L1 monoclonal antibody. ZA also has the effect of
sensitizing tumor cells to γδ T cells (93). These effects of ZA may potentiate
the effects of ICIs (Fig. 1). Li
et al (44) showed in a
mouse model of breast cancer bone metastasis that combined
treatment with ZA and anti-PD-1 antibody suppressed bone tumor
growth more effectively than either treatment alone with no
apparent toxicity. The combined treatment increased the
infiltration of CD8+ T cells and decreased that of
myeloid-derived suppressor cells in bone tumors, although the
mechanisms remain to be determined.
RANKL is currently best known for its role in
osteoclast lineage cells; however, RANKL was originally identified
as a dendritic cell-specific survival factor, which was upregulated
in activated T cells (94). In
addition to dendritic cells, myeloid cells, such as macrophages and
myeloid-derived suppressor cells, also express RANK in the tumor
microenvironment and RANKL-RANK signaling provides immunomodulatory
signals (95). However, in several
mouse models, RANKL inhibition alone exhibited minimal efficacy in
controlling subcutaneous tumor growth and experimental lung
metastases (95).
The abnormal vascular network in cancer tissue
disrupts both the trafficking of immune effectors and the delivery
of ICIs (102). Angiogenic
factors, including vascular endothelial growth factor,
angiopoietin, hepatocyte growth factor and platelet-derived growth
factor, also directly affect immune cell function and impair
optimal anti-tumor immunity. Therefore, it is expected that
anti-angiogenic agents may be able to restore both cellular and
molecular pathways in favor of improved efficacy of ICIs. In
support of this notion, several clinical trials have demonstrated
beneficial effects of these combinations (103). A study by Xie et al
(104) showed that combined
treatment with ICIs and anti-angiogenic agents significantly
prolonged median bone PFS compared with ICIs alone, whereas median
PFS and OS were not changed in patients with NSCLC with bone
metastases.
Each of these agents not only has its own
immunologic effects, but also affects the microenvironment of bone
metastases, both of which are likely to contribute to the improved
efficacy of ICIs when used in combination. Extensive studies are
still needed to elucidate the precise mechanisms of action of these
combinations. Further understanding the effects of ICIs on bone
metastases may also lead us to find better candidates for
combination therapy, including not only bone-targeted therapy but
also chemotherapy, radiotherapy and other immunotherapeutic
agents.
As described above, a number of studies have been
conducted at the clinical and preclinical levels to investigate the
effects of ICIs on bone metastases. However, these reports have
several limitations that may affect the interpretation of the
results.
The most critical issue is that our understanding of
the bone immune microenvironment and the roles of immune cells in
bone metastases is immature. As presented in Table III, the response to
anti-PD-1/PD-L1 antibody treatment is different among metastatic
sites, suggesting that the immune response varies among organs.
Preclinical studies focusing on bone-specific immunology are
definitely needed to properly interpret the effects of
immunotherapies on bone metastases. For instance, Yin et al
(106) proposed that the
transcription factor basic helix-loop-helix family member e22 is
upregulated in bone metastatic prostate cancer and drives an
immunosuppressive bone microenvironment. Since the majority of
clinical studies have been conducted in patients with NSCLC, the
study using animal models of NSCLC may aid in the interpretation of
clinical data. A significant number of patients are known to be
resistant to ICI treatment (107). Some of these patients are
primarily resistant and others acquire resistance after an initial
response. To investigate the mechanisms of resistance, animal
models of bone metastases that mimic not only primary but also
acquired resistance need to be developed.
Immunotherapies targeting immune checkpoint
molecules have been shown to be effective in several types of
cancer and have led to a paradigm shift in cancer treatment.
However, therapies with ICIs have several challenges that need to
be addressed to broaden their application, including bone
metastases (6). Current ICIs are
not effective against cancers with low inherent immunogenicity. In
addition, a considerable proportion of cancer patients exhibit
resistance to ICIs (107). Both
tumor-cell-intrinsic and tumor-cell-extrinsic mechanisms have been
suggested to contribute to the resistance, but the precise
mechanisms are still unclear. To overcome these issues and maximize
the clinical efficacy of ICIs, numerous clinical trials of ICIs in
combination with other therapeutic modalities, including
chemotherapy, radiotherapy and other immunotherapeutic agents, are
ongoing (108). With respect to
bone metastases, although an immunologically cold microenvironment
is thought to be one of the possible reasons for the limited
efficacy of ICIs, there is still a lack of studies that
comprehensively investigate the immune microenvironment and the
regulation of immune checkpoint molecules of bone metastases.
Clinical data on the effects of ICIs on bone metastases of cancers
that frequently metastasize to bone, such as breast and prostate
cancer, are also limited. Future translational and reverse
translational approaches, combined with knowledge from other cancer
fields, are expected to facilitate our understanding of the
immunology of bone metastases. The study of the bone immune
microenvironment and the roles of immune cells in bone metastases
is still in its infancy. It is esteemed that the accumulated
knowledge will lead to improved efficacy of ICIs on bone
metastases.
Not applicable.
This work was supported by JSPS KAKENHI, Japan (grant no.
JP21K09863).
Not applicable.
TH drafted the manuscript, collected and assembled
the data, and read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The author declares that he has no competing
interests.
|
1
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang JF, Shen J, Li X, Rengan R,
Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, et al:
Incidence of patients with bone metastases at diagnosis of solid
tumors in adults: A large population-based study. Ann Transl Med.
8:4822020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Svensson E, Christiansen CF, Ulrichsen SP,
Rørth MR and Sørensen HT: Survival after bone metastasis by primary
cancer type: A Danish population-based cohort study. BMJ Open.
7:e0160222017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Chan TA, Kroemer G, Wolchok JD
and López-Soto A: The hallmarks of successful anticancer
immunotherapy. Sci Transl Med. 10:eaat78072018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morad G, Helmink BA, Sharma P and Wargo
JA: Hallmarks of response, resistance, and toxicity to immune
checkpoint blockade. Cell. 184:5309–5337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott EC, Baines AC, Gong Y, Moore R Jr,
Pamuk GE, Saber H, Subedee A, Thompson MD, Xiao W, Pazdur R, et al:
Trends in the approval of cancer therapies by the FDA in the
twenty-first century. Nat Rev Drug Discov. 22:625–640. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pitter MR and Zou W: Uncovering the
immunoregulatory function and therapeutic potential of the
PD-1/PD-L1 axis in cancer. Cancer Res. 81:5141–5143. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagahama K, Aoki K, Nonaka K, Saito H,
Takahashi M, Varghese BJ, Shimokawa H, Azuma M, Ohya K and Ohyama
K: The deficiency of immunoregulatory receptor PD-1 causes mild
osteopetrosis. Bone. 35:1059–1068. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brom VC, Strauss AC, Sieberath A, Salber
J, Burger C, Wirtz DC and Schildberg FA: Agonistic and antagonistic
targeting of immune checkpoint molecules differentially regulate
osteoclastogenesis. Front Immunol. 14:9883652023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K, Gu Y, Liao Y, Bang S, Donnelly CR,
Chen O, Tao X, Mirando AJ, Hilton MJ and Ji RR: PD-1 blockade
inhibits osteoclast formation and murine bone cancer pain. J Clin
Invest. 130:3603–3620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pantano F, Tramontana F, Iuliani M, Leanza
G, Simonetti S, Piccoli A, Paviglianiti A, Cortellini A, Spinelli
GP, Longo UG, et al: Changes in bone turnover markers in patients
without bone metastases receiving immune checkpoint inhibitors: An
exploratory analysis. J Bone Oncol. 37:1004592022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo H and Wan Y: Inhibition of myeloid
PD-L1 suppresses osteoclastogenesis and cancer bone metastasis.
Cancer Gene Ther. 29:1342–1354. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Comazzetto S, Shen B and Morrison SJ:
Niches that regulate stem cells and hematopoiesis in adult bone
marrow. Dev Cell. 56:1848–1860. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu
Y, Wang G and Zou W: Bone marrow and the control of immunity. Cell
Mol Immunol. 9:11–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumagai S, Togashi Y, Kamada T, Sugiyama
E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y,
Matsui S, et al: The PD-1 expression balance between effector and
regulatory T cells predicts the clinical efficacy of PD-1 blockade
therapies. Nat Immunol. 21:1346–1358. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang K, Kim S, Cremasco V, Hirbe AC,
Collins L, Piwnica-Worms D, Novack DV, Weilbaecher K and Faccio R:
CD8+ T cells regulate bone tumor burden independent of
osteoclast resorption. Cancer Res. 71:4799–7808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo-Saito C, Fuwa T, Murakami K and
Kawakami Y: Targeting FSTL1 prevents tumor bone metastasis and
consequent immune dysfunction. Cancer Res. 73:6185–6193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bidwell BN, Slaney CY, Withana NP, Forster
S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et
al: Silencing of Irf7 pathways in breast cancer cells promotes bone
metastasis through immune escape. Nat Med. 18:1224–1231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arellano DL, Juárez P, Verdugo-Meza A,
Almeida-Luna PS, Corral-Avila JA, Drescher F, Olvera F, Jiménez S,
Elzey BD, Guise TA and Fournier PGJ: Bone
microenvironment-suppressed T cells increase osteoclast formation
and osteolytic bone metastases in mice. J Bone Miner Res.
37:1446–1463. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan
B, Miura Y and Sharma P: Differences in tumor microenvironment
dictate T helper lineage polarization and response to immune
checkpoint therapy. Cell. 179:1177–1190.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hiraga T, Nishida D and Horibe K: Primary
tumor-induced immunity suppresses bone metastases of breast cancer
in syngeneic immunocompetent mouse models. Bone. 178:1169442024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feuerer M, Rocha M, Bai L, Umansky V,
Solomayer EF, Bastert G, Diel IJ and Schirrmacher V: Enrichment of
memory T cells and other profound immunological changes in the bone
marrow from untreated breast cancer patients. Int J Cancer.
92:96–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feuerer M, Beckhove P, Bai L, Solomayer
EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V
and Umansky V: Therapy of human tumors in NOD/SCID mice with
patient-derived reactivated memory T cells from bone marrow. Nat
Med. 7:452–458. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao X, Zhang Y, Zheng C, Huang Q, Lu J,
Pulver EM, Houthuijzen J, Hutten S, Luo R, He J and Sun P:
Metastasis of breast cancer to bones alters the tumor immune
microenvironment. Eur J Med Res. 28:1192023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sawant A, Hensel JA, Chanda D, Harris BA,
Siegal GP, Maheshwari A and Ponnazhagan S: Depletion of
plasmacytoid dendritic cells inhibits tumor growth and prevents
bone metastasis of breast cancer cells. J Immunol. 189:4258–4265.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao E, Wang L, Dai J, Kryczek I, Wei S,
Vatan L, Altuwaijri S, Sparwasser T, Wang G, Keller ET and Zou W:
Regulatory T cells in the bone marrow microenvironment in patients
with prostate cancer. Oncoimmunology. 1:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Daud AI, Wolchok JD, Robert C, Hwu WJ,
Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al:
Programmed death-ligand 1 expression and response to the
anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin
Oncol. 34:4102–4109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hellmann MD, Paz-Ares L, Bernabe Caro R,
Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A,
Lupinacci L, de la Mora Jimenez E, et al: Nivolumab plus ipilimumab
in advanced non-small-cell lung cancer. N Engl J Med.
381:2020–2031. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirsch FR, McElhinny A, Stanforth D,
Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P,
Hanks D, Vennapusa B, et al: PD-L1 immunohistochemistry assays for
lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay
comparison project. J Thorac Oncol. 12:208–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rugo HS, Loi S, Adams S, Schmid P,
Schneeweiss A, Barrios CH, Iwata H, Diéras V, Winer EP, Kockx MM,
et al: PD-L1 immunohistochemistry assay comparison in atezolizumab
plus nab-paclitaxel-treated advanced triple-negative breast cancer.
J Natl Cancer Inst. 113:1733–1743. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge
JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A
prospective, multi-institutional, pathologist-based assessment of 4
immunohistochemistry assays for PD-L1 expression in non-small cell
lung cancer. JAMA Oncol. 3:1051–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun Q, Hong Z, Zhang C, Wang L, Han Z and
Ma D: Immune checkpoint therapy for solid tumours: Clinical
dilemmas and future trends. Signal Transduct Target Ther.
8:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou Y, Hu X, Zheng S, Yang A, Li X, Tang
H, Kong Y and Xie X: Discordance of immunotherapy response
predictive biomarkers between primary lesions and paired metastases
in tumours: A systematic review and meta-analysis. EBioMedicine.
63:1031372021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rozenblit M, Huang R, Danziger N, Hegde P,
Alexander B, Ramkissoon S, Blenman K, Ross JS, Rimm DL and Pusztai
L: Comparison of PD-L1 protein expression between primary tumors
and metastatic lesions in triple negative breast cancers. J
Immunother Cancer. 8:e0015582020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fankhauser CD, Schüffler PJ, Gillessen S,
Omlin A, Rupp NJ, Rueschoff JH, Hermanns T, Poyet C, Sulser T, Moch
H and Wild PJ: Comprehensive immunohistochemical analysis of PD-L1
shows scarce expression in castration-resistant prostate cancer.
Oncotarget. 9:10284–10293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Yin X, Zhang H, Sun G, Yang Y,
Chen J, Zhu X, Zhao P, Zhao J, Liu J, et al: Differential
expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic
sites in renal cell carcinoma. BMC Cancer. 19:3602019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu YJ, Chang XS, Zhou R, Chen YD, Ma HC,
Xiao ZZ, Qu X, Liu YH, Liu LR, Li Y, et al: Bone metastasis
attenuates efficacy of immune checkpoint inhibitors and displays
‘cold’ immune characteristics in Non-small cell lung cancer. Lung
Cancer. 166:189–196. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mehdi A, Attias M, Arakelian A, Piccirillo
CA, Szyf M and Rabbani SA: Co-targeting luminal B breast cancer
with S-Adenosylmethionine and immune checkpoint inhibitor reduces
primary tumor growth and progression, and metastasis to lungs and
bone. Cancers (Basel). 15:482022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Du Y, Sun T, Xue H, Jin Z and Tian
J: PD-1 blockade in combination with zoledronic acid to enhance the
antitumor efficacy in the breast cancer mouse model. BMC Cancer.
18:6692018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamaoka T, Madewell JE, Podoloff DA,
Hortobagyi GN and Ueno NT: Bone imaging in metastatic breast
cancer. J Clin Oncol. 22:2942–2953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asano Y, Yamamoto N, Demura S, Hayashi K,
Takeuchi A, Kato S, Miwa S, Igarashi K, Higuchi T, Yonezawa H, et
al: The therapeutic effect and clinical outcome of immune
checkpoint inhibitors on bone metastasis in advanced non-small-cell
lung cancer. Front Oncol. 12:8716752022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asano Y, Yamamoto N, Demura S, Hayashi K,
Takeuchi A, Kato S, Miwa S, Igarashi K, Higuchi T, Taniguchi Y, et
al: Novel predictors of immune checkpoint inhibitor response and
prognosis in advanced non-small-cell lung cancer with bone
metastasis. Cancer Med. 12:12425–12437. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bongiovanni A, Foca F, Menis J, Stucci SL,
Artioli F, Guadalupi V, Forcignanò MR, Fantini M, Recine F,
Mercatali L, et al: Immune checkpoint inhibitors with or without
bone-targeted therapy in NSCLC patients with bone metastases and
prognostic significance of neutrophil-to-lymphocyte ratio. Front
Immunol. 12:6972982021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
De Giglio A, Deiana C and Di Federico A:
Bone-specific response according to MDA criteria predicts
immunotherapy efficacy among advanced non-small cell lung cancer
(NSCLC) patients. J Cancer Res Clin Oncol. 149:1835–1847. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakata E, Sugihara S, Sugawara Y, Kozuki
T, Harada D, Nogami N, Nakahara R, Furumatsu T, Tetsunaga T,
Kunisada T and Ozaki T: Early response of bone metastases can
predict tumor response in patients with non-small-cell lung cancer
with bone metastases in the treatment with nivolumab. Oncol Lett.
20:2977–2986. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Botticelli A, Salati M, Di Pietro FR,
Strigari L, Cerbelli B, Zizzari IG, Giusti R, Mazzotta M, Mazzuca
F, Roberto M, et al: A nomogram to predict survival in non-small
cell lung cancer patients treated with nivolumab. J Transl Med.
17:992019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cortellini A, Tiseo M, Banna GL, Cappuzzo
F, Aerts JGJV, Barbieri F, Giusti R, Bria E, Cortinovis D, Grossi
F, et al: Clinicopathologic correlates of first-line pembrolizumab
effectiveness in patients with advanced NSCLC and a PD-L1
expression of ≥50%. Cancer Immunol Immunother. 69:2209–2221. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Debieuvre D, Juergens RA, Asselain B,
Audigier-Valette C, Auliac JB, Barlesi F, Benoit N, Bombaron P,
Butts CA, Dixmier A, et al: Two-year survival with nivolumab in
previously treated advanced non-small-cell lung cancer: A
real-world pooled analysis of patients from France, Germany, and
Canada. Lung Cancer. 157:40–47. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Deng J, Gao M, Gou Q, Xu C, Yan H, Yang M,
Li J, Yang X, Wei X and Zhou Q: Organ-specific efficacy in advanced
non-small cell lung cancer patients treated with first-line
single-agent immune checkpoint inhibitors. Chin Med J (Engl).
135:1404–1413. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Du W, Chen C, Luo LF, He LN, Wang Y, Zhang
X, Zhou Y, Lin Z and Hong S: Optimizing the tumor shrinkage
threshold for evaluating immunotherapy efficacy for advanced
non-small-cell lung cancer. J Cancer Res Clin Oncol. 149:1103–1113.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Garde-Noguera J, Martin-Martorell P, De
Julián M, Perez-Altozano J, Salvador-Coloma C, García-Sanchez J,
Insa-Molla A, Martín M, Mielgo-Rubio X, Marin-Liebana S, et al:
Predictive and prognostic clinical and pathological factors of
nivolumab efficacy in non-small-cell lung cancer patients. Clin
Transl Oncol. 20:1072–1079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hosoya K, Fujimoto D, Morimoto T, Kumagai
T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Matsumoto H, Hirano
K, et al: Clinical factors associated with shorter durable
response, and patterns of acquired resistance to first-line
pembrolizumab monotherapy in PD-L1-positive non-small-cell lung
cancer patients: A retrospective multicenter study. BMC Cancer.
21:3462021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kawachi H, Tamiya M, Tamiya A, Ishii S,
Hirano K, Matsumoto H, Fukuda Y, Yokoyama T, Kominami R, Fujimoto
D, et al: Association between metastatic sites and first-line
pembrolizumab treatment outcome for advanced non-small cell lung
cancer with high PD-L1 expression: A retrospective multicenter
cohort study. Invest New Drugs. 38:211–218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Landi L, D'Incà F, Gelibter A, Chiari R,
Grossi F, Delmonte A, Passaro A, Signorelli D, Gelsomino F, Galetta
D, et al: Bone metastases and immunotherapy in patients with
advanced non-small-cell lung cancer. J Immunother Cancer.
7:3162019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li X, Wang L, Chen S, Zhou F, Zhao J, Zhao
W and Su C: Adverse impact of bone metastases on clinical outcomes
of patients with advanced non-small cell lung cancer treated with
immune checkpoint inhibitors. Thorac Cancer. 11:2812–2819. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma SC, Tang XR, Long LL, Bai X, Zhou JG,
Duan ZJ, Wang J, Fu QJ, Zhu HB, Guo XJ, et al: Integrative
evaluation of primary and metastatic lesion spectrum to guide
anti-PD-L1 therapy of non-small cell lung cancer: Results from two
randomized studies. Oncoimmunology. 10:19092962021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mouritzen MT, Junker KF, Carus A, Ladekarl
M, Meldgaard P, Nielsen AWM, Livbjerg A, Larsen JW, Skuladottir H,
Kristiansen C, et al: Clinical features affecting efficacy of
immune checkpoint inhibitors in pretreated patients with advanced
NSCLC: A Danish nationwide real-world study. Acta Oncol.
61:409–416. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Petrova MP, Eneva MI, Arabadjiev JI, Conev
NV, Dimitrova EG, Koynov KD, Karanikolova TS, Valev SS, Gencheva
RB, Zhbantov GA, et al: Neutrophil to lymphocyte ratio as a
potential predictive marker for treatment with pembrolizumab as a
second line treatment in patients with non-small cell lung cancer.
Biosci Trends. 14:48–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qin A, Zhao S, Miah A, Wei L, Patel S,
Johns A, Grogan M, Bertino EM, He K, Shields PG, et al: Bone
metastases, skeletal-related events, and survival in patients with
metastatic non-small cell lung cancer treated with immune
checkpoint inhibitors. J Natl Compr Cancer Netw. 19:915–921. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rounis K, Makrakis D, Papadaki C,
Monastirioti A, Vamvakas L, Kalbakis K, Gourlia K, Xanthopoulos I,
Tsamardinos I, Mavroudis D and Agelaki S: Prediction of outcome in
patients with non-small cell lung cancer treated with second line
PD-1/PDL-1 inhibitors based on clinical parameters: Results from a
prospective, single institution study. PLoS One. 16:e02525372021.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi Y, Ji M, Jiang Y, Yin R, Wang Z, Li H,
Wang S, He K, Ma Y, Wang Z, et al: A cohort study of the efficacy
and safety of immune checkpoint inhibitors plus anlotinib versus
immune checkpoint inhibitors alone as the treatment of advanced
non-small cell lung cancer in the real world. Transl Lung Cancer
Res. 11:1051–1068. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tamiya M, Tamiya A, Inoue T, Kimura M,
Kunimasa K, Nakahama K, Taniguchi Y, Shiroyama T, Isa S, Nishino K,
et al: Metastatic site as a predictor of nivolumab efficacy in
patients with advanced non-small cell lung cancer: A retrospective
multicenter trial. PLoS One. 13:e01922272018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang T, Cheng J, Fu S, Sun T, Yang K, You
J and Li F: Pretreatment levels of serum alkaline phosphatase are
associated with the prognosis of patients with non-small cell lung
cancer receiving immune checkpoint inhibitors. Oncol Lett.
25:1542023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yao J, Wang Z, Sheng J, Wang H, You L, Zhu
X, Pan H and Han W: Efficacy and safety of combined immunotherapy
and antiangiogenic therapy for advanced non-small cell lung cancer:
A two-center retrospective study. Int Immunopharmacol. 89((Pt A)):
1070332020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yoneda T, Sone T, Koba H, Shibata K,
Suzuki J, Tani M, Nishitsuji M, Nishi K, Kobayashi T, Shirasaki H,
et al: Long-term survival of patients with non-small cell lung
cancer treated with immune checkpoint inhibitor monotherapy in
real-world settings. Clin Lung Cancer. 23:467–476. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zeng H, Huang W, Liu YJ, Huang Q, Zhao SM,
Li YL, Tian PW and Li WM: Development and validation of a nomogram
for predicting prognosis to immune checkpoint inhibitors plus
chemotherapy in patients with non-small cell lung cancer. Front
Oncol. 11:6850472021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee S, Shim HS, Ahn BC, Lim SM, Kim HR,
Cho BC and Hong MH: Efficacy and safety of atezolizumab, in
combination with etoposide and carboplatin regimen, in the
first-line treatment of extensive-stage small-cell lung cancer: A
single-center experience. Cancer Immunol Immunother. 71:1093–1101.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou J, Lu X, Zhu H, Ding N, Zhang Y, Xu
X, Gao L, Zhou J, Song Y and Hu J: Resistance to immune checkpoint
inhibitors in advanced lung cancer: Clinical characteristics,
potential prognostic factors and next strategy. Front Immunol.
14:10890262023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tanaka K, Tanabe H, Sato H, Ishikawa C,
Goto M, Yanagida N, Akabane H, Yokohama S, Hasegawa K, Kitano Y, et
al: Prognostic factors to predict the survival in patients with
advanced gastric cancer who receive later-line nivolumab
monotherapy-The Asahikawa Gastric Cancer Cohort Study (AGCC).
Cancer Med. 11:406–416. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gambale E, Palmieri VE, Rossi V, Francini
E, Bonato A, Salfi A, Galli L, Mela MM, Pillozzi S and Antonuzzo L:
Bone metastases in renal cell carcinoma: Impact of immunotherapy on
survival. Cancer Diagnosis Progn. 3:538–542. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rebuzzi SE, Signori A, Banna GL, Maruzzo
M, De Giorgi U, Pedrazzoli P, Sbrana A, Zucali PA, Masini C,
Naglieri E, et al: Inflammatory indices and clinical factors in
metastatic renal cell carcinoma patients treated with nivolumab:
the development of a novel prognostic score (Meet-URO 15 study).
Ther Adv Med Oncol. 13:175883592110196422021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Velev M, Dalban C, Chevreau C, Gravis G,
Negrier S, Laguerre B, Gross-Goupil M, Ladoire S, Borchiellini D,
Geoffrois L, et al: Efficacy and safety of nivolumab in bone
metastases from renal cell carcinoma: Results of the
GETUG-AFU26-NIVOREN multicentre phase II study. Eur J Cancer.
182:66–76. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shimizu T, Miyake M, Nishimura N, Inoue K,
Fujii K, Iemura Y, Ichikawa K, Omori C, Tomizawa M, Maesaka F, et
al: Organ-specific and mixed responses to pembrolizumab in patients
with unresectable or metastatic urothelial carcinoma: A multicenter
retrospective study. Cancers (Basel). 14:17352022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Makrakis D, Talukder R, Lin GI,
Diamantopoulos LN, Dawsey S, Gupta S, Carril-Ajuria L, Castellano
D, de Kouchkovsky I, Koshkin VS, et al: Association between sites
of metastasis and outcomes with immune checkpoint inhibitors in
advanced urothelial carcinoma. Clin Genitourin Cancer.
20:e440–e452. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Raggi D, Giannatempo P, Marandino L,
Pierantoni F, Maruzzo M, Lipari H, Banna GL, De Giorgi U, Casadei
C, Naglieri E, et al: Role of bone metastases in patients receiving
immunotherapy for pre-treated urothelial carcinoma: The
multicentre, retrospective meet-URO-1 Bone Study. Clin Genitourin
Cancer. 20:155–164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hoshi Y, Shirakura S, Yamada M, Sugiyama
T, Koide N, Tamii S, Kamata K, Yokomura M, Osaki S, Ohno T, et al:
Site of distant metastasis affects the prognosis with
recurrent/metastatic head and neck squamous cell carcinoma patients
treated with nivolumab. Int J Clin Oncol. 28:1139–1146. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Bilen MA, Shabto JM, Martini DJ, Liu Y,
Lewis C, Collins H, Akce M, Kissick H, Carthon BC, Shaib WL, et al:
Sites of metastasis and association with clinical outcome in
advanced stage cancer patients treated with immunotherapy. BMC
Cancer. 19:8572019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Botticelli A, Cirillo A, Scagnoli S,
Cerbelli B, Strigari L, Cortellini A, Pizzuti L, Vici P, De
Galitiis F, Di Pietro FR, et al: The agnostic role of site of
metastasis in predicting outcomes in cancer patients treated with
immunotherapy. Vaccines (Basel). 8:2032020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Qin Q, Jun T, Wang B, Patel VG, Mellgard
G, Zhong X, Gogerly-Moragoda M, Parikh AB, Leiter A, Gallagher EJ,
et al: Clinical factors associated with outcome in solid tumor
patients treated with immune-checkpoint inhibitors: a single
institution retrospective analysis. Discov Oncol. 13:732022.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA,
Atkins MB, Leming PD, et al: Five-year survival and correlates
among patients with advanced melanoma, renal cell carcinoma, or
non-small cell lung cancer treated with nivolumab. JAMA Oncol.
5:1411–1420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Debien V, De Caluwé A, Wang X,
Piccart-Gebhart M, Tuohy VK, Romano E and Buisseret L:
Immunotherapy in breast cancer: An overview of current strategies
and perspectives. NPJ Breast Cancer. 9:72023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sridaran D, Bradshaw E, DeSelm C,
Pachynski R, Mahajan K and Mahajan NP: Prostate cancer
immunotherapy: Improving clinical outcomes with a multi-pronged
approach. Cell Rep Med. 4:1011992023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li HS, Lei SY, Li JL, Xing PY, Hao XZ, Xu
F, Xu HY and Wang Y: Efficacy and safety of concomitant
immunotherapy and denosumab in patients with advanced non-small
cell lung cancer carrying bone metastases: A retrospective chart
review. Front Immunol. 13:9084362022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Qiang H, Lei Y, Shen Y, Li J, Zhong H,
Zhong R, Zhang X, Chang Q, Lu J, Feng H, et al: Pembrolizumab
monotherapy or combination therapy for bone metastases in advanced
non-small cell lung cancer: A real-world retrospective study.
Transl Lung Cancer Res. 11:87–99. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zheng Y, Wang PP, Fu Y, Chen YY and Ding
ZY: Zoledronic acid enhances the efficacy of immunotherapy in
non-small cell lung cancer. Int Immunopharmacol. 110:1090302022.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ishikawa T: Differences between zoledronic
acid and denosumab for breast cancer treatment. J Bone Miner Metab.
41:301–306. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dieli F, Gebbia N, Poccia F, Caccamo N,
Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano
C, et al: Induction of gammadelta T-lymphocyte effector functions
by bisphosphonate zoledronic acid in cancer patients in vivo.
Blood. 102:2310–2311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Iwasaki M, Tanaka Y, Kobayashi H,
Murata-Hirai K, Miyabe H, Sugie T, Toi M and Minato N: Expression
and function of PD-1 in human γδ T cells that recognize
phosphoantigens. Eur J Immunol. 41:345–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wong BR, Josien R, Lee SY, Sauter B, Li
HL, Steinman RM and Choi Y: TRANCE (tumor necrosis factor
[TNF]-related activation-induced cytokine), a new TNF family member
predominantly expressed in T cells, is a dendritic cell-specific
survival factor. J Exp Med. 186:2075–2080. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ahern E, Smyth MJ, Dougall WC and Teng
MWL: Roles of the RANKL-RANK axis in antitumour
immunity-implications for therapy. Nat Rev Clin Oncol. 15:676–693.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ahern E, Harjunpää H, O'Donnell JS, Allen
S, Dougall WC, Teng MWL and Smyth MJ: RANKL blockade improves
efficacy of PD1-PD-L1 blockade or dual PD1-PD-L1 and CTLA4 blockade
in mouse models of cancer. Oncoimmunology. 7:e14310882018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Batlle E and Massagué J: Transforming
growth factor-β signaling in immunity and cancer. Immunity.
50:924–940. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nixon BG, Gao S, Wang X and Li MO: TGFβ
control of immune responses in cancer: A holistic immuno-oncology
perspective. Nat Rev Immunol. 23:346–362. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Trivedi T, Pagnotti GM, Guise TA and
Mohammad KS: The role of TGF-β in bone metastases. Biomolecules.
11:16432021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang B, Bai J, Tian B, Chen H, Yang Q,
Chen Y, Xu J, Zhang Y, Dai H, Ma Q, et al: Genetically engineered
hematopoietic stem cells deliver TGF-β Inhibitor to enhance bone
metastases immunotherapy. Adv Sci (Weinh). 9:e22014512022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu W, Yang Y, Hu Z, Head M, Mangold KA,
Sullivan M, Wang E, Saha P, Gulukota K, Helseth DL, et al:
LyP-1-modified oncolytic adenoviruses targeting transforming growth
factor β inhibit tumor growth and metastases and augment immune
checkpoint inhibitor therapy in breast cancer mouse models. Hum
Gene Ther. 31:863–880. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khan KA and Kerbel RS: Improving
immunotherapy outcomes with anti-angiogenic treatments and vice
versa. Nat Rev Clin Oncol. 15:310–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Brest P, Mograbi B, Pagès G, Hofman P and
Milano G: Checkpoint inhibitors and anti-angiogenic agents: A
winning combination. Br J Cancer. 129:1367–1372. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xie X, Zhou M, Wang L, Wang F, Deng H,
Yang Y, Sun N, Li R, Chen Y, Lin X, et al: Effects of combining
immune checkpoint inhibitors and anti-angiogenic agents on bone
metastasis in non-small cell lung cancer patients. Hum Vaccin
Immunother. 19:22413102023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Castello A and Lopci E: Response
assessment of bone metastatic disease: Seeing the forest for the
trees RECIST, PERCIST, iRECIST, and PCWG-2. Q J Nucl Med Mol
Imaging. 63:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yin C, Wang M, Wang Y, Lin Q, Lin K, Du H,
Lang C, Dai Y and Peng X: BHLHE22 drives the immunosuppressive bone
tumor microenvironment and associated bone metastasis in prostate
cancer. J Immunother Cancer. 11:e0055322023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nagasaki J, Ishino T and Togashi Y:
Mechanisms of resistance to immune checkpoint inhibitors. Cancer
Sci. 113:3303–3312. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Butterfield LH and Najjar YG:
Immunotherapy combination approaches: Mechanisms, biomarkers and
clinical observations. Nat Rev Immunol. December 6–2023.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|