Cell senescence is a process that permanently
arrests cellular proliferation, which not only triggers tissue
remodelling during organismal development and after body injury but
also leads to inflammation, tumourigenesis and decreased
regenerative function of tissues (1-3).
Cellular senescence is classified into the following types: i)
Replicative senescence; ii) stress-induced premature senescence
(SIPS), including DNA damage- and oxidative stress-induced
senescence; iii) oncogene-induced senescence (OIS); iv) paracrine
senescence; v) therapy-induced senescence (TIS); vi) mitochondrial
dysfunction-associated senescence; and vii) epigenetically induced
senescence (3,4).
Although cell senescence is generally considered
beneficial, malignancy is also associated with senescence, both of
which are caused by accumulation of time-dependent cellular damage
(5). Senescent cells can secrete
cytokines [including interleukin (IL)-6, IL-8, tumour necrosis
factor (TNF)α and interferon-(IFN)γ], chemokines [C-C motif ligand
2 and C-X-C motif chemokine ligand (CXCL)1], growth factors
(granulocyte-macrophage colony-stimulating factor) and matrix
metalloprotein (matrix metalloproteinase 9), into the ageing
environment and gradually induce a senescent-associated secretory
phenotype (SASP) (6,7). In tissues, the short-term
accumulation of aged cells is beneficial for renewing the in
vivo environment; however, the long-term accumulation of aged
tumour cells may induce disequilibrium in an organism or local
organs and gradually result in the formation of an aged tumour
microenvironment (TME) (8). Among
lymphatic system malignancies, lymphoma development, including
histopathology, clinical treatment and prognosis, differs between
young and old adults (9). In
Hodgkin's lymphoma (HL), Reed-Sternberg (RS) cells retain the
characteristics of senescent cells, staining specifically for
senescence-associated β-galactosidase (SA-β-gal) and exhibiting
abnormal expression of cell cycle inhibitors
p21Cip1/p16INK4a/p53, and negative Ki-67
expression. In addition, RS-like cells exhibit a SASP in their
microenvironments (10).
Drug resistance is one of the main challenges
encountered by clinicians in lymphoma treatment. When treated with
a single drug, tumours can resist multiple chemically unrelated
drugs, resulting in multidrug resistance (MDR) (11). Common factors involved in MDR
include: i) Expression of efflux transporters, such as
P-glycoprotein [P-gp; also called ATP binding cassette subfamily B
member 1 (ABCB1)], ATP binding cassette subfamily G member 2
[ABCG2, breast cancer resistance (BCR) protein], and multidrug
resistance-associated protein 1. P-gp, encoded by MDR1, has
a crucial role in lymphoma multidrug resistance (12). Anthracycline resistance is
mediated by the overexpression of P-gp, a transmembrane protein
that functions as an efflux pump, actively transporting
anthracyclines out of cancer cells, thereby reducing their
intracellular concentration and efficacy (13). ii) Tumour stem-like cells (also
known as side populations) (14).
However, the additional mechanisms underlying lymphoma resistance
remain to be explored. Hanahan (15) reported that tumour cells undergo
transient ageing in cases of therapeutic resistance. These
senescent tumour cells appear dormant and evade targeted drug
attacks (16). In such cases,
SASP have a crucial role in the development of lymphoma resistance
(17). In murine models of
Burkitt's lymphoma, DNA damage has been found to induce the release
of paracrine factors [such as IL-6 and metalloproteinase tissue
inhibitor 1 (Timp-1)], which contribute to lymphoma cell survival
in the TME after chemotherapy and create a 'chemoresistant niche'
(18). These studies suggest that
the ageing microenvironment, in which the SASP is involved, may
have a critical role in the mechanisms of chemotherapy resistance
in lymphoma.
The present review, from the perspective of
preclinical research and clinical applications, focuses on the
interactions between the ageing TME and lymphocyte carcinogenesis
and discusses how the ageing microenvironment is reprogrammed and
promotes the development of lymphoma resistance. Finally,
opportunities and challenges in solving the problem of drug
resistance in lymphoma were discussed from the perspective of an
ageing microenvironment.
The expression of SASP markers, such as cytokines
TNF-α/IL-6/IL-8 and microRNA-155, -16 and -93, have been detected
in memory B cells (19). Compared
to young mice, older mice showed considerable differences in B-cell
composition in lymphatic tissues. This part of the B lymphocyte
lineage is termed age-associated B cells, which secrete TNF-α and
suppress pro-B-cell survival and B lymphopoiesis (20,21).
Changes in the ageing host and senescent cell
metabolism can seriously affect T-cell development, maintenance and
function (22). For instance, T
cells expressing T cell immunoglobulin and mucin domain-containing
protein 3 include a subset of senescent cells arrested in the G1/S
phase (23). In old mouse models,
memory CD8+ T cells with
CD44lowCD62Lhigh express high levels of stem
cell antigen-1 (24,25). Furthermore, γδ T-cells exert the
function of killing lymphoma cells in the TME. However, in an
ageing microenvironment, a large number of changes in the
composition of the γδ T-cell pool in peripheral lymph nodes (pLNs)
may lead to an imbalance of γδ T-cell responses in tumours and
accelerate tumour growth. Ageing alters the function of the T cell
receptor δ chain and the clonal structure of γδ T-cell subsets. In
old mice, increased IL-7 expression mediates the expansion of the
γδ17 T-cell compartment, which is related to lymphoma growth
(26). Furthermore, human type 17
T-helper (Th17) cells possess stem cell-like properties and
intervention with Th17 stemness may help address Th17-associated
drug resistance, microenvironmental disturbances and organ
disabilities (27). Failure of
the T-cell metabolism leads to the accumulation of circulating
cytokines that act as systemic inducers of ageing. Immune
dysregulation ultimately leads to T cell-derived lymphoid tissue
neoplasms (28).
There is a close relationship between abnormal
NK-cell function, carcinogenesis and the ageing microenvironment. A
key feature of NK cells is the expression of human leukocyte
antigen class I-specific receptors, which manifests as the
downregulation of NK group 2A (NKG2A) receptor and upregulation of
killer cell immunoglobulin-like receptor (KIR) family members.
However, the ageing microenvironment affects the function and
phenotype of KIR/NKG2A repertoires, which cause abnormal or
cancerous functions in NK cells (29). In addition, NK cells from patients
with germline gain-of function mutations in PIK3CD (encoding the
PI3K p110δ catalytic subunit) exhibit abnormal differentiation,
which is associated with the overexpression of
immunosenescence/depletion-related markers. Immunosenescence may
lead to a decline in the ability of the immune system to monitor
and clear abnormal cells, thus providing a more favourable
environment for NK cells to become cancerous (30). In summary, the complex mechanism
between NK-cell cancer and the immunosenescent microenvironment
needs to be further explored, as it is crucial for the treatment
and prevention of NK cell-derived lymphoma.
SA-β-gal is widely recognised as a critical
biomarker for senescent cells, including lymphoma. Milanovic et
al (32) selected SA-β-gal
and 5-bromo-2'-deoxyuridine/propidium iodide (BrdU/PI) as markers
to analyse senescent cells with stemness in B-cell lymphoma in both
human cell lines and mouse models. Senescent lymphoma cells were
detected by SA-β-gal staining in the ageing environment of relapsed
or refractory (R/R) diffuse large B-cell lymphoma with elevated
expression of the SENEX gene (33). SA-β-gal expression is important in
the study of drug resistance mechanisms, as it allows researchers
to identify whether cellular senescence has been induced and
understand how senescent lymphoma cells resist treatment, which may
potentially be applied in lymphoma treatment.
The SASP has a critical role in lymphoma
progression, enhances pro-cancerous effects on surrounding lymphoma
cells and tissues via paracrine and autocrine pathways, and may
affect the immune response in human cell lines and mouse models
(34). SASPs act as both
promoters and inhibitors of lymphoma development. Certain SASP
components promote tumour-cell formation and development; however,
other components may have a role in the immune response, helping to
fight cancer cells. All these functions of the SASP components
depend on the antitumour drug action time, drug intensity and
immune microenvironment of the organisms involved. The relationship
between SASP and lymphoma is a complex area of research and
scientists are working to develop new treatments or intervention
strategies to improve treatment outcomes in lymphoma cases
involving drug resistance (35).
Trimethylation of histone H3 lysine 9 (H3K9me3) and
the H3K9me3-specific histone methyltransferase SUV39H1 have been
reported as biomarkers for senescent lymphoma cells in human cell
lines and mouse models (36).
H3K9me-mediated ageing blocks the response to the oncogene
Ras. Loss of SUV39H1 activates Ras expression and
enhances lymphoma aggressiveness (36). In addition, TIS relies on SUV39H1,
which promotes lymphoma (37).
Overexpressed MYC is a well-known lymphoma oncogene that can
induce self-renewal of stem cells and prevent cell differentiation
(38-40). The function of MYC is limited by
TGF-β regulation and the development of lymphoma is inhibited by
SUV39H1-dependent cellular senescence (41).
RAS represents a class of signalling proteins
belonging to the small GTPase family and includes various subtypes,
such as H-RAS, K-RAS and N-RAS (42). RAS proteins have key regulatory
roles in normal cell growth and development; however, aberrant RAS
signalling pathway activity has been implicated in various tumours
and other diseases. RAS is also involved in the regulation of
cellular senescence in human cell lines and mouse models (43-45).
The ABC protein family is closely associated with
drug resistance in tumours. Lymphoma stem-like cells
(CD34+ and CD44+) with high ABCG2 expression
exhibited a senescent phenotype in mouse models (37). Multidrug resistance protein 4
(ABCC4) is overexpressed in human NK/T-cell lymphoma (NKTCL) cells,
which modifies the sensitivity of chemotherapy to epirubicin and
cisplatin and may be a functional therapeutic target (48). In senescent cells, ATP-binding box
subfamily B member 4 mediates the synthesis and release of small
extracellular vesicles and enhances drug resistance in cancer cells
(49). ABCA1 participates in the
export of free cholesterol by specific cells, such as macrophages
and foam cells, into the lymphoma microenvironment, which in turn
promotes lymphoma cell proliferation (50). ABCG2 enhances the multidrug
resistance profile of human NKTCL side-population cells (51). Thus, ABC protein family members
combined with senescence-associated biomarkers (e.g. SA-β-gal), may
be used as indicators to evaluate senescence-related drug-resistant
traits.
The relationship between senescence and lymphoma
resistance involves complex signalling pathways. It should be noted
that lymphomas are a group of highly heterogeneous diseases; thus,
different molecular subtypes and individual idiosyncrasies may have
different regulatory mechanisms involving their signalling
pathways. Therefore, the mechanisms underlying the signalling
pathways involved in cellular senescence in lymphoma must be
studied in depth according to specific lymphoma subtypes. Certain
key signalling pathways that may have critical roles in
senescence-related lymphoma resistance were discussed in the
following sections.
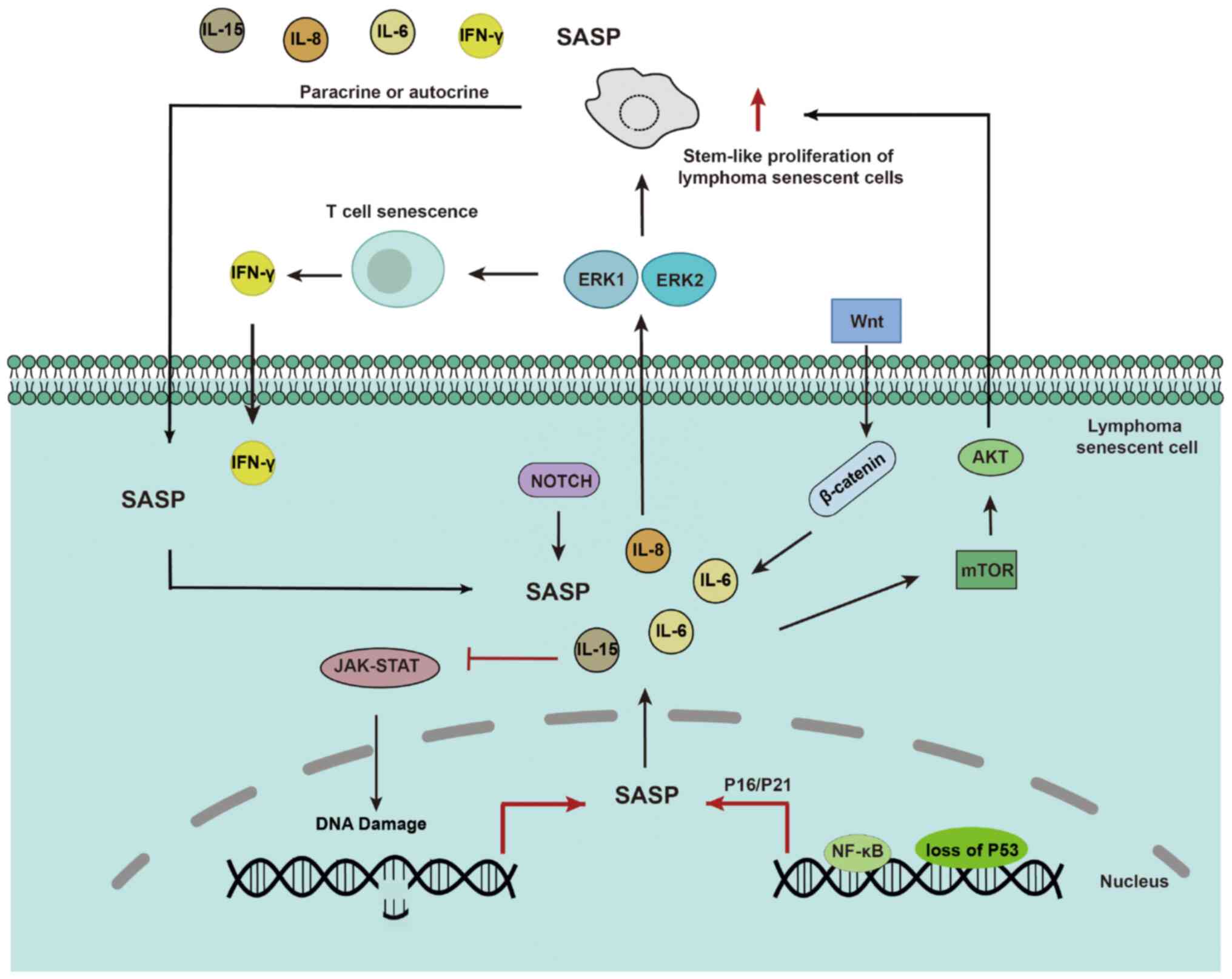
The SASP depends on paracrine mechanisms involving
interleukins IL-6 and IL-8 to induce epithelial-mesenchymal
transition and aggressive malignancy. Loss of p53 exacerbates the
paracrine activity of the SASP (47). Proteomic analyses have identified
NF-κB as a critical regulator of SASP in senescent chromatin.
Subunit p65 of NF-κB is a major transcription factor and
accumulates on senescent chromatin. NF-κB inhibition triggers
escape from immune recognition by NK cells and bypasses the
senescence mechanism by collaborating with inactivated p53. In
mouse lymphoma models, NF-κB inhibition alters the microenvironment
of treatment-induced senescence by controlling SASP, promoting drug
resistance and early recurrence of lymphoma, and shortening
survival time (17). In HL, RS
cellular senescence with abnormal expression of SA-β-gal and
p21Cip1/p16INK4a/p53 may also be the origin
of the pro-inflammatory microenvironment and gradual development of
SASP and promote HL drug resistance. NF-κB inhibitors (such as
JSH-23 and curcumin) decrease the secretion of IL-6 from RS-like
cells in HL (10).
The Wnt signalling pathway has a critical role in
cell fate determination and differentiation. In senescent lymphoma
cells, the aberrant activation of Wnt signalling may lead to the
maintenance of a stem cell-like state, inhibiting normal cell
differentiation. Abnormal Wnt/β-catenin signalling promotes tumour
stem-like cell proliferation and thus has a critical role in tumour
resistance and treatment responses (52). Wnt signalling has been reported to
participate in the SASP-mediated proliferation of stem-like cells
in the B-cell lymphoma SASP (32). The application of Wnt protein
pathway inhibitors can potentially enable more precise and
personalised treatment of drug-resistant lymphoma.
Extracellular signal-regulated protein kinases
(ERK), such as ERK1 and ERK2, act on cytotoxic T cells (CTLs) and
participate in IFN-mediated resistance of T lymphoma cells
(53). Furthermore, numerous
aneuploid senescent cells often exist as double-hit or
double-expressing diffuse large B-cell lymphomas (DH/DE-DLBCLs)
after treatment. This mechanism of mitotic escape leads to drug
resistance. These aneuploid senescent cells increase ERK/MAPK
activity via Bruton's tyrosine kinase (BTK) signalling and the
chronically active BCR pathway, resulting in elevated metabolism
(54).
The PI3K/AKT/mTOR pathway is a potent cell
proliferation and growth signalling pathway that is also involved
in the regulation of the cell cycle and apoptosis. In lymphoma,
overactivation of this pathway is frequently observed under
repeated stress conditions, leading to abnormal cell proliferation
(55,56). Senescent cells escape the treated
DH/DE-DLBCL microenvironment and acquire improved metabolic
properties via the AKT/mTOR pathway, resulting in drug resistance
(54). Downregulation of this
pathway may help to restore the apoptotic capacity of cells and
regulate the cell cycle, thereby inhibiting lymphoma-cell
proliferation.
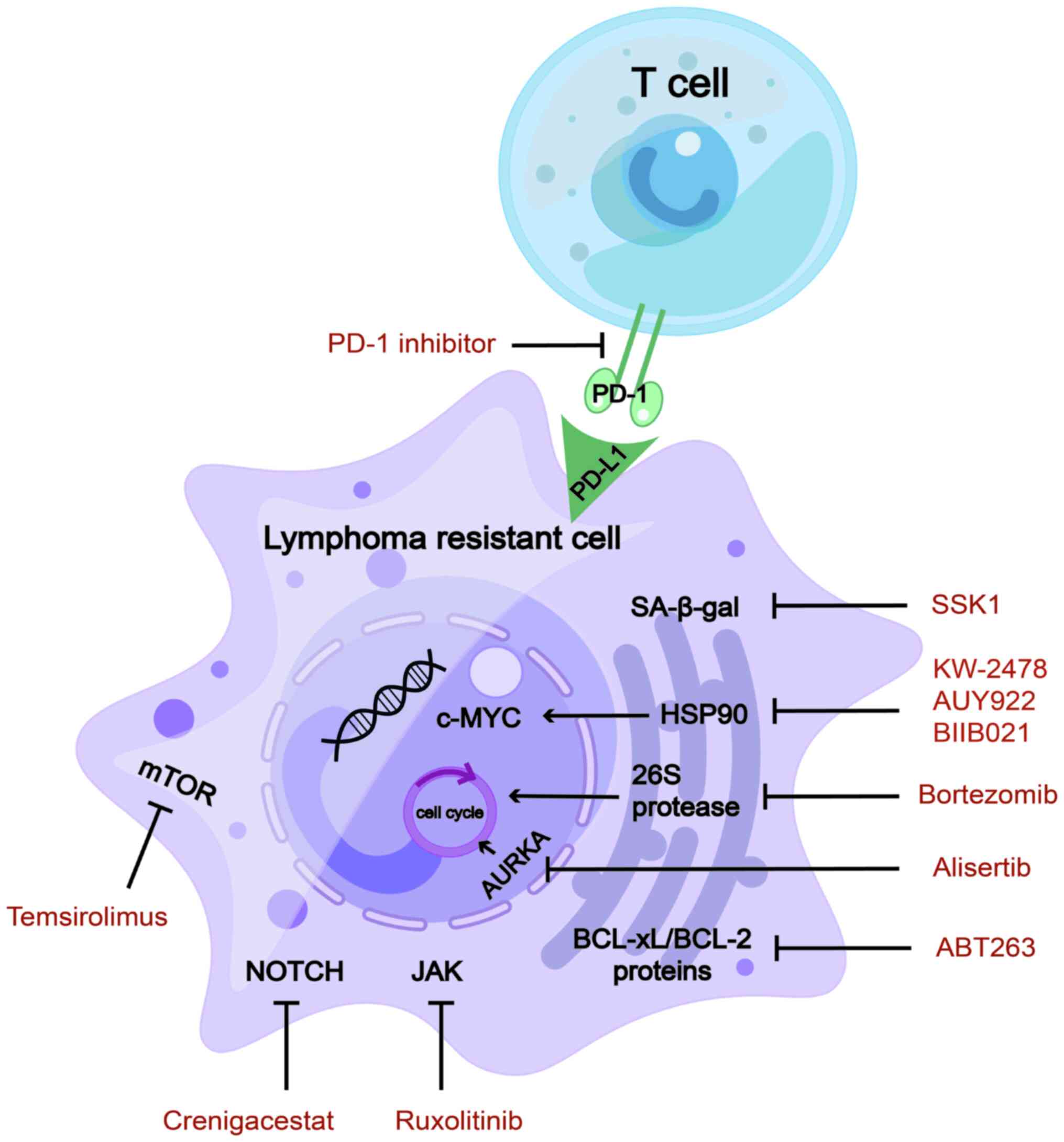
In lymphoma, the aberrant activation of the Notch
signalling pathway is closely associated with disease onset and
progression. In senescent lymphoma cells, Notch signalling cascades
with fibroblast growth factor and Wnt signalling to maintain
self-renewal of tumour stem cells and remodel the TME (57). Currently, research on the
mechanism of action of Notch in lymphoma-cell senescence is
expanding, and future studies will help to reveal the underlying
molecular mechanisms in greater detail.
The JAK-STAT signalling pathway is a key pathway
involved in cell proliferation, differentiation and immune
responses. Senescent lymphoma cells may evade detection and attack
by the immune system by manipulating the JAK-STAT signalling
pathway (58). The JAK/3
signalling pathway is required for lymphoma cell survival after
doxorubicin (DOX) treatment in vivo and in vitro
(18). Certain cytokines have a
role in JAK-STAT signalling. IL-15 stimulates JAK3-STAT5 signalling
and inhibits the expression of DNA damage response genes, thus
delaying CD8+ T-cell senescence (59). IL-6 reverses DOX resistance in
nasal NKTCL by downregulating ABCC4 and inactivating the
JAK2/STAT3/NF-κB/P65 pathway (Fig.
1) (60). Therefore,
inhibition of the JAK-STAT pathway by interfering with the
expression of SASP to kill drug-resistant lymphoma cells with a
senescent phenotype is a promising direction for future
research.
Cell and animal models have revealed several
mechanisms through which senescent cells develop drug resistance,
facilitating the development of additional senolytic drugs based on
these models. Different types of senescence have been observed in
various cellular and animal models. In this chapter, various
research models for lymphoma senescence-associated resistance are
discussed.
For TIS, the induction time has a critical role for
constructing cell models of lymphoma senescence-associated
resistance. In clinical patients, after completion of a cancer
treatment regimen, TIS in tumours and normal tissues can gradually
develop within 10 days to 6 weeks (31). In an in vitro assay, tumour
cell lines treated with various chemotherapeutic drugs were used as
cell models to study senescence-associated resistance. The
senescent phenotype can be identified via SA-β-gal assays and
SASP-related cytokine detection (such as IL-4, IL6, IL-1β)
(61). One study reported that
β-gal-positive cells could be induced in non-small cell lung cancer
cell lines after 2-6 days of treatment with cisplatin (62). To date, limited research has been
conducted on TIS-cell lymphoma models.
For SIPS, excision repair cross complement 1
knockout/deletion mice have been used as a DNA-damaging model to
study senolytic drugs (63,64). In OIS, BUB1 mitotic checkpoint
serine/threonine kinase B (BubR1) encodes a vital component of
mitotic spindle assembly. The BubR1H/H mouse is an ideal model of
cellular senescence (63,65). Mdr2-/- mice treated
with navitoclax (ABT263) are good models for primary lymphoma
resistance research (66).
Telomerase knockout mice are ideal models for studying
senescence-associated mechanisms of immune escape (67). In the case of TIS, primary
Eμ-Myc transgenic Bcl2-overexpressing lymphomas treated with
adriamycin are a recognised mouse model for the study of TIS of
B-cell lymphoma. SUV39H1 inhibits the conversion of normal cells
into induced pluripotent stem cells. SUV39H1-deficient
Eμ-Myc mice have been demonstrated to be a suitable model of
senescence-associated resistance in B-cell lymphoma (32,68).
In one study, a patient-derived DLBCL xenograft
model (PDX) was successfully established to screen for drug
sensitivity and explore resistance mechanisms (69). Cell-derived mouse xenograft models
have been frequently used to study the mechanisms underlying
drug-resistant lymphoma (70).
Multiple lymphoid organs have been designed in the
field of immune engineering, such as the engineering of bone marrow
and thymus tissue (71). The lack
of understanding of the factors that regulate lymphoma resistance
and the establishment of predictors is mainly due to the need for
in vitro lymphoma models to accurately study the lymphoma
microenvironment. In contrast to other tumour types, the
development of lymphoma organoids is slow (72).
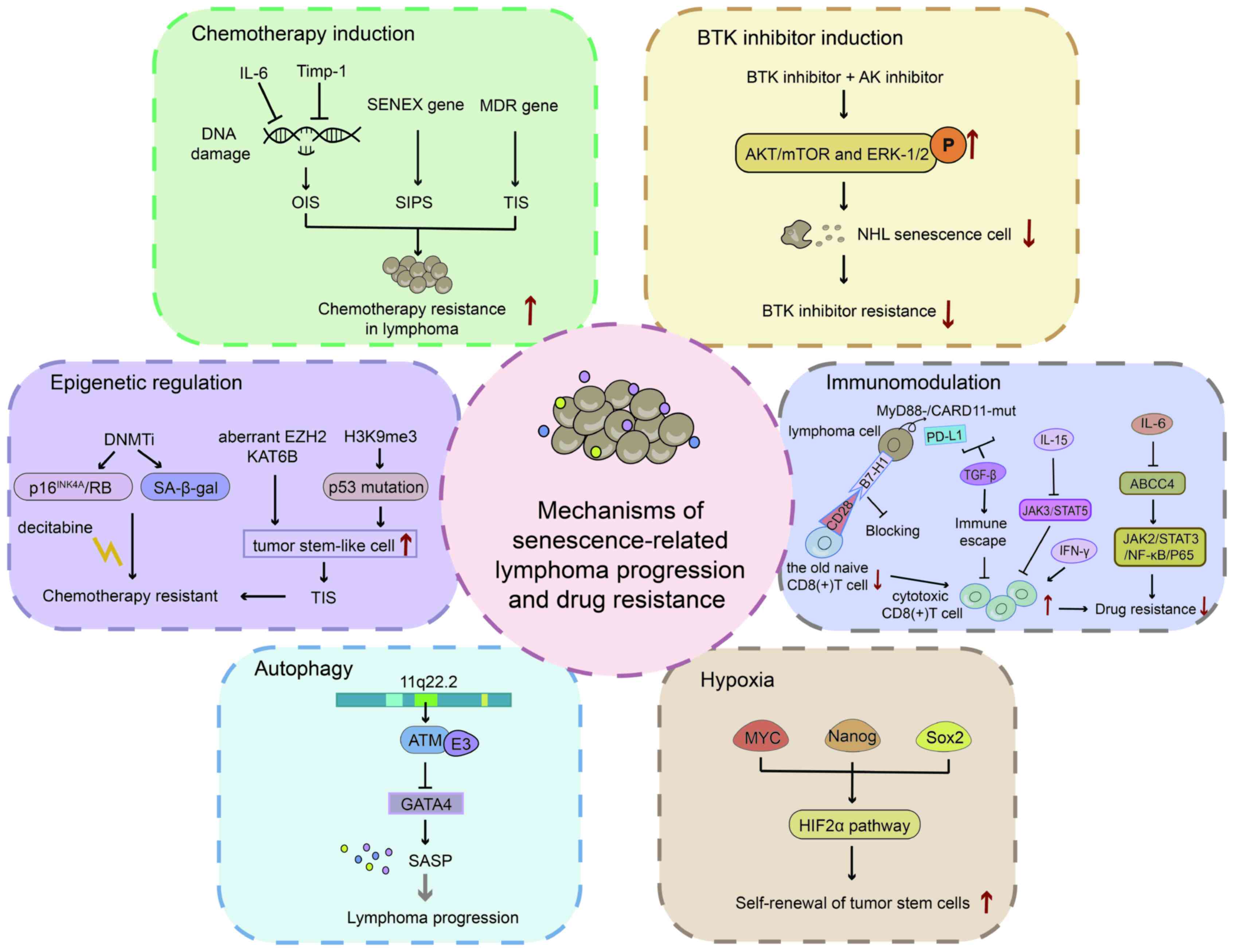
Different chemotherapeutic drugs have different
mechanisms of action. Moderate doses of chemotherapy increase the
likelihood of SASP and higher doses can directly lead to cell death
(75). The autocrine IL-6
signalling loop is induced when the oncogene RAS is activated, and
this autocrine loop enhances OIS (36,47). Gilbert and Hemann (18) used mature Burkitt's lymphoma mouse
models to demonstrate that the paracrine factors recombinant IL-6
and Timp-1 in the ageing microenvironment are released into the
thymus to resist tumour cell DNA damage, resulting in minimal
residual tumour lesions. Researchers have suggested that patients
with extranodal NKTCL exhibit primary resistance to anthracycline
regimens, mainly because of the overexpression of the MDR
gene (76). Increasing evidence
has confirmed that DOX can induce an ageing microenvironment,
leading to lymphoma resistance. For instance, in DLBCL, SIPS can
activate SENEX and mediate DOX resistance (33,77). Additional ageing
microenvironment-related drug resistance mechanisms in various
types of lymphomas need to be further explored.
Specific inhibitors targeting B-cell
receptor-associated kinases (BAKs), such as BTK and PI3K, have
revolutionised the treatment of B lymphoid malignancies. Evidence
suggests that BAK inhibitors are a class of drugs that modulate the
cancer (haematopoietic) microenvironment (78,79). However, at times, ibrutinib alone
has a poor effect in the treatment of DH/DE-DLBCL. This may be
related to the formation of drug-resistant senescent cells in the
TME. Alisertib is a senolytic aurora kinase (AK) inhibitor that
functions as a senolytic (80).
This can disrupt the escape mechanism of the immune
microenvironment and block senescent cells. Thus, a BTK inhibitor
combined with an AK inhibitor may overcome drug resistance in
DH/DE-DLBCL (54).
In the treatment of certain peripheral T-cell
lymphomas (PTCL), such as NKTCL, drug resistance often develops to
cyclophosphamide, DOX, vincristine and prednisone (CHOP) regimens
containing DOX. Furthermore, asparaginase-containing regimens, such
as cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) or
pegaspargase, gemcitabine and oxaliplatin (P-Gemox) are often
chosen by clinicians (81).
This may be because asparaginase does not depend on
classical resistance mechanisms; for example, MDR-associated ABC
family members lead to drug resistance. Asparagine has long been
considered a tumour metabolite (82). It is related to mTORC1 activity
and intracellular asparagine can be exchanged with extracellular
amino acids, thereby regulating the uptake of amino acids,
particularly serine, arginine, and histidine, which are important
regulators of amino acid homeostasis, anabolism and the
proliferation of cancer cells (83). Because asparaginase, the
cornerstone of extranodal NKTCL and acute lymphoblastic leukaemia
(ALL) therapy, is irreplaceable, exploring the mechanisms involved
in its action is important (84).
Decitabine (5-aza-CdR), a DNA methyltransferase
inhibitor, was reported to inhibit the progression of anaplastic
large cell lymphoma (ALCL) and promote the expression of
p16INK4A in the retinoblastoma protein and SA-β-gal
pathways (87). This may be a
crucial mechanism in decitabine treatment-resistant ALCL (88).
Zeste homologue 2 is a histone methyltransferase
enhancer whose aberrant expression directs cells towards the cancer
stem cell state by regulating the balance between self-renewal and
cell differentiation (89).
Histone acetyltransferases (KATs) have essential
roles in various organisms. KAT6A (MOZ or MYST3) and KAT6B (MORF or
QKF) belong to the KAT family of oncogenes that inhibit cellular
senescence. Allele deletion of KAT6A prolongs the median survival
of Myc transgenic mice with lymphoma. Selective inhibitors
of KAT6B, such as WM-1119, suppress lymphoma development in mice.
These results suggest that KAT6A and KAT6B inhibitors may be
effective therapeutic agents for lymphoma (90,91).
However, various drugs are still in preclinical
stages. Epigenetic modifications involving H3K9me3 were indicated
to drive stem cell-like functions in B-cell lymphomas in a
senescent model employing Eμ-Myc transgenic mice.
Specifically, H3K9me3 modifications or p53 mutations interfere with
cellular senescence in lymphoma and cause cells to re-enter the
cell cycle, which manifests as a stem cell-like function in
lymphomas with the failure of chemotherapy-induced senescence
(32). In addition,
senescence-associated epigenetic genes mediated by H3K9me3 in mice
helped successfully predict the prognosis of patients with lymphoma
(92). Furthermore, RNA
modifications may also influence stem cell function. For instance,
increased m6A reader insulin-like growth factor 2 mRNA binding
protein 2 expression determines the transcriptional level and
function of hematopoietic stem cells (HSCs) (93).
T-cell immune responses are compromised in ageing
environments. B7-H1, a member of the B7-family, is highly expressed
in older naïve CD8+ T cells and is considered to
negatively regulate CD8+ T cells. In lymphoma
immunotherapy, CD8+ CTLs are the main immune cells that
attack tumours. The downregulation of CD8+ T cells in
ageing environments is a critical reason for unsuccessful treatment
(94). Adaptive T-cell immunity
controls senescence-prone myeloid differentiation factor 88 (MyD88)
or caspase recruitment domain-containing protein 11 (CARD11)-mutant
B-cell lymphomas (95).
Programmed cell death protein 1 (PD-1) inhibitors
are effective immunotherapeutic drugs that improve the prognosis of
patients with relapsed/refractory lymphoma (96). Senescent cells express the immune
checkpoint protein PD-1 ligand 1 (PD-L1). PD-L1-containing cells
are sensitive to T-cell surveillance and exhibit resistance even in
the presence of SASPs. Blocking PD-L1 and eliminating senescent
cells using immune checkpoints represent promising therapeutic
strategies (97). Furthermore,
PD-1 is highly expressed in the effector memory T cells of older
mice, exhibiting proliferative hyporesponsiveness (98). In aged mice, blocking the
PD-1/PD-L1 pathway may restore T-cell function (99). Furthermore, a study investigating
the reduction in immune-related adverse events (irAEs) caused by
immune checkpoint blockade (ICB) reported that anti-PD-1 therapy
induces irAE-like multiple organ dysfunction in older mice with
tumours, but not in young mice. However, ICB-induced irAEs are
mitigated by blocking CD4+ T cell-dependent IL-21 or
CXCL13 activity and by B-cell depletion (100).
Chemotherapy and radiation therapy induce
tumour-cell senescence and contribute to the generation of SASPs,
which affect the immune response. Numerous senescent cells secrete
excessive amounts of SASP, leading to drug resistance. Chimeric
antigen receptor T-cell therapy is a new treatment option to
overcome this resistance (101).
However, the underlying mechanism remains to be fully
elucidated.
Cytokines in the ageing microenvironment are a
double-edged sword in lymphoma treatment. These are also regarded
as part of the SASP. Specific CTLs lead to the emergence of
drug-resistant cancer in the presence of IFN-γ in the TME. In
E.G7-OVA murine T-cell lymphoma models, ERK-specific CTLs combine
to release IFN-γ into the TME, resulting in the clonal
proliferation of resistant cells. Based on these findings,
scientists hypothesised that IFN-γ mediates CTL-dependent
immunoediting. ERK-specific CTLs combined with IFN-γ alter the copy
number of DNA damage response (53). IL-15 increases CD8+
T-cell function by delaying JAK3-STAT5-associated senescence
(59). IL-6 reverses DOX
resistance in nasal NKTCL by downregulating ABCC4 and inactivating
the JAK2/STAT3/NF-κB/P65 pathway (60). Increased expression of IL-7 in
aged mouse T-cell regions mediates the expansion of γδ 17 T-cell
compartments, alters the cellular composition of the γδ T-cell pool
in pLNs and leads to accelerated tumour growth (26).
Ataxia-telangiectasia mutated (ATM) kinase has a
pivotal role in DNA damage response and is a critical molecule in
regulating the connection between autophagy and senescence. ATM
promotes SASP formation by suppressing selective autophagy of GATA
binding protein 4 (102,103). ATM has been identified in mantle
cell lymphoma (MCL) as a mutated gene in the 11q22.2 region and
engages in crucial interactions with E3 ubiquitin ligase in B cell
lymphomas (104,105). ATM deletion induces cell
malignancy (106).
Hypoxia induces tumour tolerance to radiotherapy and
chemotherapy, and promotes malignant biological behaviours, such as
tumour-cell growth, invasion and metastasis. The hypoxia-inducible
factor 2α stemness pathway is regulated by MYC and mediated by
Nanog and Sox2 to maintain a state of self-renewal in tumour stem
cells. The stemness pathway also plays a crucial role in overcoming
drug resistance (Fig. 2)
(107).
Treatment resistance often develops after multi-line
chemotherapy. Senotherapeutics may represent a promising
therapeutic strategy, as certain senescent cells are reprogrammed
and exhibit resistance to treatment. The National Cancer Institute
has proposed the 'one-two punch cancer therapy' strategy to first
induce senescent cells and then clear such cells in the ageing
environment of tumours. For individualised resistant lymphoma,
cellular senescence drugs may be chosen in combination with other
treatments. For instance, immunotherapy or epigenetic drugs
combined with senolytics are more effective in clearing senescent
cells (31,32). From the perspective of an ageing
environment to overcome drug resistance in lymphoma, it may be
concluded that these drugs provide more treatment options for
patients with drug-resistant lymphoma.
Heat shock protein 90 (Hsp90) is an oncogene that
promotes c-MYC mRNA stability (108), regulates tumour immunology
(109) and participates in
aberrant metabolism of lymphoma cells (110). Hsp90 inhibitors act as novel
senolytic agents (111) that
have rapidly developed in preclinical research on lymphomas. They
downregulate DNA replication in MCL, indicating a senolytic
function (112). Hsp90
inhibitors sensitise lymphoma cells to the chemotherapeutic drugs
cisplatin and the BTK inhibitor ibrutinib (113,114). BIIB021, a classic Hsp90
inhibitor, functions as a senolytic agent and overcame multidrug
resistance in a preclinical study on lymphomas (115).
In 2015, a phase I clinical trial of KW-2478, a
novel Hsp90 inhibitor, reported that 96% of patients with R/R
B-cell malignancy temporarily achieved stable disease. Long-term
survival outcomes were not assessed. The most common AEs were
diarrhoea, fatigue, headache and hypertension. Most patients showed
generally good tolerance (116).
The phase II study of AUY922 enrolled 14 patients with DLBCL and 6
patients with PTCL. Across the whole group, the response rate stood
at 10%, encompassing complete response (CR) in 7% of DLBCL cases
and partial response (PR) in 17% of patients with PTCL. In the
majority of cases, the toxicity profile appeared to be satisfactory
(117).
Hsp90 inhibitors have demonstrated favorable
efficacy and tolerable side effects in a subset of patients with
R/R lymphoma, with significant interindividual variability.
However, the majority of patients experience disease progression
after administration, indicating that monotherapy with Hsp90
inhibitors may not promptly control disease progression,
necessitating combination with other drugs to enhance therapeutic
efficacy. However, as they are agents targeting cellular
senescence, further investigation is needed to elucidate the
therapeutic biomarkers and long-term survival rates in patients
treated with Hsp90 inhibitors.
AK inhibitors are promising inducers of aneuploidy
and senescence in patients with haematological malignancies. Aurora
kinase A (AURKA) is a key enzyme involved in cell-cycle regulation
(118), which may be applied to
the first step in 'one-two punch therapy' to induce cellular
senescence. In preclinical studies, AURKA was found to be
overexpressed in various haematological malignancies, including
DH/DE-DLBCL (118), multiple
myeloma (MM) (119) and NKTCL
(120). MK-8745 (a
small-molecule AURKA inhibitor) significantly increased apoptosis
of NKTCL cells and induced cell-cycle arrest (120).
Currently, AK inhibitors are in the clinical trial
stage for relapsed/refractory lymphomas. In 2014, monotherapy with
the AURKA inhibitor alisertib achieved a clinical response in a
phase II clinical trial for a total of 48 patients, including those
with aggressive B- and T-cell lymphomas. The objective response
rate (ORR) of 48 patients in this study was 27%. Most common AEs
were neutropenia and leukopenia (121). In a study from 2018, an AURKA
inhibitor combined with rituximab or rituximab/vincristine showed
synergistic effects in a phase I clinical trial of 45 patients in
total with aggressive B-cell lymphoma. It is worth noting that
among the 45 patients, the regimen only demonstrated activity in 20
patients with non-germinal center B-cell (non-GCB) DLBCL. Out of 20
patients, 9 (45%) showed a response (4 CRs, 5 PRs). The most common
AE was neutropenia. Of note, all of the patients with non-GCB DLBCL
showed good tolerance (122).
Overall, AK inhibitors are promising senolytics for
the development of new therapies for patients with lymphoma.
However, there is currently limited data on AK inhibitors
specifically targeting subtypes of lymphoma, with numerous studies
being descriptive clinical investigations lacking statistical and
evidence-based medicine support (121,122). Further research is warranted to
provide more comprehensive insight into this area.
Bortezomib is a proteasome inhibitor and a
senolytic agent. The primary mechanism of action of bortezomib is
to suppress chymotrypsin-like sites in 26S protease, which then
promotes cell-cycle arrest and, ultimately, apoptosis (123). Because endoplasmic reticulum
(ER) homeostasis is strictly controlled, the level of ER stress
beyond a certain point can change from a pro-survival pathway to a
pro-apoptotic pathway, ultimately leading to ER stress-induced
apoptosis. Bortezomib is a clinically available senolytic drug that
targets ER homeostasis (62) and
has been approved as a second-line treatment for MCL and MM
(123,124).
Furthermore, in 2018, phase III clinical trials
investigating bortezomib in 487 patients newly diagnosed with stage
II-IV MCL reported a significantly prolonged median overall
survival (OS) in the bortezomib treatment arms compared to those
without bortezomib (90.7 vs. 55.7 months). AEs occurred in 42 and
57% of patients who died in the bortezomib and non-bortezomib
groups, respectively, with progressive disease emerging as the
predominant cause of mortality (125). In 2022, in a phase III clinical
study, 209 patients with T-cell lymphoblastic lymphoma (T-LL) were
included, comparing those not on bortezomib to those on bortezomib.
The four-year OS rate of patients with T-LL stood at 89.5% when
treated with bortezomib, in contrast to 78.3% in the absence of
bortezomib. The general toxicity rates of grade 3 and above were
comparable in both groups (126).
In summary, the safety profile of bortezomib was
found to be well-managed and predictable. These findings provided
evidence for the efficacy of bortezomib as an active therapeutic
agent in MCL and T-LL.
Senescence-associated pathway inhibitors for the
treatment of R/R lymphoma are currently undergoing clinical trials.
Below, the pathway inhibitors currently under study in this field
are summarised.
mTOR inhibitors have been comprehensively studied
to extend the lifespan. mTOR affects T- and B-lymphocyte
differentiation, function and cell death (127). In a phase I/II clinical trial
investigating the mTOR inhibitor temsirolimus in combination with
lenalidomide for R/R lymphomas, an ORR of 26% (13% CR) was achieved
for patients with DLBCL. Among patients with classical HL (cHL),
many of whom had relapsed following both brentuximab vedotin and
autologous stem cell transplantation, the ORR was 80% (35% CR).
Grade 3/4 hematologic AEs were frequent and included anemia,
lymphopenia, neutropenia, thrombocytopenia and leucocytosis
(128). The combination regimen
involving temsirolimus and lenalidomide proved to be viable and
showed promising efficacy in the treatment of lymphoma.
The JAK/STAT signalling pathway is closely
associated with senescence-related resistant lymphoma. SB1518 is a
selective inhibitor of JAK2, a member of the JAK family,
demonstrating its function as an antitumour agent in mutant
JAK2-driven lymphocyte lines (130). A phase II clinical trial with
the JAK inhibitor ruxolitinib was performed in 45 patients with
PTCL and 7 patients with mycosis fungoides. The ORR and clinical
benefit rate were 25 and 35%, respectively. AEs predominantly
encompassed cytopenias, within the controllable range (131). In addition, a phase II clinical
trial involving ruxolitinib was conducted for R/R cHL. The median
progression-free survival was 3.6 months and the estimated 1-year
OS rate was 50.6% (median OS not reached). A single grade IV AE
(7.1%) indicated the toxicity was tolerated (132). In short, ruxolitinib monotherapy
appears to have limited efficacy. Focusing on combined treatments
is warranted to improve the efficiency and durability of
responses.
The BCL-2 family of protein inhibitors has achieved
success in the treatment of lymphoma. The use of these inhibitors
has also been associated with senescence-associated drug resistance
in lymphomas, particularly relapsed or refractory lymphomas. Bcl-2
family inhibitor navitoclax (ABT263, a BCL-xL/BCL-2 inhibitor) has
senolytic functions in R/R ALL or LBL, and senescent bone marrow
HSCs (133,134). In a phase I study of the
selective BCL-2 inhibitor, the efficacy of venetoclax in
combination with low-dose navitoclax and chemotherapy was evaluated
in pediatric and adult patients with R/R ALL/LBL. The ORR was 60%
and the treatment was well tolerated by patients (131). In conclusion, dual inhibition of
BCL-2 and BCL-XL by venetoclax and narvesone in combination with
chemotherapy is associated with significant response rates and a
good safety profile, which is a suitable treatment option for R/R
ALL or LBL.
PD-1/PD-L1 inhibitors restore T-cell function in
aged mice. From the perspective of the ageing microenvironment,
PD-1 inhibitors used to treat relapsed and refractory T-cell
lymphomas have achieved good treatment results in preclinical
studies (99). In MyD88-
or CARD11-mutant B-cell lymphomas, senescent tumour cells
express high levels of PD-L1, thus mediating age-related immune
evasion (95). In recent years,
PD-1 inhibitors have been used to treat various relapsed or
refractory lymphomas, such as R/R cHL (135) and R/R follicular lymphoma
(136). However, PD-1 inhibitors
targeting senescent lymphoma cells have not been studied in
clinical trials.
Reversing T-cell senescence is another means of
treating senescence-related drug-resistant cells. Studies have
shown that T cells with mitochondrial dysfunction, owing to
mitochondrial transcription factor A (TFAM) deficiency, can act as
senescence accelerators. Blocking the TNF-α signalling pathway with
nicotinamide adenine dinucleotide precursors may partially prevent
premature senescence in TFAM-deficient mice (28). From the perspective of clinical
transformation, the NAD+ precursor nicotinamide riboside is a
promising drug to reverse immune-cell or lymphoma-cell senescence
and may represent an emerging treatment for drug resistance in
lymphoma.
Alternative medicines, particularly Traditional
Chinese Medicines (TCM), have an important role in lymphoma
ageing-related drug resistance by regulating immune function,
improving the TME and regulating antioxidant and anti-inflammatory
pathways. However, TCM therapy requires an individualised regimen
and should be combined with modern medical therapy regimens in
clinical treatment to achieve the best therapeutic effect (137,138). For instance, dasatinib combined
with quercetin as a classic senolytic agent is effective in the
senescence-associated treatment of solid tumours (75,139,140). Dasatinib is a vital tyrosine
kinase inhibitor used for targeted leukaemia and lymphoma therapy
(141,142). Quercetin, a TCM drug, also
exhibits anti-lymphoma effects (143). When combined with dasatinib, it
may serve as an efficacious senolytic agent in patients with
clinically resistant lymphoma. Further research on the use of TCM
for the treatment of senescence-related drug resistance in
lymphomas is necessary.
The β-gal-targeting drug SSK1 can selectively clear
senescent cells and may represent a novel drug to intervene in
senescence-related drug resistance in lymphoma cases (144).
Bromodomain and extra-terminal inhibitors
(JQ1/RVX2135) have been found to synergise with ATR inhibitors
(AZ20 and VE-821) to induce DNA damage, apoptosis, SASP pathways
and ER stress in Myc-induced lymphoma cells in preclinical research
(28) and represent promising
senolytic agents for ageing-associated drug resistance in lymphoma
treatment (Table I, Fig. 3).
In the application of the 'one-two punch cancer
therapy', it is necessary to distinguish the differentiation
outcome of senescent cells at different periods. In the early
stages, cells gradually become senescent and move towards cell
death (through processes such as apoptosis, ferroptosis and
cuproptosis). In later stages, a large number of senescent cells
form an ageing microenvironment and move towards treatment
resistance through SASP reprogramming. The senescent messaging
secretome has an important role in the mechanisms underlying
chemotherapy/radiotherapy, targeted therapy and immunotherapy
resistance in lymphomas. In the early stages, senescent cells can
be treated with pro-cellular senescence drugs, and in the later
stages, drug-resistant senescent cells can be removed using
senolytics or SASP inhibitors in conjunction with SASP
reprogramming. Furthermore, senolytics combined with immunotherapy
and epigenetic drugs may be more effective for patients with
relapsed or refractory lymphoma.
It should be noted that the type of lymphoma and
specific circumstances of the patient may influence the choice of
treatment. Therefore, the treatment of lymphoma often requires
individualised and condition-based decision-making, including the
consideration of factors such as senescence-related drug
resistance. Currently, the development of several senolytic drugs
is limited to preclinical studies and clinical trials. The
heterogeneity of senescent cells is a major obstacle to their
clinical transformation. However, the development of
senescence-associated targeted drugs may lead to important
breakthroughs in overcoming lymphoma drug resistance.
Not applicable.
Investigation: YZ, JC, QH and SQ. Software: YG and
MM. Supervision: MZ, XZ and QC. Writing-original draft: YZ and JC.
Writing-review and editing: QH, SQ, ZW, QY, WS, LD, ZS, XZ and QC.
All of the authors have read and approved the final version of the
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by the Henan Provincial Science and
Technology Research Project (grant no. SBGJ202001008), the National
Natural Science Foundation of China (grant no. 82070210) and the
Outstanding Youth Item of the Henan Health-Related Technological
and Innovative Talents Project (grant no. 11459).
|
1
|
Di Micco R, Krizhanovsky V, Baker D and
d'Adda di Fagagna F: Cellular senescence in ageing: From mechanisms
to therapeutic opportunities. Nat Rev Mol Cell Biol. 22:75–95.
2021.
|
|
2
|
Munoz-Espin D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014.
|
|
3
|
Hernandez-Segura A, Nehme J and Demaria M:
Hallmarks of cellular senescence. Trends Cell Biol. 28:436–453.
2018.
|
|
4
|
Hu D, Yuan S, Zhong J, Liu Z, Wang Y, Liu
L, Li J, Wen F, Liu J and Zhang J: Cellular senescence and
hematological malignancies: From pathogenesis to therapeutics.
Pharmacol Ther. 223:1078172021.
|
|
5
|
Fane M and Weeraratna AT: How the ageing
microenvironment influences tumour progression. Nat Rev Cancer.
20:89–106. 2020.
|
|
6
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
|
|
7
|
Hao X, Wang C and Zhang R: Chromatin basis
of the senescence-associated secretory phenotype. Trends Cell Biol.
32:513–526. 2022.
|
|
8
|
Yin Y, Chen H, Wang Y, Zhang L and Wang X:
Roles of extracellular vesicles in the aging microenvironment and
age-related diseases. J Extracell Vesicles. 10:e121542021.
|
|
9
|
Newell GR, Spitz MR and Sider JG: Cancer
and age. Semin Oncol. 16:3–9. 1989.
|
|
10
|
Gopas J, Stern E, Zurgil U, Ozer J,
Ben-Ari A, Shubinsky G, Braiman A, Sinay R, Ezratty J, Dronov V, et
al: Reed-Sternberg cells in Hodgkin's lymphoma present features of
cellular senescence. Cell Death Dis. 7:e24572016.
|
|
11
|
Aouali N, Eddabra L, Macadre J and Morjani
H: Immunosuppressors and reversion of multidrug-resistance. Crit
Rev Oncol Hematol. 56:61–70. 2005.
|
|
12
|
Kang YK, Zhan Z, Regis J, Alvarez M, Robey
R, Meadows B, Dickstein B, Lee JS, Otsuki T, Stetler-Stevenson M,
et al: Expression of mdr-1 in refractory lymphoma: quantitation by
polymerase chain reaction and validation of the assay. Blood.
86:1515–1524. 1995.
|
|
13
|
Karai E, Szebenyi K, Windt T, Feher S,
Szendi E, Dekay V, Vajdovich P, Szakacs G and Furedi A: Celecoxib
prevents doxorubicin-induced multidrug resistance in canine and
mouse lymphoma cell lines. Cancers (Basel). 12:11172020.
|
|
14
|
Zhang X, Fu X, Dong M, Yang Z, Wu S, Ma M,
Li Z, Wang X, Li L, Li X, et al: Conserved cell populations in
doxorubicin-resistant human nasal natural killer/T cell lymphoma
cell line: Super multidrug resistant cells? Cancer Cell Int.
18:1502018.
|
|
15
|
De Blander H, Morel AP, Senaratne AP,
Ouzounova M and Puisieux A: Cellular plasticity: A route to
senescence exit and tumorigenesis. Cancers (Basel).
13:45612021.
|
|
16
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022.
|
|
17
|
Chien Y, Scuoppo C, Wang X, Fang X,
Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, et al:
Control of the senescence-associated secretory phenotype by NF-κB
promotes senescence and enhances chemosensitivity. Genes Dev.
25:2125–2136. 2011.
|
|
18
|
Gilbert LA ands Hemann MT: DNA
damage-mediated induction of a chemoresistant niche. Cell.
143:355–366. 2010.
|
|
19
|
Frasca D, Diaz A, Romero M and Blomberg
BB: Human peripheral late/exhausted memory B cells express a
senescent-associated secretory phenotype and preferentially utilize
metabolic signaling pathways. Exp Gerontol. 87(Pt A): 113–120.
2017.
|
|
20
|
Riley RL, Khomtchouk K and Blomberg BB:
Age-associated B cells (ABC) inhibit B lymphopoiesis and alter
antibody repertoires in old age. Cell Immunol. 321:61–67. 2017.
|
|
21
|
Jia X, Bene J, Balazs N, Szabo K, Berta G,
Herczeg R, Gyenesei A and Balogh P: Age-Associated B cell features
of the murine high-grade B Cell Lymphoma Bc.DLFL1 and its
extranodal expansion in abdominal adipose tissues. J Immunol.
208:2866–2876. 2022.
|
|
22
|
Han S, Georgiev P, Ringel AE, Sharpe AH
and Haigis MC: Age-associated remodeling of T cell immunity and
metabolism. Cell Metab. 35:36–55. 2023.
|
|
23
|
Huang X, Bai X, Cao Y, Wu J, Huang M, Tang
D, Tao S, Zhu T, Liu Y, Yang Y, et al: Lymphoma endothelium
preferentially expresses Tim-3 and facilitates the progression of
lymphoma by mediating immune evasion. J Exp Med. 207:505–520.
2010.
|
|
24
|
Zhang Y, Joe G, Hexner E, Zhu J and
Emerson SG: Host-reactive CD8+ memory stem cells in
graft-versus-host disease. Nat Med. 11:1299–1305. 2005.
|
|
25
|
Zhou B, Zhao Z, Zhang X, Deng W and Li Y:
Effect of allogenic bone marrow mesenchymal stem cell
transplantation on t cells of old mice. Cell Reprogram. 22:30–35.
2020.
|
|
26
|
Chen HC, Eling N, Martinez-Jimenez CP,
O'Brien LM, Carbonaro V, Marioni JC, Odom DT and de la Roche M:
IL-7-dependent compositional changes within the γδ T cell pool in
lymph nodes during ageing lead to an unbalanced anti-tumour
response. EMBO Rep. 20:e473792019.
|
|
27
|
Crespo J, Sun H, Welling TH, Tian Z and
Zou W: T cell anergy, exhaustion, senescence, and stemness in the
tumor microenvironment. Curr Opin Immunol. 25:214–221. 2013.
|
|
28
|
Desdin-Mico G, Soto-Heredero G, Aranda JF,
Oller J, Carrasco E, Gabande-Rodriguez E, Blanco EM, Alfranca A,
Cusso L, Desco M, et al: T cells with dysfunctional mitochondria
induce multimorbidity and premature senescence. Science.
368:1371–1376. 2020.
|
|
29
|
Manser AR and Uhrberg M: Age-related
changes in natural killer cell repertoires: Impact on NK cell
function and immune surveillance. Cancer Immunol Immunother.
65:417–426. 2016.
|
|
30
|
Edwards ESJ, Bier J, Cole TS, Wong M, Hsu
P, Berglund LJ, Boztug K, Lau A, Gostick E, Price DA, et al:
Activating PIK3CD mutations impair human cytotoxic lymphocyte
differentiation and function and EBV immunity. J Allergy Clin
Immunol. 143:276–291.e6. 2019.
|
|
31
|
Prasanna PG, Citrin DE, Hildesheim J,
Ahmed MM, Venkatachalam S, Riscuta G, Xi D, Zheng G, Deursen JV,
Goronzy J, et al: Therapy-Induced Senescence: Opportunities to
improve anticancer therapy. J Natl Cancer Inst. 113:1285–1298.
2021.
|
|
32
|
Milanovic M, Fan DNY, Belenki D, Dabritz
JHM, Zhao Z, Yu Y, Dorr JR, Dimitrova L, Lenze D, Monteiro Barbosa
IA, et al: Senescence-associated reprogramming promotes cancer
stemness. Nature. 553:96–100. 2018.
|
|
33
|
Wang J, Tao Q, Pan Y, Wanyan Z, Zhu F, Xu
X, Wang H, Yi L, Zhou M and Zhai Z: Stress-induced premature
senescence activated by the SENEX gene mediates apoptosis
resistance of diffuse large B-cell lymphoma via promoting
immunosuppressive cells and cytokines. Immun Inflamm Dis.
8:672–683. 2020.
|
|
34
|
Chen LS, Balakrishnan K and Gandhi V:
Inflammation and survival pathways: Chronic lymphocytic leukemia as
a model system. Biochem Pharmacol. 80:1936–1945. 2010.
|
|
35
|
Birch J and Gil J: Senescence and the
SASP: Many therapeutic avenues. Genes Dev. 34:1565–1576. 2020.
|
|
36
|
Braig M, Lee S, Loddenkemper C, Rudolph C,
Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T and
Schmitt CA: Oncogene-induced senescence as an initial barrier in
lymphoma development. Nature. 436:660–665. 2005.
|
|
37
|
Dorr JR, Yu Y, Milanovic M, Beuster G,
Zasada C, Dabritz JH, Lisec J, Lenze D, Gerhardt A, Schleicher K,
et al: Synthetic lethal metabolic targeting of cellular senescence
in cancer therapy. Nature. 501:421–425. 2013.
|
|
38
|
Scafuro M, Capasso L, Carafa V, Altucci L
and Nebbioso A: Gene Transactivation and Transrepression in
MYC-Driven Cancers. Int J Mol Sci. 22:34582021.
|
|
39
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008.
|
|
40
|
Cao J, Li L, Chen C, Lv C, Meng F, Zeng L,
Li Z, Wu Q, Zhao K, Pan B, et al: RNA interference-mediated
silencing of NANOG leads to reduced proliferation and self-renewal,
cell cycle arrest and apoptosis in T-cell acute lymphoblastic
leukemia cells via the p53 signaling pathway. Leuk Res.
37:1170–1177. 2013.
|
|
41
|
Reimann M, Lee S, Loddenkemper C, Dorr JR,
Tabor V, Aichele P, Stein H, Dorken B, Jenuwein T and Schmitt CA:
Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via
Suv39h1-dependent senescence. Cancer Cell. 17:262–272. 2010.
|
|
42
|
Moore AR, Rosenberg SC, McCormick F and
Malek S: RAS-targeted therapies: Is the undruggable drugged? Nat
Rev Drug Discov. 19:533–552. 2020.
|
|
43
|
Harrell Stewart DR and Clark GJ: Pumping
the brakes on RAS-negative regulators and death effectors of RAS. J
Cell Sci. 133:jcs2388652020.
|
|
44
|
Moiseeva O, Guillon J and Ferbeyre G:
Senescence: A program in the road to cell elimination and cancer.
Semin Cancer Biol. 81:48–53. 2022.
|
|
45
|
Caceres-Gutierrez RE, Alfaro-Mora Y,
Andonegui MA, Diaz-Chavez J and Herrera LA: The influence of
oncogenic RAS on chemotherapy and radiotherapy resistance through
DNA repair pathways. Front Cell Dev Biol. 10:7513672022.
|
|
46
|
Punekar SR, Velcheti V, Neel BG and Wong
KK: The current state of the art and future trends in RAS-targeted
cancer therapies. Nat Rev Clin Oncol. 19:637–655. 2022.
|
|
47
|
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008.
|
|
48
|
Zhang X, Zhao L, Li X, Wang X, Li L, Fu X,
Sun Z, Li Z, Nan F, Chang Y and Zhang M: ATP-binding cassette
sub-family C member 4 (ABCC4) is overexpressed in human NK/T-cell
lymphoma and regulates chemotherapy sensitivity: Potential as a
functional therapeutic target. Leuk Res. 39:1448–1454. 2015.
|
|
49
|
Han L, Long Q, Li S, Xu Q, Zhang B, Dou X,
Qian M, Jiramongkol Y, Guo J, Cao L, et al: Senescent stromal cells
promote cancer resistance through SIRT1 Loss-potentiated
overproduction of small extracellular vesicles. Cancer Res.
80:3383–3398. 2020.
|
|
50
|
Yano H, Fujiwara Y, Horlad H, Pan C, Kai
K, Niino D, Ohsawa K, Higashi M, Nosaka K, Okuno Y, et al: Blocking
cholesterol efflux mechanism is a potential target for antilymphoma
therapy. Cancer Sci. 113:2129–2143. 2022.
|
|
51
|
Wu S, Zhang X, Dong M, Yang Z, Zhang M and
Chen Q: sATP-binding cassette subfamily G member 2 enhances the
multidrug resistance properties of human nasal natural killer/T
cell lymphoma side population cells. Oncol Rep. 44:1467–1478.
2020.
|
|
52
|
Zhang Y and Wang X: Targeting the
Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol.
13:1652020.
|
|
53
|
Takeda K, Nakayama M, Hayakawa Y, Kojima
Y, Ikeda H, Imai N, Ogasawara K, Okumura K, Thomas DM and Smyth MJ:
IFN-gamma is required for cytotoxic T cell-dependent cancer genome
immunoediting. Nat Commun. 8:146072017.
|
|
54
|
Islam S, Qi W, Morales C, Cooke L, Spier
C, Weterings E and Mahadevan D: Disruption of aneuploidy and
senescence induced by aurora inhibition promotes intrinsic
apoptosis in double hit or double expressor diffuse large B-cell
lymphomas. Mol Cancer Ther. 16:2083–2093. 2017.
|
|
55
|
Tewari D, Patni P and Bishayee A, Sah AN
and Bishayee A: Natural products targeting the PI3K-Akt-mTOR
signaling pathway in cancer: A novel therapeutic strategy. Semin
Cancer Biol. 80:1–17. 2022.
|
|
56
|
Pi M, Kuang H, Yue C, Yang Q, Wu A, Li Y,
Assaraf YG, Yang DH and Wu S: Targeting metabolism to overcome
cancer drug resistance: A promising therapeutic strategy for
diffuse large B cell lymphoma. Drug Resist Updat.
61:1008222022.
|
|
57
|
Katoh M and Katoh M: Precision medicine
for human cancers with Notch signaling dysregulation (Review). Int
J Mol Med. 45:279–297. 2020.
|
|
58
|
Patil K, Sher G, Kuttikrishnan S, Moton S,
Alam M, Buddenkotte J, Ahmad A, Steinhoff M and Uddin S: The
cross-talk between miRNAs and JAK/STAT pathway in cutaneous T cell
lymphoma: Emphasis on therapeutic opportunities. Semin Cell Dev
Biol. 154(Pt C): 239–249. 2024.
|
|
59
|
Weng J, Moriarty KE, Baio FE, Chu F, Kim
SD, He J, Jie Z, Xie X, Ma W, Qian J, et al: IL-15 enhances the
antitumor effect of human antigen-specific CD8(+) T cells by
cellular senescence delay. Oncoimmunology. 5:e12373272016.
|
|
60
|
Wang L, Li LR, Zhang L and Wang JW: The
landscape of new drugs in extranodal NK/T-cell lymphoma. Cancer
Treat Rev. 89:1020652020.
|
|
61
|
Ge H, Ke J, Xu N, Li H, Gong J, Li X, Song
Y, Zhu H and Bai C: Dexamethasone alleviates pemetrexed-induced
senescence in non-small-cell lung cancer. Food Chem Toxicol.
119:86–97. 2018.
|
|
62
|
Ei ZZ, Choochuay K, Tubsuwan A, Pinkaew D,
Suksomtip M, Vinayanuwattikun C, Chanvorachote P and Chunhacha P:
GRP78/BiP determines senescence evasion cell fate after
cisplatin-based chemotherapy. Sci Rep. 11:224482021.
|
|
63
|
Robbins PD, Jurk D, Khosla S, Kirkland JL,
LeBrasseur NK, Miller JD, Passos JF, Pignolo RJ, Tchkonia T and
Niedernhofer LJ: Senolytic Drugs: Reducing senescent cell viability
to extend health Span. Annu Rev Pharmacol Toxicol. 61:779–803.
2021.
|
|
64
|
Azim HA Jr, Pruneri G, Raviele PR,
Steffanoni S, Martinelli G and Peccatori FA: ERCC1 Expression in
Diffuse Large B-Cell lymphoma patients treated with a
cisplatin-based regimen : A brief communication. J Egypt Natl Canc
Inst. 19:176–177. 2007.
|
|
65
|
Lee H: Impaired phosphorylation and
mis-localization of Bub1 and BubR1 are responsible for the
defective mitotic checkpoint function in Brca2-mutant thymic
lymphomas. Exp Mol Med. 35:448–453. 2003.
|
|
66
|
Moncsek A, Al-Suraih MS, Trussoni CE,
O'Hara SP, Splinter PL, Zuber C, Patsenker E, Valli PV, Fingas CD,
Weber A, et al: Targeting senescent cholangiocytes and activated
fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates
fibrosis in multidrug resistance 2 gene knockout (Mdr2(-/-)) mice.
Hepatology. 67:247–259. 2018.
|
|
67
|
Matthe DM, Thoma OM, Sperka T, Neurath MF
and Waldner MJ: Telomerase deficiency reflects age-associated
changes in CD4+ T cells. Immun Ageing. 19:162022.
|
|
68
|
Reimann M, Loddenkemper C, Rudolph C,
Schildhauer I, Teichmann B, Stein H, Schlegelberger B, Dorken B and
Schmitt CA: The Myc-evoked DNA damage response accounts for
treatment resistance in primary lymphomas in vivo. Blood.
110:2996–3004. 2007.
|
|
69
|
Vidal-Crespo A, Matas-Cespedes A,
Rodriguez V, Rossi C, Valero JG, Serrat N, Sanjuan-Pla A, Menendez
P, Roue G, Lopez-Guillermo A, et al: Daratumumab displays in vitro
and in vivo anti-tumor activity in models of B-cell non-Hodgkin
lymphoma and improves responses to standard chemo-immunotherapy
regimens. Haematologica. 105:1032–1041. 2020.
|
|
70
|
Fontan L, Goldstein R, Casalena G, Durant
M, Teater MR, Wilson J, Phillip J, Xia M, Shah S, Us I, et al:
Identification of MALT1 feedback mechanisms enables rational design
of potent antilymphoma regimens for ABC-DLBCL. Blood. 137:788–800.
2021.
|
|
71
|
Kim S, Shah SB, Graney PL and Singh A:
Multiscale engineering of immune cells and lymphoid organs. Nat Rev
Mater. 4:355–378. 2019.
|
|
72
|
Tian YF, Ahn H, Schneider RS, Yang SN,
Roman-Gonzalez L, Melnick AM, Cerchietti L and Singh A:
Integrin-specific hydrogels as adaptable tumor organoids for
malignant B and T cells. Biomaterials. 73:110–119. 2015.
|
|
73
|
Ceccato J, Piazza M, Pizzi M, Manni S,
Piazza F, Caputo I, Cinetto F, Pisoni L, Trojan D, Scarpa R, et al:
A bone-based 3D scaffold as an in-vitro model of
microenvironment-DLBCL lymphoma cell interaction. Front Oncol.
12:9478232022.
|
|
74
|
Shah SB, Carlson CR, Lai K, Zhong Z,
Marsico G, Lee KM, Felix Velez NE, Abeles EB, Allam M, Hu T, et al:
Combinatorial treatment rescues tumour-microenvironment-mediated
attenuation of MALT1 inhibitors in B-cell lymphomas. Nat Mater.
22:511–523. 2023.
|
|
75
|
Wyld L, Bellantuono I, Tchkonia T, Morgan
J, Turner O, Foss F, George J, Danson S and Kirkland JL: Senescence
and cancer: A review of clinical implications of senescence and
senotherapies. Cancers (Basel). 12:21342020.
|
|
76
|
Wang B, Li XQ, Ma X, Hong X, Lu H and Guo
Y: Immunohistochemical expression and clinical significance of
P-glycoprotein in previously untreated extranodal NK/T-cell
lymphoma, nasal type. Am J Hematol. 83:795–799. 2008.
|
|
77
|
Wang J, Wang Z, Wang H, Wanyan Z, Pan Y,
Zhu F, Tao Q and Zhai Z: Stress-Induced premature senescence
promotes proliferation by activating the SENEX and
p16(INK4a)/Retinoblastoma (Rb) pathway in diffuse large B-Cell
lymphoma. Turk J Haematol. 36:247–254. 2019.
|
|
78
|
Nguyen PH, Niesen E and Hallek M: New
roles for B cell receptor associated kinases: when the B cell is
not the target. Leukemia. 33:576–587. 2019.
|
|
79
|
Alu A, Lei H, Han X, Wei Y and Wei X: BTK
inhibitors in the treatment of hematological malignancies and
inflammatory diseases: Mechanisms and clinical studies. J Hematol
Oncol. 15:1382022.
|
|
80
|
Chibaya L, Snyder J and Ruscetti M:
Senescence and the tumor-immune landscape: Implications for cancer
immunotherapy. Semin Cancer Biol. 86(Pt 3): 827–845. 2022.
|
|
81
|
Wang X, Zhang L, Liu X, Li X, Li L, Fu X,
Sun Z, Wu J, Zhang X, Yan J, et al: Efficacy and safety of a
pegasparaginase-based chemotherapy regimen vs an
L-asparaginase-Based chemotherapy regimen for newly diagnosed
advanced extranodal natural Killer/T-Cell lymphoma: A randomized
clinical trial. JAMA Oncol. 8:1035–1041. 2022.
|
|
82
|
Kidd JG: Regression of transplanted
lymphomas induced in vivo by means of normal guinea pig serum. II.
Studies on the nature of the active serum constituent: histological
mechanism of the regression: tests for effects of guinea pig serum
on lymphoma cells in vitro: Discussion. J Exp Med. 98:583–606.
1953.
|
|
83
|
Krall AS, Xu S, Graeber TG, Braas D and
Christofk HR: Asparagine promotes cancer cell proliferation through
use as an amino acid exchange factor. Nat Commun. 7:114572016.
|
|
84
|
Wang L, Yang J, Wang HN, Fu RY, Liu XD,
Piao YS, Wei LQ, Wang JW and Zhang L: LncRNA BCYRN1-induced
autophagy enhances asparaginase resistance in extranodal NK/T-cell
lymphoma. Theranostics. 11:925–940. 2021.
|
|
85
|
Zhdanov DD, Pokrovsky VS, Pokrovskaya MV,
Alexandrova SS, Eldarov MA, Grishin DV, Basharov MM, Gladilina YA,
Podobed OV and Sokolov NN: Inhibition of telomerase activity and
induction of apoptosis by Rhodospirillum rubrum L-asparaginase in
cancer Jurkat cell line and normal human CD4+ T lymphocytes. Cancer
Med. 6:2697–2712. 2017.
|
|
86
|
Zhdanov DD, Pokrovsky VS, Pokrovskaya MV,
Alexandrova SS, Eldarov MA, Grishin DV, Basharov MM, Gladilina YA,
Podobed OV and Sokolov NN: Rhodospirillum rubruml-asparaginase
targets tumor growth by a dual mechanism involving telomerase
inhibition. Biochem Biophys Res Commun. 492:282–288. 2017.
|
|
87
|
Hassler MR, Klisaroska A, Kollmann K,
Steiner I, Bilban M, Schiefer AI, Sexl V and Egger G:
Antineoplastic activity of the DNA methyltransferase inhibitor
5-aza-2'-deoxycytidine in anaplastic large cell lymphoma.
Biochimie. 94:2297–2307. 2012.
|
|
88
|
Arosio G, Sharma GG, Villa M, Mauri M,
Crespiatico I, Fonta na D, Ma nf roni C, Mastini C, Zappa M,
Magistroni V, et al: Synergistic drug combinations prevent
resistance in ALK+ anaplastic large cell lymphoma. Cancers (Basel).
13:44222021.
|
|
89
|
Lund K, Adams PD and Copland M: EZH2 in
normal and malignant hematopoiesis. Leukemia. 28:44–49. 2014.
|
|
90
|
Baell JB, Leaver DJ, Hermans SJ, Kelly GL,
Brennan MS, Downer NL, Nguyen N, Wichmann J, McRae HM, Yang Y, et
al: Inhibitors of histone acetyltransferases KAT6A/B induce
senescence and arrest tumour growth. Nature. 560:253–257. 2018.
|
|
91
|
Huang F: New KAT6 inhibitors induce
senescence and arrest cancer growth. Synth Syst Biotechnol.
3:244–245. 2018.
|
|
92
|
Schleich K, Kase J, Dorr JR, Trescher S,
Bhattacharya A, Yu Y, Wailes EM, Fan DNY, Lohneis P, Milanovic M,
et al: H3K9me3-mediated epigenetic regulation of senescence in mice
predicts outcome of lymphoma patients. Nat Commun. 11:36512020.
|
|
93
|
Yin R, Chang J, Li Y, Gao Z, Qiu Q, Wang
Q, Han G, Chai J, Feng M, Wang P, et al: Differential m(6)A RNA
landscapes across hematopoiesis reveal a role for IGF2BP2 in
preserving hematopoietic stem cell function. Cell Stem Cell.
29:149–159 e7. 2022.
|
|
94
|
Mirza N, Duque MA, Dominguez AL, Schrum
AG, Dong H and Lustgarten J: B7-H1 expression on old CD8+ T cells
negatively regulates the activation of immune responses in aged
animals. J Immunol. 184:5466–5474. 2010.
|
|
95
|
Reimann M, Schrezenmeier J,
Richter-Pechanska P, Dolnik A, Hick TP, Schleich K, Cai X, Fan DNY,
Lohneis P, Masswig S, et al: Adaptive T-cell immunity controls
senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood.
137:2785–2799. 2021.
|
|
96
|
Kline J, Godfrey J and Ansell SM: The
immune landscape and response to immune checkpoint blockade therapy
in lymphoma. Blood. 135:523–533. 2020.
|
|
97
|
Wang TW, Johmura Y, Suzuki N, Omori S,
Migita T, Yamaguchi K, Hatakeyama S, Yamazaki S, Shimizu E, Imoto
S, et al: Blocking PD-L1-PD-1 improves senescence surveillance and
ageing phenotypes. Nature. 611:358–364. 2022.
|
|
98
|
Shimada Y, Hayashi M, Nagasaka Y,
Ohno-Iwashita Y and Inomata M: Age-associated up-regulation of a
negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp
Gerontol. 44:517–522. 2009.
|
|
99
|
Lages CS, Lewkowich I, Sproles A,
Wills-Karp M and Chougnet C: Partial restoration of T-cell function
in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging
Cell. 9:785–798. 2010.
|
|
100
|
Tsukamoto H, Komohara Y, Tomita Y, Miura
Y, Motoshima T, Imamura K, Kimura T, Ikeda T, Fujiwara Y, Yano H,
et al: Aging-associated and CD4 T-cell-dependent ectopic CXCL13
activation predisposes to anti-PD-1 therapy-induced adverse events.
Proc Natl Acad Sci USA. 119:e22053781192022.
|
|
101
|
Etxebeste-Mitxeltorena M, Del Rincon-Loza
I and Martin-Antonio B: Tumor secretome to adoptive cellular
immunotherapy: Reduce me before I make you my partner. Front
Immunol. 12:7178502021.
|
|
102
|
Stagni V, Ferri A, Cirotti C and Barila D:
ATM kinase-dependent regulation of autophagy: A key player in
senescence? Front Cell Dev Biol. 8:5990482021.
|
|
103
|
Kang C, Xu Q, Martin TD, Li MZ, Demaria M,
Aron L, Lu T, Yankner BA, Campisi J and Elledge SJ: The DNA damage
response induces inflammation and senescence by inhibiting
autophagy of GATA4. Science. 349:aaa56122015.
|
|
104
|
Bea S, Valdes-Mas R, Navarro A, Salaverria
I, Martin-Garcia D, Jares P, Gine E, Pinyol M, Royo C, Nadeu F, et
al: Landscape of somatic mutations and clonal evolution in mantle
cell lymphoma. Proc Natl Acad Sci USA. 110:18250–18255. 2013.
|
|
105
|
Sarkar A, Stellrecht CM, Vangapandu HV,
Ayres M, Kaipparettu BA, Park JH, Balakrishnan K, Burks JK, Pandita
TK, Hittelman WN, et al: Ataxia-telangiectasia mutated interacts
with Parkin and induces mitophagy independent of kinase activity.
Evidence from mantle cell lymphoma. Haematologica. 106:495–512.
2021.
|
|
106
|
Milanovic M, Shao Z, Estes VM, Wang XS,
Menolfi D, Lin X, Lee BJ, Xu J, Cupo OM, Wang D and Zha S: FATC
domain deletion compromises ATM protein stability, blocks
lymphocyte development, and promotes lymphomagenesis. J Immunol.
206:1228–1239. 2021.
|
|
107
|
Das B, Pal B, Bhuyan R, Li H, Sarma A,
Gayan S, Talukdar J, Sandhya S, Bhuyan S, Gogoi G, et al: MYC
Regulates the HIF2α stemness pathway via Nanog and Sox2 to Maintain
Self-Renewal in cancer stem cells versus non-stem cancer cells.
Cancer Res. 79:4015–4025. 2019.
|
|
108
|
Zhou X, Wen Y, Tian Y, He M, Ke X, Huang
Z, He Y, Liu L, Scharf A, Lu M, et al: Heat Shock Protein
90α-Dependent B-Cell-2-Associated Transcription Factor 1 promotes
hepatocellular carcinoma proliferation by regulating MYC
Proto-Oncogene c-MYC mRNA Stability. Hepatology. 69:1564–1581.
2019.
|
|
109
|
Albakova Z, Mangasarova Y, Albakov A,
Nikulina E, Kravchenko S and Sapozhnikov A: Aberrant HSP90
expression in lymphocytes and HSP90 Response to Anti-PD-1 therapy
in lymphoma patients. Front Immunol. 13:8931372022.
|
|
110
|
Calvo-Vidal MN, Zamponi N, Krumsiek J,
Stockslager MA, Revuelta MV, Phillip JM, Marullo R, Tikhonova E,
Kotlov N, Patel J, et al: Oncogenic HSP90 facilitates metabolic
alterations in aggressive B-cell lymphomas. Cancer Res.
81:5202–5216. 2021.
|
|
111
|
Fuhrmann-Stroissnigg H, Ling YY, Zhao J,
McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL,
Dorronsoro A, et al: Identification of HSP90 inhibitors as a novel
class of senolytics. Nat Commun. 8:4222017.
|
|
112
|
Liu H, Lu Z, Shi X, Liu L, Zhang P,
Golemis EA and Tu Z: HSP90 inhibition downregulates DNA replication
and repair genes via E2F1 repression. J Biol Chem.
297:1009962021.
|
|
113
|
Schmidt L, Issa II, Haraldsdottir H, Hald
JL, Schmitz A, Due H and Dybkaer K: Hsp90 inhibition sensitizes
DLBCL cells to cisplatin. Cancer Chemother Pharmacol. 89:431–440.
2022.
|
|
114
|
Lu Z, Wang Z, Tu Z and Liu H: HSP90
inhibitor ganetespib enhances the sensitivity of mantle cell
lymphoma to bruton's tyrosine kinase inhibitor ibrutinib. Front
Pharmacol. 13:8641942022.
|
|
115
|
He W and Hu H: BIIB021, an Hsp90
inhibitor: A promising therapeutic strategy for blood malignancies
(Review). Oncol Rep. 40:3–15. 2018.
|
|
116
|
Yong K, Cavet J, Johnson P, Morgan G,
Williams C, Nakashima D, Akinaga S, Oakervee H and Cavenagh J:
Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with
B-cell malignancies. Br J Cancer. 114:7–13. 2016.
|
|
117
|
Oki Y, Younes A, Knickerbocker J,
Samaniego F, Nastoupil L, Hagemeister F, Romaguera J, Fowler N,
Kwak L and Westin J: Experience with HSP90 inhibitor AUY922 in
patients with relapsed or refractory non-Hodgkin lymphoma.
Haematologica. 100:e272–e274. 2015.
|
|
118
|
Marumoto T, Zhang D and Saya H: Aurora-A-a
guardian of poles. Nat Rev Cancer. 5:42–50. 2005.
|
|
119
|
Farag SS: The potential role of Aurora
kinase inhibitors in haematological malignancies. Br J Haematol.
155:561–579. 2011.
|
|
120
|
Iqbal J, Weisenburger DD, Chowdhury A,
Tsai MY, Srivastava G, Greiner TC, Kucuk C, Deffenbacher K, Vose J,
Smith L, et al: Natural killer cell lymphoma shares strikingly
similar molecular features with a group of non-hepatosplenic
gammadelta T-cell lymphoma and is highly sensitive to a novel
aurora kinase A inhibitor in vitro. Leukemia. 25:348–358. 2011.
|
|
121
|
Friedberg JW, Mahadevan D, Cebula E,
Persky D, Lossos I, Agarwal AB, Jung J, Burack R, Zhou X, Leonard
EJ, et al: Phase II study of alisertib, a selective Aurora A kinase
inhibitor, in relapsed and refractory aggressive B- and T-cell
non-Hodgkin lymphomas. J Clin Oncol. 32:44–50. 2014.
|
|
122
|
Kelly KR, Friedberg JW, Park SI, McDonagh
K, Hayslip J, Persky D, Ruan J, Puvvada S, Rosen P, Iyer SP, et al:
Phase I study of the investigational aurora a kinase inhibitor
alisertib plus rituximab or rituximab/vincristine in
relapsed/refractory aggressive B-cell lymphoma. Clin Cancer Res.
24:6150–6159. 2018.
|
|
123
|
Tan CRC, Abdul-Majeed S, Cael B and Barta
SK: Clinical pharmacokinetics and pharmacodynamics of bortezomib.
Clin Pharmacokinet. 58:157–168. 2019.
|
|
124
|
Robak T, Jin J, Pylypenko H, Verhoef G,
Siritanaratkul N, Drach J, Raderer M, Mayer J, Pereira J, Tumyan G,
et al: Frontline bortezomib, rituximab, cyclophosphamide,
doxorubicin, and prednisone (VR-CAP) versus rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)
in transplantation-ineligible patients with newly diagnosed mantle
cell lymphoma: final overall survival results of a randomised,
open-label, phase 3 study. Lancet Oncol. 19:1449–1458. 2018.
|
|
125
|
Teachey DT, Devidas M, Wood BL, Chen Z,
Hayashi RJ, Hermiston ML, Annett RD, Archer JH, Asselin BL, August
KJ, et al: Children's Oncology Group Trial AALL1231: A Phase III
clinical trial testing bortezomib in newly diagnosed T-Cell acute
lymphoblastic leukemia and lymphoma. J Clin Oncol. 40:2106–2118.
2022.
|
|
126
|
Hurez V, Dao V, Liu A, Pandeswara S,
Gelfond J, Sun L, Bergman M, Orihuela CJ, Galvan V, Padron A, et
al: Chronic mTOR inhibition in mice with rapamycin alters T, B,
myeloid, and innate lymphoid cells and gut flora and prolongs life
of immune-deficient mice. Aging Cell. 14:945–956. 2015.
|
|
127
|
Major A, Kline J, Karrison TG, Fishkin
PAS, Kimball AS, Petrich AM, Nattam S, Rao K, Sleckman BG, Cohen K,
et al: Phase I/II clinical trial of temsirolimus and lenalidomide
in patients with relapsed and refractory lymphomas. Haematologica.
107:1608–1618. 2022.
|
|
128
|
Borthakur G, Martinelli G, Raffoux E,
Chevallier P, Chromik J, Lithio A, Smith CL, Yuen E, Oakley GJ III,
Benhadji KA and DeAngelo DJ: Phase 1 study to evaluate
Crenigacestat (LY3039478) in combination with dexamethasone in
patients with T-cell acute lymphoblastic leukemia and lymphoma.
Cancer. 127:372–380. 2021.
|
|
129
|
Hart S, Goh KC, Novotny-Diermayr V, Hu CY,
Hentze H, Tan YC, Madan B, Amalini C, Loh YK, Ong LC, et al:
SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the
treatment of myeloid and lymphoid malignancies. Leukemia.
25:1751–1759. 2011.
|
|
130
|
Moskowitz AJ, Ghione P, Jacobsen E, Ruan
J, Schatz JH, Noor S, Myskowski P, Vardhana S, Ganesan N, Hancock
H, et al: A phase 2 biomarker-driven study of ruxolitinib
demonstrates effectiveness of JAK/STAT targeting in T-cell
lymphomas. Blood. 138:2828–2837. 2021.
|
|
131
|
Gillessen S, Pluetschow A, Vucinic V,
Ostermann H, Kobe C, Brockelmann PJ, Boll B, Eichenauer DA, Heger
JM, Borchmann S, et al: JAK inhibition with ruxolitinib in relapsed
or refractory classical Hodgkin lymphoma: Final results of a phase
II, open label, multicentre clinical trial (JeRiCHO). Eur J
Haematol. 109:728–735. 2022.
|
|
132
|
Pullarkat VA, Lacayo NJ, Jabbour E,
Rubnitz JE, Bajel A, Laetsch TW, Leonard J, Colace SI, Khaw SL,
Fleming SA, et al: Venetoclax and navitoclax in combination with
chemotherapy in patients with relapsed or refractory acute
lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov.
11:1440–1453. 2021.
|
|
133
|
Chang J, Wang Y, Shao L, Laberge RM,
Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W,
et al: Clearance of senescent cells by ABT263 rejuvenates aged
hematopoietic stem cells in mice. Nat Med. 22:78–83. 2016.
|
|
134
|
Mei MG, Lee HJ, Palmer JM, Chen R, Tsai
NC, Chen L, McBride K, Smith DL, Melgar I, Song JY, et al:
Responseadapted anti-PD-1-based salvage therapy for Hodgkin
lymphoma with nivolumab alone or in combination with ICE. Blood.
139:3605–3616. 2022.
|
|
135
|
Nastoupil LJ, Chin CK, Westin JR, Fowler
NH, Samaniego F, Cheng X, Ma MCJ, Wang Z, Chu F, Dsouza L, et al:
Safety and activity of pembrolizumab in combination with rituximab
in relapsed or refractory follicular lymphoma. Blood Adv.
6:1143–1151. 2022.
|
|
136
|
Rybicka M, Zhao J, Piotrowicz K, Ptasnik
S, Mitka K, Kocot-Kępska M and Hui KK: Promoting whole person
health: Exploring the role of traditional Chinese medicine in
Polish healthcare. J Integr Med. 21:509–517. 2023.
|
|
137
|
Luo J, Shen S, Xia J, Wang J and Gu Z:
Mitochondria as the Essence of Yang Qi in the human body.
Phenomics. 2:336–348. 2022.
|
|
138
|
Zhu Y, Tchkonia T, Pirtskhalava T, Gower
AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M,
et al: The Achilles' heel of senescent cells: From transcriptome to
senolytic drugs. Aging Cell. 14:644–658. 2015.
|
|
139
|
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H,
Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO,
Giles CB, et al: Identification of a novel senolytic agent,
navitoclax, targeting the Bcl-2 family of anti-apoptotic factors.
Aging Cell. 15:428–435. 2016.
|
|
140
|
Yoshimura S, Panetta JC, Hu J, Li L, Gocho
Y, Du G, Umezawa A, Karol SE, Pui CH, Mullighan CG, et al:
Preclinical pharmacokinetic and pharmacodynamic evaluation of
dasatinib and ponatinib for the treatment of T-cell acute
lymphoblastic leukemia. Leukemia. 37:1194–1203. 2023.
|
|
141
|
Senapati J, Sasaki K, Issa GC, Lipton JH,
Radich JP, Jabbour E and Kantarjian HM: Management of chronic
myeloid leukemia in 2023-common ground and common sense. Blood
Cancer J. 13:582023.
|
|
142
|
Fujii K, Idogawa M, Suzuki N, Iwatsuki K
and Kanekura T: Functional Depletion of HSP72 by siRNA and
quercetin enhances vorinostat-induced apoptosis in an
HSP72-overexpressing cutaneous T-Cell lymphoma cell line, Hut78.
Int J Mol Sci. 22:112582021.
|
|
143
|
Cai Y, Zhou H, Zhu Y, Sun Q, Ji Y, Xue A,
Wang Y, Chen W, Yu X, Wang L, et al: Elimination of senescent cells
by β-galactosidase-targeted prodrug attenuates inflammation and
restores physical function in aged mice. Cell Res. 30:574–589.
2020.
|
|
144
|
Muralidharan SV, Bhadury J, Nilsson LM,
Green LC, McLure KG and Nilsson JA: BET bromodomain inhibitors
synergize with ATR inhibitors to induce DNA damage, apoptosis,
senescence-associated secretory pathway and ER stress in
Myc-induced lymphoma cells. Oncogene. 35:4689–4697. 2016.
|