Obesity is a metabolic disease caused by the
overaccumulation of subcutaneous or abdominal adipose tissue
characterized by chronic low-grade inflammation (1). This status ultimately leads to the
development of diseases such as cancer, cardiovascular disease,
type 2 diabetes, hypertension, dyslipidemia and reproductive
disorders (2-5).
Adipocytes release proinflammatory mediators such as
tumor necrosis factor (TNF)-α, interferon (IFN), interleukin
(IL)-1β and leptin, (6), which
also play a key role in tumor dissemination and metastasis
(7,8). Proinflammatory molecules are
involved in the recruitment of suppressive cells, including
myeloid-derived suppressor cells (MDSCs), in the tumor
microenvironment (TME). MDSCs are a heterogeneous population of
immature myeloid cells (IMCs) with potent immunosuppressive effects
in cancer (9).
According to the literature, obesity and cancer
create similar chronic inflammatory environments. Indeed, evidence
has been provided of the impact of obesity-associated MDSCs on
cancer progression. However, the mechanisms by which this occurs
have remained to be fully elucidated. This paper addresses the
relevant role that MDSCs play in the immune system. There is
evidence supporting the protumorigenic role of MDSCs in obese
murine models and patients. Some authors have described the
mechanisms of action of obesity-associated MDSCs and suggest
different therapeutic approaches targeting MDSCs in obesity-related
cancer. The protective role of obesity-associated MDSCs in
maintaining immune homeostasis is also briefly mentioned in this
paper.
MDSCs are IMCs developed during myelopoiesis by a
variety of cytokines, such as granulocyte-macrophage
colony-stimulating factor (GM-CSF), granulocyte colony-stimulating
factor (G-CSF), IL-17 and TNF-α (10). In normal conditions, hematopoietic
stem cells differentiate into common myeloid progenitors, which, in
turn, differentiate into neutrophils, monocytes, dendritic cells
(DCs) or macrophages. However, myelopoiesis is disrupted in
pathological settings, thereby leading to abnormal development of
mature myeloid cells. This leads to the accumulation of IMCs at
specific sites, depending on the disease (11).
Two main MDSC subpopulations have been described in
the literature, namely monocytic MDSCs (M-MDSCs) and granulocytic
MDSCs (G-MDSCs). Both cell subsets were first identified in
lymphoma BW-Sp3- and chicken ovalbumin-transfected EL-4 thymoma
EG7-bearing mice as cell subfractions with different morphological,
molecular and functional characteristics. MDSCs have been
documented to resemble inflammatory monocytes (M-MDSCs) and
immature neutrophil-like or low-density polymorphonuclear cells
(G-MDSCs) (12). Characterization
standards for MDSCs suggest defining mouse MDSCs as granulocytic
marker 1 (Gr1)+CD11b+ cells expressing
Ly6ChighLy6G- (M-MDSCs) or
Ly6ClowLy6G+ (G-MDSCs), whereas human MDSCs
are CD11b+human leukocyte antigen
(HLA)-DRlow/- cells that express
CD14+CD15- (M-MDSCs) or
CD14-CD15+ (G-MDSCs), although G-MDSCs may
also express CD66b (13). Other
markers such as CD45 and CD33 have also been used to define human
MDSCs (14,15). Of note, there is another MDSC
subset known as 'early stage MDSCs', defined as immature
Lin-(CD3, CD14, CD15, CD19, CD56)
HLA-DR-CD33+ MDSCs (13).
The main role of MDSCs is to suppress immune
responses, particularly T-cell responses, and it has been
extensively described in a variety of settings (16-19). Of note, M-MDSCs mainly express
nitric oxide synthase 2, whereas G-MDSCs express high levels of
arginase type 1 (ARG1) (20,21). This means that each MDSC subset
may use different mechanisms and effector molecules to inhibit
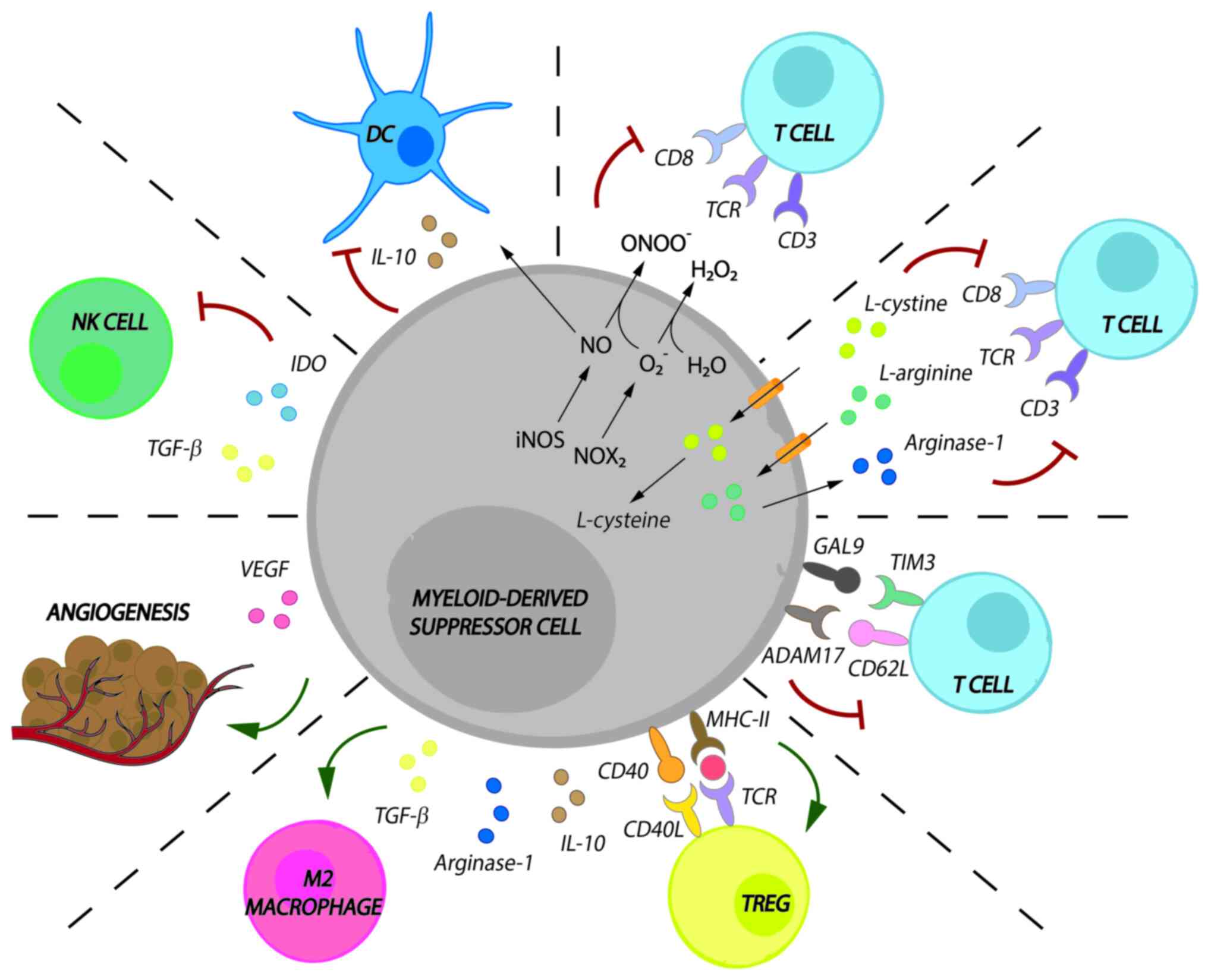
immune responses. MDSC activity in cancer is as follows (Fig. 1):
MDSCs promote both the loss of the T-cell receptor
(TCR)ζ-chain and cell cycle arrest in T cells. MDSCs exert this
effect by taking up L-arginine and L-cysteine, which are essential
amino acids for T-cell proliferation and expansion;
MDSCs release reactive oxygen species (ROS), such as
hydrogen peroxide and peroxynitrite, to promote the loss of the
TCRζ-chain;
MDSCs downregulate T-cell migration to draining
lymph nodes and induce apoptosis via disintegrin and
metalloproteinase domain-containing protein 17/CD62L interaction
and galectin 9/T-cell immunoglobulin and mucin domain-containing
protein 3 interactions;
MDSCs induce the development of M2 macrophages by
releasing ARG1, IL-10 and transforming growth factor (TGF)-β;
MDSCs induce regulatory T cells (Tregs) by releasing
ARG1, IL-10 and TFG-β and after CD40/CD40L and major
histocompatibility complex class II/TCR interaction;
MDSCs prevent DC migration by downregulating DC
maturation and antigen uptake via IL-10 and nitric oxide;
MDSCs inhibit the cytotoxic capacity of natural
killer (NK) cells via TGF-β or indoleamine 2,3-dioxygenase;
MDSCs promote angiogenesis via vascular endothelial
growth factor, which, in turn, promotes Treg and MDSC
proliferation.
Similarly, the macrophage inflammatory protein-1 γ
(best known as CCL9) is upregulated in murine models with obesity
(30) and is considered another
important agent in the recruitment of MDSCs in the TME (31). CCL9 may induce
CD11b+Gr1+ MDSC expansion after binding CCR1
(particularly in G-MDSCs from spleen and bone marrow) in oral
squamous cell carcinoma (OSCC)-bearing mice after treatment with a
high-fat diet (HFD). This diet boosted the immunosuppressive
effects of MDSCs via intracellular fatty acid uptake (32).
In, thymus-expressed chemokine (best known as
CCL25), together with GM-CSF, S100A8 and S100A9 (both upregulated
by IL5 and IL-6), were indicated to be involved in MDSC recruitment
and expansion in peripheral blood, the bone marrow and tumor tissue
from obese ovarian cancer-bearing mice (33). The key role of IL-6 has also been
demonstrated in a pancreatic cancer mouse model with HFD-induced
obesity. The HFD led to G-MDSC and M-MDSC expansion, tumor growth
and proliferation, and failure of T-cell responses (34).
MDSCs also accumulated in the TME of E0771 and
Py8119 BC-bearing mice using an HFD via the growth-regulated α
protein [C-X-C motif chemokine ligand 1 (CXCL1)]/CXCR2 signaling
pathway. This axis was associated with the body mass index (BMI)
and poor survival (35). Adipose
tissue from obese patients with prostate cancer also mobilized IL-8
[known as CXCL8 and an important chemoattractant for MDSCs
(36)] to recruit adipose stromal
cells into the TME and induce tumor growth and progression. Of
note, CXCL1 silencing in vivo promoted CD11b+Gr1+ MDSC
depletion in obese and lean mice (37).
It has been estimated that excess adipose tissue
increases the risk of human cancer by 20%. The underlying
mechanisms that link cancer to obesity need to be completely
understood for adequate treatments to be developed (38). For these reasons, most of the
studies focus on the long-term effectiveness of treatments
(39) instead of the impact of
immunosuppressive cells on obesity-related cancer. Only two studies
analyzed MDSC-like cell populations in obese cancer patients
(Table II).
Of note, obesity is an independent risk factor for
the development of non-alcoholic fatty liver disease (NAFLD) and
non-alcoholic steatohepatitis (NASH)-related hepatocellular
carcinomas (HCC) (49). MDSCs,
together with T cells and tumor-associated macrophages, are highly
infiltrated in patients with NAFLD-/NASH-related HCC (50). Certain mechanisms have been
suggested to explain the activation and recruitment of MDSCs in
these diseases. For instance, it has been shown that the release of
IL-13 could activate MDSCs to promote tumor progression (51). MDSCs expressing PD-L1 and
inducible T-cell co-stimulator have been suggested to interact with
exhausted programmed cell death 1 (PD-1)+CD8+
T cells to induce immunosuppression in these patients (50). The m6A reader protein YTH
N6-methyladenosine RNA binding protein F1 promoted the signaling
axis between the enhancer of zeste homolog 2 protein and IL-6 to
recruit MDSCs; as a result, CD8 T-mediated immune responses were
inhibited (52). In a mouse
model, it has been demonstrated that CD11b+
Ly6CIntLy6G+ MDSCs, but not M-MDSCs, can be
recruited into the liver TME by hepatic cyclin-dependent kinase 20
(also known as CCRK) signaling through mTORC1-dependent G-CSF
expression (53). In this case,
CD11b+ Ly6CIntLy6G+ MDSCs
positively correlated with tumor weight. Also, the administration
of anti-Ly6G antibody significantly suppressed CCRK-induced MDSCs
and reduced liver-related HCC tumorigenicity (53).
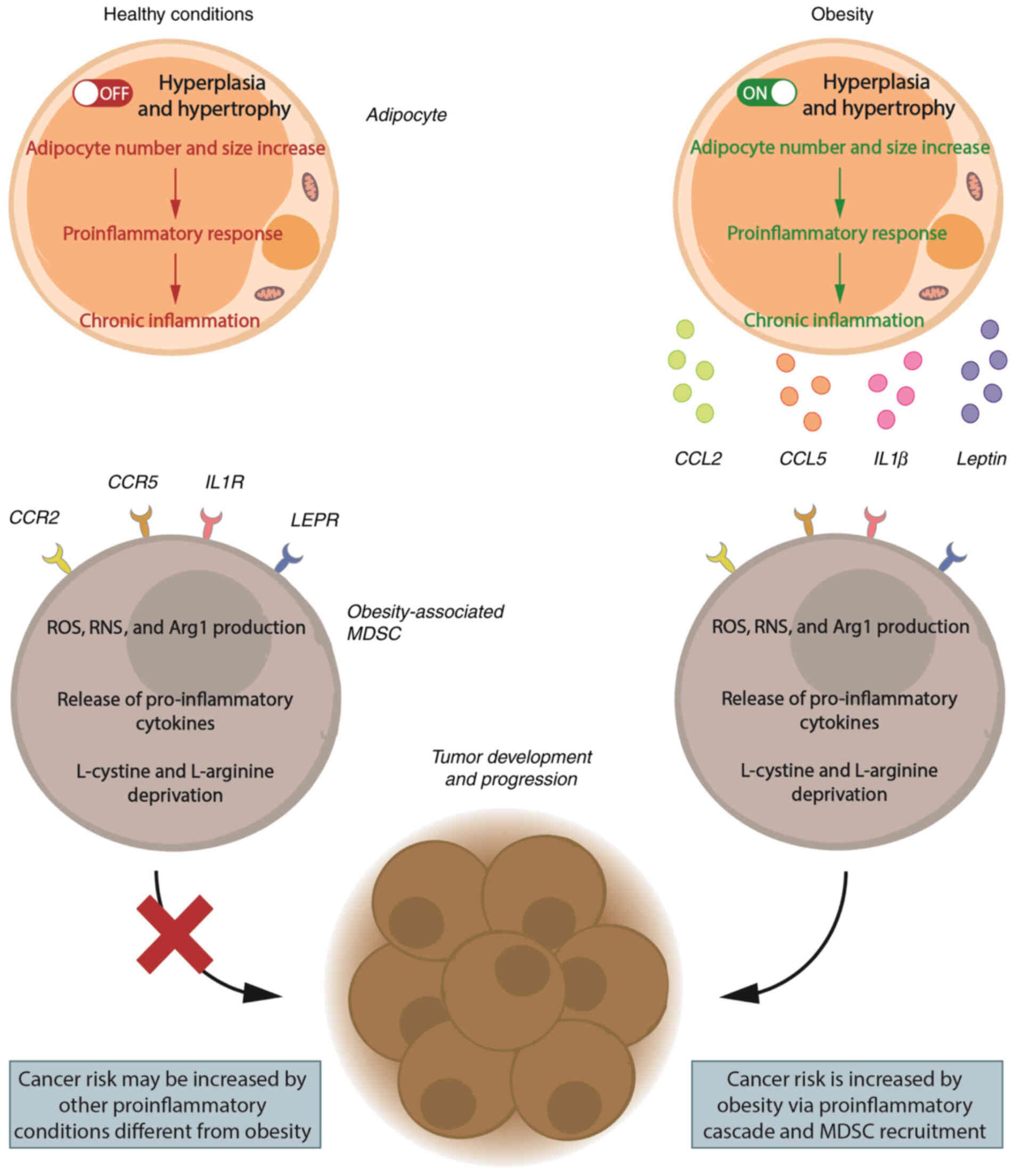
Based on experimental evidence from obese cancer
murine models and obese patients with cancer, the milieu produced
by chronic inflammation during obesity is likely to increase MDSC
recruitment and expansion. This environment promotes cancer
development and increases cancer risk, as shown in Fig. 2.
Chronic inflammation occurs when the immune system
is under permanent, non-resolving stimulation, which can lead to
the development of several health conditions, including obesity
(54). During this prolonged
process, leukocytes and plasma proteins are continually recruited
into affected tissues to promote vascularization and phagocytosis
via proinflammatory signaling (55,56). Chronic inflammation also induces
lymphocyte and macrophage recruitment (57-59).
Adipose tissue has been traditionally known for its
capacity to store energy, but it is currently considered a bona
fide immune organ due to its interactions with the immune
system (60). Obesity may
increase the risk of different conditions and diseases such as
cancer, cardiovascular disease, type 2 diabetes, hypertension,
dyslipidemia and reproductive disorders (2-5).
These effects are induced by the release of a variety of
proinflammatory mediators, including TNF-α, IFN, IL-1β, IL-6,
leptin, resistin, CCL2, CXCL5 and prostaglandin E2, during
adipocyte hypertrophy and hyperplasia [extensively reviewed in
(61,62)]. Similarly, chronic inflammation
can predispose to cancer development, tumor cell dissemination and
metastasis (7,8) by promoting mutagenic DNA damage via
ROS or reactive nitrogen species production (63). The resulting epigenetic
alterations, such as DNA methylation or microRNA dysregulation
(64), increase oxidative stress
and mediate molecular pathways, including NF-κB or signal
transducer and activator of transcription (STAT)3 signaling
(65). These epigenetic
alterations also induce the release of proinflammatory cytokines
(e.g., TNF-α, IL-1β, IL-6 and IL-17) (66).
As mentioned above, cancer and obesity both create
similar chronic inflammatory environments. In a cancer-related
environment, MDSCs are recruited (67), thus leading to tumor growth,
resistance to cancer therapies and poor outcomes (11,68,69). In obese patients, MDSCs are
recruited (70) partly through
the migration of inhibitory factor-related protein (MRP)-8 (best
known as S100A8) and MRP-14 (S100A9), which regulate MDSC-mediated
immunosuppression (40,71). Of note, lipid and fatty acid
metabolisms have been associated with the modulation of MDSC
activity (72). Adeshakin et
al (73) reported that fatty
acid transport protein 2 (FATP2) confers a protumorigenic
environment. This effect is exerted by FATP2 by regulating MDSC and
ROS activity and inducing the accumulation of lipids via the
upregulation of arachidonic acid metabolism (73). Furthermore, the proto-oncogene
serine/threonine-protein kinase Pim-1 has been reported to strongly
correlate with fatty acid oxidation, and its targeting resulted in
MDSC depletion from the TME (74).
Although low levels of circulating MDSCs are found
in healthy individuals, these cells play a major role (see section
on MDSCs) in maintaining immune homeostasis. Thus, MDSCs limit the
hyperactivation of pro-inflammatory cells and reduce tissue damage
(75). In obesity conditions,
maintaining immune homeostasis is crucial to avoid or prevent
adipose tissue dysfunction (76).
Obesity is associated with metabolic disorders such as diabetes,
which is characterized by high glucose levels resulting from the
inability of insulin to suppress hepatic glucose production
(77). The role of MDSCs in these
settings is scarcely known. To date, only a couple of studies have
demonstrated the protective role of MDSCs in obesity-driven
metabolic disorders. Xia et al (70) demonstrated that Gr-1+CD11b+ MDSCs
are highly expressed in the peripheral tissue of obese mice. This
high expression protected mice from inflammation and improved
insulin sensitivity and glucose tolerance (70). The authors suggested that MDSCs
from obese mice induced not only the apoptosis of cytotoxic CD8+ T
cells, but also the polarization of M1 macrophages into their M2
phenotype, thereby exerting insulin-sensitizing effects (70). Similarly, Clements et al
(78) reported that MDSCs from
obese mice reduced inflammation and had protective effects against
metabolic disorders. In parallel, MDSCs increased fat accumulation
and the risk of tumor progression and metastasis (78).
Leptin is a non-glycosylated hormone consisting of
167 amino acids that has been described in leptin-deficient (ob/ob)
and leptin receptor (LEPR)-deficient (db/db) mice (79,80) as the product of the 'obese gene'
(81). Leptin has pleiotropic
effects, and it is not only expressed in adipose tissue, which is
considered the main source of leptin, but also in a variety of
tissues such as the brain, kidney, liver and skeletal muscle
(82). Leptin can also bind
different LEPRs [extensively reviewed in (83)] to transduce activation signals
into cells through the Janus kinase 2/STAT3, MAPK/ERK1/2 and/or
PI3K/AKT signaling pathways (84).
In normal conditions, leptin expression is regulated
to restore physiological functions and homeostasis. This hormone
has a critical role during chronic inflammation (85) in different settings, such as
obesity (86), fertility
(87) and pregnancy (88), and when interacting with immune
cells via proinflammatory mediators (89,90). Closely related to the role of
obesity in cancer, leptin has also been suggested to be involved in
all stages of tumorigenesis. Specifically, leptin binds LEPRs to
induce a variety of processes, including systemic inflammation,
promoting cancer stem cell phenotypes, epithelial-mesenchymal
transition, antiapoptotic proteins, hypoxia and angiogenic factors.
As a result, leptin enhances cancer cell survival, proliferation
and migration, and inhibits T-cell responses (91-96). Hence, leptin correlates with
cancer risk and poor survival rates in numerous types of cancer,
including breast (97),
esophageal (98) and colorectal
cancer (99).
Leptin levels have been documented to be increased
in cancer-bearing mice treated with an HFD. Similar conclusions
have been drawn from cancer patients with obesity (26,34). Specifically, Koprivčić et
al (100) demonstrated that
women with malignant breast tumors exhibited significantly higher
leptin levels (22.24±22.58 ng/ml), as compared to those with benign
tumors (11.21±9.46 ng/ml). In addition, patients with
triple-negative breast tumors (the most aggressive type of BC)
showed higher leptin concentrations (36.11±17.95 ng/ml) than
patients with other breast tumor subtypes (100). Lower concentrations of leptin
have also been reported in invasive BC and breast carcinoma in
situ (10.4±7.0 and 8.7±5.3 ng/ml, respectively), particularly
in postmenopausal women. Of note, leptin concentrations were still
higher in these patients, as compared to healthy donors (8.4±5.3
ng/ml) (101). Another study
comparing leptin levels in patients with prostate cancer and a
healthy cohort revealed serum leptin levels of 14.18 and 1.63
ng/ml, respectively (102). Of
note, Tong et al (103)
did not find any relationship between serum leptin levels and lung
cancer; however, the authors found a significant association
between leptin expression in tissue and lung cancer. In the same
way, LEPR has been reported to be overexpressed in cancer tissue,
as compared with normal mucosa (104).
In line with the previous results, leptin has been
suggested to be involved in the induction and accumulation of MDSCs
(78). Apart from circulating
pro-inflammatory cytokines, adipose tissue also releases other
pro-inflammatory mediators such as leptin. This adipokine, in turn,
may partially elevate the concentration of those cytokines.
Therefore, leptin is involved in a feedback loop that may induce
the inflammatory milieu typically found in a variety of cancers
(105,106). The pro-inflammatory mediators
elevated by leptin are also inducers of both, MDSC differentiation
and accumulation in the TME (107-109).
MDSC depletion in mice is commonly induced using
antibodies against Gr1, which is located on the surface of murine
MDSCs (13). In cancer-bearing
mice with diet-induced obesity, different anti-Gr1 therapies have
been shown to reduce MDSC expression in the TME (32,34,35,78). Chemotherapeutic agents have also
been used to deplete MDSCs (115); however, according to the
American Society of Clinical Oncology guidelines, evidence remains
limited regarding the toxicity and efficacy of chemotherapy in
obese cancer patients (113).
This is probably due to the dysregulation of pharmacokinetics and
metabolism (116). Consequently,
there are no studies reporting the effects of chemotherapy on MDSCs
in obese patients with cancer.
The inhibition of MDSC-mediated immunosuppression
and the blocking of MDSC recruitment are the most widely used
strategies in cancer immunotherapy. In this strategy, the immune
checkpoint PD-1/anti-PD-L1 axis is blocked in one of the treatment
groups. Anti-PD-1 treatments have shown promising survival rates in
obese patients with renal cell carcinoma (26); therefore, it could be a
therapeutic approach to reduce MDSC concentrations and improve
T-cell responses (117,118). Also, the IL-6 inhibitor LMT28
has been demonstrated to reduce ovarian tumor growth in murine
models of obesity and may also be a potential therapy for obese
patients with cancer (33). IL-6
is a crucial regulator of MDSC activity and a target for cancer
immunotherapy (108).
Chemokine-targeted therapies are also useful to deplete the TME of
MDSCs. In this sense, CCR2+ MDSCs have been found in
high proportion in renal cancer murine models treated with an HFD
(29). CCR2 binds CCL2, which is
secreted by adipose tissue to promote MDSC recruitment. These
findings suggest that the CCR2/CCL2 signaling pathway may be a
pivotal target for MDSC depletion (27). Similarly, anti-CXCR1 and
anti-CXCR2 have been successfully used in patients with prostate
cancer with obesity due to it having an involvement in the
CXCR1/CXCL1 and CXCR2/CXCL8 axis; however, levels of MDSCs were not
measured in this study (37). By
contrast, SX-682, a CXCR1 and CXCR2 inhibitor, has been
demonstrated to abrogate tumor trafficking of MDSCs in head and
neck cancer murine models (119); thus, SX-682 may be another
potential therapeutic approach to inhibit MDSC-mediated
immunosuppression.
MDSC differentiation into mature non-suppressive
cells has also been tested. For instance,
cytosine-phosphate-guanosine oligodeoxynucleotides (CPG-ODNs) can
stimulate plasmacytoid dendritic cells to produce IFN-α and, in
turn, promote MDSC maturation in vitro (120). CPG-ODNs have been proven to
improve the efficacy of adenovirus 5 carrying the TNF-related
apoptosis-inducing ligand (Ad5-TRAIL/CpG) in obesity-related renal
cell carcinoma (28). In this
sense, TRAIL was demonstrated to potently induce tumor-cell
apoptosis and the activation of tumor-specific cytotoxic T cells
(121); furthermore, the
addition of Ad5-TRAIL/CpG prolonged TRAIL production (122). Of note, TRAIL receptors can be
considered potential targets to selectively inhibit MDSCs without
them affecting mature myeloid or lymphoid cells (123,124).
Other therapeutic approaches to induce MDSC
differentiation into mature cells in cancer patients include STAT3
inhibition (125) and the use of
all-trans retinoic acid (ATRA) (126) or vitamin D3 (127). Of these, only STAT3 inhibitors
have been successfully used in cancer mouse models of obesity
(128,129). However, the impact of this
therapy on MDSCs remains to be evaluated. ATRA therapy may be
optimal to prevent obesity-related metabolic syndrome (130), although its role in
obesity-related cancer has not yet been tested. Conversely, the
clinical applications of vitamin D in obese patients remain
controversial because of the confounding factors and limitations
reported in numerous studies (131). Hence, the use of vitamin D still
cannot be recommended for obese patients with cancer, despite the
promising results achieved in the depletion of tumor MDSCs.
Based on the evidence provided by preclinical and
clinical studies, obesity may be potentially associated with
carcinogenesis. The chronic inflammatory environment promoted by
obesity leads to MDSC recruitment, which has a key role in cancer
development and progression.
MDSC depletion in obesity-related tumors has been
successfully evaluated and associated with improved survival rates
and lower tumor progression. However, most of the results have been
obtained in murine models. It is worth mentioning that Gr1
inhibitors can only be tested in murine models, since Gr1 is a
typical marker of mouse MDSCs. Therefore, its translation into
clinical practice must be further investigated. Specific treatments
for oncological patients may include anti-PD-1, anti-IL-6,
anti-CCL2, anti-CXCR1, anti-CXCR2 and Ad5-TRAIL/CpG. However, there
are a variety of MDSC-targeted options that still need to be
tested, such as ATRA, vitamin D or STAT3 inhibitors, anti-CCR5,
anti-VEGFR and chemotherapeutic agents (114).
Special emphasis has been placed on the role of
obesity as a protective factor in tumorigenesis (the so-called
'obesity paradox') (132,133),
which has been documented in a variety of tumors, including
lymphomas (134,135); acute myeloid leukemia (136); hepatocellular cancer (137); colorectal cancer (138,139); renal cell carcinoma (140-143); lung cancer (144-146); or melanoma (147-149). In this sense, certain studies
have demonstrated the inhibitory effects of leptin in vitro
(150,151). Evidence has also been provided
of an inverse relationship with cancer risk and a positive
association with improved outcomes (151,152), which has been termed the 'leptin
paradox' (96).
The reasons for the positive association between
high amounts of adipose tissue and improved response to cancer
immunotherapies remain unknown (153). These phenomena could be
explained by the fact that BMI estimation does not only consider
adipose tissue but also protective muscle mass; consequently, the
BMI should be dismissed as a tool for measuring adiposity (154). Finding optimal parameters may be
difficult, since there are many variables that hinder the accurate
measurement of adiposity, such as sex, ethnicity, age and
pathophysiological group (155).
Cancer immunosuppression driven by
obesity-associated MDSCs seems clear, but further research is
needed, particularly in cancer patients. There are still various
gaps of knowledge, including i) the mechanisms involved in the
relationship between obesity, cancer and MDSCs; ii) the mechanisms
by which obesity or leptin exert protective effects in certain
types of cancer and the role of MDSCs in these settings; and iii)
the efficacy and safety of other MDSC-targeted therapies.
Not applicable.
CJC, CGG, FSJ, TVG, RFC, APP, CG, MLSL, DJGD, LHP
and NPC contributed to the literature search. CJC and CGG wrote the
manuscript draft. LdlCM and VSM were involved in the
conceptualization of the study. All authors contributed to the
revision of the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors acknowledge Mrs María Palazón-Carrión
(Oncology Service, Virgen Macarena University Hospital, School of
Medicine, University of Seville, Seville, Spain) for project
administration work.
The study was supported by PAIDI Fonds to Resarch Groups
(CTS-151) Junta de Andalucía (Andalucía, Spain).
|
1
|
Lee H, Lee IS and Choue R: Obesity,
inflammation and diet. Pediatr Gastroenterol Hepatol Nutr.
16:143–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ben-Shmuel S, Rostoker R, Scheinman EJ and
LeRoith D: Metabolic Syndrome, type 2 diabetes, and cancer:
Epidemiology and potential mechanisms. Handb Exp Pharmacol.
233:355–372. 2016. View Article : Google Scholar
|
|
3
|
Jiang SZ, Lu W, Zong XF, Ruan HY and Liu
Y: Obesity and hypertension. Exp Ther Med. 12:2395–2399. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klop B, Elte JW and Cabezas MC:
Dyslipidemia in obesity: Mechanisms and potential targets.
Nutrients. 5:1218–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broughton DE and Moley KH: Obesity and
female infertility: Potential mediators of obesity's impact. Fertil
Steril. 107:840–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanchez-Pino MD, Gilmore LA, Ochoa AC and
Brown JC: Obesity-Associated myeloid immunosuppressive cells, key
players in cancer risk and response to immunotherapy. Obesity
(Silver Spring). 29:944–953. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munn LL: Cancer and inflammation. Wiley
Interdiscip Rev Syst Biol Med. 9: View Article : Google Scholar : 2017.
|
|
8
|
Greten FR and Grivennikov SI: Inflammation
and Cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jimenez-Cortegana C, Palazon-Carrion N,
Martin Garcia-Sancho A, Nogales-Fer nandez E, Carnicero-Gonzalez F,
Rios-Herranz E, de la Cruz-Vicente F, Rodríguez-García G,
Fernández-Álvarez R, Rueda Dominguez A, et al: Circulating
myeloid-derived suppressor cells and regulatory T cells as
immunological biomarkers in refractory/relapsed diffuse large
B-cell lymphoma: Translational results from the R2-GDP-GOTEL trial.
J Immunother Cancer. 9:e0023232021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karin N: The development and homing of
myeloid-derived suppressor cells: From a two-stage model to a
multistep narrative. Front Immunol. 11:5575862020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-Derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Movahedi K, Guilliams M, Van den Bossche
J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P and Van
Ginderachter JA: Identification of discrete tumor-induced
myeloid-derived suppressor cell subpopulations with distinct T
cell-suppressive activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jimenez-Cortegana C, Liro J,
Palazon-Carrion N, Salamanca E, Sojo-Dorado J, de la Cruz-Merino L,
Pascual Á, Rodríguez-Baño J and Sánchez-Margalet V: Increased blood
monocytic myeloid derived suppressor cells but low regulatory T
lymphocytes in patients with mild COVID-19. Viral Immunol.
34:639–645. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jimenez-Cortegana C, Sanchez - Martinez P
M, Palazon-Carrion N, Nogales-Fernandez E, Henao-Carrasco F, Martin
Garcia-Sancho A, Rueda A, Provencio M, de la Cruz-Merino L and
Sánchez-Margalet V: Lower survival and increased circulating
suppressor cells in patients with relapsed/refractory diffuse large
B-Cell lymphoma with deficit of vitamin D Levels Using R-GDP Plus
Lenalidomide (R2-GDP): Results from the R2-GDP-GOTEL Trial. Cancers
(Basel). 13:46222021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farshidpour M, Ahmed M, Junna S and
Merchant JL: Myeloid-derived suppressor cells in gastrointestinal
cancers: A systemic review. World J Gastrointest Oncol. 13:1–11.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Connor MA, Rastad JL and Green WR: The
role of myeloid-derived suppressor cells in viral infection. Viral
Immunol. 30:82–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan L, Liang M, Yang T, Ji J, Jose Kumar
Sreena GS, Hou X, Cao M and Feng Z: The immunoregulatory role of
myeloid-derived suppressor cells in the pathogenesis of Rheumatoid
arthritis. Front Immunol. 11:5683622020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Guo J, Weng L, Tang W, Jin S and
Ma W: Myeloid-derived suppressor cells-new and exciting players in
lung cancer. J Hematol Oncol. 13:102020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arner E, Mejhert N, Kulyte A, Balwierz PJ,
Pachkov M, Cormont M, Lorente-Cebrián S, Ehrlund A, Laurencikiene
J, Hedén P, et al: Adipose tissue microRNAs as regulators of CCL2
production in human obesity. Diabetes. 61:1986–1993. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oo MW, Kawai H, Takabatake K, Tomida S,
Eguchi T, Ono K, Shan Q, Ohara T, Yoshida S, Omori H, et al:
Resident stroma-secreted chemokine CCL2 governs myeloid-derived
suppressor cells in the tumor microenvironment. JCI Insight.
7:e1489602022. View Article : Google Scholar :
|
|
24
|
Martinez-Chacon G, Yatkin E, Polari L,
Deniz Dinc D, Peuhu E, Hartiala P, Saarinen N and Mäkelä S: CC
chemokine ligand 2 (CCL2) stimulates aromatase gene expression in
mammary adipose tissue. FASEB J. 35:e215362021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friesenhengst A, Pribitzer-Winner T, Miedl
H, Prostling K and Schreiber M: Elevated aromatase (CYP19A1)
expression is associated with a poor survival of patients with
estrogen receptor positive breast cancer. Horm Cancer. 9:128–138.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boi SK, Orlandella RM, Gibson JT, Turbitt
WJ, Wald G, Thomas L, Buchta Rosean C, Norris KE, Bing M, Bertrand
L, et al: Obesity diminishes response to PD-1-based immunotherapies
in renal cancer. J Immunother Cancer. 8:e0007252020. View Article : Google Scholar :
|
|
27
|
Liu Y, Tiruthani K, Wang M, Zhou X, Qiu N,
Xiong Y, Pecot CV, Liu R and Huang L: Tumor-targeted gene therapy
with lipid nanoparticles inhibits tumor-associated adipocytes and
remodels the immunosuppressive tumor microenvironment in
triple-negative breast cancer. Nanoscale Horiz. 6:319–329. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
James BR, Anderson KG, Brincks EL, Kucaba
TA, Norian LA, Masopust D and Griffith TS: CpG-mediated modulation
of MDSC contributes to the efficacy of Ad5-TRAIL therapy against
renal cell carcinoma. Cancer Immunol Immunother. 63:1213–1227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hale M, Itani F, Buchta CM, Wald G, Bing M
and Norian LA: Obesity triggers enhanced MDSC accumulation in
murine renal tumors via elevated local production of CCL2. PLoS
One. 10:e01187842015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiao P, Chen Q, Shah S, Du J, Tao B,
Tzameli I, Yan W and Xu H: Obesity-related upregulation of monocyte
chemotactic factors in adipocytes: Involvement of nuclear
factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes.
58:104–115. 2009. View Article : Google Scholar :
|
|
31
|
Li B, Zhang S, Huang N, Chen H, Wang P,
Yang J and Li Z: CCL9/CCR1 induces myeloidderived suppressor cell
recruitment to the spleen in a murine H22 orthotopic hepatoma
model. Oncol Rep. 41:608–618. 2019.
|
|
32
|
Peng J, Hu Q, Chen X, Wang C, Zhang J, Ren
X, Wang Y, Tao X, Li H, Song M, et al: Diet-induced obesity
accelerates oral carcinogenesis by recruitment and functional
enhancement of myeloid-derived suppressor cells. Cell Death Dis.
12:9462021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Q, Yu B, Kang J, Li A and Sun J:
Obesity promotes tumor immune evasion in ovarian cancer through
increased production of myeloid-derived suppressor cells via IL-6.
Cancer Manag Res. 13:7355–7363. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turbitt WJ, Collins SD, Meng H and Rogers
CJ: Increased adiposity enhances the accumulation of MDSCs in the
tumor microenvironment and adipose tissue of pancreatic
tumor-bearing mice and in immune organs of tumor-free hosts.
Nutrients. 11:30122019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gibson JT, Orlandella RM, Turbitt WJ,
Behring M, Manne U, Sorge RE and Norian LA: Obesity-Associated
myeloid-derived suppressor cells promote apoptosis of
tumor-infiltrating CD8 T cells and immunotherapy resistance in
breast cancer. Front Immunol. 11:5907942020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alfaro C, Teijeira A, Onate C, Perez G,
Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A,
Rodriguez-Paulete A, et al: Tumor-Produced interleukin-8 attracts
human myeloid-derived suppressor cells and elicits extrusion of
neutrophil extracellular traps (NETs). Clin Cancer Res.
22:3924–3936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang T, Tseng C, Zhang Y, Sirin O, Corn
PG, Li-Ning-Tapia EM, Troncoso P, Davis J, Pettaway C, Ward J, et
al: CXCL1 mediates obesity-associated adipose stromal cell
trafficking and function in the tumour microenvironment. Nat
Commun. 7:116742016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Pergola G and Silvestris F: Obesity as
a major risk factor for cancer. J Obes. 2013:2915462013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ross KH, Gogineni K, Subhedar PD, Lin JY
and McCullough LE: Obesity and cancer treatment efficacy: Existing
challenges and opportunities. Cancer. 125:1588–1592. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bao Y, Mo J, Ruan L and Li G: Increased
monocytic CD14(+) HLADRlow/-myeloid-derived suppressor cells in
obesity. Mol Med Rep. 11:2322–2328. 2015. View Article : Google Scholar
|
|
41
|
Rudolph BM, Loquai C, Gerwe A, Bacher N,
Steinbrink K, Grabbe S and Tuettenberg A: Increased frequencies of
CD11b(+) CD33(+) CD14(+) HLA-DR(low) myeloid-derived suppressor
cells are an early event in melanoma patients. Exp Dermatol.
23:202–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verschoor CP, Johnstone J, Millar J,
Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL and
Bowdish DM: Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells
are increased with age and a history of cancer. J Leukoc Biol.
93:633–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Margaroli C, Cardenas MA, Jansen CS, Moon
Reyes A, Hosseinzadeh F, Hong G, Zhang Y, Kissick H, Tirouvanziam R
and Master VA: The immunosuppressive phenotype of
tumor-infiltrating neutrophils is associated with obesity in kidney
cancer patients. Oncoimmunology. 9:17477312020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hafida S, Mirshahi T and Nikolajczyk BS:
The impact of bariatric surgery on inflammation: Quenching the fire
of obesity? Curr Opin Endocrinol Diabetes Obes. 23:373–378. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grzywa TM, Sosnowska A, Matryba P,
Rydzynska Z, Jasinski M, Nowis D and Golab J: Myeloid cell-derived
arginase in cancer immune response. Front Immunol. 11:9382020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deryugina E, Carre A, Ardi V, Muramatsu T,
Schmidt J, Pham C and Quigley JP: Neutrophil elastase facilitates
tumor cell intravasation and early metastatic events. iScience.
23:1017992020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lerman I, Garcia-Hernandez ML,
Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, Krolewski JJ, Sen
A and Hammes SR: Infiltrating myeloid cells exert protumorigenic
actions via neutrophil elastase. Mol Cancer Res. 15:1138–1152.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saitta C, Pollicino T and Raimondo G:
Obesity and liver cancer. Ann Hepatol. 18:810–815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li M, Wang L, Cong L, Wong CC, Zhang X,
Chen H, Zeng T, Li B, Jia X, Huo J, et al: Spatial proteomics of
immune microenvironment in nonalcoholic steatohepatitis-associated
hepatocellular carcinoma. Hepatology. 79:560–574. 2024. View Article : Google Scholar
|
|
51
|
Ponziani FR, Bhoori S, Castelli C,
Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D,
Paroni Sterbini F, Petito V, et al: Hepatocellular carcinoma is
associated with gut microbiota profile and inflammation in
nonalcoholic fatty liver disease. Hepatology. 69:107–120. 2019.
View Article : Google Scholar
|
|
52
|
Wang L, Zhu L, Liang C, Huang X, Liu Z,
Huo J, Zhang Y, Zhang Y, Chen L, Xu H, et al: Targeting
N6-methyladenosine reader YTHDF1 with siRNA boosts antitumor
immunity in NASH-HCC by inhibiting EZH2-IL-6 axis. J Hepatol.
79:1185–1200. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun H, Yang W, Tian Y, Zeng X, Zhou J, Mok
MTS, Tang W, Feng Y, Xu L, Chan AWH, et al: An inflammatory-CCRK
circuitry drives mTORC1-dependent metabolic and immunosuppressive
reprogramming in obesity-associated hepatocellular carcinoma. Nat
Commun. 9:52142018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Furman D, Campisi J, Verdin E,
Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW,
Fasano A, Miller GW, et al: Chronic inflammation in the etiology of
disease across the life span. Nat Med. 25:1822–1832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Muller WA: Getting leukocytes to the site
of inflammation. Vet Pathol. 50:7–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Klevebro S, Bjorkander S, Ekstrom S, Merid
SK, Gruzieva O, Malarstig A, Johansson Å, Kull I, Bergström A and
Melén E: Inflammation-related plasma protein levels and association
with adiposity measurements in young adults. Sci Rep. 11:113912021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sakai Y and Kobayashi M: Lymphocyte
'homing' and chronic inflammation. Pathol Int. 65:344–354. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ingersoll MA, Platt AM, Potteaux S and
Randolph GJ: Monocyte trafficking in acute and chronic
inflammation. Trends Immunol. 32:470–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wensveen FM, Valentic S, Sestan M,
Wensveen TT and Polic B: Interactions between adipose tissue and
the immune system in health and malnutrition. Semin Immunol.
27:322–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kumar DP, Koka S, Li C and Rajagopal S:
Inflammatory mediators in obesity. Mediators Inflamm.
2019:94818192019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Howe LR, Subbaramaiah K, Hudis CA and
Dannenberg AJ: Molecular pathways: Adipose inflammation as a
mediator of obesity-associated cancer. Clin Cancer Res.
19:6074–6083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kawanishi S, Ohnishi S, Ma N, Hiraku Y and
Murata M: Crosstalk between DNA damage and inflammation in the
multiple steps of carcinogenesis. Int J Mol Sci. 18:18082017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fan Y, Mao R and Yang J: NF-ĸB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Del Prete A, Allavena P, Santoro G,
Fumarulo R, Corsi MM and Mantovani A: Molecular pathways in
cancer-related inflammation. Biochem Med (Zagreb). 21:264–275.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang Y, Li C, Liu T, Dai X and Bazhin AV:
Myeloid-Derived suppressor cells in tumors: From mechanisms to
antigen specificity and microenvironmental regulation. Front
Immunol. 11:13712020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ma P, Beatty PL, McKolanis J, Brand R,
Schoen RE and Finn OJ: Circulating myeloid derived suppressor cells
(MDSC) that accumulate in premalignancy share phenotypic and
functional characteristics with MDSC in cancer. Front Immunol.
10:14012019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Veglia F, Sanseviero E and Gabrilovich DI:
Myeloid-derived suppressor cells in the era of increasing myeloid
cell diversity. Nat Rev Immunol. 21:485–498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xia S, Sha H, Yang L, Ji Y,
Ostrand-Rosenberg S and Qi L: Gr-1+ CD11b+ myeloid-derived
suppressor cells suppress inflammation and promote insulin
sensitivity in obesity. J Biol Chem. 286:23591–23599. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Srikrishna G: S100A8 and S100A9: New
insights into their roles in malignancy. J Innate Immun. 4:31–40.
2012. View Article : Google Scholar :
|
|
72
|
Siddiqui S and Glauben R: Fatty acid
metabolism in myeloid-derived suppressor cells and tumor-associated
macrophages: Key factor in cancer immune evasion. Cancers (Basel).
14:2502022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Adeshakin AO, Liu W, Adeshakin FO, Afolabi
LO, Zhang M, Zhang G, Wang L, Li Z, Lin L, Cao Q, et al: Regulation
of ROS in myeloid-derived suppressor cells through targeting fatty
acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy.
Cell Immunol. 362:1042862021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xin G, Chen Y, Topchyan P, Kasmani MY,
Burns R, Volberding PJ, Wu X, Cohn A, Chen Y, Lin CW, et al:
Targeting PIM1-Mediated metabolism in myeloid suppressor cells to
treat cancer. Cancer Immunol Res. 9:454–469. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sanchez-Pino MD, Dean MJ and Ochoa AC:
Myeloid-derived suppressor cells (MDSC): When good intentions go
awry. Cell Immunol. 362:1043022021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhen Y, Shu W, Hou X and Wang Y: Innate
immune system orchestrates metabolic homeostasis and dysfunction in
visceral adipose tissue during obesity. Front Immunol.
12:7028352021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Klein S, Gastaldelli A, Yki-Jarvinen H and
Scherer PE: Why does obesity cause diabetes? Cell Metab. 34:11–20.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Clements VK, Long T, Long R, Figley C,
Smith DMC and Ostrand-Rosenberg S: Frontline Science: High fat diet
and leptin promote tumor progression by inducing myeloid-derived
suppressor cells. J Leukoc Biol. 103:395–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ingalls AM, Dickie MM and Snell GD: Obese,
a new mutation in the house mouse. J Hered. 41:317–318. 1950.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hummel KP, Dickie MM and Coleman DL:
Diabetes, a new mutation in the mouse. Science. 153:1127–1278.
1966. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Munzberg H and Heymsfield SB: New insights
into the regulation of leptin gene expression. Cell Metab.
29:1013–1014. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gorska E, Popko K, Stelmaszczyk-Emmel A,
Ciepiela O, Kucharska A and Wasik M: Leptin receptors. Eur J Med
Res. 15(Suppl 2): S50–S54. 2010. View Article : Google Scholar
|
|
84
|
Park HK and Ahima RS: Leptin signaling.
F1000Prime Rep. 6:732014. View
Article : Google Scholar : PubMed/NCBI
|
|
85
|
Perez-Perez A, Sanchez-Jimenez F,
Vilarino-Garcia T and Sanchez-Margalet V: Role of leptin in
inflammation and vice versa. Int J Mol Sci. 21:58872020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Obradovic M, Sudar-Milovanovic E, Soskic
S, Essack M, Arya S, Stewart AJ, Gojobori T and Isenovic ER: Leptin
and Obesity: Role and Clinical Implication. Front Endocrinol
(Lausanne). 12:5858872021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Vilarino-Garcia T, Perez-Perez A,
Santamaria-Lopez E, Prados N, Fernandez-Sanchez M and
Sanchez-Margalet V: Sam68 mediates leptin signaling and action in
human granulosa cells: Possible role in leptin resistance in PCOS.
Endocr Connect. 9:479–488. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Perez-Perez A, Toro A, Vilarino-Garcia T,
Maymo J, Guadix P, Duenas JL, Fernández-Sánchez M, Varone C and
Sánchez-Margalet V: Leptin action in normal and pathological
pregnancies. J Cell Mol Med. 22:716–727. 2018. View Article : Google Scholar :
|
|
89
|
Fernandez-Riejos P, Najib S,
Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C
and Sánchez-Margalet V: Role of leptin in the activation of immune
cells. Mediators Inflamm. 2010:5683432010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Perez-Perez A, Vilarino-Garcia T,
Fernandez-Riejos P, Martin-Gonzalez J, Segura-Egea JJ and
Sanchez-Margalet V: Role of leptin as a link between metabolism and
the immune system. Cytokine Growth Factor Rev. 35:71–84. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dutta D, Ghosh S, Pandit K, Mukhopadhyay P
and Chowdhury S: Leptin and cancer: Pathogenesis and modulation.
Indian J Endocrinol Metab. 16(Suppl 3): S596–S600. 2012. View Article : Google Scholar
|
|
92
|
Ando S and Catalano S: The multifactorial
role of leptin in driving the breast cancer microenvironment. Nat
Rev Endocrinol. 8:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feldman DE, Chen C, Punj V, Tsukamoto H
and Machida K: Pluripotency factor-mediated expression of the
leptin receptor (OB-R) links obesity to oncogenesis through
tumor-initiating stem cells. Proc Natl Acad Sci USA. 109:829–834.
2012. View Article : Google Scholar :
|
|
94
|
Ghasemi A, Saeidi J, Azimi-Nejad M and
Hashemy SI: Leptin-induced signaling pathways in cancer cell
migration and invasion. Cell Oncol (Dordr). 42:243–260. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ray A and Cleary MP: The potential role of
leptin in tumor invasion and metastasis. Cytokine Growth Factor
Rev. 38:80–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jimenez-Cortegana C, Lopez-Saavedra A,
Sanchez-Jimenez F, Perez-Perez A, Castineiras J, Virizuela-Echaburu
JA, de la Cruz-Merino L and Sánchez-Margalet V: Leptin, both bad
and good actor in cancer. Biomolecules. 11:9132021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sanchez-Jimenez F, Perez-Perez A, de la
Cruz-Merino L and Sanchez-Margalet V: Obesity and breast cancer:
Role of leptin. Front Oncol. 9:5962019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Greer KB, Falk GW, Bednarchik B, Li L and
Chak A: Associations of serum adiponectin and leptin with barrett's
esophagus. Clin Gastroenterol Hepatol. 13:2265–2272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li C, Quan J, Wei R, Zhao Z, Guan X, Liu
Z, Zou S, Wang X and Jiang Z: Leptin overexpression as a poor
prognostic factor for colorectal cancer. Biomed Res Int.
2020:75325142020.PubMed/NCBI
|
|
100
|
Koprivčić I, Marjanovic K, Matic A,
Tolusic Levak M, Lovric I, Pauzar B, Erić I and Wertheimer V: Serum
leptin level in breast cancer. Acta Clin Croat. 61:79–85. 2022.
|
|
101
|
Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH,
Yu CP, Yu JC and Sun CA: Circulating levels of leptin, adiposity
and breast cancer risk. Br J Cancer. 100:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Singh SK, Grifson JJ, Mavuduru RS, Agarwal
MM, Mandal AK and Jha V: Serum leptin: A marker of prostate cancer
irrespective of obesity. Cancer Biomark. 7:11–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tong X, Ma Y, Zhou Q, He J, Peng B, Liu S,
Yan Z, Yang X and Fan H: Serum and tissue leptin in lung cancer: A
meta-analysis. Oncotarget. 8:19699–19711. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chludzinska-Kasperuk S, Lewko J,
Sierzantowicz R, Krajewska-Kulak E and Reszec-Gielazyn J: The
effect of serum leptin concentration and leptin receptor expression
on colorectal cancer. Int J Environ Res Public Health. 20:49512023.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Inacio Pinto N, Carnier J, Oyama LM, Otoch
JP, Alcantara PS, Tokeshi F and Nascimento CM: Cancer as a
proinflammatory environment: Metastasis and cachexia. Mediators
Inflamm. 2015:7910602015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ostrand-Rosenberg S: Myeloid
derived-suppressor cells: Their role in cancer and obesity. Curr
Opin Immunol. 51:68–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng
J, Wu H, Xu X, Erben U, Wu P, et al: TNF signaling drives
myeloid-derived suppressor cell accumulation. J Clin Invest.
122:4094–4104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Weber R, Groth C, Lasser S, Arkhypov I,
Petrova V, Altevogt P, Utikal J and Umansky V: IL-6 as a major
regulator of MDSC activity and possible target for cancer
immunotherapy. Cell Immunol. 359:1042542021. View Article : Google Scholar
|
|
109
|
Elkabets M, Ribeiro VS, Dinarello CA,
Ostrand-Rosenberg S, Di Santo P, Apte RN and Vosshenrich CA: IL-1β
regulates a novel myeloid-derived suppressor cell subset that
impairs NK cell development and function. Eur J Immunol.
40:3347–3357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Avgerinos KI, Spyrou N, Mantzoros CS and
Dalamaga M: Obesity and cancer risk: Emerging biological mechanisms
and perspectives. Metabolism. 92:121–135. 2019. View Article : Google Scholar
|
|
111
|
Lauby-Secretan B, Scoccianti C, Loomis D,
Grosse Y, Bianchini F and Straif K; International Agency for
Research on Cancer Handbook Working Group: Body Fatness and
Cancer-Viewpoint of the IARC Working Group. N Engl J Med.
375:794–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Griggs JJ, Mangu PB, Anderson H, Balaban
EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD,
Rosner GL, et al: Appropriate chemotherapy dosing for obese adult
patients with cancer: American Society of Clinical Oncology
clinical practice guideline. J Clin Oncol. 30:1553–1561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Griggs JJ, Bohlke K, Balaban EP, Dignam
JJ, Hall ET, Harvey RD, Hecht DP, Klute KA, Morrison VA, Pini TM,
et al: Appropriate systemic therapy dosing for obese adult patients
with cancer: ASCO Guideline Update. J Clin Oncol. 39:2037–2048.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
De Cicco P, Ercolano G and Ianaro A: The
new Era of cancer immunotherapy: Targeting myeloid-derived
suppressor cells to overcome immune evasion. Front Immunol.
11:16802020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang Y, Jia A, Bi Y, Wang Y, Yang Q, Cao
Y, Li Y and Liu G: Targeting myeloid-derived suppressor cells in
cancer immunotherapy. Cancers (Basel). 12:26262020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Horowitz NS and Wright AA: Impact of
obesity on chemotherapy management and outcomes in women with
gynecologic malignancies. Gynecol Oncol. 138:201–206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li X, Zhong J, Deng X, Guo X, Lu Y, Lin J,
Huang X and Wang C: Targeting myeloid-derived suppressor cells to
enhance the antitumor efficacy of immune checkpoint blockade
therapy. Front Immunol. 12:7541962021. View Article : Google Scholar
|
|
118
|
Pingili AK, Chaib M, Sipe LM, Miller EJ,
Teng B, Sharma R, Asemota S, Al Abdallah Q, Mims TS, Marion TN, et
al: Immune checkpoint blockade reprograms systemic immune landscape
and tumor microenvironment in obesity-associated breast cancer.
Cell Rep. 35:1092852021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Greene S, Robbins Y, Mydlarz WK, Huynh AP,
Schmitt NC, Friedman J, Horn LA, Palena C, Schlom J, Maeda DY, et
al: Inhibition of MDSC Trafficking with SX-682, a CXCR1/2
Inhibitor, Enhances NK-cell immunotherapy in head and neck cancer
models. Clin Cancer Res. 26:1420–1431. 2020. View Article : Google Scholar
|
|
120
|
Zoglmeier C, Bauer H, Noerenberg D,
Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S and
Bourquin C: CpG blocks immunosuppression by myeloid-derived
suppressor cells in tumor-bearing mice. Clin Cancer Res.
17:1765–1775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
VanOosten RL and Griffith TS: Activation
of tumor-specific CD8+ T Cells after intratumoral Ad5-TRAIL/CpG
oligodeoxynucleotide combination therapy. Cancer Res.
67:11980–11990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Griffith TS and Broghammer EL: Suppression
of tumor growth following intralesional therapy with TRAIL
recombinant adenovirus. Mol Ther. 4:257–266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Condamine T, Kumar V, Ramachandran IR,
Youn JI, Celis E, Finnberg N, El-Deiry WS, Winograd R, Vonderheide
RH, English NR, et al: ER stress regulates myeloid-derived
suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin
Invest. 124:2626–2639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dominguez GA, Condamine T, Mony S,
Hashimoto A, Wang F, Liu Q, Forero A, Bendell J, Witt R, Hockstein
N, et al: Selective targeting of myeloid-derived suppressor cells
in cancer patients using DS-8273a, an Agonistic TRAIL-R2 Antibody.
Clin Cancer Res. 23:2942–2950. 2017. View Article : Google Scholar :
|
|
125
|
Zou S, Tong Q, Liu B, Huang W, Tian Y and
Fu X: Targeting STAT3 in Cancer Immunotherapy. Mol Cancer.
19:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Nefedova Y, Fishman M, Sherman S, Wang X,
Beg AA and Gabrilovich DI: Mechanism of all-trans retinoic acid
effect on tumor-associated myeloid-derived suppressor cells. Cancer
Res. 67:11021–11028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY,
Chen WC and Chen MF: 1α,25-Dihydroxyvitamin D3 inhibits esophageal
squamous cell carcinoma progression by Reducing IL6 Signaling. Mol
Cancer Ther. 14:1365–1375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chang CC, Wu MJ, Yang JY, Camarillo IG and
Chang CJ: Leptin-STAT3-G9a signaling promotes obesity-mediated
breast cancer progression. Cancer Res. 75:2375–2386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Park JW, Han CR, Zhao L, Willingham MC and
Cheng SY: Inhibition of STAT3 activity delays obesity-induced
thyroid carcinogenesis in a mouse model. Endocr Relat Cancer.
23:53–63. 2016. View Article : Google Scholar
|
|
130
|
Berry DC and Noy N: All-trans-retinoic
acid represses obesity and insulin resistance by activating both
peroxisome proliferation-activated receptor beta/delta and retinoic
acid receptor. Mol Cell Biol. 29:3286–3296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Karampela I, Sakelliou A, Vallianou N,
Christodoulatos GS, Magkos F and Dalamaga M: Vitamin D and Obesity:
Current evidence and controversies. Curr Obes Rep. 10:162–180.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lennon H, Sperrin M, Badrick E and Renehan
AG: The obesity paradox in cancer: A review. Curr Oncol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lee DH and Giovannucci EL: The obesity
paradox in cancer: Epidemiologic insights and perspectives. Curr
Nutr Rep. 8:175–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Weiss L, Melchardt T, Habringer S,
Boekstegers A, Hufnagl C, Neureiter D, Hopfinger G, Greil R and
Egle A: Increased body mass index is associated with improved
overall survival in diffuse large B-cell lymphoma. Ann Oncol.
25:171–176. 2014. View Article : Google Scholar
|
|
135
|
Stevenson JKR, Qiao Y, Chan KKW, Beca J,
Isaranuwatchai W, Guo H, Schwartz D, Arias J, Gavura S, Dai WF, et
al: Improved survival in overweight and obese patients with
aggressive B-cell lymphoma treated with rituximab-containing
chemotherapy for curative intent. Leuk Lymphoma. 60:1399–1408.
2019. View Article : Google Scholar
|
|
136
|
Brunner AM, Sadrzadeh H, Feng Y, Drapkin
BJ, Ballen KK, Attar EC, Amrein PC, McAfee SL, Chen YB, Neuberg DS
and Fathi AT: Association between baseline body mass index and
overall survival among patients over age 60 with acute myeloid
leukemia. Am J Hematol. 88:642–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tsang NM, Pai PC, Chuang CC, Chuang WC,
Tseng CK, Chang KP, Yen TC, Lin JD and Chang JT: Overweight and
obesity predict better overall survival rates in cancer patients
with distant metastases. Cancer Med. 5:665–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Schlesinger S, Siegert S, Koch M, Walter
J, Heits N, Hinz S, Jacobs G, Hampe J, Schafmayer C and Nöthlings
U: Postdiagnosis body mass index and risk of mortality in
colorectal cancer survivors: A prospective study and meta-analysis.
Cancer Causes Control. 25:1407–1418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Amptoulach S, Gross G and Kalaitzakis E:
Differential impact of obesity and diabetes mellitus on survival
after liver resection for colorectal cancer metastases. J Surg Res.
199:378–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Parker AS, Lohse CM, Cheville JC, Thiel
DD, Leibovich BC and Blute ML: Greater body mass index is
associated with better pathologic features and improved outcome
among patients treated surgically for clear cell renal cell
carcinoma. Urology. 68:741–746. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Waalkes S, Merseburger AS, Kramer MW,
Herrmann TR, Wegener G, Rustemeier J, Hofmann R, Schrader M, Kuczyk
MA and Schrader AJ: Obesity is associated with improved survival in
patients with organ-confined clear-cell kidney cancer. Cancer
Causes Control. 21:1905–1910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hakimi AA, Furberg H, Zabor EC, Jacobsen
A, Schultz N, Ciriello G, Mikklineni N, Fiegoli B, Kim PH, Voss MH,
et al: An epidemiologic and genomic investigation into the obesity
paradox in renal cell carcinoma. J Natl Cancer Inst. 105:1862–1870.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Albiges L, Hakimi AA, Xie W, McKay RR,
Simantov R, Lin X, Lee JL, Rini BI, Srinivas S, Bjarnason GA, et
al: Body mass index and metastatic renal cell carcinoma: Clinical
and biological correlations. J Clin Oncol. 34:3655–3663. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lam VK, Bentzen SM, Mohindra P, Nichols
EM, Bhooshan N, Vyfhuis M, Scilla KA, Feigenberg SJ, Edelman MJ and
Feliciano JL: Obesity is associated with long-term improved
survival in definitively treated locally advanced non-small cell
lung cancer (NSCLC). Lung Cancer. 104:52–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Shepshelovich D, Xu W, Lu L, Fares A, Yang
P, Christiani D, Zhang J, Shiraishi K, Ryan BM, Chen C, et al: Body
Mass Index (BMI), BMI change, and overall survival in patients with
SCLC and NSCLC: A pooled analysis of the International lung cancer
consortium. J Thorac Oncol. 14:1594–1607. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ardesch FH, Ruiter R, Mulder M, Lahousse
L, Stricker BHC and Kiefte-de Jong JC: The obesity paradox in lung
cancer: Associations with body size versus body shape. Front Oncol.
10:5911102020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hayes AJ and Larkin J: BMI and outcomes in
melanoma: More evidence for the obesity paradox. Lancet Oncol.
19:269–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
McQuade JL, Daniel CR, Hess KR, Mak C,
Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al:
Association of body-mass index and outcomes in patients with
metastatic melanoma treated with targeted therapy, immunotherapy,
or chemotherapy: A retrospective, multicohort analysis. Lancet
Oncol. 19:310–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Smith LK, Arabi S, Lelliott EJ, McArthur
GA and Sheppard KE: Obesity and the impact on cutaneous melanoma:
Friend or Foe? Cancers (Basel). 12:15832020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Somasundar P, Yu AK, Vona-Davis L and
McFadden DW: Differential effects of leptin on cancer in vitro. J
Surg Res. 113:50–55. 2013. View Article : Google Scholar
|
|
151
|
Thompson KJ, Lau KN, Johnson S, Martinie
JB, Iannitti DA, McKillop IH and Sindram D: Leptin inhibits
hepatocellular carcinoma proliferation via p38-MAPK-dependent
signalling. HPB (Oxford). 13:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Paik SS, Jang SM, Jang KS, Lee KH, Choi D
and Jang SJ: Leptin expression correlates with favorable
clinicopathologic phenotype and better prognosis in colorectal
adenocarcinoma. Ann Surg Oncol. 16:297–303. 2009. View Article : Google Scholar
|
|
153
|
Murphy WJ and Longo DL: The surprisingly
positive association between obesity and cancer immunotherapy
efficacy. JAMA. 321:1247–1248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Cespedes Feliciano EM, Kroenke CH and Caan
BJ: The obesity paradox in cancer: How important is muscle? Annu
Rev Nutr. 38:357–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Donini LM, Pinto A, Giusti AM, Lenzi A and
Poggiogalle E: Obesity or BMI Paradox? Beneath the Tip of the
Iceberg. Front Nutr. 7:532020. View Article : Google Scholar : PubMed/NCBI
|