Introduction
According to the International Agency for Research
on Cancer, bladder cancer (BC) is a major global health concern,
with >200,000 deaths and ~550,000 new cases reported annually
worldwide (1). The mortality rate
associated with BC varies considerably, with 5-year survival rates
ranging from 96% for early-stage disease to only 5% for
advanced-stage disease (2).
Cancer recurrence within 5 years of diagnosis occurs in 60-70% of
all patients with BC (3). Common
treatment approaches for BC include transurethral resection,
radiotherapy, intravesical chemotherapy and immunotherapy (4). Despite their efficacy against BC,
these approaches have some limitations (5). In some patients with advanced BC,
treatment leads to suboptimal outcomes (6); moreover, chemoresistance often
complicates disease management (7). The rate of tumor response to immune
checkpoint inhibitor-based immunotherapy is low at ~20% (8). Therefore, an improved therapeutic
approach is needed for the effective management of BC.
Hyperthermia (HT) is a type of cancer therapy that
involves exposing patients to high temperatures (41-45°C) (9). This treatment approach includes
local, regional and whole-body HT (10). The objective of HT treatment is to
specifically target and damage or destroy cancer cells, with
minimal damage to normal cells. Mechanistically, high temperatures
directly damage cancer cells by denaturing proteins, destroying
cell membrane and DNA and disrupting cellular metabolism (11). HT sensitizes cancer cells,
enhancing their response to chemotherapy and radiotherapy (12,13). In patients with high-grade
superficial BC, the combination of HT and intravesical mitomycin C
is beneficial for inhibiting recurrence (14). Furthermore, HT facilitates bladder
preservation and function, demonstrating its viability as a
therapeutic option for elderly patients with muscle-invasive BC who
are unsuitable candidates for surgery or chemoradiotherapy
(15). In patients with head and
neck cancer, the combined use of HT and radiotherapy has been
reported to increase the likelihood of achieving a complete
response by ~25% compared with that observed when using
conventional radiotherapy alone; notably, the combination therapy
had no major acute or delayed side effects (16). Furthermore, HT can create a
favorable microenvironment for antitumor immune responses (17), which makes it an attractive
treatment option for use in combination with immunotherapies, such
as intratumoral dendritic cell-based immunotherapy and natural
killer (NK) cell-based adaptive immunotherapy (18,19). Overall, HT is promising as an
adjunct to various standard treatments for cancers.
BC is a common malignancy of the urinary tract
(20). The multifaceted molecular
mechanisms underlying the progression of BC include cell
proliferation, apoptosis regulation, cancer cell invasion,
angiogenesis and metastasis (21-23). Matrix metalloproteinases (MMPs),
which constitute a zinc-dependent endopeptidase family, play vital
roles in different stages of cancer progression (24). Through the proteolytic degradation
of the extracellular matrix and the release of matrix-bound
proangiogenic factors (for example, vascular endothelial growth
factor-A), MMPs facilitate the invasion of cancer cells into
neighboring tissues (25,26). Moreover, MMPs play a role in the
pathways involved in cell proliferation and survival signaling and
thus promote tumor growth and metastasis (27).
The present study investigated the anti-tumoral
effects of HT on human BC. HT inhibited the cell proliferation,
suppressed cell migration and invasion through MMP2 downregulation,
stimulated the antitumor immune response and reduced the
chemoresistance of BC cells. These findings underscore the
potential of HT as a viable adjunctive therapy for BC.
Materials and methods
Cell culture
The human BC cell lines 5637, UMUC3 and T24 and the
normal epithelial cell line SV-HUC-1 were obtained from Bioresource
Collection and Research Center. Each cell line was cultured in a
specific medium: 5637, Roswell Park Memorial Institute-1640 (Gibco;
Thermo Fisher Scientific, Inc.; Thermo Fisher Scientific, Inc.);
UMUC3, Minimum Essential Medium (Gibco; Thermo Fisher Scientific,
Inc.); T24, McCoy's 5A (Gibco; Thermo Fisher Scientific, Inc.); and
SV-HUC-1, F12K (Gibco; Thermo Fisher Scientific, Inc.). All media
were supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 2 mM GlutaMAX-1 and
Penicillin/Streptomycin/Amphotericin B Solution (Sigma-Aldrich;
Merck KGaA). The cells were incubated at 37°C under 5%
CO2.
HT treatment in vitro
For the in vitro HT treatment, BC cells and
normal epithelial cells were pretreated with varying concentrations
of cisplatin (DDP; 0-25 µM) and exposed to 43°C for 1 h, as
previously described (28,29).
Subsequently, all cells were maintained at 37°C for 24 h. Following
treatment, cell viability and colony formation were assessed.
Western blotting
Immunoblotting was performed using a previously
reported method (30,31). Proteins from BC cells were
extracted using RIPA lysis and extraction buffer (Thermo Fisher
Scientific, Inc.) supplemented with 2% protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc.). Protein
concentration was determined using the BCA Protein Assay Kit
(Thermo Fisher Scientific, Inc.). A total of 30 µg of
protein was applied to a 10-15% SDS-polyacrylamide gel for
separation and then transferred onto PVDF membranes
(MilliporeSigma). The membranes were blocked at room temperature
for 1 h with 5% skimmed milk prepared in Tris-buffered saline with
0.1% Tween-20 (TBST), then washed three times with TBST. After
blocking, the membranes were incubated overnight at 4°C with the
primary antibodies. Primary antibodies against the following
proteins were used to measure their levels: heat shock protein
(HSP)70, AKT, phosphorylated (p-)AKT, MMP2, MMP9, cadherin 11
(CDH11), programmed death ligand 1 (PD-L1), β-actin and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Following three
washes with TBST, the membranes were incubated at room temperature
for 1 h with HRP-conjugated secondary antibodies (anti-rabbit or
anti-mouse; 1:3,000; GeneTex, Inc.). The proteins were detected by
performing an enhanced chemiluminescence assay (Kodak X-OMAT LS
Film; Kodak). Protein levels were quantified using a computing
densitometer with ImageQuant LAS 4000 (Ge Healthcare Life
Sciences). Additional information on the antibodies used for
western blotting is presented in Table I.
 | Table IAntibodies used in the present
study. |
Table I
Antibodies used in the present
study.
| Antibody | Application | Supplier | Catalog number |
|---|
| HSP70 | Western
blotting | Cell Signaling
Technology, Inc. | 4873 |
| p-AKT | Western
blotting | GeneTex, Inc. | GTX128414 |
| AKT | Western
blotting | GeneTex, Inc. | GTX121937 |
| MMP2 | Western
blotting | Cell Signaling
Technology, Inc. | 40994 |
| CDH11 | Western
blotting | Sigma-Aldrich;
Merck KGaA | 3112087 |
| PD-L1 | Western
blotting | Cell Signaling
Technology, Inc. | 13684 |
| Ki-67 |
Immunofluorescence | Cell Signaling
Technology, Inc. | 9449 |
| β-actin | Western
blotting | Sigma-Aldrich;
Merck KGaA | A5441 |
| GAPDH | Western
blotting | Proteintech Group,
Inc. | 60004-1-lg |
Transfection
The negative control short hairpin (sh)RNA (shCon;
5′-CGC GAT CGT AAT CAC CCG AGT-3′) and CDH11 shRNA (shCDH11; 5′-GCA
GAT TTG TAT GGT TCC AAA-3′) were both purchased from RNAiCore,
while pcDNA3.1(+)-scrambled plasmid and PD-L1 overexpressed plasmid
were obtained from Addgene. Transient transfection was carried out
using a ViaFect Transfection Reagent (Promega Corporation) with
Opti-MEM (Thermo Fisher Scientific, Inc.) medium following to the
manufacturer's instructions. BC cells were transfected with or
without 2 µg/µl of each of the aforementioned
plasmid, combined with 4 µl of transfection reagent, and
incubated for 24 h at 37°C in an incubator. Cell samples were then
evaluated for the indicated protein expression, as well as their
cell viability and adhesion abilities.
Reverse transcription-quantitative (RT-q)
PCR
BC cells were seeded in a 6-well plate
(3×105 cells/well) and treated with HT. Total RNA was
extracted from BC cells using TRIzol® manufacturer's
protocol (Thermo Fisher Scientific, Inc.). RNA concentration was
measured using Nanodrop (Thermo Fisher Scientific, Inc.). A total
of 1 µg of RNA was reverse-transcribed using M-MLV Reverse
Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. The obtained
complementary DNA was subjected to RT-qPCR, which was performed
using the SYBR Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following primer pairs were used: human
CDH11 (forward, 5′-ACC CTC ACC ATC AAA GTC TG-3′; reverse, 5′-TCA
GGG TCA CAA ACA ATA CT-3′), human E-cadherin (forward, 5′-CGA GAG C
TA CAC GTT CAC GG-3′; reverse, 5′-GG GTG TCG AGG GAA AAATAG G-3′),
human cytokeratin-8 (forward, 5′-ACA AGG TAG AGC TGG AGT CTC G-3′;
reverse, 5′-AGC ACC ACA GAT GTG TCC GAG A-3′), human snail1
(forward, 5′-TCG GAAG CCT AAC TAC AGC GA-3′; reverse, 5′-AGA TGA
GCA TTG GCA GCG AG-3′), human ZEB1 (forward, 5′-GAT GAT GAA TGC GAG
TCA GAT GC-3′; reverse, 5′-ACAG CAG TGT CTT GTT GTT GT-3′), human
ZEB2 (forward, 5′-CAA GAG GCG CAAA CAA GCC-3′; reverse, 5′-GGT TGG
CAA TAC CGT CAT CC-3′) and human β-actin (forward, 5′-CAC CAT TGG
CAA TGA GCG GTTC-3′; reverse, 5′-AGG TCT TT G CGG ATG TCC ACG
T-3′). The reaction parameters were the same as those reported
previously (31). The
thermocycling protocol was as follows: Initial denaturation at 95°C
for 10 min, followed by 40 cycles of denaturation at 95°C for 15
sec, and annealing at 60°C for 360 sec. The melting curve analysis
was conducted with denaturation at 95°C for 15 sec, annealing at
60°C for 1 min, and a final high-resolution melt at 95°C for 15
sec. Each RT-qPCR reaction was performed with three technical
replicates. The expression levels of the indicated EMT markers were
assessed using the 2−ΔΔCq method to quantify transcript
levels, with β-actin serving as the internal control (32).
Cell viability
BC cells were cultured in 48-well plates
(2×104 cells/well). Then, they were subjected to HT and
treated with different concentrations of DDP (0, 3.125, 6.25, 12.5
and 25 µM) for 24 h. Cell viability was assessed using a
resazurin reagent (Biotium, Inc.). Briefly, the resazurin solution
was added to the well, constituting 10% of the initial volume and
the plate incubated for 6 h at 37°C under 5% CO2.
Subsequently, the fluorescence signal was measured using a
multimode microplate reader (Varioskan LUX Plate Reader; Thermo
Fisher Scientific, Inc.) at an excitation wavelength of 550 nm and
an emission wavelength of 600 nm.
Colony formation assay
BC cells were seeded in a 6-well plate
(3×103 cells/well) and treated with HT and DDP. After 7
days of incubation, a colony was defined as having ≤50 BC cells.
The cell colonies were then fixed with 3.7% formaldehyde for 20 min
at room temperature, followed by staining with 0.05% crystal violet
(w/v) for another 20 min at room temperature. The primary stain was
extracted with 10% acetic acid. The absorbance of the resultant
solution was measured to quantify the number of cells in the
colonies.
Transwell migration and invasion
assays
Transwell inserts (pore size, 8 µm; Costar;
Corning, Inc.) were used in 24-well plates to perform cell
migration and invasion assays. For the cell invasion assay, the
upper chamber was precoated with 30 µl of Corning Matrigel
matrix (Corning, Inc.) and incubated for 30 min at 37°C. The upper
chamber was seeded with 1×104 BC cells suspended in 200
µl of serum-free medium, whereas the lower chamber was
filled with 300 µl of 1% FBS medium. After 24 h of
incubation at 37°C, the cells that had migrated and invaded into
the lower chamber were stained with 0.05% crystal violet for 30 min
at room temperature, which was followed by the quantification of
cell numbers.
Immunofluorescence (IF) assay
BC cells were placed on a chamber slide
(Sigma-Aldrich; Merck KGaA) and treated as indicated. BC cells were
incubated with a primary antibody against Ki-67 (1:50 dilution;
Cell Signaling Technology, Inc.) for 1 h at room temperature and
then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5
min at room temperature. Ki-67-positive BC cells were visualized
under the Nikon Ti2 fluorescence microscope (Nikon Corporation).
Further information about the Ki-67 antibody is presented in
Table I.
Cytotoxicity assay
BC cells were seeded in a 48-well plate
(1×104 cells/well) and treated as indicated. The cells
were pre-stained with calcein AM (a green fluorescent dye; 1
µg/µl) for 1 h at 37°C in an incubator, then washed
three times with phosphate-buffered saline (PBS). Next, the cells
were mixed with NK-92MI cells at a ratio of 1:5 and incubated for 4
h at 37°C in an incubator. Viable BC cells were identified based on
the green fluorescence signal of calcein AM and the cytotoxicity of
NK cells was assessed by measuring the corresponding fluorescence
signal using a Varioskan LUX Plate Reader (Thermo Fisher
Scientific, Inc.).
Cell adhesion assay
A cell monolayer was formed by seeding BC cells
(3.5×105 cells/well) in a 6-well plate. The cells were
then subjected to HT treatment with or without PD-L1 plasmid (1
µg/µl) transfection. After 24 h, calcein
AM-prestained NK-92MI cells were cocultured with the BC cells for 1
h at a 37°C incubator. Nonadherent NK cells were removed by washing
with PBS three times. Subsequently, the cell monolayer was fixed in
3.7% formaldehyde for 30 min at room temperature and the number of
green fluorescence-emitting NK-92MI cells was counted to quantify
NK cells adhesion on BC cells.
Statistical analysis
Each experiment was performed thrice in triplicate.
Data are presented in terms of mean ± standard deviation (SD)
values. Student's t-test was used to compare mean values between
two experimental groups, whereas one-way analysis of variance
followed by Bonferroni's post hoc test was used to compare mean
values between >2 groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
HT inhibits the proliferation of human BC
cells
The present study first investigated the inhibitory
effects of HT on proliferation of bladder epithelial cells
(SV-HUC-1) and BC cells at various stages of cancer (5637, grade
II; T24, grade III; UMUC3, grade III). Effective HT treatment was
confirmed based on an increase in the level of HSP70 compared with
the level at normal temperature (NT; 37°C), as shown in Fig. 1A-D. It was observed that BC cells
subjected to HT displayed a reduction in the expression level of
Ki-67, a key biomarker of cell proliferation (Fig. 1E-G). Considering that AKT is
essential for cell proliferation (33), it was further investigated whether
HT affected the levels of AKT protein expression. The resulting
data showed that HT inhibited phosphorylated and total AKT protein
expression in BC cells (Fig.
1I-K). However, bladder epithelial cells did not show such
effects (Fig. 1H and L). The
colony formation assay revealed that HT reduced the survival rate
of BC cells, while an AKT activator suppressed this phenomenon
(Fig. 1M-O). Taken together, HT
inhibited the proliferation of BC cells by downregulating the
expression of AKT.
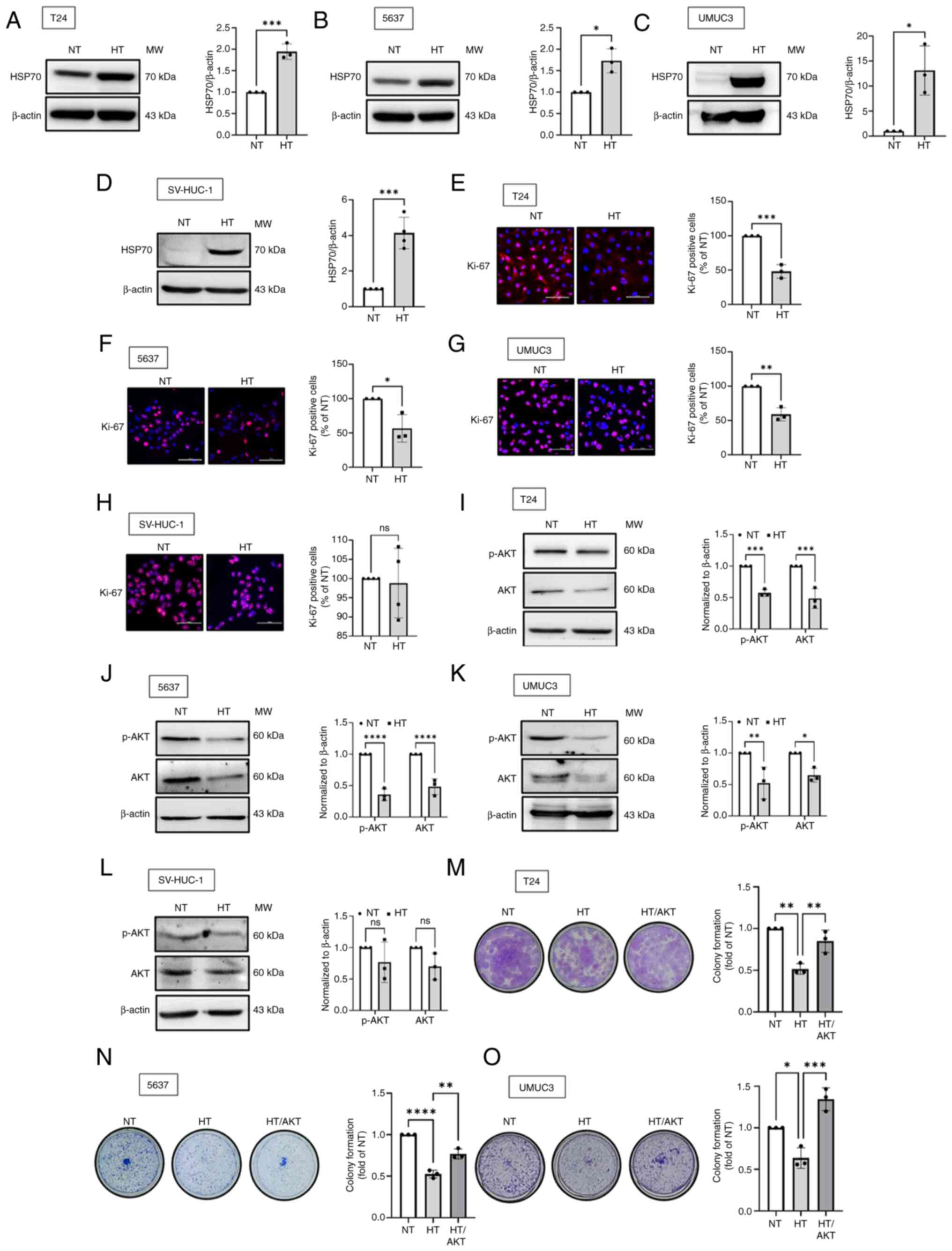 | Figure 1HT inhibits the proliferation of
human BC cells. (A-D) The normal bladder epithelial cell line
(SV-HUC-1) and BC cell lines (T24, 5637 and UMUC3) were subjected
to HT for 1 h, followed by incubation at NT for 24 h. The level of
HSP70 protein expression was evaluated through western blotting.
(E-H) IF staining was performed to measure the expression level of
Ki-67 in BC cells and normal bladder epithelial cell; Ki-67 was
stained red, whereas the nuclei were stained blue (DAPI). (I-L)
Western blotting was performed to measure the levels of AKT and
p-AKT protein expression. (M-O) Colony formation assays were
performed to assess the viability of BC cells subjected to HT and
AKT activator (SC79; 0.5 µg/ml). Data are presented in terms
of mean ± SD values. Two-groups comparisons were performed using
Student's t-test, whereas one-way ANOVA followed by Bonferroni's
post hoc test was used to compare mean values among multiple
groups. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001, vs. NT
group. HT, hyperthermia; BC, bladder cancer; NT, normal
temperature; HSP70, heat shock protein 70; DAPI,
4′,6-diamidino-2-phenylindole; ns, not significant; p-,
phosphorylated. |
HT enhances the sensitivity of human BC
cells to DDP
Platinum-based chemotherapy drugs are the preferred
first-line treatment and are often effective in eliciting positive
treatment responses (34,35). However, the adverse effects of
chemotherapy remain a major concern. The present study found that
HT enhanced the therapeutic efficacy of DDP for BC. The combination
of HT and DDP dose-dependently reduced the survival rate of BC
cells (Fig. 2A-C). To assess the
effect of HT on the long-term effectiveness of DDP treatment, a
colony formation assay was conducted. The resulting data
demonstrated that HT enhanced the sensitivity of BC cells to DDP
(Fig. 2E-G). As expected, the
co-administration of HT and DDP showed minimal effects on normal
epithelial cells (Fig. 2D and H).
In conclusion, HT showed promise as a complementary approach
alongside chemotherapy for human BC.
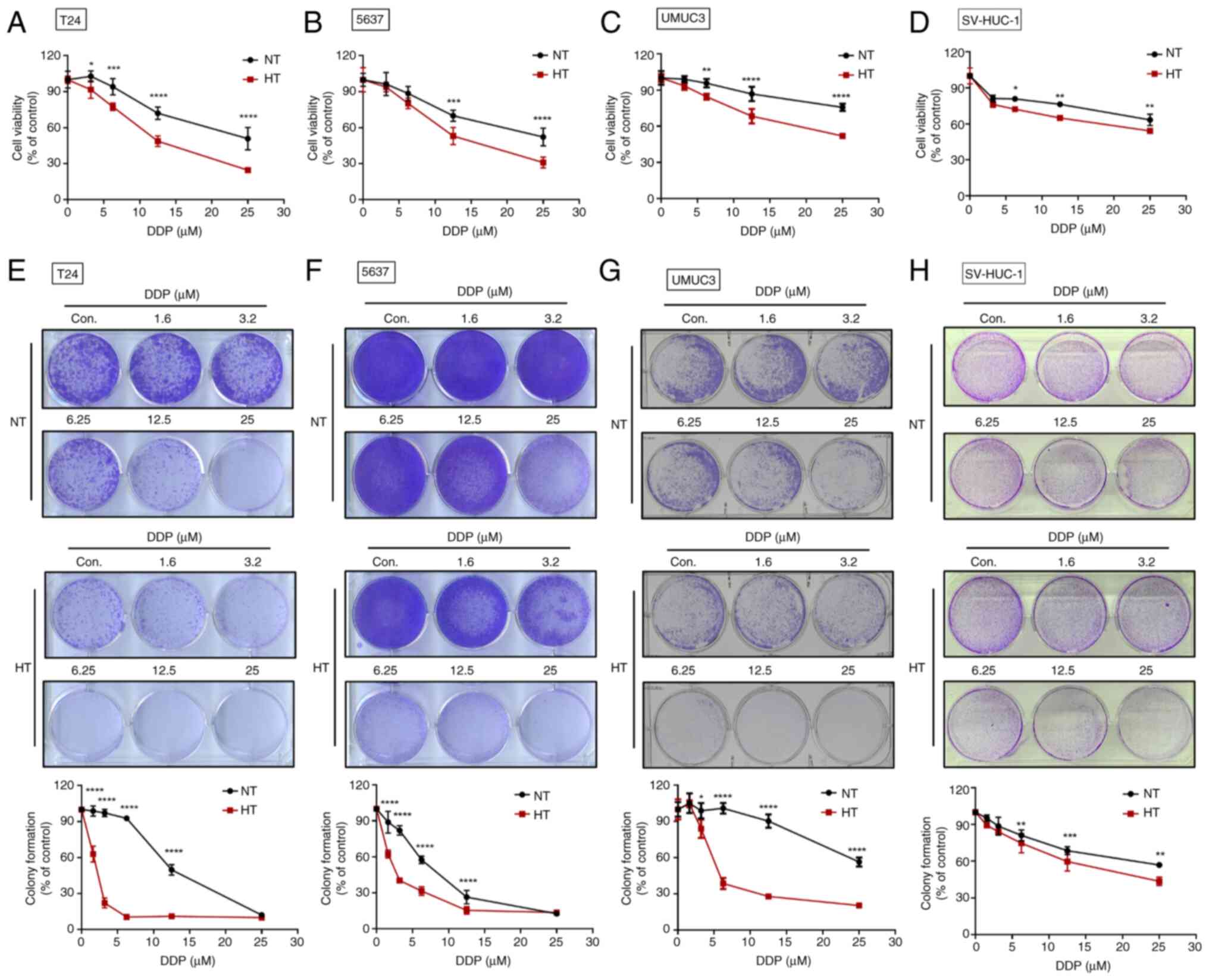 | Figure 2HT improves the sensitivity of human
BC cells to DDP. (A-D) The BC cell lines (T24, 5637 and UMUC3) and
normal bladder epithelial cell line (SV-HUC-1) were subjected to HT
for 1 h, followed by incubation with varying concentrations of DDP
for 24 h. Cell viability was assessed using a resazurin reagent.
(E-H) After 1 week of incubation, the survival rate of cells
treated with both HT and DDP was evaluated through colony formation
assays. Data are presented in terms of mean ± SD values. Two-groups
comparisons were performed using Student's t-test, whereas one-way
ANOVA followed by Bonferroni's post hoc test was used to compare
mean values among multiple groups. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001, vs. NT group. HT, hyperthermia; BC,
bladder cancer; DDP, cisplatin; NT, normal temperature. |
HT suppresses the DDP resistance in human
BC cells
Morphological transition from a mesenchymal to an
epithelial phenotype was observed in BC cells with HT treatment
(Fig. 3A). Considering that
epithelial-mesenchymal transition (EMT) activity is known to
regulate drug resistance (36),
it was hypothesized that HT treatment would counteract
chemoresistance by promoting mesenchymal-epithelial transition
(MET). Through HT treatment, DDP-resistant BC cells (UMUC3/DDP)
exhibited an increase in chemosensitivity to DDP (Fig. 3B). It was found that HT reduced
the levels of CDH11 expression (Fig.
3C and D), while the expressions of E-cadherin, cytokeratin-8,
snail1 and ZEB1/2 showed no such effect (Fig. S1A). In bladder epithelial cells,
neither CDH11 nor other EMT markers showed significant differences
(Fig. S1B and C). CDH11
knockdown significantly increased the sensitivity of BC cells to
DDP (Fig. 3E), doxorubicin and
epirubicin (Fig. S1B and C).
Furthermore, the expression level of CDH11 protein was higher in
UMUC3/DDP cells than in wild-type UMUC3 cells (Fig. 3F). Finally, through cell viability
and colony formation assays, it was validated that the inhibition
of CDH11 was associated with the enhanced chemosensitivity in
UMUC3/DDP cells (Fig. 3G and H).
Notably, the combination of low-dose DDP with CDH11 shRNA
demonstrated anticancer effects comparable to those exerted by
high-dose DDP alone (Fig. 3H),
indicating the critical role of CDH11 in regulating chemoresistance
in BC.
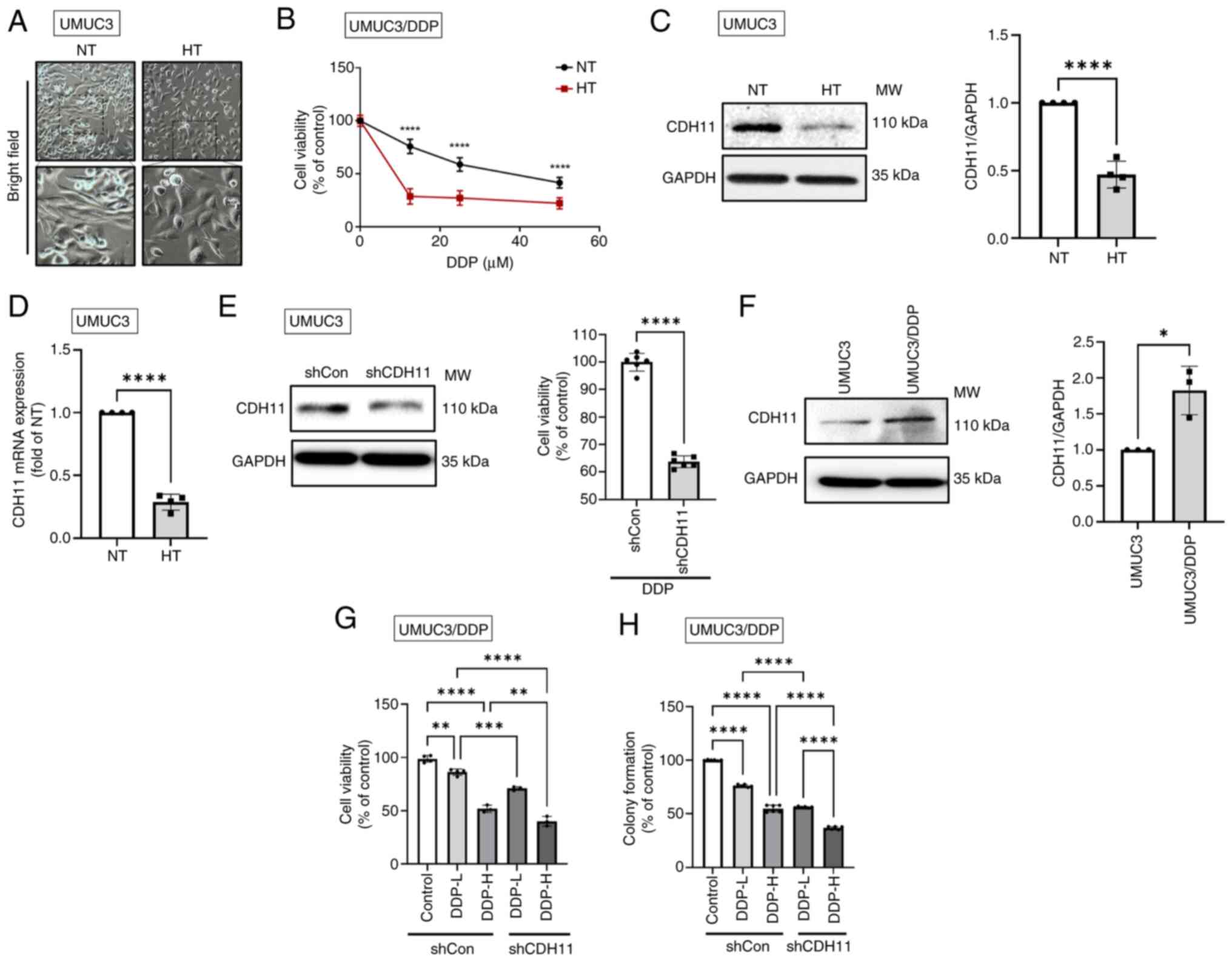 | Figure 3HT mitigates the DDP resistance of
human BC cells by downregulating CDH11 expression. (A)
Morphological characteristics of cells subjected to HT were
observed under a bright-field microscope. (B) Following HT
treatment for 1 h, DDP-resistant UMUC3 cells (UMUC3/DDP) were
incubated with varying concentrations of cisplatin for 24 h. Cell
viability was assessed using a resazurin reagent. (C) Western
blotting and (D) RT-qPCR and were performed to evaluate the levels
of CDH11 mRNA and protein in UMUC3 cells, respectively. (E) UMUC3
cells were transfected with either control shRNA (shCon; 1
µg/µl) or CDH11 shRNA (shCDH11; 1
µg/µl) for 24 h and then incubated with DDP (100
µM) for another 24 h. Cell viability was assessed using a
resazurin reagent. (F) Western blotting was performed to measure
the protein levels of CDH11 in both UMUC3 and UMUC3/DDP cells. (G)
UMUC3/DDP cells were transfected with shCon or shCDH11 for 24 h and
then incubated with high-dose (100 µM) and low-dose (25
µM) DDP for another 24 h. Cell viability was assessed using
a resazurin reagent. (H) UMUC3/DDP cells were cotreated with
shCDH11 and high-dose (6 µM) or low-dose (2 µM) DDP
for 1 week; colony formation assays were performed to estimate the
rate of cell survival. Data are presented in terms of mean ± SD
values. Two-groups comparisons were performed using Student's
t-test, whereas one-way ANOVA followed by Bonferroni's post hoc
test was used to compare mean values among multiple groups.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001, vs.
control group. HT, hyperthermia; DDP, CDH11, cadherin 11; BC,
bladder cancer; cadherin 11 NT, normal temperature; RT-qPCR,
reverse transcription-quantitative PCR; sh, short hairpin; shCon,
control shRNA; CDH11 shRNA, shCDH11. |
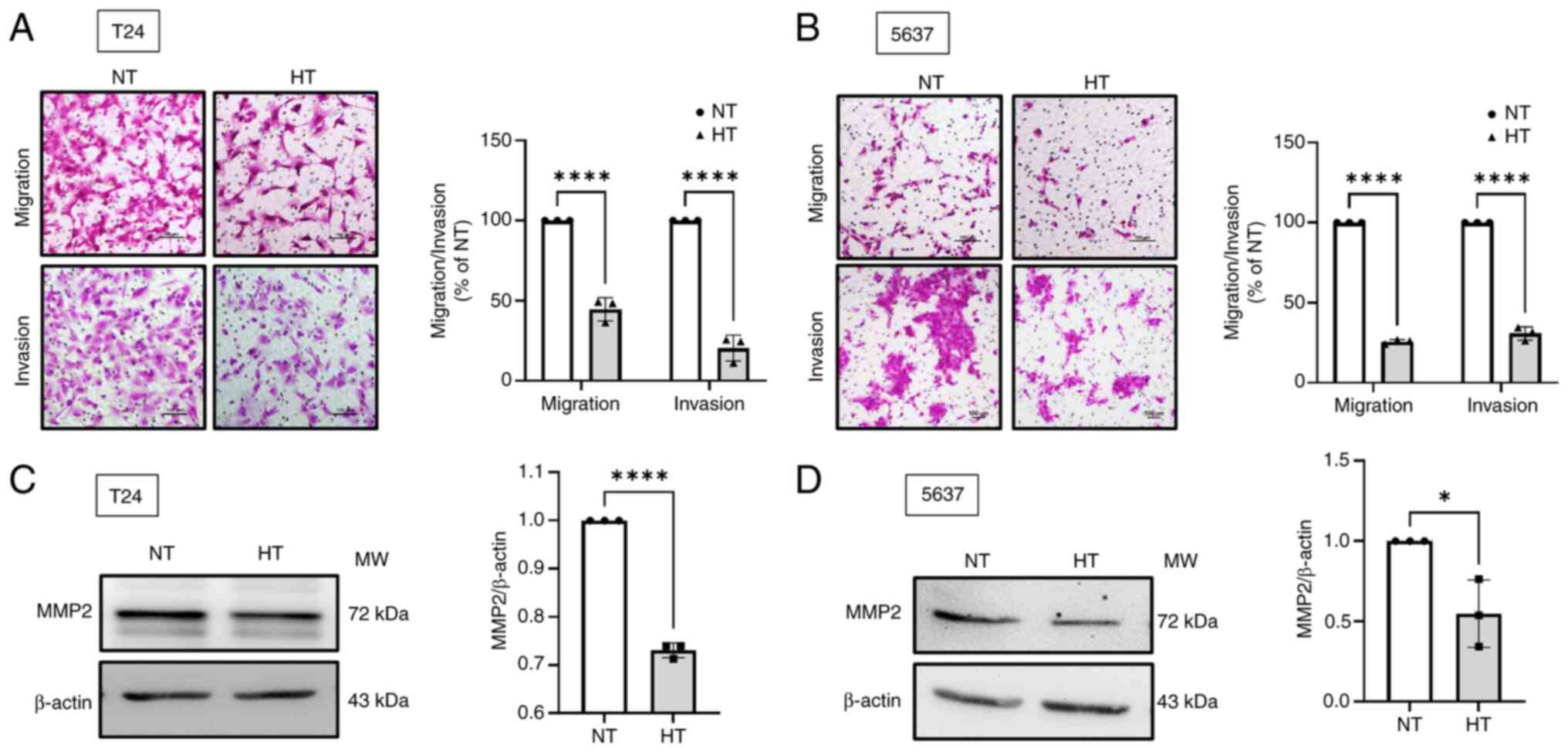
HT suppresses the migration and invasion
of human BC cells
Treating cancer poses challenges, given the capacity
of cancer cells to metastasize to distant locations (27). The Transwell migration and
invasion assay demonstrated that HT significantly inhibited the
migratory and invasive abilities of BC cells (Fig. 4A and B). MMP2 and MMP9 are crucial
mediators of BC progression (37-39). The resulting data indicated that
HT comprehensively suppressed MMP2 expression but had no effect on
MMP9 in BC cells (Fig. 4C and D;
Fig. S2A-D). In bladder
epithelial cells, the expression of both MMP2 and MMP9 was
unaffected by HT (Fig. S2E and
F). Taken together, it appeared that HT might inhibit the
migration and invasion of BC cells by downregulating the expression
of MMP2.
HT enhances the cytotoxicity of NK
cells
PD-L1 is a crucial immune checkpoint protein that
inhibits the cytotoxicity of NK cells in cancer (40). The present study found that HT
significantly suppressed the levels of PD-L1 protein expression
(Fig. 5A-C), while bladder
epithelial cells were not affected (Fig. S2G). Next, BC cells were
overexpressed with PD-L1 (Fig.
S2H-J) and it was found that the HT-enhanced adhesion and
cytotoxicity of NK cells towards BC cells were impeded by the
overexpression of PD-L1 in these cells (Fig. 5D and E). Therefore, HT enhances
the cytotoxicity of NK cells against BC cells by downregulating the
expression of PD-L1.
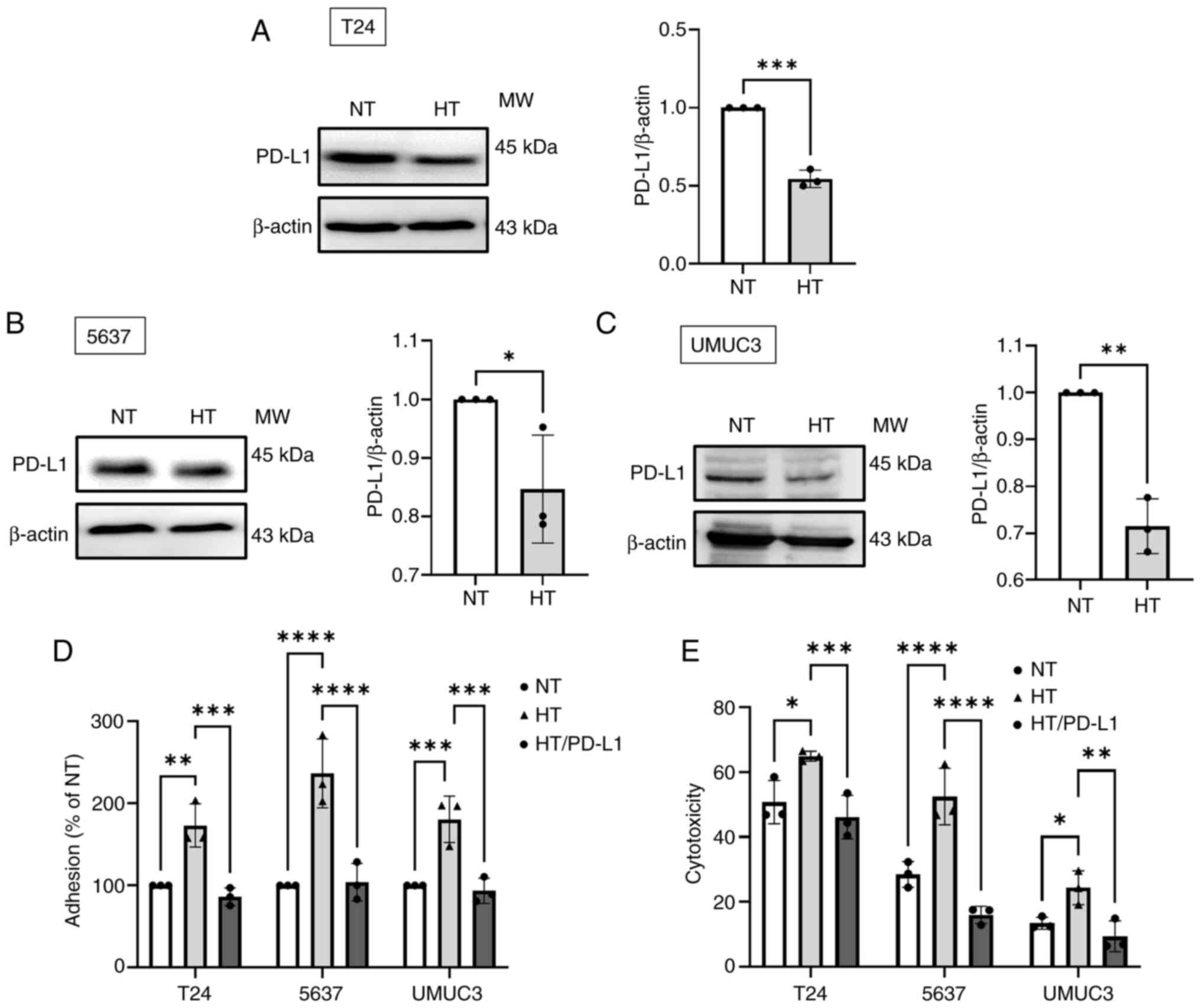 | Figure 5HT increases the cytotoxicity of NK
cells against human BC cells by suppressing PD-L1 expression. (A-C)
Western blotting was performed to measure the level of PD-L1
protein expression in BC cells subjected to HT. (D) BC cells were
subjected to HT treatment for 24 h, with or without PD-L1 plasmid
transfection. Subsequently, a cell adhesion assay was performed to
evaluate the level of NK cell adhesion to BC cells. (E) The
cytotoxicity of NK cells against BC cells was measured by detecting
the fluorescence signal. Data are presented in terms of mean ± SD
values. Two-groups comparisons were performed using Student's
t-test, whereas one-way ANOVA followed by Bonferroni's post hoc
test was used to compare mean values among multiple groups.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001, vs. NT
group. HT, hyperthermia; NK, natural killer; BC, bladder cancer;
PD-L1, programmed cell death 1 ligand 1; NT, normal
temperature. |
Discussion
As HT affects various biological processes in tumor
cells and their surrounding microenvironments (41), it has emerged as a promising
adjunctive therapy for different cancers. Clinical and preclinical
studies have indicated that HT combined with chemotherapy enhances
the rates of treatment response and overall survival in various
cancers, such as non-small cell lung cancer (42), esophageal cancer (43) and soft tissue sarcomas (44). The clinical bladder HT systems
comprise external regional hyperthermia therapy (RHT) achieved
through tissue penetration heating, microwave heating facilitated
by a miniature antenna inserted via an intravesical catheter and
intravesical circulation of heated fluid to warm the interior wall
of the bladder (45). The
BSD-2000, one of the most widely used devices for RHT, has been
used as an adjunctive treatment in conjunction with surgery,
radiation and chemotherapy for BC patients (46,47). A study in which patients were
followed up for 2 years revealed that the efficacy of intravesical
thermochemotherapy in treating of superficial transitional cell
carcinoma of the bladder was higher than that of traditional
intravesical therapy delivered using mitomycin C (48). Although the combination therapy
results in an increase in the level of local toxicity, the level
remains manageable and within acceptable thresholds (48). Furthermore, a study exploring the
benefits of intravesical thermochemotherapy for patients with
non-muscle invasive bladder cancer reported promising long-term
outcomes; notably, 53% of all patients were tumor-free 10 years
after treatment completion and 86% of all patients were able to
retain their bladders (49). In
terms of tumor recurrence, HT combined with intravesical
chemotherapy is more effective than conventional bladder
chemotherapy and equally effective as bacillus Calmette-Guérin
perfusion (50). These findings
further support the use of HT as an adjunctive therapy, which is
effective in managing BC over extended periods.
Chemotherapy serves as the first-line treatment for
human BC (51); however,
chemoresistance remains a major hurdle in achieving effective
treatment outcomes (7,52). A key factor contributing to
chemoresistance is EMT, which refers to cellular transformation
from an epithelial phenotype to a mesenchymal phenotype with
reduced chemosensitivity (53,54). EMT mediates chemoresistance
through a number of mechanisms, such as the activation of efflux
pumps (55), inhibition of
apoptosis and upregulation of the survival signaling pathway,
ultimately leading to the acquisition of stem cell-like features
with inherent chemoresistance potential (56-58). Therefore, targeting EMT may help
overcome the challenges associated with the chemoresistance of BC
cells. The present study revealed that HT increased the sensitivity
of BC cells to the chemotherapy drugs DDP, doxorubicin and
mitomycin C. Furthermore, this therapy mitigated the
chemoresistance of DDP-resistant cells to DDP by negatively
regulating EMT. The expression of CDH11, a biomarker of EMT, was
upregulated in DDP-resistant BC cells. DDP resistance can be
mitigated by reducing the level of CDH11 through the shRNA-mediated
knockdown of CDH11; this indicates the importance of CDH11
in regulating DDP resistance. Notably, HT reduced the levels of
CDH11 mRNA and protein in BC cells, increasing their susceptibility
to chemotherapy drugs. In summary, the modulation of CDH11 by HT
optimized the response of BC cells to chemotherapy. Thus, HT is an
attractive adjunctive treatment strategy for BC. A study indicated
that the induction of miR-409-3p expression through HT prevents EMT
by reducing the expression levels of β-cadherin, vimentin and
N-cadherin (59). Furthermore, HT
can sensitize gemcitabine-resistant pancreatic cancer cells by
reversing EMT through the regulation EMT-associated factors, such
as the epithelial marker E-cadherin (upregulated) and the
mesenchymal marker vimentin (downregulated) (60).
HT exerts direct and indirect effects on the immune
cells present in tumor microenvironments (61). HSP70 and HSP90, released by cancer
cells subjected to HT, activate various immune cells, such as
macrophages, dendritic cells and T cells, thereby modulating immune
response through HSP-mediated signaling pathways (62-64). The findings of the present study
indicated that HT can reduce the expression level of PD-L1 in BC
cells, thus enhancing the cytotoxicity of NK cells against the
cancer cells. HT can upregulate the expression of the adhesion
molecules L-selectin, P-selectin and intercellular cell adhesion
molecule-1 on endothelial cells that favor T-cell infiltration into
the tumor site (65).
Additionally, HT can activate NK cells by increasing the activation
of receptors such as NK group 2D, thereby enhancing the ability of
NK cells to efficiently target and eliminate cancer cells (66). HT can also induce macrophages to
secrete cytokines, such as interleukin-6 and tumor necrosis
factor-alpha, into the tumor microenvironment, thereby activating
the response of immune cells to tumors (62). Moreover, HT increases the blood
flow to the affected tissues, thus enhancing delivery of antitumor
agents and immune effectors to the cancer site (67,68). Collectively, HT can strengthen the
immune response against malignant neoplasms by inhibiting the
suppression of immune response and enhancing the ability of immune
cells to recognize and target cancer cells.
The association between MMP2 and an unfavorable
prognosis is well established (37) and the potential of MMP2 as a
prognostic biomarker has been demonstrated in patients with BC
(69). Suppressing MMP2 activity
can reduce the invasiveness of BC cells, both in vitro
(70) and in vivo
(71). Approaches aimed at
inhibiting various MMPs (such as, MMP2) hold promise for preventing
cancer progression and distant metastasis. The present study
demonstrated that HT limited the migration and invasion of BC cells
by downregulating the expression of MMP2. HT has been reported to
reduce cell invasiveness in various cancers, including breast
cancer (72), pancreatic cancer
(60), glioma (73), gastric cancer (59) and glioblastoma (74). An in vivo study conducted
by Ma et al (75) revealed
that the combination of a chemokine (C-C motif) ligand 3 derivative
(enhanced macrophage inflammatory protein) and HT markedly
inhibited the growth of colon adenocarcinoma cells and
significantly reduced distant metastases to the lungs. In a study
conducted using an in vivo breast tumor model, the
combination of ultrasound-stimulated microbubble exposure with HT
increased apoptosis and vascular disruption compared with the
outcomes of ultrasound-stimulated microbubble exposure alone
(76). This highlights the wide
applicability of HT in inhibiting cell invasion and distant
metastasis across cancers.
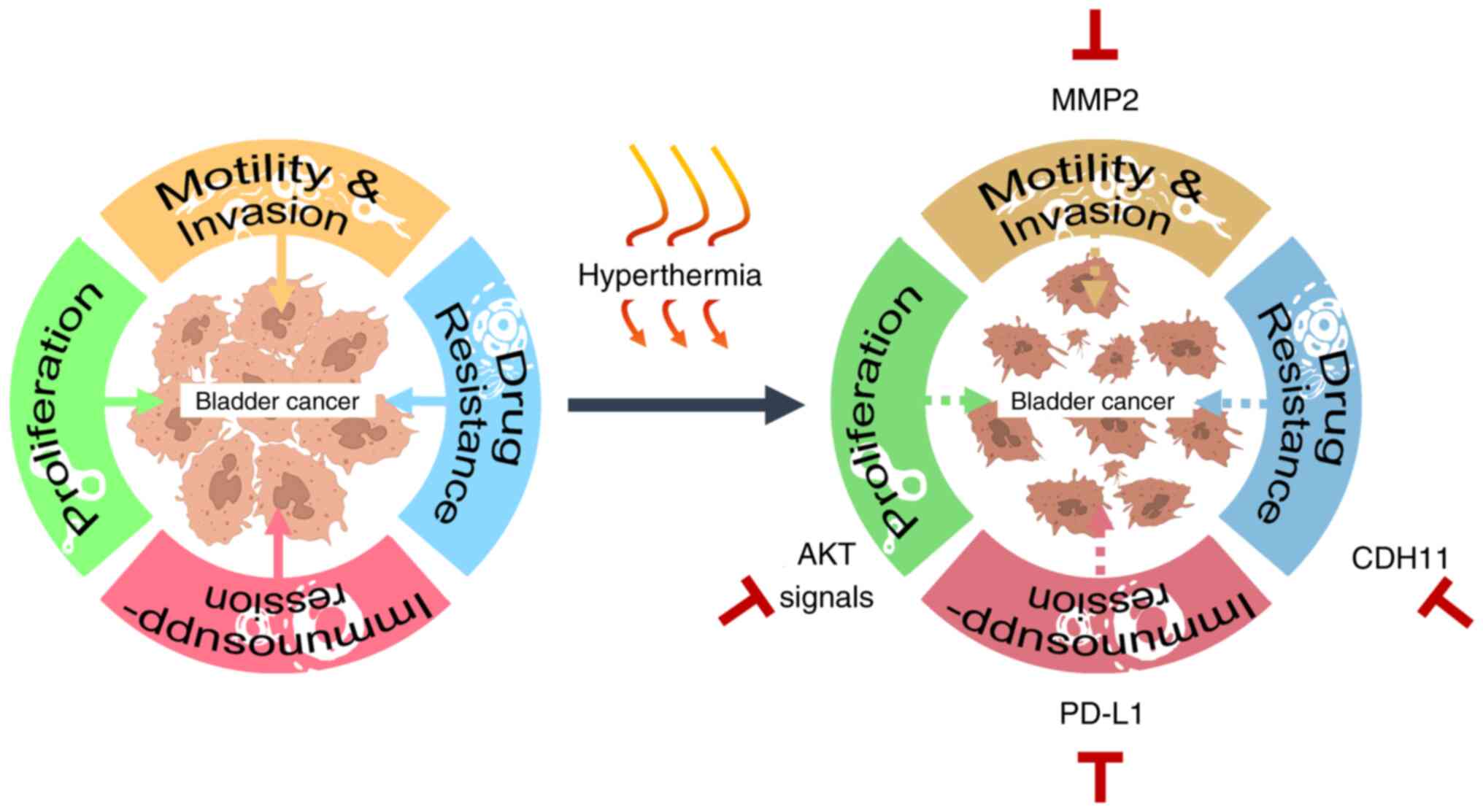
The present study offered a comprehensive analysis
of the anticancer effects of HT in BC, highlighting its potential
as an adjunctive therapy. Specifically, it demonstrated that HT
inhibited cancer cell growth by reducing both phosphorylated AKT
and total AKT signaling proteins, while decreasing the resistance
of BC cells to DDP-based medications by suppressing CDH11, which
could be beneficial for combination chemotherapy. Additionally, HT
reduced cancer cell motility by inhibiting MMP2 expression.
Finally, HT enhanced the immunotoxicity of NK cells against cancer
cells by downregulating PD-L1 expression. It was hypothesized that
these findings offered significant insights into the multifaceted
antitumor effects of HT and highlighted its potential as a
supplementary treatment in bladder cancer therapy (Fig. 6). However, further studies are
needed to include normal bladder epithelial cells as a control
group for comparison with bladder cancer cells, to demonstrate that
HT primarily affects cancer cells rather than normal cells.
Additionally, since the present study was conducted in vitro
and did not fully simulate the complex tumor microenvironment, this
is a limitation. Moreover, future research should focus on in
vivo experiments for an improved understanding of the efficacy
and potential side effects of HT in a more realistic biological
context.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization was by TH and AC. Data curation
was by TT and KC. Formal analysis was by TT and CH. Investigation
was by TH and AC. Methodology was by KC and YC. Project
administration was by HC and CH. Resource management was by PC and
TT. Software was by YC and PC. Supervision was by AC and TH.
Writing the original draft was by TT and PC. Writing, reviewing and
editing was by AC and TT. AC and TT confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Science and
Technology Council, Taiwan (grant nos. NSTC 112-2314-B-341-002-MY3,
NSTC-110-2314-B-341-001-MY2, NSTC-110-2314-B-341-004 and
NSTC-111-2314-B-341-004) and Shin Kong Wu Ho-Su Memorial Hospital
(grant nos. 2021SKHADR011 and 2022SKHBDR001).
References
|
1
|
Richters A, Aben KKH and Kiemeney LALM:
The global burden of urinary bladder cancer: An update. World J
Urol. 38:1895–1904. 2020. View Article : Google Scholar :
|
|
2
|
Saginala K and Barsouk A, Aluru JS, Rawla
P, Padala SA and Barsouk A: Epidemiology of bladder cancer. Med Sci
(Basel). 8:152020.PubMed/NCBI
|
|
3
|
Ikeda A, Kojima T, Kawai K, Hinotsu S,
Keino N, Shiga K, Miyake H, Miyata Y, Enomoto Y, Shimizu F, et al:
Risk for intravesical recurrence of bladder cancer stratified by
the results on two consecutive UroVysion fluorescence in situ
hybridization tests: A prospective follow-up study in Japan. Int J
Clin Oncol. 25:1163–1169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheung G, Sahai A, Billia M, Dasgupta P
and Khan MS: Recent advances in the diagnosis and treatment of
bladder cancer. BMC Med. 11:132013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wołącewicz M, Hrynkiewicz R, Grywalska E,
Suchojad T, Leksowski T, Roliński J and Niedźwiedzka-Rystwej P:
Immunotherapy in bladder cancer: Current methods and future
perspectives. Cancers (Basel). 12:11812020. View Article : Google Scholar
|
|
6
|
Reesink DJ, van de Garde EMW, Peters BJM,
van der Nat PB, Los M, Horenblas S and van Melick HHE: Treatment
patterns and clinical outcomes of chemotherapy treatment in
patients with muscle-invasive or metastatic bladder cancer in the
Netherlands. Sci Rep. 10:158222020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mari A, D'Andrea D, Abufaraj M, Foerster
B, Kimura S and Shariat SF: Genetic determinants for chemo- and
radiotherapy resistance in bladder cancer. Transl Androl Urol.
6:1081–1089. 2017. View Article : Google Scholar
|
|
8
|
Rijnders M, de Wit R, Boormans JL, Lolkema
MPJ and van der Veldt AAM: Systematic review of immune checkpoint
inhibition in urological cancers. Eur Urol. 72:411–423. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugarbaker PH, Sugarbaker C, Stephens AD
and Chang D: Radiofrequency hyperthermia in the palliative
treatment of mucinous carcinomatosis of appendiceal origin:
Optimizing and monitoring heat delivery in western patients. Int J
Hyperthermia. 16:429–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maluta S and Kolff MW: Role of
hyperthermia in breast cancer locoregional recurrence: A review.
Breast Care (Basel). 10:408–412. 2015. View Article : Google Scholar
|
|
11
|
Oei AL, Vriend LE, Crezee J, Franken NA
and Krawczyk PM: Effects of hyperthermia on DNA repair pathways:
One treatment to inhibit them all. Radiat Oncol. 10:1652015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De-Colle C, Weidner N, Heinrich V, Brucker
S, Hahn M, MacMillan K, Lamprecht U, Gaupp S, Voigt O and Zips D:
Hyperthermic chest wall re-irradiation in recurrent breast cancer:
A prospective observational study. Strahlenther Onkol. 195:318–326.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Issels R, Kampmann E, Kanaar R and Lindner
LH: Hallmarks of hyperthermia in driving the future of clinical
hyperthermia as targeted therapy: Translation into clinical
application. Int J Hyperthermia. 32:89–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gofrit ON, Shapiro A, Pode D, Sidi A,
Nativ O, Leib Z, Witjes JA, van der Heijden AG, Naspro R and
Colombo R: Combined local bladder hyperthermia and intravesical
chemotherapy for the treatment of high-grade superficial bladder
cancer. Urology. 63:466–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Datta NR, Stutz E, Puric E, Eberle B,
Meister A, Marder D, Timm O, Rogers S, Wyler S and Bodis S: A pilot
study of radiotherapy and local hyperthermia in elderly patients
with muscle-invasive bladder cancers unfit for definitive surgery
or chemoradiotherapy. Front Oncol. 9:8892019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Datta NR, Rogers S, Ordóñez SG, Puric E
and Bodis S: Hyperthermia and radiotherapy in the management of
head and neck cancers: A systematic review and meta-analysis. Int J
Hyperthermia. 32:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adnan A, Muñoz NM, Prakash P, Habibollahi
P, Cressman ENK and Sheth RA: Hyperthermia and tumor immunity.
Cancers (Basel). 13:25072021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsang YW, Huang CC, Yang KL, Chi MS,
Chiang HC, Wang YS, Andocs G, Szasz A, Li WT and Chi KH: Improving
immunological tumor microenvironment using electro-hyperthermia
followed by dendritic cell immunotherapy. BMC Cancer. 15:7082015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vancsik T, Máthé D, Horváth I, Várallyaly
AA, Benedek A, Bergmann R, Krenács T, Benyó Z and Balogh A:
Modulated electro-hyperthermia facilitates NK-cell infiltration and
growth arrest of human A2058 melanoma in a xenograft model. Front
Oncol. 11:5907642021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farling KB: Bladder cancer: Risk factors,
diagnosis, and management. Nurse Pract. 42:26–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fus ŁP and Górnicka B: Role of
angiogenesis in urothelial bladder carcinoma. Cent European J Urol.
69:258–263. 2016.PubMed/NCBI
|
|
22
|
Shinagare AB, Ramaiya NH, Jagannathan JP,
Fennessy FM, Taplin ME and Van den Abbeele AD: Metastatic pattern
of bladder cancer: correlation with the characteristics of the
primary tumor. AJR Am J Roentgenol. 196:117–122. 2011. View Article : Google Scholar
|
|
23
|
Biswas PK, Kwak Y, Kim A, Seok J, Kwak HJ,
Lee M, Dayem AA, Song K, Park JY, Park KS, et al: TTYH3 modulates
bladder cancer proliferation and metastasis via
FGFR1/H-Ras/A-Raf/MEK/ERK pathway. Int J Mol Sci. 23:104962022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31(Suppl 1):
S177–S183. 2016. View Article : Google Scholar
|
|
26
|
Lee S, Jilani SM, Nikolova GV, Carpizo D
and Iruela-Arispe ML: Processing of VEGF-A by matrix
metalloproteinases regulates bioavailability and vascular
patterning in tumors. J Cell Biol. 169:681–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Heijden AG, Jansen CF, Verhaegh G,
O'donnell MA, Schalken JA and Witjes JA: The effect of hyperthermia
on mitomycin-C induced cytotoxicity in four human bladder cancer
cell lines. Eur Urol. 46:670–674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Heijden AG, Verhaegh G, Jansen CF,
Schalken JA and Witjes JA: Effect of hyperthermia on the
cytotoxicity of 4 chemotherapeutic agents currently used for the
treatment of transitional cell carcinoma of the bladder: An in
vitro study. J Urol. 173:1375–1380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang AC, Lien MY, Tsai MH, Hua CH and
Tang CH: WISP-1 promotes epithelial-mesenchymal transition in oral
squamous cell carcinoma cells via the miR-153-3p/Snail axis.
Cancers (Basel). 11:19032019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang AC, Chen PC, Lin YF, Su CM, Liu JF,
Lin TH, Chuang SM and Tang CH: Osteoblast-secreted WISP-1 promotes
adherence of prostate cancer cells to bone via the VCAM-1/integrin
α4β1 system. Cancer Lett. 426:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–108. 2001.
View Article : Google Scholar
|
|
33
|
Xu N, Lao Y, Zhang Y and Gillespie DA:
Akt: A double-edged sword in cell proliferation and genome
stability. J Oncol. 2012:9517242012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ismaili N, Amzerin M and Flechon A:
Chemotherapy in advanced bladder cancer: Current status and future.
J Hematol Oncol. 4:352011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Las Rivas J, Brozovic A, Izraely S,
Casas-Pais A, Witz IP and Figueroa A: Cancer drug resistance
induced by EMT: Novel therapeutic strategies. Arch Toxicol.
95:2279–2297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fouad H, Salem H, Ellakwa DE and
Abdel-Hamid M: MMP-2 and MMP-9 as prognostic markers for the early
detection of urinary bladder cancer. J Biochem Mol Toxicol.
33:e222752019. View Article : Google Scholar
|
|
38
|
Chou KY, Chang AC, Ho CY, Tsai TF, Chen
HE, Chen PC and Hwang TI: Thrombospondin-4 promotes bladder cancer
cell migration and invasion via MMP2 production. J Cell Mol Med.
25:6046–6055. 2021.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reis ST, Leite KR, Piovesan LF,
Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP,
Srougi M and Dall'Oglio MF: Increased expression of MMP-9 and IL-8
are correlated with poor prognosis of bladder cancer. BMC Urol.
12:182012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
41
|
Oei AL, Kok HP, Oei SB, Horsman MR,
Stalpers LJA, Franken NAP and Crezee J: Molecular and biological
rationale of hyperthermia as radio- and chemosensitizer. Adv Drug
Deliv Rev. 163-164:84–97. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang Z, Yan W, Ming J and Yu Y: Docetaxel
weekly regimen in conjunction with RF hyperthermia for pretreated
locally advanced non-small cell lung cancer: A preliminary study.
BMC Cancer. 7:1892007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kitamura K, Ishida M, Kimura Y, Saeki H,
Maehara Y and Sugimachi K: Early report of correlation between the
thermal dosage and the treatment effect of hyperthermia in
combination with chemoradiotherapy for esophageal cancer patients.
Hepatogastroenterology. 49:1560–1562. 2002.PubMed/NCBI
|
|
44
|
Trabulsi NH, Patakfalvi L, Nassif MO,
Turcotte RE, Nichols A and Meguerditchian AN: Hyperthermic isolated
limb perfusion for extremity soft tissue sarcomas: Systematic
review of clinical efficacy and quality assessment of reported
trials. J Surg Oncol. 106:921–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stauffer PR and van Rhoon GC: Overview of
bladder heating technology: Matching capabilities with clinical
requirements. Int J Hyperthermia. 32:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wittlinger M, Rödel CM, Weiss C, Krause
SF, Kühn R, Fietkau R, Sauer R and Ott OJ: Quadrimodal treatment of
high-risk T1 and T2 bladder cancer: Transurethral tumor resection
followed by concurrent radiochemotherapy and regional deep
hyperthermia. Radiother Oncol. 93:358–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Juang T, Stauffer PR, Craciunescu OA,
Maccarini PF, Yuan Y, Das SK, Dewhirst MW, Inman BA and Vujaskovic
Z: Thermal dosimetry characteristics of deep regional heating of
non-muscle invasive bladder cancer. Int J Hyperthermia. 30:176–183.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Colombo R, Da Pozzo LF, Salonia A, Rigatti
P, Leib Z, Baniel J, Caldarera E and Pavone-Macaluso M:
Multicentric study comparing intravesical chemotherapy alone and
with local microwave hyperthermia for prophylaxis of recurrence of
superficial transitional cell carcinoma. J Clin Oncol.
21:4270–4276. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Colombo R, Salonia A, Leib Z,
Pavone-Macaluso M and Engelstein D: Long-term outcomes of a
randomized controlled trial comparing thermochemotherapy with
mitomycin-C alone as adjuvant treatment for non-muscle-invasive
bladder cancer (NMIBC). BJU Int. 107:912–918. 2011. View Article : Google Scholar
|
|
50
|
Duan H, Deng Z, Zou J, Zhang G, Zou X and
Xie T: The efficacy and safety of Hyperthermia intravesical
chemotherapy in the treatment non-muscle invasive bladder cancer:A
meta analysis. Urol Int. 108:322–333. 2024. View Article : Google Scholar
|
|
51
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu D, Abbosh P, Keliher D, Reardon B,
Miao D, Mouw K, Weiner-Taylor A, Wankowicz S, Han G, Teo MY, et al:
Mutational patterns in chemotherapy resistant muscle-invasive
bladder cancer. Nat Commun. 8:21932017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ashrafizadeh M, Zarrabi A, Hushmandi K,
Kalantari M, Mohammadinejad R, Javaheri T and Sethi G: Association
of the epithelial-mesenchymal transition (EMT) with cisplatin
resistance. Int J Mol Sci. 21:40022020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hill C and Wang Y: The importance of
epithelial-mesenchymal transition and autophagy in cancer drug
resistance. Cancer Drug Resist. 3:38–47. 2020.PubMed/NCBI
|
|
55
|
Wilson C, Nicholes K, Bustos D, Lin E,
Song Q, Stephan JP, Kirkpatrick DS and Settleman J: Overcoming
EMT-associated resistance to anti-cancer drugs via Src/FAK pathway
inhibition. Oncotarget. 5:7328–7341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chang TH, Tsai MF, Su KY, Wu SG, Huang CP,
Yu SL, Yu YL, Lan CC, Yang CH, Lin SB, et al: Slug confers
resistance to the epidermal growth factor receptor tyrosine kinase
inhibitor. Am J Respir Crit Care Med. 183:1071–107. 2011.
View Article : Google Scholar
|
|
57
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shibue T and Weinberg RA: EMT, CSCs, and
drug resistance: The mechanistic link and clinical implications.
Nat Rev Clin Oncol. 14:611–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Feng J, Li K, Liu G, Feng Y, Shi H and
Zhang X: Precision hyperthermia-induced miRNA-409-3p upregulation
inhibits migration, invasion, and EMT of gastric cancer cells by
targeting KLF17. Biochem Biophys Res Commun. 549:113–119. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jin H, Zhao Y, Zhang S, Yang J, Zhang X
and Ma S: Hyperthermia inhibits the motility of
gemcitabine-resistant pancreatic cancer PANC-1 cells through the
inhibition of epithelial-mesenchymal transition. Mol Med Rep.
17:7274–7280. 2018.PubMed/NCBI
|
|
61
|
Skitzki JJ, Repasky EA and Evans SS:
Hyperthermia as an immunotherapy strategy for cancer. Curr Opin
Investig Drugs. 10:550–558. 2009.PubMed/NCBI
|
|
62
|
Lee S, Son B, Park G, Kim H, Kang H, Jeon
J, Youn H and Youn B: Immunogenic effect of hyperthermia on
enhancing radiotherapeutic efficacy. Int J Mol Sci. 19:27952018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Torigoe T, Tamura Y and Sato N: Heat shock
proteins and immunity: Application of hyperthermia for
immunomodulation. Int J Hyperthermia. 25:610–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ito A, Shinkai M, Honda H, Yoshikawa K,
Saga S, Wakabayashi T, Yoshida J and Kobayashi T: Heat shock
protein 70 expression induces antitumor immunity during
intracellular hyperthermia using magnetite nanoparticles. Cancer
Immunol Immunother. 52:80–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Z, Deng J, Sun J and Ma Y: Hyperthermia
targeting the tumor microenvironment facilitates immune checkpoint
inhibitors. Front Immunol. 11:5952072020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dayanc BE, Beachy SH, Ostberg JR and
Repasky EA: Dissecting the role of hyperthermia in natural killer
cell mediated anti-tumor responses. Int J Hyperthermia. 24:41–56.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sen A, Capitano ML, Spernyak JA,
Schueckler JT, Thomas S, Singh AK, Evans SS, Hylander BL and
Repasky EA: Mild elevation of body temperature reduces tumor
interstitial fluid pressure and hypoxia and enhances efficacy of
radiotherapy in murine tumor models. Cancer Res. 71:3872–3880.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Song CW: Effect of local hyperthermia on
blood flow and microenvironment: A review. Cancer Res. 44(10
Suppl): 4721s–4730s. 1984.PubMed/NCBI
|
|
69
|
Vasala K, Pääkkö P and
Turpeenniemi-Hujanen T: Matrix metalloproteinase-2 immunoreactive
protein as a prognostic marker in bladder cancer. Urology.
62:952–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chou KY, Chang AC, Tsai TF, Lin YC, Chen
HE, Ho CY, Chen PC and Hwang TI: MicroRNA-34a-5p serves as a tumor
suppressor by regulating the cell motility of bladder cancer cells
through matrix metalloproteinase-2 silencing. Oncol Rep.
45:911–920. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hwang TI, Chen PC, Tsai TF, Lin JF, Chou
KY, Ho CY, Chen HE and Chang AC: Hsa-miR-30a-3p overcomes the
acquired protective autophagy of bladder cancer in chemotherapy and
suppresses tumor growth and muscle invasion. Cell Death Dis.
13:3902022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lee TH, Bu J, Kim BH, Poellmann MJ, Hong S
and Hyun SH: Sub-lethal hyperthermia promotes
epithelial-to-mesenchymal-like transition of breast cancer cells:
implication of the synergy between hyperthermia and chemotherapy.
RSC Adv. 9:52–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang DC, Zhang Y, Chen HY, Li XL, Qin LJ,
Li YJ, Zhang HY and Wang S: Hyperthermia promotes apoptosis and
suppresses invasion in C6 rat glioma cells. Asian Pac J Cancer
Prev. 13:3239–3245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jo Y, Han YI, Lee E, Seo J, Oh G, Sung H,
Gi Y, Kim H, Park S and Yoon M: The combination of tumor treating
fields and hyperthermia has synergistic therapeutic effects in
glioblastoma cells by downregulating STAT3. Am J Cancer Res.
12:1423–1432. 2022.PubMed/NCBI
|
|
75
|
Ma L, Kambe R, Tsuchiya T, Kanegasaki S
and Takahashi A: Anti-metastatic benefits produced by hyperthermia
and a CCL3 derivative. Cancers (Basel). 11:17702019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sharma D, Cartar H, Law N, Giles A, Farhat
G, Oelze M and Czarnota GJ: Optimization of microbubble enhancement
of hyperthermia for cancer therapy in an in vivo breast tumour
model. PLoS One. 15:e02373722020. View Article : Google Scholar : PubMed/NCBI
|