Ovarian cancer (OC) has the highest mortality rate
(>314,000 new cases and around 207,000 new deaths worldwide in
2020) among all gynecological malignancies in the world due to lack
of symptoms and effective makers at early stages (1). If the cancer is restricted to the
ovaries (stage I), up to 90% of patients can be treated with
currently available treatments (surgery, chemotherapy,
anti-angiogenic therapy, immunotherapy). Even if the disease has
spread to the pelvic organs (stage II), up to 70% of patients
survive for >10 years. However, if it further disseminates into
the peritoneum or the surface of abdominal organs (stage III) or
outside the abdomen (stage IV), 5-year survival rate declines to
≤20% (2). For most patients with
OC, tumor cytoreduction surgery is their last option at the
advanced stage with intraperitoneal and extensive pelvic
implantation metastasis. Even with aggressive first-line
chemotherapy following optimal debulking surgery, the initial cure
rate is 80%. The majority of patients with advanced-stage OC
exhibit unsatisfactory response due to acquired chemoresistance,
which results in recurrence and chemotherapy failure (3).
Increase in the amount of fluid in the abdominal
cavity >200 ml is termed ascites. Ascites formation serves a
vital role in the progression of OC, serving as a transporter of
tumor cells from the primary location to metastatic sites (4). The incidence of ascites varies
between the four stages of OC, ranging from 49.4 at stage I to 62.5
in stage II and 90.1 and 100.0% in stages III and IV, respectively
(5). Malignant ascites (MA)
contains cellular and acellular components. Cellular components
include cancer, immune and mesothelial cells and fibroblasts, while
acellular components include proteins, such as cytokines and growth
factors, metabolites and exosomes (6). Massive ascites may cause abdominal
distension, respiratory compromise, anorexia and cachexia (7). Decreased lymphatic absorption and
increased fluid production via high vascular permeability are the
primary factors contributing to MA formation. To date, a gold
standard for clinical management of MA has not been clearly
defined. Paracentesis and diuretics relieve the accumulation of
ascites, but their efficacy is often partial and temporary
(8).
Unlike other solid cancers, OC rarely exhibits
hematogenous and lymphatic metastasis. Peritoneal (trancoelomic)
metastasis is more frequent and can be detected in ~70% of OC cases
(9). Tumor cells implant directly
into adjacent organs after detachment from the primary site or
spread to the omentum, parietal and visceral peritoneum via
peritoneal fluid or ascites. In OC ascites, cancer cells float as
individual cells or multicellular aggregates (also called
spheroids). These malignant cells accumulate as globular
structures, thus resisting anoikis and helping to spread throughout
the abdominal cavity (10).
Spheroids, exerting higher tumorigenic and chemoresistant
properties than individual cancer cells (11), are considered metastatic units of
peritoneal dissemination (12).
Beside tumor cells, OC ascites also contains non-tumor cellular
components, such as macrophages, lymphocytes, fibroblasts,
adipocytes and mesothelial cells (13). The interaction of tumor and
non-tumor cells in ascites leads to formation of heterogeneous
spheroids. Such heterospheroids are more invasive and more
resistant to anoikis and chemotherapeutic drugs than homospheroids
composed of cancer cells alone (14).
Tumor-associated macrophages (TAMs) are the most
frequent cell type (up to 50% of the total) in the ascitic
microenvironment (15). Recently
(16), it has been revealed that
TAMs exist in the center of spheroids, regulating spheroid
formation, survival and adhesion to peritoneum and then regulating
peritoneal metastasis. TAMs are key for OC progression (17). Thus, it is of importance to study
the role and molecular mechanisms of TAMs in spheroid formation and
intraperitoneal implantation (18). Compared with previous reviews,
which primarily focused on the immunosuppressive role of TAMs in
cancer (15,19,20) or only briefly mentioned TAMs in OC
spheroids (21-23), the present review investigates the
role of TAMs in spheroid formation and dissemination in OC
progression, the molecular mechanisms by which TAMs participate in
peritoneal metastasis of OC, including spheroid formation, survival
and dissemination, as well as strategies targeting TAMs in OC in
clinical or preclinical research.
OC cells metastasize directly from the primary site
to the abdominal cavity, where they survive and travel as
individual cells or multicellular aggregates in the peritoneal
fluid or ascites, then adhere to peritoneal tissue, anchor to the
submesothelial matrix, and proliferate to form secondary lesions
(24). These spheroids exist in,
and can be isolated, from OC ascites. The role of spheroids in OC
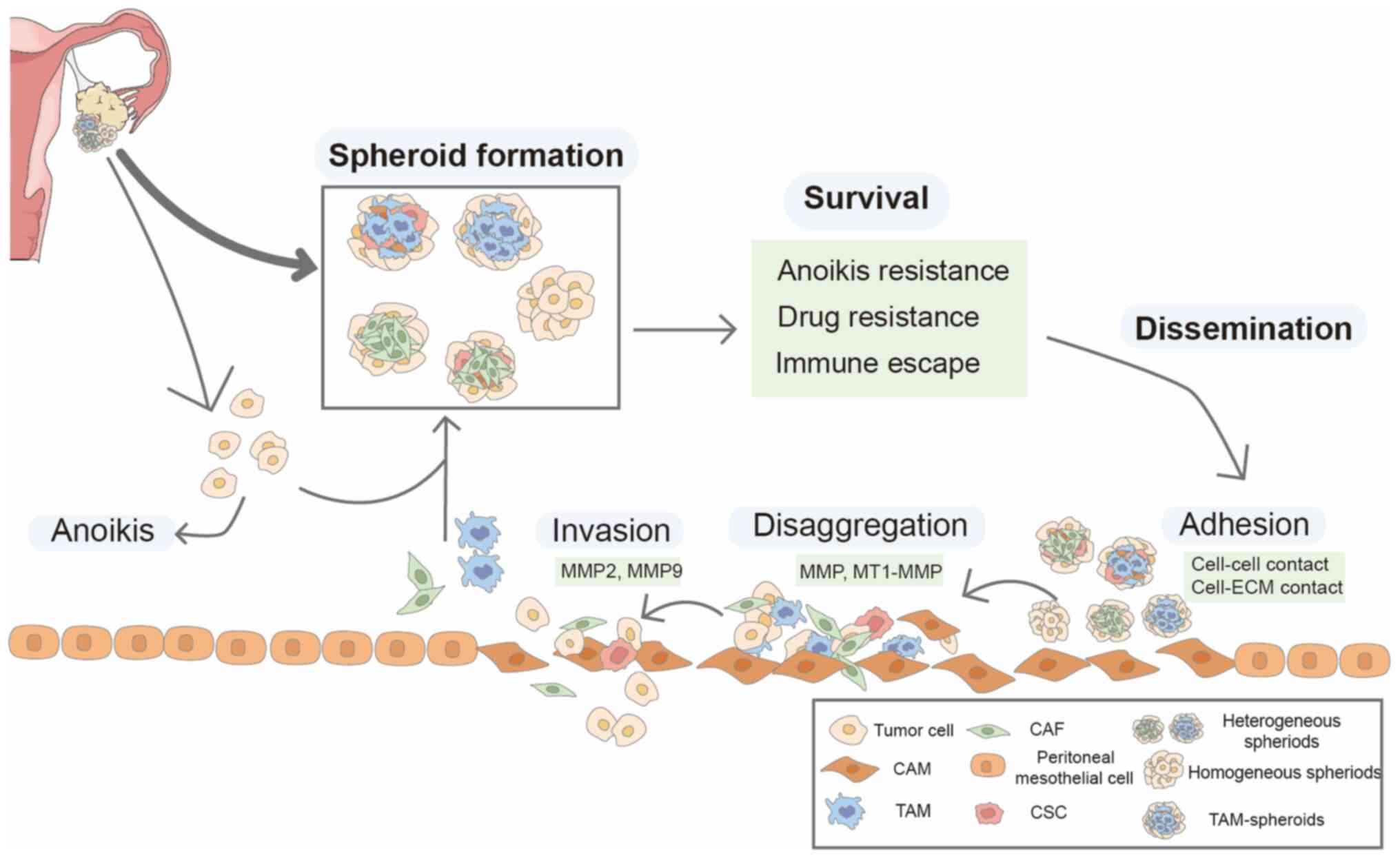
progression is summarized in Fig.
1.
Spheroids contain cancer and non-cancer cells such
as cancer-associated fibroblasts (CAFs), TAMs and rare cancer stem
cells (CSCs). Spheroids have different sizes and structures
(25). Spheroids may be formed by
aggregation of individual stromal and cancer cells in ascites or
clusters detached from the primary tumor. In mice, intraperitoneal
aggregation is not the main mechanism of spheroid formation and 80%
of OC spheroids are produced by clustered cells separating from the
primary tumor, while intraperitoneal aggregative cells account for
only a small fraction (10).
Anoikis is a specific form of apoptosis due to
insufficient or poor cellular adhesion (40). Anoikis is regulated by integrins,
which interact with ECM components to form adhesion complexes
(41). Either through intrinsic
or extrinsic apoptotic pathways, anoikis prevents epithelial cells
from detaching from their original location and colonizing new
sites (42). Tumor cells are less
sensitive to anoikis than normal epithelial cells. Cancer cells
downregulate intercellular adhesion molecules via
epithelial-mesenchymal transition (EMT), inhibiting E-cadherin
expression to decrease cadherin-dependent intercellular contact and
allow cancer cells to resist anoikis (43). Cancer cells develop several
mechanisms for abrogating anoikis, such as activating Src/AKT/ERK
signaling, which is involved in anoikis resistance via blocking the
mitochondrial pathway and glycolysis (44). The Notch signaling pathway, which
is initiated by receptor (Notch1-4)-ligand (Δ and Jagged)
interaction, has been considered a potential therapeutic target for
OC (45). High expression of
Notch3 is associated with poor prognosis in epithelial OC (46). Elevated Notch3 expression promotes
anoikis resistance via upregulation of type IV α2 collagen (COL4A2)
gene, a key component of the basement membrane that allows OC cells
to maintain survival-friendly signaling by spoofing proteins
responsible for detecting ECM contact, such as integrins, without
making contact with ECM; FAK/AKT/ERK1/2 activation is the key
mechanism (47). Hepatocyte
growth factor (HGF) receptor c-Met is frequently highly expressed
in OC and contributes to anoikis resistance. The effects are
dependent on both phosphatidylinositol 3-kinase (PI3K)/AKT and
ERK1/2 signaling pathways and Ras serves as a central role for the
cross talk (48). In addition,
cancer cells commonly evade apoptosis by upregulating
anti-apoptotic Bcl-2 family proteins and/or downregulating
pro-apoptotic proteins (49).
Frizzled family receptor 7 (FZD7), which mediates both classical
and non-classical Wnt signaling, plays an important role in
maintaining SC properties as well as tumor development (50). A study (51) demonstrated the regulatory role of
FZD7 on spheroid proliferation of CSCs in OC via activation of the
Wnt/β-catenin pathway. TWIST1 is an important regulatory molecule
of the Wnt3a/Wnt1/β-catenin signaling pathway, which is closely
related to mesenchymal and tumor stem cell phenotypes (52). Tan et al (53) found that the FZD7/TWIST1/Bcl-2
signaling pathway played a role in the maintenance of mesenchymal
phenotype and anoikis resistance and was involved in OC spheroid
formation. FZD7 promoted TWIST1 expression via epigenetic
modifications of H3K4me3 and H3K27ac at the TWIST1 proximal
promoter; TWIST1 regulated the expression of Bcl-2, an
anti-apoptotic protein (53).
In ascites, individually suspended tumor cells are
more prone to anoikis than clustered cells. Multicellular spheroids
consisting of tumor cells surrounded by immune and stromal cells
show enhanced survival compared with individual tumor cells
(54). Long non-coding RNA HOTAIR
is a key indicator of poor prognosis in patients with OC. Dai et
al (55) found that HOTAIR is
upregulated in OC cells in suspension culture and allows cells to
acquire anoikis resistance. Silencing of HOTAIR in SKOV3 cells
inhibits spheroid formation, decreases aggressiveness and enhances
chemosensitivity. HOTAIR promotes enhancer of zeste homolog 2
(EZH2) expression; EZH2-mediated methylation of lysine 27 on
histone H3 (H3K27) contribute to the formation of spheroids
(55). HOTAIR can also serve as a
competitive endogenous RNA to regulate phosphoinositide-3-kinase
regulatory subunit 3 (PIK3R3) and promotes proliferation, migration
and invasion of OC cells. PIK3R3 is a subunit of PI3K and
activation of the PI3K/AKT signaling pathway is key for cell
survival (56,57). Activation of caspase-3 is a common
event in both intrinsic and extrinsic anoikis. In spherical OC
cells, AKT kinase is activated and promotes OC survival through
inhibiting caspase-3 (58).
Tropomysin-related kinase B (TrkB), a neurotrophic tyrosine kinase
receptor, is overexpressed in OC tissue, particularly in greater
omentum metastatic lesions and multicellular spheroids in ascites.
TrkB mediates suppression of anoikis via activating the PI3K/AKT
pathway (59). Furthermore,
clustered cancer cells express specific adhesion molecule
αv-integrin to activate survival signaling pathways. αv-integrin
maintains cell survival via ERK1/2 activation, thereby enhancing
the resistance of OC tumor spheroids to anoikis (60). Moreover, there are genetic
differences between tumor cells in primary tissue and ascites.
After tumor cells detach from primary tissue and disseminate into
the abdominal cavity, tumor cells undergo independent clonal
evolution. KRAS mutation in ascites leads to acquired anoikis
resistance, which increases the survival of tumor cells in ascites
(61).
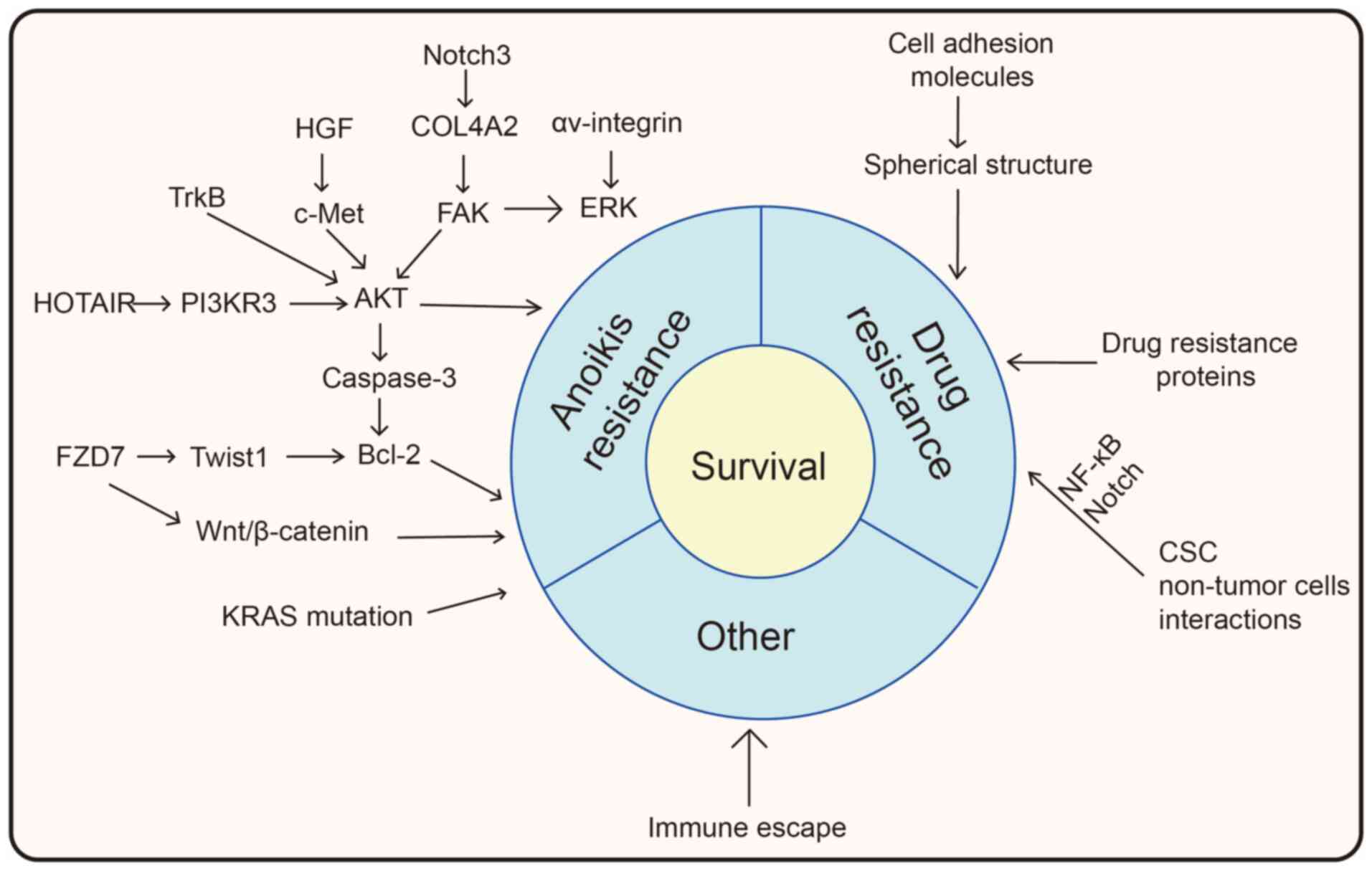
The mechanisms by which spheroids acquire resistance
to anoikis and chemotherapeutic drugs are summarized in Fig. 2. After acquiring anoikis and
chemotherapeutic resistance, as well as immune escape in the
immunosuppressive ascitic microenvironment, spheroids may survive
in the ascites, and then disseminate to the peritoneum.
The peritoneum, the largest serous membrane of the
human body, covering the abdominal and pelvic cavities and visceral
organs, is a preferred location for trancoelomic metastasis of
numerous types of epithelial malignancy, including ovarian, colonic
and gastric cancer (77). The
peritoneum is composed of a layer of mesothelial cells and
associated underlying ECM. These mesothelial cells serve as initial
barriers for cancer cells. However, they can be induced to
cancer-associated mesothelial cells (CAMs) by cancer cells.
Mesothelial cells undergo to mesothelial-mesenchymal transition
induced by HGF secreted by OC cells, then CAMs promote the
expression of pro-tumor factors such as IL-8 and C-X-C motif
chemokine ligand 5 (CXCL5) to facilitate dissemination of OC cells
(78). The greater omentum is the
most common metastatic site of OC, since it lacks basement membrane
and mesothelial cells on the surface of milky spots (79,80).
Mesothelial cells retract during peritoneal
metastasis. Unlike normal peritoneal mesothelial cells, which are
flattened and spread over the entire surface of the peritoneal
cavity, the mesothelial cells are rounded and separated from each
other during peritoneal metastasis, exposing the submesothelial
surface. OC spheroids detach from the primary tumor and disseminate
to the peritoneum though ascites. These spheroids adhere to the
mesothelial cells through adhesion molecules such as CD44, α5β1,
αvβ1 and α2β1 integrins and mesothelial cells undergo localized
retracement and detachment (81).
E-cadherin loss is often associated with metastasis of OC. In OC
cells, inhibited E-cadherin expression significantly upregulates
the expression of α5-integrin, a subunit of fibronectin receptor
α5β1-integrin, which binds with fibronectin, mediating adhesion to
the peritoneal ECM (29). Once
the spheroids spread over the monolayer of mesothelial cells,
mesothelial cells move out from directly beneath the spreading
spheroids. This is termed mesothelial clearance (82). Spheroid-induced mesothelial
clearance depends on α5β1 integrin, talin I and myosin II.
Following binding of mesothelial cells, spherical cancer cells
utilize integrin- and talin-dependent myosin activation and
traction, promoting mesothelial cells to migrate from beneath the
spheroids (82).
Mesothelial clearance leads to exposure of
underlying ECM and promotes further attachment of cancer cells.
Cancer cells express CD44 on the cell membrane, which binds to
hyaluronan in ECM to strengthen the link with peritoneal
mesothelium (83), and express
integrins that bind the basement membrane composed of laminin,
fibronectin and types I and IV collagen (84). Spheroids readily adhere to and
disaggregate from ECM substrates, particularly fibronectin and
collagen I (85). Disaggregation
of spheroids into individual cells is necessary for invasion of the
mesothelium (86). α2β1 integrin
serves a vital role in the dissemination of ovarian carcinoma
spheroids: α2β1 integrin on cancer cells adheres to type I
collagen, followed by secretion of serine and metalloproteinases,
and contributes to the metastasis of OC into the abdominal cavity
(87). In an in vitro
spreading homozygous spheroid model of OC, α2β1 integrin was
upregulated in spheroids and associated with disaggregation from
ECM and invasion by activating MMPs such as MMP2/MMP9 (88). Blockade of α2β1 integrin using
monoclonal antibodies decreases disaggregation and proteolysis of
spheroids (88).
In ascites, the majority of immune cells are
macrophages, which constitute >50% of MA cellular components
(89). A study (17) showed that macrophages are present
within all spheroids in ascites of 128 patients with OC at stage
III. Compared with primary tumors, the amount of macrophages in
spheroids is significantly increased and positively associated with
proliferation while inversely associated with the prognosis of OC
(17). In OC ascites, macrophages
float in the peritoneal cavity or in the center of tumor spheroids.
These macrophages may originate either from tissue-resident
macrophages derived from the embryonic yolk sac or from
infiltrating macrophages recruited from bone marrow-derived
monocytes. They are induced to TAMs in the tumor microenvironment
(TME), serving as an immunosuppressive cellular population,
promoting tumor growth, immune escape, angiogenesis and metastasis.
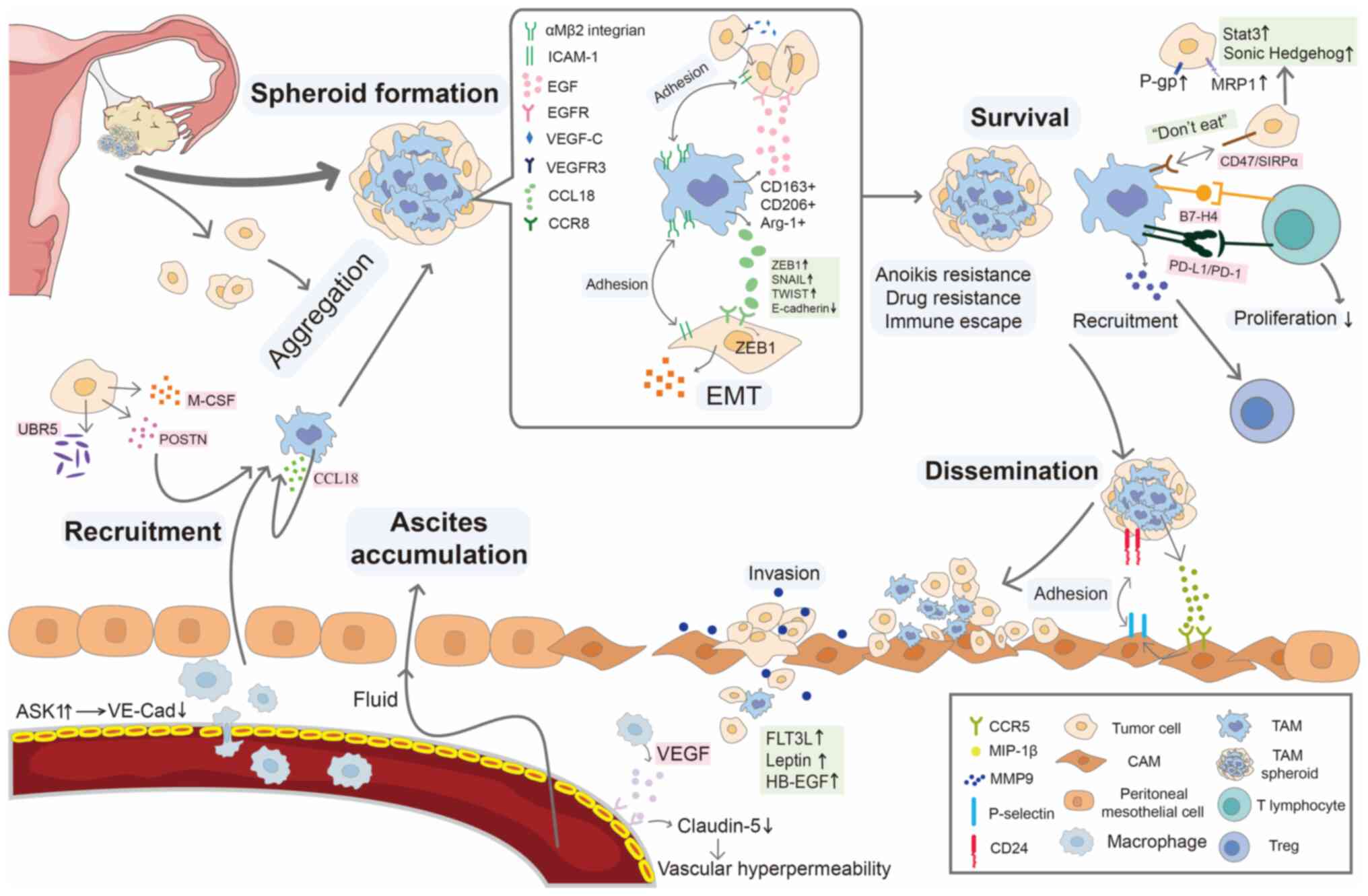
The role of TAMs in spheroid formation, survival and metastasis is
summarized in Fig. 3.
Increased permeability of vessels is a key
pathophysiological process involved in ascites accumulation. TAMs
are more likely to congregate at poorly vascularized sites
(90) and promote angiogenesis in
cancer by secreting VEGF and triggering revascularization (91). VEGF plays an essential role in
angiogenesis and lymphangiogenesis by binding to receptors
including kinase insert domain receptor (KDR)/fetal liver kinase
(Flk)-1 and FMS-like tyrosine kinase (Flt)-1 (92). Han et al (93) found that TAM-derived chemokine
CCL23 upregulates KDR/Flk-1 receptor expression in endothelial
cells and promotes VEGF-mediated angiogenesis. VEGF levels are
elevated significantly in OC ascites (94) and anti-VEGF treatment effectively
suppressed tumor growth in a xenograft mouse model of OC and
reduced ascites formation (95).
Moreover, VEGF-A, VEGF-C and VEGF-D secreted by macrophages are
involved in lymphangiogenesis dysfunction. Blockade of the
VEGF-A/C/D pathway significantly inhibits the formation of chylous
ascites in advanced OC mice (96). However, M2 macrophages
downregulate the expression of very late antigen-4 (VLA4) when
co-cultured with endothelial cells, decrease the levels of vascular
cell adhesion molecule 1 (VCAM1) in endothelial cells and
downregulate RAS-related C3 botulinum substrate 1 and reactive
oxygen species, which resulted in decreased phosphorylation of
proline-rich tyrosine kinase 2 and VE-cadherin. Therefore, M2
macrophages enhanced adhesion of endothelial cells and induced
hypopermeability. Moreover, targeting the VLA4/VCAM1 axis enhances
vascular integrity and eliminated the formation of ascites in
vivo (97).
Due to the plasticity of macrophages,
undifferentiated macrophages (M0) can be polarized into two types,
M1 and M2, which are distinguished by surface receptor expression,
secretion pattern and function (107). The terms M1 and M2 were proposed
by Mills et al (108)
based on differences in arginine metabolism in macrophages from
C57BL/6 and BALB/c mice, the effects of which are associated with
differences between T helper (Th)1 and Th2 cell response. M1
macrophages produce a large number of pro-inflammatory cytokines
under stimulation of Th1 cytokines such as IFN-γ and toll-like
receptor (TLR) agonists such as lipopolysaccharide (LPS), and serve
an essential role in anti-tumor response. M2 macrophages are
stimulated by Th2 cytokines such as IL-4, IL-10 and TGF-β,
promoting angiogenesis, and tissue repair (109). Due to the diversity of stimuli,
M2 macrophages are further divided into M2a (IL-4 and IL-13), M2b
(immune complex and LPS/IL-1), M2c (glucocorticoids, IL-10, TGF-β)
and M2d (adenosine A2A receptor agonists and LPS) subtypes
(109,110). Although the definition of M1-M2
macrophages provides a simplified paradigm for studying macrophage
phenotype and function, this may oversimplify the complexity and
diversity of macrophages, which exhibit mixed or unique phenotypes
in many pathological conditions (111,112).
In the TME, polarized macrophages are known as TAMs.
TAMs are composed of heterogeneous subpopulations, including M1 and
M2 macrophages. TAMs predominantly express M2 macrophage markers
and cytokines, such as CD206, CD163 and IL-10, and exhibit
pro-tumor effects, and are therefore referred to as M2-like TAMs
(113,114). By contrast, few TAMs in the TME
express CD86 and CD80 markers and are referred to as M1-like TAMs,
which typically exhibit anti-tumor effects (115,116). The ratio of M1/M2 is a
prognostic indicator for OC. Patients with high M1/M2 ratio have a
significantly longer overall and progression-free survival and
platinum-free interval than patients with low M1/M2 (117). Plasticity is a key feature of
macrophages. The phenotype of polarized M2-like TAMs can be
reversed to M1-like TAMs to some extent (118). Therefore, shifting M2- to
M1-like TAMs, rather than depleting TAMs, may serve as a treatment
for cancer.
In ascites of OC, TAMs either float alone or are
present in the center of spheroids encircled by cancer cells,
primarily displaying M2-like phenotype with high expression of
CD163 and CD206 (17).
CD163+ TAM is rarely found outside the spheroids
(119). TAMs participate in
formation of spheroids, and are associated with prognosis of OC.
CD68, a transmembrane glycoprotein that is widely expressed in
monocytes, is considered a marker of TAMs in the TME (120). OC cases with high percentages of
CD68+ TAMs (>14.5%) in spheroids have a significantly
lower 5-year overall survival than those with low proportion
(<14.5%) of CD68+ TAMs (17). In 3D co-culture system, CCL18/zinc
finger E-box binding protein 1 (ZEB1)/M-CSF axis facilitates
spheroid formation. During the spheroid formation stage, OC cells
secretes M-CSF to induce TAMs to M2 polarization, and M2-like TAMs
induce EMT of cancer cells, characterized by increased expression
of mesenchymal markers (including ZEB1, SNAIL and TWIST) and
decreased expression of E-cadherin. Mechanistically, TAMs release
chemokine CCL18, which interacts with chemokine receptor 8 (CCR8)
on the surface of OC cells, subsequently increasing the expression
of ZEB1, a transcriptional factor which binds the promoter of
M-CSF, and enhanced M-CSF expression. Overexpression of ZEB1 in OC
cells promotes cancer cell-TAM spheroid formation in vitro
and in mice (16). Since EMT is a
key factor involved in invasion, metastasis and chemotherapy
resistance (121), the
CCL18/ZEB1/M-CSF feedback loop between OC cells and TAMs not only
promoted formation of spheroids in ascites, but also led to faster
and earlier transcoelomic metastasis of OC (16).
TAMs can secrete various cytokines which are
essential for tumor cell proliferation and survival. EGF is one of
these cytokines that form homodimers or heterodimers on the cell
surface and mediate cell proliferation signal transduction. TAMs
are key cellular sources of EGF secretion in tumor tissues
(15). EGF/EGFR signaling between
TAMs and cancer cells was essential for spheroid formation. Within
large spheroids, TAMs displaying M2-polarization markers (including
CD163, CD206 and arginase 1) are located in the center of spheroids
and surrounded by EGFR+ cancer cells. TAM-derived EGF
activated EGFR on cancer cells, then increases expression of VEGF-C
and VEGFR3 in cancer cells. EGFR blockade with erlotinib may
inhibit spheroid formation and transcoelomic metastasis in
vivo (17). Moreover, EGF
secreted by TAMs increases the expression of αMβ2 integrin in TAMs
and ICAM-1 in cancer cells to facilitate adhesion between TAMs and
cancer cells (17). Therefore,
the EGF/EGFR/VEGF-C/VEGFR3/αMβ2/ICAM-1 signaling pathway serves a
key role in OC progression (17).
Once floating in ascites, OC cells need to resist to
anoikis, and multicellular spherical cells are more resistant to
anoikis compared with individual cancer cells (60). TAMs serve a key role in anoikis
resistance. Centrally located TAMs promote spheroid formation to
provide a structural support OC cells to evade anoikis (17). STAT3 is an essential signal
transduction molecule at the intersection of numerous pro-tumor
signaling pathways (122), as
well as mediating macrophage induction to M2-poralization (123). TAMs protected OC cells against
anoikis via releasing several soluble factors, such as IL-6 and
IL-10, which activated the STAT3 signaling in cancer cells, and
promoted cell proliferation and peritoneal dissemination (123). When co-cultured with macrophages
in vitro, especially M2 macrophages stimulated by M-CSF,
ovarian cell line SKOV3 cells exhibit activation of the STAT3
signaling pathway (123). Hence,
TAMs promote OC cell survival though enhanced anoikis
resistance.
TAMs may promote spheroid resistance to
chemotherapeutic drugs in ascites. In a 3D culture model of canine
mammary gland tumor cell lines with or without macrophages,
compared with homogeneous spheroids composed only of tumor cells,
homogeneous spheroids composed of tumor cells and macrophages
displayed increased cell viability when treated with doxorubicin.
Compared with monolayer tumor cells, expression levels of VEGF,
TGF-β, tumor necrosis factor-α-stimulated gene/protein-6 and drug
resistance-related proteins such as P-glycoprotein and multidrug
resistance-associated protein 1 are significantly increased in
spheroids. Furthermore, doxorubicin-induced apoptosis and G2/M cell
cycle arrest are decreased in the presence of tumor cells
co-cultured with macrophages (69). CSCs are involved in tumorigenicity
and drug resistance. TAMs regulated CSC activities by releasing
milk-fat globule-epidermal growth factor-VIII, which activates
STAT3 and Sonic hedgehog pathways in CSCs and contributes to their
resistance to cisplatin in vivo (124). During the interaction between M2
macrophages and ovarian CSCs, paracrine Wnt is stimulated, which
may enhance the aggressive phenotype of macrophages and cancer
cells (125).
The presence of TAMs enhances drug resistance in OC
spheroids, and TAMs can serve as a potential therapeutic target for
treatment of OC. It is possible to reduce tumor cell resistance and
improve cytotoxicity of drug therapy by blocking macrophage
recruitment into the TME and resetting macrophage polarization
(126). Recent studies revealed
that blocking M2 macrophage polarization makes OC cells more
sensitive to chemotherapeutic drugs including cisplatin and poly
(ADP-ribose) polymerase inhibitors in vitro and in
vivo (127-129).
CD47-signal receptor protein-α (SIRPα) is the main
innate immune checkpoint between macrophages and cancer cells. CD47
on the surface of tumor cells can release the signal 'don't eat me'
by binding to the SIRPα receptor on the surface of macrophages,
helping tumor cells to evade immune killing (130). Blocking the CD47/SIRPα signaling
pathway can effectively promote phagocytosis of tumor cells by
macrophages in vitro and in vivo (131). Blocking CD47 signaling with an
oncolytic adenovirus carrying a SIRPα-IgG1Fc fusion gene (SG635-SF)
significantly increases macrophage infiltration into the tumor and
suppresses tumor growth in OC mice (132).
In the TME, TAMs have a markedly immunosuppressive
effect on adaptive immune cells by releasing many cytokines,
chemokines and enzymes, such as IL-10, TGF-β1, CCL12 and Arg-1.
IL-10 causes naive CD4+ T cells to differentiate into
Th2 cells, which suppress adaptive immunity, to allow malignant
cells to escape immune surveillance (133). TGF-β1 may suppress T cell
expansion through Smad3-dependent and -independent pathways
(134). CCL22 secreted by TAMs
establishes a chemokine gradient to induce regulatory T (Treg) cell
migration into the local microenvironment, thereby increasing the
proportion of Tregs (135).
Tregs exist in ascites abundantly and accelerate tumor growth and
progression via suppressing anti-tumor immunity. Macrophage-derived
CCL23 induces CD8+ T cell exhaustion by upregulating
molecules related to immune checkpoints, including cytotoxic T
lymphocyte-associated antigen-4 (CTLA4), T cell immunoreceptor with
immunoglobulin and immune receptor tyrosine-based inhibitory domain
(TIGIT), T cell immunoglobulin and mucin domain 3 (TIM-3) and T
cell immunoglobulin and mucin domain 3 (LAG-3) (136). TAMs express programmed cell
death ligand 1 (PD-L1), PD-L2, CD80, and CD86, which restrict
CD8+ T cell activation by binding to their receptors,
PD1 and CTLA4 (137). B7-H3 not
only serves as a costimulatory molecule that modulates T cell
function directly (138), but
also serves as an immune checkpoint involved in indirect T cell
suppression via the CCL2-CCR2-M2 macrophage axis (139). B7-H4 is a recently identified B7
family molecule (140). A
subpopulation of macrophages in OC ascites express B7-H4, a
costimulatory molecule which decreases the proliferation and
cytokine production of T cells (141).
TAMs accelerate tumor metastasis by promoting
expression of peritoneal mesothelial cell adhesion molecules and
releasing growth factors and invasive proteases (142,143). During the metastasis of OC,
P-selectin is overexpressed on the surface of mesothelial cells and
cancer cells attach to P-selectin through CD24, resulting in
increased adhesion between cancer and mesothelial cells (142). In a co-culture model with cancer
and mesothelial cells and M2 macrophages, expression of P-selectin
was regulated by M2 macrophages which secrete macrophage
inflammatory pro1tein-1β (MIP-1β) that activates CCR5/PI3K
signaling in mesothelial cells, resulting in upregulation of
P-selectin on the mesothelial cell surface. MIP-1β treatment
increases P-selectin expression in peritoneal mesothelial cells of
mice and enhances OC cell adhesion in vitro and in
vivo (142). Analysis of
samples from patients with high-grade serous OC confirmed increased
MIP-1β and P-selectin expression, suggesting that TAMs in ascites
secrete MIP-1β, which increases expression of P-selectin on the
surface of mesothelial cells (142).
OC spheroids depart from the primary tumor and
reattach throughout the peritoneal cavity. Once cancer cells are
implanted at new sites, formation of metastatic lesions is
dependent on disintegration of spheroids and subsequent spread
across the ECM. Soluble factors such as FMS-like tyrosine kinase 3
ligand, leptin, or heparin-binding EGF derived from TAMs are
responsible for spheroids spreading across the underlying ECM. The
common signaling pathway of these soluble factors is Janus kinase 2
(JAK2)/STAT3 activation followed by MMP-9 mediated spreading
(144). In addition, macrophages
increase the invasion ability of cancer cells though TNF-α and
NF-κB pathways. Macrophages can release VEGF to promote the
dissemination of OC cells onto the peritoneum of mice. In mice,
deletion of macrophages alone results in decreased expression of
VEGF, inhibition of ascites formation and peritoneal metastasis
(143). Moreover, TAMs also
secrete EGF to facilitate OC metastasis via activation of EGFR/ERK
signaling and suppression of long non-coding (lnc)RNA inhibiting
metastasis (LIMT) expression. Following co-cultured with M2
macrophages, OC cells showed greater migration capacity, and these
effects could be reversed by inhibiting EGF and overexpressing
lncRNA LIMT (145).
MA and spheroids are key for OC progression and
recurrence, leading to relapse following classical therapy. TAMs
play a critical role in spheroid formation and dissemination, as
well as ascites formation. In addition, TAMs contribute to
chemotherapeutic resistance and the suppression of the immune
microenvironment characterized by enriched Tregs and exhausted
CD8+ T cells, thereby promoting survival of cancer
cells. Therefore, TAMs are a promising target in OC treatment.
Strategies to target TAMs include blocking the recruitment of
macrophages to the TME, shifting TAM polarization from M2 to M1
type, increasing phagocytosis of TAMs, blocking formation and
intraperitoneal metastasis of TAM-tumor cell spheroids and
improving chemotherapeutic sensitivity. Effects of TAM-based
treatment for OC are summarized in Table I.
Cancer cells in OC ascites exist in two forms,
individual cells and multicellular spheroids. Spheroids are divided
into homospheroids and heterotypical spheroids, which contain tumor
and non-tumor cells. The spheroids are involved in progression of
OC, since they are more resistant to anoikis and chemotherapeutic
drugs and are considered to be metastatic units. TAMs serve an
essential role in OC progression. Recently, some groups
demonstrated functions of TAMs in the formation of spheroids and
dissemination (16,21,156). Although some researchers have
revealed the discrete molecular mechanisms of spheroid formation,
survival and metastasis (16,142), there are no data showing
crosstalk between these pathways. Studies investigating the role of
TAMs in OC spheroids typically use co-culture of TAMs and tumor
cells in 3D culture model in the presence or absence of Matrigel or
other scaffolds (16,157). Most studies have utilized OC
cell lines and M2 macrophages to mimic OC cells and TAMs,
respectively (16,142,144).These findings needed to be
further clarified in patients. The majority of studies only
co-cultured TAMs and tumor cells, and lack of other cellular
components and non-cellular components present in the TME (16,144). These components are likely to
influence TAM effects on the spheroids, making the results less
convincing.
More molecules and signal pathways need to be
investigated in spheroid formation, survival and metastasis, and
the network among these molecules should be identified.
Standardized methods are required for 3D culture model, such as
description of size of spheroids, and presence or absence of
scaffolds. Results obtained in primary cancer cells and TAMs from
patients with OC are more persuasive than cell lines.
Patient-derived organoids may mimic the TME. Cellular and
non-cellular components in ascites can be separated from patients
with OC and used to mimic the TME.
Not applicable.
YL and YX designed the review. YL and HX wrote the
manuscript. HZ and YX revised the manuscript. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant no. 81602303) and Start Fund of First
Affiliated Hospital of Yangtze University (grant no.
2022DIF01).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC, Han CY, Lu Z and Lu KH: Next
steps in the early detection of ovarian cancer. Commun Med (Lond).
1:362021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Xie HJ, Li YY, Wang X, Liu XX and
Mai J: Molecular mechanisms of platinum-based chemotherapy
resistance in ovarian cancer (review). Oncol Rep. 47:822022.
View Article : Google Scholar
|
|
4
|
Almeida-Nunes DL, Mendes-Frias A,
Silvestre R, Dinis-Oliveira RJ and Ricardo S: Immune tumor
microenvironment in ovarian cancer ascites. Int J Mol Sci.
23:106922022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang H, Li YJ, Lan CY, Huang QD, Feng YL,
Huang YW and Liu JH: Clinical significance of ascites in epithelial
ovarian cancer. Neoplasma. 60:546–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim S, Kim B and Song YS: Ascites
modulates cancer cell behavior, contributing to tumor heterogeneity
in ovarian cancer. Cancer Sci. 107:1173–1178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan Q, Zhou S, Liu Y, Yin W, Liao Q, Ren
S, Zhang F, Meng Y and Mu X: Relationship between ascites volume
and clinical outcomes in epithelial ovarian cancer. J Obstet
Gynaecol Res. 47:1527–1535. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cavazzoni E, Bugiantella W, Graziosi L,
Franceschini MS and Donini A: Malignant ascites: Pathophysiology
and treatment. Int J Clin Oncol. 18:1–9. 2013. View Article : Google Scholar
|
|
9
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al Habyan S, Kalos C, Szymborski J and
McCaffrey L: Multicellular detachment generates metastatic
spheroids during intra-abdominal dissemination in epithelial
ovarian cancer. Oncogene. 37:5127–5135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed N and Stenvers KL: Getting to know
ovarian cancer ascites: Opportunities for targeted therapy-based
translational research. Front Oncol. 3:2562013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dhaliwal D and Shepherd TG: Molecular and
cellular mechanisms controlling integrin-mediated cell adhesion and
tumor progression in ovarian cancer metastasis: A review. Clin Exp
Metastasis. 39:291–301. 2022. View Article : Google Scholar :
|
|
13
|
Worzfeld T, Pogge von Strandmann E, Huber
M, Adhikary T, Wagner U, Reinartz S and Müller R: The unique
molecular and cellular microenvironment of ovarian cancer. Front
Oncol. 7:242017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Q, Yang Z, Xu S, Li X, Yang X, Jin P,
Liu Y, Zhou X, Zhang T, Gong C, et al: Heterotypic CAF-tumor
spheroids promote early peritoneal metastatis of ovarian cancer. J
Exp Med. 216:688–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan Y, Yu Y, Wang X and Zhang T:
Tumor-associated macrophages in tumor immunity. Front Immunol.
11:5830842020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long L, Hu Y, Long T, Lu X, Tuo Y, Li Y
and Ke Z: Tumor-associated macrophages induced spheroid formation
by CCL18-ZEB1-M-CSF feedback loop to promote transcoelomic
metastasis of ovarian cancer. J Immunother Cancer. 9:e0039732021.
View Article : Google Scholar
|
|
17
|
Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone
S, Xu X, Zhang H, Santin AD, Lou G and Min W: Tumor-associated
macrophages drive spheroid formation during early transcoelomic
metastasis of ovarian cancer. J Clin Invest. 126:4157–4173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song M, Yeku OO, Rafiq S, Purdon T, Dong
X, Zhu L, Zhang T, Wang H, Yu Z, Mai J, et al: Tumor derived UBR5
promotes ovarian cancer growth and metastasis through inducing
immunosuppressive macrophages. Nat Commun. 11:62982020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Arabey AA, Alkhalil SS, Al-Shouli ST,
Awadalla ME, Alhamdi HW, Almanaa TN, Mohamed SSEM and Abdalla M:
Revisiting macrophages in ovarian cancer microenvironment:
Development, function and interaction. Med Oncol. 40:1422023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larionova I, Tuguzbaeva G, Ponomaryova A,
Stakheyeva M, Cherdyntseva N, Pavlov V, Choinzonov E and
Kzhyshkowska J: Tumor-associated macrophages in human breast,
colorectal, lung, ovarian and prostate cancers. Front Oncol.
10:5665112020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyamoto T, Murphy B and Zhang N:
Intraperitoneal metastasis of ovarian cancer: New insights on
resident macrophages in the peritoneal cavity. Front Immunol.
14:11046942023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin M, Shen J, Yu S, Fei J, Zhu X, Zhao J,
Zhai L, Sadhukhan A and Zhou J: Tumor-associated macrophages
(TAMs): A critical activator in ovarian cancer metastasis. Onco
Targets Ther. 12:8687–8699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jazwinska DE, Kulawiec DG and
Zervantonakis IK: Cancer-mesothelial and cancer-macrophage
interactions in the ovarian cancer microenvironment. Am J Physiol
Cell Physiol. 325:C721–C730. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klymenko Y, Johnson J, Bos B, Lombard R,
Campbell L, Loughran E and Stack MS: Heterogeneous cadherin
expression and multicellular aggregate dynamics in ovarian cancer
dissemination. Neoplasia. 19:549–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ford CE, Werner B, Hacker NF and Warton K:
The untapped potential of ascites in ovarian cancer research and
treatment. Br J Cancer. 123:9–16. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habanjar O, Diab-Assaf M, Caldefie-Chezet
F and Delort L: 3D cell culture systems: Tumor application,
advantages, and disadvantages. Int J Mol Sci. 22:122002021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matte I, Legault CM, Garde-Granger P,
Laplante C, Bessette P, Rancourt C and Piché A: Mesothelial cells
interact with tumor cells for the formation of ovarian cancer
multicellular spheroids in peritoneal effusions. Clin Exp
Metastasis. 33:839–852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu S, Yang Y, Dong L, Qiu W, Yang L, Wang
X and Liu L: Construction and characteristics of an
E-cadherin-related three-dimensional suspension growth model of
ovarian cancer. Sci Rep. 4:56462014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawada K, Mitra AK, Radjabi AR, Bhaskar V,
Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A,
Kenny HA, et al: Loss of E-cadherin promotes ovarian cancer
metastasis via alpha 5-integrin, which is a therapeutic target.
Cancer Res. 68:2329–2339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casey RC, Burleson KM, Skubitz KM,
Pambuccian SE, Oegema TR Jr, Ruff LE and Skubitz AP: Beta
1-integrins regulate the formation and adhesion of ovarian
carcinoma multicellular spheroids. Am J Pathol. 159:2071–2080.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han Q, Huang B, Huang Z, Cai J, Gong L,
Zhang Y, Jiang J, Dong W and Wang Z: Tumor cell-fibroblast
heterotypic aggregates in malignant ascites of patients with
ovarian cancer. Int J Mol Med. 44:2245–2255. 2019.PubMed/NCBI
|
|
32
|
Hassn Mesrati M, Syafruddin SE, Mohtar MA
and Syahir A: CD44: A multifunctional mediator of cancer
progression. Biomolecules. 11:18502021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen MW, Yang ST, Chien MH, Hua KT, Wu CJ,
Hsiao SM, Lin H, Hsiao M, Su JL and Wei LH: The STAT3-miRNA-92-Wnt
signaling pathway regulates spheroid formation and malignant
progression in ovarian cancer. Cancer Res. 77:1955–1967. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li SS, Ma J and Wong AST: Chemoresistance
in ovarian cancer: Exploiting cancer stem cell metabolism. J
Gynecol Oncol. 29:e322018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Casagrande N, Borghese C, Agostini F,
Durante C, Mazzucato M, Colombatti A and Aldinucci D: In ovarian
cancer multicellular spheroids, platelet releasate promotes growth,
expansion of ALDH+ and CD133+ cancer stem cells, and protection
against the cytotoxic effects of cisplatin, carboplatin and
paclitaxel. Int J Mol Sci. 22:30192021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Condello S, Morgan CA, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-Catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:2297–2308. 2015.
View Article : Google Scholar
|
|
37
|
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick
R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al:
Myeloid-derived suppressor cells enhance stemness of cancer cells
by inducing microRNA101 and suppressing the corepressor CtBP2.
Immunity. 39:611–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suganuma T, Ino K, Shibata K, Kajiyama H,
Nagasaka T, Mizutani S and Kikkawa F: Functional expression of the
angiotensin II type 1 receptor in human ovarian carcinoma cells and
its blockade therapy resulting in suppression of tumor invasion,
angiogenesis, and peritoneal dissemination. Clin Cancer Res.
11:2686–2694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q, Yu S, Lam MMT, Poon TCW, Sun L,
Jiao Y, Wong AST and Lee LTO: Angiotensin II promotes ovarian
cancer spheroid formation and metastasis by upregulation of lipid
desaturation and suppression of endoplasmic reticulum stress. J Exp
Clin Cancer Res. 38:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Adeshakin FO, Adeshakin AO, Afolabi LO,
Yan D, Zhang G and Wan X: Mechanisms for modulating anoikis
resistance in cancer and the relevance of metabolic reprogramming.
Front Oncol. 11:6265772021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grossmann J: Molecular mechanisms of
'detachment-induced apoptosis-Anoikis'. Apoptosis. 7:247–260. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Taddei ML, Giannoni E, Fiaschi T and
Chiarugi P: Anoikis: An emerging hallmark in health and diseases. J
Pathol. 226:380–393. 2012. View Article : Google Scholar
|
|
44
|
Cai Q, Yan L and Xu Y: Anoikis resistance
is a critical feature of highly aggressive ovarian cancer cells.
Oncogene. 34:3315–3324. 2015. View Article : Google Scholar :
|
|
45
|
Cancer Genome Atlas Research Network:
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park JT, Shih IeM and Wang TL:
Identification of Pbx1, a potential oncogene, as a Notch3 target
gene in ovarian cancer. Cancer Res. 68:8852–8860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brown CW, Brodsky AS and Freiman RN:
Notch3 overexpression promotes anoikis resistance in epithelial
ovarian cancer via upregulation of COL4A2. Mol Cancer Res.
13:78–85. 2015. View Article : Google Scholar :
|
|
48
|
Tang MKS, Zhou HY, Yam JW and Wong AS:
c-Met overexpression contributes to the acquired apoptotic
resistance of nonadherent ovarian cancer cells through a cross talk
mediated by phosphatidylinositol 3-kinase and extracellular
signal-regulated kinase 1/2. Neoplasia. 12:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lopez A, Reyna DE, Gitego N, Kopp F, Zhou
H, Miranda-Roman MA, Nordstrøm LU, Narayanagari SR, Chi P, Vilar E,
et al: Co-targeting of BAX and BCL-XL proteins broadly overcomes
resistance to apoptosis in cancer. Nat Commun. 13:11992022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mei H, Nakatsu MN, Baclagon ER and Deng
SX: Frizzled 7 maintains the undifferentiated state of human limbal
stem/progenitor cells. Stem Cells. 32:938–945. 2014. View Article : Google Scholar
|
|
51
|
Condello S, Sima L, Ivan C, Cardenas H,
Schiltz G, Mishra RK and Matei D: Tissue tranglutaminase regulates
interactions between ovarian cancer stem cells and the tumor niche.
Cancer Res. 78:2990–3001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qin Q, Xu Y, He T, Qin C and Xu J: Normal
and disease-related biological functions of Twist1 and underlying
molecular mechanisms. Cell Res. 22:90–106. 2012. View Article : Google Scholar :
|
|
53
|
Tan M, Asad M, Heong V, Wong MK, Tan TZ,
Ye J, Kuay KT, Thiery JP, Scott C and Huang RY: The FZD7-TWIST1
axis is responsible for anoikis resistance and tumorigenesis in
ovarian carcinoma. Mol Oncol. 13:757–780. 2019. View Article : Google Scholar :
|
|
54
|
Motohara T, Masuda K, Morotti M, Zheng Y,
El-Sahhar S, Chong KY, Wietek N, Alsaadi A, Carrami EM, Hu Z, et
al: An evolving story of the metastatic voyage of ovarian cancer
cells: Cellular and molecular orchestration of the adipose-rich
metastatic microenvironment. Oncogene. 38:2885–2898. 2019.
View Article : Google Scholar :
|
|
55
|
Dai ZY, Jin SM, Luo HQ, Leng HL and Fang
JD: LncRNA HOTAIR regulates anoikis-resistance capacity and
spheroid formation of ovarian cancer cells by recruiting EZH2 and
influencing H3K27 methylation. Neoplasma. 68:509–518. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang L, Huang J, Yang N, Greshock J,
Liang S, Hasegawa K, Giannakakis A, Poulos N, O'Brien-Jenkins A,
Katsaros D, et al: Integrative genomic analysis of
phosphatidylinositol 3′-kinase family identifies PIK3R3 as a
potential therapeutic target in epithelial ovarian cancer. Clin
Cancer Res. 13:5314–5321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dong L and Hui L: HOTAIR promotes
proliferation, migration, and invasion of ovarian cancer SKOV3
cells through regulating PIK3R3. Med Sci Monit. 22:325–331. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dolinschek R, Hingerl J, Benge A, Zafiu C,
Schüren E, Ehmoser EK, Lössner D and Reuning U: Constitutive
activation of integrin αvβ3 contributes to anoikis resistance of
ovarian cancer cells. Mol Oncol. 15:503–522. 2021. View Article : Google Scholar
|
|
59
|
Yu X, Liu L, Cai B, He Y and Wan X:
Suppression of anoikis by the neurotrophic receptor TrkB in human
ovarian cancer. Cancer Sci. 99:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carduner L, Picot CR, Leroy-Dudal J, Blay
L, Kellouche S and Carreiras F: Cell cycle arrest or survival
signaling through αv integrins, activation of PKC and ERK1/2 lead
to anoikis resistance of ovarian cancer spheroids. Exp Cell Res.
320:329–342. 2014. View Article : Google Scholar
|
|
61
|
Kim S, Kim S, Kim J, Kim B, Kim SI, Kim
MA, Kwon S and Song YS: Evaluating tumor evolution via genomic
profiling of individual tumor spheroids in a malignant ascites. Sci
Rep. 8:127242018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Allen HJ, Porter C, Gamarra M, Piver MS
and Johnson EA: Isolation and morphologic characterization of human
ovarian carcinoma cell clusters present in effusions. Exp Cell
Biol. 55:194–208. 1987.PubMed/NCBI
|
|
63
|
Azharuddin M, Roberg K, Dhara AK, Jain MV,
Darcy P, Hinkula J, Slater NKH and Patra HK: Dissecting multi drug
resistance in head and neck cancer cells using multicellular tumor
spheroids. Sci Rep. 9:200662019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Świerczewska M, Sterzyńska K, Ruciński M,
Andrzejewska M, Nowicki M and Januchowski R: The response and
resistance to drugs in ovarian cancer cell lines in 2D monolayers
and 3D spheroids. Biomed Pharmacother. 165:1151522023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sun Y, Li S, Yang L, Zhang D, Zhao Z, Gao
J and Liu L: CDC25A facilitates chemo-resistance in ovarian cancer
multicellular spheroids by promoting E-cadherin expression and
arresting cell cycles. J Cancer. 10:2874–2884. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shen T and Huang S: The role of Cdc25A in
the regulation of cell proliferation and apoptosis. Anticancer
Agents Med Chem. 12:631–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Broggini M, Buraggi G, Brenna A, Riva L,
Codegoni AM, Torri V, Lissoni AA, Mangioni C and D'Incalci M: Cell
cycle-related phosphatases CDC25A and B expression correlates with
survival in ovarian cancer patients. Anticancer Res. 20:4835–4840.
2000.
|
|
68
|
Green SK, Francia G, Isidoro C and Kerbel
RS: Antiadhesive antibodies targeting E-cadherin sensitize
multicellular tumor spheroids to chemotherapy in vitro. Mol Cancer
Ther. 3:149–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lim GH, An JH, Park SM, Youn GH, Oh YI,
Seo KW and Youn HY: Macrophage induces anti-cancer drug resistance
in canine mammary gland tumor spheroid. Sci Rep. 13:103942023.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Makhija S, Taylor DD, Gibb RK and
Gerçel-Taylor C: Taxol-induced bcl-2 phosphorylation in ovarian
cancer cell monolayer and spheroids. Int J Oncol. 14:515–521.
1999.PubMed/NCBI
|
|
71
|
Yvon AM, Wadsworth P and Jordan MA: Taxol
suppresses dynamics of individual microtubules in living human
tumor cells. Mol Biol Cell. 10:947–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Makovec T: Cisplatin and beyond: Molecular
mechanisms of action and drug resistance development in cancer
chemotherapy. Radiol Oncol. 53:148–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kwon MJ and Shin YK: Regulation of ovarian
cancer stem cells or tumor-initiating cells. Int J Mol Sci.
14:6624–6648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD and Odunsi K: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
McAuliffe SM, Morgan SL, Wyant GA, Tran
LT, Muto KW, Chen YS, Chin KT, Partridge JC, Poole BB, Cheng KH, et
al: Targeting Notch, a key pathway for ovarian cancer stem cells,
sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA.
109:E2939–E2948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kang H, Jeong JY, Song JY, Kim TH, Kim G,
Huh JH, Kwon AY, Jung SG and An HJ: Notch3-specific inhibition
using siRNA knockdown or GSI sensitizes paclitaxel-resistant
ovarian cancer cells. Mol Carcinog. 55:1196–1209. 2016. View Article : Google Scholar
|
|
77
|
van Baal JOAM, Van de Vijver KK, Nieuwland
R, van Noorden CJF, van Driel WJ, Sturk A, Kenter GG, Rikkert LG
and Lok CAR: The histophysiology and pathophysiology of the
peritoneum. Tissue Cell. 49:95–105. 2017. View Article : Google Scholar
|
|
78
|
Nakamura M, Ono YJ, Kanemura M, Tanaka T,
Hayashi M, Terai Y and Ohmichi M: Hepatocyte growth factor secreted
by ovarian cancer cells stimulates peritoneal implantation via the
mesothelial-mesenchymal transition of the peritoneum. Gynecol
Oncol. 139:345–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yousefi M, Dehghani S, Nosrati R, Ghanei
M, Salmaninejad A, Rajaie S, Hasanzadeh M and Pasdar A: Current
insights into the metastasis of epithelial ovarian cancer-hopes and
hurdles. Cell Oncol (Dordr). 43:515–538. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cui L, Johkura K, Liang Y, Teng R, Ogiwara
N, Okouchi Y, Asanuma K and Sasaki K: Biodefense function of
omental milky spots through cell adhesion molecules and leukocyte
proliferation. Cell Tissue Res. 310:321–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Niedbala MJ, Crickard K and Bernacki RJ:
Interactions of human ovarian tumor cells with human mesothelial
cells grown on extracellular matrix. An in vitro model system for
studying tumor cell adhesion and invasion. Exp Cell Res.
160:499–513. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Iwanicki MP, Davidowitz RA, Ng MR, Besser
A, Muranen T, Merritt M, Danuser G, Ince TA and Brugge JS: Ovarian
cancer spheroids use myosin-generated force to clear the
mesothelium. Cancer Discov. 1:144–157. 2011. View Article : Google Scholar
|
|
83
|
Cannistra SA, Kansas GS, Niloff J,
DeFranzo B, Kim Y and Ottensmeier C: Binding of ovarian cancer
cells to peritoneal mesothelium in vitro is partly mediated by
CD44H. Cancer Res. 53:3830–3838. 1993.PubMed/NCBI
|
|
84
|
Witz CA, Montoya-Rodriguez IA, Cho S,
Centonze VE, Bonewald LF and Schenken RS: Composition of the
extracellular matrix of the peritoneum. J Soc Gynecol Investig.
8:299–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Burleson KM, Casey RC, Skubitz KM,
Pambuccian SE, Oegema TR Jr and Skubitz AP: Ovarian carcinoma
ascites spheroids adhere to extracellular matrix components and
mesothelial cell monolayers. Gynecol Oncol. 93:170–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Burleson KM, Boente MP, Pambuccian SE and
Skubitz AP: Disaggregation and invasion of ovarian carcinoma
ascites spheroids. J Transl Med. 4:62006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Moser TL, Pizzo SV, Bafetti LM, Fishman DA
and Stack MS: Evidence for preferential adhesion of ovarian
epithelial carcinoma cells to type I collagen mediated by the
alpha2beta1 integrin. Int J Cancer. 67:695–701. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shield K, Riley C, Quinn MA, Rice GE,
Ackland ML and Ahmed N: Alpha2beta1 integrin affects metastatic
potential of ovarian carcinoma spheroids by supporting
disaggregation and proteolysis. J Carcinog. 6:112007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gupta V, Yull F and Khabele D: Bipolar
tumor-associated macrophages in ovarian cancer as targets for
therapy. Cancers (Basel). 10:3662018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Leek RD, Lewis CE, Whitehouse R, Greenall
M, Clarke J and Harris AL: Association of macrophage infiltration
with angiogenesis and prognosis in invasive breast carcinoma.
Cancer Res. 56:4625–4629. 1996.PubMed/NCBI
|
|
91
|
Lewis JS, Landers RJ, Underwood JC, Harris
AL and Lewis CE: Expression of vascular endothelial growth factor
by macrophages is up-regulated in poorly vascularized areas of
breast carcinomas. J Pathol. 192:150–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar
|
|
93
|
Han KY, Kim CW, Lee TH, Son Y and Kim J:
CCL23 up-regulates expression of KDR/Flk-1 and potentiates
VEGF-induced proliferation and migration of human endothelial
cells. Biochem Biophys Res Commun. 382:124–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hefler LA, Zeillinger R, Grimm C, Sood AK,
Cheng WF, Gadducci A, Tempfer CB and Reinthaller A: Preoperative
serum vascular endothelial growth factor as a prognostic parameter
in ovarian cancer. Gynecol Oncol. 103:512–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Byrne AT, Ross L, Holash J, Nakanishi M,
Hu L, Hofmann JI, Yancopoulos GD and Jaffe RB: Vascular endothelial
growth factor-trap decreases tumor burden, inhibits ascites, and
causes dramatic vascular remodeling in an ovarian cancer model.
Clin Cancer Res. 9:5721–5728. 2003.PubMed/NCBI
|
|
96
|
Jeon BH, Jang C, Han J, Kataru RP, Piao L,
Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, et al: Profound
but dysfunctional lymphangiogenesis via vascular endothelial growth
factor ligands from CD11b+ macrophages in advanced ovarian cancer.
Cancer Res. 68:1100–1109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang S, Xie B, Wang L, Yang H, Zhang H,
Chen Y, Wang F, Liu C and He H: Macrophage-mediated vascular
permeability via VLA4/VCAM1 pathway dictates ascites development in
ovarian cancer. J Clin Invest. 131:e1403152021. View Article : Google Scholar :
|
|
98
|
Reinartz S, Schumann T, Finkernagel F,
Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U,
Müller-Brüsselbach S and Müller R: Mixed-polarization phenotype of
ascites-associated macrophages in human ovarian carcinoma:
Correlation of CD163 expression, cytokine levels and early relapse.
Int J Cancer. 134:32–42. 2014. View Article : Google Scholar :
|
|
99
|
Moughon DL, He H, Schokrpur S, Jiang ZK,
Yaqoob M, David J, Lin C, Iruela-Arispe ML, Dorigo O and Wu L:
Macrophage blockade using CSF1R inhibitors reverses the vascular
leakage underlying malignant ascites in late-stage epithelial
ovarian cancer. Cancer Res. 75:4742–4752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Clancy JL, Henderson MJ, Russell AJ,
Anderson DW, Bova RJ, Campbell IG, Choong DY, Macdonald GA, Mann
GJ, Nolan T, et al: EDD, the human orthologue of the hyperplastic
discs tumour suppressor gene, is amplified and overexpressed in
cancer. Oncogene. 22:5070–5081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shearer RF, Iconomou M, Watts CK and
Saunders DN: Functional roles of the E3 ubiquitin ligase UBR5 in
cancer. Mol Cancer Res. 13:1523–1532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tang M, Liu B, Bu X and Zhao P: Cross-talk
between ovarian cancer cells and macrophages through periostin
promotes macrophage recruitment. Cancer Sci. 109:1309–1318. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Durlanik S, Fundel-Clemens K, Viollet C,
Huber HJ, Lenter M, Kitt K and Pflanz S: CD276 is an important
player in macrophage recruitment into the tumor and an upstream
regulator for PAI-1. Sci Rep. 11:148492021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Worzfeld T, Finkernagel F, Reinartz S,
Konzer A, Adhikary T, Nist A, Stiewe T, Wagner U, Looso M, Graumann
J and Müller R: Proteotranscriptomics reveal signaling networks in
the ovarian cancer microenvironment. Mol Cell Proteomics.
17:270–289. 2018. View Article : Google Scholar :
|
|
105
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yin M, Zhou HJ, Zhang J, Lin C, Li H, Li
X, Li Y, Zhang H, Breckenridge DG, Ji W and Min W: ASK1-dependent
endothelial cell activation is critical in ovarian cancer growth
and metastasis. JCI Insight. 2:e918282017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wen Y and Crowley SD: The varying roles of
macrophages in kidney injury and repair. Curr Opin Nephrol
Hypertens. 29:286–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Garofalo RS, Orena SJ, Rafidi K, Torchia
AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks
JR, et al: Severe diabetes, age-dependent loss of adipose tissue,
and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin
Invest. 112:197–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gao J, Liang Y and Wang L: Shaping
polarization of tumor-associated macrophages in cancer
immunotherapy. Front Immunol. 13:8887132022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J,
Huang J, He Q, Wu B, Zhang Z, et al: Irradiated tumor cell-derived
microparticles mediate tumor eradication via cell killing and
immune reprogramming. Sci Adv. 6:eaay97892020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Shu Y and Cheng P: Targeting
tumor-associated macrophages for cancer immunotherapy. Biochim
Biophys Acta Rev Cancer. 1874:1884342020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
He J, Yin P and Xu K: Effect and molecular
mechanisms of traditional Chinese medicine on tumor targeting
tumor-associated macrophages. Drug Des Devel Ther. 14:907–919.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Macciò A, Gramignano G, Cherchi MC, Tanca
L, Melis L and Madeddu C: Role of M1-polarized tumor-associated
macrophages in the prognosis of advanced ovarian cancer patients.
Sci Rep. 10:60962020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Guiducci C, Vicari AP, Sangaletti S,
Trinchieri G and Colombo MP: Redirecting in vivo elicited tumor
infiltrating macrophages and dendritic cells towards tumor
rejection. Cancer Res. 65:3437–3446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Capellero S, Erriquez J, Battistini C,
Porporato R, Scotto G, Borella F, Di Renzo MF, Valabrega G and
Olivero M: Ovarian cancer cells in ascites form aggregates that
display a hybrid epithelial-mesenchymal phenotype and allows
survival and proliferation of metastasizing cells. Int J Mol Sci.
23:8332022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sun S, Pan X, Zhao L, Zhou J, Wang H and
Sun Y: The expression and relationship of CD68-tumor-associated
macrophages and microvascular density with the prognosis of
patients with laryngeal squamous cell carcinoma. Clin Exp
Otorhinolaryngol. 9:270–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sadrkhanloo M, Entezari M, Orouei S,
Ghollasi M, Fathi N, Rezaei S, Hejazi ES, Kakavand A, Saebfar H,
Hashemi M, et al: STAT3-EMT axis in tumors: Modulation of cancer
metastasis, stemness and therapy response. Pharmacol Res.
182:1063112022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liang R, Chen X, Chen L, Wan F, Chen K,
Sun Y and Zhu X: STAT3 signaling in ovarian cancer: A potential
therapeutic target. J Cancer. 11:837–848. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Takaishi K, Komohara Y, Tashiro H, Ohtake
H, Nakagawa T, Katabuchi H and Takeya M: Involvement of
M2-polarized macrophages in the ascites from advanced epithelial
ovarian carcinoma in tumor progression via Stat3 activation. Cancer
Sci. 101:2128–2136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jinushi M, Chiba S, Yoshiyama H, Masutomi
K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A and Tahara H:
Tumor-associated macrophages regulate tumorigenicity and anticancer
drug responses of cancer stem/initiating cells. Proc Natl Acad Sci
USA. 108:12425–12430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Raghavan S, Mehta P, Xie Y, Lei YL and
Mehta G: Ovarian cancer stem cells and macrophages reciprocally
interact through the WNT pathway to promote pro-tumoral and
malignant phenotypes in 3D engineered microenvironments. J
Immunother Cancer. 7:1902019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ruffell B and Coussens LM: Macrophages and
therapeutic resistance in cancer. Cancer Cell. 27:462–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li L, He D, Guo Q, Zhang Z, Ru D, Wang L,
Gong K, Liu F, Duan Y and Li H: Exosome-liposome hybrid
nanoparticle codelivery of TP and miR497 conspicuously overcomes
chemoresistant ovarian cancer. J Nanobiotechnology. 20:502022.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li H, Luo F, Jiang X, Zhang W, Xiang T,
Pan Q, Cai L, Zhao J, Weng D, Li Y, et al: CircITGB6 promotes
ovarian cancer cisplatin resistance by resetting tumor-associated
macrophage polarization toward the M2 phenotype. J Immunother
Cancer. 10:e0040292022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ding L, Wang Q, Martincuks A, Kearns MJ,
Jiang T, Lin Z, Cheng X, Qian C, Xie S, Kim HJ, et al: STING
agonism overcomes STAT3-mediated immunosuppression and adaptive
resistance to PARP inhibition in ovarian cancer. J Immunother
Cancer. 11:e0056272023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Willingham SB, Volkmer JP, Gentles AJ,
Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin
R, Cohen JD, et al: The CD47-signal regulatory protein alpha
(SIRPa) interaction is a therapeutic target for human solid tumors.
Proc Natl Acad Sci USA. 109:6662–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Sikic BI, Lakhani N, Patnaik A, Shah SA,
Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S,
Papadopoulos K, et al: First-in-human, first-in-class phase I trial
of the anti-CD47 antibody Hu5F9-G4 in patients with advanced
cancers. J Clin Oncol. 37:946–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Huang Y, Lv SQ, Liu PY, Ye ZL, Yang H, Li
LF, Zhu HL, Wang Y, Cui LZ, Jiang DQ, et al: A SIRPα-Fc fusion
protein enhances the antitumor effect of oncolytic adenovirus
against ovarian cancer. Mol Oncol. 14:657–668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Batchu RB, Gruzdyn OV, Kolli BK,
Dachepalli R, Umar PS, Rai SK, Singh N, Tavva PS, Weaver DW and
Gruber SA: IL-10 signaling in the tumor microenvironment of ovarian
cancer. Adv Exp Med Biol. 1290:51–65. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
McKarns SC, Schwartz RH and Kaminski NE:
Smad3 is essential for TGF-beta 1 to suppress IL-2 production and
TCR-induced proliferation, but not IL-2-induced proliferation. J
Immunol. 172:4275–4284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wertel I, Surówka J, Polak G, Barczyński
B, Bednarek W, Jakubowicz-Gil J, Bojarska-Junak A and Kotarski J:
Macrophage-derived chemokine CCL22 and regulatory T cells in
ovarian cancer patients. Tumour Biol. 36:4811–4817. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kamat K, Krishnan V and Dorigo O:
Macrophage-derived CCL23 upregulates expression of T-cell
exhaustion markers in ovarian cancer. Br J Cancer. 127:1026–1033.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Noy R and Pollard JW: Tumor-associated
macrophages: From mechanisms to therapy. Immunity. 41:49–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chapoval AI, Ni J, Lau JS, Wilcox RA,
Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K and Chen L:
B7-H3: A costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Miyamoto T, Murakami R, Hamanishi J,
Tanigaki K, Hosoe Y, Mise N, Takamatsu S, Mise Y, Ukita M, Taki M,
et al: B7-H3 suppresses antitumor immunity via the CCL2-CCR2-M2
macrophage axis and contributes to ovarian cancer progression.
Cancer Immunol Res. 10:56–69. 2022. View Article : Google Scholar
|
|
140
|
Liu Z, Jin K, Zeng H, Shao F, Chang Y,
Wang Y, Xu L, Wang Z, Cui X, Zhu Y and Xu J: B7-H4 correlates with
clinical outcome and immunotherapeutic benefit in muscle-invasive
bladder cancer. Eur J Cancer. 171:133–142. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Carroll MJ, Fogg KC, Patel HA, Krause HB,
Mancha AS, Patankar MS, Weisman PS, Barroilhet L and Kreeger PK:
Alternatively-activated macrophages upregulate mesothelial
expression of P-selectin to enhance adhesion of ovarian cancer
cells. Cancer Res. 78:3560–3573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Robinson-Smith TM, Isaacsohn I, Mercer CA,
Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM and
Drew AF: Macrophages mediate inflammation-enhanced metastasis of
ovarian tumors in mice. Cancer Res. 67:5708–5716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fogg KC, Olson WR, Miller JN, Khan A,
Renner C, Hale I, Weisman PS and Kreeger PK: Alternatively
activated macrophage-derived secretome stimulates ovarian cancer
spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett.
458:92–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zeng XY, Xie H, Yuan J, Jiang XY, Yong JH,
Zeng D, Dou YY and Xiao SS: M2-like tumor-associated
macrophages-secreted EGF promotes epithelial ovarian cancer
metastasis via activating EGFR-ERK signaling and suppressing lncRNA
LIMT expression. Cancer Biol Ther. 20:956–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sandhu SK, Papadopoulos K, Fong PC,
Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA,
Balkwill F, et al: A first-in-human, first-in-class, phase I study
of carlumab (CNTO 888), a human monoclonal antibody against
CC-chemokine ligand 2 in patients with solid tumors. Cancer
Chemother Pharmacol. 71:1041–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wesolowski R, Sharma N, Reebel L, Rodal
MB, Peck A, West BL, Marimuthu A, Severson P, Karlin DA, Dowlati A,
et al: Phase Ib study of the combination of pexidartinib (PLX3397),
a CSF-1R inhibitor, and paclitaxel in patients with advanced solid
tumors. Ther Adv Med Oncol. 11:17588359198542382019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zeng Y, Li B, Liang Y, Reeves PM, Qu X,
Ran C, Liu Q, Callahan MV, Sluder AE, Gelfand JA, et al: Dual
blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival
of ovarian tumor-bearing mice by prevention of immunosuppression in
the tumor microenvironment. FASEB J. 33:6596–6608. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wanderley CW, Colón DF, Luiz JPM, Oliveira
FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL,
et al: Paclitaxel reduces tumor growth by reprogramming
tumor-associated macrophages to an M1 Profile in a TLR4-dependent
manner. Cancer Res. 78:5891–5900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang Q, Li Y, Miao C, Wang Y, Xu Y, Dong
R, Zhang Z, Griffin BB, Yuan C, Yan S, et al: Anti-angiogenesis
effect of Neferine via regulating autophagy and polarization of
tumor-associated macrophages in high-grade serous ovarian
carcinoma. Cancer Lett. 432:144–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang F, Parayath NN, Ene CI, Stephan SB,
Koehne AL, Coon ME, Holland EC and Stephan MT: Genetic programming
of macrophages to perform anti-tumor functions using targeted mRNA
nanocarriers. Nat Commun. 10:39742019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Chen D, Xie J, Fiskesund R, Dong W, Liang
X, Lv J, Jin X, Liu J, Mo S, Zhang T, et al: Chloroquine modulates
antitumor immune response by resetting tumor-associated macrophages
toward M1 phenotype. Nat Commun. 9:8732018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bellora F, Castriconi R, Dondero A,
Pessino A, Nencioni A, Liggieri G, Moretta L, Mantovani A, Moretta
A and Bottino C: TLR activation of tumor-associated macrophages
from ovarian cancer patients triggers cytolytic activity of NK
cells. Eur J Immunol. 44:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ma L, Zhu M, Gai J, Li G, Chang Q, Qiao P,
Cao L, Chen W, Zhang S and Wan Y: Preclinical development of a
novel CD47 nanobody with less toxicity and enhanced anti-cancer
therapeutic potential. J Nanobiotechnology. 18:122020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Rakina M, Kazakova A, Villert A, Kolomiets
L and Larionova I: Spheroid formation and peritoneal metastasis in
ovarian cancer: The role of stromal and immune components. Int J
Mol Sci. 23:62152022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Long L, Yin M and Min W: 3D co-culture
system of tumor-associated macrophages and ovarian cancer cells.
Bio Protoc. 8:e28152018.PubMed/NCBI
|