Introduction
Esophageal cancer is a highly malignant and lethal
disease, with a particularly high incidence in China (1). To the best of our knowledge, a
significant improvement of the 5-year survival rate was reported
due to the advances in the therapeutic methods over the last 3
decades. However, its overall 5-year survival remains low, at ∼19%
(2,3). Therefore, an effective modality for
the treatment of this malignancy is required.
Esophagectomy, with or without other adjuvant
modalities, is preferred for patients with resectable esophageal
cancer. For those patients who do not undergo surgical treatment
for any reason, the Radiation Therapy Oncology Group (RTOG) trial
85-01 established concurrent chemoradiotherapy as the standard
treatment (4). Hyperthermia is a
modality that elevates tumor temperature to a supraphysiological
level (40–44°C), is a well-established radio- and chemosensitizer
and widely accepted as an important adjuvant therapy to chemo- and
radiotherapy (5). Hyperthermia, as
part of a combination regimen, has demonstrated improved clinical
response, local control and survival in numerous phase II studies
and randomized trials in patients with breast, cervical, head and
neck cancers, melanoma and glioblastoma multiforme (6–8).
However, studies focusing on the application of hyperthermia on
esophageal cancer treatment, which may be of interest to radiation
oncologists, are limited. In this study, data on the combination of
hyperthermia with chemo- and radiotherapy as a trimodal treatment
for patients with locally advanced esophageal cancer are
reported.
Patients and methods
Patients
A total of 78 patients hospitalized in our
department between May, 2008 and December, 2009 were enrolled in
the present study. Inclusion criteria were: pathologically
diagnosed esophageal cancer, first-treated at our institution, no
evidence of distant metastasis other than supraclavicular
locoregional lymph nodes, Karnofsky Performance Status (KPS) ≥70,
no evidence of tracheoesophageal fistula and normal hepatic and
renal function tests. This study was approved by the Institutional
Review Board of Yancheng Third People’s Hospital. Signed informed
consent was provided by all the patients.
Radiotherapy
All patients were immobilized within a thermoplastic
mold and underwent CT stimulation. A total dose of 60–66 Gy was
delivered to the 95% isodose line, which completely encompassed the
planning target volume (PTV). A dose-volume histogram (DVH) was
used to evaluate the dose received by PTV and adjacent critical
tissue and organ. In the treatment plans, the dose variation in the
PTV did not exceed ±7%, the dose received by the spinal cord was
>40 Gy, the percentage of lung volume that received 20 Gy
compared to the total lung volume (V20) was ≤25% and the mean dose
to the heart was <30 Gy. The treatment plan was implemented
following location and dose distribution verification, using 6-MV
photons generated by a linear accelerator, in a normal delivery
schedule of 1.8–2.1 Gy/fraction, 5 fractions/week. All treatment
procedures were completed within 6–7 weeks.
Chemotherapy
All enrolled patients received 4–6 courses of
monthly cycled concurrent cisplatin and 5-fluorouracil chemotherapy
(PF therapy). Cisplatin (25 mg/m2) with hydration
therapy was administered on days 1–5 (at which time the
radiotherapy was also initiated) and 450 mg/m2
5-flurouracil was infused intravenously on days 1–5. A total of 4–6
cycles of chemotherapy were administered, depending on the
individual physical conditions of the patients.
Hyperthermia
Hyperthermia was applied by the BSD-2000
hyperthermia system (BSD Medical Corporation, Salt Lake City, UT,
USA), which is able to heat locoregional lesions located deep in
the body. Precise treatment planning was based on the lesion
location as shown on the thoracic CT images, ensuring that the
tumor was entirely located within the therapeutic thermal field.
The objective of the hyper-thermia treatment was to achieve an
intratumoral temperature of ≥42.5°C for 60 min. Hyperthermia was
performed twice a week, within 2 h of the irradiation session
during the period of radiotherapy. A total of 6–12 sessions of
hyperthermia were performed, depending on the individual physical
conditions of the patients.
Primary tumor response assessment
To evaluate the primary tumor response, thoracic CT
scans, barium meal and ultrasound imaging were performed following
the delivery of a dose of 40 Gy during the course of radiotherapy
and at 3 and 6 months following the initiation of treatment. The
treatment response was evaluated according to the revised RECIST
guidelines (9).
Toxicity evaluation
Patients were carefully examined weekly throughout
the duration of the treatment, or more often if clinically
indicated. Patient physical profiles such as symptoms, physical
signs, KPS and body weight were recorded in detail. Routine blood
examination was performed weekly. A serum chemistry profile was
performed prior to and following each chemotherapy cycle, to
monitor hepatic and renal function. The toxicities were defined and
graded according to the CTCAE, version 3.0 (10).
Statistical analysis
Descriptive results such as means, medians and
proportions were calculated to characterize patient, disease and
treatment characteristics, in addition to toxicities following
treatment. The survival curves were estimated using the
Kaplan-Meier product-limit method (11). P<0.05 was considered to indicate
a statistically significant difference. Statistical software SPSS
19.0 (IBM SPSS Statistics, Chicago, IL, USA) was used for
performing statistical analyses, manipulating data and generating
data-summarizing graphs.
Results
Patient characteristics
As shown in Table
I, the majority of the patients were male and the mean age was
65.1±5.2 years. The predominant histological type was squamous cell
carcinoma, with only one case of adenocarcinoma. The majority of
cases were T3 or T4, N1 and M0 and clinical stage II or III.
 | Table I.Patient pretreatment
characteristics. |
Table I.
Patient pretreatment
characteristics.
| Characteristics | No. of patients |
|---|
| Total patient
no. | 78 |
| Age (years) | |
| Mean | 65 |
| Range | 41–79 |
| Gender | |
| Male | 56 |
| Female | 22 |
| Pathological
type | |
| Squamous cell
carcinoma | 77 |
| Adenocarcinoma | 1 |
| Lesion site | |
| Cervical | 6 |
| Upper thoracic | 28 |
| Middle
thoracic | 36 |
| Lower thoracic | 8 |
| Primary lesion length
(cm) | |
| ≤5 | 20 |
| >5 | 58 |
| Clinical stage | |
| II | 58 |
| III | 16 |
| IV | 4 |
Clinical primary tumor response
The clinical primary tumor response to the trimodal
treatment was significant. Of the 78 patients enrolled in this
study, 31 (39.7%) exhibited a complete response (CR) of the primary
lesions, 43 (56.4%) exhibited a partial response (PR) and 3 (3.9%)
cases exhibited stable disease (SD). The total response rate (CR +
PR) of the primary tumor to the trimodal treatment was as high as
96.1%.
Disease control and survival
The mean follow-up duration was 20.1 months (range,
1.8–51.5 months). The clinical outcomes of locoregional control
(LRC), distant metastasis-free survival (DMFS) and overall survival
(OS) are shown in Table II. The
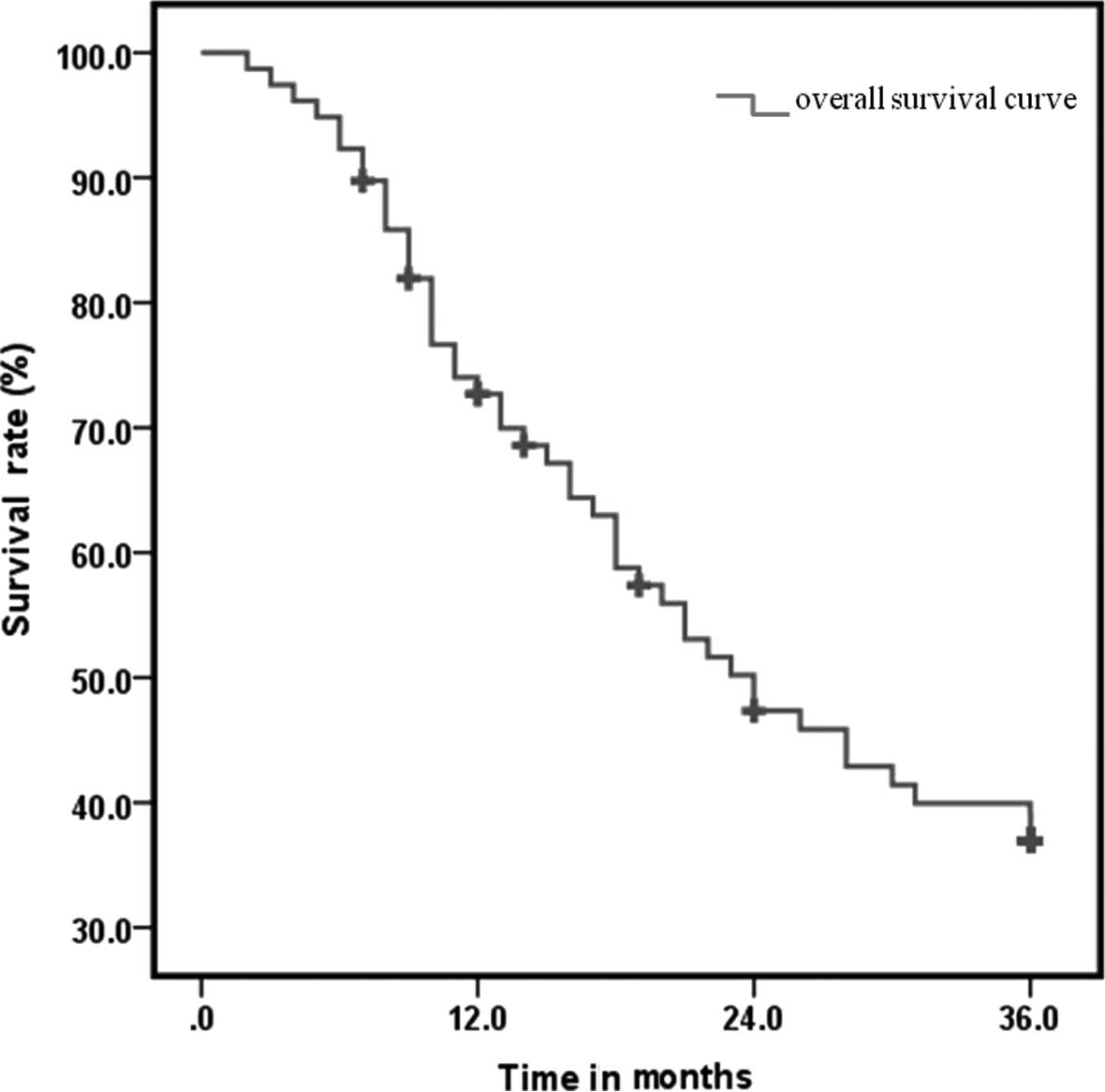
1-, 2- and 3-year LRC was 76.9, 55.1 and 47.4%, respectively; the
DMFS was 67.9, 38.5 and 30.8% respectively; with regards to OS, the
median survival time was 24 months and the 1-, 2- and 3-year
survival rate was 67.9, 41.0 and 33.3%, respectively (Fig. 1). Compared to previous studies on
concurrent chemoradiotherapy for esophageal cancer (4,12,13),
the OS outcome in our study was slightly improved. However,
additional investigations are required to establish its statistical
accuracy.
 | Table II.OS, LRC and DMFS rates during the
3-year follow-up. |
Table II.
OS, LRC and DMFS rates during the
3-year follow-up.
| Factors | 1-year (%) | 2-year (%) | 3-year (%) |
|---|
| OS | 67.9 | 41.0 | 33.3 |
| LRC | 76.9 | 55.1 | 47.4 |
| DMFS | 67.9 | 38.5 | 30.8 |
Treatment side-effects
The main toxicity of this trimodal treatment
presented as hematological toxicity due to bone marrow suppression.
Of the 78 follow-up patients, grade 3 or higher hematological
side-effects included 32 cases of leucopenia (41.0%), 6 of
thrombocytopenia (7.7%) and 2 of anemia (2.6%). Non-hematological
toxicity mainly included radiation esophagitis, nausea or vomiting,
pneumonitis and liver dysfunction (Table III). All patients succeeded in
completing the treatment and no treatment-related mortality
occurred within 90 days after the end of treatment. In general, the
side-effects of the trimodal regimen were well-tolerated and no
significant difference was observed between our therapy regimen and
those of previous studies (4,12).
 | Table III.Adverse effects of the trimodality
therapy. |
Table III.
Adverse effects of the trimodality
therapy.
| Adverse effect | Grade
|
|---|
| 0 | 1 | 2 | 3 | 4 |
|---|
| Leucopenia | 4 | 12 | 30 | 30 | 2 |
| Thrombocytopenia | 57 | 9 | 6 | 6 | - |
| Anemia | 21 | 32 | 23 | 2 | - |
| Esophagitis | 13 | 31 | 26 | 8 | - |
| Nausea, vomiting | 68 | 6 | 3 | 1 | - |
| Pneumonitis | 76 | 2 | - | - | - |
| Liver
dysfunction | 50 | 20 | 7 | 1 | - |
Discussion
For patients with esophageal cancer, the efficacy of
radiotherapy alone is not satisfactory, as demonstrated by the high
incidence of treatment failure, presenting as locoregional tumor
persistence or recurrence and distant metastasis. Over the past
decades the medical community has endeavored to optimize treatment
strategies, with the aim of reducing local and distant treatment
failure. Concurrent chemoradiotherapy was established as the
standard treatment for esophageal cancer, as recommended by the
RTOG trial 85-01 (4) in addition
to other studies (2), with a
notable improvement in treatment efficacy. However, disease
persistence remains the most common cause of treatment failure and
a significant predictor of worse OS (13). Therefore, improving LRC is critical
in the treatment of esophageal cancer.
A trimodal treatment combining intensity-modulated
radiation therapy (IMRT), chemotherapy and hyperthermia was applied
at our institution to improve the LRC and ultimately the OS rates
of esophageal cancer. IMRT is a product of the ongoing advances in
radiotherapeutic technology. Compared to the conventional radiation
dose-delivering technologies, IMRT has the ability to deliver
higher doses to the tumor target, while limiting the irradiation of
the surrounding normal tissues (14). Therefore, higher radiation doses
may be delivered and an improved LRC may be achieved. With the use
of this technology at our institution, the prescribed dose
delivered to the PTV was 10–15 Gy higher than the dose used in the
RTOG trial 85-01, with the aim of achieving optimal LRC benefits
for the patients. With regards to the chemotherapy for esophageal
cancer, concurrent administration of fluorouracil and cisplatin (PF
therapy) was established as the standard adjuvant therapy and was
applied at our institution. Locoregional hyperthermia is an
additional modality for treating clinical malignancies, with no
reported severe side-effects. The effect of hyperthermia on
improving the local tumor control rate was significant and
well-tolerated (6–8). Although hyperthermia alone may exert
an antitumor effect, its synergistic effect with radiation and
chemotherapy was the rationale for combining hyperthermia with
chemoradiation therapy. Therefore, hyperthermia was included in the
treatment regimen at our institution with the aim to improve the
LCR of malignancies.
Our 3-year observation of the outcome of the
trimodal treatment indicated a sound clinical efficacy. The primary
tumor response rate was significant, reaching 96.1%. This finding
demonstrates the radiosensitizing effect of hyper-thermia and
chemotherapy and establishes the superiority of trimodal therapy.
The clinical outcome of LRC, DMFS and OS in our study was
satisfactory. The 1-, 2- and 3-year OS was 67.9, 41.0 and 33.3%,
respectively. Notably, compared with previous studies (4,12,13),
our preliminary results demonstrated that trimodal therapy
exhibited a slightly improved long-term clinical outcome regarding
the 3-year OS (33% in our study vs. ∼25% in chemoradiation therapy
for locally advanced esophageal cancer), although additional
investigations are required to verify this finding and establish
its statistical accuracy. However, our results are similar to those
reported by another study on the synergistic effect of hyperthermia
and chemoradiation (15).
In our study, despite the higher radiation dose
delivered and the application of hyperthermia, the toxicity was not
significantly elevated. The most significant adverse effect of this
regimen was hematological toxicity: ≥grade 3 leucopenia was
observed in ∼40% of the patients. However, there were no
hematological toxicity-related mortalities, due to timely medical
intervention and supportive care. There were no significant
differences in the adverse effects between our trimodal treatment
study and other similar studies (4,12,16).
Therefore, we concluded that the toxicity of the trimodal treatment
was well-tolerated.
In conclusion, this study presents the
single-institutional 3-year outcome of the radio-, chemo- and
hyperthermotherapy combined trimodality on locally advanced
esophageal cancer. The preliminary results have demonstrated that
this regimen provides a good clinical outcome, presented by the
high primary tumor response rate, as well as a slightly improved
3-year OS.
References
|
1.
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kleinberg L, Gibson MK and Forastiere AA:
Chemoradiotherapy for localized esophageal cancer: regimen
selection and molecular mechanisms of radiosensitization. Nat Clin
Pract Oncol. 4:282–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
4.
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group JAMA. 281:1623–1627. 1999.PubMed/NCBI
|
|
5.
|
Wust P, Hildebrandt B, Sreenivasa G, et
al: Hyperthermia in combined treatment of cancer. Lancet Oncol.
3:487–497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Franckena M, Lutgens LC, Koper PC, et al:
Radiotherapy and hyperthermia for treatment of primary locally
advanced cervix cancer: results in 378 patients. Int J Radiat Oncol
Biol Phys. 73:242–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moyer HR and Delman KA: The role of
hyperthermia in optimizing tumor response to regional therapy. Int
J Hyperthermia. 24:251–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jones EL, Oleson JR, Prosnitz LR, et al:
Randomized trial of hyperthermia and radiation for superficial
tumors. J Clin Oncol. 23:3079–3085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
10.
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar
|
|
11.
|
Dinse GE and Lagakos SW: Nonparametric
estimation of lifetime and disease onset distributions from
incomplete observations. Biometrics. 38:921–932. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Minsky BD, Pajak TF, Ginsberg RJ, et al:
INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: high-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang Z, Liao Z, Jin J, et al:
Dose-response relationship in locoregional control for patients
with stage II–III esophageal cancer treated with concurrent
chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys.
61:656–664. 2005.
|
|
14.
|
Galvin JM, Ezzell G, Eisbrauch A, et al:
Implementing IMRT in clinical practice: a joint document of the
American Society for Therapeutic Radiology and Oncology and the
American Association of Physicists in Medicine. Int J Radiat Oncol
Biol Phys. 58:1616–1634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kitamura K, Kuwano H, Watanabe M, et al:
Prospective randomized study of hyperthermia combined with
chemoradiotherapy for esophageal carcinoma. J Surg Oncol. 60:55–58.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liu M, Shi X, Guo X, et al: Long-term
outcome of irradiation with or without chemotherapy for esophageal
squamous cell carcinoma: a final report on a prospective trial.
Radiat Oncol. 7:1422012. View Article : Google Scholar : PubMed/NCBI
|