Introduction
Tumor-promoting inflammation by innate immune cells
fosters multiple hallmarks of cancer (1). The neutrophil-to-lymphocyte ratio
(NLR) is a widely utilized marker of host inflammation (2). High baseline NLR (>4) has been
demonstrated to have prognostic significance for overall survival
in solid tumors, with a hazard ratio of 1.8(3). Our group has previously validated the
NEAT model, including the number of active tumors (‘N’), Eastern
Cooperative Oncology Group (ECOG) performance status (‘E’), albumin
(‘A’), and primary tumor site (‘T’) as important prognostic factors
for patients with metastatic cancer referred to radiation oncology
(4,5). Other validated models identified age,
extent of prior chemotherapy, recent hospitalization, liver
metastases and bone-only metastases as prognostic factors for
radiation oncology patients with metastatic cancer (6,7).
However, despite extensive research, these validated models only
accounted for 30-50% of observed survival (5).
The prognostic role of baseline NLR has been
demonstrated in 100 studies for several types of solid tumors
(3). To date, limited data has been
provided for NLR in patients treated with radiation, particularly
in those with metastatic solid tumors (8,9).
Previous studies have demonstrated that patients with metastatic
disease treated with radiation tend to have a baseline NLR of 4-5
(8,9). By contrast, the baseline NLR for the
general population is 1.65-2.15 (10,11).
Since NLR is a widely available and cost-effective measure of
systemic inflammation, the current study hypothesized that elevated
NLR may add further prognostic value, thereby improving predictive
accuracy.
Materials and methods
Inclusion criteria
The present retrospective study included 320
consecutive patients (age range, 23-97 years), with metastatic
stage IV solid tumor who were referred to a single physician in a
large community hospital-based radiation Οncology Department
between May 2012 and October 2015. This minimal risk study was
approved by the Good Samaritan Hospital Medical Center
Institutional Review Board.
Data collection
The charts and electronic records of all patients
were assessed for previously validated prognostic factors,
including ECOG performance status score (ECOG 0-1 vs. ECOG 2 vs.
ECOG 3-4), primary tumor type (breast, prostate, kidney, lung or
other), number of tumors (1-5 vs. ≥6), serum albumin (≥3.4 vs.
2.4-3.3 vs. ≤2.4 g/dl), location of metastases (bone only vs. liver
vs. other) and age (>60 years vs. ≤60 years). Additionally, the
relative contribution of baseline NLR (>4 vs. ≤4) at the time of
consultation was explored.
Statistical analysis
Statistical analysis was performed with Stata 13.1
(StataCorp LP). The primary outcome was overall survival, defined
as the time from the initial radiation oncology consultation to the
date of death from any cause. Patients were included regardless of
treatment received, and patients who were lost to follow-up were
censored at the date of the last follow-up. Survival data were
analyzed using the Kaplan-Meier method and summarized by median and
6-month survival. Differences in survival were assessed through the
log-rank method. The association of individual variables with
survival was assessed using the Cox proportional hazards model that
was verified by tests of correlations over time with examination of
residual plots. The Pearson χ2 test was performed to
determine whether there were baseline differences in variables that
were associated with high or low NLR. Statistical tests were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Patient characteristics
The study population included 320 patients with
distant metastases who were referred for radiation oncology
evaluation, with 55% inpatient and 45% outpatient consultations.
The median age was 68 years (interquartile range, 60-78 years), the
median NLR was 4.4 (interquartile range, 2.8-7.2) and the median
albumin was 3.4 g/dl (interquartile range, 2.8-3.8 g/dl). In terms
of performance status, 39% of the patients were ECOG 0-1, 29% were
ECOG 2 and 32% were ECOG 3-4. The most common types of cancer
included non-small cell lung cancer (34%), breast (14%), small cell
lung cancer (10%), prostate (9%), colorectal (6%), kidney (4%),
endometrial (4%) and unknown primary (4%). With respect to the
extent of the disease, 7% of the patients had only 1 site of active
disease, 7% had 2 active tumors and 13% had 3-5 active tumors.
Liver metastases were present in 21% of the patients and 16% had
bone-only metastases. Only 12% of the patients had previously
received ≥2 lines of palliative chemotherapy.
Radiation therapy and systemic
therapy
Among the 320 patients evaluated, 81% were treated
with radiation therapy. Treatment was personalized to account for
disease site and clinical status and goals of treatment. To
summarize, the majority of the patients were treated with standard
palliative regimens, most commonly 30 Gy in 10 fractions. Patients
with a poor prognosis were either not treated or treated with
short-course radiation (8-20 Gy in 1-5 fractions). Patients with
limited brain metastatic disease (typically 1 to 4 brain
metastases) were often treated with fractionated stereotactic
radiotherapy (27 Gy in 3 fractions) or single-fraction stereotactic
radiosurgery (21-24 Gy in 1 fraction). Patients with good
performance status and oligometastatic disease (defined as ≤5
metastases) were treated with curative intent dose-intensive
treatment regimens (12,13).
Systemic therapy was administered at the discretion
of the treating medical oncologist and, depending on the diagnosis,
consisted of chemotherapy, hormonal therapy, biologically targeted
therapy, immunotherapy and/or supportive care.
Results
Predictors of survival on univariate
analysis
The median survival for all patients was 6.7 months,
with a median follow-up for surviving patients of 20.6 months. On
univariate analysis, baseline NLR, number of tumors, ECOG
performance status, serum albumin, primary tumor site, prior
hospitalization within the last 3 months, liver metastases,
bone-only and liver metastases, were found to be significant
predictors of survival (Table I).
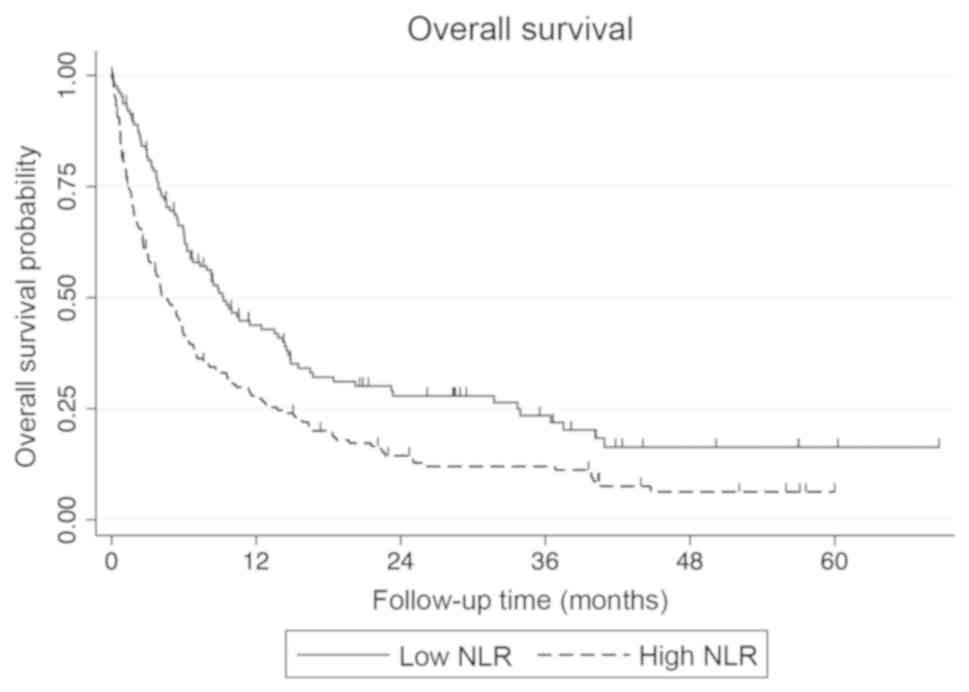
The median survival for patients with an NLR of ≤4 was 9.3 months
vs. 4.1 months for those with an NLR of >4 (Fig. 1). Age and number of prior palliative
chemotherapy cycles were not found to be statistically significant
in predicting survival.
 | Table IPredictors of overall survival. |
Table I
Predictors of overall survival.
| Variables | Number (%) | P-value | Median survival
(months) | 6-month survival
(%) |
|---|
| NLR | | <0.001 | | |
|
≤4 | 129(53) | | 9.3 | 64.6 |
|
>4 | 169(40) | | 4.1 | 41.4 |
|
Unknown | 22(7) | | | |
| ECOG performance
status score | | <0.001 | | |
|
0-1 | 125(39) | | 16.4 | 81.4 |
|
2 | 93(29) | | 5.9 | 48.0 |
|
3-4 | 102(32) | | 1.7 | 20.9 |
| Primary tumor | | <0.001 | | |
|
Breast,
prostate or kidney | 83(26) | | 14.3 | 76.3 |
|
Other | 237(74) | | 5.2 | 45.3 |
| Number of active
tumors | | <0.001 | | |
|
≤5 | 87(27) | | 18.3 | 80.8 |
|
>5 | 233(73) | | 4.8 | 43.1 |
| Albumin (g/dl) | | <0.001 | | |
|
>3.3 | 155(52) | | 12.7 | 69.9 |
|
2.4-3.3 | 114(38) | | 3.8 | 33.2 |
|
<2.4 | 30(10) | | 1.5 | 20.8 |
|
Unknown | 21(7) | | | |
| Age (years) | | 0.06 | | |
|
>60 | 81(25) | | 6.1 | 51.6 |
|
≤60 | 239(75) | | 9.5 | 58.4 |
| Liver metastases | | <0.001 | | |
|
No | 254(79) | | 7.9 | 58.2 |
|
Yes | 66(21) | | 4.0 | 35.1 |
| Bone-only
metastases | | <0.001 | | |
|
No | 270(84) | | 5.9 | 49.0 |
|
Yes | 50(16) | | 15.5 | 77.2 |
| Recent
hospitalization | | <0.001 | | |
|
No | 113(35) | | 13.8 | 75.8 |
|
Yes | 207(65) | | 4.1 | 40.6 |
| Lines of palliative
chemotherapy ≥2 | | 0.75 | | |
|
No | 281(88) | | 6.7 | 53.4 |
|
Yes | 39(12) | | 6.2 | 53.4 |
Multivariate analysis
On Cox regression analysis, the statistically
significant predictors of survival included ECOG performance status
(P<0.001), number of tumors (P<0.001), primary tumor site
(P<0.001), albumin (P<0.001), liver metastases (P=0.013) and
baseline NLR (P=0.042). Age (P=0.98), number of prior chemotherapy
cycles (P=0.93), recent hospitalization (P=0.59) and bone-only
metastases (P=0.79) were not found to be statistically significant
in predicting survival (Table
II).
 | Table IICox multivariable analysis. |
Table II
Cox multivariable analysis.
| Variables | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Primary tumor site
(breast, kidney, or prostate vs. other) | 3.30 | 2.24-4.86 | <0.001 |
| Number of active
tumors (1-5 vs. ≥6) | 2.90 | 2.04-4.11 | <0.001 |
| ECOG performance
status score (0-1 vs. 2 vs. 3-4) | 2.24 | 1.85-2.73 | <0.001 |
| Serum albumin (≥3.4
vs. 2.4-3.3 vs. <2.4 g/dl) | 1.52 | 1.22-1.90 | <0.001 |
| Liver metastasis
(no vs. yes) | 1.52 | 1.09-2.11 | 0.01 |
| NLR (≤4 vs.
>4) | 1.34 | 1.01-1.78 | 0.04 |
| Prior
hospitalizations within the last 3 months (0 vs. ≥1) | 1.10 | 0.78-1.54 | 0.59 |
| Metastasis location
(bone only vs. other) | 0.94 | 0.62-1.45 | 0.79 |
| No. of prior
palliative chemotherapy courses (0-1 vs. ≥2) | 0.98 | 0.65-1.48 | 0.93 |
| Age (≤60 years vs.
>60 years) | 1.00 | 0.81-1.23 | 0.98 |
Discussion
The present study demonstrated that baseline NLR is
an independent predictor of survival in patients with metastatic
cancer, even when accounting for potential confounding variables,
including performance status, albumin, tumor type and extent of the
disease. While statistically significant, the P-value and hazard
ratio (P=0.042; hazard ratio, 1.34) indicated that only small
improvements were observed when compared with the previously
published and validated NEAT model, defined as the number of active
tumors (‘N’), Eastern Cooperative Oncology Group (ECOG) performance
status (‘E’), albumin (‘A’), and primary tumor site (‘T’) (5). In terms of a predictive model, adding
NLR did not significantly improve the performance of the NEAT
model, having little effect on the C-index or modified r2
coefficient of determination (unpublished data).
The mechanism underlying the association of high
baseline NLR with poor survival remains elusive. The impact of host
antitumor immunity on patient clinical outcome with advanced solid
tumors is of great interest to practicing oncologists. Tumors
accompanied by significant systemic inflammation behave more
aggressively, which likely reflects the contribution of the
microenvironment to disease progression (14). Neutrophils are not only antagonists
of microbial infection and facilitators of wound healing, but also
promoters of cancer initiation, progression and metastasis
(15). In tumors, neutrophils
suppress T cell function, leading to tumor progression (16). Tumor-infiltrating lymphocytes,
particularly those with Th1 polarization, are associated with
improved prognosis (17).
Although neutrophils and lymphocytes are
non-specific parameters that are affected by concurrent infection,
inflammation, corticosteroids, chemotherapy and radiation therapy,
baseline NLR is a promising, readily available and cost-effective
biomarker that adds prognostic value to known clinical and
laboratory prognostic factors (3,8,18). In
the present study, patients with an NLR >4 with metastatic
disease that were referred to radiation oncology had a median
survival of 4.1 months. Further investigation of NLR in specific
subsets of patients, including those with specific tumor types,
oligometastatic and widespread metastatic disease treated with
radiation therapy, is warranted.
Acknowledgements
Not applicable.
Funding
Research support was provided by The Good Samaritan
Hospital Medical Center Foundation (West Islip, NY, USA).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AW, AZ, DL, PK, M-KJ and JK made substantial
contributions to conception and design, acquisition of data, and
analysis and interpretation of data. AW, AZ, DL, PK, M-KJ and JK
agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. AW, AZ, DL,
PK and JK been involved in drafting the manuscript and revised it
critically for important intellectual content. All the authors have
read and approved the final version of this manuscript to be
published.
Ethics approval and consent to
participate
The Good Samaritan Hospital Medical Center IRB (no.
16-016) approved this minimal risk registry study with waiver of
informed consent. There were no vulnerable populations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guthrie GJ, Charles KA, Roxburgh CS,
Horgan PG, McMillan DC and Clarke SJ: The systemic
inflammation-based neutrophil-lymphocyte ratio: Experience in
patients with cancer. Crit Rev Oncol Hematol. 88:218–230.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kao J, Gold KD, Zarrili G, Copel E,
Silverman AJ, Ramsaran SS, Yens D and Ryu S: Clinical predictors of
survival for patients with stage IV cancer referred to radiation
oncology. PLoS One. 10(e0124329)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zucker A, Tsai CJ, Loscalzo J, Calves P
and Kao J: The NEAT predictive model for survival in patients with
advanced cancer. Cancer Res Treat. 50:1433–1443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chow E, Abdolell M, Panzarella T, Harris
K, Bezjak A, Warde P and Tannock I: Predictive model for survival
in patients with advanced cancer. J Clin Oncol. 26:5863–5869.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Krishnan MS, Epstein-Peterson Z, Chen YH,
Tseng YD, Wright AA, Temel JS, Catalano P and Balboni TA:
Predicting life expectancy in patients with metastatic cancer
receiving palliative radiotherapy: The TEACHH model. Cancer.
120:134–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kao J, Timmins J, Ozao-Choy J and Packer
S: Effects of combined sunitinib and extracranial stereotactic
radiotherapy on bone marrow hematopoiesis. Oncol Lett.
12:2139–2144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Golden EB, Chhabra A, Chachoua A, Adams S,
Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg
J, et al: Local radiotherapy and granulocyte-macrophage
colony-stimulating factor to generate abscopal responses in
patients with metastatic solid tumours: A proof-of-principle trial.
Lancet Oncol. 16:795–803. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Azab B, Camacho-Rivera M and Taioli E:
Average values and racial differences of neutrophil lymphocyte
ratio among a nationally representative sample of United States
subjects. PLoS One. 9(e112361)2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Forget P, Khalifa C, Defour JP, Latinne D,
Van Pel MC and De Kock M: What is the normal value of the
neutrophil-to-lymphocyte ratio? BMC Res Notes.
10(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gomez DR, Blumenschein GR Jr, Lee JJ,
Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE,
Gibbons DL, et al: Local consolidative therapy versus maintenance
therapy or observation for patients with oligometastatic
non-small-cell lung cancer without progression after front-line
systemic therapy: A multicentre, randomised, controlled, phase 2
study. Lancet Oncol. 17:1672–1682. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hong JC, Ayala-Peacock DN, Lee J,
Blackstock AW, Okunieff P, Sung MW, Weichselbaum RR, Kao J, Urbanic
JJ, Milano MT, et al: Classification for long-term survival in
oligometastatic patients treated with ablative radiotherapy: A
multi-institutional pooled analysis. PLoS One.
13(e0195149)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nicolas-Avila JA, Adrover JM and Hidalgo
A: Neutrophils in homeostasis, immunity, and cancer. Immunity.
46:15–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen HM, Ma G, Gildener-Leapman N,
Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M,
Sung M, et al: Myeloid-derived suppressor cells as an immune
parameter in patients with concurrent sunitinib and stereotactic
body radiotherapy. Clin Cancer Res. 21:4073–4085. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chowdhary M, Switchenko JM, Press RH,
Jhaveri J, Buchwald ZS, Blumenfeld PA, Marwaha G, Diaz A, Wang D,
Abrams RA, et al: Post-treatment neutrophil-to-lymphocyte ratio
predicts for overall survival in brain metastases treated with
stereotactic radiosurgery. J Neurooncol. 139:689–697.
2018.PubMed/NCBI View Article : Google Scholar
|