Introduction
Bladder cancer is the tenth most common cancer in
the world, with approximately 550,000 new cases and 200,000 deaths
in 2018(1). Approximately 25% of
bladder cancer patients present with muscle-invasive bladder cancer
at the time of diagnosis. This indicates a high possibility of
metastasis and of affecting survival (2).
Radical cystectomy is an aggressive, conventional
and standard therapeutic option for patients with muscle-invasive
or bacillus Calmette-Guérin (BCG)-refractory non-muscle-invasive
bladder cancer. Postoperative survival time has a high association
with the pathological status at the time of radical cystectomy. In
patients with stage II or III bladder carcinoma, the 5-year
survival rates range from 50 to 80% (3-5).
However, clinicopathological data alone has been insufficient for
deciding the optimal treatment option (6). Based on poor surgical outcomes after
radical cystectomy, the optimal postoperative monitoring option for
high-risk bladder carcinoma is required.
There has been accumulating evidence supporting the
rationale for the association of systemic inflammatory response
(SIR) with tumor development and progression (7). Proinflammatory cytokines and growth
factors are released into the systemic circulation as factors for
SIR. In addition, it has been speculated that tumor growth factors
expedite the release of cytokines from tissues. SIR may be
activated secondary to local tissue damage caused by mutual
responses between tumor necrosis factors and original cancer cells
(8). These SIR markers include
C-reactive protein (CRP), the Glasgow Prognostic Score (GPS),
neutrophil-to-lymphocyte ratio (NLR), and platelet to lymphocyte
ratio (PLR) (9-12).
In addition, nutritional status, such as lower levels of serum
albumin (Alb) and low body mass index, is associated with worse
clinical outcomes postoperatively in patients with carcinoma, and
CRP to Alb ratio (CAR), in combination with the systemic status of
inflammation and nutrition, has been reported as an independent
prognosticator in some types of carcinoma (13-16).
SIR markers have been measured preoperatively
(13-16),
but nutritional or inflammatory status needs to be stable before
surgery or other invasive treatment. However, reports suggest
postoperative SIR markers have potential prognostic significance,
especially in patients with urothelial carcinoma. To the best of
our knowledge, only a few studies have investigated the association
of postoperative NLR and poor prognosis in patients with upper
urinary tract and bladder urothelial carcinoma (17-19).
Therefore, we sought to examine postoperative, as well as
preoperative SIR markers, including CAR, to determine independent
factors of poor prognosis in locally advanced bladder cancer.
In the present study, we retrospectively
investigated the clinicopathological and laboratory data of
patients with bladder carcinoma who underwent radical cystectomy at
National Defense Medical College (single center) to clarify whether
postoperative CAR could be an independent prognostic factor for
shorter time to cancer-specific death and/or extraurothelial
recurrence.
Patients and methods
Patients
The medical records of 102 patients who underwent
radical cystectomy between January 2007 and January 2020 and who
were pathologically diagnosed with urothelial carcinoma before or
at the time of cystectomy were retrospectively reviewed. They were
followed for at least 6 months postoperatively at our institution.
The study protocol (ID 2734) was accepted on June 14, 2017, by
National Defense Medical College Ethics Committee, and an opt-out
approach on the web page of the National Defense Medical College
was used instead of collecting written informed consent from all
participants.
A total of 80 males and 22 females with a median age
of 71 years (range, 49-83 years) were included in the present
study. The median follow-up period after surgery was 38.9 months
(range, 6.1-162.2 months). Table I
shows additional clinicopathological and laboratory data. The
absence of tumor from the histopathological examination of the
cystectomy specimen is interpreted as pT0, which translated to 22
pT0 patients.
 | Table IClinicopathological and laboratory
parameters. |
Table I
Clinicopathological and laboratory
parameters.
| Parameters | Patients, n |
|---|
|
Clinicopathologic | |
|
Sex | |
|
Men | 80 |
|
Women | 22 |
|
Urine
cytology | |
|
Positive | 26 |
|
Negative | 47 |
|
Unknown | 29 |
|
Smoking
history | |
|
Present | 71 |
|
Absent | 31 |
|
History of
UTUC | |
|
Present | 11 |
|
Absent | 91 |
|
Recurrent or
primary tumor | |
|
Recurrent | 36 |
|
Primary | 66 |
|
Reason for
cystectomy | |
|
Muscle-invasive | 87 |
|
BCG-refractory | 15 |
|
Histology | |
|
UC
alone | 57 |
|
UC
and other subtypes | 23 |
|
No
tumor | 22 |
|
Pathological
T stage | |
|
≥T3 | 48 |
|
≤T2 | 54 |
|
Tumor
grade | |
|
High | 75 |
|
PUNLMP/low | 27 |
|
Lymph node
metastasis | |
|
Positive | 21 |
|
Negative | 81 |
|
Ureteral
involvement | |
|
Positive | 6 |
|
Negative | 96 |
|
Surgical
margins | |
|
Positive | 8 |
|
Negative | 94 |
|
Lymphovascular
invasion | |
|
Positive | 50 |
|
Negative | 52 |
|
CIS | |
|
Positive | 10 |
|
Negative | 92 |
| Laboratory | |
|
Preoperative
eGFR, ml/min/1.73 m2 | |
|
<45 | 27 |
|
≥45 | 75 |
|
<45 | 29 |
|
≥45 | 73 |
|
Preoperative
CAR | |
|
≥0.17 | 32 |
|
<0.17 | 70 |
|
Postoperative
CAR | |
|
≥0.27 | 25 |
|
<0.27 | 77 |
|
Preoperative
NLR | |
|
≥2.46 | 45 |
|
<2.46 | 57 |
|
Postoperative
NLR | |
|
≥2.19 | 55 |
|
<2.19 | 47 |
|
Preoperative
PLR | |
|
≥94.80 | 83 |
|
<94.80 | 19 |
|
Postoperative
PLR | |
|
≥105.23 | 85 |
|
<105.23 | 17 |
Extraurothelial recurrence after radical cystectomy
indicates tumor recurrence outside the bladder or distant
metastasis. In the present study, we defined recurrence-free
survival as extraurothelial recurrence-free survival (ERFS).
The method of lymphadenectomy was the same as that
described in earlier studies (20,21).
All patients received regional lymphadenectomy, and 21 showed
positive lymph node metastasis. Neoadjuvant chemotherapy was
administered to 47 patients, 22 of whom developed postoperative
recurrence or distant metastasis.
As part of postoperative chemotherapy, adjuvant
chemotherapy was administered to 11 patients with histologically
confirmed lymphovascular invasion (LVI) or pathologically
determined pT3 or pT4 cancer, and salvage chemotherapy was
administered to 26 patients who developed recurrence and/or distant
metastasis after surgery. Both adjuvant and salvage chemotherapy
were administered to 5 patients. The dose of cisplatin was adjusted
according to the renal function of the patient.
All surgical specimens were processed according to
standard pathological procedures and were histologically confirmed
to be urothelial carcinoma with or without other tumor cell types.
The pathological staging of the primary tumor (pT) was determined
according to the American Joint Committee on Cancer TNM
Classification (22), whereas tumor
grading was determined according to the 2004 WHO classification of
urothelial tumors (23). Tumor
specimens were evaluated by two pathologists, and the patients were
divided into two groups on the basis of the 2004 WHO classification
system for tumor grading.
Each patient was monitored for local recurrence or
distant metastasis every 3-6 months for the first 5 years after
cystectomy and 6-12 months thereafter.
Estimated glomerular filtration rate
and inflammatory indices
Preoperative laboratory tests were performed within
1 week before cystectomy, and postoperative laboratory tests was
measured 1 to 2 months after surgery. In cases requiring
postoperative chemotherapy, the postoperative blood examination was
performed prior to postoperative chemotherapy. None of the patients
enrolled in the present study had inflammatory diseases or
hematological disorders at the time of blood tests.
The estimated glomerular filtration rate (eGFR) was
calculated by computation using the following formula:
eGFR (ml/min/1.73 m2)=194x(serum
creatinine)-1.094 x Age-0.287 x(0.739, for
women).
This formula is the isotope dilution mass
spectrometry (IDMS)-traceable 4-variable Modification of Diet in
Renal Disease (MDRD) Study equation, a modified equation from the
IDMS MDRD Study with a coefficient derived from data by the
Japanese Society of Nephrology-Chronic Kidney Disease Initiatives
(JSN-CKDI). This formula has been reported to be more accurate for
hospitalized Japanese patients with an eGFR of <60 ml/min/1.73
m2 than the original IDMS MDRD Study Equation (24).
Inflammatory indices were calculated as follows. CAR
was calculated by dividing the CRP value (mg/dl) by the Alb value
(g/dl) (10,14,15).
The NLR was calculated by dividing the absolute neutrophil count by
the absolute lymphocyte count (9,19). The
platelet to lymphocyte ratio (PLR) was calculated by dividing the
absolute platelet count by the absolute lymphocyte count (11).
Statistical analysis
Univariate and multivariate analyses were performed
using a Cox proportional hazards model to identify independent
factors for shorter cancer-specific survival (CSS) and ERFS time.
In addition, receiver operator characteristic (ROC) analysis was
performed to determine the cut-off values of eGFR, CAR, NLR and PLR
according to the method shown in earlier studies (9,19,25).
We also applied the cut-off values determined on the basis of CSS
rate, according to previous reports (9,19,25).
Fisher's exact probability test was performed to examine the
relationship between postoperative CAR ≥0.27 and any other
pathological factors or postoperative laboratory markers. Survival
curves were constructed using the Kaplan-Meier method, and the
statistical differences among them were evaluated using the
log-rank test. Statistical analyses were performed with JMP Pro 14
(SAS Institute). A P-value <0.05 was considered statistically
significant.
Results
Independent factors for shortened CSS
and ERFS time in all patients
ROC analysis revealed that patients with
preoperative eGFR ≤46.88, postoperative eGFR ≤48.02, preoperative
CAR ≥0.17, postoperative CAR ≥0.27, preoperative NLR ≥2.46,
postoperative NLR ≥2.19, preoperative PLR ≥94.80 and postoperative
PLR ≥105.23 had a higher association with cancer-specific death
than patients without these conditions. As to eGFR, we considered
45 ml/min/1.73 m2 was a practical threshold in the
present study. The ROC curves for postoperative CAR for
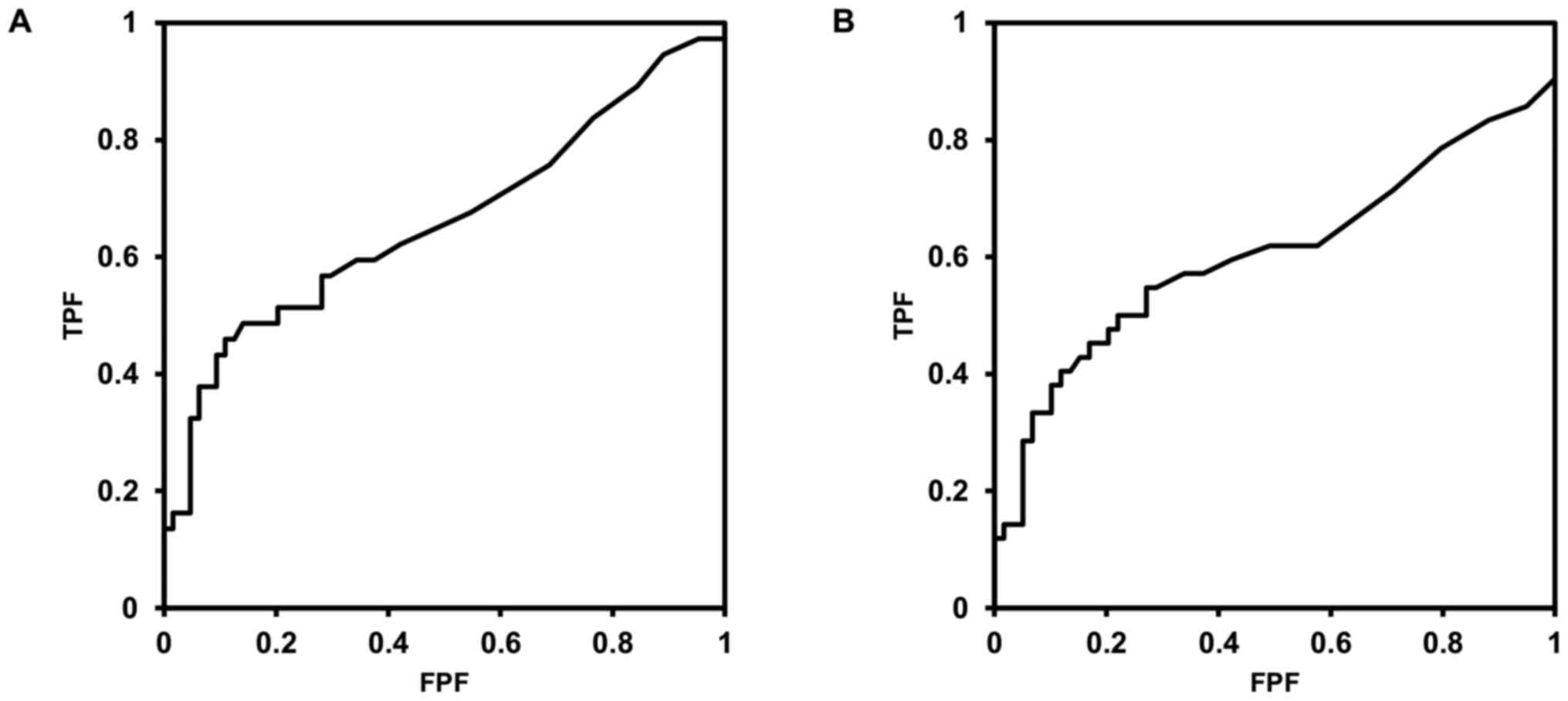
cancer-specific death and extraurothelial recurrence are shown in
Fig. 1A and B. The postoperative CAR threshold was 0.27
for both CSS and ERFS.
A Cox proportional hazards model was performed to
prove CAR was a more useful marker than CRP alone or Alb alone. On
a univariate analysis, significantly shorter cancer-specific
survival was associated with postoperative CRP ≥0.6 [hazard ratio
(HR), 3.249; 95% confidence interval (CI), 1.713-6.204;
P<0.001], postoperative Alb ≤3.4 [HR, 3.624; 95% CI,
1.822-6.938; P<0.001], and postoperative CAR ≥0.27 [HR, 4.209;
95% CI, 2.201-7.992; P<0.001]. Therefore, postoperative CAR was
used according to HR. Additionally, preoperative CAR was also
used.
Another Cox proportional hazards model was
constructed to detect the independent clinicopathological factors
and laboratory parameters for shortened CSS time. Among these
factors, in a univariate analysis, pT stage, tumor grade, LVI,
preoperative eGFR, preoperative CAR, and postoperative CAR were
found to be independent factors for shortened CSS time (P<0.001,
P=0.014, P<0.001, P=0.013, P=0.017, P<0.001, respectively).
Among these independent factors in univariate analysis, only
postoperative CAR (≥0.27) was an independent factor for worse CSS
rate in the multivariate analysis [HR, 3.368; 95% CI, 1.674-6.731;
P<0.001] (Table II).
 | Table IIUnivariate and multivariate analyses
of independent factors for cancer-specific survival. |
Table II
Univariate and multivariate analyses
of independent factors for cancer-specific survival.
| | Univariate | Multivariate |
|---|
| Parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Clinicopathological | | | | | | |
|
Pathological
T stage (≥T3 or ≤T2) | 3.943 | 1.996-8.344 | <0.001 | 2.149 | 0.974-5.070 | 0.058 |
|
Tumor grade
(high or PUNLMP/low) | 2.839 | 1.211-8.305 | 0.014 | 2.363 | 0.930-7.277 | 0.072 |
|
Lymph node
metastasis (positive or negative) | 2.069 | 0.979-4.079 | 0.056 | | | |
|
Ureter
involvement (positive or negative) | 1.501 | 0.361-4.170 | 0.524 | | | |
|
Surgical
margin (positive or negative) | 1.405 | 0.419-3.530 | 0.539 | | | |
|
Lymphovascular
invasion (positive or negative) | 3.655 | 1.844-7.992 | <0.001 | 1.615 | 0.712-3.942 | 0.258 |
|
Carcinoma
in situ (positive or negative) | 0.442 | 0.072-1.448 | 0.203 | | | |
| Laboratory | | | | | | |
|
Preoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 2.364 | 1.205-4.501 | 0.013 | 1.972 | 0.949-3.982 | 0.068 |
|
Postoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.767 | 0.874-3.407 | 0.110 | | | |
|
Preoperative
CAR (≥ or <0.17) | 2.240 | 1.163-4.254 | 0.017 | 1.320 | 0.635-2.680 | 0.451 |
|
Postoperative
CAR (≥ or <0.27) | 4.209 | 2.201-7.992 | <0.001 | 3.368 | 1.674-6.731 | <0.001 |
|
Preoperative
NLR (≥ or <2.46) | 1.863 | 0.977-3.606 | 0.059 | | | |
|
Postoperative
NLR (≥ or <2.19) | 1.667 | 0.872-3.309 | 0.123 | | | |
|
Preoperative
PLR (≥ or <94.80) | 2.091 | 0.994-4.112 | 0.052 | | | |
|
Postoperative
PLR (≥ or <105.23) | 1.419 | 0.606-2.950 | 0.396 | | | |
In another univariate analysis, ERFS time was found
to be shorter in patients with higher pT stage, high tumor grade,
lymph node metastasis, LVI, preoperative eGFR <45, preoperative
CAR ≥0.17, postoperative CAR ≥0.27, and preoperative PLR ≥94.80
(P=0.002, P=0.011, P=0.039, P=0.007, P=0.015, P=0.032, P<0.001,
P=0.037, respectively). Among these independent factors in
univariate analysis, only postoperative CAR ≥0.27 was found to act
as an independent factor for worse ERFS rate in the multivariate
analysis (HR, 2.401; 95% CI, 1.196-4.684; P=0.015) (Table III). Age, sex, body mass index,
urine cytology, smoking history, history of upper urinary tract
urothelial carcinoma, recurrent or primary tumor, reason for
radical cystectomy, and the presence of histological subtypes were
not independent predictors of worse CSS or ERFS rates in univariate
analysis (data not shown).
 | Table IIIUnivariate and multivariate analyses
of independent factors for extraurothelial recurrence-free
survival. |
Table III
Univariate and multivariate analyses
of independent factors for extraurothelial recurrence-free
survival.
| | Univariate | Multivariate |
|---|
| Parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Clinicopathological | | | | | | |
|
Pathological
T stage (≥T3 or ≤T2) | 2.602 | 1.412-4.954 | 0.002 | 1.746 | 0.819-3.883 | 0.151 |
|
Tumor grade
(high or PUNLMP/low) | 2.724 | 1.238-7.187 | 0.011 | 1.867 | 0.800-5.107 | 0.156 |
|
Lymph node
metastasis (positive or negative) | 2.114 | 1.041-4.021 | 0.039 | 1.664 | 0.764-3.466 | 0.194 |
|
Ureter
involvement (positive or negative) | 1.858 | 0.557-4.622 | 0.278 | | | |
|
Surgical
margin (positive or negative) | 1.239 | 0.372-3.079 | 0.692 | | | |
|
Lymphovascular
invasion (positive or negative) | 2.323 | 1.256-4.469 | 0.007 | 1.050 | 0.486-2.346 | 0.902 |
|
Carcinoma
in situ (positive or negative) | 0.844 | 0.253-2.099 | 0.741 | | | |
| Laboratory
parameters | | | | | | |
|
Preoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 2.209 | 1.173-4.055 | 0.015 | 1.699 | 0.858-3.276 | 0.126 |
|
Postoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.586 | 0.824-2.933 | 0.162 | | | |
|
Preoperative
CAR (≥ or <0.17) | 1.987 | 1.065-3.637 | 0.032 | 1.327 | 0.651-2.623 | 0.427 |
|
Postoperative
CAR (≥ or <0.27) | 3.147 | 1.684-5.766 | <0.001 | 2.401 | 1.196-4.684 | 0.015 |
|
Preoperative
NLR (≥ or <2.46) | 1.504 | 0.823-2.756 | 0.183 | | | |
|
Postoperative
NLR (≥ or <2.19) | 1.597 | 0.870-3.030 | 0.132 | | | |
|
Preoperative
PLR (≥ or <94.80) | 2.133 | 1.049-4.060 | 0.037 | 1.888 | 0.868-3.886 | 0.106 |
|
Postoperative
PLR (≥ or <105.23) | 1.407 | 0.633-2.814 | 0.379 | | | |
Postoperative CAR ≥0.27 was observed in 25 (24.5%)
patients. A statistically significant association was observed
between postoperative CAR ≥0.27 and postoperative NLR ≥2.19
(P=0.012), but there was not any significant association between
postoperative CAR ≥0.27 and any other pathological factors or
postoperative laboratory markers (Table IV).
 | Table IVAssociation between postoperative CAR
and clinicopathological parameters. |
Table IV
Association between postoperative CAR
and clinicopathological parameters.
| | Postoperative
CAR | |
|---|
| Parameters | Total (%)
(n=102) | ≥0.27, n (%) | <0.27, n
(%) | P-value |
|---|
| Pathological T
stage | | | | |
|
≥T3 | 48 (47.1) | 14 (29.2) | 34 (70.8) | 0.360 |
|
≤T2 | 54 (52.9) | 11 (20.4) | 43 (79.6) | |
| Tumor grade | | | | |
|
High | 75 (73.5) | 19 (25.3) | 56 (74.7) | >0.999 |
|
PUNLMP/Low | 27 (26.5) | 6 (22.2) | 21 (77.8) | |
| Lymph node
metastasis | | | | |
|
Positive | 21 (20.6) | 7 (33.3) | 14 (66.7) | 0.393 |
|
Negative | 81 (79.4) | 18 (22.2) | 63 (77.8) | |
| Lymphovascular
invasion | | | | |
|
Positive | 50 (49.0) | 15 (30.0) | 35 (70.0) | 0.253 |
|
Negative | 52 (51.0) | 10 (19.2) | 42 (80.8) | |
| Postoperative eGFR,
ml/min/1.73 m2 | | | | |
|
<45 | 29 (28.4) | 9 (31.0) | 20 (69.0) | 0.444 |
|
≥45 | 73 (71.6) | 16 (21.9) | 57 (78.1) | |
| Postoperative
NLR | | | | |
|
≥2.19 | 55 (53.9) | 19 (34.5) | 36 (65.5) | 0.012 |
|
<2.19 | 47 (46.1) | 6 (12.8) | 41 (87.2) | |
| Postoperative
PLR | | | | |
|
≥105.23 | 85 (83.3) | 20 (23.5) | 65 (76.5) | 0.758 |
|
<105.23 | 17 (16.7) | 5 (29.4) | 12 (70.6) | |
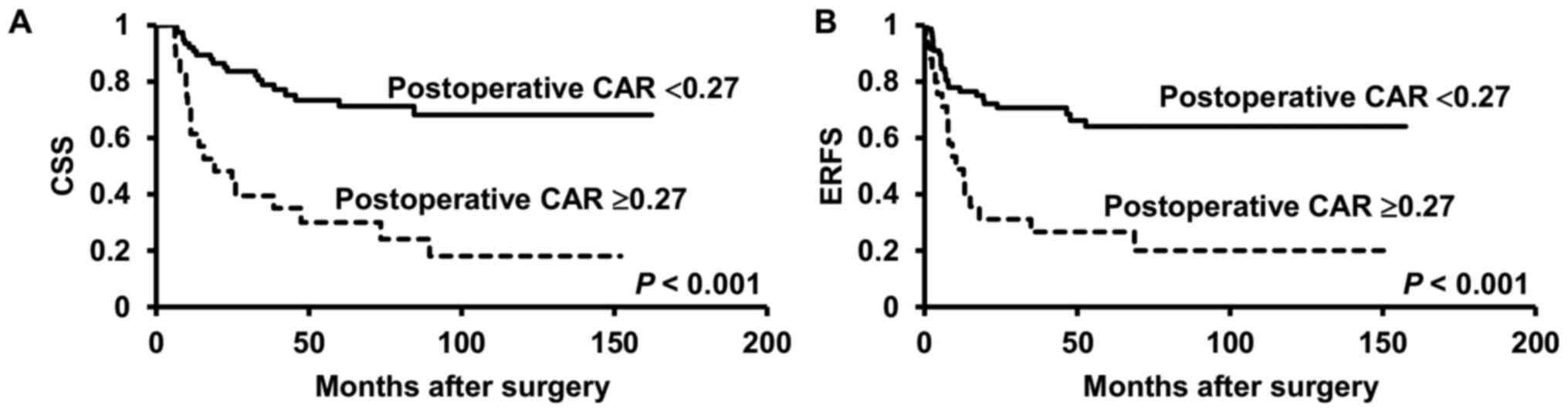
The Kaplan-Meier curves and the results of the
log-rank test revealed a significant difference in the CSS and ERFS
rates between all patients with and without postoperative CAR ≥0.27
(both P<0.001) (Fig. 2A and
B).
Independent factors for shortened CSS
and ERFS time in patients with advanced pT stage
Next, we sought to establish the independent factors
of shorter CSS rates in patients with higher pT stage (≥T3). A Cox
proportional hazards model was generated to determine the
independent factors of shortened CSS rates among
clinicopathological factors and laboratory parameters in 48
advanced pT stage (≥pT3) patients. In a univariate analysis,
postoperative CAR and preoperative PLR were found to be independent
factors for shortened CSS time (P=0.006, P=0.038, respectively).
Among these independent factors in univariate analysis, only
postoperative CAR (≥0.27) was an independent factor for worse CSS
rate in the multivariate analysis (HR, 2.600; 95% CI, 1.126-5.833;
P=0.026) (Table V).
 | Table VUnivariate and multivariate analyses
of independent factors for cancer-specific survival in 48 patients
with advanced pT stage (≥T3). |
Table V
Univariate and multivariate analyses
of independent factors for cancer-specific survival in 48 patients
with advanced pT stage (≥T3).
| | Univariate | Multivariate |
|---|
| Parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Clinicopathological | | | | | | |
|
Tumor grade
(high or PUNLMP/low) | 1.527 | 0.447-9.570 | 0.546 | | | |
|
Lymph node
metastasis (positive or negative) | 1.204 | 0.529-2.599 | 0.647 | | | |
|
Ureter
involvement (positive or negative) | 1.164 | 0.346-7.239 | 0.833 | | | |
|
Surgical
margin (positive or negative) | 1.069 | 0.410-3.653 | 0.902 | | | |
|
Lymphovascular
invasion (positive or negative) | 1.822 | 0.636-7.675 | 0.291 | | | |
|
Carcinoma
in situ (positive or negative) | 0.729 | 0.041-3.467 | 0.745 | | | |
| Laboratory | | | | | | |
|
Preoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.848 | 0.846-3.951 | 0.121 | | | |
|
Postoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.434 | 0.631-3.087 | 0.376 | | | |
|
Preoperative
CAR (≥ or <0.17) | 1.843 | 0.854-3.952 | 0.118 | | | |
|
Postoperative
CAR (≥ or <0.27) | 3.067 | 1.398-6.588 | 0.006 | 2.600 | 1.126-5.833 | 0.026 |
|
Preoperative
NLR (≥ or <2.46) | 1.270 | 0.592-2.826 | 0.541 | | | |
|
Postoperative
NLR (≥ or <2.19) | 1.521 | 0.706-3.456 | 0.287 | | | |
|
Preoperative
PLR (≥ or <94.80) | 2.586 | 1.059-5.761 | 0.038 | 1.841 | 0.231-1.381 | 0.190 |
|
Postoperative
PLR (≥ or <105.23) | 1.489 | 0.545-3.485 | 0.409 | | | |
Another univariate analysis of Cox proportional
hazards model revealed that ERFS time was found to be shorter in
patients with postoperative CAR ≥0.27 and preoperative PLR ≥94.80
(P=0.012, P=0.038, respectively). Among these independent factors
in univariate analysis, only postoperative CAR ≥0.27 was found to
act as an independent factor for worse ERFS rate in the
multivariate analysis (HR, 2.399; 95% CI, 1.062-5.297; P=0.036)
(Table VI).
 | Table VIUnivariate and multivariate analyses
of independent factors for extraurothelial recurrence-free survival
in 48 patients with advanced pT stage (≥T3). |
Table VI
Univariate and multivariate analyses
of independent factors for extraurothelial recurrence-free survival
in 48 patients with advanced pT stage (≥T3).
| | Univariate | Multivariate |
|---|
| Parameters | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
|
Clinicopathological | | | | | | |
|
Tumor grade
(high or PUNLMP/low) | 2.560 | 0.542-45.733 | 0.284 | | | |
|
Lymph node
metastasis (positive or negative) | 1.560 | 0.702-3.342 | 0.266 | | | |
|
Ureter
involvement (positive or negative) | 1.051 | 0.313-6.536 | 0.945 | | | |
|
Surgical
margin (positive or negative) | 1.050 | 0.403-3.582 | 0.928 | | | |
|
Lymphovascular
invasion (positive or negative) | 1.770 | 0.616-7.465 | 0.317 | | | |
|
Carcinoma
in situ (positive or negative) | 1.121 | 0.063-5.302 | 0.913 | | | |
| Laboratory | | | | | | |
|
Preoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.736 | 0.793-3.727 | 0.164 | | | |
|
Postoperative
eGFR (< or ≥45 ml/min/1.73 m2) | 1.315 | 0.579-2.830 | 0.499 | | | |
|
Preoperative
CAR (≥ or <0.17) | 1.329 | 0.599-2.841 | 0.473 | | | |
|
Postoperative
CAR (≥ or <0.27) | 2.777 | 1.265-5.971 | 0.012 | 2.399 | 1.062-5.297 | 0.036 |
|
Preoperative
NLR (≥ or <2.46) | 1.004 | 0.469-2.165 | 0.992 | | | |
|
Postoperative
NLR (≥ or <2.19) | 1.711 | 0.786-4.003 | 0.179 | | | |
|
Preoperative
PLR (≥ or <94.80) | 2.613 | 1.061-5.902 | 0.038 | 2.058 | 0.208-1.230 | 0.122 |
|
Postoperative
PLR (≥ or <105.23) | 1.416 | 0.518-3.316 | 0.469 | | | |
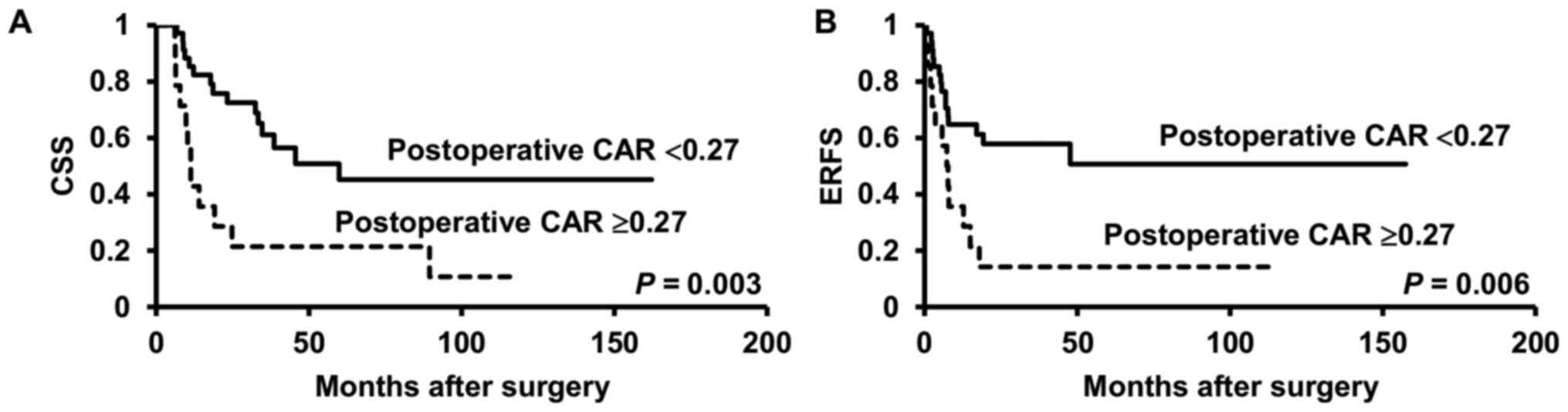
The Kaplan-Meier curves and the results of the
log-rank test revealed significant differences in the CSS and ERFS
rates between ≥pT3 patients with and without postoperative CAR
≥0.27 (P=0.003, P=0.006, respectively) (Fig. 3A and B).
In total, 28 (58.3%) patients with advanced pT stage
(≥T3) received postoperative chemotherapy. Adjuvant chemotherapy
was administered to 7 patients, and salvage chemotherapy was
administered to 16 patients. Both adjuvant and salvage chemotherapy
were administered to 5 patients. A total of 24 patients (85.7%)
received cisplatin plus gemcitabine, 2 (7.1%) received docetaxel
plus gemcitabine, and 2 (7.1%) received carboplatin plus
gemcitabine. The median number of chemotherapy cycles was three.
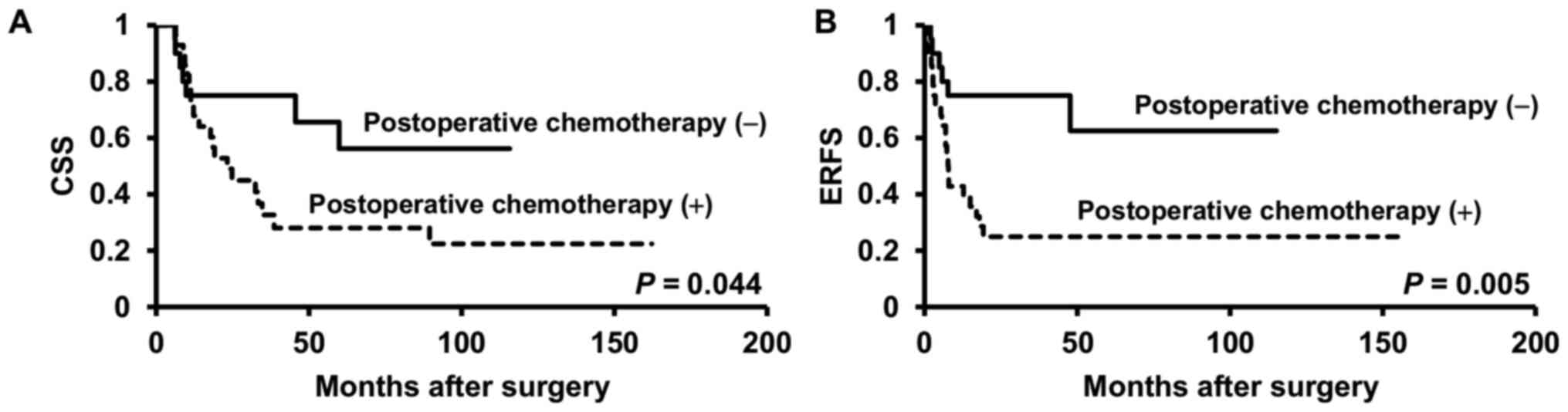
Compared with those who underwent surgical treatment alone,
patients with advanced pT stage (≥T3) who received postoperative
chemotherapy showed significantly worse CSS or ERFS rates (P=0.044,
P=0.005, respectively) (Fig. 4A and
B).
Discussion
In the present study, only postoperative CAR ≥0.27
was an independent predictor of worse CSS as well as ERFS rates.
Other laboratory parameters, such as eGFR, NLR, and PLR were found
to be independent factors for shortened CSS time in univariate
analyses but not independent factors in multivariate analyses.
Although we added clinicopathological factors such as pathological
T stage, tumor grade, lymph node metastasis, and LVI, postoperative
CAR ≥0.27 was designated as an only prognostic factor for worse CSS
and ERFS rates in multivariate analysis of Cox proportional hazards
model. Postoperative CAR ≥0.27 did not have any significant
association with pathological factors or postoperative laboratory
parameters besides preoperative NLR, which means postoperative CAR
is independent of pathological factors. We also performed
multivariate analysis in patients with advanced pT stage (≥T3) and
found that higher postoperative CAR was the only independent
predictor of poor CSS (HR, 2.600; 95% CI, 1.126-5.833; P=0.026) and
ERFS rates (HR, 2.399; 95% CI, 1.062-5.297; P=0.036). We also found
that postoperative chemotherapy did not improve prognosis in
patients with advanced pT stage. This may be because 21 out of 28
patients (75%) received salvage chemotherapy, and a higher
postoperative CAR could facilitate monitoring of patients with ≥pT3
after radical cystectomy.
CIS at radical cystectomy is known to increase the
risk of recurrence, especially in organ-confined patients (26-28).
However, out of 10 patients with CIS, two had advanced pT stage
(≥T3), one showed LVI, and other two had higher postoperative CAR
(≥0.27) in the present study. This may explain why patients with
CIS did not show worse survival in this study.
Numerous studies have demonstrated an association
between preoperative NLR and survival (9,29,30).
Conversely, some studies investigated whether postoperative SIR
markers could be an effective prognostic marker for patients with
clinically localized upper urinary tract urothelial carcinoma
undergoing radical nephroureterectomy (19,31).
Subsequently, postoperative NLR has been suggested as a beneficial
prognostic marker not only in urothelial carcinoma (17,18),
but also in other carcinomas (32,33).
Preoperative NLR only reflects the balance host protumor
inflammatory status and antitumor immune status before surgery,
whereas postoperative NLR indicates the balance between tumor
inflammatory response and host immune response after surgical
removal of the tumor, which should provide a more precise
indication of treatment response (33).
On a similar note to papers reporting on the
prognostic value of NLR, several studies have investigated
presurgical or pretreatment CAR as a predictor of poor prognosis in
patients with several types of tumors (14-16).
The prognostic value of postoperative CAR has remained unclear in
patients with bladder cancer undergoing radical cystectomy,
although several reports have implied clinical significance of
postoperative CRP (31,34). The present study demonstrated that
high postoperative CAR levels were significantly associated with
poor prognosis in patients with locally advanced bladder cancer.
Patients with higher postoperative CAR also exhibited a
significantly worse prognosis compared with those with lower
postoperative CAR, even among those with advanced pT stage (≥T3).
Based on this finding and in relation to earlier suggestions
(19,33), this may be because higher
postoperative CAR possibly indicates potential residual cancer,
including micrometastases, whereas preoperative CAR values indicate
the presence of the primary tumor and potential micrometastatic
lesions. This is the reason why higher preoperative CAR is an
independent factor only in univariate analysis, whereas higher
postoperative CAR is an independent predictor of worse CSS and ERFS
rates in multivariate analysis. Thus, if postoperative CAR reflects
micrometastases, it is potentially a significant prognostic marker
in patients with locally advanced bladder cancer undergoing
surgical treatment.
Approximately 20% of all cancer-related deaths are
attributable to malnutrition (35).
In addition, malnutrition is present in 40-80% of all patients with
cancer at some stage during the clinical course of their disease
(35). Malnutrition and
inflammation suppress the synthesis of serum Alb, which is an
indicator of the nutritional status of patients, as well as the
severity, progression, and prognosis of the disease (36). Serum Alb is an independent predictor
of clinical outcomes in various cancers (37,38).
Moreover, many studies have combined CRP and Alb to create new SIR
markers, such as GPS and CAR. In fact, both GPS and CAR are
independent indicators of poor survival in various types of cancer
(12,14-16).
In the present study, CAR was applied as a variable because CAR is
a continuous value compared with GPS, which is a dichotomous
variable, although it was required to determine the cut-off values
for cancer-specific death and/or extraurothelial recurrence.
This study has some potential limitations. First,
our sample size was relatively small, although the CSS and ERFS
rates were evaluated as our clinical endpoint. With a longer
follow-up and a larger sample population, the statistical strength
of our study can be reinforced, leading to more precise prognosis.
Second, we could not clarify the background between postoperative
CAR and worse survival rates in patients with advanced bladder
cancer, although the possible implications for patients with a
higher postoperative CAR value are suggested. Third, several other
factors that may have influenced the inflammatory status, such as
diabetes mellitus and/or hyperlipidemia, were not included in the
present study. However, we deemed these conditions had only minimal
influence on the values of inflammatory indices because body mass
index did not have any significant association with prognosis (data
not shown). Notwithstanding these limitations, our data indicate
that a higher postoperative CAR can be an independent prognostic
factor for worse survival rates.
In summary, a higher postoperative CAR value can
provide additional information about the possibility of worse CSS
and ERFS rates.
In conclusion, among the patients with bladder
cancer undergoing radical cystectomy, survival rates are worse in
those with higher postoperative CAR values than in those with lower
postoperative CAR values. Therefore, patients with higher
postoperative CAR should undergo additional treatment or at least
careful follow-up.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK, ST, AH and KI were involved in the conception
and design of the study. KK, ST, AH and KI collected the data, and
KK analyzed the data. KK drafted the manuscript. KK and KI reviewed
and edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the studies were in
accordance with the ethical standards of the National Defense
Medical College (Saitama, Japan; ID 2734). The study protocol was
accepted on June 14, 2017, by the National Defense Medical College
Ethics Committee, and an opt-out approach on the web page of the
National Defense Medical College was used instead of collecting
written informed consent from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
National collaborating centre for cancer
(UK): Bladder cancer: Diagnosis and management, 2015.
|
|
3
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ,
Sagalowsky AI, et al: Outcomes of radical cystectomy for
transitional cell carcinoma of the bladder: A contemporary series
from the Bladder Cancer Research Consortium. J Urol. 176:2414–2422.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hautmann RE, de Petriconi RC, Pfeiffer C
and Volkmer BG: Radical cystectomy for urothelial carcinoma of the
bladder without neoadjuvant or adjuvant therapy: Long-term results
in 1100 patients. Eur Urol. 61:1039–1047. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Canter D, Long C, Kutikov A, Plimack E,
Saad I, Oblaczynski M, Zhu F, Viterbo R, Chen DY, Uzzo RG, et al:
Clinicopathological outcomes after radical cystectomy for clinical
T2 urothelial carcinoma: Further evidence to support the use of
neoadjuvant chemotherapy. BJU Int. 107:58–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Scott HR, McMillan DC, Forrest LM, Brown
DJ, McArdle CS and Milroy R: The systemic inflammatory response,
weight loss, performance status and survival in patients with
inoperable non-small cell lung cancer. Br J Cancer. 87:264–267.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: Preoperative neutrophil-lymphocyte ratio can significantly
predict mortality outcomes in patients with non-muscle invasive
bladder cancer undergoing transurethral resection of bladder tumor.
Oncotarget. 8:12891–12901. 2016.
|
|
10
|
Omae K, Kondo T and Tanabe K: High
preoperative C-reactive protein values predict poor survival in
patients on chronic hemodialysis undergoing nephrectomy for renal
cancer. Urol Oncol. 33:e9–e13. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gunduz S, Mutlu H, Tural D, Yildiz O,
Uysal M, Coskun HS and Bozcuk H: Platelet to lymphocyte ratio as a
new prognostic for patients with metastatic renal cell cancer. Asia
Pac J Clin Oncol. 11:288–292. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuksel OH, Akan S, Uukmez A, Yildirim C,
Sahin A and Verit A: Preoperative Glasgow prognostic score as a
predictor of primary bladder cancer recurrence. Mol Clin Oncol.
5:201–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morgan TM, Tang D, Stratton KL, Barocas
DA, Anderson CB, Gregg JR, Chang SS, Cookson MS, Herrell SD, Smith
JA Jr and Clark PE: Preoperative nutritional status is an important
predictor of survival in patients undergoing surgery for renal cell
carcinoma. Eur Urol. 59:923–928. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou T, Zhan J, Hong S, Hu Z, Fang W, Qin
T, Ma Y, Yang Y, He X, Zhao Y, et al: Ratio of C-reactive
protein/albumin is an inflammatory prognostic score for predicting
overall survival of patients with small-cell lung cancer. Sci Rep.
5(10481)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu X, Sun X, Liu J, Kong P, Chen S, Zhan
Y and Xu D: Preoperative C-reactive protein/albumin ratio predicts
prognosis of patients after curative resection for gastric cancer.
Transl Oncol. 8:339–345. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H and Matsushima M:
The C-reactive protein/albumin ratio, a novel inflammation-based
prognostic score, predicts outcomes in patients with hepatocellular
carcinoma. Ann Surg Oncol. 22:803–810. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Morizawa Y, Miyake M, Shimada K, Hori S,
Tatsumi Y, Nakai Y, Anai S, Tanaka N, Konishi N and Fujimoto K:
Neutrophil-to-lymphocyte ratio as a detection marker of tumor
recurrence in patients with muscle-invasive bladder cancer after
radical cystectomy. Urol Oncol Semin Orig Investig. 34:257.e11–e17.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: The prognostic significance of the early postoperative
neutrophil-to-lymphocyte ratio in patients with urothelial
carcinoma of the bladder undergoing radical cystectomy. Ann Surg
Oncol. 23:335–342. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nishihara K, Suekane S, Ueda K, Nakiri M,
Matsuo M and Igawa T: High postoperative neutrophil-to-lymphocyte
ratio as a poorprognostic marker in patients with upper tract
urothelial carcinoma. Oncol Lett. 17:5241–5250. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Herr HW: Extent of pelvic lymph node
dissection during radical cystectomy: Where and Why! Eur Urol.
57:212–213. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ghoneim MA, Abdel-Latif M, El-Mekresh M,
Abol-Enein H, Mosbah A, Ashamallah A and El-Baz MA: Radical
cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5
years later. J Urol. 180:121–127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM classification of malignant tumours, 7th edition, 2009.
|
|
23
|
John E, Guido S, Jonathan E and Isabell S:
Pathology and genetics: Tumours of the urinary system and male
genital system, 2004.
|
|
24
|
Imai E, Horio M, Nitta K, Yamagata K,
Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A and Matsuo S:
Modification of the modification of diet in renal disease (MDRD)
Study equation for Japan. Am J Kidney Dis. 50:927–937.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen Y, Wang H, Li W and Chen J:
Prognostic significance of the CRP/Alb and neutrophil to lymphocyte
ratios in hepatocellular carcinoma patients undergoing TACE and
RFA. J Clin Lab Anal. 33(e22999)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Utz DC, Hanash KA and Farrow GM: The
plight of the patient with carcinoma in situ of the bladder. J
Urol. 103:160–164. 1970.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stanisic TH, Donovan JM, Lebouton J and
Graham AR: 5-Year experience with intravesical therapy of carcinoma
in situ: An inquiry into the risks of ‘conservative’ management. J
Urol. 138:1158–1161. 1987.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lamm D, Herr H, Jakse G, Kuroda M, Mostofi
FK, Okajima E, Sakamoto A, Sesterhenn I and da Silva FC: Updated
concepts and treatment of carcinoma in situ. Urol Oncol. 4:130–138.
1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Uemura K, Kawahara T, Yamashita D, Jikuya
R, Abe K, Tatenuma T, Yokomizo Y, Izumi K, Teranishi JI, Makiyama
K, et al: Neutrophil-to-lymphocyte ratio predicts prognosis in
castration-resistant prostate cancer patients who received
cabazitaxel chemotherapy. Biomed Res Int.
2017(7538647)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kasuga J, Kawahara T, Takamoto D, Fukui S,
Tokita T, Tadenuma T, Narahara M, Fusayasu S, Terao H, Izumi K, et
al: Increased neutrophil-to-lymphocyte ratio is associated with
disease-specific mortality in patients with penile cancer. BMC
Cancer. 16(396)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tanaka N, Kikuchi E, Shirotake S, Kanao K,
Matsumoto K, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, et
al: The predictive value of C-reactive protein for prognosis in
patients with upper tract urothelial carcinoma treated with radical
nephroureterectomy: A multi-institutional study. Eur Urol.
65:227–234. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tanaka H, Tamura T, Toyokawa T, Muguruma
K, Miki Y, Kubo N, Sakurai K, Hirakawa K and Ohira M: Clinical
relevance of postoperative neutrophil-lymphocyte ratio (NLR) to
recurrence after adjuvant chemotherapy of S-1 for gastric cancer.
Anticancer Res. 38:3745–3751. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jin F, Han A, Shi F, Kong L and Yu J: The
postoperative neutrophil-to-lymphocyte ratio and changes in this
ratio predict survival after the complete resection of stage I
non-small cell lung cancer. Onco Targets Ther. 9:6529–6537.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Albisinni S, Moussa I, Aoun F, Quackels T,
Assenmacher G, Peltier A and Roumeguère T: The impact of
postoperative inflammatory biomarkers on oncologic outcomes of
bladder cancer. Prog Urol. 29:270–281. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Obermair A, Simunovic M, Isenring L and
Janda M: Nutrition interventions in patients with gynecological
cancers requiring surgery. Gynecol Oncol. 145:192–199.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ballmer PE, Ochsenbein AF and
Schütz-Hofmann S: Transcapillary escape rate of albumin positively
correlates with plasma albumin concentration in acute but not in
chronic inflammatory disease. Metabolism. 43:697–705.
1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xiao Y, Ren YK, Cheng HJ, Wang L and Luo
SX: Modified Glasgow prognostic score is an independent prognostic
factor in patients with cervical cancer undergoing
chemoradiotherapy. Int J Clin Exp Pathol. 8:5273–5281.
2015.PubMed/NCBI
|
|
38
|
Ayhan A, Günakan E, Alyazıcı İ, Haberal N,
Altundağ Ö and Dursun P: The preoperative albumin level is an
independent prognostic factor for optimally debulked epithelial
ovarian cancer. Arch Gynecol Obstet. 296:989–995. 2017.PubMed/NCBI View Article : Google Scholar
|