Introduction
Malignant ascites (MA) or malignant pleural
effusions (MPE) are a common clinical manifestation in patients
with advanced neoplasia and confer a poor prognosis (1,2). It is
known that MA and MPE stimulate an aggressive cellular phenotype
and generate a pro-inflammatory environment that promotes
immunosuppression and allows the proliferation and dissemination of
cancer cells (3-5).
Growth factors, cytokines, and glycoproteins have been found to
have higher concentrations in MA and MPE than in plasma (6-9).
Such biomolecules include vascular endothelial growth factor,
angiogenin, epidermal growth factor, interleukin-6, monocyte
chemoattractant protein-1, transforming growth factor beta-1, and
secreted phosphoprotein-1 (10-12).
All of these molecules play an important role in tumor growth,
angiogenesis, and metastasis, which shorten the survival of
patients with cancer. Other studies have found elevated levels of
several proteases in malignant effusions (13,14).
Our group previously reported a macrophage-activation inhibitory
factor (MAIF), which was purified from mouse ascites by L5178Y
murine lymphoma cells and inhibited lipopolysaccharide
(LPS)-induced macrophage activation (15). MAIF also allowed the development of
hepatic abscesses in vivo when BALB/c mice were inoculated
with Entamoeba histolytica or Listeria monocytogenes
(16,17).
Nevertheless, some reports have demonstrated the
anti-tumor role of MA and MPE. Cohen et al described the
pro-apoptotic effect of cell-free ascites by activation of the JNK
pathway and induction of BRCA1, Fas, and FasL expression in SKOV3
cells (18). Other studies have
shown the existence of angiogenesis and migration inhibitors in
ascites and pleural effusion from patients with breast cancer,
ovarian carcinoma, lung carcinoma, and mesothelioma (19-22).
These findings indicate that the biochemical compositions of MA and
MPE are widely diverse and that these effusions can play dual roles
in tumor progression.
Macrophage activation by LPS polarizes them to the
M1 phenotype and can produce nitrogen-based radicals by stimulating
inducible nitric oxide synthase (iNOS) (23-25).
Thus, increased nitric oxide (NO) production can reflect
polarization to a proinflammatory phenotype.
The present study sought to explore whether the
MA-MPE-derived acellular fraction could modulate the production of
NO by peripheral blood mononuclear cells (PBMCs) and whether NO
influences the viability of healthy and cancerous cells.
Materials and methods
Clinical specimens
Forty-one malignant effusion samples were collected
from patients diagnosed with primary neoplasia and 34 samples were
derived from patients with non-cancer diagnoses. All samples were
obtained at Instituto Mexicano del Seguro Social, in Monterrey,
Mexico. The study was approved by the Institutional Ethics Board
with the registration number R-2008-1908-2, and written informed
consent was obtained from each patient before participation.
Patients with thrombocytopenia, abnormal clotting time, HIV/AIDS,
or primary immunodeficiency diseases were excluded.
Collection of biological samples
The pleural effusion and ascitic fluids used in this
study were collected by thoracentesis or paracentesis,
respectively, at the time of the therapeutic protocol.
Approximately 20 ml was taken for each specimen under aseptic
conditions. All samples were stored at -20˚C until analysis.
Purification of the <10 kDa
fraction
To guarantee the exclusive presence of
low-molecular-weight biomolecules, all samples were depleted of
cells by centrifugation at 30,000 g for 20 min, and each cell-free
supernatant was purified using centrifugal filter units with
membranes having a nominal molecular weight cutoff of 10 kDa (Merck
Millipore). The <10 kDa fraction was aliquoted into 1 ml vials,
and protein concentration was determined using the Lowry test. The
samples were stored at -20˚C until analysis.
Stimulation of peripheral whole
blood
To analyze the production of NO by PBMCs, the whole
blood of a healthy volunteer was recollected into plastic blood
collection tubes with sodium citrate (Becton, Dickinson and
Company). Aliquots (3 ml) were made within the first 60 min of
blood collection and were then stimulated with 30 µg/ml of the
<10 kDa fraction at 37˚C with 5% CO2 for 2 h, with
constant agitation. Subsequently, without removing the <10 kDa
fraction, each sample was treated with 50 ng/ml E. coli
serotype O12B:B12 LPS (Sigma-Aldrich; Merck KGaA) and incubated for
5 h under the conditions previously described. After that, we
obtained the plasma by centrifugation at 2,000 g for 10 min and
stored the samples at -80˚C until analysis. In addition, the three
control groups were shaped: a) a group with LPS-unstimulated blood,
b) an LPS-treated group as positive control, and c) an LPS-treated
group treated with 100 ng/ml of NG-monomethyl-L-arginine acetate
(Sigma-Aldrich; Merck KGaA) as NO inhibitor.
Nitric oxide assay
NO concentration was measured using the total nitric
oxide assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, to convert nitrate to
nitrite, 50 µl of plasma was plated in 96-well plates with the
nitrate reductase enzyme for 30 min at 37˚C. Nitrite was detected
as a colored azo dye product at 540 nm in a microplate reader
(BioTek Instruments, Inc.), and the results were expressed in
micrometers.
Cell lines and cell culture
Human lung fibroblast 55x and MCF-7 breast cancer
cells were obtained from the American Type Culture Collection
(ATCC). They were cultured at 37˚C with 5% CO2 in DMEM
supplemented with 10% heat-inactivated fetal bovine serum
Gibco™ (Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA).
The medium was changed every three days, and the cells were
passaged twice weekly.
Cytotoxicity assay
Cell growth inhibition was measured using the MTT
assay (Abcam) at 24 h post exposure. Briefly, 1x104
cells were seeded into 96-well culture plates and cultured for 24
h. After exposure to each sample at 2% v/v, cells were washed twice
with phosphate-buffered saline (Gibco™; Thermo Fisher
Scientific, Inc.) twice, and 100 µl of MTT solution (5 mg/ml in
medium) was added to each well. Then, the formazan in viable cells
was dissolved in acidified isopropanol solution and measured at 570
nm using a microplate reader Elx 800 (BioTek Instruments, Inc.).
The absorbance value of cells incubated with culture medium
(untreated group) was set to 100% cell viability and compared with
treated cells. We used 1% Triton X-100™ (Sigma-Aldrich;
Merck KGaA) and vincristine (500 µg/ml) as the cytotoxic
control.
Statistical analysis
Each experimental protocol was tested in triplicate
and repeated three times in independent experiments, and the
average was used for the analysis. Data are expressed as mean and
standard deviation. Student's t-test or Fischer's exact test were
used to compare the characteristics of patients with MA and
malignant pleural effusion. One-way ANOVA with Tukey's post hoc
test was used for comparisons among multiple groups. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Subjects
The clinical characteristics of the patients with
cancer are shown in Table I.
Twenty-one ascite samples from patients with primary tumor
diagnoses and 20 samples of malignant pleural effusion were
examined. The majority of patients were categorized as stage IV at
the time of sample collection. In patients with MA, the more
frequent metastatic sites were the peritoneum (13/21) and liver
(5/21), followed by the lungs (2/21) and spleen (1/21), while all
MPEs were obtained from patients with thoracic metastases. Benign
ascites (BA) samples were collected from 18 cirrhotic patients, of
which 16 patients were male. We obtained benign pleural effusion
(BPE) from patients with congestive heart failure (n=6), chronic
kidney disease (n=4), pneumothorax (n=1), pancreatitis (n=2),
panlobular emphysema (n=1), rib fracture (n=1), and penetrating
abdominal trauma (n=1).
 | Table IClinical characteristics of
oncological patients. |
Table I
Clinical characteristics of
oncological patients.
| Characteristic | Malignant
ascites | Malignant pleural
effusion | P-value |
|---|
| Age, years | | | |
|
Mean ±
SD | 51.33±12.09 | 67.45±14.86 | 0.004a |
|
Range | 35-75 | 21-87 | |
| Sex, n (%) | | | |
|
Male | 7 (33.3) | 10 (50.0) | 0.279b |
|
Female | 14 (66.6) | | 10 (50.0) |
| Diagnosis (n) | Ovarian cancer (6),
lung cancer (1), hepatocellular carcinoma (3), lymphoma (2), breast
cancer (1), mesothelioma (2), melanoma (1), gastric cancer (1),
cancer of unknown primary (3), pancreatic cancer (1) | Ovarian cancer (1),
lung cancer (10), bone and soft tissue tumors (3), lymphoma (1),
breast cancer (3), mesothelioma (1), renal cell carcinoma (1) | |
| Clinical stage, n
(%) | | | |
|
III | 3 (14.2) | 1 (5.0) | 0.317b |
|
IV | 18 (85.7) | | 19 (95.0) |
| ECOG score, n
(%) | | | |
|
2 | 0 (0.0) | 12 (60.0) | |
|
3 | 15 (71.4) | 5 (25.0) | 0.003b |
|
4 | 6 (28.5) | 2 (10.0) | 0.134b |
|
5 | 0 (0.0) | 1 (5.0) | |
| Treatment, n
(%) | | | |
|
Chemotherapy | 13 (61.9) | 4 (20.0) | 0.006b |
|
Radiotherapy | | 0 (0.0) | 4 (20.0) |
|
Both | | 1 (4.7) | 0 (0.0) |
|
None | 7 (33.3) | 12 (60.0) | 0.087b |
Patients with MA were younger than patients with
malignant pleural effusions (51.33±12.09 vs. 67.45±14.86;
P<0.01). There were no differences between the proportion of
male/female samples or clinical stage among patients with MA and
malignant pleural effusions. However, MA samples were more frequent
from patients with a history of chemotherapy or with a ECOG grade 3
(Eastern Cooperative Oncology Group scale) (Table I).
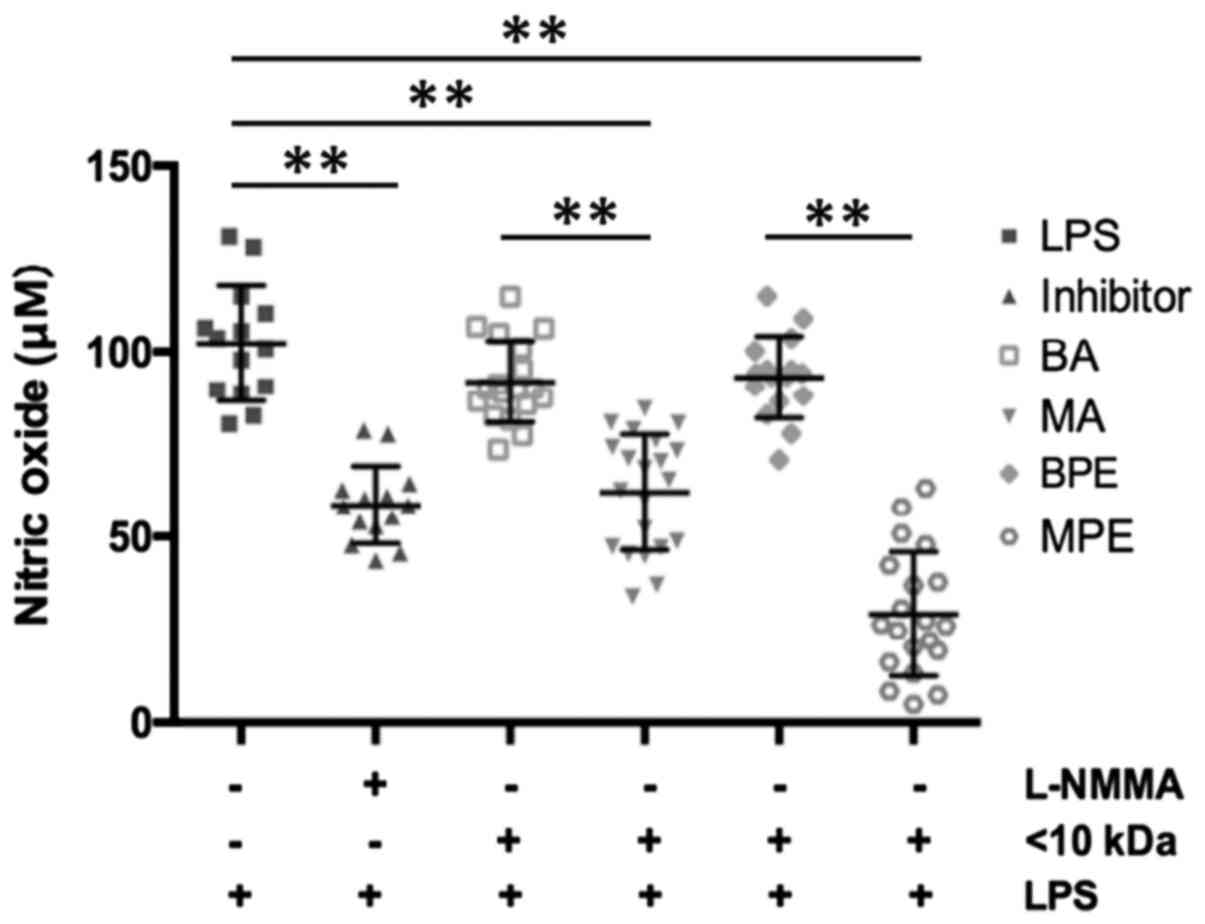
NO production
In the LPS-stimulated group, NO production was twice
as high as in the inhibitor group (102.2±15.50 vs. 58.6±10.41 µM;
P<0.001). Similarly, the amounts of NO differed between benign
(91.87±10.97 µM; P<0.001) and malignant (62.06±15.63 µM) ascites
samples, and also BPE and MPE samples differed (Fig. 1).
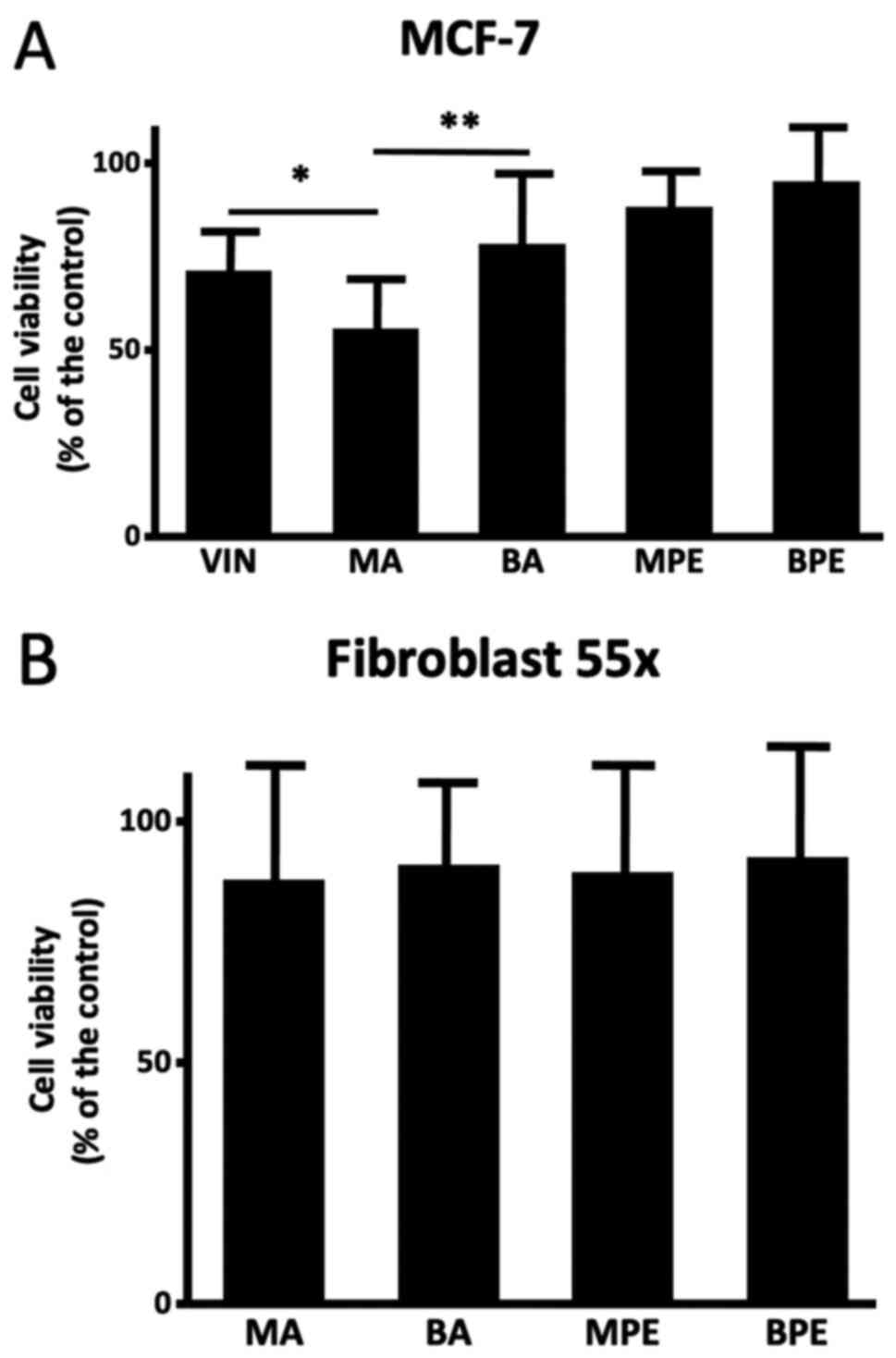
MA and MPE modulated cytotoxicity in
breast cancer cells
A cell viability assessment was performed on some
samples from MA (n=12), BA (n=10), MPE (n=8), and BPE (n=8). MA
samples induced reduction of MCF-7 cell viability in comparison
with BA (55.82±16.11 vs. 78.47±21.52; P<0.01); also, the
cytotoxic effect of MA was higher than that of vincristine
(71.20±13.67; P<0.05), and there was no difference with MPE or
BPE (88.36±11.05 and 95.15±14.31) (Fig.
2A). None of the samples, either malignant or benign, affected
the viability of Fibroblast 55x cells (Fig. 2B).
Discussion
In this study, we evaluated the NO production by
PBMCs exposed to an acellular fraction derived from MA/MPE. Our
results demonstrated that the acellular fraction of MA/MPE can
reduce NO production in PBMCs stimulated with LPS. We also
determined that the addition of MA/MPE decreased cancer cell
viability in vitro, but did not affect healthy
fibroblasts.
MA effusions are created by the tumor and act as a
unique environment that is dominated by tumor-induced interactions.
They provide a framework that orchestrates cellular and molecular
changes that contribute to aggressiveness and disease progression
(26,27). These effusions are rich in
cytokines, chemokines, growth factors, and immune effector cells
(25-27);
however, their antitumor functions have been reported to be
negatively regulated (27). Our
results are in accordance with this finding, and the NO production
in LPS-stimulated macrophages decreased when they were incubated
with the <10 kDa fraction. This macrophage activation failure
contributes to the survival of tumor cells despite the
proinflammatory environment. This is supported by our previous
observations that MA derived from L5178Y murine lymphoma fails to
activate macrophages when the cells are pre-treated with cell-free
MA before stimulation with LPS (14). However, there is evidence that
macrophages exposed to different environments can change their
polarization, and perhaps the phenotypic change from M1 to M2 could
explain the lower production of NO when the PBMCs were
pre-incubated with malignant effusion extracts (23-25).
There is evidence that NO has a dual role, where a
low NO concentration inhibits proliferation in some tissues while
in others it inhibits apoptosis, and its effects are dose-, cell-,
and even cancer stage-dependent (28-31),
we observed a decrease in the viability of tumor cells that could
be related to the decrease in NO.
Unlike MA, in patients with cancer, pleural
effusions can develop as a result of the interference with the
integrity of the lymphatic system, direct tumor involvement of the
pleura, and local inflammatory changes in response to tumor
invasion (32). Furthermore, like
MA, the presence of cancer cells in pleural effusion defines MPE.
Soini et al (33) reported
higher NO production by iNOS in MPEs than in benign ones. Our MPE
samples inhibited macrophage NO release in a similar way as MA
samples, but its effect on cancer cell survival was less
evident.
Although some studies have shown the heterogeneity
of the soluble components in the malignant fluid (34-36)
and heterogeneity in the type of cancer that produced our samples,
the decrease in cancer cell viability upon incubation with the
<10 kDa fraction and its innocuity in healthy cells, reveals the
presence of a common anti-tumorigenic molecule in all malignant
effusions. According to our data, we can speculate that the <10
kDa fractions derived from MA and MPE contain biological molecules
that modulate the activation of PBMCs and regulate breast cancer
proliferation. The next step is to profile the biochemical
composition of the <10 kDa fractions derived from malignant
fluids.
Limitations
All blood samples came from the same subject;
however, we recognize that plasma protein concentration before or
after stimulation was not considered and could affect the
cytotoxicity assay. It is also worth considering that we did not
perform a cytotoxicity assay on PBMC, nor did we evaluate the
cytotoxicity of the PBMC-stimulated extract. We only performed a
cytotoxicity assay using the <10 kDa fraction.
In conclusion, independent of cellular origin, low
molecular weight fractions derived from MA and MPE had molecules
that inhibited PBMC defense mechanisms and decreased the viability
of breast cancer cells in vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Instituto Mexicano del
Seguro Social (grant no. FIS/IMSS/PROT/G 2006/1A/I/080).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JVV, MCR and RPC conceived the original research
idea and developed the experimental design. AEO, HGH and EDDLG
designed the study. AEO, HGH, EDDLG, OMD, CAMA, FGS and MGMT
performed the experiments and acquired the data. FJGDLG, JVV, MCR
and RPC conducted data analysis and revised the manuscript. FJGDLG,
JVV and MCR were responsible for interpretation of data and
manuscript writing. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the National Committee for
Scientific Research of Instituto Mexicano del Seguro Social (Mexico
City, Mexico; registration no. R-2008-1908-2). Written informed
consent was obtained from all patients before their
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kipps E, Tan DSP and Kaye SB: Meeting the
challenge of ascites in ovarian cancer: New avenues for therapy and
research. Nat Rev Cancer. 13:273–282. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Psallidas I, Kalomenidis I, Porcel JM,
Robinson BW and Stathopoulos GT: Malignant pleural effusion: From
bench to bedside. Eur Respir Rev. 25:189–198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Egan AM, McPhillips D, Sarkar S and Breen
DP: Malignant pleural effusion. QJM. 107:179–184. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mikuła-Pietrasik J, Uruski P, Szubert S,
Moszyński R, Szpurek D, Sajdak S, Tykarski A and Książek K:
Biochemical composition of malignant ascites determines high
aggressiveness of undifferentiated ovarian tumors. Med Oncol.
33(94)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yin T, Wang G, He S, Shen G, Su C, Zhang
Y, Wei X, Ye T, Li L, Yang S, et al: Malignant pleural effusion and
ascites induce epithelial-mesenchymal transition and cancer
stem-like cell properties via the vascular endothelial growth
factor (VEGF)/Phosphatidylinositol 3-kinase (PI3K)/Akt/mechanistic
target of rapamycin (mTOR) pathway. J Biol Chem. 291:26750–26761.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu DW, Chang WA, Liu KT, Yen MC and Kuo
PL: Vascular endothelial growth factor and protein level in pleural
effusion for differentiating malignant from benign pleural
effusion. Oncol Lett. 14:3657–3662. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chudecka-Glaz A, Cymbaluk-Płoska A,
Menkiszak J, Pius-Sadowska E, Machaliński B, Sompolska-Rzechuła A
and Rzepka-Górska I: Assessment of selected cytokines, proteins,
and&nbsp;growth factors in the peritoneal fluid of patients
with ovarian cancer and benign gynecological conditions. Onco
Targets Ther. 8:471–485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kucukgoz Gulec U, Paydas S, Guzel AB,
Buyukkurt S, Seydaoglu G and Vardar MA: Comparative analysis of CA
125, ferritin, beta-2 microglobulin, lactic dehydrogenase levels in
serum and peritoneal fluid in patients with ovarian neoplasia. Med
Oncol. 29:2937–2943. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng D, Liang B and Kong H: Clinical
significance of vascular endothelial growth factor and endostatin
levels in the differential diagnosis of malignant and benign
ascites. Med Oncol. 29:1397–1402. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matte I, Lane D, Laplante C, Rancourt C
and Piché A: Profiling of cytokines in human epithelial ovarian
cancer ascites. Am J Cancer Res. 2:566–580. 2012.PubMed/NCBI
|
|
11
|
Kolomeyevskaya N, Eng KH, Khan ANH,
Grzankowski KS, Singel KL, Moysich K and Segal BH: Cytokine
profiling of ascites at primary surgery identifies an interaction
of tumor necrosis factor-α and interleukin-6 in predicting reduced
progression-free survival in epithelial ovarian cancer. Gynecol
Oncol. 138:352–357. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Saraya T, Ohkuma K, Watanabe T, Mikura S,
Kobayashi F, Aso J, Nunokawa H, Honda K, Ogawa Y, Tamura M, et al:
Diagnostic value of vascular endothelial growth factor,
transforming growth factor-β, Interleukin-8, and the ratio of
lactate dehydrogenase to adenosine deaminase in pleural effusion.
Lung. 196:249–254. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van Hensbergen Y, Broxterman HJ,
Hanemaaijer R, Jorna AS, van Lent NA, Verheul HM, Pinedo HM and
Hoekman K: Soluble aminopeptidase N/CD13 in malignant and
nonmalignant effusions and intratumoral fluid. Clin Cancer Res.
8:3747–3754. 2002.PubMed/NCBI
|
|
14
|
Fiorelli A, Ricci S, Feola A, Mazzella A,
D'Angelo L, Santini M, Di Domenico M and Di Carlo A: Matrix
metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in
diagnosis of pleural effusion of malignant origin. Interact
Cardiovasc Thorac Surg. 22:411–418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Palacios-Corona R, Ortı́z-Navarrete VF,
Said-Fernández S, Rodrı́guez-Padilla C and González-Garza MT:
Detection of a factor released by L5178Y lymphoblasts that inhibits
mouse macrophage-activation induced by lipopolysaccharides. Arch
Med Res. 30:298–302. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
González-Garza MT, Palacios-Corona R,
Ortiz-Navarrete V, Castro-Garza J and Said-Fernandez S: The
macrophage-activation inhibitory factor (MAIF) from L5178Y murine
lymphoma favors experimental amebic hepatic abscess development in
Balb/c mice. Arch Med Res. 31 (Suppl 4):S104–S105. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palacios-Corona R, Ortiz-Navarrete V,
Castro-Garza J, Said-Fernandez S, Moreno-Cuevas J, Guzmán-Delgado N
and González-Garza MT: Macrophage-activation inhibitor factor from
L5178Y murine lymphoma and formation of hepatic abscesses in BALB/c
Mice. Arch Med Res. 37:474–478. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cohen M, Pierredon S, Wuillemin C, Delie F
and Petignat P: Acellular fraction of ovarian cancer ascites induce
apoptosis by activating JNK and inducing BRCA1, Fas and FasL
expression in ovarian cancer cells. Oncoscience. 1:262–271.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Richardson M, Gunawan J, Hatton MWC,
Seidlitz E, Hirte HW and Singh G: Malignant ascites fluid (MAF),
including ovarian-cancer-associated MAF, contains angiostatin and
other factor(s) which inhibit angiogenesis. Gynecol Oncol.
86:279–287. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ruiz E, Alemán C, Alegre J, Monasterio J,
Segura RM, Armadans L, Vázquez A, Soriano T and Fernández de
Sevilla T: Angiogenic factors and angiogenesis inhibitors in
exudative pleural effusions. Lung. 183:185–195. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jandu N, Richardson M, Singh G, Hirte H
and Hatton MW: Human ovarian cancer ascites fluid contains a
mixture of incompletely degraded soluble products of fibrin that
collectively possess an antiangiogenic property. Int J Gynecol
Cancer. 16:1536–1544. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Puiffe ML, Le Page C, Filali-Mouhim A,
Zietarska M, Ouellet V, Tonin PN, Chevrette M, Provencher DM and
Mes-Masson AM: Characterization of ovarian cancer ascites on cell
invasion, proliferation, spheroid formation, gene expression in an
in vitro model of epithelial ovarian cancer. Neoplasia. 9:820–829.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Flannagan R, Heit B and Heinrichs D:
Antimicrobial mechanisms of macrophages and the immune evasion
strategies of staphylococcus aureus. Pathogens. 4:826–868.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lam GY, Huang J and Brumell JH: The many
roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin
Immunopathol. 32:415–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shapouri-Moghaddam A, Mohammadian S,
Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi
A, Afshari JT and Sahebkar A: Macrophage plasticity, polarization,
and function in health and disease. J Cell Physiol. 233:6425–6440.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Piché A: Malignant peritoneal effusion
acting as a tumor environment in ovarian cancer progression: Impact
and significance. World J Clin Oncol. 9:167–171. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bal-Price A, Gartlon J and Brown GC:
Nitric oxide stimulates PC12 cell proliferation via cGMP and
inhibits at higher concentrations mainly via energy depletion.
Nitric Oxide. 14:238–246. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Villalobo A: Nitric oxide and cell
proliferation. FEBS J. 273:2329–2344. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Keshet R and Erez A: Arginine and the
metabolic regulation of nitric oxide synthesis in cancer. Dis Model
Mech. 11(dmm033332)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng H, Wang L, Mollica M, Re AT, Wu S
and Zuo L: Nitric oxide in cancer metastasis. Cancer Lett. 353:1–7.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lat T and Paul M: Malignant Effusion. In:
StatPearls. Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK519522/ Accessed
July 19, 2020.
|
|
33
|
Soini Y, Kahlos K, Puhakka A, Lakari E,
Säily M, Pääkkö P and Kinnula V: Expression of inducible nitric
oxide synthase in healthy pleura and in malignant mesothelioma. Br
J Cancer. 83:880–886. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li Y, Lian H, Jia Q and Wan Y: Proteome
screening of pleural effusions identifies IL1A as a diagnostic
biomarker for non-small cell lung cancer. Biochem Biophys Res
Commun. 457:177–182. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li H, Tang Z, Zhu H, Ge H, Cui S and Jiang
W: Proteomic study of benign and malignant pleural effusion. J
Cancer Res Clin Oncol. 142:1191–1200. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jin J, Son M, Kim H, Kim H, Kong SH, Kim
HK, Kim Y and Han D: Comparative proteomic analysis of human
malignant ascitic fluids for the development of gastric cancer
biomarkers. Clin Biochem. 56:55–61. 2018.PubMed/NCBI View Article : Google Scholar
|