Introduction
Multiple myeloma (MM) is characterized by clonal
proliferation of plasma cells in the bone marrow. Extramedullary
plasmacytomas (EMPs) may also develop, and they may either be
present at diagnosis or develop later during the disease course
(1). EMP has a cumulative incidence
of 9% among all patients with MM and is usually associated with a
poor prognosis (2,3). Serum monoclonal immunoglobulin (Ig)
allows distinguishing between different types of MM, and a
multicentre analysis reported that IgD-subtype myeloma accounts for
6.5% of all cases in China (4). IgD
MM presenting with muscle infiltration at diagnosis and central
nervous system (CNS) involvement after complete response (CR) are
rare occurrences. Due to the rarity of this subtype and incomplete
understanding of its clinical course, the optimal diagnostic and
management strategies have yet to be determined.
In the present study, a case of IgD-λ MM with CNS
involvement was retrospectively analyzed and a systematic review of
the literature was performed to provide a contemporary update on
the incidence, demographics, management and outcome of EMP with CNS
involvement.
Case report
A 47-year-old male patient was admitted to the
Putian Hospital (Putian, China) in March 2019 with a chief
complaint of blepharoptosis for 4 months and fatigue for 2 months.
There were no other complaints, and the routine blood tests were
normal. An enhanced magnetic resonance imaging (MRI) brain scan
performed in March 2019 revealed soft tissue masses in the sphenoid
and bilateral cavernous sinuses, and further examinations were
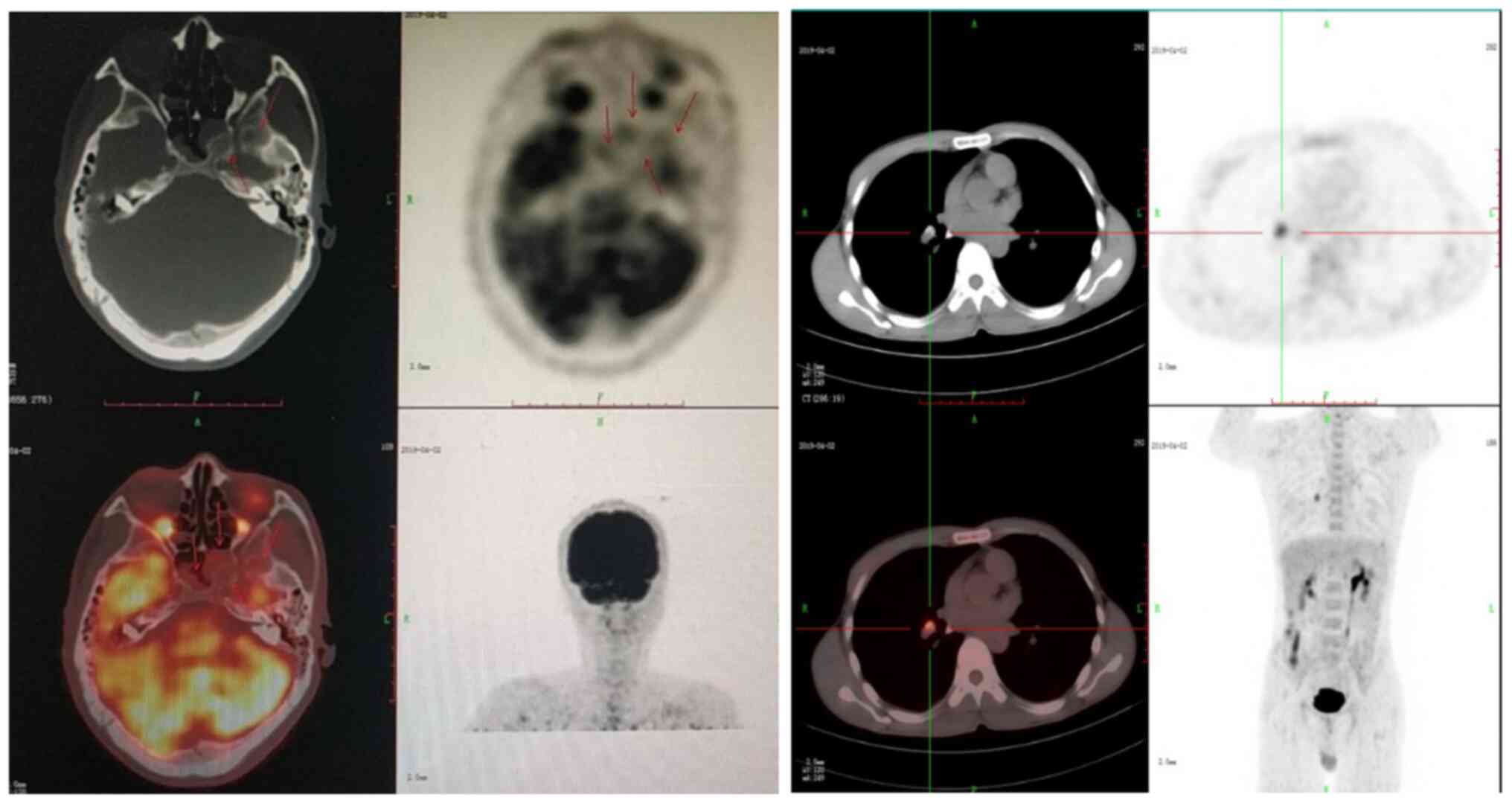
recommended. Positron emission tomography/computer tomography
(PET-CT) performed in April 2019 revealed abnormal bone density of
the whole body, multiple soft tissue masses, and a pathological
fracture of the 10th rib on the left side (Fig. 1). Biopsy of the chest wall mass was
performed in April 2019, and examination of the bioptic material
revealed abnormal plasma cell infiltration of muscle tissue, after
which time the patient was referred to the Fujian Medical
University Union Hospital (Fuzhou, China) for further investigation
and treatment.
Following admission in April 2019, further
evaluations were performed. Blood and biochemistry tests revealed
normal levels of haemoglobin, calcium, lactate dehydrogenase,
albumin, globulin, creatinine and serum β2-microglobulin (Table I). Serum immunofixation
electrophoresis indicated IgD-λ light chains, and the levels of
serum free λ and κ light chains were 151.0 and 14.3 mg/l,
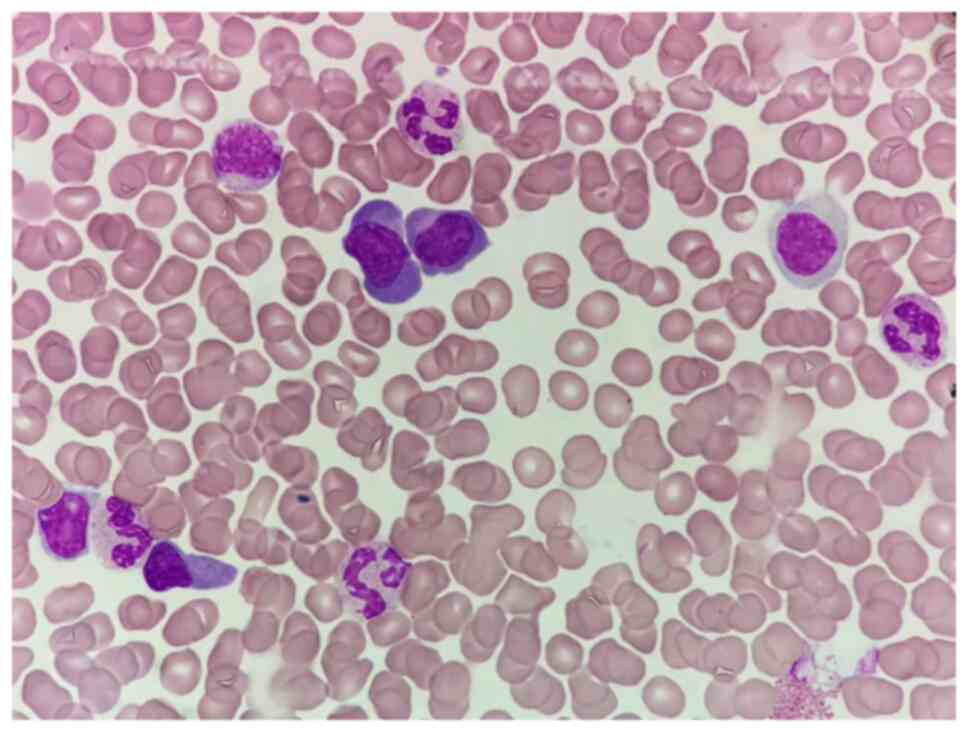
respectively. Bone marrow aspiration revealed that the proportion
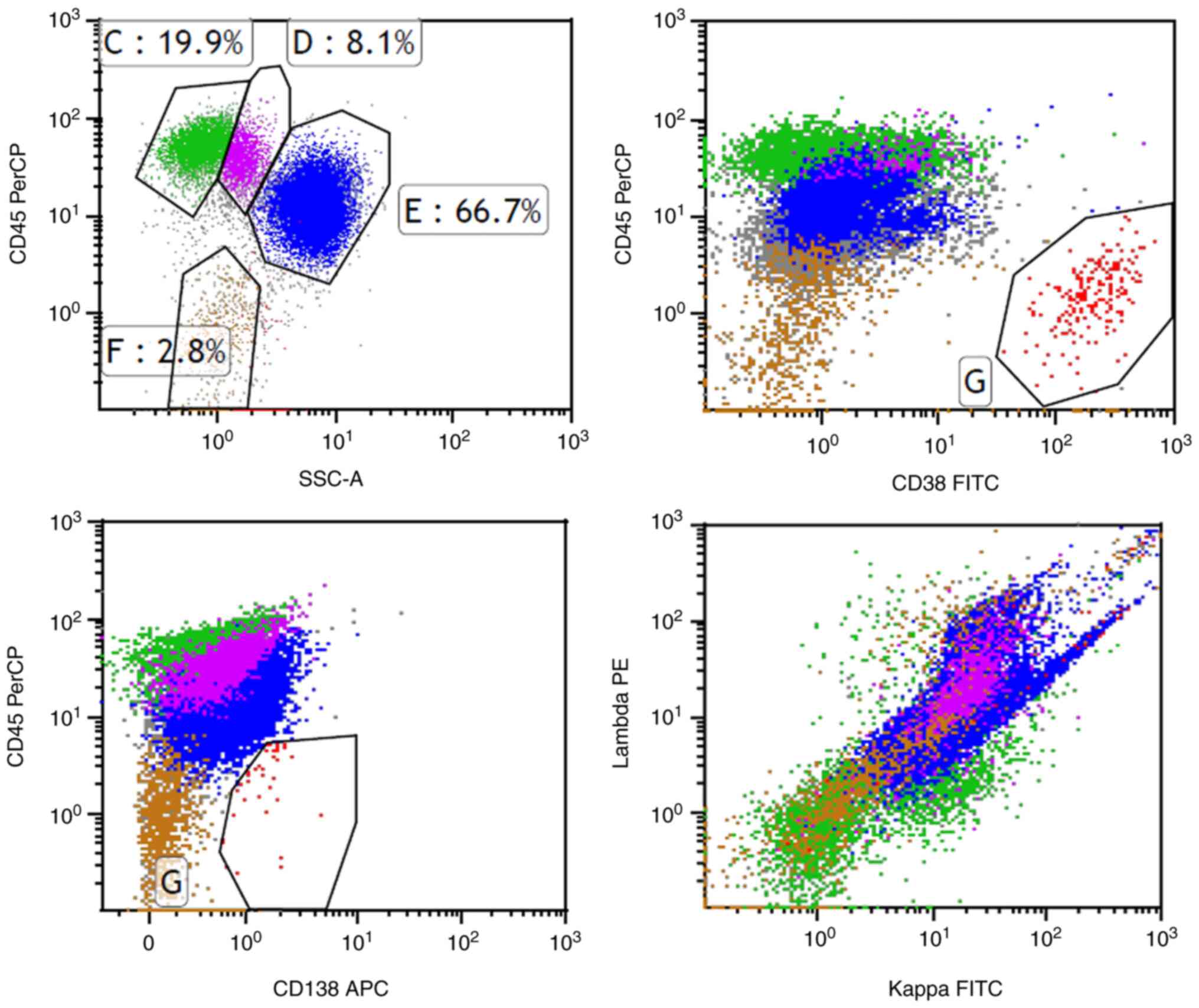
of plasma cells was 32.0%, as shown in Fig. 2. Flow cytometry revealed abnormal
clonal plasma cells that expressed CD38, CD138 and λ light chains,
and were negative for CD19, CD56, CD117 and κ light chains
(Fig. 3). A large number of
immature plasma cells were identified on bone marrow biopsy. The
results of immunohistochemistry (Fig.
4) were as follows: CD38 (3+), CD138 (3+), λ (3+), CD56 (-),
CD20 (±) and κ (-). Furthermore, the biopsy of the chest wall mass
was reviewed, and a mass of immature plasma cells was identified in
the muscle tissue. The following antibodies were ready to use and
applied on the Lumatas automated immunostainer (Fuzhou Maixin
Biotech. Co., Ltd.) with EDTA heat retrieval solution (Fuzhou
Maixin Biotech. Co., Ltd.): Anti-CD20, anti-CD79α, anti-CD138,
anti-κ, anti-λ and anti-multiple myeloma oncogene (MUM)1, and the
details are listed in Table II.
Staining intensity level was defined as follows: -, not stained; +,
weakly stained; ++, moderately stained; and +++, strongly stained.
Flow cytometry was performed with BD FACSCanto (Becton, Dickinson
and Company). The results of immunohistochemistry (Fig. 5) were as follows: CD38 (3+), CD138
(3+), λ (3+), κ (±), MUM1 (2+), CD56 (-), CD20 (-), Pax5 (-) and
CD79a (±). The results of the chromosome and MM fluorescence in
situ hybridization assays were normal.
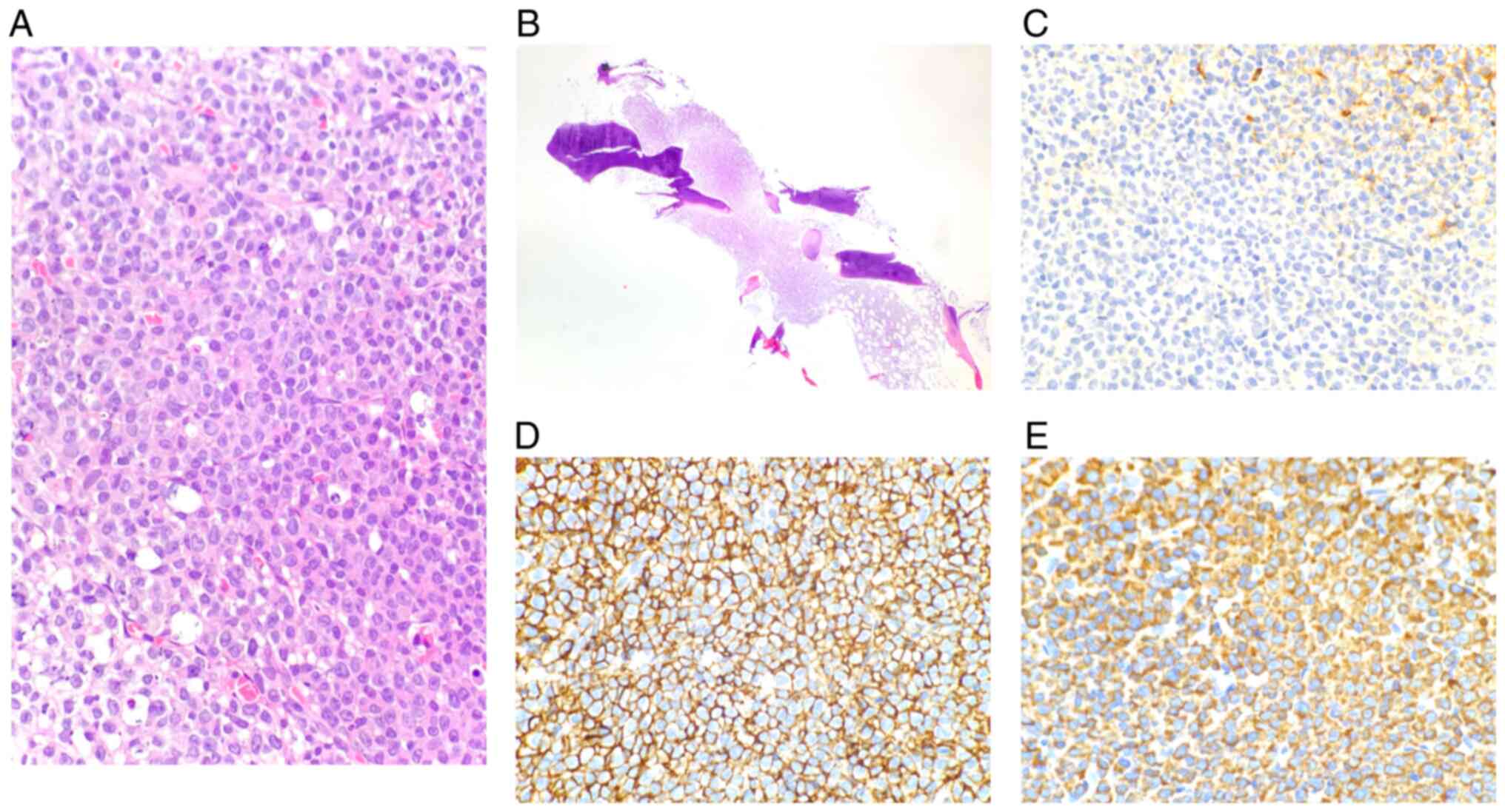
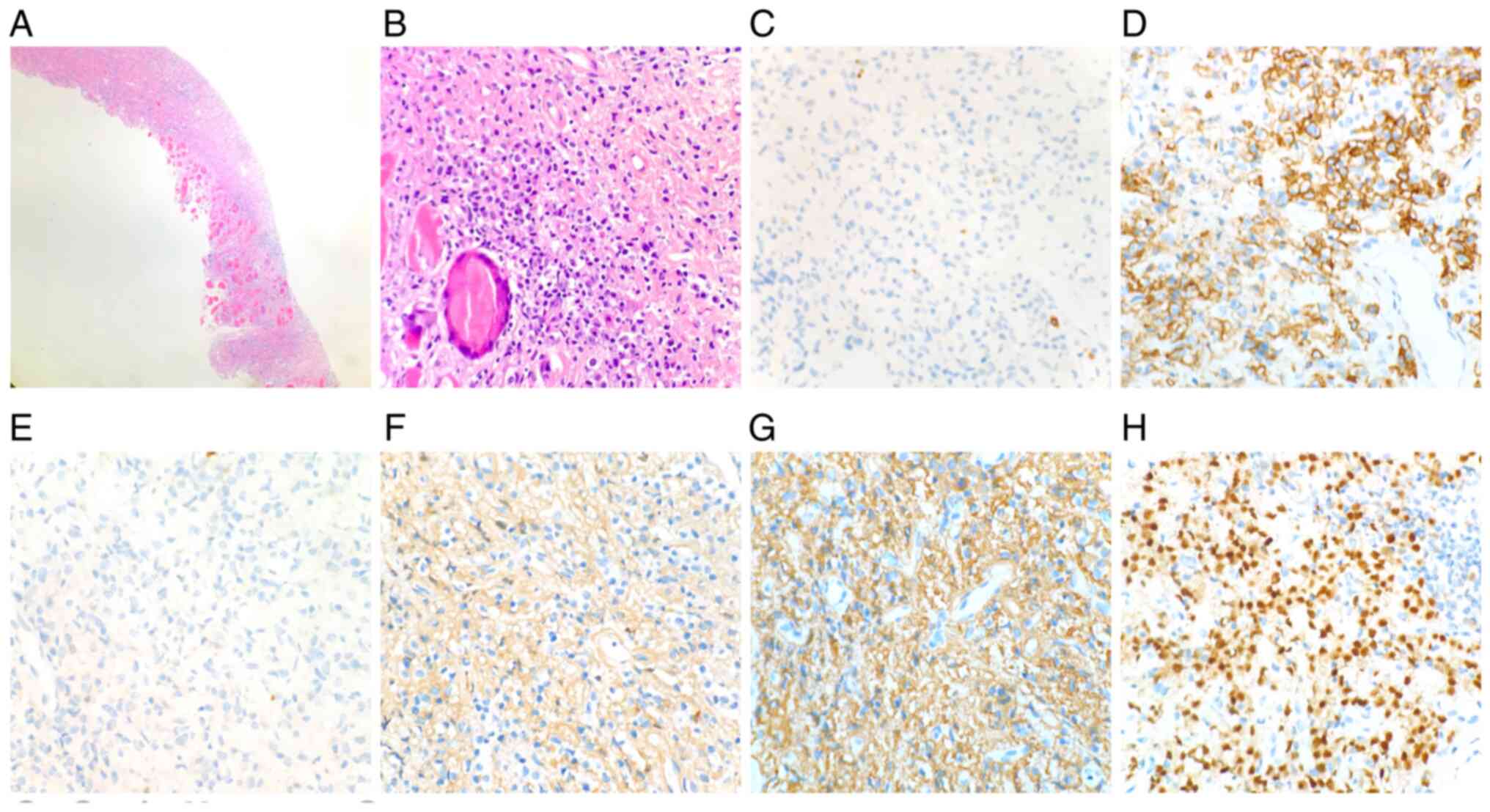 | Figure 5Chest wall mass biopsy. On
pathological examination, a mass composed of immature plasma cells
was found in muscular tissue. The plasma cells were positive for
CD38 (3+), CD138 (3+), λ (3+) and MUM1 (2+), and negative for
CD79a, CD20 and κ. (A and B) HE staining; magnification, (A) x20
and (B) x200; (C) CD20; magnification, x200; (D) CD138;
magnification, x200; (E) CD79a; magnification, x200; (F) κ;
magnification, x200; (G) λ; magnification, x200; (H) MUM1;
magnification, x200. MUM1, multiple myeloma oncogene 1. |
 | Table IBiochemical parameters at diagnosis
and at the time of recurrence. |
Table I
Biochemical parameters at diagnosis
and at the time of recurrence.
| Parameters | Diagnosis | Recurrence |
|---|
| Leukocyte count
(4-10x109/l) | 4.9 | 7.5 |
| Haemoglobin
(g/l) | 134 | 107 |
| Platelet count
(100-300x109/l) | 142 | 155 |
| Immunoglobulin (20-35
g/l) | 27.7 | 21.8 |
| Albumin (35-54
g/l) | 42 | 43 |
| Creatinine (40-135
µmol/l) | 65 | 78 |
| Calcium (2.1-2.7
µmol/l) | 2.34 | 2.2 |
| Lactate dehydrogenase
(109-245 IU/l) | 208 | 309 |
| Serum
β2-microglobulin (1.09-2.53 mg/l) | 2.5 | 2.37 |
| Bone marrow plasma
cell (%) | 32 | 1 |
| Chromosome | 46, XY | - |
| MM fluorescence in
situ hybridization | Negative | - |
 | Table IIAntibodies used for
immunohistochemistry. |
Table II
Antibodies used for
immunohistochemistry.
| Antibody | Manufacturer | Clone no. | Species | Type | Platform |
|---|
| CD20 | Roche Diagnostics
GmbH | L26 | Mouse | Monoclonal | Ventana BenchMark
XT |
| CD79a | Roche Diagnostics
GmbH | SP18 | Rabbit | Monoclonal | Ventana BenchMark
XT |
| CD138 | Fuzhou Maixin
Biotech. Co., Ltd. | MI15 | Mouse | Monoclonal | Ventana BenchMark
XT |
| κ | Fuzhou Maixin
Biotech. Co., Ltd. | RAB-0111 | Rabbit | Monoclonal | Ventana BenchMark
XT |
| λ | Fuzhou Maixin
Biotech. Co., Ltd. | LAM03 + HP6054 | Mouse | Monoclonal | Ventana BenchMark
XT |
| MUM1 | Fuzhou Maixin
Biotech. Co., Ltd. | MUM1p | Mouse | Monoclonal | Ventana BenchMark
XT |
Based on the aforementioned findings, a diagnosis of
active MM with multiple extramedullary foci, Revised International
Staging System stage I, low-risk group, was confirmed for this
patient according to the International Myeloma Working Group
criteria, version 2014 (5,6). Thus, bortezomib, lenalidomide and
dexamethasone (BRD regimen: Bortezomib 1.3 mg/m2, days
1, 4, 8 and 11; lenalidomide 25 mg/day, days 1-21; and
dexamethasone 40 mg/day, days 1-4 and 8-11) were administered to
this patient as the initial treatment. After 4 cycles of the BRD
regimen, the multiple extramedullary lesions disappeared, serum
immunofixation electrophoresis became negative, and the proportion
of plasma cells in the bone marrow decreased to 1%. Thus, the
interim efficacy assessment was CR, and 5 continuous cycles of the
BRD regimen were administered. Considering that the patient had IgD
MM with multiple extramedullary lesions at diagnosis, autologous
haematopoietic stem-cell transplantation (auto-HSCT) was
recommended several times during the treatment. However, the
patient refused to receive auto-HSCT. After 9 cycles of the BRD
regimen, the serum levels of free λ and κ light chains were 21.8
and 53.2 mg/l, respectively. Subsequently, lenalidomide (25 mg/day,
days 1-21, 28 days per cycle) was administered as maintenance
therapy.
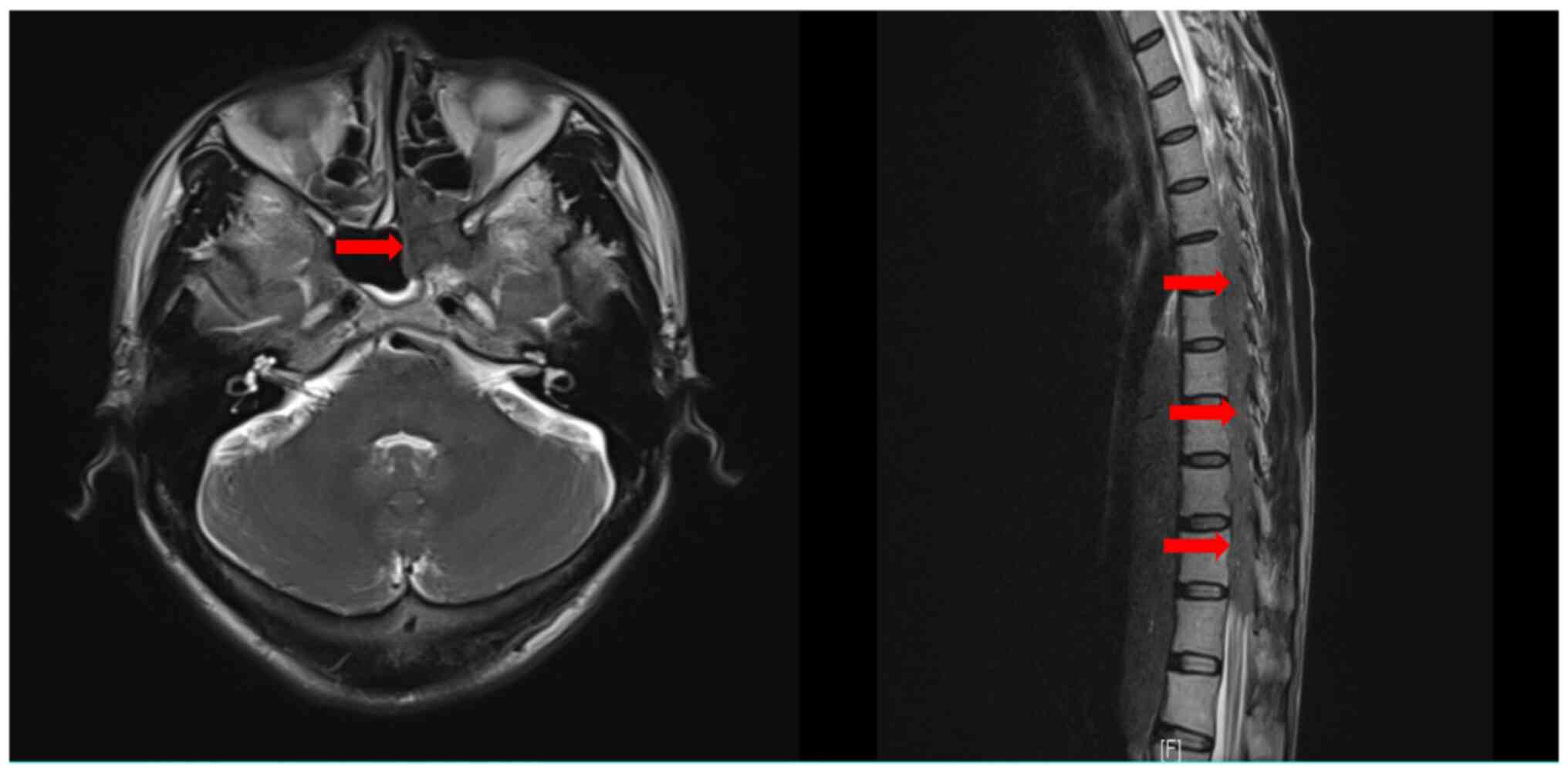
At 3 months after the last chemotherapy cycle, the
patient developed weakness of both lower limbs and urinary
incontinence. No neurological symptoms indicating cranial nerve
abnormalities were present. Contrast-enhanced MRI scans of the
brain and whole spine were immediately performed, and
space-occupying lesions in the sphenoid sinus and ethmoidal
cellules, multiple foci the T6-12 vertebral body and appendix, and
T5-11 intraspinal space-occupying lesions were identified (Fig. 6); the whole-brain contrast-enhanced
MRI scan revealed no suspicious lesions. Based on the clinical
history and imaging characteristics, MM involvement of the
aforementioned sites was considered. The results of blood tests,
biochemical detection, serum immunofixation electrophoresis and
bone marrow examination were normal (Table I). Thus, taking all these findings
into account, CNS involvement and extramedullary relapse were
considered in this case. Subsequently, the IRD regimen, which
includes ixazomib (4 mg/day, days 1, 8 and 15), lenalidomide (25
mg/day, days 1-21) and dexamethasone (40 mg/day, days 1-4 and
8-11), was administered as a salvage therapy, and radiotherapy for
extramedullary lesions was scheduled for this patient.
Unfortunately, despite the second-line treatment strategy, the
patient succumbed to the disease within 1 month after recurrence
detection.
Discussion
MM is a plasma cell neoplasia characterized by
diffuse tumour infiltration of the bone marrow, resulting in
anaemia, bone damage with hypercalcaemia, and bone pathological
fractures. Occasionally, neoplastic plasma cells acquire a
different growth pattern, generating tumour masses, which is
referred to as EMP. CNS involvement is a rare extramedullary
manifestation of MM, with a reported incidence of ~1% (7,8). MM of
the CNS is strongly associated with high-risk chromosomal
abnormalities, plasmablastic morphology and extramedullary
manifestations (9,10). Extramedullary infiltration may be
identified at the time of MM diagnosis or during disease
progression. However, it is more common in refractory disease or
during relapse.
In recent decades, the prognosis of patients with MM
has significantly improved with the introduction of
immunomodulatory (IMiD) agents, proteasome inhibitors (PIs) and
monoclonal antibodies (11,12). Currently, PIs in combination with
IMiD agents, such as those used in the BRD regimen, are considered
as the first-line treatment option for active MM. However, CNS
involvement remains a terminal event in most cases, with a median
survival of <6 months (13-15).
This may be attributed to the lack of effective intrathecal (IT)
therapy, the limited effectiveness of radiotherapy and the limited
availability of blood brain barrier (BBB)-penetrating systemic
therapies (16).
Dias et al (17) performed a retrospective cohort
study, enrolling 20 patients with a median follow-up of 33.5 months
after CNS infiltration. The median overall survival in the group
with CNS infiltration at relapse was 7.4 months, and the patients
with leptomeningeal involvement had a median overall survival of
5.8 months. Varga et al (18) reported the findings from 13 MM
patients with CNS involvement, in whom the overall treatment
outcome was poor; 1 patient responded to daratumumab-based
treatment, whereas the effectiveness of pomalidomide and marizomib
have shown some promising results.
Marizomib, a novel irreversible PI, has demonstrated
promising anti-MM activity in patients with highly refractory MM
(19). Previous studies have
demonstrated that marizomib localizes to the CNS and significantly
inhibits proteasome activity in the brain. Recent data from an
ongoing phase 1 trial on malignant glioma evaluating weekly dosing
demonstrated that marizomib is well tolerated and shows promising
antitumour activity (20). Badros
et al (21) reported the
cases of 2 patients with refractory CNS-MM who benefited from
marizomib-based therapy, providing additional evidence for the CNS
activity of this PI and underscoring the need for further
evaluation of this drug in CNS-MM.
Daratumumab is a humanized monoclonal antibody
specific for CD38 that targets tumour cells via antibody-dependent
cell-mediated cytotoxicity, complement-dependent cytotoxicity and
phagocytosis. There are currently no available data to suggest that
daratumumab can cross the BBB; however, the fact that this barrier
may become more permeable in disease states and that there is no
barrier in the meninges raises this possibility. Elhassadi et
al (22) reported that the
combined approach of craniospinal irradiation, triple IT
chemotherapy and anti-CD38 monoclonal antibody produced a durable
response in a MM patient with CNS involvement. Thus, the role of
daratumumab in this disease status deserves further evaluation.
There were some potential limitations to this case
study. First, we only had the qualitative analysis of IgD, so we
could not calculate the M protein. Second, the percentage of plasma
cells was not high and the finding of CD138 positivity was not
particularly clear on flow cytometry for bone marrow dilution.
Third, there was no imaging evaluation for extramedullary lesions
after CR. Fourth, biopsy of extramedullary foci and lumbar puncture
to examine the cerebrospinal fluid were not performed at the time
of relapse.
In conclusion, MM with CNS involvement is associated
with extremely poor survival, and sufficient assessment of EMD is
necessary during the entire course of the disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Construction Project of
Fujian Medical Center Of Hematology (grant no. Min201704), the
Fujian Provincial Key Special Projects (grant nos. 2016Y9032,
2016B041 and 2017Y4005), the Fujian Provincial Public Health
Project (grant no. WKJ2016-2-06) and the National Key Research and
Development Program of China (grant no. 2016YFC0902800).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC analyzed and interpreted the patient data and
wrote this manuscript; YQ collected the materials; HF and JL were
responsible for clinical diagnosis and treatment; LC was
responsible for pathological diagnosis; SL was responsible for
imaging diagnosis; TL was the chief physician responsible for this
case. YC, YQ and TL have seen and can confirm the authenticity of
the raw data. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present case report was approved by the local
Ethics Committee. The patient received standard treatment for his
disease, and this case report had no impact on the treatment
protocol.
Patient consent for publication
The patient's son provided informed consent for the
publication of this case report and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bhutani M, Foureau DM, Atrash S, Voorhees
PM and Usmani SZ: Extramedullary multiple myeloma. Leukemia.
34:1–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sevcikova S, Minarik J, Stork M, Jelinek
T, Pour L and Hajek R: Extramedullary disease in multiple
myeloma-controversies and future directions. Blood Rev. 36:32–39.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu J, Lu J, Chen W, Huo Y, Huang X and Hou
J: Chinese Medical Doctor Association Hematology Branch. Clinical
features and treatment outcome in newly diagnosed Chinese patients
with multiple myeloma: Results of a multicenter analysis. Blood
Cancer J. 4(e239)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International myeloma working group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised International staging system
for multiple myeloma: A report from International myeloma working
group. J Clin Oncol. 33:2863–2869. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Paludo J, Painuly U, Kumar S, Gonsalves
WI, Rajkumar V, Buadi F, Lacy MQ, Dispenzieri A, Kyle RA, Mauermann
ML, et al: Myelomatous involvement of the central nervous system.
Clin Lymphoma Myeloma Leuk. 16:644–654. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fassas AB, Muwalla F, Berryman T,
Benramdane R, Joseph L, Anaissie E, Sethi R, Desikan R, Siegel D,
Badros A, et al: Myeloma of the central nervous system: Association
with high-risk chromosomal abnormalities, plasmablastic morphology
and extramedullary manifestations. Br J Haetomal. 117:103–108.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fassas AB, Ward S, Muwalla F, Van Hemert
R, Schluterman K, Harik S and Tricot G: Myeloma of the central
nervous system: Strong association with unfavorable chromosomal
abnormalities and other high-risk disease features. Leuk Lymphoma.
45:291–300. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sonneveld P, Avet-Loiseau H, Lonial S,
Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle
RA, et al: Treatment of multiple myeloma with high-risk
cytogenetics: A consensus of the International myeloma working
group. Blood. 127:2955–2962. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Raza S, Safyan RA and Lentzsch S:
Immunomodulatory drugs (IMiDs) in multiple myeloma. Curr Cancer
Drug Targets. 17:846–857. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Scalzulli E, Grammatico S, Vozella F and
Petrucci MT: Proteasome inhibitors for the treatment of multiple
myeloma. Expert Opin Pharmacother. 19:375–386. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Usmani SZ, Heuck C, Mitchell A, Szymonifka
J, Nair B, Hoering A, Alsayed Y, Waheed S, Haider S, Restrepo A, et
al: Extramedullary disease portends poor prognosis in multiple
myeloma and is over-represented in high-risk disease even in the
era of novel agents. Haematologica. 97:1761–1767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Katodritou E, Terpos E, Kastritis E,
Delimpasis S, Symeonidis AS, Repousis P, Kyrtsonis MC, Vadikolia C,
Michalis E, Polychronidou G, et al: Lack of survival improvement
with novel anti-myeloma agents for patients with multiple myeloma
and central nervous system involvement: The Greek myeloma study
group experience. Ann Hematol. 94:2033–2042. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nieuwenhuizen L and Biesma DH: Central
nervous system myelomatosis: Review of the literature. Eur J
Haematol. 80:1–9. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Egan PA, Elder PT, Deighan WI, O'Connor
SJM and Alexander HD: Multiple myeloma with central nervous system
relapse. Haematologica. 105:1780–1790. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dias ALMS, Higashi F, Peres ALM, Cury P,
Crusoé EQ and Hungria VTM: Multiple myeloma and central nervous
system involvement: Experience of a Brazilian center. Rev Bras
Hematol Hemoter. 40:30–36. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Varga G, Mikala G, Gopcsa L, Csukly Z,
Kollai S, Balázs G, Botond T, Wohner N, Horváth L, Szombath G, et
al: Multiple myeloma of the central nervous system: 13 cases and
review of the literature. J Oncol. 2018(3970169)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Richardson PG, Zimmerman TM, Hofmeister
CC, Talpaz M, Chanan-Khan AA, Kaufman JL, Laubach JP, Chauhan D,
Jakubowiak AJ, Reich S, et al: Phase 1 study of marizomib in
relapsed or relapsed and refractory multiple myeloma: NPI-0052-101
Part 1. Blood. 127:2693–2700. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Millward M, Price T, Townsend A, Sweeney
C, Spencer A, Sukumaran S, Longenecker A, Lee L, Lay A, Sharma G,
et al: Phase 1 clinical trial of the novel proteasome inhibitor
marizomib with the histone deacetylase inhibitor vorinostat in
patients with melanoma, pancreatic and lung cancer based on in
vitro assessments of the combination. Invest New Drugs.
30:2303–2317. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Badros A, Singh Z, Dhakal B, Kwok Y,
MacLaren A, Richardson P, Trikha M and Hari P: Marizomib for
central nervous system-multiple myeloma. Br J Haetomal.
177:221–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Elhassadi E, Murphy M, Hacking D and
Farrell M: Durable treatment response of relapsing CNS plasmacytoma
using intrathecal chemotherapy, radiotherapy, and Daratumumab. Clin
Case Rep. 6:723–728. 2018.PubMed/NCBI View Article : Google Scholar
|