Introduction
World wide, thyroid cancer is found in ~5% of women
and 1% of men (1). Thyroid cancer
is frequently identified during routine physical examinations using
ultrasound imaging or fine needle aspiration biopsy (FNAB)
(2). The final diagnosis of
malignant thyroid nodules requires confirmation using histological
examination of the excised thyroid tumor via surgery or FNAB
(3). Therefore, a few methods,
including specific gene detection using PCR, have been developed to
preoperatively differentiate benign thyroid nodules (4). Thyroid cancers are divided into
papillary thyroid cancer (PTC), follicular thyroid cancer (FTC),
medullary thyroid cancer (MTC), poorly differentiated thyroid
cancer (PDTC) and anaplastic thyroid cancer based on their
histopathological characteristics and original tissue resources
(5). Normally, PTC and FTC
together can be classified as differentiated thyroid cancer (DTC),
which arises from follicular cells of the thyroid gland and has a
more favorable prognosis than other type of thyroid cancer
(6,7). By contrast, MTC, PDTC, and anaplastic
thyroid cancers (8) arise from
parafollicular cells and have a neuroendocrine origin. Treatments
for patients with thyroid cancer include surgery, radioactive
iodine-131 therapy, chemotherapy, hormone therapy and targeted
therapy (9). Surgical resection
and radioactive iodine-131 are no doubt effective therapies for
non-metastatic thyroid cancers. However, radioactive iodine-131
therapy has little benefit in the treatments of MTC, PDTC and
anaplastic thyroid cancers. Patients with unresectable nodules can
be treated with external irradiation, which relieves pain in
patients with bone metastases. The prognosis of thyroid cancer is
associated with its histopathological type and
tumor-node-metastasis (TNM) staging. Normally, the overall
prognoses of PTC and FTC are excellent. The overall 5-year survival
rate is 85% for women and 74% for men at stages I-III, respectively
(10,11). By contrast, patients with stage IV
thyroid cancer and anaplastic thyroid cancer have a poor prognoses
(11,12) because these patients with advanced
thyroid cancer or low differentiated thyroid cancer do not respond
to surgery or insensitive to radioactive iodine-131 treatment
(13). Therefore, there is an
urgent need to identify more sensitive and accurate biomarkers at
an early stage for determining prognosis in patients with thyroid
cancer.
Clinically, thyroglobulin can be used as a sensitive
biomarker for monitoring DTC and most PDTC development. If a
patient's thyroglobulin level is >0.3 ng/ml in serum after
thyroidectomy, the change of relapse ismarkedly higher (14), but serial measurement in a
shorttime limits its extensive application andanaplastic thyroid
cancer, metastatic DTC and metastatic MTC may not produce
thyroglobulin because of their poorly differentiated status. Recent
studiesshowed that circulating tumor cells (CTCs) originate from
the primary tumor and are released into the bloodstream, giving
rise to tumor metastasis (15,16).
Studies have demonstrated that CTC counts are a sensitive biomarker
to predict tumor progression andhelp make treatment decisions
(17-19).
For example, Wang et al (20) show that higher CTC levels in
patients with liver cancer are strongly correlated with early
relapse. A review by Micalizzi et al (21) reported that epithelial cells from
primary tumors can enter adjacent tissues via
epithelial-mesenchymal transition (EMT) mechanism. CTCs are
classified into epithelial, MCTC, and mixed types according to
their surface markers (22). So
far, CTC evaluation for thyroid cancer has little data to support
its use, but available data revealed a slight correlation with CTC
number (4,22,23).
Therefore, new biomarkers need to be validated. Studies revealed
that CD133, a glycoprotein encoded by the PROM1 gene
(24), is the most common marker
of cancer stem cells (CSCs) from different carcinomas (25) and may be a good biomarker for
predicting the prognosis of young patients with thyroid cancer
(26). However, the detailed
mechanisms remain to be elucidated.
The present study detected CD133 gene
expression and CTC levels in blood samples from patients with
thyroid cancer and aimed to investigate the prognostic value of
CTCs and CD133 expression inthyroid cancer.
Materials and methods
Patient samples
A total of 394 patients, including 270 cases
papillary thyroid cancer (PTC), 60 follicular thyroid cancer (FTC),
30 of medullary thyroid cancer (MTC), 15 of poorly differentiated
thyroid cancer (PDTC) and 19 of anaplastic thyroid cancer (8) as classified based on their
histopathological characteristics, were involved in the present
study between January 2018 and September 2020. Another 10 patients
without thyroid tumors were used as the negative controls. Their
age ranged between 9-82 years. Males made up 149 of the cases and
females 245 cases. All samples were collected from patients'
peripheral blood at diagnosis and before treatment. All patients
were followed up at every five months. Patients with possible
recurrence were followed up every two months. Overall survival (OS)
was calculated as the time from initial diagnosis to patient
mortality. The study protocol was approved by the ethics committee
of the Affiliated Cancer Hospital of Zhengzhou University (approval
no. 2022-KY-0009-001). Written informed consent was obtained from
all the participating patients prior to sample collection.
Characterization of CTCs using
CanPatrol and tricolor RNA-ISH methods
Characterization strategies using CTC in patients
with thyroid cancer were followed as described in the literature
(27). A total of 5 ml of
peripheral blood was collected from the patients at diagnosis and
the control participants. Each sample was then spun for 5 min at
300 x g at room temperature (RT) for 4 h after collection. The
upper plasma phase was discarded and CTCs were isolated using
CanPatrol CTC enrichment technique (SurExam Bio-Tech Co., Ltd.).
For CanPatrol CTC enrichment procedure, the above cells were mixed
with 15 ml erythrocyte lysis buffer (cat. no. 00-4333-57; Thermo
Fisher Scientific, Inc.) and incubated for 30 min at RT. Then, it
was centrifuged for 5 min at 350 x g at RT and the supernatants
discarded. Cells were fixed for 15 min with cytofix/cytoperm fix
solution (cat. no. 554722, BD Biosciences) at 4˚C and were
transferred to a filter tube with an 8 µm pore size filter membrane
for filtering with a vacuum pump. Cells were further fixed at RT
for 1 h by 4% paraformaldehyde (PFA).
Following CTC enrichment, Alexa Fluor 594 labeled
epithelial makers (EpCAM, CK8/18/19), Alexa Fluor 488 conjugated
mesenchymal markers (vimentin and twist) and nuclear makers
(4',6-diamidino-2-phenylindole, DAPI) were used to identify CTCs
along with a tri-color RNA in situ hybridization technique
(28). Briefly, the enriched CTCs
were treated with 0.1% mg/ml proteinase K to increase cell membrane
permeability. Then, capture probes were mixed for hybridization at
40˚C for 2 h and unbound probes were washed with 0.1X SSC solution.
To amplify the probe signal, the pre-amplification and the
amplification solution were added to the hybridization solution.
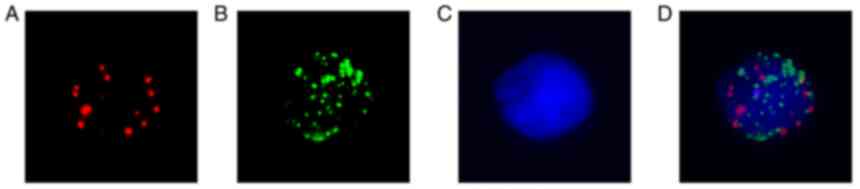
CTCs were classified into epithelial, mesenchymal and mixed types
according to the combination of their surface markers with DAPI
(Fig. 1). Following capture probes
hybridization and signal amplification, cells were stained with
DAPI and counted under a fluorescence microscope (Olympus BX53;
Olympus Corporation). Epithelial CTCs were identified with Alexa
Fluor 594 labeled epithelial makers (EpCAM/CK8/18/19) and showed
red color dots under the microscope (Fig. 1A). MCTCs were counted with Alexa
Fluor 488 conjugated mesenchymal markers (vimentin and twist)
probes and revealed green color dots under the microscope (Fig. 1B). If there were red and green
mixed dots in cells, these cells were counted as mixed CTCs
(Fig. 1D). Cell nuclear images
were marked with DAPI staining (Fig.
1C).
CTCs counting criteria
Following the above CTCs markers, different CTC
subset criteria were set up. The red dots, green dots and mixed
dots were counted at x100 magnification using different wavelengths
with an automated imaging fluorescent microscope (Carl Zeiss AG).
Positive and negative cells were counted for the three types of
CTCs in seven fields of view (DAPI positive cells) manually under
the fluorescent microscope.
CD133 expression by reverse
transcription-quantitative (RT-q) PCR
Whole peripheral blood (5 ml) was obtained from the
patients with thyroid cancer and negative control. Mononuclear
cells (MNC) were isolated via lymphocyte Ficoll separation solution
at 300 x g for 90 min at RT and cell density adjusted to
1x106/ml for total 2 ml. Next, 1 ml TRIzol (Thermo
Fisher Scientific, Inc.) was added. Total RNA was extracted using
RNeasy kit (cat. no. 74004; QiagenGmbH) and cDNA synthesis
performed using commercial reagents (cat. no. K1621; Thermo Fisher
Scientific, Inc.) following manufacture's protocol. The CD133 gene
PCR reagents were purchased from Thermo Fisher Scientific and were
performed quantitative PCR using SYBR Green Master Mix (Thermo
Fisher Scientific, Inc.). For human CD 133 and GAPDH primer
sequences were following: CD133 forward primer,
5'-AGTCGGAAACTGGCAGATAGC-3'; reverse primer:
5'-GGTAGTGTTGTACTGGGCCAAT-3'; GAPDH forward primer,
5'-GGAGCGAGATCCCTCCAAAAT-3'; GAPDH reverse primer:
5'-GGCTGTTGTCATACTTCTCATGG-3'. Human CD133 and GAPDH gene ID
#:NM_001145847 and NM_001256799 were entered into pga.mgh.harvard.edu/cgi-bin/primerbank. Thermocycling
conditions were: Denaturing 95˚C for 5 min; 95˚C for 30 sec, 56˚C
for 30 sec, 72˚C for 1.5 min, 35 cycles; 72˚C for 5 min; 4˚C for 1
h. CD133 expression was calculated by the 2-ΔΔCq method
and normalized to GAPDH (29). The
results were from three independent experiments.
Statistical analysis
The association between CTC levels and
clinicopathological profiles were evaluated by the χ2
test. CTCs levels were compared by Dunn's test following
Kruskal-Wallis test. OS was calculated as the time from initial
diagnosis to death at cut-off time using the Kaplan-Meier method
and log-rank test. CD133 expression in different thyroid cancer
subsets was performed using χ2 test. All results were
analyzed using GraphPad Prism 8 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics
The present study enrolled 394 thyroid cancer
patients with T1-4TNM stages and 10 negative controls.
Clinicopathological features of the patients are presented in
Table I. The clinical parameters
included age, sex, histology, differentiation grade and TNM stages.
Among the patients, most patients were diagnosed with papillary
thyroid cancer (PTC; 69.8%) and follicular thyroid cancer (FTC;
14.2%). In addition, patients with medullary thyroid cancer (MTC;
7.6%), poorly differentiated thyroid cancer (PDTC; 3.7%) and
anaplastic thyroid cancer (4.7%) were also included the present
study. Among the different subtypes, the number of female patients
(265; 67.3%) was almost double than that of the male patients (129;
32.7%). There were no significant differences in patient age. Tumor
sizes ranged from 0.123-33.2 cm3.
 | Table IBasic demographic and
clinicopathological characteristics. |
Table I
Basic demographic and
clinicopathological characteristics.
| Clinicopathological
characteristic | No of patients | Age | Sex, female
(%) | Tumor size range
(cm) |
|---|
| Control | 10 | 43.8 (19-75) | 67 | 2.0 (0.2-1.0) |
| MAL | 394 | 44.6 (9-82) | 66.5 | 2.0 (1.2-2.8) |
| MAL-papillary | 270 | 48.6 (9-75) | 74 | 2.3 (1.0-4.8) |
| MAL-follicular | 60 | 46.5 (15-81) | 68 | 2.3 (1.1-5.5) |
| MAL-medually | 30 | 44.5 (13-75) | 75 | 2.1 (1.0-3.0) |
| MAL-poorly
differentiated | 15 | 40.7 (15-68) | 60.3 | 2.0 (1.3-2.8) |
| MAL-anaplastic | 19 | 41.5 (15-65) | 67 | 2.5 (1.8-3.5) |
| I | 154 | 42.7 (9-82) | 64.6 | |
| II | 50 | 46.7 (13-75) | 68 | |
| III | 54 | 47.1 (15-76) | 63.7 | |
| IV | 136 | 44.9 (15-81) | 65.7 | |
Identification of CTC subtypes in
patients with thyroid cancer
Peripheral blood (5 ml) from 394 patients with
thyroid cancer and 10 healthy controls were used to identify CTC
subtypes using CanPatrol and tricolor RNA-ISH method. This method
has some advantages over the other techniques for CTC detection: i)
It can measure the frequency of relatively fewer cells; ii) it
allows for detection of multiple genes in a single CTC; and iii) it
can assess the EMT of CTCs and predict the prognosis of cancer
(30). CTCs were classified into
epithelial, mesenchymal and mixed subtypes based on their surface
markers with different immunofluorescent dye staining (Fig. 1). The data revealed that most
patients had only one type of CTC. Few epithelial CTCs were
detected in the benign control group. CTCs were found 376 out of
394 thyroid cancer patients (95.4%). All patients with MTC, PDTC
and ATC had detectable levels of CTCs.
Characteristics of CTCs in patients
with thyroid cancer
To assess the clinical significance of CTC number in
patients with different types of thyroid cancer, the total CTCs and
CTC subtypes of major differentiated thyroid cancers PTC and FTC
were compared because MTC, PDTC and ATC are undifferentiated
thyroid cancer and have a short OS. The results are shown in
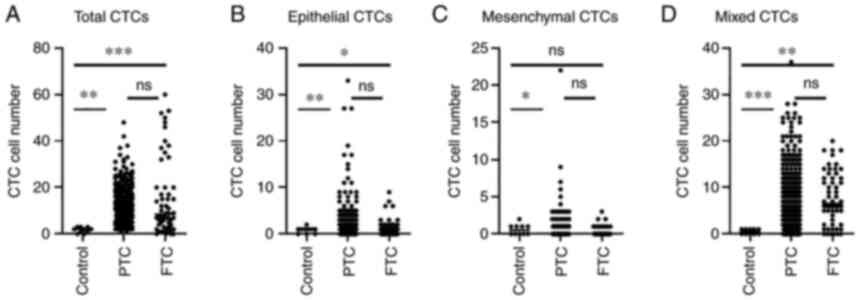
Fig. 2. Total CTCs and mixed CTCs
in either PTC or FTC are dramatically higher than those in control
(Fig. 2A, P<0.01). However,
there were no significant differences between the PTC and FTC
groups (Fig. 2A). In epithelial
CTCs (Fig. 2B), dramatically
higher than the control (P<0.01) and FTC also was markedly more
than the control (P<0.05). By contrast, MCTC (Fig. 2C), MCTC count in PTC patients were
significantly higher than those in the controls, but there was no
obvious difference between FTC patients and controls. Similar to
total CTCs, mixed CTCs (Fig. 2D)
in either the PTC or FTC group were significantly higher than those
in the control group.
Prognostic significance of CTC counts
and subtypes
The prognosis of patients with DTC is generally
excellent. Therefore, the clinical significance of CTC subtypes in
poorly differentiated thyroid cancers, such as MTC, PATC and ATC
was further investigated and followed up to 60 months for patient
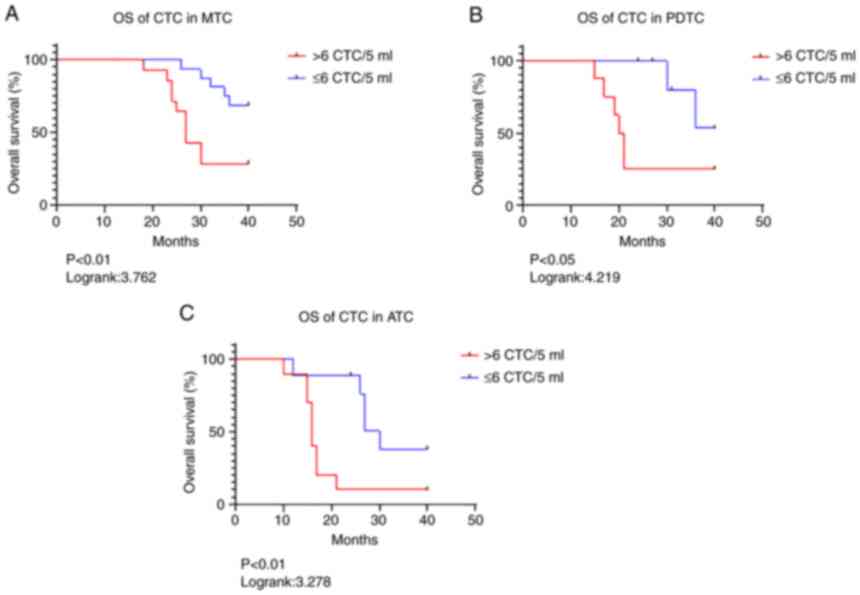
prognosis. The results are presented in Fig. 3 and Table II. The OS in the patients with MTC
(Fig. 3A); PDTC (Fig. 3B) and ATC (Fig. 3C) was investigated. In MTC
patients, OS when CTCs >6 was significantly shorter (P<0.01)
compared with patients with CTCs ≤6 according to the Kaplan-Meier's
survival curve analysis. The hazard ratio (HR) (31) and 95% confidence interval (CI) were
3.762 and 1.299 to 10.89, respectively. In the patients with PDTC,
the HRs and 95% CIs were 4.219 and 1.034 to 17.2 (P<0.05). By
contrast, HR and 95% CI were 3.278 and 1.077 to 9.8 (P<0.01) in
ATC patients (Table II). These
results indicated that high CTC numbers in patients with PDTC were
a powerful biomarker for predicting the prognosis of thyroid
cancer.
 | Table IIComparison of OS on MTC, PDTC and ATC
patients. |
Table II
Comparison of OS on MTC, PDTC and ATC
patients.
| Variables | HR | 95% CI | P-value |
|---|
| CTC in MTC >6
vs. ≤6/5 ml | 3.762 | 1.299-10.89 | <0.01 |
| CTC in PDTC >6
vs. ≤6/5 ml | 4.219 | 1.034-13.2 | <0.05 |
| CTC in ATC >6
vs. ≤6/5 ml | 3.278 | 1.077-9.8 | <0.01 |
CD133 expression is significant
relevant to thyroid cancer differentiation
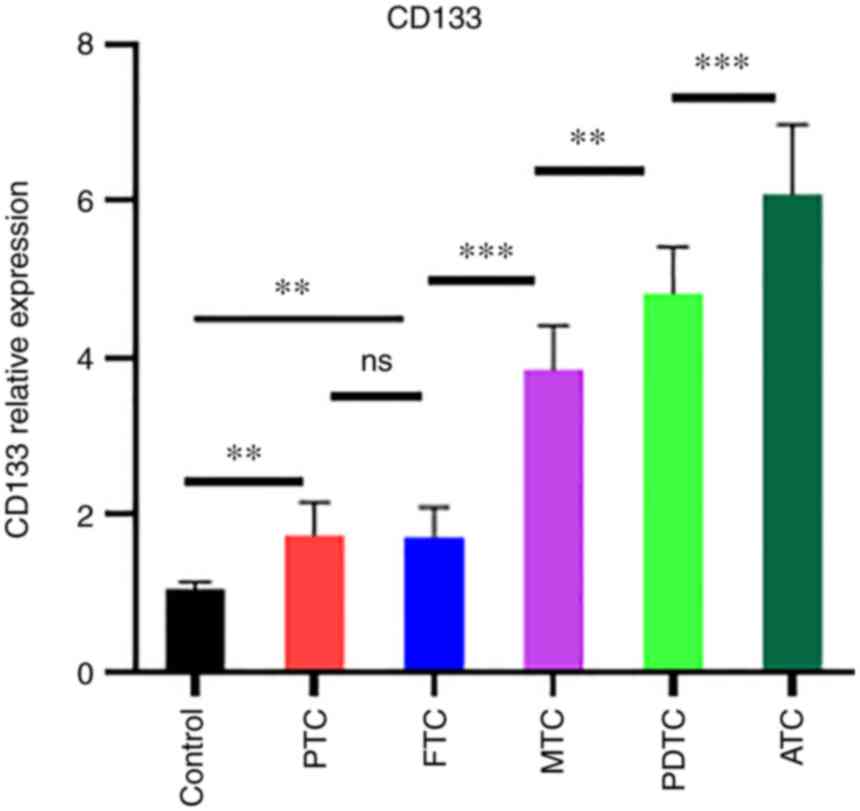
To evaluate the relationship between CD133
expression and thyroid cancer differentiation, CD133 gene
expression was measured using qPCR in the thyroid cancer subtype.
The results are shown in Fig. 4.
CD133 expression was higher in poorly differentiated cells. ATC and
PDTC showed robust expression compared to the control (P<0.001).
By contrast, PTC or FTC showed high expression compared to control
(P<0.01). Notably, CD133 expression was also significantly
higher in ATC than in PDTC (P<0.001). CD133 expression in PDTC
was higher than that in PTC and FTC (P<0.01). These results
revealed that CD133 expression is strongly associated with the
degree of thyroid cancer differentiation.
Discussion
Studies show that CTCs are strongly associated with
cancer development (21,32). A number of clinical studies have
revealed that the CTC count of the peripheral blood in patients
with advanced stages of cancer is an important guideline for
predicting patient prognosis (17-19).
A few reports have indicated that CTCs of patients with thyroid
cancer are involved in disease progression (22,23,33).
However, data investigating CTCs in patients with thyroid cancer at
early stages are limited. The present study showed that the total
CTCs and their subtypes had a significant clinical association with
the prediction of thyroid cancer prognosis.
CTCs in the blood stream can often be identified as
epithelial, mesenchymal, or both mixed types according to their
surface markers with different immunofluorescence stains (34,35).
Studies indicate that EMT marker expression in CTCs in a number of
types of cancer, such as gastric cancer, colorectal cancer,
non-small cell lung cancer, breast cancer and prostate cancer are
relevant to invasion and metastasis (36-39).
Previous studies show that CTCs in thyroid cancer can be detected
with immunochemical staining of EpCAM epithelial marker (23) or based on cell size (40) and antibody capture (41). However, these methods have low
sensitivity and are less reliable than RNA-ISH. Antibody capture
needs ≤27.5 ml peripheral blood. By contrast, the RNA-ISH has very
high sensitivity and specificity in only 5 ml peripheral blood. It
was found that if the total CTC and MCTC counts were high at
diagnosis, patients were more likely to have rapid tumor
progression. The present study also showed that if patient blood
samples had <6 MCTCs, OS of patients with thyroid cancer were
significantly longer than that of patients with >6 CTCs.
Similarly, patients with an increased MCTC percentage following
surgery relapse earlier in hepatocellular cancers (42). Finding from past studies support
that CTC monitoring identifies not only the nature of the tumor,
but also provides the underlying biology of tumor recurrence and
metastasis (43-45).
For example, the presence of CTCs is closely associated with
metastasis of small cell lung cancer (46). de Sousa e Melo et al
(47) found that a high number of
CTCs indicates relapse in patients with colorectal cancer.
Therefore, the detection of CTCs, EMT CTCs and changes in patients
with thyroid cancer may provide another predictor of recurrence
compared with conventional clinical parameters.
In addition to the clinical significance of CTCs, a
number of studies have also explored other biomarkers for the
prognosis of thyroid cancer (48-50).
Among these biomarkers, CD133 is an interesting gene because it is
involved in a number of types of cancers (51-53).
Ge et al (54) reveal that
targeting CD133 may greatly improve the prognosis of ATC. This
result shows that high CD133 expression promotes thyroid cancer
proliferation. Indeed, the present study indicated that higher
CD133 levels were strongly associated with poorly differentiated
thyroid cancer. These data confirm that CD133 is also a good
biomarker for the diagnosis and therapy of thyroid cancer.
The present study indicated that CTCs in peripheral
blood were strongly associated with OS in patients with thyroid
cancer. High levels of CTCs or MCTCs were significantly correlated
with early recurrence or metastasis. CD133 is a new biomarker for
the diagnosis and therapy of thyroid cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Henan Provincial
Commission of Health (grant no. TLHGJ20190623).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD conceived and designed this study; DL and NL
performed the experiments and analyzed the data. DL, NL and YD
drafted the manuscript. DL and YD confirm the authenticity of all
the raw data. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
All human thyroid cancer blood samples were approved
by the ethical committees of the Affiliated Cancer Hospital of
Zhengzhou University (approval no. 2022-KY-0009-001). A written
informed consent was obtained before study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tunbridge WM, Evered DC, Hall R, Appleton
D, Brewis M, Clark F, Evans JG, Young E, Bird T and Smith PA: The
spectrum of thyroid disease in a community: The Whickham survey.
Clin Endocrinol (Oxf). 7:481–493. 1977.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Haugen BR: 2015 American thyroid
association management guidelines for adult patients with thyroid
nodules and differentiated thyroid cancer: What is new and what has
changed? Cancer. 123:372–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Al-Hilli Z, Strajina V, McKenzie TJ,
Thompson GB, Farley DR and Richards ML: The role of lateral neck
ultrasound in detecting single or multiple lymph nodes in papillary
thyroid cancer. Am J Surg. 212:1147–1153. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jee HG, Kim BA, Kim M, Yu HW, Choi JY, Kim
SJ and Lee KE: Expression of SLC5A5 in circulating tumor cells may
distinguish follicular thyroid carcinomas from adenomas:
Implications for blood-based preoperative diagnosis. J Clin Med.
8(257)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chin PD, Zhu CY, Sajed DP, Fishbein GA,
Yeh MW, Leung AM and Livhits MJ: Correlation of ThyroSeq results
with surgical histopathology in cytologically indeterminate thyroid
nodules. Endocr Pathol. 31:377–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nix P, Nicolaides A and Coatesworth AP:
Thyroid cancer review 2: Management of differentiated thyroid
cancers. Int J Clin Pract. 59:1459–1463. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nix PA, Nicolaides A and Coatesworth AP:
Thyroid cancer review 3: Management of medullary and
undifferentiated thyroid cancer. Int J Clin Pract. 60:80–84.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Executive summary: Heart disease and stroke statistics-2012 update:
A report from the American Heart Association. Circulation.
125:188–197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lamartina L, Grani G, Durante C and
Filetti S: Recent advances in managing differentiated thyroid
cancer. F1000Res. 7(86)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Patrone R, Velotti N, Masone S, Conzo A,
Flagiello L, Cacciatore C, Filardo M, Granata V, Izzo F, Testa D,
et al: Management of low-risk thyroid cancers: Is active
surveillance a valid option? A systematic review of the literature.
J Clin Med. 10(3569)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Capdevila J, Galofre JC, Grande E, Zafon
Llopis C, Ramón Y, Cajal Asensio T, Navarro Gonzalez E,
Jiménez-Fonseca P, Santamaría Sandi J, Gómez Sáez JM and Riesco
Eizaguirre G: Consensus on the management of advanced radioactive
iodine-refractory differentiated thyroid cancer on behalf of the
Spanish society of endocrinology thyroid cancer working group
(GTSEEN) and Spanish rare cancer working group (GETHI). Clin Transl
Oncol. 19:279–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barbet J, Campion L, Kraeber-Bodere F and
Chatal JF: Group GTES. Prognostic impact of serum calcitonin and
carcinoembryonic antigen doubling-times in patients with medullary
thyroid carcinoma. J Clin Endocrinol Metab. 90:6077–6084.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Perros P, Boelaert K, Colley S, Evans C,
Evans RM, Gerrard Ba G, Gilbert J, Harrison B, Johnson SJ, Giles
TE, et al: Guidelines for the management of thyroid cancer. Clin
Endocrinol (Oxf). 81 (Suppl 1):S1–S122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miyauchi A, Kudo T, Miya A, Kobayashi K,
Ito Y, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Inoue H,
et al: Prognostic impact of serum thyroglobulin doubling-time under
thyrotropin suppression in patients with papillary thyroid
carcinoma who underwent total thyroidectomy. Thyroid. 21:707–716.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aya-Bonilla CA, Morici M, Hong X, McEvoy
AC, Sullivan RJ, Freeman J, Calapre L, Khattak MA, Meniawy T,
Millward M, et al: Detection and prognostic role of heterogeneous
populations of melanoma circulating tumour cells. Br J Cancer.
122:1059–1067. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sawabata N, Nakamura T, Kawaguchi T,
Watanabe T, Ouji NS, Ito T and Taniguchi S: Circulating tumor cells
detected only after surgery for non-small cell lung cancer: Is it a
predictor of recurrence? J Thorac Dis. 12:4623–4632.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hayes DF, Cristofanilli M, Budd GT, Ellis
MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV and
Terstappen LW: Circulating tumor cells at each follow-up time point
during therapy of metastatic breast cancer patients predict
progression-free and overall survival. Clin Cancer Res.
12:4218–4224. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang PX, Xu Y, Sun YF, Cheng JW, Zhou KQ,
Wu SY, Hu B, Zhang ZF, Guo W, Cao Y, et al: Detection of
circulating tumor cells enables early recurrence prediction in
hepatocellular carcinoma patients undergoing liver transplantation.
Liver Int. 41:562–573. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Micalizzi DS, Haber DA and Maheswaran S:
Cancer metastasis through the prism of epithelial-to-mesenchymal
transition in circulating tumor cells. Mol Oncol. 11:770–780.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ehlers M, Allelein S, Schwarz F, Hautzel
H, Kuebart A, Schmidt M, Haase M, Dringenberg T and Schott M:
Increased numbers of circulating tumor cells in thyroid cancer
patients. Horm Metab Res. 50:602–608. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xu JY, Handy B, Michaelis CL, Waguespack
SG, Hu MI, Busaidy N, Jimenez C, Cabanillas ME, Fritsche HA Jr,
Cote GJ and Sherman SI: Detection and prognostic significance of
circulating tumor cells in patients with metastatic thyroid cancer.
J Clin Endocrinol Metab. 101:4461–4467. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Corbeil D, Fargeas CA and Huttner WB: Rat
prominin, like its mouse and human orthologues, is a pentaspan
membrane glycoprotein. Biochem Biophys Res Commun. 285:939–944.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Baker M: Cancer stem cells tracked.
Nature. 488:13–14. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Decaussin-Petrucci M, Deladoey J,
Hafdi-Nejjari Z, Sassolas G, Borson-Chazot F, Abu-Khudir R, Fusco
A, Descotes F, Cournoyer S and Sartelet H: group of Pathologists of
the Rhône Alpes Region. Expression of CD133 in differentiated
thyroid cancer of young patients. J Clin Pathol. 68:434–440.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang ZL, Zhang P, Li HC, Yang XJ, Zhang
YP, Li ZL, Xue L, Xue YQ, Li HL, Chen Q and Chong T: Dynamic
changes of different phenotypic and genetic circulating tumor cells
as a biomarker for evaluating the prognosis of RCC. Cancer Biol
Ther. 20:505–512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li J, Liao Y, Ran Y, Wang G, Wu W, Qiu Y,
Liu J, Wen N, Jing T, Wang H and Zhang S: Evaluation of sensitivity
and specificity of CanPatrol™ technology for detection of
circulating tumor cells in patients with non-small cell lung
cancer. BMC Pulm Med. 20(274)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hassan S, Blick T, Williams ED and
Thompson EW: Applications of RNA from circulating tumor cells.
Front Biosci (Landmark Ed). 25:874–892. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Syahrani RA, Yunita E and Wanandi SI:
Suppression of rotenone-treated human breast cancer stem cell
survival using survivin inhibitor YM155 is associated to oxidative
stress modulation. Asian Pac J Cancer Prev. 21:2631–2637.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moon DH, Lindsay DP, Hong S and Wang AZ:
Clinical indications for, and the future of, circulating tumor
cells. Adv Drug Deliv Rev. 125:143–150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Qiu ZL, Wei WJ, Sun ZK, Shen CT, Song HJ,
Zhang XY, Zhang GQ, Chen XY and Luo QY: Circulating tumor cells
correlate with clinicopathological features and outcomes in
differentiated thyroid cancer. Cell Physiol Biochem. 48:718–730.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Topa J, Gresner P, Zaczek AJ and
Markiewicz A: Breast cancer circulating tumor cells with
mesenchymal features-an unreachable target? Cell Mol Life Sci.
79(81)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Russo GI, Musso N, Romano A, Caruso G,
Petralia S, Lanzano L, Broggi G and Camarda M: The role of
dielectrophoresis for cancer diagnosis and prognosis. Cancers
(Basel). 14(198)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Satelli A, Batth I, Brownlee Z, Mitra A,
Zhou S, Noh H, Rojas CR, Li H, Meng QH and Li S: EMT circulating
tumor cells detected by cell-surface vimentin are associated with
prostate cancer progression. Oncotarget. 8:49329–49337.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hyun KA, Koo GB, Han H, Sohn J, Choi W,
Kim SI, Jung HI and Kim YS: Epithelial-to-mesenchymal transition
leads to loss of EpCAM and different physical properties in
circulating tumor cells from metastatic breast cancer. Oncotarget.
7:24677–24687. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li TT, Liu H, Li FP, Hu YF, Mou TY, Lin T,
Yu J, Zheng L and Li GX: Evaluation of epithelial-mesenchymal
transitioned circulating tumor cells in patients with resectable
gastric cancer: Relevance to therapy response. World J
Gastroenterol. 21:13259–13267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang Y, Liu Y, Zhang L, Tong L, Gao Y, Hu
F, Lin PP, Li B and Zhang T: Vimentin expression in circulating
tumor cells (CTCs) associated with liver metastases predicts poor
progression-free survival in patients with advanced lung cancer. J
Cancer Res Clin Oncol. 145:2911–2920. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vona G, Sabile A, Louha M, Sitruk V,
Romana S, Schutze K, Capron F, Franco D, Pazzagli M, Vekemans M, et
al: Isolation by size of epithelial tumor cells: A new method for
the immunomorphological and molecular characterization of
circulatingtumor cells. Am J Pathol. 156:57–63. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Riethdorf S, Fritsche H, Muller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qi LN, Xiang BD, Wu FX, Ye JZ, Zhong JH,
Wang YY, Chen YY, Chen ZS, Ma L, Chen J, et al: Circulating tumor
cells undergoing EMT provide a metric for diagnosis and prognosis
of patients with hepatocellular carcinoma. Cancer Res.
78:4731–4744. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Marcuello M, Vymetalkova V, Neves RPL,
Duran-Sanchon S, Vedeld HM, Tham E, van Dalum G, Flügen G,
Garcia-Barberan V, Fijneman RJ, et al: Circulating biomarkers for
early detection and clinical management of colorectal cancer. Mol
Aspects Med. 69:107–122. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Allard WJ and Terstappen LW: CCR 20th
anniversary commentary: Paving the way for circulating tumor cells.
Clin Cancer Res. 21:2883–2885. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tieng FYF, Baharudin R, Abu N, Mohd Yunos
RI, Lee LH and Ab Mutalib NS: Single cell transcriptome in
colorectal cancer-current updates on its application in metastasis,
chemoresistance and the roles of circulating tumor cells. Front
Pharmacol. 11(135)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
de Sousa e Melo F, Kurtova AV, Harnoss JM,
Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z,
Koeppen H, et al: A distinct role for Lgr5(+) stem cells in primary
and metastatic colon cancer. Nature. 543:676–680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Cefalu AB, Giammanco A, Noto D, Spina R,
Cabibi D, Barbagallo CM and Averna M: Effectiveness and safety of
lomitapide in a patient with familial chylomicronemia syndrome.
Endocrine. 71:344–350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Proskurnina EV, Fedorova MV, Sozarukova
MM, Mitichkin AE, Panteleev IV and Svetlov EV: Microsomal reductase
activity in patients with thyroid neoplasms. Endocrine. 72:735–743.
2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zou X, Gao F, Wang ZY, Zhang H, Liu QX,
Jiang L, Zhou X and Zhu W: A three-microRNA panel in serum as novel
biomarker for papillary thyroid carcinoma diagnosis. Chin Med J
(Engl). 133:2543–2551. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Riegg F, Lutz MS, Schmied BJ, Heitmann JS,
Queudeville M, Lang P, Jung G, Salih HR and Märklin M: An
Fc-Optimized CD133 antibody for induction of NK cell reactivity
against B cell acute lymphoblastic leukemia. Cancers (Basel).
13(1632)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu Y, Yao X, Wang C, Wang M, Wang Y, Ye M
and Liu Y: Peptide-based 68Ga-PET radiotracer for imaging CD133
expression in colorectal cancer. Nucl Med Commun. 42:1144–1150.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Acikgoz E, Mukhtarova G, Alpay A, Avci CB,
Bagca BG and Oktem G: Sonic hedgehog signaling is associated with
resistance to zoledronic acid in CD133high/CD44high prostate cancer
stem cells. Mol Biol Rep. 48:3567–3578. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ge MH, Zhu XH, Shao YM, Wang C, Huang P,
Wang Y, Jiang Y, Maimaitiyiming Y, Chen E, Yang C and Naranmandura
H: Synthesis and characterization of CD133 targeted aptamer-drug
conjugates for precision therapy of anaplastic thyroid cancer.
Biomater Sci. 9:1313–1324. 2021.PubMed/NCBI View Article : Google Scholar
|