Introduction
Lung cancer is the most common cause of cancer
mortality in the world, and non-small cell lung cancer accounts for
approximately 80% of all lung cancers. Non-small lung cancer
predominantly comprises adenocarcinomas and squamous cell
carcinomas (1–3). Despite advances in multimodality
treatment, including surgical management, chemotherapy,
radiotherapy and biological therapy, the overall 5-year survival
rate of lung cancer in many countries is less than 15% (4). In addition, metastasis is one of the
reasons for the lower survival rate following the radical resection
of lung cancers (5). Cancer
metastasis is a complex, multistep process that involves cell
adhesion, invasion and migration, proliferation and vessel
formation (6,7). Therefore, the prevention or
inhibition of lung cancer metastasis has important clinical
applications for prolonging life and enhancing the quality of life
of patients.
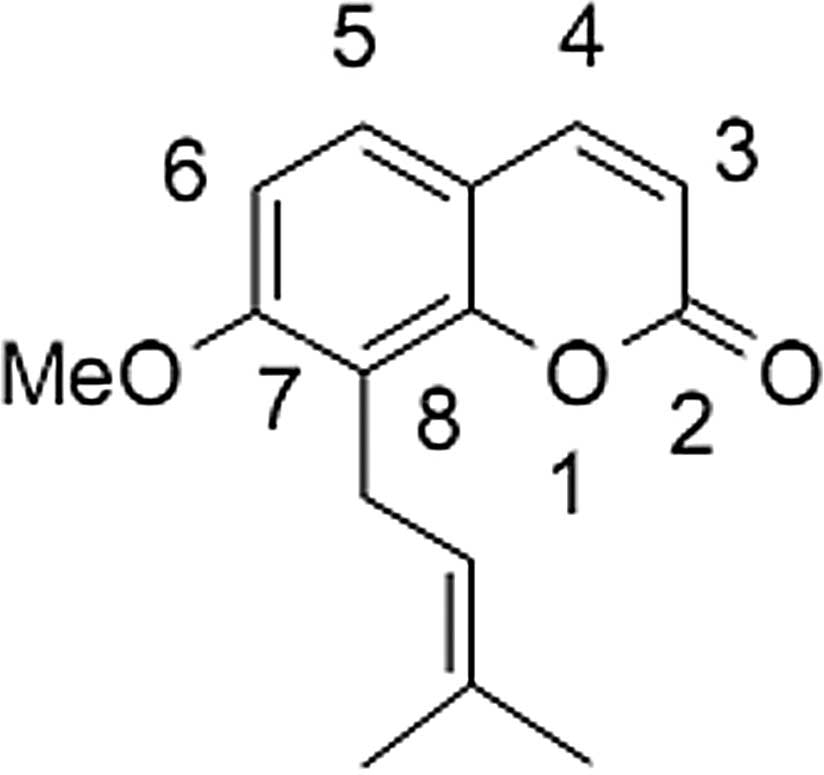
Osthole, 7-methoxy-8-(3-methyl-2-butenyl)coumarin
(Fig. 1), a natural compound, may
be extracted from Cnidium monnieri and other medicinal
plants. Previous studies have revealed that osthole has
antiproliferative (8),
vasorelaxant (9),
anti-inflammatory (10),
antimicrobacterial (11) and
antiallergic (12) properties.
Furthermore, the anticancer effect of osthole has been described.
It has been reported that osthole is able to abrogate HGF-induced
cell scattering, migration and invasion in MCF-7 breast cancer
cells (13). Osthole is also
capable of inducing apoptosis in HeLa cells and HL-60 leukemia
cells (14,15).
In a previous study, we reported that osthole
induces G2/M arrest and apoptosis in A549 human lung cancer cells
by modulating the PI3K/Akt pathway (16). However, the effects of osthole on
the migration and invasion of human lung cancer cells remain
unclear. The purpose of the current study was to investigate the
effects of osthole on the induction of migration and invasion in
A549 human lung cancer cells. We also aimed to investigate whether
the effects of osthole on the migration and invasion of A549 cells
were mediated through the inhibition of matrix metalloproteinase-2
(MMP-2) and matrix metallopeptidase-9 (MMP-9). The findings should
indicate whether osthole has the ability to inhibit the metastasis
of human lung cancer.
Materials and methods
Reagents and chemicals
Osthole was purchased from the National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China). A 50 mM stock solution of osthole was dissolved in dimethyl
sulfoxide (DMSO) and stored at −20°C. RPMI-1640, trypsin,
penicillin and streptomycin were purchased from Biological
Industries (Kibbutz Beit Haemek, Israel). Fetal bovine serum (FBS)
and Giemsa were purchased from Solarbio Science and Technology
(Beijing, China).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Matrigel and antibodies were purchased from BD Biosciences (San
Jose, CA, USA). All other reagents were procured locally.
Cell culture
The A549 human lung cancer cell line was obtained
from the China Center for Type Culture Collection (Wuhan, China)
and maintained in RPMI-1640 supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
MTT assay
The proliferation of A549 cells following treatment
with osthole was measured using the MTT assay. Briefly, A549 cells
were plated at a density of 1×104 cells/well in 96 well
plates overnight and then treated with various concentrations of
osthole (0, 20, 40, 60 and 80 μmol/l). Following a 24 h treatment,
20 μl MTT solution (2 mg/ml in PBS) was added to each well and the
cells were cultured for another 4 h at 37°C. The medium was then
totally removed and 150 μl DMSO was added to solubilize the MTT
formazan crystals. Finally, the plates were shaken and the optical
density was determined at 570 nm (OD570) using an ELISA plate
reader (Model 550, Bio-Rad, Hercules, CA, USA). At least three
independent experiments were performed.
Cell migration assay
For the cell migration assay, Transwell chambers
were used. Briefly, A549 cells (1×105 cells/well) were
placed in the upper chambers of 8 μm Transwells and treated with
various concentrations of osthole (0, 40 and 80 μmol/l). The bottom
chambers of the Transwells were filled with 0.6 ml RPMI-1640 with
10% FBS as a chemoattractant. After 24 h, non-migratory cells were
carefully removed with a cotton swab. The filter membrane was fixed
with cold methanol and acetic acid (3/1, v/v) for 30 min, then
stained with Giemsa. Images were captured using an Olympus inverted
microscope using ×200 magnification and cell migration was
quantified by counting the number of cells in 5 random fields. The
percentage inhibition of migratory cells was quantified and
expressed in relation to the untreated control cells. All
experiments were repeated three times.
Cell invasion assay
The invasion assay was performed using the same
Transwells as were used in the migration assay. Briefly, A549 cells
(1×105 cells/well) were placed in the upper chambers of
matrigel-coated 8 μm Transwells and treated with various
concentrations of osthole (0, 40 and 80 μmol/l). The bottom
chambers of the Transwells were filled with 0.6 ml RPMI-1640 with
10% FBS as a chemoattractant. Following incubation for 24 h,
non-invading cells were carefully removed with a cotton swab. Cells
that had penetrated through the matrigel located on the underside
of the filter were fixed with cold methanol and acetic acid (3/1,
v/v) for 30 min, then stained with Giemsa. The degree of
invasiveness was quantified by counting the number of cells in 5
random fields. All experiments were repeated three times.
Western blot analysis
The expression of cellular proteins was evaluated by
western blotting. A549 cells were plated onto 6 well plates and
starved overnight, then treated with various concentrations of
osthole (0, 40 and 80 μmol/l). Following treatment for 24 h, the
total proteins were solubilized and extracted with lysis buffer (20
mM HEPES, pH 7.9, 20% glycerol, 200 mM KCl, 0.5 mM EDTA, 0.5%
NP-40, 0.5 mM DTT and 1% protease inhibitor cocktail). Protein
concentrations were determined by bicinchoninic acid (BCA) protein
assay. All samples were separated by SDS-PAGE to determine the
proteins associated with cell invasion and migration, MMP-2 and
MMP-9.
Statistical analysis
All experiments were conducted three times. Data
were expressed as the mean ± SD. Statistical correlation of data
was checked for significance by ANOVA and the Student’s t test.
P<0.05 was considered to indicate a statistically significance
result. The analyses were performed using SPSS 13.0 software.
Results
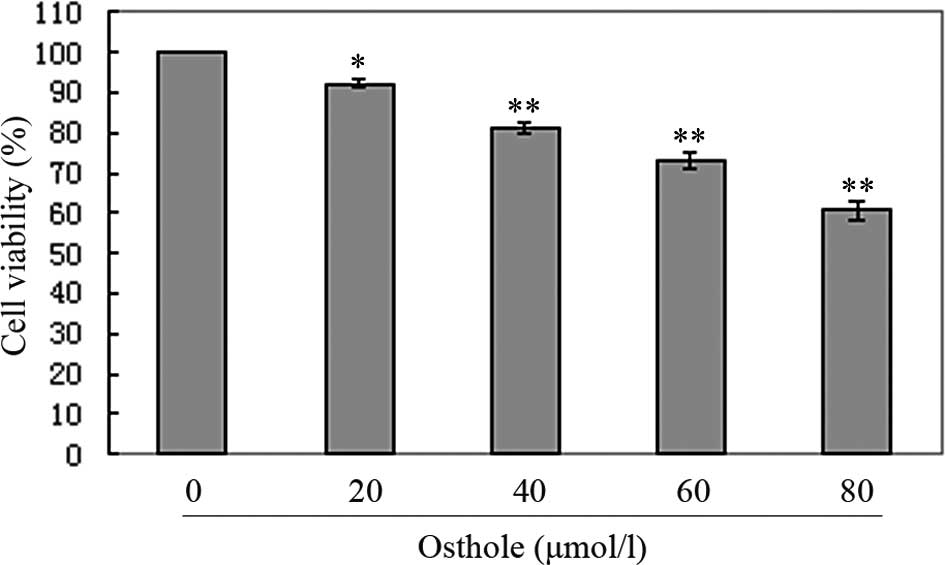
Osthole inhibited A549 cell
proliferation
In order to investigate the growth inhibitory
effects of osthole, A549 cells were treated with various
concentrations of osthole for 24 h and the rate of inhibition was
determined by MTT assay. We observed that the growth of the A549
cells was suppressed in a dose-dependent manner (Fig. 2).
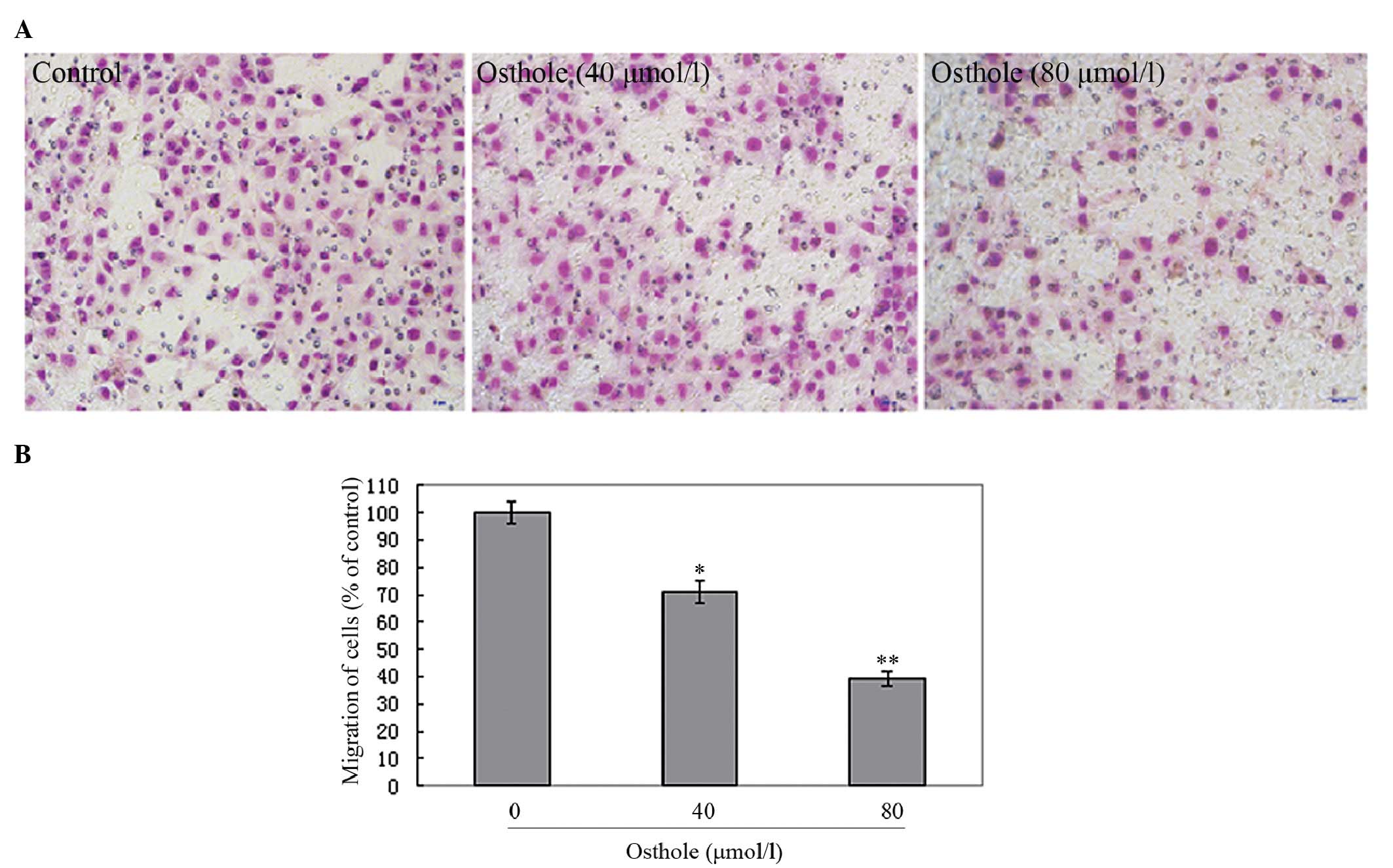
Osthole inhibited the migration and
invasion of A549 cells in vitro
Transwell assays were performed to investigate the
effects of osthole on lung cancer cell migration and invasion. A549
cells were treated with various concentrations of osthole (0, 40
and 80 μmol/l) in order to perform the Transwell migration and
matrigel-based Transwell invasion assays. As shown in Fig. 3, the A549 cells migrated from the
upper to the lower chamber and this was inhibited by osthole. As
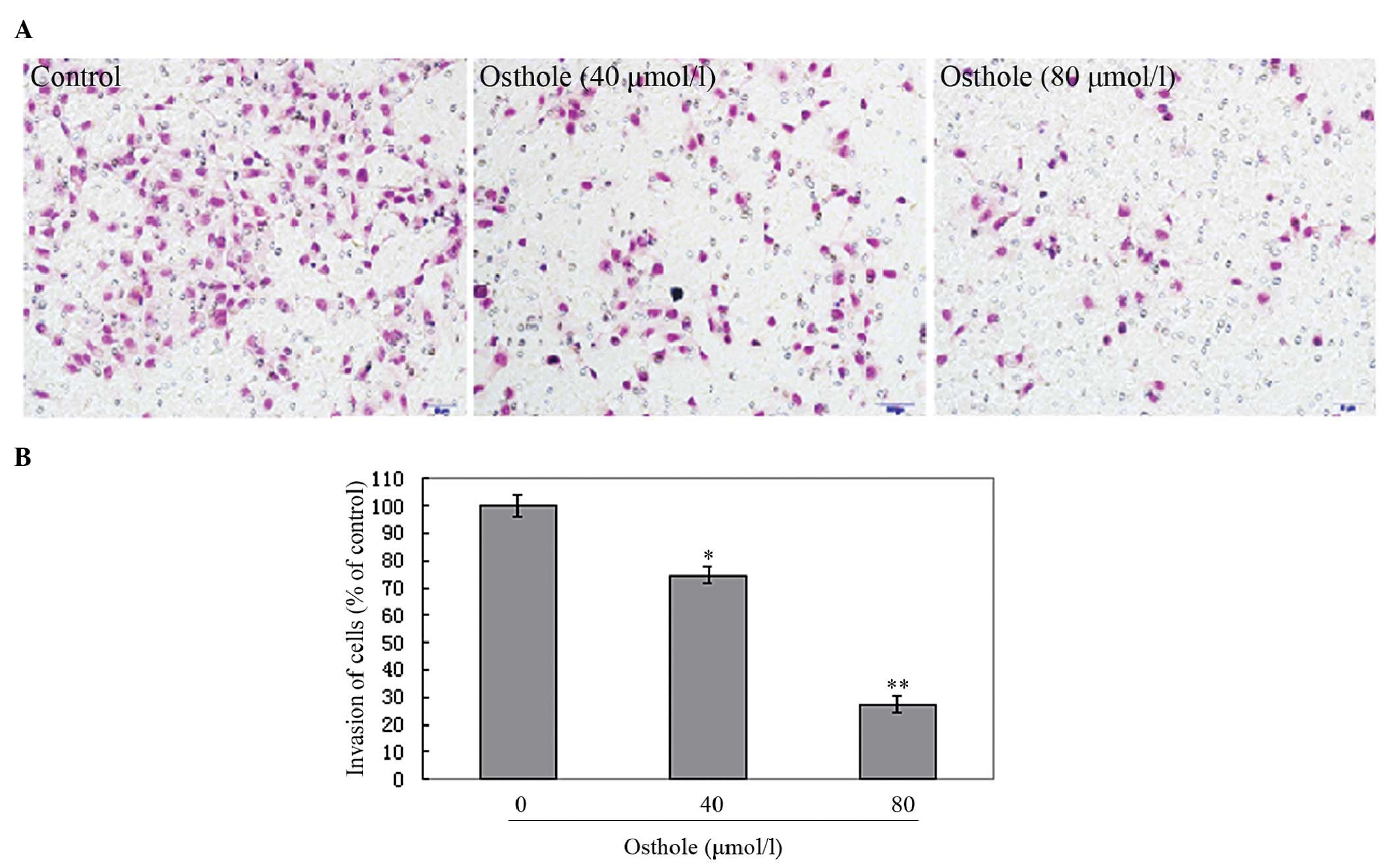
shown in Fig. 4, the penetration
of the A549 cells through the matrigel to the lower surface of the
filter was also inhibited by osthole. These inhibitory effects were
higher at an osthole concentration of 80 μmol/l than of 40 μmol/l.
Our results indicate that osthole significantly inhibits lung
cancer cell migration and invasion in a dose-dependent manner,
suggesting a crucial role for osthole in the suppression of lung
cancer metastasis.
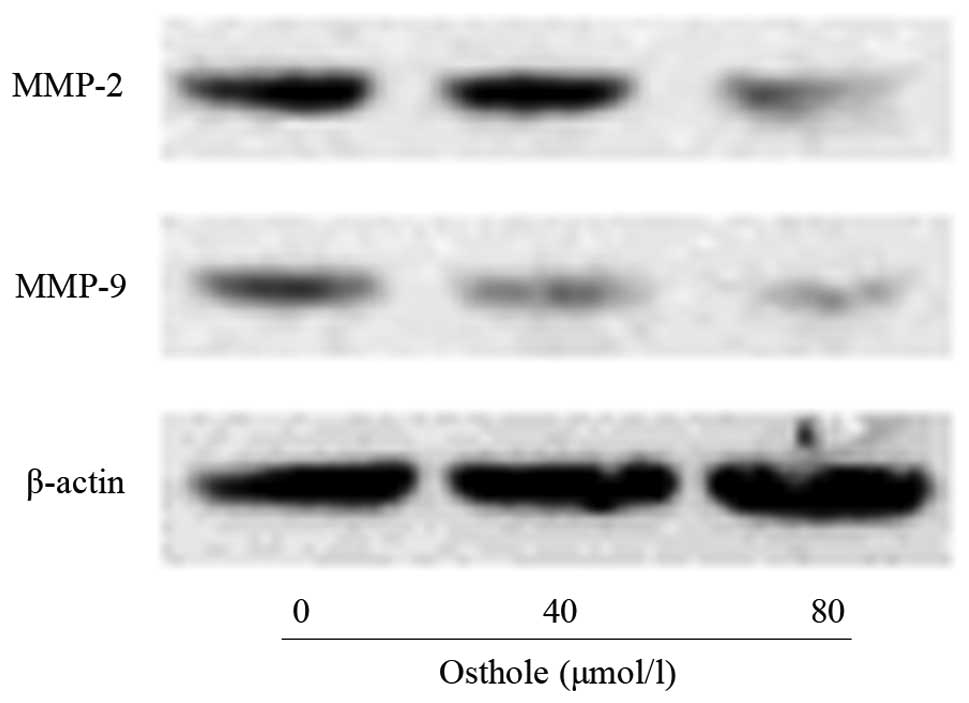
Osthole inhibited levels of MMP-2 and
MMP-9 in A549 cells
The levels of migration- and invasion-associated
proteins during the treatment with osthole were examined by western
blotting. As shown in Fig. 5, the
levels of MMP-2 and MMP-9 in the osthole-treated cells were lower
than those in the control cells. MMP-2 and MMP-9 are significant in
lung cancer cell invasion and migration. The inhibitory effects on
MMP-2 and MMP-9 may be responsible for the inhibition of the
invasion and migration of A549 cells following exposure to
osthole.
Discussion
The anticancer effects of osthole have been well
documented in numerous types of human cancers (13–15).
However, the actions of osthole on the migration and invasion of
A549 lung cancer cells and the associated mechanisms have not been
reported. In the current study, we investigated the effects of
osthole on the migration and invasion of A549 cells by Transwell
assays and western blot analyses. Our results indicate that osthole
inhibited the migration and invasion of the A549 cells and that
these effects were dose-dependent. Moreover, the results from the
western blot analyses revealed that the mechanism underlying these
effects was related to the inhibition of the expression of MMP-2
and MMP-9 in the A549 cells.
Metastasis, the most common cause of treatment
failure and death in cancer patients, is a complex biological
process in the later stages of cancer progression (17,18).
At present, there are no effective therapeutic drugs that are able
to specifically treat cancer metastasis, and little is known
concerning the molecular mechanisms that regulate the process of
metastasis (19,20). Several studies have shown that
metastasis is associated with the ability of cells to migrate and
invade, and that the inhibition of cell migration and invasion may
decrease metastasis (21–23). Therefore, the discovery of drugs
that are able to inhibit cancer cell migration and invasion is
important for the prevention and treatment of metastasis in lung
cancer. In the current study, Transwell migration and matrigel
Transwell invasion assays revealed that osthole clearly inhibited
the migration and invasion of cells in a concentration-dependent
manner. Osthole may have the ability to inhibit the metastasis of
human lung cancer.
MMPs comprise a rapidly growing family of
structurally related endopeptidases capable of degrading all known
components of the extracellular matrix (ECM). Among MMPs, MMP-2 and
MMP-9 are vital in the degradation of the ECM due to their
substrate specificity toward type IV collagen, the major component
of basement membranes (24,25).
High expression levels of MMP-2 and MMP-9 have frequently been
correlated with increased cancer metastasis in lung cancer
(26,27). To further elucidate the mechanisms
by which osthole inhibits the migration and invasion of human lung
cancer cells, we investigated the effects of osthole on MMP-2 and
MMP-9 in A549 cells. In our experiment, treatment with osthole
decreased the expression levels of MMP-2 and MMP-9 in a
dose-dependent manner. Our results revealed that osthole inhibited
the levels of MMP-2 and MMP-9 involved in the migration and
invasion in A549 cells, which is in agreement with reports that
osthole inhibited the migration and invasion of breast cancer cells
via inhibition of the expression of MMP-2 (28).
In conclusion, our data indicate for the first time
that osthole inhibits the migration and invasion of A549 human lung
cancer cells by inhibiting the expression of MMP-2 and MMP-9.
Osthole should be considered as a possible therapeutic agent for
inhibiting the metastasis of lung cancer. Further investigations
will be required to assess the potential of osthole in the
treatment of cancer.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008.
|
|
3
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.
|
|
4
|
Erridge SC, Møller H, Price A and Brewster
D: International comparisons of survival from lung cancer: pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007.
|
|
5
|
Wang T, Nelson RA, Bogardus A and Grannis
FW Jr: Five-year lung cancer survival: which advanced stage
nonsmall cell lung cancer patients attain long-term survival?
Cancer. 116:1518–1525. 2010.
|
|
6
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
|
|
7
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007.
|
|
8
|
Guh JH, Yu SM, Ko FN, Wu TS and Teng CM:
Antiproliferative effect in rat vascular smooth muscle cells by
osthole, isolated from Angelica pubescens. Eur J Pharmacol.
298:191–197. 1996.
|
|
9
|
Ko FN, Wu TS, Liou MJ, Huang TF and Teng
CM: Vasorelaxation of rat thoracic aorta caused by osthole isolated
from Angelica pubescens. Eur J Pharmacol. 219:29–34. 1992.
View Article : Google Scholar
|
|
10
|
Zimecki M, Artym J, Cisowski W, Mazol I,
Włodarczyk M and Gleńsk M: Immunomodulatory and anti-inflammatory
activity of selected osthole derivatives. Z Naturforsch C.
64:361–368. 2009.
|
|
11
|
Cai J, Yu B, Xu G and Wu J: Studies on the
quality of fructus Cnidii-comparison of antibacterial
action. Zhongguo Zhong Yao Za Zhi. 16:451–453. 5101991.(In
Chinese).
|
|
12
|
Matsuda H, Tomohiro N, Ido Y and Kubo M:
Anti-allergic effects of cnidii monnieri fructus (dried
fruits of Cnidium monnieri) and its major component, osthol.
Biol Pharm Bull. 25:809–812. 2002.
|
|
13
|
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT and
Way TD: Osthole suppresses hepatocyte growth factor (HGF)-induced
epithelial-mesenchymal transition via repression of the
c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food
Chem. 59:9683–9690. 2011.
|
|
14
|
Chou SY, Hsu CS, Wang KT, Wang MC and Wang
CC: Antitumor effects of Osthol from Cnidium monnieri: an in
vitro and in vivo study. Phytother Res. 21:226–230. 2007.PubMed/NCBI
|
|
15
|
Yang LL, Wang MC, Chen LG and Wang CC:
Cytotoxic activity of coumarins from the fruits of Cnidium
monnieri on leukemia cell lines. Planta Med. 69:1091–1095.
2003.
|
|
16
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res.
30:332011.
|
|
17
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: from dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009.
|
|
18
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: new insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010.
|
|
19
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006.
|
|
20
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352.
2007.
|
|
21
|
Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai
KC, Ma CY, Wood WG and Chung JG: Berberine suppresses in vitro
migration and invasion of human SCC-4 tongue squamous cancer cells
through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and
-9. Cancer Lett. 279:155–162. 2009. View Article : Google Scholar
|
|
22
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010.
|
|
23
|
Ni L, Feng Y, Wan H, Ma Q, Fan L, Qian Y,
Li Q, Xiang Y and Gao B: Angiotensin-(1–7) inhibits the migration
and invasion of A549 human lung adenocarcinoma cells through
inactivation of the PI3K/Akt and MAPK signaling pathways. Oncol
Rep. 27:783–790. 2012.
|
|
24
|
Hua H, Li M, Luo T, Yin Y and Jiang Y:
Matrix metalloproteinases in tumorigenesis: an evolving paradigm.
Cell Mol Life Sci. 68:3853–3868. 2011.
|
|
25
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Modulation of MMP-2 and MMP-9 by
cytokines, mitogens and inhibitors in lung cancer and malignant
mesothelioma cell lines. Oncol Rep. 22:1283–1291. 2009.
|
|
26
|
Park JK, Park SH, So K, Bae IH, Yoo YD and
Um HD: ICAM-3 enhances the migratory and invasive potential of
human non-small cell lung cancer cells by inducing MMP-2 and MMP-9
via Akt and CREB. Int J Oncol. 36:181–192. 2010.
|
|
27
|
Hung WC, Tseng WL, Shiea J and Chang HC:
Skp2 overexpression increases the expression of MMP-2 and MMP-9 and
invasion of lung cancer cells. Cancer Lett. 288:156–161. 2010.
|
|
28
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010.
|