Introduction
Curcumin is a natural yellow-pigmented polyphenol
component of the spice turmeric, which is derived from the roots of
the Curcuma longa plant which is indigenous to Southeast
Asia. Curcumin has potent antioxidant, anti-mutagenic and antitumor
properties. In recent years, studies have shown that curcumin is
able to inhibit the growth, invasion and metastasis of a variety of
tumor cells, induce apoptosis through a variety of mechanisms and
increase the sensitivity of tumor cells to chemotherapeutic drugs
and radiation (1,2).
Breast cancer is the most common malignant disease
among women worldwide. Triple-negative breast cancer (TNBC), which
represents approximately 15% of all breast cancers (3) and shows high recurrence and poor
survival rates (4), is defined by
the lack of estrogen receptor (ER), progesterone receptor (PR) and
epidermal growth factor receptor 2 (HER2/cerbB2/EGFR2) expression
(5). Thus, to date, TNBC lacks
effective targeted therapies. Endocrine therapy is also
ineffective. Chemotherapy remains the only possible therapeutic
option in the adjuvant or metastatic setting, but TNBC is
frequently resistant to standard chemotherapeutic regimens.
Therefore, TNBC has the worst prognosis of all breast cancer
subtypes. TNBC cells are often accompanied by high expression
levels of EGFR and abnormal activation of MAPK signaling pathways
(6,7). However, it has not been reported
whether curcumin is able to inhibit the proliferation of TNBC cells
and induce apoptosis through the inhibition of EGFR-MAPK signaling
pathways. In this study, we studied the effects of curcumin on TNBC
cells and the possible mechanism.
Materials and methods
Materials and reagents
The MDA-MB-231
(ER−/PR−/HER2−, EGFR+)
breast cancer cells were purchased from the Shanghai Cell Bank of
the Chinese Academy of Sciences (Shanghai, China). The study was
approved by the ethics committee of Zhejiang Provincial People’s
Hospital, Hangzhou, China. Curcumin was purchased from Sigma (St.
Louis, MO, USA). Rabbit anti-human ERK1/2, pERK1/2 antibody and
rabbit anti-human EGFR, pEGFR antibody products were obtained from
Cell Signaling Technology (Danvers, MA, USA) and mouse anti-rabbit
secondary antibody was purchased from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China). The Annexin V/PI
apoptosis detection kit was purchased from United Biotechnology
Co., Ltd. (Shanghai, China) and DMEM and fetal bovine serum were
acquired from Gibco-BRL (Carlsbad, CA, USA). MTT was purchased from
Sigma.
MTT assay of breast cancer cell
proliferation
To detect the rate of cell proliferation, MDA-MB-231
cells in DMEM media containing 10% FBS with penicillin and
streptomycin (100 μ/ml) were incubated at 37°C in a humidified
atmosphere of 5% CO2. After digestion with 0.25%
trypsin, 1×104/ml cells were inoculated into 96-well
culture plates at 37°C overnight. The cells were divided into
control and curcumin (10, 20 and 30 μmol/ml) treatment groups and
cultured in 96-well plates for 48 h. The cells were then
centrifuged for 3 min at 500 × g and 100 μl supernatant was
removed. MTT 10 μl (5 mg/ml) was added, the cells were incubated
for 4 h at 37°C and 100 μl DMSO was added. After shocking for 10
min, the OD490 value was detected using an enzyme immunoassay
instrument. The rate of inhibition of cell proliferation was
calculated as follows: inhibition rate = (1 − Atreatment
group/Acontrol group) × 100%.
Curcumin-induced apoptosis of breast
cancer cells
An Annexin V-FITC/PI double staining method was
carried out according to the kit’s instructions: MDA-MB-231 cells
were routinely cultured with DMEM. After digestion with 0.25%
trypsin, 1×105/ml cells were inoculated into 6-well
culture plates at 37°C overnight. The cells were divided into
control and curcumin (10, 20 and 30 μmol/ml) treatment groups.
After a 48-h treatment, the cells were collected following trypsin
digestion, centrifuged for 5 min at 800 × g and then washed three
times with PBS. Buffer (50 μl) was added followed by V-FITC (5 μl)
and PI (10 μl). The cells were incubated in the dark for >5 min
and then evaluated by flow cytometry.
Effect of curcumin on EGFR and ERK1/2
phosphorylation
The expression levels of ERK1/2, pERK1/2, EGFR and
pEGFR were detected by western blot analysis. MDA-MB-231 cells were
cultured with DMEM. After digestion with 0.25% trypsin,
1×105/ml cells were inoculated into 6-well culture
plates at 37°C overnight. The cells were divided into control and
curcumin (30 μmol/ml) treatment groups; 48 h later, the cells were
collected, subjected to one-step cleavage and after 13,000 rpm
high-speed centrifugation for 10 min, the supernatant was taken for
protein quantification. The 10% SDS-PAGE separating gel and
laminated gel were prepared conventionally. Cells in the above
groups were collected separately, and total proteins in each group
were extracted using the one-shot method. After mixing with 4X
loading buffer, boiling for 5 min and centrifuging at 12000 rpm for
3 min, 20 μl of the resulting sample was used for SDS-PAGE analysis
at 100 V. The gel was gently removed, washed once with Millipore
H2O and electrophoretically transferred to a membrane at
100 V for 2 h. After blocking with a blocking buffer at 37°C for 2
h, the membrane was incubated with the corresponding primary
antibody (1:1,000) overnight at 4°C, washed with TBST four times at
10-min intervals and then incubated with the corresponding
horseradish peroxidase-labeled secondary antibody (1:5,000) at room
temperature for 1 h. After washing with TBST 4 times at 10-min
intervals, an ECL reagent was added and the results were detected
using an X-ray film. With GAPDH as an internal control, the gray
scales of the bands were analyzed semi-quantitatively using Band
Leader software.
Statistical analysis
SPSS 13.0 software was used for statistical
analysis, all data are shown as the mean ± SD. Statistical
differences were determined by t-test analysis and P<0.05 was
considered to indicate a statistically significant result.
Results
Cell proliferation inhibition rate
detected by MTT assay
The results showed that as the curcumin
concentration increased its inhibitory effect on MDA-MB-231 cell
proliferation also increased; at a concentration of 30 μmol/ml, the
proliferation inhibiting effect of curcumin on the MDA-MB-231 cells
was significantly higher than that of the other groups (P<0.01;
Table I).
 | Table IGrowth inhibition rates of MDA-MB-231
cells detected by MTT (%, mean ± SD). |
Table I
Growth inhibition rates of MDA-MB-231
cells detected by MTT (%, mean ± SD).
| Group | MDA-MB-231 cells |
|---|
| Control | 0 |
| Curcumin
treatment |
| (10 μmol/ml) | 14.67±3.26 |
| (20 μmol/ml) | 18.53±3.59 |
| (30 μmol/ml)a | 58.76±4.97 |
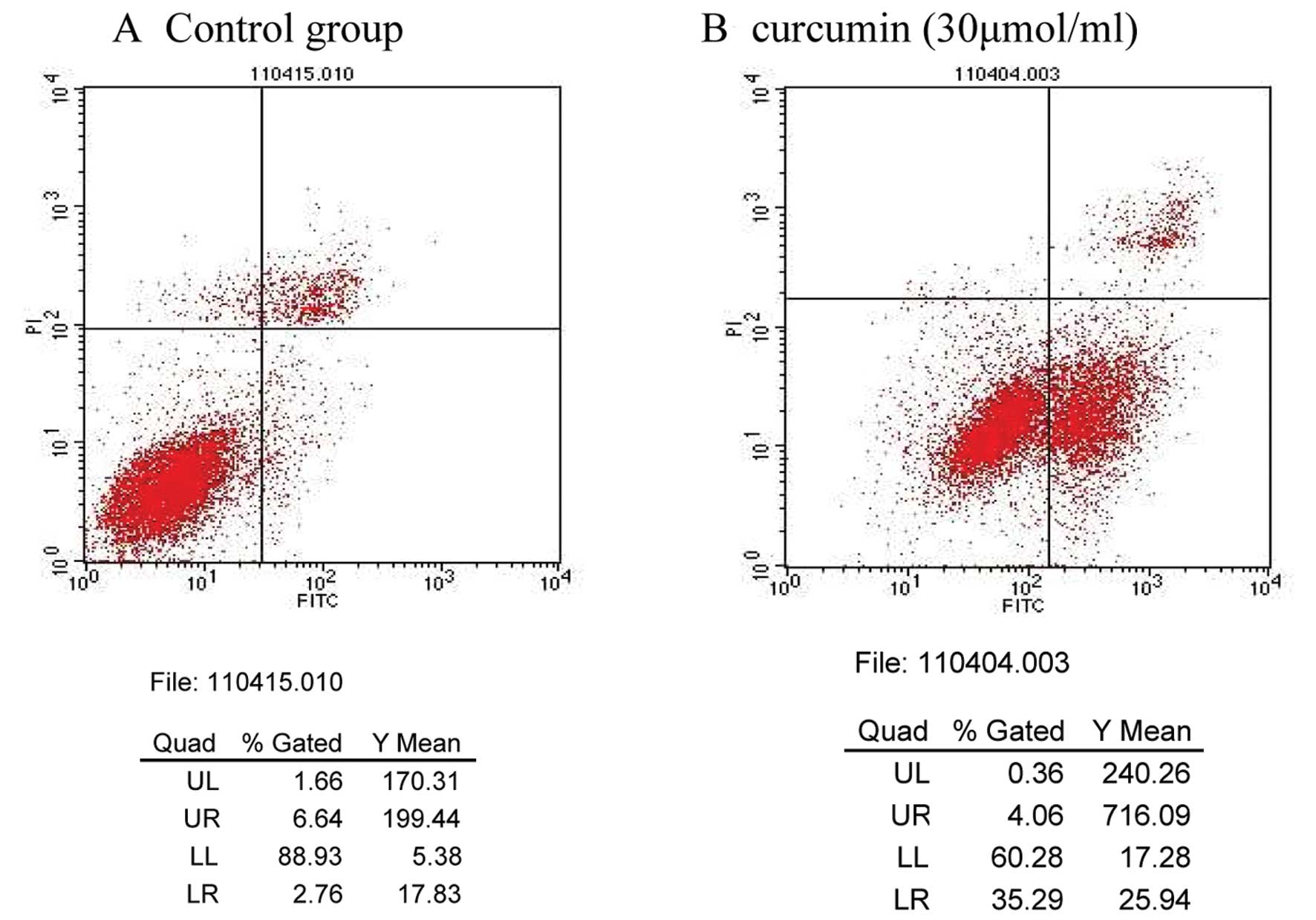
Apoptosis detected by flow cytometry
The curcumin-induced effects on MDA-MB-231 cell
apoptosis were determined by flow cytometry. The apoptosis rates of
the control and 30 μmol/ml curcumin treatment groups were 2.76 and
26.34%, respectively; these results were significantly different
(P<0.01; Table II; Fig. 1).
 | Table IIApoptosis rate of MDA-MB-231 cells
detected by flow cytometery (%, mean ± SD). |
Table II
Apoptosis rate of MDA-MB-231 cells
detected by flow cytometery (%, mean ± SD).
| Group | MDA-MB-231 cells |
|---|
| Control | 2.76±0.29 |
| Curcumin
treatment |
| (10 μmol/ml) | 8.62±0.78 |
| (20 μmol/ml) | 9.24±0.81 |
| (30 μmol/ml)a | 26.34±1.26 |
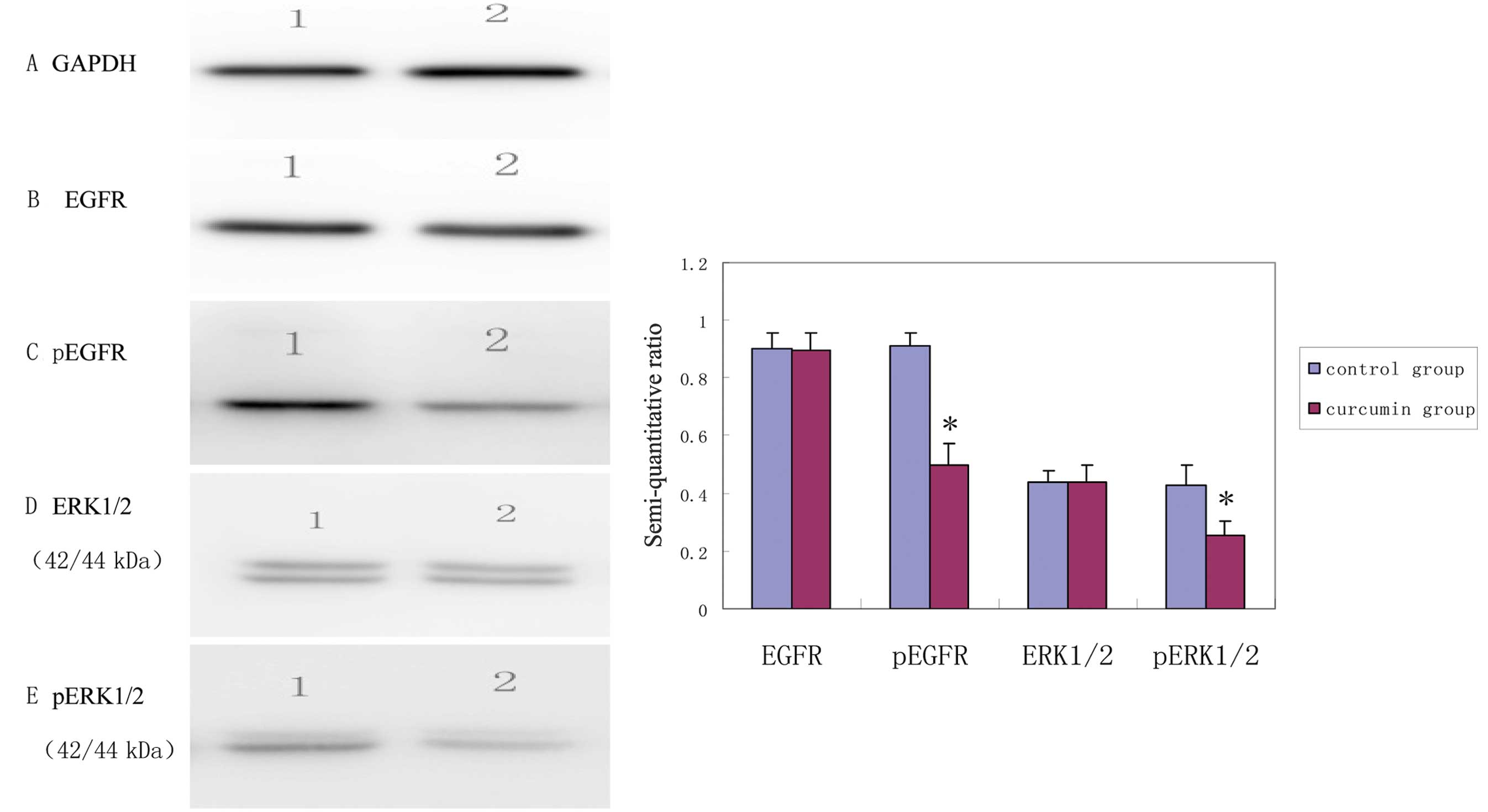
EGFR and ERK1/2 phosphorylation levels in
MDA-MB-231 cells inhibited by curcumin
Using GAPDH as internal control, the results were
obtained by semi-quantitative analysis of the gray scales of the
bands using Band Leader software. The results revealed that the
expression level of EGFR in the curcumin treatment group was not
significantly different from that in the control group (t=7.91,
P=0.92) while the expression level of pEGFR in the curcumin
treatment group was significantly decreased compared with that in
the control group (t=10.59, P<0.001). The expression level of
ERK1/2 in the curcumin treatment group was not significantly
different from that in the control group (t=0.06, P=0.95), while
the expression level of pERK1/2 in the curcumin treatment group was
significantly decreased compared with that in control group
(t=4.80, P=0.002). The results indicated that, after curcumin
treatment for 48 h, the expression levels of pEGFR and pERK1/2 in
the MDA-MB-231 cells were decreased, suggesting that curcumin
inhibited the activation of EGFR and its downstream signaling
molecules (Fig. 2).
Discussion
Curcumin has a variety of therapeutic properties,
including antioxidant, analgesic, anti-inflammatory,
anti-proliferative and antiseptic activities (8). Curcumin has been well-studied as a
potential anticancer agent for the past decade (1). It has been shown that curcumin
prevents tumor initiation, proliferation and metastasis in breast,
colon, oral, ovarian and a number of other human cancers (9). Numerous studies have confirmed that
curcumin is able to inhibit the growth of various tumor cell lines
and induce tumor cell apoptosis. Multiple mechanisms of action have
been proposed, including inhibition of NF-κB and STAT3
transcription factor activities, regulation of tumor suppressor
genes, cancer genes and their protein expression, induction of cell
cycle arrest and regulation of apoptosis signaling (10,11).
Curcumin is able to reduce the activation levels of NF-κB in KCP-4
and MDA-MB-231 cells, and suppress the expression levels of Bcl-2,
Bcl-xL and survivin, which are apoptosis-related proteins regulated
by NF-κB (12–14). The effect of curcumin on human
cancer cell lines is multi-functional and the inhibition of
telomerase expression followed by induction of apoptosis may be one
of the major mechanisms by which curcumin inhibits the
proliferation of cancer cells (15). Recent studies have shown that
curcumin inhibits the EGFR, Her-2, Hh/Gli, Wnt/B-catenin and Notch
signaling pathways (16,17). Curcumin has been reported to
potentiate the antitumor activity of gefitinib in cell lines and a
xenograft mice model of NSCLC through inhibition of proliferation,
EGFR phosphorylation and induction of EGFR ubiquitination and
apoptosis (18). Curcumin has also
been shown to reduce EGFR mRNA transcription and protein
expression, thus inhibiting the proliferation of bladder cancer
cells (19).
In our study, we found that when MDA-MB-231 TNBC
cells were cocultured with gradually increasing concentrations of
curcumin, the MDA-MB-231 cell proliferation activity gradually
decreased; 30 μmol/ml curcumin significantly inhibited the
MDA-MB-231 cell proliferation. The level of apoptosis in the
curcumin-treated group was significantly different from that in the
control group. The results indicate that curcumin is able to induce
MDA-MB-231 cell apoptosis and inhibit cell proliferation in
vitro. The expression levels of pERK1/2 and pEGFR in the
curcumin-treated group were lower than those in the control group.
The EGFR is highly expressed in approximately 60% of TNBCs
(3). MAPK and PI3K-AKT signaling
pathways were over-activated, suggesting that TNBC cell growth
depends on the EGFR signaling pathway (20). High intratumoral EGFR and CK5/6
expression levels may have a role in the development of nodal or
distant metastases in TNBC and may be predictive of metastatic
disease (21). An EGFR inhibitor
has been reported to induce a change from the mesenchymal to the
epithelial phenotype in TNBC cells; the EGFR tyrosine kinase
inhibitor erlotinib inhibited tumor growth and metastasis in a
SUM149 xenograft mouse model (22). Our study identified that curcumin
was able to inhibit EGFR and extracellular regulated protein kinase
(ERK1/2) phosphorylation in MDA-MB-231 cells; ERK1/2 is one of the
major signaling molecules downstream of EGFR. This suggests that
curcumin inhibited the activation of EGFR and its downstream
signaling molecules, thus inhibiting MDA-MB-231 cell proliferation.
Anti-EGFR therapeutic strategies, including monoclonal antibodies
(cetuximab, panitumumab) and small molecule inhibitors (gefitinib,
erlotinib), may be of potential benefit in the treatment of TNBC
(23).
Our result indicate that curcumin is able to inhibit
the proliferation of MDA-MB-231 TNBC cells and induce their
apoptosis in vitro by inhibiting the EGFR signaling
pathway.
References
|
1
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Basnet P and Skalko-Basnet N: Curcumin: an
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siziopikou KP, Ariga R, Proussaloglou KE,
et al: The challenging estrogen receptor-negative/progesterone
receptor-negative/HER-2-negative patient: a promising candidate for
epidermal growth factor receptor-targeted therapy? Breast J.
12:360–362. 2006. View Article : Google Scholar
|
|
4
|
De Ruijter TC, Veeck J, de Hoon JP, et al:
Characteristics of triple-negative breast cancer. J Cancer Res Clin
Oncol. 137:183–192. 2011.PubMed/NCBI
|
|
5
|
Thike AA, Cheok PY, Jara-Lazaro AR, et al:
Triple-negative breast cancer: clinicopathological characteristics
and relationship with basal-like breast cancer. Mod Pathol.
23:123–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan DS, Marchió C, Jones RL, et al: Triple
negative breast cancer: molecular profiling and prognostic impact
in adjuvant anthracycline-treated patients. Breast Cancer Res
Treat. 111:27–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eralp Y, Derin D, Ozluk Y, et al: MAPK
overexprssion is associated with anthracycline resistance and
increased risk for recurrence in patients with triple-negative
breast cancer. Ann Oncol. 19:669–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: a review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:12–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reuter S, Eifes S, Dicato M, et al:
Modulation of anti-apoptotic and survival pathways by curcumin as a
strategy to induce apoptosis in cancer cells. Biochem Pharmacol.
76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
11
|
Rowe DL, Ozbay T, O’Regan RM and Nahta R:
Modulation of the BRCA1 protein and induction of apoptosis in
triple negative breast cancer cell lines by the polyphenolic
compound curcumin. Breast Cancer (Auckland). 3:61–75.
2009.PubMed/NCBI
|
|
12
|
Oiso S, Ikeda R, Nakamura K, et al:
Involvement of NF-κB activation in the cisplatin resistance of
human epidermoid carcinoma KCP-4 cells. Oncol Rep. 28:27–32.
2012.
|
|
13
|
Chiu TL and Su CC: Curcumin inhibits
proliferation and migration by increasing the Bax to Bcl-2 ratio
and decreasing NFκBp65 expression in breast cancer MDA-MB-231
cells. Int J Mol Med. 23:469–475. 2009.PubMed/NCBI
|
|
14
|
Chakraborty G, Jain S, Kale S, et al:
Curcumin suppresses breast tumor angiogenesis by abrogating
osteopontin-induced VEGF expression. Mol Med Report. 1:641–646.
2008.PubMed/NCBI
|
|
15
|
Cui SX, Qu XJ, Xie YY, et al: Curcumin
inhibits telomerase activity in human cancer cell lines. Int J Mol
Med. 18:227–231. 2006.PubMed/NCBI
|
|
16
|
Mimeault M and Batra SK: Potential
applications of curcumin and its novel synthetic analogs and
nanotechnology-based formulations in cancer prevention and therapy.
Chin Med. 6:312011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirose H, Ishii H, Mimori K, et al: Notch
pathway as candidate therapeutic target in Her2/Neu/ErbB2
receptor-negative breast tumors. Oncol Rep. 23:35–43.
2010.PubMed/NCBI
|
|
18
|
Lee JY, Lee YM, Chang GC, et al: Curcumin
induces EGFR degradation in lung adenocarcinoma and modulates p38
activation in intestine: the versatile adjuvant for gefitinib
therapy. PLoS One. 6:e237562011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chadalapaka G, Jutooru I, Burghardt R and
Safe S: Drugs that target specificity proteins downregulate
epidermal growth factor receptor in bladder cancer cells. Mol
Cancer Res. 8:739–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Billar JA, Dueck AC, Stucky CC, et al:
Triple-negative breast cancers: unique clinical presentations and
outcomes. Ann Surg Oncol. 17(Suppl 3): 384–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sutton LM, Han JS, Molberg KH, et al:
Intratumoral expression level of epidermal growth factor receptor
and cytokeratin 5/6 is significantly associated with nodal and
distant metastases in patients with basal-like triple-negative
breast carcinoma. Am J Clin Pathol. 134:782–787. 2010. View Article : Google Scholar
|
|
22
|
Ueno NT and Zhang D: Targeting EGFR in
triple negative breast cancer. J Cancer. 2:324–328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sánchez-Muñoz A, Gallego E, de Luque V, et
al: Lack of evidence for KRAS oncogenic mutations in
triple-negative breast cancer. BMC Cancer. 10:1362010.PubMed/NCBI
|