Introduction
Human zinc finger and BTB domain-containing 7A
(Zbtb7A), also known as POK erythroid myeloid ontogenic factor
(pokemon) (1), factor that binds
to IST, the HIV-1 inducer of short transcripts (FBI-1) (2), leukemia/lymphoma-related factor mouse
homolog (LRF) (3) and
osteoclast-derived zinc finger rat homolog (OCZF) (4), was originally identified as a
cellular factor that binds specifically to the human
immunodeficiency virus type 1 promoter element (5). Zbtb7A belongs to the POK protein
family (6) and plays critical
roles in differentiation (7),
oncogenesis (1,8) and adipogenesis (9). The overexpression of Zbtb7A is
observed in various human cancers (1). Zbtb7A suppresses the p14ARF, Rb tumor
suppressor gene (1,10), p21CIP1 (11), E2F4 and cyclin A (9), indicating that Zbtb7A may be a
molecular target for cancer therapy.
Many Zbtb7A downstream targets have been identified,
but little is known about the mitogens that regulate the expression
of Zbtb7A. Roh et al reported that Zbtb7A activity was
regulated by sumoylation (12). In
addition, we have demonstrated previously that Sp1, a well-known
effector of insulin in cancer cells (13), elevates Zbtb7A expression by
directly binding to its promoter (14). Based on this study, we hypothesized
that there is a correlation between insulin and Zbtb7A
expression.
Apart from its role in the regulation of glucose
uptake, insulin also acts as a mitogen and differentiation factor
in a variety of cells and tissues (15–18).
As a mitogen, insulin regulates the transcription of >100 genes
(19,20) and promotes cell growth (21,22).
The actions of insulin are initiated through the activation of the
insulin receptor, which is a receptor tyrosine kinase, and
downstream serine/threonine kinase-signaling pathways including PI
3-kinase (PI3K/AKT) and Ras-MAPK cascades (23,24).
Boyd reported that the bioavailability of insulin contributes to
tumor development (25). It has
also been reported that insulin is able to stimulate the expression
of the oncogene PTTG (26). In the
current study, we demonstrate that the mRNA and protein levels of
Zbtb7A are significantly upregulated by insulin via the PI3K/AKT
pathway and the transcription factor Sp1.
Materials and methods
Materials
Insulin and polyclonal anti-Zbtb7A were obtained
from Sigma (St. Louis, MO, USA). Goat anti-rabbit IgG horseradish
peroxidase (HRP)-linked antibody, goat anti-mouse HRP-linked
antibody, the PI3K/AKT inhibitor LY294002 and the MAPK inhibitor
PD98059 were purchased from Beyotime Biotech (Jiangsu, China). The
nylon membrane Hybond-N was obtained from Pall Corporation (Port
Washington, NY, USA). The TRIzol reagent was purchased from
Invitrogen Life Technologies (Carslbad, CA, USA).
Cell culture
HepG2 cells were cultured in Dullbecco’s modified
Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 μg/ml streptomycin in humidified air containing
5% CO2 at 37°C. Cells were trypsinized and seeded in
6-well plates and grown to 50–70% confluence. They were then
stimulated with various reagents as described in the figure
legends.
RNA extraction and real-time PCR
analysis
The total RNA of the HepG2 cells was isolated using
TRIzol reagent. Reverse transcription was performed using a RT-PCR
kit according to the instructions of the manufacturer (Toyobo,
Osaka, Japan). The quantification of the Zbtb7A mRNA (1 μg) was
performed using an ABI 7500 real-time PCR system with the Zbtb7A
specific primers: 5′-GAAGCCCTACGAGTGCAACATC-3′ (forward) and
5′-GTGGTTCTTCAGGTCGTAGTTGTG-3′ (reverse). GAPDH served as the
internal control and was amplified with the following primers:
5′-GGTGGTCTCCTCT GACTTCAACA-3′ (forward) and 5′-GTTGCTGTAGCCAAA
TTCGTTGT-3′ (reverse). The volume of real-time RT-PCR system was 20
μl, containing: 10 μl SYBR-buffer, 0.4 μl forward primer (10
μmol/l), 0.4 μl reverse primer (10 μmol/l), 0.4 μl SYBR-Green II
dye, 1 μl cDNA and 7.8 μl water. Thermal cycling conditions were
denaturation at 95°C for 10 sec, and then 5 sec denaturation at
95°C, 34 sec annealing at 60°C for 40 cycles, followed by a
dissociation stage (95°C for 15 sec, 60°C for 1 min and 95°C for 15
sec) to monitor the specificity of the primers. All samples were
run in triplicate.
Site directed mutagenesis
The Sp1 recognition site of the Zbtb7A promoter was
GGGCGG, -641-636 bp relative to the transcription start site. The
mutation of this site was obtained by preparing the mutated
promoter Sp1M, using mutated primers within GC-boxes. The plasmids
with mutation were generated using a site-directed mutagenesis
system (Promega, Madison, WI, USA) according to the instructions
provided by the manufacturer and a pLuc1000 construct was used as a
template. The oligonucleotide used for mutagenesis (mutations
indicated with inclined form and bold letters) is:
5′-AATGATCCAAAAAAAACTGCCTCCCAAG-3′. DNA sequencing
was performed to confirm that the sequences of the PCR products
were correct as compared with the Zbtb7A promoter published in the
Human Genome database.
Plasmid transfection and luciferase
assay
Human pGL4.10-Zbtb7A promoter pLuc1000 (1000 bp,
wild-type) or mutated promoter Sp1M was used for transient
transfection. Cells were plated in 24-well plates. After culturing
for 18 h, the cells were transfected with Lipofectamine 2000
according to the instructions of the manufacturer (Invitrogen Life
Technologies). Wild-type or mutated Zbtb7A promoter-luciferase
construct (1 μg/well) was used and 30 ng/well of pRLTK plasmid was
co-transfected as a control reporter plasmid. Six hours after
transfection, the cells were washed with PBS twice, cultured in
DMEM containing 10% FBS for 24 h and then treated with the
indicated concentrations of insulin for 24 h.
Cells were harvested by lysis buffer (Promega). Cell
lysates (10 μl) were analyzed to determine the promoter activity.
Three independent transfection experiments were carried out for
each assay.
Western blot analysis
Following treatment with the indicated reagents, the
cells were lysed with RIPA buffer containing 10 mM KCl, 0.1%
Nonidet P-40, 10 mM HEPES, pH 7.9, 1 mM ethylenediamine tetraacetic
acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.5 mM
Na3VO4 and 1 mM dithiothreitol (DTT). The
protein concentration was determined by Bradford protein assay.
Equal amounts of each protein sample (30 μg) were resolved by
SDS-PAGE and transferred to a nitrocellulose membrane. Blots were
incubated in 5% non-fat milk at room temperature for 1 h, washed
three times with 0.5% Tween-20 Tris-buffered saline (TBST) and
probed with a suitable primary antibody at room temperature for 2
h. The membrane was washed and incubated with secondary antibody
for 1 h at room temperature and detected using Quantity One
software (Bio-Rad Laboratories, Hercules, CA, USA). β-actin was
used to monitor the equal loading of each sample.
Statistical analysis
Statistical analysis was performed using ANOVA.
Values of *P<0.05, **P<0.01 and
***P<0.001 were considered to indicate statistically
significant differences.
Results
Insulin regulates Zbtb7A expression in a
dose-dependent manner
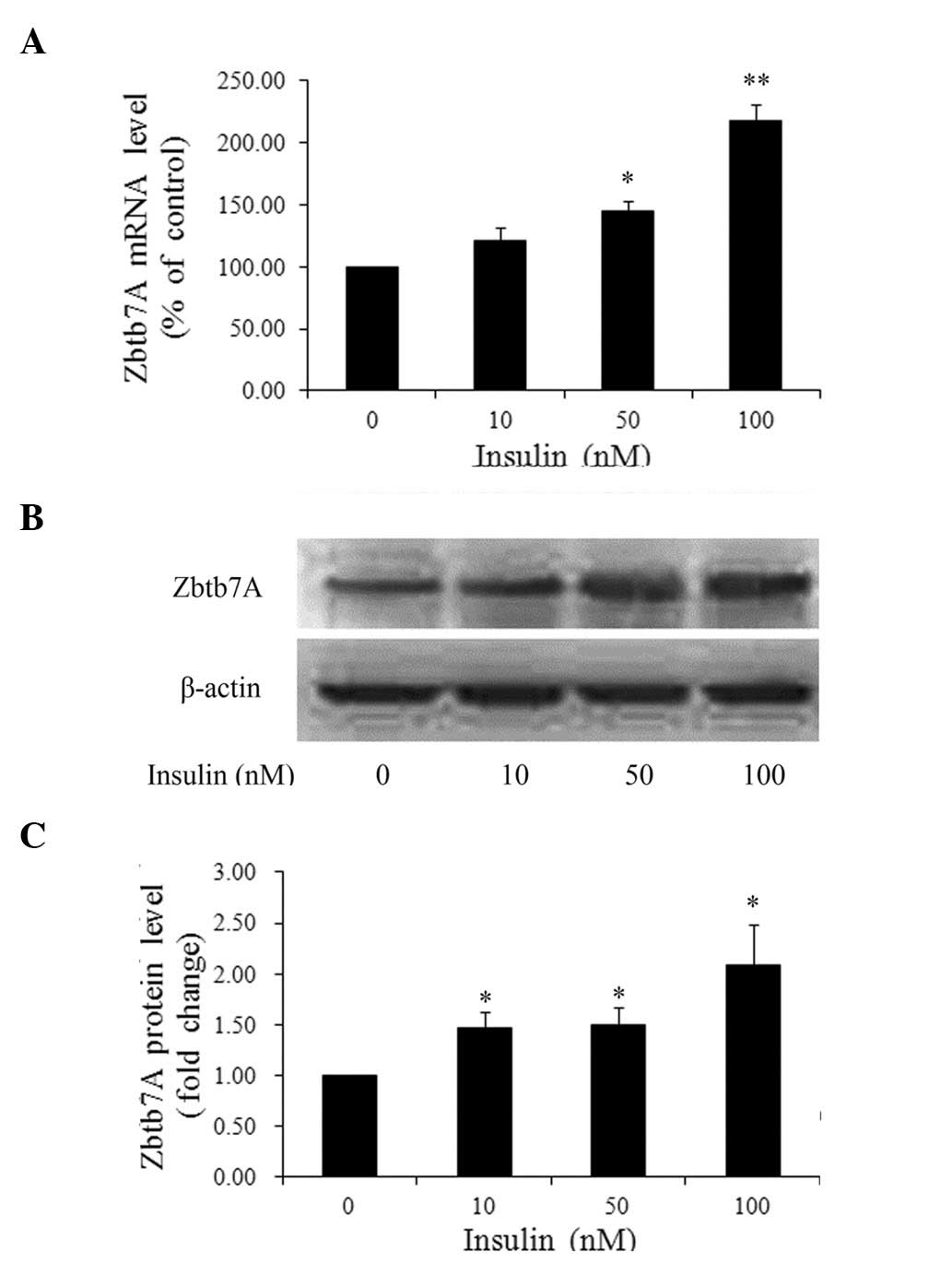
To determine whether insulin enhances Zbtb7A
expression, HepG2 cells were treated with various concentrations of
insulin or left untreated for 24 h. The Zbtb7A mRNA and protein
levels were analyzed by real-time PCR and western blot analysis,
respectively. As shown in Fig. 1,
insulin activated Zbtb7A expression in a dose-dependent manner.
Compared with the control group, when the cells treated with 10, 50
and 100 nM insulin, the Zbtb7A mRNA levels were increased 1.21-,
1.45- (P<0.05) and 2.17-fold (P<0.01), respectively;
similarly, the Zbtb7A protein levels were increased 1.47-, 1.49-
and 2.09-fold (all P<0.05), respectively (Fig. 1B and C).
Insulin stimulates Zbtb7A expression in a
time-dependent manner
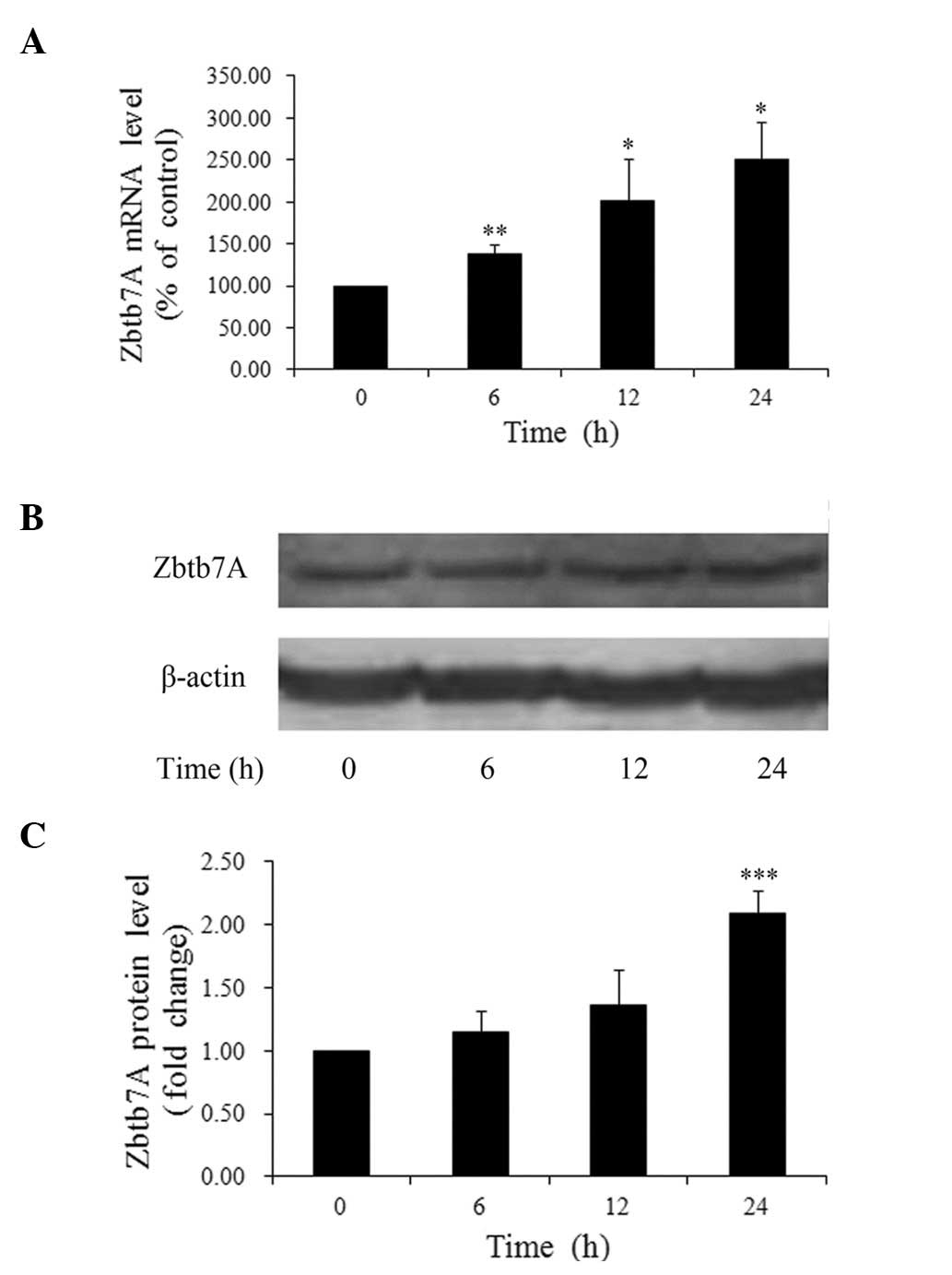
We then sought to discover the time-dependent
effects of insulin on Zbtb7A expression. HepG2 cells treated with
100 nM insulin were harvested at different time-points. It was
found that the insulin-induced upregulation of Zbtb7A mRNA levels
was time-dependent (Fig. 2A). The
expression levels of Zbtb7A in the cells treated with 100 nM
insulin for 6, 12 and 24 h were increased 1.38- (P<0.01), 2.01-
(P<0.05) and 2.51-fold (P<0.05), respectively, compared with
untreated cells. In addition, a maximum 2.09-fold (P<0.001)
increase in Zbtb7A protein levels was observed 24 h after insulin
treatment, but no marked changes in Zbtb7A protein levels were
detected at 6 and 12 h (Fig. 2B and
C). These results suggest that insulin upregulates the mRNA and
protein levels of Zbtb7A in HepG2 cells in a time-dependent
manner.
Insulin-induced upregulation of Zbtb7A
expression is mediated by the PI3K/AKT pathway
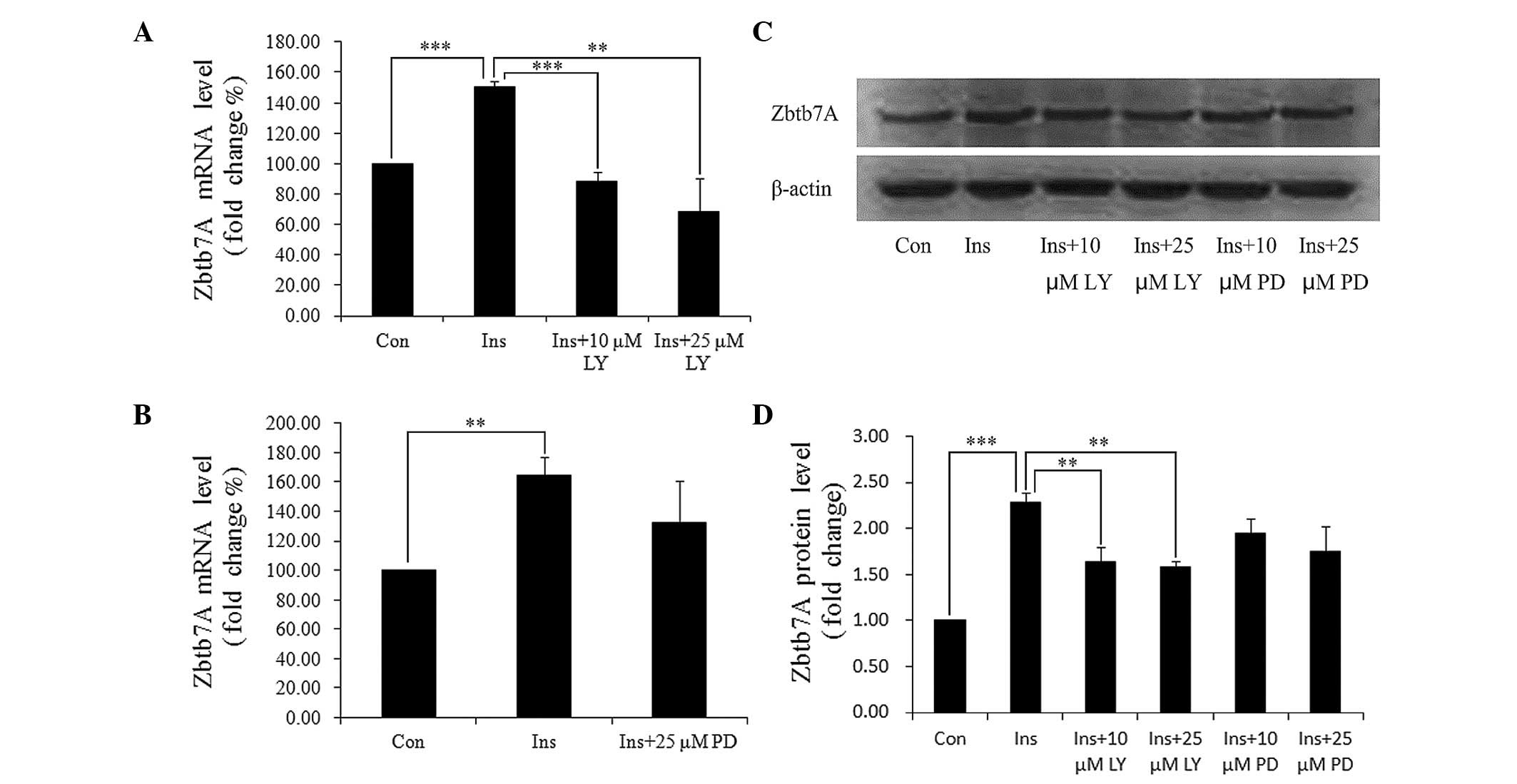
Insulin mediates target proteins through two major
pathways: PI3K/AKT and MAPK. We used specific inhibitors of the two
pathways to identify which of these pathways are involved in the
insulin-induced Zbtb7A expression. HepG2 cells were pre-treated
with LY294002 (a PI3K inhibitor) or PD98059 (a MAPK inhibitor) for
30 min, and then incubated with DMEM containing 100 nM insulin and
the indicated inhibitors. It was identified that LY294002
completely blocked the insulin-induced Zbtb7A expression at the
mRNA and protein levels, and that PD98059 partially attenuated the
effect of insulin on Zbtb7A expression (Fig. 3). The results indicate that insulin
enhances Zbtb7A expression predominantly through the PI3K/AKT
pathway.
Insulin enhances Zbtb7A promoter activity
through Sp1
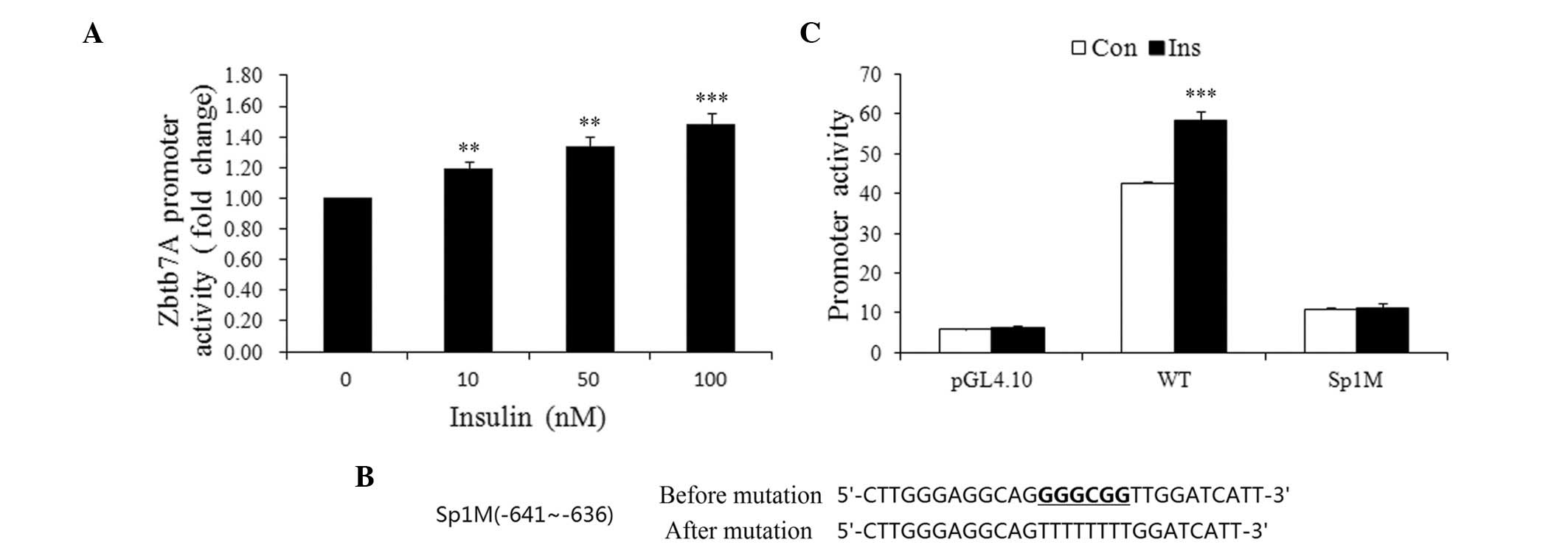
To elucidate the mechanism of the insulin-induced
Zbtb7A expression, wild-type and mutated Zbtb7A promoter-luciferase
constructs were developed and transiently transfected into HepG2
cells prior to treatment of the cells with various concentrations
of insulin. As shown in Fig. 4A,
the activity of the Zbtb7A promoter was increased by insulin in a
concentration-dependent manner. Insulin (100 nM) increased Zbtb7A
promoter activity by 48.3% compared with the control group.
Since Sp1 is an effector that mediates the action of
insulin on gene expression, we attempted to determine whether Sp1
plays a role in the insulin-induced Zbtb7A expression by using a
wild-type promoter of Zbtb7A and an Sp1 binding site mutated
promoter (Sp1M). Cells transfected with the wild-type or mutated
promoters were treated with or without 100 nM insulin for 48 h. As
shown in Fig. 4C, the activity of
the wild-type promoter was enhanced by insulin, while Sp1M
demonstrated no clear response to insulin treatment. Therefore, it
may be inferred that insulin enhances Zbtb7A promoter activity
through Sp1 binding sites.
Discussion
Our results demonstrate that insulin upregulates
Zbtb7A expression in HepG2 cells. To our knowledge, this is the
first report that insulin stimulates Zbtb7A promoter activity and
enhances endogenous Zbtb7A mRNA and protein levels in a dose- and
time-dependent manner, and that the PI3K/AKT cascade and
transcription factor Sp1 are responsible for the insulin-induced
Zbtb7A expression in HepG2 cells.
To validate the effect of insulin on Zbtb7A
expression, we treated HepG2 cells with various concentrations of
insulin for 24 h. Cells incubated with 100 nM insulin revealed a
maximum 2.17-fold increase in the Zbtb7A mRNA level and a 2.09-fold
increase in the Zbtb7A protein level. We then cultured the cells in
DMEM with 100 nM insulin for various times, and tested the mRNA and
protein levels of Zbtb7A at the indicated time-points. The highest
Zbtb7A protein and mRNA levels were induced by insulin in the HepG2
cells at 24 h. Zbtb7A mRNA was elevated significantly at 6 and 12
h, however, the corresponding protein level showed no evident
increase at these times. These differences may be due to the
interval between gene transcription and mRNA translation. We used
specific inhibitors of different insulin pathways to determine
which pathway may be involved in the insulin-induced Zbtb7A
expression. The observation that LY294002 completely blocked the
insulin-induced Zbtb7A expression, while PD98059 attenuated the
effect of insulin on Zbtb7A expression to a lesser extent than
LY294002, suggests that insulin regulates Zbtb7A expression via the
PI3K/AKT pathway. A 1-kb Zbtb7A promoter luciferase construct was
developed to identify whether insulin has an effect on Zbtb7A gene
promoter activity. The promoter activity was increased 1.48-fold by
treatment with 100 nM insulin. However, insulin was not able to
boost the activity of a Zbtb7A promoter with an Sp1 binding site
mutation. These results suggest that insulin upregulates Zbtb7A
mRNA synthesis through the activation of the Zbtb7A gene promoter
via Sp1.
Insulin has the effect of modulating carbohydrate
metabolism and regulating certain oncogenes, including c-myc and
PTTG (26–29). Messina reported that insulin
initially inhibits the proto-oncogene c-myc, but this initial
decrease in c-myc is followed by an approximately 3-fold increase
in c-myc expression by 60–120 min (27). Thompson and Kakar demonstrated that
insulin and IGF-1 stimulate the expression of the oncogene PTTG in
a time- and dose-dependent manner through the PI3K/AKT pathway, and
they also suggested that insulin-related signaling pathways are a
potential molecular target for cancer therapy (26). Since the increased expression of
Zbtb7A in non-small cell lung cancer (NSCLC) resulted in
carcinogenesis and Zbtb7A had some clinical significance for the
prognostic evaluation of patients with NSCLC (30), the stimulation of Zbtb7A by insulin
provides a possible mechanism by which insulin contributes to tumor
development and/or progression.
Hyperinsulinemia is a common symptom in obese and
type 2 diabetic patients. Hepatocellular cancer has increased
incidence and prevalence in obesity (31). Compared with the general
population, diabetic patients have an increased frequency of
hepatitis C, which may contribute to prolonged insulin resistance
and liver cancer (32). As a
growth factor, insulin is crucial in liver regeneration (33), promotes the growth of the human
hepatoma cell line PLC/PRF/5 (34), and reverses the
dexamethasone-induced inhibition of rat hepatoma-cell growth and
cell-cycle traverse (35). It has
been reported that HepG2 cells have intact insulin signaling
(36), so we selected HepG2 cells
as a cell model to investigate the effects of insulin on Zbtb7A
expression. We present evidence of a possible relationship between
type 2 diabetes and hepato-carcinoma and our results imply that
Zbtb7A might not only be a downstream target of insulin but also an
effector of insulin in tumorigenesis.
In conclusion, the present study demonstrates that
insulin enhances Zbtb7A expression via activation of the PI3K/AKT
cascade and the transcription factor Sp1 in HepG2 cells. This is
the first report concerning the regulation of Zbtb7A expression by
insulin, which may have significant implications for the
development of diabetes-associated carcinoma and provide insight
into the importance of Zbtb7A in clinical treatment.
Acknowledgements
We thank the financial support from the Ministry of
Science and Technology of China (2012ZX09506001-010, 2012CB722605,
2012AA020305 and 2011DFA30620).
References
|
1
|
Maeda T, Hobbs RM, Merghoub T, et al: Role
of the proto-oncogene Pokemon in cellular transformation and ARF
repression. Nature. 433:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morrison DJ, Pendergrast PS, Stavropoulos
P, Colmenares SU, Kobayashi R and Hernandez N: FBI-1, a factor that
binds to the HIV-1 inducer of short transcripts (IST), is a POZ
domain protein. Nucleic Acids Res. 27:1251–1262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies JM, Hawe N, Kabarowski J, et al:
Novel BTB/POZ domain zinc-finger protein, LRF, is a potential
target of the LAZ-3/BCL-6 oncogene. Oncogene. 18:365–375. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kukita A, Kukita T, Ouchida M, Maeda H,
Yatsuki H and Kohashi O: Osteoclast-derived zinc finger (OCZF)
protein with POZ domain, a possible transcriptional repressor, is
involved in osteoclastogenesis. Blood. 94:1987–1997.
1999.PubMed/NCBI
|
|
5
|
Pessler F, Pendergrast PS and Hernandez N:
Purification and characterization of FBI-1, a cellular factor that
binds to the human immunodeficiency virus type 1 inducer of short
transcripts. Mol Cell Biol. 17:3786–3798. 1997.PubMed/NCBI
|
|
6
|
Apostolopoulou K, Pateras IS, Evangelou K,
et al: Gene amplification is a relatively frequent event leading to
ZBTB7A (Pokemon) overexpression in non-small cell lung cancer. J
Pathol. 213:294–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merghoub T, Cattoretti G, Piazza F, et al:
Pokemon is required for cellular differentiation in multiple
tissues. Blood. 98:792a2001.
|
|
8
|
Maeda T, Hobbs R, Merghoub T, et al:
POKEMON is a proto-oncogene which plays a key role in
lymphomagenesis. Blood. 104:951a2004.
|
|
9
|
Laudes M, Bilkovski R, Oberhauser F, et
al: Transcription factor FBI-1 acts as a dual regulator in
adipogenesis by coordinated regulation of cyclin-A and E2F-4. J Mol
Med (Berlin). 86:597–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeon BN, Yoo JY, Choi WI, Lee CE, Yoon HG
and Hur MW: Proto-oncogene FBI-1 (Pokemon/ZBTB7A) represses
transcription of the tumor suppressor Rb gene via binding
competition with Sp1 and recruitment of co-repressors. J Biol Chem.
283:33199–33210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi WI, Jeon BN, Yun CO, et al:
Proto-oncogene FBI-1 represses transcription of p21CIP1 by
inhibition of transcription activation by p53 and Sp1. J Biol Chem.
284:12633–12644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roh HE, Lee MN, Jeon BN, et al: Regulation
of Pokemon 1 activity by sumoylation. Cell Physiol Biochem.
20:167–180. 2007.PubMed/NCBI
|
|
13
|
Samson SL and Wong NC: Role of Sp1 in
insulin regulation of gene expression. J Mol Endocrinol.
29:265–279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zu X, Yu L, Sun Q, et al: SP1 enhances
Zbtb7A gene expression via direct binding to GC box in HePG2 cells.
BMC Res Notes. 2:1752009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calera MR and Pilch PF: Induction of Akt-2
correlates with differentiation in Sol8 muscle cells. Biochem
Biophys Res Commun. 251:835–841. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roche S, Downward J, Raynal P and
Courtneidge SA: A function for phosphatidylinositol 3-kinase beta
(p85alpha-p110beta) in fibroblasts during mitogenesis: requirement
for insulin- and lysophosphatidic acid-mediated signal
transduction. Mol Cell Biol. 18:7119–7129. 1998.
|
|
17
|
Rother KI, Imai Y, Caruso M, Beguinot F,
Formisano P and Accili D: Evidence that IRS-2 phosphorylation is
required for insulin action in hepatocytes. J Biol Chem.
273:17491–17497. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slieker LJ, Sloop KW and Surface PL:
Differentiation method-dependent expression of leptin in adipocyte
cell lines. Biochem Biophys Res Commun. 251:225–229. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosen OM: After insulin binds. Science.
237:1452–1458. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O’Brien RM and Granner DK: Regulation of
gene expression by insulin. Physiol Rev. 76:1109–1161. 1996.
|
|
21
|
Koontz JW and Iwahashi M: Insulin as a
potent, specific growth factor in a rat hepatoma cell line.
Science. 211:947–949. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Block GD, Locker J, Bowen WC, et al:
Population expansion, clonal growth, and specific differentiation
patterns in primary cultures of hepatocytes induced by HGF/SF, EGF
and TGF alpha in a chemically defined (HGM) medium. J Cell Biol.
132:1133–1149. 1996. View Article : Google Scholar
|
|
23
|
Scassa ME, Guberman AS, Varone CL and
Cánepa ET: Phosphatidylinositol 3-kinase and Ras/mitogen-activated
protein kinase signaling pathways are required for the regulation
of 5-aminolevulinate synthase gene expression by insulin. Exp Cell
Res. 271:201–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saltiel AR and Pessin JE: Insulin
signaling pathways in time and space. Trends Cell Biol. 12:65–71.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyd DB: Insulin and cancer. Integr Cancer
Ther. 2:315–329. 2003. View Article : Google Scholar
|
|
26
|
Thompson AD III and Kakar SS: Insulin and
IGF-1 regulate the expression of the pituitary tumor transforming
gene (PTTG) in breast tumor cells. FEBS Lett. 579:3195–3200. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Messina JL: Inhibition and stimulation of
c-myc gene transcription by insulin in rat hepatoma cells. Insulin
alters the intragenic pausing of c-myc transcription. J Biol Chem.
266:17995–18001. 1991.PubMed/NCBI
|
|
28
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J and Jin T: Both Wnt and mTOR
signaling pathways are involved in insulin-stimulated
proto-oncogene expression in intestinal cells. Cell Signal.
20:219–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao ZH, Wang SF, Yu L, et al:
Overexpression of Pokemon in non-small cell lung cancer and
foreshowing tumor biological behavior as well as clinical results.
Lung Cancer. 62:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flegal KM, Carroll MD, Kuczmarski RJ and
Johnson CL: Overweight and obesity in the United States: prevalence
and trends, 1960–1994. Int J Obes Relat Metab Disord. 22:39–47.
1998.
|
|
32
|
Mason AL, Lau JY, Hoang N, et al:
Association of diabetes mellitus and chronic hepatitis C virus
infection. Hepatology. 29:328–333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar
|
|
34
|
Motoo Y, Kobayashi K and Hattori N: Effect
of insulin on the growth of a human hepatoma cell line PLC/PRF/5: a
possible role of insulin receptor. Tohoku J Exp Med. 156:351–357.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spydevold O, Sørensen H, Clausen OP and
Gautvik KM: Dexamethasone inhibition of rat hepatoma cell growth
and cell cycle traverse is reversed by insulin. Biochim Biophys
Acta. 1052:221–228. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Au WS, Kung HF and Lin MC: Regulation of
microsomal triglyceride transfer protein gene by insulin in HepG2
cells: roles of MAPKerk and MAPKp38. Diabetes. 52:1073–1080. 2003.
View Article : Google Scholar : PubMed/NCBI
|