Introduction
Based on the GLOBOCAN 2008 estimates, breast cancer
is the leading cause of cancer-related death in women worldwide.
New cases of breast cancer are estimated to be 1,383,500 with
458,400 deaths (1). Many
prescriptions for chemotherapy have been derived from plants
(2). Danshen (Salviae
miltiorrhizae radix) has been used in traditional Chinese
medicine for cardiovascular disease and appeared in the Chinese
book, Shennong Bencao Jing (ca. 100 A.D.) (3). Tanshinone IIA (Tan-IIA) was extracted
from danshen and was first described in 1968. It is known to have
antioxidant (4) and
anti-inflammatory properties (5).
Our previous studies showed that Tan-IIA induces apoptosis in
certain human cancer cells, such as human colon cancer colo 205
cells (6,7), human hepatocellular carcinoma hep-J5
cells (8), small-cell lung cancer
H146 cells (9) and non-small-cell
lung cancer A549 cells (10). Our
previous study also showed that Tan-IIA inhibited MDA-MB-231 cells
through inducing apoptosis by increasing Bax to Bcl-xL ratios and
the protein levels of p21 and caspase-8 in vitro (11). The anticancer effects of Tan-IIA on
human breast cancer cell lines in vitro and in vivo
have been well documented (12–15).
However, the molecular mechanisms have not been fully elucidated.
In the present study, we evaluated the effects and molecular
mechanisms of Tan-IIA on MDA-MB-231 cells in vitro using a
human breast cancer xenograft model in SCID mice.
Materials and methods
Chemicals
Tan-IIA (molecular formula:
C19H18O3, purity >96% by HPLC)
was purchased from Herbasin Co. (Shenyang, China). Corn oil,
aprotinin, antipain, sodium deoxycholate, leupeptin, sodium
orthovanadate, Triton X-100, Tris-HCl, ribonuclease-A and MTT
[3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide] were
obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dimethyl
sulfoxide (DMSO), potassium phosphates and TE buffer were purchased
from Merck Co. (Darmstadt, Germany). L-15 medium, fetal bovine
serum (FBS), penicillin-streptomycin, trypsin-EDTA and glutamine
were obtained from Gibco BRL (Grand Island, NY, USA). The
MDA-MB-231 human breast cancer cell line was obtained from the Food
Industry Research and Development Institute (Hsinchu, Taiwan).
Cell culture
The MDA-MB-231 human breast cancer cells were placed
into 75-cm3 tissue culture flasks and grown at 37˚C in a
humidified atmosphere (CO2 was not present) in L-15
medium (Sigma Chemical Co.) containing 10% heat-inactivated FBS, 1%
penicillin-streptomycin (10,000 U/ml penicillin and 10 mg/ml
streptomycin). The data presented herein are from a minimum of
three independent experiments.
The protein expression of LC3-II and Erb-B2 in
MDA-MB-231 cells treated with Tan-IIA was determined by western
blotting. The cytotoxicity of Tan-IIA on MDA-MB-231 cells has been
well documented (11). The result
showed that the proliferation of MDA-MB-231 cells was inhibited by
Tan-IIA in a dose- and time-dependent manner. The IC50
was 11.85±0.29 μg/ml when MDA-MB-231 cells were treated with
Tan-IIA for 48 h.
Protein preparation
Approximately 1×106 cells/10-cm dish were
incubated with Tan-IIA at 0, 3, 10 and 25 μg/ml concentrations for
48 h before the cells were harvested by centrifugation. Protein was
extracted as described (16).
Briefly, cell pellets were resuspended in modified RIPA buffer (50
mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium
deoxycholate, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 1 mM sodium
orthovanadate, 1 mM sodium fluoride, 5 μg/ml aprotinin, 5 μg/ml
leupeptin and 5 μg/ml antipain) for 30 min at 4˚C. Lysates were
immediately centrifuged at 13,000 × g for 20 min at 4˚C, and the
supernatant was collected, aliquoated (50 μg/tube) and stored at
−80˚C before being assayed. The protein concentrations were
measured using the Bradford method (17).
Effects of Tan-IIA on the expression of
LC3-II, Erb-B2 and β-actin in MDA-MB-231 cells
All samples were separated by sodium dodecyl sulfate
polyacrylamide (SDS-PAGE) gel electrophoresis (Bio-Rad Life Science
Products, Hercules, CA, USA) as previously described (16). The SDS separated proteins were
followed by equilibration in transfer buffer (50 mM Tris, pH 9.0,
40 mM glycine, 0.375% SDS and 20% methanol) and electro-transferred
to Immobilon-P Transfer membranes (Millipore Corporation, Bedford,
MA, USA). The blot was then blocked with a solution containing 5%
nonfat dry milk in Tris-buffered saline [10 mM Tris and 150 mM NaCl
(Sigma Chemical Co.)], containing 0.05% Tween-20 for 1 h. It was
then washed and incubated with antibodies to Erb-B2 (R&D),
LC3-II (Sigma Chemical Co.) and β-actin (Upstate, Lake Placid, NY,
USA) at 4˚C overnight. After incubating with anti-mouse
peroxidase-conjugated antibody (Santa Cruz, Santa Cruz, CA, USA),
the signal was visualized by enhanced chemiluminescence (ECL;
Amersham Pharmacia Biotech). The detection of β-actin was used as
an internal control in all the data for western blotting.
In vivo MDA-MB-231 cell tumor xenograft
model
Three-week-old female nude SCID mice (NOD.CB
17-Prkdcscid/Tcu) were xenografted with MDA-MB-231 human breast
cancer cells (3×106/0.2 ml) and maintained in a
pathogen-free environment (Laboratory Animal Center of Tzu Chi
University, Hualien, Taiwan). On day 28 onwards, the mice were
divided randomly into three groups (each group n=7) to be treated
with Tan-IIA (20 and 60 mg/kg of body weight, dissolved in corn
oil). As a control, xenografted tumors were separately treated with
corn oil (0.1 ml/10 g body weight). Each agent was administered
orally every other day. At the end of the 3-month dosing schedule,
the SCID mice were sacrificed by CO2 inhalation on the
97th day. After xenograft transplantation, mice exhibiting tumors
were monitored, and the tumor size was measured once every 2 days
using calipers. The tumor volume in each animal was estimated
according to the formula: Tumor volume (mm3) = L ×
W2/2 (where L is the length and W is the width), with
the final measurement taken 3 months after tumor cell inoculation.
At the same time, the body weight of each animal was measured once
every 2 days. The xenograft tumors were dissected and weighed
individually, then the proteins were extracted for western
blotting. The protein expression of NF-κBp65, caspase-3 and β-actin
was measured by western blotting. Immunoreactive bands were scanned
and analyzed using a digital scanning densitometer (Molecular
Dynamics, Sunnyvale, CA, USA).
Statistical analysis
Values are presented as the means ± SD. The
Student's t-test was used to analyze the statistical significance
and p<0.05 was considered significant for all tests.
Results and Discussion
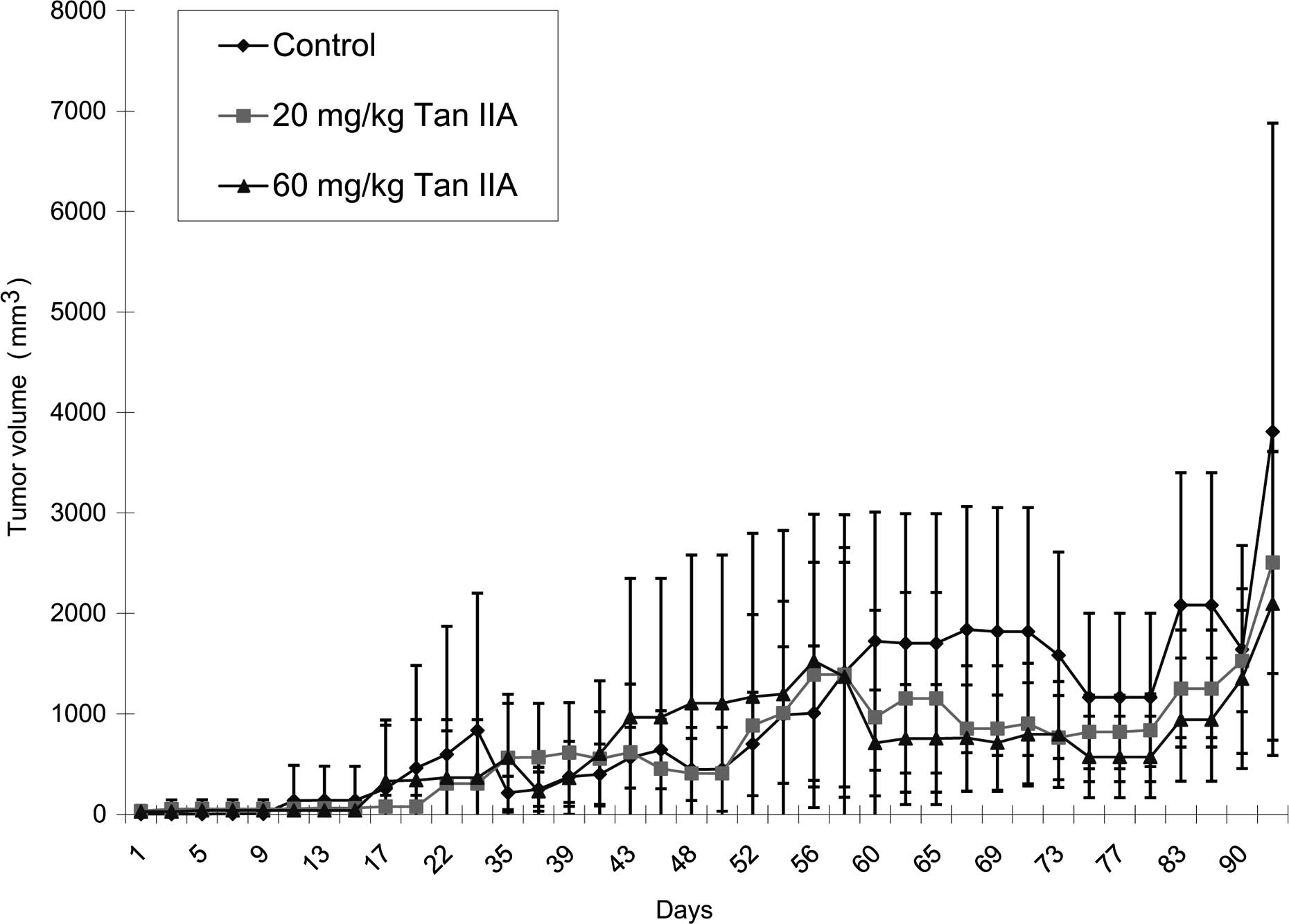
The results showed that Tan-IIA significantly
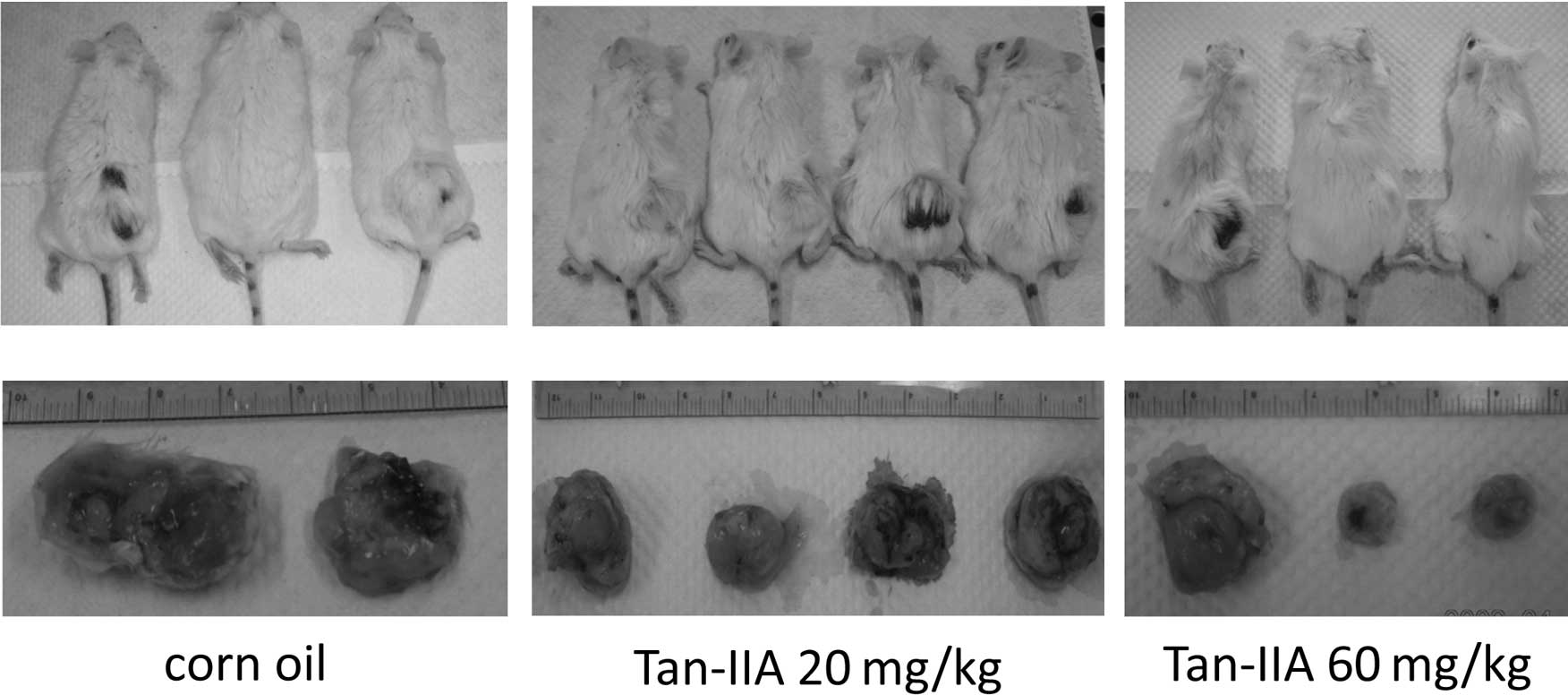
decreased the MDA-MB-231 cell xenograft tumor size (Fig. 1). An illustration of a
representative animal treated with Tan-IIA relative to the control
is shown in Fig. 2A. The results
also showed that Tan-IIA (60 mg/kg) significantly decreased the
weight of MDA-MB-231 cell xenograft tumors when compared to the
control group (Fig. 2B). All
MDA-MB-231 cell xenograft tumors appeared only at the inoculation
sites (data not shown). Based on these results, Tan-IIA treatment
(both 20 and 60 mg/kg body weight/per day) by oral administration
inhibited MDA-MB-231 cell xenograft tumor growth. In the present
study, mice with MDA-MB-231 xenograft tumors received Tan-IIA
treatment; these tumors continue to grow slowly, indicating that
multiple treatments are necessary to achieve a complete response.
However, the results showed that a high dose of Tan-IIA had
therapeutic potential in MDA-MB-231 cells in vivo. This is
in agreement with other studies (12,13).
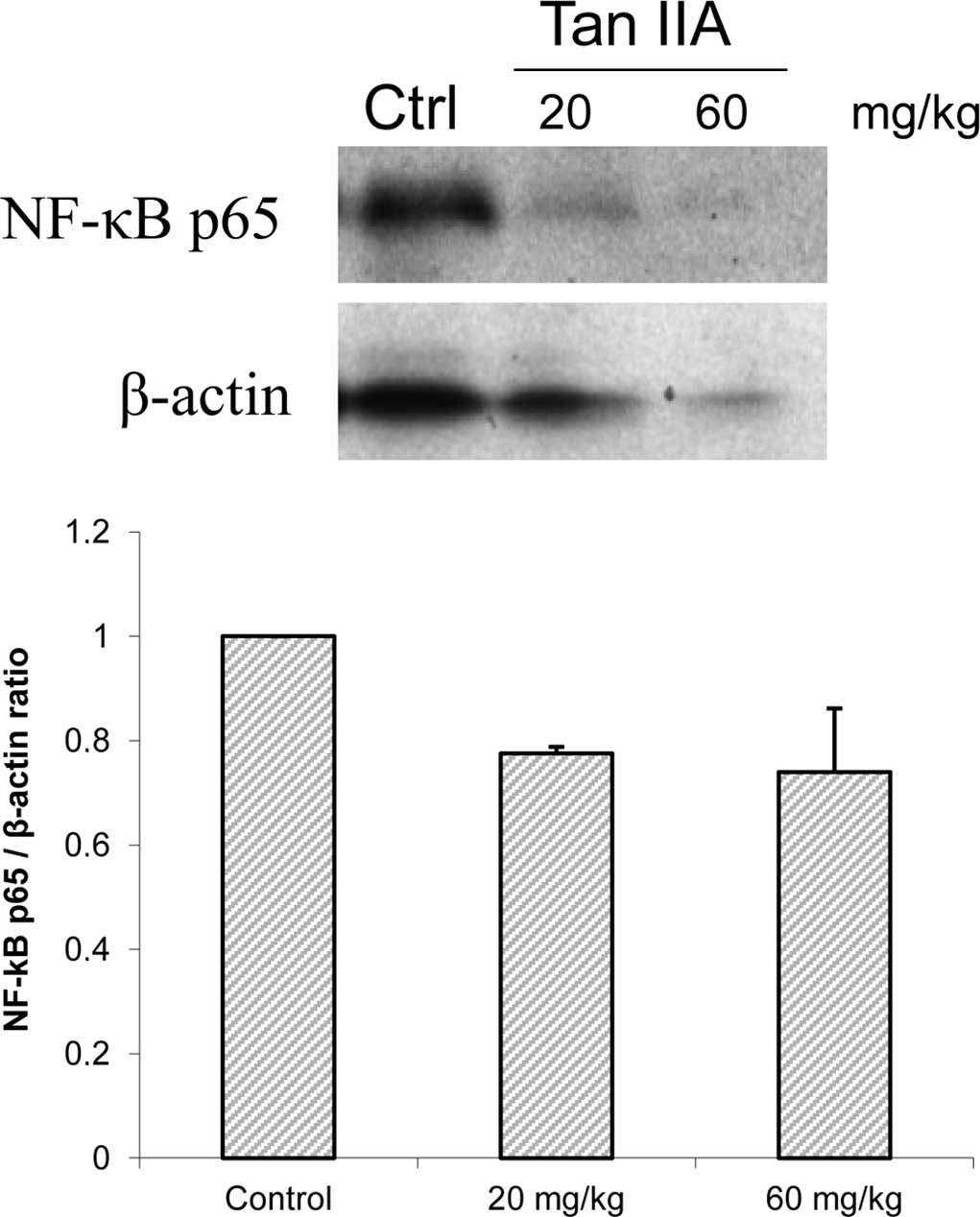
Our results showed that MDA-MB-231 cell xenograft tumors treated
with Tan-IIA decreased NF-κBp65 (Fig.
3A), but increased caspase 3 expression (Fig. 3B) when compared to the control
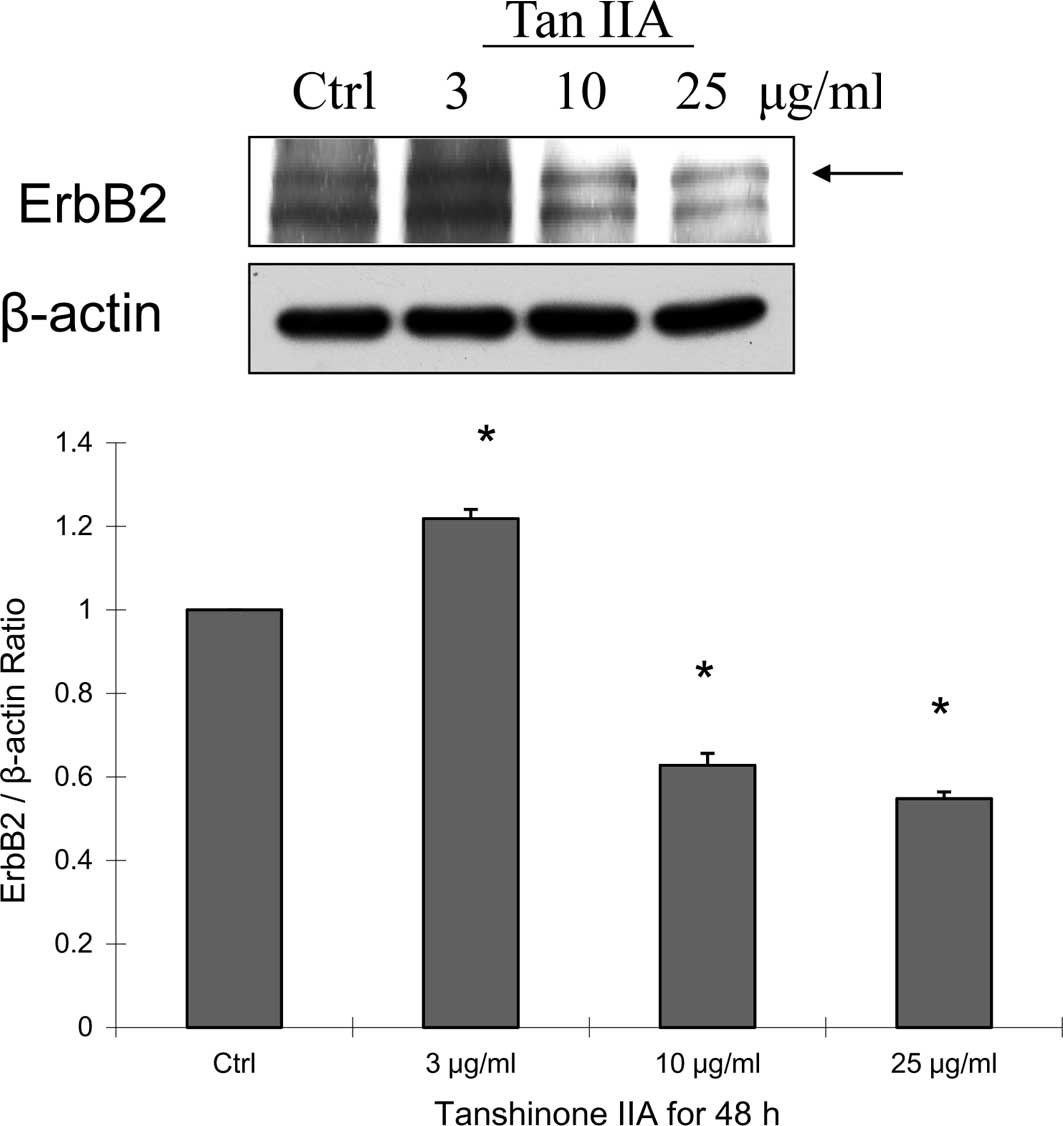
group. Our results also showed that MDA-MB-231 cells treated with
Tan-IIA for 48 h decreased the protein expression of Erb-B2
(Fig. 4A) and LC3-II (Fig. 4B), but increased caspase 3
(Fig. 4C) expression. This is not
in agreement with other studies, which showed that xenograft tumors
treated with Tan-IIA decreased P53 and bcl-2 expression, but not of
cerbB-2 (12,13). The present study provides the first
report of Tan-IIA anti-human breast cancer activity through a
decrease in Erb-B2 and LC3-II expression in vitro and a
decrease in NF-κBp65 expression in an MDA-MB-231 cell xenograft
tumor model.
Acknowledgements
This study was supported by the grant NSC
98-2314-B-303-004 from the National Science Council, Department of
Health, Executive Yuan, Taiwan, R.O.C.
References
|
1
|
A JemalF BrayMM CenterJ FerlayE WardD
FormanGlobal Cancer StatisticsCA Cancer J
Clin616990201110.3322/caac.20107
|
|
2
|
TW LeungPJ JohnsonSystemic therapy for
hepatocellular carcinomaSemin
Oncol28514520200110.1016/S0093-7754(01)90144-711685744
|
|
3
|
ZT LiBJ YangGE MaChemical studies of
Salvia miltiorrhiza f. albaYaoxue Xuebao262092131991(In
Chinese).
|
|
4
|
TJ LinAntioxidation mechanism of
schizandrin and tanshinonatic acid A and their effects on the
protection of cardio-toxic action of adriamycinShengli Kexue
Jinzhan223423451991(In Chinese).
|
|
5
|
Y LiangYM YangSL YuanStudies on Pharmic
mechanism and clinic application of TanshinoneTradit Herbal
Drugs313043062000
|
|
6
|
CC SuYH LinTanshinone IIA down-regulates
the protein expression of ErbB-2 and up-regulates TNF-α in colon
cancer cells in vitro and in vivoInt J Mol
Med22847851200819020785
|
|
7
|
CC SuGW ChenJC KangMH ChanGrowth
inhibition and apoptosis induction by tanshinone IIA in human colon
adenocarcinoma cellsPlanta
Med7413571362200810.1055/s-2008-108129918622903
|
|
8
|
CY ChengCC SuTanshinone IIA inhibits
Hep-J5 cells by increasing calreticulin, caspase 12 and GADD153
protein expressionInt J Mol Med26379385201020664954
|
|
9
|
CY ChengCC SuTanshinone IIA may inhibit
the growth of small cell lung cancer H146 cells by up-regulating
the Bax/Bcl-2 ratio and decreasing mitochondrial membrane
potentialMol Med Rep3645650201021472292
|
|
10
|
TL ChiuCC SuTanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potentialInt J Mol Med25231236201020043132
|
|
11
|
CC SuYH LinTanshinone IIA inhibits human
breast cancer cells through increased Bax to Bcl-xL ratiosInt J Mol
Med22357361200818698495
|
|
12
|
X ZhangPR ZhangJ ChenQA LüStudy on the
effect of Tanshinone II A against human breast cancer in
vivoSichuan Da Xue Xue Bao Yi Xue Ban4162672010(In Chinese).
|
|
13
|
Q LuP ZhangX ZhangJ ChenExperimental study
of the anti-cancer mechanism of tanshinone IIA against human breast
cancerInt J Mol Med24773780200919885617
|
|
14
|
PR ZhangQA Lüstudy on anticancer activity
of tanshinone IIA against human breast cancerSichuan Da Xue Xue Bao
Yi Xue Ban402452492009(In Chinese).
|
|
15
|
X WangY WeiS YuanG LiuY LuJ ZhangW
WangPotential anticancer activity of tanshinone IIA against human
breast cancerInt J Cancer116799807200510.1002/ijc.2088015849732
|
|
16
|
HC ChenWT HsiehWC ChangJG ChungAloe-emodin
induced in vitro G2/M arrest of cell cycle in human promyelocytic
leukemia HL-60 cellsFood Chem
Toxicol4212511257200410.1016/j.fct.2004.03.00215207375
|
|
17
|
MM BradfordA rapid and sensitive method
for the quantitation of microgram quantities of protein using the
principle of protein-dye bindingAnal
Biochem72248254197610.1016/0003-2697(76)90527-3942051
|