Introduction
Gastric cancer is the most common malignant tumor of
the digestive tract. It has a poor prognosis and results in
frequent death caused by postoperative relapse and metastasis
(1,2). Peritoneal implantation metastasis of
gastric cancers constitutes nearly 50% of postoperative relapses
and is a leading cause of death in patients with gastric cancer
(3,4). However, the detailed mechanisms of
peritoneal metastasis of gastric cancer have not been fully
understood. A better strategy to prevent and treat peritoneal
metastasis is also required.
Peritoneal immune cells, including T lymphocytes,
neutrophils, natural killer cells and macrophages, are cellular
components of innate immunity which protect against tumor cells
(5). Peritoneal macrophages are
the most important immune cells in the abdominal cavity, and the
function of macrophages is critical to prevent the peritoneal
implantation metastasis of gastric cancer cells (6). Classically activated M1 macrophages
are capable of phagocytosing microorganisms and tumor cells,
antigen processing and presentation and producing proinflammatory
cytokines (7). Therefore, they are
involved in the peritoneal immunity against infection and tumor
cell invasion and play a critical role in the cellular immunity
against gastric cancer. On the contrary, alternatively activated M2
macrophages display a distinct function from M1 macrophages. M2
macrophages cannot prevent tumor progression, but rather facilitate
tumor cell proliferation, angiogenesis and tissue remodeling
(7,8). It has been reported that tumor cells
can secrete inhibitory cytokines to evade immune surveillance
(9). Peritumoral macrophages
exhibit alternative activation possibly through the action of
various cytokines secreted by tumor cells.
In this study, mouse forestomach cells (MFCs)
(10) and isolated peritoneal
macrophages were recruited to investigate the immunosuppressive
effects of gastric carcinoma cells on macrophages. MFC conditioned
medium (CM) was collected and used to treat peritoneal macrophages.
The phagocytotic ability, cytokine secretion and M1/M2 macrophage
markers were analyzed. Further examination disclosed that
transforming growth factor (TGF)-β1 may be the key cytokine through
which MFCs modify macrophage functions.
Materials and methods
Isolation of peritoneal macrophages and
cell culture
Eight-week-old male C57BL6 mice were purchased from
the Institute of Laboratory Animal Science of the Chinese Academy
of Medical Science. Mice were sacrificed by cervical dislocation.
Five milliliters of precooled RPMI-1640 medium was injected into
the abdominal cavity using a syringe. After injection, gentle
massage was performed on the peritoneum to dislodge attached cells.
The peritoneal fluid was collected into another syringe and the
peritoneal cavity was washed twice. The fluid and wash solution
were centrifuged and the macrophages were purified using the
adherence method. All the protocols were reviewed and approved by
the Animal Care and Use Committee of the Third Military Medical
University.
Both macrophages and MFCs were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 μg/ml streptomycin at 37˚C in a humidified, 5%
CO2 atmosphere. MFCs (1×106) were plated into
a 6-well plate. After culture for 2 days, the supernatant of the
MFCs was collected as MFC CM and stored at −20˚C until use.
Macrophages were treated with CM alone or together with 1 μg/ml
TGF-β1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 2
days and then underwent subsequent analysis.
Phagocytosis assay
The phagocytotic ability of macrophages was measured
by the CytoSelect phagocytosis assay kit (Cell Biolabs, Inc., San
Diego, CA) according to the manufacturer's instructions. Zymosan
was used as a substrate in this assay. The absorbance of each
sample was read at 405 nm.
Measurement of cytokine secretion
After treatment, the supernant of the macrophages
was collected and the concentrations of tumor necrosis factor
(TNF)-α, interleukin (IL)-1β, IL-10 and vascular endothelial growth
factor (VEGF) were measured by ELISA kit (Invitrogen, Carlsbad,
CA).
RNA extraction and real-time RT-PCR
Total RNA was isolated from the macrophages by
TRIzol reagent (Invitrogen) and first strand cDNA was generated by
AMV reverse transcriptase (Takara, Dalian, China) with Oligo
dT-Adaptor Primer (Takara). Gene specific primers for inducible
nitric oxide synthase (iNOS) (forward, 5′-TTCTGTGCTGTCCCA GTGAG-3′;
reverse, 5′-TGAAGAAAACCCCTTGTGCT-3′), chemokine (C-X-C motif)
ligand (CXCL) 11 (forward, 5′-CGCCCCTGTTTGAACATAAG-3′; reverse,
5′-CTGCTGA GATGAACAGGAAGG-3′), arginase-1 (forward, 5′-TTTTTC
CAGCAGACCAGCTT-3′; reverse, 5′-AGAGATTATCGG AGCGCCTT-3′) and found
in inflammatory zone 1 (Fizz1) (forward,
5′-CTGGATTGGCAAGAAGTTCC-3′; reverse, 5′-CCCTTCTCATCTGCATCTCC-3′)
were used for expression analysis by real-time PCR on the ABI 7500
thermocycler (Applied Biosystem, Foster City, CA) using SYBR-Green
mix (Applied Biosystem). β-actin (forward, 5′-ATGGAGGGGAAT
ACAGCCC-3′; reverse, 5′-TTCTTTGCAGCTCCTTCGTT-3′) was used for
normalization.
Western blot analysis
After treatment, macrophages were washed with
ice-cooled phosphate-buffered saline twice and lysed with M-PER
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific,
Inc., Rockford, IL). Then, 20 μg of total protein was separated by
10% SDS-PAGE and transferred to PVDF membranes (Millipore,
Billerica, MA). After blocking with 5% skim milk, antibodies for
Smad2 or phosphorylated-Smad2 (p-Smad2) (Santa Cruz Biotechnology)
were incubated with the membranes overnight at 4˚C. Horseradish
peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology)
was used to detect the protein levels.
Statistical analysis
All values are expressed as the means ± SEM.
Statistical analyses were performed using the Student's t-test.
Differences having a p-value <0.05 were considered statistically
significant.
Results
MFC CM induces immunosuppression of
macrophages
Mouse peritoneal macrophages were isolated, and flow
cytometric assay revealed that >90% of the total cells were
CD68-positive cells, which indicated that the isolated cells were
macrophages (data not shown). To determine the indirect effects of
MFCs on macrophages, the MFC CM was used to the treat macrophages
for 2 days. Phagocytosis assay was performed to evaluate the
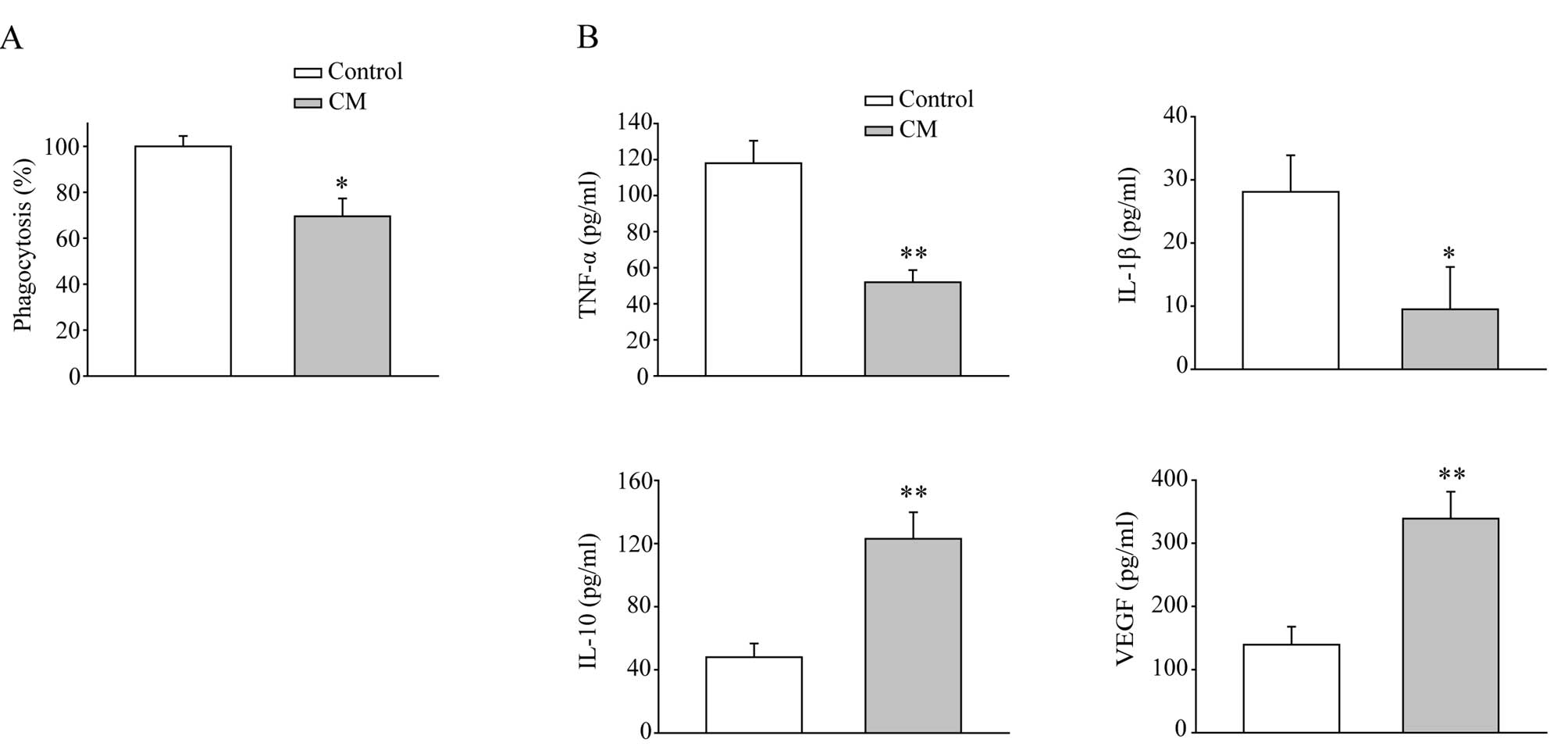
functional change in macrophages. As shown in Fig. 1A, the CM significantly reduced the
phagocytotic capability of macrophages. Macrophages also secreted
much less TNF-α and IL-1β after CM treatment (Fig. 1B). Meanwhile, secretion of IL-10,
which is an immunosuppressive cytokine, was elevated nearly 3-fold
compared to the control cells. Notably, VEGF, which is able to
promote angiogenesis and accelerate tumor growth, was also greatly
increased by CM treatment. These data suggest that MFC-derived
soluble factors generate a microenvironment which suppresses the
innate immunity of macrophages and induces angiogenesis, thus
supporting tumor progression.
MFC CM induces macrophage
polarization
The immunosuppressive state of macrophages is always
accompanied by the increase in alternatively activated macrophages,
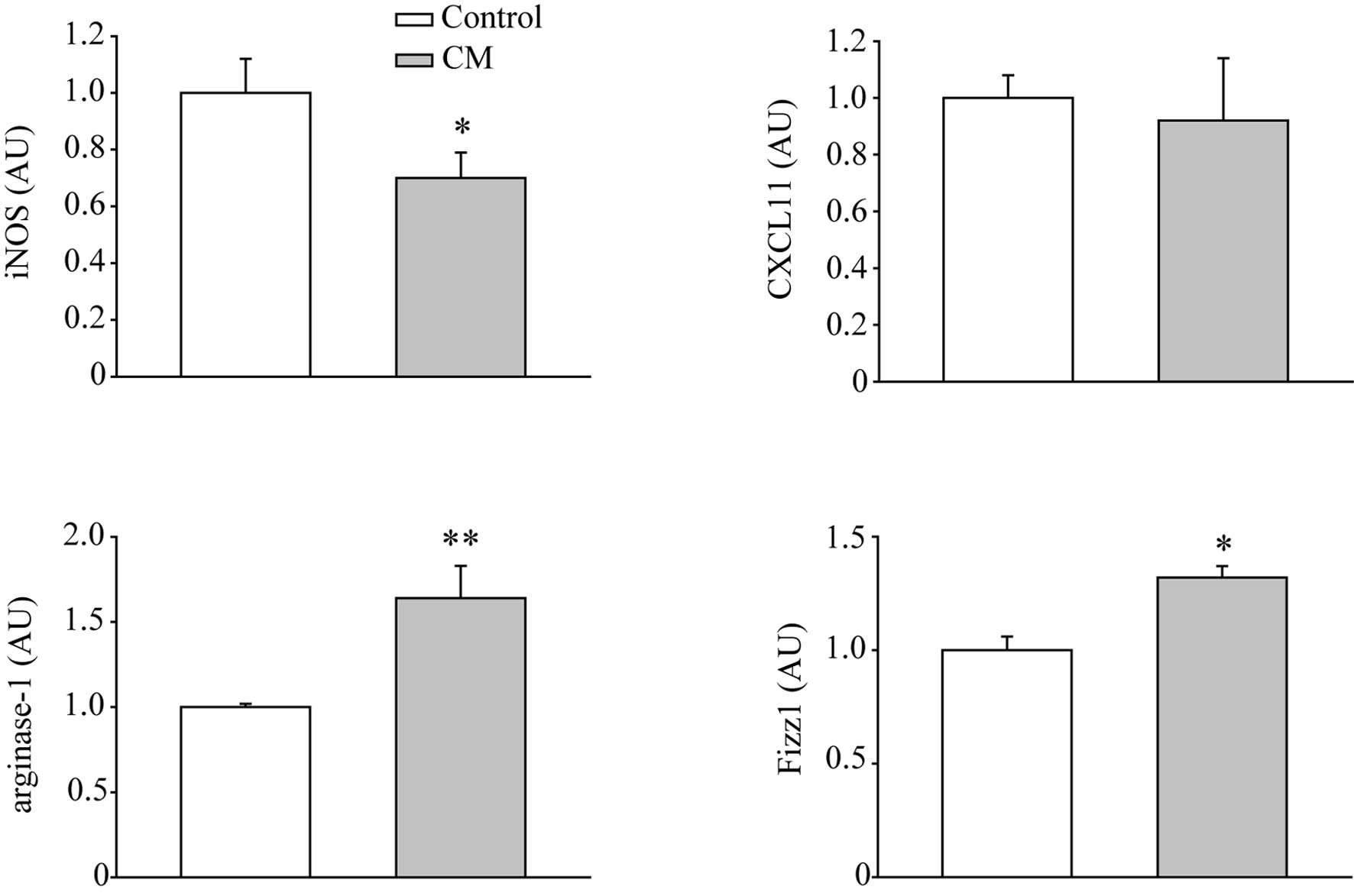
also called M2 macrophages. Therefore, real-time RT-PCR was
performed to examine the markers of M1 and M2 macrophages. As shown
in Fig. 2, CM down-regulated the
mRNA levels of M1 macrophage marker, iNOS, but not CXCL11. The M2
macrophage markers, arginase-1 and Fizz1, were increased by
treatment of CM for 2 days, indicating CM stimulated the M2
macrophage polarization.
Increased level of TGF-β1 in CM activates
TGF-β1 signaling in macrophages
Many different types of cytokines respond to
macrophage immunosuppression. We further measured the concentration
of these cytokines in the CM, including macrophage inhibitory
cytokine-1, soluble colony-stimulating factor, TGF-β1, IL-4 and
IL-10. Among them, only the TGF-β1 level was dramatically elevated
in the CM (3.05±0.58 pg/ml in RPMI-1640 medium, 107.56±4.82 pg/ml
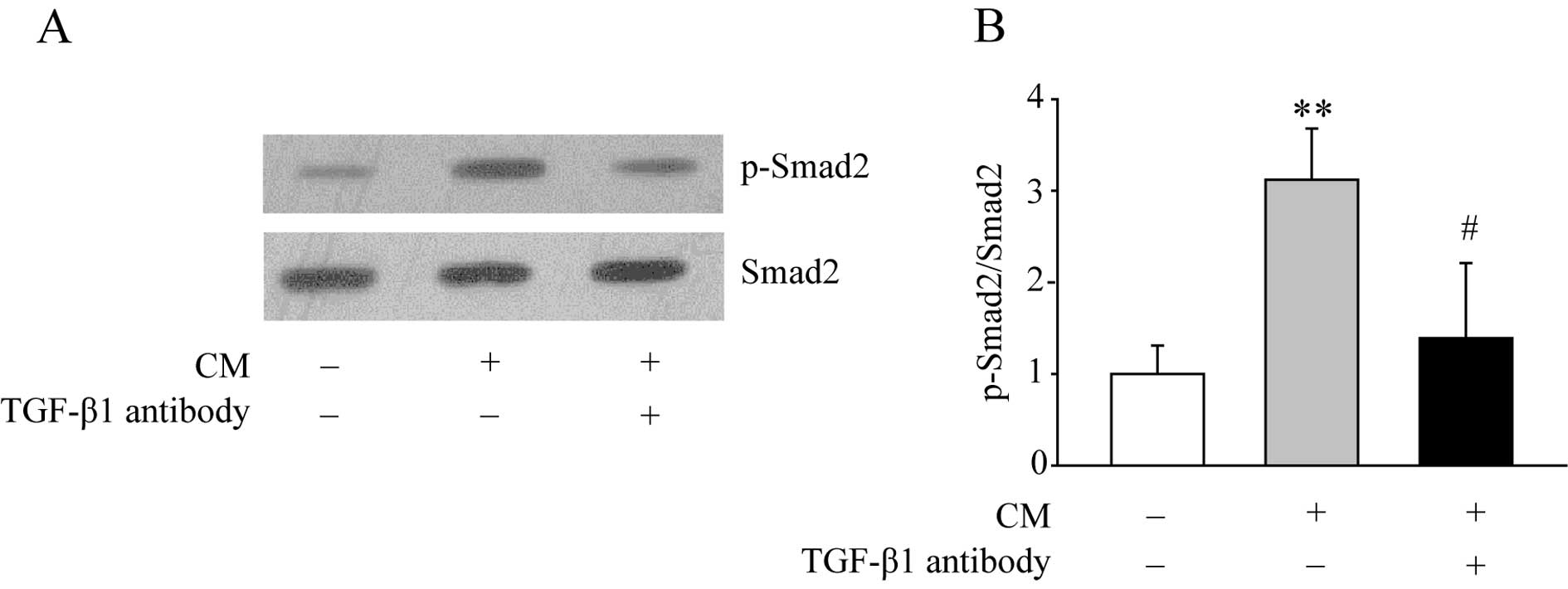
in CM, n=4, p<0.01 and data not shown). Next, the downstream
TGF-β1 signaling was examined by western blotting. As shown in
Fig. 3, CM treatment increased the
expression level of p-Smad2. Moreover, when macrophages were
treated with CM and the TGF-β1 antibody together, the increased
level of p-Smad2 was significantly diminished.
Neutralization of TGF-β1 restores
macrophage functions
Since CM was collected 2 days after culturing with
MFCs, the nutrient contents and growth factors in the CM may have
been depleted due to MFC consumption, consequently interfering with
the macrophage functions. To further confirm the specific role of
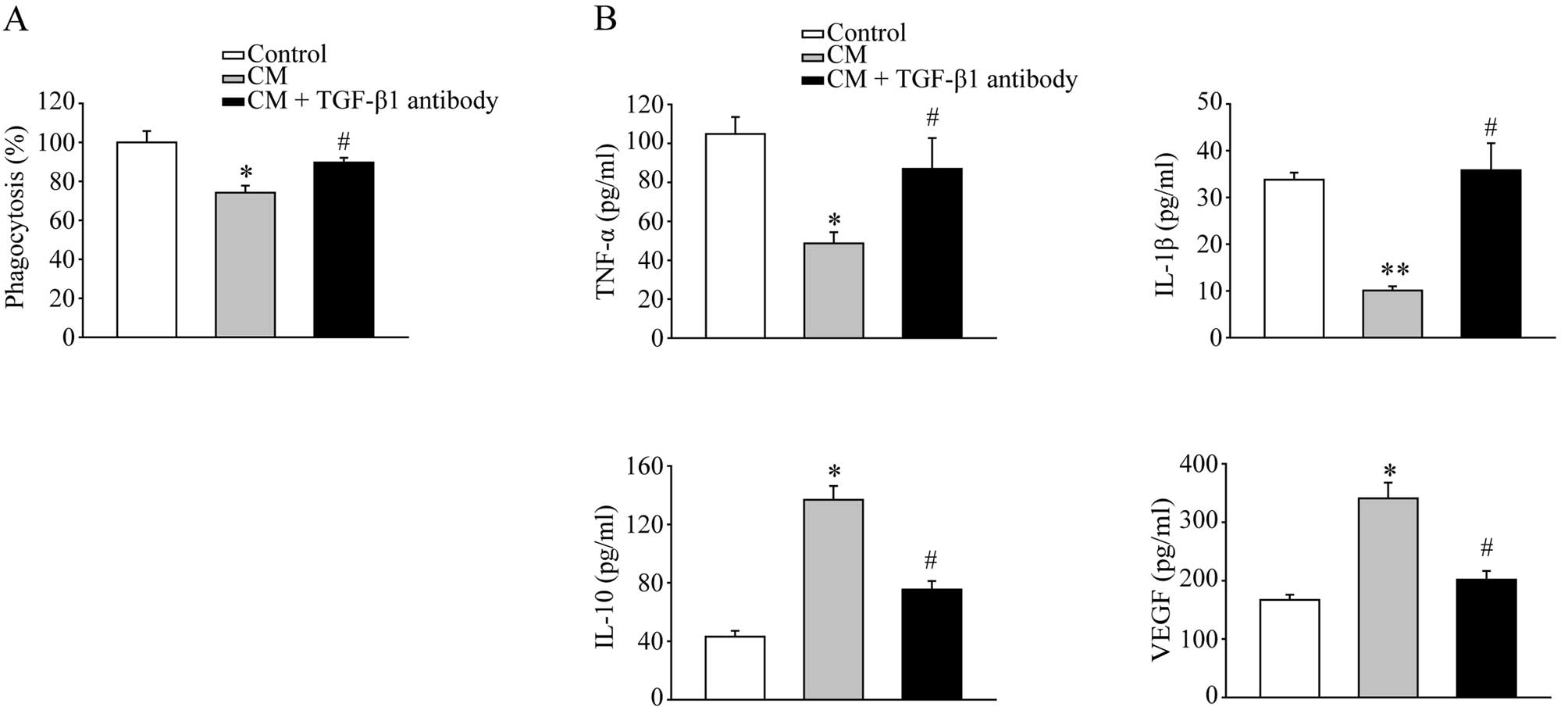
TGF-β1 in the inhibitory effects of CM on macrophages, the TGF-β1
antibody was added to the CM to neutralize TGF-β1. The phagocytosis
assay revealed that the TGF-β1 antibody reversed the suppressive
effects of CM on macrophages (Fig.
4A). The effects of CM on cytokine secretion was also blocked
by the TGF-β1 antibody (Fig. 4B).
These data suggest that TGF-β1 is the key cytokine secreted by MFCs
to induce immunosuppressive macrophages.
Discussion
In the present study, we demonstrated that the
immune functions of isolated peritoneal macrophages was restrained
by CM, which contained inhibitory cytokines secreted by MFCs. The
macrophages treated with CM showed weaker phagocytosis, less TNF-α
and IL-1β production, increased secretion of IL-10 and VEGF and
gain of M2 macrophage phenotypes. Among the different cytokines,
the level of TGF-β1 in the CM was greatly increased and the TGF-β1
signaling was activated in the macrophages, evidenced by the
phosphorylation of Smad2. Neutralization of TGF-β1 by its antibody
helped macrophages retain their functions.
Macrophages are characterized by their remarkable
versatility, heterogeneity and plasticity. They can response to
different cytokines and certain microbial products that are present
in the microenvironment (11).
Activated macrophages induced by interferon (IFN)-γ, either alone
or in combination with LPS, were found to produce a large amount of
toxic agents, such as nitric oxide and reactive oxygen species,
with strong antigen presentation capability and they further
activated type I immune response (7,8,11).
Macrophages which can be alternatively activated by IL-4 and IL-10,
are known as M2 macrophages (12).
M2 macrophages restrict inflammation and type I adaptive immunity,
scavenge residues, promote angiogenesis and participate in tissue
remodeling and repair (7). In the
present study the increased expression levels of arginase-1 and
Fizz1, as well as reduced iNOS expression, indicated the
polarization of M2. However, the M1 macrophage marker CXCL11, which
is an IFN-γ inducible gene (13),
did not show a difference between the two groups. Several other
types of tumors are also capable of inducing M2 macrophages and
promoting tumor progression, including glioma cancer (14), breast cancer (15) and hepatocellular carcinoma
(16).
Our data suggest that TGF-β1 played a central role
in the CM to regulate macrophage functions. Blocking TGF-β1
signaling by its antibody decreased p-Smad2 expression and
antagonized the immunosuppressive effects of the CM. TGF-β1 is a
member of a class of multifunctional polypeptide growth factors
that play important regulatory roles in cell proliferation and
differentiation, extracellular matrix production, angiogenesis,
apoptosis and the immune system. TGF-β1 regulates cellular
processes by binding to its cell-surface receptors and initiates
intracellular signaling by phosphorylating several transcription
factors known as Smads (17).
TGF-β1 has dual roles during tumorigenesis, both as a tumor
suppressor and a tumor promoter. In the early stage, TGF-β1
controls cell growth and cell cycle progression. As tumor cells
acquire certain genetic and epigenetic changes in the genome, they
switch the TGF-β1 response from inhibition of proliferation to
promotion of growth, motility and invasion (18). The interference of phagocytosis by
MFC-derived TGF-β1, together with reduced levels of proinflammatory
cytokines and elevated levels of anti-inflammatory cytokines,
contribute to the escape of immune surveillance. Moreover, the CM
induced higher VEGF secretion from the macrophages, suggesting that
macrophages ameliorate tumor growth by enhanced angiogenesis. In
gastric cancer patients, an elevated serum TGF-β1 was observed and
correlated with venous invasion (19). In addition, TGF-β1 receptor
inhibitors down-regulated the invasion, migration and
epithelial-to-mesenchymal transition of scirrhous gastric cancer
cells, suggesting the autocrine role of TGF-β1 during tumorigenesis
(20). Approaches targeting TGF-β1
signaling may have beneficial effects on both tumor cells and
macrophages.
In summary, the secreted factors by MFCs were able
to induce immunosuppression of macrophages, thus avoiding immune
restrictions and transforming macrophages into the tumor-promoting
phenotype. These effects were mostly, if not totally, through
TGF-β1 secretion. The present study provided preliminary in
vitro evidence of the central role of TGF-β1 in the interaction
between MFCs and peritoneal macrophages. The components of TGF-β1
signaling may be promising candidates for the prevention and
management of the peritoneal metastasis of gastric cancers.
Abbreviations:
|
MFCs
|
mouse forestomach cells
|
|
CM
|
conditioned medium
|
|
TGF
|
transforming growth factor
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
VEGF
|
vascular endothelial growth factor
|
|
iNOS
|
inducible nitric oxide synthase
|
|
CXCL
|
chemokine (C-X-C motif) ligand
|
|
Fizz1
|
found in inflammatory zone 1
|
|
IFN
|
interferon
|
References
|
1
|
S MoriguchiY MaeharaD KorenagaK SugimachiY
NoseRisk factors which predict pattern of recurrence after curative
surgery for patients with advanced gastric cancerSurg
Oncol1341346199210.1016/0960-7404(92)90034-I1341269
|
|
2
|
U Ribeiro JrJJ Gama-RodriguesAV
Safatle-RibeiroPrognostic significance of intraperitoneal free
cancer cells obtained by laparoscopic peritoneal lavage in patients
with gastric cancerJ Gastrointest
Surg2244249199810.1016/S1091-255X(98)80019-X9841981
|
|
3
|
E BandoY YonemuraY TakeshitaIntraoperative
lavage for cytological examination in 1,297 patients with gastric
carcinomaAm J
Surg178256262199910.1016/S0002-9610(99)00162-210527450
|
|
4
|
CH YooSH NohDW ShinSH ChoiJS MinRecurrence
following curative resection for gastric carcinomaBr J
Surg87236242200010.1046/j.1365-2168.2000.01360.x10671934
|
|
5
|
K MaruyamaZ SelmaniH IshiiK
YamaguchiInnate immunity and cancer therapyInt
Immunopharmacol11350357201110.1016/j.intimp.2010.09.012
|
|
6
|
SJ OosterlingGJ van der BijM BogelsJR van
der SijpRH BeelenS MeijerM van EgmondInsufficient ability of
omental milky spots to prevent peritoneal tumor outgrowth supports
omentectomy in minimal residual diseaseCancer Immunol
Immunother5510431051200610.1007/s00262-005-0101-y
|
|
7
|
A MantovaniS SozzaniM LocatiP AllavenaA
SicaMacrophage polarization: tumor-associated macrophages as a
paradigm for polarized M2 mononuclear phagocytesTrends
Immunol23549555200210.1016/S1471-4906(02)02302-512401408
|
|
8
|
A MantovaniA SicaS SozzaniP AllavenaA
VecchiM LocatiThe chemokine system in diverse forms of macrophage
activation and polarizationTrends
Immunol25677686200410.1016/j.it.2004.09.01515530839
|
|
9
|
A MantovaniA SicaMacrophages, innate
immunity and cancer: balance, tolerance, and diversityCurr Opin
Immunol22231237201010.1016/j.coi.2010.01.00920144856
|
|
10
|
SS QianJ GaoJX WangY LiuHY
DongEstablishment of a mouse forestomach carcinoma cell line with
spontaneous hematogenous metastasis and preliminary study of its
biological characteristicsZhonghua Zhong Liu Za
Zhi926126419873678016
|
|
11
|
DM MosserJP EdwardsExploring the full
spectrum of macrophage activationNat Rev
Immunol8958969200810.1038/nri244819029990
|
|
12
|
S GoerdtCE OrfanosOther functions, other
genes: alternative activation of antigen-presenting
cellsImmunity10137142199910.1016/S1074-7613(00)80014-X10072066
|
|
13
|
SA BensonJD ErnstTLR2-dependent inhibition
of macrophage responses to IFN-γ is mediated by distinct,
gene-specific mechanismsPLoS One4e63292009
|
|
14
|
A WuJ WeiLY KongGlioma cancer stem cells
induce immunosuppressive macrophages/microgliaNeuro
Oncol1211131125201010.1093/neuonc/noq08220667896
|
|
15
|
J O'BrienP SchedinMacrophages in breast
cancer: do involution macrophages account for the poor prognosis of
pregnancy-associated breast cancer?J Mammary Gland Biol
Neoplasia14145157200910.1007/s10911-009-9118-819350209
|
|
16
|
YW LiSJ QiuJ FanTumor-infiltrating
macrophages can predict favorable prognosis in hepatocellular
carcinoma after resectionJ Cancer Res Clin
Oncol135439449200910.1007/s00432-008-0469-018781326
|
|
17
|
GC BlobeWP SchiemannHF LodishRole of
transforming growth factor β in human diseaseN Engl J
Med342135013582000
|
|
18
|
GJ InmanSwitching TGFβ from a tumor
suppressor to a tumor promoterCurr Opin Genet Dev2193992011
|
|
19
|
Y LinS KikuchiY ObataK YagyuSerum levels
of transforming growth factor β1 are significantly correlated with
venous invasion in patients with gastric cancerJ Gastroenterol
Hepatol214324372006
|
|
20
|
O ShintoM YashiroH KawajiriK ShimizuT
ShimizuA MiwaK HirakawaInhibitory effect of a TGFβ receptor type-I
inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer
cellsBr J Cancer1028448512010
|