Introduction
Dengue virus (DENV), with 4 serologic types, is one
of the most prevalent flaviviruses to have re-emerged during recent
decades. The virus has been identified globally and is no longer
associated with tropical and subtropical countries only. Due to
lack of vaccines and specific treatments, ~2.5 billion individuals
from >110 countries are at risk of DENV infection each year and
>500,000 individuals are affected by the potentially fatal
dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS)
(1). Therefore, studies on
non-vaccine surrogates to control the spread of DENV and eradicate
severe forms of the infection are urgently required.
Although the pathogenesis of DHF/DSS remains to be
adequately elucidated, immune enhancement, cytokine storm,
secondary infection and virulence variation all appear to
contribute to the devastating physiopathology of DENV infection. It
is hypothesized that a number of clinical complications are closely
associated with the host immune response (2,3). In
specific cases of secondary infection, a burst of cytokines and
additional inflammatory molecules from cross-reactive T cells, mast
or endothelial cells has been observed (4). A complex interactive network, that
includes synergistic and antagonistic effects of the initial
cytokines, in turn induces a cascade of additional cytokines and
inflammatory mediators (4).
The expression of cytokine encoding genes is
regulated and affected by a number of endogenous and exogenous
factors. Recently, microRNAs (miRNAs) were identified as key
components of immune regulation (5). Mature miRNAs, ~22–25 nucleotides in
length, downregulate mRNA expression through the assembly of a
miRNA-induced silencing complex known as RISC, which binds
complementary sequences in target mRNAs and subsequently causes
catalytic cleavage or transcriptional repression of target mRNAs
(6). Several miRNAs were
demonstrated to regulate downstream inflammatory responses by
directly targeting genes encoding molecules that are secreted by or
are expressed on the surface of immune cells (7–9).
Specific miRNAs are capable of targeting genes that control
epigenetic pathways. These miRNAs affect the expression of certain
epigenetic regulators, including histone deacetylases, DNA
methyltransferases and polycomb group genes. This network between
miRNAs and epigenetic pathways appears to form an epigenetic-miRNA
regulatory circuit and regulate genome expression (10).
In the present study, we hypothesized that,
following DENV infection, excessive secretion of cytokines and
inflammatory mediators occurs, in part, as a result of the activity
of miRNAs that target the corresponding genes or regulators. Using
a cytokine array and miRNA microarray, we examined the expression
of inflammatory cytokines and profile of miRNAs, respectively, in
DENV serotype 2 (DENV2)-infected peripheral blood mononuclear cells
(PBMCs). Data analysis was performed using several online databases
to elucidate the potential molecular mechanisms underlying the
pathology of DENV infection. This in-depth study provides novel
insight into potential therapeutic treatments to attenuate the
vigorous host immune responses in DENV infection.
Materials and methods
Collection of blood samples from
donors
All blood samples and procedures in this study were
approved by the Ethics Committee of Sun Yat sen University. Healthy
volunteers provided voluntary informed consent. Blood samples were
provided by healthy volunteers (n=6) at the Zhongshan School of
Medicine (Sun Yat-sen University, China). Samples were processed
independently. PBMCs were isolated from each sample by
Ficoll-Hypaque density gradient centrifugation. PBMCs were then
washed once in RPMI-1640 medium and resuspended in the same medium
containing 10% inactivated fetal calf serum. PBMCs were cultured in
24-well culture plates (all obtained from Gibco, Carlsbad, CA, USA)
and incubated at 37°C in 5% CO2 for 6 or 12 h.
Expansion and titration of DENV2
The DENV2 serotype used in this study originated
from the New Guinea C strain. The virus was bred in C6/36 mosquito
cells that were cultured in Eagle’s Minimal Essential Medium
containing 10% fetal bovine serum (FBS). The viral suspension was
added to the cell cultures and incubated for 3–5 days. The virus
was harvested from the culture supernatant by centrifugation to
separate the cells and cell debris. The supernatant was stored at
−80°C until required. To determine the plaque-forming units (PFU)
of the viral suspension, C6/36 cells were first seeded in a 24-well
plate at a concentration of 1×106 cells/well and then
infected with serial dilutions of the viral suspension. The plate
was incubated at 37°C for 5 days. Plaques were visualized by
staining with 0.5% crystal violet and PFU/ml was estimated using a
previously described method (11).
Infection of PBMCs with DENV2
Six of the PBMC cultures obtained from blood samples
of the healthy volunteers were used for DENV2 infection. Confluent
cultures of PBMCs in 24-well plates were rinsed with RPMI-1640
medium and infected with the viral suspension in the same medium,
at a multiplicity of infection of 4 PFU/106 cells. Cells
were infected for 2 h at 37°C. Following cell infection, the medium
was discarded and replaced with RPMI-1640 medium containing 4% FBS
and the plates were incubated further for 6 or 12 h. Culture
supernatants containing DENV2 were separated from cells and cell
debris by centrifugation at 1,000 × g.
Cytokine protein array
Culture supernatants of DENV2- infected and
-uninfected PBMCs were collected, as described, at 0, 6 and 12 h
post-DENV2 infection. Equal volumes (1 ml) of each culture
supernatant were incubated with the precoated Proteome Profiler
array membrane (ARY005, Human Cytokine Antibody Array kit; R&D
Systems, Minneapolis, MN, USA) and processed according to the
manufacturer’s instructions. Densitometric analysis of dot blots
was performed using Image J software (National Institutes of
Health, Bethesda, MD, USA), as described previously (12).
Total RNA extraction
Total RNA was isolated from cultured DENV2-infected
and -uninfected (both n=6) PBMCs using TRIzol (Invitrogen,
Carlsbad, CA, USA) and miRNeasy mini kit (Qiagen, Dusseldorf,
Germany) according to the manufacturer’s instructions. RNA quality
and quantity was measured using a Nanodrop spectrophotometer
(ND-1000; Nanodrop Technologies, Wilmington, DE, USA). RNA
integrity was confirmed by gel electrophoresis.
miRNA microarray analysis
RNA samples were labeled using the miRCURY™
Hy3™/Hy5™ Power labeling kit according to the manufacturer’s
instructions. The Hy3™-labeled samples were hybridized to the
miRCURY™ LNA Array (v.16.0) according to the manufacturer’s
instructions. Following hybridization, the slides were washed 3
times using wash buffer from the kit and dried by centrifugation
for 5 min at 1,000 × g (all obtained from Exiqon, Vedbaek,
Denmark). Slides were scanned using the Axon GenePix 4000B
microarray scanner (Axon Instruments, Inc., Foster City, CA, USA).
Scanned images were imported into GenePix Pro 6.0 software for grid
alignment and data extraction. Replicate miRNA in duplicate samples
was averaged and miRNAs with intensities of ≥50 in all samples from
each group (DENV2-infected or control groups) were selected for
calculation of the normalization factor. Expressed data were
median-normalized. Following normalization, significantly
differentially expressed miRNAs were identified through Volcano
Plot filtering. Hierarchical clustering was performed using MEV
software (v4.6; TIGR, http://compbio.dfci.harvard.edu/tgi/software/).
Quantitative reverse transcription
polymerase chain reaction analysis (qRT-PCR) of miRNAs
Microarray data on differential expression were
verified for selected genes by qRT-PCR using extracted total RNA
samples as templates. Primers were synthesized at Life Technologies
(Life Technologies, Grand Island, NY, USA). Gene Amp PCR System
9700 (Applied Biosystems, Foster City, CA, USA) was used to
synthesize cDNAs for RT-PCR. RT-PCR amplification was performed on
the ABI PRISM 7900 System (Applied Biosystems). Total RNA from the
donors used for the miRNA microarrays was used to assay hsa-let-7e,
hsa-miR-4290, -4279, -451, -720, -491-3P, -451, -4286, -3653, -30b,
-20a, -106b, -222, -320c, -767-5p, -1246, -33a, -1290, -3686,
-625*, by qRT-PCR (Table
I). Briefly, 500 ng total RNA was reverse-transcribed to cDNA
(60°C for 5 min, 37°C for 60 min) using MMLV reverse transcriptase
(Epicentre Biotechnologies, Madison, WI, USA). For quantification
of each miRNA, a quantitative PCR step (cycle program: denaturation
for 10 min at 95°C and 40 cycles of denaturation, 10 sec at 95°C
and amplification, 1 min at 60°C) was performed on the Takara PCR
Thermal Cycler (Takara Bio, Inc., Shiga, Japan). RT-PCR was
performed using the sample mi-RNA (miR-RT) and the internal
reference was non-coding small nuclear RNA (snRNA U6) as previously
described (13).
 | Table IqRT-PCR and RT-PCR primers. |
Table I
qRT-PCR and RT-PCR primers.
| Experiment | Gene name | Direction | Primer sequence |
|---|
| qRT-PCR | snRNA U6 | F |
5′-CCCTGCGCAAGGATGAC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-4290 | GSP |
5′-TGCCCTCCTTTCTTCCC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-4279 | GSP |
5′-GCTCTCCTCCCGGCTT-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-720 | GSP |
5′-GGTTCTCGCTGGGGC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-491-3p | GSP |
5′-GGCTTATGCAAGATTCCCT-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-451 | GSP |
5′-GGAAACCGTTACCATTACTGAG-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-4286 | GSP |
5′-GGGACCCCACTCCTG-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-3653 | GSP |
5′-GGGGGCTAAGAAGTTGACT-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-30b | GSP |
5′-GGGTGTAAACATCCTACACTCA-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-20a | GSP |
5′-GGGGTAAAGTGCTTATAGTGC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-106b | GSP |
5′-GGGGTAAAGTGCTGACAGTG-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-320c | GSP |
5′-GGAAAAGCTGGGTTGAGAG-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-767-5p | GSP |
5′-TGCACCATGGTTGTCTGAG-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-1246 | GSP |
5′-GGGAATGGATTTTTGGAGC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-33a | GSP |
5′-GGGGTGCATTGTAGTTGC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-1290 | GSP |
5′-GGGGTGGATTTTTGGATC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-miR-3686 | GSP |
5′-GGGGATCTGTAAGAGAAAGTAAAT-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| hsa-let-7e | GSP |
5′-GGGTGAGGTAGGAGGTTGTAT-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
|
hsa-miR-625* | GSP |
5′-CCGACTATAGAACTTTCCCC-3′ |
| | R |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| RT-PCR | snRNA U6 | - |
5′-GTCGGTGTCGTGGAGTCG-3′ |
| miR-RT | - |
5′-GTCGGTGTCGTGGAGTCGTTTGCAATTGC |
| | |
ACTGGATTTTTTTTTTTTTTTTTTV-3 |
Bioinformatics analysis
To predict target genes of highly differentially
expressed miRNAs by correlating their abundance with expression of
cytokine genes, the correlation of miRNA and target genes was
examined using three web-based databases: Sanger mibase (http://www.mirbase.org/), miRanda (http://www.microrna.org/microrna/home.do/) and
TargetScan (http://www.targetscan.org/). Target genes were
overlapped with sequences from each database to identify common
predicted genes and develop a mechanistic understanding of cytokine
secretion in PBMCs following DENV infection. To analyze the
pathways that these targets may collectively regulate, ingenuity
pathway analysis (IPA) software was used to identify specific
pathways predicted to be modulated by these miRNAs. P<0.05 was
considered statistically significant.
Results
Identification of differentially
expressed cytokines during DENV2 infection in PBMCs
The excessive release of cytokines following DENV
infection is hypothesized to be responsible for enhanced vascular
permeability that results in fatal plasma leakage and shock in DHF
and DSS. Therefore, a human cytokine array was performed to
identify the differentially expressed cytokines in DENV-infected
PBMCs. PBMCs were isolated from blood samples of healthy volunteers
and cultured in vitro. Supernatants were collected at 0, 6
and 12 h following DENV2-infection. Protein extracts from culture
supernatants were allowed to react with a cocktail of anti-cytokine
antibodies and subsequently incubated with the array of capture
antibodies that detect 36 cytokines. A large immune response was
evident in the infected PBMCs compared with control, from the
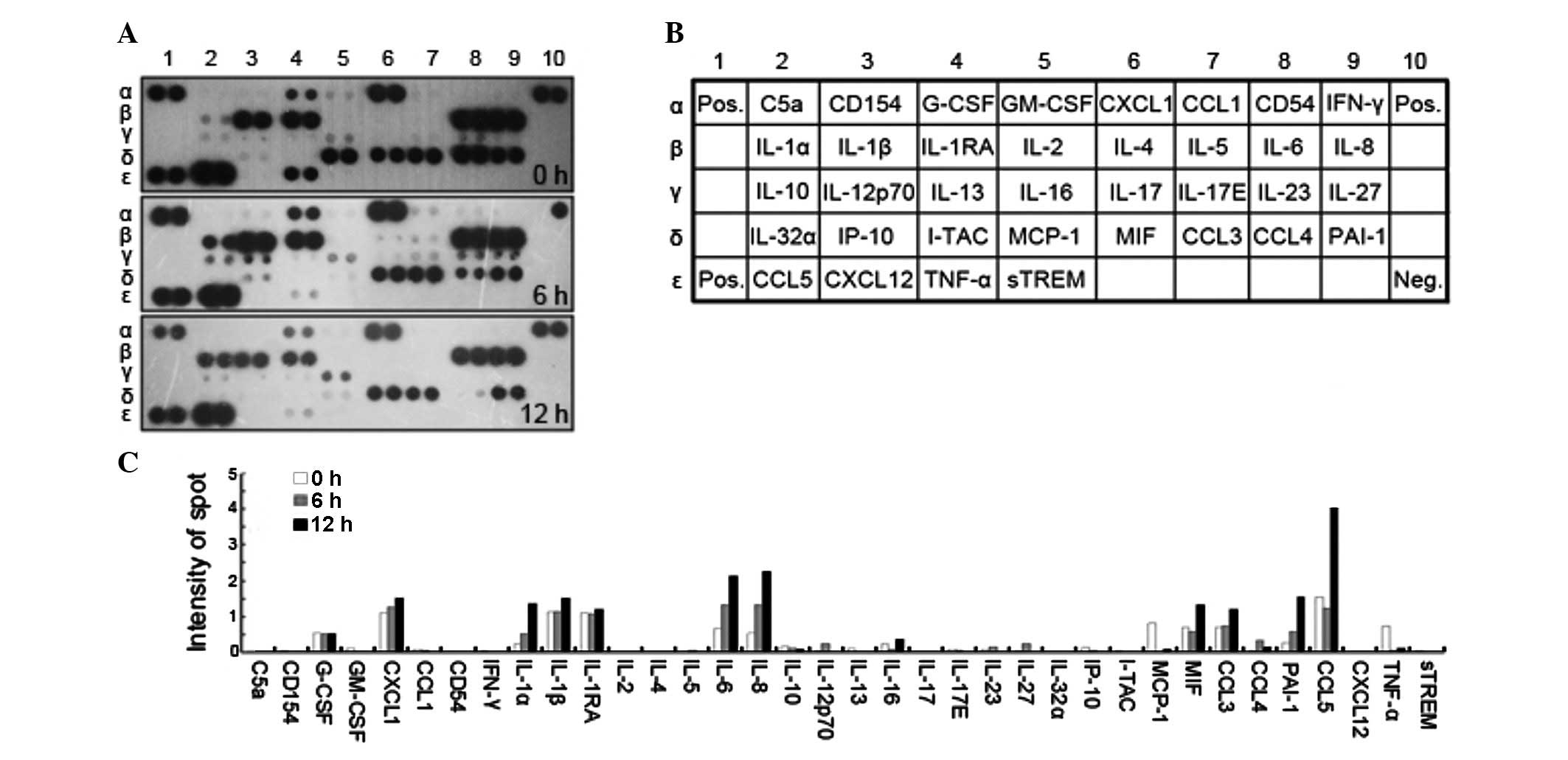
capture of cytokines on the array (Fig. 1A). Overexpressed cytokines
included: CCL5 and 3, PAI-1 and IL-6, -8, -1α and -1β.
Downregulated cytokines included: TNF-α, CCL4, MCP-1 and IL-10.
Based on the results of the cytokine array, CCL5 and IL-6 and -8
were the most highly expressed immunomodulators (Fig. 1C), expressed at least four times
higher in DENV2-infected PBMCs than in DENV2-uninfected cells.
Differential miRNA expression between
DENV2-infected and -uninfected PBMCs
To test our hypothesis that miRNAs are involved in
gene expression changes during DENV infection, total RNA was
extracted from the DENV2-infected and control PBMCs and analyzed by
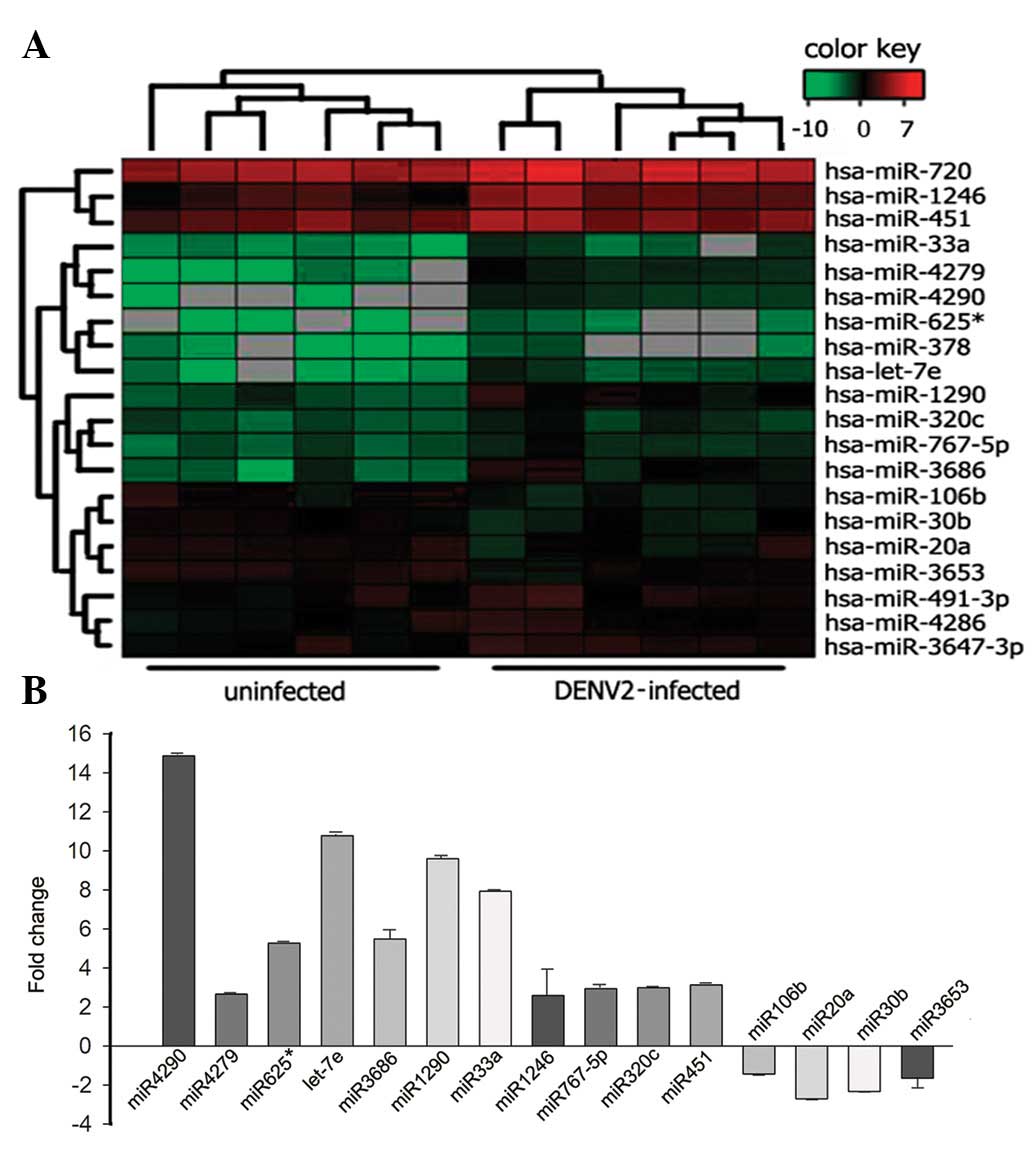
microarray (Fig. 2A). miRNAs that
were differentially expressed during DENV2 infection were
identified (Table II). Among
these, upregulated miRNA subsets included: miR-4290, -4279,
-625*, -let-7e, -1290, -33a, -378, -1246, -767-5p,
-320c, -720, -491-3p, -3647, -451 and -4286. Downregulated miRNA
subsets included: miR-106b, -20a, -30b and -3653 (Table II). miRNAs that exhibited a fold
change (FC) >1.5 and a P-value <0.05 for the comparison of
levels in infected and control RNAs were analyzed further by
qRT-PCR (Fig. 2B).
 | Table IIComparison of miRNA expression in
DENV2-infected and control PBMCs. |
Table II
Comparison of miRNA expression in
DENV2-infected and control PBMCs.
| miRNA | Chromosomal
location | Fold change
(I/C) | Parametric
P-value |
|---|
| hsa-miR-4290 | 9 | 36.627 | 2.85E-02 |
| hsa-miR-4279 | 5 | 21.815 | 1.29E-02 |
|
hsa-miR-625* | 14q23.3 | 8.987 | 3.77E-02 |
| hsa-let-7e | 19q13.41 | 8.585 | 2.53E-02 |
| hsa-miR-3686 | 8 | 7.728 | 4.63E-03 |
| hsa-miR-1200 | 1 | 6.929 | 1.62E-03 |
| hsa-miR-33a | 22q13.2 | 6.244 | 1.16E-02 |
| hsa-miR-378a | 5q32 | 4.773 | 2.90E-02 |
| hsa-miR-1246 | 2q31.1 | 3.295 | 7.33E-03 |
| hsa-miR-767-5p | Xq28 | 3.184 | 1.67E-02 |
| hsa-miR-320c | 18Q11.2 | 3.014 | 2.33E-02 |
| hsa-miR-720 | 3 | 2.038 | 1.21E-02 |
| hsa-miR-491-3p | 9q21.3 | 1.98 | 1.15E-02 |
| hsa-miR-451 | 17q11.2 | 1.918 | 4.32E-02 |
| hsa-miR-4286 | 8 | 1.861 | 4.63E-03 |
| hsa-miR-106b | 7q22.1 | 0.411 | 5.00E-03 |
| hsa-miR-20a | 13q31.3 | 0.494 | 6.14E-04 |
| hsa-miR-30b | 8q24.22 | 0.542 | 1.51E-02 |
| hsa-miR-3653 | 22 | 0.603 | 3.56E-03 |
Authentication of the miRNA signatures by
qRT-PCR
To confirm differential expression of the miRNAs
identified in the microarray described, qRT-PCR analysis was
performed on the RNA samples isolated from DENV2-infected or
-uninfected PBMCs. qRT-PCR results were normalized using an
internal control (snRNA U6) to reduce sample variation (data not
shown). Expression of miR-4290, -1290 and-33a and let-7e revealed
significant upregulation with a high FC ranging between 7.92 and
14.87. By contrast, the downregulation of miR-106b, -20a and -30b
was identified with FCs ranging between 0.41 and 0.60 (Fig. 2B).
Target gene and pathway prediction by
bioinformatic analysis
The function of miRNAs that were highly
differentially expressed between DENV2-infected and -uninfected
PBMCs was analyzed. The online databases of human miRNAs, Sanger
mibase, miRanda and TargetScan v4.2 were used to predict target
groups of the differentially expressed miRNAs by correlating their
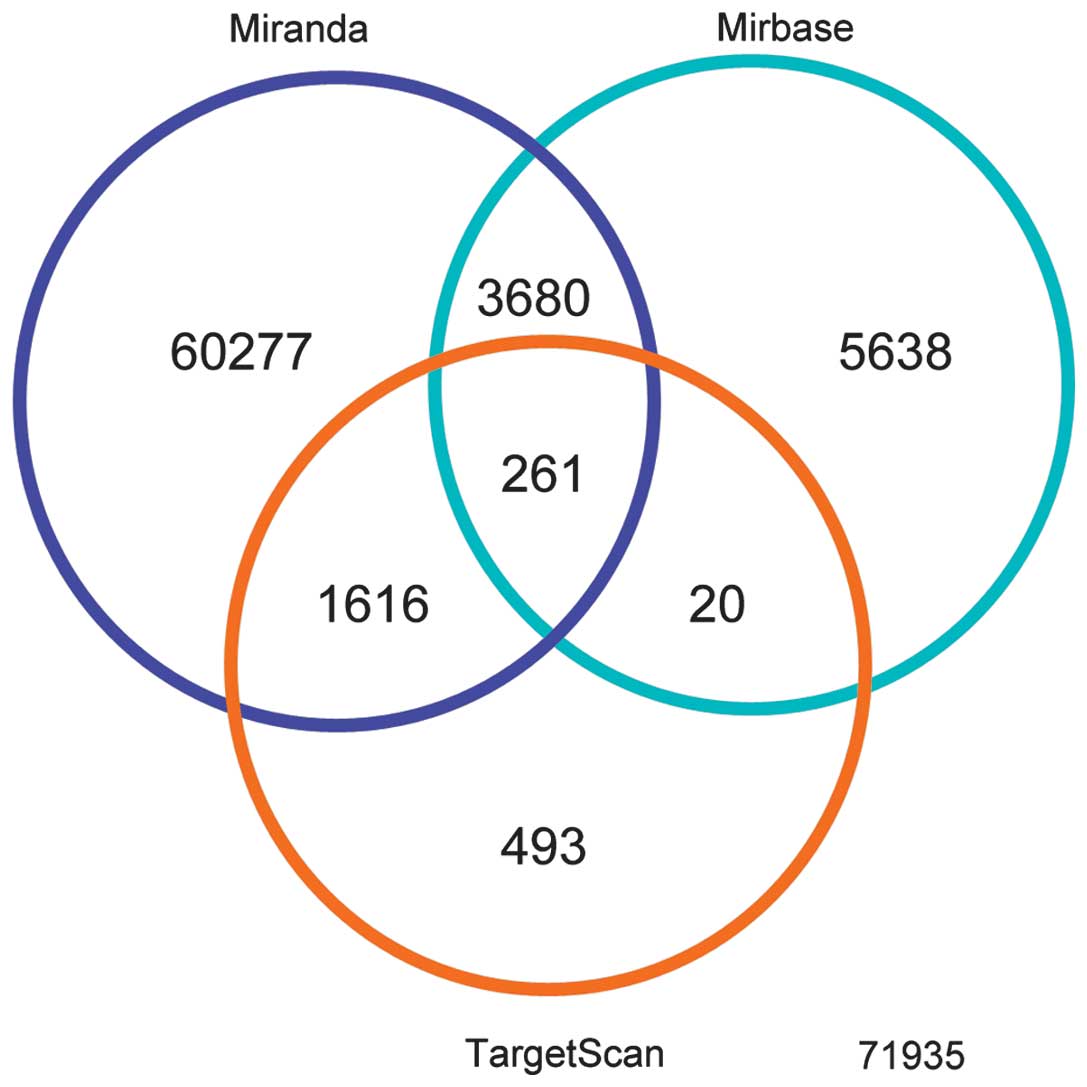
abundance with the expression of cytokine genes. When targets were
overlapped with each database, 261 common predicted genes were
detected (Fig. 3). Combined
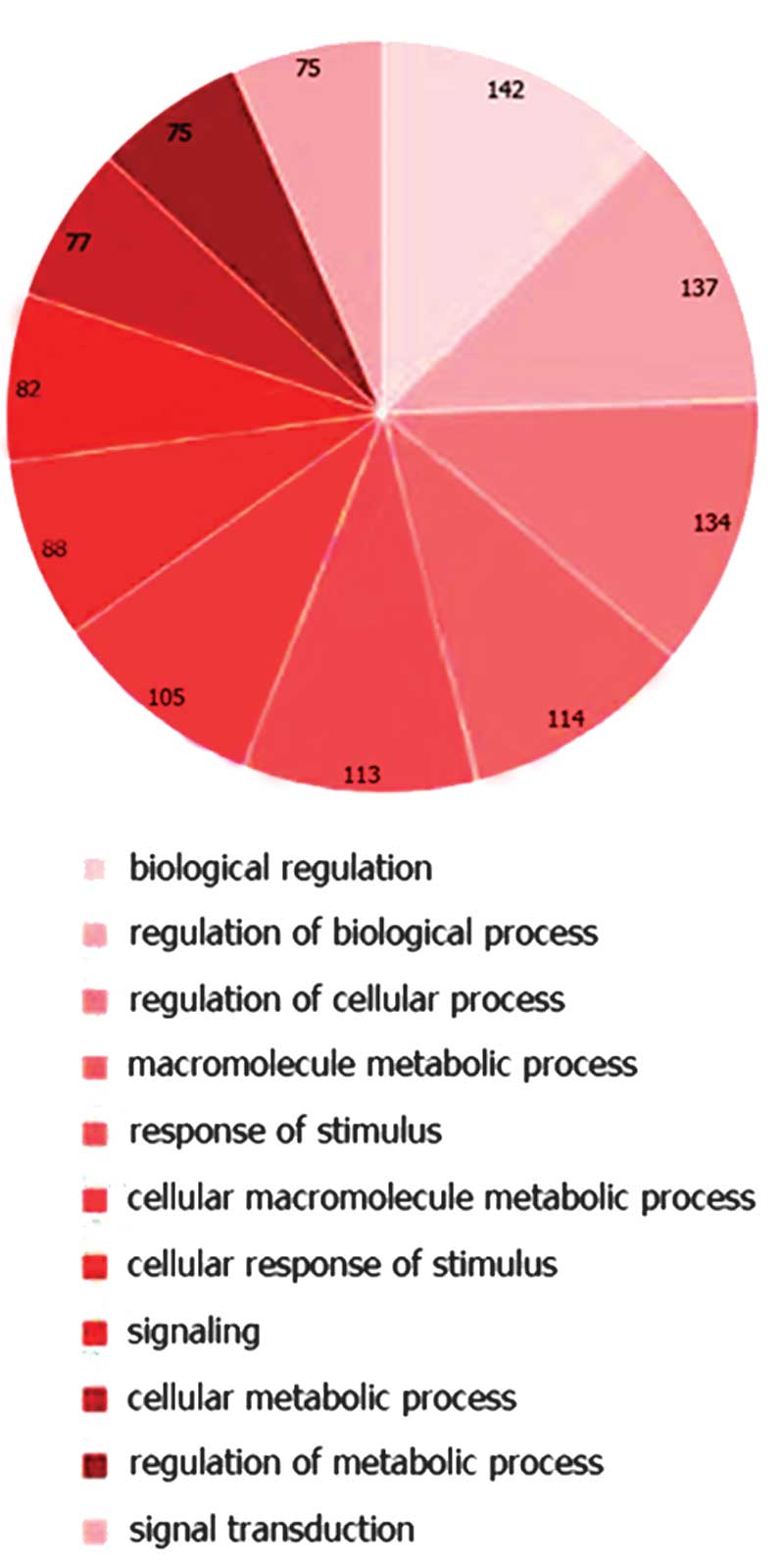
targets were submitted to computational analysis to identify
pathways that may be collectively regulated. Using IPA software,
canonical pathways, including pathways associated with biological
regulation, cell response to stimulus, signal transduction and
metabolism were predicted to be markedly modulated by these miRNAs
(Fig. 4).
Cytokine storm-related miRNAs and
epigenetic regulators
To examine whether following DENV2 infection,
differential expression of miRNAs is associated with elevated
levels of cytokines, we performed specific sequence homology
searches to identify a match between the sequences of
chemokine-encoding genes and miRNAs that were differentially
expressed during DENV2-infection (Fig.
2B). Of note, the 5′ region of the miR-let-7e (low expression)
had a sequence that was complementary to the 3′-untranslated region
(UTR) elements of IL-6 and CCL3. Similarly, mRNA corresponding to
MIF and CCL5 appeared to be targeted by miR-451 and -106b,
respectively. In addition, CXCL1 was revealed as a potential target
of miR-4279 that was differentially expressed during
DENV2-infection (Table III). The
miR-let-7e sequence was also found to be complementary to the
target sequence of EZH2 mRNA, a conserved catalytic subunit within
PRC2 that initiates silencing by catalyzing histone H3 Lysine 27
methylation in an initial step. The miR-30b sequence was
complementary to the target sequence in the mRNA of DNA
methyltransferase 3A (Table
III).
 | Table IIIAlignment of sequences from mRNA of
target genes encoding cytokines with miRNA sequences that were
differentially expressed in DENV2-infected compared with
-uninfected PBMCs. |
Table III
Alignment of sequences from mRNA of
target genes encoding cytokines with miRNA sequences that were
differentially expressed in DENV2-infected compared with
-uninfected PBMCs.
| Sequence in target
mRNA/miRNA | Matching
sequence |
|---|
| IL-6 3′
UTR/hsa-let-7e |
5′-UGGAAAGUGUAGGCUUACCUCAA-3′
3′-UUGAUAUGUUGGAGGAUGGAGU-5′ |
| CCL3 3′
UTR/hsa-let-7e |
5′-GUGUGACCUCCACAGCUACCUCU-3′
3′-UUGAUAUGUUGGAGGAUGGAGU-5′ |
| CXCL1 3′
UTR/hsa-miR-4279 |
5′-GUUUGAGCAUCGCUUAGGAGAAG-3′
3′-CUUCGGCCCUCCUCUC-5′ |
| MIF 3′
UTR/hsa-miR-451 |
5′-UGGUGGGGAGAAAUAAACGGUUU-3′
3′-UUGAGUCAUUACCAUUGCCAAA-5′ |
| CCL5 3′
UTR/hsa-miR-106b |
5′-GCCUGUAAUCCCAGCACUUUG-3′
3′-GACGUGACAGUCGUGAAAU-5′ |
| EZH2 3′
UTR/hsa-let-7e |
5′-NNNNNNNNNNCAUCUGCUACCUCC-3′
3′-UUGAUAUGUUGGAGGAUGGAGU-5′ |
| DNMT3A 3′
UTR/hsa-miR-30b |
5′-GUACAGGGCUUCACAGUUUACAA-3′
3′-UCGACUCACAUCCUACAAAUGU-5′ |
Discussion
Currently, it is hypothesized that excessive release
of pro-inflammatory molecules, largely by T cells and mast and
endothelial cells, is responsible for damage to endothelial cells
in DHF and DSS. Cytokines, rather than viruses, bind to vascular
endothelial cells directly, resulting in pleiotropic effects which
include enhanced inflammation and induction of vascular leakage
(4). Although cytokine storm is
primarily attributed to massive T-cell activation following
secondary DENV infection, a number of studies indicate that innate
immune response-related cytokines are also involved, particularly
in severe cases of primary dengue infection (14). In particular, high concentrations
of cytokines, including IFN-γ, TNF-α and IL-10, in the serum of
patients with severe DENV infections have been widely reported in
Vietnam (15), India (16) and Cuba (17), as well as elevated levels of IL-6
in children with ascites (18).
Additional studies have demonstrated that endothelial cells induce
a high level of immune cell-mediated recruitment of cytokines
IL-6/8, CXCL9/10/11, CCL5 and IL-7 (19). In the present study, levels of
specific immunomodulators in DENV2-infected PBMCs were elevated,
including CCL5 and IL-6 and -8, while those of TNF-α, IL-10, MCP-1
and CCL4 were decreased compared with uninfected PBMCs. In this
study, PBMCs were isolated from blood samples of healthy donors
with no history of DENV infection and PBMC cultures were infected
with DENV2 in vitro. This procedure avoided any interference
or variability in samples that may be introduced by secondary
infections or differential severity of infection.
Mature miRNAs are small, non-coding RNAs that are
largely identified in mammalian cells, functioning in a similar
manner to small interfering RNAs. Single-stranded miRNAs bind to
the 3′-UTR of target mRNAs through partial sequence homology and
induce translation blockage or, less frequently, mRNA degradation
(20). A previous study indicated
that miRNAs are able to silence gene expression primarily by
destabilizing target mRNA, thereby reducing their abundance
(21). In addition to functioning
as tumor suppressor genes, miRNAs are widely recognized to be
associated with physiological processes, including growth,
differentiation, apoptosis, stress responses and T-cell
homeostasis, as well as pathological processes, including
initiation, progression and prognosis of numerous diseases
(22).
miRNAs were previously reported to be closely
associated with pathogenesis of flaviviruses. miR-124a, -128a, -218
and -let-7c may be important for neurological symptoms caused by a
chimeric tick-borne encephalitis/dengue virus (23). Additional studies have demonstrated
that miR-122 (24) and -142
(25) of the host cells are
involved in restricting dengue virus replication. Tolfvenstam et
al identified high levels of secretion of cytokines, including
MCP-1, CCL8, IP-10 and CCL3, in dengue-infected peripheral blood
cells, hypothesizing that these cytokines may be negatively
regulated by miR-147 (26). In the
present study, miR-106b, -20a and -30b were observed to be
downregulated during DENV2 infection with an FC ranging between
0.41 and 0.60. We hypothesized that decreased miRNAs may relieve
inhibition of target genes, leading to the observed increase in
levels of pro-inflammatory cytokines. In support of this
hypothesis, bioinformatic analyses reveal that abundance of
miR-106b, which may bind CCL5 mRNA, was decreased during DENV2
infection. Therefore, it is likely that during DENV2 infection,
decreased miR-106b may lead to derepression of CCL5 expression and
enhance secretion of this cytokine.
In contrast to miR-106b, other miRNAs, including
miR-4290, -let-7e, -1290 and-33a were highly expressed during
DENV2-infection-induced cyctokine activation. Of these, miR-let-7e
may bind CCL3 and IL-6 mRNA, levels of which are also elevated
during DENV2 infection. As discussed, miRNAs may regulate gene
expression not only by destabilizing mRNAs by direct binding, but
also via epigenetic mechanisms (10). Transcription initiation may be
regulated via epigenetic processes that are dependent on chromatin
structure. Open and accessible chromatin (euchromatin) facilitates
active and selective gene transcription, whereas closed and
condensed chromatin (heterochromatin) is unable to be translated
into mRNA. These chromatin structures are markedly affected by
DNA-binding proteins, including histones and chromatin remodeling
processes i.e., histone modifications and DNA methylation. It is
known that miRNAs target specific epigenetic regulatory proteins,
including EZH2. Therefore, we propose that epigenetic modulation
may account for differential miRNA expression during the DENV
cytokine storm.
In the present study, molecular changes in PBMCs
post-DENV2 infection were analyzed by microarray and cytokine
array. A number of cytokines were identified to be abundantly
secreted and differentially expressed miRNAs were revealed between
DENV2-infected and control samples. In addition, putative targets
of specific differentially expressed miRNAs were identified using
bioinformatics analysis. Based on previous studies and data
presented in the current study, specific differentially expressed
miRNA appear to cause de-repression of cytokine expression, while
additional miRNAs target epigenetic modulators of cytokine
expression. Further investigations should be performed to determine
molecules that interact with these miRNAs and elucidate the
molecular mechanisms that trigger cytokine storm, a major
pathophysiological event following DENV infection.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (no. 30872350), Natural Science
Foundation of Guangdong Province (nos. 81510008901000017 and
S2012010009050), Guangdong Province Scientific Technology Project
(nos. 2010B050700008 and 2011B040300022), Guangzhou City Scientific
Technology Project (nos. 2011J4100084 and 2008Z1-E221) and The
Fundamental Research Funds for the Central Universities (no.
10YKPY31). The authors thank additional members of the laboratory
for their valuable comments.
Abbreviations:
|
DENV
|
dengue virus
|
|
DF
|
dengue fever
|
|
DHF
|
dengue hemorrhagic fever
|
|
DSS
|
dengue shock syndrome
|
|
IPA
|
ingenuity pathway analysis
|
|
m.o.i.
|
multiplicity of infection
|
|
miRNAs
|
microRNAs
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Guzman MG and Vazquez S: The complexity of
antibody-dependent enhancement of dengue virus infection. Viruses.
2:2649–2662. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martina BE, Koraka P and Osterhaus AD:
Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev.
22:564–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Green S and Rothman A: Immunopathological
mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect
Dis. 19:429–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pang T, Cardosa MJ and Guzman MG: Of
cascades and perfect storms: the immunopathogenesis of dengue
haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol Cell
Biol. 85:43–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gracias DT and Katsikis PD: MicroRNAs: key
components of immune regulation. Adv Exp Med Biol. 780:15–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lieber D and Haas J: Viruses and
microRNAs: a toolbox for systematic analysis. Wiley Interdiscip Rev
RNA. 2:787–801. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Curtale G, Citarella F, Carissimi C, et
al: An emerging player in the adaptive immune response:
microRNA-146a is a modulator of IL-2 expression and
activation-induced cell death in T lymphocytes. Blood. 115:265–273.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashimi ST, Fulcher JA, Chang MH, Gov L,
Wang S and Lee B: MicroRNA profiling identifies miR-34a and miR-21
and their target genes JAG1 and WNT1 in the coordinate regulation
of dendritic cell differentiation. Blood. 114:404–414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ceppi M, Pereira PM, Dunand-Sauthier I, et
al: MicroRNA-155 modulates the interleukin-1 signaling pathway in
activated human monocyte-derived dendritic cells. Proc Natl Acad
Sci USA. 106:2735–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sato F, Tsuchiya S, Meltzer SJ and Shimizu
K: MicroRNAs and epigenetics. FEBS J. 278:1598–1609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao H, Xu J and Huang J: FasL/Fas pathway
is involved in dengue virus induced apoptosis of the vascular
endothelial cells. J Med Virol. 82:1392–1399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neseliler S, Narayanan D, Fortis-Santiago
Y, Katz DB and Birren SJ: Genetically induced cholinergic
hyper-innervation enhances taste learning. Front Syst Neurosci.
5:972011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luers AJ, Loudig OD and Berman JW:
MicroRNAs are expressed and processed by human primary macrophages.
Cell Immunol. 263:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Espada-Murao LA and Morita K: Dengue and
soluble mediators of the innate immune system. Trop Med Health.
39:53–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen TH, Nguyen TL, Lei HY, et al:
Association between sex, nutritional status, severity of dengue
hemorrhagic fever and immune status in infants with dengue
hemorrhagic fever. Am J Trop Med Hyg. 72:370–374. 2005.PubMed/NCBI
|
|
16
|
Chakravarti A and Kumaria R: Circulating
levels of tumour necrosis factor-alpha and interferon-gamma in
patients with dengue and dengue haemorrhagic fever during an
outbreak. Indian J Med Res. 123:25–30. 2006.PubMed/NCBI
|
|
17
|
Perez AB, Garcia G, Sierra B, et al: IL-10
levels in Dengue patients: some findings from the exceptional
epidemiological conditions in Cuba. J Med Virol. 73:230–234. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Juffrie M, Meer GM, Hack CE, et al:
Inflammatory mediators in dengue virus infection in children:
interleukin-6 and its relation to C-reactive protein and secretory
phospholipase A2. Am J Trop Med Hyg. 65:70–75. 2001.PubMed/NCBI
|
|
19
|
Dalrymple NA and Mackow ER: Endothelial
cells elicit immune-enhancing responses to dengue virus infection.
J Virol. 86:6408–6415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar
|
|
21
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong Y and Han JH: MicroRNA: biological
and computational perspective. Genomics Proteomics Bioinformatics.
3:62–72. 2005.PubMed/NCBI
|
|
23
|
Heiss BL, Maximova OA and Pletnev AG:
Insertion of microRNA targets into the flavivirus genome alters its
highly neurovirulent phenotype. J Virol. 85:1464–1472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee TC, Lin YL, Liao JT, et al: Utilizing
liver-specific microRNA-122 to modulate replication of dengue virus
replicon. Biochem Biophys Res Commun. 396:596–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pham AM, Langlois RA and TenOever BR:
Replication in cells of hematopoietic origin is necessary for
Dengue virus dissemination. PLoS Pathog. 8:e10024652012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tolfvenstam T, Lindblom A, Schreiber MJ,
et al: Characterization of early host responses in adults with
dengue disease. BMC Infect Dis. 11:2092011. View Article : Google Scholar : PubMed/NCBI
|