Introduction
Chronic obstructive pulmonary disease (COPD) is a
largely irreversible progressive lung disease and is one of the
leading causes of death and disability worldwide (1–3). It
is estimated that COPD may be the third leading cause of death in
the world by 2020 (3,4). COPD is a common disease with high
morbidity and mortality rates in China and several other developing
countries (5,6). COPD is characterized by chronic
inflammation of the airways, chronic airflow limitation and
compromised immune function in the body (7,8).
Malnutrition develops in 25–65% of COPD patients; COPD-associated
malnutrition is attributed not only to the disease condition, but
also to a number of endogenous factors, including leptin levels
(9–12). Leptin is an adipocyte-derived
hormone that is important in energy homeostasis; its role is to
inform the brain of the volume of adipose tissue stored in the body
(13,14). Leptin is also a proinflammatory
factor that promotes the release of other inflammatory factors and
positively regulates the immune function by exerting protective
effects (15). Therefore, leptin
may be important in the occurrence and development of COPD. To
clarify the pathogenesis of COPD, it may be useful to determine the
leptin levels in biopsy sample tissues or blood and to evaluate the
prognosis and treatment of COPD patients.
Thus, extensive investigation into the association
of leptin and inflammatory factors with the pathological processes
of COPD is required. In this study, the levels of leptin and
inflammatory factor interleukin (IL)-6 were determined in patients
with an acute exacerbation of COPD (AECOPD) and stable COPD, and in
rat models of acute COPD, which have the potential to significantly
impact the future treatment strategies of COPD.
Materials and methods
Clinical study
A total of 51 outpatients and inpatients with COPD
and 20 healthy subjects undergoing physical examinations in the
Fourth Affiliated Hospital of Harbin Medical University were
included in this study. Serum and sputum samples were collected
from the subjects and radioimmunoassay (RIA) kits (Beijing Furui
Biotech Co., Ltd., Beijing, China) were used to determine the
levels of leptin and IL-6. Fasting venous blood was obtained to
determine white blood cell (WBC) counts and serum albumin (ALB)
levels. Lung function was assessed in each subject using spirometry
(MS-IOS; Jaeger, Hoechberg, Germany); the forced expiratory volume
in one second (FEV1) was recorded and spirometry was performed
three times. Differences of <10% between two measures of FEV1
were used as the final FEV1 result, the average was calculated and
the FEV1 percentage (FEV1%) was determined.
Animal experiments
Eight-week-old male Sprague-Dawley (SD) specific
pathogen-free rats weighing 200±20 g were purchased from the
Experimental Animal Center of Norman Bethune University of Medical
Science (Changchun, China). For tobacco smoke exposure, Daguang
filter cigarettes were used (tar content, 15 mg; nicotine content,
1.1 mg; Kunming Hongta Tobacco Co., Ltd., Kunming, China).
Enzyme-linked immunosorbent assay (ELISA) kits for leptin and IL-6
were purchased from Beijing Biosen Biotech Co., Ltd. (Beijing,
China), RIA kits were from Beijing Furui Biotech Co., Ltd.
(Beijing, China) and lipopolysaccharide (LPS) was from Harbin
Hongbo Xinye Biotech Co., Ltd. (Harbin, China).
Study groups
Clinical study
A total of 51 inpatients and outpatients with COPD
who were admitted to the Department of Respiratory Diseases in the
Fourth Affiliated Hospital of Harbin Medical University between
December 2010 and June 2011 were included in this study. There were
27 patients with AECOPD and 24 with stable COPD. All the patients
were male, <80 years of age and had a normal body mass index
(BMI). COPD was diagnosed according to the GOLD Guidelines for COPD
(2008) and additional metabolic diseases were excluded. Support
therapy was provided to inpatients and glucocorticoids were
administered prior to the study. The control group included 20
healthy subjects who underwent physical examinations in our
hospital. All the subjects were male, <80 years of age and had a
normal BMI. A review of the medical histories, physical
examinations and accessory examinations of patients excluded the
presence of diabetes and organic diseases of the heart, brain,
liver and kidney. Additionally, patients had no systemic infections
during the last 2 months. During the study, all the subjects kept a
routine diet. The present study was approved by the Ethics
Committee on Human Research of our hospital and written informed
consent was obtained from the patients.
Animal experiments
A total of 36 male SD rats were randomly allocated
to 3 groups (12 rats/group); the healthy control, COPD1 and COPD2
groups. The animals were housed in the Animal Center of The Fourth
Hospital of Harbin Medical University at 20±2°C with a humidity of
40–70% under a 12:12-h light-dark cycle. Animals were given ad
libitum access to general rat chow and water. The passive
smoking equipment was prepared as previously described (automatic
combustion-supporting ashtray). Rats in the COPD1 group were
intratracheally administered LPS (200 μg) on days 1 and 14, and
then exposed to 5% smoke for 2 h daily for 4 consecutive weeks
(with the exception of days 1 and 14). Rats in the COPD2 group were
exposed to 5% smoke for 2 h daily for 12 consecutive weeks. Rats in
all three groups were housed in the same environment; however, rats
in the control group were not exposed to smoke. All the
experimental protocols were approved by the Animal Care and Use
Committee of our Institute.
Sample collection
Human clinical study
Blood and sputum samples were collected, centrifuged
and stored for future use. RIA kits were used to determine leptin
and IL-6 levels in the serum and sputum. The fasting venous blood
was used for WBC counts and ALB level determination. Lung function
was also assessed in each subject.
Animal experiments
After the COPD model was established in the rats,
they were intraperitoneally anesthetized with 25% urethane (1,000
mg/kg). Subsequently, 4 ml of blood was collected from the
abdominal aorta and centrifuged at 3,000 rpm for 15 min. Serum was
collected and stored in aliquots at −70°C for later use. The lungs
were harvested, fixed in formalin solution for 35 days, embedded
and sectioned, followed by hematoxylin and eosin (H&E) staining
and the immunohistochemical analysis of leptin and IL-6. RIA kits
were used to determine the serum leptin and IL-6 levels according
to the manufacturer’s instructions.
Immunohistochemical analysis
The following criteria were used for determining
protein expression: At a high magnification, cells with
yellow-brown granules in the cytoplasm and nucleus were evaluated
as positive for a target protein. The PA800 computerized
pathological image analyzer (Beckman Coulter, Miami, FL, USA) was
used for analysis and bronchioles with a diameter of 100–200 μm
with a surrounding alveolar area were selected under a light
microscope (magnification, ×400). Three fields were randomly
selected and the positive signal optical density was
determined.
Statistical analysis
SPSS version 17.0 software was used for statistical
analysis. Results for quantitative variables are expressed as the
mean ± standard deviation and results for ranked data are presented
as medians and interquartile ranges (possible outliers were
determined using the Q test). Means among the different groups were
compared using analysis of variance or the rank sum test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient serum and sputum sample
analysis
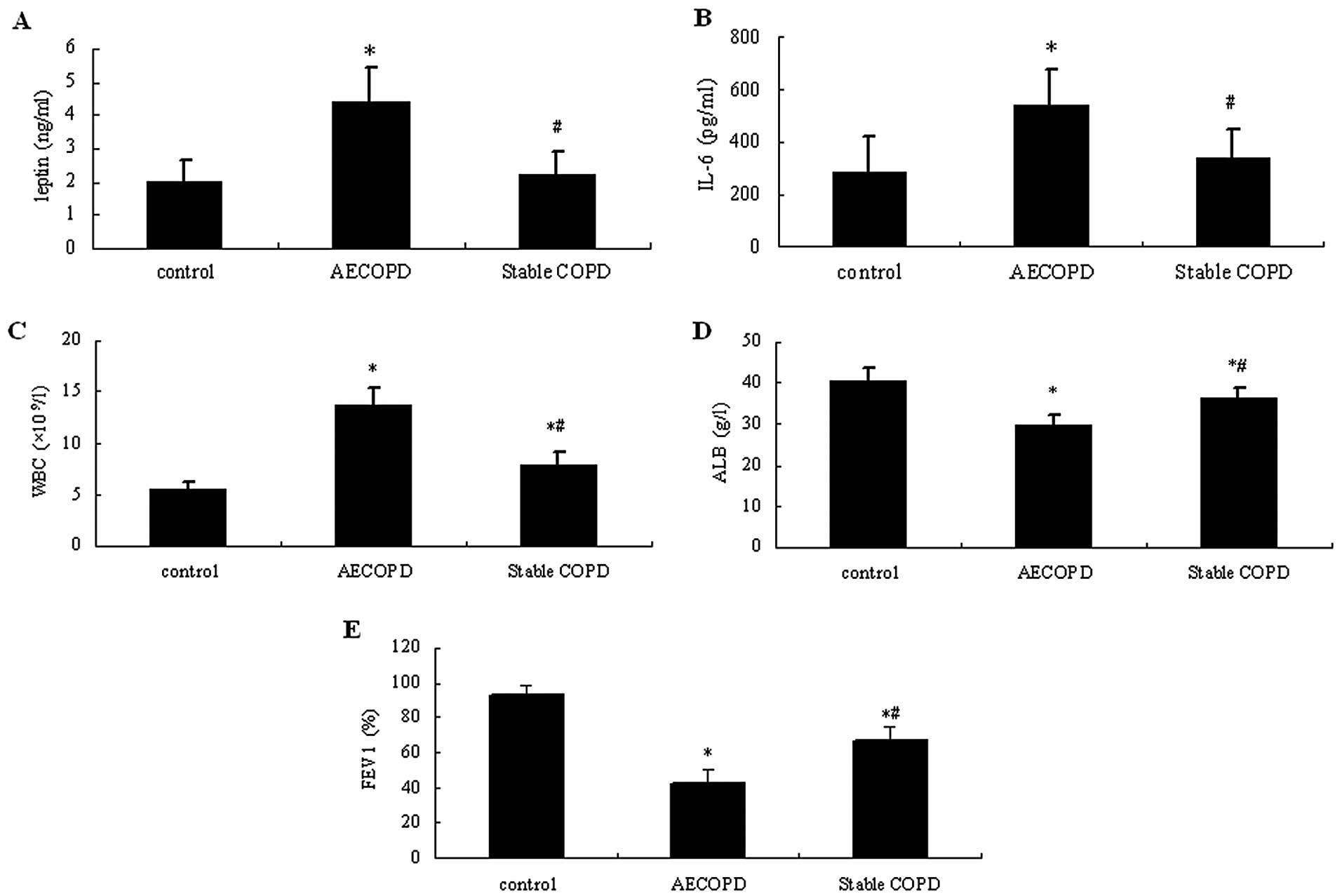
As shown in Fig. 1,
the serum levels of leptin and IL-6 and WBC counts in the AECOPD
patients were significantly higher compared with those in patients
with stable COPD and healthy controls (P<0.05). These were only
slightly increased in patients with stable COPD compared with
healthy controls (P>0.05). In the control group, ALB levels and
FEV1% were markedly higher compared with those in the COPD groups
(P<0.05), while they were significantly lower in the AECOPD
group compared with stable COPD patients (P<0.05).
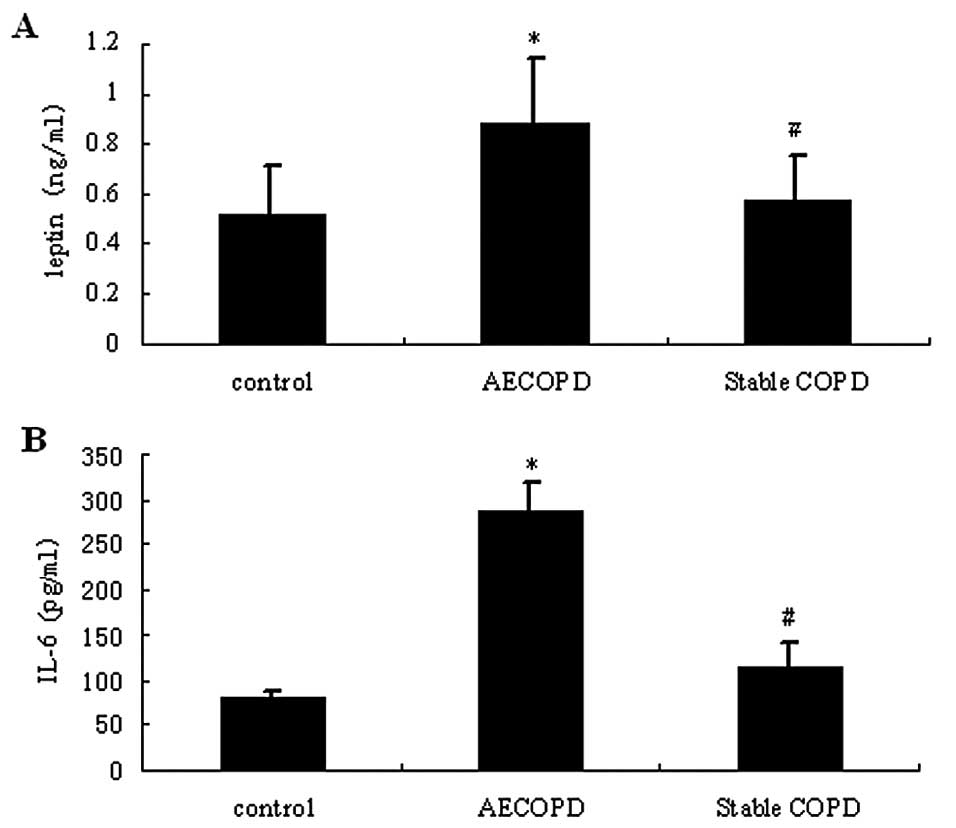
As shown in Fig. 2,
the sputum levels of leptin and IL-6 in patients with AECOPD were
significantly higher compared with those in stable COPD and healthy
control patients (P<0.05). In the serum samples, leptin and IL-6
levels were slightly increased in patients with COPD compared with
healthy control patients (P>0.05).
Rat COPD models
General status
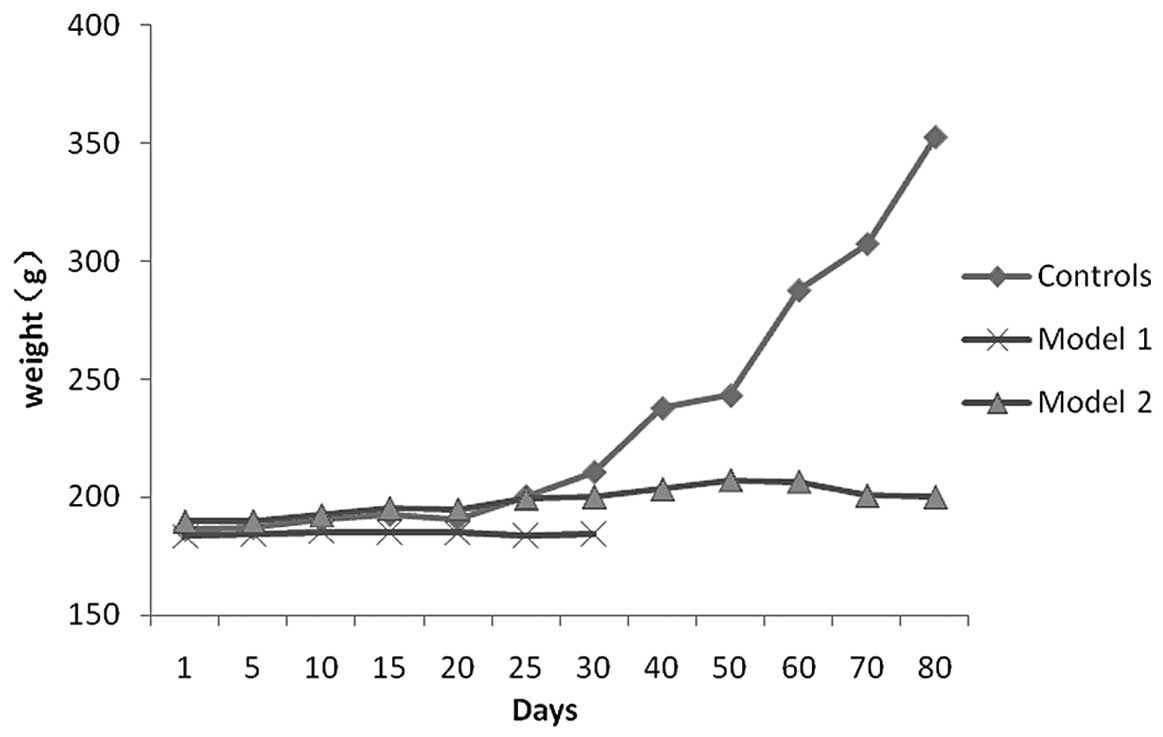
In COPD rats, the rate of body weight gain was
slower compared with that in control rats (Fig. 3). Additionally, the hair of COPD
rats was lusterless and became yellow. These animals were largely
unanimated, had reduced activities and flaring nares. Cyanosis,
tachypnea and wheezing were also noted.
Lung pathology
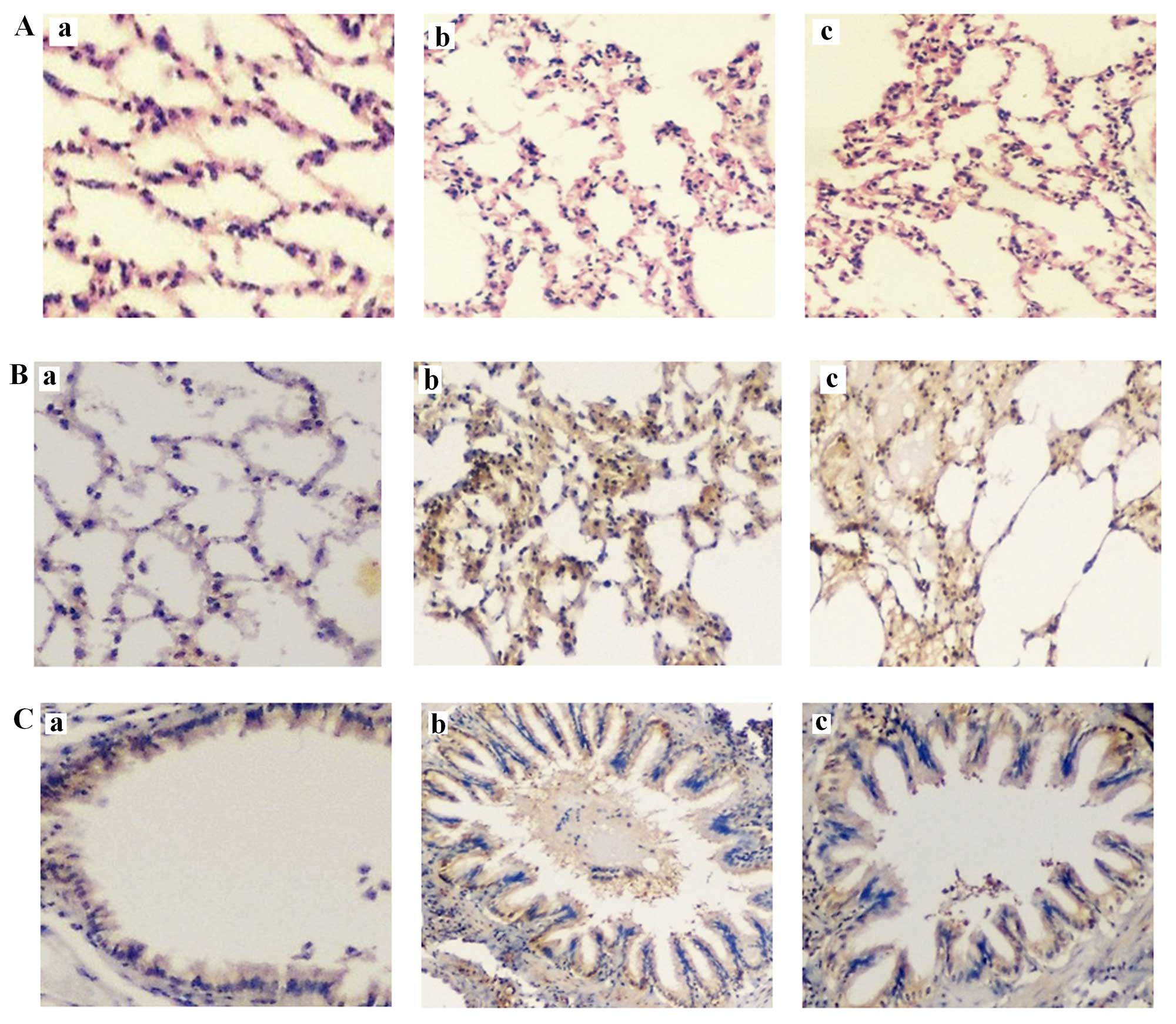
Representative rat lung histology sections after
H&E staining are shown in Fig.
4Aa (control), Fig. 4Ab
(COPD1) and Fig. 4Ac (COPD2). With
regard to the macroscopic assessment of the lungs, rats in the COPD
groups had significantly larger lungs than rats in the control
group. Additionally, the lungs of rats in the COPD groups were pale
and had bullae, although hemorrhage and exudation were not
observed.
In terms of the microscopic findings, the alveolar
walls of COPD rats were partially thickened and large numbers of
neutrophils, lymphocytes and mononuclear macrophages were observed
around the trachea and blood vessels. Focal hemorrhage was noted
and exudates were observed in the trachea. Furthermore, the
alveolar walls were thin, those close to capsules were interrupted
and several bullae were observed (emphysema).
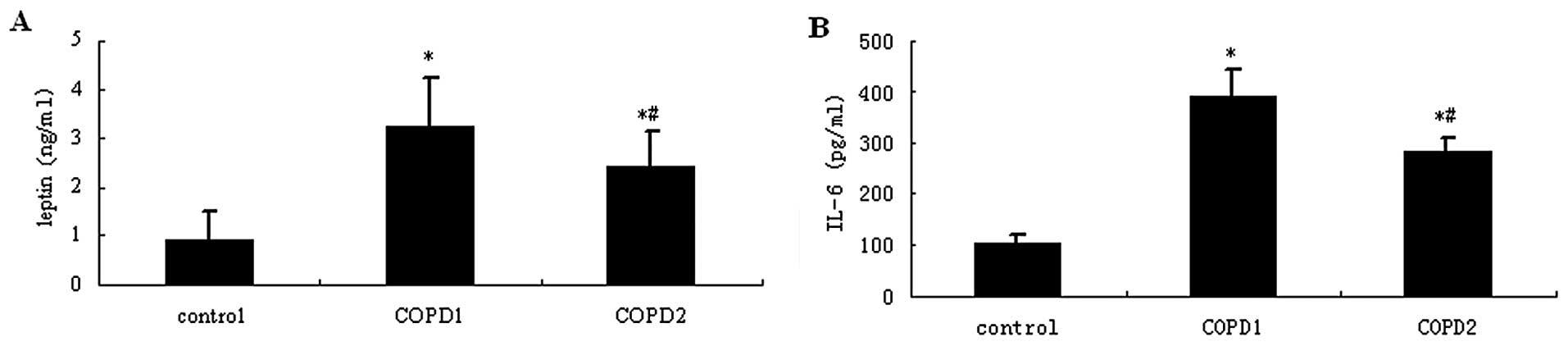
Immunohistochemical analysis
Target protein expression in lung tissues was
determined as described in Materials and methods. Representative
rat lung histology sections following staining for leptin are shown
in Fig. 4Ba (control), Fig. 4Bb (COPD1) and Fig. 4Bc (COPD2) and comparisons for
leptin expression among the groups of rats are summarized in
Fig. 5. Leptin expression was
detected in bronchial epithelial cells, the alveolar wall and all
the inflammatory cells. In the control group, a notably small
fraction of bronchial epithelial cells were positive for leptin. In
the COPD groups, leptin expression was significantly increased
compared with that of the control group (P<0.05). Furthermore, a
significant difference in leptin expression was identified between
the COPD1 and COPD2 groups (P<0.05).
Representative rat lung histology sections following
staining for IL-6 are shown in Fig.
4Ca (control), Fig. 4Cb
(COPD1) and Fig. 4Cc (COPD2) and
comparisons for IL-6 expression among the groups of rats are
summarized in Fig. 5. A small
number of bronchial epithelial and inflammatory cells in the
alveolar wall expressed IL-6. IL-6 expression was also detected in
blood vessels. In the COPD groups, IL-6 expression levels were
markedly increased compared with those of the control group
(P<0.05). A significant difference in IL-6 expression was also
observed between the COPD1 and COPD2 groups (P<0.05).
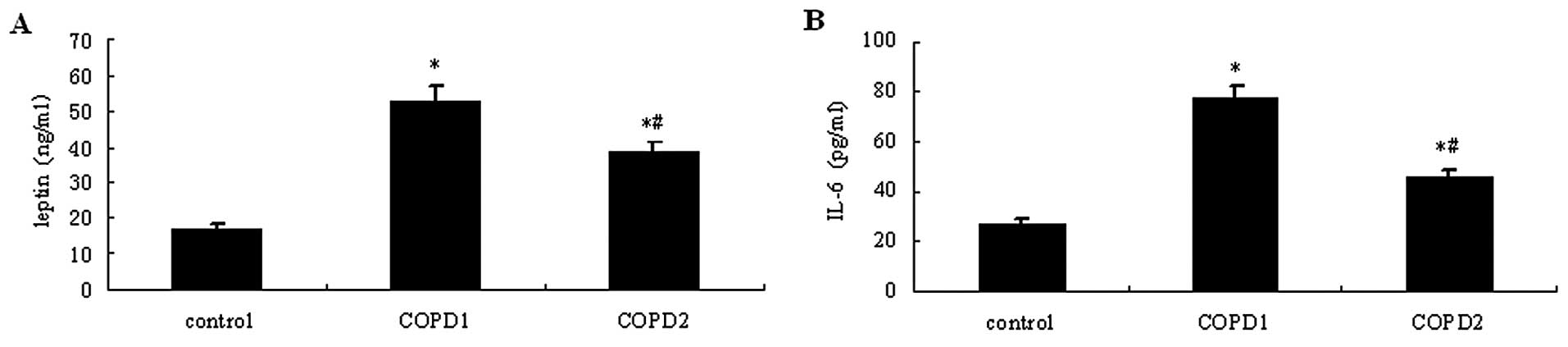
Following COPD induction in the rats, blood was
collected from the aorta and used to determine the leptin and IL-6
serum levels. As shown in Fig. 6,
leptin and IL-6 serum levels were higher in the two COPD groups
compared with the control group (P<0.05). The serum levels of
leptin and IL-6 were higher in COPD1 rats compared with those of
COPD2 rats (P<0.05).
Discussion
COPD is a chronic inflammation of the airways and
lungs that is characterized by the infiltration of macrophages,
neutrophils and T lymphocytes. This inflammation may involve a
variety of proinflammatory cytokines, including C reactive protein
(CRP), IL-6, IL-8 and tumor necrosis factor-α (TNF-α) (11). Leptin, which was first identified
in 1994, is a protein hormone encoded by the obese (ob) gene
and synthesized in adipose tissues. It is closely associated with
energy metabolism and insulin resistance. It mainly regulates body
weight by inhibiting appetite and reducing energy intake. Thus, it
is important in regulating energy balance, the metabolism of
glucose and fat and maintaining the body fat balance. Leptin has
also been revealed as a proinflammatory factor (16) and is important in inflammatory
responses. Leptin expression has been shown to be increased with
infections and inflammation (17),
and the leptin receptor has been identified in human alveoli and
bronchial epithelial cells (18).
Leptin expression has been identified in human
airway epithelial cells and type II alveolar cells. Under
physiological conditions, leptin inhibits the appetite, increases
energy expenditure and suppresses fat synthesis, which ultimately
reduces fat deposition. Under pathological conditions, serum leptin
has been suggested to be associated with the initiation and
development of certain diseases (19). Leptin is a proinflammatory factor
that promotes the release of proinflammatory cytokines (IL-6, IL-1
and TNF-α) from mononuclear macrophages, and it is regulated by a
number of inflammatory factors. Thus, leptin has the
characteristics of endocrine hormones and inflammatory cytokines
and may interact with other inflammatory cytokines.
During COPD development, obstruction of the
peripheral airways, destruction of pulmonary parenchyma and
abnormal pulmonary blood vessels reduce the alveolar surface area
available for gas exchange, leading to hypoxemia and hypoxia in
organs and tissues. Subsequently, cytokines are released,
particularly TNF-α and its receptor (TNF-αR) (20). Excessive energy expenditure and a
high metabolic rate are partially attributable to increases in
respiratory force and oxygen consumption, since the increase in
respiratory force is not able to explain body weight loss. Thus,
the uncorrected malnutrition that is observed in COPD patients may
involve other mechanisms.
Nutrition status is directly associated with the
development of COPD and there is evidence showing that malnutrition
is an independent risk factor for a poor COPD prognosis. Schols
et al(21) showed that the
presence of certain cytokines and leptin was the main cause of
severe malnutrition and the poor response to nutrition support in
COPD patients. In the present study, the clinical investigation and
animal experiments demonstrated that the impact of COPD on body
weight and nutrition was associated with leptin levels, suggesting
that leptin was important in this process.
Bai and Xu (22)
revealed that serum leptin levels were significantly reduced in
rats exposed to cigarette smoke and were inversely associated with
the amount of smoke exposure. This may have been associated with
increased serum catecholamine levels following cigarette smoke
exposure, which increases the degradation of fat and leads to a
subsequent reduction in leptin via a cAMP-dependent pathway.
Additionally, cigarette smoke may alter the sensitivity of leptin
receptors in the hypothalamus to regulate leptin synthesis
(23). Our study showed that the
leptin levels of COPD rats were markedly increased following
long-term exposure to cigarette smoke compared with those of the
control rats. Furthermore, the rate of body weight gain in these
rats increased more slowly than in control rats; in some cases,
there was even a body weight loss. This may be attributed to
chronic inflammation characterized by the infiltration of
macrophages into the alveoli, leading to the production of numerous
inflammatory mediators, including TNF-α and IL-6.
After IL-6 enters the circulation, it stimulates the
synthesis of leptin by adipose tissues and promotes the synthesis
of CRP by hepatocytes (24). IL-6
also facilitates IL-6 synthesis (25) and promotes the degradation of fat
tissues. Some studies have suggested that the reduction of leptin
levels occurs as a result of a protective response, although the
specific mechanisms are yet to be determined.
Our results showed that the levels of leptin and
IL-6 in the serum and sputum and the WBC counts of AECOPD patients
were higher than those in healthy controls, and these values in the
AECOPD patients were increased compared with those of stable COPD
patients. Additionally, these variables were inversely related to
FEV1% and ALB levels. These results suggest that leptin levels are
associated with the severity of inflammation and that leptin
expression increases with increased severity. High leptin levels
are usually accompanied by a high rate of catabolism and increased
energy expenditure, which may result in hypoproteinemia in COPD
patients during the acute exacerbation stage.
Furthermore, the expression levels of leptin and
IL-6 in the lungs of the COPD rats were markedly increased, as
demonstrated by immunohistochemical analysis and ELISA. These
findings suggest that leptin is involved in the occurrence and
development of COPD and that it is positively associated with COPD
severity. Additionally, the increased leptin expression was
accompanied by elevated IL-6 production, suggesting a correlation
between leptin and IL-6.
The interaction between IL-6 and leptin forms a
positive feedback loop. IL-6 is primarily secreted by macrophages,
lymphocytes and vascular endothelial cells. It has been shown that
IL-6 is an important mediator in inflammation and a series of
pathophysiological processes, and that it is closely associated
with the activity of these diseases (26). IL-6 is a proinflammatory and
immune-regulating cytokine that is important in local and systemic
inflammatory responses (27). High
IL-6 expression may cause damage to vascular endothelial cells,
promote immune adhesion and the formation of microthrombi and
inhibit the repair of endothelial cells. This results in damage to
blood vessels and an increase in their permeability, which may
damage the lungs and impair lung function.
IL-6 levels may reflect the severity of inflammation
in COPD (28). Walter et
al(29) showed that plasma
IL-6 levels, together with age and smoking, are independent risk
factors for reduced FEV1. Foschino et al(30) demonstrated that IL-6 expression
levels in the airway epithelial cells of COPD patients in the acute
exacerbation stage were higher compared with those in stable COPD
and healthy control patients, and that IL-6 expression levels in
stable COPD patients were significantly increased compared with
those of healthy controls. These findings are consistent with those
of the present study, with the exception that IL-6 levels in
patients with stable COPD were only slightly higher compared with
those in healthy controls. This suggests that inflammatory
mediators and cytokines are maintained at a low level in patients
with stable COPD and that leptin and IL-6 are markers of
inflammation during acute COPD exacerbations that may be used to
evaluate the severity of COPD during the early stage.
The acute recurrence of respiratory infections often
leads to COPD development and lung function impairment. LPS is the
primary toxic component of endotoxin. It causes direct damage to
airway epithelial cells, promotes the chemotaxis of neutrophils and
subsequently activates these cells and facilitates the expression
of inflammatory cytokines in the bronchi and alveoli. LPS also
promotes goblet cell proliferation in the airways, airway
remodeling and contraction of the bronchus, which may eventually
lead to bronchial inflammation and the development of emphysema
within a short period of time.
The investigation of leptin expression in rat COPD
models following exposure to LPS and smoke or smoke alone is
important for evaluating the association between leptin and COPD.
Additionally, the rate of body weight gain in rats exposed to LPS
and smoke was slower compared with rats exposed to smoke alone.
Pathological examinations indicated that the infiltration of
inflammatory cells and emphysema in rats exposed to LPS and smoke
were more severe and the expression of leptin and IL-6 were
increased compared with rats exposed to smoke alone. This may be
due to an increase in leptin following LPS stimulation. COPD
induced by exposure to LPS and smoke in animals more closely
resembles human COPD.
In COPD patients, the expression levels of leptin
and IL-6 were markedly increased and these increases were
positively associated with one another. Leptin and IL-6 may form a
complex regulatory network to affect the occurrence and development
of COPD. Our results also showed that the alveolar wall was
negative for IL-6. This suggests that IL-6 is mainly involved in
the occurrence of chronic inflammation in the airways.
However, the airways and alveolar walls were
positive for leptin. This indicates that leptin may act as an
inflammatory mediator in the development of emphysema and promotes
the occurrence of local inflammation that is involved in emphysema
development. Blood vessel walls were negative for leptin, while the
airways and alveolar walls were positive for leptin. It is
estimated that leptin levels in the sputum were higher compared
with those in the serum; however, these types of samples are not
directly comparable.
Our results indicate that levels of leptin and IL-6
in the sputum of AECOPD patients were higher compared with those in
stable COPD and healthy control patients, but were similar in
stable COPD and healthy control patients. Thus, the detection of
leptin and IL-6 levels in the sputum is more clinically important.
LPS promotes the expression of leptin and IL-6, suggesting that
leptin and IL-6 may serve as biomarkers for airway inflammation in
AECOPD patients. Thus, the detection of leptin and IL-6 levels may
be important for the determination of COPD severity and
prognosis.
References
|
1
|
Vogelmeier C, Hederer B, Glaab T, et al:
Tiotropium versus salmeterol for the prevention of exacerbations of
COPD. N Engl J Med. 364:1093–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calverley PM, Anderson JA, Celli B, et al:
Salmeterol and fluticasone propionate and survival in chronic
obstructive pulmonary disease. N Engl J Med. 356:775–789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Struik FM, Duiverman ML, Bladder G and
Wijkstra PJ: Effects of non-invasive positive pressure ventilation
(NIPPV) in stable chronic obstructive pulmonary disease (COPD).
Respir Med COPD update. 4:94–100. 2008. View Article : Google Scholar
|
|
4
|
Murray CJ and Lopez AD: Alternative
projections of mortality and disability by cause 1990–2020: Global
Burden of Disease Study. Lancet. 349:1498–1504. 1997.PubMed/NCBI
|
|
5
|
Fang X, Wang X and Bai C: COPD in China:
the burden and importance of proper management. Chest. 139:920–929.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon HI and Sin DD: Confronting the
colossal crisis of COPD in China. Chest. 139:735–736. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siafakas NM, Vermeire P, Pride NB, et al:
Optimal assessment and management of chronic obstructive pulmonary
disease (COPD). The European Respiratory Society Task Force. Eur
Respir J. 8:1398–1420. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Boer WI, Sont JK, van Schadewijk A,
Stolk J, van Krieken JH and Hiemstra PS: Monocyte chemoattractant
protein 1, interleukin 8, and chronic airways inflammation in COPD.
J Pathol. 190:619–626. 2000.PubMed/NCBI
|
|
9
|
Lewis MI and Belman MJ: Nutrition and the
respiratory muscles. Clin Chest Med. 9:337–348. 1988.
|
|
10
|
Nishimura Y, Tsutsumi M, Nakata H,
Tsunenari T, Maeda H and Yokoyama M: Relationship between
respiratory muscle strength and lean body mass in men with COPD.
Chest. 107:1232–1236. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wouters EF, Groenewegen KH, Dentener MA
and Vernooy JH: Systemic inflammation in chronic obstructive
pulmonary disease: the role of exacerbations. Proc Am Thorac Soc.
4:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Broekhuizen R, Vernooy JH, Schols AM,
Dentener MA and Wouters EF: Leptin as local inflammatory marker in
COPD. Respir Med. 99:70–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Auwerx J and Staels B: Leptin. Lancet.
351:737–742. 1998. View Article : Google Scholar
|
|
14
|
Yang YM, Sun TY and Liu XM: The role of
serum leptin and tumor necrosis factor-alpha in malnutrition of
male chronic obstructive pulmonary disease patients. Chin Med J
(Engl). 119:628–633. 2006.
|
|
15
|
Lam QL and Lu L: Role of leptin in
immunity. Cell Mol Immunol. 4:1–13. 2007.PubMed/NCBI
|
|
16
|
Bellmeyer A, Martino JM, Chandel NS, et
al: Leptin resistance protects mice from hyperoxia-induced acute
lung injury. Am J Respir Crit Care Med. 175:587–594. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otero M, Lago R, Lago F, et al: Leptin,
from fat to inflammation: old questions and new insights. FEBS
Lett. 579:295–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vernooy JH, Drummen NE, van Suylen RJ, et
al: Enhanced pulmonary leptin expression in patients with severe
COPD and asymptomatic smokers. Thorax. 64:26–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meier U and Gressner AM: Endocrine
regulation of energy metabolism: review of pathobiochemical and
clinical chemical aspects of leptin, ghrelin, adiponectin, and
resistin. Clin Chem. 50:1511–1525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sevenoaks MJ and Stockley RA: Chronic
Obstructive Pulmonary Disease, inflammation and co-morbidity - a
common inflammatory phenotype? Respir Res. 7:702006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schols AM, Creutzberg EC, Buurman WA, et
al: Plasma leptin is related to proinflammatory status and dietary
intake in patients with chronic obstructive pulmonary disease. Am J
Respir Crit Care Med. 160:1220–1226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai XL and Xu JY: Smoking to rat blood
lean meat, adiponectin, IL-6 and c-reactive protein influence. Chin
J Respir Crit Care Med. 9:298–299. 2010.(In Chinese).
|
|
23
|
Sull JW, Kim HJ, Yun JE, et al: Serum
adiponectin is associated with smoking status in healthy Korean
men. Endocr J. 56:73–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trujillo ME, Sullivan S, Harten I, et al:
Interleukin-6 regulates human adipose tissue lipid metabolism and
leptin production in vitro. J Clin Endocrinol Metab. 89:5577–5582.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fantuzzi G: Adipose tissue, adipokines,
and inflammation. J Allergy Clin Immunol. 115:911–919; quiz 920.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rose-John S, Waetzig GH, Scheller J, et
al: The IL-6/sIL-6R complex as a novel target for therapeutic
approaches. Expert Opin Ther Targets. 11:613–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanbaeva DG, Dentener MA, Creutzberg EC
and Wouters EF: Systemic inflammation in COPD: is genetic
susceptibility a key factor? COPD. 3:51–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wouters EF: Local and systemic
inflammation in chronic obstructive pulmonary disease. Proc Am
Thorac Soc. 2:26–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walter RE, Wilk JB, Larson MG, et al:
Systemic inflammation and COPD: the Framingham Heart Study. Chest.
133:19–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foschino Barbaro MP, Carpagnano GE,
Spanevello A, et al: Inflammation, oxidative stress and systemic
effects in mild chronic obstructive pulmonary disease. Int J
Immunopathol Pharmacol. 20:753–763. 2007.PubMed/NCBI
|