Introduction
Diabetes mellitus has become an increasingly
important public health problem worldwide. However, the etiology of
diabetes mellitus has not yet been fully elucidated. Reactive
oxygen species (ROS)-induced oxidative stress is known to be
important in the pathogenic process of diabetes mellitus. ROS that
are particularly responsible for oxidative stress include
superoxide (O2−), hydroxyl radical
(•OH), singlet oxygen (1O2),
hydrogen peroxide (H2O2), nitric oxide (NO)
and peroxynitrite (ONOO−) (1). Oxidative stress may induce the
dysfunction of pancreatic β cells, decreased insulin secretion
(2) and the development of
diabetic complications, including retinopathy, nephropathy,
neuropathy and vascular damage (3,4).
Generally, various antioxidative compounds exist in mammalian
cells, including low-molecular mass antioxidants such as
glutathione (GSH), uric acid, vitamin C, vitamin E and various
endogenous antioxidant enzymes against oxidative stress. It is
widely accepted that superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GSH-px) are three important endogenous
antioxidant enzymes for the protection of living organs against
ROS-induced oxidative stress. Among these antioxidant enzymes, SOD
catalyzes the dismutation of O2− into
H2O2, which may be transformed into
H2O and O2 by CAT. GSH-px is crucial for
removing lipid hydroperoxides and reducing free
H2O2 to water (1).
A number of drugs used in clinical diabetes mellitus
treatment have been associated with side-effects, including
gastrointestinal disturbances, edema, myocardial infarction and
risk of cardiovascular disease (5–8). To
date, >400 traditional plant treatments for diabetes mellitus
have been identified (9). The
anti-diabetic components of these natural plants may constitute
ancillary medication for diabetes treatment.
Lagerstroemia speciosa (Lythraceae), also
named banaba, is a tropical plant that grows in several parts of
southeast Asia, including southern China, Vietnam, Malaysia and the
Philippines. Lagerstroemia speciosa has been used as a
traditional folk medicine for the treatment of diabetes and
kidney-related diseases in the Philippines for ~1,000 years
(10,11). A number of studies have reported
that Lagerstroemia speciosa has antioxidant (12,13),
anti-inflammatory (13),
anticancer (14), anti-obesity
(15) and antidiabetic (12,16)
activities. Tannic and triterpene acids are the main components of
Lagerstroemia speciosa leaf extracts and have been shown to
downregulate blood glucose and possess apparent antidiabetic
properties in vivo and in vitro(16–19).
The present study aimed to investigate the potential
cytoprotective effects of hot water extracts from Lagerstroemia
speciosa leaves (LWE) on 3-morpholinosydnonimine
(SIN-1)-induced oxidative stress in HIT-T15 cells and to elucidate
the underlying mechanisms involved in this process.
Materials and methods
Plant extract preparation
Fresh Lagerstroemia speciosa leaves were
purchased from a local market in Chongqing, China. LWE was prepared
by boiling 160 g air-dried Lagerstroemia speciosa leaves in
1 l distilled water for 2 h, followed by ultracentrifugation at
30,000 × g for 30 min, filtration with a 0.4-μm filter,
concentration by heat evaporation and freeze-drying. LWE was
redissolved in dimethyl sulfoxide (DMSO) at a concentration of 50
mg/ml and stored at 4°C until future use.
Cell culture
Syrian hamster insulin-secreting HIT-T15 cells were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). The cells were routinely maintained in RPMI-1640 medium
supplemented with 10% (v/v) fetal bovine serum (FBS) and 1%
penicillin-streptomycin in a humidified CO2 incubator
(model 3154; Forma Scientific, Inc., Marietta, OH, USA) with 5%
CO2 at 37°C.
Cell viability assay
Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
method. The cells were seeded in 96-well plates at a density of
5×103 cells/well. Following 24-h incubation, the cells
were primarily treated with SIN-1 (50 μM) for 24 h and then
incubated with LWE (1–100 μg/ml) for 48 h. Next, 100 μl MTT reagent
(final concentration, 0.5 mg/ml) was added to each well and the
cells were incubated in a humidified incubator at 37°C to allow MTT
to be metabolized. After 4 h, 100 μl DMSO was added to each well to
dissolve formazan deposits. The absorbance of the samples was
measured at a wavelength of 540 nm using a microplate reader (model
680; Bio-Rad, Hercules, CA, USA).
Determination of intracellular ROS
Intracellular ROS levels were measured using the
fluorescent probe dihydrodichlorofluorescein (H2DCFDA).
Following treatment, HIT-T15 cells were washed with calcium- and
magnesium-free phosphate-buffered saline (PBS) and incubated in
H2DCFDA (20 μM) containing serum- and phenol red-free
Dulbecco’s modified Eagle’s medium (DMEM) for 30 min. Following
incubation, the medium was removed and cells were washed with PBS
twice. Fluorescence was measured using a FLUOstar OPTIMA
fluorescence plate reader (BMG Labtech, Ortenberg, Germany);
excitation was read at 485 nm and emission at 535 nm. Relative ROS
production (calculated as a percentage of the control) was
expressed as the ratio of fluorescence in the treated samples over
the response in the appropriate controls:
(fluorescencetreatment/fluorescencecontrol)
×100.
Lipid peroxidation levels
Lipid peroxidation was evaluated using a
thiobarbituric acid reactive substance (TBARS) assay (20). Briefly, the cultured cells were
washed with cooled PBS, scraped into trichloroacetic acid (TCA;
2.8%, w/v) and sonicated; total protein was determined using a
bicinchoninic acid (BCA) assay. The suspension was mixed with 1 ml
TBA (0.67%, w/v) and 1 ml TCA (25%, w/v), heated (30 min at 95°C)
and centrifuged (22,000 × g; 10 min at 4°C). TBA reacted with the
oxidative degradation products of lipids, yielding red complexes
that absorbed at 535 nm. The level of TBARS was determined using a
UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan).
Antioxidant enzyme activity
HIT-T15 cells grown in a 10-cm cell culture dish
were co-incubated with SIN-1 (50 μM) for 24 h and then treated with
LWE (2.5–50 μg/ml) for 48 h for further assessment. The cells were
washed with PBS, removed by scraping and centrifuged, and the
resulting cell pellet was stored at −80°C. Cell pellets were
thawed, resuspended in 300 ml cold lysis buffer (PBS and 1 mM
EDTA), homogenized and centrifuged (22,000 × g; 10 min at 4°C). The
resulting supernatants were used for activity measurements. CAT
activity (U/mg protein) was assessed according to the method
described by Nelson and Kiesow (21), in which the disappearance of the
substrate H2O2 was measured
spectophotometrically at 240 nm. SOD activity (U/mg protein) was
assayed using a modified auto-oxidation of pyrogallol method
(22). One unit of SOD activity
was defined as the amount of enzyme that inhibited the
auto-oxidation rate of pyrogallol by 50%. GSH-px activity (U/mg
protein) was assayed according to the method described by Hafemen
et al(23). Protein
contents were determined using the Bio-Rad protein assay kit
according to the manufacturer’s instructions.
Insulin secretion assay
Insulin secretion was measured using a
radioimmunoassay (RIA) method. The cells were seeded at a density
of 5×105 cells/well in a 96-well plate and primarily
treated with SIN-1 (50 μM) for 24 h, followed by LWE (2.5–50 μg/ml)
for 48 h. To determine the level of insulin secreted, aliquots of
samples (10 μl/well) were collected from the experimental medium at
the indicated time points (48 h) and subjected to an insulin
antiserum immunoassay within 5 min, according to the manufacturer’s
instructions (Linco Research, Inc., St. Charles, MO, USA). The
absorbance was read at 450 and 590 nm using a model 680 microplate
reader (Bio-Rad) and results were recorded.
Statistical analysis
Data are presented as the mean ± SD. The differences
between the mean values of individual groups were assessed using
one-way ANOVA and Duncan’s multiple range tests. P<0.05 was
considered to indicate a statistically significant difference. The
SAS v9.1 statistical software package (SAS Institute, Inc., Cary,
NC, USA) was used for analysis.
Results
Effects of LWE on SIN-1-induced oxidative
damage in HIT-T15 cells
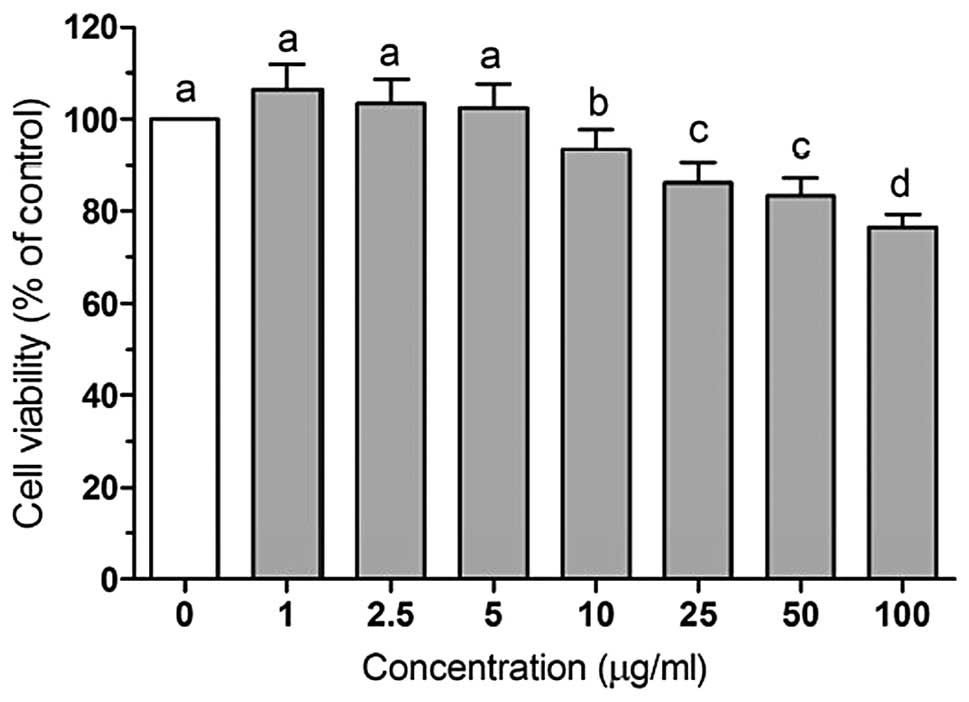
To investigate LWE-induced cytotoxicity, HIT-T15
cells were first treated with various concentrations of LWE (1–100
μg/ml) for 48 h and the cell viability was determined using an MTT
assay. Treatment with LWE at doses of 1–50 μg/ml at 37°C for 48 h
did not cause significant cytotoxicity and cell viability was
>80%. A dose of 100 μg/ml LWE induced cell damage (cell
viability, 76%; Fig. 1). Based on
these results, concentrations of 2.5–50 μg/ml LWE were used for
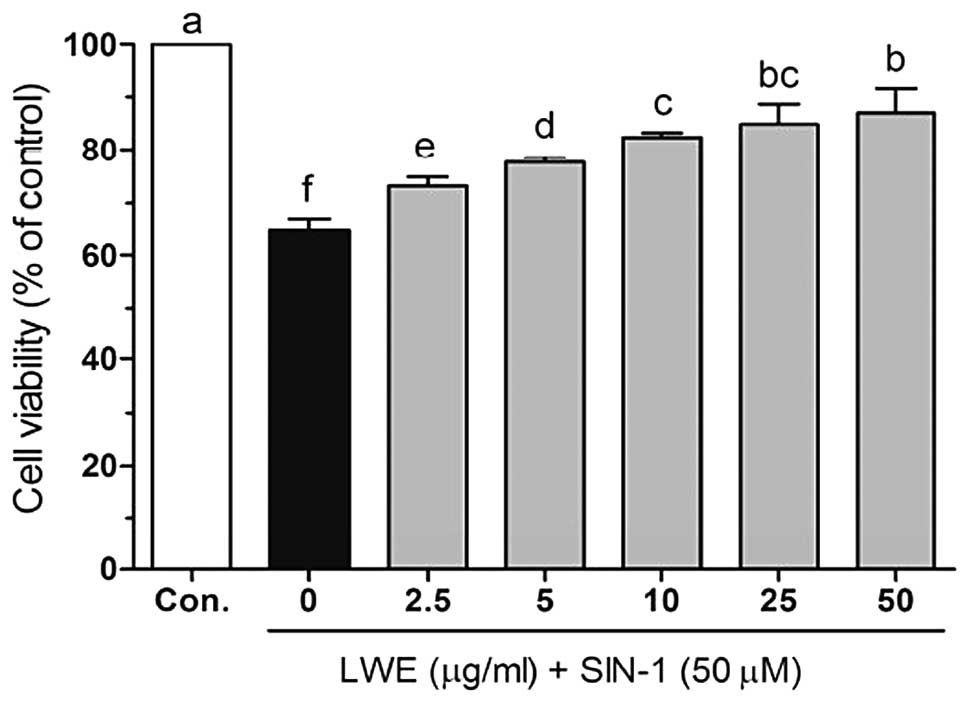
further assessment. As shown in Fig.
2, SIN-1 (50 μM) significantly induced cell death in HIT-T15
cells. However, following treatment with various concentrations of
LWE, the cell viability increased in a dose-dependent manner.
Effects of LWE against SIN-1-induced
intracellular ROS levels in HIT-T15 cells
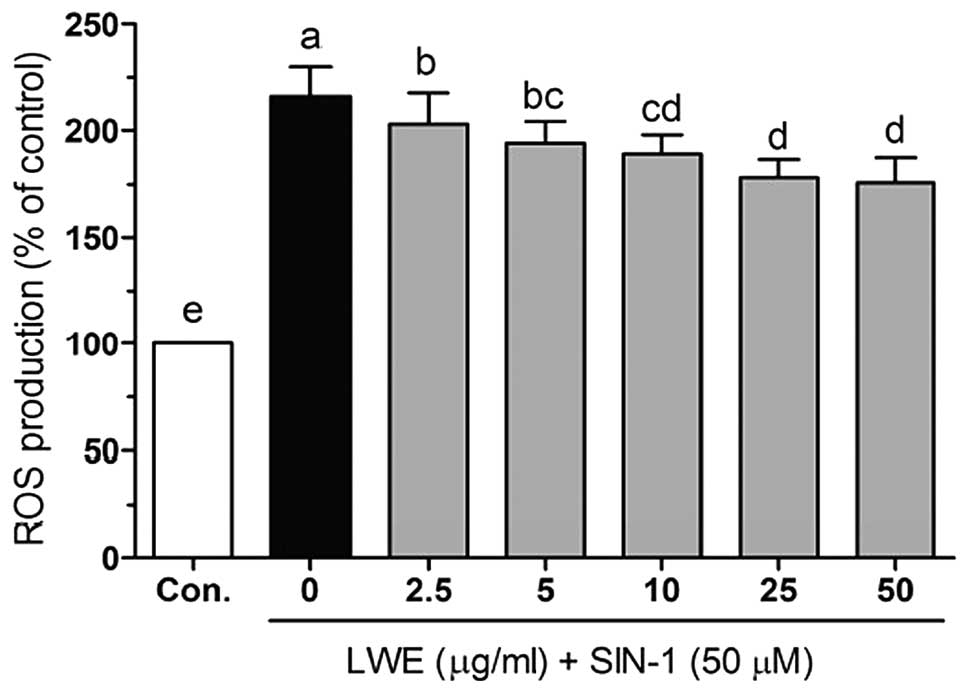
To investigate the protective effects of LWE in
SIN-1-treated HIT-T15 cells, the intracellular ROS levels were
determined using the fluorescent probe H2DCFDA. As shown
in Fig. 3, SIN-1 significantly
increased ROS levels compared with those in the control cells. In
the presence of SIN-1, LWE at doses of 2.5–50 μg/ml significantly
reduced ROS generation in a dose-dependent manner. The
intracellular ROS levels were 203.2±14.6, 194.3±9.9, 188.3±9.8,
178.3±7.5 and 175.7±10.8% when the cells were treated with 2.5, 5,
10, 25 and 50 μg/ml LWE, respectively. Treatment with the same
concentrations of LWE alone did not significantly increase the
intracellular ROS levels (date not shown). These results suggest
that LWE is a free radical scavenger.
Effects of LWE on lipid peroxidation in
SIN-1-treated HIT-T15 cells
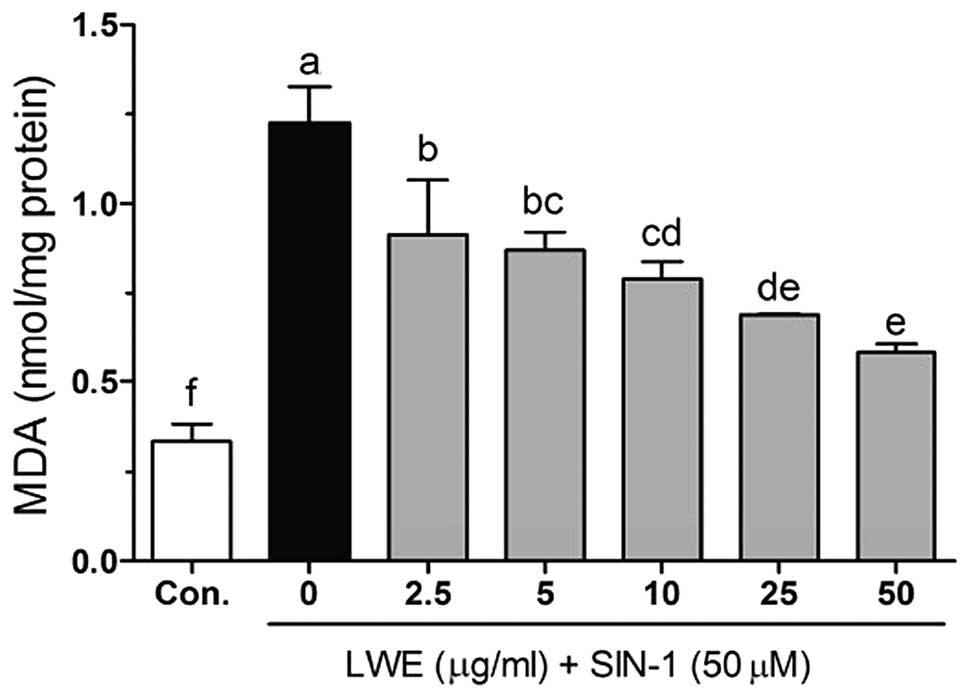
Free radical- and ROS-induced oxidative damage has
been strongly associated with the lipid peroxidation of cell
membranes and increased levels of malondialdehyde (MDA), which is a
biomarker of cell membrane lipid peroxidation. As shown in Fig. 4, SIN-1 significantly increased the
level of MDA (1.23±0.10 nmol/mg protein) compared with that in the
control cells (0.33±0.05 nmol/mg protein). LWE at doses of 2.5–50
μg/ml significantly reduced MDA levels in a dose-dependent manner.
The MDA levels were 0.91±0.15, 0.87±0.05, 0.79±0.05, 0.69±0.01 and
0.58±0.02 nmol/mg protein when the cells were treated with 2.5, 5,
10, 25 and 50 μg/ml LWE, respectively.
Effects of LWE on the activity of
antioxidant enzymes in SIN-1-treated HIT-T15 cells
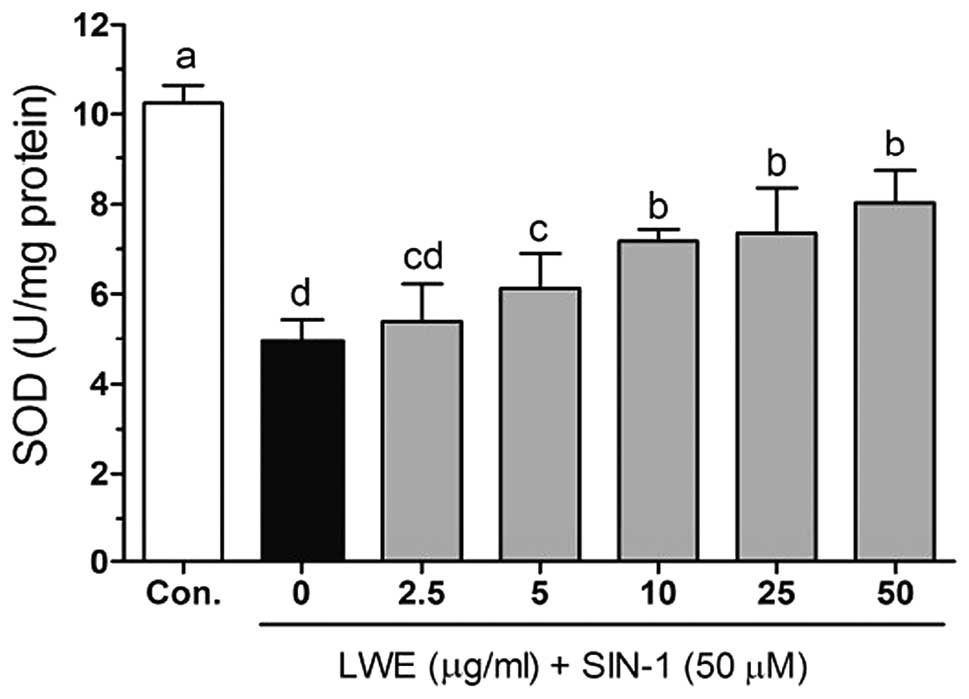
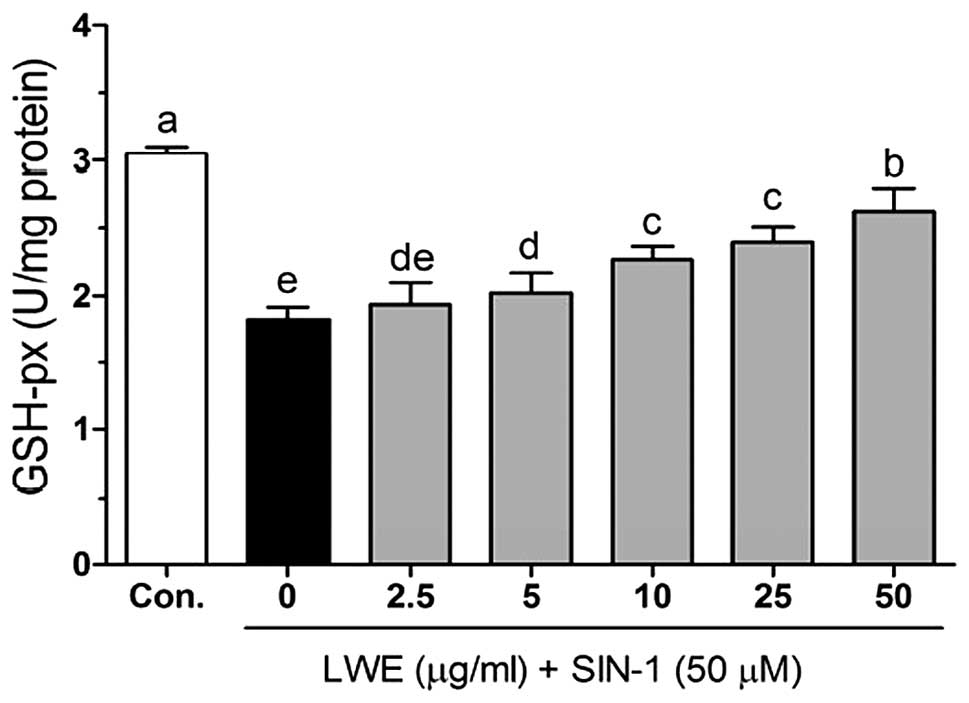
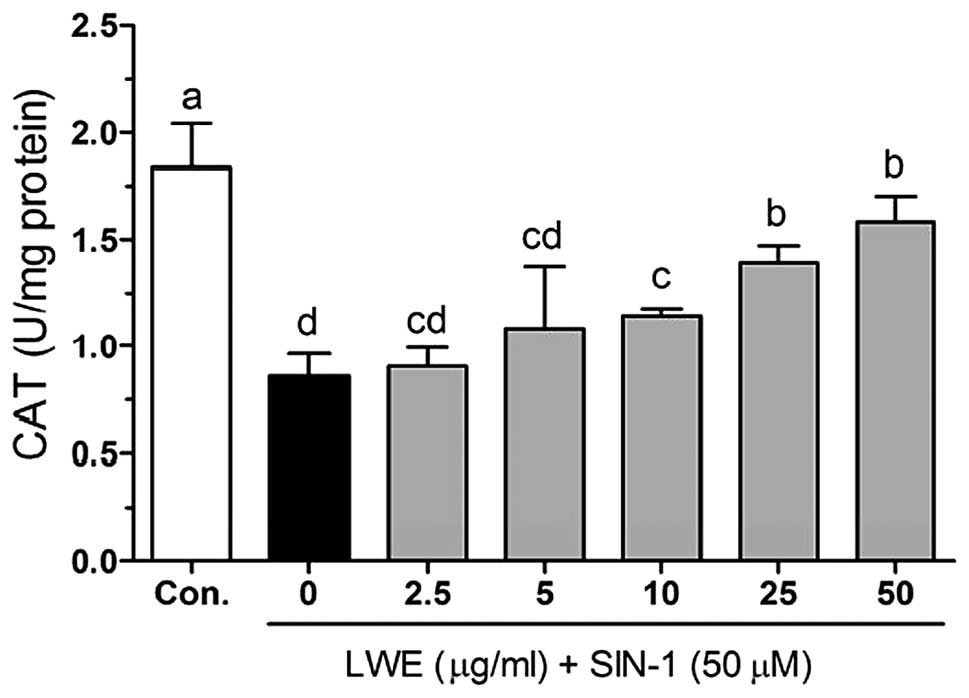
Figs. 5–7 demonstrate the intracellular
antioxidant enzyme activities with LWE in SIN-1-treated HIT-T15
cells. The activity of SOD was decreased with SIN-1 treatment
(4.96±0.46 U/mg protein) compared with that in the control cells;
however, this recovered to 5.39±0.83, 6.13±0.78, 7.19±0.26,
7.37±0.99 and 8.04±0.73 U/mg protein when the cells were treated
with 2.5, 5, 10, 25 and 50 μg/ml LWE, respectively. Following
treatment with SIN-1, cellular CAT was decreased (0.86±0.10 U/mg
protein) compared with that in the control cells (1.83±0.21 U/mg
protein). However, CAT activity was significantly increased
(P<0.05) following treatment with LWE (Fig. 6). Additionally, LWE reduced the
SIN-1-induced decrease in GSH-px in HIT-T15 cells. The GSH-px
activity was identified to be significantly increased from
1.93±0.17 to 2.63±0.17 U/mg protein when the cells were treated
with LWE (Fig. 7).
Effects of LWE on insulin secretion in
SIN-1-treated HIT-T15 cells
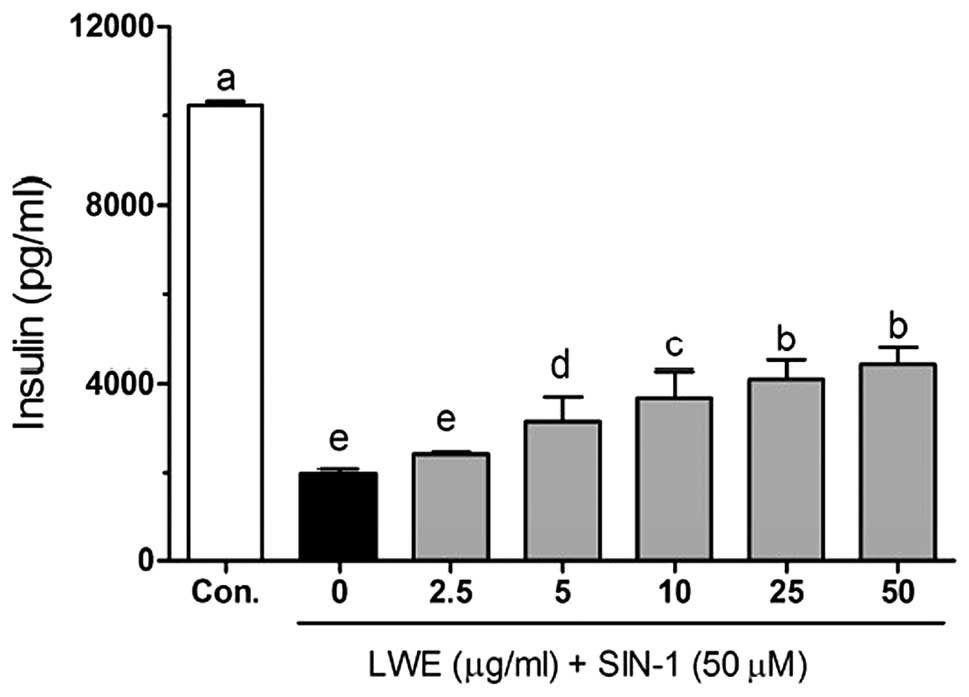
As shown in Fig. 8,
SIN-1 significantly decreased insulin levels (2095.4±105.0 pg/ml)
compared with those in the control cells (10236.7±98.9 pg/ml).
Following treatment with 2.5, 5, 10, 25 and 50 μg/ml LWE, the
insulin levels were 2433.7±34.5, 2824.2±150.3, 3565.4±223.3,
4730.9±140.3 and 5069.2±131.5 pg/ml, respectively. These results
suggest that LWE treatment is effective in increasing pancreatic β
cell survival and maintaining normal biological functions in
ROS-induced diabetes.
Discussion
ROS-induced oxidative damage in pancreatic β cells
is considered to be important in the pathological process of
diabetes. A number of studies have shown that reducing ROS levels
and treatment with antioxidants (including NAC, vitamin C and
vitamin E) improved β cell structure and function in
vitro(24,25). However, whether LWE protects
pancreatic β cells against SIN-1-induced oxidative damage has not
yet been elucidated. In the present study, we demonstrated that LWE
protected HIT-T15 cells against ROS-induced cell damage. The
cytoprotective effects of LWE were mainly mediated by increased
intracellular antioxidant enzyme activity.
The results of this study clearly showed that LWE
prevented SIN-1-induced cell death, as assessed using the MTT
assay. Additionally, LWE alone was not significantly cytotoxic to
cells at the concentrations used. Treatment with LWE was shown to
have a significant protective effect, which may be attributed to
the free radical scavenging activity of the extract.
To evaluate the role of free radicals in the
protective activity of LWE, the effect of LWE on SIN-1-induced ROS
generation was analyzed using the H2DCFDA assay. SIN-1
treatment alone significantly increased intracellular ROS
generation. Following treatment with LWE, ROS generation was found
to decline in a dose-dependent manner. This decrease in the
SIN-1-induced ROS generation may account for the decline in the
observed cytoprotective effect of LWE.
Lipid peroxidation is the most extensively
investigated process induced by free radicals. ROS participate in
the toxic actions that lead to apoptosis in insulin-producing cells
(26). In the present study,
increased lipid peroxidation levels were observed in SIN-1-treated
HIT-T15 cells. However, treatment with LWE resulted in a decrease
in lipid peroxidation, indicating that oxidative
stress-related damage was reduced in LWE-treated cells. The ability
of LWE to reduce lipid peroxidation may be due to its function as a
preventive antioxidant to scavenge initiating radicals.
The overproduction and consequently increased levels
of free radicals may be scavenged by endogenous antioxidant
enzymes, including SOD and GSH-px. In cells, SOD catalyzes the
conversion of O2− to
H2O2, and H2O2 is
further reduced to H2O by the activity of CAT or GSH-px.
Pancreatic β cells have been reported to contain low levels of
endogenous antioxidant enzymes, particularly GSH-px and CAT
(27). In the present study,
SIN-1-treated HIT-T15 cells were shown to have decreased GSH-px and
CAT activities, which may be due to the increased oxidative damage
induced by SIN-1. However, LWE treatment caused an increase in the
activity of these antioxidant enzymes in HIT-T15 cells, indicating
that LWE reduced SIN-1-induced oxidative stress. A number of
studies have reported that the overexpression of CuZnSOD had a
protective effect in NO-induced human islets, INS-1
insulin-secreting cells (28) and
alloxan- and streptozotocin-induced diabetes (29,30).
CAT has also exhibited a protective effect against
H2O2 and streptozotocin-induced oxidative
stress in vivo(31).
Additionally, combinatorial overexpression of CAT and GSH-px has
been shown to have a protective effect against ROS-induced
oxidative stress through improving the activity of CuZnSOD or MnSOD
(32–35).
In conclusion, the present study showed that LWE had
protective activity against SIN-1-induced cell death in Syrian
hamster HIT-T15 insulin-secreting cells. LWE effectively scavenged
the products of SIN-1-induced intracellular ROS generation and
reduced pancreatic β cell death through increasing the activity of
intracellular antioxidant enzymes, including SOD, CAT and GSH-Px.
LWE also promoted insulin secretion in SIN-1-treated HIT-T15
cells.
Acknowledgements
This study was supported by the Natural Science
Foundation Project of CQ CSTC (No. CSTC2012jjA80002) and the
Science and Technology Research Project of Chongqing Municipal
Education Commission (No. KJ121504)
References
|
1
|
Halliwell B: Reactive species and
antioxidants. Redox biology is a fundamental theme of aerobic life.
Plant Physiol. 141:312–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Are oxidative stress-activated signaling pathways
mediators of insulin resistance and beta-cell dysfunction?
Diabetes. 52:1–8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahimi R, Nikfar S, Larijani B and
Abdollahi M: A review on the role of antioxidants in the management
of diabetes and its complications. Biomed Pharmacother. 59:365–373.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robertson RP and Harmon JS: Diabetes,
glucose toxicity, and oxidative stress: a case of double jeopardy
for the pancreatic islet beta cell. Free Radic Biol Med.
41:177–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bell DS: Do sulfonylurea drugs increase
the risk of cardiac events? CMAJ. 174:185–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Home PD, Pocock SJ, Beck-Nielsen H, Gomis
R, Hanefeld M, Jones NP, Komajda M and McMurray JJ; RECORD Study
Group. Rosiglitazone evaluated for cardiovascular outcomes - an
interim analysis. N Engl J Med. 357:28–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Psaty BM and Furberg CD: The record on
rosiglitazone and the risk of myocardial infarction. N Engl J Med.
357:67–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nathan DM: Rosiglitazone and
cardiotoxicity - weighing the evidence. N Engl J Med. 357:64–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bailey CJ and Day C: Traditional plant
medicines as treatments for diabetes. Diabetes Care. 12:553–564.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia F: Distribution and deterioration
of insulin-like principle in Lagerstroemia speciosa
(banaba). Acta Med Philippina. 3:99–104. 1941.
|
|
11
|
Klein G, Kim J, Himmeldirk K, Cao Y and
Chen X: Antidiabetes and anti-obesity activity of Lagerstroemia
speciosa. Evid Based Complement Alternat Med. 4:401–407. 2007.
View Article : Google Scholar
|
|
12
|
Mishra Y, Khan MSY, Zafar R and Agarwal
SS: Hypoglycemic activity of leaves of Lagerstroemia
speciosa (L) Pers. Indian J Pharmacol. 22:174–176. 1990.
|
|
13
|
Priya TT, Sabu MC and Jolly CI: Free
radical scavenging and anti-inflammatory properties of
Lagerstroemia speciosa (L). Inflammopharmacology.
16:182–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan MT, Lampronti I, Martello D, Bianchi
N, Jabbar S, Choudhuri MS, Datta BK and Gambari R: Identification
of pyrogallol as an antiproliferative compound present in extracts
from the medicinal plant Emblica officinalis: effects on in
vitro cell growth of human tumor cell lines. Int J Oncol.
21:187–192. 2002.PubMed/NCBI
|
|
15
|
Suzuki Y, Unno T, Ushitani M, Hayashi K
and Kakuda T: Antiobesity activity of extracts from
Lagerstroemia speciosa L. leaves on female KK-Ay mice. J
Nutr Sci Vitaminol (Tokyo). 45:791–795. 1999. View Article : Google Scholar
|
|
16
|
Kakuda T, Sakane I, Takihara T, Ozaki Y,
Takeuchi H and Kuroyanagi M: Hypoglycemic effect of extracts from
Lagerstroemia speciosa L. leaves in genetically diabetic
KK-AY mice. Biosci Biotechnol Biochem. 60:204–208. 1996.
|
|
17
|
Liu F, Kim J, Li Y, Liu X, Li J and Chen
X: An extract of Lagerstroemia speciosa L. has insulin-like
glucose uptake-stimulatory and adipocyte differentiation-inhibitory
activities in 3T3-L1 cells. J Nutr. 131:2242–2247. 2001.PubMed/NCBI
|
|
18
|
Liu X, Kim JK, Li Y, Li J, Liu F and Chen
X: Tannic acid stimulates glucose transport and inhibits adipocyte
differentiation in 3T3-L1 cells. J Nutr. 135:165–171.
2005.PubMed/NCBI
|
|
19
|
Liu J, Sun H, Duan W, Mu D and Zhang L:
Maslinic acid reduces blood glucose in KK-Ay mice. Biol Pharm Bull.
30:2075–2078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fraga CG, Leibovitz BE and Tappel AL:
Lipid peroxidation measured as thiobarbituric acid-reactive
substances in tissue slices: characterization and comparison with
homogenates and microsomes. Free Radic Biol Med. 4:155–161. 1988.
View Article : Google Scholar
|
|
21
|
Nelson DP and Kiesow LA: Enthalpy of
decomposition of hydrogen peroxide by catalase at 25 degrees C
(with molar extinction coefficients of H2O2
solutions in the UV). Anal Biochem. 49:474–478. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autoxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hafeman DG, Sunde RA and Hoekstra WG:
Effect of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J Nutr. 104:580–587. 1974.PubMed/NCBI
|
|
24
|
Robertson RP, Harmon J, Tran PO, Tanaka Y
and Takahashi H: Glucose toxicity in beta-cells: type 2 diabetes,
good radicals gone bad, and the glutathione connection. Diabetes.
52:581–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng Q, Law PK, de Gasparo M and Leung
PS: Combination of the dipeptidyl peptidase IV inhibitor LAF237
[(S)-1-[(3-hydroxy-1-adamantyl)ammo]acetyl-2-cyanopyrrolidine] with
the angiotensin II type 1 receptor antagonist valsartan
[N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-4-yl]methyl]-L-valine]
enhances pancreatic islet morphology and function in a mouse model
of type 2 diabetes. J Pharmacol Exp Ther. 327:683–691. 2008.
|
|
26
|
Lenzen S: Oxidative stress: the vulnerable
beta-cell. Biochem Soc Trans. 36:343–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Ollinger K and Brunk U:
Insulinoma cells in culture show pronounced sensitivity to
alloxan-induced oxidative stress. Diabetologia. 38:635–641. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriscot C, Pattou F, Kerr-Conte J,
Richard MJ, Lemarchand P and Benhamou PY: Contribution of
adenoviral-mediated superoxide dismutase gene transfer to the
reduction in nitric oxide-induced cytotoxicity on human islets and
INS-1 insulin-secreting cells. Diabetologia. 43:625–631. 2000.
View Article : Google Scholar
|
|
29
|
Kubisch HM, Wang J, Bray TM and Phillips
JP: Targeted overexpression of Cu/Zn superoxide dismutase protects
pancreatic beta-cells against oxidative stress. Diabetes.
46:1563–1566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubisch HM, Wang J, Luche R, Carlson E,
Bray TM, Epstein CJ and Phillips JP: Transgenic copper/zinc
superoxide dismutase modulates susceptibility to type I diabetes.
Proc Natl Acad Sci USA. 91:9956–9959. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu B, Moritz JT and Epstein PN:
Overexpression of catalase provides partial protection to
transgenic mouse beta cells. Free Radic Biol Med. 27:830–837. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amstad P, Moret R and Cerutti P:
Glutathione peroxidase compensates for the hypersensitivity of
Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol
Chem. 269:1606–1609. 1994.PubMed/NCBI
|
|
33
|
Lortz S and Tiedge M: Sequential
inactivation of reactive oxygen species by combined overexpression
of SOD isoforms and catalase in insulin-producing cells. Free Radic
Biol Med. 34:683–688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lepore DA, Shinkel TA, Fisicaro N, Mysore
TB, Johnson LE, d’Apice AJ and Cowan PJ: Enhanced expression of
glutathione peroxidase protects islet beta cells from
hypoxia-reoxygenation. Xenotransplantation. 11:53–59. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mysore TB, Shinkel TA, Collins J, Salvaris
EJ, Fisicaro N, Murray-Segal LJ, Johnson LE, Lepore DA, Walters SN,
Stokes R, Chandra AP, O’Connell PJ, d’Apice AJ and Cowan PJ:
Overexpression of glutathione peroxidase with two isoforms of
superoxide dismutase protects mouse islets from oxidative injury
and improves islet graft function. Diabetes. 54:2109–2116. 2005.
View Article : Google Scholar : PubMed/NCBI
|