Introduction
Progranulin (PGRN), also termed proepithelin,
acrogranin or prostate cancer cell-derived growth factor, is a
growth modulating factor involved in multiple biological functions
(1). The mutations within the PGRN
gene result in the partial loss of the PGRN protein, which is the
leading cause of frontotemporal lobar degeneration (FTLD) (2). It was demonstrated that PGRN
exhibited high expression levels during early neural development
(3). However, its expression was
restricted to defined neuronal populations, including cortical and
hippocampal pyramidal neurons and Purkinje cells in the adult brain
(4). Although the expression of
PGRN in motor neurons and neuroblastoma cell lines has been
investigated (5,6), the involvement of PGRN in the central
nervous system (CNS) remains to be elucidated.
Neural stem cells (NSCs) are the undifferentiated
neural cells that possess multi-potential and self-renewal
capacity, and differentiate into three cell lineages in the CNS:
neurons, astrocytes and oligodendrocytes (7,8).
NSCs were predominantly separated and cultured from the
subventricular zone (SVZ) of the lateral ventricles and subgranular
zone (SGZ) of the hippocampus in the adult nervous system (9). Investigation of the involvement of
ectogenic and endogenic factors in NSCs aid in the understanding of
how to modulate the proliferation and differentiation of NSCs
during neurogenesis (10,11). Recent studies have demonstrated
that PGRN is a neurotrophic factor that modulates neurite outgrowth
and enhances neuronal survival (12), and exogenous PGRN promotes neural
progenitor cell proliferation (13). However, the expression profiles of
PGRN in neurospheres and its differentiated subpopulations are not
fully understood.
Considering the essential role of NSCs in
neurogenesis, further insight into the association between PGRN and
NSCs may aid in the clarification of the involvement of PGRN in the
pathogenesis of neurodegenerative diseases and provide a basis for
future novel therapies. In the present study, primary NSCs were
isolated and cultured from the neonatal rat brain and were used to
investigate the expression of PGRN in NSCs and differentiated
subpopulations.
Materials and methods
Primary culture of rat neural stem
cells
All animal related experiments were conducted in
accordance with the guidelines of the Experimental Laboratory
Animal Committee of Sun Yat-sen University Health Science Center
(Guangzhou, China) and under the approval of the principles of the
National Institutes of Health’s Guide for the Care and Use of
Laboratory Animals. NSCs were prepared from the SVZ of the brain of
neonatal Sprague Dawley (SD) rats. Neonatal SD pups (age, 1 day)
were sacrificed and their brains were removed. The meninges and
blood vessels were removed with fine forceps; a thin tissue layer
surrounding the lateral ventricles was isolated from coronal slices
of the brain and cut into small sections in ice-cold D-Hanks medium
(Genom Co., Hangzhou, China) containing 100 U/ml penicillin and 100
mg/ml streptomycin. Following two washes in D-Hanks medium, the
tissues were dissociated mechanically with a Pasteur pipette
(Biolegix, Philadelphia, PA, USA) into coarse single-cell
suspensions, which were further centrifuged and resuspended prior
to being passed through a cell strainer (40-μm, 352340, BD Falcon™;
BD Biosciences, Franklin Lakes, NJ, USA) to remove the debris. When
the homogenate was centrifuged at 80 × g for 5 min, the cells were
resuspended in supplemented culture medium that consisted of DMEM:
nutrient mixture F12 (DMEM/F12) (1:1) (Gibco-BRL, Carlsbad, CA,
USA) supplemented with 1% B27 (Invitrogen Life Technologies,
Carlsbad, CA, USA), 1% N2 supplement (17502-048; Invitrogen Life
Technologies), 20 ng/ml epidermal growth factor (EGF) (Invitrogen
Life Technologies) and 20 ng/ml basic fibroblast growth factor
(bFGF) (Invitrogen Life Technologies). Cells were incubated at 37ºC
in a 5% CO2 atmosphere and half the volume of the
culture medium was replaced every 2 days. After 7 days, NSCs
aggregated into neurospheres. For passaging, neurospheres were
resuspended and replated into non-coated culture flasks at a
density of 2×105 cells/ml following mechanical
dissociation.
Differentiation of neural stem cells
To induce the differentiation of NSCs, neurospheres
were seeded onto poly-L-lysine-coated glass coverslips (NEST
Biotechnology Co., Ltd., Shanghai, China) following three passages
and then cultured in differentiation medium containing DMEM/F12
(1:1), 1% N2, and 10% fetal bovine serum (FBS) (Gibco-BRL). Under
this environment, the sedimentary neurospheres attached to the
poly-L-lysine-coated surface and began to differentiate into
neurons, astrocytes and oligodendrocytes. The cells were maintained
at 37ºC in a 5% CO2 atmosphere and the medium was
replaced every 2 days. The differentiation of NSCs was observed
using a phase-contrast microscope (DMI4000B; Leica, Wetzlar,
Germany), cells were photographed and analyzed for morphology at
day 7.
Preparation of tissue sections
The brains of neonatal SD rats (age, 1 and 7 days)
were used to investigate the expression and localization of PGRN
during development. The rats were anesthetized with a peritoneal
injection of 10% chloral hydrate (Sigma-Aldrich, St. Louis, MO,
USA) and perfused transcardially with cold phosphate saline buffer
(PBS) followed by ice-cold 4% paraformaldehyde in 0.1 M PBS. The
brains were removed and postfixed in 4% paraformaldehyde solution
at 4ºC overnight, after which the brains were dehydrated serially
in 10, 20 and 30% sucrose in PBS overnight at 4ºC. When the the
brain sediment reached the bottom of the tubes, the brains were
embedded in Optimal Cutting Temperature compound (Sakura Finetek
Co., Ltd., Tokyo, Japan). Serial coronal sections were cut using a
freezing microtome at 10 μm and mounted onto poly-L-lysine-coated
glass slides. PGRN was detected using immunohistochemistry.
Immunocytochemistry staining
To investigate the expression of PGRN in NSCs, the
neurospheres were collected by centrifugation and then washed three
times with PBS and fixed in 4% paraformaldehyde solution for 30 min
at room temperature. Following three washes with PBS, the
neurospheres were blocked using 10% normal goat serum for 1 h at
room temperature to reduce non-specific binding. Neurospheres were
then incubated with primary antibody against nestin (dilution,
1:1,000; Abcam, Hong Kong, China) for NSCs and PGRN (dilution,
1:500; R&D Systems, Inc., Minneapolis, MN, USA) in PBS
containing 0.3% Triton X-100 at 4ºC overnight. Following sufficient
washing, cells were incubated with appropriate
fluorescence-conjugated secondary antibodies for 1 h at 37ºC. For
the identification of PGRN in differentiated NSCs, the cells were
grown on poly-L-lysine-coated round glass coverslips, rinsed three
times with PBS following 7 days of differentiation, fixed with 4%
paraformaldehyde solution for 30 min at room temperature and
blocked with 10% normal goat serum for 1 h at room temperature. The
cells were then incubated with primary antibody against
βIII-tubulin for neurons (dilution, 1:1,000; Abcam), glial
fibrillary acidic protein (GFAP) for astrocytes (dilution, 1:1,000;
Abcam), Oli for oligodendrocytes (dilution, 1:1,000; Abcam) and
PGRN (dilution, 1:500; R&D Systems, Inc.) in PBS containing
0.3% Triton X-100 at 4ºC overnight. The cells were washed with PBS
and incubated with the corresponding fluorescence-conjugated
secondary antibodies [Alexa Fluor®488 donkey anti-sheep
IgG (H+L), A-11015; Alexa Fluor®594 goat anti-mouse IgG
(H+L), A-11005; and Alexa Fluor®594 goat anti-rabbit IgG
(H+L), A-11012; Invitrogen Life Technologies)] for 1 h at 37ºC.
Nuclei were stained for 5 min with the nuclear dye Hoechst 33342
(20 ng/ml; Biotium, Hayward, CA, USA). Images were captured with a
fluorescence microscope (BX51WI; Olympus, Tokyo, Japan).
Double immunofluorescent staining
The expression of PGRN was also detected in the
brain of neonatal SD rats (age, 1 and 7 days). Frozen sections were
heated to 56ºC for 10 min, washed with PBS and incubated with
blocking solution with 10% normal goat serum containing 0.3% Triton
X-100 for 1 h at room temperature. The sections were then incubated
with primary antibodies (anti-PGRN, 1:600; anti-βIII-Tubulin,
1:1,000; anti-GFAP, 1:1,000; and anti-Oli, 1:1,000) overnight at
4ºC. When the sections had been thoroughly washed with PBS,
appropriate secondary antibodies Alexa Flour 488 and Alexa Flour
594 (1:1,000 dilution, Invitrogen Life Technologies) were added and
incubated for 2 h at 37ºC, and the sections were washed three times
with PBS. Nuclei were stained with the nuclear dye Hoechst 33342
(20 ng/ml) for 5 min and rinsed with PBS, and the coverslips were
mounted on slides with FluorSave reagent (Beyotime, Jiangsu,
China).
Results
Neural stem cells aggregate into
neurospheres and express PGRN
Neurospheres were clearly observed at day 3.
Numerous neurospheres proliferated in suspension at day 7 following
primary culture (Fig. 1A).
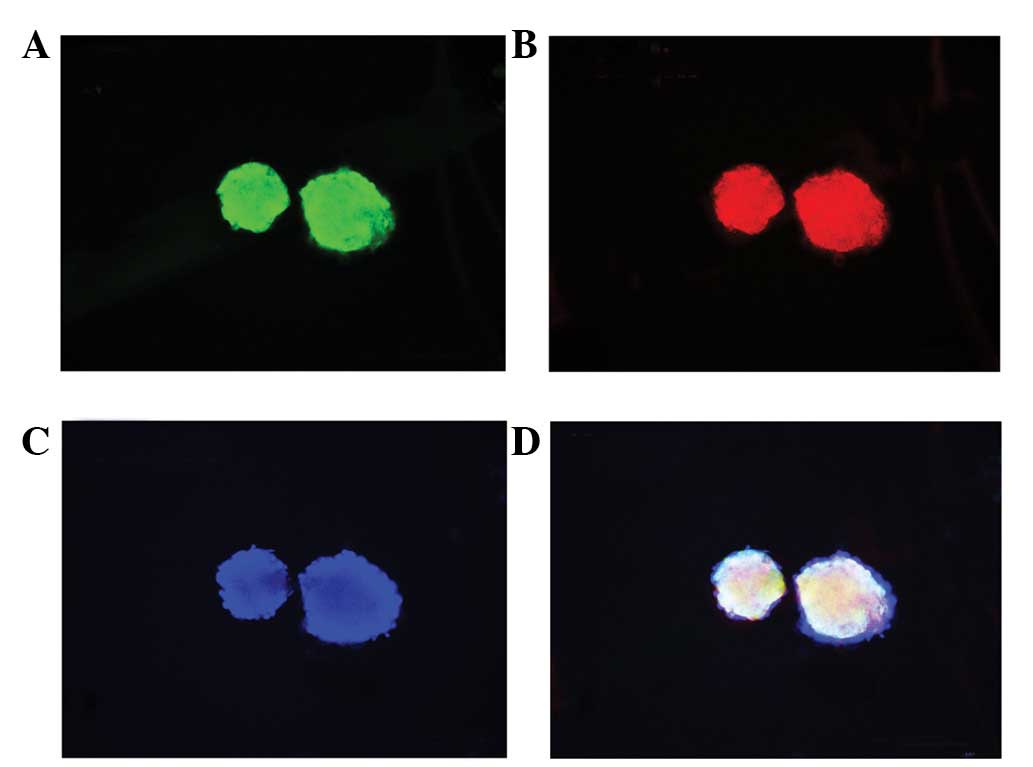
Immunocytochemistry results showed that neurospheres appeared
positive for nestin and PGRN expression (Fig. 2A–D). These results indicated that
NSCs were successfully isolated and cultured and expressed
PGRN.
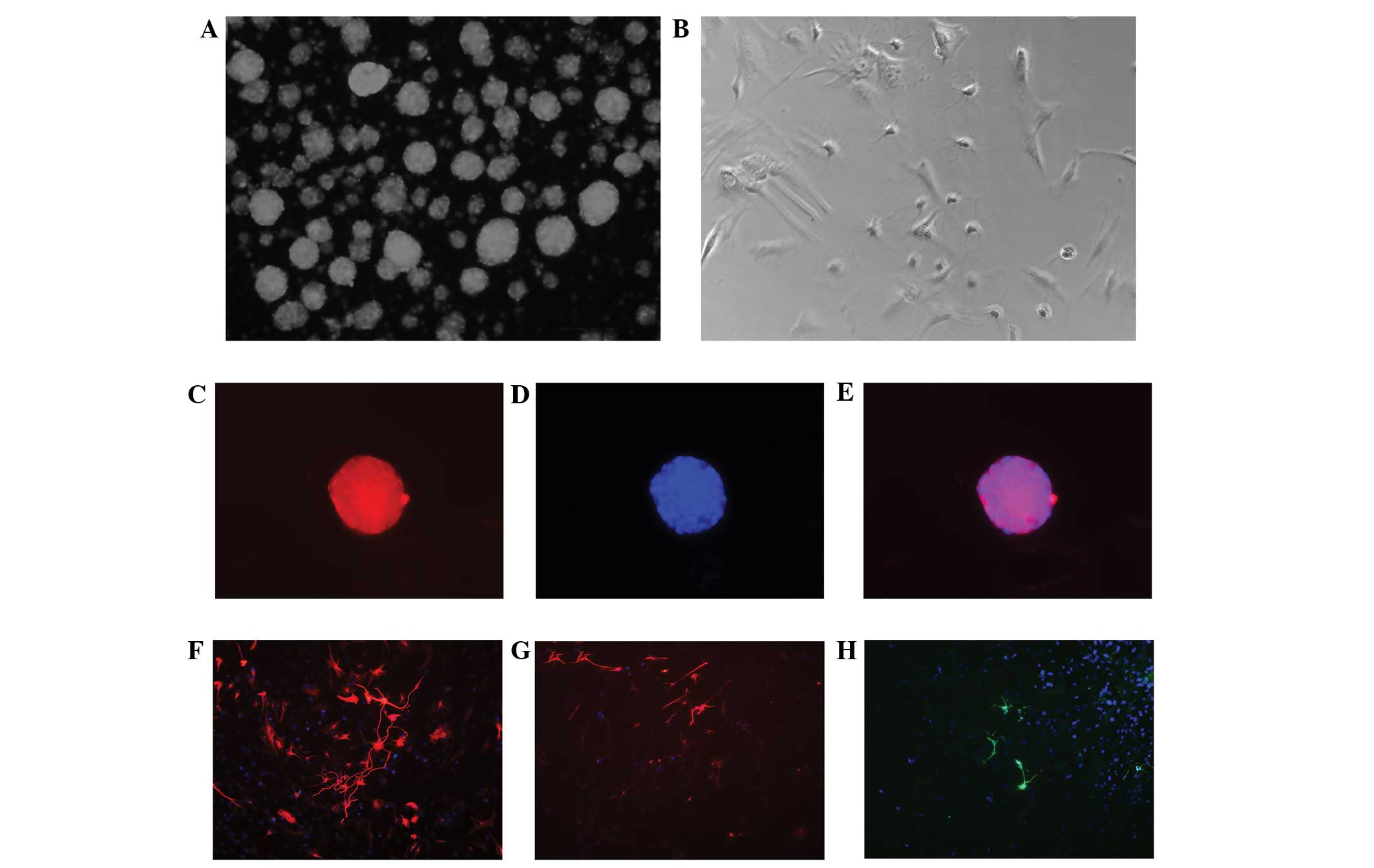 | Figure 1(A,C–E) Characteristics of
neurospheres isolated and cultured from the subventricular zone of
neonatal SD rats, (magnification, ×100). Immunocytochemical
staining showed that neurospheres appeared positive when tested for
nestin, an intermediate filament characteristic of undifferentiated
neuroepithelial cells. (A) Neurospheres maintained in the culture
medium for 7 days. (B) When neurospheres were maintained in the
differential medium for 7 days, the neurosphere-derived cells grow
onto the coverslips and gradually showed the morphological features
of neural cells. (C) Nestin (red) expression was observed in the
neurospheres in primary cultures. (D and H) Cell nuclei were
staining with Hoechst 33342 (blue). (B,F–H) Neural stem cells
(NSCs) differentiate into neurons, astrocytes and oligodendrocytes,
(magnification, ×200). The immunocytochemical staining shows that
the NSCs differentiate into (F) βIII-tubulin+ neurons,
(G) glial fibrillary acidic protein+ astrocytes and (H)
Oli+ oligodendrocytes. |
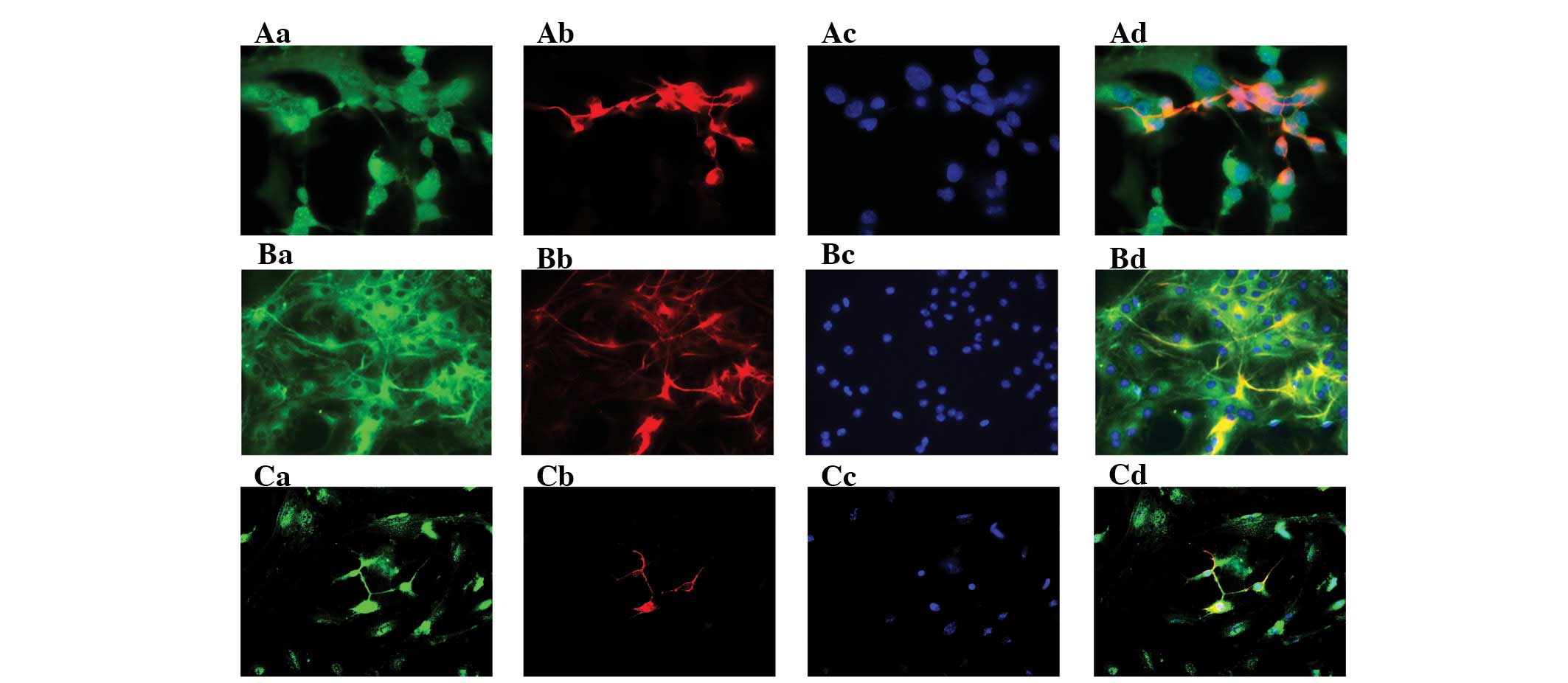
NSC-derived subpopulations express
PGRN
After three passages, the neurospheres were
transferred in 24-well plates containing poly-L-lysine-coated glass
coverslips and were maintained in differential medium, containing
DMEM/F12 (1:1), 1% N2 supplement and 10% FBS, for a further 7 days.
The neurospheres began to attach to the coverslips and gradually
showed the morphological features of neural cells (Fig. 1B). The immunocytochemical staining
showed that the NSCs differentiated into βIII-tubulin+
neurons, GFAP+ astrocytes and Oli+
oligodendrocytes (Fig. 1F–H).
Moreover, all NSC-derived cells expressed PGRN in vitro
(Fig. 3).
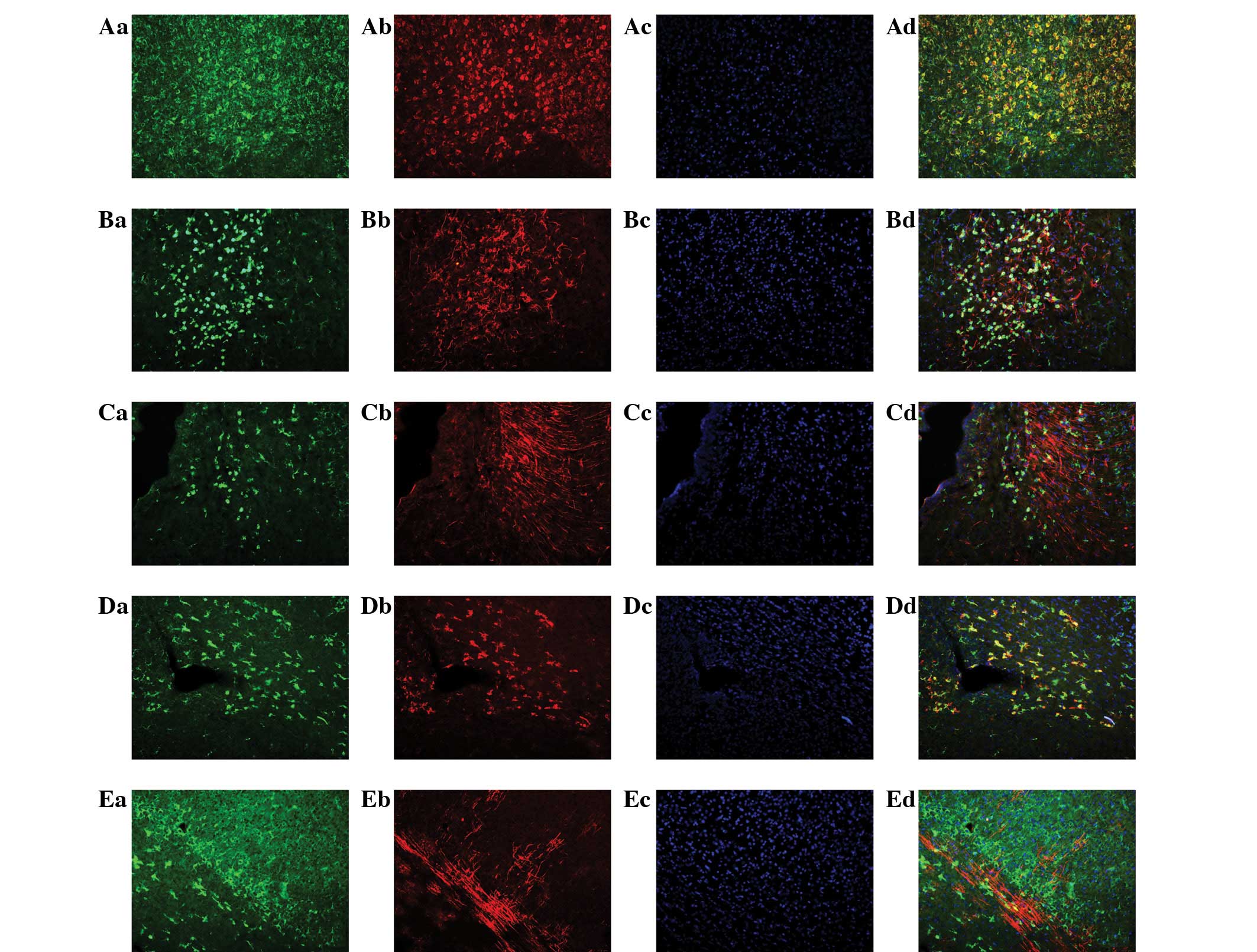
PGRN is expressed in neurons and
microglia but marginally in NSCs, astrocytes and oligodendrocytes
in the brains of neonatal rats
The expression of PGRN was also analyzed by
immunofluorescent staining in postnatal rat brains at day 1 (P1)
and day 7 (P7). PGRN immunoreactivity was observed predominantly in
the cortex, corpus callosum and hippocampus. To analyze the cell
type-specific expression of PGRN in the neonatal brain,
immunofluorescent double labeling was performed with specific
markers for neurons (βIII-tubulin), astrocytes (GFAP), microglia
(OX-42), NSCs (Nestin) and oligodendrocytes (Oli). The results
indicated that the mature neuronal marker, βIII-tubulin (Fig. 4Aa–Ad) and microglia marker, OX-42
(Fig. 4Da–Dd) were double-labeled
with PGRN, which indicated that PGRN was predominantly expressed in
neurons and microglia during the early stages of rat brain
development. By contrast, PGRN immunoreactivity was marginal in
astrocytes (Fig. 4Ab–Db), NSCs
(Fig. 4Ca–Cd) and oligodendrocytes
(Fig. 4Ea–Ed). Thus, these results
suggest that PGRN was predominantly expressed in neurons and
microglia rather than NSCs, astrocytes and oligodendrocytes in the
brain at P1 and P7.
Discussion
Numerous studies have demonstrated that the 68.5-kDa
secreted protein PGRN is a growth factor (14), which functions as a potential
mediator of wound healing and cancer progression (1). More recently, PGRN was suggested to
be associated with neurodegenerative diseases and psychiosis
(15,16). Mutations in the progranulin gene
have been identified as a predominant cause of frontotemporal lobar
degeneration with ubiquitin-positive inclusions (17). Previous studies have indicated that
the overexpression of PGRN was cytoprotective in cultured cells
(5) and in vivo in
transgenic mice (18). PGRN was
observed to be an endogenous neuroprotectant against apoptosis and
inflammatory responses. Conversely, extracellular PGRN also acts as
a neuroprotective factor in the regulation of neurite outgrowth,
enhanced neuronal survival and protection against excitotoxicity
and oxidative stress which leads to the exacerbation of neuronal
cell death (12,19). Taken together, these results
demonstrated that PGRN may be a therapeutic target for
neurodegenerative diseases.
It was demonstrated that PGRN also enhances neural
progenitor cell proliferation (13). In the present study, the expression
of PGRN was analyzed in NSCs cultured from the SVZ of neonatal rat
brain and their differentiated cell lineages. The expression and
cell location of PGRN was also detected in the neonatal rat brain.
The results indicated that PGRN was abundantly expressed in
neurospheres and their differentiated cell lineages. The in
vivo studies showed that PGRN was highly expressed in neurons
and microglia but only marginally in NSCs, astrocytes and
oligodendrocytes in neonatal rat brain. These data provided useful
information concerning the involvement of PGRN in the regulation of
NSCs under the physiological conditions during development.
PGRN was identified in motor neurons, neuroblastoma
cell lines and fibroblasts (5,20,21).
Our data indicated that PGRN may be important in regulating the
differentiation of NSCs under physiological conditions during
development. In the present study, the expression of PGRN was
analyzed in NSCs in vitro, and the results showed that PGRN
was co-expressed with nestin in the NSC cytosol during
proliferation. The immunostaining for PGRN by different neural cell
markers nestin, βIII-Tubulin, GFAP and Oli was also used to analyze
the in vitro NSC differentiation. The in vivo studies
suggested that PGRN was co-expressed with neuron and microglia
markers, but not with markers of NSCs, astrocytes and
oligodendrocytes. The differential PGRN expression between in
vitro and in vivo studies may be due to the different
environmental stimulus. The data also demonstrated the expression
of PGRN in neurons increased during the cell maturation, whereas
the expression level in microglia was dependent upon the activation
state of the cells, as it was upregulated in microglia in response
to excitotoxic injuries (22).
Astrocytes differentiated from in vitro NSCs exhibited a
high level of PGRN, indicating that the astrocytes are a potential
source for cell transplantation during the treatment of FTLD.
In conclusion, the present data suggest that PGRN is
likely to be involved in regulating the differentiation of NSCs.
PGRN expression may be altered following oxidative stress or other
environmental stimuli. Therefore, further studies should focus on
the mechanism underlying the regulation of the differentiation and
maturation of NSCs and its subpopulations by PGRN during
development.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China for Youth (grant no. 81000562), the
National innovation experiment program for University students
(grant no. 101055827) and the Medical Scientific Research
Foundation of Guangdong Province, China (grant no. B2010068).
Abbreviations:
|
PGRN
|
progranulin
|
|
NSCs
|
neural stem cells
|
|
EGF
|
epidermal growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
FTLD
|
frontotemporal lobar degeneration
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium,
FBS, fetal bovine serum
|
|
PBS
|
phosphate- buffered saline
|
References
|
1
|
He Z and Bateman A: Progranulin
(granulin-epithelin precursor, P-cell-derived growth factor,
acrogranin) mediates tissue repair and tumorigenesis. J Mol Med
(Berl). 10:600–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gass J, Lee WC, Cook C, et al: Progranulin
regulates neuronal outgrowth independent of sortilin. Mol
Neurodegener. 7:332012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daniel R, He Z, Carmichael KP, Halper J
and Bateman A: Cellular localization of gene expression for
progranulin. J Histochem Cytochem. 48:999–1009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daniel R, Daniels E, He Z and Bateman A:
Progranulin (acrogranin/PC cell-derived growth
factor/granulin-epithelin precursor) is expressed in the placenta,
epidermis, microvasculature, and brain during murine development.
Dev Dyn. 227:593–599. 2003. View Article : Google Scholar
|
|
5
|
Ryan CL, Baranowski DC, Chitramuthu BP,
Malik S, Li Z, Cao M, Minotti S, Durham HD, Kay DG, Shaw CA,
Bennett HP and Bateman A: Progranulin is expressed within motor
neurons and promotes neuronal cell survival. BMC Neurosci.
10:1302009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piscopo P, Rivabene R, Adduci A, Mallozzi
C, Malvezzi-Campeggi L, Crestini A and Confaloni A: Hypoxia induces
up-regulation of progranulin in neuroblastoma cell lines. Neurochem
Int. 57:893–898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanagisawa M and Yu RK: The expression and
functions of glycoconjugates in neural stem cells. Glycobiology.
17:57R–74R. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JH, Choi MR, Park KS, Kim SH, Jung KH
and Chai YG: The characterization of gene expression during mouse
neural stem cell differentiation in vitro. Neurosci Lett.
506:50–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergström T and Forsberg-Nilsson K: Neural
stem cells: brain building blocks and beyond. Ups J Med Sci.
117:132–142. 2012.PubMed/NCBI
|
|
10
|
Trujillo CA, Schwindt TT, Martins AH,
Alves JM, Mello LE and Ulrich H: Novel perspectives of neural stem
cell differentiation: from neurotransmitters to therapeutics.
Cytometry A. 75:38–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alvarez-Buylla A and Lim DA: For the long
run: maintaining germinal niches in the adult brain. Neuron.
41:683–686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Damme P, Van Hoecke A, Lambrechts D,
Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L
and Robberecht W: Progranulin functions as a neurotrophic factor to
regulate neurite outgrowth and enhance neuronal survival. J Cell
Biol. 181:37–41. 2008.PubMed/NCBI
|
|
13
|
Nedachi T, Kawai T, Matsuwaki T,
Yamanouchi K and Nishihara M: Progranulin enhances neural
progenitor cell proliferation through glycogen synthase kinase 3β
phosphorylation. Neuroscience. 185:106–115. 2011.PubMed/NCBI
|
|
14
|
Bateman A and Bennett HP: Granulins: the
structure and function of an emerging family of growth factors. J
Endocrinol. 158:145–151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Muynck L and Van Damme P: Cellular
effects of progranulin in health and disease. J Mol Neurosci.
45:549–560. 2011.PubMed/NCBI
|
|
16
|
Galimberti D, Dell’Osso B, Fenoglio C, et
al: Progranulin gene variability and plasma levels in bipolar
disorder and schizophrenia. PLoS One. 7:e321642012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gass J, Prudencio M, Stetler C and
Petrucelli L: Progranulin: an emerging target for FTLD therapies.
Brain Res. 1462:118–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tao J, Ji F, Wang F, Liu B and Zhu Y:
Neuroprotective effects of progranulin in ischemic mice. Brain Res.
1436:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Xilouri M, Bruban J, Shioi J, Shao
Z, Papazoglou I, Vekrellis K and Robakis NK: Extracellular
progranulin protects cortical neurons from toxic insults by
activating survival signaling. Neurobiol Aging.
32:23262011.PubMed/NCBI
|
|
20
|
Guerra RR, Kriazhev L, Hernandez-Blazquez
FJ and Bateman A: Progranulin is a stress-response factor in
fibroblasts subjected to hypoxia and acidosis. Growth Factors.
25:280–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naphade SB, Kigerl KA, Jakeman LB, Kostyk
SK, Popovich PG and Kuret J: Progranulin expression is upregulated
after spinal contusion in mice. Acta Neuropathol. 119:123–133.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petkau TL, Neal SJ, Orban PC, MacDonald
JL, Hill AM, Lu G, Feldman HH, Mackenzie IR and Leavitt BR:
Progranulin expression in the developing and adult murine brain. J
Comp Neurol. 518:3931–3947. 2010. View Article : Google Scholar : PubMed/NCBI
|