Introduction
Periodontitis is one of the most widespread
infectious diseases in humans. It is the predominant cause of tooth
loss and is associated with a number of systemic diseases, such as
diabetes and cardiovascular disease (1). Periodontitis is stimulated by a
variety of factors. For example, lipopolysaccharide (LPS), which
acts as an endotoxin and elicits strong immune responses, is
important in the pathogenesis of periodontitis. LPS directly
induces tumor necrosis factor-α release from macrophages, and is
the leading stimulus that initiates the host response in the
periodontal pocket and activates macrophages to release
proinflammatory cytokines (2–5).
Moreover, LPS was observed to exhibit a significant cytotoxic
effect on periodontal ligament stem cells (PDLSCs), and affect the
self-renewal and osteogeneic differentiation potential of PDLSCs
(6).
Alkaline phosphatase (ALP) activity in periodontal
ligament cells (PDLCs) is an index for osteogeneic differentiation
(7). The activity of ALP
isoenzymes were observed to be correlated with cementum formation
and root development, and mice lacking ALP demonstrated cementum
formation inhibition (8–10). In addition, high ALP activity was
observed in the periodontal ligament due to the constant renewal of
the tissue or pathological conditions. Furthermore, patients with
chronic periodontitis exhibited increased ALP activity in the serum
(11), which indicated the
possible association between ALP activity in PDLCs and
periodontitis. Previous studies have demonstrated that LPS
diminishes ALP activity in PDLCs, induces the subtype change and
inhibits PDLC differentiation (12). Increased ALP activity appeared to
be correlated with subclinical recurrent inflammation and further
healing or remodeling of the periodontal tissue (13). Thus, improving ALP activity in
periodontal ligaments may be applicable for periodontal tissue
regeneration and repair.
Icariin (ICA) is the predominant active ingredient
of Herba Epimedii, which is a herb used in traditional Chinese and
alternative medicine. ICA increases trabecular bone mineral density
in ovariectomized rats and stimulates osteoblastic cell
proliferation and differentiation (14). Other studies have demonstrated that
ICA and its glycosides accelerated osteoblastic but suppressed
osteoclastic differentiation (15). In the present study, the effect of
ICA on human PDLCs (hPDLCs) inhibited by LPS was investigated, with
the aim of identifying a therapeutic agent for the treatment of
periapical disease resulting from bacterial infection.
Material and methods
hPDLC isolation and culture
Human tissue samples were collected from the
clinically healthy teeth of 11–14 adolescents who had undergone
teeth extraction for orthodontic treatment, no history of
periodontal disease and a relatively healthy periodontium. The
periodontal ligament tissues were obtained as remnants or discarded
tissues following routine dental procedures at the Second
Affiliated Hospital of. All protocols for the handling of human
tissue were approved by the Research Ethics Committee of Harbin
Medical University (Harbin, China) and written informed consent was
obtained from the families of the patients. The isolation and
culture of human mesenchymal stem cells from healthy periodontal
ligament tissues was performed as previously described (16). Briefly, tissues were treated
aseptically and incubated overnight at 4°C with 2 mg/ml dispase
(Sigma-Aldrich, St. Louis, Mo, USA) and 4 mg/ml collagenase IV
(Worthington Biochemical, Lakewood, NJ, USA). The dissociated cell
suspension was filtered through a 70-μm cell strainer (Falcon; BD
Biosciences, Franklin Lakes, NJ, USA), plated on nontreated 10-cm
petri dishes (VWR International, West Sussex, UK) with complete
α-minimal essential medium (Invitrogen Life Technologies, Carlsbad,
CA, USA) containing 20% fetal bovine serum (Clontech Laboratories
Inc., Mountain View, CA, USA), 100 U/ml penicillin, 100 μg/ml
streptomycin (Invitrogen Life Technologies), 2 M L-glutamine, 100
mM nonessential amino acid and 550 μM 2-mercaptoethanol
(Sigma-Aldrich). The suspension was cultured at 37°C in a
humidified tissue culture incubator with 5% CO2 and 95%
O2. After 72 h, the nonadherent cells were removed. The
plastic-adherent confluent cells were passaged with 0.05% trypsin
containing 1 mM EDTA and continuously subcultured and maintained in
complete growth medium. Cells from the fourth to sixth passages
were used in the experiments. Cells from the third passage were
fixed by 10% formaldehyde solution and stained with vimentin and
keratin according to standard immunohistochemical methods.
MTT proliferation assay
hPDLCs were seeded in a 96-well plate
(2×103 cells/well) with varied doses of ICA (0,
10−5, 10−6, 10−7, 10−8
and 10−9 mol/l). After 96 h, 20 μl of MTT was added and
the cells were incubated for 4 h. Following the addition of 150 μl
dimethylsulfoxide, the cells were agitated for 10 min, and the
concentration was analyzed by measuring the absorbance at 490 nm
with an iMark microplate reader (Bio-Rad, Hercules, CA, USA).
ALP activity assay
An ALP staining kit (Sigma-Aldrich) was used.
Subsequent to fixation with 70% ethanol, cells were incubated with
a solution of 0.25% naphthol AS-BI phosphate and 0.75% Fast Red
Violet LB Base dissolved in 0.1 M Tris buffer (pH 9.3). The ALP
activity assay was conducted according to the manufacturer’s
instructions and normalized on the basis of protein
concentrations.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cultured cells
undergoing osteogenic differentiation using an RNeasy Mini kit
(Qiagen, Valencia, CA, USA). Adipocyte- and osteocyte-specific
genes were amplified using the One-Step RT-PCR kit (Qiagen). The
specific primers used were as follows: Forward,
5′-AGGGCTGTAAGGACATCG-′3 and reverse, 5′-GGAGTGCTTGTATCTCGGTT-3′
for ALP; and forward, 5′-CATTGCCGACAGGATGCA-3′ and reverse,
5-′CATCTGCTGGAAGGTGGACAG-3′ for β-actin.
Western blot analysis
Cells were lysed with buffer containing 50 mM
Tris-HCl (pH 7.5), 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100, 10 mM
sodium fluoride, 20 mM 2-ME, 250 μM sodium orthovanadate, 1 mM
phenylmethylsulfonyl fluoride and complete protease inhibitor
mixture (Sigma-Aldrich), and were incubated at 4°C for 1 h. The
lysates were ultrasonicated (AIS92-IIDL ultrasonicator; Aismir,
Beijing, China) and centrifuged at 12,000 × g for 10 min. Protein
concentrations were determined using the bicinchoninic acid method.
Proteins (50–100 μg) were separated on 8–10% polyacrylamide-sodium
dodecyl sulfate gels and electroblotted onto nitrocellulose
membranes (Hybond ECL; Amersham Pharmacia, Picastaway, NJ, USA).
Subsequent to blocking with Tris-buffered saline and 5% nonfat dry
milk for 2 h, the membrane was incubated overnight at 4°C with
antibodies against human ALP (mouse ant-human; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) followed by incubation
with a horseradish peroxidase-conjugated secondary antibody (goat
anti-mouse; 1:2,000; Pierce, Rockford, IL, USA) for 45 min at room
temperature, and the signals were visualized by enhanced
chemiluminescence detection. As a loading control, the blots were
reprobed with a specific antibody against human β-actin (mouse
anti-human; dilution, 1:5,000; Santa Cruz Biotechnology, Inc.).
Statistical analysis
Statistical significance was assessed by two-tailed
Student’s t-test or analysis of variance. P<0.05 and P<0.01
were considered to indicate a statistically significant
difference.
Results
Isolation of hPDLCs
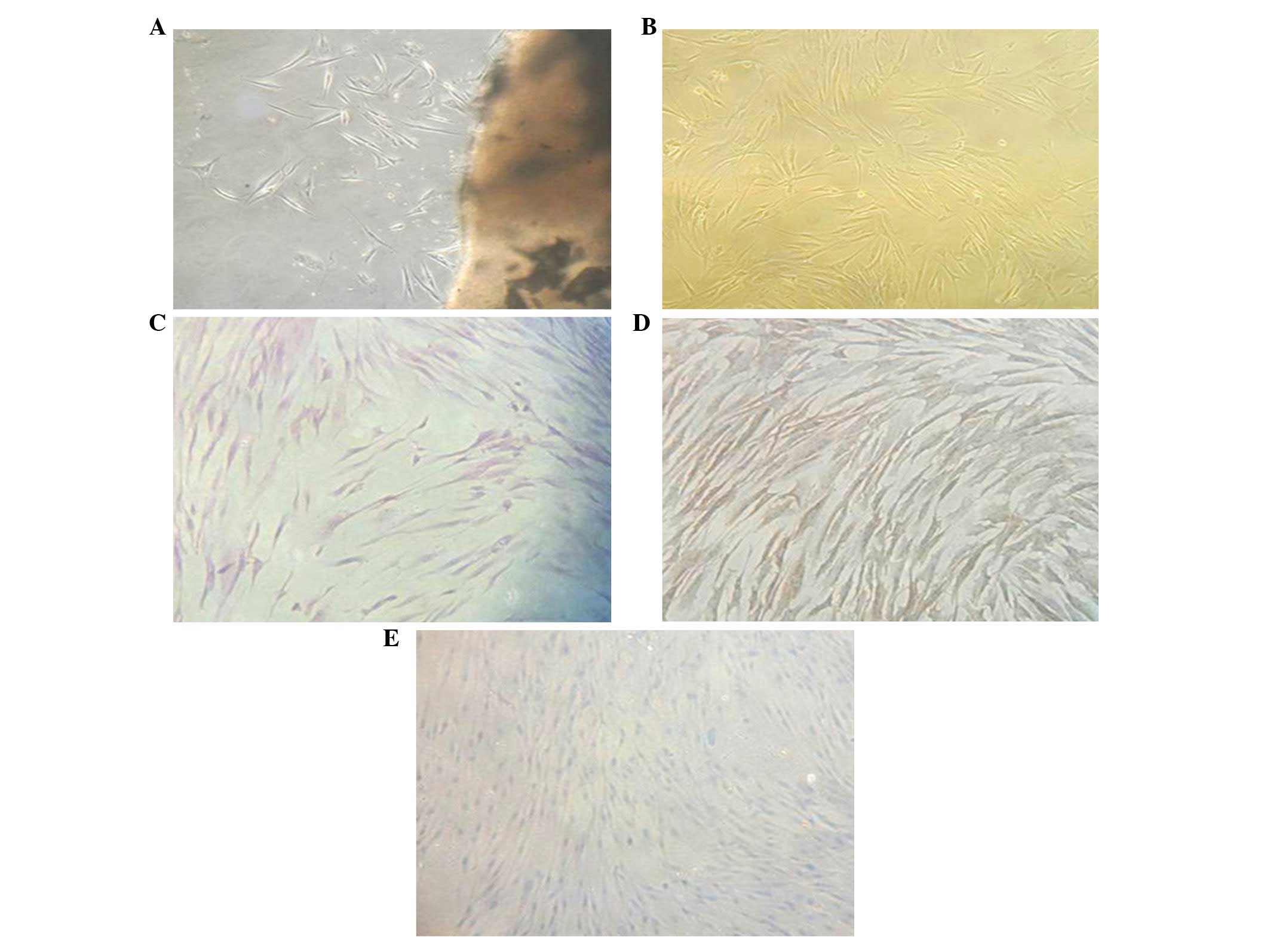
Following primary culture for 4–10 days,
fibroblast-like cells with a long fusiform shape emerged beside the
tissue block (Fig. 1A). When
subcultured to the fourth generation, cells adhered to the bottom
of the culture plate and were observed to exhibit a star- or long
fusiform-like shape under the microscope. Cell cytoplasm was plump
with round or oval nuclei. Cell arrangement was observed to be in a
gyrate or radial shape (Fig. 1B).
To confirm the origin of cultured cells, various types of stains
were applied. Hematoxylin and eosin-stained cell bodies showed long
fusiform- or star-like shapes. Nuclei were rounded or oval-shaped
and located in the center of the cell body. Cytoplasm stained with
anti-vimentin polyclonal antibody appeared brown, while keratin was
not stained (Fig. 1D and E). These
results suggested that isolated cells had an interstitial opposed
to an epithelial origin.
Effects of icariin on hPDLC
proliferation
As shown in Table
I, icariin accelerated the proliferation of hPDLCs when the
concentration was between 10−5 and 10−6 mol/l
(P<0.01 compared with the control group). The effect appeared to
be concentration-dependent within this concentration range. Lower
icariin concentrations exhibited no significant effect on hPDLC
proliferation while higher concentrations inhibited the division of
hPDLCs (P<0.05).
 | Table IEffect of ICA concentration on hPDLC
proliferation. |
Table I
Effect of ICA concentration on hPDLC
proliferation.
| ICA concentration
(mol/l) | Proliferation ability
(OD490) |
|---|
| 0 | 0.36±0.04 |
| 10−5 |
0.32±0.04a |
| 10−6 |
0.49±0.04b |
| 10−7 |
0.40±0.05a |
| 10−8 | 0.36±0.02 |
| 10−9 | 0.36±0.03 |
Icariin promotes differentiation of
hPDLCs inhibited by LPS
High ALP activity is a well-known index for hPDLC
ossification. Previous studies have shown that LPS inhibited hPDLC
differentiation and this process was associated with ALP activity
decreases. When icariin (10−5 mol/l) was added to media
containing LPS, the ALP activity was not changed at ~24 h; however,
following incubation for 96 h, hPDLCs exhibited high ALP activity
(Table II).
 | Table IIALP activity of different groups after
96 h incubation.. |
Table II
ALP activity of different groups after
96 h incubation..
| Groups | ALP activity (U/μl/μg
protein) |
|---|
| N | 3.55±0.41 |
| LPS | 1.18±0.38 |
| ICA+LPS | 2.1±0.44 |
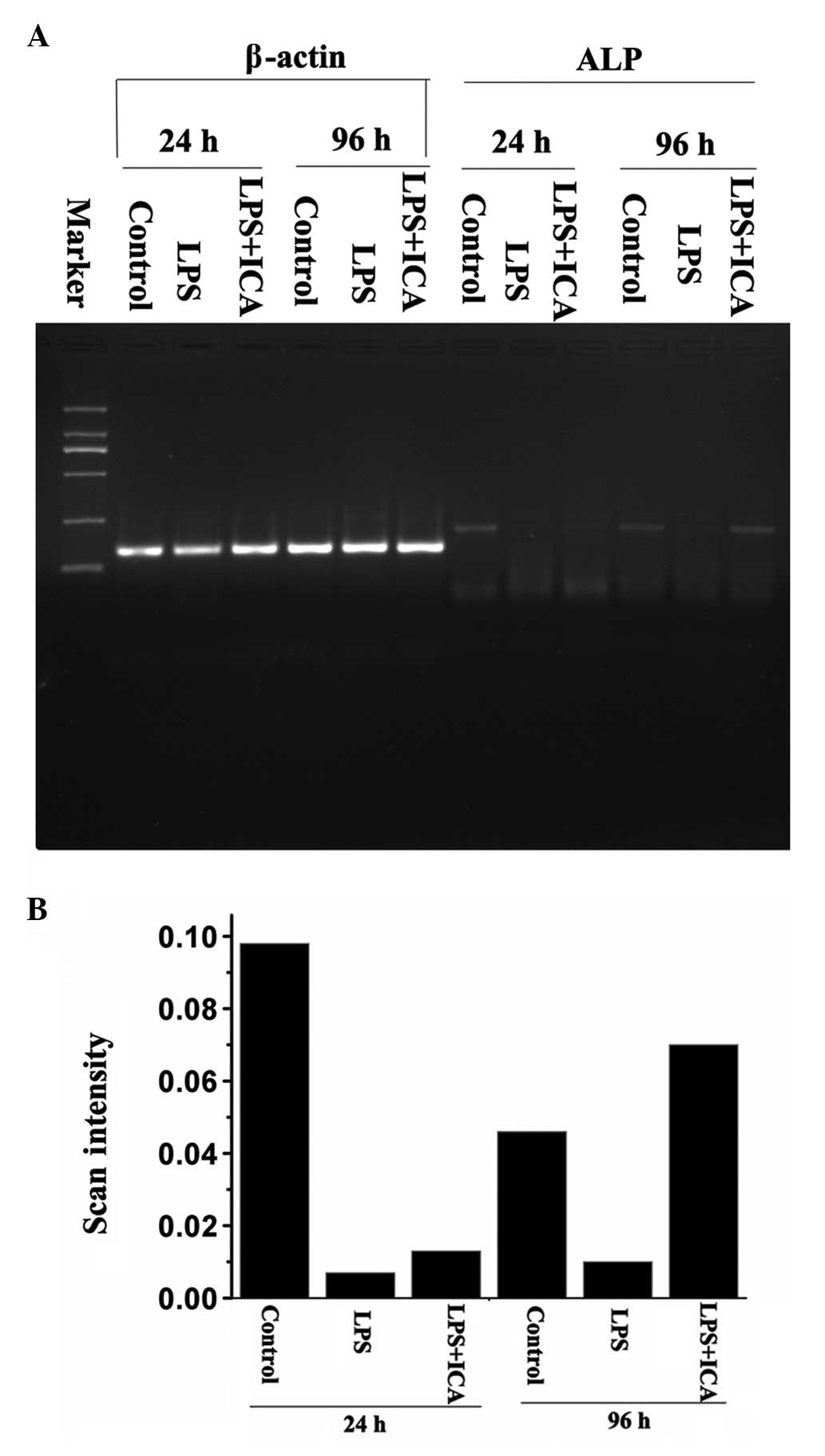
To identify at which level ALP activity returned to
normal levels, RT-PCR and western blot analysis were used to
measure the gene expression and protein levels of ALP,
respectively. The RT-PCR results showed that no significant
ALP expression difference was detected after 24 h.
Differentiation emerged between the LPS group and icarrin-treated
group after 96 h. The ALP activity of the icariin group was greater
than that observed in the control group (P<0.01) (Fig. 2).
These results suggested that the ALP gene
expression level that had been inhibited by LPS was recovered by
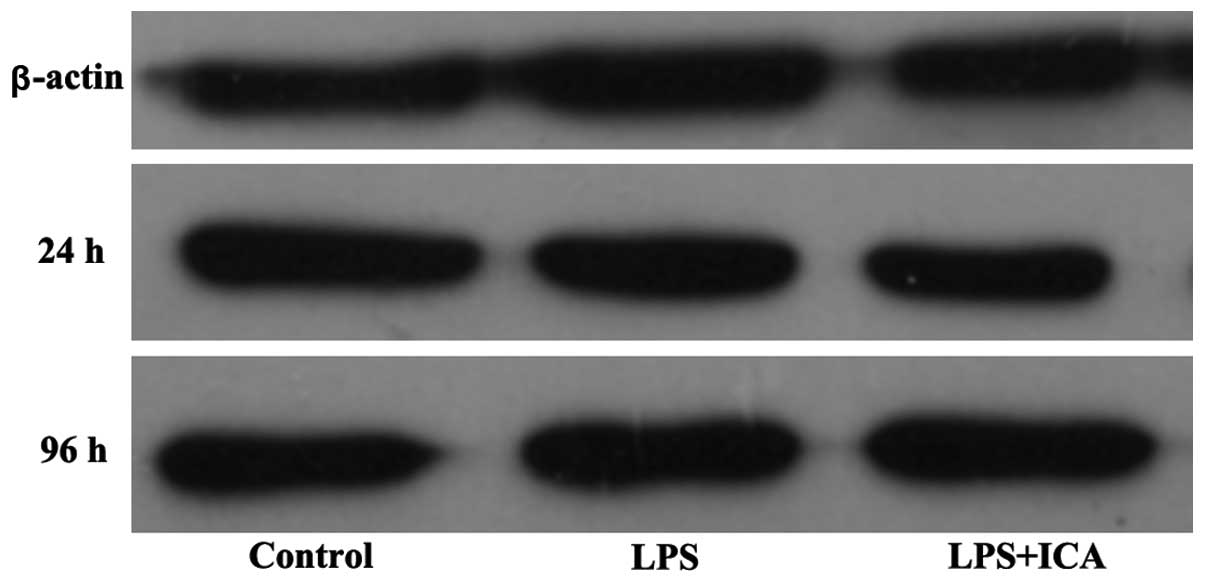
icariin following prolonged exposure. These results were similar in
the western blot analysis (Fig.
3). LPS suppressed ALP expression at 24 and 96 h, while icariin
accelerated this inhibition at 24 h, but markedly increased the
expression at 96 h, exceeding the level of the control group
(P<0.01). Thus, the ALP activity fluctuation was directly
correlated with the protein level of the enzyme, which was
controlled by the ALP expression.
Discussion
The apex dentis lesion of the un-develped permanent
teeth of teenagers could be medically induced to apical foramen
occlusion. Yang et al(17)
observed that the occlusion was hindered by the accumulation of
dentin, osteoid dentin, cementum and mineralization, which
differentiated from parodontium connective tissue. Parodontium
predominantly consists of PDLCs and collagenous fiber (18). PDLCs are heterogenic multipotential
stem cells that possess important biological functions. PDLCs
proliferate and differentiate into osteoblasts, which synthesize
periodontal ligament, alveolar bone and cementum, thus repairing
and regenerating periapical tissue (19).
Herba epimedii is a traditional Chinese herb that
has multiple medical effects. Modern pharmacological studies have
suggested that icariin extracted from herba epimedii inhibited
osteoclast function and accelerated the proliferation and
differentiation of osteoblasts (20). Icariin also decreased the
cytotoxicity resulting from LPS; however, the detailed mechanism of
this action remains to be elucidated. In the present study, the
effects of icariin on PDLC proliferation in an in vitro
culture were investigated. The results suggested that icariin
significantly accelerated PDLC proliferation at a concentration
range between 10−7 and 10−6 mol/l in a
concentration-dependent manner. A concentration of 10−6
mol/l exhibited the strongest effect.
ALP activity is one of the most important markers
for ossification (7). ALP conducts
dephosphorylation, destroys inhibitors of mineralization and acts
as a calcium binding protein or phosphate transporter to promote
ossification. ALP activity in PDLCs indicates ossification
transformation. LPS, a pathogenic sugar residue isolated
specifically on Gram negative bacteria, is a toxic antigen to cells
in the periapical tissue (21).
LPS is important in the development of periapical diseases
(7). In hPDLCs, LPS was observed
to decrease the ALP activity, leading to a loss of potential to
differentiate into osteoblasts and cementoblasts. Thus, increasing
the ALP activity in inhibited PDLCs is crucial for PDLC
regeneration and differentiation. The addition of icariin to
LPS-inhibited PDLCs increased ALP activity following exposure for
>96 h, while a short exposure period (~24 h) did not show a
marked increase. The molecular mechanism underlying the antagonism
was suggested to be mediated by icariin increasing ALP gene and
protein expression levels, which promotes ALP activity. Improved
ALP activity induced PDLC renewal and the potential for
differentiation. This provided insight into the use of icariin as a
treatment for periapical disease resulting from LPS.
Acknowledgements
This study was supported by the Natural Science
Foundation of Heilongjiang province (grant
no.41400649-6-12281).
Abbreviations:
|
hPDLCs
|
human periodontal ligament cells
|
|
ALP
|
alkaline phosphatase
|
|
ICA
|
icariin
|
|
LPS
|
lipopolysaccharide
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
References
|
1
|
Kinane DF and Marshall GJ: Periodontal
manifestations of systemic disease. Aust Dent J. 46:2–12. 2001.
View Article : Google Scholar
|
|
2
|
Roberts FA, Hockett RD Jr, Bucy RP, et al:
Quantitative assessment of inflammatory cytokine gene expression in
chronic adult periodontitis. Oral Microbiol Immunol. 12:336–344.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gamonal J, Acevedo A, Bascones A, et al:
Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival
crevicular fluid and cell populations in adult periodontitis
patients and the effect of periodontal treatment. J Periodontol.
71:1535–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thammasitboon K, Goldring SR and Boch JA:
Role of macrophages in LPS-induced osteoblast and PDL cell
apoptosis. Bone. 38:845–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumada H, Haishima Y, Umemoto T, et al:
Structural study on the free lipid A isolated from
lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol.
177:2098–2106. 1995.PubMed/NCBI
|
|
6
|
Cho JH, Lee SK, Lee JW and Kim EC: The
role of heme oxygenase-1 in mechanical stress-and
lipopolysacharide-induced osteogenic differentiation in human
periodontal ligament cells. Angle Orthod. 80:552–559. 2010.
|
|
7
|
Murakami Y, Kojima T, Nagasawa T, et al:
Novel isolation of alkaline phosphatase-positive subpopulation from
periodontal ligament fibroblasts. J Periodontol. 74:780–786. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Groeneveld MC, Everts V and Beertsen W:
Alkaline phosphatase activity in the periodontal ligament and
gingiva of the rat molar: its relation to cementum formation. J
Dent Res. 74:1374–1381. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beersten W, vandenBos T and Everts V: Root
development in mice lacking functional tissue non-specific alkaline
phosphatase gene: inhibition of acellullar cementum formation. J
Dent Res. 78:1221–1229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anan H, Akamine A and Maeda K: An enzyme
histochemical study of the behavior of rat bone cells during
experimental apical periodontitis. J Endod. 19:83–86. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibert P, Tramini P, Sieso V and Piva MT:
Alkaline phosphatase isoenzyme activity in serum from patients with
chronic periodontitis. J Periodontal Res. 38:362–365. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng-Qiu Zhang, Zhi-Fen Wu, Ling Wan, et
al: Effect of lipopolysaccharides on proliferation and alkaline
phosphatase activity of periodontal ligament cells. Chinese Journal
of Conservative Dentistry. 13:27–19. 2003.(In Chinese).
|
|
13
|
Perinetti G, Paolantonio M, Femminella B,
Serra E and Spoto G: Gingival crevicular fluid alkaline phosphatase
activity reflects periodontal healing/recurrent inflammation phases
in chronic periodontitis patients. J Periodontol. 79:1200–1207.
2008. View Article : Google Scholar
|
|
14
|
Mok SK, Chen WF, Lai WP, et al: Icariin
protects against bone loss induced by oestrogen deficiency and
activates oestrogen receptor-dependent osteoblastic functions in
UMR 106 cells. Br J Pharmacol. 159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Yuan L, Wang X, Zhang TL and Wang
K: Icariin and its glycosides enhance osteoblastic, but suppress
osteoclastic, differentiation and activity in vitro. Life Sci.
81:832–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Xu J, Liu O, Fan Z, et al:
Mesenchymal stem cells derived from inflamed periodontal ligaments
exhibit impaired immunomodulation. J Clin Periodontol.
39:1174–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SF, Yang ZP and Chang KW: Continuing
root formation following apexifiction treatment. Endod Dent
Traumatol. 6:232–235. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lekic P and McCulloch CA: Periodontal
ligament cell populations: the central role of fibroblasts in
creating a unique tissue. Anat Rec. 245:327–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chantarawaratit P, Sangvanich P, Banlunara
W, Soontornvipart K and Thunyakitpisal P: Acemannan sponges
stimulate alveolar bone, cementum and periodontal ligament
reneberation in a canine class II furcation defect model. J
Periodontal Res. 48:224–232. 2013.PubMed/NCBI
|
|
20
|
Hseih TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011.PubMed/NCBI
|
|
21
|
Hong CY, Lin SK, Kok SH, et al: The role
of lipopolysaccharide in infectious bone resorption of periapical
lesion. J Oral Pathol Med. 33:162–169. 2004. View Article : Google Scholar : PubMed/NCBI
|