Introduction
Streptococcus pneumoniae (SP), discovered in
1881, is a gram-positive Streptococcus and a predominant
pathogen that results in community-acquired pneumonia, otitis
media, meningitis, abscesses and other infections. These infections
occur particularly in infants, young children and elderly adults
(1). In developing countries, up
to 1 million children under the age of five suffer from fatal
pneumonia annually, in which SP is the predominant pathogen
(2). SP infections may result in
high levels of morbidity and mortality; however, SP often exists as
an opportunistic pathogen in the upper respiratory tract of
asymptomatic carriers (3) and it
is difficult to determine when it will become pathogenic. During
the colonization and invasion, particularly in the development of
blood-borne (bb) infections, such as infections of the blood and
cerebrospinal fluid (CSF), there are complex interactions between
SP and the host immune system. Various components of SP are
involved in the immune response, such as capsular and virulence
factors (4–6). Furthermore, a variety of cells from
the host immune system (such as neutrophils, monocytes/macrophages
and dendritic cells) are also involved in the response (5–8), and
a variety of factors [such as interleukin (IL)-1α, IL-1β, IL-6,
interferon (IFN)-α, IL-8 and intracellular adhesion molecule-1
(ICAM-1)] are released (3,8,9).
Currently, studies are focusing on the mechanisms of
SP resistance and pathogenesis. Sepsis and meningitis accounted for
~5% of the total number of SP infections; however, the resulting
morbidity is markedly greater (>30%) than that of other types of
SP infection (10,11). Neurological complications are the
predominant cause of death in children, while systemic
complications are the predominant cause in the elderly. There are
only a few studies that have analyzed the mechanism of SP invasion
into the circulatory and nervous systems, which may be due to the
small number of clinical cases available to observe. As described
previously, the progression of the SP infection involves the
interaction between the host immune system and various SP virulence
factors. SP has >10 types of virulence factors, including NanA,
CpsA, CbpA, PsaA, PspA, PspC and Ply (2). The virulence factors are involved in
different processes and may contribute to the different types of SP
infection (6,12,13).
THP-1 cells are monocytes derived from human
monocytic leukemia, and are currently predominantly used for the
study of the pathogenesis of SP. Thornton and McDaniel (9) confirmed that Ply upregulated ICAM-1
secretion in THP-1 cells. The majority of current studies focus on
individual cytokines or virulence factors; however, usually more
than one virulence factor is involved in SP invasion and
pathogenesis. The differences among SP invasions are predominantly
due to two key factors. The response of different hosts to SP and
the reaction of SP to different host cells results in different
invasion outcomes. For example, it may determine whether cells are
not infected, are carriers or exhibit an apparent infection. With
an apparent infection, ~80–90% of patients present with pneumonia
symptoms, while 5% of patients suffer from SP bacteremia, sepsis or
SP CSF infection, the mortality of which is markedly higher than
that of the SP pneumonia infection. The interaction between SP and
the host is complex, thus, in this study, representative cytokines
and adhesion molecules were selected and different sources of SP
were used as stimuli for THP-1 cells. Changes in the levels of the
secretion of IL-8 and IL-10 cytokines and ICAM-1 adhesion molecule
were investigated, and the biological characteristics of SP from
different sources were compared.
Materials and methods
Strains and cells
The ATCC 49619 SP standard strain was provided by
the China Medical Culture Collection Center (Beijing, China).
Twenty-three clinical SP strains were isolated in 2009 from the
Second Affiliated Hospital of Wenzhou Medical College (Wenzhou,
Zhejiang, China), among which 11 were isolated from blood samples,
marked as bb strains and 12 from sputum specimens, marked as
sputum-borne (sb) strains. The species of the strains were
identified by a VITEK-32 (bioMérieux Inc., Marcy-Etoile, France)
automatic microbial analyzer GPI card (bioMérieux Inc.), and
confirmed by an SP species-specific PCR assay. THP-1 cells were
purchased from the Shanghai Cell Bank of Chinese Academy of
Sciences (Shanghai, China).
Reagents and instruments
The following reagents and instruments were used in
the study: Fetal bovine serum (Zhejiang Tianhang, Hangzhou, China);
RPMI-1640 culture medium and F-12K culture medium (Gibco-BRL,
Carlsbad, CA, USA); PCR primers (Shanghai Shanjing, China); 3111
type CO2 incubator (Thermo Electron Corp., Madison, WI,
USA); VITEK-32 automatic microorganism analyzer (bioMérieux Inc.,
Marcy-Etoile, France); Mastercycler PCR instrument (Eppendorf,
Germany); Wellscan MK 3-type microplate reader and the Wellwash 4
MK2 washer (Thermo Electron Corp.); WD-9413A gel imaging analyzer
(Beijing Liuyi Instrument Factory, Fengtai, Beijing, China); and
the PAC3000 type electrophoresis apparatus (Bio-Rad, Hercules, CA,
USA).
Cell culture
THP-1 cells were cultured in RPMI-1640 culture
medium (supplemented with 1% penicillin and 10% fetal calf serum)
and incubated at 37ºC in a 5% CO2 atmosphere. The cells
(less than seven generations) were collected and RPMI-1640 medium
(supplemented with 10% inactivated fetal bovine serum) was added to
adjust the concentration to 4.0×108 cells/l.
The 23 clinical SP strains and the ATCC 49619
standard strain were cultured and suspended in normal saline (at a
turbidity of 1.0 McFarland).
THP-1 cell stimulation
The THP-1 cell suspension (1.2 ml) was distributed
into 60 wells of the cell culture plate, wherein 12 wells were
selected as controls. SP suspension (100 μl) was added to each
well, the control wells were filled with 100 μl saline and the
plate was incubated at 37ºC in a 5% CO2 atmosphere.
After 4 h, the solutions in 30 wells (including six controls) were
transferred to 1.5-ml microcentrifuge tubes and centrifuged at
1,789 × g for 10 min. The supernatants were stored at −80ºC for
further determination. After 8 h, solutions in the other 30 wells
(including six controls) were treated as described previously.
ELISA assay for IL-8, ICAM-1 and
IL-10
A double antibody sandwich ABC-ELISA method was
used. The anti-human IL-8, ICAM-1 and IL-10 monoclonal antibodies
(mAbs) were coated onto a microtiter plate and were allowed to
combine with the IL-8, ICAM-1 and IL-10 in the standards and
samples. Subsequent to this, biotinylated anti-human IL-8, ICAM-1
and IL-10 mAbs were added. The immune complexes formed and the
streptavidin-labeled horseradish peroxidase combined with the
complexes. The chromogenic substrate was then added followed by the
stop solution. The optical density (OD) values at 450 nm were
measured. The IL-8, ICAM-1 and IL-10 concentrations were
proportional to the OD values, thus the IL-8, ICAM-1 and IL-10
concentrations in the specimens were determined according to the
obtained standard curve. This was conducted according to the
manufacturer’s instructions.
pbp2B detection
To prepare DNA templates, 15–20 pure colonies of SP
were isolated from blood agar and placed into 0.5-ml centrifuge
tubes containing 200 μl dH2O. The isolates were then
placed in a boiling water bath for 30 min, removed and placed in a
−20ºC freezer for 10 min. Subsequent to this, they were centrifuged
at 16,099 × g for 30 sec and the resulting supernatants were
transferred to 0.5-ml centrifuge tubes. For PCR amplification, the
primers used were as previously reported (14): Forward:
5′-CTGACCATTGATTTGGCTTTCCAA-3′ and reverse:
5′-TTTGCAATAGTTGCTACATACTG-3′ for the target gene pbp2B, 682 bp.
The PCR reaction conditions were as follows: 94ºC denaturation for
5 min, 94ºC for 30 sec, 55ºC for 1 min and 72ºC for 1 min for 30
cycles, and 72ºC for 10 min. Electrophoresis was conducted using 5
μl PCR product, 1 μl 6X loading buffer was loaded onto 2.0% agarose
gel and 150 V and 75 mA electrophoresis was conducted for 25 min.
The electrophoresis results were acquired by a gel camera system
(WD-9413A gel imaging analyzer).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Single-factor analysis of variance was performed using SPSS,
version l7.0 (SPSS, Inc., Chicago, IL, USA). Normality was analyzed
using the Kolmogorov-Smirnov test, and homogeneity of variance was
analyzed using the Levene test. Clinical SP (including the bb and
sb strains) and the blank control group or ATCC group were compared
using a one-sample t-test. When variances were unequal, comparisons
between the bb-SP and sb-SP groups were performed using the
Satterthwaite approximate two-sample t-test. When variances were
equal, comparisons between the bb-SP and sb-SP groups was performed
using variance analysis. The following criteria were applied:
Normality test, P>0.10 and homogeneity of variance test,
P>0.10. P<0.05 was considered to indicate a statistically
significant difference.
Results
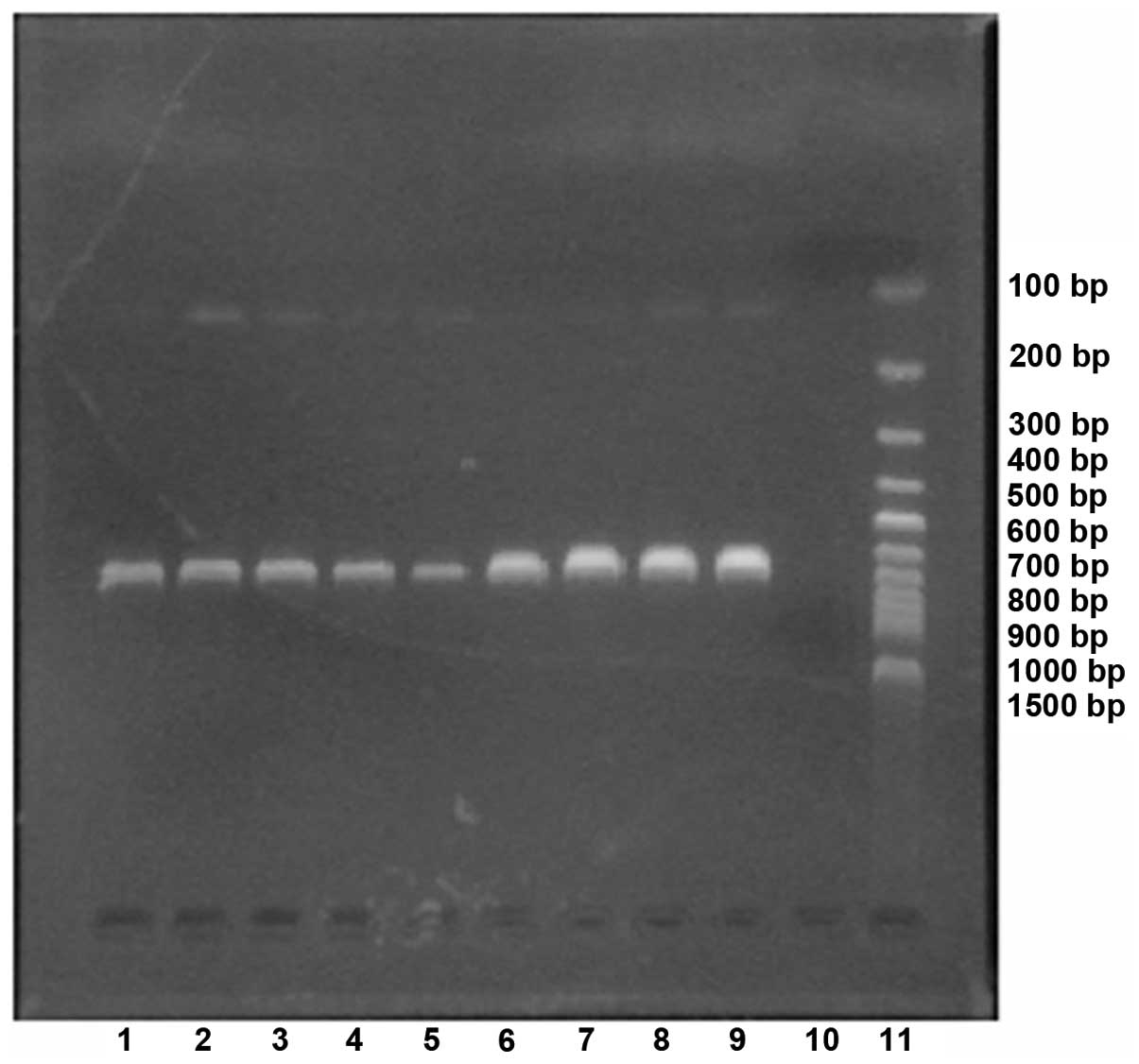
PCR of the SP pbp2B gene
The results of the electrophoresis for the PCR
product of the SP pbp2B gene is shown in Fig. 1.
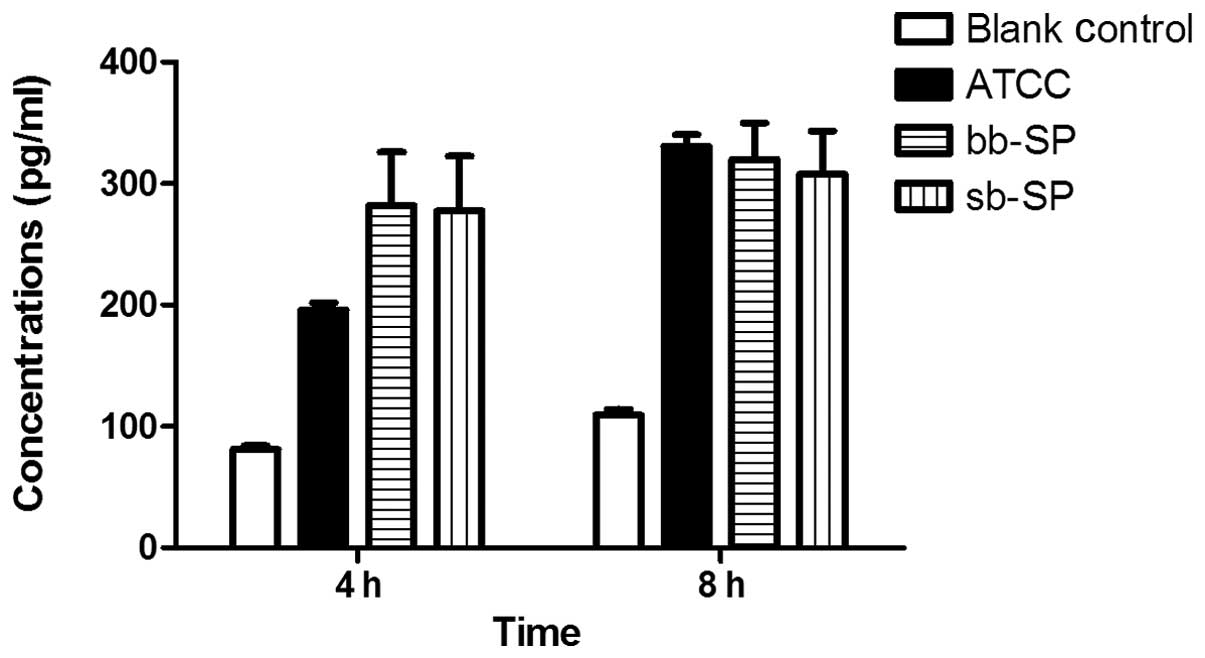
IL-8 secretion by THP-1 cells following
SP stimulation
Compared with the blank control group, the levels of
IL-8 secreted by the THP-1 cells were significantly increased in
the bb-SP and sb-SP groups following 4 and 8 h treatment. Compared
with the cells treated with the ATCC 49619 positive control, THP-1
cells treated with bb-SP and sb-SP for 4 h secreted greater levels
of IL-8 (P<0.05); however, no significant difference was
observed after 8 h. Levels of IL-8 secreted by the THP-1 cells
following 8 h stimulation with SP were higher than those at 4 h
(P<0.05), indicating that the IL-8 concentration increased with
stimulation time. At the two time points, there was no
statistically significant difference between the bb-SP and sb-SP
groups (Fig. 2).
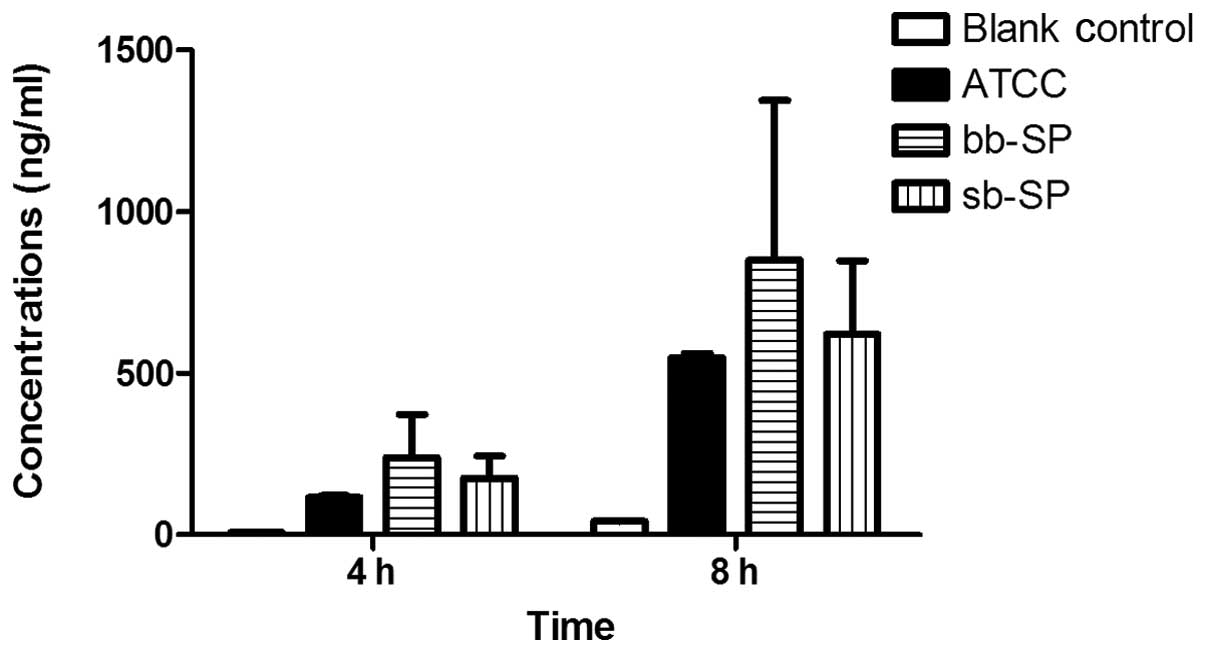
ICAM-1 secretion by THP-1 cells following
SP stimulation
The results for the ICAM-1 secretion were similar to
those for IL-8. Compared with the blank control group, the levels
of ICAM-1 secreted by the THP-1 cells were significantly increased
in the bb-SP and sb-SP groups following treatment for 4 and 8 h. In
addition, compared with the cells treated with the ATCC 49619
positive control, THP-1 cells treated with bb-SP and sb-SP for 4 h
secreted greater levels of ICAM-1 (P<0.05); however, there were
no significant differences after 8 h. The levels of ICAM-1 secreted
by the THP-1 cells subsequent to 8 h of stimulation with SP were
greater than those at 4 h (P<0.05), indicating that the ICAM-1
concentration increased with stimulation time. At the two time
points, there was no significant difference between the bb-SP and
sb-SP groups (Fig. 3).
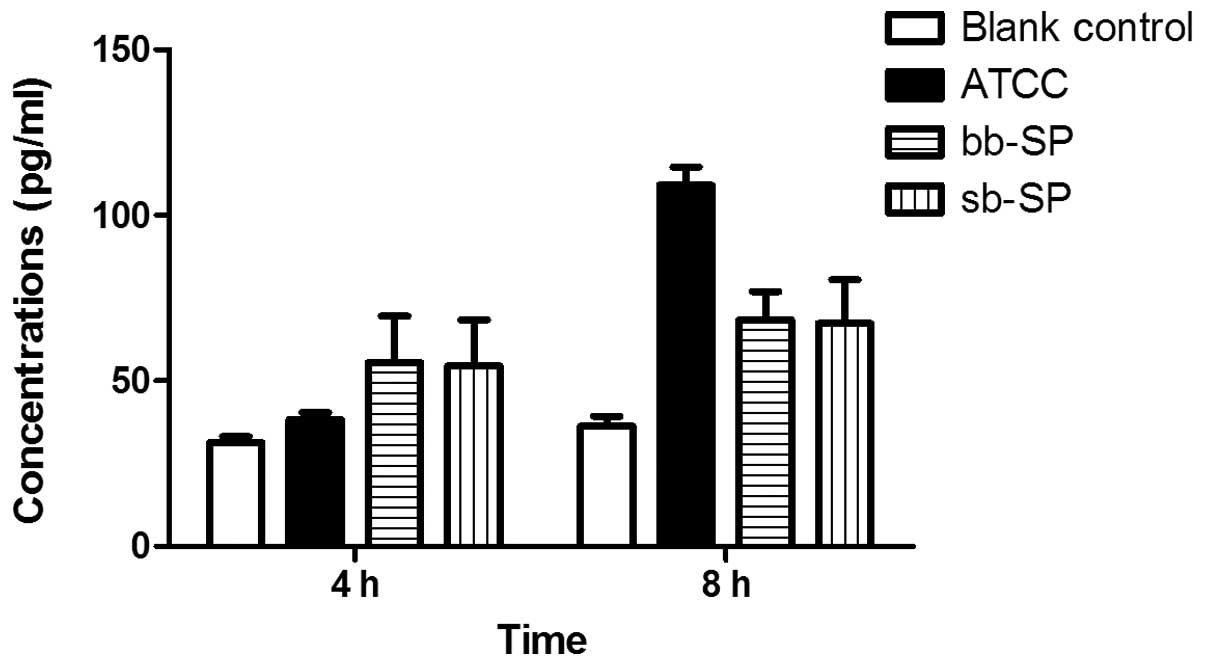
IL-10 secretion by THP-1 cells following
SP stimulation
Compared with the blank control group, the levels of
IL-10 secreted by the THP-1 cells were significantly increased in
the bb-SP and sb-SP groups following treatment for 4 and 8 h.
Compared with the cells treated with the ATCC 49619 positive
control, THP-1 cells treated with bb-SP and sb-SP for 4 h secreted
more IL-10; however, after 8 h these cells secreted less
(P<0.05). Levels of IL-10 secreted by the THP-1 cells following
8 h stimulation with SP were higher than those for 4 h (P<0.05),
indicating that IL-10 concentration increased with stimulation
time. At the two time points, there was no significant difference
between the bb-SP and sb-SP groups (Fig. 4).
Discussion
IL-8, also termed chemokine factor 8, is secreted by
monocytes/macrophages and epithelial cells. It promotes the
accumulation and activation of inflammatory cells, the release of
inflammatory mediators and aids in neutrophil chemotaxis to sites
of inflammation to regulate the inflammatory response (15,16).
This process is an important response to SP infection and is
essential for the body to maintain stability and remove foreign
microbes. As shown in Fig. 2, the
bb-SP and sb-SP increased the level of IL-8 secretion in THP-1
cells, consistent with the results of previous studies (17,18),
and significant differences were observed between clinical SP and
ATCC 49619 groups at 4 h. bb-SP demonstrated a similar ability to
sb-SP with regard to stimulation of THP-1 cells to secrete IL-8. It
is suggested that the IL-8 levels secreted by THP-1 cells may not
be elevated infinitely following SP stimulation (19). THP-1 cells which were not
stimulated also secreted a low level of IL-8, and the concentration
increased slowly with the incubation time; therefore, this low
level of IL-8 may aid in the proliferation of THP-1 cells.
ICAM-1 is one of the earliest discovered immune
protein superfamily adhesion molecules and it combines with the
lymphocyte function-associated antigen 1 (LFA-1) (4). ICAM-1 is widely distributed in
numerous types of cells, including lymph node and tonsil vascular
endothelial cells, thymic dendritic cells, tonsil and glomerular
epithelial cells, leukocytes, macrophages and fibroblasts. IL-1,
TNF-α, IFN and LPS are able to promote the expression of ICAM-1
molecules. As shown in Fig. 3, the
bb-SP and sb-SP increased the levels of IL-10 secretion in the
THP-1 cells, which has been previously demonstrated to enhance the
interaction of ICAM-1 with its receptor and promote leukocyte
accumulation in infected lesions, particularly the extravascular
lesions (20,21). This is important for the body to
remove foreign microbes and maintain stability. Secretion of ICAM-1
from THP-1 cells stimulated by SP increased with time, and reached
a peak at ~8 h (9). However, it is
considered that the ICAM-1 concentration following SP stimulation
of THP-1 cells is not infinitely increased, as it would result in
significant damage to the body (22). In the present study, significant
differences existed between the clinical SP and ATCC 49619 groups
at 4 h. bb-SP showed a similar ability to as sb-SP to with regard
to stimulation of THP-1 cells. In addition, THP-1 cells which were
not stimulated also secreted a low level of ICAM-1, and the
concentration increased slowly with the incubation time. This low
level of ICAM-1 is the basis for the implementation of the immune
defense functions.
IL-10 is predominantly produced by T helper cell 2
(TH2) cells. In addition, IL-10 is also be secreted by TH0 cells,
monocytes/macrophages and B cells. IL-10 is predominantly
anti-inflammatory and is involved in the inhibition of the
antigen-presenting function of macrophages, inhibition of the
response of TH1 cells, promotion of B cell proliferation,
differentiation and antibody production, and the indirect
inhibition of NK cell activity. It therefore exerts a protective
effect on the body by preventing an excessive inflammatory
reaction. As shown in Fig. 4, the
bb-SP and sb-SP increased the IL-10 secretion by THP-1 cells and
significant differences existed between the clinical SP and ATCC
49619 groups. bb-SP demonstrated a similar ability to sb-SP in
stimulating THP-1 cells to secrete IL-10.
In conclusion, the bb-SP and sb-SP exhibited similar
abilities in increasing the levels of secretion of IL-8, ICAM-1 and
IL-10 by THP-1 cells, however, this differed from that of ATCC
49619. In conclusion, the host’s reaction did not appear to be the
predominant factor in the cause of different types of SP infection
(such as lung and blood infections); therefore, the biological
characteristics of SP itself may have an important involvement and
thus require further investigation.
Acknowledgements
This study was supported by a grant from the Science
and Technology Bureau of Taizhou Jiaojiang (grant no. 10275).
References
|
1
|
Jin P, Xiao M, Kong F, et al: Simple,
accurate, serotype-specific PCR assay to differentiate
Streptococcus pneumoniae serotypes 6A, 6B, and 6C. J Clin
Microbiol. 47:2470–2474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahdi LK, Ogunniyi AD, LeMessurier KS and
Paton JC: Pneumococcal virulence gene expression and host cytokine
profiles during pathogenesis of invasive disease. Infect Immun.
76:646–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki H and Ikeda K: Mode of action of
long-term low-dose macrolide therapy for chronic sinusitis in the
light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy.
1:117–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morona JK, Morona R and Paton JC:
Attachment of capsular polysaccharide to the cell wall of
Streptococcus pneumoniae type 2 is required for invasive
disease. Proc Natl Acad Sci USA. 103:8505–8510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cockeran R, Mitchell TJ, Feldman C and
Anderson R: Pneumolysin induces release of matrix
metalloproteinase-8 and -9 from human neutrophils. Eur Respir J.
34:1167–1170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uchiyama S, Carlin AF, Khosravi A, et al:
The surface-anchored NanA protein promotes pneumococcal brain
endothelial cell invasion. J Exp Med. 206:1845–1852. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hyams C, Camberlein E, Cohen JM, et al:
The Streptococcus pneumoniae capsule inhibits complement
activity and neutrophil phagocytosis by multiple mechanisms. Infect
Immun. 78:704–715. 2010.
|
|
8
|
Robson RL, Reed NA and Horvat RT:
Differential activation of inflammatory pathways in A549 type II
pneumocytes by Streptococcus pneumoniae strains with
different adherence properties. BMC Infect Dis. 6:712006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thornton J and McDaniel LS: THP-1
monocytes up-regulate intercellular adhesion molecule 1 in response
to pneumolysin from Streptococcus pneumoniae. Infect Immun.
73:6493–6498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koedel U, Scheld WM and Pfister HW:
Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet
Infect Dis. 2:721–736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weisfelt M, van de Beek D, Spanjaard L, et
al: Clinical features, complications, and outcome in adults with
pneumococcal meningitis: a prospective case series. Lancet Neurol.
5:123–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schachern PA, Tsuprun V, Cureoglu S, et
al: Virulence of pneumococcal proteins on the inner ear. Arch
Otolaryngol Head Neck Surg. 135:657–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Littmann M, Albiger B, Frentzen A, et al:
Streptococcus pneumoniae evades human dendritic cell
surveillance by pneumolysin expression. EMBO Mol Med. 1:211–222.
2009. View Article : Google Scholar
|
|
14
|
du Plessis M, Smith AM and Klugman KP:
Rapid detection of penicillin-resistant Streptococcus
pneumoniae in cerebrospinal fluid by a seminested-PCR strategy.
J Clin Microbiol. 36:453–457. 1998.PubMed/NCBI
|
|
15
|
Baggiolini M, Walz A and Kunkel SL:
Neutrophil-activating peptide-1/interleukin 8, a novel cytokine
that activates neutrophils. J Clin Invest. 84:1045–1049. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahuja SK, Ozçelik T, Milatovitch A, et al:
Molecular evolution of the human interleukin-8 receptor gene
cluster. Nat Genet. 2:31–36. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spanaus KS, Nadal D, Pfister HW, et al:
C-X-C and C-C chemokines are expressed in the cerebrospinal fluid
in bacterial meningitis and mediate chemotactic activity on
peripheral blood-derived polymorphonuclear and mononuclear cells in
vitro. J Immunol. 158:1956–1964. 1997.
|
|
18
|
Martner A, Skovbjerg S, Paton JC and Wold
AE: Streptococcus pneumoniae autolysis prevents phagocytosis
and production of phagocyte-activating cytokines. Infect Immun.
77:3826–3837. 2009. View Article : Google Scholar
|
|
19
|
Yoon BN, Choi NG, Lee HS, et al: Induction
of interleukin-8 from nasal epithelial cells during bacterial
infection: the role of IL-8 for neutrophil recruitment in chronic
rhinosinusitis. Mediators Inflamm. 2010:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Etzioni A: Adhesion molecules in leukocyte
endothelial interaction. Adv Exp Med Biol. 408:151–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Springer TA: Traffic signals for
lymphocyte recirculation and leukocyte emigration: the multistep
paradigm. Cell. 76:301–314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerr JR: Cell adhesion molecules in the
pathogenesis of and host defence against microbial infection. Mol
Pathol. 52:220–230. 1999. View Article : Google Scholar : PubMed/NCBI
|