Introduction
In females, breast cancer is the most frequently
diagnosed type of cancer and the leading cause of cancer-related
mortality worldwide (1).
Approximately half of the breast cancer patients and 60% of the
mortalities were presented in economically developing countries in
2008 (1). Cancers
characteristically develop rapidly in primary and metastatic
locations, accompanied by novel blood vessel development and poor
blood flow, resulting in a progressively hypoxic and hypoglycemic
microenvironment for cancer cells (2,3).
Hypoxia is identified as a physiological abnormality in solid
tumors and is involved in the malignant progression of a number of
cancers (4).
Although hypoxia is toxic to cancer and normal
cells, cancer cells undergo genetic and adaptive changes that allow
them to survive and metastasize in a hypoxic environment. These
processes contribute to the malignant phenotype and to aggressive
tumor behavior. In solid tumors, hypoxia can promote malignant
growth, and confer resistance to chemotherapy and metastasis by
altering gene expression, particularly in breast cancer (5). However, the mechanisms of
hypoxia-induced tumor metastasis in breast cancer requires further
clarification.
Gankyrin, also known as p28GANK and PSMD10, is a 25
KDa protein with 226 amino acids, which is comprised of seven
ankyrin repeats (6). Gankyrin has
been confirmed as a bridging factor between the proteasome and
various tumor-associated substrates, including pRb and p53
(7). Gankyrin increases the
hyperphosphorylation of Rb by activating CDK4 and therefore
activates E2F-dependent transcription of DNA synthesis genes. It
can also activate the ubiquitin protein ligase murine double minute
2 and lead to the proteasomal degradation of p53 (8). Previous studies showed that gankyrin
was overexpressed in various human cancers, including
hepatocellular carcinoma (9) and
esophageal squamous cell (10),
colorectal (11), pancreatic
(12) and oral (13) cancer. In breast cancer, gankyrin is
frequently overexpressed and is associated with ErbB2 expression
(14), which also promotes breast
cancer cell metastasis by regulating Rac1 activity (15). Previously, a study found that
gankyrin may bind to and sequester factor inhibiting hypoxia
inducible factor-1 (FIH-1), resulting in a decreased interaction
between FIH-1 and hypoxia-inducible factor-1α (HIF-1α), which
increased the activity of HIF-1 to promote vascular endothelial
growth factor (VEGF) production (16). Therefore, gankyrin may be
significant in the hypoxia-induced malignant progression of human
cancer.
In the present study, the capability of hypoxia to
increase gankyrin mRNA and protein expression in breast cancer cell
lines, and the roles of gankyrin in the hypoxia induced invasion
and metastasis of breast cancer cells was investigated using
lentivirus-mediated siRNA targeting gankyrin.
Materials and methods
Cell lines and tissue samples
BT474 and MCF7 human breast cancer cell lines were
provided by colleagues in the Department of Pharmacology and
Toxicology, Beijing Institute of Radiation Medicine (Beijing,
China), and cell lines were routinely cultured in RPMI 1640 medium
containing 10% fetal calf serum. For the hypoxic condition, cells
were cultured in a modular incubator chamber (Billups-Rothenberg,
San Diego, CA, USA), which was provided with 1% O2, 5%
CO2 and 94% N2, and for normoxic conditions,
cells were maintained under (20% O2, 5% CO2,
and 75% N2).
Quantitative polymerase chain reaction
(qPCR)
Total RNA of breast cancer cell lines in normal
conditions and undergoing hypoxia treatment for 0, 12, 24 and 36 h
were extracted using TRIzol (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
The primer sequences used were as follows: Forward:
5′-TCTTCAAGCCATCCTGTGTG-3′ and reverse: 5′-TGGTGATGTTGGACTCCTCA-3′
for gankyrin; and forward: 5′-ATGATATCGCCGCGCTCGTC-3′ and reverse:
5′-CGCTCGGTGAGGATCTTCA-3′ for β-actin. The qPCR assays were
performed and results were calculated as previously described
(13).
Western blotting
Total protein of breast cancer cell lines under
normal conditions and hypoxia treatment for 0, 12, 24 and 36 h were
extracted following a previous study (11). The total protein was separated on a
12% (for gankyrin and β-actin) or 8% (for E-cadherin)
polyacrylamide gels, and electrotransferred on to a nitrocellulose
membrane. Mouse polyclonal anti-gankyrin (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA; 1:100), mouse monoclonal anti-E-cadherin
(Santa Cruz Biotechnology Inc.; 1:100), and mouse monoclonal
anti-beta actin (Sigma, St. Louis, MO, USA; 1:3,000).
Lentivirus-mediated siRNA construction
and transfection
The lentivirus-mediated siRNA targeting gankyrin was
subcloned into the PGC-LV system with enhanced green fluorescent
protein (EGFP). The siRNA interfering sequence was
5′-CTGACCAGGACAGCAGAAC-3′. The control lentivirus was also enhanced
with EGFP. The siRNA and control lentivirus were transfected into
BT474 cells, and the GFP-positive cells were purified by flow
cytometry (FACScan; Becton Dickinson, San Jose, CA, USA), and
labeled si- and con-BT474.
Wound-healing experiment
The wound-healing assay was used to detect the
migration of cells as described previously (17). Briefly, 2×106 cells of
each cell line were plated in a 60-mm-diameter dish, and cultured
until the cells reached confluency. A plastic pipette tip was then
drawn across the center of the plate to produce clean 1-mm-wide
wound areas. Following 48 h culturing in normoxia or hypoxic
conditions, a phase-contrast microscope (Olympus, Tokyo, Japan) was
used to detect the cells in the wound areas.
Transwell assays
Cell migration and invasion assays were performed
using transwells (8-μl pore size; Corning Inc, Acton, MA, USA). For
transwell migration assays, 1×105 cells were plated in
the top chamber lined with a non-coated membrane. For invasion
assays, the chamber inserts were coated at a concentration of 200
mg/ml in Matrigel (BD Biosciences, San Jose, CA, USA) and dried
under sterile conditions for 10 h. Cells were prepared at
concentration of 1×105 cells in RPMI 1640 culture
solution without fetal bovine serum or growth factors, and 200 μl
mixed liquor was plated into the top chamber. Next, 400 μl medium
supplemented with 20% fetal bovine serum was plated into the lower
chamber. Following incubation in normal culture conditions for 24
h, cells in the top chambers were wiped to remove the non-invasive
cells, and invaded cells on the underside membrane were incubated
with 10% paraformaldehyde for 10 min and stained in 0.1% crystal
violet. Following three washes with phosphate-buffered saline and
air-drying, cells were counted by phase-contrast microscopy at
magnification, ×200 on 10 random visual fields in each well. Each
experimental condition was repeated in triplicate.
Statistical analysis
The SPSS 17.0 software (SPSS Inc., Chicago, IL, USA)
was used to evaluate the statistical differences, and P<0.05 was
considered to indicate a statistically significant difference. A
t-test was performed to analyze the difference between two
groups.
Results
Expression of gankyrin in breast cancer
cell lines
qPCR and western blot analysis were used to detect
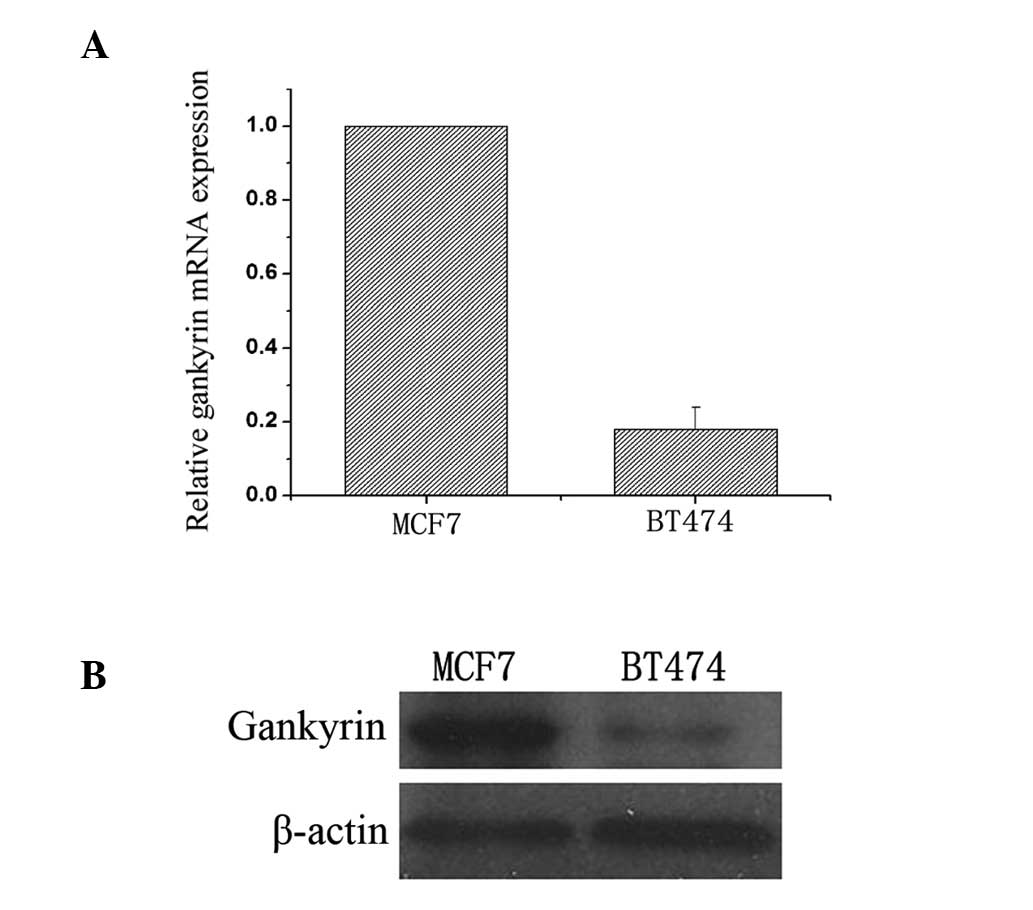
the expression of gankyrin in MCF7 and BT474 breast cancer cell
lines under normoxic conditions. The mRNA of gankyrin expression
was markedly higher in MCF7 compared with BT474 cells (Fig. 1A). Consistent with mRNA expression,
the gankyrin protein expression was markedly higher in MCF7
compared with BT474 cells (Fig.
1B). Therefore, the BT474 cell line was used for further
investigation under hypoxic conditions.
Hypoxia induces overexpression of
gankyrin in BT474 cells
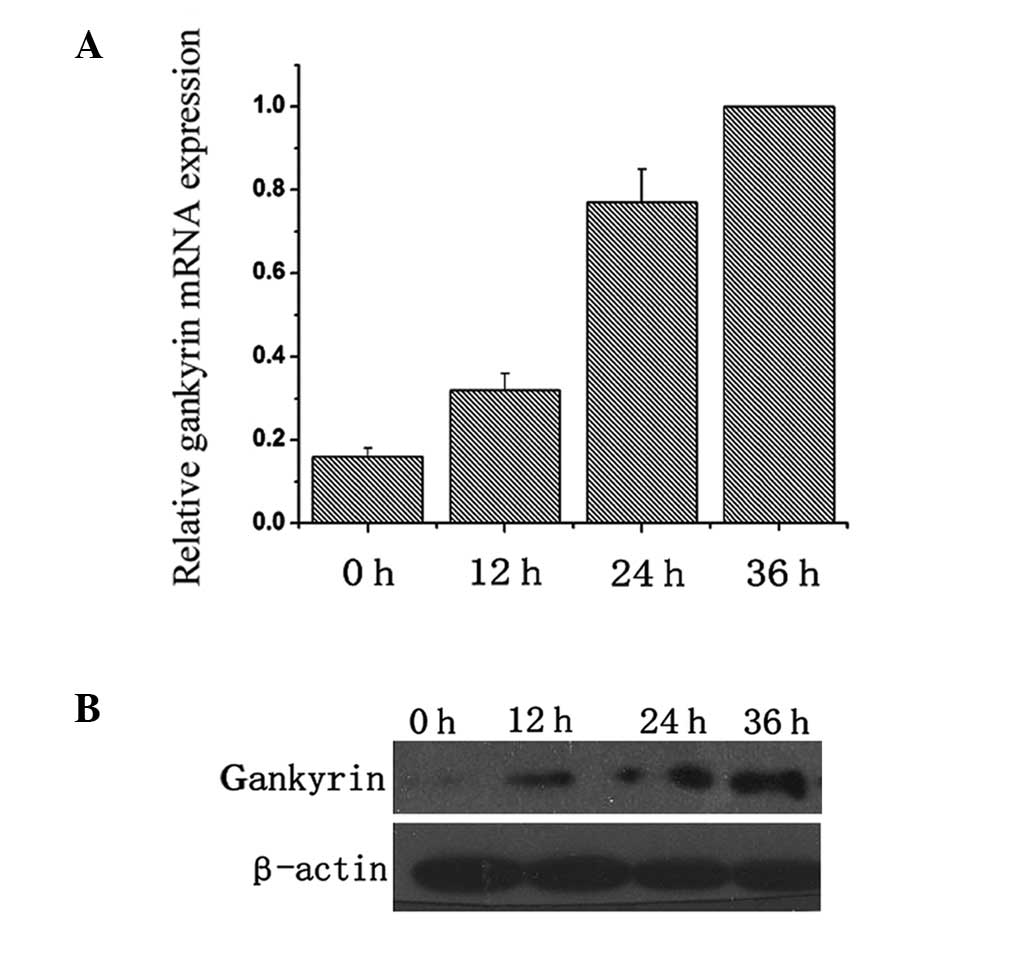
Under normoxic conditions, low expression of
gankyrin was detected in BT474 cells. Following induction of
hypoxia for 0, 12, 24 and 36 h, the mRNA and protein were collected
for qPCR and western blot analyses. As shown in Fig. 2A, gankyrin mRNA expression
exhibited a marked overexpression tendency under hypoxic conditions
in BT474 cells. Consistent with results of qPCR, western blot
analysis showed a gradual increase in gankyrin expression under
hypoxic conditions (Fig. 2B),
indicating that hypoxia induced the overexpression of gankyrin in
BT474 cells.
Hypoxia increases the migration and
invasion of BT474 cells
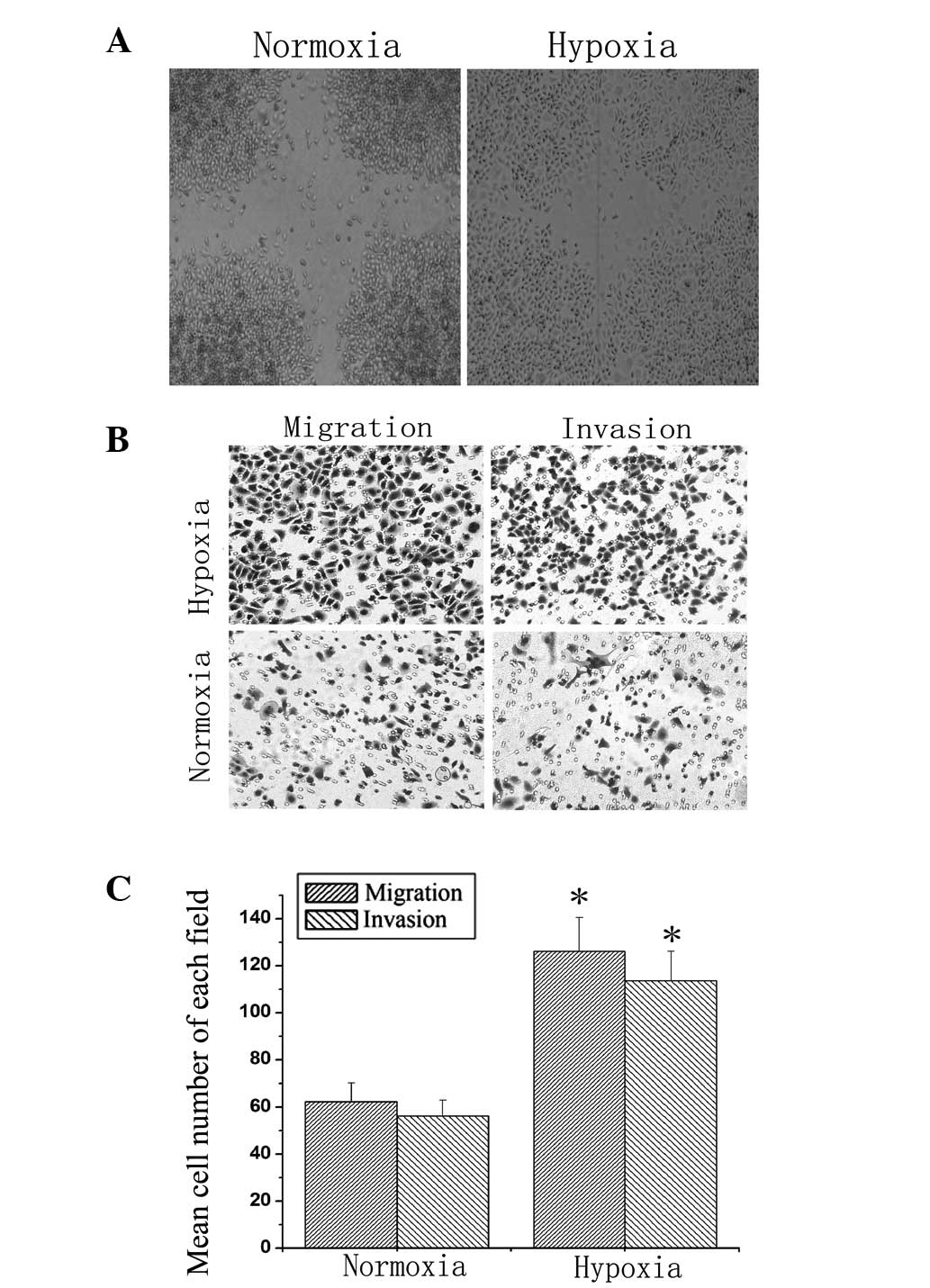
Wound-healing assays were performed to compare the
migration rate of the BT474 cells under normoxic and hypoxic
conditions. As shown in Fig. 3A,
hypoxia markedly increased BT474 cell migration from the edge of
the wound compared with normoxic conditions (Fig. 3A). Next, transwell assays were used
to detect the migration and invasion of BT474 cells. Following
culturing in normoxic or hypoxic conditions for 36 h, hypoxia
markedly increased the migration and invasion ability of BT474
cells compared with normoxia (Fig. 3B
and C). These data indicated that hypoxia increased the
migration and invasion of BT474 cells.
Gankyrin deletion abrogates the increased
metastatic potential of BT474 cells due to hypoxia
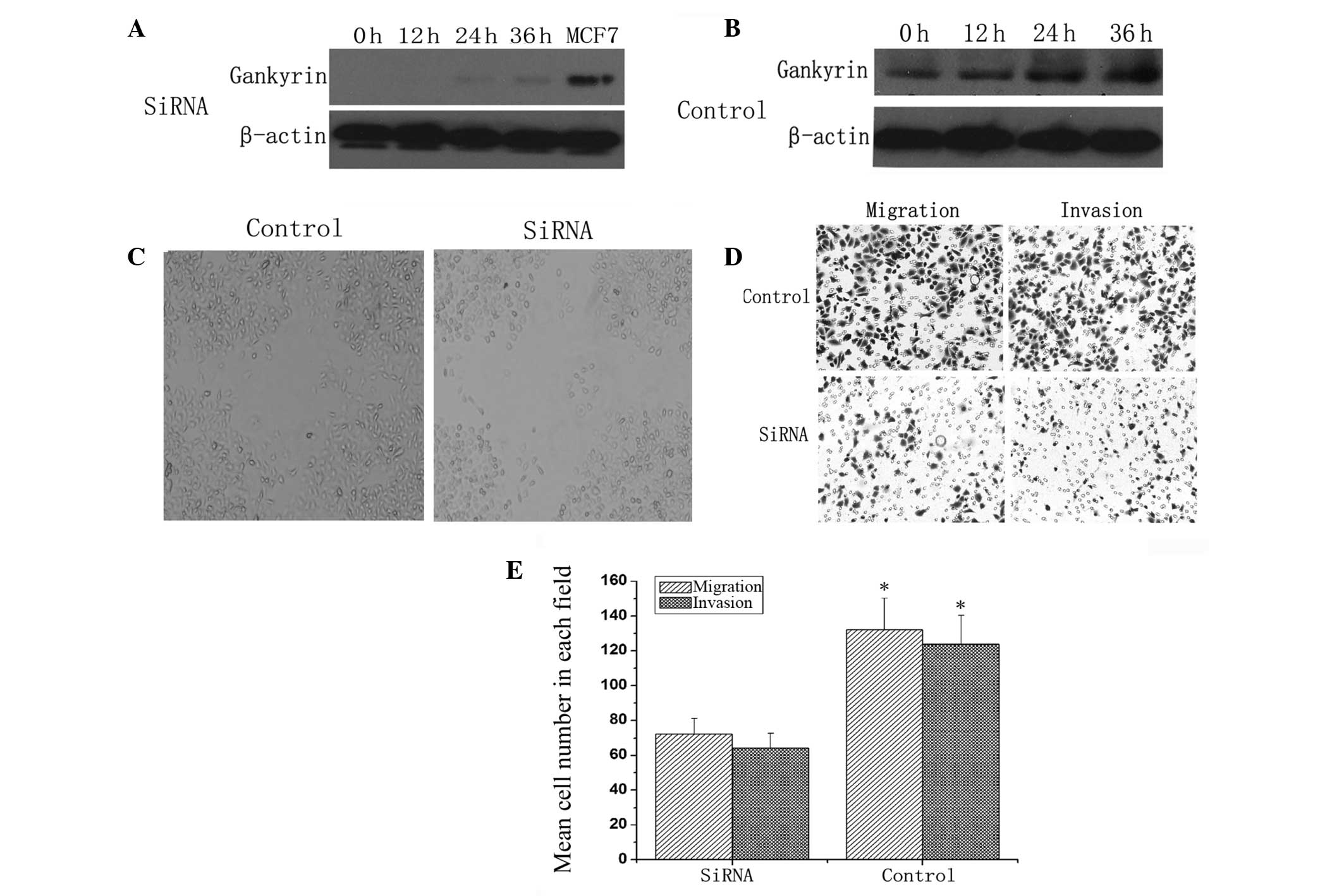
To investigate the roles of gankyrin in the
hypoxia-induced metastatic potential of BT474 cells,
lentivirus-mediated siRNA targeting gankyrin was transfected into
BT474 cells. As shown in Fig. 4A,
hypoxia could not induce the overexpression of gankyrin in BT474
cells transfected with siRNA compared with normoxic conditions
(Fig. 4B). Wound-healing (Fig. 4C) and transwell assays assays
(Fig. 4D and E) showed that the
migration and invasion of gankyrin siRNA-transfected cells were
significantly inhibited compared with control siRNA transfected
cells. These findings suggest that gankyrin deletion abrogates the
increased metastatic potential of BT474 cells due to hypoxia.
E-cadherin is involved in the
gankyrin-induced invasion of breast cancer cells due to
hypoxia
A previous study showed that E-cadherin expression
was markedly inhibited under hypoxic conditions in breast cancer
cells (18). In addition, gankyrin
was reported to be involved in the metastasis of hepatocellular
carcinoma invasiveness and metastasis (19). Therefore, the role of E-cadherin in
the gankyrin-induced invasion of breast cancer cells due to hypoxia
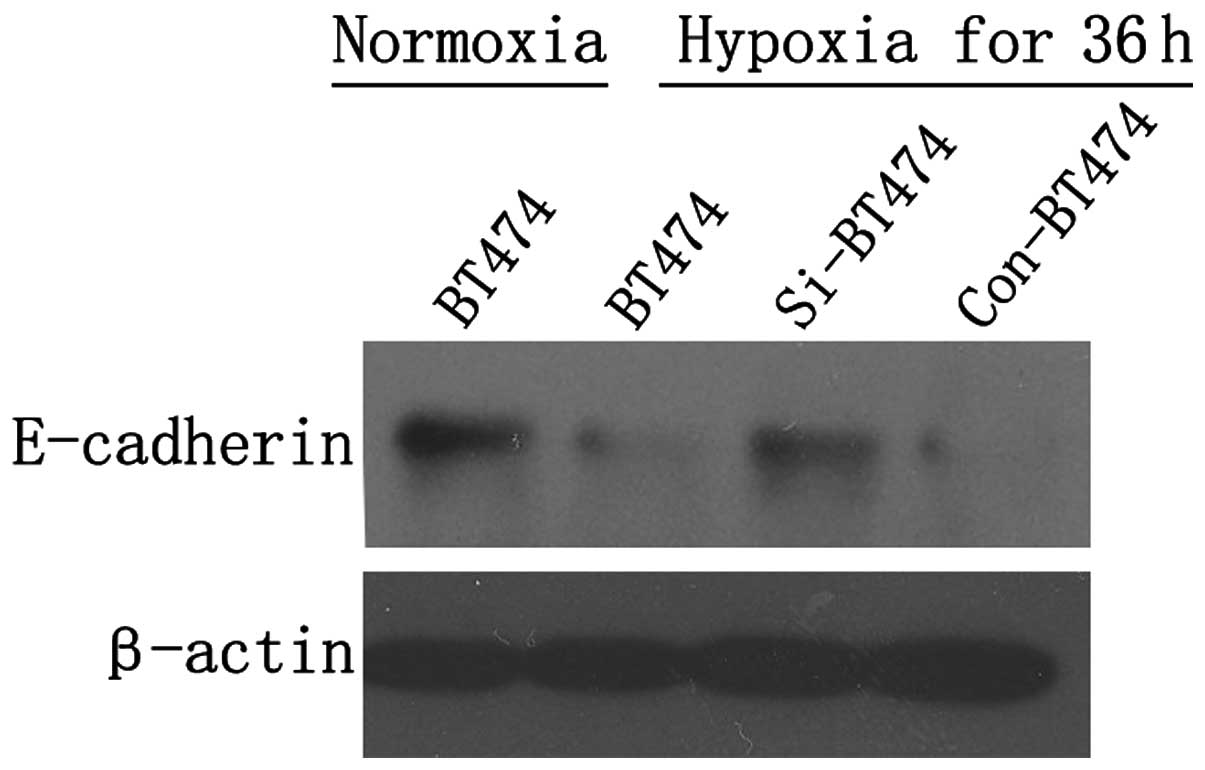
was investigated. Western blotting showed that E-cadherin
expression was markedly decreased following culturing for 36 h
under hypoxic conditions in BT474 cells compared with normoxic
conditions. Under hypoxic conditions, the expression of E-cadherin
was markedly higher in si-BT474 cells compared with con-BT474 cells
(Fig. 5), indicating that hypoxia
may inhibit the expression of E-cadherin, and gankyrin deletions
may abrogate the inhibition of E-cadherin expression in BT474 cells
due to hypoxia.
Discussion
In breast cancer, hypoxia is a critical regulator
for the transcriptional activation of several pathways, including
angiogenesis, immortalization, invasion and metastasis (5). A previous study showed that HIF-1α
and HIF-2α accumulated in breast cancer cells with hypoxia and
potentiated Notch signaling (20).
The hypoxic conditions increased the expression of BlyS in human
breast cancer cell lines, and upregulation of BlyS led to
activation and nuclear translocation of NF-κB p65, which also
increased the migration and invasion of breast cancer cells
(21). A recent study showed that
gankyrin binds to and sequesters factors inhibiting FIH-1,
resulting in a decreased interaction between FIH-1 and HIF-1α,
which increased activity of HIF-1 to promote VEGF production
(16), indicating that gankyrin is
significant in the hypoxia-associated phenotype in breast cancer
cells. However, the role and mechanism of gankyrin in hypoxic
environments remain unknown.
In the present study, the mRNA and protein of
gankyrin were observed to be increased in breast cancer cells with
hypoxia, accompanied with the increased migration and invasion in
BT474 cells. To investigate the roles of gankyrin in the
hypoxia-induced metastatic potential, lentiviral-mediated siRNA
targeting gankyrin was transfected into BT474 cells. Compared with
control siRNA transfection, the expression of gankyrin did not
increase under hypoxic conditions in gankyrin siRNA transfected
cells, indicating that siRNA is capable of inhibiting the increased
gankyrin expression due to hypoxia in BT474 cells. Wound-healing
and transwell assays confirmed that gankyrin deletion abrogated the
increased metastatic potential of BT474 cells due to hypoxia. These
findings indicated enhanced breast cancer cell migration in
response to gankyrin under hypoxic conditions, which may be a
potential therapeutic target for breast cancer metastasis
treatment. According to a previous report, gankyrin expression may
be modulated by growth factors, including epidermal growth factor
or hepatocyte growth factor stimulation, and Ras activation through
the activation of phosphoinositide 3-kinase signaling (22). The present study showed that
hypoxia is another factor that results in stimulation of gankyrin
expression.
Epithelial-to-mesenchymal transition is induced by
the loss of cell adhesion, repression of E-cadherin expression and
increased cell migration and invasion. E-cadherin expression was
repressed during the metastasis of breast cancer. A previous study
showed that E-cadherin expression was markedly inhibited under
hypoxic conditions in breast cancer cells (18). Overexpression of gankyrin
significantly downregulated the expression of E-cadherin, and
suppression of gankyrin expression using adenovirus-delivered siRNA
markedly increased the expression of E-cadherin in HCC cell lines
(19). Therefore, we further
investigated the associations between gankyrin and E-cadherin
involved in promoting the migration and invasion of breast cancer
cells under hypoxic conditions. Consistent with a previous study,
western blot analyses showed that E-cadherin expression was
decreased under hypoxic conditions (18). However, when gankyrin was deleted
by siRNA, the expression of E-cadherin was not markedly altered and
no marked metastatic potential change was observed in BT474 cells
under hypoxic conditions. This indicated that E-cadherin was
involved in the gankyrin-induced invasion of breast cancer cells
due to hypoxia.
In addition, to investigate the significance of
gankyrin expression in breast cancer tissues, 104 pairs of breast
cancer and matched non-tumor tissues were used for
immunohistochemical staining. Further analyses revealed that
gankyrin expression was associated with a high histological tumor
grade, estrogen/progesterone receptors and axillary lymph node
status. These data were consistent with previous studies of the
significance of gankyrin expression in breast cancer tissues
(14,15).
The present study demonstrated that the increased
expression of gankyrin was significant in the hypoxia-enhanced
metastatic potential in breast cancer cells partly through the
regulation of E-cadherin. Further studies are required to determine
the mechanism by which hypoxia induces the overexpression of
gankyrin in breast cancer cells.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
30900675).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Onozuka HK, Tsuchihara K and Esumi H:
Hypoglycemic/hypoxic condition in vitro mimicking the tumor
microenvironment markedly reduced the efficacy of anticancer drugs.
Cancer Sci. 102:975–982. 2011.PubMed/NCBI
|
|
3
|
Louie E, Nik S, Chen JS, et al:
Identification of a stem-like cell population by exposing
metastatic breast cancer cell lines to repetitive cycles of hypoxia
and reoxygenation. Breast Cancer Res. 12:R942010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006.PubMed/NCBI
|
|
5
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krzywda S, Brzozowski AM, Higashitsuji H,
et al: The crystal structure of gankyrin, an oncoprotein found in
complexes with cyclin-dependent kinase 4, a 19 S proteasomal ATPase
regulator, and the tumor suppressors Rb and p53. J Biol Chem.
279:1541–1545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lozano G and Zambetti GP: Gankyrin: an
intriguing name for a novel regulator of p53 and RB. Cancer Cell.
8:3–4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Higashitsuji H, Higashitsuji H, Itoh K, et
al: The oncoprotein gankyrin binds to MDM2/HDM2, enhancing
ubiquitylation and degradation of p53. Cancer Cell. 8:75–87. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu XY, Wang HY, Tan L, Liu SQ, Cao HF and
Wu MC: Overexpression of p28/gankyrin in human hepatocellular
carcinoma and its clinical significance. World J Gastroenterol.
8:638–643. 2002.PubMed/NCBI
|
|
10
|
Ortiz CM, Ito T, Tanaka E, et al: Gankyrin
oncoprotein overexpression as a critical factor for tumor growth in
human esophageal squamous cell carcinoma and its clinical
significance. Int J Cancer. 122:325–332. 2008. View Article : Google Scholar
|
|
11
|
Tang S, Yang G, Meng Y, et al:
Overexpression of a novel gene gankyrin correlates with the
malignant phenotype of colorectal cancer. Cancer Biol Ther.
9:88–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng Y, He L, Guo X, et al: Gankyrin
promotes the proliferation of human pancreatic cancer. Cancer Lett.
297:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Knobloch TJ, Kresty LA, et al:
Gankyrin, a biomarker for epithelial carcinogenesis, is
overexpressed in human oral cancer. Anticancer Res. 31:2683–2692.
2011.PubMed/NCBI
|
|
14
|
Kim YH, Kim JH, Choi YW, et al: Gankyrin
is frequently overexpressed in breast cancer and is associated with
ErbB2 expression. Exp Mol Pathol. 94:360–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhen C, Chen L, Zhao Q, et al: Gankyrin
promotes breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Higashitsuji H, Higashitsuji H, et
al: Overexpression of gankyrin in mouse hepatocytes induces
hemangioma by suppressing factor inhibiting hypoxia-inducible
factor-1 (FIH-1) and activating hypoxia-inducible factor-1. Biochem
Biophys Res Commun. 432:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu N, Zhang G, Bi F, et al: RhoC is
essential for the metastasis of gastric cancer. J Mol Med (Berl).
85:1149–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Tang SQ, Huang DS, Wang JW, Liu Y
and Wang JY: Aplastic anemia transformed into acute myeloblastic
leukemia M1 8 years later: a case report. Zhonghua Er Ke Za Zhi.
43:2212005.(In Chinese).
|
|
19
|
Fu J, Chen Y, Cao J, et al: p28GANK
overexpression accelerates hepatocellular carcinoma invasiveness
and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible
factor-1alpha pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar
|
|
20
|
Chen J, Imanaka N, Chen J and Griffin JD:
Hypoxia potentiates Notch signaling in breast cancer leading to
decreased E-cadherin expression and increased cell migration and
invasion. Br J Cancer. 102:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu J, Sun L, Lin S, et al: BlyS is
up-regulated by hypoxia and promotes migration of human breast
cancer cells. J Exp Clin Cancer Res. 31:312012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong LW, Yang GZ, Pan YF, et al: The
oncoprotein p28GANK establishes a positive feedback loop in
beta-catenin signaling. Cell Res. 21:1248–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|