Introduction
Tissue expansion is one of the most significant
innovations of the 20th century in plastic surgery (1). It is a process which involves
implanting a silicon sac subcutaneously and regularly injecting
saline into it, resulting in the formation of new skin under the
mechanical stretch and providing a supply of tissue similar in
color, structure and adnexal distribution to the adjacent skin in
order to offer highly effective repair. Therefore, tissue expansion
is an extremely useful technique and has a wide range of
applications in plastic surgery, particularly for defective repair
following large scar resection (2).
The tissue expanders are mainly composed of silicone
and following implantation into the body, will initiate a host
response named the foreign body response (FBR). This leads to the
formation of a fibrous capsule around the expander in order to
‘seal it off’ (3). In the clinic,
the thick fibrous capsule formed around the implant prolongs the
period of tissue expansion and makes the expanded skin contract
markedly. Thus, 4–6 months are always required to achieve the
needed expansion; in addition, limited expanded skin could be
harvested and used in a repair surgery in a period of treatment and
re-expansion is required. The length of time required for the
expansion is disadvantageous since it causes inconvenience in the
daily lives of the patients. In addition, it increases the
incidence of complications, including infections, rupture and
exposure of the tissue expander.
Due to the wide application of the tissue expander,
it is of high diagnostic and clinical significance to clarify the
exact molecular mechanisms behind the phenomenon of peri-silicone
implant capsule formation. In other words, the identification of a
method to inhibit the formation of the fibrous capsule may provide
a novel therapy to accelerate tissue expansion and to harvest more
regenerative tissue. In order to provide more specific information
with regard to the immune cells and cytokines, known to be critical
to the progress of FBR, the present study reports their dynamic
change by a subcutaneous implantation model. We hypothesize that
this knowledge is essential as it would offer a proper intervention
time to decrease the harmful effects of the FBR on the application
of tissue expanders in the clinic.
Materials and methods
Ethics statement
All the animal procedures were approved under the
guidelines of Shanghai Jiao Tong University Medical Center, the
Institutional Animal Care and Use Committee (Shanghai, China).
Implant model and tissue collection
A tissue expansion model was used to analyze the
dynamic changes of the immune cells and cytokines. A total of 20
Lewis rats (six weeks old; male; body weight, 110–120 g; Shanghai
Experimental Animal Center, Shanghai, China) were anesthetized with
3% sodium pentobarbital at 0.13 ml/100 g and shaved. A single
incision was made at the back of rats, and a subcutaneous pocket of
3.0×6.0 cm was created into which the tissue expander was placed.
The expander pot was set under the head skin. Next, 15 ml
physiological saline was injected through these pots and an
additional volume of 3 ml was injected into the expander pocket
once a week.
Identification of immune cells in
peri-implant tissue
The immune cells in peri-implant tissue were
identified using immunohistochemistry. The tissues harvested on day
1, 3, 7 14, 28 and 90 post-implantation were fixed and embedded in
paraffin for a histological assay. Following blocking with hydrogen
peroxide (H2O2) and with a protein blocking
agent, the primary antibodies, mouse anti-rat CD68, CD4 and CD11c
(Abcam, Cambridge, UK) were applied directly at 4°C overnight.
Subsequent to washing in phosphate-buffered saline (PBS), the
sections were incubated in phycoerythrin-coupled anti-mouse
antibody (DAKO, Glostrup, Denmark) for 1 h at 37°C and the cell
nuclei were counterstained with hematoxylin. The control samples
were processed following the same protocol but with the omission of
the primary antibody.
Fibrous capsule and collagen
deposition
Hematoxylin-eosin (HE) staining was applied to
observe the fibrous capsule formed around the tissue expander.
Collagen deposition in the tissue around the implant was studied by
Masson’s trichrome staining. Briefly, they were fixed in preheated
Bouin’s solution at 56°C for 15 min, then in Harris’ hematoxylin
for 5 min and stained with Masson’s trichrome stain (trichrome
stain LG solution; Sigma-Aldrich, St. Louis, MO, USA) for 5 min.
The stained sections were observed for collagen deposition by
bright field microscopy. The α-smooth muscle actin (SMA) positive
cells in the peri-implant tissue were stained by
immumohistochemical staining with anti-α-SMA antibody (Abcam) as
described in the aforementioned method.
Protein level of cytokines
For detection of growth factors, 0.5 g tissue from
the above area was collected from each group (n=4 for each group).
The tissues were homogenized in 500 μl tissue protein extraction
reagent (CWBIO, Beijing, China) and 5 μl phenylmethanesulfonyl
fluoride (Sigma-Aldrich). Subsequent to centrifugation at 11,176 ×
g for 10 min, the supernatant was collected for the assay of
interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-13, monocyte
chemoattractant protein-1 (MCP-1), interferon-γ (IFN-γ) and tumor
necrosis factor (TNF)-α using a Protein Quantibody array kit
according to the manufacturer’s instructions (R&D Systems,
Minneapolis, MN, USA). The signals can be visualized through the
use of a laser scanner equipped with a Cy3 wavelength (Thermo
Fisher, Waltham, MA, USA). The data extraction can be performed
with the majority of the microarray analysis software (GenePix,
ScanArray Express, ArrayVision or MicroVigene).
Western blotting for NF-κB, JNK and P38
MAPK
In order to determine the levels of NF-κB, JNK and
p38 MAPK, nuclear extracts were prepared from the expanded tissue
and were totally resolved on 10% SDS-PAGE. Following
electrophoresis, the proteins were electrotransferred onto
nitrocellulose filters, probed with rabbit polyclonal Abs against
NF-κB, JNK and p38 MAPK (Cell Signaling Technology, Inc., Boston,
MA, USA), and detected by chemiluminescence (ECL, Amersham,
Piscataway, NJ, USA). The bands obtained were quantitated with
Personal Densitometer Scan version 1.30 using Image Quant software
version 3.3 (Molecular Dynamics, Inc., Sunnyvale, CA, USA).
Results
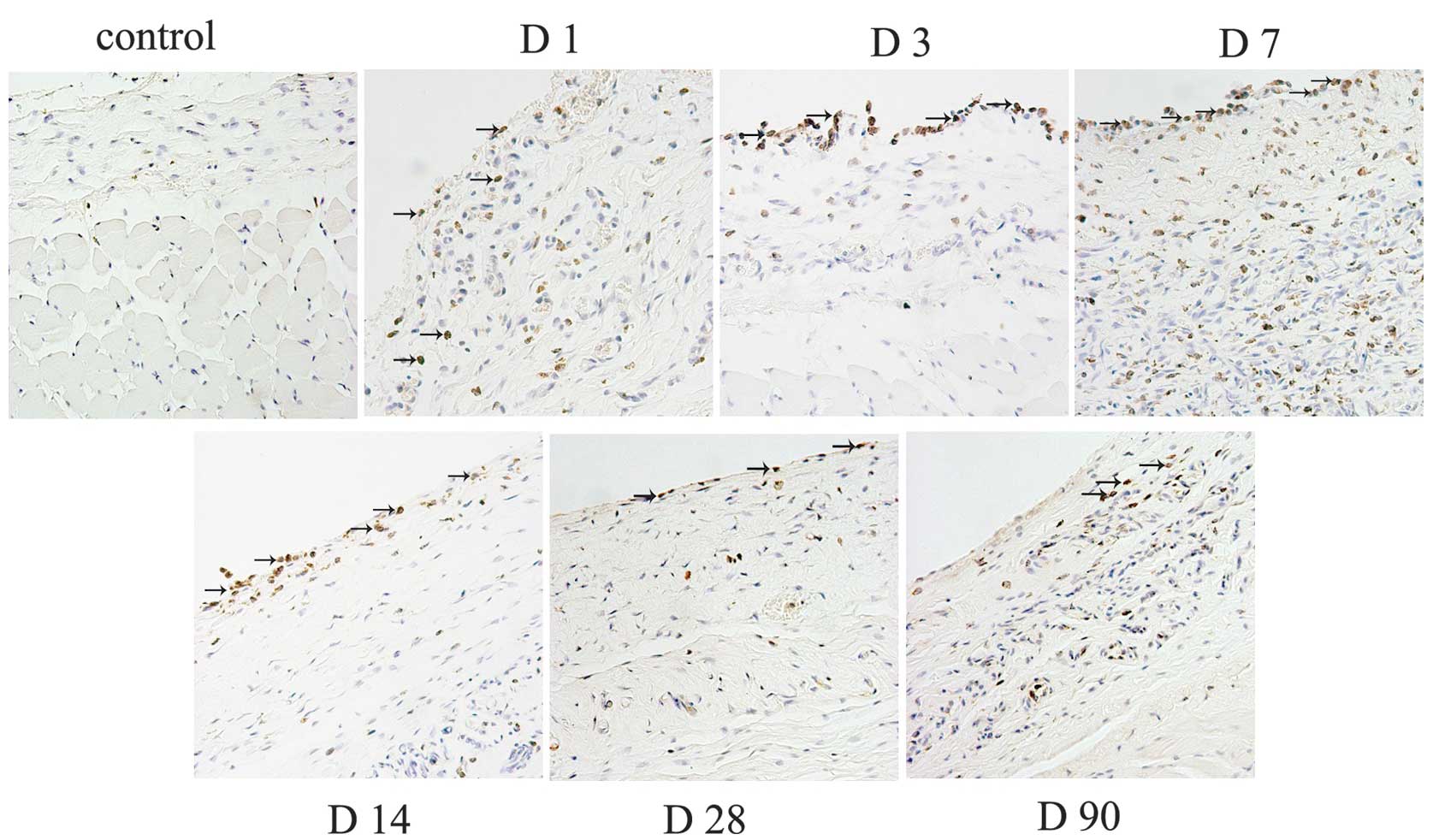
Infiltration of immune cells
The macrophages revealed a persistent infiltration,
and mainly located at the tissue-material interface. The number of
macrophages increased steadily around the tissue expander since day
1, and the highest amount of positive cells were observed in the
tissue at day 7. Next, the number decreased gradually until day 90
(Fig. 1). However, there were a
few CD4+ lymphocytes and CD11c+ dendritic
cells observed (data not shown).
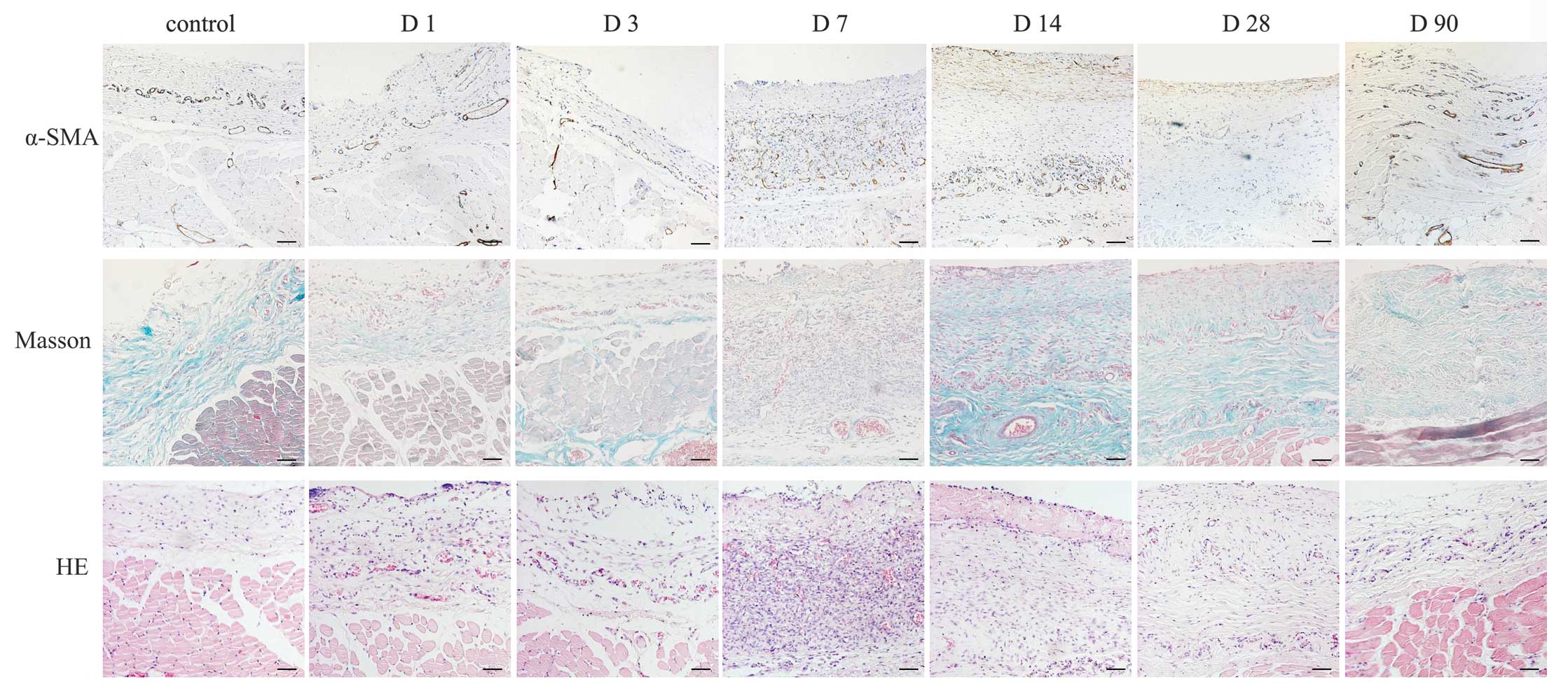
Formation of the fibrous capsule at the
tissue-material interface
HE staining revealed that fibrous tissue began to
form around the tissue expander at day 14, increased to maximum at
30 days and decreased gradually with a persistent thin layer at day
90. Masson’s trichrome staining revealed the most evident
deposition of collagen around the implants at day 14, which
maintained with a gradual decrease over time until day 90. It has
been previously reported that myofibroblasts contributed to walling
off the foreign body in chronic inflammation and were transformed
from fibroblasts in this process (4). In the present study, a few of α-SMA
positive myofibroblasts were present around silicone at 14 and 30
days and a decrease was observed at 90 days (Fig. 2).
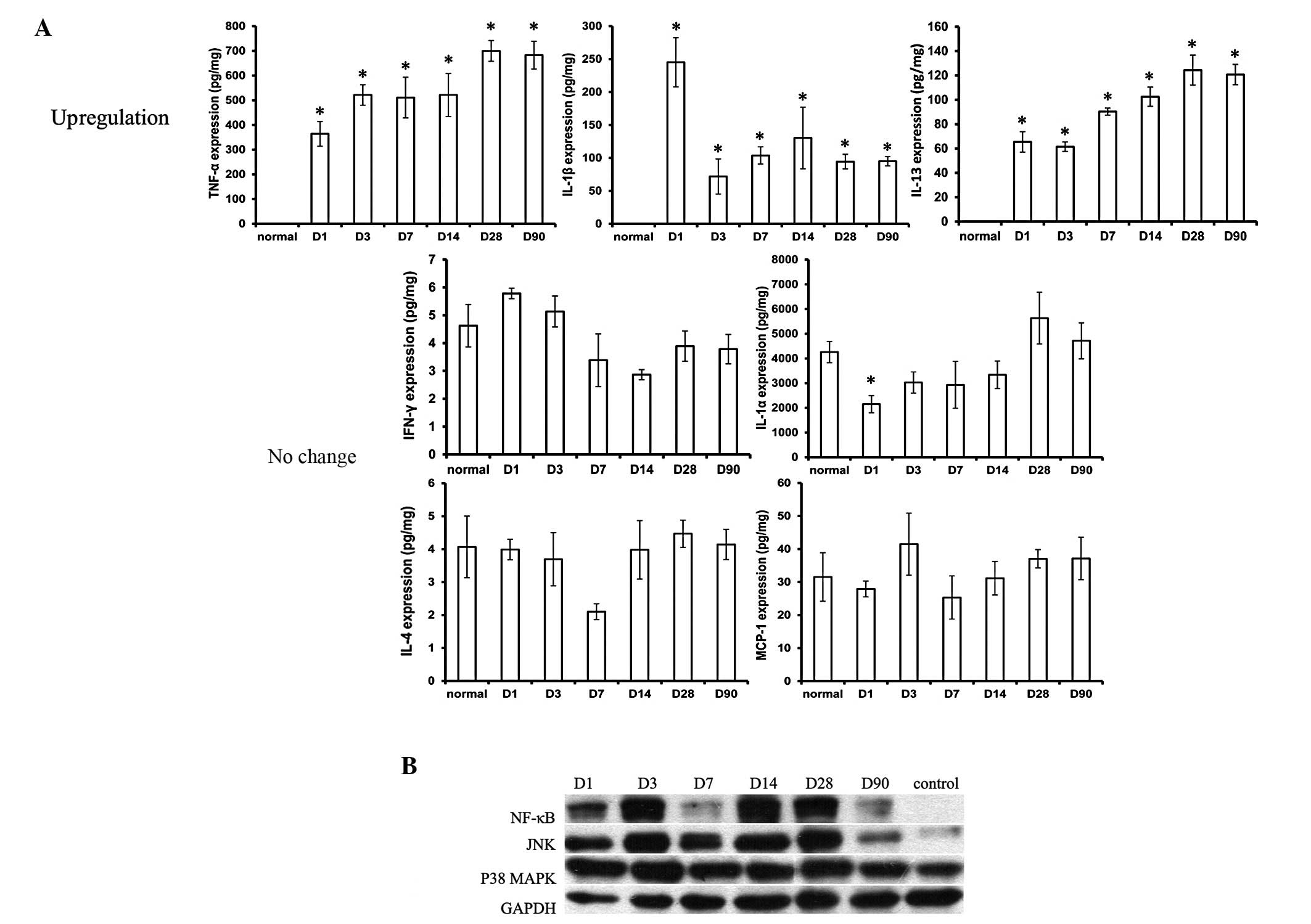
Cytokines in peri-implant tissue
Among all these inflammatory cytokines, TNF-α
revealed the highest expression and IL-1β and IL-13 moderately
increased in the expanded tissue at all the time points when
compared with the tissue which was without an implantation of a
tissue expander. The level of the cytokines IL-1α, IL-4, IFN-γ and
MCP-1 was almost at similar levels to that in the normal tissue
(Fig. 3).
Expression of NF-κB, JNK and p38
MAPK
In order to gain an improved understanding of the
potential mechanisms underlying the initiation of the inflammatory
response by inflammatory cytokines, western blotting of NF-κB, JNK
and p38 MAPK was performed. As revealed in the present study, NF-κB
and JNK were expressed from day 1–90 in the peri-implant tissue,
and p38 MAPK did not show any difference in expression when
compared with the control group (Fig.
3).
Discussion
Silicone implants are widely used in the field of
plastic surgery for wound repair and cosmetic augmentation. The
formation of a thick fibrous capsule around the implant limits its
potential maximum effect. The implantation of a tissue expander and
injury caused by the surgical procedure triggers a tissue reaction,
including the secretion of pro-inflammatory cytokines and
chemoattractants. As a result, an inflammatory response is caused
and infiltration of macrophages, neutrophils and lymphocytes to the
site of the implantation.
The macrophage, which is the dominant cell in FBR,
mainly located at the tissue/material interface throughout the time
points. It has been revealed that cytokines including the
transforming growth factor-β, platelet derived growth factor,
chemokine (C-X-C motif) ligand 4 (CXCL4) and IL-1 in tissues could
direct macrophages to the wound site (5). A previous study indicated that mast
cell degranulation and the release of histamine may play an
integral role in recruiting macrophages to the implantation site
(6). Next, infiltrated macrophages
could secrete more inflammatory cytokines and chemokines, including
MCP-1, granulocyte and granulocyte-macrophage colony stimulating
factors, attracting more macrophages to the wound site, and
engaging in the subsequent events of the FBR (7–9).
However, in the present study, there was no difference observed in
the expression of MCP-1. This is consistent with a previous study
in which it was demonstrated that the recruitment of monocytes to
subcutaneous implant sites was not affected by MCP-1 (10).
Previously, it has been demonstrated that besides
the macrophages, CD4+ T lymphocytes also participate in
the FBR and are located at the tissue/material interface (4). Rodriguez et al hypothesized
that they are present in the FBR due to adaptive immunity (11). However, in the present study, there
was no positive staining observed. This is contradictory with the
study by Joseph et al (4)
which reported CD4+ T lymphocytes appeared around
silicone expander implants. The presence of lymphocytes has been
demonstrated to promote macrophage adhesion via paracrine effects
and direct signaling (12,13). The absence of the proinflammatory
cytokine IFN-γ in the present study correlates with the lack of the
Th1 type inflammatory response observed. During FBR, Th2-polarized
T cells were originally hypothesized to be the source of the
cytokines IL-10, IL-4 and IL-13 (12,14).
However, it was demonstrated that macrophage- or neutrophil-derived
cells may serve as a source of IL-13 at the beginning of
inflammation and also maintain IL-13 production during the chronic
inflammatory response to the biomaterial (15,16).
In other words, CD4+ T lymphocytes did not affect the
secretion of IL-13. It has been shown that IL-4 and IL-13 play
significant roles in determining the extent and degree of the
subsequent development of the FBR. In the present study, moderately
expressed IL-13 demonstrated more important roles than IL-4 due to
IL-4 not showing significant changes. Besides the macrophages and
CD4+ T lymphocytes, there was a lack of expression of
CD11c in the covering tissue (data not shown), indicating that
dendritic cells do not play a significant role in the progress of
FBR in vivo, which is consistent with a previous study
(3). However, in a previous in
vitro study, it was demonstrated that biomaterials can affect
the maturation of dendritic cells (17).
In the present study, the macrophages predominated
over the first two weeks, then the cells decreased, followed by an
accumulation of collagen and myofibroblasts around the implant
resulting from cytokines released by the macrophages. This led to
the formation of a fibrous capsule around the implant from the
second week. The main cell types found in fibrotic tissue are
fibroblasts and myofibroblasts. Transformation of fibroblasts to
phenotypically different myofibroblasts occurs in FBR (4). An in vitro experiment with
silicone particles by Granchi et al has led to a hypothesis
that the macrophage ingestion of these particles induces a state of
activation leading to the release of cytokines with high fibrogenic
activity (18). In the present
study, α-SMA-positive myofibroblasts appeared at day 14 and 28,
accompanied by the deposition of collagen.
As the main source of inflammatory cytokines, the
persistent existence of macrophages caused the continuous high
expression of TNF-α and IL-1β. It has been proven that
pro-inflammatory cytokines, including TNF-α and IL-1β, could
initiate the inflammatory response through the main inflammatory
pathways, including JNK, p38 MAPK and NF-κB (19–22).
JNK and NF-κB are preferentially activated by cytokines, growth
factors or cellular damage, and p38 MAPK is potently activated by
environmental stress (23).
Activation of these pathways triggers downstream signaling cascades
that lead to the production of pro-inflammatory cytokines which are
important mediators of acute and chronic inflammation, FBR and cell
apoptosis (24,25). In addition, a previous study has
reported that TNF-α expression was associated with an increased
Baker grade of periprosthetic capsular contracture following breast
implant surgery, and positive TNF-α staining in breast capsules was
localized to fibroblasts, macrophages and were extracellularly
close to the prosthesis (26).
A number of published models have provided a basic
cellular and cytokine signaling knowledge with regard to FBR
development. However, to the best of our knowledge, there are no
published studies examining the dynamic changes of the immune cells
and inflammatory cytokines caused by implantation of the tissue
expander. The requirement to study the change lies in finding an
appropriate time point to inhibit the immune reaction in order to
further interfere with the formation of the fibrous capsule. In the
clinic, it is of great significance to gain such an understanding
and the reduction of the thickness of the fibrous capsule may be a
novel direction to accelerate the process of tissue expansion.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30730092 and 30925034) and
the National Key Project of Scientific and Technical Supporting
Programs Funded by the Ministry of Science & Technology of
China (no. 2012BAI11B03).
References
|
1
|
Radovan C: Breast reconstruction after
mastectomy using the temporary expander. Plast Reconstr Surg.
69:195–208. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheng L, Yang M, Du Z, Yang Y and Li Q:
Transplantation of stromal vascular fraction as an alternative for
accelerating tissue expansion. J Plast Reconstr Aesthet Surg.
66:551–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins DM, Basaraba RJ, Hohnbaum AC, Lee
EJ, Grainger DW and Gonzalez-Juarrero M: Localized
immunosuppressive environment in the foreign body response to
implanted biomaterials. Am J Pathol. 175:161–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joseph J, Mohanty M and Mohanan PV: Role
of immune cells and inflammatory cytokines in regulation of
fibrosis around silicone expander implants. J Mater Sci Mater Med.
21:1665–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Broughton G 2nd, Janis JE and Attinger CE:
The basic science of wound healing. Plast Reconstr Surg.
117:12S–34S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang L, Jennings TA and Eaton JW: Mast
cells mediate acute inflammatory responses to implanted
biomaterials. Proc Natl Acad Sci USA. 95:8841–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo Y and Murakami M: Monitoring of
intracellular ammonium in perfused rat salivary gland by
nitrogen-14 nuclear magnetic resonance spectroscopy. Proc Biol Sci.
244:191–6. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brodbeck WG, Nakayama Y, Matsuda T, Colton
E, Ziats NP and Anderson JM: Biomaterial surface chemistry dictates
adherent monocyte/macrophage cytokine expression in vitro.
Cytokine. 18:311–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brodbeck WG, Voskerician G, Ziats NP,
Nakayama Y, Matsuda T and Anderson JM: In vivo leukocyte cytokine
mRNA responses to biomaterials are dependent on surface chemistry.
J Biomed Mater Res A. 64:320–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kyriakides TR, Foster MJ, Keeney GE, Tsai
A, Giachelli CM, Clark-Lewis I, Rollins BJ and Bornstein P: The CC
chemokine ligand, CCL2/MCP1, participates in macrophage fusion and
foreign body giant cell formation. Am J Pathol. 165:2157–2166.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez A, Voskerician G, Meyerson H,
MacEwan SR and Anderson JM: T cell subset distributions following
primary and secondary implantation at subcutaneous biomaterial
implant sites. J Biomed Mater Res A. 85:556–565. 2008. View Article : Google Scholar
|
|
12
|
Brodbeck WG, Macewan M, Colton E, Meyerson
H and Anderson JM: Lymphocytes and the foreign body response:
lymphocyte enhancement of macrophage adhesion and fusion. J Biomed
Mater Res A. 74:222–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang DT, Colton E and Anderson JM:
Paracrine and juxtacrine lymphocyte enhancement of adherent
macrophage and foreign body giant cell activation. J Biomed Mater
Res A. 89:490–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anderson JM, Rodriguez A and Chang DT:
Foreign body reaction to biomaterials. Semin Immunol. 20:86–100.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brandt E, Woerly G, Younes AB, Loiseau S
and Capron M: IL-4 production by human polymorphonuclear
neutrophils. J Leukoc Biol. 68:125–130. 2000.PubMed/NCBI
|
|
16
|
Woerly G, Lacy P, Younes AB, Roger N,
Loiseau S, Moqbel R and Capron M: Human eosinophils express and
release IL-13 following CD28-dependent activation. J Leukoc Biol.
72:769–779. 2002.PubMed/NCBI
|
|
17
|
Yoshida M, Mata J and Babensee JE: Effect
of poly(lactic-co-glycolic acid) contact on maturation of murine
bone marrow-derived dendritic cells. J Biomed Mater Res A. 80:7–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granchi D, Cavedagna D, Ciapetti G, Stea
S, Schiavon P, Giuliani R and Pizzoferrato A: Silicone breast
implants: the role of immune system on capsular contracture
formation. J Biomed Mater Res. 29:197–202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manna SK and Aggarwal BB: Vesnarinone
suppresses TNF-induced activation of NF-kappa B, c-Jun kinase, and
apoptosis. J Immunol. 164:5815–5825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogata M: p38 MAP kinase in the immune
response. Tanpakushitsu Kakusan Koso. 47:2261–2267. 2002.(In
Japanese).
|
|
21
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI
|
|
22
|
Zandi E, Rothwarf DM, Delhase M, Hayakawa
M and Karin M: The IkappaB kinase complex (IKK) contains two kinase
subunits, IKKalpha and IKKbeta, necessary for IkappaB
phosphorylation and NF-kappaB activation. Cell. 91:243–252. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho PJ, Chou CK and Yeh SF: Role of JNK and
p38 MAPK in Taiwanin A-induced cell death. Life Sci. 91:1358–1365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huh JE, Kang KS, Chae C, Kim HM, Ahn KS
and Kim SH: Roles of p38 and JNK mitogen-activated protein kinase
pathways during cantharidin-induced apoptosis in U937 cells.
Biochem Pharmacol. 67:1811–1818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang HJ, Soh Y, Kim MS, Lee EJ, Surh YJ,
Kim HR, Kim SH and Moon A: Roles of JNK-1 and p38 in selective
induction of apoptosis by capsaicin in ras-transformed human breast
epithelial cells. Int J Cancer. 103:475–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan KT, Wijeratne D, Shih B, Baildam AD
and Bayat A: Tumour necrosis factor-α expression is associated with
increased severity of periprosthetic breast capsular contracture.
Eur Surg Res. 45:327–332. 2010.
|