Introduction
Peroxisome proliferator-activated receptor-γ
(PPAR-γ) is an essential nuclear receptor that acts as a key
regulator of energy balance and is associated with
hyperandrogenemia (1,2). Moreover, the PPAR-γ protein plays a
pivotal role in modulating adipocyte differentiation, glucose and
lipid homeostasis. Activation of PPAR-γ improves the action of
insulin and reduces the risk of obesity by modulating adipocyte
differentiation, glucose and lipid homeostasis (3,4).
Deregulation of PPAR-γ was associated with metabolic diseases,
including obesity, type 2 diabetes, and obesity-associated
hypertension (5).
Polycystic ovary syndrome (PCOS) is a common
endocrine disorder in women of fertile age. PCOS is characterized
by menstrual irregularity, ovarian and adrenal androgen
overproduction, and insulin resistance (IR) (6). IR was recently recognized as a key
etiological factor in metabolic disorders such as obesity, type 2
diabetes, and obesity-associated hypertension. A previous study
indicated that gene polymorphisms in the PPAR-γ gene are
associated with PCOS occurrence in different ethnic backgrounds
(7). The PPAR-γ gene is
mainly expressed in the adipose tissue, where it promotes the
differentiation of preadipocytes into adipocytes and affects
insulin sensitivity (8). A study
exploring the correlation between the PPAR-γ level and
hyperinsulinemia suggested that hyperandrogenemia might be involved
in the development of PCOS (9).
The study showed that PPAR-γ associates with PCOS pathogenesis.
However, the expression of PPAR-γ in adipose tissue of women with
PCOS has not been fully understood.
The level of the androgen dehydroepiandrosterone
(DHEA) is high in the blood of women with PCOS, and thus, DHEA is
applied to establish animal models of PCOS (10). The DHEA-PCOS murine model exhibits
many of the salient features of human PCOS such as
hyperandrogenemia, IR, endocrine disturbance, follicle maturation
disorders, and infertility (11).
Moreover, the DHEA-PCOS model has been widely used to study a
number of adipocytokines from adipose tissue, such as adiponectin,
resistin, leptin and tumor necrosis factor-α (TNF-α), which may
influence the pathogenesis of IR in the PCOS (12).
The present study was designed to investigate PPAR-γ
expression in adipose tissue, and whether PPAR-γ induces or
attenuates, through the lipid metabolism pathway, the PCOS
stimulated by DHEA. Evaluating this aspect would allow
understanding of the relationship between adipocyte differentiation
and the development of PCOS, as well as provide information
relevant to the improvement of the efficacy of treatment of PCOS in
certain conditions.
Materials and methods
Animals
Sixteen sexually immature Sprague-Dawley female rats
(21 days old) were purchased from the Experimental Animal Center of
Guangdong Province (Guangzhou, China). The rats were kept in a
light-controlled room under a 12 h/12 h light/dark cycle and
controlled temperature (23–25°C), and had free access to food and
water. The animals were randomly divided into the DHEA (n=8) or
control (n=8) group. The rats in the DHEA group were subcutaneously
injected with 6 mg/100 g body weight DHEA (Hubei Fangtong
Pharmaceutical Co., Ltd., Huangshi, Hubei, China) dissolved in 0.2
ml of sesame oil. Injections were performed daily for 20
consecutive days according to the method of Henmi et al
(13). The rats in the control
group received a standard laboratory diet for 20 consecutive days.
During the experiment, the 4-day ovarian cycle of rats was
monitored daily using vaginal cytology. The protocol was approved
by the Ethics Committee of the First Affiliated Hospital of Jinan
University (Guangzhou, China).
Tissue collection
All rats were sacrificed by decapitation 24 h
following administration of the last DHEA dose. Parametrial adipose
tissue was rapidly excised and immediately frozen in liquid
nitrogen or stored at −70°C until further use for reverse
transcription polymerase chain reaction (RT-PCR) and western
blotting.
RT-PCR
Total mRNA from adipose tissue was extracted from 16
fresh adipose tissue samples from the DHEA group (n=8) and the
control group (n=8) using an RNA extraction kit (Omega Bioteh,
Norcross, GA, USA) according to the manufacturer’s instructions.
The adipose tissue were pulverized with a pestle and mortar in
liquid nitrogen. The mRNA concentration and purity were determined
by a 1.0% agarose gel electrophoresis and spectrophotometric
measurements; the optical density (OD) ratio at 260/280 nm was
>1.8. Aliquots of mRNA (20 μg) from each sample were reverse
transcribed using Oligo(dT)18 primer and Moloney murine
leukemia virus (MMLV) reverse transcriptase. The gene encoding
β-actin was used as an internal control to normalize the results
for variation in RNA quantities or differencies in the efficiency
of reverse transcription. The primers used for amplification were
PPAR-γ, forward 5′-GGTGAAACTCTGGGAGATCCTCC-3′ and reverse
5′-AGCAACCATTGGGTCAGCTCT-3′; β-actin, forward
5′-CCTAAGGCCAACCGTAAAG-3′ and reverse
5′-GGTCCACATTCTTTTCCTGATACTG-3′. Forty cycles of amplification were
performed and each cycle consisted of denaturation at 95°C for 30
sec, annealing at 60°C for 1 min, and extension at 70°C for 1 min,
with an additional extension at 72°C for 10 min. Five microliters
of each RT-PCR product were loaded onto a 1.5% agarose gel, and
subsequently visualized and quantified using GDS-8000 Gel
Scientific Image System and ImageQuant analysis software (Amersham
Pharmacia Biotech, Hong Kong, China). Densitometrical values were
used to calculate the ratio of PPAR-γ to β-actin.
Western blotting
The adipose tissue samples were lysed on ice using
cell lysis buffer and a protease inhibitor cocktail. After
centrifugation at 10,000 × g for 20 min at 4°C, protein
concentrations were determined using the Pierce Bicinchoninic Acid
Protein Assay kit by Thermo Scientific (Rockford, IL, USA). Total
protein from each sample was denatured in loading buffer,
fractionated on a 10% 1-dimensional SDS-PAGE gel, and transferred
to a polyvinylidene difluoride membrane (Immobilon-P; Millipore
Corp., Bedford, MA, USA). Blots were blocked for 2 h in TBST
solution (20 mmol/l Tris pH 7.6, 137 mmol/l sodium chloride, 0.1%
Tween-20) supplemented with 10% non-fat dry milk. The blots were
then incubated with antibodies against human PPAR-γ (1:1,000; Cell
Signaling Technology, Danvers, MA, USA) overnight at 4°C, and
against β-actin (1:3,000; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) for 1 h at room temperature by agitating. The blots
were washed three times for 7 min each in TBST, followed by
incubation for 1 h at room temperature with anti-rabbit and
anti-mouse IgG horseradish peroxidase-conjugated species-specific
secondary antibodies. The bound antibodies were detected using the
BeyoECL Plus enhanced chemiluminescence system (Beyotime, Shanghai,
China). Band intensities were quantified by scanning densitometry
using the Quantity One software (Bio-Rad Laboratories, Hercules,
CA, USA).
Immunohistochemistry
Paraffin-embedded sections of adipose tissue from
the DHEA group (n=8) and the control group (n=8) were used for
immunohistochemistry. Positive staining was evaluated with the
standard streptavidin-biotin system (Maixin Bio, Fuzhou, China).
Sections (4-μm) were deparaffinized in xylene, hydrated through
graded alcohol, and incubated in antigen retrieval solution
(0.01-mol/l sodium citrate buffer, pH 6.0) at 60°C for 16 min.
Endogenous peroxidase activity was blocked by incubating the
samples in 3% hydrogen peroxide for 10 min. Non-specific antibody
binding was blocked by incubation in normal goat serum for 10 min.
The PPAR-γ monoclonal antibody (1:100, Maixin Bio) was used as the
primary antibody, and the sections were incubated with this
antibody overnight at 4°C. As negative control, we used sections of
the same tissues incubated without the primary antibody. As
secondary antibodies, biotinylated anti-mouse immunoglobulins were
used, and the reaction was developed using the
streptavidin-peroxidase system. The diaminobenzidine
substrate-chromogen system (Maixin Bio, Fuzhou, China) was used as
the color-developing substrate.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). The difference between the two groups was evaluated by a
t-test. P<0.05 was considered to indicate statistical
significance. Statistical analysis was performed using the SPSS
16.0 (SPSS, Chicago, IL, USA).
Results
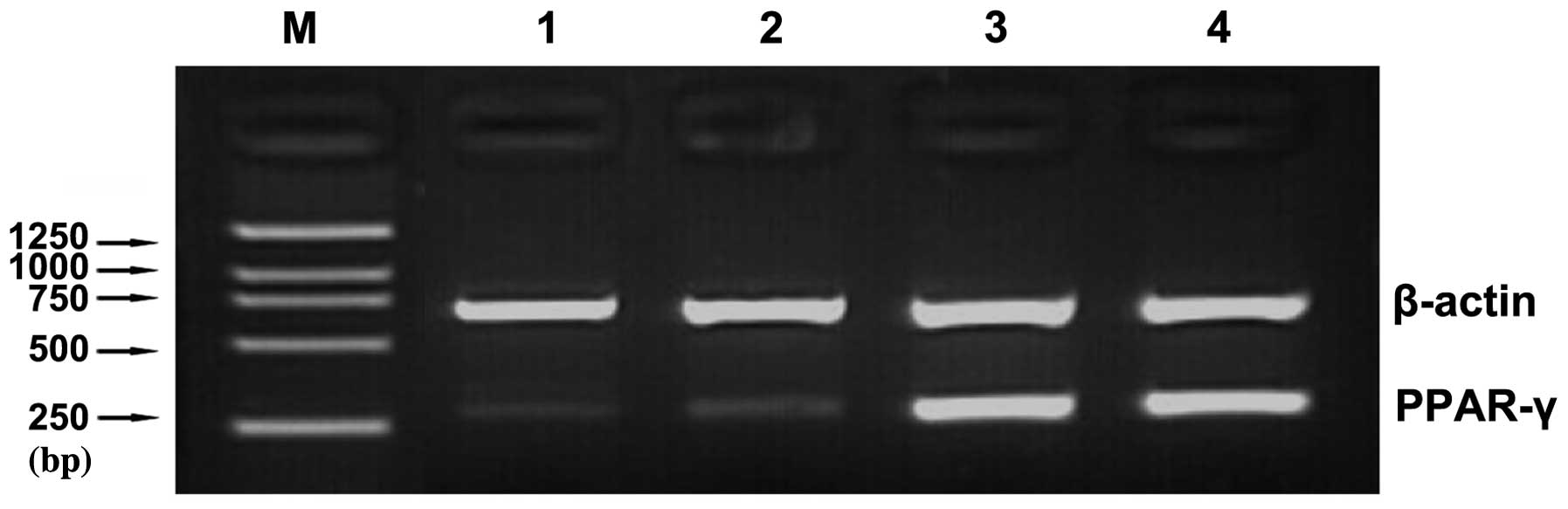
RT-PCR analysis
PPAR-γ gene expression in the adipose tissue
was analyzed by RT-PCR. A total of 16 adipose tissue samples were
positive for the β-actin mRNA, and thus considered for subsequent
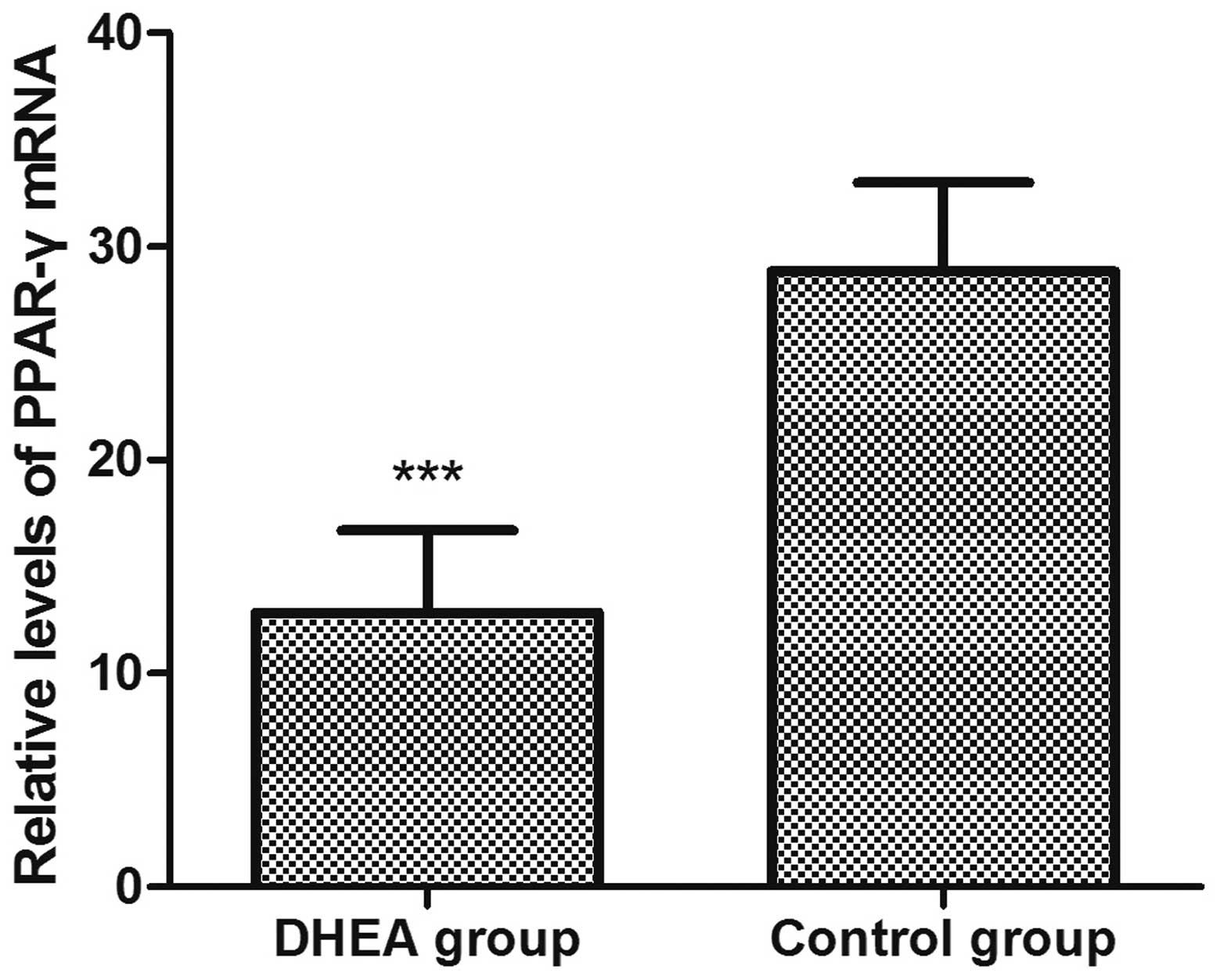
examination of the mRNA level of PPAR-γ (Fig. 1). The expression level of
PPAR-γ in the DHEA group (12.83±3.87) was significantly
(P<0.01) lower (28.83±4.15) compared to the control (Fig. 2).
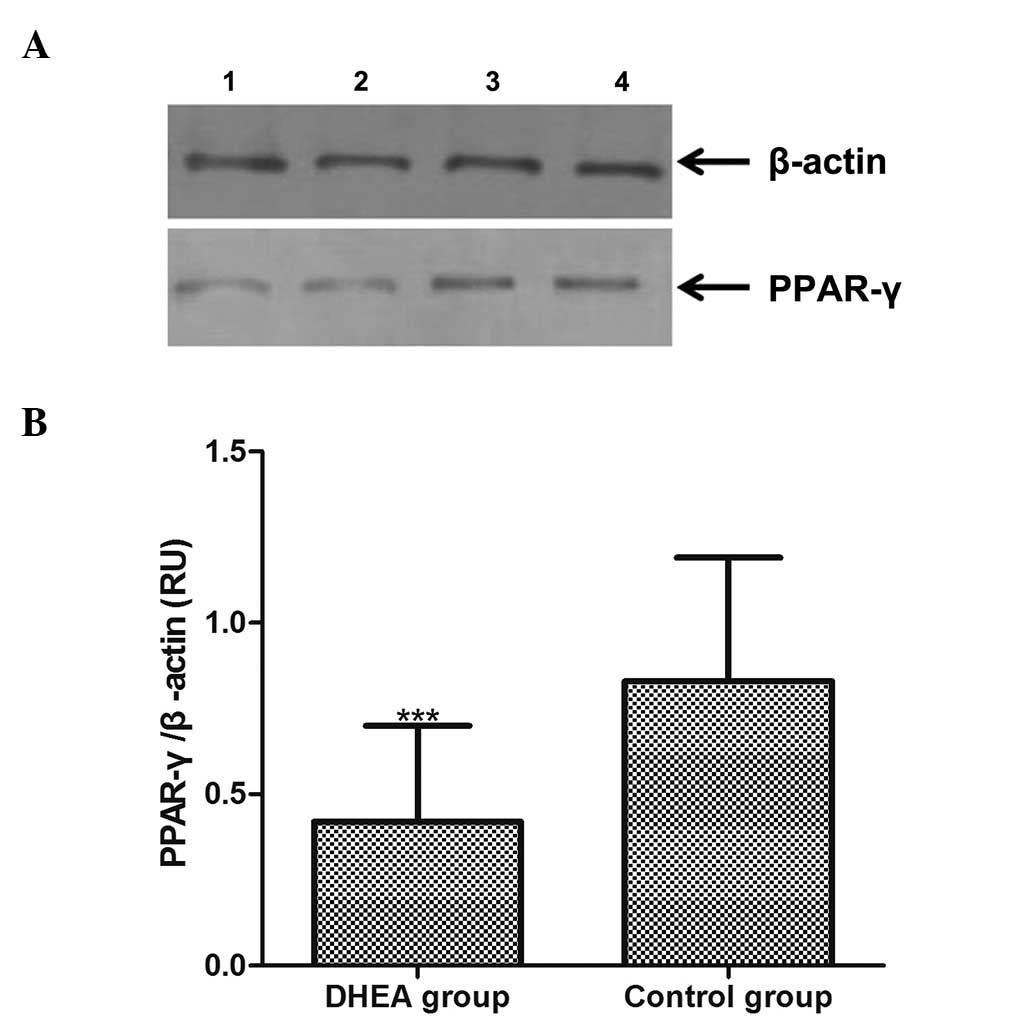
Western blot analysis
PPAR-γ protein expression in adipose tissue was
analyzed by western blotting, and the level of the protein was
normalized to that of β-actin (Fig.
3A). The result of quantitative analysis of the 16 adipose
tissue samples is shown in Fig.
3B. The relative optical densities of the PPAR-γ protein in the
DHEA and the control group were 0.42±0.28 and 0.83±0.36,
respectively (Fig. 3B). The
expression level of PPAR-γ was thus lower in the DHEA group, and
this decrease was significant (P<0.01).
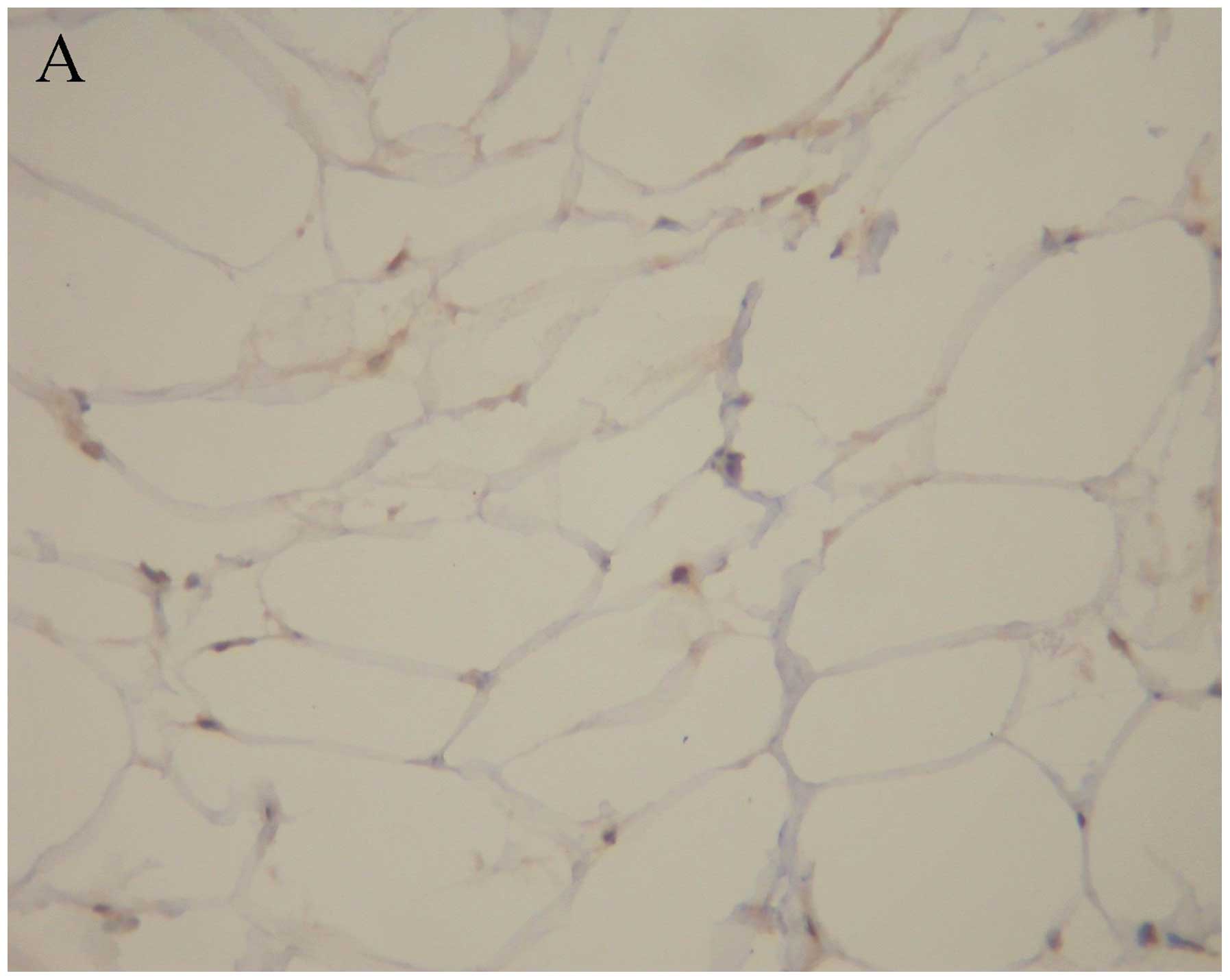
Immunostaining of PPAR-γ protein
PPAR-γ-positive immunostaining appeared in the
adipose tissue of the two groups (Fig.
4). PPAR-γ staining mainly appeared in the cytoplasm near the
membrane of adipose cells. Positive staining of PPAR-γ was also
observed in the nuclei of adipose cells. PPAR-γ-positive
immunostaining was strong in the control group and weak in the DHEA
group.
Discussion
DHEA was found to be an abundant circulating
androgen in women with PCOS (10).
A mechanism underlying DHEA-induced PCOS involves endocrine
disorder, which was related to abnormal levels of sex hormones
(14). In previous studies, we
successfully induced PCOS in rats using DHEA (15,16).
The nuclear hormone receptor PPAR-γ has been widely used to
elucidate the potential metabolic mechanisms underlying the
induction of PCOS by DHEA. In the present study, PPAR-γ expression
was significantly lower in the DHEA group compared to the control
group. This finding indicates that downregulation of PPAR-γ,
induced by DHEA, might affect the pathogenesis of PCOS via yet
undefined mechanisms.
The PPAR-γ gene is suspected to be involved
in the regulation of adipose metabolism in humans, and is also a
susceptibility gene for the development of both obesity and IR,
frequently associated with PCOS (17). Association of polymorphisms in the
PPAR-γ gene with PCOS in women remains a controversial
issue, with significance of correlations depending on the
population (18,19). Therefore, whether there is a
correlation between the expression of PPAR-γ and PCOS, and
whether there is another pathway via which the PPAR-γ protein may
exert its physiological effects on PCOS pathogenesis, independently
of gene mutation or variation, is uncertain. We have demonstrated
that expression of PPAR-γ was significantly decreased in rats with
PCOS induced by DHEA. The results of this study on the effect of
PPAR-γ in peripheral adipose tissue are consistent with the
hypothesis that, apart from the direct endocrine function of
androgen, hyperandrogenemia may induce metabolic abnormalities
through regulation of PPAR-γ in women with PCOS (2,8).
Moreover, previous studies have demonstrated that IR and
hyperandrogenemia play a pathogenetic role in PCOS (10,12,20).
IR is a common feature of metabolic disorders including PCOS
without exception of alterations in sensitivity to insulin which
could be originated from PPAR-γ (4,8,16).
Based on this study and the literature (8,16,20,21),
we present a model for the potential association between DHEA,
PPAR-γ and IR during the development of PCOS (Fig. 5). If some endogenous factors (e.g.,
hormone disturbance) or exogenous factors (e.g., environmental
contamination by androgens and estrogens) raise the androgen level
to a certain degree, the endocrine balance may be altered.
Androgens, as DHEA in this study, may directly affect the action of
insulin by inhibiting the activity and expression of
glucose-6-phosphatase and phosphoenolpyruvate carboxykinase
(PEPCK). DHEA increases glucose uptake in hepatocytes and increases
insulin binding to its own receptor leading to hyperinsulinism, and
eventually, IR (22). It is
generally accepted that IR is likely to aggravate clinical features
of PCOS. On the other hand, DHEA may contribute to PCOS via another
pathway, through reduction of the expression levels of PPAR-γ.
In vivo and in vitro studies have demonstrated that
DHEA activates phosphatidylinositol 3-kinase and atypical protein
kinase C (PKC) via C/EBPα transcription factors to decrease the
expression of PPAR-γ. The reduction in PPAR-γ protein levels might
attenuate other adipocyte-specific phenotypes through changes in
the spatial conformation of proteins such as glyceraldehyde
3-phosphate dehydrogenase (GAPDH), adipocyte lipid-binding protein
(aP2) and sterol regulatory element binding protein (SREBP)
(23,24). These proteins are transcriptional
factors associated with hyperinsulinism, inevitably promoting PCOS.
In addition, IR in PCOS reduces the concentration of peripheral
tissues in PPAR-γ via TNF-α, which is another PPAR-γ-reducing
agonist (25). The subsequent
decrease in PPAR-γ expression further initiates the occurrence of a
number of IR symptoms. Thus, we suggest that a complex and multiple
network of interactions may characterize and regulate PPAR-γ,
hyperandrogenemia, IR and PCOS. As shown in Fig. 5, hyperandrogenemia induces PCOS
through the classical IR pathway. Based on our results, the
occurrence or development of PCOS in conditions of androgen excess
may also correlate to the lipid metabolism pathway associated with
the decrease in PPAR-γ. There is number of limitations in the
present study, such as the limited sample size and the fact that we
used an animal model. Therefore, whether additional pathways or
negative feedback loops allow to bypass the regulation scheme
presented in Fig. 5 remains to be
investigated.
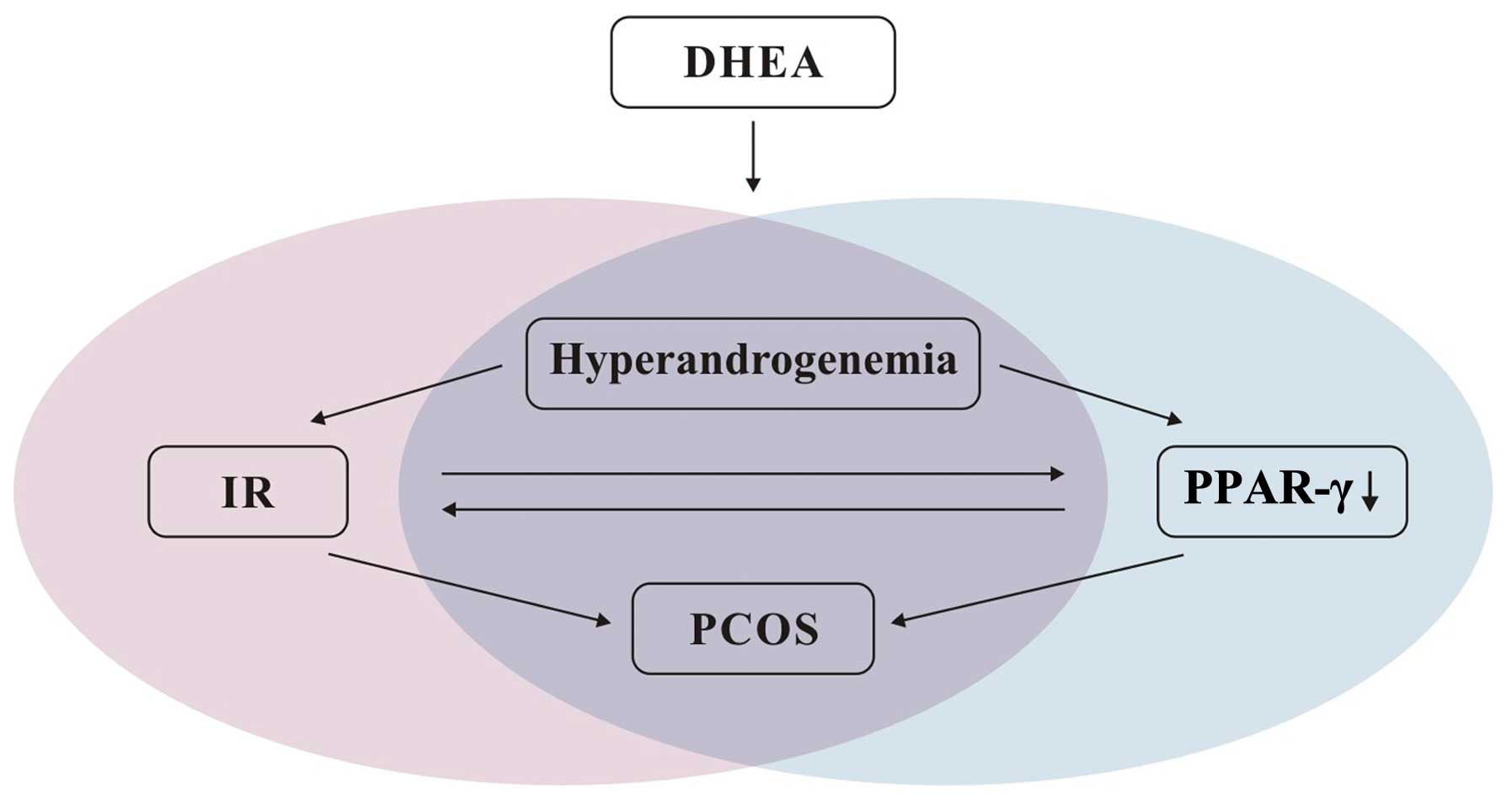 | Figure 5Model for the association between
DHEA, PPAR-γ and IR in PCOS. Exogenous DHEA increases the androgen
level. On the one hand, hyperandrogenemia leads to IR, which can
aggravate clinical features of PCOS. On the other hand,
hyperandrogenemia can decrease the expression of PPAR-γ in adipose
tissue via the lipid metabolism pathway, further contributing to
PCOS pathogenesis. The reduction in PPAR-γ can further result in
hyperinsulinism, initiating the occurrence of several symptoms of
IR, and IR in PCOS may decrease the PPAR-γ level. Therefore,
multiple interactions and two pathways are associated with the
occurrence or development of PCOS under conditions of excess of
DHEA. DHEA, dehydroepiandrosterone; IR, insulin resistance; PCOS,
polycystic ovary syndrome; PPAR-γ, peroxisome
proliferator-activated receptor-γ. |
Expression patterns of PPAR-γ differ
substantially in numerous diseases and tissues. In rat models of
diabetes, the PPAR-γ gene was downregulated in the renal
cortex and retina (26), while it
was upregulated in the aorta (27). By contrast, in the present study,
only a reduction in the transcription and translation of the gene
was observed in DHEA-induced rats with PCOS, with concordant
results from three independent methods of assessment of the
expression of PPAR-γ. This indicates that PPAR-γ may exert various
biological effects dependent on the tissue and other biological
features. Thus, to understand the exact physiological effects of
PPAR-γ on infertility of PCOS women, the variability and complexity
of PPAR-γ expression need to be thoroughly studied. Although PPAR-γ
is primarily expressed in adipose tissue, it can have direct or
indirect effects on regulation of the function of ovarian granulosa
cells, by affecting related adipocytokines that affect the normal
release of oocytes (28).
Moreover, because of the alteration in lipid metabolism caused by
impaired insulin signaling, regulation, by PPAR-γ, of its
downstream targets is also impaired (29). These two effects induced by PPAR-γ
on ovary and lipid metabolism may constitute important factors
contributing to the infertility of women with PCOS. PPAR-γ may thus
play a role in the molecular linking of lipid metabolism to
reproduction, with inactivation of PPAR-γ promoting infertility in
women with PCOS. Thus, treating PCOS requires focalizing on, and
understanding the PPAR-γ-related pathways, and potential
therapeutic agents targeting this protein to enhance its activity
have the potential to improve fertility.
In summary, results of this study have shown that
DHEA excess can induce an adipose-specific reduction in PPAR-γ
expression, which is associated with PCOS. The characteristics of
PPAR-γ expression in adipose tissue of the PCOS rat model indicated
that PPAR-γ may induce or promote PCOS through the lipid metabolism
pathway. A complex network might promote development of PCOS, which
in this study was associated with an increase in DHEA and a
decrease in PPAR-γ levels. Both the metabolic and endocrine
pathways are related to the pathogenesis of PCOS. Further studies
are needed to elucidate the etiology of infertility and its
association with PPAR-γ expression in PCOS. Improved therapeutic
treatment in the clinic may involve developing agents specifically
targeting PPAR-γ to increase fertility of women with PCOS.
Acknowledgements
This study was supported by Science and Technology
Planning Project of Guangdong Province, China (no.
2012B031800400).
References
|
1
|
Ryan KK, Li B, Grayson BE, Matter EK,
Woods SC and Seeley RJ: A role for central nervous system PPAR-γ in
the regulation of energy balance. Nat Med. 17:623–626. 2011.
|
|
2
|
Cipolletta D, Feuerer M, Li A, Kamei N,
Lee J, Shoelson SE, Benoist C and Mathis D: PPAR-γ is a major
driver of the accumulation and phenotype of adipose tissue Treg
cells. Nature. 486:549–553. 2012.
|
|
3
|
Sun K and Scherer PE: The PPARγ-FGF1 axis:
an unexpected mediator of adipose tissue homeostasis. Cell Res.
22:1416–1418. 2012.
|
|
4
|
Jonker JW, Suh JM, Atkins AR, Ahmadian M,
Li P, Whyte J, He M, Juguilon H, Yin YQ, Phillips CT, Yu RT,
Olefsky JM, Henry RR, Downes M and Evans RM: A PPARγ-FGF1 axis is
required for adaptive adipose remodelling and metabolic
homeostasis. Nature. 485:391–394. 2012.
|
|
5
|
Tyagi S, Gupta P, Saini AS, Kaushal C and
Sharma S: The peroxisome proliferator-activated receptor: a family
of nuclear receptors role in various diseases. J Adv Pharm Technol
Res. 2:236–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Svendsen PF, Christiansen M, Hedley PL,
Nilas L, Pedersen SB and Madsbad S: Adipose expression of
adipocytokines in women with polycystic ovary syndrome. Fertil
Steril. 98:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasgupta S, Sirisha P, Neelaveni K,
Anuradha K, Sudhakar G and Reddy BM: Polymorphisms in the IRS-1 and
PPAR-γ genes and their association with polycystic ovary syndrome
among South Indian women. Gene. 503:140–146. 2012.
|
|
8
|
Saraf N, Sharma PK, Mondal SC, Garg VK and
Singh AK: Role of PPARg2 transcription factor in
thiazolidinedione-induced insulin sensitization. J Pharm Pharmacol.
64:161–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amalfi S, Velez LM, Heber MF, Vighi S,
Ferreira SR, Orozco AV, Pignataro O and Motta AB: Prenatal
hyperandrogenization induces metabolic and endocrine alterations
which depend on the levels of testosterone exposure. PLoS One.
7:e376582012. View Article : Google Scholar
|
|
10
|
Lenarcik A, Bidzińska-Speichert B,
Tworowska-Bardzińska U and Krępuła K: Hormonal abnormalities in
first-degree relatives of women with polycystic ovary syndrome
(PCOS). Endokrynol Pol. 62:129–133. 2011.PubMed/NCBI
|
|
11
|
Abramovich D, Irusta G, Bas D, Cataldi NI,
Parborell F and Tesone M: Angiopoietins/TIE2 system and VEGF are
involved in ovarian function in a DHEA rat model of polycystic
ovary syndrome. Endocrinology. 153:3446–3456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glintborg D, Andersen M, Hagen C, Frystyk
J, Hulstrøm V, Flyvbjerg A and Hermann AP: Evaluation of metabolic
risk markers in polycystic ovary syndrome (PCOS). Adiponectin,
ghrelin, leptin and body composition in hirsute PCOS patients and
controls. Eur J Endocrinol. 155:337–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henmi H, Endo T, Nagasawa K, Hayashi T,
Chida M, Akutagawa N, Iwasaki M, Kitajima Y, Kiya T, Nishikawa A,
Manase K and Kudo R: Lysyl oxidase and MMP-2 expression in
dehydroepiandrosterone-induced polycystic ovary in rats. Biol
Reprod. 64:157–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenfield RL, Mortensen M, Wroblewski K,
Littlejohn E and Ehrmann DA: Determination of the source of
androgen excess in functionally atypical polycystic ovary syndrome
by a short dexamethasone androgen-suppression test and a low-dose
ACTH test. Hum Reprod. 26:3138–3146. 2011.PubMed/NCBI
|
|
15
|
Wang YX, Xie XM and Zhu WJ: Serum
adiponectin and resistin levels in patients with polycystic ovarian
syndrome and their clinical implications. J Huazhong Univ Sci
Technolog Med Sci. 30:638–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YX, Sun YY and Qiu HY: Expression of
resistin mRNA in adipose tissue of rat model with polycystic
ovarian syndrome and its implication. J Huazhong Univ Sci Technolog
Med Sci. 24:621–624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knebel B, Lehr S, Janssen OE, Hahn S,
Nitzgen U, Jacob S, Haas J, Muller-Wieland D and Kotzka J: Genetic
variants in central metabolic genes influence some but not all
relations of inflammatory markers in a collective with polycystic
ovary syndrome. Arch Physiol Biochem. 118:219–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang H, Bi Y, Hu C, Lu W and Zhu D:
Association between the Pro12Ala polymorphism of PPAR-γ gene and
the polycystic ovary syndrome: a meta-analysis of case-control
studies. Gene. 503:12–17. 2012.
|
|
19
|
San-Millán JL and Escobar-Morreale HF: The
role of genetic variation in peroxisome proliferator-activated
receptors in the polycystic ovary syndrome (PCOS): an original
case-control study followed by systematic review and meta-analysis
of existing evidence. Clin Endocrinol (Oxf). 72:383–392. 2010.
|
|
20
|
Brettenthaler N, De Geyter C, Huber PR and
Keller U: Effect of the insulin sensitizer pioglitazone on insulin
resistance, hyperandrogenism, and ovulatory dysfunction in women
with polycystic ovary syndrome. J Clin Endocrinol Metab.
89:3835–3840. 2004. View Article : Google Scholar
|
|
21
|
Yildiz BO, Yarali H, Oguz H and Bayraktar
M: Glucose intolerance, insulin resistance, and hyperandrogenemia
in first degree relatives of women with polycystic ovary syndrome.
J Clin Endocrinol Metab. 88:2031–2036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan K, Huang A and Azziz R:
Dehydroepiandrosterone sulfate and insulin resistance in patients
with polycystic ovary syndrome. Fertil Steril. 91:1848–1852. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kajita K, Ishizuka T, Mune T, Miura A,
Ishizawa M, Kanoh Y, Kawai Y, Natsume Y and Yasuda K:
Dehydroepiandrosterone down-regulates the expression of peroxisome
proliferator-activated receptor gamma in adipocytes. Endocrinology.
144:253–259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yogosawa S, Mizutani S, Ogawa Y and Izumi
T: Activin receptor-like kinase 7 suppresses lipolysis to
accumulate fat in obesity through downregulation of peroxisome
proliferator-activated receptor γ and C/EBPα. Diabetes. 62:115–123.
2012.PubMed/NCBI
|
|
25
|
Swaroop JJ, Rajarajeswari D and Naidu JN:
Association of TNF-α with insulin resistance in type 2 diabetes
mellitus. Indian J Med Res. 135:127–130. 2012.
|
|
26
|
Wang F, Gao L, Gong B, Hu J, Li M, Guan Q
and Zhao J: Tissue-specific expression of PPAR mRNAs in diabetic
rats and divergent effects of cilostazol. Can J Physiol Pharmacol.
86:465–471. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma AK, Bharti S, Ojha S, Bhatia J,
Kumar N, Ray R, Kumari S and Arya DS: Up-regulation of PPARγ, heat
shock protein-27 and -72 by naringin attenuates insulin resistance,
β-cell dysfunction, hepatic steatosis and kidney damage in a rat
model of type 2 diabetes. Br J Nutr. 106:1713–1723. 2011.
|
|
28
|
Kim J, Sato M, Li Q, Lydon JP, Demayo FJ,
Bagchi IC and Bagchi MK: Peroxisome proliferator-activated receptor
gamma is a target of progesterone regulation in the preovulatory
follicles and controls ovulation in mice. Mol Cell Biol.
28:1770–1782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Asrih M, Lerch R, Papageorgiou I, Pellieux
C and Montessuit C: Differential regulation of stimulated glucose
transport by free fatty acids and PPARα or -δ agonists in cardiac
myocytes. Am J Physiol Endocrinol Metab. 302:E872–E884.
2012.PubMed/NCBI
|