Introduction
Cardiac transplant is a useful therapeutic tool for
the treatment of patients with end-stage heart failure or severe
coronary artery disease (1).
Despite marked effort and advances made during the past few years,
with improved immunosuppression regimens and post-operative care,
there are a number of problems that remain to be resolved,
including poor long-term cardiac allograft outcomes, infections and
malignancies (2–4). The introduction of cyclosporine (CsA)
in immunosuppressive therapy has greatly improved the clinical
outcome of cardiac transplantation (5,6).
However, the side effects of CsA, including irreversible pulmonary
hypertension, infection and cancer, are serious limitations due to
the high dosage required (7,8).
More recent strategies have focused on using cell therapies from
different sources to facilitate stimulating the regeneration of
cells inside the transplanted heart. However, the effect of
differentiation on the expression profile of MHC proteins in
allogeneic cell therapies is largely unknown (9). The development of a novel, short-term
and effective immunomodulatory strategy is urgently required.
Melatonin, secreted by the pineal gland, is a
widespread physiological mediator (10). Melatonin is a multifunctional gene,
affecting various systems, including regulating circadian rhythm,
immunoregulation, anti-oxidative effect and infection (11). Early evidence indicated that
melatonin regulates the expression of Th1/2 cytokines, including
tumor necrosis factor-α (TNF-α), γ-interferon, interleukin (IL)-12,
IL-1, IL-2 and macrophage colony-stimulating factor (11,12).
Rats supplemented with high (50 mg/kg) doses of melatonin exhibited
significantly elevated survival times due to the abrogation of
alloimmune responses (13).
Further investigation has demonstrated that immunosuppressive
maintenance therapy with CsA and rapamycin affected endogenous
melatonin secretion (14).
Melatonin has exhibited a protective action against CsA-induced
oxidative stress, nephrotoxic effects and autophagy in vivo
or in vitro (15–17). Previous studies have demonstrated
that high-dose melatonin treatment prolonged cardiac allograft
survival without side effects (13). However, the combined effect of
melatonin plus CsA remains to be elucidated and the molecular
regulatory mechanisms are unclear.
In the present study, it was determined whether
co-treatment with melatonin and CsA was able to improve the
survival of cardiac allografts. In addition, the possible
mechanisms underlying this effect were investigated.
Materials and methods
Animal care
Specific pathogen-free male Sprague-Dawley (SD) rats
and Wistar rats, weighing 200–250 g, were obtained from Shanghai
Slaccas Laboratory Animal Company Ltd. (Shanghai, China). The
animals were housed in standard polypropylene cages (three rats per
cage) in a constant temperature (22±2°C) with a 12-h light and dark
cycle. The care of the animals and experimental protocols was
approved by the Animal Care and Use Committee of Fudan College of
Science (Shanghai, China).
Animal experiments
Inbred SD rats and inbred Wistar rats were used as
the recipients and donors, respectively. A total of 40 allogenic
heart-transplanted SD rats were randomly divided into four groups
of ten. Donor heart grafts were transplanted into the abdominal
cavity of SD recipients using standard microsurgical techniques
under sterile conditions. The saline group and the other three
groups were administered 200 mg/kg/day melatonin (Sigma, St. Louis,
MO, USA), 20 mg/kg/day CsA and 50 mg/kg/day melatonin with 5
mg/kg/day CsA, respectively. The rate and strength of allograft
pulsations were determined each day to check the survival times.
Rejection was defined as heartbeat cessation for one day.
TdT-mediated dUTP-biotin nick end
labeling (TUNEL) assay
For the detection of DNA strand breaks,
paraffin-embedded sections were stained with the in situ
cell death detection kit (Promega Corporation, Madison, WI, USA)
according to the manufacturer’s instructions. Briefly,
formalin-fixed sections were dehydrated in alcohol and defatted in
xylenes. Following being permeabilized in proteinase K for 10 min,
endogenous peroxidase was deactivated by 0.3% hydrogen peroxide.
Next, the sections were incubated with 10 μl TUNEL reaction
mixture, containing terminal deoxynucleotidyl transferase and
fluorescein isothiocyanate dUTP, for 60 min at 37°C. These sections
were then stained with 3, 3′-diaminobenzidine, following
hematoxylin post-staining, and observed under an optical microscope
(4XC-MS; Shanghai Lunjie Mechanical and Electronical Instrument
Co., Ltd., Shanghai, China). TUNEL-positive cells were counted in
twelve randomly selected fields from each slide at a magnification
of ×200. The percentage of TUNEL-positive cells was analyzed in ten
cardiac sections from ten different rats.
Immunohistochemistry
All of the fresh tissues were fixed in 10% neutral
buffered formalin and embedded in paraffin. A total of 5-μm
paraffin-embedded sections were cut on a microtome and stained with
hematoxylin and eosin (H&E) and Masson’s trichrome according to
the standard procedures. Generally, the 5-μm paraffin-embedded
sections were de-paraffinized in a series of xylene and hydrated in
a graded series of ethanol. The sections were washed briefly in
distilled water and stained in hematoxylin solution for 10 min.
Histological specimens were evaluated by the International Society
of Heart and Lung Tranplantation (ISHLT) grading system (18) with an optical microscope (4XC-MS;
Shanghai Lunjie Mechanical and Electronical Instrument Co.,
Ltd.).
IL-2 and TNF-α determination
Serum was prepared from the blood collected five
days following heart transplantation. The levels of IL-2 and TNF-α
were measured in the plasma acquired from the ELISA assay, which
was stimulated with saline, melatonin, CsA or melatonin with CsA.
IL-2 ELISAs were performed using the reagents and instructions
provided by the manufacturer (Rat IL-2 Quantikine ELISA kit;
R&D Systems Inc., Minneapolis, MN, USA). TNF-α ELISAs were
performed using the reagents and instructions provided by the
manufacturer (Rat TNF-α Quantikine ELISA kit; R&D Systems
Inc.). All of the plasma specimens were examined in duplicate and
expressed in pg/ml.
Western blot analysis
The lysis of the frozen left ventricle tissue
samples were harvested in RIPA lysis buffer with proteinase
inhibitor (Roche Diagnostics, Ltd., Lewes, UK), sonicated for 30
sec and finally centrifuged for 30 min at 16,200 × g. The proteins
(~60 μg protein) from the total tissue samples were resolved by the
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
then transferred to the pure nitrocellulose membrane (Bio-Rad,
Hercules, CA, USA). Blocking was performed with 5% non-fat dry milk
overnight at 4°C and incubated with mouse monoclonal Bcl-2 antibody
(Proteintech Group Inc., Wuhan, China; cat no. 60178–1-Ig), mouse
monoclonal IL-1β antibody (Cell Signaling Technology, Inc.,
Beverly, MA, USA; cat no. 8689), rabbit polyclonal Phospho-NF-κB
p65 (Ser536) antibody (Cell Signaling Technology, Inc.; cat no.
3031), rabbit polyclonal NF-κB p65 (C22B4; Cell Signaling
Technology, Inc.; cat no. 4764) and rabbit polyclonal β-actin
(Sigma; cat no. A2066) overnight at 4°C. The western blots were
processed using a horseradish peroxidase goat anti-rabbit IgG
(Shanghai Kangcheng Bioengineering Co., Ltd., Shanghai, China). The
Pierce enhanced chemiluminescence Western Blotting Substrate was
used for chemiluminescence detection according to the
manufacturer’s instructions (Pierce Biotechnology, Inc., Rockford,
IL, USA). Densitometric analysis of protein was conducted using
Bio-Rad Quantity One® 1-D software (Bio-Rad).
Statistical analysis
Each experiment was performed at least three times
and the representative data were collected. Data are presented as
the mean ± standard deviation. Differences between the four groups
were determined using one-way analysis of variance. The statistical
analysis was performed using the GraphPad Prism V5.03 software
(GraphPad, San Diego, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
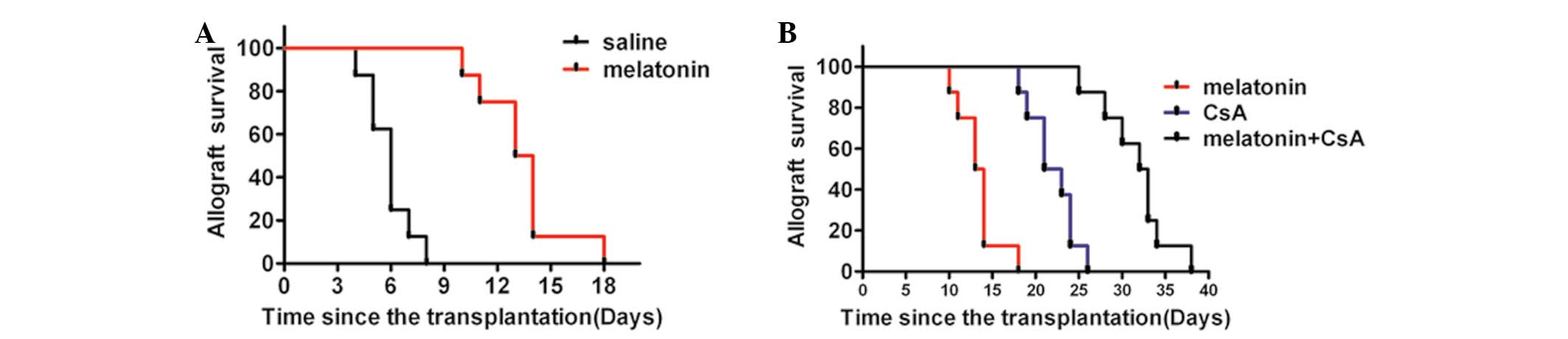
Melatonin with CsA markedly prolongs
cardiac allograft survival
Previous studies have demonstrated that melatonin is
able to suppress acute cardiac allograft rejection in vivo.
The present study investigated the effects on acute cardiac
allograft rejection of melatonin only, CsA only and melatonin with
CsA. As revealed in Fig. 1, the
survival times of hearts transplanted to animals treated with
melatonin were significantly prolonged compared with the untreated
control in a rat cardiac allograft model starting at the day of
transplantation. However its efficacy was less than that of 20
mg/kg CsA. By contrast, 50 mg/kg melatonin in combination with 5
mg/kg CsA was more effective than the sole administration of 20
mg/kg CsA (Fig. 1). These data
indicated that melatonin exhibits a beneficial effect on allograft
survival and may offer the possibility of reducing the dose of CsA
used in patients, thereby limiting the potential for severe side
effects.
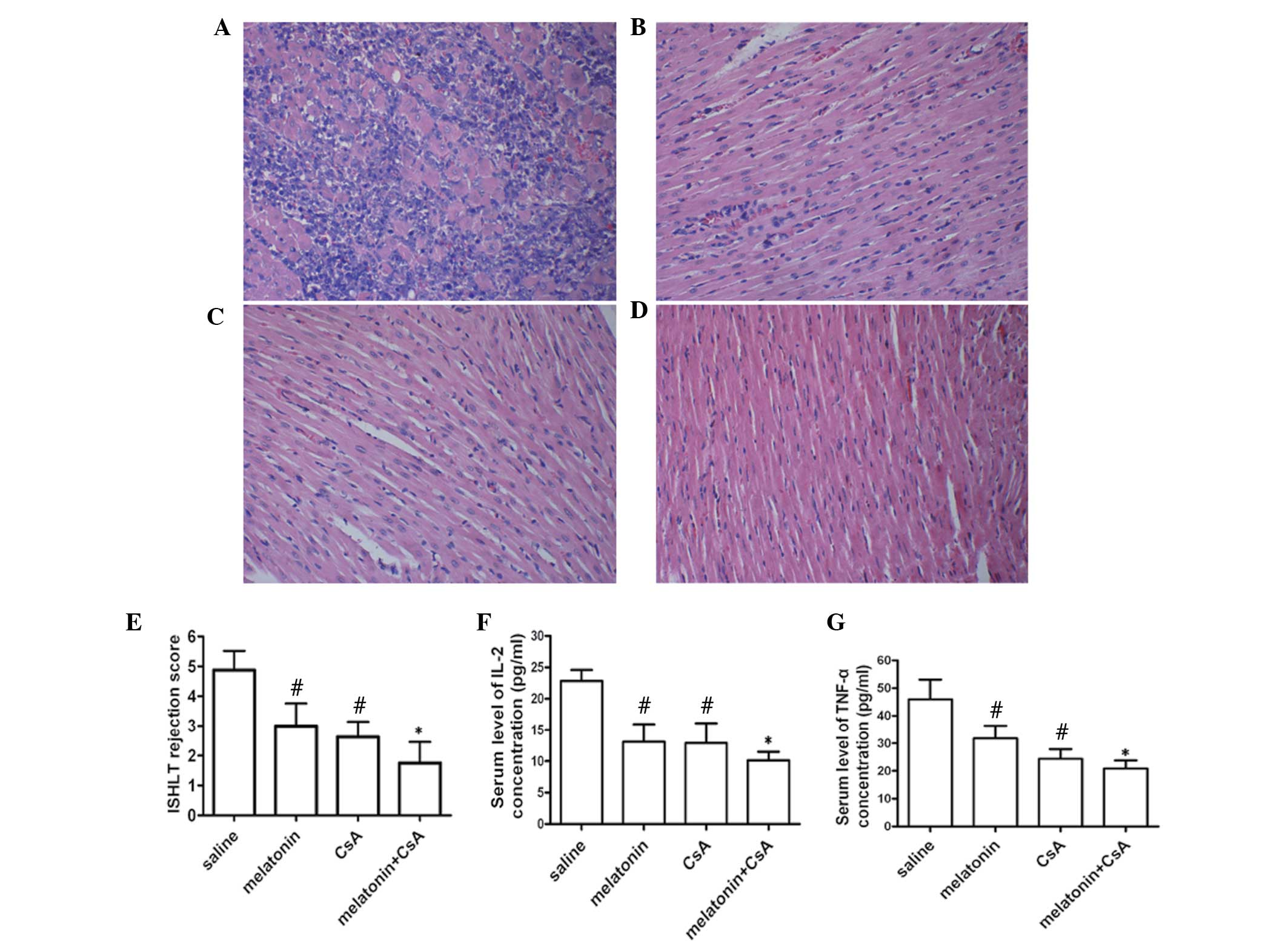
Melatonin suppresses inflammatory
responses in vivo
Imbalances in inflammatory processes are important
in the pathogenesis of immune rejection. In order to examine acute
immune rejection in the myocardium, H&E-stained sections were
evaluated using the ISHLT grading system (18). As demonstrated in Fig. 2B and C, the cardiac histopathology
revealed that 50 mg/kg melatonin and 20 mg/kg CsA was able to
extenuate the inflammatory reaction. While 50 mg/kg melatonin
co-administrated with 5 mg/kg CsA significantly alleviated heart
congestion (Fig. 2D). The
rejection grade diagnosed by ISHLT confirmed the histological
results (Fig. 2E). Further
analysis revealed that treatment with melatonin significantly
attenuated the release of inflammatory cytokines, including IL-2
and TNF-α (Fig. 2F and G). These
results suggested that melatonin attenuated immune rejection by
reducing the activation of inflammatory responses.
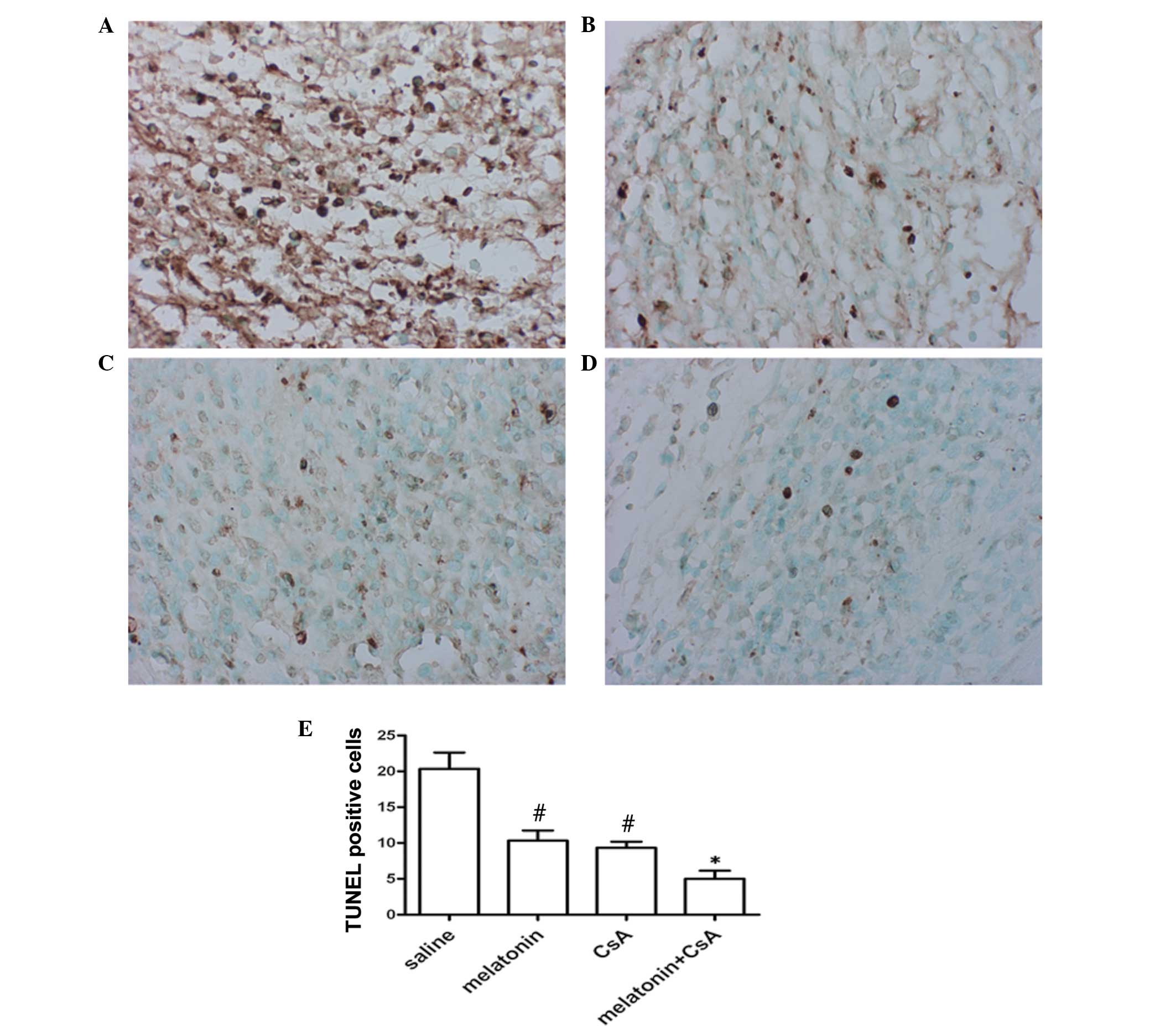
Melatonin protects against apoptosis of
cardiac myocytes
The number of apoptotic cardiac myocytes increased
markedly in association with the increased infiltration of
macrophages into the myocardial tissue during the course of cardiac
allograft rejection. Melatonin has been reported to exhibit an
antiapoptotic effect in other tissues. Thus, the present study
investigated the role of melatonin on apoptosis in myocardial
tissue on day five following transplantation. Apoptosis was
observed in all myocardial tissue and the number of apoptotic
cardiac myocytes significantly reduced in the allografts treated
with 200 mg/kg melatonin or 20 mg/kg CsA, compared with in the
allografts administered saline (Fig.
3A–C). When 50 mg/kg melatonin with less CsA 5 mg/kg was
administered to the animals, the proportion of apoptotic cardiac
myocytes was 6-fold lower than the saline group and 2-fold lower
than the rats treated with melatonin or CsA alone (Fig. 3D). These data suggested that
melatonin significantly reduces the number of TUNEL-positive
cells.
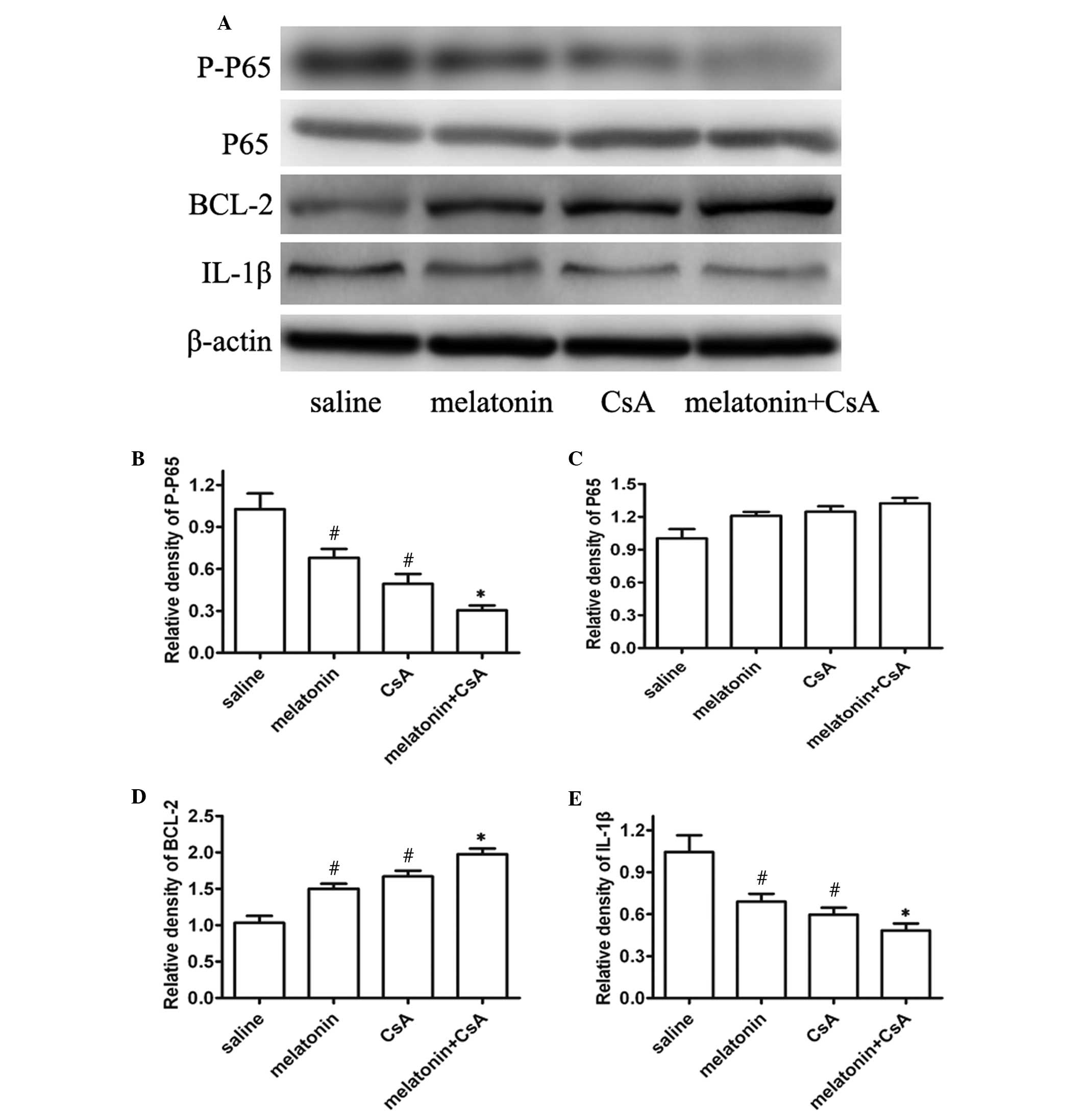
Expression levels of p65, Bcl-2 and IL-1β
in left ventricular tissue
To examine the possible mechanisms underlying the
improvement of allograft survival in melatonin-treated rats, the
present study focused on the expression of p65, Bcl-2 and IL-1β,
which are key genes in inflammation and apoptosis. As expected, it
was identified that the expression of p-p65 (Ser536) also
significantly decreased in the 200 mg/kg melatonin or 20 mg/kg
CsA-treated rats in comparison with the saline-treated animals
(Fig. 4A). Consistent with p-p65,
the expression of IL-1β was also reduced and the level of Bcl-2 was
upregulated (Fig. 4). Notably, 50
mg/kg melatonin with 5 mg/kg CsA have an effective inhibition on
the expression of p-p65 (Ser536) and IL-1β (Fig. 4). These data indicated that
melatonin may inhibit immune rejection to reduce the release of
inflammatory cytokines that induce apoptosis.
Discussion
In the present study, it was demonstrated that
allograft viability is prolonged by direct modulation of host
immunity via concomitant immunosuppression with melatonin-treatment
by utilizing a well-characterized experimental rat cardiac
transplantation model. Melatonin ameliorated prolonged inflammation
and the associated release of proinflammatory cytokines and
chemokines, which lead to transplant pathology. Serum levels of
TNF-α and IL-2 were significantly reduced in vitro in the
presence of melatonin and/or CsA, which may contribute to enhanced
cardiac allograft survival.
These data in a heart transplant model suggested
that melatonin effectively prolongs the survival of cardiac
allografts and reduces the dose of CsA. CsA has a marked beneficial
effect in organ transplantation in vivo and in vitro,
however, there are a number of significant side effects. In the
present study, the rats were treated with melatonin and CsA, which
effectively suppressed the activity of the adaptive immune
response, including the release of IL-2 and TNF-α in serum, which
was consistent with previous studies (13,19).
The protein levels of p-p65 and IL-1β also markedly declined.
Several studies have demonstrated that melatonin increases the
production of TNF-α (20). The
discrepancy may be as a result of the dose of melatonin or the
experimental model used.
Apoptosis is the process of programmed cell death
distinct from necrosis (21,22).
Apoptotic myocytes are mainly localized adjacent to areas of
inflammatory infiltration (23,24),
so the coercion of apoptosis protects the cardiac myocytes. The
results indicated that melatonin was able to reduce the number of
apoptotic myocytes and the upregulation of Bcl-2. The combination
of melatonin and CsA had an additive effect, which provided an
effective approach to alleviate the proinflammatory state
associated with acute graft rejection.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grant no 81270326).
References
|
1
|
Gill JS: Cardiovascular disease in
transplant recipients: current and future treatment strategies.
Clin J Am Soc Nephrol. 3(Suppl 2): S29–S37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruschi G, Colombo T, Oliva F, et al:
Heart transplantation: 25 years’ single-centre experience. J
Cardiovasc Med (Hagerstown). 14:637–647. 2013.
|
|
3
|
Fiorelli AI, Branco JN, Dinkhuysen JJ, et
al: Risk factor analysis of late survival after heart
transplantation according to donor profile: a multi-institutional
retrospective study of 512 transplants. Transplant Proc.
44:2469–2472. 2012. View Article : Google Scholar
|
|
4
|
Suzuki J, Ogawa M, Hirata Y, Nagai R and
Isobe M: Effects of immunoglobulin to prevent coronary allograft
vasculopathy in heart transplantation. Expert Opin Ther Targets.
16:783–789. 2012. View Article : Google Scholar
|
|
5
|
Tedoriya T, MacDonald PS, Keogh AM, Wilson
M and Spratt PM: Reversal of chronic cyclosporin nephrotoxicity
after heart transplantation. J Heart Lung Transplant. 20:247–248.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aumente MD, Arizón JM, Segura J, et al:
Relationship between pharmacokinetic parameters of cyclosporin and
the incidence of acute rejection after heart transplantation.
Transplant Proc. 37:4014–4017. 2005. View Article : Google Scholar
|
|
7
|
Baan CC, Vaessen LM, Balk AH, et al:
Cyclosporin A sensitivity of allo-specific precursor and committed
cytotoxic T lymphocytes after clinical heart transplantation.
Transplant Proc. 26:2849–2851. 1994.PubMed/NCBI
|
|
8
|
Werkö L: Cyclosporin as a cause of
hypertension in patients with heart transplantation.
Lakartidningen. 88:5031991.(In Swedish).
|
|
9
|
Huang XP, Sun Z, Miyagi Y, et al:
Differentiation of allogeneic mesenchymal stem cells induces
immunogenicity and limits their long-term benefits for myocardial
repair. Circulation. 122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Espino J, Pariente JA and Rodríguez AB:
Oxidative stress and immunosenescence: therapeutic effects of
melatonin. Oxid Med Cell Longev. 2012:6702942012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fildes JE, Yonan N and Keevil BG:
Melatonin - a pleiotropic molecule involved in pathophysiological
processes following organ transplantation. Immunology. 127:443–449.
2009. View Article : Google Scholar
|
|
12
|
Gilad E, Wong HR, Zingarelli B, et al:
Melatonin inhibits expression of the inducible isoform of nitric
oxide synthase in murine macrophages: role of inhibition of
NFkappaB activation. Faseb J. 12:685–693. 1998.PubMed/NCBI
|
|
13
|
Jung FJ, Yang L, Härter L, et al:
Melatonin in vivo prolongs cardiac allograft survival in rats. J
Pineal Res. 37:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cardell M, Jung FJ, Zhai W, et al: Acute
allograft rejection and immunosuppression: influence on endogenous
melatonin secretion. J Pineal Res. 44:261–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eşrefoğlu M1, Kuruş M and Sahna E: The
beneficial effect of melatonin on chronic cyclosporin A
nephrotoxicity in rats. J Int Med Res. 31:42–44. 2003.PubMed/NCBI
|
|
16
|
Rezzani R, Buffoli B, Rodella L,
Stacchiotti A and Bianchi R: Protective role of melatonin in
cyclosporine A-induced oxidative stress in rat liver. Int
Immunopharmacol. 5:1397–1405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo YM and Jeung EB: Melatonin suppresses
cyclosporine A-induced autophagy in rat pituitary GH3 cells. J
Pineal Res. 48:204–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jessup M, Banner N, Brozena S, et al:
Optimal pharmacologic and non-pharmacologic management of cardiac
transplant candidates: approaches to be considered prior to
transplant evaluation: International Society for Heart and Lung
Transplantation guidelines for the care of cardiac transplant
candidates - 2006. J Heart Lung Transplant. 25:1003–1023. 2006.
|
|
19
|
Carrillo-Vico A, Lardone PJ, Naji L, et
al: Beneficial pleiotropic actions of melatonin in an experimental
model of septic shock in mice: regulation of pro-/anti-inflammatory
cytokine network, protection against oxidative damage and
anti-apoptotic effects. J Pineal Res. 39:400–408. 2005. View Article : Google Scholar
|
|
20
|
Santello FH, Frare EO, Caetano LC,
AlonsoToldo MP and do Prado JC Jr: Melatonin enhances
pro-inflammatory cytokine levels and protects against Chagas
disease. J Pineal Res. 45:79–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang EH, Libby P, Vanhoutte PM and Xu A:
Anti-inflammation therapy by activation of prostaglandin EP4
receptor in cardiovascular and other inflammatory diseases. J
Cardiovasc Pharmacol. 59:116–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolters SL, Corsten MF, Reutelingsperger
CP, Narula J and Hofstra L: Cardiovascular molecular imaging of
apoptosis. Eur J Nucl Med Mol Imaging. 34(Suppl 1): S86–S98. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szabolcs M, Michler RE, Yang X, et al:
Apoptosis of cardiac myocytes during cardiac allograft rejection.
Relation to induction of nitric oxide synthase. Circulation.
94:1665–1673. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gedik HS, Korkmaz K, Erdem H, Karakilic E,
Lafci G and Ankarali H: Protective effect of heparin in the end
organ ischemia/reperfusion injury of the lungs and heart. J
Cardiothorac Surg. 7:1232012. View Article : Google Scholar : PubMed/NCBI
|