Introduction
Catechins are important components of green tea
polyphenols with numerous favorable biological functions, including
anti-inflammatory, anti-oxidative, anti-atherosclerotic,
anticarcinogenic and anti-arthritic effects in humans (1–3).
Green tea mainly contains four catechins, epicatechin,
epigallocatechin, epicatechin gallate and (−)-epigallocatechin
gallate (EGCG), and among them EGCG is the most abundant (4).
Platelets are important in primary hemostasis and
the repair of vascular injury. Platelet adhesion and platelet
aggregation are important steps in thrombus formation. Platelets
initially adhere to the vessel wall at the sites of endothelial
cell activation and develop into chronic atherosclerosis via
adhesive receptors, including glycoprotein Ib/IX/V receptors, which
mediate rolling and tethering of the platelets to von Willebrand
factor at the sites of vascular injury and induce glycoprotein
IIb/IIIa activation and the release of adenosine diphosphate (ADP),
resulting in platelet aggregation (5–7). In
addition, platelets engage collagen in the vessel wall through
their adhesion receptors glycoprotein Ia/IIa (8). The interaction of von Willebrand
factor and glycoprotein Ib/IX/V is known to be induced by
ristocetin, an activator of glycoprotein Ib/IX/V (9). It has been reported that
ristocetin-induced glycoprotein Ib/IX/V activation leads to the
generation of thromboxane A2 (TXA2) in platelets (7). ADP enhances platelet activation by
engaging specific GTP-binding protein coupled receptors, P2Y1/P2Y12
receptors, and activates glycoprotein IIb/IIIa and the
cyclooxygenase (COX)-1 pathway, resulting in the stimulation of
platelet aggregation (10,11). Then activated platelets release
inflammatory agents, including soluble CD40 (sCD40) ligand and
secrete mitogenic mediators, including platelet-derived growth
factor (PDGF)-AB from granules into the local microenvironment.
Previous studies (12,13) demonstrated that ADP induces heat
shock protein 27 (HSP27) phosphorylation via p38 mitogen-activated
protein (MAP) kinase and p44/p42 MAP kinase in human platelets, and
is also associated with the secretion of PDGF-AB and the sCD40
ligand. In addition, it has been demonstrated that ristocetin
stimulates the release of the sCD40 ligand from human platelets
through TXA2-mediated activation of the TXA2 receptor and that
release of the sCD40 ligand via TXA2 generation from platelets in
atherosclerotic patients is elevated (14). Regarding the effect of EGCG on
human platelets, it has been reported that EGCG exhibits a potent
antithrombotic effect by the inhibition of platelet aggregation
(15). However, its precise
mechanism in human platelets has not yet been elucidated.
The present study examined the effects of EGCG on
human platelet activation by various stimulators, including ADP,
collagen, ristocetin and the TXA2 receptor agonist, and the exact
mechanisms underlying the effect of EGCG. The present study also
demonstrated that EGCG selectively inhibits ADP-stimulated human
platelet activation and that EGCG reduces PDGF-AB secretion and
sCD40 ligand release due to the suppression of HSP27
phosphorylation via p38 MAP kinase.
Materials and methods
Materials
EGCG, ADP, U46619 and ristocetin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Collagen was purchased from
Nycomed Pharma GmbH (Munich, Germany). PDGF-AB enzyme-linked
immunosorbent assay (ELISA) kits and sCD40 ligand ELISA kits were
purchased from R&D Systems (Minneapolis, MN, USA).
Phospho-specific anti-p38 MAP kinase antibodies, p38 MAP kinase
antibodies and phospho-HSP27 (Ser-78) antibodies were obtained from
Cell Signaling Technology, Inc. (Beverly, MA, USA). Anti-HSP27
antibodies and GAPDH antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The other materials and
chemicals were obtained from commercial sources.
Preparation of platelets
Human blood was donated from healthy volunteers into
1/10 volume of 3.8% sodium citrate. Platelet-rich plasma (PRP) was
obtained from blood samples by centrifugation at 155 × g for 12 min
at room temperature. Platelet-poor plasma (PPP) was prepared from
the residual blood by centrifugation at 2,500 × g for 5 min. All
participants signed an informed consent agreement following
receiving a detailed explanation and the study was approved by the
Committee of Ethics in Gifu University Graduate School of Medicine
(Gifu, Japan).
Platelet aggregation
Platelet aggregation using citrated PRP was followed
in a PA-200 aggregometer (Kowa Co. Ltd., Tokyo, Japan), which is
able to determine the size of platelet aggregates based upon
particle counting using laser scattering methods (small size, 9–25
μm; medium size, 25–50 μm; large size, 50–70 μm) (16), at 37°C with a stirring speed of 800
rpm. The platelets were pre-incubated for 1 min and then platelet
aggregation was monitored for 4 min. The percentage of
transmittance of the isolated platelets was recorded as 0% and that
of the appropriate PPP (blank) was recorded as 100%. When
indicated, PRP was pretreated with EGCG for 15 min.
Protein preparation following
stimulation
Following stimulation with ADP, collagen, ristocetin
or U46619, platelet aggregation was terminated by the addition of
an ice-cold EDTA (10 mM) solution. The mixture was centrifuged at
10,000 × g at 4°C for 2 min. In order to measure PDGF-AB and the
sCD40 ligand as described below, the supernatant was isolated and
stored at −30°C for subsequent ELISA. For the western blot analysis
of p38 MAP kinase and HSP27, the pellet was washed twice with
phosphate-buffered saline and then lysed and immediately boiled in
lysis buffer containing 62.5 mM Tris/Cl, pH 6.8; 2% sodium dodecyl
sulfate (SDS), 50 mM dithiothreitol and 10% glycerol.
Western blot analysis
A western blot analysis was performed as described
previously (17). Briefly,
SDS-PAGE was performed by the method described by Laemmli (18) in a 10% polyacrylamide gel. The
proteins in the gel were transferred onto polyvinylidene fluoride
(PVDF) membranes, which were then inhibited with 5% fat-free dry
milk in Tris-buffered saline with 0.1% Tween-20 (TBST; 20 mM Tris;
pH 7.6; 137 mM NaCl; 0.1% Tween-20) for 2 h prior to incubation
with the indicated primary antibodies. The primary antibodies used
in the present study were anti-phospho-specific p38 MAP kinase, p38
MAP kinase, phospho-HSP27 (Ser-78), HSP27 or GAPDH antibodies.
Peroxidase-labeled anti-mouse IgG (Santa Cruz Biotechnology, Inc.)
or anti-rabbit IgG antibodies (KPL, Gaithersburg, MD, USA) were
used as secondary antibodies. The primary and secondary antibodies
were diluted for optimum concentration, respectively, with 5%
fat-free dry milk in TBST. The peroxidase activity on the PVDF
membranes was visualized on X-ray film by means of an enhanced
chemiluminescent western blotting detection system (GE Healthcare,
Little Chalfont, UK) according to the manufacturer’s
instructions.
Measurement of PDGF-AB and the sCD40
ligand
The PDGF-AB and sCD40 ligand levels in the samples
were determined using each ELISA kit according to the
manufacturer’s instructions.
Statistical analysis
All figures are shown from representative results of
five independent experiments. The data are presented as the mean ±
standard error of the mean. The data were analyzed by Student’s
t-test and values of P<0.05 were considered to indicate a
statistically significant difference.
Results
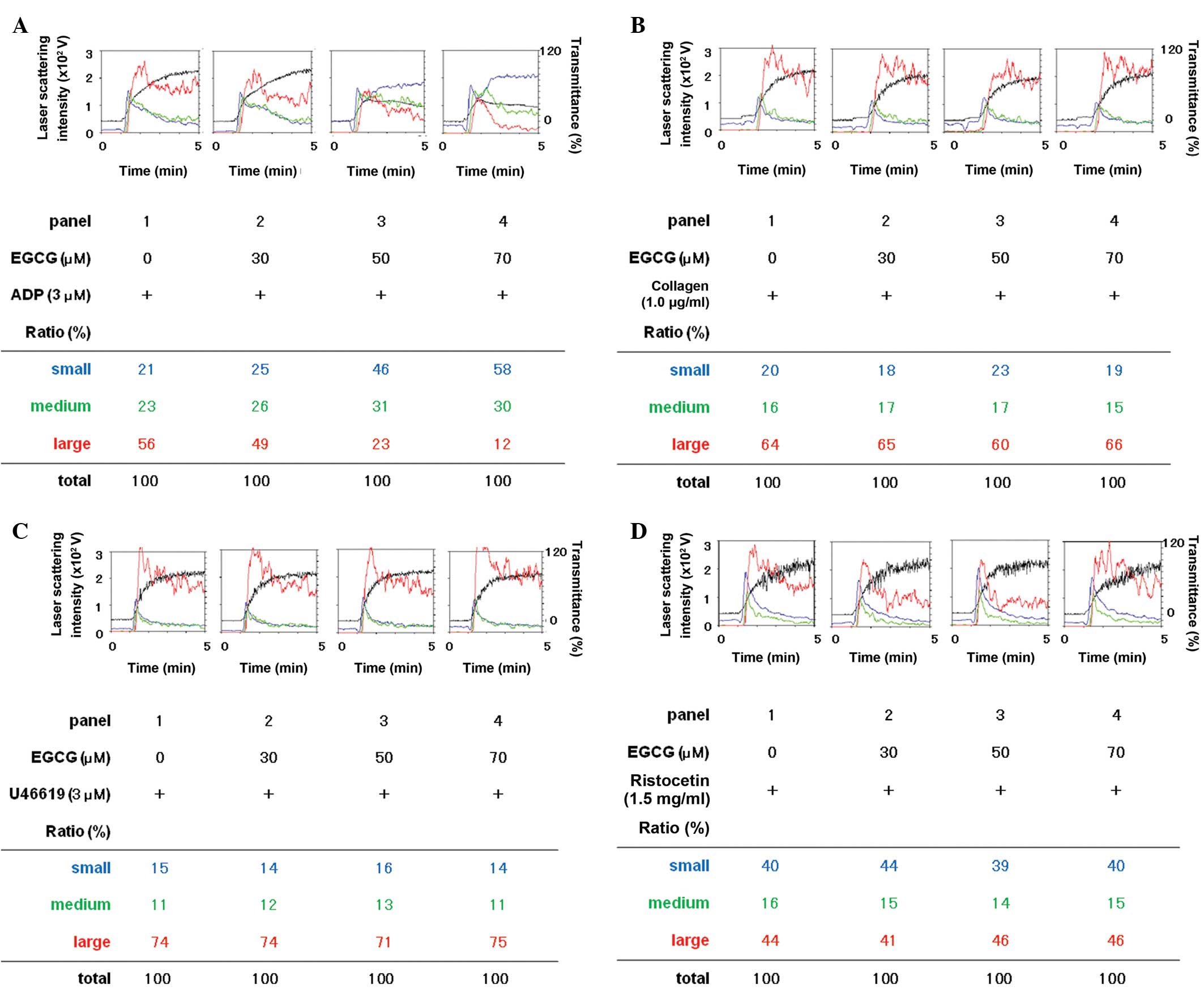
Effects of EGCG on platelet aggregation
induced by ADP, collagen, U46619 or ristocetin
The effects of EGCG on platelet aggregation
stimulated by ADP, collagen, U46619 (a TXA2 receptor agonist) or
ristocetin (an activator of glycoprotein Ib/IX/V) were examined
using a laser scattering system. ADP-stimulated platelet
aggregation in percentage of transmittance was markedly reduced by
EGCG in a dose-dependent manner in the range between 30 and 70 μM
(Fig. 1). EGCG dose dependently
decreased the formation of large aggregates (50–70 μm) according to
the analysis of the size of platelet aggregates whereas small
aggregates (9–25 μm) and medium aggregates (25–50 μm) were markedly
increased by EGCG (Fig. 1A).
By contrast, EGCG failed to affect the platelet
aggregation stimulated by collagen, U46619 or ristocetin (Fig. 1B–D). In addition, EGCG had little
effect on the ratio of the platelet aggregate size induced by
collagen, U46619 or ristocetin (Fig.
1B–D).
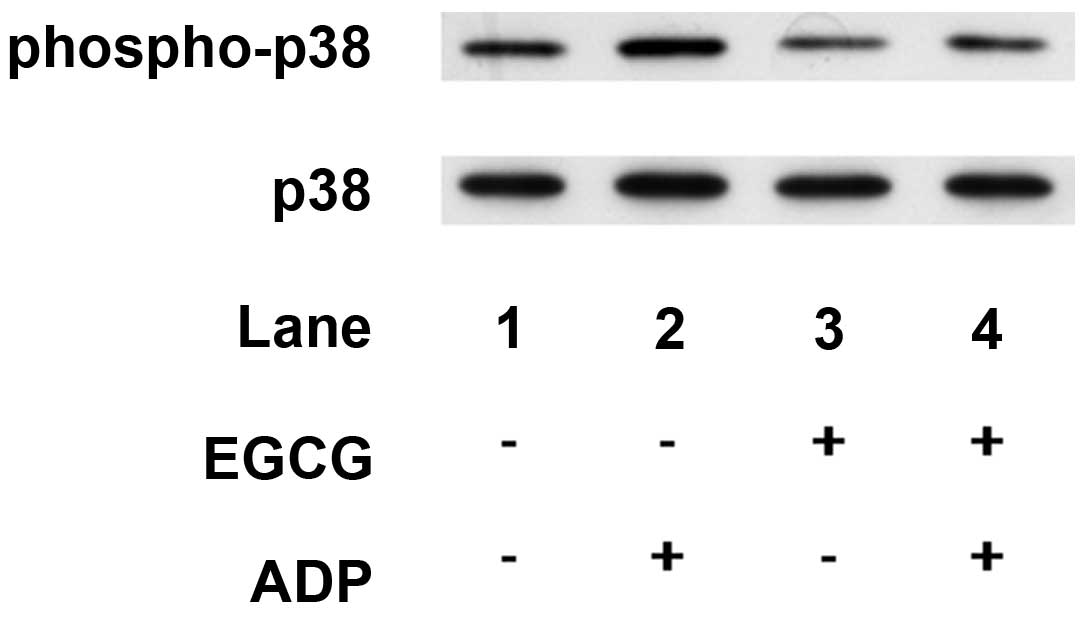
Effects of EGCG on the ADP-induced
phosphorylation of p38 MAP kinase or HSP27 in human platelets
Previously, it has been demonstrated that ADP
induces HSP27 phosphorylation via p38 MAP kinase activation in
human platelets (12). Therefore,
in order to examine how EGCG affects ADP-induced platelet
aggregation, the effect of EGCG on the ADP-induced phosphorylation
of p38 MAP kinase and HSP27 was examined. EGCG, which alone had
little effect on p38 MAP kinase phosphorylation, markedly
attenuated the ADP-induced phosphorylation of p38 MAP kinase
(Fig. 2).
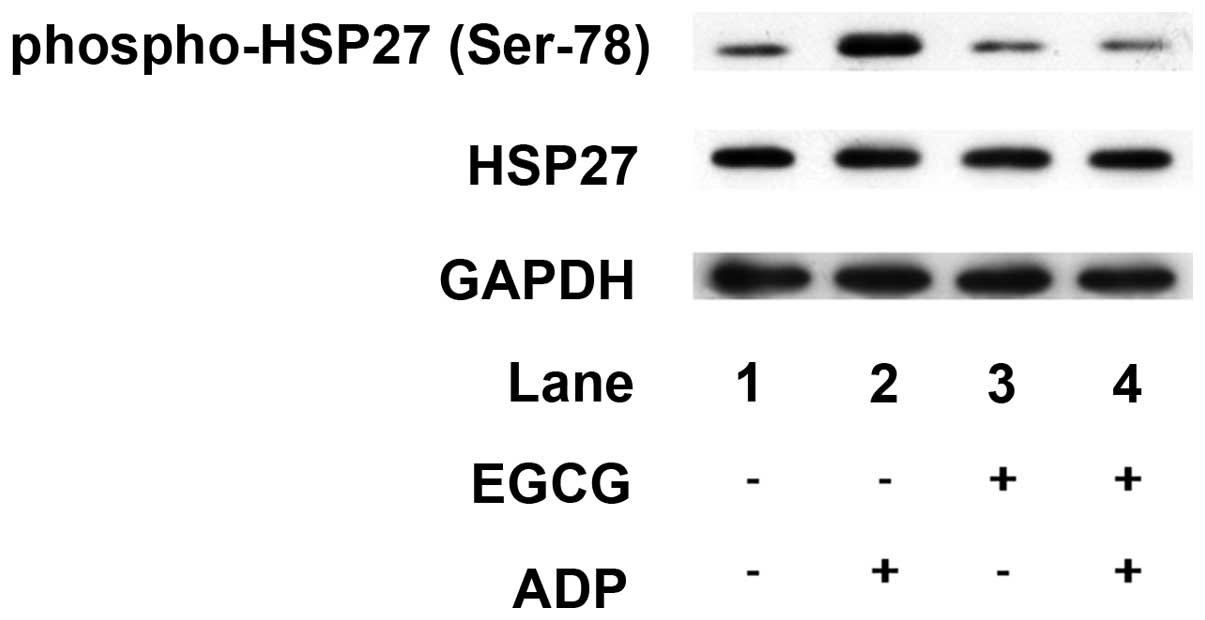
In addition, EGCG, which alone did not affect HSP27
phosphorylation, markedly suppressed the ADP-induced
phosphorylation of HSP27 (Ser-78; Fig.
3).
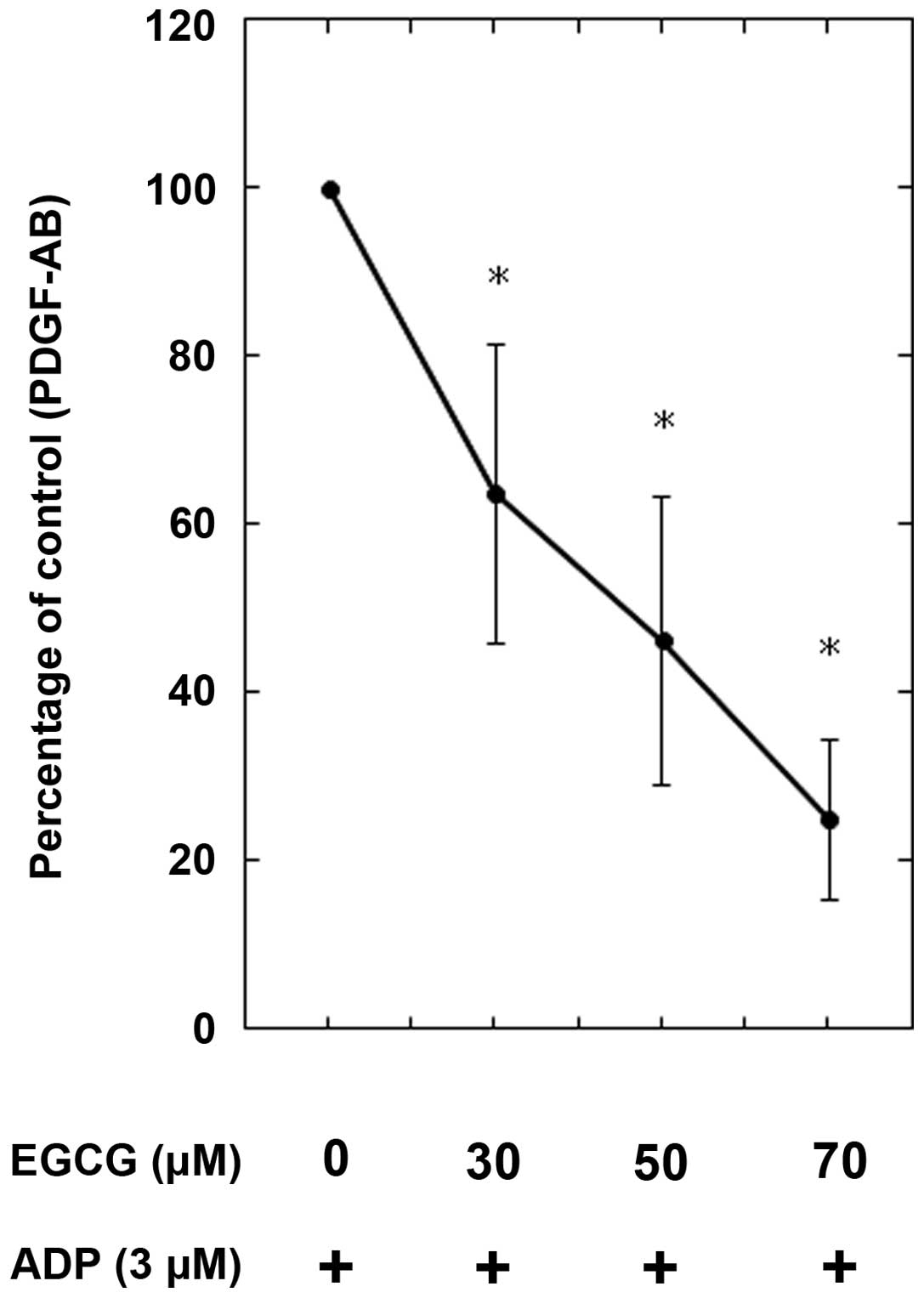
Effects of EGCG on ADP-induced PDGF-AB
secretion or sCD40 ligand release from human platelets
In our previous studies (12,13),
ADP stimulated PDGF-AB secretion and sCD40 ligand release through
HSP27 phosphorylation via p38 MAP kinase activation in human
platelets. Thus, the effect of EGCG on the ADP-stimulated PDGF-AB
secretion or sCD40 ligand release was examined. EGCG significantly
reduced ADP-induced PDGF-AB secretion in a dose-dependent manner
between 30 and 70 μM (Fig. 4).
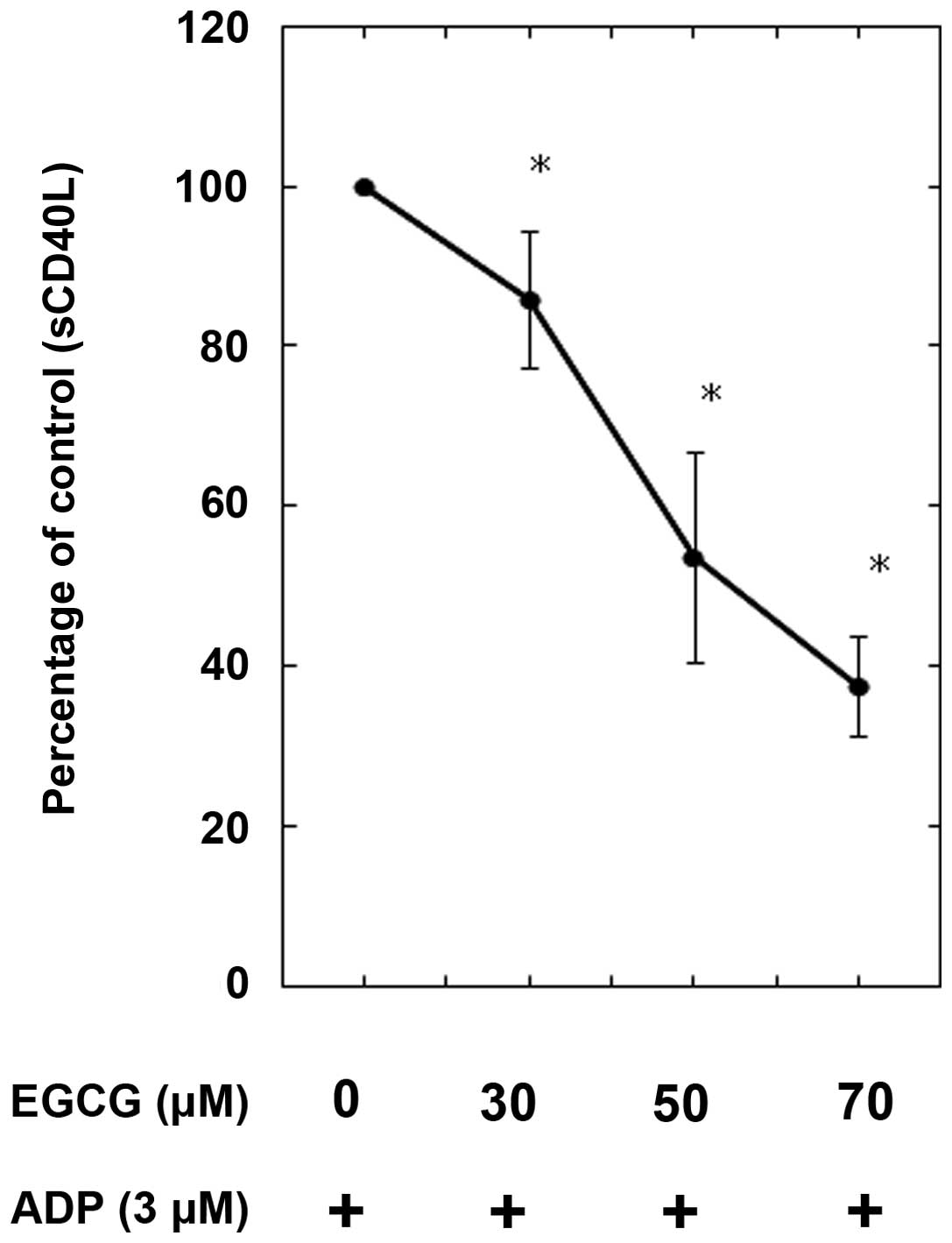
Additionally, the release of the sCD40 ligand stimulated by ADP was
dose-dependently suppressed by EGCG (Fig. 5).
Discussion
The present study demonstrated that EGCG, a
predominant polyphenolic compound in green tea (4), markedly suppressed the human platelet
aggregation induced by ADP, however, not by collagen, ristocetin or
U46619 (a TXA2 receptor agonist). It is generally recognized that
platelets initially interact with subendothelium. Under high shear
stress conditions, von Willebrand factor binds to the platelet
membrane glycoprotein Ib/IX/V, which mediates initial tethering of
platelets and initiates signals leading to platelet adhesion. This
interaction is able to be induced by ristocetin, an activator of
glycoprotein Ib/IX/V (9). It has
been demonstrated that glycoprotein Ib/IX/V activation first
generates TXA2 by COX-1, leading to ADP secretion (19). In addition, at the injured vascular
sites, collagen in the vessel wall induces platelet activation
through their adhesion receptor glycoprotein Ia/IIa, a major
collagen receptor (8). TXA2
potently activates glycoprotein IIb/IIIa through signal
transduction from the TXA2 receptor, TP (20). Subsequently, platelet aggregation
develops by the induction of glycoprotein IIb/IIIa activation,
resulting in thrombus formation. Platelet aggregation is important
in the development of thrombus formation. Based on our findings
that demonstrated that EGCG selectively suppressed ADP-induced
platelet aggregation, it appears unlikely that EGCG inhibits human
platelet adhesion, the first step of platelet activation. ADP is
recognized to be a weak stimulator in comparison with other
platelet activating agonists, including collagen (10). However, ADP is a necessary cofactor
for the normal activation of human platelets by other stimulators.
Low concentrations of ADP enhance or potentiate the effects of
agonists for platelet activation (10). Therefore, it is most likely that
EGCG suppresses human platelet aggregation, which is amplified by
ADP, the second step of platelet activation.
Thrombus formation is associated with the release of
granule contents, including PDGF-AB and serotonin, and the release
of inflammatory substances, including sCD40 ligand from platelets.
It has previously been reported that ADP stimulates the
phosphorylation of HSP27 via p38 MAP kinase activation in human
platelets and that the ADP-induced HSP27 phosphorylation via p38
MAP kinase correlates with PDGF-AB secretion and sCD40 ligand
release from human platelets (12,13).
Therefore, the present study examined the effect of EGCG on the
phosphorylation of p38 MAP kinase and HSP27 induced by ADP in human
platelets. It was demonstrated that EGCG markedly attenuated the
ADP-induced phosphorylation levels of p38 MAP kinase and HSP27.
Based on these findings, it is possible that the suppression of
ADP-stimulated platelet aggregation by EGCG is at least in part
mediated by the attenuation of HSP27 phosphorylation through the
p38 MAP kinase signaling pathway in human platelets. It has
previously been reported that EGCG inhibits phospholipase C
activity in human platelets (15);
however, the exact mechanism remains unclear. Further investigation
is required to clarify the details regarding the effects of EGCG on
human platelets.
It is firmly established that the materials stored
in the specific granules, including α-granules are secreted from
activated platelets. Large adhesive and healing proteins, including
PDGF-AB, are stored in α-granules (21). PDGF-AB released from platelet
α-granules is a potent mitogenic growth factor, which mainly acts
on connective tissue, including vascular smooth muscle cells and
promotes arteriosclerosis (22).
In addition, activated platelets release inflammatory mediators of
atherosclerosis, including the sCD40 ligand. The CD40 ligand is
stored in the cytoplasm of unstimulated platelets and rapidly
translocated on the surface following platelet activation by
agonists, including collagen (23,24).
The CD40 ligand expressed on the activated platelet surface
undergoes a cleavage that generates a functional soluble fragment
termed the sCD40 ligand. It is recognized that the sCD40 ligand
released from platelets induces inflammatory responses via CD40,
which is expressed on vascular endothelial cells and neutrophils
(25). It has been demonstrated
that the elevation of plasma sCD40 ligand is associated with an
increased risk of cardiovascular events in patients with unstable
coronary artery disease (26). The
present study demonstrated that EGCG significantly inhibited the
ADP-induced secretion of PDGF-AB and release of the sCD40 ligand
from human platelets. Taking these findings into account, it is
most likely that EGCG is important as an agent of
anti-atherosclerosis and anti-inflammation through diminishing the
levels of PDGF-AB and the release of the sCD40 ligand. The present
study was able to provide a possible mechanism of the
anti-inflammatory and anti-atherogenic effects of EGCG, the most
abundant catechin in green tea.
In conclusion, the present findings suggest that
EGCG selectively inhibits ADP-stimulated human platelet activation
and that EGCG reduces the release of PDGF-AB and the sCD40 ligand
by suppressing HSP27 phosphorylation via p38 MAP kinase.
Acknowledgements
The authors would like to thank Yumiko Kurokawa for
her skillful technical assistance. This study was supported in part
by a Grant-in-Aid for Scientific Research (grant nos. 20590565 and
20591825) from the Ministry of Education, Science, Sports and
Culture of Japan and the Research Grant for Longevity Sciences
(grant no. 22-4) from the National Center for Geriatrics and
Gerontology, Japan.
References
|
1
|
Jankun J, Selman SH, Swiercz R and
Skrzypczak-Jankun E: Why drinking green tea could prevent cancer.
Nature. 387:5611997. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang WS, Lim IH, Yuk DY, et al:
Antithrombotic activities of green tea catechins and
(−)-epigallocatechin gallate. Thromb Res. 96:229–237. 1999.
|
|
3
|
Oyama J, Maeda T, Kouzuma K, et al: Green
tea catechins improve human forearm endothelial dysfunction and
have antiatherosclerotic effects in smokers. Circ J. 74:578–588.
2010. View Article : Google Scholar
|
|
4
|
Harborne JB and Williams CA: Advances in
flavonoid research since 1992. Phytochemistry. 55:418–504. 2000.
View Article : Google Scholar
|
|
5
|
Ruggeri ZM: The role of von Willebrand
factor in thrombus formation. Thromb Res. 120:S5–S9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berndt MC, Shen Y, Dopheide SM, Gardiner
EE and Andrews RK: The vascular biology of the glycoprotein Ib-IX-V
complex. Thromb Haemost. 86:178–188. 2001.PubMed/NCBI
|
|
7
|
Garcia A, Quinton TM, Dorsam RT and
Kunapuli SP: Src family kinase-mediated and Erk-mediated
thromboxane A2 generation are essential for VWF/GPIb-induced
fibrinogen receptor activation in human platelets. Blood.
106:3410–3414. 2005. View Article : Google Scholar
|
|
8
|
Jennings LK: Mechanisms of platelet
activation: need for new strategies to protect against
platelet-mediated atherothrombosis. Thromb Haemost. 102:248–257.
2009.
|
|
9
|
Dong JF, Berndt MC, Schade A, McIntire LV,
Andrews RK and López JA: Ristocetin-dependent, but not
botrocetin-dependent, binding of von Willebrand factor to the
platelet glycoprotein Ib-IX-V complex correlates with
shear-dependent interactions. Blood. 97:162–168. 2001. View Article : Google Scholar
|
|
10
|
Hechler B, Léon C, Vial C, Vigne P, Frelin
C, Cazenave JP and Gachet C: The P2Y1 receptor is necessary for
adenosine 5′-diphosphate-induced platelet aggregation. Blood.
92:152–159. 1998.
|
|
11
|
Daniel JL, Dangelmaier C, et al: Role of
intracellular signaling events in ADP-induced platelet aggregation.
Thromb Haemost. 82:1322–1326. 1999.PubMed/NCBI
|
|
12
|
Kato H, Takai S, Matsushima-Nishiwaki R,
Adachi S, Minamitani C, Otsuka T, Tokuda H, Akamatsu S, Doi T,
Ogura S and Kozawa O: HSP27 phosphorylation is correlated with
ADP-induced platelet granule secretion. Arch Biochem Biophys.
475:80–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doi T, Adachi S, Matsushima-Nishiwaki R,
Kato H, Enomoto Y, Minamitani C, Otsuka T, Tokuda H, Akamatsu S,
Iwama T, Kozawa O and Ogura S: Antithrombin III suppresses
ADP-induced platelet granule secretion: inhibition of HSP27
phosphorylation. Arch Biochem Biophys. 489:62–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Enomoto Y, Adachi S, Matsushima-Nishiwaki
R, Doi T, Niwa M, Akamastu S, Tokuda H, Ogura S, Yoshimura S, Iwama
T and Kozawa O: Thromboxane A(2) promotes soluble CD40 ligand
release from human platelets. Atherosclerosis. 209:415–421. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin YR, Im JH, Park ES, Cho MR, Han XH,
Lee JJ, Lim Y, Kim TJ and Yun YP: Antiplatelet activity of
epigallocatechin gallate is mediated by the inhibition of PLCgamma2
phosphorylation, elevation of PGD2 production, and maintaining
calcium-ATPase activity. J Cardiovasc Pharmacol. 51:45–54. 2008.
View Article : Google Scholar
|
|
16
|
Fabre JE, Nguyen M, Latour A, Keifer JA,
Audoly LP, Coffman TM and Koller BH: Decreased platelet
aggregation, increased bleeding time and resistance to
thromboembolism in P2Y1-deficient mice. Nat Med. 5:1199–1202. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and αB-crystallin by cyclic AMP in C6 rat glioma cells. J
Neurochem. 66:946–950. 1996.
|
|
18
|
Laemmli UK: Cleavage of structural
proteins during assembly of the head of bacteriophage T4. Nature.
227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Pestina TI, Berndt MC, Steward SA,
Jackson CW and Gartner TK: The roles of ADP and TXA2 in
botrocetin/VWF-induced aggregation of washed platelets. J Thromb
Haemost. 2:2213–2222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakahata N: Thromboxane A2:
physiology/pathophysiology, cellular signal transduction and
pharmacology. Pharmacol Ther. 118:18–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rendu F and Brohard-Bohn B: The platelet
release reaction: granules’ constituents, secretion and functions.
Platelets. 12:261–273. 2001.
|
|
22
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI
|
|
23
|
Hermann A, Rauch BH, Braun M, Schrör K and
Weber AA: Platelet CD40 ligand (CD40L) - subcellular localization,
regulation of expression, and inhibition by clopidogrel. Platelets.
12:74–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
André P, Nannizzi-Alaimo L, Prasad SK and
Phillips DR: Platelet-derived CD40L: the switch-hitting player of
cardiovascular disease. Circulation. 106:896–899. 2002.PubMed/NCBI
|
|
25
|
Henn V, Slupsky JR, Gräfe M,
Anagnostopoulos I, Förster R, Müller-Berghaus G and Kroczek RA:
CD40 ligand on activated platelets triggers an inflammatory
reaction of endothelial cells. Nature. 391:591–594. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heeschen C, Dimmeler S, Hamm CW, van den
Brand MJ, Boersma E, Zeiher AM and Simoons ML; CAPTURE Study
Investigators. Soluble CD40 ligand in acute coronary syndromes. N
Engl J Med. 348:1104–1111. 2003. View Article : Google Scholar : PubMed/NCBI
|