Introduction
Epidemiological investigation has shown that
nonalcoholic fatty liver disease (NAFLD) is an important public
health problem. NAFLD develops and progresses to more advanced
forms, including steatohepatitis, fibrosis and cirrhosis (1,2).
Surplus lipid, particularly triglyceride deposition, is a
prerequisite for the development of NAFLD. Furthermore, a
persistent increase in triglyceride synthesis is an important
factor in the accumulation of fatty acids in the liver.
Mammalian acetyl-CoA carboxylase 1 (ACC1) catalyzes
the carboxylation of acetyl-CoA to form malonyl-CoA, an
intermediate in the de novo synthesis of fatty acids
(3). Chang et al (4) found that the activity of ACC1 was
markedly increased in obese mice. It has been proposed that the
phosphorylation and/or dephosphorylation of target serine residues
is important for the short-term regulation of ACC1 (5–7).
Moreover, AMP-activated protein kinase (AMPK) phosphorylates ACC1
on serine 79, which further inhibits its activity, while
dephosphorylation of ACC1 on serine 79 increases its activity
(8,9). Various studies have reported that
protein phosphatases may dephosphorylate ACC1 (8–10);
however, only a few have shown that ACC1 may be dephosphorylated by
protein phosphatase-2A (PP2A) (8).
It is not clear whether other protein phosphatases are involved in
ACC1 dephosphorylation.
The serine/threonine phosphatase phosphatase 4 (PP4)
is an important member of the PP2A family. PP4 is highly conserved
from invertebrates to vertebrates (11). PP4 dephosphorylates a series of
downstream substrates through its phosphatase activity and
participates in various signaling pathways, including the nuclear
factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway,
apoptosis, hepatic gluconeogenesis and histone modification
(12–15). However, whether PP4 is involved in
lipid metabolism has yet to be elucidated.
Therefore, the present study investigated the
expression of PP4, ACC1 and pACC1-Ser79 in the livers of db/db mice
and mouse primary hepatocytes.
Materials and methods
Animals
Six to eight week-old male db/db mice were purchased
from the National Resource Center for Mutant Mice (Nanjing, China)
which were originally purchased from The Jackson Laboratory (Bar
Harbor, ME, USA). C57BL/6 mice were purchased from Vital River
Laboratory Animal Technology Co., Ltd. China (Beijing, China).
Animals were fed a standard laboratory diet for two weeks in a
temperature- (20–24°C) and humidity-controlled (45–55%) environment
under a 12-h light/dark cycle. All animal protocols were approved
by the Animal Ethics Committee at the Beijing Institute of
Geriatrics (Beijing, China).
Adenovirus vector construction
The pAdxsi-GFP-PP4 adenovirus vector (PP4-Ad) and
the corresponding control adenovirus vector (Control-Ad) were
purchased from the Chinese National Human Genome Center (Beijing,
China).
Cell culture
HepG2 cells (American Type Culture Collection,
Rockville, MD, USA) were cultured in low-glucose Eagle’s minimum
essential medium (Invitrogen Life Technologies, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (Hyclone, Waltham, MA,
USA), 100 U/ml penicillin (Invitrogen Life Technologies) and 0.1
mg/ml streptomycin (Invitrogen Life Technologies). Cells were
maintained at 37°C in humidified air with 5% CO2.
Primary hepatocyte isolation
The isolation of primary hepatocytes was performed
as described previously (16,17).
In brief, the mice were anesthetized using intraperitoneal
injection with 4% chloral hydrate (100 μl/100 g body weight) and
perfused through the vena porta hepatis with D-Hanks solution, then
with collagenase solution at 37°C for 10 min. Livers were carefully
removed and transferred onto a Petri dish, then cut into small
pieces and pressed through a sieve and flushed with warm Dubecco’s
modified Eagle’s medium/F12. Centrifugation was performed at 24 × g
for 5 min at 4°C. The supernatant was discarded and the hepatocyte
cell pellet was retrieved.
Western blot analysis
Cell lysates (10–30 μg protein) were separated using
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore Corporation, Billerica, MA, USA), blocked using 5%
non-fat dry milk for 2 h and probed with primary antibodies at 4°C
overnight. The blots were incubated with horseradish
peroxidase-conjugated anti-immunoglobuin G (Millipore Corporation),
followed by detection using a Fusion SL 3500 enhanced
chemiluminescence system (Vilber Lourmat, Marne-la-Vallée, France).
Rabbit monoclonal anti-ACC1 and anti-pACC1 (Abcam, Cambridge, MA,
USA) and goat polyclonal anti-PP4 (Santa Cruz Biotechnology,
Dallas, TX, USA) antibodies were purchased. Antibodies against
β-actin were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA).
Co-immunoprecipitation
Cells were harvested and lysed 24 h after
transfection [lysate buffer: 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 6
mM ethylene glycol tetraacetic acid, 1% NP-40, 120 mM NaCl, 1 mM
dithiothreitol, 50 μM phenylmethylsulfonyl fluoride and 2 μg/ml
aprotinin]. Endogenous PP4 was immunoprecipitated using the
anti-PP4 antibody and the endogenous pACC1-Ser79 was
immunoprecipitated using an anti-pACC1-Ser79 antibody at 4°C
overnight. The immunoprecipitates were washed three times with
buffer containing 50 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4), 0.1%
Triton X-100 and 500 mM NaCl. The immunoprecipitates were then
separated and analyzed using western blot analysis.
Triglyceride analysis
Intracellular triglyceride concentration was
measured as described previously (18–20).
Cells were washed three times with ice-cold phosphate-buffered
saline and added to an isovolumic mixture of chloroform and
methanol (2:1 v/v). Subsequently, 0.05% H2SO4
was added and the samples were vortexed and centrifuged to split
the phases. The bottom phase was removed to 1% Triton X-100 (1:1
v/v), dried and dissolved in deionized water and analyzed using a
Triglyceride Reagent kit (ShenSuoYouFu Medical Diagnostic Products
Co., Ltd., Shanghai, China).
Statistics
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analysis. All values are
presented as the mean ± standard deviation of the indicated number
of measurements. Differences were analyzed using the Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
PP4 is highly expressed in the livers of
db/db mice
The db/db mice exhibited increased levels of
glucose, total triglyceride and low density lipoprotein-cholesterol
in the serum compared with the control groups (Fig. 1A). Severe lipid accumulation was
observed in the livers of the db/db mice as demonstrated through
the elevated triglyceride levels in the livers (Fig. 1B). The expression of PP4, as well
as molecules of lipid metabolism, including fatty acid synthase
(FAS), sterol regulatory element-binding protein (SREBP) 1 and low
density lipoprotein receptor (LDLR) were analyzed in the livers of
db/db mice and C57BL/6 mice. As shown in Fig. 1C, the levels of PP4, FAS, SREBP1
and LDLR were found to be significantly increased in the livers of
the db/db mice. Thus, PP4 may be involved in the regulation of
triglyceride metabolism in the liver.
PP4 overexpression induces the
accumulation of triglycerides in mouse primary hepatocytes
To determine whether PP4 overexpression induces
triglyceride accumulation in mouse primary hepatocytes,
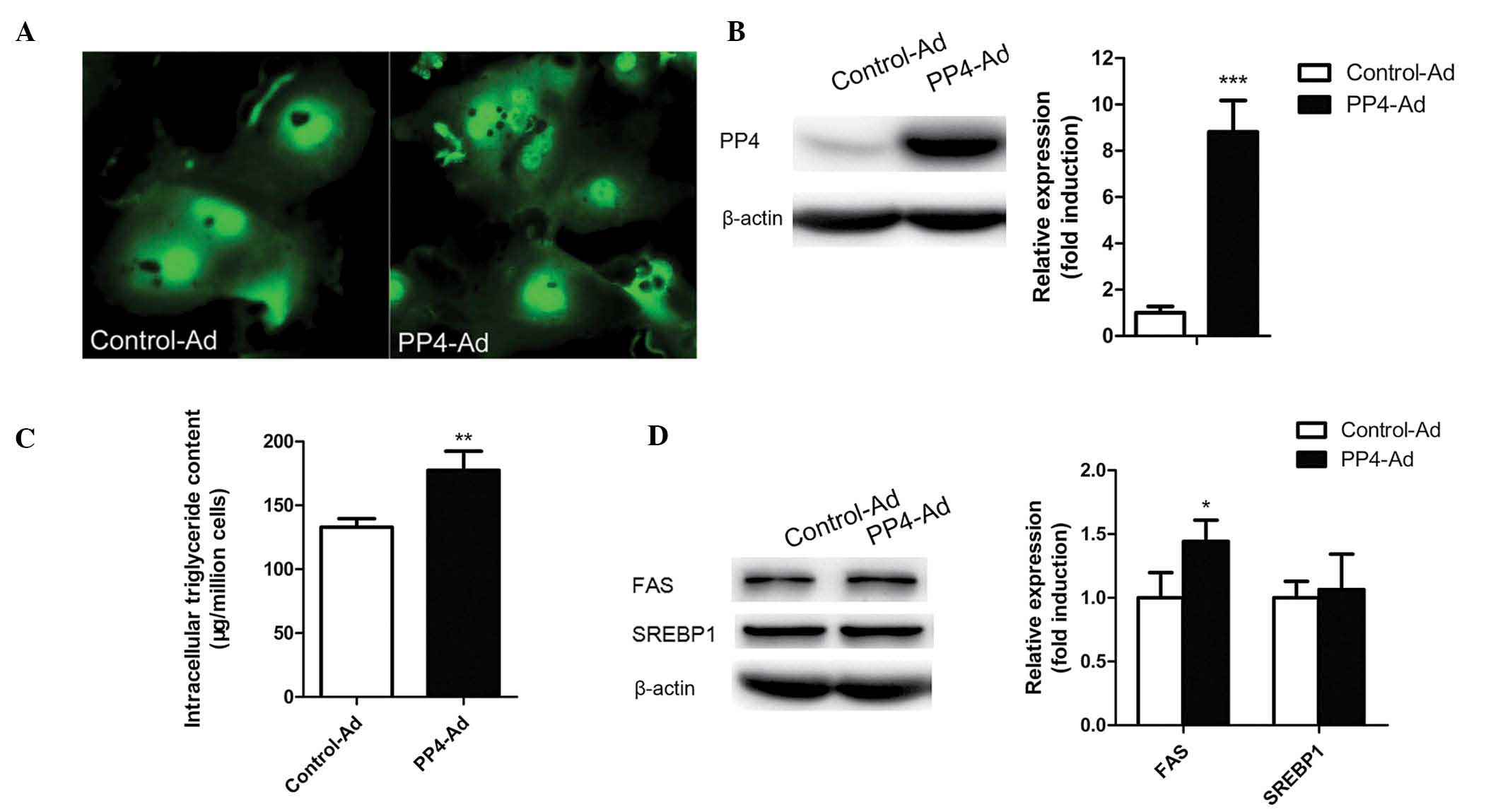
adenovirus-mediated PP4 overexpression was used. As shown in
Fig. 2A and B, PP4 was highly
overexpressed in the mouse primary hepatocytes following treatment
with PP4-Ad compared with treatment with control-Ad. In addition to
an increase in PP4 expression, an increase in triglyceride content
was observed (Fig. 2C), indicating
that PP4 may have an important role in triglyceride
accumulation.
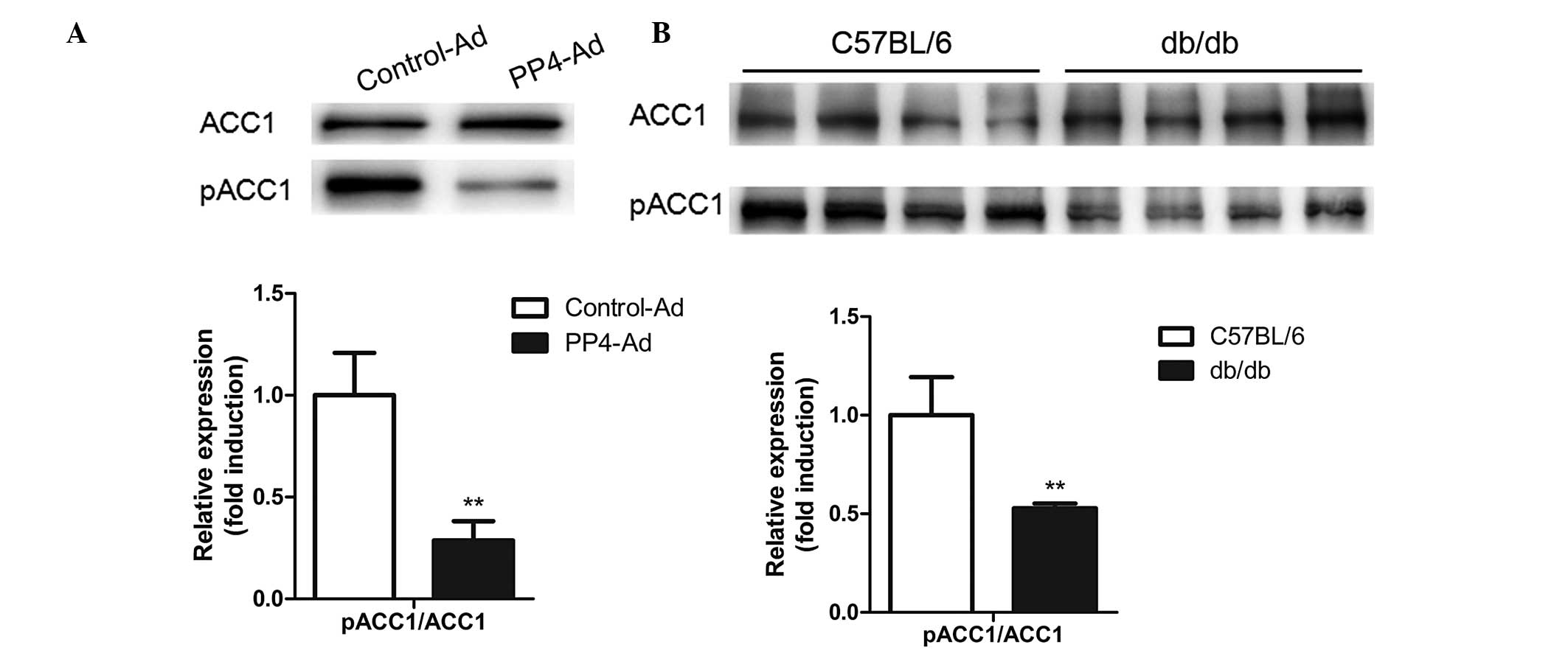
PP4 regulates hepatic triglyceride
content through regulating the dephosphorylation of ACC1
Considering the critical importance of lipogenic
genes in hepatic lipid homeostasis, the levels of FAS, SREBP1, ACC1
and pACC1-Ser79 were analyzed following PP4 overexpression. As
shown in Fig. 3A, PP4
overexpression in the primary hepatocytes led to a decrease in the
levels of pACC1-Ser79/ACC1, while no significant change occurred in
the levels of SREBP1 (Fig. 2D).
Furthermore, reduced levels of pACC1-Ser79/ ACC1 were also detected
in the livers of db/db mice (Fig.
3B), suggesting that PP4 may be involved in triglyceride
metabolism through regulating the dephosphorylation of ACC1.
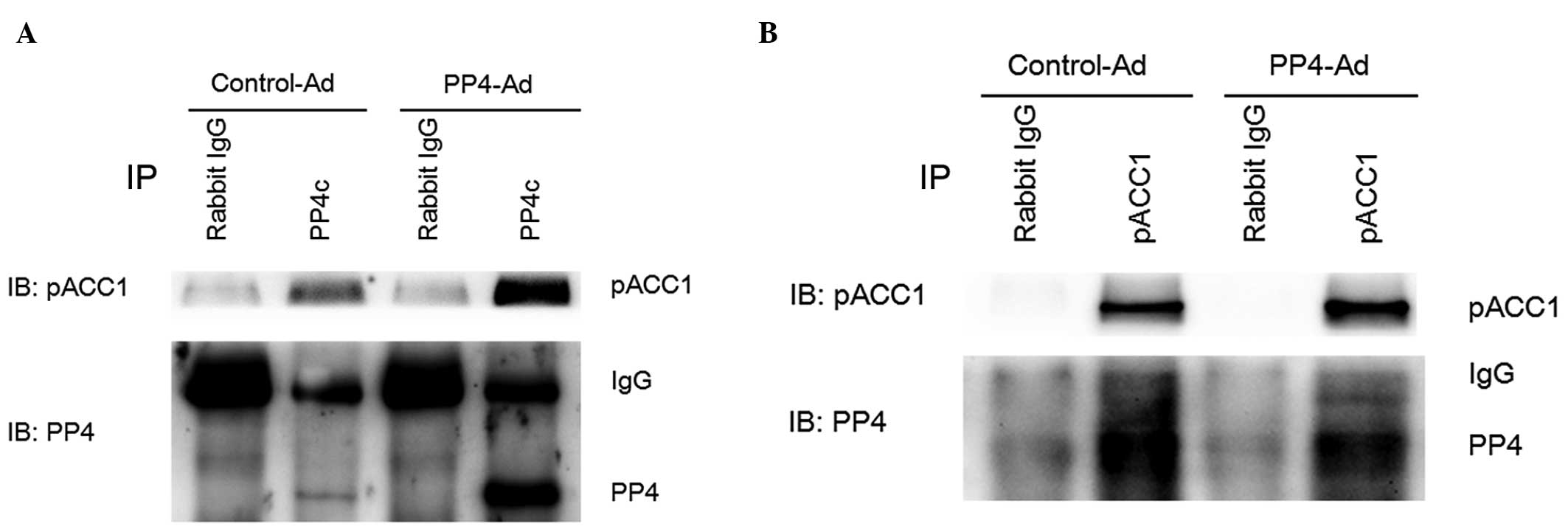
PP4 dephosphorylates ACC1 directly
through interacting with ACC1
The present study investigated whether PP4 was able
to dephosphorylate ACC1 directly. Co-immunoprecipitation revealed
that PP4 was capable of dephosphorylating ACC1 directly through
direct interaction with ACC1 (Fig. 4A
and B).
Discussion
ACC1 is a key enzyme involved in fatty acid
biosynthesis from acetyl CoA to malonyl-CoA, which is an important
step in fatty acid synthesis (21–23).
ACC1 is highly expressed in tissues involved in lipogenesis,
including the liver and adipose tissues (24). The activity of ACC1 is regulated by
Mn2+, insulin, citrate and certain other conditions
(21,25,26).
Previous studies of ACC1 have shown that ACC1 is phosphorylated on
approximately eight serine residues by six different protein
kinases in hepatocytes (9,27). However, the phosphorylation of ACC1
at Ser-79 by AMPK has been identified to be the main site and is
capable of reducing the activity of ACC1 in vitro and in
vivo (27,28).
By contrast, dephosphorylation of ACC1 at Ser-79 has
been found to increase the catalytic activity of ACC1. Evidence has
shown that phosphorylated ACC1 is dephosphorylated by PP2Ac
(8). Moreover, in the present
study, PP4, a member of the PP2A family which only shares 65% amino
acid homology with PP2A, was observed to directly dephosphorylate
ACC1 at Ser-79 through interacting with ACC1, which increased the
activity of ACC1, resulting in the accumulation of intracellular
triglycerides in hepatocytes. However, when the hepatocytes were
treated with a higher dose of PP4-Ad, the concentration of
intracellular triglycerides markedly decreased (data not shown),
suggesting that PP4 may be involved in other mechanisms in lipid
metabolism.
As a ubiquitously expressed serine/threonine protein
phosphatase, PP4 dephosphorylates a wide range of substrates at
serine/threonine residues and is involved in various signaling
pathways (12–14,29).
The findings of the present study widen the range of the biological
effects of PP4. In the present study, PP4 was observed to directly
dephosphorylate the key active site of ACC1, resulting in an
increase in FAS, a target of ACC1, as well as the biosynthesis and
accumulation of intracellular triglycerides. However, no such
effect was observed on SREBP1.
As a classic animal model, db/db mice are widely
used in studies of NAFLD and insulin resistance. In the present
study, PP4 was observed to be highly expressed (2.5-fold) in the
livers of db/db mice and the ratio of pACC1 and ACC1 was found to
be decreased, suggesting that PP4 may dephosphorylate ACC1 and
increase ACC1 activity. This may suggest that PP4 is responsible,
at least in part, for triglyceride accumulation in the livers of
db/db mice. However, pACC1 was also highly expressed in the livers
of db/db mice, indicating other factors may be involved in reducing
the activity of ACC1 in lipid metabolism disorders.
A previous study showed that the majority of lipids
(>90%) are synthesized through de novo lipogenesis in
cancer cells (30), suggesting
that the biosynthesis of fatty acids is required for
carcinogenesis. In the present study, ACC1, an important enzyme for
de novo lipogenesis, was found to be strongly upregulated by
PP4. Thus, PP4 may be a target for cancer therapy in the
future.
In conclusion, the present study investigated the
direct interaction between PP4 and pACC1-Ser79. PP4 was found to
enhance ACC1 activity through dephosphorylation, leading to
intracellular triglyceride accumulation. The results of the present
study may provide insight into novel regulatory mechanisms of the
activity of ACC1 in physiological and/or pathological lipogenesis.
Moreover, the findings of the present study have enhanced the
understanding of the functionality of PP4 in lipid metabolism.
However, the versatile role of PP4 in cellular processes, as well
as its physiological functions and underlying mechanisms, have yet
to be elucidated.
Acknowledgements
The present study was supported by grants from the
National Basic Research Program of China (no. 2012CB517502) and the
National Natural Science Foundation of China (nos. 81270495,
81170381 and 81270887).
References
|
1
|
Collantes RS, Ong JP and Younossi ZM: The
metabolic syndrome and nonalcoholic fatty liver disease. Panminerva
Med. 48:41–48. 2006.PubMed/NCBI
|
|
2
|
Ruhl CE and Everhart JE: Epidemiology of
nonalcoholic fatty liver. Clin Liver Dis. 8:501–519. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abu-Elheiga L, Matzuk MM, Kordari P, et
al: Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically
lethal. Proc Natl Acad Sci USA. 102:12011–12016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang HC, Seidman I, Teebor G and Lane MD:
Liver acetyl CoA carboxylase and fatty acid synthetase: relative
activities in the normal state and in hereditary obesity. Biochem
Biophys Res Commun. 28:682–686. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thampy KG and Wakil SJ: Regulation of
acetyl-coenzyme A carboxylase. I Purification and properties of two
forms of acetyl-coenzyme A carboxylase from rat liver. J Biol Chem.
263:6447–6453. 1988.PubMed/NCBI
|
|
6
|
Mabrouk GM, Helmy IM, Thampy KG and Wakil
SJ: Acute hormonal control of acetyl-CoA carboxylase. The roles of
insulin, glucagon, and epinephrine. J Biol Chem. 265:6330–6338.
1990.PubMed/NCBI
|
|
7
|
Mohamed AH, Huang WY, Huang W,
Venkatachalam KV and Wakil SJ: Isolation and characterization of a
novel acetyl-CoA carboxylase kinase from rat liver. J Biol Chem.
269:6859–6865. 1994.PubMed/NCBI
|
|
8
|
Gaussin V, Hue L, Stalmans W and Bollen M:
Activation of hepatic acetyl-CoA carboxylase by glutamate and
Mg2+ is mediated by protein phosphatase-2A. Biochem J.
316:217–224. 1996.PubMed/NCBI
|
|
9
|
Davies SP, Sim AT and Hardie DG: Location
and function of three sites phosphorylated on rat acetyl-CoA
carboxylase by the AMP-activated protein kinase. Eur J Biochem.
187:183–190. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carling D and Hardie DG: The substrate and
sequence specificity of the AMP-activated protein kinase.
Phosphorylation of glycogen synthase and phosphorylase kinase.
Biochim Biophys Acta. 1012:81–86. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen PT, Philp A and Vázquez-Matin C:
Protein phosphatase 4 - from obscurity to vital fuctions. FEBS
Lett. 579:3278–3286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mourtada-Maarabouni M and Williams GT:
Protein phosphatase 4 regulates apoptosis in leukemic and primary
human T-cells. Leuk Res. 1539–1551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia S, Dai F, Wu D, et al: Protein
phosphatase 4 cooperates with Smads to promote BMP signaling in
dorsoventral patterning of zebrafish embryos. Dev Cell.
22:1065–1078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Ozawa Y and Lee H: Histone
deacetylase 3 (HDAC3) activity is regulated by interaction with
protein serine/threonine phosphatase 4. Genes Dev. 19:827–839.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu MC, Tang-Oxley Q, Qiu WR, et al:
Protein phosphatase X interacts with c-Rel and stimulates
c-Rel/nuclear factor kappaB activity. J Biol Chem. 273:33561–33565.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang R, Kong X, Cui A, et al:
Sterol-regulatory-element-binding protein 1c mediates the effect of
insulin on the expression of Cidea in mouse hepatocytes. Biochem J.
430:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Jia S, Wang C, et al: FAM3A is a
target gene of peroxisome proliferator-activated receptor gamma.
Biochim Biophys Acta. 1830:4160–4170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han CC, Wang JW, Pan ZX, et al: Effect of
liver X receptor activation on the very low density lipoprotein
secretion and messenger ribonucleic acid level of related genes in
goose primary hepatocytes. Poult Sci. 90:402–409. 2011. View Article : Google Scholar
|
|
19
|
Tang W, Ma Y, Jia L, Ioannou YA, Davies JP
and Yu L: Genetic inactivation of NPC1L1 protects against
sitosterolemia in mice lacking ABCG5/ABCG8. J Lipid Res.
50:293–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naranmandura H, Xu S, Koike S, et al: The
endoplasmic reticulum is a target organelle for trivalent
dimethylarsinic acid (DMAIII)-induced cytotoxicity. Toxicol Appl
Pharmacol. 260:241–249. 2012. View Article : Google Scholar
|
|
21
|
Hardie DG: Regulation of fatty acid
synthesis via phosphorylation of acetyl-CoA carboxylase. Prog Lipid
Res. 28:117–146. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KH: Regulation of mammalian
acetyl-coenzyme A carboxylase. Annu Rev Nutr. 17:77–99. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shui JW, Hu MC and Tan TH: Conditional
knockout mice reveal an essential role of protein phosphatase 4 in
thymocyte development and pre-T-cell receptor signaling. Mol Cell
Biol. 27:79–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bianchi A, Evans JL, Iverson AJ, Nordlund
AC, Watts TD and Witters LA: Identification of an isozymic form of
acetyl-CoA carboxylase. J Biol Chem. 265:1502–1509. 1990.PubMed/NCBI
|
|
25
|
Thampy KG and Wakil SJ: Activation of
acetyl-CoA carboxylase. Purification and properties of a
Mn2+-dependent phosphatase. J Biol Chem. 260:6318–6323.
1985.PubMed/NCBI
|
|
26
|
Haystead TA and Hardie DG: Evidence that
activation of acetyl-CoA carboxylase by insulin in adipocytes is
mediated by a low-Mr effector and not by increased phosphorylation.
Biochem J. 240:99–106. 1986.PubMed/NCBI
|
|
27
|
Hardie DG, Carling D and Sim ATR: The
AMP-activated protein kinase: a multisubstrate regulator of lipid
metabolism. Trends Biochem Sci. 14:20–23. 1989. View Article : Google Scholar
|
|
28
|
Ha J, Daniel S, Broyles SS and Kim KH:
Critical phosphorylation sites for acetyl-CoA carboxylase activity.
J Biol Chem. 269:22162–22168. 1994.PubMed/NCBI
|
|
29
|
Yoon YS, Lee MW, Ryu D, et al: Suppressor
of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C)
is a key regulator of hepatic gluconeogenesis. Proc Natl Acad Sci
USA. 107:17704–17709. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.PubMed/NCBI
|