Introduction
Inflammation has an important role in the
progression of numerous pathologies, including cardiovascular
disease, cancer and autoimmune diseases (1–3). The
inhibition of the inflammatory response has become a therapeutic
priority for the treatment of these diseases. Previous studies have
demonstrated that macrophages are important in the pathological
process of inflammation (4–5).
In the inflammatory response, macrophages have three
main functions: Antigen-presentation, phagocytosis and the
production of various cytokines and active substances that regulate
immune functions, including, tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and nitric oxide (NO) (6).
Chitin is a homopolymer of β-1,4-linked
N-acetylglucosamine, and chitosan is the product of partial
deacetylation of chitin. Chitin and chitosan are the second most
abundant polysaccharides occurring in nature, and are the main
components found in the exoskeletons of crabs, shrimp and insects,
as well as fungi (7). It has
previously been reported that both chitin and chitosan have various
biological functions, including anti-inflammatory,
immuno-enhancing, and antitumor effects (8–10).
However, their insoluble and high viscous properties limit their
use in vivo. Therefore, research has focused on low
molecular weight oligochitosan, which is water-soluble, nontoxic,
biocompatible and possesses versatile functional properties.
However, the mechanism of the anti-inflammatory effects of
oligochitosan remains to be elucidated. Macrophages produce TNF-α,
IL-1β and NO, which have important roles in the occurrence and
development of inflammation. Therefore, the aims of the present
study were to investigate the effects of oligochitosan on the
production of inflammatory cytokines and the activation of NF-κB in
lipopolysaccharide-induced RAW264.7 cells in order to illuminate
the initial mechanisms of the anti-inflammatory effects of
oligochitosan.
Materials and methods
Preparation of oligochitosan
A total of 9 g chitosan (degree of N-acetylation
<15%; Sigma-Aldrich, St Louis, MO, USA) was dissolved in 300 ml
HCl (pH 5.0). The mixture was incubated with 1.5 g cellulase in a
reaction vessel at 50°C for 48 h. The solution was then neutralized
to pH 7.0 using 1 mol/l NaOH. Following centrifugation at 8,000 × g
for 10 min, the crude oligochitosan in the supernatant was
precipitated by the addition of ethanol. The precipitate was
dissolved in distilled water and vacuum-dried, prior to collection
of the oligochitosan sample. The components of the oligochitosan
sample were detected using high performance liquid chromatography
(HPLC). The analysis was conducted on a TSK-Gel Amide-80 (TOSOH,
Tokyo, Japan) equipped with a differential detector. The mobile
phase consisted of C2H3N:water in a ratio of
37:63, pH 4.0, 25°C, with a sample volume of 1 μl.
Cell culture
The RAW264.7 murine macrophage-like cells, were
obtained from the Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Science (Shanghai, China). The cells
were cultured in RPMI-1640 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA), supplemented with 10% heat-inactivated fetal
bovine serum (FBS), 100 IU/ml penicillin G and 100 μg/ml
streptomycin, and incubated at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air.
MTT assay
The RAW 264.7 macrophages (1×106
cells/ml) were seeded into 6-well plates in 1 ml RPMI-1640 medium,
containing 10% FBS. Following a 24 h incubation, the cells were
washed and incubated with 1 ml RPMI-1640 medium, containing various
doses of oligochitosan. Following a further 24 h incubation, MTT
reagent (Sangon, Shanghai, China) was added to each well for 4 h
and formazan crystals were solubilized in dimethyl sulfoxide.
Subsequently, the absorbance was measured at 570 nm, using a
microplate reader (Bio-Tek Instruments, Winooski, VT, USA). All of
the experiments were performed in triplicate.
Macrophage stimulation
The RAW264.7 cells (1×106 cells/ml) were
stimulated with 1 μg/ml LPS (Sigma-Aldrich), with or without
different concentrations of oligochitosan (0, 50, 100, or 500
μg/ml), for 24 h at 37°C in the presence of 5% CO2. The
cells were collected for western blot analysis, and the culture
supernatants were used for ELISA and nitrite assays.
Nitrite assay
The concentration of nitrite in the culture medium,
which correlated with the amount of NO secreted by macrophages, was
measured as described by previous methods (11). Briefly, 100 μl aliquots of the
culture supernatants were placed in triplicate in a 96-well ELISA
plate and incubated with an equal volume of Griess reagent [1%
sulfanilamide, 0.1% N-(1-naphthyl)-ethylendiamine dihydrochloride,
2.5% H3PO4] at room temperature for 10 min.
The absorbance was measured at 540 nm, using a microplate reader.
The concentration of nitrite was determined using sodium nitrite as
a standard. All of the experiments were performed in
triplicate.
ELISA
TNF-α and IL-1β assay kits (Diaclone, Besancon,
France) were used according to the manufacturer’s instructions.
Pre-coated ELISA plates were incubated with 50 μl culture
supernatants for 2 h at room temperature. Subsequently 50 μl
aliquots of biotin-conjugated antibody were added and the plates
were incubated at 37°C for 90 min. The plates were washed
thoroughly using the washing solution from the kit and 100 μl
streptavidin-horseradish peroxidase (HRP) was added, followed by a
further 30 min incubation at 37°C. The plates were washed
thoroughly and 100 μl substrate solution (freshly prepared
tetramethylbenzidine with H2O2) was added.
The plates were then incubated at 37°C for 20 min in a dark
chamber, and the optical density was measured at 450 nm.
Recombinant murine TNF-α and IL-1β were diluted and used as
standards. All of the experiments were performed in triplicate.
Western blot analysis
The culture medium was removed from the RAW264.7
macrophages, following which, a total of 1×106 cells
were suspended in 1 ml of ice-cold phosphate-buffered saline (pH
7.2). The cells were then centrifuged at 1,000 × g for 5 min,
resuspended in 400 μl of ice-cold hypotonic buffer(10 mM HEPES-KOH
pH 7.9, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT, 1
μg/ml Leupeptin, 1 mM PMSF), kept on ice for 10 min, vortexed, and
centrifuged at 15,000 × g, for 30 sec at 4°C. The pelleted nuclear
protein was resuspended in 50 μl of ice-cold saline buffer (50 mM
HEPES-KOH, pH 7.9, 10% glycerol, 300 mM NaCl, 1.5 mM KCl, 0.1 mM
EDTA, 1 mM DTT, 1 μl/ml Leupeptin, 1 mM PMSF), kept on ice for 20
min, vortexed, and centrifuged at 15,000 × g for 10 min at 4°C. The
protein concentration was determined by the BCA method (Pierce,
Rockford, IL, USA) and the aliquots were stored at −70°C, until
further use. A total of 30 μg nuclear protein was loaded onto 10%
SDS-polyacrylamide gels, separated by electrophoresis, followed by
transfer to a nitrocellulose membrane (Millipore, Bedford, MA,
USA). The membrane was then blocked with 5% skim milk in
tris-buffered saline containing Tween® (TBST) for 1 h at
room temperature, and incubated with rabbit anti-murine NF-κB p65
antibody (Rockland Immunochemical Inc., Gilbertsville, PA, USA) for
1 h. The membrane was washed three times in TBST, and incubated
with HRP-conjugated goat anti-rabbit immunoglobulin G (Rockland
Immunochemical Inc.) for 1 h. The antibody-specific protein was
visualized using an Enhanced Chemiluminesence Detection system
(Pierce, Rockford, IL, USA). The intensities of the protein bands
were analyzed using the Gel-Pro® Analyzer software
(Media Cybernetics, USA).
Statistical analysis
The results of the present study are expressed as
the means ± standard deviation of the indicated number of
experiments. The statistical significance was estimated using a
t-test for unpaired observations. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Preparation of oligochitosan
The components of the oligochitosan sample were
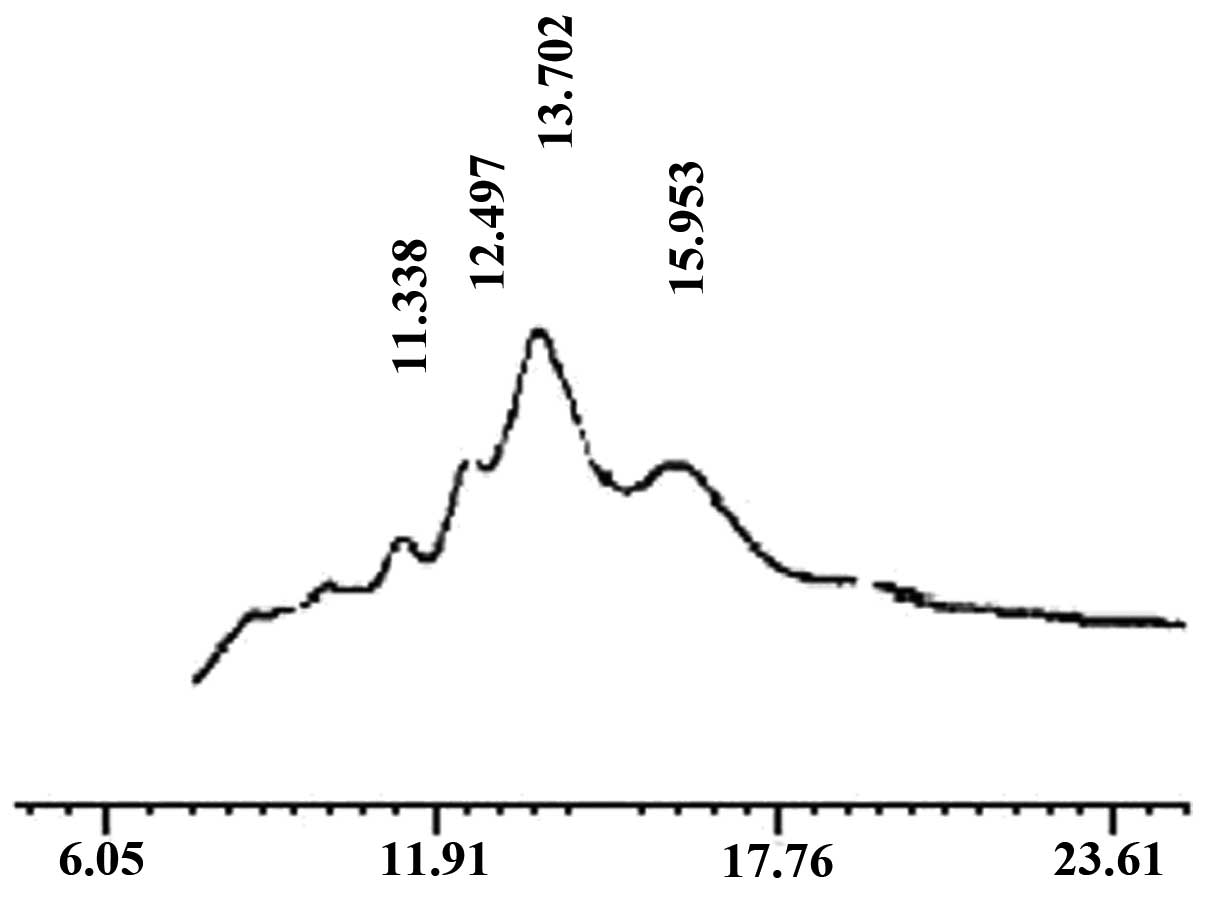
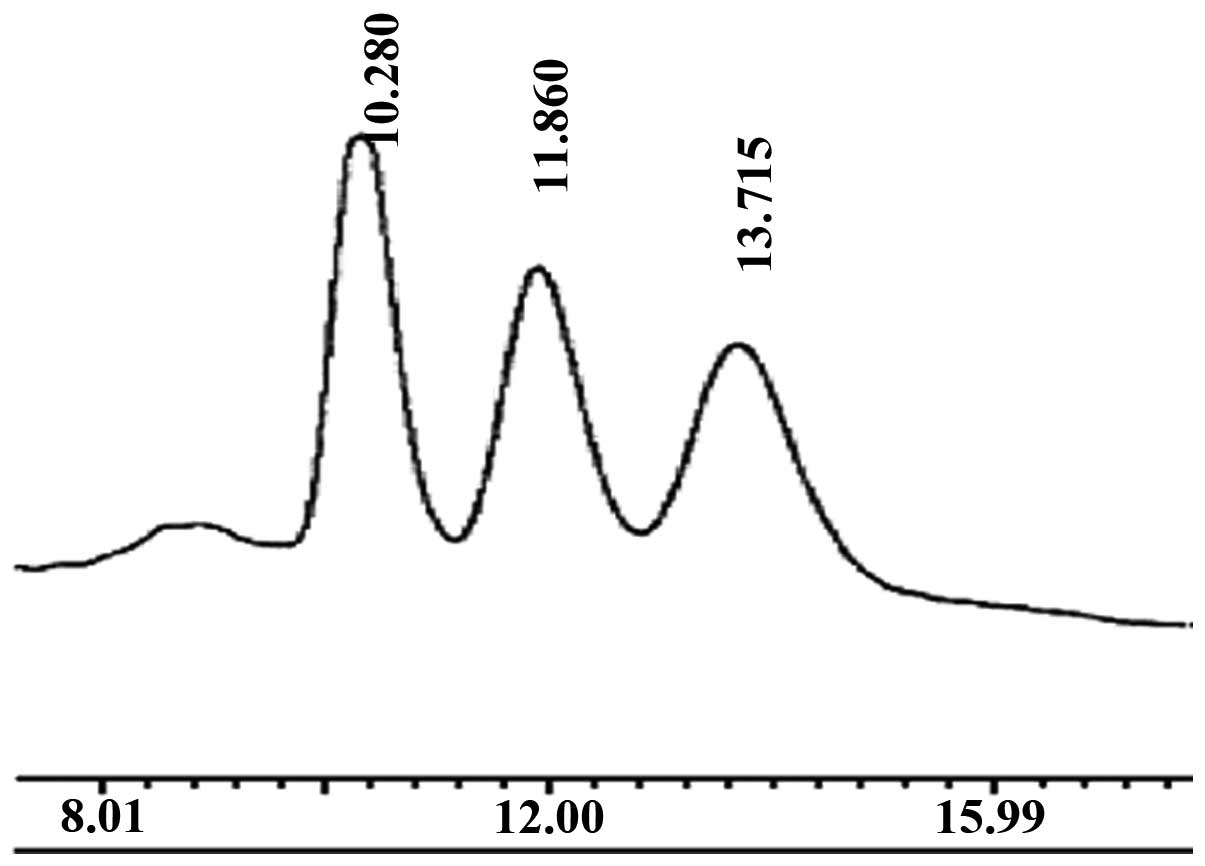
determined by HPLC (Fig. 1). The
main peak corresponded to that of standard chitohexaose (Fig. 2), which indicated that the main
component of the prepared oligochitosan sample was
chitohexaose.
The effects of oligochitosan on RAW264.7
cell viability
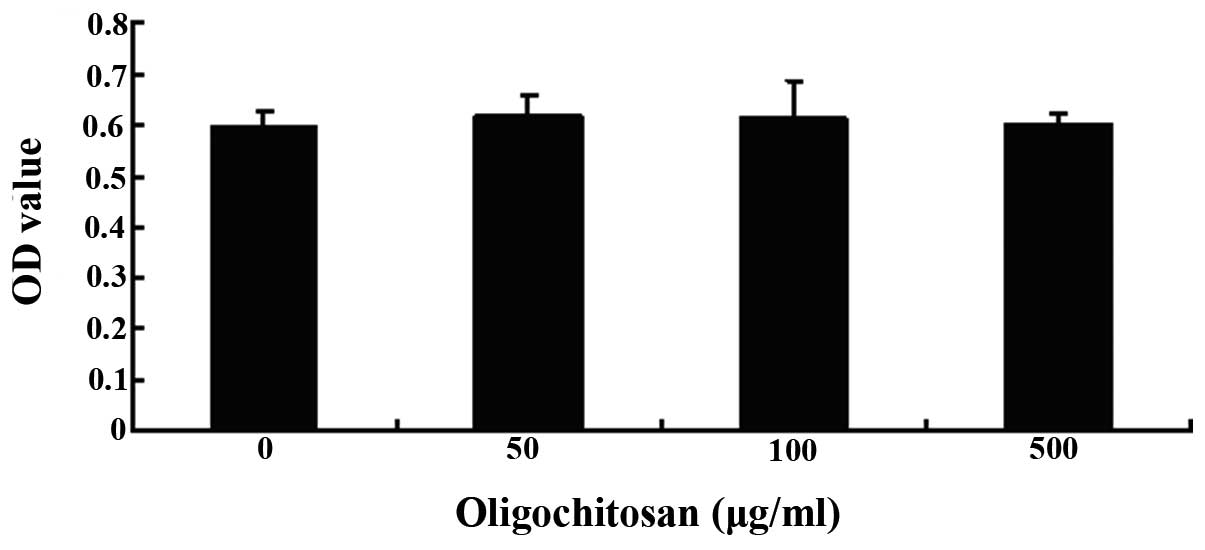
A MTT assay was used to determine the viability of
the RAW264.7 cells, following treatment with different
concentrations of oligochitosan (0, 50, 100 and 500 μg/ml). No
cellular toxicity was observed in the cells treated with 50–500
μg/ml oligochitosan, for 24 h (Fig.
3). These results suggest that nonspecific cytotoxicity could
be excluded as a factor from the results of the present study.
Oligochitosan has inhibitory effects on
LPS-induced proinflammatory cytokine production in RAW264.7
cells
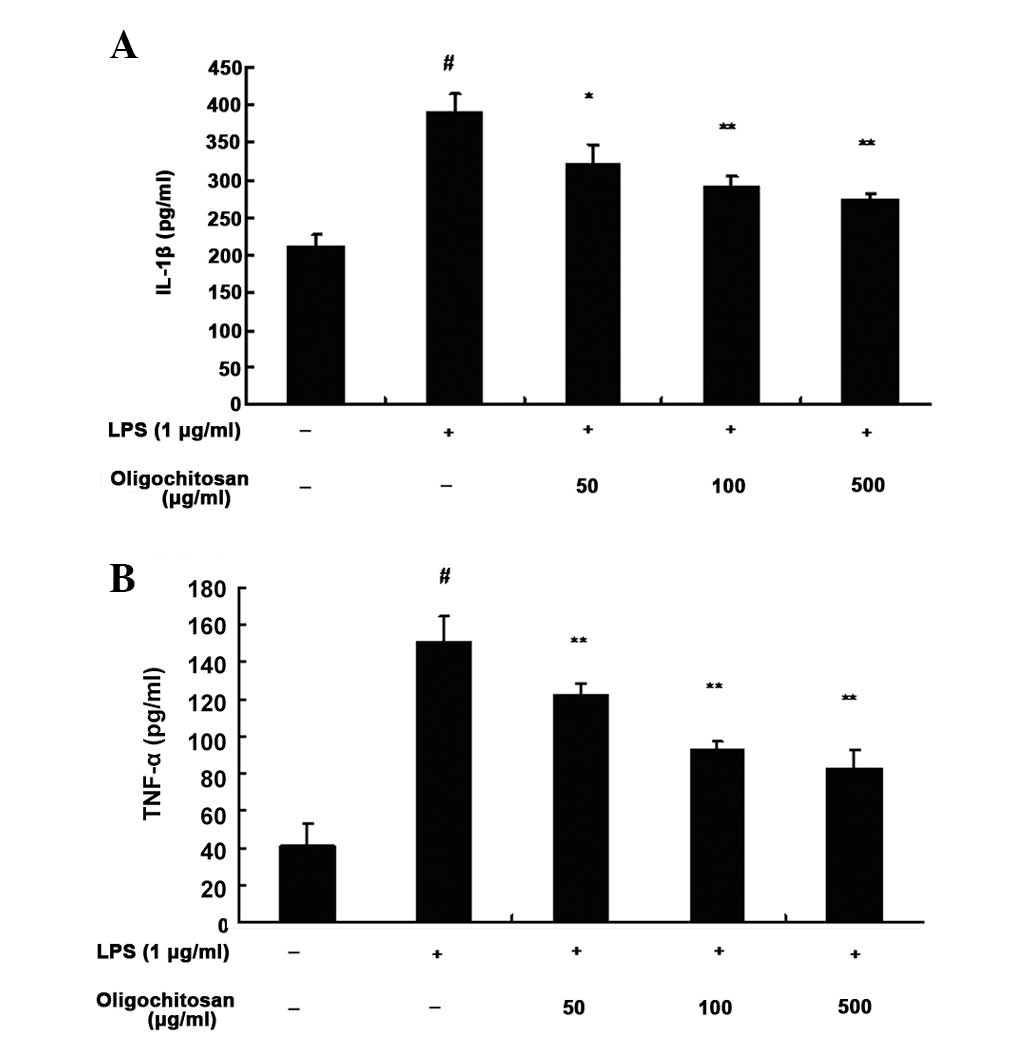
An ELISA was used to determine the inhibitory
effects of oligochitosan on LPS-induced production of TNF-α and
IL-1β in RAW264.7 cells. Following a 24 h incubation with medium
alone, the expression levels of TNF-α (n=3, P<0.01, Fig. 4B) and IL-1β (n=3, P <0.01,
Fig. 4A) were low. However, the
expression levels of TNF-α (n=3; 50, 100 and 500 μg/ml : all
P<0.01; Fig. 4B) and IL-1β
(n=3; 50, 100 and 500 μg/ml: P<0.05, P<0.01 and P<0.01,
respectively; Fig. 4A) in the
supernatant of LPS-stimulated RAW264.7 cells, were significantly
increased, as compared with the control group. Different
concentrations of oligochitosan (50, 100 and 500 μg/ml)
significantly lowered the expression levels of TNF-α and IL-1β, as
compared with the LPS group (Fig.
4).
Oligochitosan has inhibitory effects on
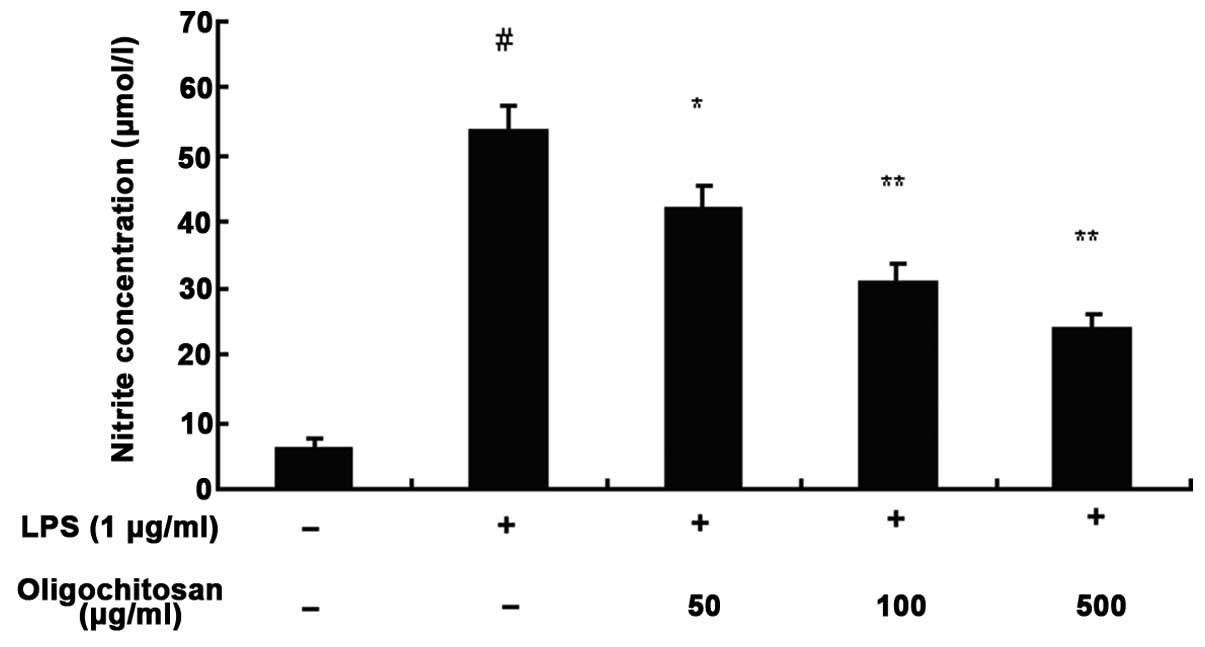
LPS-induced NO production in RAW264.7 cells
The concentration of nitrite in the culture medium
was measured using the Griess method, to assess endogenous
synthesis of NO upon LPS-stimulation of the RAW264.7 macrophages.
Following a 24 h incubation with medium alone, the production of NO
was low; however, the production of NO in the culture medium of
LPS-stimulated RAW264.7 cells was significantly increased, as
compared with the control group (n=3, P <0.01, Fig. 5). Different concentrations of
oligochitosan (50, 100 and 500 μg/ml) significantly reduced the
production of NO, as compared with the LPS group (n=3; 50, 100 and
500 μg/ml: P <0.05, P<0.01 and P<0.01, respectively)
(Fig. 5).
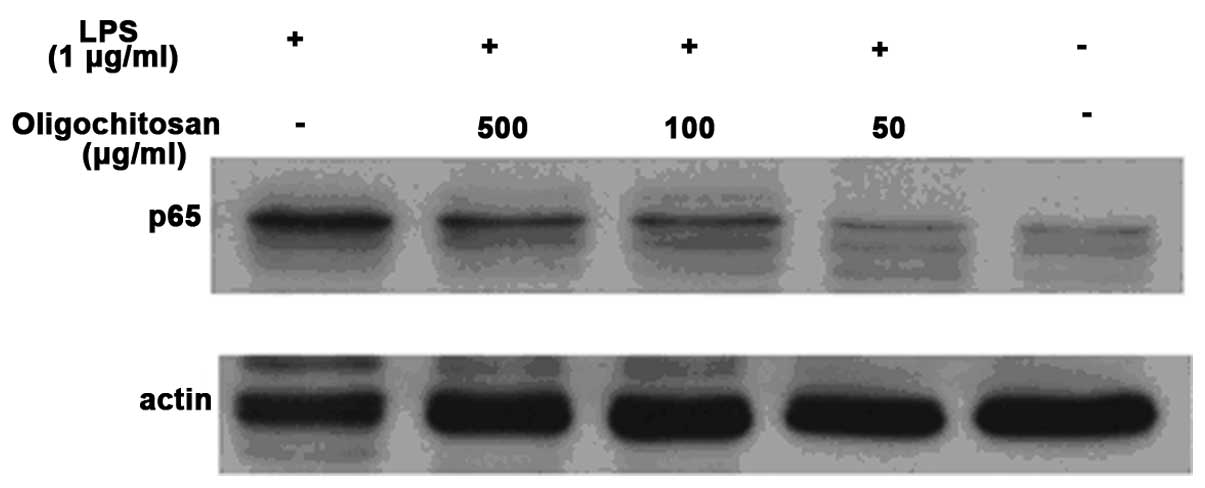
The effects of oligochitosan on NF-κB
activation in LPS-stimulated RAW264.7 cells
The NF-κB signaling pathway is involved in the
production of TNF-α, IL-1β and NO in LPS-stimulated macrophages. To
determine the mechanism of the anti-inflammatory effects of
oligochitosan in macrophages, the effects of oligochitosan on the
activation of NF-κB activation in LPS-stimulated RAW264.7 cells was
examined (Fig. 6). Western blot
analysis showed that the protein expression levels of NF-κB (p65)
in the cells cultured in the absence of LPS, were very low.
However, the protein expression levels of NF-κB (p65) in the
culture medium of LPS-stimulated RAW264.7 cells were significantly
increased, as compared with the control group. Different
concentrations of oligochitosan (50, 100 and 500 μg/mL)
significantly downregulated the expression levels of NF-κB (p65),
as compared with the LPS group.
Discussion
High molecular weight chitosan (300 kDa) with a high
degree of N-acetylation (>30%) may activate macrophages, inhibit
bacterial growth, induce apoptosis of tumor cells and promote wound
healing (12,13). Chitosan has been used in various
pharmaceutical products, however, its poor solubility in neutral
aqueous solutions has restricted its technological application
(12,13). In the present study, to facilitate
its utilization, oligochitosan, with a lower molecular weight (1
kDa) and degree of N-acetylation (<15%), was prepared by
enzymatic hydrolysis. The biochemical composition was analyzed
using HPLC and the results showed that the main component of the
oligochitosan was chitohexaose, which has good water solubility and
low viscosity. In the present study, oligochitosan was shown to be
capable of regulating the function of LPS-stimulated RAW264.7
cells, which were used as an in vitro macrophage model
system. The results of the present study suggest that oligochitosan
may inhibit the production of active molecules, including NO, IL-1β
and TNF-α in LPS-stimulated RAW264.7 cells through the NF-κB
pathway. This may provide an explanation in regards to the
mechanism of the anti-inflammatory effects of oligochitosan.
Macrophages have an important role in the
initiation, maintenance and resolution of inflammation. Activated
macrophages can directly counteract harmful pathogenic stimuli. In
response to LPS, macrophages secrete numerous pro-inflammatory
cytokines, including IL-1β and TNF-α, in order to mediate the
inflammatory response (14).
Overproduction of these pro-inflammatory mediators results in
excessive inflammation (15);
therefore, inhibition of the release of pro-inflammatory mediators
may be beneficial in attenuating the inflammatory response. During
the process of inflammation and infection, activated macrophages
are attracted to the site of inflammation and markedly increase the
production of NO around the wounded tissue (16). NO is a highly reactive oxidant that
is associated with numerous biological processes, including the
regulation of inflammation, and is thought to be a major
destructive factor in the wound healing process (17). Therefore, inhibition of the release
of NO may be beneficial in attenuation of the inflammatory
response.
NF-κB is a major transcription factor that widely
regulates the expression of genes responsible for both a series of
immune responses and inflammatory reactions. It has been proven
that NF-κB has an important role in the inflammatory response
(18). In the present study,
western blot analysis showed that oligochitosan significantly
downregulated the protein expression levels of NF-κB in
LPS-stimulated RAW264.7 macrophages. These results suggest that
oligochitosan may have a role in suppressing the NF-κB signaling
pathway in the inflammatory response-mediated production of NO,
IL-1β and TNF-α.
In conclusion, the present study demonstrated that
oligochitosan significantly inhibited the overproduction of NO,
IL-1β and TNF-α. in LPS-stimulated RAW264.7 macrophages. This
inhibition by oligochitosan may be mediated through the
downregulation of NF-κB. Due to its low toxicity and cost,
oligochitosan may be a good macrophage targeting, anti-inflammatory
drug.
Acknowledgements
The present study was supported in part by a grant
from the Zhejiang Medicine & Health Research Fund (no.
2013KYB014).
References
|
1
|
Anogeianaki A, Angelucci D, Cianchetti E,
et al: Atherosclerosis: a classic inflammatory disease. Int J
Immunopathol Pharmacol. 24:817–825. 2011.PubMed/NCBI
|
|
2
|
Mahmoudi M, Curzen N and Gallagher PJ:
Atherogenesis: the role of inflammation and infection.
Histopathology. 50:535–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani A: Cancer: Inflaming metastasis.
Nature. 457:36–37. 2009. View
Article : Google Scholar
|
|
4
|
Duffield JS: The inflammatory macrophage:
a story of Jekyll and Hyde. Clin Sci (Lond). 104:27–38. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jakobsson PJ: Pain: how macrophages
mediate inflammatory pain via ATP signaling. Nat Rev Rheumatol.
6:679–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar
|
|
7
|
Yu Zhijun, Zhao Luhang and Ke Haiping:
potential role of nuclear factor-kappaB in the induction of nitric
oxide and tumor necrosis factor-alpha by oligochitosan in
macrophages. International immunopharmacology. 4:193–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura K, Nishmura K, Nishi N, et al:
Immunological activity of chitin and its derivatives. Vaccine.
2:93–99. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokoro A, Kobayashi M, Tatewaki N, et al:
Protective effect of N-acetyl-chitohexaose on Listeria
monocytogenes infection in mice. Microbiol Immunol. 33:357–367.
1989. View Article : Google Scholar
|
|
10
|
Suzuki K, Mikami T, Okawa Y, et al:
Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose.
Carbohydr Res. 151:403–408. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong HJ, Koo HN, Oh EY, et al: Nitric
oxide production by high molecular weight water-soluble chitosan
via nuclear factor-kappaB activation. Int J Immunopharmacol.
22:923–933. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arthur Felse P and Panda T: Studies on
applications of chitin and its derivatives. Bioprocess Biosyst Eng.
20:505–512. 1999.
|
|
13
|
Synowiecki J and Al-Khateeb NA:
Production, properties, and some new applications of chitin and its
derivatives. Crit Rev Food Sci Nutr. 43:145–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immuno. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeGeorge GL, Heck DE and Laskin JD:
Arginine metabolism in keratinocytes and macrophages during nitric
oxide biosynthesis: multiple modes of action of nitric oxide
synthase inhibitors. Biochem Pharmacol. 54:103–112. 1997.
View Article : Google Scholar
|
|
17
|
Rubbo H, Tarpey M and Freeman BA: Nitric
oxide and reactive oxygen species in vascular injury. Biochem Soc
Symp. 61:33–45. 1995.PubMed/NCBI
|
|
18
|
He Xiaojuan, Shu Jun, Xu Li, et al:
inhibitoy effect of Astragalus polysaccharides on
lipopolysaccharide-induced TNF-α and IL-1β production in THP-1
cells. Molecules. 17:3155–3164. 2012.
|