Introduction
The major function of mitochondria in cells is the
production of cellular energy, ATP, by the mitochondrial
respiratory chain. In addition to energy production, the
mitochondria is also a crucial site of reactive oxygen species
(ROS) formation. The accumulation of ROS has an important role in
the development of neurological disorders, including epilepsy
(1,2). The mitochondrial electron transport
chain complex enzymes are major sites of ROS generation (3). Our previous studies demonstrated that
mitochondrial electron transport chain complex I function was
exacerbated following status epilepticus (SE; is a state of
persistent seizures, which can result in neuronal damage in the
brain), which was attenuated by peroxisome proliferator-activated
receptor coactivator 1-α (PGC-1α) signaling pathway (4). PGC-1α has been characterized as a
major regulator of glucose homeostasis, lipid catabolism,
mitochondrial biogenesis and mitochondrial functions (5). PGC-1α induces the increase of
mitochondrial numbers and intracellular ATP concentration in a
variety of cells (6). PGC-1α also
suppresses ROS production in cells through the induction of ROS
detoxifying enzymes. It has been demonstrated that decreased PGC-1α
expression increases oxidative stress and neurodegeneration
(7). It has also been reported
that PGC-1α is required in neurons for the induction of
mitochondria related ROS-detoxifying proteins, uncoupling protein 2
(UCP2) and superoxide dismutase 2 (SOD2) (8–10).
In a previous study, it was demonstrated that the PGC-1α signaling
pathway was associated with the regulation of the mitochondrial
antioxidant system, which could alleviate the oxidative stress
damages induced by SE (4).
SIRT1 is one of the mammalian sirtuins, a group of
NAD+-dependent deacetylases, which regulate a wide
variety of cellular processes, including metabolism, development,
cell survival and aging (11). In
neurons, SIRT1 prevents mitochondria loss and modulates DNA damage
responses (11). The activators of
SIRT1 may increase mitochondrial function and energy expenditure
(12). More importantly, SIRT1 has
been demonstrated to interact directly with PGC-1 to increase
PGC-1α expression and mitochondria biogenesis (13).
The exact roles of SIRT1 on neuron mitochondria
functions, particularly the mitochondria antioxidant defense system
following SE, remain to be determined. The present study examined
the SIRT1 expression in rat hippocampi following SE. In addition,
the regulation of SIRT1 on PGC-1α/mitochondria antioxidant proteins
in a rodent epileptic model was investigated.
Materials and methods
Animals and treatment
Adult male Wistar rats (Experimental Animal Center
of Shandong University, Shandong, China) weighing 250–300 g were
used. The experimental procedure conformed to the local guidelines
of the animal care and use committees of Shandong University
(Shandong, China), which were in accordance with the international
standards (NIH Publication no.80-23). All efforts were made to
minimize animal suffering. The rats were administered 1 mg/kg
scopolamine (subcutaneously; Sigma, St. Louis, MO, USA) prior to
300 mg/kg pilocarpine (intraperitoneally; Sigma) to prevent
peripheral cholinergic effects. Following pilocarpine treatment,
the seizure behavior was observed for up to 90 min. The seizure
behavior was graded according to a modified Racine scale (14). Only the rats with sustained
generalized motor seizures (stage 4 or 5) were included in the
study. The seizures were terminated by an intraperitoneal injection
of 10 mg/kg diazepam (Sigma) after 60 min. The SIRT1 activator,
resveratrol (40 mg/kg intraperitoneally; Sigma) or SIRT1 inhibitor,
sirtinol (20 μg/kg intracerebroventricully; Sigma); was
administered 30 min prior to intraperitoneal injection of
pilocarpine.
To examine the SIRT1 expression, mitochondrial
electron transport chain complex I activity and ATP content, the
rats were divided into five groups: The control and the 3, 8, 24
and 72 h following SE groups. To investigate the regulation of
SIRT1 on the mitochondria antioxidant system and the histological
changes in the hippocampi, the rats were divided into four groups:
Control, SE, SE + resveratrol and SE + sirtinol groups. The rats
were sacrificed by decapitation at 8 or 24 h following SE. Then
hippocampi were quickly removed on an ice-cooled board and stored
at −80°C. The rats in the control group were only injected with
equal volumes of dissolvent. Each experimental group consisted of
eight rats.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
RNA isolation from the snap-frozen whole hippocampi
involved use of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RNA
was reverse transcribed by use of M-MLV reverse transcriptase
(Fermentas, Glen Burnie, MD, USA). The reaction was conducted in
the Light Cycler PCR system (Roche Diagnostics, Mannheim, Germany).
The primers were used as follows: Forward:
5′-TCACCACCGAAATCCTTA-3′; and reverse: 5′-GGTGTCTGTAGTGGCTTGAT-3′
for PGC-1α; forward: 5′-ACCGAGGAGAAGTACCACGA-3′ and reverse:
5′-TAGGGCTCAGGTTTGTCCAG-3′ for SOD2; forward:
5′-AATGACCTGTTCTTTGAGGCTGAC-3′ and reverse:
5′-GCTTCGACAGTGCTCTGGTA-3′ for UCP2; and forward:
5′-TGCTGGTGCTGAGTATGTCGTG-3′ and reverse:
5′-CGGAGATGATGACCCTTTTGG-3′ for GAPDH. The standard curves for each
gene were generated by a serial dilution of RNA isolated from the
control rats. The values of the target genes were normalized using
the value of the housekeeping gene GAPDH.
Western blot analysis
The hippocampi tissues were homogenized in lysis
buffer and centrifuged at 12,000 × g at 4°C for 10 min. The
supernatants were collected as cell proteins. The concentration of
protein was determined using a bicinchoninic acid protein assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). A total of 40 μg
of protein were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis then transferred to nitrocellulose membranes.
Following blocking in Tris-buffered saline with Tween-20, the
membranes were incubated with a mouse monoclonal primary antibody
against SIRT1, PGC-1α, SOD2, UCP2 (1:1000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA), and β-actin (1:4000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight.
Next the membranes were washed and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:3000;
Santa Cruz Biotechnology, Inc.) for 1 h. The immunoreactivity was
enhanced by a chemiluminescence kit (Pierce Biotechnology, Inc.)
and exposed to film (Fuji, Tokyo, Japan). The band intensity was
analyzed with an image analyzer (Alpha Innotech 2200; Alpha
Innotech, San Leandro, CA, USA).
Measurement of SIRT1 activity
The SIRT1 activity was measured as described in the
SIRT1 Fluorimetric Activity assay/Drug Discovery kit (Biomol
Research Laboratories, Inc., Plymouth Meeting, PA, USA). Briefly,
an acetylated peptide fragment derived from p53, known to be
deacetylated by SIRT1, fluoresces upon deacetylation. Recombinant
SIRT1 was preincubated with potential activators or inhibitors of
the enzyme for 10 min. The acetylated p53-based substrate was then
added and the reaction was allowed to proceed for 45 min. The
reaction was quenched by the addition of nicotinamide and the
fluorescence was measured uaing Varioskan Flash instrument (Thermo
Fisher Scientific, Waltham, MA, USA).
Measurement of mitochondrial electron
transport chain complex I activity
The method was conducted as previously described
(4). Briefly, mitochondrial
protein was isolated from the whole hippocampi. The
rotenone-sensitive nicotinamide adenine dinucleotide diaphorase to
CoQ1 oxidoreductase (complex I) and citrate synthase activities
were measured spectrophotometrically (Sigma). All of the assays
were processed by a thermostatically regulated UV-visible
spectrophotometer (Thermo Fisher Scientific).
Measurement of ATP concentration
ATP was measured via an ATP Bioluminescence Assay
kit (Roche Diagnostics) according to the manufacturer’s
instructions. Briefly, hippocampi were washed and lysed on ice with
lysis buffer. The homogenates were immediately centrifuged at
14,000 × g for 5 min at 4°C. The supernatants were collected and
combined with an equal quantity of luciferase reagent, and the
samples were imaged immediately using an Alpha Innotech imaging
system (Alpha Innotech).
Histology
The rats were intracardially perfused with 4%
paraformaldehyde at 24 h following SE. The fixed brain tissue
blocks were paraffinized and sectioned coronally at 10 μm and Nissl
staining with Toluidine Blue was performed. Under a high
magnification (x400), the number of surviving pyramidal cells in
the hippocampal CA3 region was blindly counted.
Statistical analysis
The values are expressed as the mean ± standard
deviation. Statistical analysis of the results was performed by
one-way analysis of variance followed by the Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Seizure behavior of experimental
rats
Seizure behavior induced by pilocarpine followed the
typical course of seizures, with a gradual increase in intensity to
SE (stage 4 or 5). There was no difference of the percent incidence
or onset latency of seizure behavior in the resveratrol or sirtinol
pretreatment groups compared with that of the SE group.
SE activates SIRT1 in the rat
hippocampus
The protein level of SIRT1 and its activity were
examined in the rat hippocampus at different time points following
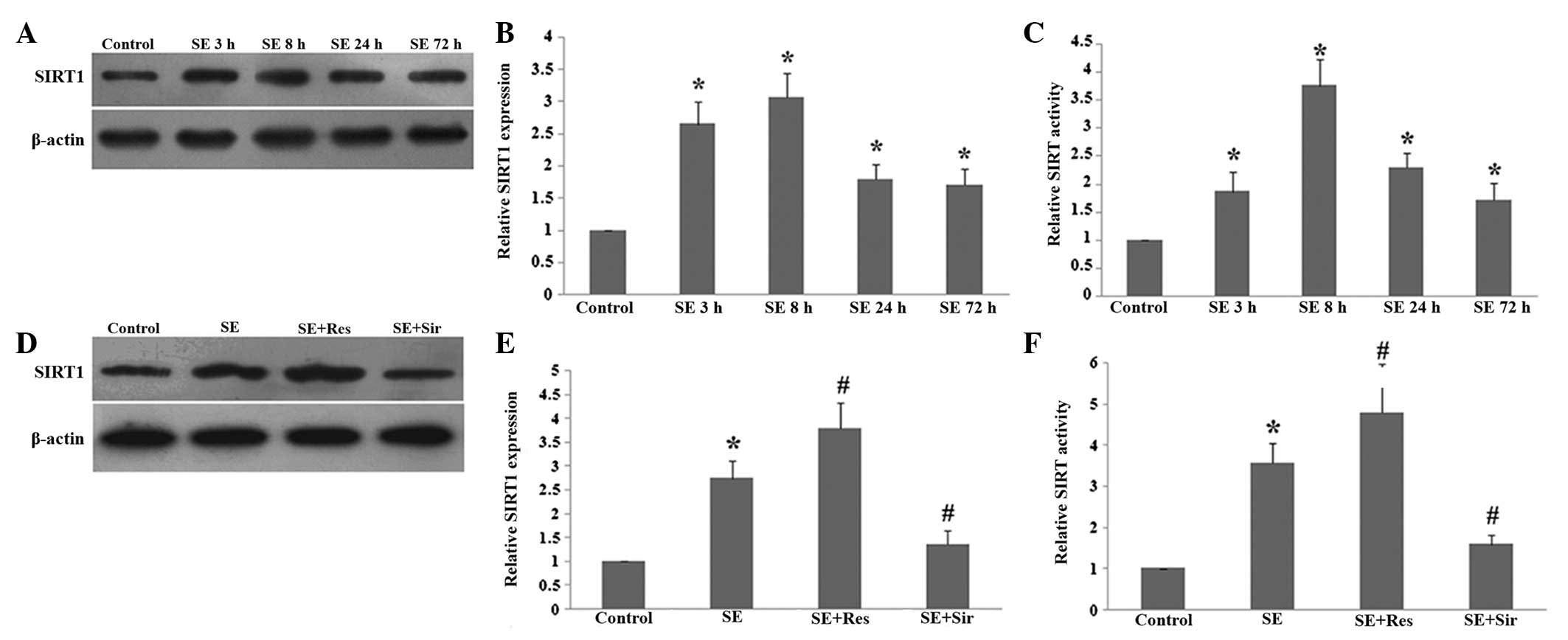
SE. As demonstrated in Fig. 1, the
protein level and activity of SIRT1 began to increase at 3 h,
reached a peak at 8 h and remained elevated 72 h following SE
(P<0.05; Fig. 1A–C).
Resveratrol significantly promoted the expression and activity of
SIRT1 at 8 h following SE (P<0.05). Additionally, sirtinol
effectively inhibited the increase in the protein level and
activity of SIRT1 in the rat hippocampus following SE (P<0.05;
Fig. 1D–F).
SIRT1 regulates PGC-1α/mitochondrial
antioxidant enzymes following SE
The present study then further examined the effects
of SIRT1 on the expression of PGC-1α, SOD2 and UCP2, which are
important mitochondrial related ROS-detoxifying enzymes. The
protein and mRNA expression of PGC-1α, SOD2 and UCP2 significantly
increased in the rat hippocampus at 8 h following
pilocarpine-induced SE induced (P<0.05). When resveratrol had
been administered, the expression of PGC-1α, SOD2 and UCP2 were
evidently enhanced (P<0.05). Consistently, sirtinol
significantly suppressed the mRNA or protein overexpression of
PGC-1α, SOD2 and UCP2 following SE (P<0.05; Fig. 2).
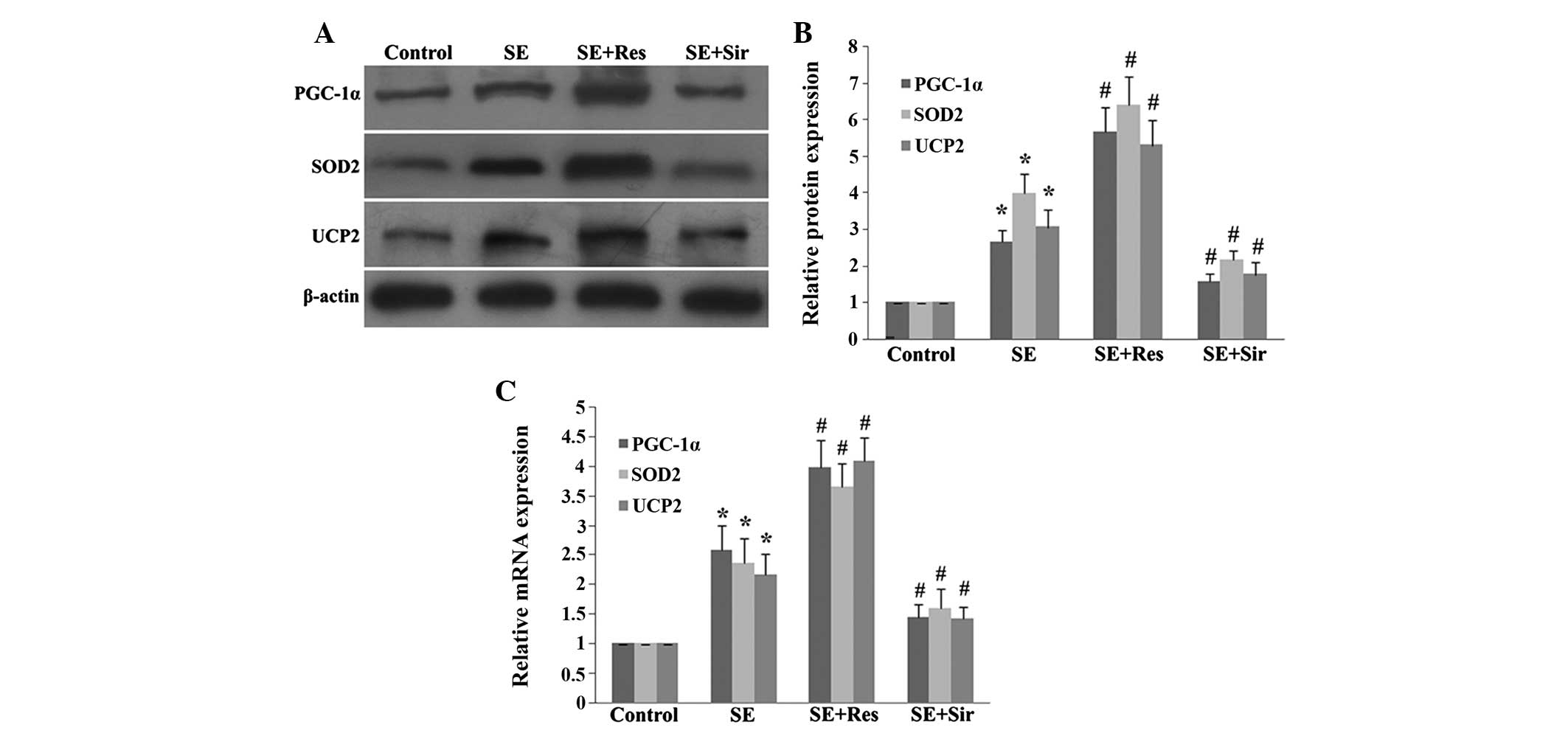 | Figure 2SIRT1 regulates levels of PGC-1α, SOD2
and UCP2 8 h following SE in the rat hippocampus. (A)
Immunoblotting analysis of resveratrol or sirtinol pretreatment on
the protein expression of PGC-1α, SOD2 and UCP2. (B) Quantitative
representation of Res or Sir pretreatment on the protein expression
of PGC-1α, SOD2 and UCP2 in the rat hippocampus. (C) Quantitative
representation of Res or Sir pretreatment on the mRNA expression of
PGC-1α, SOD2 and UCP2 in the rat hippocampus following SE. The data
are expressed as the mean ± standard deviation of six independent
animals. *P<0.05, vs. the control group;
#P<0.05, vs. the SE group. SIRT1, sirtuin 1; PGC-1α,
peroxisome proliferator-activated receptor (PPAR) coactivator-1α;
SOD2, superoxide dismutase 2; UCP2, uncoupling protein 2; SE,
status epilepticus; Res, resveratrol; Sir, sirtinol. |
SIRT1 activation enhances mitochondria
functions following SE
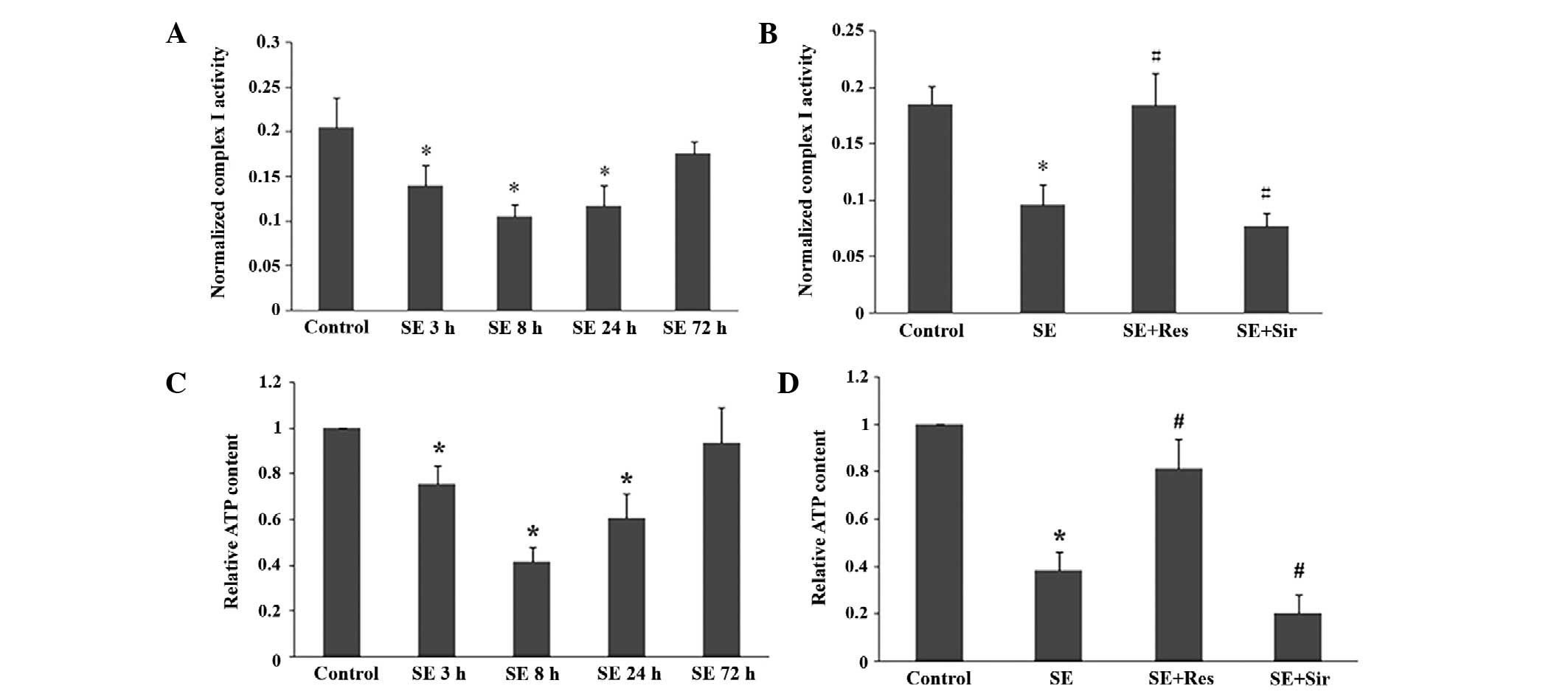
The mitochondrial electron transport chain complex I
activity and ATP content began to decline at 3 h, reached the
lowest levels at 8 h and remained decreased at 24 h in the rat
hippocampal tissues following SE (P<0.05; Fig. 3A and C). As demonstrated in
Fig. 3B and D, resveratrol
significantly attenuated the suppression of mitochondrial electron
transport chain complex I activity and ATP content induced by SE
(P<0.05). Furthermore, after sirtinol was administered, the
suppression of mitochondrial electron transport chain complex I
activity and ATP content was significantly exacerbated
(P<0.05).
SIRT1 attenuates neuron damage following
SE
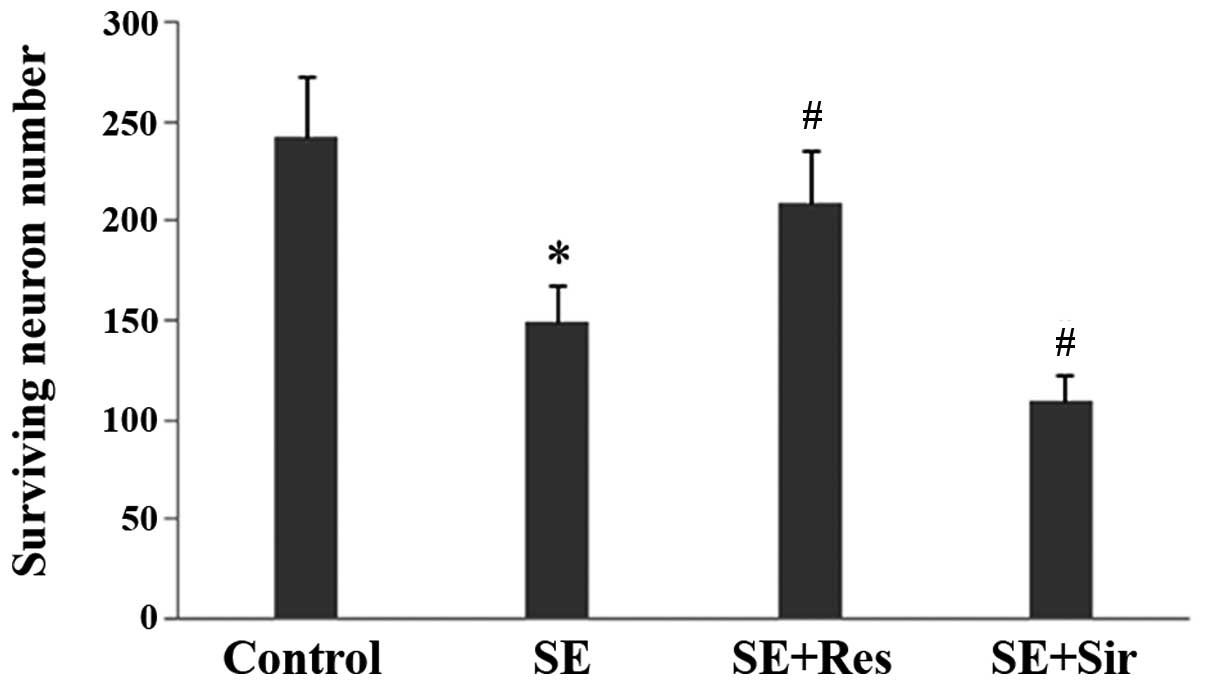
The present data demonstrated evident pyramidal
neuron damage in the CA3 region of the rat hippocampus at 24 h
following SE. The number of surviving neurons was significantly
higher in the resveratrol pretreatment group as compared with the
SE group (n=6; P<0.05; Fig. 4).
By contrast, sirtinol further aggravated the neuron damage induced
by SE.
Discussion
In the present study, it was demonstrated that SIRT1
protein expression and activity increased in the rat hippocampus at
the acute phase of pilocarpine-induced seizures. SIRT1, a class III
histone deacetylase, is an important member of the sirtuins family.
SIRT1 is known to have numerous important functions, including
stress protection and metabolism regulation. The results of the
present study suggest that SIRT1 may also be a positive regulator
of the mitochondrial antioxidant system following the development
of seizures. Previous studies demonstrated that SIRT1 activator
resveratrol had antioxidant and anti-inflammatory effects (15). Recently, we also demonstrated that
resveratrol attenuated the inflammatory responses induced by
seizures (16). Resveratrol may
reduce neurodegeneration in the rodent hippocampus by the PGC-1α
signaling pathway (17). PGC-1α is
known to be a positive regulator of mitochondrial function and
oxidative metabolism (6).
Increased PGC-1α expression accelerates the recovery of
mitochondrial and cellular functions following acute cell injury
(6,7). PGC-1α null mice are more sensitive to
the neurodegenerative effects of oxidative stress (7). PGC-1α expression is under the control
of several signaling pathways involving nitric oxide,
calcium-dependent protein kinases, calcineurin A, adenosine
monophosphate-activated protein kinase and p38 MAPK (6,18,19).
Previously, it was demonstrated that nitric oxide and AMPK regulate
the PGC-1α and mitochondrial antioxidant system in epileptic rats
(4). The present study
demonstrated that SIRT1 was also an important upstream regulator of
PGC-1α signaling in seizures induced by pilocarpine. SIRT1 is able
to deacetylate specific lysine residues on PGC-1α, mediating
gluconeogenesis and mitochondrial biogenesis (13).
PGC-1α may not only stimulate mitochondrial
biogenesis, but may also protect neurons from oxidative injury
through the induction of several ROS-detoxifying enzymes (7,8,20).
UCP2 and SOD2 are two important ROS-detoxifying mitochondrial
proteins (21). SOD2 has been
demonstrated to have protective effects against oxidative
stress-induced neuronal damage in various experimental models
(22,23). UCP2 is able to eliminate free
radicals and elevate ATP levels in neurons (24,25).
The upregulation of UCP2 decreases the release of ROS and reduces
neuronal loss in the brain tissue following ischemic or epileptic
injury (4,24,26).
Besides regulating the PGC-1α pathway, SIRT1 is reported to repress
mitochondrial UCP2 transcription by binding directly to its
promoter (27). Our previous data
demonstrated that the levels of UCP2 and SOD2 are increased
following SE in association with PGC-1α signaling (4). The present study revealed that the
expression of PGC-1α and UCP2/SOD2 were upregulated by the SIRT1
activator, resveratrol, in the rat hippocampus following seizures.
This indicates that SIRT1 may regulate UCP2 and SOD2, at least
partially, via PGC-1α signaling.
A previous study also suggested that PGC-1α may
activate components in the mitochondrial electron transport chain,
including ATP synthase and SOD2 (28). The present data revealed that the
SIRT1 activator also upregulated mitochondrial electron transport
chain complex I activity and mitochondrial ATP production in rat
hippocampi following seizures. These results indicate that SIRT1 is
possibly involved in the protection of mitochondrial energy
metabolism damage by oxidative stress induced by SE. SE may induce
oxidative stress, which leads to neuronal cell death. It was
previously demonstrated that SE caused significant neuronal cell
death and a marked increase in oxidative stress (4). In the present study, it was
identified that the neuron death in the hippocampus of rats
following SE was also alleviated by the activator of SIRT1.
In conclusion, the present study demonstrated that
SIRT1 is activated in the rat hippocampus following SE. SIRT1
activation enhanced the expression of mitochondrial antioxidant
enzymes UCP2/SOD2 and attenuated oxidative stress as revealed by
increased levels of ATP and mitochondrial electron transport chain
complex I following SE. Furthermore, SIRT1 may be a positive
regulator of the mitochondrial antioxidant system following
pilocarpine-induced SE, at least in part by regulating PGC-1α. This
may assist in understanding the role of mitochondria during
epilepsy and provide new targets for developing new antiepileptic
drugs.
Acknowledgements
The present study was supported by grants from
National Nature Science Foundation of China (grant no. 81000557)
and the Independent Innovation Foundation of Shandong University
(grant no. 2012TS145).
References
|
1
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waldbaum S and Patel M: Mitochondrial
dysfunction and oxidative stress: a contributing link to acquired
epilepsy? J Bioenerg Biomembr. 42:449–455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dröse S and Brandt U: Molecular mechanisms
of superoxide production by the mitochondrial respiratory chain.
Adv Exp Med Biol. 748:145–169. 2012.
|
|
4
|
Han YX, Lin YT, Xu JJ, et al: Status
epilepticus stimulates peroxisome proliferator-activated receptor γ
coactivator 1-α/mitochondrial antioxidant system pathway by a
nitric oxide-dependent mechanism. Neuroscience. 186:128–134.
2011.PubMed/NCBI
|
|
5
|
Wu Z and Boss O: Targeting PGC-1 alpha to
control energy homeostasis. Expert Opin Ther Targets. 11:1329–1338.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasbach KA and Schnellmann RG: PGC-1alpha
over-expression promotes recovery from mitochondrial dysfunction
and cell injury. Biochem Biophys Res Commun. 355:734–739. 2007.
View Article : Google Scholar
|
|
7
|
St-Pierre J, Drori S, Uldry M, Silvaggi JM
and Rhee J: Suppression of reactive oxygen species and
neurodegeneration by the PGC-1 transcriptional coactivators. Cell.
127:397–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valle I, Alvarez-Barrientos A, Arza E,
Lamas S and Monsalve M: PGC-1αlpha regulates the mitochondrial
antioxidant defense system in vascular endothelial cells.
Cardiovasc Res. 66:562–573. 2005.
|
|
9
|
Csiszar A, Labinskyy N, Pinto JT, et al:
Resveratrol induces mitochondrial biogenesis in endothelial cells.
Am J Physiol Heart Circ Physiol. 297:H13–H20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun AY, Wang Q, Simonyi A and Sun GY:
Resveratrol as a therapeutic agent for neurodegenerative diseases.
Mol Neurobiol. 41:375–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houtkooper RH, Pirinen E and Auwerx J:
Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol
Cell Biol. 13:225–238. 2012.PubMed/NCBI
|
|
12
|
Della-Morte D, Dave KR, DeFazio RA, et al:
Resveratrol pretreatment protects rat brain from cerebral ischemic
damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience.
159:993–1002. 2009. View Article : Google Scholar
|
|
13
|
Nemoto S, Fergusson MM and Finkel T: SIRT1
functionally interacts with the metabolic regulator and
transcriptional coactivator PGC-1{alpha}. J Biol Chem.
280:16456–16460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Udenigwe CC, Ramprasath VR, Aluko RE and
Jones PJ: Potential of resveratrol in anticancer and
anti-inflammatory therapy. Nutr Rev. 66:445–454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SJ, Bo QY, Zhao XH, et al:
Resveratrol pre-treatment reduces early inflammatory responses
induced by status epilepticus via mTOR signaling. Brain Res.
1492:122–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Nguyen MD, Dobbin MM, et al: SIRT1
deacetylase protects against neurodegeneration in models for
Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J.
26:3169–3179. 2007.
|
|
18
|
Cantó C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009.PubMed/NCBI
|
|
19
|
Rowe GC, Jiang A and Arany Z: PGC-1
coactivators in cardiac development and disease. Circ Res.
107:825–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen SD, Lin TK, Yang DI, et al:
Protective effects of peroxisome proliferator-activated receptors
gamma coactivator-1alpha against neuronal cell death in the
hippocampal CA1 subfield after transient global ischemia. J
Neurosci Res. 88:605–613. 2010.
|
|
21
|
Rubiolo JA, Mithieux G and Vega FV:
Resveratrol protects primary rat hepatocytes against oxidative
stress damage: activation of the Nrf2 transcription factor and
augmented activities of antioxidant enzymes. Eur J Pharmacol.
591:66–72. 2008. View Article : Google Scholar
|
|
22
|
Vincent AM, Russell JW, Sullivan KA, et
al: SOD2 protects neurons from injury in cell culture and animal
models of diabetic neuropathy. Exp Neurol. 208:216–227. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukui M and Zhu BT: Mitochondrial
superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical
role in protection against glutamate-induced oxidative stress and
cell death in HT22 neuronal cells. Free Radic Biol Med. 48:821–830.
2010. View Article : Google Scholar
|
|
24
|
Andrews ZB, Horvath B, Barnstable CJ, et
al: Uncoupling protein-2 is critical for nigral dopamine cell
survival in a mouse model of Parkinson’s disease. J Neurosci.
25:184–191. 2005.PubMed/NCBI
|
|
25
|
Ho PW, Ho JW, Liu HF, et al: Mitochondrial
neuronal uncoupling proteins: a target for potential
disease-modification in Parkinson’s disease. Transl Neurodegener.
1:32012.PubMed/NCBI
|
|
26
|
Mehta SL and Li PA: Neuroprotective role
of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb
Blood Flow Metab. 29:1069–1078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bordone L, Motta MC, Picard F, et al:
Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic
beta cells. PLoS Biol. 4:e312006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong X, Wang R, Xue Y, et al: Sirtuin 3, a
new target of PGC-1alpha, plays an important role in the
suppression of ROS and mitochondrial biogenesis. PLoS One.
5:e117072010. View Article : Google Scholar : PubMed/NCBI
|